Submitted:
28 November 2023
Posted:
29 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
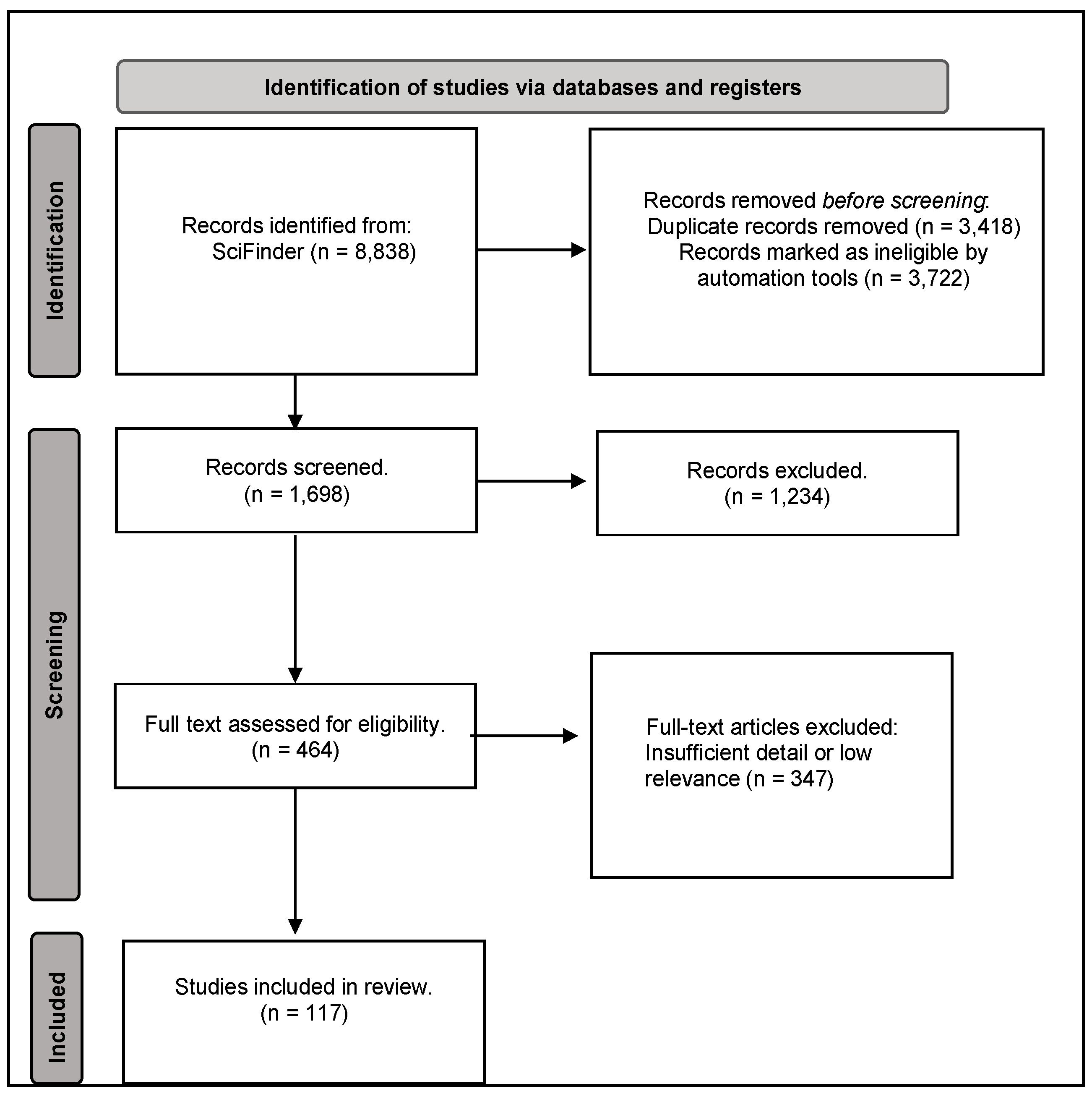
2. Included Studies
3. Plant Families
4. Plant Genera
Piper
Hypericum
Artemisia
Garcinia
Thalictrum
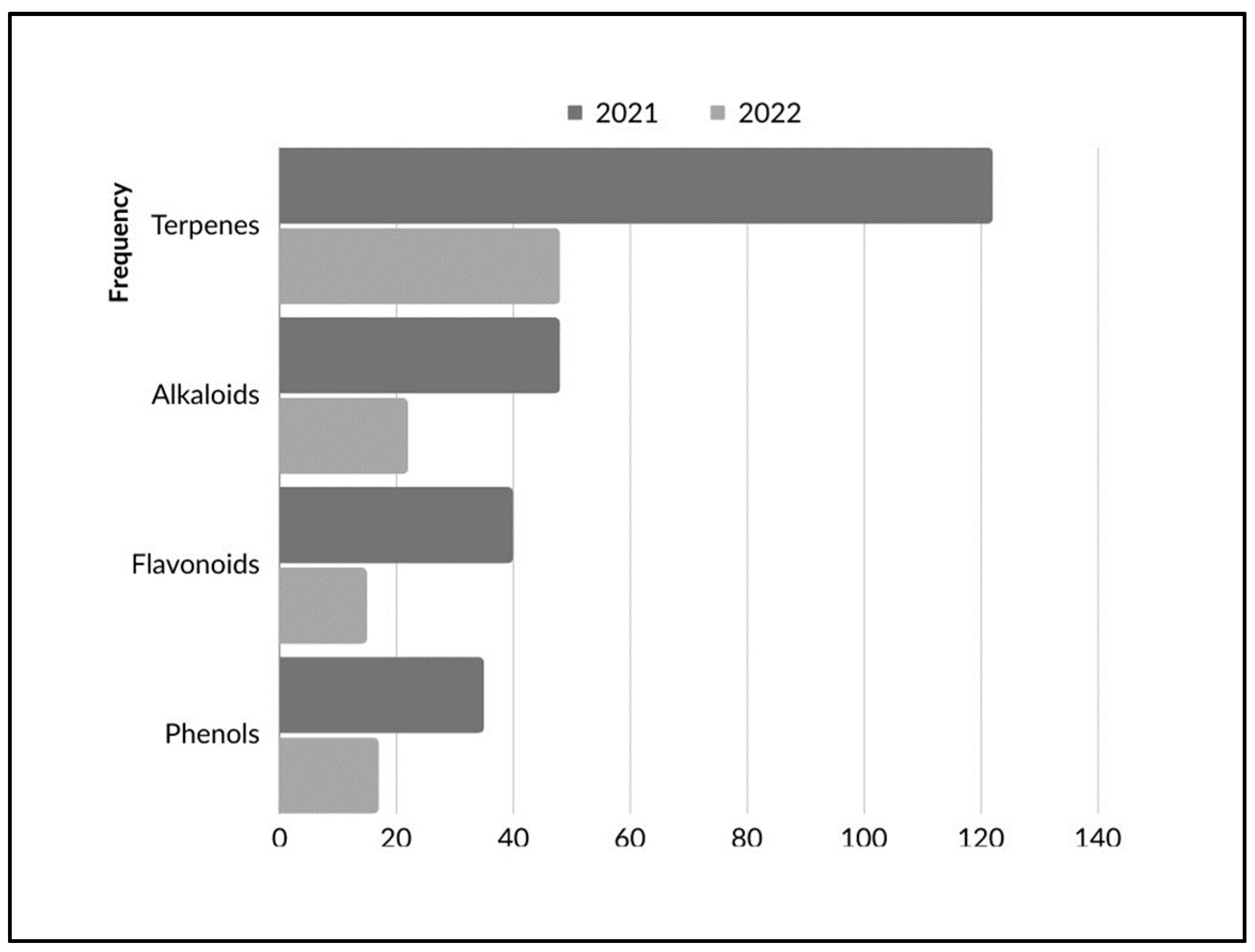
5. Compounds Classes
5.1. Terpenes
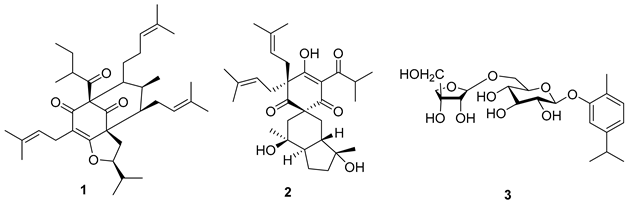
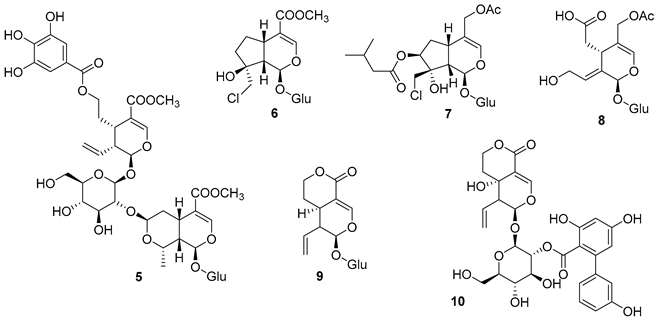
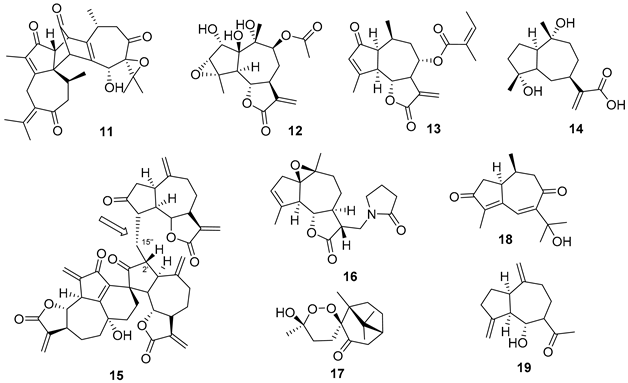
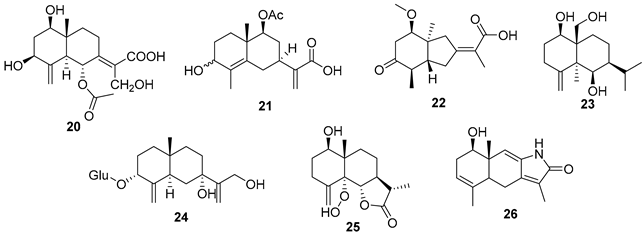
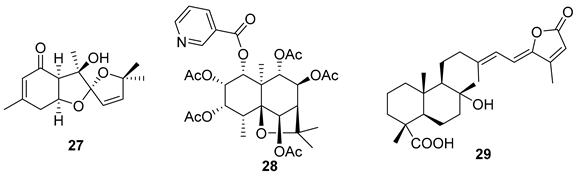
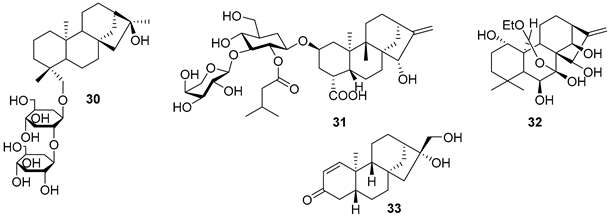
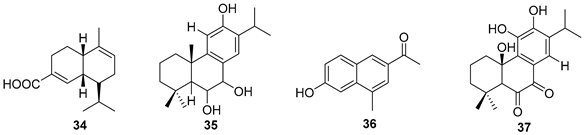
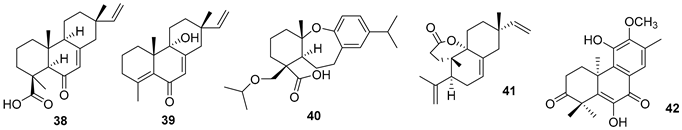
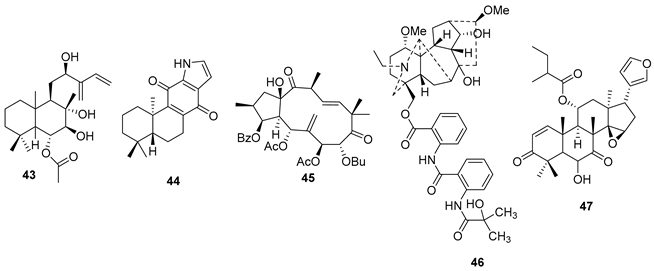
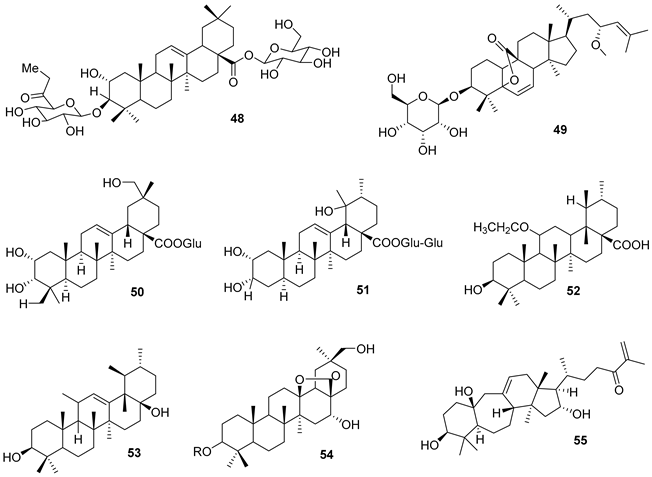
5.2. Alkaloids
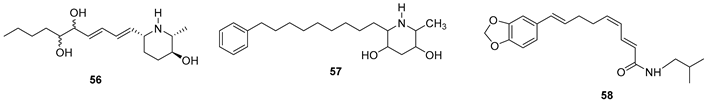
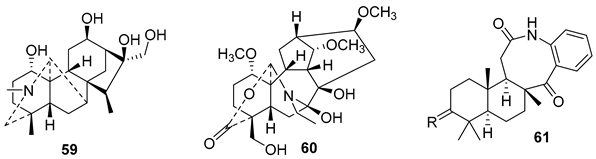
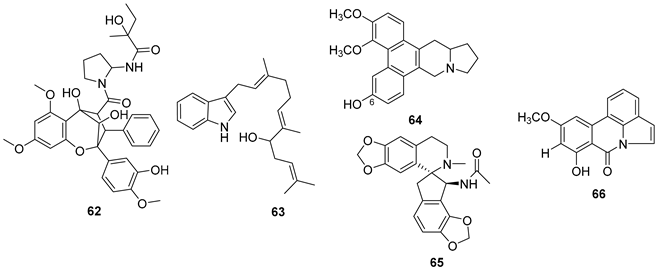
5.3. Phenols
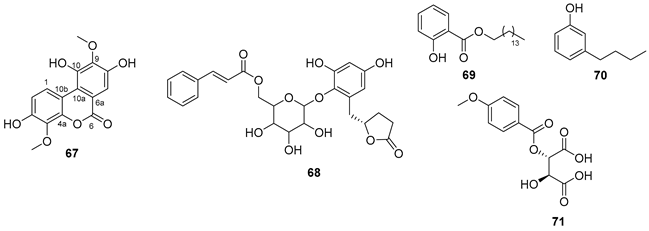
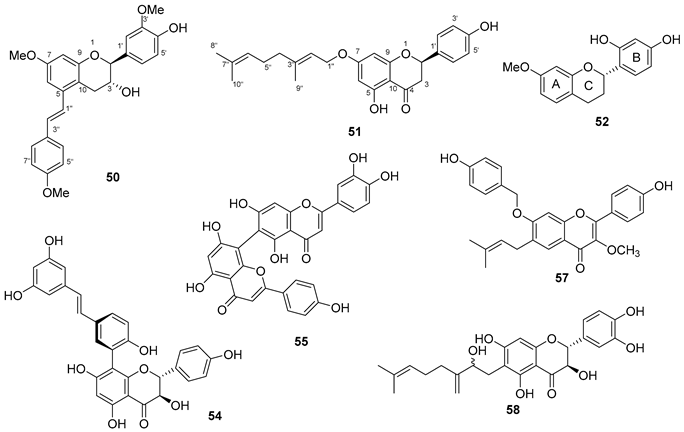
5.4. Flavonoids
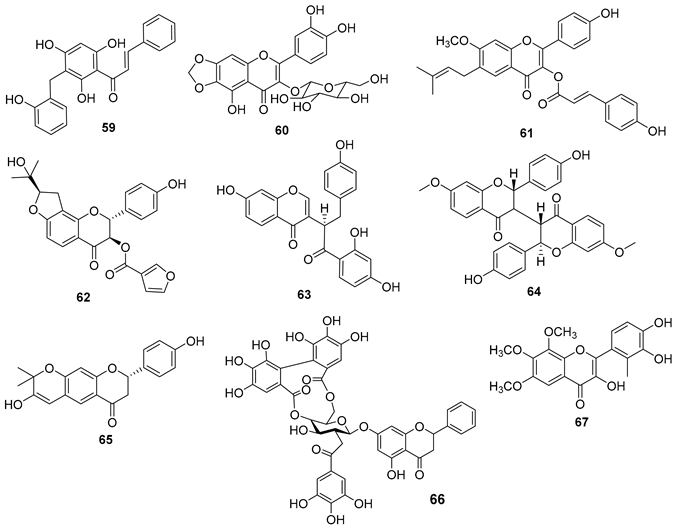
5.5. Coumarins
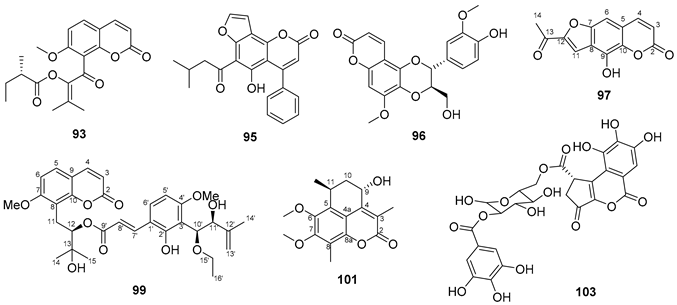
5.6. Antraquinones
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dias, D. A.; Urban, S.; Roessner, U. A Historical Overview of Natural Products in Drug Discovery. Metabolites, 2012, 2(2), 303–336. [Google Scholar] [CrossRef]
- Newman, D. J.; Cragg, G. M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod., 2020, 83(3), 770–803. [Google Scholar] [CrossRef] [PubMed]
- Pye, C. R.; Bertin, M. J.; Lokey, R. S.; Gerwick, W. H.; Linington, R. G. Retrospective Analysis of Natural Products Provides Insights for Future Discovery Trends. Proc. Natl. Acad. Sci. U. S. A., 2017, 114(22), 5601–5606. [Google Scholar] [CrossRef]
- Patridge, E.; Gareiss, P.; Kinch, M. S.; Hoyer, D. An Analysis of FDA-Approved Drugs: Natural Products and Their Derivatives. Drug Discov. Today, 2016, 21(2), 204–207. [Google Scholar] [CrossRef] [PubMed]
- Scannell, J. W.; Blanckley, A.; Boldon, H.; Warrington, B. Diagnosing the Decline in Pharmaceutical R&D Efficiency. Nat. Rev. Drug Discov., 2012, 11(3), 191–200. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A. L.; Edrada-Ebel, R.; Quinn, R. J. The Re-Emergence of Natural Products for Drug Discovery in the Genomics Era. Nat. Rev. Drug Discov., 2015, 14(2), 111–129. [Google Scholar] [CrossRef]
- Demarque, D. P.; Dusi, R. G.; de Sousa, F. D. M.; Grossi, S. M.; Silvério, M. R. S.; Lopes, N. P.; Espindola, L. S. Mass Spectrometry-Based Metabolomics Approach in the Isolation of Bioactive Natural Products. Sci. Rep., 2020, 10(1), 1–9. [Google Scholar] [CrossRef]
- Page, M. J.; Moher, D.; Bossuyt, P. M.; Boutron, I.; Hoffmann, T. C.; Mulrow, C. D.; Shamseer, L.; Tetzlaff, J. M.; Akl, E. A.; Brennan, S. E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ, 2021, 372. [Google Scholar] [CrossRef]
- Christenhusz, M. J. M.; Byng, J. W. Phytotaxa. Phytotaxa, 2016, 261(3), 201–217. [Google Scholar] [CrossRef]
- de Souza Oliveira, F. A.; Passarini, G. M.; de Medeiros, D. S. S.; Santos, A. P. de A.; Fialho, S. N.; Gouveia, A. de J.; Latorre, M.; Freitag, E. M.; de Medeiros, P. S. de M.; Teles, C. B. G.; et al. Antiplasmodial and Antileishmanial Activities of Compounds from Piper Tuberculatum Jacq Fruits. Rev. Soc. Bras. Med. Trop., 2018, 51(3), 382–386. [Google Scholar] [CrossRef]
- Oliveira, E. R.; Menini Neto, L. Levantamento Etnobotânico de Plantas Medicinais Utilizadas Pelos Moradores Do Povoado de Manejo, Lima Duarte - MG. Rev. Bras. Plantas Med., 2012, 14(2), 311–320. [Google Scholar] [CrossRef]
- Sunila, E. S.; Kuttan, G. Piper Longum Inhibits VEGF and Proinflammatory Cytokines and Tumor-Induced Angiogenesis in C57BL/6 Mice. Int. Immunopharmacol., 2006, 6(5), 733–741. [Google Scholar] [CrossRef] [PubMed]
- Wirotesangthong, M.; Inagaki, N.; Tanaka, H.; Thanakijcharoenpath, W.; Nagai, H. Inhibitory Effects of Piper Betle on Production of Allergic Mediators by Bone Marrow-Derived Mast Cells and Lung Epithelial Cells. Int. Immunopharmacol., 2008, 8(3), 453–457. [Google Scholar] [CrossRef] [PubMed]
- Santos, T. G.; Rebelo, R. A.; Dalmarco, E. M.; Guedes, A.; De Gasper, A. L.; Cruz, A. B.; Schmit, A. P.; Cruz, R. C. B.; Steindel, M.; Nunes, R. K. Composição Química e Avaliação Da Atividade Antimicrobiana Do Óleo Essencial Das Folhas de Piper Malacophyllum (C. Presl.) C. DC. Quim. Nova, 2012, 35(3), 477–481. [Google Scholar] [CrossRef]
- Atiya, A.; Salim, M. A.; Sinha, B. N.; Ranjan Lal, U. Two New Anticancer Phenolic Derivatives from Leaves of Piper Betle Linn. Nat. Prod. Res., 2021, 35(23), 5021–5029. [Google Scholar] [CrossRef] [PubMed]
