Submitted:
18 November 2023
Posted:
20 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
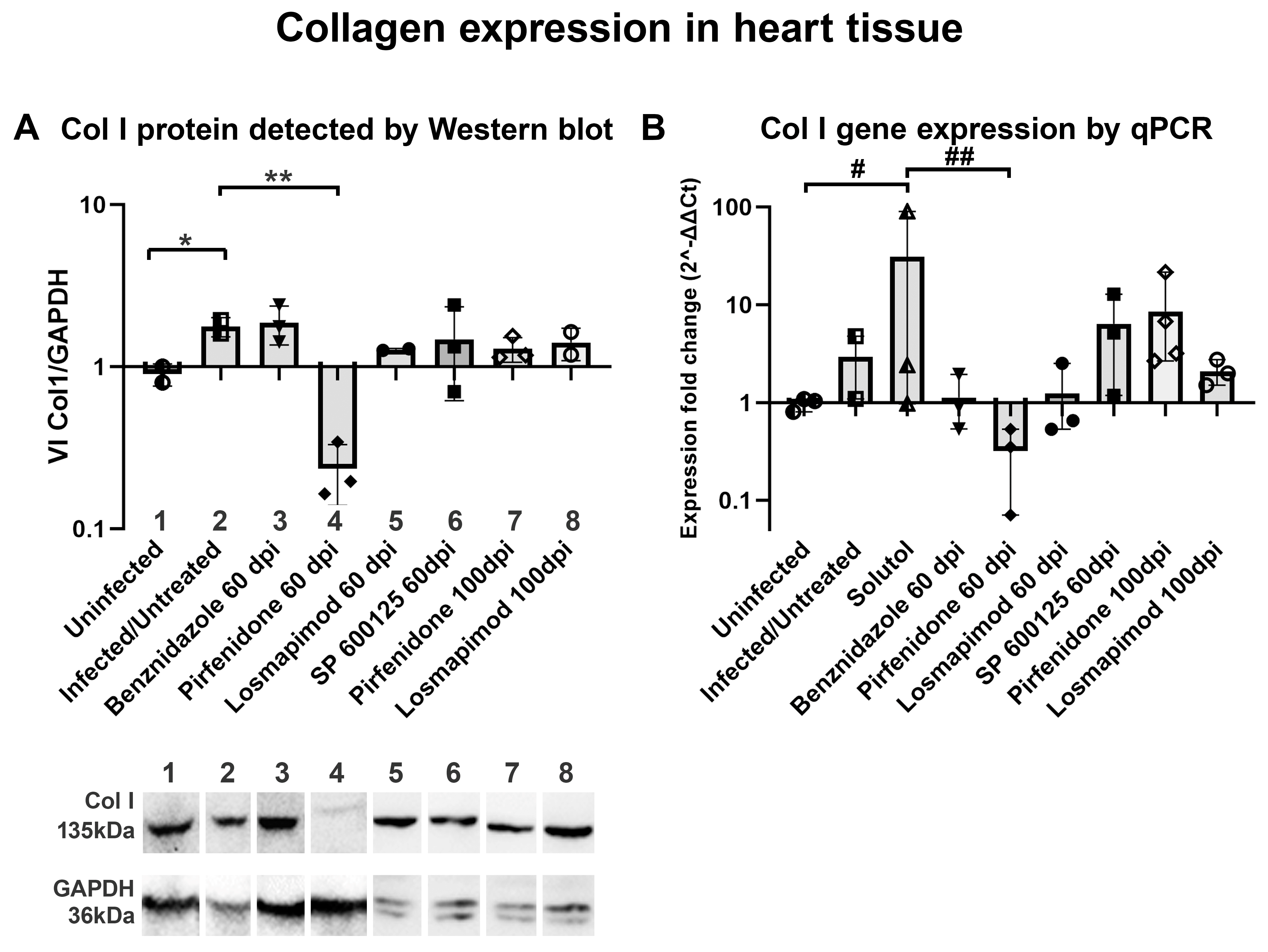
2. Results
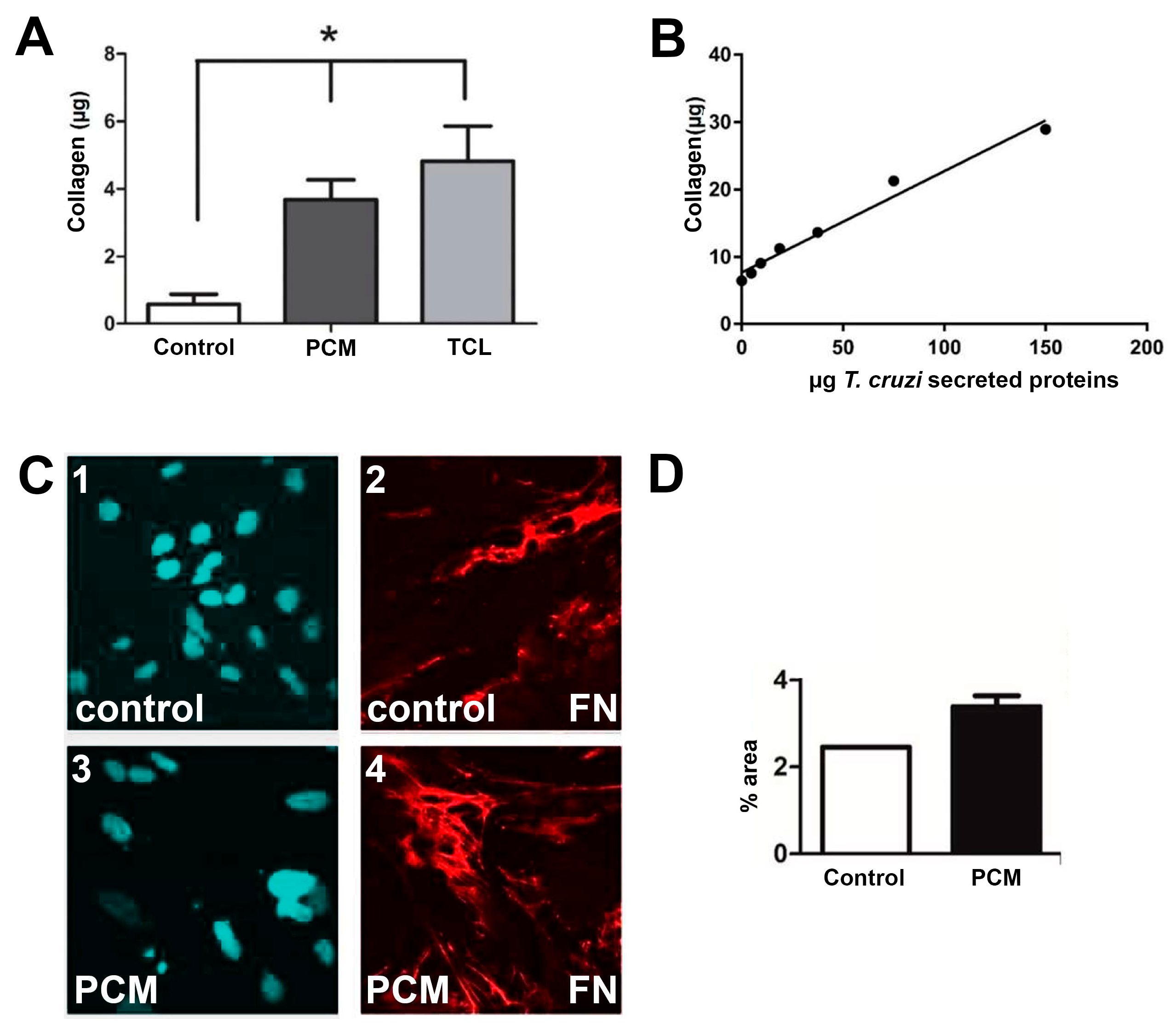
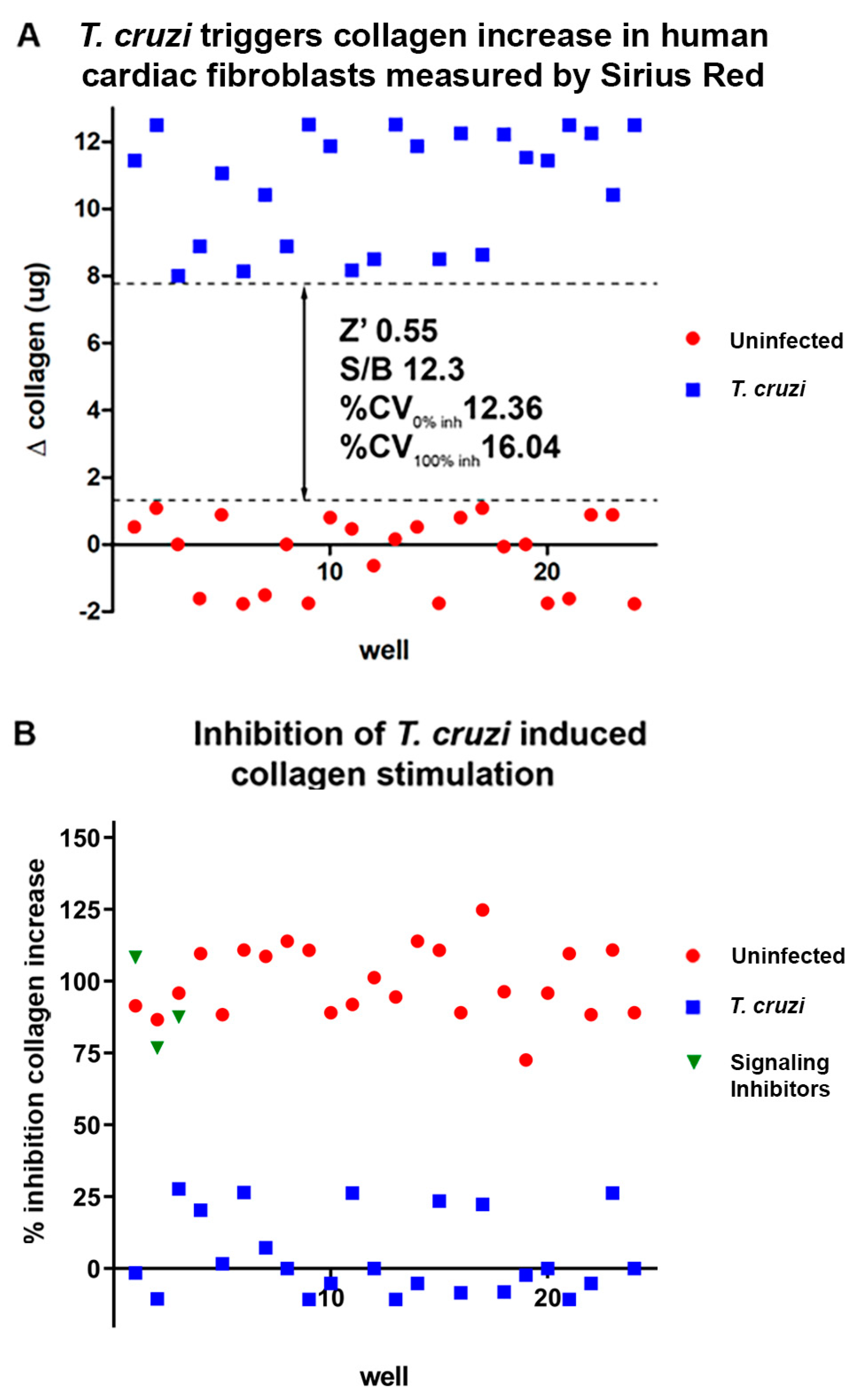
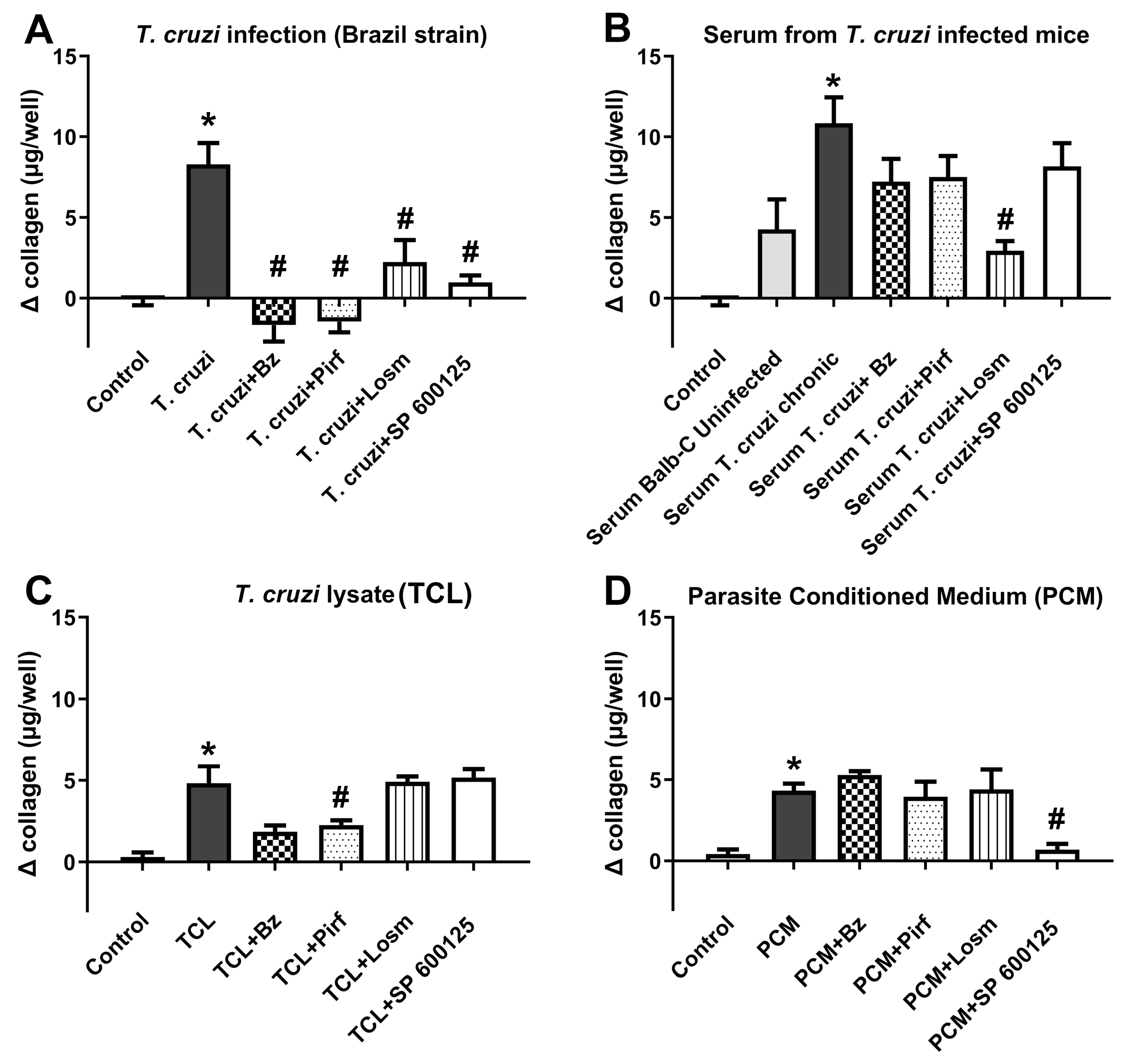
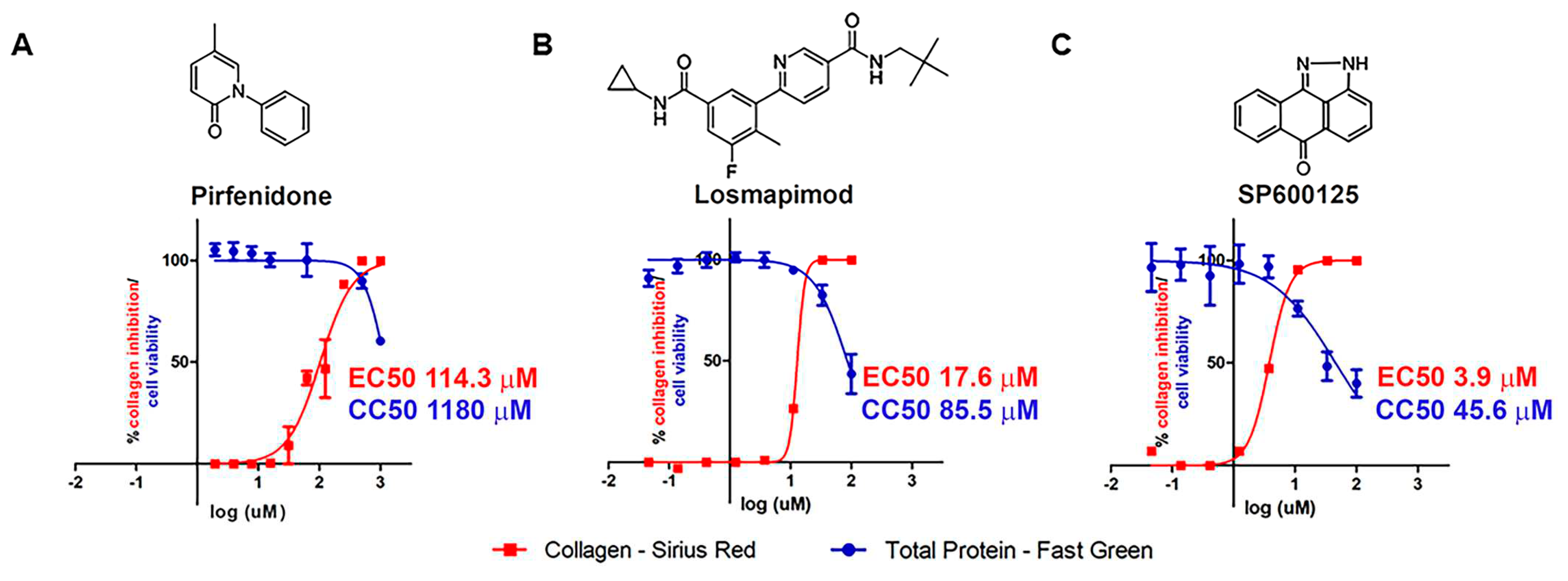
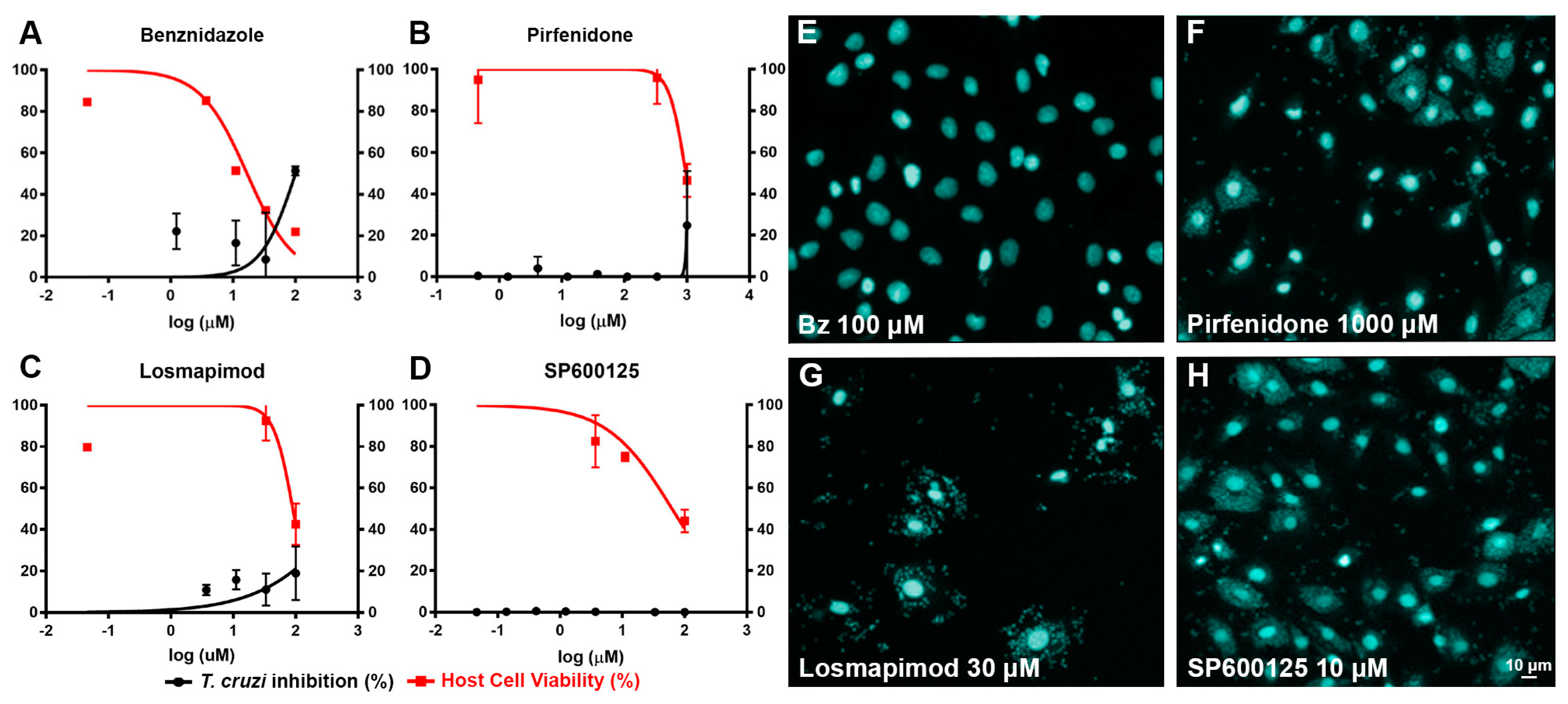
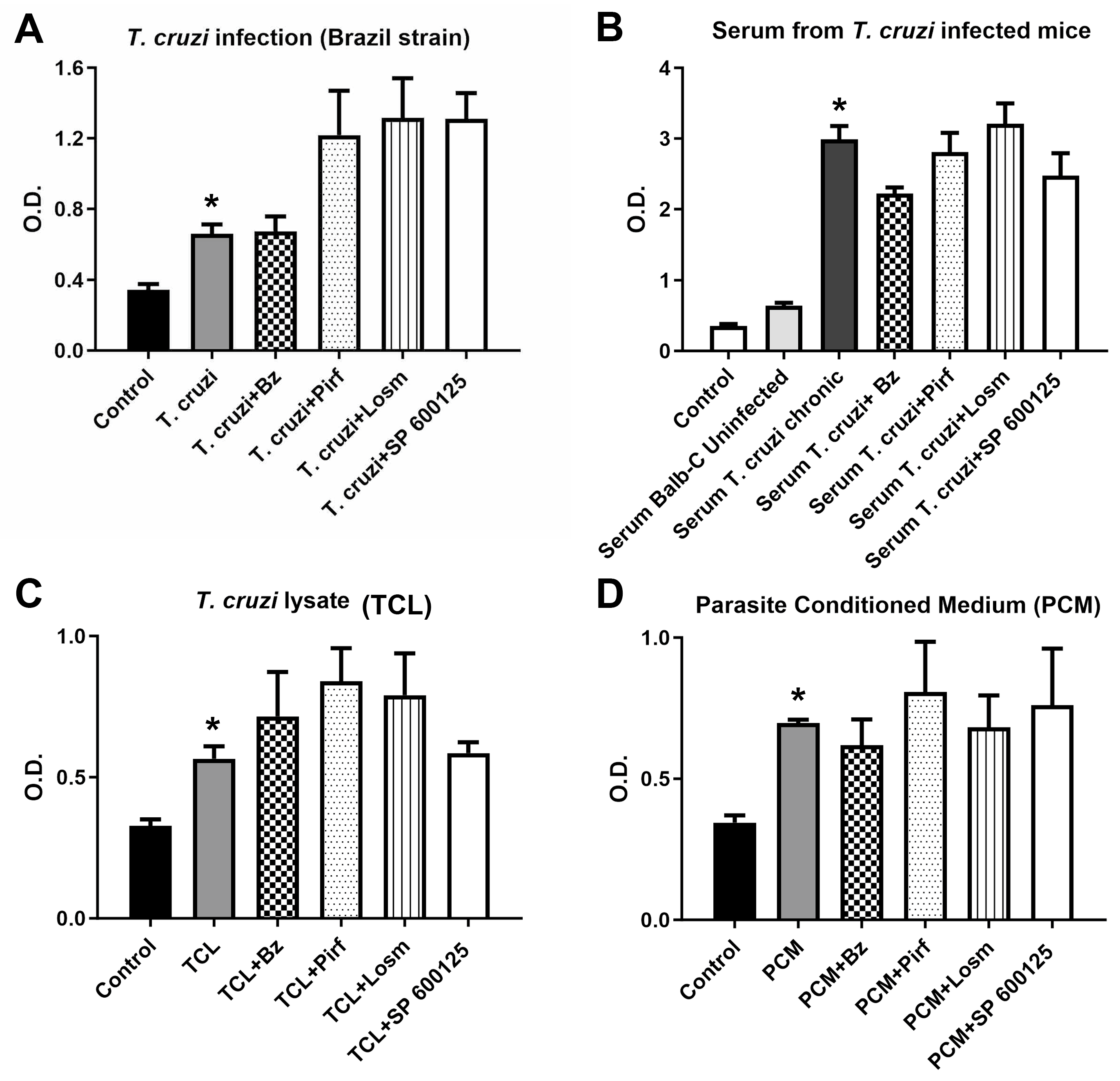
2.1. In vitro assays.
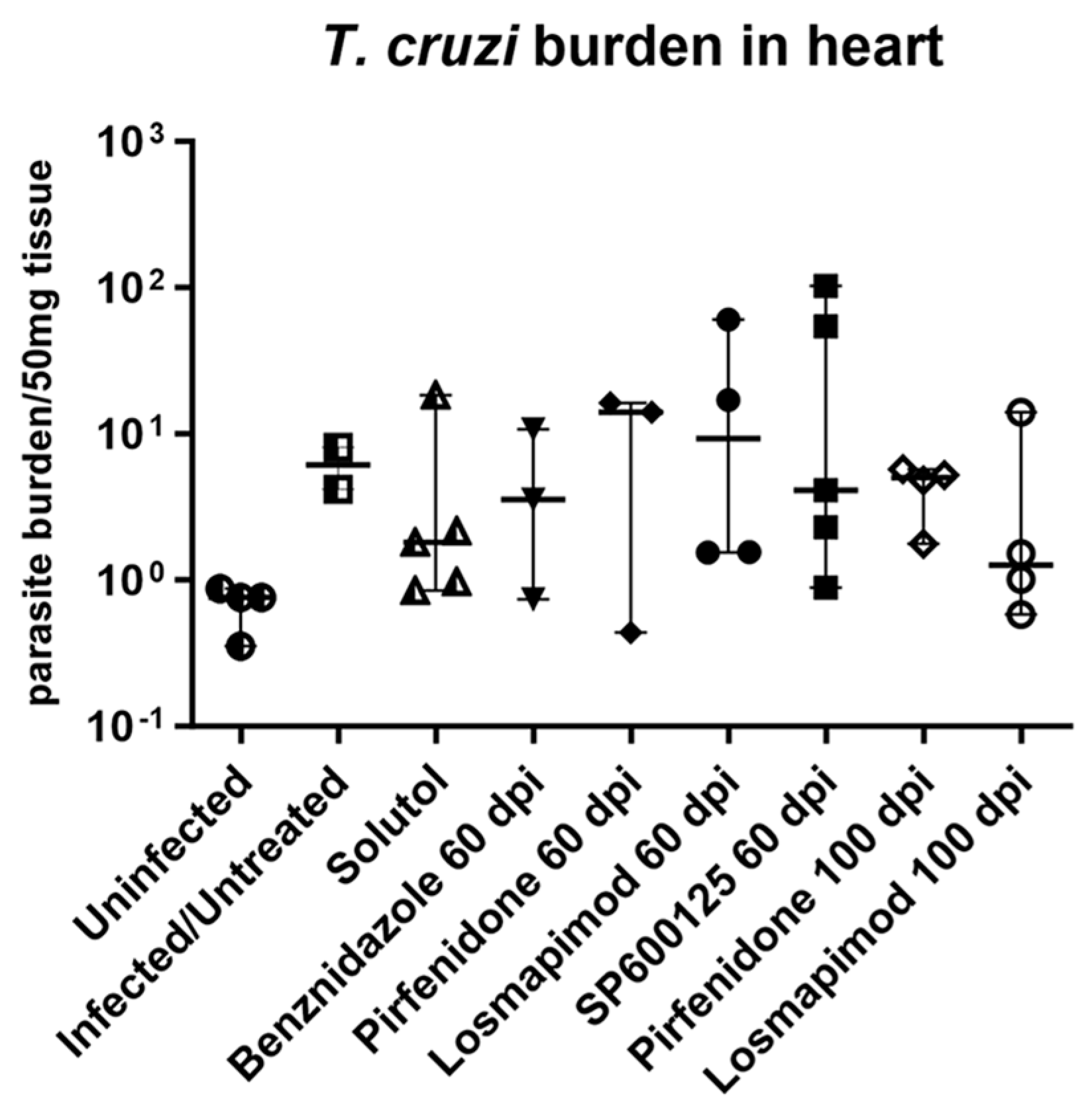
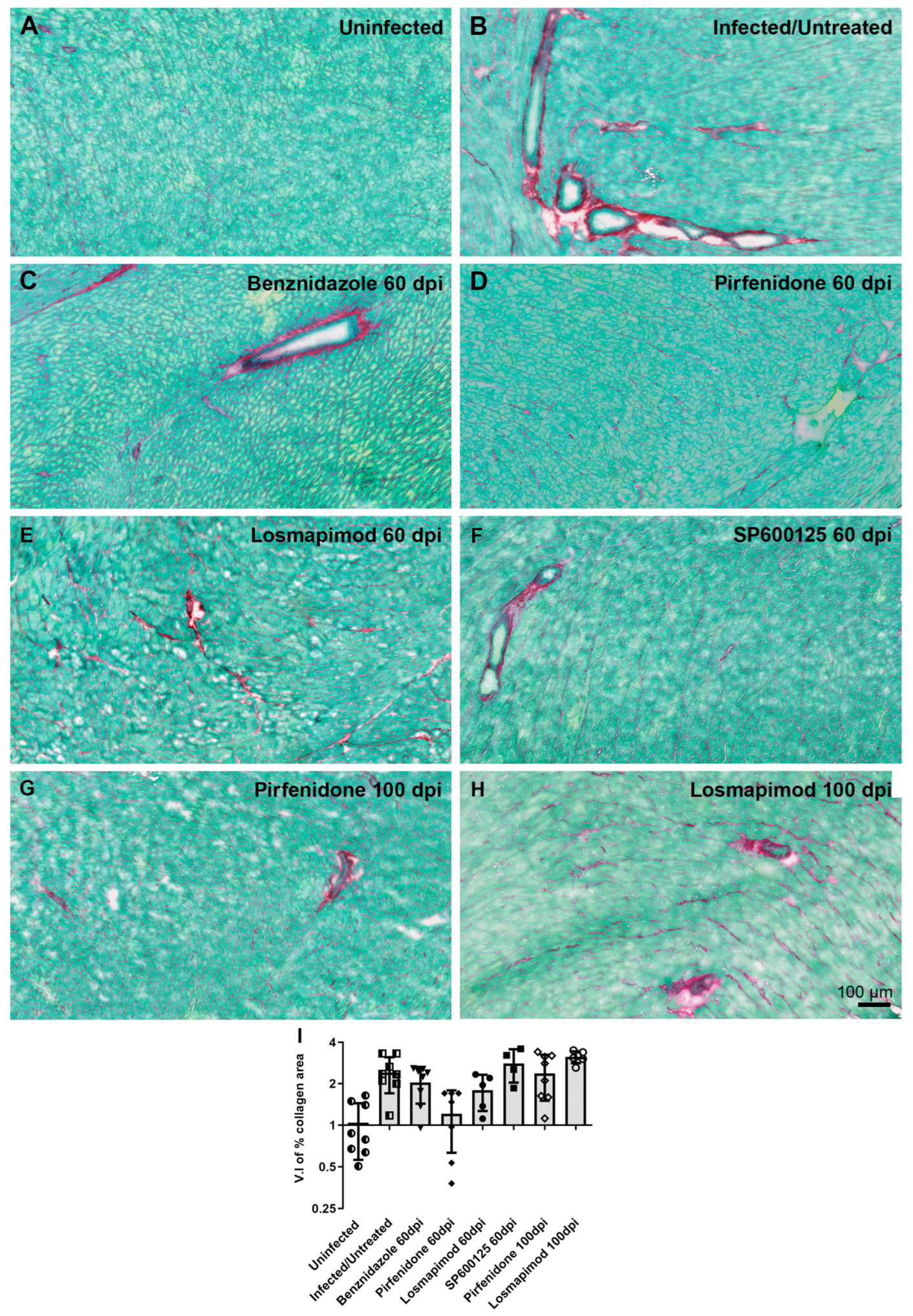
2.2. In vivo assays.
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization Chagas Disease (Also Known as American Trypanosomiasis). Available online: https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis) (accessed on 13 November 2023).
