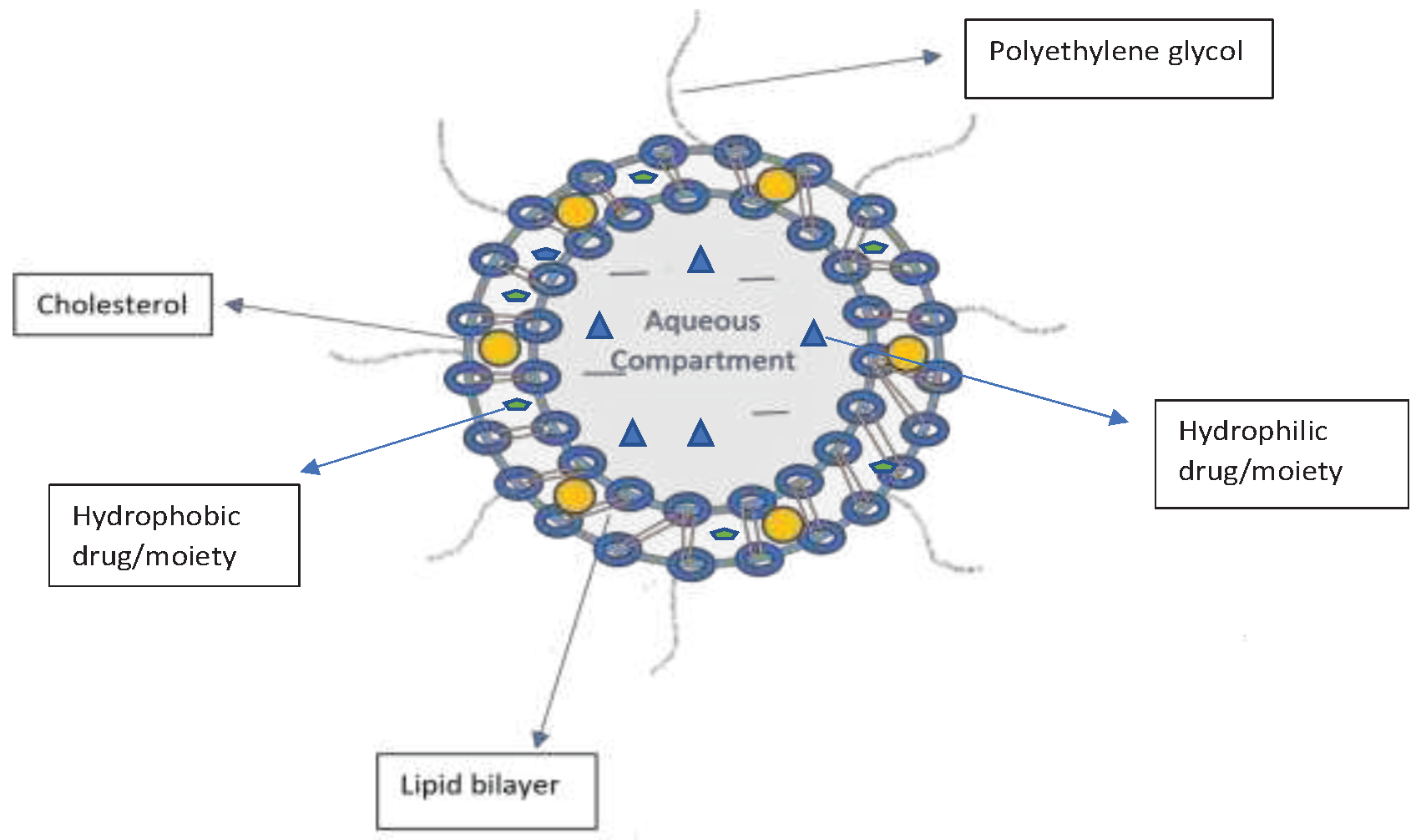
Introduction to drug delivery through Liposomes
Among the different types of nanoparticles for drug delivery, liposomes are the most developed and established clinically available drug delivery systems available clinically (Hu et al., 2010). A typical liposome structure is depicted in a review of dual-functional drug liposomes for the treatment of drug-resistant cancers (
Figure 1) (Pande S., 2023).
Liposomes are spherical vesicles comprising lipid bilayer shells surrounding aqueous interior cores that are spontaneously formed when amphiphilic lipids are dispersed in water (Ta et al., 2013). Moreover, they are non-toxic, biocompatible, and biodegradable and have been approved by the Food and Drug Administration (FDA) for the delivery of various anticancer agents (Affram et al., 2015; Nogueira et al., 2015). Among various drug delivery systems, liposomes represent versatile and advanced nanodelivery systems for a wide range of biologically active compounds (Xu et al., 2014). PEGylated liposomes are effective drug delivery vehicles because they facilitate high drug-loading capabilities, improved biocompatibility, and long-circulating properties, with improved stability (Ashley et al., 2016; Calvagno et al., 2007). Various potent drugs have been incorporated into liposomes with remarkable clinical success (Ashley et al., 2016; Chang et al., 2012). Some FDA-approved liposome formulations include Ambisome (amphotericin B), Dioxil (doxorubicin), and Marquibo (vincristine), thereby emphasizing the advantages of using liposomes as drug delivery systems (Zylberberg et al., 2016).
