Submitted:
04 October 2023
Posted:
05 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bisorca-Gassendorf, L.; Boden, K.T.; Szurman, P.; Al-Nawaiseh, S.; Rickmann, A.; Januschowski, K. Postoperative Endophthalmitis Im Spiegel Der Literatur. Der Ophthalmologe 2021, 118, 210–218. [CrossRef]
- Gentile, R.C.; Shukla, S.; Shah, M.; Ritterband, D.C.; Engelbert, M.; Davis, A.; Hu, D.-N. Microbiological Spectrum and Antibiotic Sensitivity in Endophthalmitis: A 25-Year Review. Ophthalmology 2014, 121, 1634–1642. [CrossRef]
- Bremond-Gignac, D.; Chiambaretta, F.; Milazzo, S. A European Perspective on Topical Ophthalmic Antibiotics: Current and Evolving Options. Ophthalmol Eye Dis 2011, 3, 29–43. [CrossRef]
- Simina, D.S.; Larisa, I.; Otilia, C.; Ana Cristina, G.; Liliana, M.V.; Aurelian, M.G. The Ocular Surface Bacterial Contamination and Its Management in the Prophylaxis of Post Cataract Surgery Endophthalmitis. Rom J Ophthalmol 2021, 65, 2–9. [CrossRef]
- Gutiérrez-Abejón, E.; Herrera-Gómez, F.; Ayestarán-Martínez, I.J.; Álvarez, F.J. Trend in the Use of Topical Ocular Anti-Infectives in a Region of Spain between 2015 and 2019: A Population-Based Registry Study. Rev Esp Quimioter 2020, 33, 453–458. [CrossRef]
- Yu, Z.; Zhu, J.; Jin, J.; Yu, L.; Han, G. Trends in Outpatient Prescribing Patterns for Ocular Topical Anti-Infectives in Six Major Areas of China, 2013-2019. Antibiotics (Basel) 2021, 10. [CrossRef]
- O’Gallagher, M.; Bunce, C.; Hingorani, M.; Larkin, F.; Tuft, S.; Dahlmann-Noor, A. Topical Treatments for Blepharokeratoconjunctivitis in Children. Cochrane Database Syst Rev 2017, 2, CD011965. [CrossRef]
- Wayne, P. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard-Tenth Edition; Clinical and Laboratory Standards Institute, Ed.; 10th ed.; 2015;
- Peck, T.J.; Patel, S.N.; Ho, A.C. Endophthalmitis after Cataract Surgery: An Update on Recent Advances. Curr Opin Ophthalmol 2021, 32, 62–68. [CrossRef]
- Bowen, R.C.; Zhou, A.X.; Bondalapati, S.; Lawyer, T.W.; Snow, K.B.; Evans, P.R.; Bardsley, T.; McFarland, M.; Kliethermes, M.; Shi, D.; et al. Comparative Analysis of the Safety and Efficacy of Intracameral Cefuroxime, Moxifloxacin and Vancomycin at the End of Cataract Surgery: A Meta-Analysis. British Journal of Ophthalmology 2018, 102, 1268–1276. [CrossRef]
- Grzybowski, A.; Brona, P.; Zeman, L.; Stewart, M.W. Commonly Used Intracameral Antibiotics for Endophthalmitis Prophylaxis: A Literature Review. Surv Ophthalmol 2021, 66, 98–108. [CrossRef]
- George, N.K.; Stewart, M.W. The Routine Use of Intracameral Antibiotics to Prevent Endophthalmitis After Cataract Surgery: How Good Is the Evidence? Ophthalmol Ther 2018, 7, 233–245. [CrossRef]
- Brockhaus, L.; Goldblum, D.; Eggenschwiler, L.; Zimmerli, S.; Marzolini, C. Revisiting Systemic Treatment of Bacterial Endophthalmitis: A Review of Intravitreal Penetration of Systemic Antibiotics. Clin Microbiol Infect 2019, 25, 1364–1369. [CrossRef]
- Chen, K.-J.; Sun, M.-H.; Hou, C.-H.; Chen, H.-C.; Chen, Y.-P.; Wang, N.-K.; Liu, L.; Wu, W.-C.; Chou, H.-D.; Kang, E.Y.-C.; et al. Susceptibility of Bacterial Endophthalmitis Isolates to Vancomycin, Ceftazidime, and Amikacin. Sci Rep 2021, 11, 15878. [CrossRef]
- Chen, K.-J.; Sun, M.-H.; Hou, C.-H.; Chen, H.-C.; Chen, Y.-P.; Wang, N.-K.; Liu, L.; Wu, W.-C.; Chou, H.-D.; Kang, E.Y.-C.; et al. Susceptibility of Bacterial Endophthalmitis Isolates to Vancomycin, Ceftazidime, and Amikacin. Sci Rep 2021, 11, 15878. [CrossRef]
- Relhan, N.; Albini, T.A.; Pathengay, A.; Kuriyan, A.E.; Miller, D.; Flynn, H.W. Endophthalmitis Caused by Gram-Positive Organisms with Reduced Vancomycin Susceptibility: Literature Review and Options for Treatment. Br J Ophthalmol 2016, 100, 446–452. [CrossRef]
- Babalola, O.E. Intravitreal Linezolid in the Management of Vancomycin-Resistant Enterococcal Endophthalmitis. Am J Ophthalmol Case Rep 2020, 20, 100974. [CrossRef]
- Reddy, A.K.; Reddy, R.R.; Paruvelli, M.R.; Ambatipudi, S.; Rani, A.; Lodhi, S.A.K.; Reddy, J.M.L.; Reddy, K.R.; Pandey, N.; Videkar, R.; et al. Susceptibility of Bacterial Isolates to Vancomycin and Ceftazidime from Patients with Endophthalmitis: Is There a Need to Change the Empirical Therapy in Suspected Bacterial Endophthalmitis? Int Ophthalmol 2015, 35, 37–42. [CrossRef]
- Shao, E.H.; Yates, W.B.; Ho, I.-V.; Chang, A.A.; Simunovic, M.P. Endophthalmitis: Changes in Presentation, Management and the Role of Early Vitrectomy. Ophthalmol Ther 2021, 10, 877–890. [CrossRef]
- Fliney, G.D.; Pecen, P.E.; Cathcart, J.N.; Palestine, A.G. Trends in Treatment Strategies for Suspected Bacterial Endophthalmitis. Graefes Arch Clin Exp Ophthalmol 2018, 256, 833–838. [CrossRef]
- Patel, S.N.; Storey, P.P.; Levin, H.; Pancholy, M.; Obeid, A.; Wibbelsman, T.D.; Kuley, B.; Ho, A.C.; Hsu, J.; Garg, S.J.; et al. Endophthalmitis after Cataract Surgery: Changes in Management Based on Microbiologic Cultures. Ophthalmol Retina 2021, 5, 16–22. [CrossRef]
