Submitted:
26 October 2023
Posted:
27 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
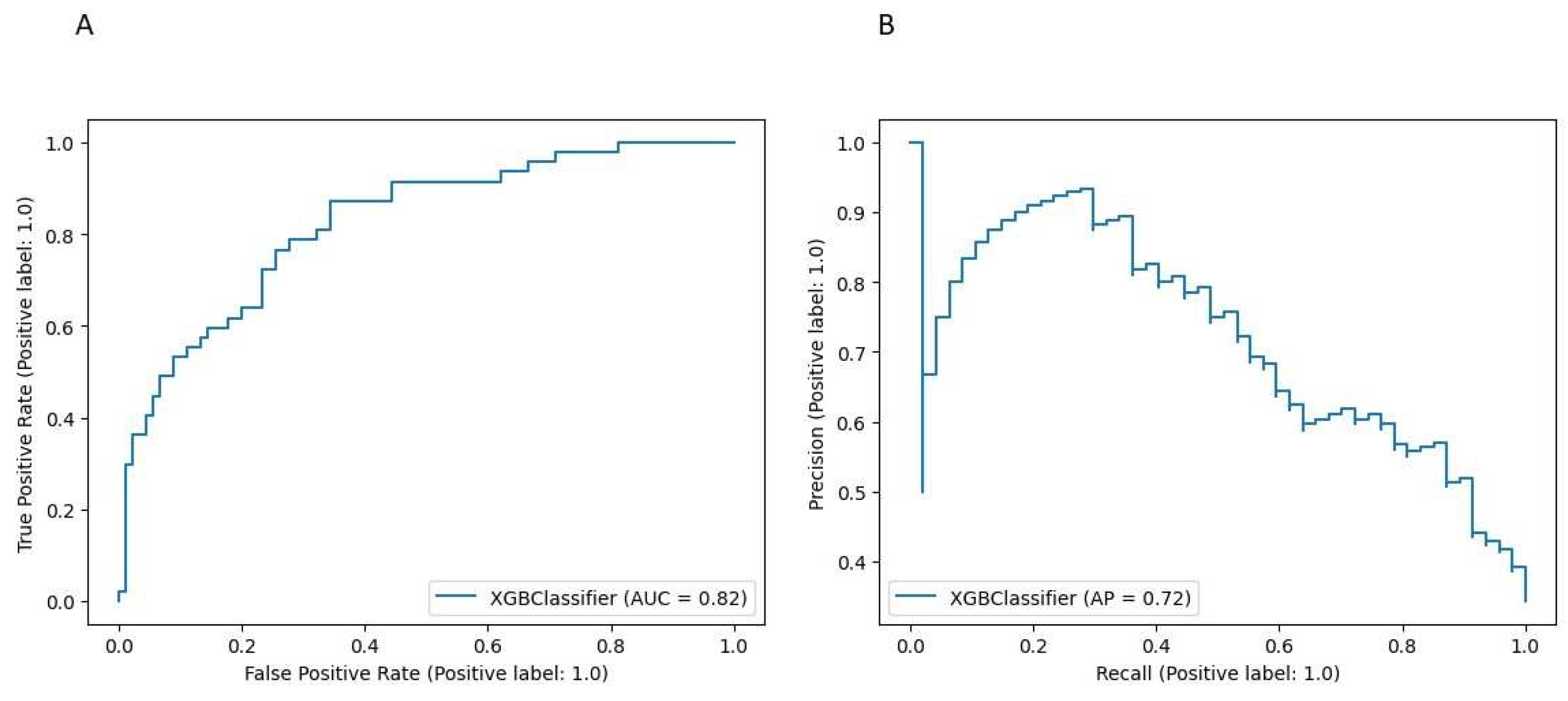
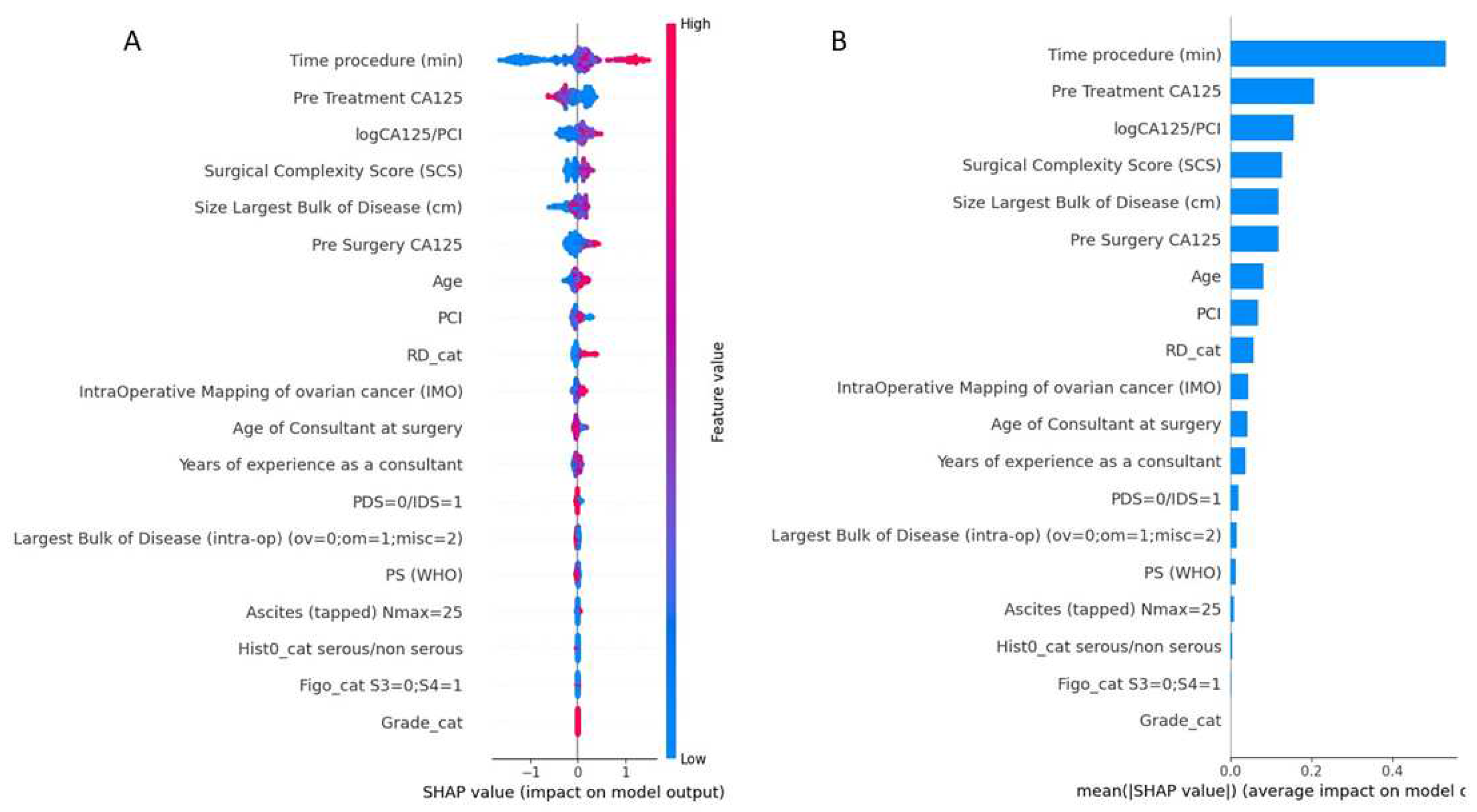
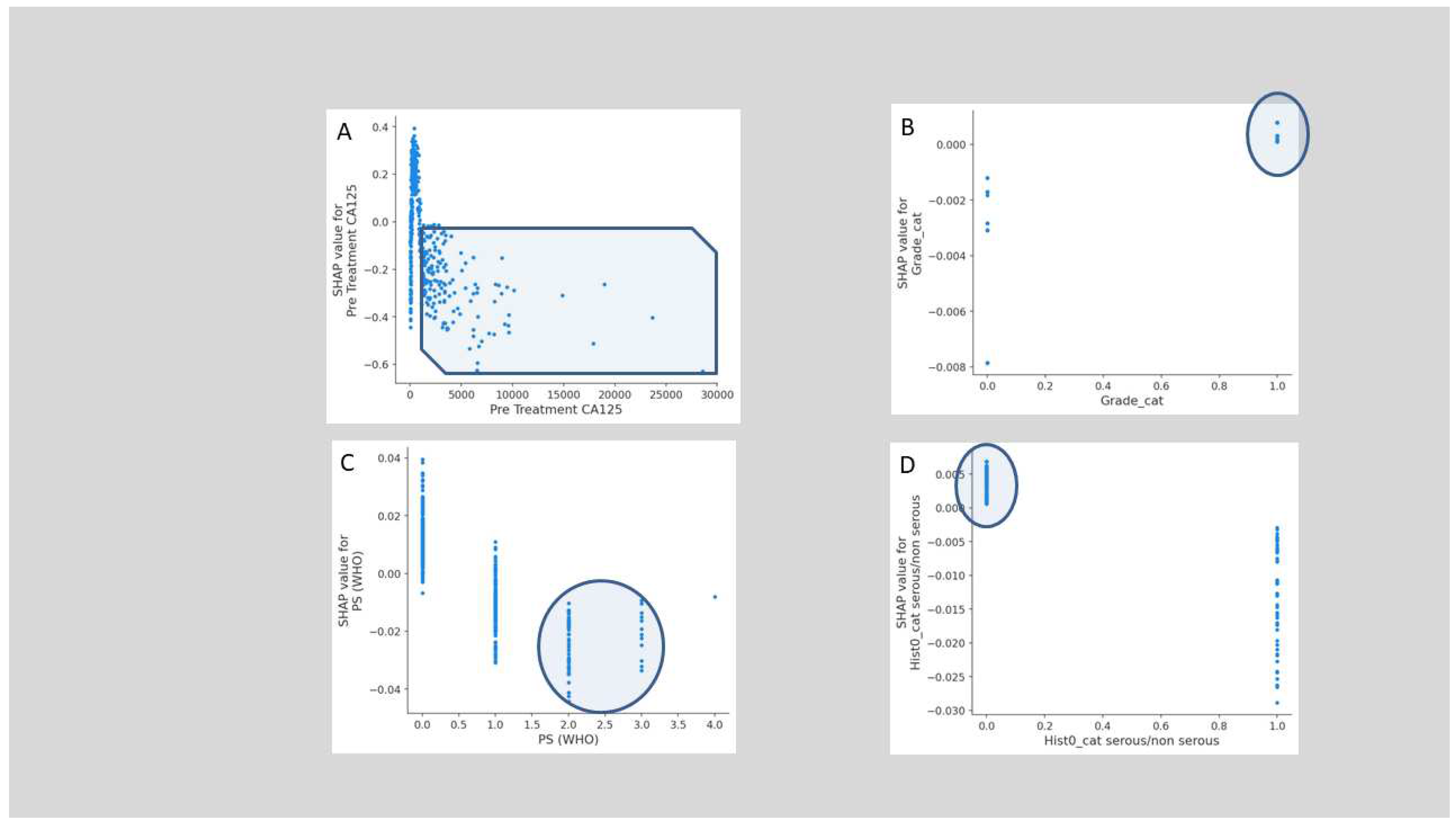
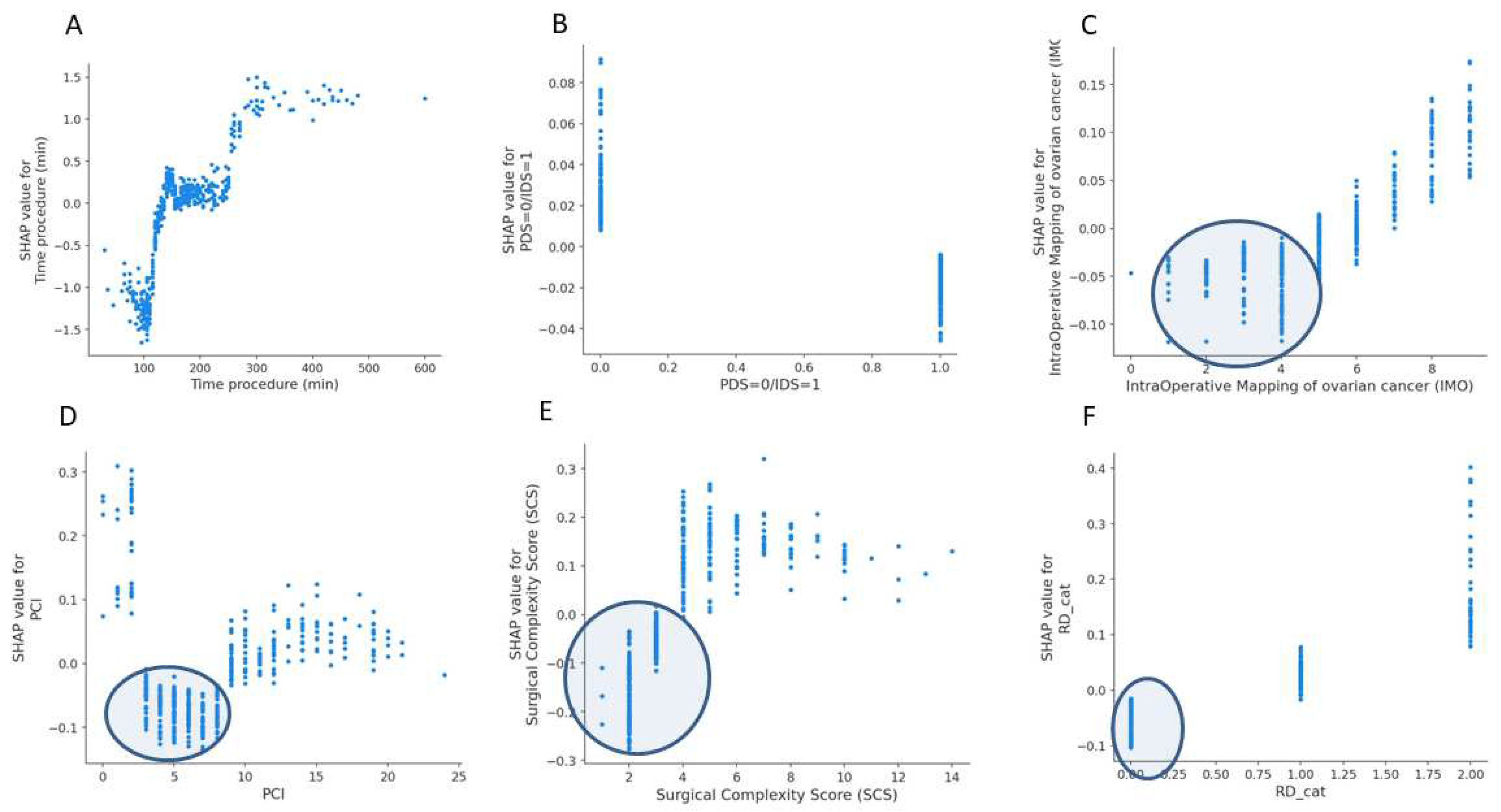
3. Results
4. Discussion
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| TLA | Three letter acronym |
| LD | Linear dichroism |
References
- Fotopoulou, C.; Planchamp, F.; Aytulu, T.; Chiva, L.; Cina, A.; Ergönül, Ö.; Fagotti, A.; Haidopoulos, D.; Hasenburg, A.; Hughes, C.; others. European Society of Gynaecological Oncology guidelines for the peri-operative management of advanced ovarian cancer patients undergoing debulking surgery. International Journal of Gynecologic Cancer 2021, 31. [Google Scholar] [CrossRef] [PubMed]
- Manning-Geist, B.L.; Alimena, S.; Del Carmen, M.G.; Goodman, A.; Clark, R.M.; Growdon, W.B.; Horowitz, N.S.; Berkowitz, R.S.; Muto, M.G.; Worley Jr, M.J. Infection, thrombosis, and oncologic outcome after interval debulking surgery: does perioperative blood transfusion matter? Gynecologic Oncology 2019, 153, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Laios, A.; Kalampokis, E.; Johnson, R.; Munot, S.; Thangavelu, A.; Hutson, R.; Broadhead, T.; Theophilou, G.; Leach, C.; Nugent, D.; others. Factors predicting surgical effort using explainable artificial intelligence in advanced stage epithelial ovarian cancer. Cancers 2022, 14, 3447. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Asch, S.M. Machine learning and prediction in medicine—beyond the peak of inflated expectations. The New England journal of medicine 2017, 376, 2507. [Google Scholar] [CrossRef] [PubMed]