- Chagas, C. Nova Tripanozomiaze Humana. Mem. Inst. Oswaldo Cruz 1909, 1, 0074–0276. [Google Scholar] [CrossRef]
- Lidani, K.C.F.; Andrade, F.A.; Bavia, L.; Damasceno, F.S.; Beltrame, M.H.; Messias-Reason, I.J.; Sandri, T.L. Chagas Disease: From Discovery to a Worldwide Health Problem. J Phys Oceanogr 2019, 49. [Google Scholar] [CrossRef] [PubMed]
- Andrade, D. V.; Gollob, K.J.; Dutra, W.O. Acute Chagas Disease: New Global Challenges for an Old Neglected Disease. PLoS Negl Trop Dis 2014, 8, e3010. [Google Scholar] [CrossRef] [PubMed]
- Bern, C.; Kjos, S.; Yabsley, M.J.; Montgomery, S.P. Trypanosoma Cruzi and Chagas’ Disease in the United States. Clin Microbiol Rev 2011, 24, 655–681. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.Y.; Bacon, K.M.; Bottazzi, M.E.; Hotez, P.J. Global Economic Burden of Chagas Disease: A Computational Simulation Model. Lancet Infect Dis 2013, 13, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ochoa, S.A.; Rojas, L.Z.; Echeverría, L.E.; Muka, T.; Franco, O.H. Global, Regional, and National Trends of Chagas Disease from 1990 to 2019: Comprehensive Analysis of the Global Burden of Disease Study. Glob Heart 2022, 17. [Google Scholar] [CrossRef] [PubMed]
- Bonney, K.M.; Luthringer, D.J.; Kim, S.A.; Garg, N.J.; Engman, D.M. Pathology and Pathogenesis of Chagas Heart Disease. Annu. Rev. Pathol. Mech. Dis. 2019 2018, 14, 421–447. [Google Scholar] [CrossRef] [PubMed]
- Tassi, E.M.; Continentino, M.A.; Nascimento, E.M. Do; Pereira, B.D.B.; Pedrosa, R.C. Relationship between Fibrosis and Ventricular Arrhythmias in Chagas Heart Disease Without Ventricular Dysfunction. Arq Bras Cardiol 2014, 102, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Barizon, G.C.; Simões, M.V.; Schmidt, A.; Gadioli, L.P.; Murta Junior, L.O. Relationship between Microvascular Changes, Autonomic Denervation, and Myocardial Fibrosis in Chagas Cardiomyopathy: Evaluation by MRI and SPECT Imaging. Journal of Nuclear Cardiology 2018, 1–11. [Google Scholar] [CrossRef]
- Melo, R.J.L.; Assunção, A.N.; Morais, T.C.; Nomura, C.H.; Scanavacca, M.I.; Martinelli-Filho, M.; Ramires, F.J.A.; Fernandes, F.; Ianni, B.M.; Mady, C.; et al. Detection of Early Diffuse Myocardial Fibrosis and Inflammation in Chagas Cardiomyopathy with T1 Mapping and Extracellular Volume. Radiol Cardiothorac Imaging 2023, 5. [Google Scholar] [CrossRef]
- Pecoul, B.; Batista, C.; Stobbaerts, E.; Ribeiro, I.; Vilasanjuan, R.; Gascon, J.; Pinazo, M.J.; Moriana, S.; Gold, S.; Pereiro, A.; et al. The BENEFIT Trial: Where Do We Go from Here? PLoS Negl Trop Dis 2016, 10, e0004343. [Google Scholar] [CrossRef] [PubMed]
- Morillo, C.; Marin-Neto, J.; Avezum, A.; Sosa-Estani, S.; Rassi Jr, A.; Rosas, F.; Vilhena, E.; Quiroz, R.; Bonilla, R.; Britto, C.; et al. Randomized Trial of Benznidazole for Chronic Chagas’ Cardiomyopathy. N Engl J Med 2015, 373, 1295–1306. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Valdéz, F.J.; Padilla, A.; Wang, W.; Orr, D.; Tarleton, R. Spontaneous Dormancy Protects Trypanosoma Cruzi during Extended Drug Exposure. Elife 2018, e34039. [Google Scholar] [CrossRef] [PubMed]
- WHO Research Priorities for Chagas Disease, Human African Trypanosomiasis and Leishmaniasis. World Health Organ Tech Rep Ser 2012, v–xii, 1–100.
