Submitted:
23 October 2023
Posted:
25 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
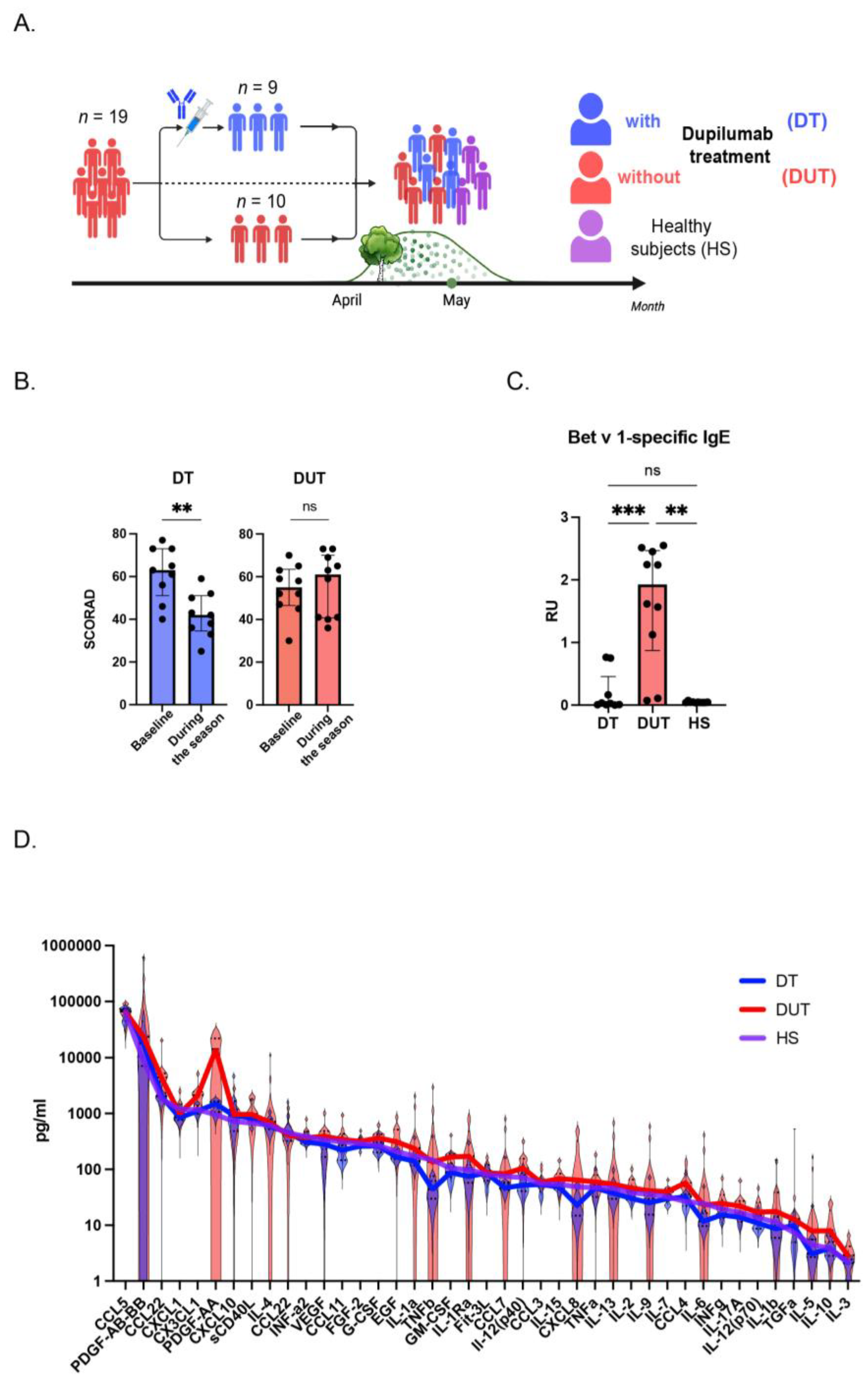
2.1. The Patients with Atopic Dermatitis Demonstrated Significant Clinical Improvements one Year after Initiation of Dupilumab Therapy During Birch Pollen Season
2.2. The Level of Bet v 1-Specific IgE was Lower in Dupilumab-Treated Patients in Comparison with Dupilumab-Untreated Group
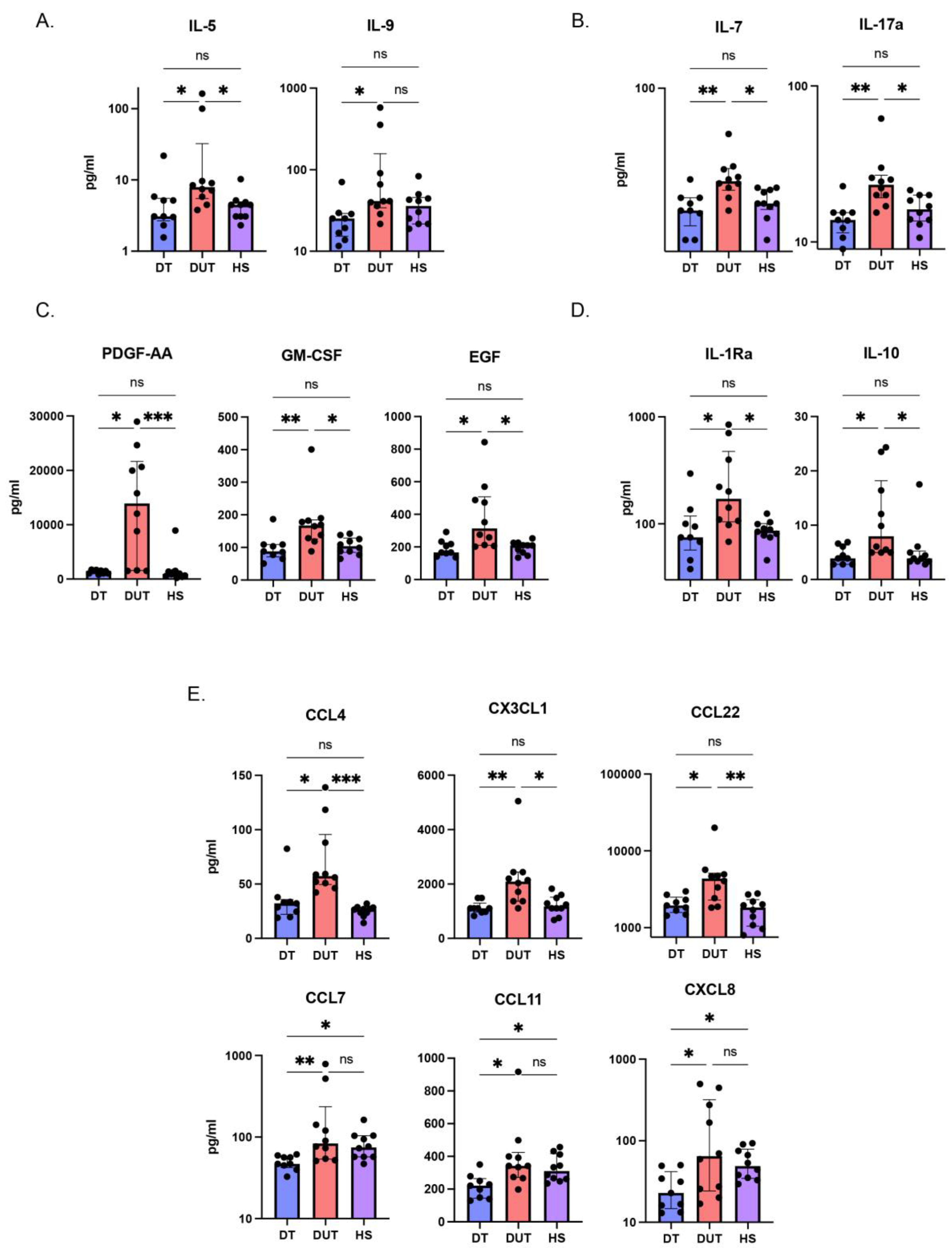
2.3. The Cytokine Profile in AD Patients Without Dupilumab Treatment Significantly Differs from Those Treated with Dupilumab, as Well as from Healthy Individuals
2.4. The Concentrations of AD Key Cytokines were Diminished in the Group of Patients Treated with Dupilumab Compared to Those in the Group That Did Not Receive Dupilumab Treatment
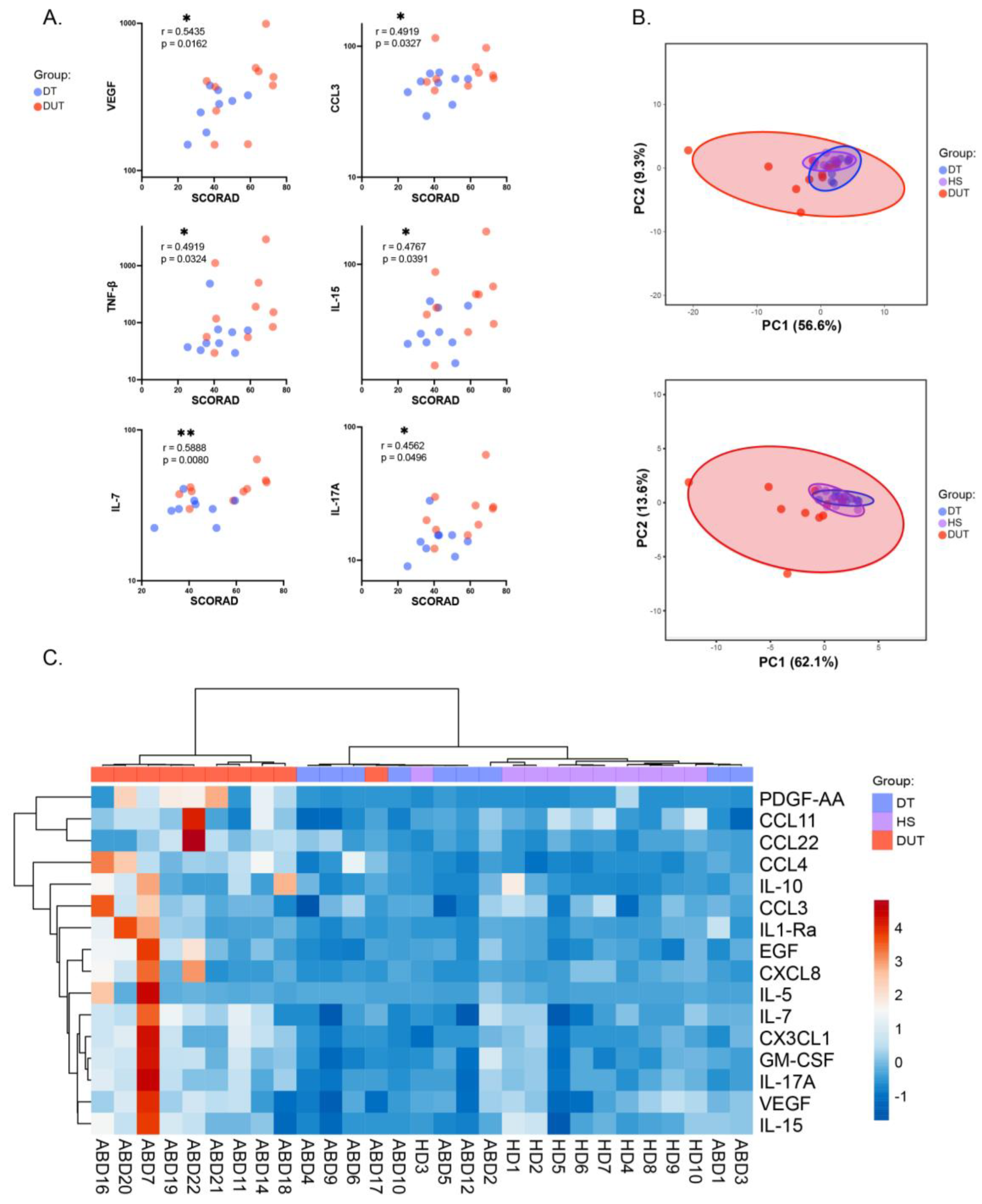
2.5. Specific Cytokine Pattern Aid in Assessing Atopic Dermatitis Patients’ Condition
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Blood Sample Collection and Cytokine Analysis
4.3. ELISA
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sroka-Tomaszewska, J.; Trzeciak, M. Molecular Mechanisms of Atopic Dermatitis Pathogenesis. International Journal of Molecular Sciences 2021, 22, 4130. [Google Scholar] [CrossRef]
- Ali, F.; Vyas, J.; Finlay, A. Counting the Burden: Atopic Dermatitis and Health-Related Quality of Life. Acta Dermato Venereologica 2020, 100, adv00161. [Google Scholar] [CrossRef]
- Nutten, S. Atopic Dermatitis: Global Epidemiology and Risk Factors. Annals of Nutrition and Metabolism 2015, 66, 8–16. [Google Scholar] [CrossRef]
- Eichenfield, L.F.; Stripling, S.; Fung, S.; Cha, A.; O’Brien, A.; Schachner, L.A. Recent Developments and Advances in Atopic Dermatitis: A Focus on Epidemiology, Pathophysiology, and Treatment in the Pediatric Setting. Pediatric Drugs 2022, 24, 293–305. [Google Scholar] [CrossRef]
