1. Introduction
Epithelial ovarian cancer (EOC) is often diagnosed at advanced stages [
1]. For over a century, the cornerstone of EOC treatment has been a combination of cytoreductive surgery (CRS) and platinum-based chemotherapy. Recent findings strongly indicate that achieving no macroscopic residual disease (CC 0) in advanced EOC leads to longer overall survival rates [
2]. Maximal effort cytoreductive surgeries aiming for CC 0 resections in these patients are frequently radical and extensive, carrying a high risk of complications [
3].
Acute Kidney Injury (AKI) is defined as a rapid (hours to days) reduction in kidney function. It is a common complication in hospitalized patients, with a reported incidence as high as 18% [
4,
5]. This risk is associated with increasing age, perioperative nephrotoxic drug use, and a greater comorbidity burden, including diabetes mellitus, liver disease, and heart failure [
5]. AKI substantially increases the risk of patient morbidity and mortality, prolongs hospital admissions, and can trigger severe complications, including hyperkalemia, pulmonary edema, metabolic acidosis, and chronic kidney disease (CKD). At its most severe, AKI can result in multi-organ failure, with mortality rates reported as high as 80% [
6]. Despite its severity, hospital-acquired AKI has historically been poorly managed. The 2009 National Confidential Enquiry into Patient Outcome and Death (NCEPOD) found that 43% of patients had delayed recognition of AKI, 21% of cases were avoidable, and only 50% of patients received good care [
4]. Given the high incidence and delays in prompt management, AKI costs NHS England approximately £1.02 billion per year [
6]. It is a costly complication for both the patient and the healthcare system.
Intraperitoneal surgery is another significant risk factor for AKI development, as midline laparotomy can result in prolonged, radical surgery with a high risk of significant blood loss, renal damage, and infection, thereby increasing the likelihood of AKI [
7,
8]. This is especially pertinent in the management of advanced EOC, where CRS aims to resect all macroscopic disease alongside adjuvant or neoadjuvant chemotherapy [
9,
10]. The incidence of AKI post-CRS for EOC widely varies in the literature. A single-centre retrospective cohort study reported that, among 282 patients undergoing CRS, 11.7% developed AKI [
5]. This incidence was higher in patients with hypertension or diabetes mellitus; ACE inhibitor or ARB use, and a baseline eGFR <60 were independent risk factors for AKI. In contrast, another single-centre retrospective study found a much higher incidence of AKI [
7]. Among 47 patients who underwent CRS, 40.4% developed AKI, with significant risk factors being age >48 years, baseline eGFR <90, the interval between neoadjuvant chemotherapy and the operation date <7 days, and intraoperative blood transfusion >2 units. Other studies identified Charlson Comorbidity Index (CCI), platinum-based chemotherapy, and high estimated blood loss (EBL) as significant predictors of AKI in the context of CRS [
11,
12].
Despite the well-documented risks of AKI post-surgery, existing literature often focuses on AKI secondary to CRS with hyperthermic intraperitoneal chemotherapy (HIPEC), which does not align with NICE clinical guidelines [
9] and presents a confounding factor given the nephrotoxic nature of platinum-based chemotherapeutic agents [
8]. The primary aim of this study was to investigate the incidence of AKI in patients undergoing CRS without HIPEC for advanced EOC and to perform an exploratory analysis of preoperative and intraoperative predictors of AKI in these patients. Secondary aims included using AI modelling to enhance data analysis and assess whether AKI affected patients’ postoperative outcomes. The study’s endpoints included AKI incidence, predictors of AKI, and secondary outcomes influenced by AKI.
2. Materials and Methods
2.1. Study Population
Retrospective data was collected for 138 patients with a confirmed or working diagnosis of advanced EOC (FIGO Stage III-IV) who underwent CRS between January 2021 and December 2022 at a UK tertiary referral centre, which is an ESGO accredited centre of excellence for ovarian cancer surgery. Inclusion criteria included women 18 years or older having a positive CT thorax/abdomen/pelvis and/or histological confirmation by omental biopsy. They were also included irrespective of their chronic kidney disease (CKD) status. Exclusion criteria included non-epithelial histology, synchronous non-ovarian primary tumours, and early-stage or borderline EOC. Patients were excluded if there was no attempt at resection due to inoperable disease. Before making treatment decisions, all cases were discussed at the central gynaecological oncology multidisciplinary meeting.