- Laios, A.; Kalampokis, E.; Johnson, R.; Thangavelu, A.; Tarabanis, C.; Nugent, D.; De Jong, D. Explainable artificial intelligence for prediction of complete surgical cytoreduction in advanced-stage epithelial ovarian cancer. Journal of personalized medicine 2022, 12, 607. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, P.; Sugarbaker, P.H. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Peritoneal carcinomatosis: principles of management, 1996; pp. 359–374. [Google Scholar]
- Sehouli, J.; Könsgen, D.; Mustea, A.; Oskay-Özcelik, G.; Katsares, I.; Weidemann, H.; Lichtenegger, W. „IMO”-Intraoperatives Mapping des Ovarialkarzinoms. Zentralblatt für Gynäkologie 2003, 125, 129–135. [Google Scholar] [PubMed]
- Aletti, G.D.; Dowdy, S.C.; Podratz, K.C.; Cliby, W.A. Relationship among surgical complexity, short-term morbidity, and overall survival in primary surgery for advanced ovarian cancer. American journal of obstetrics and gynecology 2007, 197, 676–e1. [Google Scholar] [CrossRef]
- Querleu, D.; Planchamp, F.; Chiva, L.; Fotopoulou, C.; Barton, D.; Cibula, D.; Aletti, G.; Carinelli, S.; Creutzberg, C.; Davidson, B.; others. European Society of Gynaecologic Oncology quality indicators for advanced ovarian cancer surgery. International Journal of Gynecologic Cancer 2016, 26. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.H. Greedy function approximation: a gradient boosting machine. Annals of statistics, 2001; pp. 1189–1232. [Google Scholar]
- Lewis, K.M.; Li, Q.; Jones, D.S.; Corrales, J.D.; Du, H.; Spiess, P.E.; Menzo, E.L.; DeAnda Jr, A. Development and validation of an intraoperative bleeding severity scale for use in clinical studies of hemostatic agents. Surgery 2017, 161, 771–781. [Google Scholar] [CrossRef]
- Lundberg, S.M.; Erion, G.; Chen, H.; DeGrave, A.; Prutkin, J.M.; Nair, B.; Katz, R.; Himmelfarb, J.; Bansal, N.; Lee, S.I. From local explanations to global understanding with explainable AI for trees. Nature machine intelligence 2020, 2, 56–67. [Google Scholar] [CrossRef]
