Introduction
The average functional lifespan of urban trees is similar to that of the hardscape that surrounds them (Hauer et al. 2020). Consequently, long-lived street trees are likely to encounter at least one construction event as communities work to extend or maintain their built infrastructure (Hauer et al. 2020). When construction activities take place in close proximity to trees, damage can occur both above and below ground (Matheny et al. 2023). While the latter scenario may be less visibly apparent, it remains a significant threat to the future health and stability of trees.
One common form of damage involves the linear removal of tree roots on one or more sides of a tree (Smiley 2008, Benson et al. 2019a). This pattern of belowground damage can result from various activities such as trenching, grade changes, road or sidewalk installation or replacement, or building construction. For the sake of simplicity, we will refer to these collectively as “trenching” in this article. Trenching damage can diminish both tree health (Watson 1998, Benson et al. 2019b) and stability (Smiley et al. 2014, Fini et al. 2020), with the extent of impact dependent on factors such as proximity to the tree, tree species, age, and condition (Matheny et al. 2023).
In industry best management practices and local tree preservation policies, proximity is often scaled relative to the diameter at breast height (DBH) of a tree. For example, trenching occurring 30 cm away from the base of a tree with a stem diameter of 30 cm would be considered as one times the DBH. The most recent edition of the International Society of Arboriculture’s Best Management Practices—Managing Trees During Construction recommends establishing a protection zone that extends between 6 and 18 tree diameters away from the base, taking into account the perceived tolerance of the species and the relative age of the tree (Matheny et al. 2023).
While tree protection zones can be used to preserve trees completely surrounded by construction activities (Benson et al. 2019c), often only a smaller portion of the root system is at risk of damage. Given that many roots remain intact, a common question associated with trenching and related activities is, “How close is too close?” in terms of tree health and stability. Drawing on findings from Benson et al. (2019a), current industry Best Management Practices highlight that long-term water stress can result if trenching takes place closer than six times the DBH of the tree (Matheny et al. 2023). Regarding stability, these same guidelines indicate that many trees will see a decline in structural integrity when root loss occurs at three times the DBH. Moreover, most trees will face an immediate loss in stability if this approaches one and a half times the DBH (Matheny et al. 2023).
These recommendations stem from a limited yet expanding body of research that documents the effects of root damage on mature tree health and stability. In relation to tree health, Geisler and Ferree (1984) observed that Malus spp. ‘Golden Delicious’ trees, that experienced 28% and 58% root loss through trenching, exhibited reduced photosynthesis, transpiration, leaf size, and shoot growth compared to similar trees with only 10% root loss. Similarly, a study by Miller and Neely (1993) found that over 92% of trees with trenching damage between 0.5 m to 3.3 m from their bases remained alive after five growing seasons. Only one species, Celtis occidentalis L., exhibited noticeable decreases in growth due to this root disruption. In a controlled study, Watson (1998) trenched Quercus palustris Münchh. trees on one, two, or three sides, at one DBH from the trunk base. While all trees survived, significant dieback was observed in trees that were trenched on all three sides. As the intensity of the trenching treatments increased, signs of stress became more visible (Watson 1998). Similar results were reported by Benson et al. (2019b), who found that increasing trenching intensity from one side to two, three, or four sides of Acer spp. trees increased the impact on stomatal conductance and growth parameters, including trunk diameter, leaf area, and shoot growth.
Recent experiments have assessed both industry-recommended and suboptimal tree protection zones in relation to trenching damage (Benson et al. 2019a) and other construction activities, such as site-wide grade changes (Benson et al. 2019c). In the former study, the authors observed that trenching mature Quercus virginiana Mill. trees at 3 times, 6 times, or 12 times the DBH from the tree’s base resulted in discernible water stress in the subsequent weeks. A year later, trees subjected to the 6 times and 12 times DBH treatments had recovered, but those trenched at 3 times DBH remained water-stressed (Benson et al. 2019a). In the second study where trenching was intensified to encircle the same species entirely (Benson et al. 2019c), water stress was prevented when damage was situated more than 12 times the DBH away. Shoot extension diminished when trenching took place less than 12 times the DBH. Furthermore, trunk diameter growth decreased when trenching was conducted within the tree diameter’s widths.
Beyond health and growth impacts, root loss due to trenching and construction activities can reduce tree stability. Smiley (2008) trenched young Quercus phellos L. trees, progressively cutting roots in DBH increments from 5 to 0, to determine at which point rooting strength was compromised. They found that trenching occurring closer than three times the DBH significantly reduced the force required to tilt trees to a 1-degree angle during static pull tests. In contrast, Ghani et al. (2009) observed only minor, though statistically significant, differences in whole tree stability when trenching occurred less than three times the DBH away from the base of Eugenia grandis Wight trees.
More recent findings by Fini et al. (2020) showed that Aesculus hippocastanum L. and Tilia ✕ europaea L. trees experienced long-term losses (e.g., nearly 5 years) in rooting strength when trenched 4.5 times the DBH away from the tree base. These results are supported by post-storm field observations conducted by Johnson et al. in 2019. In their study, researchers determined that street trees located adjacent to sidewalks that were replaced in the years prior were more than twice as likely to fail following a wind event compared to trees without construction damage. Similarly, Moore (2014) found that of the 80 whole tree failures inspected after storm events, 58.8% of the trees showed evidence of past trenching root damage.
Trees that survive trenching damage and do not topple will regrow roots in an effort to regain structural integrity and their capacity to absorb water and nutrients. When roots are severed, new root growth typically occurs near the cut surface (Lyford and Wilson 1966). However, roots may also initiate further back, with Gilman and Yeager (1988) noting root growth occurring more than 10 cm behind the point of cutting. Cutting often leads to the emergence of multiple roots, resulting in a ‘coppice’ of new root growth (Wajja-Musukwe et al. 2008). Additionally, research has shown that larger woody roots are less likely to produce new roots than smaller ones when damaged (Watson et al. 2014).
To gain deeper insights into the long-term effects of root loss due to trenching, we revisited the research plots from Benson et al. 2019a, five years after the initial wounding. We measured root regrowth and compared it to our original treatments to determine if providing a larger buffer resulted in benefits associated with root regrowth, in addition to the physiological benefits observed in our original study. We also looked at decay and discoloration for a subsample of the study tree population. Our findings aim to further inform policies and best management practices related to tree protection during construction activities.