- Liu, C.; Ji, J.; Li, S.; Wang, Z.; Tang, L.; Cao, W.; Sun, X. Microbiological Isolates and Antibiotic Susceptibilities: A 10-Year Review of Culture-Proven Endophthalmitis Cases. Curr Eye Res 2017, 42, 443–447. [CrossRef]
- Tabatabaei, S.A.; Aminzade, S.; Ahmadraji, A.; Soleimani, M.; Sefidan, B.B.; Kasaee, A.; Cheraqpour, K. Early and Complete Vitrectomy versus Tap and Inject in Acute Post Cataract Surgery Endophthalmitis Presenting with Hand Motion Vision; a Quasi-Experimental Study. BMC Ophthalmol 2022, 22, 16. [CrossRef]
- Muqit, M.M.; Mehat, M.; Bunce, C.; Bainbridge, J.W. Early Vitrectomy for Exogenous Endophthalmitis Following Surgery. Cochrane Database of Systematic Reviews 2022, 2022. [CrossRef]
- Montan, P.G.; Wejde, G.; Koranyi, G.; Rylander, M. Prophylactic Intracameral Cefuroxime. Efficacy in Preventing Endophthalmitis after Cataract Surgery. J Cataract Refract Surg 2002, 28, 977–981. [CrossRef]
- Ma, X.; Xie, L.; Huang, Y. <p>Intraoperative Cefuroxime Irrigation Prophylaxis for Acute-Onset Endophthalmitis After Phacoemulsification Surgery</P>. Infect Drug Resist 2020, Volume 13, 1455–1463. [CrossRef]
- Rahman, N.; Murphy, C.C. Impact of Intracameral Cefuroxime on the Incidence of Postoperative Endophthalmitis Following Cataract Surgery in Ireland. Ir J Med Sci 2015, 184, 395–398. [CrossRef]
- Röck, T.; Bramkamp, M.; Bartz-Schmidt, K.-U.; Mutlu, U.; Yörük, E.; Röck, D.; Thaler, S. Reduktion Der Postoperativen Endophthalmitisrate Durch Intrakamerale Cerfuroximgabe: Ergebnisse Aus 5 Jahren Erfahrungen an Der Universitäts-Augenklinik Tübingen. Klin Monbl Augenheilkd 2014, 231, 1023–1028. [CrossRef]
- Conci, L. da S.; Favarato, A.P.; Pinheiro, A.G. Cost Effectiveness of Intracameral Cefuroxime Prophylaxis and Its Efficacy in Preventing Endophthalmitis after Cataract Surgery in a Referral Hospital. Arq Bras Oftalmol 2022, 86. [CrossRef]
- Friling, E.; Montan, P. Bacteriology and Cefuroxime Resistance in Endophthalmitis Following Cataract Surgery before and after the Introduction of Prophylactic Intracameral Cefuroxime: A Retrospective Single-Centre Study. Journal of Hospital Infection 2019, 101, 88–92. [CrossRef]
- Ng, A.L.-K.; Tang, W.W.-T.; Li, P.S.-H.; Li, K.K.-W. Intracameral Cefuroxime in the Prevention of Postoperative Endophthalmitis: An Experience from Hong Kong. Graefe’s Archive for Clinical and Experimental Ophthalmology 2016, 254, 1987–1992. [CrossRef]
- Ng, A.L.-K.; Tang, W.W.-T.; Li, P.S.-H.; Li, K.K.-W. Intracameral Cefuroxime in the Prevention of Postoperative Endophthalmitis: An Experience from Hong Kong. Graefes Arch Clin Exp Ophthalmol 2016, 254, 1987–1992. [CrossRef]
- Bowen, R.C.; Zhou, A.X.; Bondalapati, S.; Lawyer, T.W.; Snow, K.B.; Evans, P.R.; Bardsley, T.; McFarland, M.; Kliethermes, M.; Shi, D.; et al. Comparative Analysis of the Safety and Efficacy of Intracameral Cefuroxime, Moxifloxacin and Vancomycin at the End of Cataract Surgery: A Meta-Analysis. Br J Ophthalmol 2018, 102, 1268–1276. [CrossRef]
- Arshinoff, S.A.; Felfeli, T.; Modabber, M. Aqueous Level Abatement Profiles of Intracameral Antibiotics: A Comparative Mathematical Model of Moxifloxacin, Cefuroxime, and Vancomycin with Determination of Relative Efficacies. J Cataract Refract Surg 2019, 45, 1568–1574. [CrossRef]
- Suzuki, T.; Yamamoto, T.; Torikai, T.; Ohashi, Y. Combination Effect of Cefuroxime and Levofloxacin Against Bacteria Isolated from the Healthy Conjunctival Sac and Endophthalmitis Cases Using a Fractional Inhibitory Concentration Index. Journal of Ocular Pharmacology and Therapeutics 2017, 33, 19–23. [CrossRef]
- Shorstein, N.H.; Liu, L.; Carolan, J.A.; Herrinton, L. Endophthalmitis Prophylaxis Failures in Patients Injected With Intracameral Antibiotic During Cataract Surgery. Am J Ophthalmol 2021, 227, 166–172. [CrossRef]
- Mesnard, C.; Beral, L.; Hage, R.; Merle, H.; Farès, S.; David, T. Endophthalmitis after Cataract Surgery despite Intracameral Antibiotic Prophylaxis with Licensed Cefuroxime. J Cataract Refract Surg 2016, 42, 1318–1323. [CrossRef]
- Gower, E.W.; Lindsley, K.; Tulenko, S.E.; Nanji, A.A.; Leyngold, I.; McDonnell, P.J. Perioperative Antibiotics for Prevention of Acute Endophthalmitis after Cataract Surgery. Cochrane Database Syst Rev 2017, 2, CD006364. [CrossRef]
- Sun, J.; Guo, Z.; Li, H.; Yang, B.; Wu, X. Acute Infectious Endophthalmitis After Cataract Surgery: Epidemiological Characteristics, Risk Factors and Incidence Trends, 2008-2019. Infect Drug Resist 2021, 14, 1231–1238. [CrossRef]
- Shockley, R.K.; Jay, W.M.; Friberg, T.R.; Aziz, A.M.; Rissing, J.P.; Aziz, M.Z. Intravitreal Ceftriaxone in a Rabbit Model. Dose- and Time-Dependent Toxic Effects and Pharmacokinetic Analysis. Arch Ophthalmol 1984, 102, 1236–1238. [CrossRef]
- Tiecco, G.; Laurenda, D.; Mulè, A.; Arsuffi, S.; Storti, S.; Migliorati, M.; Boldini, A.; Signorini, L.; Castelli, F.; Quiros-Roldan, E. Gram-Negative Endogenous Endophthalmitis: A Systematic Review. Microorganisms 2022, 11, 80. [CrossRef]