2.2. Study Design and Data Collection
The study was a single-centre, retrospective, exploratory cohort review of clinical case notes. The patient cohort was identified retrospectively by their CRS operative record, and data was extracted from various sources within the electronic health records (EHRs), including their preoperative assessment, operation note and discharge summary. Data on patient’s preoperative characteristics including age, body mass index (BMI) and CCI; and intraoperative factors including procedure length (PL), EBL, the ANAFI score [
14], and Aletti Surgical Complexity Score (SCS) were collected (
Table A1). Additionally, postoperative data was collected for secondary outcomes which aimed to illustrate the impact AKI had on patients and NHS workload; these included postoperative length of stay (LOS), HDU/ICU admission and short-term mortality within 90 days of the procedure. The study was conducted in accordance with the guidelines of the Declaration of Helsinki and was approved by the Institutional Review Board (23/NE/0229/328779/12.01.24). It was registered in the UMIN/CTR Trial Registry under the identifier UMIN000049480.
2.3. Identification and Classification of AKI
The Kidney Disease: Improving Global Outcomes (KDIGO) definition and staging criteria were used to identify AKI in this study, aligning with institutional AKI guidelines [
6] and NICE Guideline NG148 [
4]. KDIGO defines AKI as a rise in serum creatinine of 26.5 µmol/L or more within 48 hours, an increase in serum creatinine of 50% within one week, or urine output less than 0.5 mL/kg/hour for six consecutive hours [
15].
Patients whose biochemistry results met KDIGO criteria were automatically flagged for AKI in the EHR system, with staging calculated via an algorithm in the Lab Information Management System (LIMS) based on KDIGO definitions (see
Table A2) [
6]. In perioperative care, serum creatinine is routinely measured pre- and postoperatively, providing reliable and objective data. If preoperative serum creatinine was not recorded, the most recent value before surgery was used as the baseline. Measurement of urinary output to identify AKI incidence was excluded in the planning phase due to the prediction of missingness in retrospective data collection from case notes. If an AKI alert was triggered, clinical teams implemented the STOP AKI care bundle to confirm or refute the clinical diagnosis of AKI. Patients with end-stage kidney disease on dialysis trigger the alert and patients who have been commenced on diuretics or ACEi/ ARBs for heart failure may experience a rise in creatinine that is acceptable but not clinically AKI. Clinical data is routinely collected, analysed, and reviewed at a specialty level to recommend service changes where appropriate.
2.4. Statistical Analysis
Microsoft Excel was used to calculate the percentage incidence of AKI. For preoperative, intraoperative and postoperative variables, numerical data was expressed using medians and categorical data was expressed using percentages. SPSS V24® was used to perform Mann-Whitney U tests to identify differences in preoperative and intraoperative variables and postoperative secondary outcomes between the AKI and non-AKI groups. Spearman’s Rank tests identified significant associations between preoperative, intraoperative, and postoperative variables, and correlation heatmaps were generated. Univariate logistic regression analyses identified important predictors of AKI. A p-value of <0.05 was considered to indicate statistical significance.
The extreme gradient boost (XGBoost) classifier was employed to model all variables pertaining to AKI events [
13]. Receiver operating characteristic (ROC) curves were used to test model performance. Explainable Artificial Intelligence (XAI) was used to explain the prediction, and SHAP Force Plots were generated to illustrate an individual patient’s feature importance and to identify their protective and risk factors to calculate their probability of developing an AKI [
14].
3. Results
138 patients were listed for CRS for advanced EOC in Leeds Teaching Hospitals Trust in the years 2021–2022. Four patients were excluded from data analysis as no attempt at resection was made. Of the 134 patients included in the study, the total incidence of AKI post-CRS was 6.72% (). Of the AKI patients, 88.88% () had KDIGO AKI Stage 1, no patients had Stage 2 and 11.11% () had Stage 3. 44.44% of patients () with AKI had a pre-renal cause (hypovolaemia , infection ) and 11.11% () had a renal cause (nephrectomy ). 44.44% () had no suggested cause of AKI given in their discharge summaries.
With regards to preoperative patient characteristics (
Table 1), the cohort median age was 65.5 years. The AKI group had a younger median age of 55 years compared to the non-AKI group’s median age of 66 years (
). The median BMI was higher in the AKI group (29.7) compared to non-AKI (25.85), though not significantly different. The CCI was significantly lower in the AKI patients (
) versus non-AKI patients (
) (
). Seven patients had a diagnosis of CKD, and none of them went on to develop a postoperative AKI.
There were no significant differences in the percentage of patients using ACE inhibitors, ARBs, NSAIDs or diuretics who developed an AKI compared to those who did not. 33.3% of AKI patients reported being regular smokers in their preoperative assessment compared to 12.8% in non-AKI patients, although this was not a significant difference (). The median Eastern Cooperative Oncology Group Performance Score (ECOG PS) was zero in both groups, indicating a normal functional baseline across the cohort before surgery. The median baseline eGFR was slightly decreased from in the non-AKI group to 88 in the AKI group (). Differences between baseline creatinine and baseline albumin were negligible. The percentage of patients undergoing neoadjuvant chemotherapy was similar (55.6% vs. 52%, ).