- Sacotte, R.; Silverberg, J.I. Epidemiology of Adult Atopic Dermatitis. Clinics in Dermatology 2018, 36, 595–605. [Google Scholar] [CrossRef]
- Cabanillas, B.; Brehler, A.-C.; Novak, N. Atopic Dermatitis Phenotypes and the Need for Personalized Medicine. Current Opinion in Allergy & Clinical Immunology 2017, 17, 309–315. [Google Scholar] [CrossRef]
- Han, H.; Roan, F.; Ziegler, S.F. The Atopic March: Current Insights into Skin Barrier Dysfunction and Epithelial Cell-derived Cytokines. Immunological Reviews 2017, 278, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Elhaji, Y.; Sasseville, D.; Pratt, M.; Asai, Y.; Matheson, K.; McLean, W.H.I.; Hull, P.R. Filaggrin Gene Loss-of-function Mutations Constitute a Factor in Patients with Multiple Contact Allergies. Contact Dermatitis 2019, 80, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Campana, R.; Dzoro, S.; Mittermann, I.; Fedenko, E.; Elisyutina, O.; Khaitov, M.; Karaulov, A.; Valenta, R. Molecular Aspects of Allergens in Atopic Dermatitis. Current Opinion in Allergy & Clinical Immunology 2017, 17, 269–277. [Google Scholar] [CrossRef]
- Tamagawa-Mineoka, R.; Katoh, N. Atopic Dermatitis: Identification and Management of Complicating Factors. International Journal of Molecular Sciences 2020, 21, 2671. [Google Scholar] [CrossRef]
- Kihlström, A.; Lilja, G.; Pershagen, G.; Hedlin, G. Exposure to Birch Pollen in Infancy and Development of Atopic Disease in Childhood. Journal of Allergy and Clinical Immunology 2002, 110, 78–84. [Google Scholar] [CrossRef]
- Meinke, M.; Fölster-Holst, R.; Galecka, J.; Weißmantel, S.; Dickschat, U.; Rippke, F.; Bohnsack, K.; Werfel, T.; Wichmann, K.; Buchner, M.; et al. Birch Pollen Influence the Severity of Atopic Eczema &Ndash; Prospective Clinical Cohort Pilot Study and Ex Vivo Penetration Study. Clinical, Cosmetic and Investigational Dermatology 2015, 539. [Google Scholar] [CrossRef] [PubMed]