- Brockhaus, L.; Goldblum, D.; Eggenschwiler, L.; Zimmerli, S.; Marzolini, C. Revisiting Systemic Treatment of Bacterial Endophthalmitis: A Review of Intravitreal Penetration of Systemic Antibiotics. Clinical Microbiology and Infection 2019, 25, 1364–1369. [CrossRef]
- SCHAUERSBERGER, J.; AMON, M.; WEDRICH, A.; NEPP, J.; EL MENYAWI, I.; DERBOLAV, A.; GRANINGER, W. Penetration and Decay of Meropenem into the Human Aqueous Humor and Vitreous. Journal of Ocular Pharmacology and Therapeutics 1999, 15, 439–445. [CrossRef]
- Tappeiner, C.; Schuerch, K.; Goldblum, D.; Zimmerli, S.; Fleischhauer, J.; Frueh, B. Combined Meropenem and Linezolid as a Systemic Treatment for Postoperative Endophthalmitis. Klin Monbl Augenheilkd 2010, 227, 257–261. [CrossRef]
- Bozkurt, E.; Muhafiz, E.; Kepenek, H.S.; Bozlak, Ç.E.B.; Koç Saltan, S.; Bingol, S.A. A New Treatment Experience in Pseudomonas Keratitis: Topical Meropenem and Cefepime. Eye & Contact Lens: Science & Clinical Practice 2021, 47, 174–179. [CrossRef]
- Sueke, H.; Kaye, S.; Wilkinson, M.C.; Kennedy, S.; Kearns, V.; Zheng, Y.; Roberts, P.; Tuft, S.; Neal, T. Pharmacokinetics of Meropenem for Use in Bacterial Keratitis. Investigative Opthalmology & Visual Science 2015, 56, 5731. [CrossRef]
- Galvis, V.; Tello, A.; Sánchez, W.; Camacho, P.; Villarreal, D.; García, D. Minimum Inhibitory Concentrations and Resistance for Selected Antimicrobial Agents (Including Imipenem, Linezolid and Tigecycline) of Bacteria Obtained from Eye Infections. Rom J Ophthalmol 2020, 64, 269–279.
- Gulten, M.Ay.S.C.Akhan.S.Erturk.E.S.Aktas.S.K.Ozkara.Y.Caglar. Comparison of Intravitreal Ceftazidime and Meropenem in Treatment of Experimental Pseudomonal Posttraumatic Endophthalmitis in a Rabbit Model. J Appl Res 2004, 4, 336–345.
- Radhika, M.; Mithal, K.; Bawdekar, A.; Dave, V.; Jindal, A.; Relhan, N.; Albini, T.; Pathengay, A.; Flynn, H.W. Pharmacokinetics of Intravitreal Antibiotics in Endophthalmitis. J Ophthalmic Inflamm Infect 2014, 4, 22. [CrossRef]
- Sharma, S. Antibiotic Resistance in Ocular Bacterial Pathogens. Indian J Med Microbiol 2011, 29, 218–222. [CrossRef]
- McDonald, M.; Blondeau, J.M. Emerging Antibiotic Resistance in Ocular Infections and the Role of Fluoroquinolones. J Cataract Refract Surg 2010, 36, 1588–1598. [CrossRef]
- Grandi, G.; Bianco, G.; Boattini, M.; Scalabrin, S.; Iannaccone, M.; Fea, A.; Cavallo, R.; Costa, C. Bacterial Etiology and Antimicrobial Resistance Trends in Ocular Infections: A 30-Year Study, Turin Area, Italy. Eur J Ophthalmol 2021, 31, 405–414. [CrossRef]
- Miller, D. Update on the Epidemiology and Antibiotic Resistance of Ocular Infections. Middle East Afr J Ophthalmol 2017, 24, 30–42. [CrossRef]
- Milder, E.; Vander, J.; Shah, C.; Garg, S. Changes in Antibiotic Resistance Patterns of Conjunctival Flora Due to Repeated Use of Topical Antibiotics after Intravitreal Injection. Ophthalmology 2012, 119, 1420–1424. [CrossRef]
- Baudin, F.; Benzenine, E.; Mariet, A.-S.; Ghezala, I. Ben; Bron, A.M.; Daien, V.; Gabrielle, P.-H.; Quantin, C.; Creuzot-Garcher, C. Topical Antibiotic Prophylaxis and Intravitreal Injections: Impact on the Incidence of Acute Endophthalmitis—A Nationwide Study in France from 2009 to 2018. Pharmaceutics 2022, 14, 2133. [CrossRef]
- Sanfilippo, C.M.; Morrissey, I.; Janes, R.; Morris, T.W. Surveillance of the Activity of Aminoglycosides and Fluoroquinolones Against Ophthalmic Pathogens from Europe in 2010–2011. Curr Eye Res 2015, 1–9. [CrossRef]
- Chatterjee, S.; Agrawal, D.; Gomase, S.; Parchand, S.; Gangwe, A.; Mishra, M. Fluoroquinolone Resistance in Bacterial Isolates from Ocular Infections: Trend in Antibiotic Susceptibility Patterns between 2005-2020. Indian J Ophthalmol 2022, 70, 4391. [CrossRef]
- Iwasaki, T.; Nejima, R.; Miyata, K. Ocular Surface Flora and Prophylactic Antibiotics for Cataract Surgery in the Age of Antimicrobial Resistance. Jpn J Ophthalmol 2022. [CrossRef]
- Schechter, B.A.; Sheppard, J.D.; Sanfilippo, C.M.; DeCory, H.H.; Asbell, P.A. An Evaluation of Staphylococci from Ocular Surface Infections Treated Empirically with Topical Besifloxacin: Antibiotic Resistance, Molecular Characteristics, and Clinical Outcomes. Ophthalmol Ther 2020, 9, 159–173. [CrossRef]
- Durrani, A.F.; Atta, S.; Bhat, A.K.; Mammen, A.; Dhaliwal, D.; Kowalski, R.P.; Jhanji, V. Methicillin-Resistant Staphylococcus Aureus Keratitis: Initial Treatment, Risk Factors, Clinical Features, and Treatment Outcomes. Am J Ophthalmol 2020, 214, 119–126. [CrossRef]
- Ahmad, A.; Rehman, M. EFFICACY OF INJECTING INTRA-VITREAL MOXIFLOXACIN IN ACUTE POST-OPERATIVE ENDOPHTHALMITIS. Journal of Ayub Medical College Abbottabad 2023, 35. [CrossRef]
- Kuriakose, R.K.; Cho, S.; Nassiri, S.; Hwang, F.S. Comparative Outcomes of Standard Perioperative Eye Drops, Intravitreal Triamcinolone Acetonide-Moxifloxacin, and Intracameral Dexamethasone-Moxifloxacin-Ketorolac in Cataract Surgery. J Ophthalmol 2022, 2022, 1–8. [CrossRef]
- Lucena, N. de P.; Pereira, I.M. de S.; Gaete, M.I.L.; Ferreira, K.S.A.; Mélega, M.V.; Lira, R.P.C. Intracameral Moxifloxacin after Cataract Surgery: A Prospective Study. Arq Bras Oftalmol 2018, 81. [CrossRef]
