Submitted:
13 November 2023
Posted:
13 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Why Ferrocene?
 |
Solvent | Electrolyte |
Em vs. Fc0/+ (V) |
Ref |
|---|---|---|---|---|
| R1-10: H | DCM | 0.1 M [Bu4N][ClO4] | 0.000 | [2] |
| R1-10: CH3 | DCM | 0.1 M [Bu4N][ClO4] | -0.570 | [2] |
| R1-5: CH3; R6-10: H | DCM | 0.1 M [Bu4N][ClO4] | -0.270 | [2] |
| R1,10: CH3; R2-9: H | CH3CN | 0.1 M [Bu4N][ClO4] | -0.113 | [39] |
| CH3CN | 0.1 M [Bu4N][PF6] | -0.096 | [40] | |
| MeOH | 0.1 M [Bu4N][ClO4] | -0.104 | [39] | |
| Toluene | 0.5 M [Hex4N][ClO4] | -0.075 | [39] | |
| R2-9: CH3; R1,10: H | CH3CN | 0.1 M [Bu4N][PF6] | -0.406 | [40] |
| R1,3,7,10: t-Bu; R2,4,5,6,8,9: H | CH3CN | 0.1 M [Bu4N][ClO4] | -0.238 | [39] |
| CH3CN | 0.1 M [Bu4N][PF6] | -0.233 | [40] | |
| MeOH | 0.1 M [Bu4N][ClO4] | -0.229 | [39] | |
| Toluene | 0.5 M [Hex4N][ClO4] | -0.097 | [39] | |
| R1: n-Bu; R2-10: H | CH3CN | 0.1 M [Bu4N][ClO4] | -0.062 | [39] |
| MeOH | 0.1 M [Bu4N][ClO4] | -0.055 | [39] | |
| Toluene | 0.5 M [Hex4N][ClO4] | -0.073 | [39] | |
| R1-10: CH2Ph | DCM | 0.1 M [Bu4N][ClO4] | -0.070 | [2] |
| R1,10: CF3; R2-9: H | DCM | 0.1 M [Bu4N][ClO4] | 0.640 | [2] |
| R1: CH=CH2; R2-10: H | CH3CN | 0.1 M [Et4N][ClO4] | 0.022 | [41] |
| R1: CH2OH; R2-10: H | CH3CN | 0.1 M [Et4N][ClO4] | 0.029 | [41] |
| CH3CN | 0.1 M [Bu4N][ClO4] | -0.012 | [39] | |
| CH3CN | 0.1 M [Bu4N][PF6] | 0.016 | [38] | |
| MeOH | 0.1 M [Bu4N][ClO4] | 0.005 | [39] | |
| Toluene | 0.5 M [Hex4N][ClO4] | -0.044 | [39] | |
| R1: (CH2)2OH; R2-10: H | CH3CN | 0.1 M [Bu4N][PF6] | -0.046 | [38] |
| R1: (CH2)3OH; R2-10: H | CH3CN | 0.1 M [Bu4N][PF6] | -0.052 | [38] |
| R1: (CH2)4OH; R2-10: H | CH3CN | 0.1 M [Bu4N][PF6] | -0.054 | [38] |
| R1: CH(CH3)OH; R2-10: H | CH3CN | 0.1 M [Et4N][ClO4] | -0.008 | [41] |
| R1,10: CH(CH3)OH; R2-9: H | CH3CN | 0.1 M [Et4N][ClO4] | -0.013 | [41] |
| R1: CH2CONH2; R2-10: H | CH3CN | 0.1 M [Bu4N][PF6] | -0.003 | [38] |
| R1: (CH2)2CONH2; R2-10: H | CH3CN | 0.1 M [Bu4N][PF6] | -0.027 | [38] |
| R1: (CH2)3CONH2; R2-10: H | CH3CN | 0.1 M [Bu4N][PF6] | -0.049 | [38] |
| R1: COOH; R2-10: H | CH3CN | 0.1 M [Et4N][ClO4] | 0.239 | [41] |
| CH3CN | 0.1 M [Bu4N][ClO4] | 0.234 | [39] | |
| CH3CN | 0.1 M [Li][ClO4] | 0.239 | [37] | |
| MeOH | 0.1 M [Bu4N][ClO4] | 0.233 | [39] | |
| Toluene | 0.5 M [Hex4N][ClO4] | 0.157 | [39] | |
| R1: CH2COOH; R2-10: H | CH3CN | 0.1 M [Li][ClO4] | -0.006 | [37] |
| R1: (CH2)2COOH; R2-10: H | CH3CN | 0.1 M [Li][ClO4] | -0.022 | [37] |
| R1: (CH2)3COOH; R2-10: H | CH3CN | 0.1 M [Li][ClO4] | -0.047 | [37] |
| R1: COOCH3; R2-10: H | CH3CN | 0.1 M [Et4N][ClO4] | 0.243 | [41] |
| CH3CN | 0.1 M [Bu4N][ClO4] | 0.237 | [39] | |
| MeOH | 0.1 M [Bu4N][ClO4] | 0.263 | [39] | |
| Toluene | 0.5 M [Hex4N][ClO4] | 0.214 | [39] | |
| R1,10: COOCH3; R2-9: H | CH3CN | 0.1 M [Et4N][ClO4] | 0.470 | [41] |
| R1: COCH3; R2-10: H | CH3CN | 0.1 M [Bu4N][ClO4] | 0.244 | [39] |