- Waghabi, M.C.; Ferreira, R.R.; Da Silva Abreu, R.; Degrave, W.; De Souza, E.M.; Bailly, S.; Feige, J.J.; De Araújo-Jorge, T.C. Transforming Growth Factor-ß as a Therapeutic Target for the Cardiac Damage of Chagas Disease. Mem Inst Oswaldo Cruz 2022, 117. [Google Scholar] [CrossRef] [PubMed]
- Torres, D.J.L.; Arruda, T.R. De; Barros, M. da S.; Gonçales, J.P.; Soares, A.K.A.; Oliveira, K.K. dos S.; Moreira, L.R.; Medeiros, C.; Cavalcanti, M. da G.A.M.; Martins, S.M.; et al. Is a Negative Correlation between STNFR1 and TNF in Patients with Chronic Chagas Disease the Key to Clinical Progression? Immunobiology 2022, 227. [CrossRef]
- Cunha-Neto, E.; Dzau, V.J.; Allen, P.D.; Stamatiou, D.; Benvenutti, L.; Higuchi, M.L.; Koyama, N.S.; Silva, J.S.; Kalil, J.; Liew, C.-C. Cardiac Gene Expression Profiling Provides Evidence for Cytokinopathy as a Molecular Mechanism in Chagas’ Disease Cardiomyopathy. Am J Pathol 2005, 167, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Calvet, C.M.; Oliveira, F.O.R.; Araújo-Jorge, T.C.; Pereira, M.C.S. Regulation of Extracellular Matrix Expression and Distribution in Trypanosoma Cruzi-Infected Cardiomyocytes. International Journal of Medical Microbiology 2009, 299, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Calvet, C.M.; Meuser, M.; Almeida, D.; Meirelles, M.N.L.; Pereira, M.C.S. Trypanosoma Cruzi-Cardiomyocyte Interaction: Role of Fibronectin in the Recognition Process and Extracellular Matrix Expression in Vitro and in Vivo. Exp Parasitol 2004, 107, 20–30. [Google Scholar] [CrossRef]
- Silva, T.A.; Ferreira, L.F. de C.; Pereira, M.C. de S.; Calvet, C.M. Differential Role of TGF-Β in Extracellular Matrix Regulation during Trypanosoma Cruzi-Host Cell Interaction. Int J Mol Sci 2019, 20. [CrossRef]
- Araújo-Jorge, T.C.; Waghabi, M.C.; Hasslocher-moreno, A.M.; Xavier, S.; Higuchi, M.D.L.; Keramidas, M.; Bailly, S.; Feige, J. Implication of Transforming Growth Factor – Β1 in Chagas Disease Myocardiopathy. J Infect Dis 2002, 186, 1823–1828. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, L.G.; Santos, R.H.B.; Fiorelli, A.I.; Mairena, E.C.; Benvenuti, L.A.; Bocchi, E.A.; Stolf, N.A.; Kalil, J.; Cunha-Neto, E.; Gabriel Nogueira, L.; et al. Myocardial Gene Expression of T-Bet, GATA-3, Ror- γ t, FoxP3, and Hallmark Cytokines in Chronic Chagas Disease Cardiomyopathy: An Essentially Unopposed T H 1-Type Response. Mediators Inflamm 2014, 2014, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Gudey, S.K.; Landström, M. Non-Smad Signaling Pathways. Cell Tissue Res 2012, 347, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, A.; Heldin, C. Non-Smad TGF-β Signals. J Cell Sci 2005, 118, 3573–3584. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Hang, W.; Shu, H.; Zhou, N. Pirfenidone Alleviates Cardiac Fibrosis Induced by Pressure Overload via Inhibiting TGF-Β1/Smad3 Signalling Pathway. J Cell Mol Med 2022, 26, 4548–4555. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, C.J.; Ruhrmund, D.W.; Pan, L.; Seiwert, S.D.; Kossen, K. Antifibrotic Activities of Pirfenidone in Animal Models. European respiratory review 2011, 20, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Newby, L.K.; Marber, M.S.; Melloni, C.; Sarov-Blat, L.; Aberle, L.H.; Aylward, P.E.; Cai, G.; de Winter, R.J.; Hamm, C.W.; Heitner, J.F.; et al. Losmapimod, a Novel P38 Mitogen-Activated Protein Kinase Inhibitor, in Non-ST-Segment Elevation Myocardial Infarction: A Randomised Phase 2 Trial. The Lancet 2014, 384, 1187–1195. [Google Scholar] [CrossRef]
- Mellion, M.L.; Ronco, L.; Berends, C.L.; Pagan, L.; Brooks, S.; van Esdonk, M.J.; van Brummelen, E.M.J.; Odueyungbo, A.; Thompson, L.A.; Hage, M.; et al. Phase 1 Clinical Trial of Losmapimod in Facioscapulohumeral Dystrophy: Safety, Tolerability, Pharmacokinetics, and Target Engagement. Br J Clin Pharmacol 2021, 87, 4658–4669. [Google Scholar] [CrossRef]
- Guo, W.; Cao, S.; Yan, B.; Zhang, G.; Li, J.; Zhao, Y.; Zhang, S. Myocardial Protective Effects of a C-Jun N-Terminal Kinase Inhibitor in Rats with Brain Death. J Cell Mol Med 2016, 20, 1214–1218. [Google Scholar] [CrossRef]
- Bennett, B.L.; Sasaki, D.T.; Murray, B.W.; O’Leary, E.C.; Sakata, S.T.; Xu, W.; Leisten, J.C.; Motiwala, A.; Pierce, S.; Satoh, Y.; et al. SP600125, an Anthrapyrazolone Inhibitor of Jun N-Terminal Kinase. Proceedings of the National Academy of Sciences 2001, 98, 13681–13686. [Google Scholar] [CrossRef]
- Zhang, J.-H.; Chung, T.D.Y.; Oldenburg, K.R. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J Biomol Screen 1999, 4, 67–73. [Google Scholar] [CrossRef]
- George, P.M.; Wells, A.U. Pirfenidone for the Treatment of Idiopathic Pulmonary Fibrosis. Expert Rev Clin Pharmacol 2017, 10, 483–491. [Google Scholar] [CrossRef]
- Tucci, A.R.; de Oliveira, F.O.R.; Lechuga, G.C.; Oliveira, G.M.; Eleuterio, A.C.; de Mesquita, L.B.; Farani, P.S.G.; Britto, C.; Moreira, O.C.; Pereira, M.C.S. Role of FAK Signaling in Chagasic Cardiac Hypertrophy. Brazilian Journal of Infectious Diseases 2020, 24, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Jelicks, L.; Chandra, M.; Shirani, J.; Shtutin, V.; Tang, B.; Christ, G.J.; Factor, S.M.; Wittner, M.; Huang, H.; Weiss, L.M.; et al. Cardioprotective Effects of Phosphoramidon on Myocardial Structure and Function in Murine Chagas’ Disease. Int J Parasitol 2002, 32, 1497–1506. [Google Scholar] [CrossRef] [PubMed]
- Tanowitz, H.B.; Huang, H.; Jelicks, L.A.; Chandra, M.; Loredo, M.L.; Weiss, L.M.; Factor, S.M.; Shtutin, V.; Mukherjee, S.; Kitsis, R.N.; et al. Role of Endothelin 1 in the Pathogenesis of Chronic Chagasic Heart Disease. 2005, 73, 2496–2503.