- San, T. T.; Wang, Y. H.; Hu, D. B.; Yang, J.; Zhang, D. D.; Xia, M. Y.; Yang, X. F.; Yang, Y. P. A New Sesquineolignan and Four New Neolignans Isolated from the Leaves of Piper Betle, a Traditional Medicinal Plant in Myanmar. Bioorganic Med. Chem. Lett., 2021, 31(October 2020), 127682. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C. Y.; Sun, Z. L.; Huang, J.; Li, R. S.; He, J. M.; Gibbons, S.; Ju, D. W.; Mu, Q. Neolignans from Piper Betle Have Synergistic Activity against Antibiotic-Resistant Staphylococcus Aureus. J. Org. Chem., 2021, 86(16), 11072–11085. [Google Scholar] [CrossRef] [PubMed]
- Viet Phong, N.; Thi Nguyet Anh, D.; Yeong Chae, H.; Young Yang, S.; Jeong Kwon, M.; Sun Min, B.; Ah Kim, J. Anti-Inflammatory Activity and Cytotoxicity against Ovarian Cancer Cell Lines by Amide Alkaloids and Piperic Esters Isolated from Piper Longum Fruits: In Vitro Assessments and Molecular Docking Simulation. Bioorg. Chem., 2022, 128(June), 106072. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y. K.; Su, B. J.; Wang, Y. Q.; Wang, H. S.; Liao, H. B.; Liang, D. New Tyramine- And Aporphine-Type Alkamides with NO Release Inhibitory Activities FromPiper Puberulum. J. Nat. Prod., 2021, 84(4), 1316–1325. [Google Scholar] [CrossRef]
- Zheng, Y. kun; Wang, Y. qi; Su, B. jun; Wang, H. shan; Liao, H. bing; Liang, D. New Enantiomeric Lignans and New Meroterpenoids with Nitric Oxide Release Inhibitory Activity from Piper Puberulum. Bioorg. Chem., 2022, 119(June 2021), 105522. [Google Scholar] [CrossRef]
- Nongmai, C.; Kanokmedhakul, K.; Promgool, T.; Paluka, J.; Suwanphakdee, C.; Kanokmedhakul, S. Chemical Constituents and Antibacterial Activity from the Stems and Leaves of Piper Wallichii. J. Asian Nat. Prod. Res., 2022, 24(4), 344–352. [Google Scholar] [CrossRef] [PubMed]
- Meseguer, A. S.; Aldasoro, J. J.; Sanmartín, I. Bayesian Inference of Phylogeny, Morphology and Range Evolution Reveals a Complex Evolutionary History in St. John’s Wort (Hypericum). Mol. Phylogenet. Evol., 2013, 67(2), 379–403. [Google Scholar] [CrossRef] [PubMed]
- Nürk, N. M.; Scheriau, C.; Madriñán, S. Explosive Radiation in High Andean Hypericum-Rates of Diversification among New World Lineages. Front. Genet., 2013, 4(SEP). [Google Scholar] [CrossRef] [PubMed]
- Alves, A. C. S.; Moraes, D. C.; De Freitas, G. B. L.; Almeida, D. J. Botanical, Chemical, Pharmacological and Therapeutic Aspects of Hypericum Perforatum L. Rev. Bras. Plantas Med., 2014, 16(3), 593–606. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, Y.; Xu, Q.; Shi, Z.; Xiang, M.; Li, H.; Wang, Y.; Qi, C.; Zhang, Y. (±)-Hyperpyran A: Terpenoid-Based Bicyclic Dihydropyran Enantiomers with Hypoglycemic Activity from Hypericum Perforatum (St. John’s Wort). Fitoterapia, 2022, 161(May), 105221. [Google Scholar] [CrossRef] [PubMed]
- Zong, J. F.; Zhang, M. M.; Zhou, Y. B.; Li, J.; Hou, A. J.; Lei, C. Polyprenylated Acylphloroglucinol Meroterpenoids with PTP1B Inhibition from Hypericum Forrestii. Fitoterapia, 2021, 153(April), 104959. [Google Scholar] [CrossRef] [PubMed]
- Lu, W. J.; Xu, W. J.; Zhang, M. H.; Zhang, Y. Q.; Li, Y. R.; Zhang, H.; Luo, J.; Kong, L. Y. Diverse Polycyclic Polyprenylated Acylphloroglucinol Congeners with Anti-Nonalcoholic Steatohepatitis Activity FromHypericum Forrestii. J. Nat. Prod., 2021, 84(4), 1135–1148. [Google Scholar] [CrossRef]
- Matsumoto, T.; Watanabe, T. Isolation and Structure Elucidation of Constituents of Citrus Limon, Isodon Japonicus, and Lansium Domesticum as the Cancer Prevention Agents. Genes Environ., 2020, 42(1), 1–9. [Google Scholar] [CrossRef]
- Yan, S.; Feng, H.; Sun, L.; Shi, Z.; Hu, H.; Duan, Y.; Guo, Y.; Tan, X.; Chen, G.; Qi, C.; et al. Discovery of Immunosuppressive Lupane-Type Triterpenoids from Hypericum Longistylum. Nat. Prod. Res., 2022, 36(17), 4394–4400. [Google Scholar] [CrossRef]
- Peng, X.; Tan, Q.; Zhou, H.; Xu, J.; Gu, Q. Discovery of Phloroglucinols from Hypericum Japonicum as Ferroptosis Inhibitors. Fitoterapia, 2021, 153(June), 104984. [Google Scholar] [CrossRef]
- Cao, Y.; Zang, Y.; Huang, X.; Cheng, Z. Chemical Constituents from Artemisia Rupestris and Their Neuraminidase Inhibitory Activity. Nat. Prod. Res., 2021, 35(11), 1775–1782. [Google Scholar] [CrossRef]
- Ribnicky, D.; Beom Kim, S.; Poulev, A.; Wang, Y.; Boudreau, A.; Raskin, I.; Bisson, J.; Ray, G. J.; Chen, S. N.; Richard, A.; et al. Prenylated Coumaric Acids FromArtemisia ScopariaBeneficially Modulate Adipogenesis. J. Nat. Prod., 2021, 84(4), 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, Q.; Bao, W.; Pa, B.; Xu, Y. Two New Compounds from Supercritical Fluid Extract of Artemisia Integrifolia L. Nat. Prod. Res., 2021, 35(14), 2365–2369. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Q.; Bao, W.; Pa, B. Antihyperlipidemic Effect, Identification and Isolation of the Lipophilic Components from Artemisia Integrifolia. Molecules, 2019, 24(4). [Google Scholar] [CrossRef] [PubMed]