- Leung, E.H.; Gibbons, A.; Stout, T.J.; Koch, D.D. Intracameral Moxifloxacin for Endophthalmitis Prophylaxis after Cataract Surgery: Cost-Effectiveness Analysis. J Cataract Refract Surg 2018, 44, 971–978. [CrossRef]
- Mitchell, W.; Tom, L.; Durai, I.; Rajagopal, S.; Vimalanathan, M.; Rengaraj, V.; Srinivasan, K.; Zebardast, N. The Effectiveness of Intracameral Moxifloxacin Endophthalmitis Prophylaxis for Trabeculectomy. Ophthalmol Glaucoma 2021, 4, 11–19. [CrossRef]
- Bispo, P.J.M.; Sahm, D.F.; Asbell, P.A. A Systematic Review of Multi-Decade Antibiotic Resistance Data for Ocular Bacterial Pathogens in the United States. Ophthalmol Ther 2022, 11, 503–520. [CrossRef]
- Petrillo, F.; Pignataro, D.; Di Lella, F.M.; Reibaldi, M.; Fallico, M.; Castellino, N.; Parisi, G.; Trotta, M.C.; D’Amico, M.; Santella, B.; et al. Antimicrobial Susceptibility Patterns and Resistance Trends of Staphylococcus Aureus and Coagulase-Negative Staphylococci Strains Isolated from Ocular Infections. Antibiotics 2021, 10, 527. [CrossRef]
- Asbell, P.A.; DeCory, H.H. Antibiotic Resistance among Bacterial Conjunctival Pathogens Collected in the Antibiotic Resistance Monitoring in Ocular Microorganisms (ARMOR) Surveillance Study. PLoS One 2018, 13, e0205814. [CrossRef]
- Cagini, C.; Piccinelli, F.; Lupidi, M.; Messina, M.; Cerquaglia, A.; Manes, S.; Fiore, T.; Pellegrino, R.M. Ocular Penetration of Topical Antibiotics: Study on the Penetration of Chloramphenicol, Tobramycin and Netilmicin into the Anterior Chamber after Topical Administration. Clin Exp Ophthalmol 2013, 41, 644–647. [CrossRef]
- Herrinton, L.J.; Shorstein, N.H.; Paschal, J.F.; Liu, L.; Contreras, R.; Winthrop, K.L.; Chang, W.J.; Melles, R.B.; Fong, D.S. Comparative Effectiveness of Antibiotic Prophylaxis in Cataract Surgery. Ophthalmology 2016, 123, 287–294. [CrossRef]
- Ma, W.; Hou, G.; Wang, J.; Liu, T.; Tian, F. Evaluation of the Effect of Gentamicin in Surgical Perfusion Solution on Cataract Postoperative Endophthalmitis. BMC Ophthalmol 2022, 22, 410. [CrossRef]
- Torres-Costa, S.; Ramos, D.; Brandão, E.; Carneiro, Â.; Rosas, V.; Rocha-Sousa, A.; Falcão-Reis, F.; Falcão, M. Incidence of Endophthalmitis after Intravitreal Injection with and without Topical Antibiotic Prophylaxis. Eur J Ophthalmol 2021, 31, 600–606. [CrossRef]
- Baudin, F.; Benzenine, E.; Mariet, A.-S.; Ghezala, I. Ben; Bron, A.M.; Daien, V.; Gabrielle, P.-H.; Quantin, C.; Creuzot-Garcher, C. Topical Antibiotic Prophylaxis and Intravitreal Injections: Impact on the Incidence of Acute Endophthalmitis-A Nationwide Study in France from 2009 to 2018. Pharmaceutics 2022, 14. [CrossRef]
- Kareem Rhumaid, A.; Alak Mahdi Al-Buhilal, J.; Al-Rubaey, N.K.F.; Yassen Al-Zamily, K. Prevalence and Antibiotic Susceptibility of Pathogenic Bacteria Associated with Ocular Infections in Adult Patients. Arch Razi Inst 2022, 77, 1917–1924. [CrossRef]
- Oong, G.C.; Tadi, P. Chloramphenicol; 2023;
- Bale, B.I.; Elebesunu, E.E.; Manikavasagar, P.; Agwuna, F.O.; Ogunkola, I.O.; Sow, A.U.; Lucero-Prisno, D.E. Antibiotic Resistance in Ocular Bacterial Infections: An Integrative Review of Ophthalmic Chloramphenicol. Trop Med Health 2023, 51, 15. [CrossRef]
- Nithya, V.; Rathinam, S.; Siva Ganesa Karthikeyan, R.; Lalitha, P. A Ten Year Study of Prevalence, Antimicrobial Susceptibility Pattern, and Genotypic Characterization of Methicillin Resistant Staphylococcus Aureus Causing Ocular Infections in a Tertiary Eye Care Hospital in South India. Infect Genet Evol 2019, 69, 203–210. [CrossRef]
- Lorenzo, D. Chloramphenicol Resurrected: A Journey from Antibiotic Resistance in Eye Infections to Biofilm and Ocular Microbiota. Microorganisms 2019, 7. [CrossRef]
- Grandi, G.; Bianco, G.; Boattini, M.; Scalabrin, S.; Iannaccone, M.; Fea, A.; Cavallo, R.; Costa, C. Bacterial Etiology and Antimicrobial Resistance Trends in Ocular Infections: A 30-Year Study, Turin Area, Italy. Eur J Ophthalmol 2021, 31, 405–414. [CrossRef]
- Wong, E.S.; Chow, C.W.Y.; Luk, W.K.; Fung, K.S.C.; Li, K.K.W. A 10-Year Review of Ocular Methicillin-Resistant Staphylococcus Aureus Infections: Epidemiology, Clinical Features, and Treatment. Cornea 2017, 36, 92–97. [CrossRef]
- Harford, D.A.; Greenan, E.; Knowles, S.J.; Fitzgerald, S.; Murphy, C.C. The Burden of Methicillin-Resistant Staphylococcus Aureus in the Delivery of Eye Care. Eye (Lond) 2022, 36, 1368–1372. [CrossRef]
- Croghan, C.; Lockington, D. Management of MRSA-Positive Eye Swabs and the Potential Advantages of Chloramphenicol Availability in the United Kingdom. Eye (Lond) 2018, 32, 157–159. [CrossRef]
- Bhattacharya, P.; Singha, M.; Senapati, K.; Saha, S.; Mandal, S.; Mandal, S.M.; Ghosh, A.K.; Basak, A. Chloramphenicol-Borate/Boronate Complex for Controlling Infections by Chloramphenicol-Resistant Bacteria. RSC Adv 2018, 8, 18016–18022. [CrossRef]
- Andaluz-Scher, L.; Medow, N.B. Chloramphenicol Eye Drops: An Old Dog in a New House. Ophthalmology 2020, 127, 1289–1291.