| MeOH | 0.1 M [Bu4N][ClO4] | 0.271 | [39] | |
| Toluene | 0.5 M [Hex4N][ClO4] | 0.191 | [39] | |
| R1,10: COCH3; R2-9: H | CH3CN | 0.1 M [Et4N][ClO4] | 0.482 | [41] |
| R1: COPh; R2-10: H | CH3CN | 0.1 M [Bu4N][ClO4] | 0.250 | [39] |
| MeOH | 0.1 M [Bu4N][ClO4] | 0.272 | [39] | |
| Toluene | 0.5 M [Hex4N][ClO4] | 0.214 | [39] | |
| R1: CONH2; R2-10: H | CH3CN | 0.1 M [Bu4N][PF6] | 0.183 | [38] |
| R1: CHO; R2-10: H | CH3CN | 0.1 M [Bu4N][ClO4] | 0.285 | [39] |
| MeOH | 0.1 M [Bu4N][ClO4] | 0.304 | [39] | |
| Toluene | 0.5 M [Hex4N][ClO4] | 0.259 | [39] | |
| R1: CH2NH2; R2-10: H | CH3CN | 0.1 M [Bu4N][PF6] | -0.014 | [38] |
| R1: (CH2)2NH2; R2-10: H | CH3CN | 0.1 M [Bu4N][PF6] | -0.037 | [38] |
| R1: (CH2)3NH2; R2-10: H | CH3CN | 0.1 M [Bu4N][PF6] | -0.046 | [38] |
| R1: (CH2)4NH2; R2-10: H | CH3CN | 0.1 M [Bu4N][PF6] | -0.060 | [38] |
| R1: CH2N(CH3)2; R2-10: H | CH3CN | 0.1 M [Bu4N][ClO4] | -0.004 | [39] |
| MeOH | 0.1 M [Bu4N][ClO4] | 0.046 | [39] | |
| Toluene | 0.5 M [Hex4N][ClO4] | 0.009 | [39] | |
| CH3CN | 0.1 M [Bu4N][PF6] | -0.023 | [42] | |
| DCM:CH3CN 1:4 | 0.1 M [Bu4N][PF6] | 0.003 | [7] | |
| R1,10: CH2N(CH3)2; R2-9: H | CH3CN | 0.1 M [Bu4N][PF6] | -0.017 | [42] |
| R1,10: (CH2)2N(CH3)2; R2-9: H | CH3CN | 0.1 M [Bu4N][PF6] | -0.077 | [42] |
| R1,10: CH2N(CH2Ph)2; R2-9: H | DCM | 0.2 M [Bu4N][PF6] | -0.001 | [9] |
| R1,10: C(CH3)=N(CH2)5CH3; R2-9: H | DCM:CH3CN 1:1 | 0.1 M [Bu4N][PF6] | 0.211 | [43] |
R1,10: 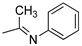 ; R2-9: H ; R2-9: H |
DCM:CH3CN 1:1 | 0.1 M [Bu4N][PF6] | 0.289 | [43] |
R1,10: 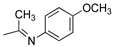 ; R2-9: H ; R2-9: H |
DCM:CH3CN 1:1 | 0.1 M [Bu4N][PF6] | 0.245 | [43] |
R1,10: 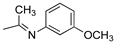 ; R2-9: H ; R2-9: H |
DCM:CH3CN 1:1 | 0.1 M [Bu4N][PF6] | 0.261 | [43] |
R1,10: 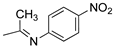 ; R2-9: H ; R2-9: H |
DCM:CH3CN 1:1 | 0.1 M [Bu4N][PF6] | 0.435 | [43] |
R1,10: 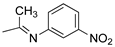 ; R2-9: H ; R2-9: H |
DCM:CH3CN 1:1 | 0.1 M [Bu4N][PF6] | 0.390 | [43] |
| R1,10: SH; R2-9: H | DCM | 0.1 M [Bu4N][ClO4] | 0.200 | [2] |
| R1: S(CH2)2OH; R2-10: H | CH3CN | 0.1 M [Et4N][ClO4] | 0.010 | [41] |
| R1: SCH2CH(CH3)COOH; R2-10: H | CH3CN | 0.1 M [Et4N][ClO4] | 0.039 | [41] |
| R1: CH(CH3)SPh; R2-10: H | CH3CN | 0.1 M [Et4N][ClO4] | 0.020 | [41] |
| R1: CH(Ph)SPh; R2-10: H | CH3CN | 0.1 M [Et4N][ClO4] | 0.043 | [41] |
R1: 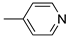 ; R2-10: H ; R2-10: H |
CH3CN | 0.1 M [Bu4N][ClO4] | 0.180 | [44] |
R1,10: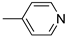 ; R2-9: H ; R2-9: H |
CH3CN | 0.1 M [Bu4N][ClO4] | 0.350 | [44] |
R1: 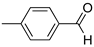 ; R2-10: H ; R2-10: H |
CH3CN | 0.1 M [Bu4N][ClO4] | 0.170 | [44] |
R1,10: 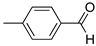 ; R2-9: H ; R2-9: H |
CH3CN | 0.1 M [Bu4N][ClO4] | 0.180 | [44] |