Materials and Methods
This study was conducted at the University of Florida Gulf Coast Research and Education Center in Balm, Florida, USA (27° 45′ 41.76” N, 82° 13′ 41.01” W). The site is situated in peninsular Florida, a region bounded to the west by the Gulf of Mexico, to the south by the Caribbean Sea, and to the east by the Atlantic Ocean. The research site experiences a subtropical climate and falls within USDA Hardiness Zone 9b, with a minimum average temperature range of 25 to 30 degrees F (-4 to -1 degrees C). The trees used in this study were planted in loamy sand soil as part of the research center’s landscaping 12 years before the study’s start.
In 2017, a total of 31 Quercus virginiana Mill. trees were chosen for a study examining the physiological effects, such as growth and water stress, resulting from root loss due to linear trenching (Benson et al. 2019a). Among these, 24 trees were deliberately subjected to trenching treatment, while the remaining 7 served as undisturbed controls. At the time of the study, the average trunk diameter at 1.4 m for this sample population measured 34.2 cm and the average height stood at 8.86 m.
Trees were randomly assigned to one of three trenching treatments: trenching at three times the diameter from the base of the trunk, trenching at 6 times the diameter from the base of the trunk, or trenching at 12 times the diameter from the base of the trunk. The trenches were dug on one side of each tree and measured approximately 10 meters in length, 10 centimeters in width, and 50 centimeters in depth. An air excavator (Air Spade, Guardair, Chicopee, MA, USA) was employed to create the trenches, and any exposed roots were cleanly cut using a hand pruning saw or secateurs. All cuts were made perpendicular to the direction of root growth, resulting in a flat pruning wound.
In September 2022, 15 out of the 24 wounded trees from the 2017 trial were re-excavated using the air excavator. Labor and cost constraints prevented us from visiting all 24 treated trees. By referring to the original plot map (Benson et al. 2019c), we were able to locate the initial trench lines and cut roots for remeasurement. Cut roots were measured to determine their CSA, based on two perpendicular caliper measurements of the original root. These were made just behind the regrowth. Furthermore, we recorded the number of new roots >1 mm in diameter emerging from each cut, along with the CSA of each newly formed root and the combined CSA of all roots emerging from the cut root. We also assessed the Euclidean distance between the cut root end and the tree’s base and recorded the current DBH of the tree.
The presence or absence of root regrowth was assessed as a mixed effects logistic regression model. We employed the glmer() function in the R package ‘lme4’ (Bates et al. 2015) to conduct the analysis, considering cut root CSA and either the initial treatment (i.e., trenching 3, 6, or 12 times the stem diameter away from the base of the tree) or the distance from the cut root end to the tree as predictor variables (
Figure 1). We included the tree identification number as a random effect.
We also explored the influence of our three predictor variables (distance, treatment, and cut root CSA;
Figure 1) on the level of root regrowth observed after five years. To achieve this, we established a “percent root regrowth” variable, which we calculated by dividing the combined CSA of all the new roots by the 2022 measurement of CSA of the cut root from which they originated and then multiplying the result by 100. Modeling was performed using the lme() function in the R package ‘nlme’ (Pinheiro and Bates 2000, Pinheiro et al. 2023). Our percent root regrowth variable was not strictly limited to the range between 0 and 100, as the combined CSA sometimes exceeded that of the cut root. Similar to our logistic regression analysis, we modeled this response twice. The first model incorporated treatment and cut root CSA as predictors, while the second focused on cut root CSA and the distance from the cut root end to the tree (
Figure 1). An alpha level of 0.05 for type I error was employed to determine statistical significance in all the above-mentioned models.
In addition to measuring root regrowth, we also harvested roots to assess discoloration; our assumption was that discoloration was an indication of decay. Our initial plan was to harvest 3 roots from each of the 15 trees in the study. However, this process proved more time-consuming than we had anticipated, and we were only able to excavate 19 roots from 6 trees before needing to return the air compressor rented for this project. The roots were cut from the tree in 10 cm sections until no discoloration was detected in the cut cross-section. Subsequently, these cross-sections were split radially to enable us to measure the depth of the discoloration from the cut surface. We provide some basic summary statistics of this data in our results for the benefit of those interested in root decay. However, due to the limited size of this dataset, we refrained from including it in any formal hypothesis testing.
Figures were generated using slide presentation software (Powerpoint, Microsoft Corporation, Redmond, WA, USA) and a data visualization application (DataGraph, Visual Data Tools, Inc., Chapel Hill, NC). All original written content was authored by the lead author, though an AI-powered language model (ChatGPT, OpenAI, Inc., San Francisco, CA, USA) was employed for copy editing purposes.
Results and Discussion
Out of the 557 roots remeasured for this study, only 38 (6.82%) did not regrow (
Table 1). In our logistic regression modeling, we were unable to detect any significant impact of treatment (minimum
P-value = 0.841) or the original root CSA (
P-value = 0.945) on the presence or absence of tree root regrowth. Similarly, the distance between the cut root and the trunk did not serve as a predictor for root regrowth presence/absence when exchanged with treatment in our second model (P-value = 0.892).