- Cave, J.A. Chloramphenicol Eye Drops, Boron, Infants and Fertility. Drug Ther Bull 2021, 59, 98.
- Evans, J.R.; Solomon, A.W.; Kumar, R.; Perez, Á.; Singh, B.P.; Srivastava, R.M.; Harding-Esch, E. Antibiotics for Trachoma. Cochrane Database Syst Rev 2019, 9, CD001860. [CrossRef]
- Ta, C.N.; Chang, R.T.; Singh, K.; Egbert, P.R.; Shriver, E.M.; Blumenkranz, M.S.; Miño de Kaspar, H. Antibiotic Resistance Patterns of Ocular Bacterial Flora: A Prospective Study of Patients Undergoing Anterior Segment Surgery. Ophthalmology 2003, 110, 1946–1951. [CrossRef]
- Belyhun, Y.; Moges, F.; Endris, M.; Asmare, B.; Amare, B.; Bekele, D.; Tesfaye, S.; Alemayehu, M.; Biadgelegne, F.; Mulu, A.; et al. Ocular Bacterial Infections and Antibiotic Resistance Patterns in Patients Attending Gondar Teaching Hospital, Northwest Ethiopia. BMC Res Notes 2018, 11, 597. [CrossRef]
- Khan, M.; Willcox, M.D.P.; Rice, S.A.; Sharma, S.; Stapleton, F. Development of Antibiotic Resistance in the Ocular Pseudomonas Aeruginosa Clone ST308 over Twenty Years. Exp Eye Res 2021, 205, 108504. [CrossRef]
- Toribio, A.; Marrodán, T.; Fernández-Natal, I.; Martínez-Blanco, H.; Rodríguez-Aparicio, L.; Ferrero, M.Á. Conjunctival Flora in Anophthalmic Patients: Microbiological Spectrum and Antibiotic Sensitivity. Int J Ophthalmol 2019, 12, 765–773. [CrossRef]
- Petrillo, F.; Pignataro, D.; Di Lella, F.M.; Reibaldi, M.; Fallico, M.; Castellino, N.; Parisi, G.; Trotta, M.C.; D’Amico, M.; Santella, B.; et al. Antimicrobial Susceptibility Patterns and Resistance Trends of Staphylococcus Aureus and Coagulase-Negative Staphylococci Strains Isolated from Ocular Infections. Antibiotics (Basel) 2021, 10. [CrossRef]
- Gaynor, B.D.; Chidambaram, J.D.; Cevallos, V.; Miao, Y.; Miller, K.; Jha, H.C.; Bhatta, R.C.; Chaudhary, J.S.P.; Osaki Holm, S.; Whitcher, J.P.; et al. Topical Ocular Antibiotics Induce Bacterial Resistance at Extraocular Sites. Br J Ophthalmol 2005, 89, 1097–1099. [CrossRef]
- Jabbehdari, S.; Memar, O.M.; Caughlin, B.; Djalilian, A.R. Update on the Pathogenesis and Management of Ocular Rosacea: An Interdisciplinary Review. Eur J Ophthalmol 2021, 31, 22–33. [CrossRef]
- Stone, D.U.; Chodosh, J. Ocular Rosacea: An Update on Pathogenesis and Therapy. Curr Opin Ophthalmol 2004, 15, 499–502.
- Stone, D.U.; Chodosh, J. Oral Tetracyclines for Ocular Rosacea: An Evidence-Based Review of the Literature. Cornea 2004, 23, 106–109. [CrossRef]
- Sharma, A.; Kroumpouzos, G.; Kassir, M.; Galadari, H.; Goren, A.; Grabbe, S.; Goldust, M. Rosacea Management: A Comprehensive Review. J Cosmet Dermatol 2022, 21, 1895–1904. [CrossRef]
- Schaller, M.; Kemény, L.; Havlickova, B.; Jackson, J.M.; Ambroziak, M.; Lynde, C.; Gooderham, M.; Remenyik, E.; Del Rosso, J.; Weglowska, J.; et al. A Randomized Phase 3b/4 Study to Evaluate Concomitant Use of Topical Ivermectin 1% Cream and Doxycycline 40-Mg Modified-Release Capsules, versus Topical Ivermectin 1% Cream and Placebo in the Treatment of Severe Rosacea. J Am Acad Dermatol 2020, 82, 336–343. [CrossRef]
- Halim, M.S.; Onghanseng, N.; Hassan, M.; Besalti, Z.; Ng, S.M.; Nguyen, Q.D. Oral Antibiotics for Chronic Blepharitis. Cochrane Database of Systematic Reviews 2020. [CrossRef]
- Garnock-Jones, K.P. Azithromycin 1.5% Ophthalmic Solution: In Purulent Bacterial or Trachomatous Conjunctivitis. Drugs 2012, 72, 361–373. [CrossRef]
- Opitz, D.L.; Harthan, J.S. Review of Azithromycin Ophthalmic 1% Solution (AzaSite(®)) for the Treatment of Ocular Infections. Ophthalmol Eye Dis 2012, 4, 1–14. [CrossRef]
- Kagkelaris, K.A.; Makri, O.E.; Georgakopoulos, C.D.; Panayiotakopoulos, G.D. An Eye for Azithromycin: Review of the Literature. Ther Adv Ophthalmol 2018, 10, 2515841418783622. [CrossRef]
- Patel, P.H.; Hashmi, M.F. Macrolides; StatPearls Publishing, Treasure Island (FL), 2023.
