Submitted:
26 October 2023
Posted:
27 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
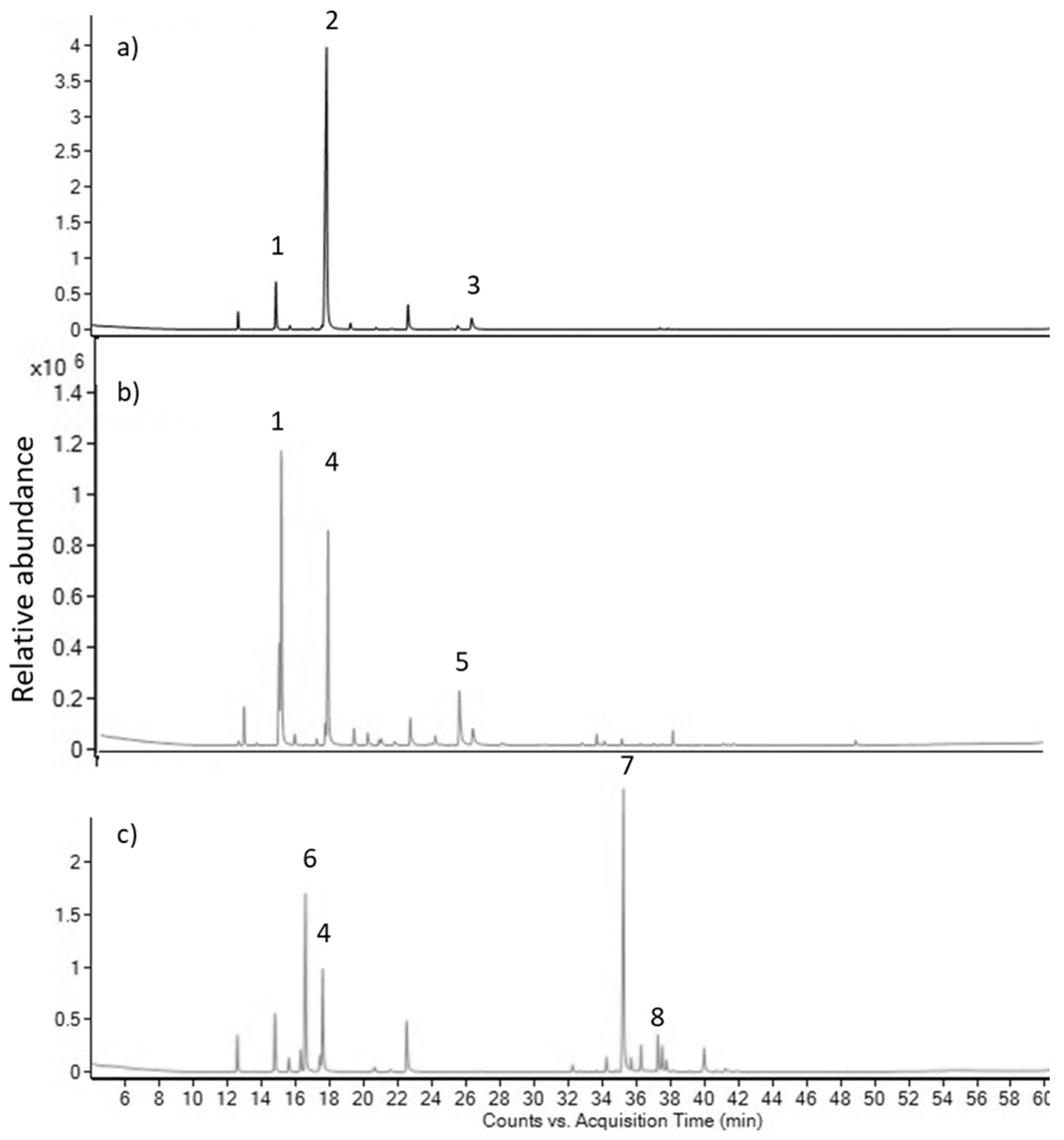
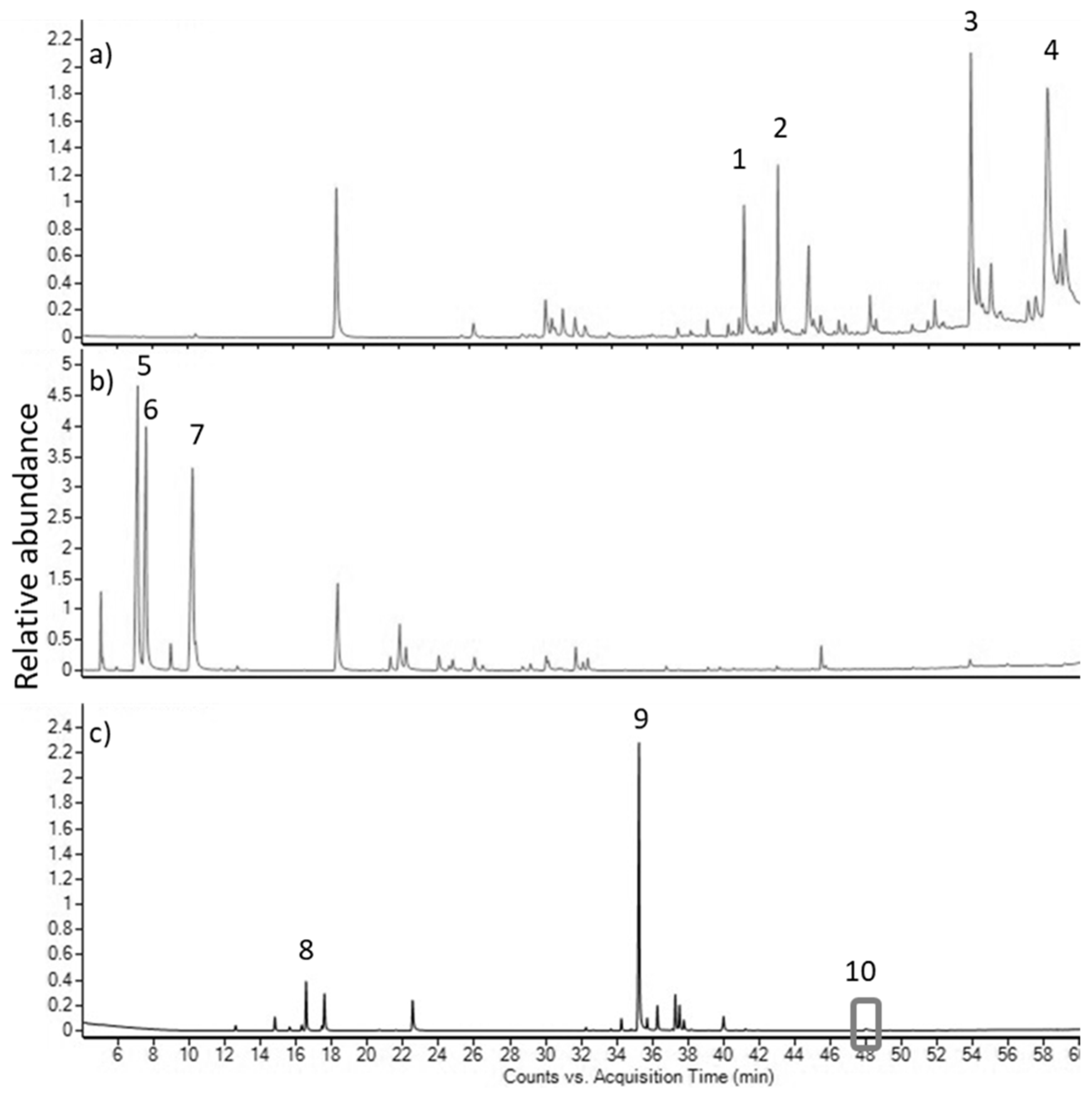
2. Results
3. Discussion
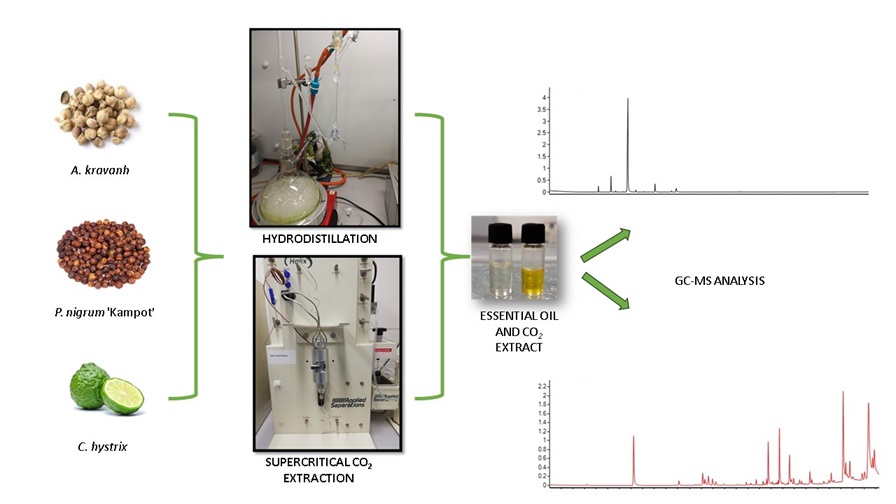
4. Materials and Methods
4.1. Plant material and sample preparation
4.2. Hydrodistillation of EOs
4.3. Supercritical CO2 extracts preparation
4.4. Gas chromatography-mass spectrometry analysis (GC-MS)
4.5. Identification of constituents, quantification, and statistical analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- E. Ceylan and D. Y. C. Fung, Antimicrobial activity of spices, J Rapid Methods Autom Microbiol 12 (1), 1–55, (2004). [CrossRef]
- K. V. Peter and M. R. Shylaja, Introduction to herbs and spices: definitions, trade and applications, in Handbook of Herbs and Spices, Elsevier, (2012), 1–24. [CrossRef]
- Market reports, available online: https://www.marketsandmarkets.com/Market-Reports/spices-market-739.html, 2021, (accessed 15th October 2022).