- Su, L. H.; Ma, Y. B.; Geng, C. A.; Li, T. Z.; Huang, X. Y.; Hu, J.; Xin Zhang; Tang, S.; Shen, C.; Gao, Z.; et al. Artematrovirenins A–P, Guaiane-Type Sesquiterpenoids with Cytotoxicities against Two Hepatoma Cell Lines from Artemisia Atrovirens. Bioorg. Chem., 2021, 114 (April), 105072. [CrossRef]
- Shao, Z.; Li, L.; Zheng, Y.; Gong, Q.; Ke, C. Q.; Yao, S.; Zhang, H.; Tang, C.; Ye, Y. Anti-Inflammatory Sesquiterpenoid Dimers from Artemisia Atrovirens. Fitoterapia, 2022, 159(April), 105199. [Google Scholar] [CrossRef]
- Wang, X.; Peng, X.; Tang, C.; Zhou, S.; Ke, C. Q.; Liu, Y.; Yao, S.; Ai, J.; Ye, Y. Anti-Inflammatory Eudesmane Sesquiterpenoids from Artemisia Hedinii. J. Nat. Prod., 2021, 84(5), 1626–1637. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Song, Z.; Li, Y.; Wang, H.; Zhang, S.; Reid, A. M.; Lall, N.; Zhang, J.; Wang, C.; Lee, D.; et al. Cytotoxic and Antiangiogenetic Xanthones Inhibiting Tumor Proliferation and Metastasis from Garcinia Xipshuanbannaensis. J. Nat. Prod., 2021, 84(5), 1515–1523. [Google Scholar] [CrossRef]
- Liu, Q.; Zheng, H.; Wang, X.; Zhou, L.; Wang, S.; Shen, T.; Ren, D. Cytotoxic New Caged-Polyprenylated Xanthonoids from Garcinia Oligantha. Fitoterapia, 2022, 156(November 2021), 105092. [Google Scholar] [CrossRef]
- Fouotsa, H.; Dzoyem, J. P.; Lannang, A. M.; Stammler, H. G.; Mbazoa, C. D.; Luhmer, M.; Nkengfack, A. E.; Allémann, É.; Delie, F.; Meyer, F.; et al. Antiproliferative Activity of a New Xanthone Derivative from Leaves of Garcinia Nobilis Engl. Nat. Prod. Res., 2021, 35(24), 5604–5611. [Google Scholar] [CrossRef]
- Auranwiwat, C.; Limtharakul, T.; Pyne, S. G.; Rattanajak, R.; Kamchonwongpaisan, S. A New Xanthone and a Biphenyl from the Flower and Twig Extracts of Garcinia Mckeaniana. Nat. Prod. Res., 2021, 35(20), 3404–3409. [Google Scholar] [CrossRef]
- Feng, Z.; Chen, J.; Feng, L.; Chen, C.; Ye, Y.; Lin, L. Polyisoprenylated Benzophenone Derivatives from Garcinia Cambogia and Their Anti-Inflammatory Activities. Food Funct., 2021, 12(14), 6432–6441. [Google Scholar] [CrossRef]
- Yin, G. Y.; Zhu, Y. N.; Wu, F.; Mi, Q. L.; Shi, J. Q.; Gao, Q.; Zhu, L. C.; Zhou, T.; Li, J.; Liu, X.; et al. Two New Antibacterial Chromeno[3,2-c]Pyridine Alkaloids from Whole Plants of Thalictrum Scabrifolium. Chem. Nat. Compd., 2022, 58(3), 506–510. [Google Scholar] [CrossRef]
- Hu, Q. F.; Zhang, L. F.; Liu, M. X.; Cai, B. B.; Li, Y.; Zhou, T.; Li, M. F.; Wang, H. S.; Xu, Y.; Kong, W. S.; et al. Two New Chromeno[3,2-c]Pyridine Derivatives from the Whole Plants of Thalictrum Finetii and Their Antirotavirus Activity. Chem. Nat. Compd., 2022, 58(3), 511–515. [Google Scholar] [CrossRef]
- Wu, Y. P.; Lin, Z. L.; Zhao, G. K.; Zhou, M.; Yao, H.; Zhang, G. H.; Li, W.; Yang, G. Y.; Li, Y. K.; Hu, Q. F.; et al. Two New Anti-Tobacco Mosaic Virus Alkaloids from the Whole Plants of Thalictrum Microgynum. Chem. Nat. Compd., 2022, 58(4), 699–703. [Google Scholar] [CrossRef]
- Xu, L.; Yang, W.; Hu, J.; Han, C. M.; Li, P. F. Three New Isoquinoline Alkaloids from the Whole Plants of Thalictrum Tenue with Cytotoxic Activities. J. Asian Nat. Prod. Res., 2020, 22(7), 618–625. [Google Scholar] [CrossRef]
- Xue, J. J.; Jiang, C. Y.; Zou, D. L.; Li, B. J.; Lu, J. C.; Li, D. H.; Lin, B.; Li, Z. L.; Hua, H. M. Baicalensines A and B, Two Isoquinoline Alkaloids from the Roots of Thalictrum Baicalense. Org. Lett., 2020, 22(19), 7439–7442. [Google Scholar] [CrossRef]
- Xia, X.; Zhang, J.; Wang, X. J.; Lu, Y.; Chen, D. F. New Phenolic Glycosides and Lignans from the Roots of Lilium Dauricum. Planta Med., 2022, 88(7), 518–526. [Google Scholar] [CrossRef] [PubMed]
- Shu, P.; Yu, M.; Zhu, H.; Luo, Y.; Li, Y.; Li, N.; Zhang, H.; Zhang, J.; Liu, G.; Wei, X.; et al. Two New Iridoid Glycosides from Gardeniae Fructus. Carbohydr. Res., 2021, 501(December 2020), 108259. [Google Scholar] [CrossRef]
- Yu, J.; Wang, K.; Zhao, H.; Chen, L.; Wang, X. Bioactive Constituents from the Leaves of Lonicera Japonica. Fitoterapia, 2022, 162(June), 105277. [Google Scholar] [CrossRef]
- Sun, S.; Fu, J.; Liu, K.; Dai, M.; Li, Y.; Liu, Y.; Ma, S.; Qu, J. Two New Iridoid Glucosides from the Whole Plant of Patrinia Scabiosifolia Link. Molecules, 2021, 26(14). [Google Scholar] [CrossRef]
- Wang, H.; Huang, H.; Lv, J.; Jiang, N.; Li, Y.; Liu, X.; Zhao, H. Iridoid Compounds from the Aerial Parts of Swertia Mussotii Franch. with Cytotoxic Activity. Nat. Prod. Res., 2021, 35(9), 1544–1549. [Google Scholar] [CrossRef]