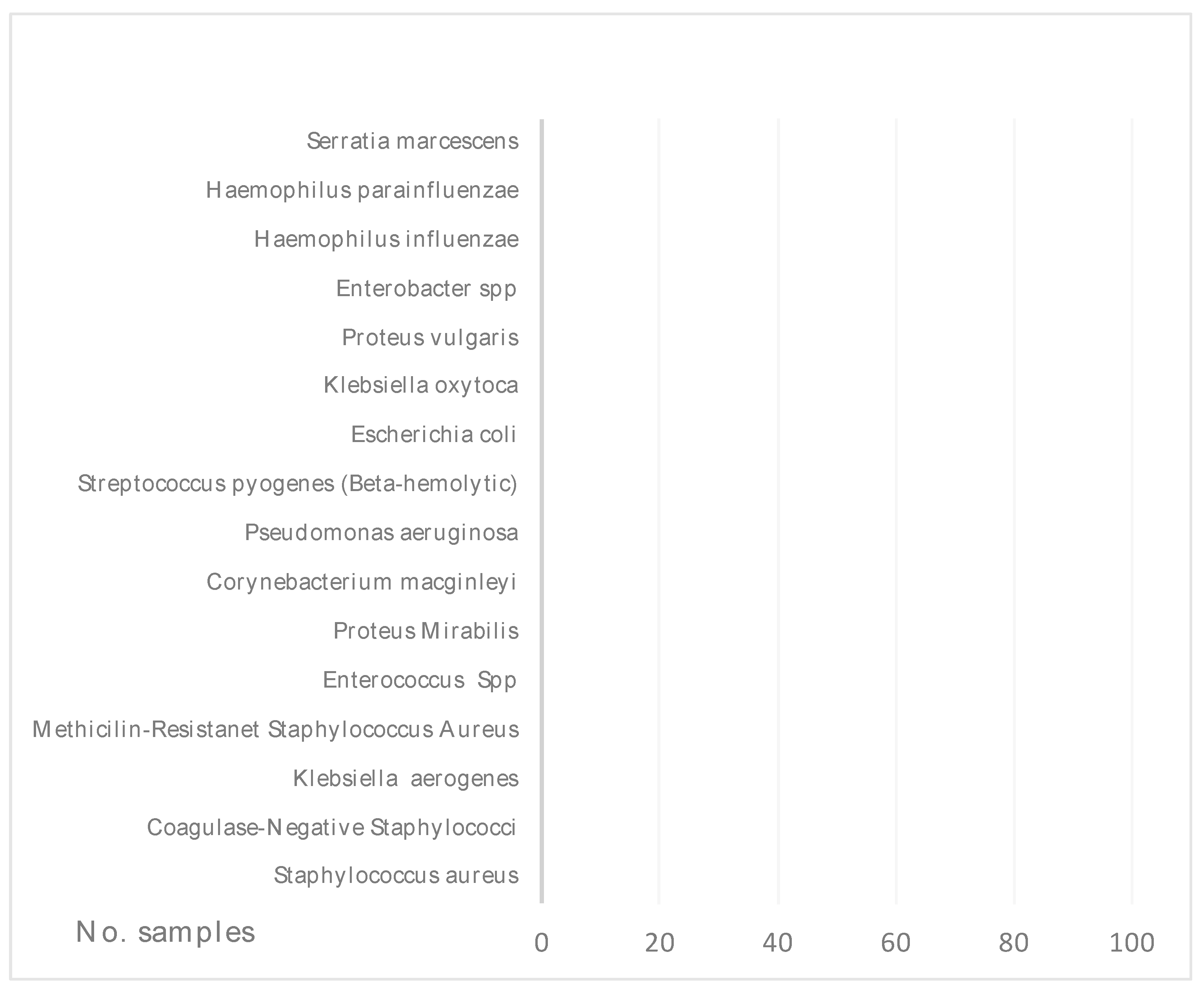
| Bacterial Isolates Species | No. | Antibiotic Sensitivity | Commonly Tested Antimicrobial, No. (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMP | AMX | CLR | CXM | CIP | ERY | GEN | LVX | OFX | TET | TOB | VAN | |||
|
Staphylococcus aureus |
90 |
S | 3 (15.7) | 6(46.15) | 12(52.17) | 19(82.6) | 54(84.37) | 34(47.22) | 55(82.08) | 29(82.85) | 15(75) | 24(52.17) | 25(75.75) | 26(100) |
| I | 0(0) | 0(0) | 0(0) | 0(0) | 5(7.81) | 4(5.55) | 2(2.98) | 4(11.42) | 1(5) | 2(4.34) | 4(12.12) | 0(0) | ||
| R | 16(84.2) | 7(53.84) | 11(47.8) | 4(17.39) | 5(7.81) | 34(47.220 | 10(14.92) | 2(5.71) | 4(20) | 20(43.47) | 4(12.12) | 0(0) | ||
|
Coagulase-Negative Staphylococci |
35 |
S | 1(12.5) | 3(60) | 5(45.45) | 10(76.92) | 19(79.16) | 7(30.43) | 21(70) | 12(85.71) | 5(100) | 7(41.17) | 9(69.23) | 11(91.67) |
| I | 0(0) | 0(0) | 0(0) | 1(7.69) | 2(8.33) | 2(8.69) | 1(3.33) | 0(0) | 0(0) | 1(5.88) | 3(23.07) | 1(8.33) | ||
| R | 7(87.5) | 2(40) | 6(54.54) | 2)15.38) | 3(12.5) | 14(60.86) | 8(26.67) | 2(14.2) | 0(0) | 9(52.94) | 1(7.69) | 0(0) | ||
|
Klebsiella Spp |
7 |
S | 0(0) | 1(100) | - | 2(33.33) | 3(100) | - | 6(100) | 4(100) | 4(100) | 1(100) | 2(100) | - |
| I | 0(0) | 0(0) | - | 0(0) | 0(0) | - | 0(0) | 0(0) | 0(0) | 0(0) | 0(0) | - | ||
| R | 5(100) | 0(0) | - | 4(66.67) | 0(0) | - | 0(0) | 0(0) | 0(0) | 0(0) | 0(0) | - | ||
|
Methicilin-ResistentStaphylococcus Aureus |
5 |
S | - | 0(0) | 1(100) | 1 | 4(80) | 1(33.33) | 2(66.67) | 1(100) | 3(100) | 0(0) | 1(33.33) | 1(100) |
| I | - | 0(0) | 0(0) | 0 | 0(0) | 0(0) | 0(0) | 0(0) | 0(0) | 0(0) | 1(33.33) | 0(0) | ||
| R | - | 1(100) | 0(0) | 2 | 1(20) | 2(66.67) | 1(33.33) | 0(0) | 0(0) | 3(100) | 1(33.33) | 0(0) | ||
|
Enterococcus Spp |
4 |
S | 1(33.33) | - | - | 0 | 3(100) | - | 1(100) | 3(100) | - | 1(50) | - | 3(75) |
| I | 0(0) | - | - | 0 | 0(0) | - | 0(0) | 0(0) | - | 0(0) | - | 0(0) | ||
| R | 2(66.7) | - | - | 0 | 0(0) | - | 0(0) | 0(0) | - | 1(50) | - | 1(25) | ||
|
Proteus Spp |
6 |
S | 1(33.33) | 0(0) | - | 1 | 5(100) | - | 5(100) | 5(100) | 1(100) | 0(0) | 2(100) | - |
| I | 0(0) | 0(0) | - | 0 | 0(0) | - | 0(0) | 0(0) | 0(0) | 1(100) | 0(0) | - | ||
| R | 2(66.67) | 1(100) | - | 1 | 0(0) | - | 0(0) | 0(0) | 0(0) | 0(0) | 0(0) | - | ||
|
Corynebacterium macginleyi |
2 |
S | - | - | - | - | 2(100) | - | - | - | - | 2(100) | - | 1(100) |
| I | - | - | - | - | 0(0) | - | - | - | - | 0(0) | - | 0(0) | ||
| R | - | - | - | - | 0(0) | - | - | - | - | 0(0) | - | 0(0) | ||
|
Pseudomonas aeruginosa |
3 |
S | 0(0) | - | - | - | 3(100) | - | 2(100) | 1(100) | 1(100) | - | 2(100) | - |
| I | 0(0) | - | - | - | 0(0) | - | 0(0) | 0(0) | 0(0) | - | 0(0) | - | ||
| R | 1(100) | - | - | - | 0(0) | - | 0(0) | 0(0) | 0(0) | - | 0(0) | - | ||
|
Streptococcus pyogenes (Beta-hemolytic) |
3 |
S | - | - | - | - | 1(100) | 2(100) | 1(100) | 1(100) | - | 1(50) | - | 2(100) |