R1: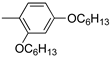 ; R2-10: H ; R2-10: H |
CH3CN | 0.1 M [Bu4N][ClO4] | 0.020 | [44] |
R1: 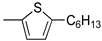 ; R2-10: H ; R2-10: H |
CH3CN | 0.1 M [Bu4N][ClO4] | 0.090 | [44] |
R1: 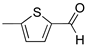 ; R2-10: H ; R2-10: H |
CH3CN | 0.1 M [Bu4N][ClO4] | 0.130 | [44] |
R1: 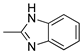 ; R2-10: H ; R2-10: H |
CH3CN | 0.1 M [Bu4N][ClO4] | 0.075 | [45] |
R1: 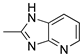 ; R2-10: H ; R2-10: H |
CH3CN | 0.1 M [Bu4N][ClO4] | 0.085 | [45] |
R1,10: 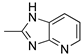 ; R2-9: H ; R2-9: H |
CH3CN | 0.1 M [Bu4N][ClO4] | 0.205 | [45] |
R1,10: 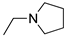 ; R2-9: H ; R2-9: H |
DCM | 0.2 M [Bu4N][PF6] | 0.002 | [9] |
R1,10: 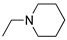 ; R2-9: H ; R2-9: H |
DCM | 0.2 M [Bu4N][PF6] | -0.001 | [9] |
R1,10: 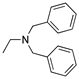 ; R2-9: H ; R2-9: H |
DCM | 0.2 M [Bu4N][PF6] | -0.001 | [9] |
R1: 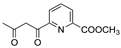 ; R2-10: H ; R2-10: H |
CH3CN | 0.1 M [Bu4N][ClO4] | 0.366 | [45] |
R1: 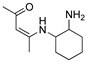 ; R2-10: H ; R2-10: H |
CH3CN | 0.1 M [Bu4N][PF6] | 0.100 | [46] |
R1: 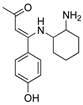 ; R2-10: H ; R2-10: H |
CH3CN | 0.1 M [BuN][PF6] | 0.120 | [46] |
3. Ferrocene-Based Electrochemical Sensors Using Oxygen-Containing Host Molecules
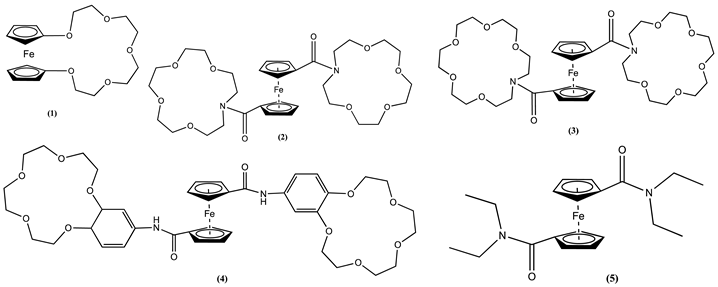
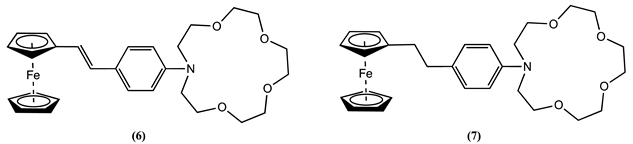
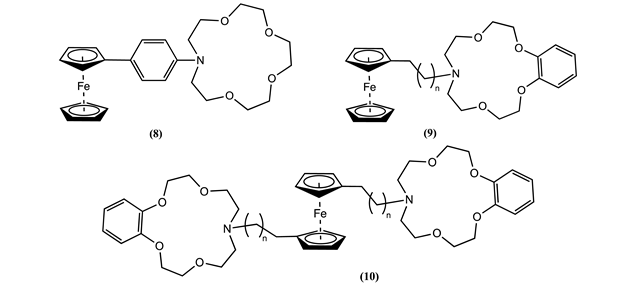
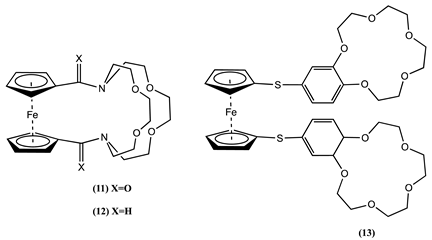
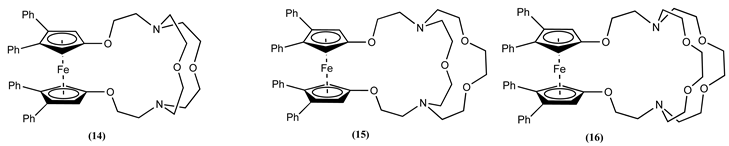
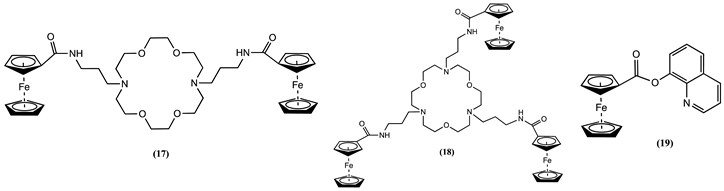
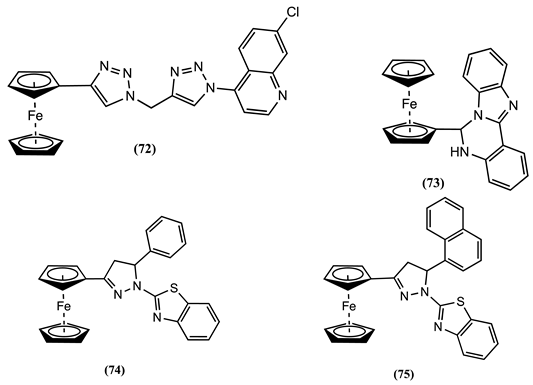
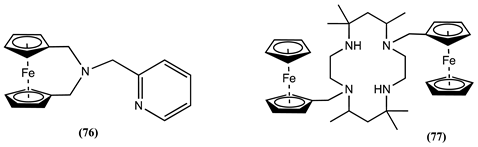
4. Ferrocene-Based Electrochemical Sensors Using Nitrogen-Containing Host Molecules
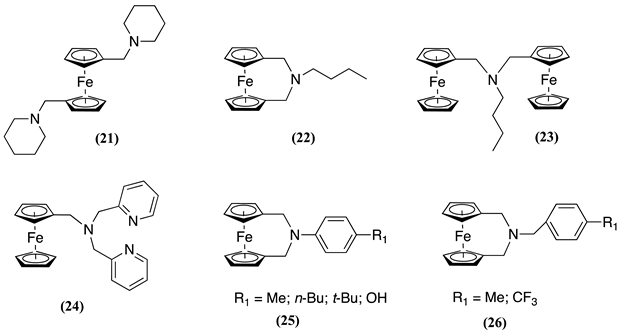
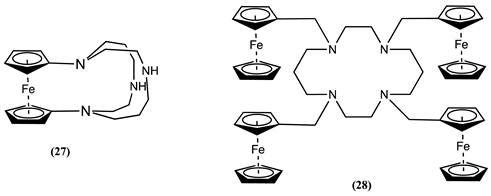
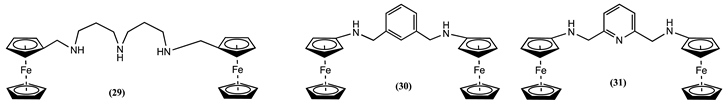

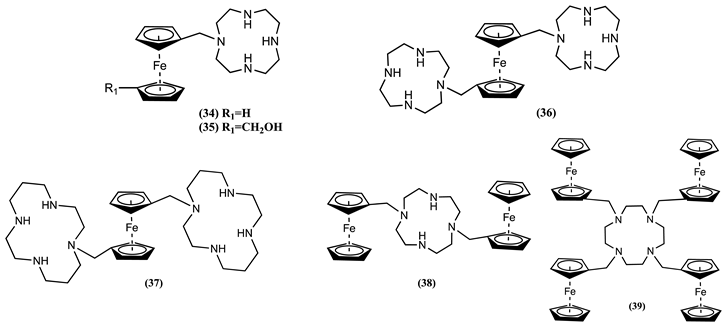
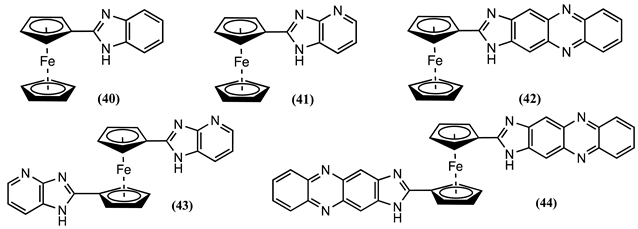
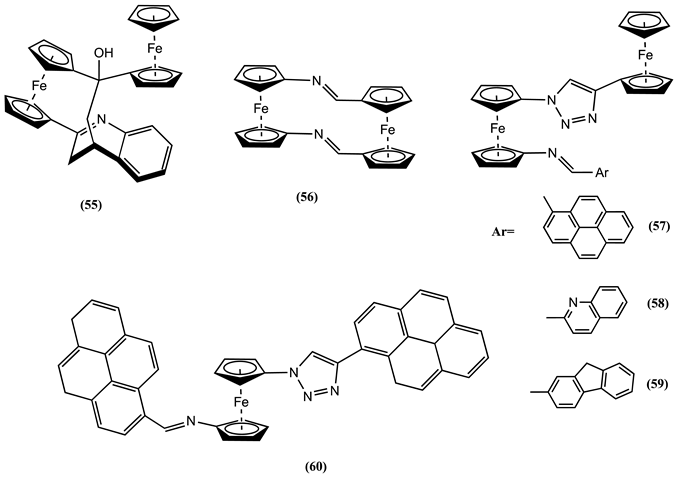
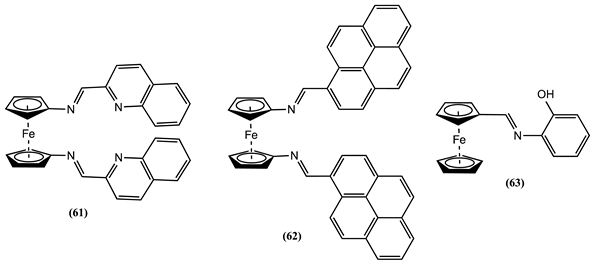
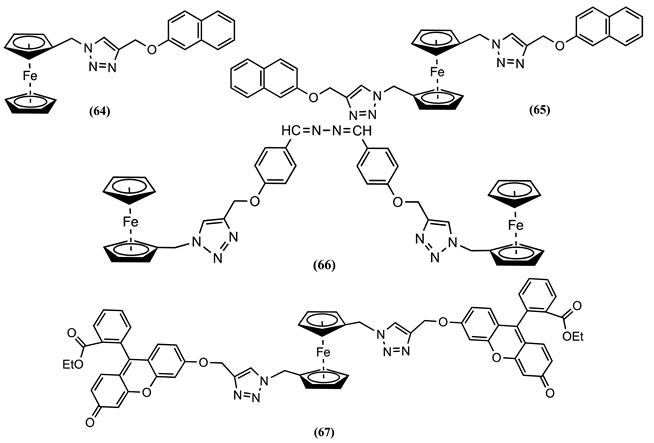
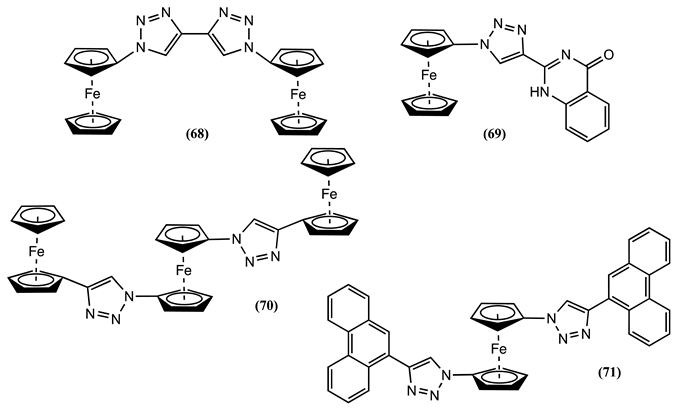
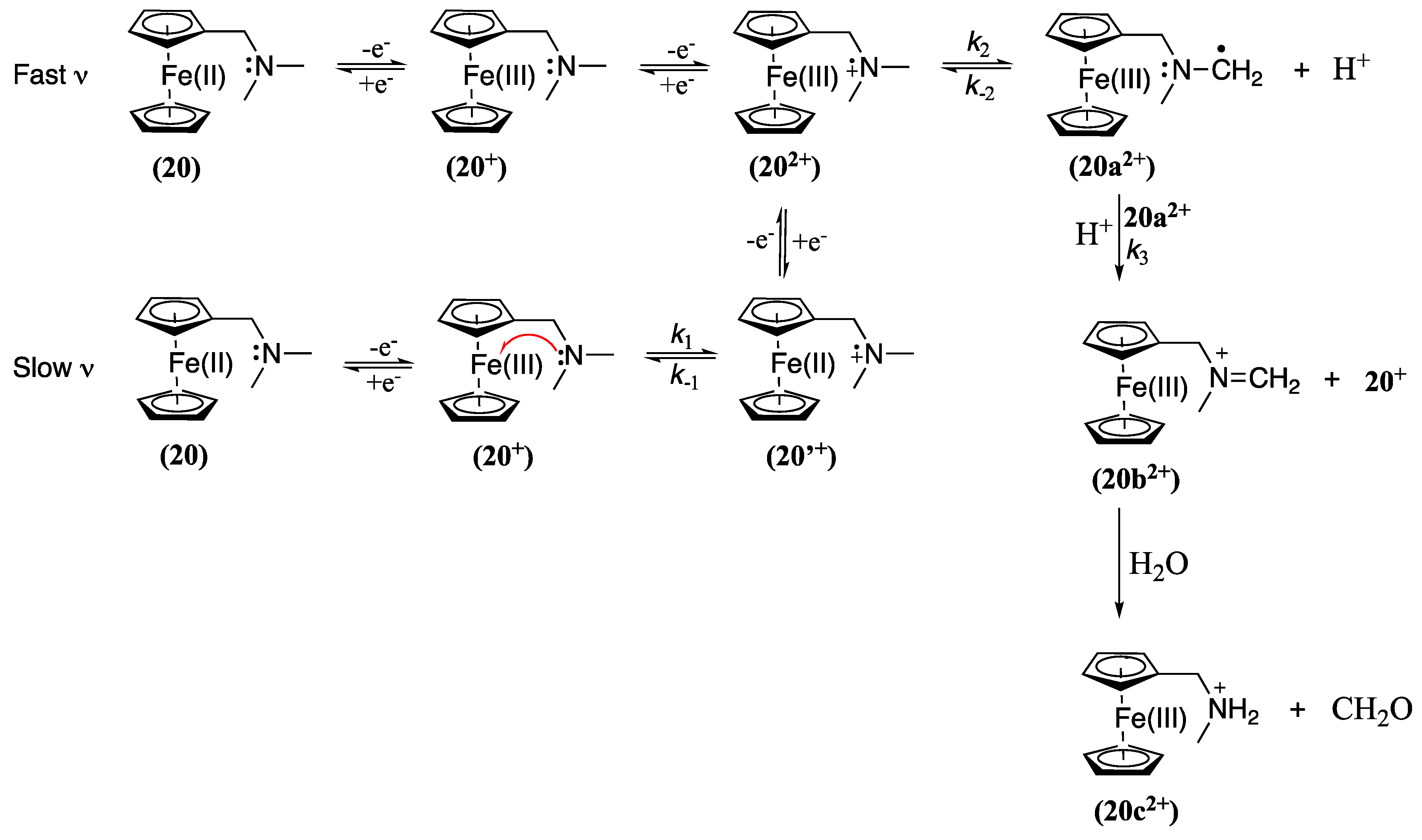
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lehn, J.M. Supramolecular Chemistry; Wiley-VCH: New York, USA, 1995; pp. I–X. [Google Scholar]
- Zanello, P. Inorganic Electrochemistry: Theory, Practice and Application; The Royal Society of Chemistry: Cambridge (UK), 2003. [Google Scholar]
- Molina, P.; Tárraga, A.; Caballero, A. Ferrocene-Based Small Molecules for Multichannel Molecular Recognition of Cations and Anions. European Journal of Inorganic Chemistry 2008, 2008, 3401–3417. [Google Scholar] [CrossRef]
- Togni, A.; Hayashi, T. , (Eds.) Ferrocenes: homogeneolls catalysis, organic synthesis, materials science. VCH: New York, USA, 1995.