- Wassmann-Otto, A.; Heratizadeh, A.; Wichmann, K.; Werfel, T. Birch Pollen-related Foods Can Cause Late Eczematous Reactions in Patients with Atopic Dermatitis. Allergy 2018, 73, 2046–2054. [Google Scholar] [CrossRef] [PubMed]
- Blauvelt, A.; Bruin-Weller, M. de; Gooderham, M.; Cather, J.C.; Weisman, J.; Pariser, D.; Simpson, E.L.; Papp, K.A.; Hong, H.C.-H.; Rubel, D.; et al. Long-Term Management of Moderate-to-Severe Atopic Dermatitis with Dupilumab and Concomitant Topical Corticosteroids (LIBERTY AD CHRONOS): A 1-Year, Randomised, Double-Blinded, Placebo-Controlled, Phase 3 Trial. The Lancet 2017, 389, 2287–2303. [Google Scholar] [CrossRef] [PubMed]
- Bruin-Weller, M. de; Thaçi, D.; Smith, C.H.; Reich, K.; Cork, M.J.; Radin, A.; Zhang, Q.; Akinlade, B.; Gadkari, A.; Eckert, L.; et al. Dupilumab with Concomitant Topical Corticosteroid Treatment in Adults with Atopic Dermatitis with an Inadequate Response or Intolerance to Ciclosporin A or When This Treatment Is Medically Inadvisable: A Placebo-Controlled, Randomized Phase III Clinical t. British Journal of Dermatology 2018, 178, 1083–1101. [Google Scholar] [CrossRef]
- Simpson, E.L.; Bieber, T.; Guttman-Yassky, E.; Beck, L.A.; Blauvelt, A.; Cork, M.J.; Silverberg, J.I.; Deleuran, M.; Kataoka, Y.; Lacour, J.-P.; et al. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. New England Journal of Medicine 2016, 375, 2335–2348. [Google Scholar] [CrossRef] [PubMed]
- Snast, I.; Reiter, O.; Hodak, E.; Friedland, R.; Mimouni, D.; Leshem, Y.A. Are Biologics Efficacious in Atopic Dermatitis? A Systematic Review and Meta-Analysis. American Journal of Clinical Dermatology 2018, 19, 145–165. [Google Scholar] [CrossRef]