- Laios, A.; Kalampokis, E.; Johnson, R.; Munot, S.; Thangavelu, A.; Hutson, R.; Broadhead, T.; Theophilou, G.; Nugent, D.; De Jong, D. Development of a Novel Intra-Operative Score to Record Diseases’ Anatomic Fingerprints (ANAFI Score) for the Prediction of Complete Cytoreduction in Advanced-Stage Ovarian Cancer by Using Machine Learning and Explainable Artificial Intelligence. Cancers 2023, 15, 966. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.; Orlick, T.; Letts, M. Mental readiness in surgeons and its links to performance excellence in surgery. Journal of Pediatric Orthopaedics 1995, 15, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.; Spooner, S.; Horne, J.; Chainrai, M.; Runau, F.; Bourne, T.; Moss, E.L.; Davies, Q.; Chattopadhyay, S.; Bharathan, R. Peri-operative variables associated with prolonged intensive care stay following cytoreductive surgery for ovarian cancer. Anticancer Research 2021, 41, 3059–3065. [Google Scholar] [CrossRef] [PubMed]
- Heddens, D.; Alberts, D.S.; Hannigan, E.V.; Williams, S.D.; Garcia, D.; Roe, D.J.; Bell, J.; Alvarez, R.D. Prediction of the need for red cell transfusion in newly diagnosed ovarian cancer patients undergoing platinum-based treatment. Gynecologic oncology 2002, 86, 239–243. [Google Scholar] [CrossRef]
- Pinheiro de Almeida, J.; Vincent, J.L.; Barbosa Gomes Galas, F.R.; Pinto Marinho de Almeida, E.; Fukushima, J.T.; Osawa, E.A.; Bergamin, F.; Lee Park, C.; Nakamura, R.E.; Fonseca, S.M.; others. Transfusion requirements in surgical oncology patients: a prospective, randomized controlled trial. Anesthesiology 2015, 122, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Kahn, R.; Boerner, T.; An, A.; Gardner, G.; Roche, K.L.; Sonoda, Y.; Zivanovic, O.; Grisham, R.; Abu-Rustum, N.; Chi, D. A preoperative tool for estimating the risk of blood transfusion over an ovarian cancer debulking surgery (113). Gynecologic Oncology 2022, 166, S74. [Google Scholar] [CrossRef]
