Submitted:
18 November 2023
Posted:
21 November 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Materials and Methods
Patients study
Data Collection
Samples Collection
Microbiological Laboratory Analysis
Ethical Approval
Statistical Analysis
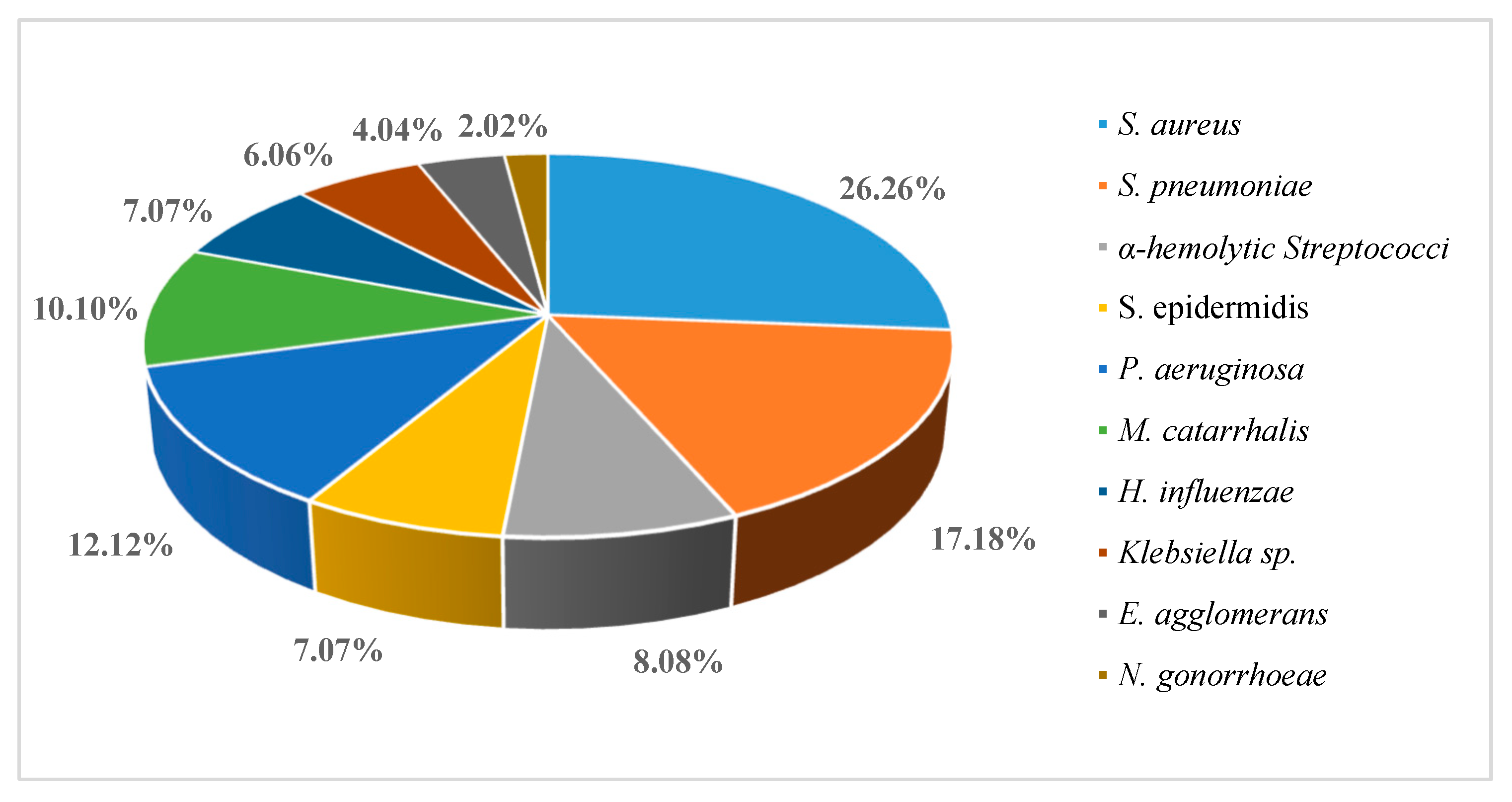
Results
Discussion
Conclusion
Funding
Acknowledgments
Conflict of Interest
References
- G. Høvding, “Acute bacterial conjunctivitis,” Acta Ophthalmologica, 86, 1: 5–17, Feb. 2008. [CrossRef]
- K. K. Y. O. Faao, “Bacterial conjunctivitis (pink eye): practice essentials, background, pathophysiology.”. Available online: https://emedicine.medscape.com/article/1191730-overview#showall.