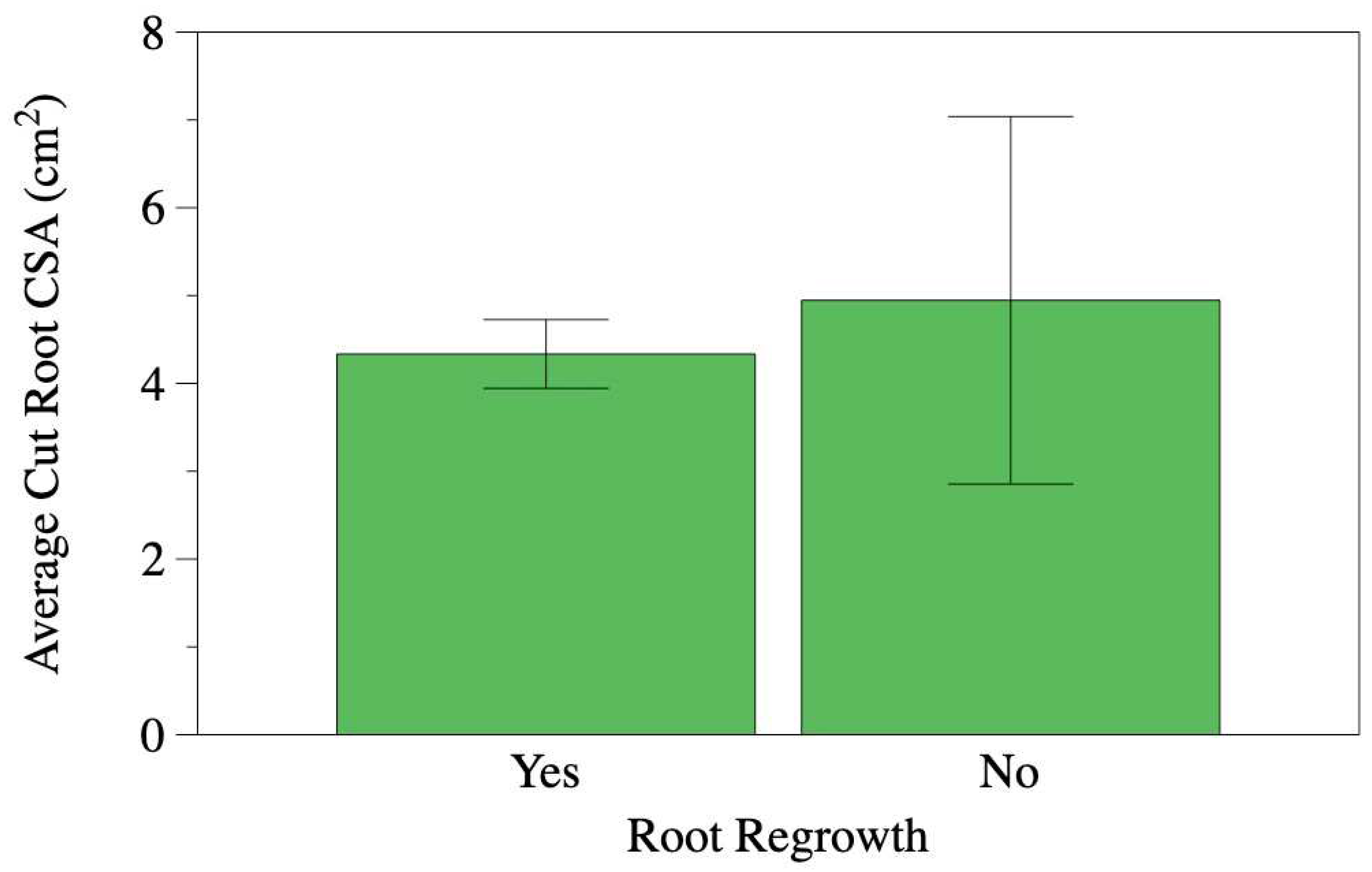
When comparing the average cut root areas between roots that exhibited regrowth and those that did not, we found that they were similar. The roots that regrew had an average cross-sectional area of 4.3 cm
2, while those that did not regrow measured an average of 4.9 cm
2 (
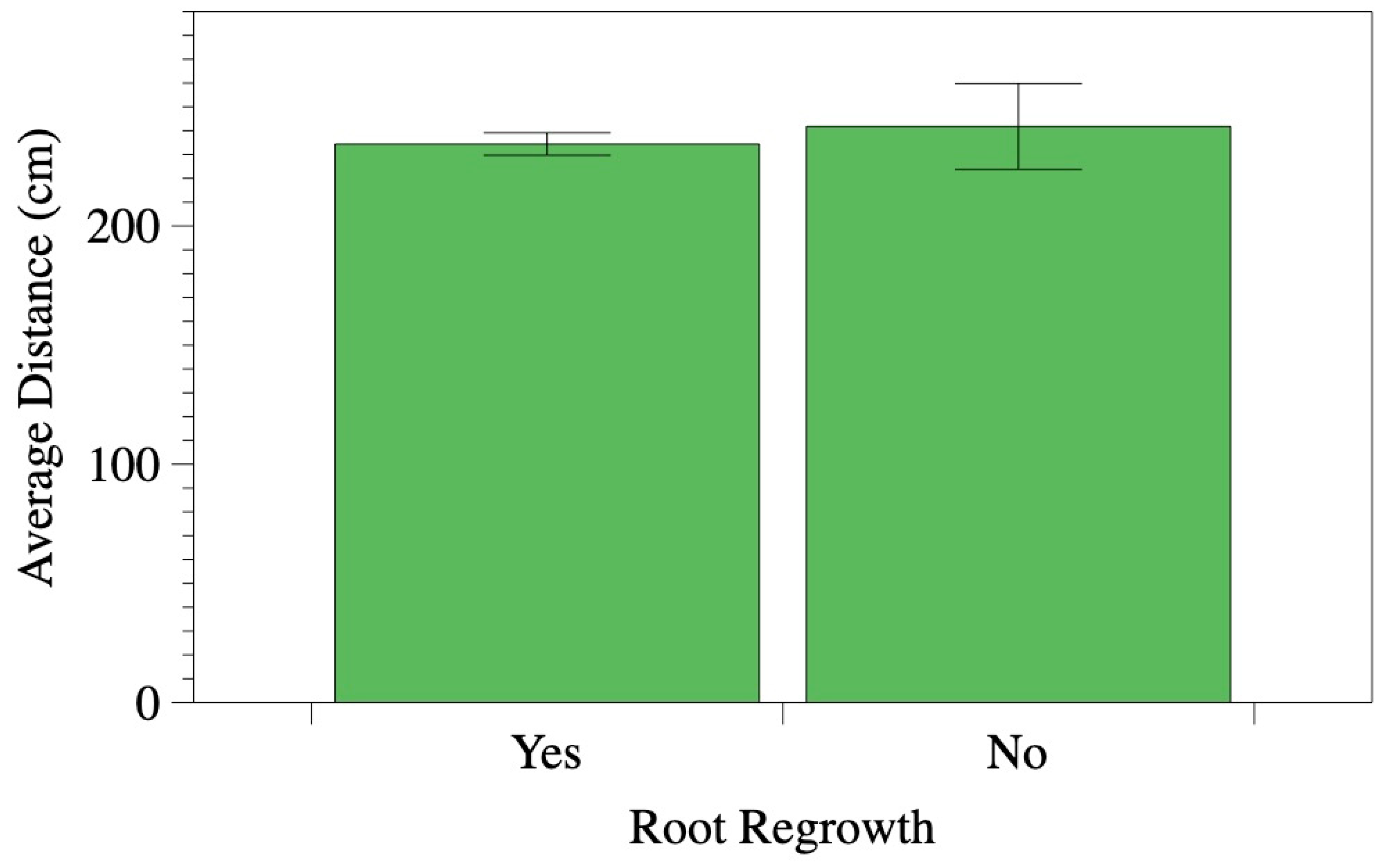
Figure 2). Similarly, the average distance from the trunk to the cutting location was comparable: 234.4 cm for roots with regrowth and 241.8 cm for roots without regrowth (
Figure 3).