The entrapment of anticancer agents and delivery through liposomes is a promising strategy that has gained tremendous potential for the treatment of cancers (Pande S., 2023). Numerous studies have been conducted on the ability of liposomes to be loaded with single or multiple drugs (Hu et al., 2010) especially for the treatment of cancer. A study describing the formulation of gemcitabine-loaded thermosensitive liposomes for antitumor activity explained the feasibility of loading and improving the release of gemcitabine into tumor cells and emphasized the potential of liposomes as drug delivery vehicles (Affram et al., 2015). Paclitaxel, a highly potent anticancer drug for breast and ovarian cancers, was delivered through liposomes to increase the total drug content in a stable formulation (Kan et al., 2011). Doxorubicin (DOX), a widely used anticancer drug for prostate cancer is known for its severe side effects including tissue cytotoxicity and cardiotoxicity was formulated into a thermosensitive liposomal formulation (Eleftheriou et al., 2020). The results showed that there was stable and controlled drug release from thermo-responsive liposomes with enhanced cell uptake owing to formulation modification and suitable release conditions (Eleftheriou et al., 2020). Thus, liposomes with their successful and versatile abilities need to be explored as delivery systems for single and dual drug-loaded formulations.
Literature review of factors for optimization using statistical experimental design
Several studies have described the specific properties and applications of drug-loaded liposomes by controlling and modifying process parameters, methods of formulation, varying drug, and lipid contents, changing the volume of the aqueous phase during formulation, and modifying liposome surface for active or passive drug loading mechanisms (Lila et al., 2017; Zylberberg et al., 2016). These studies also explain the outcomes of formulation modifications that typically improve the pharmacokinetics and therapeutic potential of drug-loaded liposomes. The entire process of conducting trials or experiments with predicted variations to achieve the desired outcome is known as optimization of the formulation. The design of the experiment is an important and efficient step in identifying the important factors that affect the outcome variable (Zoghi et al., 2016). A statistical experimental design allows the use of different statistical models to obtain the most competent results that require minimum experimental trials (Zoghi et al., 2016). To establish significance, it is necessary to explain the impact of each factor on the outcome variable. Therefore, an overview of these factors and their impact on drug loading and release, along with other response variables, is provided in this review.
Optimization with phospholipid to cholesterol ratio
The composition of lipids in liposome formulations has been well characterized and documented. Phospholipids and cholesterol generally constitute the lipid compartments of liposomal structures (Pamunuwa et al., 2016). The phospholipid:cholesterol ratio affects various properties of liposomes, including size, zeta potential, stability, drug loading, and drug release (Pamunuwa et al., 2016; Miao et al., 2015). Among the various phospholipids, phosphatidylcholine and phosphatidylethanolamine are most used in liposome preparation (Yingchoncharoen et al., 2016). Cholesterol, which is also an important component of the lipid compartment, confers rigidity to the lipid bilayer, reduces the permeability of water-soluble molecules through the liposomal membrane, and imparts stability to the liposome (Yingchoncharoen et al., 2016; Akbarzadeh et al., 2013). High drug-loading capacity and sustained drug release from liposomes are desirable and beneficial for the development of drug-loaded liposomes for clinical applications (Pereira et al., 2016). Several studies have reported the effect of optimizing phospholipid:cholesterol to improve (%) drug loading and stable drug release properties.
A study on docetaxel-loaded liposomes with respect to the effect of lipid composition and purification on drug encapsulation was performed (Pereira et al., 2016). Specifically, this study focused on the effect of varying the lipid composition on the drug loading and physicochemical properties of docetaxel-loaded liposomes (Pereira et al., 2016). Liposomes were prepared using a thin-film hydration method, followed by extrusion and size-exclusion chromatography to remove the free unencapsulated drug. Liposomes were prepared with different phospholipid and cholesterol compositions and variable drug-to-lipid ratios. The results showed that with increasing lipid content, the drug loading and encapsulation efficiency obtained was around 95%. When the lipid content was low and the drug content was high, there was a decrease in the drug loading and encapsulation of approximately 40%. The effect of lipid composition on drug-trapping efficiency and vesicle stability has been studied in dexamethasone-incorporated liposomes (Tsotas et al., 2007). Liposomes were formulated with different amounts of phosphatidylcholine (PC) and distearoyloglycero-PC (DSPC), along with two different cholesterol:lipid ratios (2:1 and 1:1). The results showed that DSPC+Cholesterol liposomes with high cholesterol content had a stable displacement of dexamethasone compared to PC+Cholesterol liposomes. The combination of PC and DSPC liposomes with cholesterol caused steady release of dexamethasone over 48 h. Based on the results obtained in this study, lipid composition had a significant effect on drug incorporation efficiency. Additionally, the study also revealed that release kinetics of drugs can be modified by varying and optimizing lipid composition. The Co-encapsulation of quercetin and resveratrol into elastic liposomes was achieved by optimizing the drug-loaded formulation (Cadena et al., 2013). Dual-drug-loaded liposomes were prepared using the thin lipid film method. The experimental design of this study consisted of two parts. The first part included a two-level fractional factorial design to evaluate the effects of phospholipid and cholesterol concentrations and drug inclusion complexes on the size, polydispersity index, zeta potential, and (%) drug encapsulation efficiency of liposomes. The second part of the experimental design was a two-level full factorial design to study the effects of drug concentration and 1:1 co-encapsulation of quercetin and resveratrol on the same outcome variables as the first part. The results of the optimization studies showed that (%) encapsulation efficiency of 97% was achieved in the optimized formulation with a slightly negative zeta potential (-13.3 mV) and particle size of 149 nm with a polydispersity index of 0.3. Liposomes loaded with the antiviral agent nevirapine have been developed using three different lipid components: PLPC, POPE, and cholesterol (Ramana et al., 2010). Liposomes were prepared using a thin-film hydration technique followed by extrusion and freeze-drying. Drug loading was performed using different ratios of drug to phospholipid. A phospholipid:cholesterol ratio of 9:1 showed maximum drug encapsulation and was influenced by the presence of cholesterol in the formulation. High cholesterol levels resulted in low drug loading and encapsulation. Lornoxicam-loaded liposomes were prepared and optimized using a central composite design (Joseph et al., 2018). Drug-loaded liposomes were prepared using the thin-film hydration method with pH-induced vesiculation. Optimization was performed using a central composite design with phospholipid and cholesterol contents as the two independent variables. The dependent variables in this study were the drug entrapment efficiency and in vitro drug release. A polynomial equation was used to relate the effects of the independent variables on the outcome of this study. The results showed that the maximum entrapment of lornoxicam was 98% at 45% cholesterol and 80% phospholipid contents. The optimized formulation showed steady drug release for 8h with a particle size of 156 nm. Hence, variation in the phospholipid:cholesterol ratio is an important factor in liposome optimization studies.