- E. Westphal, R. K. Arora, Prosea Project., Plant resources of south-east asia. Pudoc, 1989.
- B. Hammouti, A. Asehraou, A. Bouyanzer, M. Dahmani, A. Ettouhami, M. Messali, R. Touzani and I. Warad, Black pepper, the ‘king of spices’: chemical composition to applications, Arab J Chem Environ Res 6 (1), 12–56, (2019).
- Future market insights reports, available online: https://www.futuremarketinsights.com/reports/black-pepper-market, 2021, (accessed 2nd November 2022).
- M. R. Shylaja and K. V. Peter, Spices in the nutraceutical and health food industry, Acta Hortic 756, 369–378, (2007). [CrossRef]
- A. C. de Groot and E. Schmidt, Essential oils, part III: chemical composition, Dermatitis 27 (4), 161–169, (2016). [CrossRef]
- M. Moghaddam and L. Mehdizadeh, Chemistry of essential oils and factors influencing their constituents, in Soft Chemistry and Food Fermentation, Elsevier, (2017), 379–419. [CrossRef]
- M. Viuda-Martos, J. Fernandez-Lopez, J.-A. Perez-Alvarez and Y. Ruiz-Navajas, Chemical composition of the essential oils obtained from some spices widely used in mediterranean region, Acta Chim Slov 54 (4), 921–926, (2007).
- Q. M. T. Ngo, T. Q. Cao, M. T. Ha, L. S. Hoang, B. S. Min and M. H. Woo, Cytotoxic activity of alkaloids from the fruits of Piper nigrum, Nat Prod Commun 13 (1), 1934578X1801301, (2018). [CrossRef]
- A. Rivera-Pérez, A. Garrido Frenich and R. Romero-González, Determination and occurrence of alkenylbenzenes, pyrrolizidine and tropane alkaloids in spices, herbs, teas, and other plant-derived food products using chromatographic methods: review from 2010–2020, Food Rev Int, 1–27, (2021). [CrossRef]
- M. E. Embuscado, Spices and herbs: natural sources of antioxidants – a mini review, J Funct Foods 18, 811–819, (2015). [CrossRef]
- M. N. Sovilj, B. G. Nikolovski, and M. D. Spasojević, Critical review of supercritical fluid extraction of selected spice plant materials, Maced J Chem Chem Eng 30 (2), 197-220, (2011). [CrossRef]
- R. P. F. F. da Silva, A. C. Duarte and T. A. P. Rocha-Santos, Supercritical fluid extraction of bioactive compounds, TrAC - Trends Anal Chem 76, 40–51, (2016). [CrossRef]
- H. M. A. Barbosa, M. A. Coimbra, M. M. R. de Melo, C. P. Passos, and C. M. Silva, Optimization of the supercritical fluid coextraction of oil and diterpenes from spent coffee grounds using experimental design and response surface methodology, J Supercrit Fluids 85, 165–172, (2014). [CrossRef]
- T. Fornari, M. R. García-Risco, G. Reglero, E. Vázquez and G. Vicente, Isolation of essential oil from different plants and herbs by supercritical fluid extraction, J Chromatogr A 1250. 34–48, (2012). [CrossRef]
- M. G. Montalbán and G. Víllora, Supercritical fluids: properties and applications, in Phase equilibria with supercritical carbon dioxide – application to the components of a biocatalytic process, IntechOpen, (2022). [CrossRef]
- P. A. Uwineza and A. Waśkiewicz, Recent advances in supercritical fluid extraction of natural bioactive compounds from natural plant materials, Molecules 25 (17), 3847, (2020). [CrossRef]
- L. T. Danh, N. Foster, L. N. Han, R. Mammucari, N. D. A. Triet, and J. Zhao, Comparison of chemical composition, antioxidant and antimicrobial activity of lavender (lavandula angustifolia l.) essential oils extracted by supercritical CO2, hexane and hydrodistillation, Food Bioproc Tech 6 (12), 3481–3489, (2013). [CrossRef]
- B. Marongiu, C. Cavaleiro, M.A. Frau, M. J. Goncalves, A. Maxia, A. Piras, S. Porcedda, L. Salgueiro and E. Tuveri, Isolation of the volatile fraction from Apium graveolens l. (apiaceae) by supercritical carbon dioxide extraction and hydrodistillation: chemical composition and antifungal activity, Nat Prod Res 27, (17), 1521–1527, (2013). [CrossRef]
- A. Maxia, C. Cabral, C. Cavaleiro, D. Falconieri, M.A. Frau., M.J. Goncalves, B. Marongiu, A. Piras, S. Porcedda and L. Salgueiro, Chemical composition and antifungal activity of essential oils and supercritical CO2 extracts of Apium nodiflorum (l.) lag, Mycopathologia 174 (1) 61–67, (2012). [CrossRef]
- M. C. Mesomo, L. Cardozo, M. L. Corazza, O. R. Dalla Santa, P. M. Ndiaye, and A. de P. Scheer, Supercritical CO2 extracts and essential oil of ginger (Zingiber officinale r.): chemical composition and antibacterial activity, J Supercrit Fluids 80, 44–49, (2013). [CrossRef]
- B. Ooraikul, A. Sirichote, and S. Siripongvutikorn, Southeast asian diets and health promotion, in Wild-Type Food in Health Promotion and Disease Prevention, Totowa, NJ: Humana Press, (2008), 515–533. [CrossRef]
- S. In, V. Camel, C. Lambre, and M. Ouldhelkim, Regional and seasonal variations of food consumption in cambodia, Malays J Nutr 21 (2), (2015).