Table 1.
Descriptive statistics for the preoperative variables, comparing the AKI and non-AKI groups, supported with the p-value obtained from Mann-Whitney U tests. Significant p-values are marked with an *.
Table 1.
Descriptive statistics for the preoperative variables, comparing the AKI and non-AKI groups, supported with the p-value obtained from Mann-Whitney U tests. Significant p-values are marked with an *.
| Characteristic |
All (n=134) |
AKI Group (n=9) |
Non-AKI Group (n=125) |
p-value |
| Pre-operative Factors |
| Age (years) |
65.5 (58.25, 72) |
55 (52, 65) |
66 (59, 73) |
0.018* |
| BMI (kg/m2) |
26 (22.85, 29.7) |
29.7 (27.9, 33.1) |
25.85 (22.83, 29.58) |
0.102 |
| CCI |
8 (7, 9) |
7 (7, 8) |
8 (8, 9) |
0.008* |
| CKD |
7 (5.2%) |
0 (0%) |
7 (5.6%) |
0.468 |
| ACEi/ARB |
26 (19.4%) |
1 (11.1%) |
25 (20%) |
0.510 |
| Diuretic |
7 (5.2%) |
0 (0%) |
7 (5.6%) |
0.443 |
| NSAID |
10 (7.5%) |
1 (11.1%) |
9 (7.2%) |
0.720 |
| Smoking |
19 (14.2%) |
3 (33.3%) |
16 (12.8%) |
0.079 |
| ECOG PS |
0 (0, 1) |
0 (0, 1) |
0 (0, 1) |
0.719 |
| FIGO Stage |
3 (3, 3) |
3 (3, 4) |
3 (3, 3) |
0.533 |
| Baseline eGFR (mL/min/1.73m2) |
90 (77, 90) |
88 (80.25, 90) |
90 (77, 90) |
0.863 |
| Baseline Creatinine (µmol/L) |
59 (51, 67) |
60.5 (56, 67.75) |
59 (51, 67) |
0.726 |
| Baseline Albumin (g/L) |
38 (36, 40) |
38 (34.25, 40.25) |
37 (36, 39.5) |
0.983 |
| Neoadjuvant chemotherapy |
70 (52.2%) |
5 (55.6%) |
65 (52%) |
0.905 |
Of the intraoperative variables (
Table 2), there were marked differences in procedure length and surgical complexity. The AKI group underwent longer surgeries with a median length of 255 minutes compared to the non-AKI group’s median of 205 minutes (
). The AKI median SCS was 7 compared to the non-AKI median SCS of 3 (
). There was no difference between the intraoperative fluid statuses of either group, including EBL and fluid volume given. In terms of surgical outcomes, 72.4% (
) of patients had a CC 0 achieved. 17.9% (
) had a CC1, meaning that residual nodules were smaller than 2.5mm, and 9.7% (
) had a CC2, meaning residual nodules were between 2.5mm and 2.5cm.
Postoperatively, three patients from the non-AKI group died in the 90 days following CRS, although their cause of death was not examined in the scope of this study. The short-term mortality rate following CRS for advanced EOC in this study was 2.2%. A total of 20.1% (
) required more complex medical support and were admitted to HDU/ICU following surgery. The rate of HDU/ICU admission was 33.3% in the AKI group compared to 19.2% in the non-AKI group (
). Significantly, the AKI group spent twice as long in hospital post-CRS with a median stay of 12 days compared to 6 days for the non-AKI group (
) (
Table 3).
Spearman’s rank correlations between the significant factors showed associations between the preoperative, intraoperative, and postoperative outcomes (
Table 4). Age was significantly positively correlated with CCI (
,
), age being a component of CCI. Charlson Comorbidity Index was negatively correlated with procedure length (
,
), indicating less comorbid patients received longer operations. Aletti SCS and procedure length were also positively correlated (
,
), suggesting that more complex surgeries lasted longer. A longer procedure length also correlated with a longer stay postoperatively (
,
).
Univariate logistic regressions (
Table 5) for age (OR 0.942, 95% CI 0.891, 0.996) and CCI (OR 0.415, 95% CI 0.205, 0.841) indicated that they were significant predictors for AKI. Baseline eGFR, creatinine, and albumin were not significant predictors for AKI, suggesting AKI incidence was not dependent on preoperative renal function. For intraoperative variables, procedure length (OR 1.006, 95% CI 1.001, 1.012,
) and Aletti SCS (OR 1.427, 95% CI 1.104, 1.844,
) were both significant predictors of AKI outcome.