- C. Beal and B. Giordano, “Clinical evaluation of red eyes in pediatric patients,” Journal of Pediatric Health Care, 30, 5, : 506–514, Sep. 2016. [CrossRef]
- Centers for Disease Control and Prevention (CDC). Pinkeye (Conjunctivitis). January 4, 2019. Available online: https://www.cdc.gov/conjunctivitis/about/causes.html#print.
- J. Epling, “Bacterial conjunctivitis,” BMJ Clin Evid. 2012, 0704. Feb 20;2012.
- K. C. Leung, K. L. Hon, A. H. C. Wong, and A. S. Wong, “Bacterial conjunctivitis in childhood: etiology, clinical manifestations, diagnosis, and management,” Recent Patents on Inflammation & Allergy Drug Discovery, 12, 2: 120–127, Sep. 2018. Sep. [CrossRef]
- Azari and N., P. Barney, “Conjunctivitis,” JAMA, 310, 16; 1721, Oct. 2013. [CrossRef]
- F. Smith and C. Waycaster, “Estimate of the direct and indirect annual cost of bacterial conjunctivitis in the United States,” BMC Ophthalmology, 9, 1, Nov. 2009. [CrossRef]
- W. H. Edrees, “Seroprevalence and risk factors for Helicobacter pylori infection among school students in Sana'a City, Yemen,” Universal J Pharm Res, 7, 2: 67–73, May 15 2022. [CrossRef]
- W. H. Edrees, A. A. Al-Asbahi, W. A. Al-Shehari, and E. A. Qasem, “Vulvovaginal candidiasis prevalence among pregnant women in different hospitals in Ibb, Yemen,” Universal J Pharm Res, 5, 4: 1–5, Sep. 15, 2020. [CrossRef]
- W. H. Edrees, A. A. Anbar, “Prevalence and antibacterial susceptibility of bacterial uropathogens isolated from pregnant women in Sana'a, Yemen,” PSM Biol Res, 5, 4: 157–165, Oct. 31, 2020.
- Al-Halani, A.A.; Edrees, W.H.; Alrahabi, L.M.; Thabit, J.M.; Al-Bahloul, S.M.; Alwashali, F.A.; Al-Qhali, R.M.; Morshed, M.M.; Alـhossainy, A.S.; Al-Maflhi, E.A.; et al. “Prevalence of intestinal parasites, malnutrition, anemia and their risk factors among orphaned children in Sana'a City, Yemen,” Universal J Pharm Res, 8, 2: 32-39. May, 2023. [CrossRef]
- Abdullah, Q.Y.; Al-Helali, M.F.; Al-Mahbashi, A.; Qaaed, S.T.; Edrees, W.H. “Seroprevalence of dengue fever virus among suspected patients in Taiz Governorate-Yemen,” Universal J Pharm Res, 5, 5: 21–26, Nov. 15, 2020. [CrossRef]
- Al-Arosi, S.A.H.; Al-Shamahi, E.Y.; Al-Kholani, A.I.M.; Al-Jawfi, A.Y.; Al-Shamahy, H.A.; A Al-Moyed, K.A.; Al-Ankoshy, A.A.M. “Neonatal bacterial conjunctivitis in tertiary hospitals in Sana'a city, Yemen,” Universal Journal of Pharmaceutical Research, 1, 6, pp: 36-42. Jan. 2022. [CrossRef]
- L. M. De La Maza, M. T. L. M. De La Maza, M. T. Pezzlo, C. E. Bittencourt, and E. M. Peterson, Color Atlas of Medical Bacteriology. Third Edition. John Wiley and Sons, 2020.
- Adegbehingbe and, A. Onipede, “Conjunctivitis as seen in Ile-Ife,” Nigerian Journal of Ophthalmology, 13, 1, Oct. 2005. [CrossRef]
- Silvester, A.; Neal, T.; Czanner, G.; Briggs, M.; Harding, S.; Kaye, S. “Adult bacterial conjunctivitis: resistance patterns over 12 years in patients attending a large primary eye care centre in the UK,” BMJ Open Ophthalmology, 2016;1:e000006. [CrossRef]
- Faraz, and M. A. Farhan, “Microbial etiology of neonatal conjunctivitis in a general hospital in Saudi Arabia,” Al-Shifa J Ophthalmol, 15, 1: 30-34, Jan–Mar. 2019.