Our inability to predict the presence or absence of root regrowth was somewhat surprising. In their comprehensive literature review of urban tree root management, Watson et al. (2014) noted that larger roots are less likely to regrow new roots when cut (Watson et al. 2014). The cited source for this work was Balder et al. (1999), who stated that root regeneration “depends on the age and thickness of the root, an old root is not able to form new roots.” While our roots varied in diameter (i.e., thickness), the trees had been in the landscape for only 12 years before undergoing root pruning. Consequently, the roots present in the surrounding landscape were relatively young, despite the study being conducted on “mature” Q. virginiana trees (Benson et al. 2019a). In addition to root size and age, there may be a species-specific effect determining the likelihood that roots will regrow when cut.
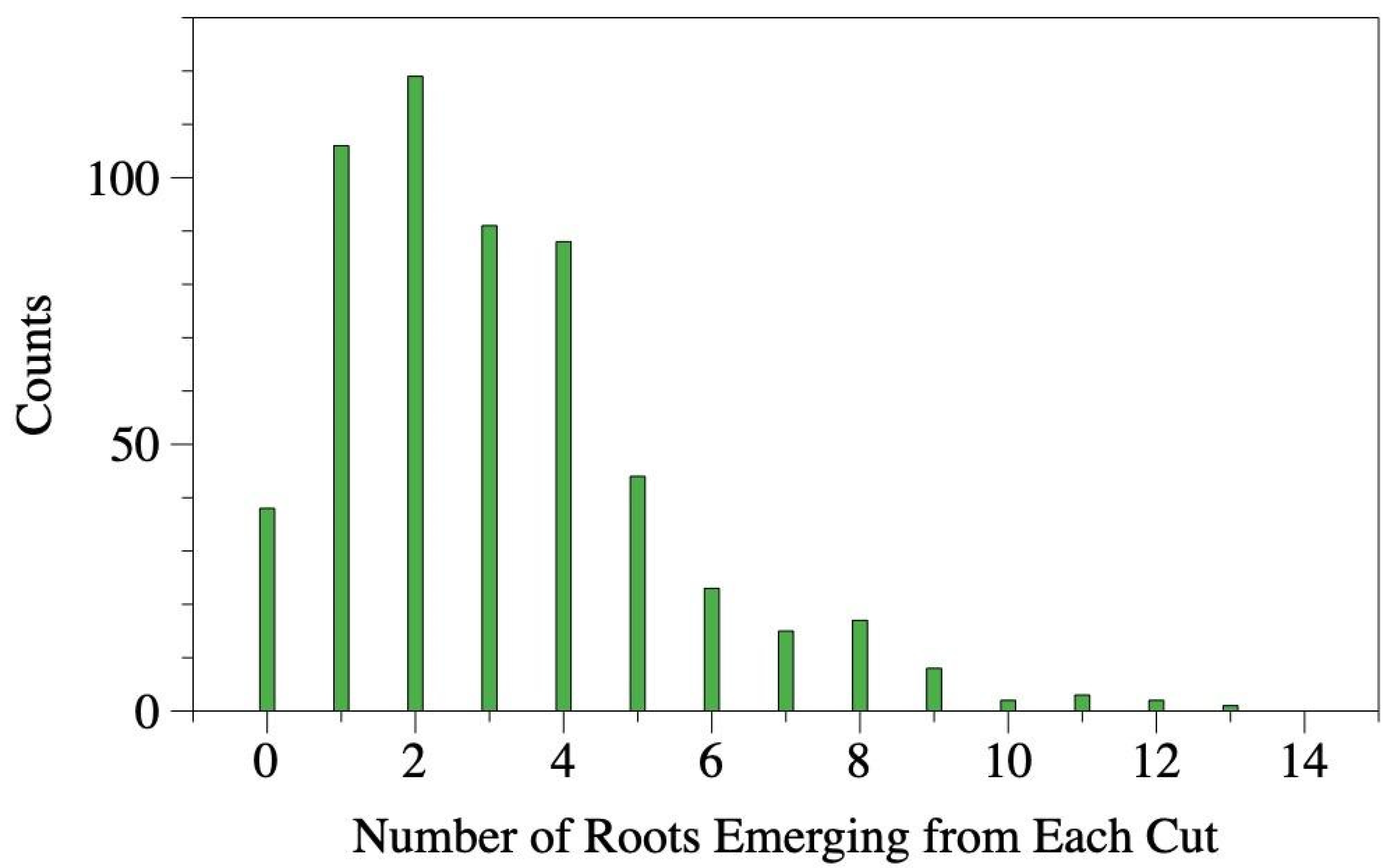
We observed the number of new roots emerging from each cut root and found an average of 3.1 with a median of three new roots (
Figure 4). While some cut roots generated as many as 13 new roots, the majority produced between 1 (Q1) and 4 (Q3) new roots (
Figure 4).
These results align with previous research, which found that root pruning or injury increased root branching (Wilson 1970). Wajja-Musukwe et al. (2008) referred to this regrowth as a “coppice”. In their study of root regrowth in five species planted in an agroforestry system, they observed an average of 4.7 to 9.6 new coppice roots for every main root, 11 months post-pruning (Wajja-Musukwe et al. 2008). Our findings showed an average of 3.1 roots per pruned root, which is slightly lower than these previously recorded counts. However, our results closely resemble those from a study by Gilman and Yeager (1988) on small container trees. In that study, root-pruned Q. virginiana seedlings exhibited three times more new root growth compared to unpruned controls.
