Submitted:
22 October 2023
Posted:
23 October 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Carbon Nanodots
| No. | Application | Description | References |
|---|---|---|---|
| 1. | Anticancer Drug Delivery | Carbon dots are utilized as drug carriers for anticancer medications, enhancing drug solubility and targeted delivery to tumor sites. They also serve as imaging agents. | [8] |
| 2. | Antibiotic Drug Delivery | Carbon dots improve the stability and bioavailability of antibiotics, aiding in the treatment of bacterial infections. They are also used for infection imaging. | [9] |
| 3. | Peptide and Protein Delivery | Carbon dots enhance the delivery of therapeutic peptides and proteins by improving their stability and protecting them from enzymatic degradation. | [12] |
| 4. | Gene Delivery | Carbon dots are used as non-viral vectors for gene delivery, facilitating the introduction of genetic material into cells for gene therapy and genetic studies. | [10] |
| 5. | Photothermal Therapy | Carbon dots are employed for photothermal therapy, where they absorb and convert light into heat to target and destroy cancer cells in a controlled manner. | [13] |
| 6. | Imaging Agents | Carbon dots are used as contrast agents in medical imaging, including fluorescence imaging, MRI, and photoacoustic imaging, for diagnostic and visualization purposes. | [14] |
| 7. | Ocular Drug Delivery | Carbon dots are explored in ophthalmic applications, serving as carriers for drugs targeted at eye diseases and as imaging agents for retinal imaging. | [11] |
| 8. | Wound Healing | Carbon dots are incorporated into wound dressings to enhance wound healing, reduce infection risk, and provide controlled drug release. | [15] |
| 9. | Real-Time Drug Release Monitoring | Carbon dots are integrated into drug delivery systems to monitor drug release in real-time, ensuring precise dosage and timing. | [13] |