- V. Verner, P. Chaloupkova, S, M. Kosova, L. Kokoska, S. Nguon and P. Van Damme, Tourists’ preferences for traditional food products as indicators of market potential of the market potential of underutilized species in Cambodia, Agriculture 13 (8), 1599, (2023) . [CrossRef]
- E. Morm, F. Debaste, B. Haut, S. Horn, S. In and K. Ma, Experimental characterization of the drying of kampot red pepper (Piper nigrum L.), Foods 9 (11,), 1532, (2020). [CrossRef]
- I. Jantan, A. S. Ahmad, A. R. Ahmad, N. A. M. Ali, and N. Ayop, Chemical composition of some citrus oils from malaysia, J Essent Oil Res 8 (6), 627–632, (1996). [CrossRef]
- A. Suresh, S. Ayyasamy, M. Rathinasamy and S. Velusamy, Techniques for essential oil extraction from kaffir lime and its application in health care products—a review, Flavour Fragr J 36 (1), 5–21, (2021). [CrossRef]
- J. Waikedre, I. Barrachina, P. Cabalion, A. Dugay, A. Fournet and C. Herrenknecht, Chemical composition and antimicrobial activity of the essential oils from new caledonian Citrus macroptera and Citrus hystrix, Chem Biodivers 7 (4), 871–877, (2010). [CrossRef]
- W.-R. Diao, S. Feng, L.-L., J.-G. Xu and Zhang, S. Chemical composition, antibacterial activity, and mechanism of action of the essential oil from Amomum kravanh, J Food Prot 77 (10), 1740–1746, (2014). [CrossRef]
- X. Feng, Z.-T. Jiang, R. Li and Y. Wang, Composition comparison of essential oils extracted by hydro distillation and microwave-assisted hydrodistillation from Amomum kravanh and Amomum compactum, J Essent Oil Bear Plants 14 (3), 354–359, (2011). [CrossRef]
- J.-S. Zhang, X.-X. Cao, and H. Zhang, Chemical constituents from the fruits of Amomum kravanh, Biochem Syst Ecol 92, 104127, (2020). [CrossRef]
- W. Yothipitak, P. Boonnoun, M. Goto, A. Shotipruk and N. Tonanon, Response surface methodology to supercritical carbon dioxide extraction of essential oil from Amormum krevanh pierre, Sep Sci Technol 44 (16,) 3923–3936, (2009). [CrossRef]
- O. Norkaew, K. Pitija, P. Pripdeevech, P. Sookwong, and S. Wongpornchai, Supercritical fluid extraction and gas chromatographic-mass spectrometric analysis of terpenoids in fresh kaffir lime leaf oil, Chiang Mai J.Sci 40 (2), 240–247, 2013.
- AOAC International. Method of Analysis–Official methods 991.14. International Official Methods of Analysis Official Methods. Gaithersburg, MD, USA (2012).
- EDQM, European Pharmacopoeia 8.0, Published in Accordance with the Convention of the Elaboration of a European Pharmacopoeia, European Treaty Series 8.0., Council of Europe, Strasbourg, France, (2013).
- R. P. Adams, Identification of essential oil components by gas chromatography / mass spectrometry, Carol Stream, IL: Allured Publishing corporation, (2007).
- N. Sabulal and S. Baby, Chemistry of Amomum essential oils, J Essent Oil Res 33 (5), 427–441, (2021). [CrossRef]
- G.-W. Yu, Q. Cheng, M. Lee, Z. Li, J. Nie, and X. Wang, Microwave hydrodistillation based on deep eutectic solvent for extraction and analysis of essential oil from three Amomum species using gas chromatography–mass spectrometry, Chromatographia 81 (4), 657–667, (2018). [CrossRef]
- F. David, P. Sandra, and A. K. Vickers, Column selection for the analysis of fatty acid methyl esters, Food analysis application 19, 19, (2005).
- S.-G. Woo, S. Bang, J. Lee, M. Lee, K. On, J. Park and K. Yoo, Comparison of fatty acid analysis methods for assessing biorefinery applicability of wastewater cultivated microalgae, Talanta 97, 103–110, (2012). [CrossRef]
- A. Sato, K. Asano, and T. Sato, The chemical composition of Citrus hystrix dc (swangi), J Essent Oil Res 2 (4), 179–183, (1990). [CrossRef]
- T. K. N. Tran, L. G. Bach, X. P. Huynh, T. C. Q. Ngo, T. H. Tran and T. T. Tran, Comparison of volatile compounds and antibacterial activity of Citrus aurantifolia, Citrus latifolia, and Citrus hystrix shell essential oils by pilot extraction, IOP Conf Ser Mater Sci Eng 1092 (1), p. 012076, (2021). [CrossRef]
- G. Li, Y. Cheng, L. Han, X. Long, Y. Pan, S. Xiang, and X. Zhao., Effects of cold-pressing and hydrodistillation on the active non-volatile components in lemon essential oil and the effects of the resulting oils on aging-related oxidative stress in mice, Front Nutr 8, (2021). [CrossRef]
- X. T. Le, P. T. H. Ha, T. T. Hien, T. T. K. Ngan and H. X. Phong, Extraction of essential oils and volatile compounds of kaffir lime (Citrus hystrix d.c) by hydrodistillation method, IOP Conf Ser Mater Sci Eng 991 (1), 012024, (2020). [CrossRef]
- Q. Lu, N. Huang, Pan, Y. Peng, and C. Zhu, Peel oils from three citrus species: volatile constituents, antioxidant activities and related contributions of individual components, J Food Sci Technol 56 (10), 4492–4502, (2019). [CrossRef]
- M. Russo, A. Arigò, P. Dugo, L. Mondello and F. Rigano, Coumarins, psoralens and polymethoxyflavones in cold-pressed citrus essential oils: a review, J Essent Oil Res 33 (3), 221–239, (2021). [CrossRef]
- M. A. Ferhat, F. Chemat and B. Y. Meklati, Comparison of different isolation methods of essential oil fromcitrus fruits: cold pressing, hydrodistillation and microwave ‘dry’ distillation, Flavour Fragr J 22 (6), 494–504, (2007). [CrossRef]
- S. V. Luca, T. Kittl, and M. Minceva, Supercritical CO2 extraction of spices: a systematic study with focus on terpenes and piperamides from black pepper (Piper nigrum l.), Food Chem 406, 135090, (2023). [CrossRef]