- Goel, R.; Kanhere, H.; Trochsler, M. The ‘Surgical Time’: a myth or reality? Surgeons’ prediction of operating time and its effect on theatre scheduling. Australian Health Review 2020, 44, 772–777. [Google Scholar] [CrossRef]
- Eisenkop, S.M.; Friedman, R.L.; Wang, H.J. Complete cytoreductive surgery is feasible and maximizes survival in patients with advanced epithelial ovarian cancer: a prospective study. Gynecologic oncology 1998, 69, 103–108. [Google Scholar] [CrossRef]
- Yitgin, Y.; Altınkaya, N.; Turaliev, N.; Guven, S.; Ergul, R.B.; Boyuk, A.; Verep, S.; Tefik, T.; Karagoz, M.A.; Ibis, M.A.; others. Evaluation of the optimal duration for retrograde intrarenal stone surgery to prevent postoperative complications. Scottish Medical Journal 2022, 67, 121–125. [Google Scholar] [CrossRef]
- Kubi, B.; Nudotor, R.; Fackche, N.; Nizam, W.; Cloyd, J.M.; Grotz, T.E.; Fournier, K.F.; Dineen, S.P.; Powers, B.D.; Veerapong, J.; others. Impact of perioperative blood transfusions on outcomes after hyperthermic intraperitoneal chemotherapy: A propensity-matched analysis. Annals of surgical oncology 2021, 28, 4499–4507. [Google Scholar] [CrossRef]
- Prescott, L.S.; Taylor, J.S.; Lopez-Olivo, M.A.; Munsell, M.F.; VonVille, H.M.; Lairson, D.R.; Bodurka, D.C. How low should we go: a systematic review and meta-analysis of the impact of restrictive red blood cell transfusion strategies in oncology. Cancer treatment reviews 2016, 46, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kietpeerakool, C.; Supoken, A.; Laopaiboon, M.; Lumbiganon, P. Effectiveness of tranexamic acid in reducing blood loss during cytoreductive surgery for advanced ovarian cancer. Cochrane Database of Systematic Reviews 2016. [Google Scholar] [CrossRef]
- Prescott, L.S.; Vergote, I.; Sun, C.C.; Bodurka, D.C.; Coleman, R.L. Transfusion use and effect on progression-free, overall survival, and quality of life in upfront treatment of advanced epithelial ovarian cancer: evaluation of the European Organization for Research and Treatment EORTC-55971 Cohort. International Journal of Gynecologic Cancer 2023, 33. [Google Scholar] [CrossRef] [PubMed]