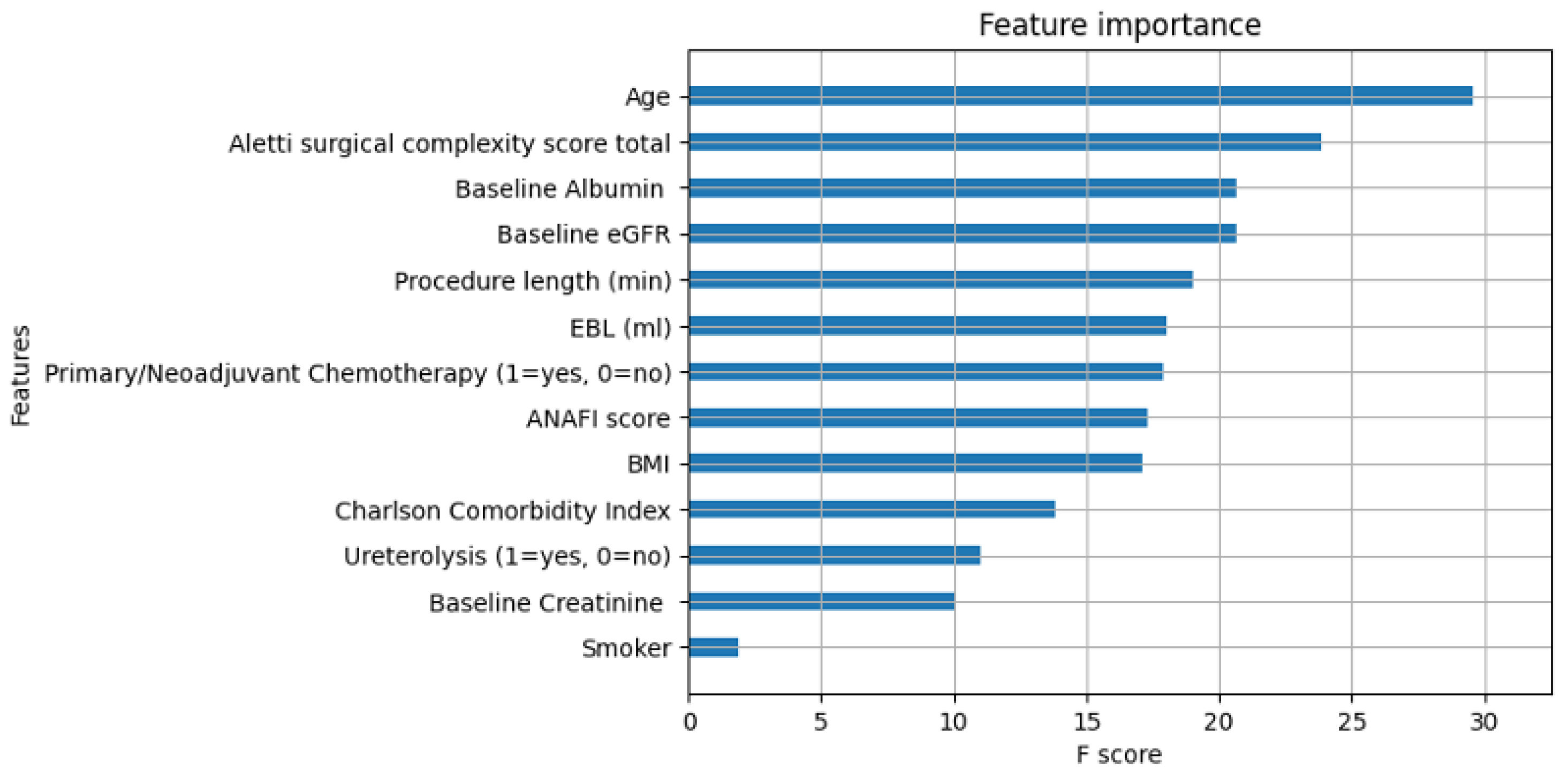
Feature importance was calculated in machine learning based on the whole cohort (
Figure A1). In line with conventional regression analysis, AI identified that age and SCS were the most important features in determining a patient’s risk of AKI development, although the feature importance plots did not indicate whether a variable was a risk factor or a protective factor. Neoadjuvant chemotherapy, CCI, and procedure length were reported as less important features compared with suggestions by traditional statistics. The feature importance of the thirteen most important predictive features is illustrated in a parallel coordinate plot (
Figure 1). More features were identified to be predictors of AKI by AI than SPSS. The XGBoost was superior to conventional regression for the AKI prediction (area under curve (AUC) = 0.85 vs 0.72).