| 10. | Antibacterial Agents | Functionalized carbon dots exhibit antibacterial properties and are studied for combating drug-resistant bacteria and infections. | [16] |
Cannabidiol (CBD): Understanding the Therapeutic Compound
Therapeutic Applications
Pain Management
Neurological Disorders
Anti-Inflammatory Effects
Synergizing CNDs and CBD
Mechanisms of Synergy
CNDs and CBD Compatibility
Impact on Solubility and Bioavailability
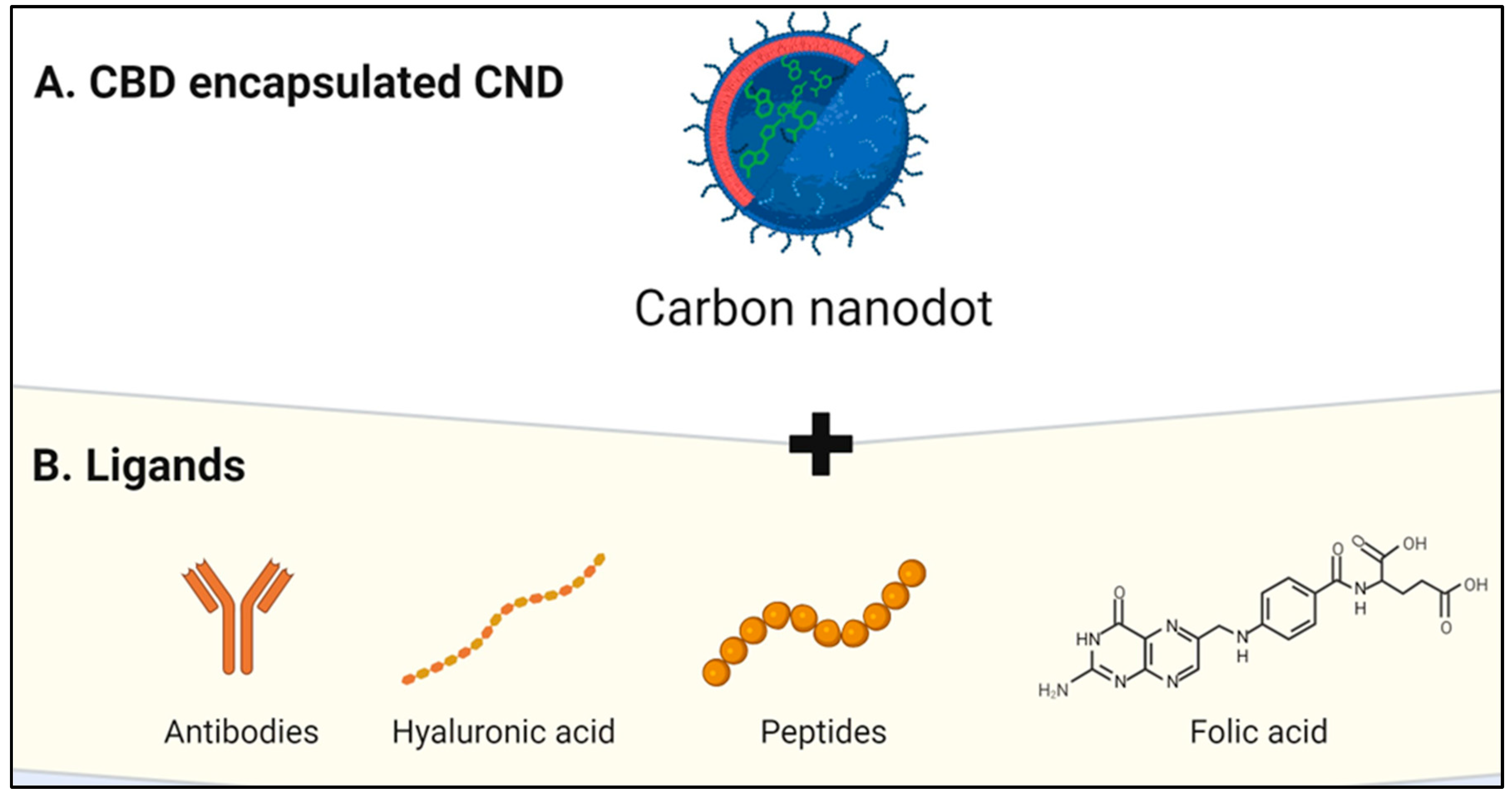
CNDs encapsulation of CBD
| Targeting Molecule | Targeted Cancer Biomarker(s) | Targeted Cancer Type(s) | References |
|---|---|---|---|
| Monoclonal Antibodies | HER2, CD20, EGFR, EpCAM, PSMA, CD133 | Breast cancer, lymphomas, various solid tumors | [93,94] |
| Aptamers | EGFR, PD-1, MUC1, PSMA | Lung cancer, melanoma, prostate cancer, ovarian cancer | [95,96] |
| Peptides | RGD, CD44, CD133 | Various solid tumors, breast cancer, glioblastoma | [97,98] |