- Casado, C.M.; Alonso, B.; García-Armada, M.P. 7.02—Ferrocenes and Other Sandwich Complexes of Iron. In Comprehensive Organometallic Chemistry IV, Parkin, G., Meyer, K., O’hare, D., Eds.; Elsevier: Oxford, 2022; pp. 3–45. [Google Scholar]
- Stepnicka, P. , (Ed.) Ferrocenes: Ligands, Materials and Biomolecules. John Wiley & Sons, Ltd.: Chichester, UK, 2008.
- Torriero, A.A.J.; Zeng, Z.; Mruthunjaya, A.K.V.; Bond, A.M. Electrochemical Properties of Cyclen and Cyclam Macrocycles Bearing Ferrocenyl Pendants and Their Transition Metal Complexes. Journal of Electroanalytical Chemistry 2023, 945, 117687. [Google Scholar] [CrossRef]
- Milaeva, E.R.; Tyurin, V.Y.; Shpakovsky, D.B.; Moiseeva, A.A.; Gracheva, Y.A.; Antonenko, T.A.; Maduar, V.V.; Osolodkin, D.I.; Palyulin, V.A.; Shevtsova, E.F. Redox-active metal complexes with 2,2′-dipicolylamine containing ferrocenyl moiety: Synthesis, electrochemical behavior and biological activity. Journal of Organometallic Chemistry 2017, 839, 60–70. [Google Scholar] [CrossRef]
- Dwadnia, N.; Allouch, F.; Pirio, N.; Roger, J.; Cattey, H.; Fournier, S.; Penouilh, M.-J.; Devillers, C.H.; Lucas, D.; Naoufal, D.; et al. Aminomethyl-Substituted Ferrocenes and Derivatives: Straightforward Synthetic Routes, Structural Characterization, and Electrochemical Analysis. Organometallics 2013, 32, 5784–5797. [Google Scholar] [CrossRef]
- Osakada, K.; Sakano, T.; Horie, M.; Suzaki, Y. Functionalized ferrocenes: Unique properties based on electronic communication between amino group of the ligand and Fe center. Coordination Chemistry Reviews 2006, 250, 1012–1022. [Google Scholar] [CrossRef]
- Fabbrizzi, L. The ferrocenium/ferrocene couple: a versatile redox switch. ChemTexts 2020, 6, 22. [Google Scholar] [CrossRef]
- Bayly, S.R.; Beer, P.D.; Chen, G.Z. Ferrocene Sensors. In Ferrocenes: Ligands, Materials and Biomolecules Stepnicka, P., Ed.; John Wiley & Sons: Chichester, England, 2008; pp. 281–318. [Google Scholar]
- Noviandri, I.; Brown, K.N.; Fleming, D.S.; Gulyas, P.T.; Lay, P.A.; Masters, A.F.; Phillips, L. The Decamethylferrocenium/Decamethylferrocene Redox Couple: A Superior Redox Standard to the Ferrocenium/Ferrocene Redox Couple for Studying Solvent Effects on the Thermodynamics of Electron Transfer. J. Phys. Chem. B 1999, 103, 6713–6722. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, L. Theoretical investigations of ferrocene/ferrocenium solvation in imidazolium-based room-temperature ionic liquids. PCCP Phys. Chem. Chem. Phys. 2013, 15, 2669–2683. [Google Scholar] [CrossRef]
- Torriero, A.A.J. Characterization of decamethylferrocene and ferrocene in ionic liquids: argon and vacuum effect on their electrochemical properties. Electrochimica Acta 2014, 137, 235–244. [Google Scholar] [CrossRef]
- Torriero, A.A.J.; Sunarso, J.; Forsyth, M.; Pozo-Gonzalo, C. Assessment of permethylated transition-metal sandwich complexes as internal reference redox systems in ionic liquids. PCCP Phys. Chem. Chem. Phys. 2013, 15, 2547–2553. [Google Scholar] [CrossRef]
- Torriero, A.A.J.; Howlett, P.C. Ionic liquid effects on the redox potential of ferrocene. Electrochemistry Communications 2012, 16, 84–87. [Google Scholar] [CrossRef]
- Torriero, A.A.; Sunarso, J.; Howlett, P.C. Critical evaluation of reference systems for voltammetric measurements in ionic liquids. Electrochimica Acta 2012, 82, 60–68. [Google Scholar] [CrossRef]
- Torriero, A.A.J. Reference systems for voltammetric measurements in ionic liquids. In Electrochemistry in Ionic Liquids. Volume 1: Fundamentals, Torriero, A.A.J., Ed.; Springer: Switzerland, 2015; Volume 1. [Google Scholar]
- Torriero, A.A.J.; Sunarso, J.; Howlett, P.C. Critical Evaluation of Reference Systems for Voltammetric Measurements in Ionic Liquids. Electrochimica Acta 2012, 82, 60–68. [Google Scholar] [CrossRef]
- Torriero, A.A.J. On Choosing Ferrocene as an Internal Reference Redox Scale for Voltammetric Measurements: A Cautionary Tale. Medicinal & Analytical Chemistry International Journal 2019, 3, 000151. [Google Scholar] [CrossRef]
- Inzelt, G.; Lewenstam, A.; Scholz, F. Handbook of reference electrodes; Springer: Berlin, 2013. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Electrochemical methods: fundamentals and applications, 2nd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2001. [Google Scholar]
- Izutzu, K. Electrochemistry in nonaqueous solutions; Wiley-VCH: NY, USA, 2002. [Google Scholar]
- Smith, T.J.; Stevenson, K.J. Reference electrodes. In Handbook of electrochemistry, Zoski, C.G., Ed.; Elsevier: Amsterdam, 2007; pp. 73–110. [Google Scholar]
- Torriero, A.A.J.; Bond, A.M. Critical Evaluation of Electrochemistry in Ionic Liquids. In Electroanalytical Chemistry Research Trends, Hayashi, K., Ed.; Nova Science Publishers, Inc.: New York, 2009. [Google Scholar]
- Torriero, A.A.J. Understanding the Differences between a Quasi-Reference Electrode and a Reference Electrode. Medicinal & Analytical Chemistry International Journal 2019, 3, 000144. [Google Scholar]
- Gritzner, G.; Kuta, J. Recommendations on reporting electrode potentials in nonaqueous solvents. Pure and Applied Chemistry 1983, 56, 461–466. [Google Scholar] [CrossRef]
- Torriero, A.A.J.; Feldberg, S.; Zhang, J.; Simonov, A.; Bond, A. On choosing a reference redox system for electrochemical measurements: a cautionary tale. Journal of Solid State Electrochemistry 2013, 17, 3021–3026. [Google Scholar] [CrossRef]