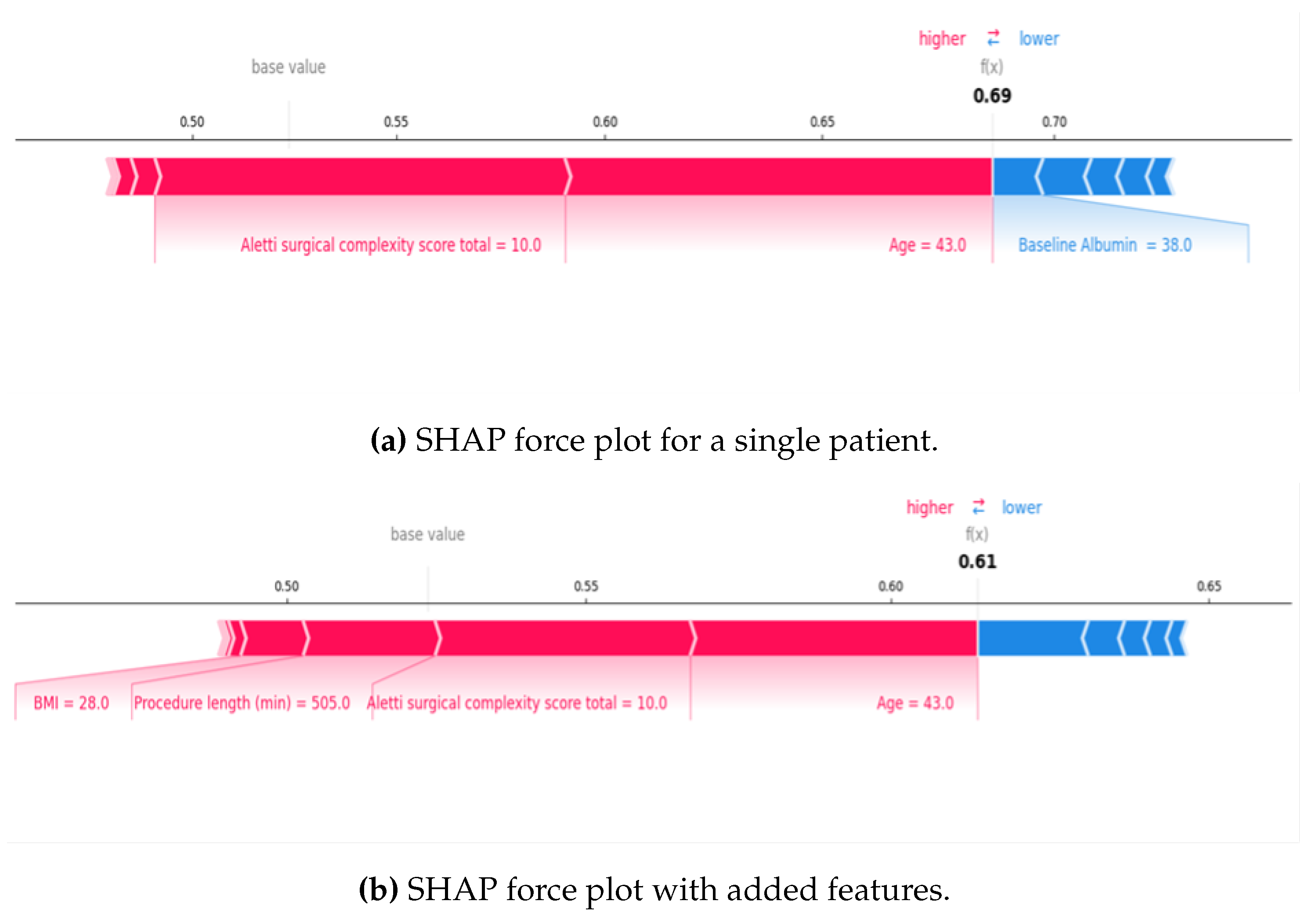
For local explainability, AI-based SHAP Force Plots displayed both risk and protective factors for individual patients and visualised the probability of AKI development for a patient based on their unique characteristics. This was ‘learnt’ from the cohort’s characteristics and then applied to one patient.
Figure 2 displays example SHAP Force Plots for a single patient. The blue features decreased the AKI risk, and the red features increased the risk. In Plot A, the calculated SHAP value risk was 0.69 due to younger age and higher surgical complexity, which were high-risk factors for the patient. Whereas for Plot B, the odds of AKI was 0.61 despite other red features being added because age and surgical complexity had a weaker predictive value for that specific patient, thus lowering the risk.