- Y. Andriana, Q.-T. Le, T. N. Quy, H.-D. Tran, and T. D. Xuan, Biological activities and chemical constituents of essential oils from Piper cubeba bojer and Piper nigrum l., Molecules 24 (10), 1876, (2019). [CrossRef]
- Y. Li, L. Chen, M. Liu, S. Pan, J. Tian, K. Yang, X. Zemg, and C. Zhang, Analysis of chemical components and biological activities of essential oils from black and white pepper (Piper nigrum l.) in five provinces of southern China, LWT 117, 108644, (2020). [CrossRef]
- I. P. S. Kapoor, C. A. N. Catalan, C. S. De Heluani, M. P. De Lampasona, B. Singh and G. Singh, Chemistry and in vitro antioxidant activity of volatile oil and oleoresins of black pepper (Piper nigrum), J Agric Food Chem 57 (12), 5358–5364, (2009). [CrossRef]
- H. Bagheri, M. Y. Bin Abdul Manap, and Z. Solati, Antioxidant activity of Piper nigrum l. essential oil extracted by supercritical CO2 extraction and hydro-distillation, Talanta 121, 220–228, (2014). [CrossRef]
- K. S. Andrade, S. R. S. Ferreira and G. Trivellin, Piperine-rich extracts obtained by high pressure methods, J Supercrit Fluids 128, 370–377, (2017). [CrossRef]
- U. Topal, M. Goto, S. Otles and M. Sasaki, Chemical compositions and antioxidant properties of essential oils from nine species of Turkish plants obtained by supercritical carbon dioxide extraction and steam distillation, Int J Food Sci Nutr 59 (7–8), 619–634, (2008). [CrossRef]
| RIa | Compoundb | Cc | Extraction type/Column type/Peak area [%] | Column type/Identification methodd | ||||||||||||||
| Essential oil | CO2 extract | |||||||||||||||||
| Obs. | Lit. | HP-5 MS | DB-Wax | HP-5 MS | DB-Wax | HP-5 MS | DB-Wax | |||||||||||
| 923 | 931 | α-Thujene | MH | 0.09 | ± | 0.01 | - | - | - | - | - | - | - | - | - | RI, GC-MS | - | |
| 929 | 932 | α-Pinene | MH | 2.3 | ± | 0.05 | 2.20 | ± | 0.03 | - | - | - | - | - | - | RI, GC-MS, Std | GC-MS, Std | |
| - | 945 | α-Fenchene | MH | - | - | - | - | - | - | - | - | - | 0.01 | ± | 0.01 | - | GC-MS | |
| 944 | 946 | Camphene | MH | 0.07 | ± | 0.01 | 0.08 | ± | 0.00 | - | - | - | - | - | - | RI, GC-MS, Std | GC-MS, Std | |
| 970 | 969 | Sabinene | MH | 0.2 | ± | 0.04 | 0.23 | ± | 0.00 | - | - | - | - | - | - | RI, GC-MS | GC-MS | |
| 973 | 974 | β-Pinene | MH | 7.68 | ± | 0.08 | 7.49 | ± | 0.09 | - | - | - | - | - | - | RI, GC-MS, Std | GC-MS, Std | |
| 989 | 988 | β-Myrcene | MH | 0.78 | ± | 0.03 | 0.86 | ± | 0.08 | - | - | - | - | - | - | RI, GC-MS | GC-MS | |
| 1003 | 1002 | α-Phellandrene | MH | 0.08 | ± | 0.01 | - | - | - | - | - | - | - | - | - | RI, GC-MS, Std | GC-MS, Std | |
| 1015 | 1009 | 4-Carene | MH | 0.24 | ± | 0.03 | 0.17 | ± | 0.12 | - | - | - | - | - | - | RI, GC-MS | GC-MS | |
| - | 1014 | α-Terpinene | MH | - | - | - | 0.22 | ± | 0.01 | - | - | - | - | - | - | - | GC-MS | |
| 1025 | 1022 | o-Cymene | MH | 0.69 | ± | 0.04 | 0.87 | ± | 0.02 | - | - | - | - | - | - | RI, GC-MS | GC-MS | |
| 1031 | 1026 | Eucalyptol | MO | 78.89 | ± | 0.42 | 72.60 | ± | 0.89 | - | - | - | 0.08 | ± | 0.01 | RI, GC-MS | GC-MS | |
| - | 1031 | D-Limonene | MH | - | - | - | 5.12 | ± | 0.09 | - | - | - | 0.01 | ± | 0.01 | - | GC-MS | |
| 1058 | 1054 | γ-Terpinene | MH | 1.05 | ± | 0.06 | 1.06 | ± | 0.02 | - | - | - | - | - | - | RI, GC-MS | GC-MS | |
| - | 1083 | Fenchone | MO | - | - | - | 0.18 | ± | 0.02 | - | - | - | - | - | - | - | GC-MS | |
| 1087 | 1086 | Isoterpinolene | MO | 0.43 | ± | 0.02 | - | - | - | - | - | - | - | - | - | GC-MS | - | |
| 1105 | 1095 | Linalool | MO | 0.45 | ± | 0.01 | 0.50 | ± | 0.01 | - | - | - | - | - | - | RI, GC-MS, Std | GC-MS, Std | |
| 1174 | 1162 | δ-Terpineol | MO | 0.39 | ± | 0.05 | 0.43 | ± | 0.01 | - | - | - | - | - | - | RI, GC-MS | GC-MS | |
| 1182 | 1174 | L-terpinen-4-ol | MO | 1.19 | ± | 0.12 | 1.32 | ± | 0.02 | - | - | - | - | - | - | RI, GC-MS | GC-MS | |
| 1196 | 1186 | α-Terpineol | MO | 4.31 | ± | 0.17 | 4.67 | ± | 0.08 | 3.68 | ± | 0.16 | 1.77 | ± | 0.15 | RI, GC-MS | GC-MS | |
| 1350 | 1346 | α-Terpinyl acetate | MO | - | - | - | - | - | - | 0.17 | ± | 0.01 | - | - | - | RI, GC-MS | - | |
| 1368 | 1356 | Eugenol | MO | - | - | - | - | - | - | 7.91 | ± | 0.18 | 5.06 | ± | 0.18 | RI, GC-MS | GC-MS | |
| - | 1416 | α-Santalene | SH | - | - | - | - | - | - | - | - | 0.08 | ± | 0.01 | - | GC-MS | ||
| 1421 | 1419 | β-Caryophyllene | SH | - | - | - | - | - | - | 1.37 | ± | 0.25 | 0.66 | ± | 0.02 | RI, GC-MS, Std | GC-MS, Std | |
| 1457 | 1452 | Humulene | SH | - | - | - | - | - | - | 0.41 | ± | 0.03 | 0.12 | ± | 0.10 | RI, GC-MS, Std | GC-MS, Std | |
| 1486 | 1465 | (Z)-muurola-4(14),5-diene | SH | 0.16 | ± | 0.01 | - | - | - | - | - | - | - | - | - | RI, GC-MS | - | |
| - | 1478 | γ-Muurolene | SH | - | - | - | 0.16 | ± | 0.02 | - | - | - | - | - | - | - | GC-MS | |
| 1484 | 1484 | Germacrene D | SH | - | - | - | - | - | - | 1.38 | ± | 0.02 | - | - | - | RI, GC-MS | - | |
| 1490 | 1489 | β-Selinene | SH | 0.35 | ± | 0.03 | 0.25 | ± | 0.01 | 2.06 | ± | 0.04 | 0.88 | ± | 0.16 | RI, GC-MS | GC-MS | |
| 1497 | 1496 | Valencene | SH | - | - | - | - | - | - | 0.69 | ± | 0.10 | 0.09 | ± | 0.01 | RI, GC-MS | GC-MS | |
| 1508 | 1505 | β-Bisabolene | SH | 0.36 | ± | 0.03 | 0.23 | ± | 0.01 | 3.59 | ± | 0.06 | 1.12 | ± | 0.10 | RI, GC-MS | GC-MS | |