Some studies have demonstrated the use of different drug loading or encapsulation techniques with different lipid compositions to assess the formulation with the best lipid composition and loading conditions. Carboplatin was loaded into preformed liposomes with different lipid compositions using a passive equilibration method (Wehbe et al., 2017). This method is applicable to liposomes prepared with high (45 mol%) or low (<20 mol%) cholesterol levels. The main goal of this study was to assess the role of ethanol in stable liposome formation and the effect of cholesterol content on ethanol-induced destabilization of liposomes. The lipid compositions used to formulate liposomes in this study were DSPC:Chol (55:45 mol ratio), DSPC:DSPG:Chol (70:20:10 mol ratio), DSPC:DSPE-PEG2000 (95:5 mol ratio), and DSPC:Chol:DSPE-PEG2000 (65:30:5 mol ratio). All liposomes were prepared using the thin-film hydration and extrusion method. The loading efficiency of carboplatin was the highest in DSPC:DSPG:Chol (70:20:10 mol ratio) compared to other combinations in the presence of ethanol as an encapsulation enhancer. Quercetin-loaded liposomes were optimized with respect to variations in lipid composition to evaluate the in vitro cytotoxic effects of quercetin (Saraswat et al., 2020). Liposomes were prepared using a thin-film hydration method, followed by sonication. Three combinations of lipids were used to prepare the liposomes. Liposomes containing 3% PEG had a phosphatidylcholine: cholesterol (67:30), liposomes with 5% PEG had a phosphatidylcholine: cholesterol (65:30) and liposomes with 7% PEG had a phosphatidylcholine: cholesterol (63:30). Results showed that Among the three combinations of formulations, the highest drug encapsulation of 90% was observed in the formulation with 3% PEG and Phosphatidylcholine:Cholesterol (67:30). Thus, variations in the phospholipid:cholesterol ratio contributed to the improved drug loading and release characteristics. Therefore, optimization of the lipid ratio is essential for achieving improved drug-loading and release properties.
Effect of volume of aqueous phase on drug loading and release characteristics
In the preparation of liposomes, the aqueous phase usually consists of buffers of varying pH depending on the requirement and method of preparation. Some studies that indicated the importance of optimizing the volume of the aqueous phase for drug loading and release properties are explained below. The effect of phosphate buffer (pH 7.4) on in vitro drug release from pilocarpine nitrate-loaded liposomes was evaluated (Rathod et al., 2010). Drug-loaded liposomes hydrated using phosphate buffer (pH 7.4) showed prolonged release for over 8 h. Additionally, optimization was performed for the volume of buffer (5 ml) used during hydration. Large volumes of buffer result in poor drug loading and subsequently affect release properties (Rathod et al., 2010; Muppidi et al., 2012). The ketorolac-loaded liposomes were optimized by considering the molar ratio of phospholipid:cholesterol, pH value of the hydration medium, volume of the aqueous hydration phase, and concentration of surfactant used (Mehanna et al., 2017). Liposomes were prepared using a thin-film hydration method and their entrapment efficiency was evaluated. The results showed that among the tested formulations, the highest (%) entrapment efficiency was observed in the formulation with a hydration volume of 2.5 mL and pH of 4.2 at 50% cholesterol concentration. Thus, optimization of the aqueous phase volume could be an important factor in improving the drug release and loading properties of liposomes.
Optimization with Lipid type and drug to lipid ratio
The drug:lipid ratio is an important characteristic in the formulation of liposomes that expresses the capacity of liposomes to accommodate the drug and can thereby play a key role in the optimization process of liposome formulation (Chountoulesi et al., 2018). A novel study explaining the production of methotrexate (MTX) loaded liposomes by double flow focusing microfluidic device focused on optimizing encapsulation efficiency, drug loading and stability parameters of the formulated liposomes (Aghaei et al., 2021). The formulation optimization was achieved by adjusting the operational and formulation parameters flow rate ratio (FRR), total flow rate (TFR), total lipid concentration and MTX concentration. Similar studies describing the variations in the drug:lipid ratio and their effects on drug loading and release characteristics are discussed below.
Clodronate, an active bisphosphonate compound used in the treatment of osteoporosis and several cancers, was loaded into liposomes using an optimized lipid to drug ratio (4:1) that gave maximum drug loading compared to the other ratios used in the study (Ailiesei et al., 2016).
A study describing the formulation of primaquine- and chloroquine-loaded liposomes was performed using varying amounts of hydrogenated soy phosphatidylcholine (hspc), cholesterol and DSPE-PEG2000 (Miatmoko et al., 2019). Primaquine and chloroquine were the antimalarial drugs of choice. Liposomes were prepared using the thin-film hydration and extrusion method. Drug loading was determined using the transmembrane gradient method. In vitro drug release experiments were performed using dialysis. The results showed that the optimal drug-to-lipid ratios for loading primaquine and chloroquine were 1:10 and 1:3, respectively. Drug release data for dual-drug-loaded liposomes showed steady drug release of 63% for primaquine and 44% for chloroquine at 48 h. Another study describing the formulation of liposomal vincristine was performed by varying the drug-to-lipid ratio to optimize the rate of drug release (Johnston et al., 2006). Vincristine was loaded into the liposomes using the ionophore-loading technique. Results showed that the formulation with slowest rate of drug release at 24hr consisted of lipid:cholesterol (55:45 mol ratio) had a t1/2 of 15.6h in vivo. The study also stated that this formulation has undergone advanced clinical trials for the treatment of non-hodgkins, which shows the potential of considering phospholipid:cholesterol and drug:lipid ratios as factors for optimization.