- A. S. Al-Atrushi, and A. Al-Brefkani, “Ophthalmia neonatorum in Duhok, north of Iraq: bacterial causative agents and their antibiotic sensitivity pattern,” Isra Med J, 4, 4, pp; 09-213, Dec 2012.
- Nsanze, H.; Dawodu, A.; Usmani, A.; Sabarinathan, K.; Varady, E. “Ophthalmia neonatorum in the United Arab Emirates,” Annals of Tropical Paediatrics, 16, 1, pp; 27-32, Mar, 1996.
- Afjeiee, S.A.; Tabatabaei, S.R.; Fallah, F.; Fard, A.T.; Shiva, F.; Adabian, S.; Karimi, A.; Rahbar, M. , “A microbiological study of neonatal conjunctivitis in two hospitals in Tehran, Iran,” Asian Pacific Journal of Tropical Disease, 3, 6: 429–433, Dec. 2013. [CrossRef]
- K. Sehgal, L. K. Sehgal, L. Kant, B. K. Jain, and K. Lal, “Prevalence of eye diseases in a semi-urban area,” Indian J Public Health, 28, 4: 189-93. Oct-Dec. 1984. [Google Scholar]
- Black, R.E.; Allen, L.H.; Bhutta, Z.A.; Caulfield, L.E.; De Onis, M.; Ezzati, M.; Mathers, C.; Rivera, J. ; Maternal and Child Undernutrition Study Group. Maternal and child undernutrition: global and regional exposures and health consequences,” The Lancet, 371, 9608: 243–260, Jan. 2008. [CrossRef]
- G. N. Rao, S. G. N. Rao, S. Sabnam, S. Pal, H. Rizwan, B. Thakur, and A. Pal, “Prevalence of ocular morbidity among children aged 17 years or younger in the eastern India,” Clinical Ophthalmology, 12: 1645–1652, Sep. 2018. [CrossRef]
- Petrillo, F.; Pignataro, D.; Lavano, M.A.; Santella, B.; Folliero, V.; Zannella, C.; Astarita, C.; Gagliano, C.; Franci, G.; Avitabile, T.; et al. “Current evidence on the ocular surface microbiota and related diseases,” Microorganisms, 8, 7; 1033, Jul. 2020. [CrossRef]
- Kumar, R.; Mehra, M.; Dabas, P.; Raha, R. A study of ocular infections amongst primary school children in Delhi,” J Commun Dis, 36, 2; 121–126, 2004.
- A, Subramanian, and S, Yessaian, “Screening and antibiogram pattern of bacterial ophthalmic infections,” Int J Pharm Pharm Sci, vol 7, 1; 412-415, 2014.
- Wen, X.; Miao, L.; Deng, Y.; Bible, P.W.; Hu, X.; Zou, Y.; Liu, Y.; Guo, S.; Liang, J.; Chen, T.; et al. “The Influence of age and sex on ocular surface microbiota in healthy adults,” Investigative Opthalmology and Visual Science, 58, 14; 6030, Dec. 2017. [CrossRef]
- S. J. Lichtenstein and M. Rinehart, “Efficacy and safety of 0.5% levofloxacin ophthalmic solution for the treatment of bacterial conjunctivitis in pediatric patients,” Journal of American Association for Pediatric Ophthalmology and Strabismus, 7, 5: 317–324, Oct. 2003. [CrossRef]
- K. M. Cavuoto, R. K. M. Cavuoto, R. Mendez, D. Miller, A. Galor, and S. Banerjee, “Effect of clinical parameters on the ocular surface microbiome in children and adults,” Clinical Ophthalmology, 12: 1189–1197, Jul. 2018. [CrossRef]
- M. F. Hashmi, “Conjunctivitis,” StatPearls - NCBI Bookshelf, Dec. 06, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK541034/.