- Thaçi, D.; Simpson, E.L.; Deleuran, M.; Kataoka, Y.; Chen, Z.; Gadkari, A.; Eckert, L.; Akinlade, B.; Graham, N.M.H.; Pirozzi, G.; et al. Efficacy and Safety of Dupilumab Monotherapy in Adults with Moderate-to-Severe Atopic Dermatitis: A Pooled Analysis of Two Phase 3 Randomized Trials (LIBERTY AD SOLO 1 and LIBERTY AD SOLO 2). Journal of Dermatological Science 2019, 94, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.-P.; Tang, X.-J.; Wei, C.-Q.; Xu, L.-R.; Mao, H.; Luo, F.-M. Dupilumab Treatment in Moderate-to-Severe Atopic Dermatitis: A Systematic Review and Meta-Analysis. Journal of Dermatological Science 2018, 90, 190–198. [Google Scholar] [CrossRef]
- Elisyutina, O.; Lupinek, C.; Fedenko, E.; Litovkina, A.; Smolnikov, E.; Ilina, N.; Kudlay, D.; Shilovskiy, I.; Valenta, R.; Khaitov, M. IgE-reactivity Profiles to Allergen Molecules in Russian Children with and without Symptoms of Allergy Revealed by Micro-array Analysis. Pediatric Allergy and Immunology 2021, 32, 251–263. [Google Scholar] [CrossRef]
- Movérare, R.; Petäys, T.; Vartiainen, E.; Haahtela, T. IgE Reactivity Pattern to Timothy and Birch Pollen Allergens in Finnish and Russian Karelia. International Archives of Allergy and Immunology 2005, 136, 33–38. [Google Scholar] [CrossRef]
- Westman, M.; Lupinek, C.; Bousquet, J.; Andersson, N.; Pahr, S.; Baar, A.; Bergström, A.; Holmström, M.; Stjärne, P.; Carlsen, K.C.L.; et al. Early Childhood IgE Reactivity to Pathogenesis-Related Class 10 Proteins Predicts Allergic Rhinitis in Adolescence. Journal of Allergy and Clinical Immunology 2015, 135, 1199–1206e11. [Google Scholar] [CrossRef]
- Geba, G.P.; Li, D.; Xu, M.; Mohammadi, K.; Attre, R.; Ardeleanu, M.; Musser, B. Attenuating the Atopic March: Meta-Analysis of the Dupilumab Atopic Dermatitis Database for Incident Allergic Events. Journal of Allergy and Clinical Immunology 2023, 151, 756–766. [Google Scholar] [CrossRef]