- Hunsicker, O.; Fotopoulou, C.; Pietzner, K.; Koch, M.; Krannich, A.; Sehouli, J.; Spies, C.; Feldheiser, A. Hemodynamic consequences of malignant ascites in epithelial ovarian cancer surgery: a prospective substudy of a randomized controlled trial. Medicine 2015, 94. [Google Scholar] [CrossRef] [PubMed]
- Tse, A.; Chow, O.; Matar, A.; Alzahrani, N.; Morris, D. Strategies for ‘bloodless’ surgery: the experience of cytoreductive surgery for peritoneal carcinomatosis in Jehovah’s Witnesses. ANZ Journal of Surgery 2020, 90, 1953–1957. [Google Scholar] [CrossRef] [PubMed]
- Miralpeix, E.; Sole-Sedeno, J.M.; Rodriguez-Cosmen, C.; Taus, A.; Muns, M.D.; Fabregó, B.; Mancebo, G. Impact of prehabilitation during neoadjuvant chemotherapy and interval cytoreductive surgery on ovarian cancer patients: a pilot study. World Journal of Surgical Oncology 2022, 20, 46. [Google Scholar] [CrossRef] [PubMed]
- Tanner, E.J.; Filippova, O.T.; Gardner, G.J.; Roche, K.C.L.; Sonoda, Y.; Zivanovic, O.; Fischer, M.; Chi, D.S. A prospective trial of acute normovolemic hemodilution in patients undergoing primary cytoreductive surgery for advanced ovarian cancer. Gynecologic oncology 2018, 151, 433–437. [Google Scholar] [CrossRef]
- Prescott, L.S.; Vergote, I.; Sun, C.C.; Bodurka, D.C.; Coleman, R.L. Transfusion use and effect on progression-free, overall survival, and quality of life in upfront treatment of advanced epithelial ovarian cancer: evaluation of the European Organization for Research and Treatment EORTC-55971 Cohort. International Journal of Gynecologic Cancer 2023, 33. [Google Scholar] [CrossRef]
- Castro, B.G.R.; Dos Reis, R.; Cintra, G.F.; de Assunção Sousa, M.M.; de Andrade Vieira, M.; da Cunha Andrade, C.E.M. Predictive factors for surgical morbidities and adjuvant chemotherapy delay for advanced ovarian cancer patients treated by primary debulking surgery or interval debulking surgery. International Journal of Gynecologic Cancer 2018, 28. [Google Scholar] [CrossRef]