- Peng, Z. C.; He, J.; Pan, X. G.; Zhang, J.; Wang, Y. M.; Ye, X. S.; Xia, C. Y.; Lian, W. W.; Yan, Y.; He, X. L.; et al. Secoiridoid Dimers and Their Biogenetic Precursors from the Fruits of Cornus Officinalis with Potential Therapeutic Effects on Type 2 Diabetes. Bioorg. Chem., 2021, 117(August), 105399. [Google Scholar] [CrossRef] [PubMed]
- Ma, L. L.; Wang, L. L.; Zhang, Y. F.; Jiang, X. F.; Zhu, X. L.; Pan, K.; Wan, C. X.; Zhou, Z. B. A New Chlorine-Containing Iridoid Glycoside from Plantago Maxima. Nat. Prod. Res., 2021, 35(9), 1491–1496. [Google Scholar] [CrossRef]
- Tang, J. X.; Quan, L. Q.; Xie, K.; Zhou, Y.; Ye, R. R.; Liu, D.; Li, R. T.; Li, H. M. Jatavaleridoids A-H, Eight New Iridoids from the Roots and Rhizomes of Valeriana Jatamansi Jones. Fitoterapia, 2022, 162(May), 105286. [Google Scholar] [CrossRef]
- Wei, W.; Hao, E. W.; Zhang, M.; Pan, X. L.; Qin, J. F.; Xie, J. L.; Zhou, J. Y.; Hou, X. T.; Deng, J. G. Chemical Constituents from Jasminum Pentaneurum Hand.-Mazz and Their Cytotoxicity against Human Cancer Cell Lines. Nat. Prod. Res., 2021, 35(6), 921–929. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q. Q.; Zhang, C.; Zhang, Y. L.; Lei, J. L.; Kong, L. Y.; Luo, J. G. Dimeric Guaianes from Leaves of Xylopia Vielana as Snail Inhibitors Identified by High Content Screening. Bioorg. Chem., 2021, 108(September 2020). [Google Scholar] [CrossRef]
- Boudermine, S.; Parisi, V.; Lemoui, R.; Boudiar, T.; Chini, M. G.; Franceschelli, S.; Pecoraro, M.; Pascale, M.; Bifulco, G.; Braca, A.; et al. Cytotoxic Sesquiterpenoids from Ammoides Atlantica Aerial Parts. J. Nat. Prod., 2022, 85(3), 647–656. [Google Scholar] [CrossRef]
- Kim, J. G.; Lee, J. W.; Le, T. P. L.; Han, J. S.; Cho, Y. B.; Kwon, H.; Lee, D.; Lee, M. K.; Hwang, B. Y. Sesquiterpenoids from Chrysanthemum Indicum with Inhibitory Effects on NO Production. J. Nat. Prod., 2021, 84(3), 562–569. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Huang, H.; He, H.; Qiu, L.; Gao, Q.; Li, Y.; Ding, W. Sesquiterpenoids from the Inflorescence of Ambrosia Artemisiifolia. Molecules, 2022, 27(18). [Google Scholar] [CrossRef]
- Ding, N.; Wang, J.; Liu, J.; Zhu, Y.; Hou, S.; Zhao, H.; Yang, Y.; Chen, X.; Hu, L.; Wang, X. Cytotoxic Guaianolide Sesquiterpenoids from Ainsliaea Fragrans. J. Nat. Prod., 2021, 84(9), 2568–2574. [Google Scholar] [CrossRef]
- Ma, G.; Chen, J.; Wang, L.; Qian, F.; Li, G.; Wu, X.; Zhang, L.; Li, Y. Eighteen Structurally Diversified Sesquiterpenes Isolated from Pogostemon Cablin and Their Inhibitory Effects on Nitric Oxide Production. Fitoterapia, 2022, 156(November 2021), 105098. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Wang, J.; Liu, J.; Ni, L.; Du, Y.; Wei, X. Sesquiterpenoids and Diterpenoids from the Flowers of Nicotiana Tabacum L. and Their Antifungal Activity. Rec. Nat. Prod., 2022, 16(5), 483–487. [Google Scholar] [CrossRef]
- Nhoek, P.; Chae, H. S.; Kim, Y. M.; Pel, P.; Huh, J.; Kim, H. W.; Choi, Y. H.; Lee, K.; Chin, Y. W. Sesquiterpenoids from the Aerial Parts of Salvia Plebeia with Inhibitory Activities on Proprotein Convertase Subtilisin/Kexin Type 9 Expression. J. Nat. Prod., 2021, 84(2), 220–229. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Song, M.; Sun, Y.; Yang, F.; Yu, H.; Wu, C.; Sun, Y.; Chang, W.; Ge, D.; Zhang, H. Antifungal and Allelopathic Activities of Sesquiterpenes from Solidago Canadensis. Curr. Org. Chem., 2021, 25(21), 2676–2682. [Google Scholar] [CrossRef]
- Hanh, T. T. H.; Cham, P. T.; My, N. T. T.; Cuong, N. T.; Dang, N. H.; Quang, T. H.; Huong, T. T.; Cuong, N. X.; Nam, N. H.; Minh, C. Van. Sesquiterpenoids from Saussurea Costus. Nat. Prod. Res., 2021, 35(9), 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Wu, H. B.; Ma, L. H.; Li, X. M.; Liu, T. T. Selective Phytotoxic Effects of Sesquiterpenoids from Sonchus Arvensis as a Preliminary Approach for the Biocontrol of Two Problematic Weeds of Wheat. J. Agric. Food Chem., 2022, 70(30), 9412–9420. [Google Scholar] [CrossRef] [PubMed]
- WANG, Y. Y.; LI, Q. R.; CHI, J.; LI, J. X.; KONG, L. Y.; LUO, J. Sesquiterpenoids from the Leaves of Sarcandra Glabra. Chin. J. Nat. Med., 2022, 20(3), 215–220. [Google Scholar] [CrossRef]
- Tian, D.; Cao, L.; Li, Q.; Huang, H.; Xu, W.; Chen, G.; Song, Z.; He, Y.; Yao, X.; Tang, J. Benzannulated 5,5-Spiroketal Sesquiterpenes from the Roots of Angelica Pubescens. Bioorg. Chem., 2021, 107(October 2020). [Google Scholar] [CrossRef]
- Ning, R.; Mu, H.; Chen, L.; Wang, T.; Xu, X.; He, S.; Jiang, M.; Zhao, W. First Report on Inhibitory Effect against Osteoclastogenesis of Dihydro-β-Agarofuran-Type Sesquiterpenoids. J. Agric. Food Chem., 2022, 70(2), 554–566. [Google Scholar] [CrossRef]