- Aranzaes, J.R.; Daniel, M.-C.; Astruc, D. Metallocenes as references for the determination of redox potentials by cyclic voltammetry—Permethylated iron and cobalt sandwich complexes, inhibition by polyamine dendrimers, and the role of hydroxy—containing ferrocenes. Canadian Journal of Chemistry 2006, 84, 288–299. [Google Scholar] [CrossRef]
- Ruiz, J.; Astruc, D. Permethylated electron-reservoir sandwich complexes as references for the determination of redox potentials. Suggestion of a new redox scale. Comptes Rendus de l’Academie des Sciences, Serie IIc: Chimie 1998, 1, 21–27. [Google Scholar] [CrossRef]
- Matsumoto, M.; Swaddle, T.W. The Decamethylferrocene(+/0) Electrode Reaction in Organic Solvents at Variable Pressure and Temperature. Inorganic Chemistry 2004, 43, 2724–2735. [Google Scholar] [CrossRef]
- Freyberg, D.P.; Robbins, J.L.; Raymond, K.N.; Smart, J.C. Crystal and molecular structures of decamethylmanganocene and decamethylferrocene. Static Jahn-Teller distortion in a metallocene. Journal of the American Chemical Society 1979, 101, 892–897. [Google Scholar] [CrossRef]
- Barriere, F.; Geiger, W.E. Use of Weakly Coordinating Anions to Develop an Integrated Approach to the Tuning of Delta E1/2 Values by Medium Effects. J. Am. Chem. Soc. 2006, 128, 3980–3989. [Google Scholar] [CrossRef]
- Bennett, M.A.; Bhargava, S.K.; Bond, A.M.; Burgar, I.M.; Guo, S.X.; Kar, G.; Priver, S.H.; Wagler, J.; Willis, A.C.; Torriero, A.A.J. Synthesis, X-ray structure and electrochemical oxidation of palladium(II) complexes of ferrocenyldiphenylphosphine. Dalton Transactions 2010, 39, 9079–9090. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Torriero, A.A.J.; Belousoff, M.J.; Bond, A.M.; Spiccia, L. Synthesis, X-ray Structure of Ferrocene Bearing bis(Zn-cyclen) Complexes and the Selective Electrochemical Sensing of TpT. Chemistry - A European Journal 2009, 15, 10988–10996. [Google Scholar] [CrossRef] [PubMed]
- Blom, N.F.; Neuse, E.W.; Thomas, H.G. Electrochemical characterization of some ferrocenylcarboxylic acids. Transition Metal Chemistry 1987, 12, 301–306. [Google Scholar] [CrossRef]
- Nonjola, P.T.N.; Siegert, U.; Swarts, J.C. Synthesis, Electrochemistry and Cytotoxicity of Ferrocene-Containing Amides, Amines and Amino-Hydrochlorides. Journal of Inorganic and Organometallic Polymers and Materials 2015, 25, 376–385. [Google Scholar] [CrossRef]
- Zhong, Z.H.; Matsumura-Inoue, T.; Ichimura, A. Solvent effect on the redox potential of ferrocene derivatives using an ultramicroelectrode. Analytical Sciences 1992, 8, 877–879. [Google Scholar] [CrossRef]
- Paul, A.; Borrelli, R.; Bouyanfif, H.; Gottis, S.; Sauvage, F. Tunable Redox Potential, Optical Properties, and Enhanced Stability of Modified Ferrocene-Based Complexes. ACS Omega 2019, 4, 14780–14789. [Google Scholar] [CrossRef] [PubMed]
- Scholl, H.; Sochaj, K. Cyclic voltammetry of some ferrocenophanes in acetonitrile. Electrochimica Acta 1991, 36, 689–694. [Google Scholar] [CrossRef]
- Plenio, H.; Yang, J.; Diodone, R.; Heinze, J. Redox-Switched Bonding of Protons to Ferrocenophanes, Ferrocene Cryptands, and Simple Ferrocene Amines. Correlation of X-ray Structural Data and Cyclic Voltammetry Derived Redox Potentials. Inorganic Chemistry 1994, 33, 4098–4104. [Google Scholar] [CrossRef]
- Lee, T.-Y.; Chiang, P.-R.; Tsai, M.-C.; Lin, C.-Y.; Huang, J.-H. From diacetylferrocene to 1,1′-ferrocenyldiimines: Substituent effects on synthesis, molecular structure, electrochemical behavior and optical absorption property. Journal of Molecular Structure 2009, 935, 102–109. [Google Scholar] [CrossRef]
- Manfredi, N.; Decavoli, C.; Boldrini, C.L.; Coluccini, C.; Abbotto, A. Ferrocene Derivatives Functionalized with Donor/Acceptor (Hetero)Aromatic Substituents: Tuning of Redox Properties. Energies 2020, 13. [Google Scholar] [CrossRef]
- Zapata, F.; Caballero, A.; Espinosa, A.; TaÌrraga, A.; Molina, P. Imidazole-Annelated Ferrocene Derivatives as Highly Selective and Sensitive Multichannel Chemical Probes for Pb(II) Cations. The Journal of Organic Chemistry 2009, 74, 4787–4796. [Google Scholar] [CrossRef] [PubMed]
- Celedón, S.; Hamon, P.; Artigas, V.; Fuentealba, M.; Kahlal, S.; Carrillo, D.; Saillard, J.-Y.; Hamon, J.-R.; Manzur, C. Ferrocene functionalized enantiomerically pure Schiff bases and their Zn(ii) and Pd(ii) complexes: a spectroscopic, crystallographic, electrochemical and computational investigation. New Journal of Chemistry 2022, 46, 3948–3960. [Google Scholar] [CrossRef]
- Paul D., Beer; Gale, P.A.; Chen, G.Z. Electrochemical molecular recognition: pathways between complexation and signalling. Journal of the Chemical Society, Dalton Transactions 1999, 1897–1910. [Google Scholar] [CrossRef]
- Beer, P.D.; Danks, J.P.; Hesek, D.; McAleer, J.F. A potassium-selective sulfide-linked redox-active ferrocene ionophore that exhibits extraordinary electrochemical recognition behaviour. Journal of the Chemical Society, Chemical Communications, 1993; 1735–1737. [Google Scholar] [CrossRef]
- Beer, P.D. Transition Metal and Organic Redox-Active Macrocycles Designed to Electrochemically Recognize Charged and Neutral Guest Species. In Advances in Inorganic Chemistry; Sykes, A.G., Ed.; Academic Press, 1992; Volume 39, pp. 79–157. [Google Scholar]
- Pedersen, C.J. Cyclic polyethers and their complexes with metal salts. Journal of the American Chemical Society 1967, 89, 7017–7036. [Google Scholar] [CrossRef]
- Lindoy, L.F. The Chemistry of Macrocyclic Ligand Complexes; Cambridge University Press: Cambridge, 1989. [Google Scholar]
- Dobler, M. Macrocyclic chemistry: aspects of organic and inorganic supramolecular chemistry by B. Dietrich, P. Viout and J.-M. Lehn. Acta Crystallographica Section B 1993, 49, 1074–1074. [Google Scholar] [CrossRef]