4. Discussion
Our study found a lower-than-expected incidence of AKI following CRS for advanced EOC. The reported incidence ranges widely between 11.4% [
5] and 40.4% [
7] in the literature. This could reflect the difficulties in accurate and prompt AKI monitoring; firstly, because AKI has multiple diagnostic criteria (KDIGO, RIFLE, AKIN and NCI-CTCAE) [
5,
7,
11] and secondly because decreasing urine output is harder to accurately measure and report than changing biochemical values, thus making comparisons between studies challenging. In that sense, the exclusion of diagnostic urine output is an accepted limitation of this study that may have resulted in under-reporting AKI incidence. Although the reasons for this discrepancy were not examined in the scope of this study, one such reason could be the absence of HIPEC, a known risk factor for AKI development [
5,
8]. Furthermore, a meta-analysis reviewing the incidence of AKI following 12,947 major gynaecological surgeries found that 7% of patients with appropriate monitoring developed an AKI [
16].
Compared to the current literature, some results from this study contradicted the initial working hypothesis. The preoperative factors expected to be predictive of AKI were increased age, use of an ACEi/ARB, a reduced baseline eGFR, an increased CCI, a low baseline albumin and neoadjuvant chemotherapy. Contrastingly, this study found younger age and decreased CCI to be predictive of AKI. Higher Aletti SCS and increased procedure length were found to be predictive of AKI in this cohort. The correlation between procedure length and increasing SCS and decreasing CCI suggests that healthier patients are selected for more radical surgeries. Longer surgeries increase exposure to potential nephrotoxic factors such as prolonged anaesthesia and intraoperative hypotension resulting in a higher risk of developing a postoperative AKI. As complete cytoreduction is required to ensure the best survival outcomes, maximal effort CRS is likely to be exerted by surgeons in younger patients with fewer comorbidities who are likely to better tolerate the surgical acuity.
Baseline eGFR, creatinine and albumin levels were not found to be predictive of AKI. We suspect that the missing baseline eGFR for five patients and the missing baseline albumin for thirty-three patients, which represents 33.3% of the AKI group, are responsible for this. It is possible that with the missing data, baseline albumin may have been a significant predictor for AKI as suggested by the literature [
7]. Additionally, within EHRs all normal kidney function values are recorded as >90 mL/min/1.73m
2; to run data analysis, this had to be taken as an eGFR of 90 which limits the accuracy of eGFR in statistical analysis. Whereas AI models could input eGFR >90 and therefore eGFR is possibly more accurately analysed in the machine learning figures. Overall, our study suggests that patients with a sub-optimal eGFR can still sustain a complex CRS without an increased risk of AKI in the postoperative window.
Neoadjuvant chemotherapy was not predictive of AKI in this cohort, suggesting that fears of chemotherapy-related nephrotoxicity contributing to AKI should not interfere with surgical planning when it comes to CRS. Blood loss was not a predictor of AKI in this study, despite hypovolaemia being the modal stated cause of AKI in discharge summaries. This is likely because the EBL and fluid balance during surgery were monitored and carefully corrected by the anaesthetic team and the hypovolaemia was likely to have occurred in the early postoperative period due to the fluid shift. Causes of postoperative hypovolaemia leading to AKI were not explored in this study.
Patients with AKI experienced a significantly longer median length of hospital admission of 12 days compared to six days for non-AKI patients, akin to the post-AKI length of stay across other disciplines [
6]. Patients with AKI also had a greater need for intensive or high dependency care (33.33% vs. 19.20%) as several common factors could be shared between HDU admission and AKI predictions [
17]. Notably, while three patients in the non-AKI group died within 90 days of admission, there were no fatalities amongst the AKI group.
Despite multiple factors risking renal function during CRS for advanced EOC, the study adds to the scarce literature regarding AKI incidence, predictors and outcomes in patients receiving CRS without HIPEC. The limitations of a single-centre retrospective study need to be acknowledged. However, the study provides meaningful insights into postoperative AKI development as a result of the primary management of EOC within the UK. This is increasingly important given the projected rise in ovarian cancer incidence of 5% by 2038 [
18]. Additionally, the strength of AI-guided data interpretation over traditional statistics allowed the identification of trends in a whole cohort and make predictive insights for patients on a population or individual level.
Most patients experienced stage 1 AKI. Rapid reductions in kidney function were automatically flagged in the EHRs, leading to better utilisation of the AKI bundle. The renal team was appropriately involved in all stage 3 AKI cases. However, event documentation was minimal in discharge letters. One stage 3 AKI case was caused by a known ureteric leak and small bowel ischemia during cytoreductive surgery. Notably, the STOP AKI bundle was not completed in any of the AKI cases, but hypovolemia was effectively managed according to protocol.