Some studies have optimized liposome formulations using different types of lipids, varying amounts of cholesterol, and polyethylene glycol, along with different drug-to-lipid ratios. Topotecan-loaded PEGylated liposomes were prepared and characterized according to the thin-film hydration-extrusion method, and optimized using a factorial design (Vali et al., 2008). This study used a fractional factorial experimental design. The independent variables included the type of lipid, molar ratio of phosphatidylglycerol to the main lipid, mole percentage of DSPE-PEG2000 and drug to lipid molar ratio. The results showed that the entrapment of hydrophilic drugs prepared by the thin-film hydration method is affected by lipid composition, the percentage of each lipid, and the drug-to-lipid molar ratio. Additionally, PEGylated liposomes showed prolonged drug release for over 48h. The study concluded by describing the role of the type of lipid, amount of DSPG, drug-to-lipid molar ratio, and interactions between these factors for improved drug encapsulation. Berberine-containing liposomes were optimized using a 32 full factorial design and evaluated for in vitro drug release (Sailor et al., 2015). Liposomes were prepared by thin-film hydration and were optimized using a full factorial design. The independent variables for this study were the drug-to-lipid and (SPC) ratios. The dependent variables were liposome size (nm) and EE (%). According to this study, a three-level two-factor design was effective in achieving the desired outcomes with a limited number of experiments. The results of the study were visually observed using a response surface (3D) and contour plots. Among all the prepared formulations, the formulation with drug:lipid (1:9.56) and SPC:Cholesterol (50:50) provided the optimum values for vesicle size and (%) entrapment efficiency. Finally, sustained in vitro drug release (24 h) was observed for the selected formulation.
Optimization using response surface methodology was performed to develop folic acid-conjugated liposomes for the delivery of 5-Fluorouracil in the treatment of colon cancer (Handali et al., 2018). Liposomes were prepared using a thin-film hydration technique followed by size reduction using sonication to evaluate the encapsulation efficiency. A central composite design was used to evaluate the influence of the amounts of phospholipids (DPPC) and 5-FU on the encapsulation efficiency and particle size of the liposomes. The optimized formulation had an encapsulation efficiency of 39.71% and particle size of 174 nm. Liposomes co-encapsulated with cabazitaxel and silibinin for targeted delivery to CD44 receptors have been optimized to improving (%) drug loading (Mahira et al., 2019). Dual drug-loaded liposomes were prepared using the ethanol injection method and were characterized for particle size, entrapment efficiency, and cytotoxicity against prostate cancer cells. The independent variables were lipid weight, phase volume ratio, and concentration of hyaluronic acid (HA). Drug loading (%) was the dependent variable. The results showed that the optimized formulation had a particle size of < 100 nm with > 90% entrapment efficiency at 10% w/w drug loading. The influence of liposomal lipid composition on vesicle size, zeta potential and liposome induced dendritic cell maturation was evaluated using design of experiment approach in peptide-containing liposomes (Soema et al., 2015). This study used four lipid types to assess the effect of lipid composition on the physicochemical properties of liposomes. A linear mixture model was used as a part of the statistical experimental design of this study. The values for every parameter were set, and a D-optimal design containing 18 runs and one central point was predicted to evaluate size, zeta potential, and dendritic cell maturation. The optimized formulation had a size of 181.1±8.7 nm, polydispersity index of 0.12±0.01 and zeta potential 30.3±6.2. Thus, the experimental design used in this study helped predict the optimized formulation with respect to the outcome variable. Finally, a besifloxacin hydrochloride-loaded liposomal gel was prepared and optimized using a 32 full factorial design (Bhattacharjee et al., 2019). Drug-loaded liposomes were prepared using a thin-film hydration technique and optimized with two independent variables: soy lecithin-to-cholesterol ratio and lipid-to-drug ratio. The outcome variables were entrapment efficiency, drug loading, and particle size. As seen earlier, a quadratic polynomial equation helps predict the relationship between independent and outcome variables. The optimized formulation had particle size of 436.8±23.4 nm; (%) drug loading of 10.84±0.46% and encapsulation efficiency of 41.01±1.22%. Thus, the use of statistical experimental designs for the optimization of liposome formulations is useful for the development of stable and effective formulations for transition into clinical practice.