- K. Cavuoto, D. K. Cavuoto, D. Zutshi, C. L. Karp, D. Miller, and W. Feuer, “Update on bacterial conjunctivitis in South Florida,” Ophthalmology, 115, 1: 51–56, Jan. 2008. [CrossRef]
- Alhazmi, A.; Abuallut, I.; Alwadani, I.; Haddad, M.; Ageeli, B.; Majrabi, H.; Muslihi, I.; AlAli, L.; Homadi, H.; Madkhli, E.; et al. “Neonatal Healthcare-Associated Conjunctivitis: A Descriptive Study from Saudi Arabia,” Medicina, 58, 10, p. 1448, Oct. 2022. [CrossRef]
- Ogunfowora, O.; Ajewole, J.; Ajibode, H. “Conjunctival bacterial infection among hospitalized neonates,” Annals of Health Research, 6, 2: 230–238, May 2020. [CrossRef]
- R. O’Callaghan, “The pathogenesis of Staphylococcus aureus eye infections,” Pathogens, 7, 1, p. 9, Jan. 2018. [CrossRef]
- Alhlale, M.F.; Humaid, A.; Saleh, A.H.; Alsweedi, K.S.; Edrees, W.H. “Effect of most common antibiotics against bacteria isolated from surgical wounds in Aden Governorate hospitals, Yemen,” Universal J Pharm Res, 5, 1: 21–24, Mar. 08, 2020. [CrossRef]
- Al-Khawlany, R.S.; Edrees, W.H.; AL-Jaufy, A.Y.; Nasher, M.A.; Al-Shehari, W.A.; Reem, A.; Almezgagi, M.M. “Prevalence of methicillin-resistant Staphylococcus aureus and antibacterial susceptibility among patients with skin and soft tissue infection at Ibb City, Yemen,” PSM Microbiol, 6, 1: 1–11, Mar. 31, 2021. Available online: https://psmjournals.org/index.php/microbiol/article/view/535.
- Edrees, W.H. Antibacterial susceptibility and Sider honey activity against isolated bacteria from wound patients attending at Al-Gmohori hospital in Hajja City, Yemen,” Al-Razi Univ J Med Sci; 5, 2: 1–8, Juan, 30, 2021. [Google Scholar]
- N. Buznach, R. N. Buznach, R. Dagan, and D. Greenberg, “Clinical and bacterial characteristics of acute bacterial conjunctivitis in children in the antibiotic resistance era,” The Pediatric Infectious Disease Journal, 24, 9: 823–828, Sep. 2005. [CrossRef]
- H. Tao, J. H. Tao, J. Wang, L. Li, H.-Z. Zhang, M.-P. Chen, and L. Li, “Incidence and antimicrobial sensitivity profiles of normal conjunctiva bacterial flora in the central area of China: A Hospital-based study,” Frontiers in Physiology, 8, 17 May 2017. [CrossRef]
- Wahl, B.; O’Brien, K.L.; Greenbaum, A.; Majumder, A.; Liu, L.; Chu, Y.; Lukšić, I.; Nair, H.; McAllister, D.A.; Campbell, H. “Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000–15,” The Lancet Global Health, 6, 7: e744–e757, Jul. 2018. [CrossRef]
- Sugita, G.; Hotomi, M.; Sugita, R.; Kono, M.; Togawa, A.; Yamauchi, K.; Funaki, T.; Yamanaka, N. “Genetic characteristics of Haemophilus influenzae and Streptococcus pneumoniae isolated from children with conjunctivitis-otitis media syndrome,” Journal of Infection and Chemotherapy, 20, 8: 493–497, Aug. 2014. [CrossRef]
- T. Tesfaye, G. T. Tesfaye, G. Beyene, Y. Gelaw, S. Bekele, and M. Saravanan, “Bacterial profile and antimicrobial susceptibility pattern of external ocular infections in Jimma University Specialized Hospital, Southwest Ethiopia,” American Journal of Infectious Diseases and Microbiology, 1, 1: 13–20, Jan. 2013.
- D. Khosravi, M. D. Khosravi, M. Mehdinejad, and M. Heidari, “Bacteriological findings in patients with ocular infection and antibiotic susceptibility patterns of isolated pathogens,” Singap Med J, 28, 8: 741–743, 2007.
- Ly, C.N.; Pham, J.N.; Badenoch, P.R.; Bell, S.M.; Hawkins, G.; Rafferty, D.L.; A McClellan, K. “Bacteria commonly isolated from keratitis specimens retain antibiotic susceptibility to fluoroquinolones and gentamicin plus cephalothin,” Clinical and Experimental Ophthalmology, 34, 1: 44–50, Jan. 2006.
- S. Jebur, A. H. S. Jebur, A. H. Al-Hamadani, and J. Sh, “Evaluation of genetic study and bacterial culture for diagnosis of Pseudomonal eye infections,” Int J Curr Microbiol App Sci, 4, 3: 348–56, 2015.