Our results demonstrated an unexpected AKI risk profile. While we cannot recommend changes to CRS procedures to reduce AKI incidence — since survival benefits potentially outweigh the perioperative risks — this research can support improved postoperative guidelines and enhance clinical education. Resident doctors must complete the STOP AKI bundle during daily rounds and document AKI events at discharge to inform GPs. Staff should distribute appropriate patient information leaflets. Advanced EOC patients present unique postoperative challenges, requiring meticulous daily documentation of fluid management plans for those with AKI. Effective management of AKI risk requires interdisciplinary collaboration. Explainable AI could help introduce a formal AKI risk assessment tool into the postoperative guideline to encourage more vigilant monitoring and timely intervention. Early recognition could also minimise the length of postoperative admission, even a reduction of one day could save the NHS £2349 per day and improve patient outcomes [
19].
There is scope for further research assessing the clinical utility of AI models in predicting AKI and the potential for the development of real-time, point-of-care online tools to enable clinicians to make more informed decisions based on a patient’s individual risk assessment.
5. Conclusions
Prediction of AKI is a complex task. The study found a lower-than-expected AKI incidence following advanced EOC cytoreductive surgery. The AKI results from a multifactorial interplay of patient-specific and surgical factors. It is linked to heightened surgical risk-taking, highlighting the scope for improved guidelines focusing on postoperative monitoring for targeted patients. Artificial Intelligence offers the potential for personalised AKI prediction in cytoreduced patients.
Author Contributions
Conceptualization, A.L.; Data curation, A.L., E.R., C.D., and M.Q.; Software, A.L., E.K., C.D., and E.R.; Supervision, A.T., and D.D.J.; Writing—original draft, E.R., C.D., and A.L.; Writing and editing, M.Q., S.M., A.T., T.B., D.N., and D.D.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
Table A1.
Summary of Pre-operative, Intraoperative, and Post-operative Variables.
Table A1.
Summary of Pre-operative, Intraoperative, and Post-operative Variables.
| Pre-operative |
|
Intraoperative |
Post-operative |
| Age |
ECOG Performance Status |
Procedure Length |
Length of hospital stay |
| BMI |
Baseline eGFR |
Estimated Blood Loss |
HDU/ICU admission |
| Charlson Comorbidity Index |
Baseline Creatinine |
Aletti Surgical Complexity Score |
Short term mortality (<90 days) |
| CKD Status |
Baseline Albumin |
Ureterolysis |
|
| ACEi/ARB |
Neoadjuvant chemotherapy |
|
|
| Diuretics |
Time between last chemotherapy & surgical date |
|
|
| NSAIDs |
FIGO Stage |
|
|
| Smoking Status |
|
|
|
Table A2.
Staging criteria for acute kidney injury (AKI) as defined by KDIGO [
14], used in the staging algorithm in LTHT’s Lab Information Management System (LIMS) [
5].
Table A2.
Staging criteria for acute kidney injury (AKI) as defined by KDIGO [
14], used in the staging algorithm in LTHT’s Lab Information Management System (LIMS) [
5].
| AKI Stage |
Serum Creatinine |
Urine Output |
| 1 |
1.5–1.9 times baseline OR ≥ 26.5 µmol/L (≥ 0.3 mg/dL) increase |
<0.5 mL/kg/h for 6–12 hours |
| 2 |
2.0–2.9 times baseline |
<0.5 mL/kg/h for ≥ 12 hours |
| 3 |
3.0 times baseline OR increase in serum creatinine to ≥ 353.6 µmol/L (≥ 4.0 mg/dL) OR initiation of renal replacement therapy |
<0.3 mL/kg/h for ≥ 24 hours OR anuria for ≥ 12 hours |
Figure A1.
Feature Importance plot generated by XGBoost Classifier for the preoperative and intraoperative variables that were the most apparent in AKI prediction. The greater the F score, the more influential that variable was in the cohort. It does not indicate whether the relationship was positive or negative in relation to AKI prediction. EGFR = Estimated Glomerular Filtration Rate, EBL = Estimated Blood Loss, ANAFI = Anatomic Fingerprints Score for the Prediction of Complete Cytoreduction, BMI = Body Mass Index.
Figure A1.
Feature Importance plot generated by XGBoost Classifier for the preoperative and intraoperative variables that were the most apparent in AKI prediction. The greater the F score, the more influential that variable was in the cohort. It does not indicate whether the relationship was positive or negative in relation to AKI prediction. EGFR = Estimated Glomerular Filtration Rate, EBL = Estimated Blood Loss, ANAFI = Anatomic Fingerprints Score for the Prediction of Complete Cytoreduction, BMI = Body Mass Index.