Studies with optimization of process parameters for improved drug release, loading and entrapment efficiencies
Some formulation studies have used process parameters/steps for liposome optimization. The preparation and optimization of quercetin-loaded liposomes evaluated the effect of the temperature of the water bath and rotation speed of the rotary evaporator on (%) encapsulation efficiency (%), drug release, and mean particle size (Jangde et al., 2016). Liposomes were prepared using a thin-film hydration method and optimized using response surface methodology. Optimization results showed that a rotational speed of 75 rpm and a water bath temperature of 46°C yielded the best particle size (146 nm), (%) encapsulation efficiency (86.5%), and in vitro drug release of 75.09% at 24 h. Similarly, the preparation of liposomes containing hydroxytyrosol (HT) for evaluation of antioxidant activity was optimized using response surface methodology (Yuan et al., 2017). The factors used for optimization were temperature, phospholipid:cholesterol ratio, Tween 80 volume, and HT mass. Liposomes were prepared using the film dispersion method. This study describes the stability problems associated with hydroxytyrosol and the need to improve its encapsulation efficiency in liposomes. Preliminary data from this study showed that among the four factors, Tween 80 volume had no effect on the encapsulation efficiency of HT. Hence, the remaining three factors were used to optimize the formulation. The results showed that the formulation with phospholipid:cholesterol ratio of 4.5:1, HT mass of 5 mg, and water bath temperature of 63°C had the highest EE (%). Moreover, HT liposomes had better stability and sustained in vitro release than free HT. Liposomes containing madecassoside were prepared and optimized using the response surface methodology to evaluate in vitro dermal permeation (Li et al., 2016). The liposomes were prepared using a two-step emulsification procedure. The factors used for optimization were the concentration of madecassoside (mg/mL), the ratio of egg yolk lecithin to cholesterol (w/w), and the stirring speed (rpm). The statistical design predicted 15 experimental runs that were part of the central composite design. A second-order polynomial equation was used to describe the effect of each factor on the outcome variable. The results showed that among the three factors, the ratio of egg yolk lecithin to cholesterol and the concentration of madecassoside were significant in achieving high drug-loading efficiency and sustained release rate of the drug. Doxycycline-, albendazole-, and diethylcarbamazepine-loaded solid lipid nanoparticles were prepared and optimized to evaluate the effects of independent variables on the size, polydispersity index, zeta potential, and encapsulation efficiency (Permana et al., 2019). Dox-loaded liposomes were prepared using a hot emulsification-ultrasonication method. Albendazole- and diethylcarbamazepine-loaded liposomes were prepared using a double emulsion technique. Different lipids, stabilizers, and surfactants have been screened for liposome preparation. The particle size and encapsulation efficiency of the liposomes were measured. Based on the results obtained from the screening studies, glycerol monostearate and Tween 80 were used in the optimization process with a central composite design. The measured values for both dependent variables were very close to the predicted values from the statistical design, indicating the accuracy of the design of the experiment. The liposomal formulation of the cytotoxic agent capecitabine was surface-modified with a tumor-homing peptide (THP) to achieve site-specific delivery to breast cancer cells (Singh et al., 2019). The formulation was optimized using a central composite design with three independent variables: amount of THP-cholesterol conjugate, amount of capecitabine, and sonication time (min). The dependent variables were particle size and encapsulation efficiency. The predicted values of the adapted design had a particle size of 114.036 nm and an encapsulation efficiency of 80.87%, which were very close to the measured values obtained in this study. Depending on the desired outcome, variations in process parameters, along with formulation content, could be beneficial in the development of drug-loaded liposomes.
Studies with multiple factors for optimization of liposomes
Multiple studies on liposome formulations have demonstrated the use of three or more factors for optimization. The development and optimization of G-1 polymeric nanoliposomes were performed using different volumes of T-80 solution, stir bar sizes, surfactant types, and sonication regimes (Listik 2018). The effects of each of these independent variables on the size, polydispersity, and zeta potential of the nanoparticles were evaluated. Similarly, a study describing the development of co-encapsulating curcumin and doxorubicin liposomes selected four factors as independent variables to optimize the formulation for evaluating anticancer effects (Tefas et al., 2017). The four factors chosen for optimization were lipid concentration, drug concentration, buffer pH, and the phospholipid:cholesterol ratio. This study utilized variations in these factors to obtain liposomes with predefined specifications by running a set of experiments predicted by the statistical experimental design. The quality-by-design approach of this study was successful in identifying the factors that significantly contributed to the outcome variable. Optimization of docetaxel loading conditions in liposomes was studied using variable cholesterol content and different phospholipids to develop stable formulations with high encapsulation efficiency (Vakili-Ghartavol et al., 2020). There were two sets of three formulations, each with variations in phospholipid and cholesterol content, loaded using active and passive loading methods. For both the active and passive loading sets, formulation with HSPC/mPEG2000-DSPE/DSPG/Chol (85/5/5/10) showed the highest encapsulation efficiency and a steady rate of drug release at 72h. The drug loading of paclitaxel-long circulating liposomes was optimized to improve the physical stability of the formulated liposomes by varying process parameters, such as the number of extrusion cycles, drug-lipid ratio, and total lipid and cholesterol content (Kannan et al., 2014). The goal of this study was to optimize the liposome formulation by testing the effect of variations in process parameters and drug-lipid content on (%) drug loading. Paclitaxel-loaded liposomes were prepared using the thin-film hydration-extrusion method and were characterized for particle size and morphology. The results showed that an increase in total phospholipid content caused an increase in the amount of paclitaxel in the formulation. However, formulations with a high total phospholipid content reduced drug entrapment. Cholesterol improved the overall stability of liposomes and a subsequent decrease in cholesterol content caused an increase in paclitaxel loading. Different drugs and lipids were tested to increase drug-loading capacity. Maximum drug loading was observed at a drug:lipid ratio of 1:30, with poor formulation stability. The optimum stability was observed at a drug:lipid ratio of 1:60. Finally, 10 extrusion cycles were used to prepare the liposomes. Liposomes containing methotrexate for enhanced skin permeation were optimized using the following factors: lipid:drug ratio, proportion of lipids used, and concentration of polymer (Sadarani et al., 2019). Liposomes were prepared using a thin-film hydration method and were optimized using the Box-Behnken design. The dependent variables were the particle size, entrapment efficiency, and transdermal flux. This study utilized a three-factor, three-level Box-Behnken statistical design experiment involving 15 trials. The results showed that the optimized formulation had a drug:lipid ratio of 1:6, the proportion of lipids used was PC:OA:LAB (9:1:1), and the polymer concentration was 1.5%. Vancomycin-loaded liposomes have been characterized and optimized to improve encapsulation efficiency (Liu et al., 2015). Liposomes were prepared using the reverse-phase evaporation-rehydration method. Optimization studies included the ratio of cholesterol to lecithin, the ratio of drug to lipid (w/w), the ratio of the water phase to the oil phase, and the hydration temperature as independent variables. Encapsulation efficiency was selected as the dependent variable. The orthogonal experimental design used in this study predicted nine formulations as a part of the screening process. The formulation with the highest EE (%) was selected as the optimized formulation. Additionally, the in vitro release of vancomycin was sustained for 48 hours. Amphotericin B-loaded liposomes have been prepared, characterized, and optimized for ocular drug delivery (Lakhani et al., 2019). Drug-loaded liposomes were prepared by hot-melt emulsification, followed by high-pressure homogenization. Liposome optimization was performed using the Box-Behnken design. The independent variables chosen for the study were the amount of amphotericin B, castor oil content, amount of mPEG-2k-DSPE, and the number of high-pressure homogenization cycles. The response variables were the particle size, zeta potential, PDI, entrapment efficiency, and loading efficiency. Results showed that the optimized formulation was prepared with 30 homogenization cycles with particle size 218±5 nm; PDI 0.3±0.02; (%) drug loading 4.6±0.1% w/w and entrapment efficiency 92.7±2.5% w/w. The use of statistical experimental design has indeed improved the optimization of liposomal formulations and is therefore described in the articles cited in this review.