- R. Henry, H. W. R. Henry, H. W. Flynn, D. Miller, R. K. Forster, and E. C. Alfonso, “Infectious keratitis progressing to endophthalmitis,” Ophthalmology, 119, 12: 2443–2449, Dec. 2012. [CrossRef]
- M. Teweldemedhin, H. M. Teweldemedhin, H. Gebreyesus, A. H. Atsbaha, S. W. Asgedom, and M. Saravanan, “Bacterial profile of ocular infections: a systematic review,” BMC Ophthalmology, Nov. 25, 2017. [CrossRef]
- Al-Haik, W.M.; Al-Haddad, A.M.; Al-Kaf, A.G.; Edrees, W.H. Antimicrobial activities for hadhrami honey on growth of some pathogenic bacteria,” Universal J Pharm Res, 2, 6: 7–12, Jan. 15, 2018. [CrossRef]
- . Alhlale, F.M. The inhibitory effect of Euphorbia hirta extracts against some wound bacteria isolated from Yemeni patients,” COPS, 3, 2: 780–786, Jan. 23, 2019.
- W. H, Edrees, and S. M. Al-Awar. Bacterial contamination of mobile phones of medical laboratory workers at Sana’a city, Yemen and their antimicrobial susceptibility. JPPRes. 2020, 8, 6: 591–599. [Google Scholar]
- Zhang, Y.; Liu, Z.-R.; Chen, H.; Fan, Y.-C.; Duo, J.; Zheng, H.; Wang, G.-J.; Li, Y.-C.; Jiachu, D.-B.; Zewang, G.-M. Comparison on conjunctival sac bacterial flora of the seniors with dry eye in Ganzi autonomous prefecture,” Int J Ophthalmol, 6, 4: 452–457. 2013.
- Moloney, T.P.; Park, J. Microbiological isolates and antibiotic sensitivities in culture-proven endophthalmitis: a 15-year review,” British Journal of Ophthalmology, 98, 11: 1492–1497, Jun. 2014. [CrossRef]
- Flores-Páez, L.A.; Zenteno, J.C.; Alcántar-Curiel, M.D.; Vargas-Mendoza, C.F.; Rodríguez-Martínez, S.; Cancino-Diaz, M.E.; Jan-Roblero, J.; Cancino-Diaz, J.C. Molecular and phenotypic characterization of Staphylococcus epidermidis isolates from healthy conjunctiva and a comparative analysis with isolates from Ocular Infection,” PLOS ONE, 10, 8; e0135964, Aug. 2015. [CrossRef]
- Mj. Bharathi, R. Mj. Bharathi, R. Ramakrishnan, C. Shivakumar, R. Meenakshi, and D. Lionalraj, “Etiology and antibacterial susceptibility pattern of community-acquired bacterial ocular infections in a tertiary eye care hospital in south India,” Indian Journal of Ophthalmology, 58, 6; 497, 2010. [CrossRef]
- J. Costumbrado, “Gonococcal Conjunctivitis,” StatPearls - NCBI Bookshelf, Sep. 12, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459289/#:~:text=Gonococcal%20conjunctivitis%20.
- Hu, Y.L.; Lee, P.I.; Hsueh, P.R.; Lu, C.Y.; Chang, L.Y.; Huang, L.M.; Chen, J.M. Predominant role of Haemophilus influenzae in the association of conjunctivitis, acute otitis media and acute bacterial paranasal sinusitis in children,” Scientific Reports, 11, 1, Jan. 2021.
- R. P. Rietveld, G. R. P. Rietveld, G. ter Riet, P. J. E. Bindels, J. H. Sloos, and H. C. P. M. van Weert, “Predicting bacterial cause in infectious conjunctivitis: cohort study on informativeness of combinations of signs and symptoms,” BMJ, 329, 7459: 206–210, Jun. 2004.