| I | - | - | - | - | 0(0) | 0(0) | 0(0) | 0(0) | - | 0(0) | - | 0(0) | ||
| R | - | - | - | - | 0(0) | 0(0) | 0(0) | 0(0) | - | 1(50) | - | 0(0) | ||
|
Escherichia coli |
2 |
S | 0(0) | 1(50) | 1(100) | 1(100) | 2(100) | - | 2(100) | 2(100) | 2(100) | 2(100) | 1(100) | - |
| I | 0(0) | 0(0) | 0(0) | 0(0) | 0(0) | - | 0(0) | 0(0) | 0(0) | 0(0) | 0(0) | - | ||
| R | 2(100) | 1(50) | 0(0) | 0(0) | 0(0) | - | 0(0) | 0(0) | 0(0) | 0(0) | 0(0) | - | ||
|
Enterobacter spp |
1 |
S | 0(0) | 1(50) | - | - | - | - | 1(100) | - | - | - | - | - |
| I | 0(0) | 0(0) | - | - | - | - | 0(0) | - | - | - | - | - | ||
| R | 1(100) | 0(0) | - | - | - | - | 0(0) | - | - | - | - | - | ||
|
Haemophilus spp |
2 |
S | 1(50) | 0(0) | - | - | 2(100) | - | - | 1(100) | 1(100) | 1(100) | - | - |
| I | 0(0) | 0(0) | - | - | 0(0) | - | - | 0(0) | 0(0) | 0(0) | - | - | ||
| R | 1(50) | 1(100) | - | - | 0(0) | - | - | 0(0) | 0(0) | 0(0) | - | - | ||
|
Serratia marcescens |
1 |
S | - | - | - | 0(0) | 1(100) | - | 1(100) | 1(100) | - | - | 1(100) | - |
| I | - | - | - | 0(0) | 0(0) | - | 0(0) | 0(0) | - | - | 0(0) | - | ||
| R | - | - | - | 1(100) | 0(0) | - | 0(0) | 0(0) | - | - | 0(0) | - | ||
| Type of Antibiotic Tested | Abbrev. | Total Number of Bacteria | Response of Bacteria to Antibiotic | |||||
|---|---|---|---|---|---|---|---|---|
| S | I | R | S % | I % | R % | |||
| Vancomycin | VAN | 46 | 44 | 1 | 1 | 97.8 | 2.2 | 0 |
| Ceftriaxone | CRO | 24 | 18 | 2 | 4 | 75 | 8.3 | 16.7 |
| Cefuroxime | CXM | 50 | 35 | 1 | 14 | 70 | 2 | 28 |
| Cefazolin | CFZ | 3 | 1 | 0 | 2 | 33.3 | 0 | 66.7 |
| Meropenem | MEM | 17 | 16 | 0 | 1 | 94.1 | 0 | 5.9 |
| Imipenem | IPM | 15 | 14 | 0 | 1 | 93.3 | 0 | 6.7 |
| Moxifloxacin | MXF | 39 | 32 | 4 | 3 | 82.1 | 7.7 | 10.3 |
| Levofloxacin | LVX | 68 | 60 | 4 | 4 | 88.2 | 5.9 | 5.9 |
| Ofloxacin | OFX | 38 | 33 | 1 | 4 | 86.8 | 2.6 | 10.5 |
| Ciprofloxacin | CIP | 117 | 100 | 7 | 10 | 85.5 | 6 | 8.5 |
| Netilmicin | NET | 20 | 19 | 1 | 0 | 95 | 5 | 0 |
| Tobramycin | TOB | 58 | 44 | 8 | 6 | 75.9 | 13.8 | 10.3 |
| Amikacin | AMK | 20 | 19 | 0 | 1 | 95 | 0 | 5 |
| Kanamycin | KAN | 9 | 6 | 0 | 3 | 66.7 | 0 | 33.3 |
| Gentamicin | GEN | 121 | 99 | 3 | 19 | 81.8 | 2.5 | 15.7 |
| Chloramphenicol | CHL | 54 | 49 | 1 | 4 | 90.7 | 7.4 | 1.9 |
| Tetracycline | TET | 77 | 39 | 4 | 34 | 50.6 | 5.2 | 44.2 |
| Doxycycline | DOX | 24 | 14 | 0 | 10 | 58.3 | 0 | 41.7 |
| Rifampicin | RIF | 41 | 38 | 1 | 2 | 92.7 | 2.4 | 4.9 |
| Azithromycin | AZM | 19 | 6 | 0 | 13 | 31.6 | 0 | 68.4 |
| Clarithromycin | CLR | 36 | 19 | 0 | 17 | 52.8 | 0 | 47.2 |
| Erythromycin | ERY | 100 | 44 | 6 | 50 | 44 | 6 | 50 |
| Ampicillin | AMP | 45 | 7 | 0 | 38 | 15.6 | 0 | 84.4 |
| Amoxicillin | AMX | 20 | 19 | 0 | 1 | 95 | 0 | 5 |
| Type of Antibiotic Tested | Abbrev. | Total Number of Gram-Positive Bacteria | Response of Bacteria to Antibiotic | |||||
|---|---|---|---|---|---|---|---|---|
| S | I | R | S % | I % | R % | |||