| 1518 | 1513 | γ-Cadinene | SH | - | - | - | - | - | - | 1.34 | ± | 0.09 | 0.73 | ± | 0.11 | RI, GC-MS | GC-MS | |
| - | 1514 | Cubebol | SO | - | - | - | - | - | - | - | - | - | 0.39 | ± | 0.01 | - | GC-MS | |
| 1525 | 1521 | β-Sesquiphellandrene | SH | 0.12 | ± | 0.02 | 0.15 | ± | 0.00 | 1.60 | ± | 0.62 | 0.65 | ± | 0.02 | RI, GC-MS | GC-MS | |
| 1531 | 1521 | Eugenol acetate | MO | - | - | - | - | - | - | 14.02 | ± | 0.74 | 5.23 | ± | 0.11 | RI, GC-MS | GC-MS | |
| 1558 | 1542 | (Z)-Sesquisabinene hydrate | SO | 0.12 | ± | 0.02 | 0.15 | ± | 0.00 | 0.51 | ± | 0.11 | 0.20 | ± | 0.01 | RI, GC-MS | GC-MS | |
| 1566 | 1561 | (E)-Nerolidol | SO | - | - | - | 0.12 | ± | 0.00 | 1.71 | ± | 0.03 | 0.61 | ± | 0.04 | RI, GC-MS | GC-MS | |
| 1595 | 1577 | (E)-Sesquisabinene hydrate | SO | - | - | - | - | - | - | 1.11 | ± | 0.30 | 0.44 | ± | 0.01 | RI, GC-MS | GC-MS | |
| - | 1577 | Spathulenol | SO | - | - | - | - | - | - | - | - | - | 0.15 | ± | 0.00 | - | GC-MS | |
| 1591 | 1582 | Caryophyllene oxide | SO | - | - | - | - | - | - | 0.39 | ± | 0.10 | 0.25 | ± | 0.02 | RI, GC-MS | GC-MS | |
| 1675 | 1674 | β-Bisabolol | SO | 0.04 | ± | 0.04 | 0.12 | ± | 0.04 | 0.21 | ± | 0.00 | 0.07 | ± | 0.00 | RI, GC-MS | GC-MS | |
| 1691 | 1685 | α-Bisabolol | SO | - | - | - | - | - | - | 0.21 | ± | 0.00 | 0.07 | ± | 0.00 | RI, GC-MS | GC-MS | |
| 1714 | 1715 | β-Santalol | SO | - | - | - | - | - | - | 0.56 | ± | 0.04 | 0.90 | ± | 0.01 | RI, GC-MS | GC-MS | |
| - | 1959 | Palmitic acid | FAD | - | - | - | - | - | - | - | - | - | 17.07 | ± | 0.23 | - | GC-MS | |
| 2086 | 2100 | Heneicosane | AH | - | - | - | - | - | - | 2.12 | ± | 0.03 | 0.76 | ± | 0.00 | RI, GC-MS | GC-MS | |
| - | 2113 | Linoleic acid | FAD | - | - | - | - | - | - | - | - | 5.17 | ± | 0.36 | - | GC-MS | ||
| 2166 | 2141 | Oleic Acid | FAD | - | - | - | - | - | - | 12.21 | ± | 0.25 | 29.26 | ± | 0.42 | RI, GC-MS | GC-MS | |
| - | 2172 | Stearic acid | FAD | - | - | - | - | - | - | - | - | - | 2.16 | ± | 0.09 | - | GC-MS | |
| 2186 | 2200 | Docosane | AH | - | - | - | - | - | - | 1.57 | ± | 0.09 | 0.51 | ± | 0.10 | RI, GC-MS | GC-MS | |
| 2286 | 2300 | Tricosane | AH | - | - | - | - | - | - | 14.74 | ± | 0.60 | 5.24 | ± | 0.09 | RI, GC-MS | GC-MS | |
| 2383 | 2400 | Tetracosane | AH | - | - | - | - | - | - | 1.94 | ± | 0.03 | 0.69 | ± | 0.05 | RI, GC-MS | GC-MS | |
| 2482 | 2500 | Pentacosane | AH | - | - | - | - | - | - | 5.19 | ± | 0.39 | 1.88 | ± | 0.08 | RI, GC-MS | GC-MS | |
| - | 2700 | Heptacosane | AH | - | - | - | - | - | - | - | - | - | 0.46 | ± | 0.02 | - | GC-MS | |
| 2096 | NA | Nonadecan-2-one | K | - | - | - | - | - | - | 0.54 | ± | 0.02 | - | - | - | GC-MS | - | |
| 2261 | NA | Tetradec-9-enal | A | - | - | - | - | - | - | 0.24 | ± | 0.01 | - | - | - | GC-MS | - | |
| 2267 | NA | Palmitoleic acid | FAD | - | - | - | - | - | - | 0.80 | ± | 0.06 | 1.94 | ± | 0.03 | GC-MS | GC-MS | |
| 2464 | NA | Hexadec-7-enal | A | - | - | - | - | - | - | 1.50 | ± | 0.13 | 0.11 | ± | 0.02 | GC-MS | GC-MS | |
| 2691 | NA | Azelaic acid bis(2-ethylhexyl) ester | E | - | - | - | - | - | - | 4.99 | ± | 0.12 | 1.96 | ± | 0.20 | GC-MS | GC-MS | |
| 2810 | NA | β-Monoolein | E | - | - | - | - | - | - | 2.88 | ± | 0.23 | - | - | - | GC-MS | - | |
| - | NA | Tricosanol | A | - | - | - | - | - | - | - | - | - | 0.38 | ± | 0.01 | - | GC-MS | |
| - | NA | Cyclopentadecanone | K | - | - | - | - | - | - | - | - | - | 0.46 | ± | 0.01 | - | GC-MS | |
| - | NA | Pentacos-1-ene | AH | - | - | - | - | - | - | - | - | - | 0.48 | ± | 0.04 | - | GC-MS | |
| - | NA | Heptacos-1-ene | AH | - | - | - | - | - | - | - | - | - | 1.31 | ± | 0.04 | - | GC-MS | |
| - | NA | Hexadec-9-enoic acid | FAD | - | - | - | - | - | - | - | - | - | 0.30 | ± | 0.04 | - | GC-MS | |
| - | NA | Octacosanol | A | - | - | - | - | - | - | - | - | - | 3.16 | ± | 0.06 | - | GC-MS | |
| - | NA | Squalene | TH | - | - | - | - | - | - | - | - | - | 1.17 | ± | 0.05 | - | GC-MS | |
| - | NA | Glyceryl linolenate | E | - | - | - | - | - | - | - | - | - | 1.43 | ± | 0.06 | - | GC-MS | |
| - | NA | β-Sitosterol | O | - | - | - | 0.17 | ± | 0.14 | - | - | - | - | - | - | - | GC-MS | |
| Total identified [%] | 99.88 | ± | 0.05 | 99.29 | ± | 0.16 | 91.64 | ± | 0.88 | 95.41 | ± | 0.46 | ||||||
| RIa | Compoundb | Cc | Extraction type/Column type/Peak area [%] | Column type/Identification methodd | ||||||||||||||
| Essential oil | CO2 extract | |||||||||||||||||
| Obs. | Lit. | HP-5 MS | DB-Wax | HP-5 MS | DB-Wax | HP-5 MS | DB-Wax | |||||||||||
| 923 | 931 | α-Thujene | MH | 0.31 | ± | 0.01 | 0.34 | ± | 0.02 | 0.32 | ± | 0.02 | 0.31 | ± | 0 | RI, GC-MS | GC-MS | |
| 930 | 937 | α-Pinene | MH | 2.93 | ± | 0.16 | 2.96 | ± | 0.09 | 2.54 | ± | 0.25 | 2.52 | ± | 0.09 | RI, GC-MS, Std | GC-MS, Std | |
| - | 945 | Fenchene | MH | - | - | - | - | - | - | - | - | - | 0.02 | ± | 0.02 | - | GC-MS | |
| 945 | 946 | Camphene | MH | 0.16 | ± | 0.01 | 0.19 | ± | 0.01 | 0.14 | ± | 0.01 | 0.15 | ± | 0 | RI, GC-MS, Std | GC-MS, Std | |
| 971 | 976 | Sabinene | MH | 9.94 | ± | 0.22 | 10.2 | ± | 0.15 | 19.36 | ± | 0.97 | 19.55 | ± | 0.33 | RI, GC-MS | GC-MS | |
| 974 | 980 | β-Pinene | MH | 29.95 | ± | 0.54 | 29.5 | ± | 0.45 | 30.2 | ± | 1.84 | 28.9 | ± | 0.55 | RI, GC-MS, Std | GC-MS, Std | |