- Beer, P.D.; Keefe, A.D.; Sikanyika, H.; Blackburn, C.; McAleer, J.F. Metallocene bis(aza-crown ether) ligands and related compounds. Their syntheses, co-ordination chemistry, and electrochemical properties. Journal of the Chemical Society, Dalton Transactions 1990, 3289–3294. [Google Scholar] [CrossRef]
- Saji, T.; Kinoshita, I. Electrochemical ion transport with ferrocene functionalized crown ether. Journal of the Chemical Society, Chemical Communications 1986, 716–717. [Google Scholar] [CrossRef]
- Beer, P.D.; Sikanyika, H.; Slawin, A.M.Z.; Williams, D.J. The synthesis, coordination and electrochemical studies of metallocene bis(crown ether) receptor molecules. Single-crystal x-ray structure of a ferrocene bis(crown ether) potassium complex. Polyhedron 1989, 8, 879–886. [Google Scholar] [CrossRef]
- Saji, T. ELECTROCHEMICALLY SWITCHED CATION BINDING IN PENTAOXA [13] FERROCENOPHANE. Chemistry Letters 1986, 15, 275–276. [Google Scholar] [CrossRef]
- Beer, P.D.; Sikanyika, H.; Blackburn, C.; McAleer, J.F.; Drew, M.G.B. Redox responsive crown ethers containing a direct link between the ferrocene redox-active centre and benzo crown ether. Crystal structure of a ferrocene benzo-15-crown-5 sodium complex. Journal of Organometallic Chemistry 1988, 356, C19–C22. [Google Scholar] [CrossRef]
- Beer, P.D.; Blackburn, C.; McAleer, J.F.; Sikanyika, H. Redox-responsive crown ethers containing a conjugated link between the ferrocene moiety and a benzo crown ether. Inorganic Chemistry 1990, 29, 378–381. [Google Scholar] [CrossRef]
- Jin, S.; Wang, D.; Jin, X.; Chen, G.Z. Intramolecular Electrostatics: Coulomb’s Law at Sub-Nanometers. ChemPhysChem 2004, 5, 1623–1629. [Google Scholar] [CrossRef]
- Hall, C.D. Macrocycles and Cryptands Containing the Ferrocene Unit. In Ferrocenes: homogeneolls catalysis, organic synthesis, materials science, Togni, A., Hayashi, T., Eds.; VCH: New York, USA, 1995; pp. 279–316. [Google Scholar]
- Hall, C.D.; Sharpe, N.W.; Danks, I.P.; Sang, Y.P. Cyclic voltammetry studies on the complexation of metal cations by cryptands containing the ferrocene unit. Journal of the Chemical Society, Chemical Communications 1989, 0, 419–421. [Google Scholar] [CrossRef]
- Dennis Hall, C.; Chu, S.Y.F. Cyclic voltammetry of cryptands and cryptates containing the ferrocene unit. Journal of Organometallic Chemistry 1995, 498, 221–228. [Google Scholar] [CrossRef]
- Medina, J.C.; Goodnow, T.T.; Rojas, M.T.; Atwood, J.L.; Lynn, B.C.; Kaifer, A.E.; Gokel, G.W. Ferrocenyl iron as a donor group for complexed silver in ferrocenyldimethyl[2.2]cryptand: a redox-switched receptor effective in water. J. Am. Chem. Soc. 1992, 114, 10583–10595. [Google Scholar] [CrossRef]
- Plenio, H.; Aberle, C. Oxaferrocene Cryptands as Efficient Molecular Switches for Alkali and Alkaline Earth Metal Ions. Organometallics 1997, 16, 5950–5957. [Google Scholar] [CrossRef]
- Beer, P.D.; Crowe, D.B.; Ogden, M.I.; Drew, M.G.B.; Main, B. Ammonium redox-responsive receptors containing multiple ferrocene and quinone redox-active centres attached to di- and tri-aza crown ether macrocycles. Journal of the Chemical Society, Dalton Transactions, 1993; 2107–2116. [Google Scholar] [CrossRef]
- Shi, L.; Song, W.; Li, Y.; Li, D.-W.; Swanick, K.N.; Ding, Z.; Long, Y.-T. A multi-channel sensor based on 8-hydroxyquinoline ferrocenoate for probing Hg(II) ion. Talanta 2011, 84, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-H.; Chen, F.-R.; Zhou, Y.-F.; Wang, J.-N.; Zhang, H.; Xu, J.-G. Enhanced fluorescence sensing of hydroxylated organotins by a boronic acid-linked Schiff base. Chemical Communications 2009, 4179–4181. [Google Scholar] [CrossRef]
- Zanello, P.; Cinquantini, A.; Fontani, M.; Giardiello, M.; Giorgi, G.; Landis, C.R.; Kimmich, B.F.M. Redox behavior of boronato-functionalized 1,1′-bis(diphenylphosphino)ferrocenes. Journal of Organometallic Chemistry 2001, 637-639, 800–804. [Google Scholar] [CrossRef]
- El Ghachtouli, S.; Cadiou, C.; Déchamps-Olivier, I.; Chuburu, F.; Aplincourt, M.; Turcry, V.; Le Baccon, M.; Handel, H. Spectroscopy and Redox Behaviour of Dicopper(II) and Dinickel(II) Complexes of Bis(cyclen) and Bis(cyclam) Ligands. European Journal of Inorganic Chemistry 2005, 2005, 2658–2668. [Google Scholar] [CrossRef]
- Mruthunjaya, A.K.V.; Torriero, A.A.J. Mechanistic Aspects of the Electrochemical Oxidation of Aliphatic Amines and Aniline Derivatives. Molecules 2023, 28, 471. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Belousoff, M.J.; Spiccia, L.; Bond, A.M.; Torriero, A.A.J. Macrocycles Bearing Ferrocenyl Pendants and their Electrochemical Properties upon Binding to Divalent Transition Metal Cations. ChemPlusChem 2018, 83, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Torriero, A.A.J.; Bond, A.M.; Spiccia, L. Fluorescent and Electrochemical Sensing of Polyphosphate Nucleotides by Ferrocene Functionalised with Two Zn(II)-(TACN)(pyrene) Complexes. Chemistry - A European Journal 2010, 16, 9154–9163. [Google Scholar] [CrossRef]
- Plenio, H.; Aberle, C. Synthesis of a ferrocene bridged cyclam: a new redox-active macrocycle and the structure of a nickel(II) complex with strongly coupled metal centers. Chemical Communications 1998, 2697–2698. [Google Scholar] [CrossRef]
- Plenio, H.; Aberle, C.; Al Shihadeh, Y.; Lloris, J.M.; Martínez-Máñez, R.; Pardo, T.; Soto, J. Ferrocene–Cyclam: A Redox-Active Macrocycle for the Complexation of Transition Metal Ions and a Study on the Influence of the Relative Permittivity on the Coulombic Interaction between Metal Cations. Chemistry – A European Journal 2001, 7, 2848–2861. [Google Scholar] [CrossRef] [PubMed]