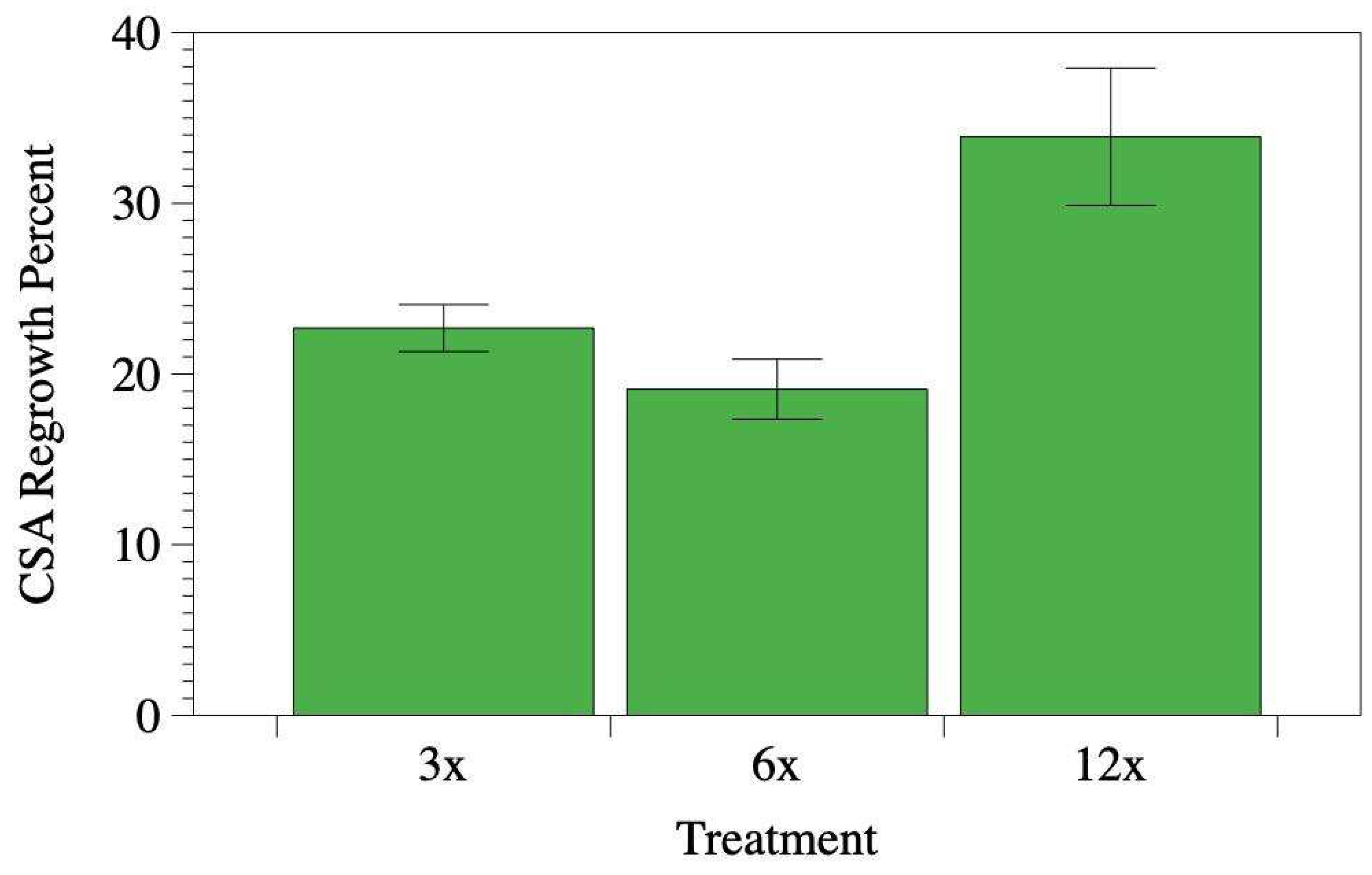
After five years, the average root CSA regrowth percentage for all 557 cut roots stood at 22.2%. In our model evaluating root regrowth percentage, treatment emerged as a significant predictor (
P-value = 0.024;
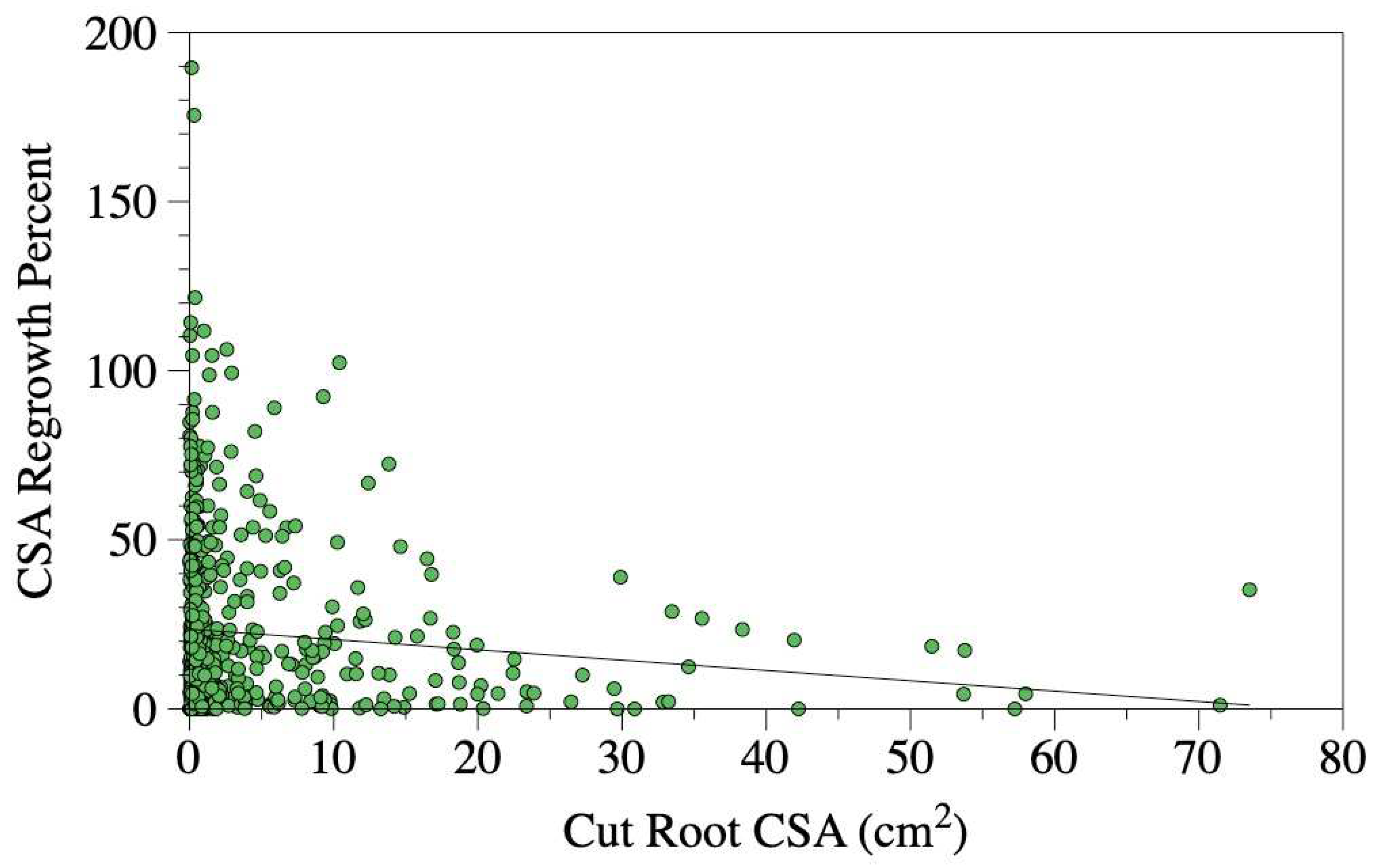
Figure 5). However, both the cut root CSA (
P-value = 0.066,
Figure 6) and the interaction between treatment and cut root CSA were not significant (
P-value = 0.123). When assessing average root CSA regrowth across treatments, roots cut 12 times the DBH from the trunk base exhibited the highest regrowth at 33.9% (
Figure 5). For comparison, roots cut at six times the DBH regrew by 22.69%, while those cut at 3 times the DBH saw a regrowth of 19.1% (
Figure 5).