- Zhong, W.; Li, M.; Han, S.; Sun, J.; Cao, L.; Mu, Z.; Du, X.; Cui, Y.; Feng, Y.; Zhong, G. Carpelipines C and D, Two Anti-Inflammatory Germacranolides from the Flowers of Carpesium Lipskyi Winkl. (Asteraceae). Chem. Biodivers., 2022, 19(7). [Google Scholar] [CrossRef]
- Aliyu, A. B.; Koorbanally, N. A.; Moodley, B.; Chenia, H. Y. Sesquiterpene Lactones from Polydora Serratuloides and Their Quorum Sensing Inhibitory Activity. Nat. Prod. Res., 2021, 35(22), 4517–4523. [Google Scholar] [CrossRef] [PubMed]
- Achoub, H.; Mencherini, T.; Esposito, T.; Luca, R.; Aquino, R.; Gazzerro, P.; Zaiter, L.; Benayache, F.; Benayache, S. New Sesquiterpenes from Asteriscus Graveolens. Nat. Prod. Res., 2021, 35(13), 2190–2198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, H.; Lin, C.; Fu, L.; Zou, Z. New 11-Methoxymethylgermacranolides from the Whole Plant of Carpesium Divaricatum. Molecules, 2022, 27(18), 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mirzania, F.; Moridi Farimani, M.; Sarrafi, Y.; Nejad Ebrahimi, S.; Troppmair, J.; Kwiatkowski, M.; Stuppner, H.; Alilou, M. New Sesterterpenoids from Salvia Mirzayanii Rech.f. and Esfand. Stereochemical Characterization by Computational Electronic Circular Dichroism. Front. Chem., 2022, 9(January), 1–11. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, D. D.; Zhou, Y. Q.; Wu, J. T.; Qi, Z. T.; Algradi, A. M.; Pan, J.; Guan, W.; Yang, B. Y.; Kuang, H. X. A New Ent-Kaurane Diterpenoid from the Pericarps of Datura Metel. J. Asian Nat. Prod. Res., 2022, 24(9), 884–890. [Google Scholar] [CrossRef] [PubMed]
- Wang, S. J.; Yu, M.; Li, H.; Zhang, G. J. Structures and Biological Activities of Polyacylated Ent-Kaurane Diterpenoid Glycosides from the Aerial Parts of Inula Hupehensis. J. Nat. Prod., 2022, 85(1), 185–195. [Google Scholar] [CrossRef] [PubMed]
- Wei, W. J.; Zhu, B.; Si, Y.; Guo, T.; Kang, J.; Dai, L. Cytotoxic Ent-Kaurane Diterpenoids from Rabdosia Rubescens. Chem. Biodivers., 2022, 19(10), 1–5. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Xu, J.; Lv, J. J.; Zhu, H. T.; Wang, D.; Yang, C. R.; Zhang, Y. J. New Ent-Kaurane and Cleistanthane Diterpenoids with Potential Cytotoxicity from Phyllanthus Acidus (L.) Skeels. Fitoterapia, 2022, 157(January). [Google Scholar] [CrossRef]
- Yan, X. L.; Zou, M. F.; Chen, B. L.; Yuan, F. Y.; Zhu, Q. F.; Zhang, X.; Lin, Y.; Long, Q. De; Liu, W. L.; Liao, S. G. Euphorane C, an Unusual C17-Norabietane Diterpenoid from Euphorbia Dracunculoides Induces Cell Cycle Arrest and Apoptosis in Human Leukemia K562 Cells. Arab. J. Chem., 2022, 15(11), 104203. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, J.; He, F.; Fu, W.; Tang, B.; Bin, Y.; Fang, M.; Wu, Z.; Qiu, Y. Anti-Inflammatory Sesquiterpenoids from the Heartwood of Juniperus Formosana Hayata. Fitoterapia, 2022, 157(November 2021), 105105. [Google Scholar] [CrossRef]
- Ntungwe, E. N.; Stojanov, S. J.; Duarte, N. M.; Candeias, N. R.; Díaz-Lanza, A. M.; Vágvölgyi, M.; Hunyadi, A.; Pešić, M.; Rijo, P. C20- nor-Abietane and Three Abietane Diterpenoids from Plectranthus Mutabilis Leaves as P-Glycoprotein Modulators. ACS Med. Chem. Lett., 2022, 13(4), 674–680. [Google Scholar] [CrossRef] [PubMed]
- Huang, X. L.; Wang, D. W.; Liu, Y. Q.; Cheng, Y. X. Diterpenoids from Blumea Balsamifera and Their Anti-Inflammatory Activities. Molecules, 2022, 27(9). [Google Scholar] [CrossRef]
- Li, W. F.; Liang, Z. M.; Zhao, C. L.; Tsang, N. Y.; Li, J. X.; Liu, Y. H.; He, K.; Pan, L. T.; Rong, L.; Zou, J.; et al. 3,4-Seco-Isopimarane Diterpenes from the Twigs and Leaves of Isodon Flavidus. Molecules, 2022, 27(10), 2–11. [Google Scholar] [CrossRef] [PubMed]
- Su, X. M.; Liang, Q.; Hu, J. X.; Zhang, X. M.; Jia, R. L.; Xu, W. H. Diterpenoids from the Whole Plants of Croton Yunnanensis and Their Bioactivities. Bioorganic Med. Chem., 2021, 51(August), 116495. [Google Scholar] [CrossRef]
- Kang, H.; Lee, D.; Kang, K. S.; Kim, K. H. A New Labdane-Type Diterpene, 6-O-Acetyl-(12R)-Epiblumdane, from Stevia Rebaudiana Leaves with Insulin Secretion Effect. Biomedicines, 2022, 10(4). [Google Scholar] [CrossRef]
- Chen, B. L.; Zhu, Q. F.; Zhang, X.; Lin, Y.; Long, Q. De; Liu, W. L.; Yan, X. L. An Unusual Indole-Diterpenoid with C-17 Norcassane Skeleton from Euphorbia Fischeriana Induces HEL Cell Cycle Arrest and Apoptosis. Fitoterapia, 2022, 159(April), 105195. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Tang, D.; Nijat, D.; Yang, H.; Aisa, H. A. Diterpenoids from Euphorbia Glomerulans with Potential Reversal Activities against P-Glycoprotein-Mediated Multidrug Resistance. Bioorg. Chem., 2021, 117(August), 105442. [Google Scholar] [CrossRef]