| Vancomycin | VAN | 45 | 44 | 1 | 0 | 97.8 | 2.2 | 0 |
| Ceftriaxone | CRO | 15 | 9 | 2 | 4 | 60 | 13.3 | 26.7 |
| Cefuroxime | CXM | 39 | 30 | 1 | 8 | 76.9 | 2.6 | 20.5 |
| Cefazolin | CFZ | 1 | 0 | 0 | 1 | 0 | 0 | 100 |
| Meropenem | MEM | 11 | 10 | 0 | 1 | 90.9 | 0 | 9.1 |
| Imipenem | IPM | 12 | 11 | 0 | 1 | 91.7 | 0 | 8.3 |
| Moxifloxacin | MXF | 36 | 29 | 3 | 4 | 80.6 | 8.3 | 11.1 |
| Levofloxacin | LVX | 54 | 46 | 4 | 4 | 85.2 | 7.4 | 7.4 |
| Ofloxacin | OFX | 28 | 23 | 1 | 4 | 82.1 | 3.6 | 14.3 |
| Ciprofloxacin | CIP | 99 | 82 | 7 | 10 | 82.8 | 7.1 | 10.1 |
| Netilmicin | NET | 19 | 18 | 1 | 0 | 94.7 | 5.3 | 0 |
| Tobramycin | TOB | 48 | 35 | 7 | 6 | 72.9 | 14.6 | 12.5 |
| Amikacin | AMK | 10 | 9 | 0 | 1 | 90 | 0 | 10 |
| Kanamycin | KAN | 8 | 5 | 0 | 3 | 62.5 | 0 | 37.5 |
| Gentamicin | GEN | 102 | 80 | 3 | 19 | 78.4 | 2.9 | 18.6 |
| Chloramphenicol | CHL | 47 | 42 | 1 | 4 | 89.4 | 2.1 | 8.5 |
| Tetracycline | TET | 70 | 33 | 3 | 34 | 47.1 | 4.3 | 48.6 |
| Doxycycline | DOX | 19 | 9 | 0 | 10 | 47.4 | 0 | 52.6 |
| Rifampicin | RIF | 38 | 36 | 1 | 1 | 94.7 | 2.6 | 2.6 |
| Azithromycin | AZM | 18 | 5 | 0 | 13 | 27.8 | 0 | 72.2 |
| Clarithromycin | CLR | 35 | 18 | 0 | 17 | 51.4 | 0 | 48.6 |
| Erythromycin | ERY | 99 | 44 | 6 | 49 | 44.4 | 6.1 | 49.5 |
| Ampicillin | AMP | 29 | 5 | 0 | 24 | 17.2 | 0 | 82.8 |
| Amoxicillin | AMX | 19 | 9 | 0 | 10 | 47.4 | 0 | 52.6 |
| Type of Antibiotic Tested | Abbrev. | Total Number of Gram-Negative Bacteria | Response of Bacteria to Antibiotic | |||||
|---|---|---|---|---|---|---|---|---|
| S | I | R | S % | I % | R % | |||
| Vancomycin | VAN | 1 | 0 | 0 | 1 | 0 | 0 | 100 |
| Ceftriaxone | CRO | 9 | 9 | 0 | 0 | 100 | 0 | 0 |
| Cefuroxime | CXM | 11 | 5 | 0 | 6 | 45.5 | 0 | 54.5 |
| Cefazolin | CFZ | 2 | 1 | 0 | 1 | 50 | 0 | 50 |
| Meropenem | MEM | 6 | 6 | 0 | 0 | 100 | 0 | 0 |
| Imipenem | IPM | 3 | 3 | 0 | 0 | 100 | 0 | 0 |
| Moxifloxacin | MXF | 3 | 3 | 0 | 0 | 100 | 0 | 0 |
| Levofloxacin | LVX | 14 | 14 | 0 | 0 | 100 | 0 | 0 |
| Ofloxacin | OFX | 10 | 10 | 0 | 0 | 100 | 0 | 0 |
| Ciprofloxacin | CIP | 18 | 18 | 0 | 0 | 100 | 0 | 0 |
| Netilmicin | NET | 1 | 1 | 0 | 0 | 100 | 0 | 0 |
| Tobramycin | TOB | 10 | 9 | 1 | 0 | 90 | 10 | 0 |
| Amikacin | AMK | 10 | 10 | 0 | 0 | 100 | 0 | 0 |
| Kanamycin | KAN | 1 | 1 | 0 | 0 | 100 | 0 | 0 |
| Gentamicin | GEN | 19 | 19 | 0 | 0 | 100 | 0 | 0 |
| Chloramphenicol | CHL | 7 | 7 | 0 | 0 | 100 | 0 | 0 |
| Tetracycline | TET | 7 | 6 | 1 | 0 | 85.7 | 14.3 | 0 |
| Doxycycline | DOX | 5 | 5 | 0 | 0 | 100 | 0 | 0 |
| Rifampicin | RIF | 3 | 2 | 0 | 1 | 66.7 | 0 | 33.3 |
| Azithromycin | AZM | 1 | 1 | 0 | 0 | 100 | 0 | 0 |
| Clarithromycin | CLR | 1 | 1 | 0 | 0 | 100 | 0 | 0 |
| Erythromycin | ERY | 1 | 0 | 0 | 1 | 0 | 0 | 100 |
| Ampicillin | AMP | 16 | 2 | 0 | 14 | 12.5 | 0 | 87.5 |
| Amoxicillin | AMX | 6 | 3 | 0 | 3 | 50 | 0 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