- Ogawa-Momohara, M.; Muro, Y.; Murase, C.; Taki, T.; Tanahashi, K.; Yamashita, Y.; Koizumi, H.; Fukaura, R.; Takeichi, T.; Akiyama, M. Allergen-Specific IgG4 Increase in Atopic Dermatitis with Long-Term Dupilumab Use. British Journal of Dermatology 2023, 189, 472–474. [Google Scholar] [CrossRef]
- Spekhorst, L.S.; Rijst, L.P. van der; Graaf, M. de; Megen, M. van; Zuithoff, N.P.A.; Knulst, A.C.; Bruin-Weller, M.S. de; Le, T.-M. Dupilumab Has a Profound Effect on <scp>specific-IgE</Scp> Levels of Several Food Allergens in Atopic Dermatitis Patients. Allergy 2023, 78, 875–878. [Google Scholar] [CrossRef]
- Cabanillas, B. Dupilumab for Atopic Dermatitis—From Clinical Trials to Molecular and Cellular Mechanisms. Dermatitis® 2023, 34, 21–28. [Google Scholar] [CrossRef]
- Kim, K.; Kim, H.; Sung, G.Y. An Interleukin-4 and Interleukin-13 Induced Atopic Dermatitis Human Skin Equivalent Model by a Skin-On-A-Chip. IJMS 2022, 23, 2116. [Google Scholar] [CrossRef]
- Fedenko, E.S.; Elisyutina, O.G.; Filimonova, T.M.; Boldyreva, M.N.; Burmenskaya, O.V.; Rebrova, O.Y.; Yarilin, A.A.; Khaitov, R.M. Cytokine Gene Expression in the Skin and Peripheral Blood of Atopic Dermatitis Patients and Healthy Individuals. Self/Nonself 2011, 2, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Reeder, K.M.; Dunaway, C.W.; Blackburn, J.P.; Yu, Z.; Matalon, S.; Hastie, A.T.; Ampleford, E.J.; Meyers, D.A.; Steele, C. The Common γ-Chain Cytokine IL-7 Promotes Immunopathogenesis during Fungal Asthma. Mucosal Immunology 2018, 11, 1352–1362. [Google Scholar] [CrossRef] [PubMed]
- Von Freeden-Jeffry, U.; Davidson, N.; Wiler, R.; Fort, M.; Burdach, S.; Murray, R. IL-7 Deficiency Prevents Development of a Non-T Cell Non-B Cell-Mediated Colitis. The Journal of Immunology 1998, 161, 5673–5680. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Tang, T.-X.; Deng, H.; Yang, X.-P.; Tang, Z.-H. Interleukin-7 Biology and Its Effects on Immune Cells: Mediator of Generation, Differentiation, Survival, and Homeostasis. Front. Immunol. 2021, 12, 747324. [Google Scholar] [CrossRef]