- Duschak, V.G. Targets and Patented Drugs for Chemotherapy of Chagas Disease in the Last 15 Years-Period. Recent Pat Antiinfect Drug Discov 2016, 74–173. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, N.S.; Montgomery, S.P. Chagas Disease. Ann Intern Med 2023, 176, ITC17–ITC32. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.L.; Wells, A.R.; Leung, K.P. Pirfenidone Reduces Profibrotic Responses in Human Dermal Myofibroblasts, in Vitro. Laboratory Investigation 2018, 98, 640–655. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Keating, G.M. Pirfenidone: A Review of Its Use in Idiopathic Pulmonary Fibrosis. Drugs 2015, 75, 219–230. [Google Scholar] [CrossRef]
- Lewis, M.D.; Francisco, A.F.; Taylor, M.C.; Jayawardhana, S.; Kelly, J.M. Host and Parasite Genetics Shape a Link between Trypanosoma Cruzi Infection Dynamics and Chronic Cardiomyopathy. Cell Microbiol 2016, 18, 1429–1443. [Google Scholar] [CrossRef]
- Huang, H.; Chan, J.; Wittner, M.; Jelicks, L. a; Morris, S. a; Factor, S.M.; Weiss, L.M.; Braunstein, V.L.; Bacchi, C.J.; Yarlett, N.; et al. Expression of Cardiac Cytokines and Inducible Form of Nitric Oxide Synthase (NOS2) in Trypanosoma Cruzi-Infected Mice. J Mol Cell Cardiol 1999, 31, 75–88. [Google Scholar] [CrossRef]
- Roman-Campos, D.; Duarte, H.L.L.; Sales, P. a; Natali, A.J.; Ropert, C.; Gazzinelli, R.T.; Cruz, J.S. Changes in Cellular Contractility and Cytokines Profile during Trypanosoma Cruzi Infection in Mice. Basic Res Cardiol 2009, 104, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Machado, F.; Martins, G.; Aliberti, J.; Mestriner, F.; Cunha, F.; Silva, J. Trypanosoma Cruzi-Infected Cardiomyocytes Produce Chemokines and Cytokines That Trigger Potent Nitric Oxide-Dependent Trypanocidal Activity. Circulation 2000, 102, 3003–3008. [Google Scholar] [CrossRef] [PubMed]
- Waghabi, M.C.; Coutinho-Silva, R.; Feige, J.-J.; Higuchi, M.D.L.; Becker, D.; Burnstock, G.; Araújo-Jorge, T.C. Gap Junction Reduction in Cardiomyocytes Following Transforming Growth Factor-Beta Treatment and Trypanosoma Cruzi Infection. Mem Inst Oswaldo Cruz 2009, 104, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Pinho, R.T.; Vannier-Santos, M.A.; Alves, C.R.; Marino, A.P.M.P.; Castello Branco, L.R.R.; Lannes-Vieira, J. Effect of Trypanosoma Cruzi Released Antigens Binding to Non-Infected Cells on Anti-Parasite Antibody Recognition and Expression of Extracellular Matrix Components. Acta Trop 2002, 83, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Serra, N.; Gualdron-Lopez, M.; Pinazo, M.J.; Torrecilhas, A.C.; Fernandez-Becerra, C. Extracellular Vesicles in Trypanosoma Cruzi Infection: Immunomodulatory Effects and Future Perspectives as Potential Control Tools against Chagas Disease. J Immunol Res 2022, 2022. [Google Scholar] [CrossRef] [PubMed]
- Brossas, J.Y.; Gulin, J.E.N.; Bisio, M.M.C.; Chapelle, M.; Marinach-Patrice, C.; Bordessoules, M.; Ruiz, G.P.; Vion, J.; Paris, L.; Altcheh, J.; et al. Secretome Analysis of Trypanosoma Cruzi by Proteomics Studies. PLoS One 2017, 12. [Google Scholar] [CrossRef]
- Nogueira, P.M.; Ribeiro, K.; Silveira, A.C.O.; Campos, J.H.; Martins-Filho, O.A.; Bela, S.R.; Campos, M.A.; Pessoa, N.L.; Colli, W.; Alves, M.J.M.; et al. Vesicles from Different Trypanosoma Cruzi Strains Trigger Differential Innate and Chronic Immune Responses. J Extracell Vesicles 2015, 4, 28734. [Google Scholar] [CrossRef] [PubMed]
- Moraes, A.V. de; Esteves-Filho, A.; Barbero-Marcial, M.; Belloti, G.; Kalil, R.; Bocchi, E.A.; Higuchi, M. de L.; Weiss, R.; Sosa, E.; Jatene, A.; et al. In Vivo Detection of Trypanosoma Cruzi Antigens in Hearts of Patients with Chronic Chagas’ Heart Disease. Am Heart J 2004, 131, 301–307. [CrossRef]
- Ferrão, P.M.; d’Avila-Levy, C.M.; Araujo-Jorge, T.C.; Degrave, W.M.; Gonçalves, A.D.S.; Garzoni, L.R.; Lima, A.P.; Feige, J.J.; Bailly, S.; Mendonça-Lima, L.; et al. Cruzipain Activates Latent TGF-β from Host Cells during T. Cruzi Invasion. PLoS One 2015, 10, e0124832. [Google Scholar] [CrossRef]
- Shi, Q.; Liu, X.; Bai, Y.; Cui, C.; Li, J.; Li, Y.; Hu, S.; Wei, Y. In Vitro Effects of Pirfenidone on Cardiac Fibroblasts: Proliferation, Myofibroblast Differentiation, Migration and Cytokine Secretion. PLoS One 2011, 6. [Google Scholar] [CrossRef]
- Wu, W.; Muchir, A.; Shan, J.; Bonne, G.; Worman, H.J. Mitogen-Activated Protein Kinase Inhibitors Improve Heart Function and Prevent Fibrosis in Cardiomyopathy Caused by Mutation in Lamin A/C Gene. Circulation 2011, 123, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Jiang, R.; Chen, M.; Dong, M.; Liu, Q.; Cheng, H.; Zhou, K.; Chen, L.; Li, M.; Jiang, C. Puerarin Decreases Collagen Secretion in AngII-Induced Atrial Fibroblasts Through Inhibiting Autophagy Via the JNK-Akt-MTOR Signaling Pathway. J Cardiovasc Pharmacol 2019, 73, 373–382. [Google Scholar] [CrossRef]
- Lu, C.; Yang, Y.; Zhu, Y.; Lv, S.; Zhang, J. An Intervention Target for Myocardial Fibrosis: Autophagy. Biomed Res Int 2018, 2018. [Google Scholar] [CrossRef]
- Schieven, G.L. The Biology of P38 Kinase: A Central Role in Inflammation; 2005; Vol. 5;