- Tendero, M.J.L.; Benito, A.; Cano, J.; Lloris, J.M.; Martínez-Máñez, R.; Soto, J.; Edwards, A.J.; Raithby, P.R.; Rennie, M.A. Host molecules containing electroactive cavities obtained by the molecular assembly of redox-active ligands and metal ions. Journal of the Chemical Society, Chemical Communications, 1995; 1643–1644. [Google Scholar] [CrossRef]
- D. Beer, P.; K. Smith, D. Tunable bis(ferrocenyl) receptors for the solution-phase electrochemical sensing of transition-metal cations. Journal of the Chemical Society, Dalton Transactions 1998, 417–424. [Google Scholar] [CrossRef]
- De Santis, G.; Fabbrizzi, L.; Licchelli, M.; Pallavicini, P.; Perotti, A. A redox-switchable ligand for which the binding ability is enhanced by oxidation of its ferrocene unit. Journal of the Chemical Society, Dalton Transactions, 1992; 3283–3284. [Google Scholar] [CrossRef]
- Zapata, F.; Caballero, A.; Espinosa, A.; Tárraga, A.; Molina, P. A Simple but Effective Ferrocene Derivative as a Redox, Colorimetric, and Fluorescent Receptor for Highly Selective Recognition of Zn2+ Ions. Organic Letters 2007, 9, 2385–2388. [Google Scholar] [CrossRef]
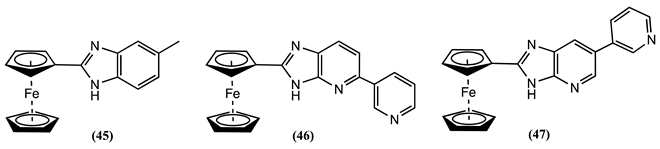
- Nedunchezhian, K.; Nallathambi, S. Mono- and di-ferrocene conjugated 5-methyl benzimidazole based multi-channel receptors for cations/anions with their antimicrobial and anticancer studies. New Journal of Chemistry 2023, 47, 4656–4666. [Google Scholar] [CrossRef]
- Zhang, B.; Suo, Q.; Li, Q.; Hu, J.; Zhu, Y.; Gao, Y.; Wang, Y. Multiresponsive chemosensors based on ferrocenylimidazo[4,5-b]pyridines: Solvent-dependent selective dual sensing of Hg2+ and Pb2+. Tetrahedron 2022, 120, 132878. [Google Scholar] [CrossRef]
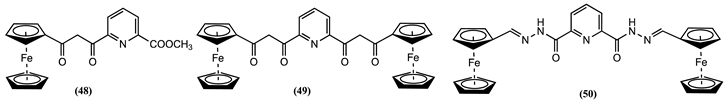
- Tian, H.-j.; Tang, R.-r.; Li, S.-f.; Luo, Y.-m. Synthesis, characterization and electrochemical recognition of metal ions of three new ferrocenyl derivatives containing pyridyl moiety. Journal of Central South University 2013, 20, 3379–3384. [Google Scholar] [CrossRef]
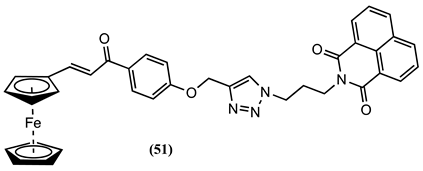
- Kaur, S.; Shalini, *!!! REPLACE !!!*; Ahmad Shiekh, B.; Kumar, V.; Kaur, I. Triazole-tethered naphthalimide-ferrocenyl-chalcone based voltammetric and potentiometric sensors for selective electrochemical quantification of Copper(II) ions. Journal of Electroanalytical Chemistry 2022, 905, 115966. [Google Scholar] [CrossRef]
- Arivazhagan, C.; Borthakur, R.; Ghosh, S. Ferrocene and Triazole-Appended Rhodamine Based Multisignaling Sensors for Hg2+ and Their Application in Live Cell Imaging. Organometallics 2015, 34, 1147–1155. [Google Scholar] [CrossRef]
- Lopez, J.L.; Tárraga, A.; Espinosa, A.; Velasco, M.D.; Molina, P.; Lloveras, V.; Vidal-Gancedo, J.; Rovira, C.; Veciana, J.; Evans, D.J.; et al. A New Multifunctional Ferrocenyl-Substituted Ferrocenophane Derivative: Optical and Electronic Properties and Selective Recognition of Mg2+ Ions. Chemistry – A European Journal 2004, 10, 1815–1826. [Google Scholar] [CrossRef]
- Otón, F.; Ratera, I.; Espinosa, A.; Wurtz, K.; Parella, T.; Tárraga, A.; Veciana, J.; Molina, P. Selective Metal-Cation Recognition by [2.2]Ferrocenophanes: The Cases of Zinc- and Lithium-Sensing. Chemistry – A European Journal 2010, 16, 1532–1542. [Google Scholar] [CrossRef]
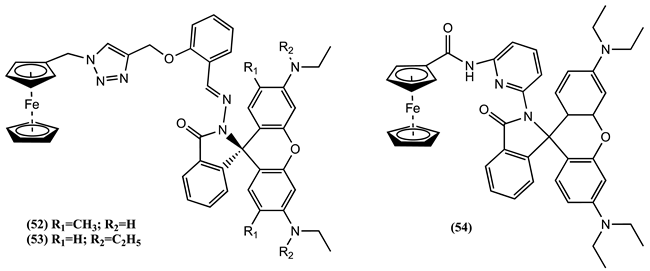
- Otón, F.; González, M.d.C.; Espinosa, A.; Tárraga, A.; Molina, P. Synthesis, Structural Characterization, and Sensing Properties of Clickable Unsymmetrical 1,1′-Disubstituted Ferrocene–Triazole Derivatives. Organometallics 2012, 31, 2085–2096. [Google Scholar] [CrossRef]
- González, M.a.d.C.; Otón, F.; Orenes, R.A.; Espinosa, A.; Tárraga, A.; Molina, P. Ferrocene–Triazole–Pyrene Triads as Multichannel Heteroditopic Recognition Receptors for Anions, Cations and Ion Pairs. Organometallics 2014, 33, 2837–2852. [Google Scholar] [CrossRef]
- Sola, A.; Otón, F.; Espinosa, A.; Tárraga, A.; Molina, P. Aldimines generated from aza-Wittig reaction between bis(iminophosphoranes) derived from 1,1′-diazidoferrocene and aromatic or heteroaromatic aldehydes: electrochemical and optical behaviour towards metal cations. Dalton Transactions 2011, 40, 12548–12559. [Google Scholar] [CrossRef]
- Guo, L.; Yan, L.; Xie, R.; Han, L.; Zhu, N. Multi-channel sensing of trivalent metal ions using a simple ferrocenyl Schiff base probe with AIE property. Journal of Molecular Structure 2024, 1295, 136629. [Google Scholar] [CrossRef]
- Bhatta, S.R.; Bheemireddy, V.; Vijaykumar, G.; Thakur, A. Triazole appended mono and 1,1′ di-substituted ferrocene-naphthalene conjugates: Highly selective and sensitive multi-responsive probes for Hg(II). Sensors and Actuators B: Chemical 2017, 240, 640–650. [Google Scholar] [CrossRef]
- Bhatta, S.R.; Mondal, B.; Lima, S.; Thakur, A. Metal-coordination driven intramolecular twisting: a turn-on fluorescent-redox probe for Hg2+ ions through the interaction of ferrocene nonbonding orbitals and dibenzylidenehydrazine. Dalton Transactions 2019, 48, 8209–8220. [Google Scholar] [CrossRef] [PubMed]
- Bhatta, S.R.; Pal, A.; Sarangi, U.K.; Thakur, A. Ferrocene appended fluorescein-based ratiomeric fluorescence and electrochemical chemosensor for Fe3+ and Hg2+ ions in aqueous media: Application in real samples analysis. Inorganica Chimica Acta 2019, 498, 119097. [Google Scholar] [CrossRef]