- Wan, L. X.; Zhang, J. F.; Zhen, Y. Q.; Zhang, L.; Li, X.; Gao, F.; Zhou, X. L. Isolation, Structure Elucidation, Semi-Synthesis, and Structural Modification of C19-Diterpenoid Alkaloids FromAconitum Apetalumand Their Neuroprotective Activities. J. Nat. Prod., 2021, 84(4), 1067–1077. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, X.; Fei, J.; Dong, X.; Wang, Y.; Yang, S.; Hao, X.; Ding, X.; Zhao, Y. Identification of Limonoids from Walsura Yunnanensis and Evaluation of Their Cytotoxicity against Cancer Cell Lines. Fitoterapia, 2022, 157(December 2021), 105120. [Google Scholar] [CrossRef]
- Bernard, D.; Hassana, Y.; Djaouda, M.; Mathieu, M.; Bouba Romeo, W.; Benoît, K.; Tul Wahab, A. Antibacterial Effects of a New Triterpenoid Saponin from Roots of Gardenia Ternifolia Schumach. & Thonn (Rubiaceae). Results Chem., 2022, 4(January), 0–6. [Google Scholar] [CrossRef]
- Deng, Y.; Ma, Y.; Liu, H.; Zhang, Y.; Wei, Z.; Liu, G.; Tang, X.; Jia, X. Structure Determination, Bitterness Evaluation and Hepatic Gluconeogenesis Inhibitory Activity of Triterpenoids from the Momordica Charantia Fruit. Food Chem., 2022, 372(June 2021), 131224. [Google Scholar] [CrossRef]
- Hoang, T. D. H.; Le, T. K. Van; Do, T. H. Triterpene Glycosides from the Aerial Parts of Elsholtzia Penduliflora W. W. Smith and Their Cytotoxic Activity. Fitoterapia, 2022, 162(May), 105264. [Google Scholar] [CrossRef] [PubMed]
- Hu, B. Y.; Zhao, Y. L.; Ma, D. Y.; Xiang, M. L.; Zhao, L. X.; Luo, X. D. Anti-Hyperuricemic Bioactivity of Alstonia Scholaris and Its Bioactive Triterpenoids in Vivo and in Vitro. J. Ethnopharmacol., 2022, 290(February), 115049. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Hu, R.; Zhou, Y.; Zhu, W.; Zhou, Y. Cytotoxic 13,28 Epoxy Bridged Oleanane-Type Triterpenoid Saponins from the Roots of Ardisia Crispa. Molecules, 2022, 27(3). [Google Scholar] [CrossRef] [PubMed]
- Zhang, C. Y.; Chu, Z. J.; Zhou, J. C.; Liu, S. G.; Zhang, J. Z.; Qian, L.; Lou, H. X. Cytotoxic Activities of 9,10-Seco-Cycloartane-Type Triterpenoids from the Chinese Liverwort Lepidozia Reptans. J. Nat. Prod., 2021, 84(12), 3020–3028. [Google Scholar] [CrossRef]
- Vitaku, E.; Smith, D. T.; Njardarson, J. T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem., 2014, 57(24), 10257–10274. [Google Scholar] [CrossRef]
- Wu, Z. L.; Zhang, W. Y.; Zhong, J. C.; Huang, X. J.; Xu, W.; Chen, M. F.; Weng, S. Q.; Zhang, D. M.; Che, C. T.; Ye, W. C.; et al. Angiogenesis-Inhibitory Piperidine Alkaloids from the Leaves of Microcos Paniculata. J. Nat. Prod., 2022, 85(2), 375–383. [Google Scholar] [CrossRef] [PubMed]
- GAO, W.; WANG, Y.; WANG, R.; WANG, Y. H.; XU, J. W.; HE, X. J. Antiproliferative Piperidine Alkaloids from Giant Taro (Alocasia Macrorrhiza). Chin. J. Nat. Med., 2022, 20(7), 541–550. [Google Scholar] [CrossRef]
- Li, H.; Xu, J.; Zhang, X.; Liu, C.; Chen, N.; Naruo, A.; Qin, Z.; Ao, M. New Napelline-Type Diterpenoid Alkaloids from Aconiti Kusnezoffii Roots: Structure Elucidation, Plausible Biogenetic Pathway and Biological Activities. Phytochem. Lett., 2021, 43(83), 53–59. [Google Scholar] [CrossRef]
- Wang, X. Y.; Zhou, Q. M.; Guo, L.; Dai, O.; Meng, C. W.; Miao, L. L.; Liu, J.; Lin, Q.; Peng, C.; Xiong, L. Cardioprotective Effects and Concentration-Response Relationship of Aminoalcohol-Diterpenoid Alkaloids from Aconitum Carmichaelii. Fitoterapia, 2021, 149(November 2020), 104822. [Google Scholar] [CrossRef]
- Ye, M. Z.; Li, X. Y.; Xie, J.; Chen, L.; Gao, F.; Zhou, X. L.; Huang, S. Gyalanunines A and B, Two New C20-Diterpenoid Alkaloids from Delphinium Gyalanum. Tetrahedron Lett., 2022, 108, 154153. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, D.; Chen, Y.; Xu, J.; Xu, Y.; Yue, X.; Jia, J.; Li, H.; Chen, L. Alkaloids of Delphinium Grandiflorum and Their Implication to H2O2-Induced Cardiomyocytes Injury. Bioorganic Med. Chem., 2021, 37(February), 116113. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Yan, Y.; Li, X.; Gong, G.; Wang, W. Three New Diterpenoid Alkaloids from Delphinium Tatsienense. Phytochem. Lett., 2021, 41(November 2020), 142–146. [Google Scholar] [CrossRef]
- Kemgni, M. F.; Chenda, L. B. N.; Tchamgoue, J.; Kenfack, P. T.; Ngandjui, Y. A. T.; Wouamba, S. C. N.; Tiani, G. L. M.; Green, I. R.; Kouam, S. F. Greenwaylactams A, B and C, the First Group of Sesquiterpene Alkaloids with an Eight-Membered Lactam Ring from Greenwayodendron Oliveri. ChemistrySelect, 2021, 6(7), 1705–1709. [Google Scholar] [CrossRef]