Recent advancements in the development of optimized liposomes
There have been significant advancements in developing optimized drug-loaded liposomes for clinical applications. A crucial study describing the liposome delivery of CRISPR/cas9 was able to inhibit HPV (Human Papillomavirus) inducing autophagy and cell death related immune activation in treatment of HPV infection-associated cervical cancer (Zhen et al., 2022). The study explained how the combination of HPV-targeting guide RNA–liposomes with immune inhibitors and death-1 antibodies produced highly effective antitumor effects especially in treatment of cervical cancer. The development of Nucleic acid drug delivery through liposomes is expanding exponentially as there are many potential targets and therapies designed for application in preclinical and clinical stages (Nsairat et al., 2023). The FDA approved drug ONPATTRO® is used clinically for the treatment of hereditary transthyretin-mediated amyloidosis (hATTR amyloidosis) thereby proving the effectiveness of RNA-based therapeutics (Nsairat et al., 2023). A summary of the current state of nucleic acid based liposomal drugs can be seen in
Table 1 (Nsairat et al., 2023).
Conclusion
Liposomes have shown potential as drug carriers for the treatment of various complex diseases. These nanocarriers are biocompatible and can help mitigate the side effects of conventional therapies. Its ability to encapsulate multiple drugs and diagnostic agents has been demonstrated in various clinically approved formulations. The studies discussed in this review clearly demonstrated the advantages of optimizing drug-loaded liposomes. The goal of this review is to highlight the significance of selecting every individual factor for optimization, which will help researchers design the statistical experimental sections of their respective projects. The importance of selecting each factor and its influence on the outcome variable should be studied in detail to represent a validated approach for conducting optimization studies.
Author Contributions
Dr. Shantanu Pande is the sole author of this review. This section certifies that Dr. Shantanu Pande (Sole Author) was responsible for the conception and design of this study. Similarly, the drafting, revision, and final approval of this article were performed by Dr. Shantanu Pande.
Funding
No Funding was used in this study.
Data availability statement
References
- Affram K, Udofot O, Cat A and Agyare E (2015): In vitro and in vivo antitumor activity of gemcitabine loaded thermosensitive liposomal nanoparticles and mild hyperthermia in pancreatic cancer, Int J Adv Res (Indore), 3, 859-874.
- Aghaei H, Solaimany Nazar A, Varshosaz J (2021): Double flow focusing microfluidic-assisted based preparation of methotrexate–loaded liposomal nanoparticles: Encapsulation efficacy, drug release and stability, Colloids and Surfaces A: Physicochemical and Engineering Aspects, 614 126166, ISSN 0927-7757. [CrossRef]
- Ahmad H, Arya A, Agrawal S, Dwivedi A (2017): Chapter 19- Novel lipid nanostructures for delivery of natural agents with antioxidant, anti-inflammatory and antistroke potential: perspectives and outcomes, Micro and Nano Technologies, 577-605. [CrossRef]
- Ailiesei I, Anuta V, Mircioiu C, Cojocaru V, Orbesteanu M and Cinteza L (2016): Application of statistical design of experiments for the optimization of Clodronate loaded Liposomes for Oral Administration, Rev. Chim (Bucharest), 67, 1566-1570.
- Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo S, Zarghami N, Hanifehpour Y, Samiei Mohammad, Kouhi Mohammad and Nejati-Koshki Kazem (2013): Liposome: Classification, Preparation and Applications, Nanoscale Research Letters, 8, 9 pages.
- Ashley J, Quinlan C, Schroeder V, Suckow M, Pizzuti V, Kiziltepe T and Bilgicer B (2016): Dual Carfilzomib and Doxorubicin-Loaded Liposomal Nanoparticles for Synergistic Efficacy in Multiple Myeloma, Mol Cancer Ther, 15, 9 pages.
- Bhattacharjee A, Das PJ, Dey S, Kumar Nayak A, Roy PK, Chakrabarti S, Marbaniang D, Das SK, Ray S, Chattopadhyay P, Mazumder B (2019): Development and optimization of besifloxacin hydrochloride loaded liposomal gel prepared by thin film hydration method using 32 full factorial design, Colloids and Surfaces A: Physicochemical and Engineering Aspects, 585, 124071.
- Cadena P, Pereira M, Cordeiro R, Cavalcanti I, Neto B, Pimentel M, Filho J, Silva V, Santos-Magalhães N (2013): Nanoencapsulation of quercetin and resveratrol into elastic liposomes, Biochimica et Biophysica Acta, 1828, 309-316.