- Romero, T.; Orenes, R.A.; Tárraga, A.; Molina, P. Preparation, Structural Characterization, Electrochemistry, and Sensing Properties toward Anions and Cations of Ferrocene-Triazole Derivatives. Organometallics 2013, 32, 5740–5753. [Google Scholar] [CrossRef]
- Kamal, A.; Kumar, S.; Kumar, V.; Mahajan, R.K. Selective sensing ability of ferrocene appended quinoline-triazole derivative toward Fe (III) ions. Sensors and Actuators B: Chemical 2015, 221, 370–378. [Google Scholar] [CrossRef]
- Pandey, R.; Gupta, R.K.; Shahid, M.; Maiti, B.; Misra, A.; Pandey, D.S. Synthesis and Characterization of Electroactive Ferrocene Derivatives: Ferrocenylimidazoquinazoline as a Multichannel Chemosensor Selectively for Hg2+ and Pb2+ Ions in an Aqueous Environment. Inorganic Chemistry 2012, 51, 298–311. [Google Scholar] [CrossRef] [PubMed]
- Kiran Kumar, C.; Trivedi, R.; Giribabu, L.; Niveditha, S.; Bhanuprakash, K.; Sridhar, B. Ferrocenyl pyrazoline based multichannel receptors for a simple and highly selective recognition of Hg2+ and Cu2+ ions. Journal of Organometallic Chemistry 2015, 780, 20–29. [Google Scholar] [CrossRef]
- Chen, L.; Cui, X.; Cheng, H.; Chen, X.; Song, M.; Tang, M.; Wei, D.; Wu, Y. Syntheses, structures of N-(substituted)-2-aza-[3]-ferrocenophanes and their application as redox sensor for Cu2+ ion. Applied Organometallic Chemistry 2012, 26, 449–454. [Google Scholar] [CrossRef]
- Krishnapriya, K.R.; Sampath, N.; Ponnuswamy, M.N.; Kandaswamy, M. Synthesis and electrochemical sensing behaviour of a new ferrocene functionalized tet ‘a’ macrocyclic receptor towards transition metal ions. Applied Organometallic Chemistry 2007, 21, 311–317. [Google Scholar] [CrossRef]
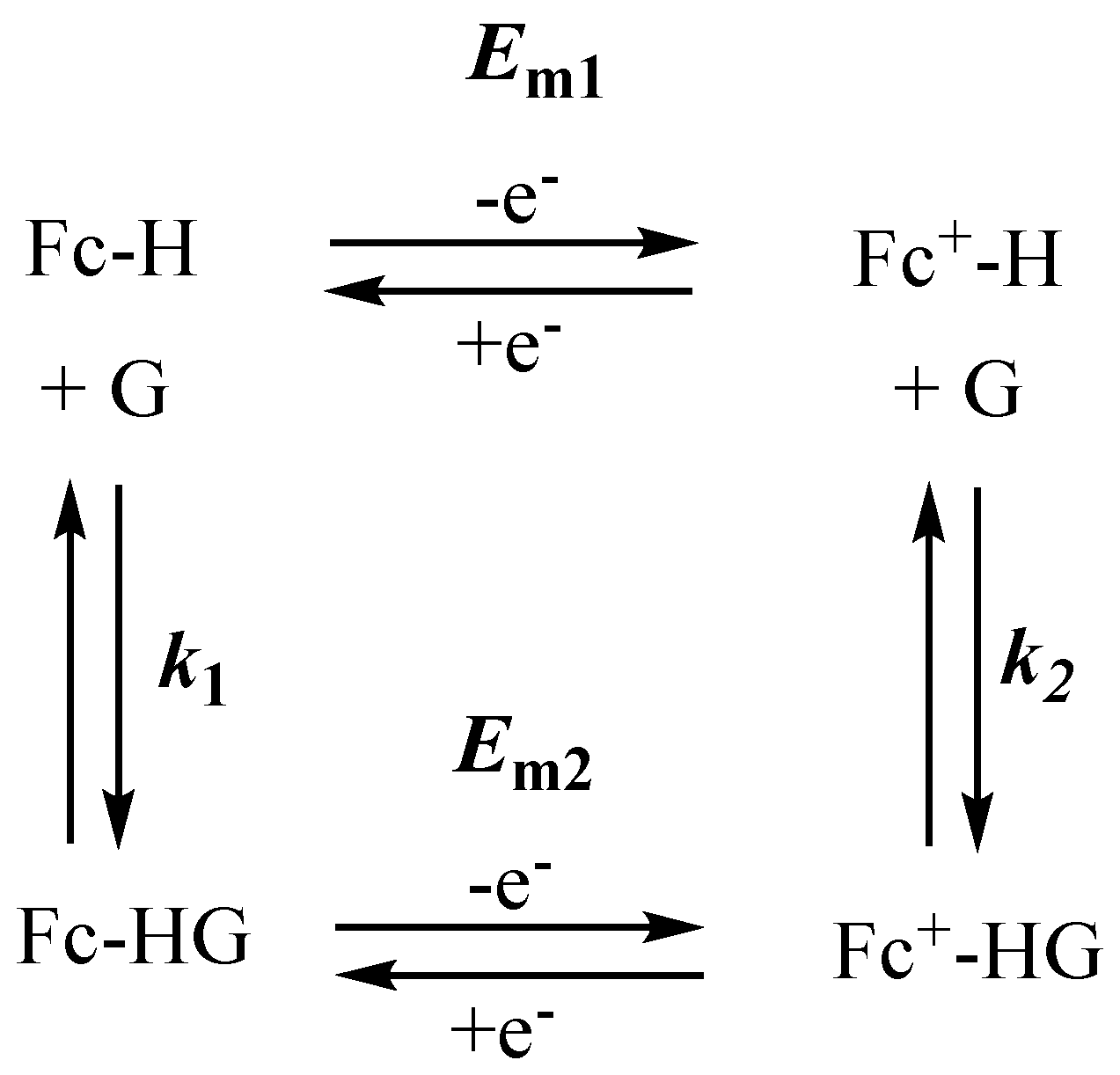
| Solvent | Electrolyte |
Em of Fc0/+ vs. DmFc0/+ (V) |
Ref. |
|---|---|---|---|
| 1,2-dibromoethane | 0.1 M [Bu4N][ClO4] | 0.475±0.007 | [13] |
| 1,2-dichloroethane | 0.1 M [Bu4N][ClO4] | 0.532±0.001 | [13] |
| 1,2-dichlorobenzene | 0.1 M [Bu4N][ClO4] | 0.535±0.001 | [13] |
| 2-propanol | 0.1 M [Bu4N][CF3SO3] | 0.455±0.003 | [13] |
| 2,2,2-trifluoroethanol | 0.1 M [Bu4N][ClO4] | 0.575±0.004 | [13] |
| Acetone | 0.1 M [Bu4N]Cl | 0.451±0.005 | [34] |
| 0.1 M [Bu4N][ClO4] | 0.479±0.004 | [13] | |
| 0.1 M [Bu4N][PF6] | 0.487±0.005 | [34] | |
| 0.1 M [Bu4N][TFAB] | 0.504±0.005 | [34] | |
| Acetonitrile (CH3CN) | 0.1 M [Bu4N]Cl | 0.501±0.005 | [34] |
| 0.1 M [Bu4N][ClO4] | 0.505±0.002 | [13] | |
| 0.1 M [Bu4N][PF6] | 0.509±0.003 | [35] | |
| 0.1 M [Bu4N][TFAB] | 0.517±0.005 | [34] | |
| Acetonitrile:dichloromethane (80:20) | 0.1 M [Bu4N][PF6] | 0.512±0.003 | [36] |
| Aniline | 0.1 M [Bu4N][ClO4] | 0.527±0.004 | [13] |
| Anisole | 0.1 M [Bu4N][PF6] | 0.518±0.005 | [34] |
| 0.1 M [Bu4N][TFAB] | 0.607±0.005 | [34] | |
| Benzonitrile | 0.1 M [Bu4N]Cl | 0.524±0.005 | [34] |
| 0.1 M [Bu4N][ClO4] | 0.523±0.001 | [13] | |
| 0.1 M [Bu4N][PF6] | 0.530±0.005 | [34] | |
| 0.1 M [Bu4N][TFAB] | 0.543±0.005 | [34] | |
| Benzyl alcohol | 0.1 M [Bu4N][ClO4] | 0.508±0.003 | [13] |
| Bromobenzene | 0.1 M [Bu4N][ClO4] | 0.489±0.005 | [13] |
| Chlorobenzene | 0.1 M [Bu4N][ClO4] | 0.497±0.001 | [13] |
| Chloroform | 0.1 M [Bu4N][ClO4] | 0.483±0.001 | [13] |
| Dichloromethane (DCM) | 0.1 M [Bu4N]Cl | 0.534±0.005 | [34] |
| 0.1 M [Bu4N][ClO4] | 0.532±0.002 | [13] | |
| 0.1 M [Bu4N][ClO4] | 0.570±0.002 | [2] | |
| 0.1 M [Bu4N][PF6] | 0.548±0.003 | [35] | |
| 0.1 M [Et4N][BF4] | 0.541±0.003 | [17] | |
| 0.1 M [Bu4N][TFAB] | 0.614±0.005 | [34] | |
| 0.1 M [C4mPyr][FAP] | 0.589±0.003 | [17] | |
| 0.1 M [C2mim][FAP] | 0.590±0.003 | [17] | |
| 0.1 M [C2mim][B(CN)4] | 0.588±0.003 | [17] | |
| 0.1 M [C4mim][NTf2] | 0.570±0.003 | [17] | |
| 0.1 M [C4mPyr][NTf2] | 0.568±0.003 | [17] | |
| 0.1 M [C2mim][FSI] | 0.569±0.003 | [17] | |
| 0.1 M [C3mim][FSI] | 0.568±0.003 | [17] | |
| 0.1 M [C4mPyr][N(CN)2] | 0.564±0.003 | [17] | |
| 0.1 M [C4mim][PF6] | 0.556±0.003 | [17] | |
| 0.1 M [C4mim][BF4] | 0.557±0.003 | [17] | |
| 0.1 M [C4mim][CF3SO3] | 0.556±0.003 | [17] | |
| Diethyl ether | 0.1 M [Bu4N][BArF24] | 0.550±0.005 | [34] |
| 0.1 M Na[BArF24] | 0.583±0.005 | [34] | |
| Dimethyl sulfoxide | 0.1 M [Bu4N][PF6] | 0.486±0.005 | [34] |
| 0.1 M [Bu4N][TFAB] | 0.493±0.005 | [34] | |
| 0.1 M [Bu4N][ClO4] | 0.468±0.001 | [13] | |
| Ethanol | 0.1 M [Bu4N][ClO4] | 0.473±0.005 | [13] |
| Formamide | 0.1 M [Bu4N][ClO4] | 0.510±0.003 | [13] |
| Methanol (MeOH) | 0.1 M [Bu4N][ClO4] | 0.497±0.002 | [13] |
| Nitrobenzene | 0.1 M [Bu4N][ClO4] | 0.514±0.002 | [13] |
| Nitromethane | 0.1 M [Bu4N]Cl | 0.505±0.005 | [34] |
| 0.1 M [Bu4N][ClO4] | 0.516±0.004 | [13] | |
| 0.1 M [Bu4N][PF6] | 0.510±0.005 | [34] | |
| 0.1 M [Bu4N][TFAB] | 0.516±0.005 | [34] | |
| N-methylformamide | 0.1 M [Bu4N][ClO4] | 0.510±0.002 | [13] |
| N,N-dimethylformamide (DMF) | 0.1 M [Bu4N]Cl | 0.475±0.005 | [34] |
| 0.1 M [Bu4N][ClO4] | 0.458±0.003 | [13] | |
| 0.1 M [Bu4N][PF6] | 0.478±0.005 | [34] | |
| 0.1 M [Bu4N][TFAB] | 0.493±0.005 | [34] | |
| N,N-dimethylacetamide | 0.1 M [Bu4N][ClO4] | 0.455±0.008 | [13] |
| Propylene carbonate | 0.1 M [Bu4N][ClO4] | 0.495±0.002 | [13] |
| Pyridine | 0.1 M [Bu4N][ClO4] | 0.517±0.004 | [13] |
| Tetrahydrofuran | 0.1 M [Bu4N][BF4] | 0.413±0.005 | [34] |
| 0.1 M [Bu4N][CF3SO3] | 0.438±0.005 | [34] | |
| 0.1 M [Bu4N][ClO4] | 0.423±0.005 | [34] | |
| 0.427±0.002 | [13] | ||
| 0.1 M [Bu4N][PF6] | 0.446±0.005 | [34] | |
| 0.1 M [Bu4N][BPh4] | 0.485±0.005 | [34] | |
| 0.1 M Na[BArF24] | 0.502±0.005 | [34] | |
| 0.1 M [Bu4N][TFAB] | 0.484±0.005 | [34] | |
| 0.1 M [Bu4N][BArF24] | 0.521±0.005 | [34] | |
| Toluene | 0.1 M [Bu4N][BF4] a | 0.430±0.005 | [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