- Xie, T. Z.; Zhao, Y. L.; Wang, H.; Chen, Y. C.; Wei, X.; Wang, Z. J.; He, Y. J.; Zhao, L. X.; Luo, X. D. New Steroidal Alkaloids with Anti-Inflammatory and Analgesic Effects from Veratrum Grandiflorum. J. Ethnopharmacol., 2022, 293(April), 115290. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W. J.; Zhu, P. Y.; Qiao, M.; Gao, W. F.; Li, G. D.; Wang, X. J.; Sheng, J. Two New Steroidal Alkaloids with Cytotoxic Activities from the Roots of Veratrum Grandiflorum Loes. Phytochem. Lett., 2021, 46(December 2020), 56–60. [Google Scholar] [CrossRef]
- Wu, P. F.; Liu, J.; Li, Y. N.; Ding, R.; Tan, R.; Yang, X. M.; Yu, Y.; Hao, X. J.; Yuan, C. M.; Yi, P. Three New Aglain Derivatives from Aglaia Odorata Lour. and Their Cytotoxic Activities. Chem. Biodivers., 2022, 19(4), 1–8. [Google Scholar] [CrossRef] [PubMed]
- Promchai, T.; Thaima, T.; Rattanajak, R.; Kamchonwongpaisan, S.; Pyne, S. G.; Limtharakul, T. (R)-3-(8′-Hydroxyfarnesyl)-Indole and Other Chemical Constituents from the Flowers of Anomianthus Dulcis and Their Antimalarial and Cytotoxic Activities. Nat. Prod. Res., 2021, 35(15), 2476–2481. [Google Scholar] [CrossRef] [PubMed]
- Wan Othman, W. N. N.; Salim, F.; Abdullah, N. N.; Abu Bakar, S. I.; Awang, K.; Jayasinghe, L.; Ismail, N. H. (R)-13aα-Densiindolizidine, A New Phenanthroindolizidine Alkaloid From Cryptocarya Densiflora Blume (Lauraceae) and Molecular Docking Against SARS-CoV-2. Nat. Prod. Commun., 2022, 17(8), 0–7. [Google Scholar] [CrossRef]
- Yuan, H. L.; Zhao, Y. L.; Qin, X. J.; Liu, Y. P.; Yang, X. W.; Luo, X. D. Diverse Isoquinolines with Anti-Inflammatory and Analgesic Bioactivities from Hypecoum Erectum. J. Ethnopharmacol., 2021, 270(November 2020), 113811. [Google Scholar] [CrossRef]
- Panthong, K.; Ingkaninan, K. Amabiloid A from Crinum × Amabile Donn Ex Ker Gawl. Nat. Prod. Res., 2021, 35(19), 3220–3225. [Google Scholar] [CrossRef]
- Liu, S. B.; Zeng, L.; Xu, Q. L.; Chen, Y. Le; Lou, T.; Zhang, S. X.; Tan, J. W. Polycyclic Phenol Derivatives from the Leaves of Spermacoce Latifolia and Their Antibacterial and α-Glucosidase Inhibitory Activity. Molecules, 2022, 27(10). [Google Scholar] [CrossRef]
- Tao, H.; Zhou, Y.; Yin, X.; Wei, X.; Zhou, Y. Two New Phenolic Glycosides with Lactone Structural Units from Leaves of Ardisia Crenata Sims with Antibacterial and Anti-Inflammatory Activities. Molecules, 2022, 27(15). [Google Scholar] [CrossRef]
- Liao, N.; Li, M. S.; Qin, F.; Jiang, J. C.; Luo, R. Da; Chen, X. L.; Zhou, M. M.; Zhu, Y. K.; Wang, H. S. A New Phenolic Acid from Zanthoxylum Nitidum Var. Tomentosum (Rutaceae) and Its Chemotaxonomic Significance. Biochem. Syst. Ecol., 2021, 99(August), 104351. [Google Scholar] [CrossRef]
- Zheng, X.; Wen, R.; Liu, Y.; Gan, L.; Zhang, Q.; Jiang, Y.; Tu, P. Nitric Oxide Inhibitory Phenolic Constituents Isolated from the Roots and Rhizomes of Notopterygium Incisum. Bioorg. Chem., 2022, 128(March), 106060. [Google Scholar] [CrossRef]
- Nhung, L. T. H.; Linh, N. T. T.; Cham, B. T.; Thuy, T. T.; Tam, N. T.; Thien, D. D.; Huong, P. T. M.; Tan, V. M.; Tai, B. H.; Hoang Anh, N. T. New Phenolics from Dianella Ensifolia. Nat. Prod. Res., 2021, 35(18), 3063–3070. [Google Scholar] [CrossRef]
- Cho, H. M.; Zhang, M.; Park, E. J.; Lee, B. W.; Park, Y. J.; Kim, H. W.; Pham, H. T. T.; Chin, Y. W.; Oh, W. K. Flavonostilbenes Isolated from the Stems of Rhamnoneuron Balansae as Potential SIRT1 Activators. J. Nat. Prod., 2022, 85(1), 70–82. [Google Scholar] [CrossRef]
- El-Nashar, H. A. S.; Mostafa, N. M.; Eldahshan, O. A.; Singab, A. N. B. A New Antidiabetic and Anti-Inflammatory Biflavonoid from Schinus Polygama (Cav.) Cabrera Leaves. Nat. Prod. Res., 2022, 36(5), 1182–1190. [Google Scholar] [CrossRef]
- Fu, H. M.; Yin, C. L.; Shen, Z. Y.; Yang, M. H. Flavonoids from the Leaves of Apocynum Venetum and Their Anti-Inflammatory Activity. J. Chem. Res., 2022, 46(1), 4–7. [Google Scholar] [CrossRef]
- Molčanová, L.; Treml, J.; Brezáni, V.; Maršík, P.; Kurhan, S.; Trávníček, Z.; Uhrin, P.; Šmejkal, K. C-Geranylated Flavonoids from Paulownia Tomentosa Steud. Fruit as Potential Anti-Inflammatory Agents. J. Ethnopharmacol., 2022, 296(June). [Google Scholar] [CrossRef]
- Qin, H.; Bi, D.; Li, F.; Luo, R.; Zhuang, H.; Wang, L. Two New C-Benzylated Chalcones and One New Mimosin-Type Homoisoflavonoid from the Twigs and Leaves of Caesalpinia Digyna. Fitoterapia, 2022, 162(June), 105279. [Google Scholar] [CrossRef]
- Shin, H.; Jang, J.; Lee, M. K.; Lee, K. Y. Cell Extraction Method Coupled with LC-QTOF MS/MS Analysis for Predicting Neuroprotective Compounds from Polygonum Tinctorium. J. Pharm. Biomed. Anal., 2022, 220(May), 114988. [Google Scholar] [CrossRef]
- Zheng, J.; Zhou, Q.; Cao, X.; Meng, Y.; Jiang, G.; Xu, P. Two New Flavonol Derivatives from the Whole Plants of Centella Asiatica and Their Cytotoxic Activities. Phytochem. Lett., 2022, 47(August 2021), 34–37. [Google Scholar] [CrossRef]