| 990 | 991 | β-Myrcene | MH | 1.20 | ± | 0.05 | 1.38 | ± | 0.02 | 1.43 | ± | 0.06 | 1.58 | ± | 0.02 | RI, GC-MS | GC-MS | |
| - | 1004 | Pseudolimonene | MH | - | - | - | 0.97 | ± | 0.07 | - | - | - | 0.97 | ± | 0.04 | - | GC-MS | |
| 1003 | 1005 | α-Phellandrene | MH | 0.07 | ± | 0.01 | 0.06 | ± | 0 | - | - | - | - | - | - | RI, GC-MS, Std | GC-MS, Std | |
| - | 1008 | 3-Carene | MH | - | - | - | 0.01 | ± | 0.02 | - | - | - | - | - | - | - | GC-MS | |
| 1015 | 1009 | 4-Carene | MH | - | - | - | - | - | - | 0.02 | ± | 0.02 | - | ± | - | RI, GC-MS | - | |
| 1015 | 1009 | α-Terpinene | MH | 0.51 | ± | 0.04 | 0.61 | ± | 0.01 | - | - | - | - | - | - | RI, GC-MS | GC-MS | |
| 1025 | 1022 | o-Cymene | MH | 1.91 | ± | 0.01 | 2.34 | ± | 0.01 | 0.1 | ± | 0.01 | 0.21 | ± | 0.02 | RI, GC-MS | GC-MS | |
| 1028 | 1031 | D-Limonene | MH | 24.54 | ± | 0.16 | 23.2 | ± | 0.1 | 23.99 | ± | 0.45 | 23.74 | ± | 0.12 | RI, GC-MS | GC-MS | |
| 1058 | 1062 | γ-Terpinene | MH | 1.67 | ± | 0.01 | 1.63 | ± | 0.02 | 0.08 | ± | 0 | 0.1 | ± | 0.01 | RI, GC-MS | GC-MS | |
| 1072 | 1065 | (Z)-Sabinene hydrate | MO | - | - | - | - | - | - | 0.78 | ± | 0.06 | 1.06 | ± | 0.02 | RI, GC-MS | GC-MS | |
| - | 1071 | β-Terpinene | MH | - | - | - | - | - | - | - | - | - | 0.03 | ± | 0.01 | - | GC-MS | |
| 1074 | 1074 | Linalool oxide | MO | 1.55 | ± | 0.02 | 1.57 | ± | 0.03 | - | - | - | 0.05 | ± | 0.01 | RI, GC-MS | GC-MS | |
| 1087 | 1086 | Terpinolene | MH | 0.54 | ± | 0.03 | 0.52 | ± | 0.01 | 0.02 | ± | 0.01 | 0.05 | ± | 0 | RI, GC-MS | GC-MS | |
| 1105 | 1095 | Linalool | MO | 0.72 | ± | 0.09 | 0.98 | ± | 0.02 | 0.52 | ± | 0.27 | 0.81 | ± | 0.07 | RI, GC-MS, Std | GC-MS, Std | |
| - | 1098 | (E)-Sabinene hydrate | MO | - | - | - | - | - | - | - | - | - | 0.35 | ± | 0.03 | - | GC-MS | |
| - | 1114 | Fenchol | MO | - | - | - | 0.02 | ± | 0.03 | - | - | - | - | - | - | - | GC-MS | |
| 1145 | 1137 | Sabinol | MO | - | - | - | - | - | - | 0.08 | ± | 0.02 | - | ± | - | RI, GC-MS | - | |
| 1150 | 1145 | L-isopulegol | MO | 0.22 | ± | 0.04 | 0.28 | ± | 0.03 | 0.07 | ± | 0.01 | 0.11 | ± | 0 | RI, GC-MS | GC-MS | |
| 1154 | 1148 | Citronellal | MO | 1.38 | ± | 0.07 | 1.05 | ± | 0.04 | 5.21 | ± | 0.31 | 4.28 | ± | 0.25 | RI, GC-MS | GC-MS | |
| 1174 | 1165 | Borneol | MO | 0.09 | ± | 0.02 | - | - | - | - | - | - | - | - | - | RI, GC-MS | - | |
| 1161 | 1167 | dl-Isopulegol | MO | 0.12 | ± | 0.02 | - | - | - | - | - | - | - | - | - | RI, GC-MS | - | |
| 1184 | 1174 | L-terpinen-4-ol | MO | 9.71 | ± | 0.16 | 9.07 | ± | 0.22 | 0.36 | ± | 0.04 | 0.42 | ± | 0.02 | RI, GC-MS | GC-MS | |
| - | 1176 | m-Cymen-8-ol | MO | - | - | - | 0.09 | ± | 0 | - | - | - | - | - | - | - | GC-MS | |
| - | 1182 | Pinocarveol | MO | - | - | - | 0.03 | ± | 0.03 | - | - | - | 0.06 | ± | 0 | - | GC-MS | |
| 1199 | 1189 | α-Terpineol | MO | 3.70 | ± | 0.09 | 3.62 | ± | 0.07 | 0.98 | ± | 0.17 | 1 | ± | 0.04 | RI, GC-MS | GC-MS | |
| - | 1194 | Myrtenol | MO | - | - | - | 0.05 | ± | 0 | - | - | - | - | - | - | - | GC-MS | |
| 1217 | 1205 | (E)-Piperitol | MO | 0.02 | ± | 0.04 | - | - | - | - | - | - | - | - | - | RI, GC-MS | - | |
| 1237 | 1228 | Citronellol | MO | 0.75 | ± | 0.15 | 0.94 | ± | 0.03 | 0.43 | ± | 0.28 | 0.92 | ± | 0.04 | RI, GC-MS | GC-MS | |
| - | 1249 | Geraniol | MO | - | - | - | 0.1 | ± | 0 | - | - | - | - | - | - | - | GC-MS | |
| 1291 | 1273 | (Z)-Ascaridole glycol | O | 0.40 | ± | 0.10 | 0.69 | ± | 0.02 | - | - | - | - | - | - | RI, GC-MS | GC-MS | |
| - | 1312 | Citronellic acid | MO | - | - | - | 0.3 | ± | 0.01 | - | - | - | 0.25 | ± | 0.01 | - | GC-MS | |
| 1351 | 1345 | α-Cubebene | SH | - | - | - | - | - | - | 0.05 | ± | 0.02 | 0.07 | ± | 0 | RI, GC-MS | GC-MS | |
| 1355 | 1354 | Citronellyl acetate | MO | 0.27 | ± | 0.03 | 0.32 | ± | 0.01 | 0.33 | ± | 0.11 | 0.44 | ± | 0.03 | RI, GC-MS | GC-MS | |
| 1384 | 1365 | Neryl acetate | MO | 0.24 | ± | 0.04 | - | - | - | 0.22 | ± | 0.14 | 0.01 | ± | 0.02 | RI, GC-MS | GC-MS | |
| 1379 | 1374 | α-Copaene | SH | 0.95 | ± | 0.03 | 0.75 | ± | 0.04 | 1.43 | ± | 0.13 | 1.21 | ± | 0.12 | RI, GC-MS | GC-MS | |
| - | 1379 | Geranyl acetate | MO | - | - | - | 0.36 | ± | 0.02 | - | - | - | 0.51 | ± | 0.03 | - | GC-MS | |
| 1391 | 1390 | β-Cubebene | SH | 0.37 | ± | 0.01 | - | - | - | 1.04 | ± | 0.15 | - | ± | - | RI, GC-MS | - | |
| 1395 | 1391 | β-Elemene | SH | 0.05 | ± | 0.00 | - | - | - | - | - | - | - | - | - | RI, GC-MS | - | |
| 1424 | 1419 | β-Caryophyllene | SH | 0.72 | ± | 0.02 | 0.57 | ± | 0.01 | 1.12 | ± | 0.09 | 1.05 | ± | 0.09 | RI, GC-MS, Std | GC-MS, Std | |
| - | 1430 | β-Copaene | SH | - | - | - | 0.31 | ± | 0.01 | - | - | - | 0.96 | ± | 0.09 | - | GC-MS | |
| 1461 | 1454 | Humulene | SH | 0.23 | ± | 0.01 | 0.2 | ± | 0.01 | 0.35 | ± | 0.03 | 0.32 | ± | 0.02 | RI, GC-MS, Std | GC-MS, Std | |
| 1488 | 1484 | D-Germacrene | SH | 0.24 | ± | 0.01 | 0.07 | ± | 0 | 0.69 | ± | 0.05 | 0.49 | ± | 0.06 | RI, GC-MS | GC-MS | |
| 1502 | 1495 | Bicyclogermacrene | SH | - | - | - | - | - | - | 0.14 | ± | 0.07 | 0.13 | ± | 0.01 | RI, GC-MS | GC-MS | |
| 1505 | 1499 | α-Muurolene | SH | 0.10 | ± | 0.01 | 0.05 | ± | 0.02 | 0.1 | ± | 0.05 | 0.09 | ± | 0.01 | RI, GC-MS | GC-MS | |
| - | 1514 | Cubebol | SO | - | - | - | - | - | - | - | - | - | 0.24 | ± | 0.02 | - | GC-MS | |
| 1528 | 1524 | β-Cadinene | SH | 1.49 | ± | 0.04 | 1.06 | ± | 0.1 | 1.75 | ± | 0.11 | 1.57 | ± | 0.07 | RI, GC-MS | GC-MS | |