- Calvagno G, Celia C, Paolino D, Cosco D, Lannone M, Castelli F, Doldo P and Fresta M (2007): Effects of Lipid Composition and Preparation Conditions on Physical-Chemical Properties, Technological Parameters and In Vitro Biological Activity of Gemcitabine-Loaded Liposomes, Current Drug Delivery, 4, 89-101.
- Chang H, Yeh M (2012): Clinical development of liposome-based drugs: formulation, characterization, and therapeutic efficacy, Int J Nanomed, 7, 49-60.
- Chountoulesi M, Naziris N, Pippa N, Demetzos C (2018): The significance of drug-to-lipid ratio to the development of optimized liposomal formulation, J Liposome Res, 28, 3, 249-258.
- Eleftheriou K, Kaminari A, Panagiotaki K, Sideratou Z, Zachariadis M, Anastassopoulou J, Tsiourvas D (2020): A combination drug delivery system employing thermosensitive liposomes for enhanced cell penetration and improved in vitro efficacy, International Journal of Pharmaceutics, 574, 118912.
- Handali S, Moghimipour E, Rezaei M, Ramezani Z, Kouchak M, Amini M, Angali K, Saremy S, Dorkoosh F (2018): A novel 5-Fluorouracil targeted delivery to colon cancer using folic acid conjugated liposomes, Biomedicine & Pharmacotherapy, 108, 1259-1273.
- Hu C-M, Aryal S, Zhang L (2010): Nanoparticle-assisted combination therapies for effective cancer treatment, Therapeutic Delivery, 1, 2, 323-334.
- Jangde R, Singh D (2016): Preparation and optimization of quercetin-loaded liposomes for wound healing, using response surface methodology, Artificial Cells, Nanomedicine, and Biotechnology, 44, 635-641.
- Jin J, Teng C, Li T (2018): Combination Therapy versus gemcitabine monotherapy in the treatment of elderly pancreatic cancer: a meta-analysis of randomized controlled trials, Drug Design, Development and Therapy, 12, 475-480.
- Johnston M, Semple S, Klimuk S, Edwards K, Eisenhardt M, Leng E, Karlsson G, Yanko D, Cullis P (2006): Therapeutically optimized rates of drug release can be achieved by varying the drug-to-lipid ratio in liposomal vincristine formulations, Biochimica et Biophysica Acta, 1758, 55-64.
- Kan P, Tsao C.W, Wang A.J, Su W.C and Liang H.F (2011): A liposomal formulation able to incorporate a high content of paclitaxel and exert promising anticancer effect, Journal of Drug delivery, 9 pages.
- Kannan V, Balabathula P, Divi M, Thoma L, Wood G (2014): Optimization of drug loading to improve physical stability of paclitaxel-loaded long-circulating liposomes, J Liposome Res, 1-8.
- Lakhani P, Patil A, Wu K, Sweeney C, Tripathi S, Avula B, Taskar P, Khan S, Majumdar S (2019): Optimization, stabilization and characterization of amphotericin B loaded nanostructured lipid carriers for ocular drug delivery, International Journal of Pharmaceutics, 572, 118771, 14 pages. [CrossRef]
- Li Z, Liu M, Wang H, Du S (2016): Increased cutaneous wound healing effect of biodegradable liposomes containing madecassoside: preparation, optimization, in vitro dermal permeation, and in vivo bioevaluation, International Journal of Nanomedicine, 11, 2995-3007.
- Lila A and Ishida T (2017): Liposomal Delivery Systems: Design Optimization and Current Applications, Biol. Pharm. Bull, 40, 1-10.
- Listik E (2018): Development and optimization of G-1 polymeric nanoparticulated and liposomal systems for central nervous system applications, Neurol Disord Therap, 2, 1, 1-5.
- Liu J, Wang Z, Li F, Gao J, Wang L, Huang G (2015): Liposomes for systematic delivery of vancomycin hydrochloride to decrease nephrotoxicity: Characterization and evaluation, Asian Journal of Pharmaceutical Sciences, 10, 212-222.
- Mahira S, Kommineni N, Husain G, Khan W (2019): Cabazitaxel and silibinin co-encapsulated cationic liposomes for CD44 targeted delivery: A new insight into nanomedicine based combinational chemotherapy for prostate cancer, Biomedicine & Pharmacotherapy, 110, 803-817.
- Mehanna M, El-Kader N, Samaha M (2017): Liposomes as potential carriers for ketorolac ophthalmic delivery: formulation and stability issues, Braz. J. Pharm. Sci, 53, 2, 10 pages.
- Miao Z.L, Deng Y.J, Du H.Y, Suo X.B, Wang X.Y, Xiao W, Li W, Cui L.J and Duan N (2015): Preparation of a liposomal delivery system and its in vitro release of rapamycin, Experimental and Therapeutic Medicine, 9, 941-946.
- Miatmoko A, Salim H, Zahro S, Annuryanti F, Sari R, Hendradi E (2019): Dual Loading Of Primaquine And Chloroquine Into Liposome, Eur. Pharm. J, 66, 2, 18-25.
- Muppidi K, Pumerantz A, Wang J and Betageri G (2012): Development and Stability Studies of Novel Liposomal Vancomycin Formulations; ISRN Pharmaceutics, 8 pages.
- Nogueira E, Gomes A, Preto A and Paulo A.C (2015); Design of liposomal formulations for cell targeting, Colloids and Surfaces B: Biointerfaces, 136, 514-526.
- Nsairat H, Alshaer W, Odeh F, Esawi E, Khater D, Al Bawab A, El-Tanani M, Awidi A, Mubarak MS(2023); Recent advances in using liposomes for delivery of nucleic acid-based therapeutics, OpenNano, 11, 100132, ISSN 2352-9520.