- Kojonazarov, B.; Novoyatleva, T.; Boehm, M.; Happe, C.; Sibinska, Z.; Tian, X.; Sajjad, A.; Luitel, H.; Kriechling, P.; Posern, G.; et al. P38 Mapk Inhibition Improves Heart Function in Pressure-Loaded Right Ventricular Hypertrophy. Am J Respir Cell Mol Biol 2017, 57, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Arabacilar, P.; Marber, M. The Case for Inhibiting P38 Mitogen-Activated Protein Kinase in Heart Failure. Front Pharmacol 2015, 6. [Google Scholar] [CrossRef]
- De Souza, A.P.; Tanowitz, H.B.; Chandra, M.; Shtutin, V.; Weiss, L.M.; Morris, S. a; Factor, S.M.; Huang, H.; Wittner, M.; Shirani, J.; et al. Effects of Early and Late Verapamil Administration on the Development of Cardiomyopathy in Experimental Chronic Trypanosoma Cruzi (Brazil Strain) Infection. Parasitol Res 2004, 92, 496–501. [Google Scholar] [CrossRef]
- Araujo-Jorge, T.C.; Rivera, M.T.; Vanderpas, J.; Garzoni, L.R.; Carvalho, A.C.C.; Waghabi, M.C.; Holanda, M.T.; Mediano, M.F.F.; Hasslocher-Moreno, A.M.; Bonecini-Almeida, M. da G.; et al. Selenium, TGF-Beta and Infectious Endemic Cardiopathy: Lessons from Benchwork to Clinical Application in Chagas Disease. Biomolecules 2022, 12.
- de Oliveira, F.L.; Araújo-Jorge, T.C.; de Souza, E.M.; de Oliveira, G.M.; Degrave, W.M.; Feige, J.-J.; Bailly, S.; Waghabi, M.C. Oral Administration of GW788388, an Inhibitor of Transforming Growth Factor Beta Signaling, Prevents Heart Fibrosis in Chagas Disease. PLoS Negl Trop Dis 2012, 6, e1696. [Google Scholar] [CrossRef]
- Ferreira, R.R.; Abreu, S.; Vilar-pereira, G.; Ferreira, C.; Degrave, W.; Meuser-batista, M.; Vale, N.; Souza, E.M. De; Ramos, I.P.; Moreira, C.; et al. TGF-β Inhibitor Therapy Decreases Fibrosis and Stimulates Cardiac Improvement in a Pre-Clinical Study of Chronic Chagas ’ Heart Disease. PLoS Negl Trop Dis 2019, 13, e0007602. [Google Scholar] [CrossRef]
- Ferreira, R.R.; de Souza, E.M.; Vilar-Pereira, G.; Degrave, W.M.S.; Abreu, R. da S.; Meuser-Batista, M.; Ferreira, N.V.C.; Ledbeter, S.; Barker, R.H.; Bailly, S.; et al. In Chagas Disease, Transforming Growth Factor Beta Neutralization Reduces Trypanosoma Cruzi Infection and Improves Cardiac Performance. Front Cell Infect Microbiol 2022, 12. [CrossRef]
- Aimo, A.; Spitaleri, G.; Panichella, G.; Lupón, J.; Emdin, M.; Bayes-Genis, A. Pirfenidone as a Novel Cardiac Protective Treatment. Heart Fail Rev 2022, 27, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.; Chen, J.; Zhao, S.; Li, H. Pirfenidone Attenuates Cardiac Fibrosis in a Mouse Model of TAC-Induced Left Ventricular Remodeling by Suppressing NLRP3 Inflammasome Formation. Cardiology (Switzerland) 2013, 126, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, X.; Wang, B.; Nie, Y.; Wen, J.; Wang, Q.; Gu, C. Pirfenidone Suppresses MAPK Signalling Pathway to Reverse Epithelial-Mesenchymal Transition and Renal Fibrosis. Nephrology 2017, 22, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, Y.; Chi, P. Pirfenidone Suppresses TGF-Β1-Induced Human Intestinal Fibroblasts Activities by Regulating Proliferation and Apoptosis via the Inhibition of the Smad and PI3K/AKT Signaling Pathway. Mol Med Rep 2018, 18, 3907–3913. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.A.; Rosala-Hallas, A.; Dodd, S.; Schelbert, E.B.; Williams, S.G.; Cunnington, C.; McDonagh, T.; Miller, C.A. Characteristics Associated With Growth Differentiation Factor 15 in Heart Failure With Preserved Ejection Fraction and the Impact of Pirfenidone. J Am Heart Assoc 2022, 11. [Google Scholar] [CrossRef]
- Gunatilleke, S.S.; Calvet, C.M.; Johnston, J.B.; Chen, C.-K.; Erenburg, G.; Gut, J.; Engel, J.C.; Ang, K.K.H.; Mulvaney, J.; Chen, S.; et al. Diverse Inhibitor Chemotypes Targeting Trypanosoma Cruzi CYP51. PLoS Negl Trop Dis 2012, 6. [Google Scholar] [CrossRef] [PubMed]
- Houghton, P.E.; Keefer, K.A.; Diegelmann, R.F.; Krummel, T.M. A Simple Method to Assess the Relative Amount of Collagen Deposition in Wounded Fetal Mouse Limbs. Wound Repair and Regeneration 1996, 4, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Calvet, C.M.; Silva, T.A.; Thomas, D.; Suzuki, B.; Hirata, K.; Siqueira-Neto, J.L.; McKerrow, J.H. Long Term Follow-up of Trypanosoma Cruzi Infection and Chagas Disease Manifestations in Mice Treated with Benznidazole or Posaconazole. PLoS Negl Trop Dis 2020, 14. [Google Scholar] [CrossRef] [PubMed]
- McCall, L.I.; Morton, J.T.; Bernatchez, J.A.; De Siqueira-Neto, J.L.; Knight, R.; Dorrestein, P.C.; McKerrow, J.H. Mass Spectrometry-Based Chemical Cartography of a Cardiac Parasitic Infection. Anal Chem 2017, 89, 10414–10421. [Google Scholar] [CrossRef]
- Hossain, E.; Khanam, S.; Dean, D.A.; Wu, C.; Lostracco-Johnson, S.; Thomas, D.; Kane, S.S.; Parab, A.R.; Flores, K.; Katemauswa, M.; et al. Mapping of Host-Parasite-Microbiome Interactions Reveals Metabolic Determinants of Tropism and Tolerance in Chagas Disease; 2020;
- Tsujita, Y.; Muraski, J.; Shiraishi, I.; Kato, T.; Kajstura, J.; Anversa, P.; Sussman, M.A. Nuclear Targeting of Akt Antagonizes Aspects of Cardiomyocyte Hypertrophy; 2006; Vol. 103.
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