- Sheehy, J.; Rutledge, H.; Acharya, U.R.; Loh, H.W.; Gururajan, R.; Tao, X.; Zhou, X.; Li, Y.; Gurney, T.; Kondalsamy-Chennakesavan, S. Gynecological cancer prognosis using machine learning techniques: A systematic review of last three decades (1990–2022). Artificial Intelligence in Medicine, 2023; p. 102536. [Google Scholar]
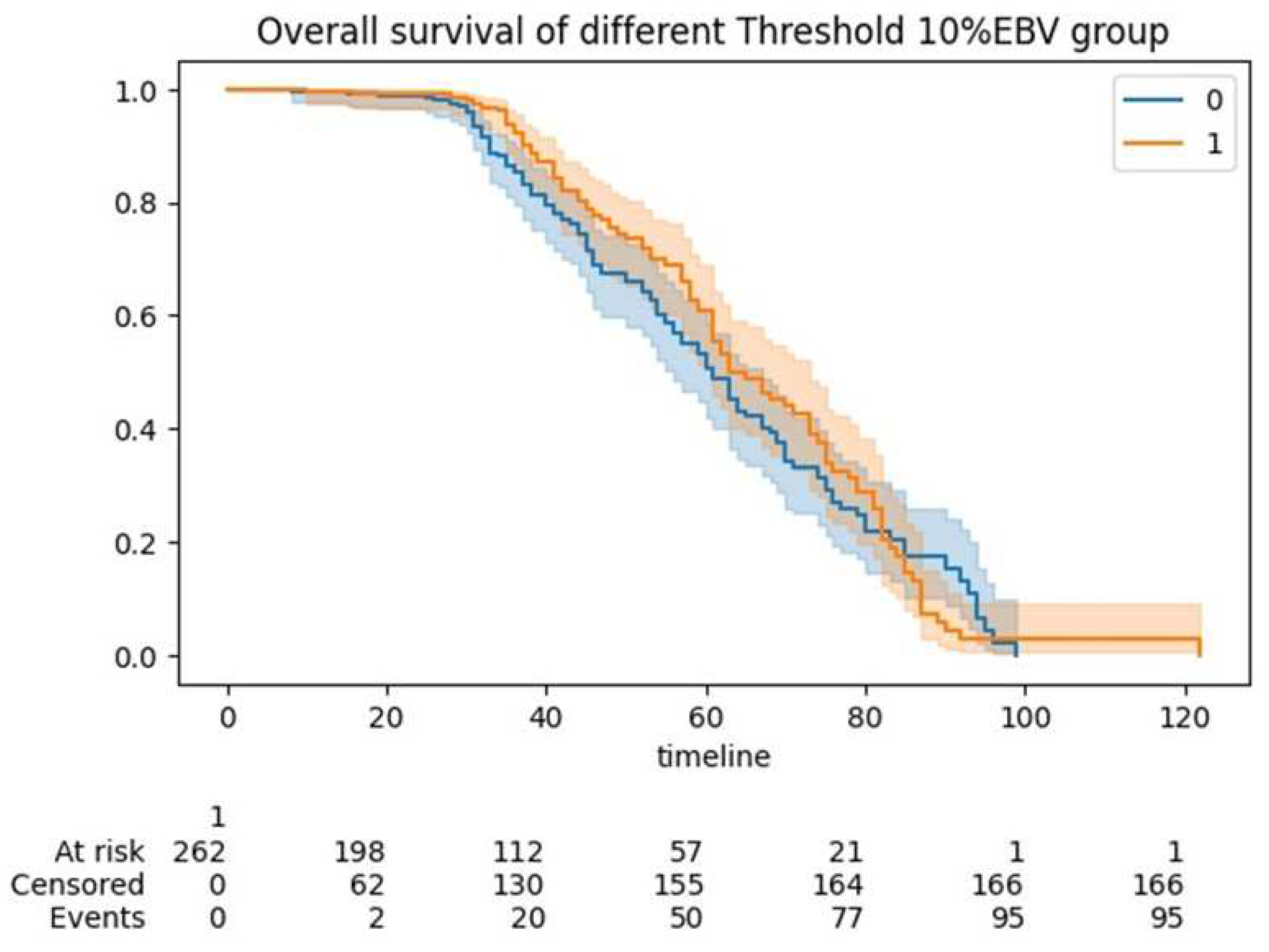
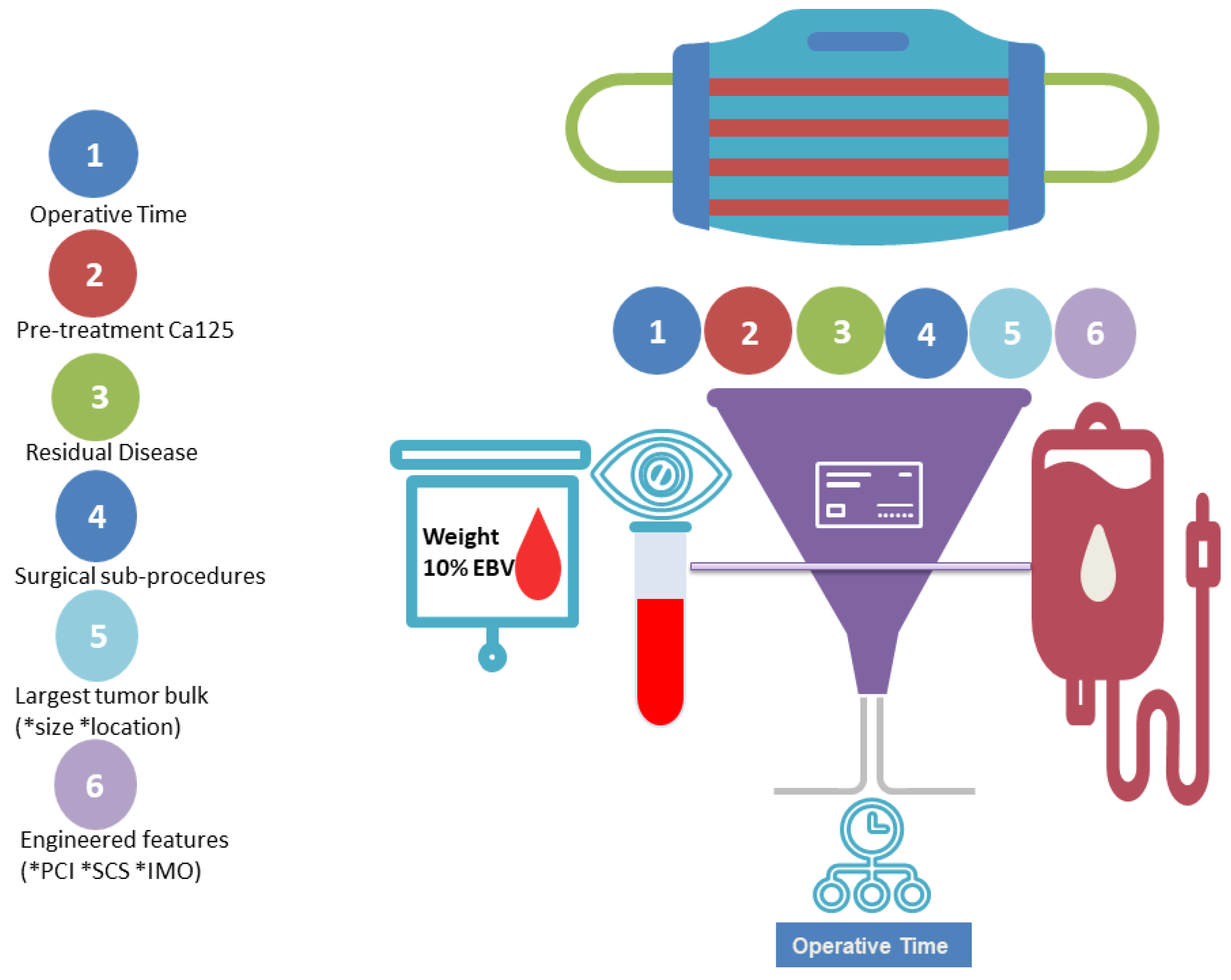
| Overall | Training Set | Testing Set | pvalue (training) | Group <10%EBV | Group >10%EBV) | pvalue (Threshold 10%EBV) | ||
|---|---|---|---|---|---|---|---|---|
| Histo_cat1 | 0 | 489 (87.32) | 394 (87.95) | 95 (84.82) | 0.465 | 264 (88.59) | 137 (87.82) | 0.929 |
| 1 | 71 (12.68) | 54 (12.05) | 17 (15.18) | 0.465 | 34 (11.41) | 19 (12.18) | 0.929 | |
| Grade_cat | 0 | 56 (10.0) | 46 (10.27) | 10 (8.93) | 0.805 | 20 (6.71) | 22 (14.1) | 0.016 |
| 1 | 504 (90.0) | 402 (89.73) | 102 (91.07) | 0.805 | 278 (93.29) | 134 (85.9) | 0.016 | |
| Figo_cat2 | 0 | 406 (72.5) | 322 (71.88) | 84 (75.0) | 0.586 | 216 (72.48) | 113 (72.44) | 1 |
| 1 | 154 (27.5) | 126 (28.12) | 28 (25.0) | 0.586 | 82 (27.52) | 43 (27.56) | 1 | |
| PS (WHO) | 0 | 266 (47.5) | 205 (45.76) | 61 (54.46) | 0.099 | 137 (45.97) | 76 (48.72) | 0.048 |
| 1 | 208 (37.14) | 175 (39.06) | 33 (29.46) | 0.099 | 111 (37.25) | 65 (41.67) | 0.048 | |
| 2 | 67 (11.96) | 56 (12.5) | 11 (9.82) | 0.099 | 43 (14.43) | 9 (5.77) | 0.048 | |
| 3 | 17 (3.04) | 11 (2.46) | 6 (5.36) | 0.099 | 7 (2.35) | 5 (3.21) | 0.048 | |
| 4 | 2 (0.36) | 1 (0.22) | 1 (0.89) | 0.099 | 0 (0.0) | 1 (0.64) | 0.048 | |
| PDS=0/ IDS=1 | 0 | 172 (30.71) | 132 (29.46) | 40 (35.71) | 0.243 | 72 (24.16) | 61 (39.1) | 0.001 |
| 1 | 388 (69.29) | 316 (70.54) | 72 (64.29) | 0.243 | 226 (75.84) | 95 (60.9) | 0.001 | |