- Pamunuwa G, Karunaratne V, Nedra Karunaratne D (2016): Effect of Lipid Composition on In Vitro Release and Skin Deposition of Curcumin Encapsulated Liposomes, Journal of Nanomaterials, 9 pages.
- Pande S (2023): Liposomes for drug delivery: review of vesicular composition, factors affecting drug release and drug loading in liposomes, Artificial Cells, Nanomedicine, and Biotechnology, 51:1, 428-440.
- Pereira S, Egbu R, Jannati G, Al-Jamal W (2016): Docetaxel-loaded liposomes: The effect of lipid composition and purification on drug encapsulation and in vitro toxicity, International Journal of Pharmaceutics, 514, 150-159.
- Permana A, Tekko I, McCrudden M, Anjani Q, Ramadon D, McCarthy H, Donnelly R (2019): Solid lipid nanoparticle-based dissolving microneedles: A promising intradermal lymph targeting drug delivery system with potential for enhanced treatment of lymphatic filariasis, Journal of Controlled Release, 316, 34-52.
- Rathod S and Deshpande S.G (2010): Design and Evaluation of Liposomal Formulation of Pilocarpine Nitrate, Indian J Pharm Sci., 72, 155-160.
- Sadarani B, Majumdar A, Paradkar S, Mathur A, Sachdev S, Mohanty B, Chaudhari P (2019): Enhanced skin permeation of Methotrexate from penetration enhancer containing vesicles: In vitro optimization and in vivo evaluation, Biomedicine and Pharmcotherapy, 114, 13 pages.
- Sailor G, Seth A, Parmar G, Chauhan S, Javia A (2015): Formulation and in vitro evaluation of berberine containing liposome optimized by 32 full factorial designs, Journal of Applied Pharmaceutical Science, 5, 7, 23-28.
- Saito T, Ishido K, Kudo D, Kimura N, Wakiya T, Nakayama Y, Hakamada K (2017): Combination Therapy with gemcitabine and nab-paclitaxel for locally advanced unresectable pancreatic cancer, Molecular and Clinical Oncology, 6, 963-967.
- Singh M, Pindiprolu S, Sanapalli B, Yele V, Ganesh G (2019): Tumor homing peptide modified liposomes of capecitabine for improved apoptotic activity and HER2 targeted therapy in breast cancer: in vitro studies, RSC Adv., 9, 24987.
- Soema P, Willems G, Jiskoot W, Amorij JP, Kersten G (2015): Predicting the influence of liposomal lipid composition on liposome size, zeta potential and liposome-induced dendritic cell maturation using a design of experiments approach, European Journal of Pharmaceutics and Biopharmaceutics, 94, 427-435. [CrossRef]
- Ta T and Porter T (2013): Thermosensitive liposomes for localized delivery and triggered release of chemotherapy, J Control Release, 169, 112-125.
- Tefas L, Sylvester B, Tomuta, Sesarman A, Licarete E, Banciu M, Porfire A (2017): Development of antiproliferative long-circulating liposomes co-encapsulating doxorubicin and curcumin, through the use of a quality-by-design approach, Drug Design, Development and Therapy, 11, 1605-1621.
- Vakili-Ghartavol R, Rezayat S M, Faridi-Majidi R, Sadri K, Jaafari M R (2020): Optimization of Docetaxel Loading Conditions in Liposomes: proposing potential products for metastatic breast carcinoma chemotherapy, Sci Rep, 10, 5569, 14 pages.
- Vali A, Toliyat T, Shafaghi B, Dadashazdeh S (2008): Preparation, Optimization and Characterization of Topotecan Loaded PEGylated Liposomes Using Factorial Design, Drug Development and Industrial Pharmacy, 34, 10-23. [CrossRef]
- Wolfram J, Suri K, Huang Y, Molinaro R, Borsoi C, Scott B, Boom K, Paolino D, Fresta M, Wang J, Ferrari M, Celia C, Shen H (2014): Evaluation of anticancer activity of celastrol liposomes in prostate cancer cells, J Microencapsul, 7 pages.
- Wehbe M, Malhotra A, Anantha M, Roosendaal J, Leung A, Plackett D, Edwards K, Gilabert-Oriol R, Bally M (2017): A simple passive equilibration method for loading carboplatin into pre-formed liposomes incubated with ethanol as a temperature dependent permeability enhancer, Journal of Controlled Release, 252, 50-61.
- Xu Y and Meng H (2014): Paclitaxel- loaded stealth liposomes: Development, Characterization, Pharmacokinetics and Biodistribution, Artificial cells, Nanomedicine and Biotechnology, 44, 350-355.
- Yingchoncharoen P, Kalinowski D and Richardson D (2016): Lipid-Based Drug delivery systems in cancer therapy: what is available and what is yet to come, Pharmacol Rev 68, 701-787.
- Yuan J, Qin F, Tu J, Li B (2017): Preparation, Characterization, and Antioxidant Activity Evaluation of Liposomes Containing Water-Soluble Hydroxytyrosol from Olive, Molecules, 22, 870, 15 pages. [CrossRef]
- Zhen, S, Qiang, R, Lu, J, Tuo, X, Yang, X, Li, X (2022): CRISPR/Cas9-HPV-liposome enhances antitumor immunity and treatment of HPV infection-associated cervical cancer. J Med Virol.; 95:e28144.
- Zylberberg C, Matosevic S (2016): Pharmaceutical liposomal drug delivery: a review of new delivery systems and a look at the regulatory landscape, Drug Delivery, 23, 9, 3319-3329.
- Zoghi A, Khosravi-Darani K, Omri A (2016): Process Variables and Design of Experiments in Liposome and Nanoliposome Research, Mini-Reviews in Medicinal Chemistry, 16, 1, 16 pages.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).