- Koga, C.; Kabashima, K.; Shiraishi, N.; Kobayashi, M.; Tokura, Y. Possible Pathogenic Role of Th17 Cells for Atopic Dermatitis. Journal of Investigative Dermatology 2008, 128, 2625–2630. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. PERSPECTIVE ARTICLE: Growth Factors and Cytokines in Wound Healing. Wound Repair and Regeneration 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Toriyama, M.; Rizaldy, D.; Nakamura, M.; Atsumi, Y.; Toriyama, M.; Fujita, F.; Okada, F.; Morita, A.; Itoh, H.; Ishii, K.J. Dendritic Cell Proliferation by Primary Cilium in Atopic Dermatitis. Frontiers in Molecular Biosciences 2023, 10. [Google Scholar] [CrossRef]
- Lee, H.J.; Hong, Y.J.; Kim, M. Angiogenesis in Chronic Inflammatory Skin Disorders. International Journal of Molecular Sciences 2021, 22, 12035. [Google Scholar] [CrossRef]
- Biedermann, T.; Skabytska, Y.; Kaesler, S.; Volz, T. Regulation of T Cell Immunity in Atopic Dermatitis by Microbes: The Yin and Yang of Cutaneous Inflammation. Front. Immunol. 2015, 6. [Google Scholar] [CrossRef]
- Zhang, L.; Wen, X.; Hou, Y.; Yang, Y.; Song, W.; Zeng, Y.; Sun, J. Integrated Metabolomics and Lipidomics Study of Patients with Atopic Dermatitis in Response to Dupilumab. Front. Immunol. 2022, 13, 1002536. [Google Scholar] [CrossRef] [PubMed]
- Bangert, C.; Rindler, K.; Krausgruber, T.; Alkon, N.; Thaler, F.M.; Kurz, H.; Ayub, T.; Demirtas, D.; Fortelny, N.; Vorstandlechner, V.; et al. Persistence of Mature Dendritic Cells, T H 2A, and Tc2 Cells Characterize Clinically Resolved Atopic Dermatitis under IL-4Rα Blockade. Science Immunology 2021, 6. [Google Scholar] [CrossRef] [PubMed]