- Kalenga, T. M.; Ndoile, M. M.; Atilaw, Y.; Munissi, J. J. E.; Gilissen, P. J.; Rudenko, A.; Bourgard, C.; Sunnerhagen, P.; Nyandoro, S. S.; Erdelyi, M. Antibacterial and Cytotoxic Biflavonoids from the Root Bark of Ochna Kirkii. Fitoterapia, 2021, 151(December 2020), 104857. [Google Scholar] [CrossRef]
- Liu, X.; Yang, J.; Yu, H.; Zhang, J.; Du, J.; Wang, X.; Wang, Y.; Chai, X. Chemical Constituents from the Fruits of Cullen Corylifolium (L.) Medik. by the Targeted Separation Mode. Nat. Prod. Res., 2021, 35(7), 1071–1076. [Google Scholar] [CrossRef]
- Zhao, W. W.; Guo, W. W.; Guo, J. F.; Wang, X.; Chen, X. Q.; Wu, X. Three New Flavonoids from Penthorum Chinense Pursh and Their Docking Studies. Nat. Prod. Res., 2021, 35(1), 49–56. [Google Scholar] [CrossRef]
- Tambewagh, U. U.; Javir, G. R.; Joshi, K. S.; Rojatkar, S. R. Two New Polyoxygenated Flavonoids from Blumea Eriantha DC, Methanol Extract and Their Anti-Proliferative Activity. Nat. Prod. Res., 2021, 35(17), 2815–2822. [Google Scholar] [CrossRef]
- Blanco Carcache, P. J.; Anaya Eugenio, G. D.; Ninh, T. N.; Moore, C. E.; Rivera-Chávez, J.; Ren, Y.; Soejarto, D. D.; Kinghorn, A. D. Cytotoxic Constituents of Glycosmis Ovoidea Collected in Vietnam. Fitoterapia, 2022, 162(June). [Google Scholar] [CrossRef]
- Chukaew, A.; Chaniad, P. Chemical Constituents from the Roots of Calophyllum Pisiferum Planch. & Triana and Their Cytotoxic and Antioxidant Activities. Rec. Nat. Prod., 2022, 16(1), 66–73. [Google Scholar] [CrossRef]
- Liu, F.; Mallick, S.; O’donnell, T. J.; Rouzimaimaiti, R.; Luo, Y.; Sun, R.; Wall, M.; Wongwiwatthananukit, S.; Date, A.; Silva, D. K.; et al. Coumarinolignans with Reactive Oxygen Species (ROS) and NF-ΚB Inhibitory Activities from the Roots of Waltheria Indica. Molecules, 2022, 27(10), 1–15. [Google Scholar] [CrossRef]
- Shen, H. yan; Zhang, M.; Ouyang, J. kui; Jia, Y. xia; Luo, Y. Two New Coumarin Derivatives from the Whole Plant of Spermacoce Latifolia. Phytochem. Lett., 2022, 51(May), 82–85. [Google Scholar] [CrossRef]
- Xia-Hou, Z. R.; Feng, X. F.; Mei, Y. F.; Zhang, Y. Y.; Yang, T.; Pan, J.; Yang, J. H.; Wang, Y. S. 5-Demethoxy-10′-Ethoxyexotimarin F, a New Coumarin with MAO-B Inhibitory Potential from Murraya Exotica L. Molecules, 2022, 27(15). [Google Scholar] [CrossRef]
- Zhou, Q.; Wu, S. Y.; Jiang, C. X.; Tong, Y. P.; Zhao, T.; Zhang, B.; Nong, X. H.; Jin, Z. X.; Hu, J. F. A New Coumarin Derivative from the Stems of the Endangered Plant Ulmus Elongata. Nat. Prod. Res., 2021, 35(21), 3562–3568. [Google Scholar] [CrossRef]
- Lee, B. Z.; Kim, C. S.; Jeong, S. K.; Lee, S.; Lee, I. S.; Hong, K. W. Anti-Inflammatory Isocoumarins from the Bark of Fraxinus Chinensis Subsp. Rhynchophylla. Nat. Prod. Res., 2021, 35(22), 4380–4387. [Google Scholar] [CrossRef]
- Hung, H. Y.; Cheng, K. C.; Kuo, P. C.; Chen, I. T.; Li, Y. C.; Hwang, T. L.; Lam, S. H.; Wu, T. S. Chemical Constituents of Hedyotis Diffusa and Their Anti-Inflammatory Bioactivities. Antioxidants, 2022, 11(2), 1–15. [Google Scholar] [CrossRef]
- Li, C.; Wang, H.; Dong, C.; Xie, J.; Lai, S.; Liu, J.; Chen, R.; Kang, J. New Quinones, a Sesquiterpene and Phenol Compounds with Cytotoxic Activity from the Aerial Parts of Morinda Umbellata L. Fitoterapia, 2022, 156(November 2021), 105089. [Google Scholar] [CrossRef]
- Hangsamai, N.; Photai, K.; Mahaamnart, T.; Kanokmedhakul, S.; Kanokmedhakul, K.; Senawong, T.; Pitchuanchom, S.; Nontakitticharoen, M. Four New Anthraquinones with Histone Deacetylase Inhibitory Activity from Ventilago Denticulata Roots. Molecules, 2022, 27(3), 1–14. [Google Scholar] [CrossRef]
- Wisetsai, A.; Lekphrom, R.; Schevenels, F. T. New Anthracene and Anthraquinone Metabolites from Prismatomeris Filamentosa and Their Antibacterial Activities. Nat. Prod. Res., 2021, 35(10), 1582–1589. [Google Scholar] [CrossRef]
| Botanical Family | Absolute Frequency | Relative Frequency (%) |
|---|---|---|
| Asteraceae | 40 | 8.5% |
| Lamiaceae | 27 | 5.7% |
| Fabceae | 26 | 5.5% |
| Ranunculaceae | 18 | 3.8% |
| Solanaceae | 16 | 3.4% |
| Rutaceae | 16 | 3.4% |
| Euphorbiaceae | 14 | 3.0% |
| Rubiaceae | 14 | 3.0% |
| Apocynaceae | 12 | 2.5% |
| Genus | Family | Order | Published Studies * | New Compounds |
|---|---|---|---|---|
| Piper | Piperaceae | Piperalis | 7 | 42 |
| Hypericum | Hypericaceaea | Malpighiales | 7 | 34 |
| Artemisia | Asteraceae | Asterales | 6 | 45 |
| Garcinia | Clusiaceae | Malpighiales | 5 | 23 |
| Thalictrum | Ranunculaceae | Ranunculales | 4 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