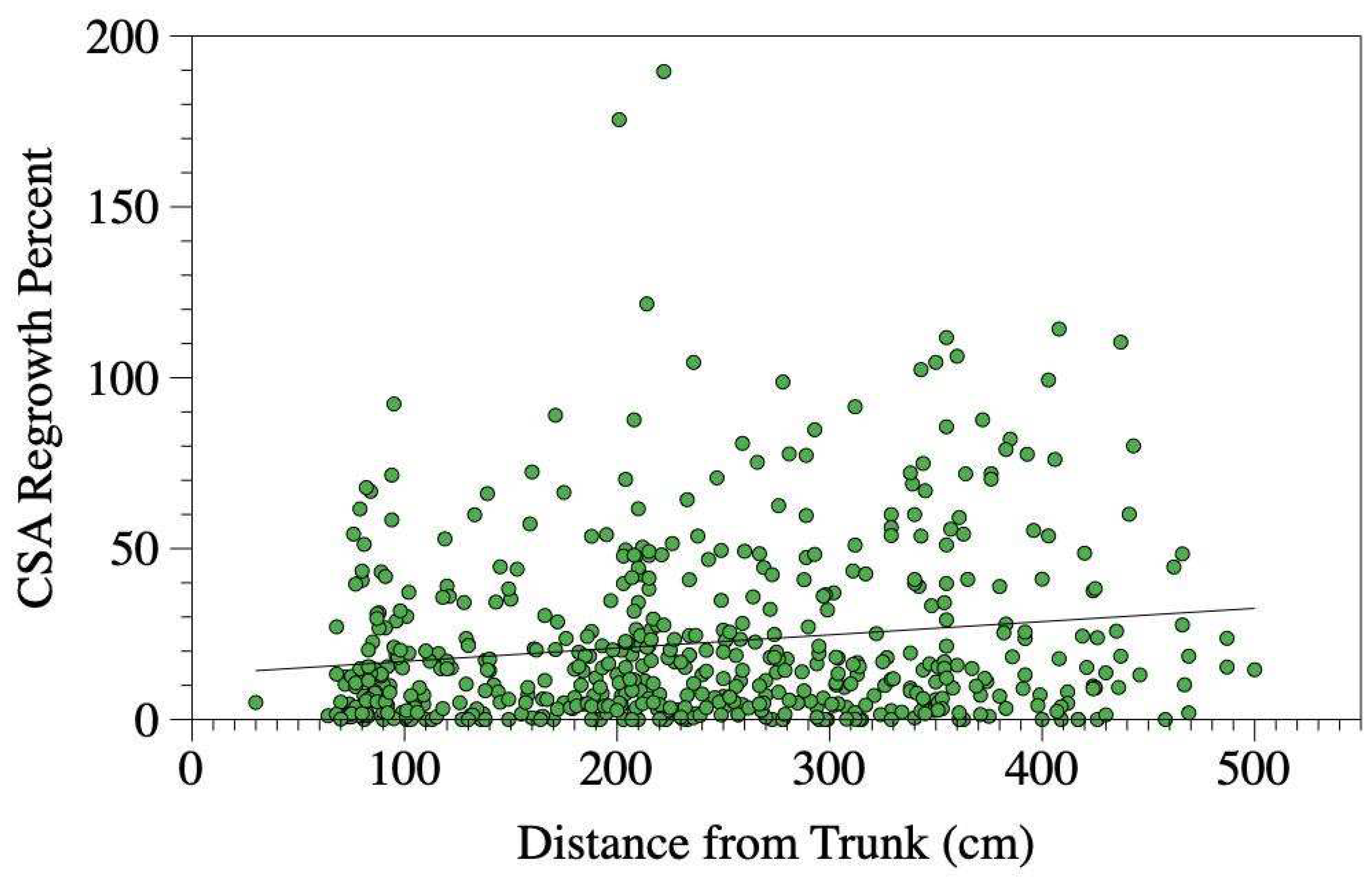
Substituting the treatment variable in the above model with the distance between the trunk and the cut root end yielded comparable results. The distance from the trunk significantly predicted the percentage of root regrowth (
P-value = 0.002,
Figure 7). However, neither the cut root CSA (
P-value = 0.125) nor the interaction between the two terms was not significant (
P-value = 0.621).
As previously mentioned, the CSA of the new root regrowth was 22.2% of the CSA of the cut roots. This is consistent with past findings by Wajja-Musukwe et al. (2008). In that study, researchers compared the CSA of root regrowth/coppice to the CSA of the main roots that grew from 11 months after pruning. Regrowth varied from 15.2% to 40.8%, depending on the species. The overall percentage of root regrowth across the five species was 25.8% (Wajja-Musukwe et al. 2008).