- Bachelez, H. Chemokine Ligand 7 ( CCL 7) and Innate Immune Cells in Psoriasis: Beyond Redundancy. Experimental Dermatology 2016, 25, 265–266. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-T.; Chen, C.; Chen, J.-W. CCL7 as a Novel Inflammatory Mediator in Cardiovascular Disease, Diabetes Mellitus, and Kidney Disease. Cardiovasc Diabetol 2022, 21, 185. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Duca, E.D.; Diaz, A.; Kim, H.J.; Gay-Mimbrera, J.; Zhang, N.; Wu, J.; Beaziz, J.; Estrada, Y.; Krueger, J.G.; et al. Mild Atopic Dermatitis Lacks Systemic Inflammation and Shows Reduced Nonlesional Skin Abnormalities. Journal of Allergy and Clinical Immunology 2021, 147, 1369–1380. [Google Scholar] [CrossRef] [PubMed]
- Del Duca, E.; Renert-Yuval, Y.; Pavel, A.B.; Mikhaylov, D.; Wu, J.; Lefferdink, R.; Fang, M.; Sheth, A.; Blumstein, A.; Facheris, P.; et al. Proteomic Characterization of Atopic Dermatitis Blood from Infancy to Adulthood. Journal of the American Academy of Dermatology 2023, 88, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Hirai, T.; Zenke, Y.; Yang, Y.; Bartholin, L.; Beura, L.K.; Masopust, D.; Kaplan, D.H. Keratinocyte-Mediated Activation of the Cytokine TGF-β Maintains Skin Recirculating Memory CD8+ T Cells. Immunity 2019, 50, 1249–1261e5. [Google Scholar] [CrossRef] [PubMed]
- Humeau, M.; Boniface, K.; Bodet, C. Cytokine-Mediated Crosstalk Between Keratinocytes and T Cells in Atopic Dermatitis. Front. Immunol. 2022, 13, 801579. [Google Scholar] [CrossRef]
- Julia, V.; Staumont-Salle, D.; Dombrowicz, D. Rôle de La Fractalkine/CX3CL1 et de Son Récepteur CX3CR1 Dans Les Pathologies Allergiques. médecine/sciences 2016, 32, 260–266. [Google Scholar] [CrossRef]
- Julia, V. CX3CL1 in Allergic Diseases: Not Just a Chemotactic Molecule. Allergy 2012, 67, 1106–1110. [Google Scholar] [CrossRef]