| - | 1528 | Calamenene | SH | - | - | - | 0.01 | ± | 0.01 | - | - | - | - | - | - | - | GC-MS | |
| - | 1548 | Elemol | SO | - | - | - | 0.09 | ± | 0.02 | - | - | - | 0.01 | ± | 0.01 | - | GC-MS | |
| 1588 | 1574 | Germacrene D-4-ol | SO | - | - | - | - | - | - | 0.07 | ± | 0.01 | 0.17 | ± | 0.01 | RI, GC-MS | GC-MS | |
| - | 1577 | Spathulenol | SO | - | - | - | 0.09 | ± | 0 | - | - | - | 0.09 | ± | 0.01 | - | GC-MS | |
| - | 1582 | Caryophyllene oxide | SO | - | - | - | - | - | - | - | - | - | 0.05 | ± | 0 | - | GC-MS | |
| - | 1608 | Humulene epoxide | SO | - | - | - | 0.01 | ± | 0.01 | - | - | - | - | - | - | - | GC-MS | |
| - | 1619 | Humulane-16-dien-3-ol | SO | - | - | - | - | - | - | - | - | - | 0.04 | ± | 0 | - | GC-MS | |
| 1641 | 1627 | Epicubenol | SO | 0.08 | ± | 0.01 | 0.08 | ± | 0.02 | - | - | - | - | - | - | RI, GC-MS | GC-MS | |
| 1647 | 1631 | γ-Eudesmol | SO | 0.26 | ± | 0.01 | 0.41 | ± | 0.03 | - | - | - | - | - | - | RI, GC-MS | GC-MS | |
| - | 1645 | Cubenol | SO | - | - | - | 0.07 | ± | 0.02 | - | - | - | - | - | - | - | GC-MS | |
| 1656 | 1645 | δ-Cadinol | SO | 0.10 | ± | 0.00 | 0 | ± | 0.01 | - | - | - | 0.02 | ± | 0.02 | RI, GC-MS | GC-MS | |
| - | 1649 | β-Selinenol | SO | - | - | - | 0.18 | ± | 0.02 | - | - | - | 0.04 | ± | 0 | - | GC-MS | |
| 1671 | 1652 | α-Eudesmol | SO | 0.41 | ± | 0.01 | 0.06 | ± | 0.01 | - | - | - | - | - | - | RI, GC-MS | GC-MS | |
| - | 1656 | Patchouli alcohol | SO | - | - | - | - | - | - | - | - | - | 0.04 | ± | 0.01 | - | GC-MS | |
| - | 1942 | Phytol | DO | - | - | - | - | - | - | - | - | - | 0.06 | ± | 0 | - | GC-MS | |
| - | 1984 | Palmitic acid | FAD | - | - | - | - | - | - | - | - | - | 0.62 | ± | 0.01 | - | GC-MS | |
| - | 2132 | Linoleic acid | FAD | - | - | - | - | - | - | - | - | - | 0.28 | ± | 0.03 | - | GC-MS | |
| 2521 | 2501 | Oxypeucedanin | O | - | - | - | - | - | - | 2.96 | ± | 0.64 | - | ± | - | RI, GC-MS | - | |
| 2707 | 2707 | β-Monolinolein | E | - | - | - | - | - | - | 0.18 | ± | 0.01 | - | ± | - | RI, GC-MS | - | |
| 1562 | NA | Hedycaryol | SO | - | - | - | - | - | - | 0.17 | ± | 0.01 | 0.17 | ± | 0.02 | GC-MS | GC-MS | |
| 2009 | NA | Thunbergol | DO | - | - | - | - | - | - | 1.79 | ± | 0.12 | 1.4 | ± | 0 | GC-MS | GC-MS | |
| 2010 | NA | trans-Geranylgeraniol | DO | 0.79 | ± | 0.02 | 0.64 | ± | 0.01 | 0.31 | ± | 0.03 | 0.19 | ± | 0 | GC-MS | GC-MS | |
| - | NA | 2-p-Menthen-1-ol | MO | - | - | - | 0.15 | ± | 0.01 | - | - | - | - | - | - | - | GC-MS | |
| - | NA | tau.-Muurolol | SO | - | - | - | 0.05 | ± | 0.01 | - | - | - | - | - | - | - | GC-MS | |
| - | NA | Tetradecanoic acid | FAD | - | - | - | - | - | - | - | - | - | 0.16 | ± | 0.01 | - | GC-MS | |
| - | NA | 17-Octadecynoic acid | FAD | - | - | - | - | - | - | - | - | - | 0.12 | ± | 0.08 | - | GC-MS | |
| - | NA | Ricinoleic acid | FAD | - | - | - | - | - | - | - | - | - | 0.06 | ± | 0.01 | - | GC-MS | |
| Total identified [%] | 98.69 | ± | 0.22 | 98 | ± | 0.39 | 99.36 | ± | 0.07 | 98.06 | ± | 0.32 | ||||||
| RIa | Compoundb | Cc | Extraction type/Column type/Peak area [%] | Identificationd | ||||||||||||||
| Essential oil | CO2 extract | |||||||||||||||||
| Obs. | Lit. | HP-5 MS | DB-Wax | HP-5 MS | DB-Wax | HP-5 MS | DB-Wax | |||||||||||
| 923 | 924 | α-Thujene | MH | 0.059 | ± | 0 | - | - | - | - | - | - | - | - | - | RI, GC-MS | - | |
| 929 | 937 | α-Pinene | MH | 2.806 | ± | 0.25 | 2.568 | ± | 0.09 | 0.649 | ± | 0.02 | 0.574 | ± | 0.04 | RI, GC-MS, Std | GC-MS, Std | |
| 944 | 946 | Camphene | MH | 0.04 | ± | 0.01 | 0.05 | ± | 0 | - | - | - | - | - | - | RI, GC-MS, Std | GC-MS, Std | |
| 970 | 976 | Sabinene | MH | 0.048 | ± | 0.03 | 0.091 | ± | 0 | - | - | - | - | - | - | RI, GC-MS | GC-MS | |
| 973 | 980 | β-Pinene | MH | 5.424 | ± | 0.45 | 5.322 | ± | 0.14 | 2.039 | ± | 0.04 | 1.996 | ± | 0.14 | RI, GC-MS, Std | GC-MS, Std | |
| 989 | 991 | β-Myrcene | MH | 1.477 | ± | 0.14 | 1.682 | ± | 0.12 | 0.681 | ± | 0.05 | - | - | - | RI, GC-MS | GC-MS | |
| - | 1001 | 2-Carene | MH | - | - | - | - | - | - | - | - | - | 0.076 | ± | 0.02 | - | GC-MS | |
| 1003 | 1005 | α-Phellandrene | MH | 1.803 | ± | 0.14 | 1.481 | ± | 0.08 | 0.762 | ± | 0.03 | 0.681 | ± | 0.03 | RI, GC-MS, Std | GC-MS, Std | |
| 1008 | 1008 | 3-Carene | MH | 18.72 | ± | 1.46 | 18.49 | ± | 0.42 | 7.395 | ± | 0.17 | 7.181 | ± | 0.4 | RI, GC-MS | GC-MS | |
| 1025 | 1022 | o-Cymene | MH | 1.399 | ± | 0.12 | 1.495 | ± | 0.04 | 0.636 | ± | 0.01 | 0.771 | ± | 0.05 | RI, GC-MS | GC-MS | |
| 1028 | 1031 | D-Limonene | MH | 11.18 | ± | 0.79 | 10.93 | ± | 0.15 | 6.265 | ± | 0.11 | 6.034 | ± | 0.39 | RI, GC-MS | GC-MS | |
| 1058 | 1062 | γ-Terpinene | MH | 0.056 | ± | 0.01 | 0.045 | ± | 0 | - | - | - | - | - | - | RI, GC-MS | GC-MS | |
| 1084 | 1086 | Isoterpinolene | MH | 0.194 | ± | 0.04 | 0.4 | ± | 0.02 | 0.09 | ± | 0 | - | - | - | RI, GC-MS | GC-MS | |
| 1087 | 1086 | Terpinolene | MH | 0.428 | ± | 0.08 | 0.169 | ± | 0 | 0.156 | ± | 0.01 | 0.191 | ± | 0.01 | RI, GC-MS | GC-MS | |
| 1104 | 1095 | Linalool | MO | 0.354 | ± | 0.03 | 0.453 | ± | 0 | 0.238 | ± | 0.05 | 0.386 | ± | 0.01 | RI, GC-MS, Std | GC-MS, Std | |
| - | 1140 | Verbenol | MO | - | - | - | 0.185 | ± | 0.02 | - | - | - | - | - | - | - | GC-MS | |
| - | 1179 | p-Cymen-8-ol | MO | - | - | - | 0.05 | ± | 0.05 | - | - | - | - | - | - | - | GC-MS | |