| RD_cat | 0 | 369 (65.89) | 294 (65.62) | 75 (66.96) | 0.168 | 209 (70.13) | 93 (59.62) | 0.051 |
| 1 | 130 (23.21) | 100 (22.32) | 30 (26.79) | 0.168 | 64 (21.48) | 41 (26.28) | 0.051 | |
| 2 | 61 (10.89) | 54 (12.05) | 7 (6.25) | 0.168 | 25 (8.39) | 22 (14.1) | 0.051 | |
| Ascites3 | 0 | 414 (73.93) | 335 (74.78) | 79 (70.54) | 0.427 | 234 (78.52) | 103 (66.03) | 0.005 |
| 1 | 146 (26.07) | 113 (25.22) | 33 (29.46) | 0.427 | 64 (21.48) | 53 (33.97) | 0.005 | |
| Largest Bulk of Disease Location 4 | PA node | 1 (0.18) | 1 (0.22) | 0 (0.0) | 0.023 | 1 (0.34) | 0 (0.0) | 0.446 |
| POD | 1 (0.18) | 0 (0.0) | 1 (0.89) | 0.023 | 1 (0.34) | 0 (0.0) | 0.446 | |
| caecum | 1 (0.18) | 0 (0.0) | 1 (0.89) | 0.023 | 1 (0.34) | 0 (0.0) | 0.446 | |
| mesentery | 2 (0.36) | 2 (0.45) | 0 (0.0) | 0.023 | 0 (0.0) | 2 (1.28) | 0.446 | |
| omentum | 249 (44.46) | 204 (45.54) | 45 (40.18) | 0.023 | 138 (46.31) | 71 (45.51) | 0.446 | |
| ovary | 300 (53.57) | 238 (53.12) | 62 (55.36) | 0.023 | 154 (51.68) | 82 (52.56) | 0.446 | |
| peritoneum | 1 (0.18) | 1 (0.22) | 0 (0.0) | 0.023 | 0 (0.0) | 1 (0.64) | 0.446 | |
| rectum | 1 (0.18) | 0 (0.0) | 1 (0.89) | 0.023 | 1 (0.34) | 0 (0.0) | 0.446 | |
| sigmoid | 1 (0.18) | 0 (0.0) | 1 (0.89) | 0.023 | 0.446 | |||
| umbilicus | 1 (0.18) | 0 (0.0) | 1 (0.89) | 0.023 | 1 (0.34) | 0 (0.0) | 0.446 | |
| Age | 63.51 ± 11.22 | 63.23 ± 11.06 | 64.64 ± 11.82 | 0.252 | 63.53 ± 11.24 | 62.78 ± 11.27 | 0.502 | |
| Consultant age5 | 49.13 ± 6.03 | 49.1 ± 6.07 | 49.25 ± 5.91 | 0.815 | 49.96 ± 6.09 | 48.43 ± 6.11 | 0.011 | |
| Years6 | 9.65 ± 5.33 | 9.65 ± 5.36 | 9.68 ± 5.22 | 0.952 | 10.03 ± 5.31 | 9.29 ± 5.47 | 0.165 | |
| SCS7 | 3.8 ± 2.11 | 3.82 ± 2.06 | 3.71 ± 2.31 | 0.648 | 3.34 ± 1.83 | 4.73 ± 2.51 | <0.001 | |
| Time procedure8 | 170.39 ± 77.55 | 172.98 ± 76.53 | 160.04 ± 81.03 | 0.129 | 147.84 ± 55.24 | 215.1 ± 97.06 | <0.001 | |