- Staumont-Sallé, D.; Fleury, S.; Lazzari, A.; Molendi-Coste, O.; Hornez, N.; Lavogiez, C.; Kanda, A.; Wartelle, J.; Fries, A.; Pennino, D.; et al. CX3CL1 (Fractalkine) and Its Receptor CX3CR1 Regulate Atopic Dermatitis by Controlling Effector T Cell Retention in Inflamed Skin. Journal of Experimental Medicine 2014, 211, 1185–1196. [Google Scholar] [CrossRef]
- Chong, S.; Lan, H.; Zeng, K.; Zhao, X. Serum Fractalkine (CX3CL1) Concentration Correlates with Clinical Severity in Pediatric Atopic Dermatitis Patients. Ann Clin Lab Sci 2016, 46, 168–173. [Google Scholar]
| Dupilumab-treated patients (DT) | Dupilumab-untreated patients (DUT) | Healthy subjects (HS) | |
|---|---|---|---|
| demographical data | Number of subjects | ||
| 9 | 10 | 10 | |
| Age, Me [Q1;Q3] years | |||
| 32.0 [23.0; 39.0] | 26.5 [22.3; 29.5] | 25.5 [24.3; 28.5] | |
| Sex(%) | |||
| m: 6 (66.7%) | m: 3 (30.0%) | m: 5 (50.0%) | |
| f: 3 (33.3%) | f: 7 (70.0%) | F: 5 (50.0%) | |
| AD characterization | AD duration (years), Me | ||
| 12 | 10 | 0 | |
| SCORAD at baseline, Me [Q1;Q3] | |||
| 63 [56.2; 72.5] | 54,65 [48.5; 61.9] | n/a | |
| SCORAD , Me [Q1;Q3] | |||
| 42,2 [35.7; 50.0] | 60,85 [40.7; 67.7] | 0 [0;0] | |
| DLQI, Me[Q1;Q3] | |||
| 5 [5.0; 12.0] | 12 [7.5; 20.0] | 0 [0;0] | |
| Itch intensity, NRS score, Me [Q1;Q3] | |||
| 4 [3.0; 6.0] | 6 [4.3; 8.8] | 0 [0;0] | |
| Allergic comorbidities | Allergic rhinitis, n(%) | ||
| 9 (100%) | 10 (100.0%) | 0 (0.0%) | |
| Asthma, n(%) | |||
| 5 (55.6%) | 5 (50.0%) | 0 (0.0%) | |
| Food allergy,n(%) | |||
| 5 (55.6%) | 8 (80.0%) | 0 (0.0%) | |
| IgE sensitization profile | House dust mites, n(%) | ||
| 8 (88.9%) | 10 (100.0%) | 0 (0.0%) | |
| Epidermal allergens, n(%) | |||
| 7 (77.8%) | 10 (100.0%) | 0 (0.0%) | |
| Trees pollen, n(%) | |||
| 9 (100%) | 10 (100.0%) | 0 (0.0%) | |
| Grass pollen, n(%) | |||
| 6 (66.7%) | 5 (50.0%) | 0 (0.0%) | |
| Weeds pollen, n(%) | |||
| 5 (55.6%) | 6 (60.0%) | 0 (0.0%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