| - | 1318 | 2,3-Pinanediol | MO | - | - | - | 0.254 | ± | 0.01 | - | - | - | - | - | - | - | GC-MS | |
| - | 1329 | Piperonal | O | - | - | - | - | - | - | - | - | - | 0.04 | ± | 0.01 | - | GC-MS | |
| 1339 | 1339 | δ-EIemene | SH | 0.559 | ± | 0.02 | 0.588 | ± | 0.01 | 0.491 | ± | 0.01 | 0.516 | ± | 0.01 | RI, GC-MS | GC-MS | |
| - | 1340 | Piperitenone | MO | - | - | - | 0.063 | ± | 0.05 | - | - | - | - | - | - | - | GC-MS | |
| 1351 | 1351 | α-Cubebene | SH | 0.097 | ± | 0 | 0.086 | ± | 0.02 | 0.144 | ± | 0.01 | 0.117 | ± | 0.01 | RI, GC-MS | GC-MS | |
| - | 1357 | Octadecanal | A | - | - | - | 0.57 | ± | 0.1 | - | - | - | - | - | - | - | GC-MS | |
| 1378 | 1374 | α-Copaene | SH | 0.194 | ± | 0.01 | 0.17 | ± | 0.01 | 0.275 | ± | 0.01 | 0.247 | ± | 0.02 | RI, GC-MS | GC-MS | |
| 1394 | 1391 | β-Elemene | SH | 1.483 | ± | 0.08 | - | - | - | 1.887 | ± | 0.03 | 1.303 | ± | 0.03 | RI, GC-MS | - | |
| 1410 | 1409 | α-Gurjunene | SH | 0.164 | ± | 0.01 | 0.131 | ± | 0 | 0.257 | ± | 0.01 | 0.239 | ± | 0.03 | RI, GC-MS | GC-MS | |
| 1416 | 1411 | α-Bergamotene | SH | 0.093 | ± | 0 | 0.01 | ± | 0.01 | 0.154 | ± | 0.01 | 0.018 | ± | 0 | RI, GC-MS | GC-MS | |
| 1425 | 1419 | β-Caryophyllene | SH | 37.84 | ± | 2.05 | 39.55 | ± | 1.12 | 54.21 | ± | 0.85 | 55.86 | ± | 1.37 | RI, GC-MS, Std | GC-MS, Std | |
| - | 1434 | γ-Elemene | SH | - | - | - | 0.057 | ± | 0 | - | - | - | 0.12 | ± | 0.01 | - | GC-MS, | |
| 1440 | 1437 | α-Guaiene | SH | 0.983 | ± | 0.07 | - | - | - | 1.363 | ± | 0.02 | - | - | - | RI, GC-MS | - | |
| 1456 | 1454 | β-Farnesene | SH | 0.101 | ± | 0.03 | 0.058 | ± | 0.05 | 0.143 | ± | 0 | 0.167 | ± | 0 | RI, GC-MS | GC-MS | |
| 1459 | 1454 | Humulene | SH | 2.572 | ± | 0.22 | 2.52 | ± | 0.07 | 3.7 | ± | 0.02 | 3.465 | ± | 0.08 | RI, GC-MS, Std | GC-MS, Std | |
| - | 1475 | γ-Gurjunene | SH | - | - | - | 0.9 | ± | 0.03 | - | - | - | - | - | - | - | GC-MS | |
| 1493 | 1485 | β-Selinene | SH | 3.653 | ± | 0.33 | 3.358 | ± | 0.15 | 5.242 | ± | 0.14 | 4.757 | ± | 0.11 | RI, GC-MS | GC-MS | |
| 1486 | 1492 | Valencene | SH | 0.136 | ± | 0.01 | - | - | - | 0.224 | ± | 0.01 | - | - | - | RI, GC-MS | - | |
| 1501 | 1494 | α-Selinene | SH | 2.409 | ± | 0.23 | 1.972 | ± | 0.29 | 3.493 | ± | 0.08 | 3.009 | ± | 0.09 | RI, GC-MS | GC-MS | |
| 1510 | 1506 | β-Bisabolene | SH | 1.131 | ± | 0.1 | 0.887 | ± | 0.1 | 1.711 | ± | 0.06 | 1.284 | ± | 0.05 | RI, GC-MS | GC-MS | |
| 1524 | 1520 | 7-epi-α-Selinene | SH | 0.114 | ± | 0.01 | - | - | - | 0.168 | ± | 0.03 | - | - | - | RI, GC-MS | - | |
| - | 1528 | Calamenene | SH | - | - | - | - | - | - | - | - | - | 0.013 | ± | 0 | - | GC-MS | |
| 1533 | 1529 | γ-Bisabolene | SH | 0.069 | ± | 0.01 | - | - | - | - | - | - | - | - | - | RI, GC-MS | - | |
| - | 1561 | Nerolidol | SO | - | - | - | - | - | - | - | - | - | 0.076 | ± | 0 | - | GC-MS | |
| - | 1577 | Spathulenol | SO | - | - | - | 0.184 | ± | 0 | - | - | - | 0.118 | ± | 0.09 | - | GC-MS | |
| - | 1579 | Isoaromadendrene epoxide | SO | - | - | - | 0.086 | ± | 0 | - | - | - | - | - | - | - | GC-MS | |
| 1593 | 1582 | Caryophylene oxide | SO | 2.941 | ± | 0.24 | 3.295 | ± | 0.26 | 2.036 | ± | 1.01 | 3.013 | ± | 0.2 | RI, GC-MS | GC-MS | |
| 1621 | 1608 | Humulene epoxide II | SO | 0.154 | ± | 0.02 | 0.158 | ± | 0 | 0.128 | ± | 0.01 | 0.139 | ± | 0 | RI, GC-MS | GC-MS | |
| 1643 | 1638 | Isospathulenol | SO | 0.492 | ± | 0.05 | 0.421 | ± | 0.32 | 0.463 | ± | 0.02 | 0.526 | ± | 0.08 | RI, GC-MS | GC-MS | |
| 1668 | 1651 | Pogostole | SO | 0.154 | ± | 0.02 | 0.215 | ± | 0.1 | - | - | - | 0.09 | ± | 0 | - | GC-MS | |
| - | 1658 | Neointermedeol | SO | - | - | - | 0.054 | ± | 0.01 | - | - | - | - | - | - | - | GC-MS | |
| 1675 | 1665 | Intermedeol | SO | 0.05 | ± | 0.02 | 0.07 | ± | 0.01 | - | - | - | - | - | - | RI, GC-MS | GC-MS | |
| 1950 | 1938 | Pellitorine | O | - | - | - | 0.006 | ± | 0.01 | 1.191 | ± | 0.09 | 1.669 | ± | 0.06 | RI, GC-MS | GC-MS | |
| - | 1953 | Hexadec-9-enoic acid | FAD | - | - | - | - | - | - | - | - | - | 0.103 | ± | 0 | - | GC-MS | |
| - | 1959 | Palmitic acid | FAD | - | - | - | - | - | - | - | - | - | 0.423 | ± | 0.04 | - | GC-MS | |
| - | 2141 | Oleic Acid | FAD | - | - | - | - | - | - | - | - | - | 0.267 | ± | 0.03 | - | GC-MS | |
| 2707 | 2707 | β-Monolinolein | E | - | - | - | - | - | - | 1.84 | ± | 0.98 | - | - | - | RI, GC-MS | - | |
| 2018 | NA | Heptadec-14-enal | A | - | - | - | - | - | - | 0.122 | ± | 0.04 | - | - | - | RI, GC-MS | - | |
| 2815 | NA | β-Monoolein | E | - | - | - | - | - | - | 0.503 | ± | 0.6 | 0.52 | ± | 0.06 | RI, GC-MS | GC-MS | |
| - | NA | Hexadec-9-en-1-ol | O | - | - | - | - | - | - | - | - | - | 0.238 | ± | 0.01 | - | GC-MS | |
| - | NA | Octadec-17-ynoic acid | FAD | - | - | - | - | - | - | - | - | - | 0.025 | ± | 0.02 | - | GC-MS | |
| - | NA | Tetradec-9-enal | A | - | - | - | - | - | - | - | - | - | 0.073 | ± | 0.01 | - | GC-MS | |
| - | NA | Kalecide | O | - | - | - | - | - | - | - | - | - | 0.181 | ± | 0 | - | GC-MS | |
| - | NA | Ricinoleic acid | FAD | - | - | - | - | - | - | - | - | - | 0.231 | ± | 0.01 | - | GC-MS | |
| - | NA | Sabinol | SO | - | - | - | 0.072 | ± | 0.01 | - | - | - | - | - | - | - | GC-MS | |
| Total identified [%] | 99.4 | ± | 0.2 | 99.2 | ± | 0.1 | 98.7 | ± | 0.7 | 96.7 | ± | 0.1 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