| Pre Treatment CA125 | 1516.14 ± 2711.14 | 1582.85 ± 2769.98 | 1249.29 ± 2455.18 | 0.212 | 1689.69 ± 3189.63 | 1420.7 ± 2071.05 | 0.279 | |
| Pre Surgery CA125 | 410.46 ± 1175.43 | 411.43 ± 944.52 | 406.56 ± 1833.3 | 0.978 | 360.46 ± 1280.81 | 614.46 ± 1298.66 | 0.048 | |
| logCA125/ PCI | 0.41 ± 0.36 | 0.4 ± 0.35 | 0.42 ± 0.4 | 0.756 | 0.41 ± 0.35 | 0.4 ± 0.41 | 0.657 | |
| IMO score9 | 4.92 ± 1.97 | 4.98 ± 1.99 | 4.7 ± 1.89 | 0.158 | 4.57 ± 1.86 | 5.6 ± 2.11 | <0.001 | |
| PCI | 7.37 ± 4.47 | 7.48 ± 4.51 | 6.92 ± 4.31 | 0.225 | 6.78 ± 4.08 | 8.79 ± 5.16 | <0.001 | |
| Largest Bulk (cm) | 8.89 ± 5.61 | 9.13 ± 5.69 | 7.96 ± 5.23 | 0.039 | 8.29 ± 5.64 | 9.98 ± 5.49 | 0.002 |
| Overall (n = 560) | CC0 (n = 368) | Non-CC0 (n = 192) | p-Value | ||
|---|---|---|---|---|---|
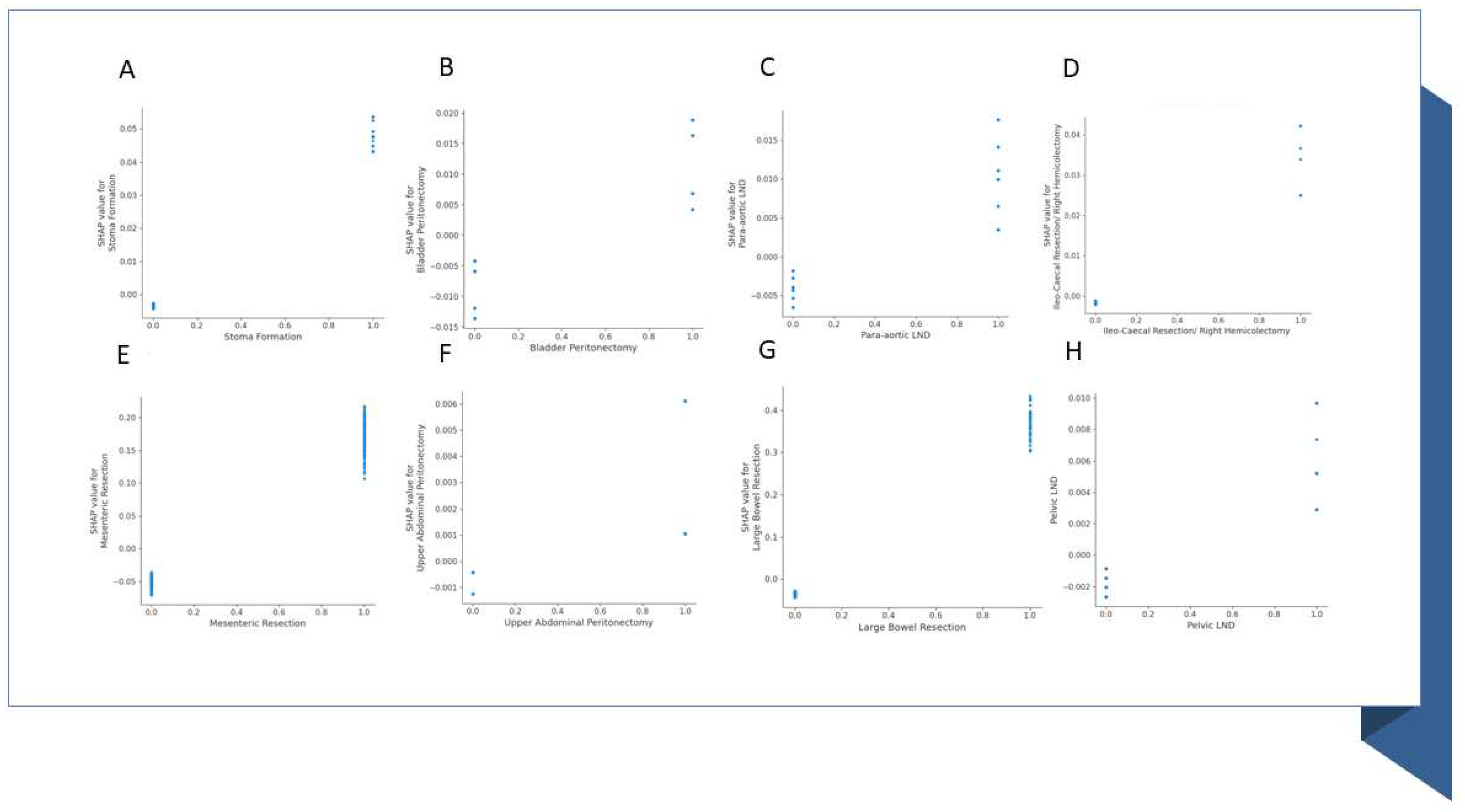
| Stoma Formation | 0 | 509 (90.89) | 334 (90.76) | 175 (91.15) | 1 |
| 1 | 51 (9.11) | 34 (9.24) | 17 (8.85) | 1 | |
| Bladder Peritonectomy | 0 | 358 (63.93) | 217 (58.97) | 141 (73.44) | 0.001 |
| 1 | 202 (36.07) | 151 (41.03) | 51 (26.56) | 0.001 | |
| Para-aortic node dissection | 0 | 381 (68.04) | 221 (60.05) | 160 (83.33) | <0.001 |
| 1 | 179 (31.96) | 147 (39.95) | 32 (16.67) | <0.001 | |
| Ileo-Caecal Resection/ Right Hemicolectomy | 0 | 539 (96.25) | 352 (95.65) | 187 (97.4) | 0.426 |
| 1 | 21 (3.75) | 16 (4.35) | 5 (2.6) | 0.426 | |
| Mesenteric Resection | 0 | 427 (76.25) | 269 (73.1) | 158 (82.29) | 0.02 |
| 1 | 133 (23.75) | 99 (26.9) | 34 (17.71) | 0.02 | |
| Upper Abdominal Peritonectomy | 0 | 481 (85.89) | 296 (80.43) | 185 (96.35) | <0.001 |
| 1 | 79 (14.11) | 72 (19.57) | 7 (3.65) | <0.001 | |
| Large Bowel Resection | 0 | 496 (88.57) | 323 (87.77) | 173 (90.1) | 0.494 |
| 1 | 64 (11.43) | 45 (12.23) | 19 (9.9) | 0.494 | |
| Pelvic node dissection | 0 | 414 (73.93) | 242 (65.76) | 172 (89.58) | <0.001 |
| 1 | 146 (26.07) | 126 (34.24) | 20 (10.42) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