References
- Thompson C. Ovarian Neoplasms. In: Magowan B, Owen P, Thomson AJ, editors. Clinical Obstetrics and Gynaecology, Fifth Edition. Edinburgh: Elsevier; 2019. p. 139-148.
- du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials. Cancer. 2009;115:1234-1244. [CrossRef]
- Chi DS, Franklin CC, Levine DA, Akserol F, Sabbatini P, Jarnagin WR, et al. Improved optimal cytoreduction rates for stages IIIC and IV epithelial ovarian, fallopian tube, and primary peritoneal cancer: a change in surgical approach. Gynecol Oncol. 2004;94:650-654. [CrossRef]
- NICE. Acute kidney injury: prevention, detection and management NG148. [Internet]. NICE; 2024 [cited 2024 Oct 24]. Available from: https://www.nice.org.uk/guidance/ng148/chapter/Recommendations#detecting-acute-kidney-injury.
- Bai Y, Du Y, Ye P, Luo Y. Acute kidney injury after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in patients with advanced ovarian cancer. Front Oncol. 2023;13:1094410.
- Lewington A. Acute Kidney Injury in Adults Guideline (Secondary Care). LTHT, Leeds; 2024.
- Sin EIL, Chia CS, Tan GHC, Soo KC, Teo MCC. Acute kidney injury in ovarian cancer patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Int J Hyperthermia. 2017;33(6):690-695.
- Cata J, Zavala A, Van Meter A, Williams U, Soliz J, Hernandez M, Owusu-Agyemang P. Risk factors for postoperative acute kidney injury after cytoreductive surgery with HIPEC. Int J Hyperthermia. 2018;34(5):538-544.
- NICE. Cytoreductive surgery with HIPEC for peritoneal carcinomatosis [Internet]; 2021 [cited 2023 Sept 7]. Available frohttps://www.nice.org.uk/guidance/ipg688.
- Chang SJ, Bristow RE, Ryu HS. Impact of complete cytoreduction on survival in advanced ovarian cancer. Ann Surg Oncol. 2012;19:4045-4067.
- Naffouje SA, Tulla K, Chorely R, Armstrong N, Salti G. AKI increases the rate of major morbidities in CRS and HIPEC. Ann Med Surg. 2018;35:163-168.
- Mitani Y, Arai Y, Mitani T, et al. Association of intraoperative gross hematuria with AKI post-CRS. Pleura Peritoneum. 2022;7(1):19-26. ed on Day Month Year).
- Laios A, Kalampokis E, Johnson R, Thangavelu A, Tarabanis C, Nugent D, et al. Explainable Artificial Intelligence for Prediction of Complete Surgical Cytoreduction in Advanced-Stage Epithelial Ovarian Cancer. J Pers Med. 2022;12(4):607. [CrossRef]
- Laios A, Kalampokis E, Johnson R, Munot S, Thangavelu A, Hutson R, et al. Development of a Novel Intra-Operative Score to Record Diseases’ Anatomic Fingerprints (ANAFI Score) for the Prediction of Complete Cytoreduction in Advanced-Stage Ovarian Cancer by Using Machine Learning and Explainable Artificial Intelligence. Cancers (Basel). 2023;15(3):966. [CrossRef]
- KDIGO. KDIGO Clinical Practice Guideline for Acute Kidney Injury. International Society of Nephrology [Internet]. 2012;2(1):8. Available from: https://kdigo.org/wp-content/uploads/2016/10/KDIGO-2012-AKI-Guideline-English.pdf.
- Reza, C.; Klarskov, N.; Amirian, I. Acute kidney injury after gynaecological surgery—a systematic review and meta-analysis. Danish Medical Journal 2023, 70, A11220733-A.
- Laios, A.; De Oliveira Silva, R.V.; Dantas De Freitas, D.L.; Tan, Y.S.; Saalmink, G.; Zubayraeva, A.; et al. Machine Learning-Based Risk Prediction of Critical Care Unit Admission for Advanced Stage High Grade Serous Ovarian Cancer Patients Undergoing Cytoreductive Surgery: The Leeds-Natal Score. J. Clin. Med. 2021, 11, 87. [CrossRef]
- Cancer Research UK. Ovarian cancer statistics [Internet]; 2023 [cited 2024 Sept 30]. Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/ovarian-cancer#heading-Four.
- UK Parliament. Hospital Beds: Costs 2023 [updated 14 March 2023]. Available from: https://questions-statements.parliament.uk/written-questions/detail/2023-03-14/165361.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).