Out of the 19 roots harvested to evaluate discoloration, the cross-sectional area (CSA) of the cut roots ranged from 0.6361 cm2 to 73.53 cm2, with an average CSA of 22.40 cm2. Distance from the base of the tree ranged from 87 cm to 407 cm with an average distance of 199.21 cm. When assessing discoloration, only 3 out of the 19 roots showed measurable signs of discoloration. In two cases, the discoloration was within a centimeter from the cut root surface. One root, with a cut root CSA of 23.88 cm2, exhibited discoloration that extended up to 17.8 cm behind the cut surface.
Previous research conducted by Watson (2008) demonstrated that the tree species can have a significant impact on the extent of decay and discoloration observed in cut roots. In Watson’s study, it was found that Gleditsia triacanthos L. exhibited an average of 77.1 cm of discoloration when roots were cut 3 meters from the tree’s base. Interestingly, the length of discoloration decreased for cuts made closer to the tree’s base for this species, although this pattern was not observed for the other three species tested.
Our limited results suggest that the behavior of Q. virginiana roots is similar to that of Fraxinus pennsylvanica Marshall roots in the study conducted by Watson. For Q. virginiana, the average discoloration did not vary based on the distance of the cut from the tree’s base, ranging from 3.2 cm of decay for cuts made at the root flare to 6.3 cm for cuts made 3 meters from the base of the tree (Watson 2008).
Our results are even more closely aligned with the research conducted by Santamour in 1985. In his study, Santamour used a tree spade to transplant 40 Platanus spp. L. and 40 Liquidambar styraciflua L. saplings, and he observed no wood discoloration or decay in their root systems that could be attributed to root pruning. Similar findings were obtained when examining 36 mature Acer rubrum L. trees with a major root severed using a chainsaw within 0.5 meters of the base of the tree (Santamour 1985). Santamour noted that in the latter case, this strong compartmentalization with regard to root wounding was somewhat at odds with the species’ reputation for being a poor stem compartmentalizer.
Though not within the scope of this study, it would be interesting to investigate how the CSA of the conductive root tissue in the larger cut root compares to the combined conductive area of the smaller new roots. The utilization of dyes or electronic impedance measurements (Benson et al. 2019d) could provide insights into the extent of recovery in the root’s ability to absorb and transport water beyond the simple 22.2% regrowth relationship measured externally.
Conclusion
In conclusion, our findings suggest that increasing the distance between a tree and trenching activities can promote increased root regrowth, as demonstrated by the relationships observed between treatment levels, distance from the tree, and percent root regrowth. Building upon our initial study on these trees five years ago, this research reinforces the notion that providing more space is advantageous for safeguarding roots from construction damage. Not only are trees spared from the initial physiological stress linked with extensive root loss, but there also seems to be a benefit for root regrowth, even when controlling for the root’s CSA.
Acknowledgements
The authors would like to thank Seth Blair, Zach Freeman, Deb Hilbert, Drew McLean, Hunter Thorn, and Elise Willis for their efforts in excavating and recording root regrowth data. This work was funded through the Tree Fund and the Florida Chapter of the International Society of Arboriculture.
References
- Balder, H. Mechanical root injuries—Compartmentalisation, pruning and wound dressing. Acta Hortic. 1999, 496, 239–244. [Google Scholar] [CrossRef]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Software 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Benson, A.R.; Koeser, A.K.; Morgenroth, J. Responses of mature roadside trees to root severance treatments. Urban For. Urban Green. 2019a, 46, 126448. [Google Scholar] [CrossRef]
- Benson, A.R.; Morgenroth, J.; Koeser, A.K. The effects of root pruning on growth and physiology of two Acer species in New Zealand. Urban For. Urban Green. 2019b, 38, 64–73. [Google Scholar] [CrossRef]
- Benson, A.R.; Koeser, A.K.; Morgenroth, J. A test of tree protection zones: Response of Quercus virginiana Mill trees to root severance treatments. Urban For. Urban Green. 2019c, 38, 54–63. [Google Scholar] [CrossRef]
- Benson, A.R.; Koeser, A.K.; Morgenroth, J. Estimating conductive sapwood area in diffuse and ring porous trees with electronic resistance tomography, Tree Phys. 2019d, 39, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Fini, A.; Frangi, P.; Mori, J.; Sani, L.; Vigevani, I.; Ferrini, F. Evaluating the effects of trenching on growth, physiology and uprooting resistance of two urban tree species over 51-months. Urban For. Urban Green. 2020, 53, 126734. [Google Scholar] [CrossRef]
- Geisler, D.; Ferree, D.C. The influence of root pruning on water relations, net photosynthesis, and growth of young ‘Golden Delicious’ apple trees. J. Amer. Soc. Hort. Sci. 1984, 109, 827–831. [Google Scholar] [CrossRef]
- Ghani, M.A.; Stokes, A.; Fourcaud, T. The effect of root architecture and root loss through trenching on the anchorage of tropical urban trees (Eugenia grandis Wight). Trees 2009, 23, 197–209. [Google Scholar] [CrossRef]
- Gilman, E.F.; Yeager, T.H. Root initiation in root-pruned hardwoods. HortScience 1988, 23, 775. [Google Scholar] [CrossRef]
- Hauer, R.; Koeser, A.; Parbs, S.; Kringer, J.; Krouse, R.; Ottman, K.; Miller, R.; Sivyer, D.; Timilsina, N.; Werner, L. Effects of a tree preservation program on tree survival, condition, and growth in Milwaukee, WI, USA. Landsc. Urban Plan. 2020, 193, 103670. [Google Scholar] [CrossRef]
- Johnson, G.; Giblin, C.; Murphy, R.; North, E.; Rendahl, A. Boulevard tree failures during wind loading events. Arborist. Urban For. 2019, 45, 259–269. [Google Scholar] [CrossRef]
- Lyford, W.H.; Wilson, B.F. Controlled growth of forest tree roots: technique and application. Harvard Forest Paper No. 16. 1966.
- Matheny, N.; Smiley, E.T.; Gilpin, R.; Hauer, R. 2023. Best Management Practices—Managing Trees During Construction. 3rd ed. Atlanta (GA): International Society of Arboriculture, 64 pp.
- Miller, F.D.; Neely, D. The effect of trenching on growth and plant health of selected species of shade trees. J. Arboric. 1993, 19, 226–229. [Google Scholar] [CrossRef]
- Moore, G.M. Wind-thrown trees: Storms or Management? Arboric. Urban For. 2014, 40, 53–69. [Google Scholar] [CrossRef]
- Pinheiro, J.C.; Bates, D.M. Mixed-Effects Models in S and S-PLUS; Springer: New York (NY), 2000; 548 pp. [Google Scholar]
- Pinheiro, J.; Bates, D. ; R Core Team. 2023. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-162, Available online: https://CRAN.R-project.org/package=nlme.