| Gender | Examined | Infected | Non-infected | ||
|---|---|---|---|---|---|
| No. (%) | No | Rate (%) | No | Rate (%) | |
| Male | 896 (52.03) | 72 | 8.04 | 824 | 91.96 |
| Female | 826 (47.97) | 126 | 15.25 | 700 | 84.75 |
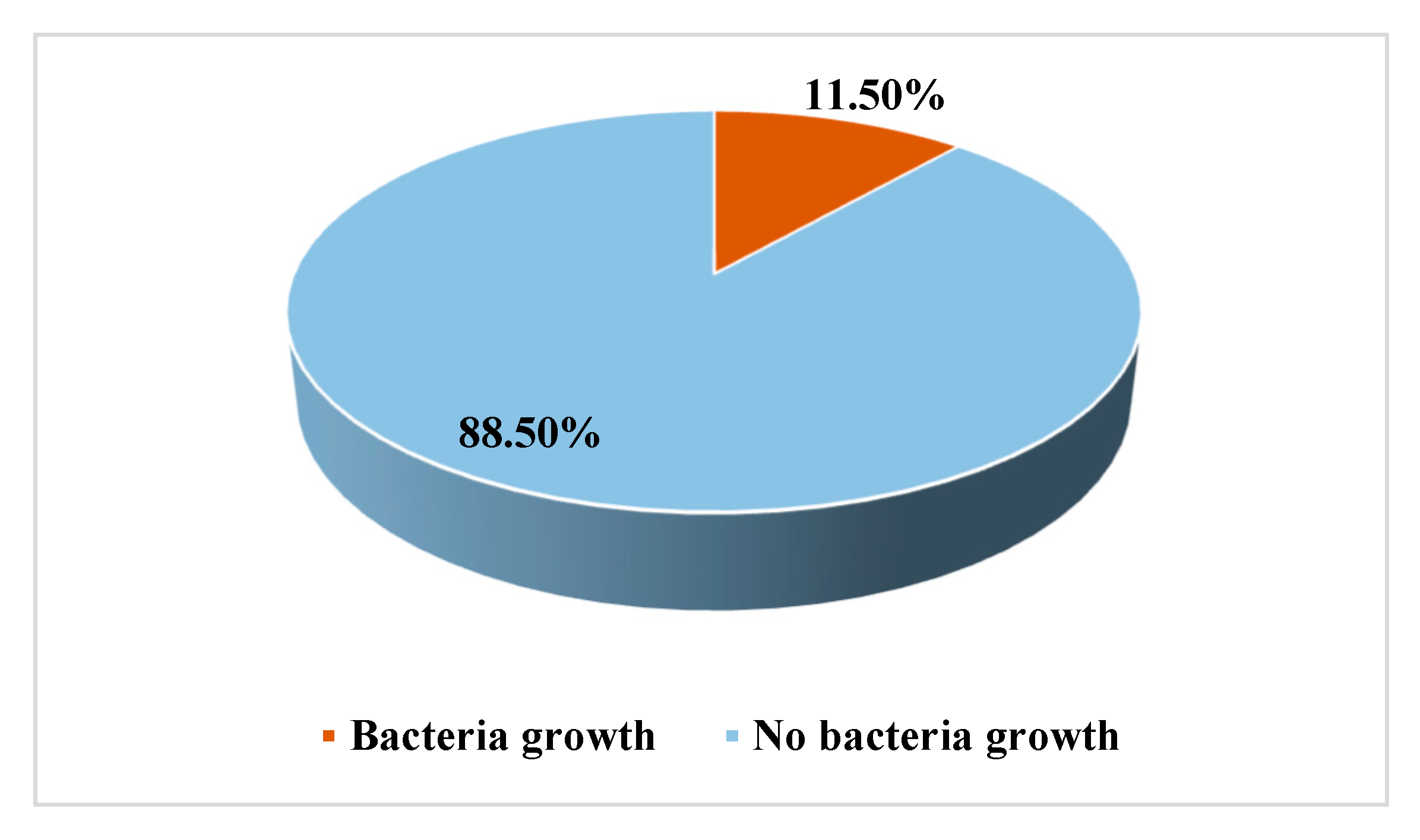
| Total | 1722 | 198 | 11.50 | 1524 | 88.5 |
| Age (years) | Patients (n=198) | Male (n=72) | Female (n=126) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No | (%) | No | (%) | No | (%) | ||||
| <1 | 4 | 2.02 | 0 | 0 | 4 | 3.17 | |||
| 1-10 | 42 | 21.21 | 20 | 27.78 | 22 | 17.46 | |||
| 11-20 | 26 | 13.13 | 10 | 13.89 | 16 | 12.70 | |||
| 21-30 | 36 | 18.19 | 14 | 19.44 | 22 | 17.46 | |||
| 31-40 | 58 | 29.29 | 18 | 25.0 | 40 | 31.75 | |||
| 41-50 | 8 | 4.04 | 0 | 0.0 | 8 | 6.35 | |||
| >50 | 24 | 12.12 | 10 | 13.89 | 14 | 11.11 | |||
| Variables | Cases (n=198) | Percentage % |
|---|---|---|
| Red eye | 198 | 100 |
| Tearing | 186 | 93.93 |
| Itching | 180 | 90.9 |
| Pain | 174 | 87.87 |
| Purulent eye | 112 | 56.56 |
| Eye discharge | 76 | 38.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