- Santamour, F.S. Trunk wood discoloration and decay following root wounding. Journal of Arboriculture 1985, 11, 257–262. [Google Scholar] [CrossRef]
- Smiley, E.T. Root pruning and stability of your willow oak. Arboric. Urban For. 2008, 34, 123–128. [Google Scholar] [CrossRef]
- Smiley, E.T.; Holmes, L.; Fraedrich, B.R. Pruning of buttress roots and stability changes of red maple (Acer rubrum). Arb. Urb. For. 2014, 40, 230–236. [Google Scholar] [CrossRef]
- Wajja-Musukwe, T.-N.; Wilson, J.; Sprent, J.I.; Ong, C.K.; Deans, D.; Okorio, J. Tree growth and management in Ugandan agroforestry systems: Effects of root pruning on tree growth and crop yield. Tree Phys. 2008, 28, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Watson, G.W. Tree growth after trenching and compensatory crown pruning. J. Arboric. 1998, 24, 47–53. [Google Scholar] [CrossRef]
- Watson, G. Discoloration and decay in severed tree roots. Arboric. Urban For. 2008, 34, 257–262. [Google Scholar] [CrossRef]
- Watson, G.W.; Hewitt, A.M.; Custic, M.; Lo, M. The management of tree root systems in urban and suburban settings II: A review of strategies to mitigate human impacts. Arboric. Urban For. 2014, 40, 249–271. [Google Scholar] [CrossRef]
- Wilson, B.F. Evidence for injury as a cause of tree root branching. Can. J. Bot. 1970, 48, 1497–1498. [Google Scholar] [CrossRef]
Figure 1.
The three measurements utilized to predict root regrowth in our study include: Treatment, which denotes one of the three levels of trenching damage inflicted on the trees five years prior to the study, with the treatments being trenching at distances of 3, 6, and 12 times the DBH from the tree’s base; Cut Root CSA, representing the cross-sectional area of the root cut during trenching, derived from the average of two perpendicular diameter measurements; and Distance, indicating the Euclidean distance from the cut root to the nearest point on the base of the tree. Given the relationship between distance and treatment, these factors were analyzed separately in conjunction with cut root CSA to determine which offered greater predictive power.
Figure 1.
The three measurements utilized to predict root regrowth in our study include: Treatment, which denotes one of the three levels of trenching damage inflicted on the trees five years prior to the study, with the treatments being trenching at distances of 3, 6, and 12 times the DBH from the tree’s base; Cut Root CSA, representing the cross-sectional area of the root cut during trenching, derived from the average of two perpendicular diameter measurements; and Distance, indicating the Euclidean distance from the cut root to the nearest point on the base of the tree. Given the relationship between distance and treatment, these factors were analyzed separately in conjunction with cut root CSA to determine which offered greater predictive power.
Figure 2.
Average cross-sectional area of roots that regrew five years post-trenching (yes, n = 519) compared to those that did not (no, n = 38). Error bars represent standard error.
Figure 2.
Average cross-sectional area of roots that regrew five years post-trenching (yes, n = 519) compared to those that did not (no, n = 38). Error bars represent standard error.
Figure 3.
Average distance from the cut root to the base of the tree (cm) of roots that regrew five years post-trenching (yes, n = 519) compared to those that did not (no, n = 38). Error bars represent standard error.
Figure 3.
Average distance from the cut root to the base of the tree (cm) of roots that regrew five years post-trenching (yes, n = 519) compared to those that did not (no, n = 38). Error bars represent standard error.
Figure 4.
Histogram depicting the number roots emerging from each cut root assessed in this study.
Figure 4.
Histogram depicting the number roots emerging from each cut root assessed in this study.
Figure 5.
Average root CSA regrowth percentage by treatment. Regrowth was determined by dividing the combined cross-sectional area of all new roots by the cross-sectional area of the originating cut root and then multiplying the result by 100. ‘Treatments’ denote trenching activities where roots were cut at distances of 3, 6, and 12 times the diameter of the respective tree. Error bars represent standard error.
Figure 5.
Average root CSA regrowth percentage by treatment. Regrowth was determined by dividing the combined cross-sectional area of all new roots by the cross-sectional area of the originating cut root and then multiplying the result by 100. ‘Treatments’ denote trenching activities where roots were cut at distances of 3, 6, and 12 times the diameter of the respective tree. Error bars represent standard error.
Figure 6.
Relationship between the cross-sectional area of the root cut during trenching and the percentage of root regrowth observed five years later. Regrowth was calculated by dividing the combined cross-sectional area of all new roots by the cross-sectional area of the original cut root, then multiplying the result by 100. A linear trend line is included for reference; however, the relationship was not significant when factored into models with other predictors.
Figure 6.
Relationship between the cross-sectional area of the root cut during trenching and the percentage of root regrowth observed five years later. Regrowth was calculated by dividing the combined cross-sectional area of all new roots by the cross-sectional area of the original cut root, then multiplying the result by 100. A linear trend line is included for reference; however, the relationship was not significant when factored into models with other predictors.
Figure 7.
Relationship between the distance between the trunk and the cut root end and the percentage of root regrowth observed five years later. Regrowth was calculated by dividing the combined cross-sectional area of all new roots by the cross-sectional area of the original cut root, then multiplying the result by 100. A linear trend line is included for reference. The relationship was significant (P-value = 0.002) when modeled with cut root cross sectional area (P-value = 0.125).
Figure 7.
Relationship between the distance between the trunk and the cut root end and the percentage of root regrowth observed five years later. Regrowth was calculated by dividing the combined cross-sectional area of all new roots by the cross-sectional area of the original cut root, then multiplying the result by 100. A linear trend line is included for reference. The relationship was significant (P-value = 0.002) when modeled with cut root cross sectional area (P-value = 0.125).
Table 1.
Percentage of tree roots that either regrew after cutting or did not regrow separated by initial trenching treatments.
Table 1.
Percentage of tree roots that either regrew after cutting or did not regrow separated by initial trenching treatments.
| |
Trenching Treatment |
| Root Regrowth |
3x DBH |
6x DBH |
12x DBH |
| Yes |
239 (92.3%) |
223 (93.7%) |
57 (95%) |
| No |
20 (7.7%) |
15 (6.3%) |
3 (5.0%) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).