| Folic Acid (Folate) | Folate Receptors | Ovarian cancer, lung cancer, brain tumors | [99] |
| Transferrin | Transferrin Receptors | Brain cancer, leukemia, lymphoma | [100] |
| Antigen-Binding Fragments (scFv) | EGFR, EpCAM, CD133 | Head and neck cancer, colorectal cancer, liver cancer | [101,102] |
| Hyaluronic Acid | CD44 | Breast cancer, ovarian cancer, pancreatic cancer | [103] |
| Glycyrrhetinic Acid | Glycyrrhetinic Acid Receptors | Hepatocellular carcinoma | [104] |
| PSMA Ligands | PSMA | Prostate cancer | [105,106] |
| CD133-Targeting Peptides | CD133 | Various solid tumors, cancer stem cells | [107] |
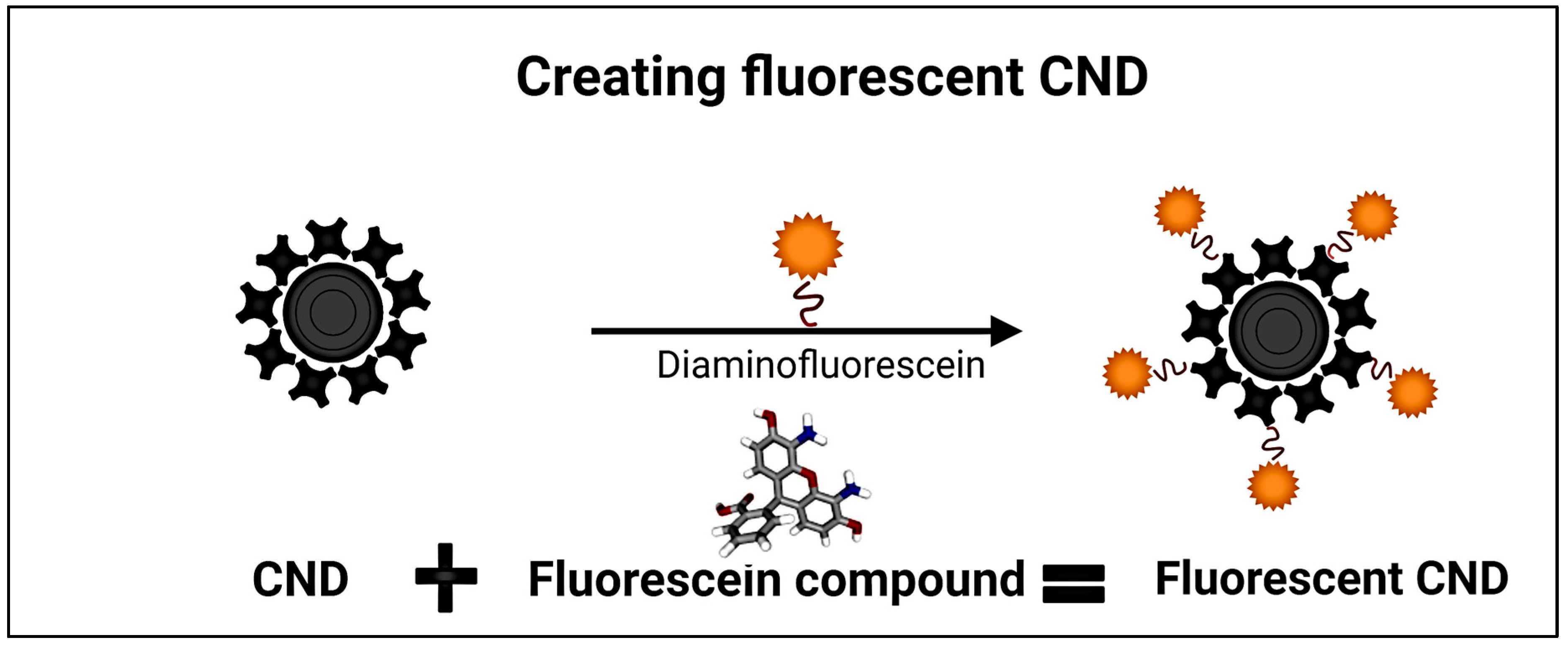
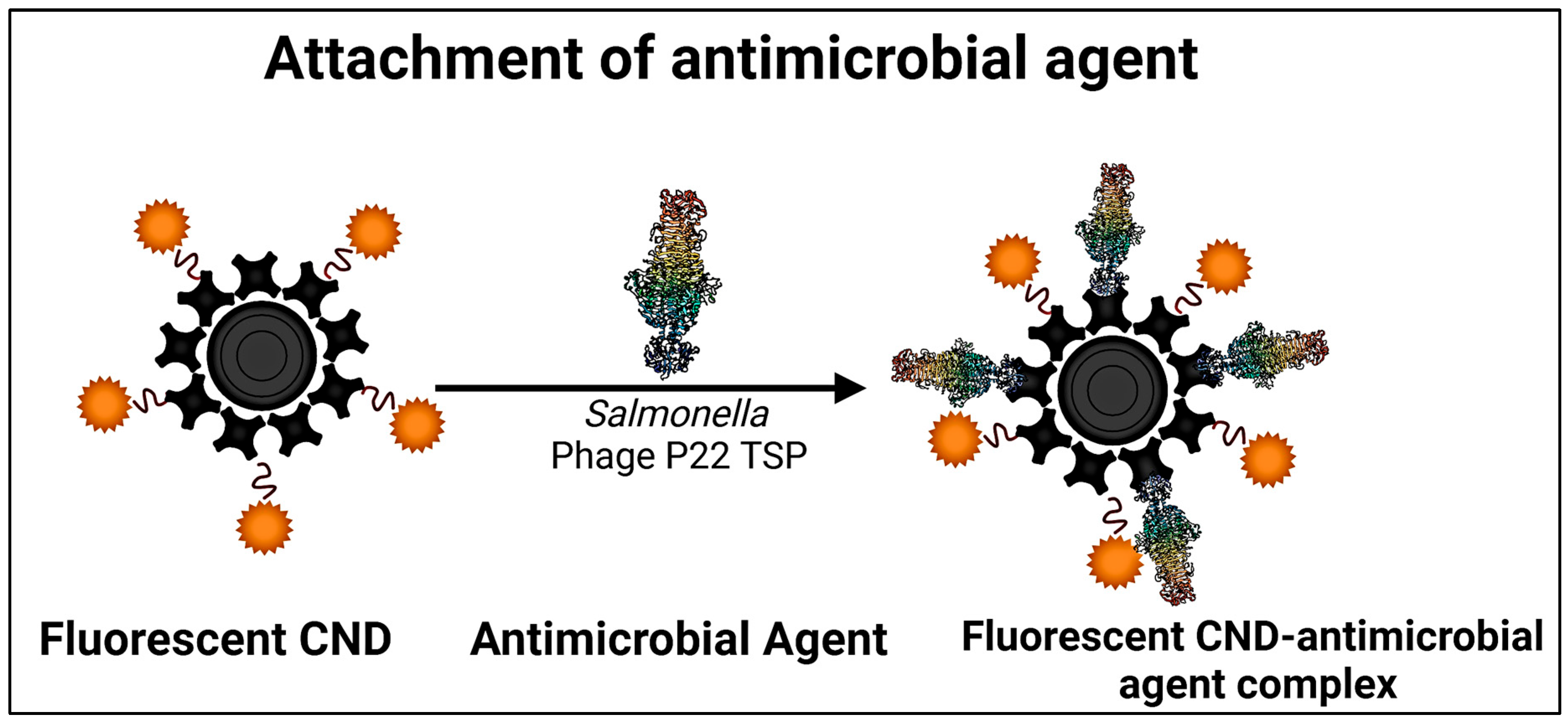
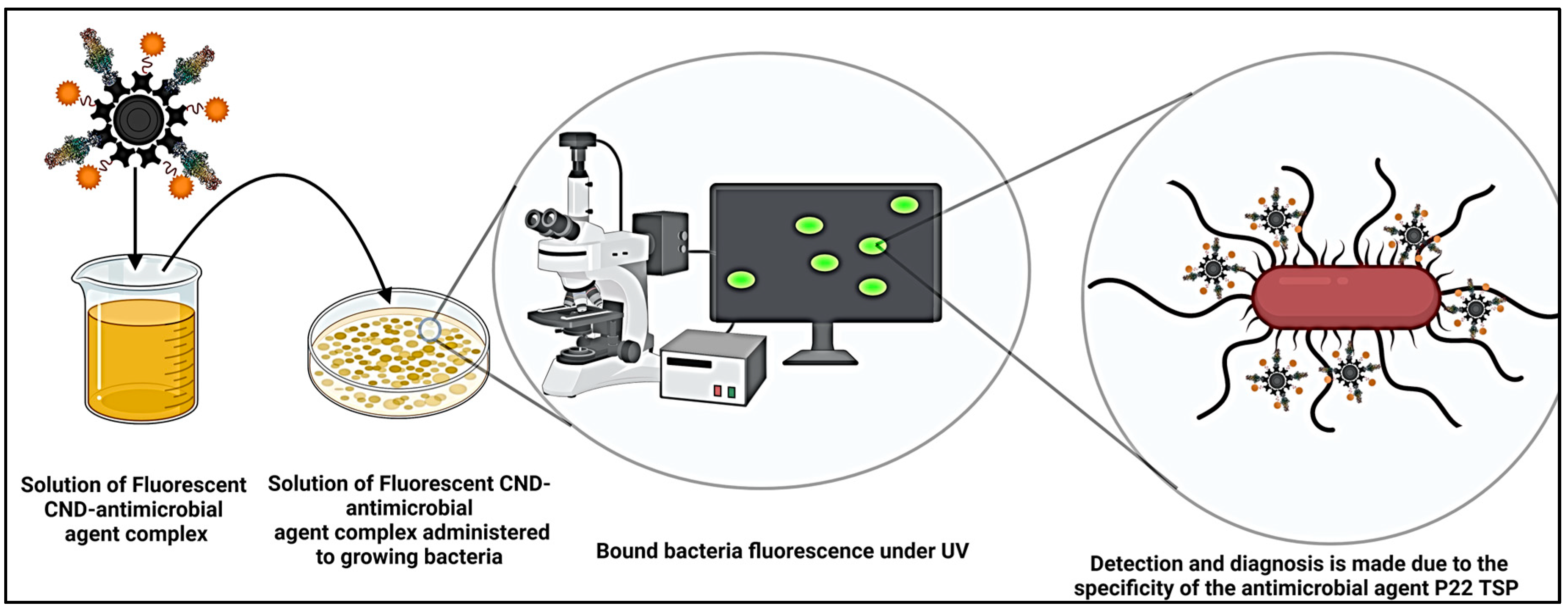
| No. | Marker | Diagnostic Application | References |
|---|---|---|---|
| 1 | Epidermal Growth Factor (EGF) | Detection and diagnosis of various cancers, including lung and breast cancers. | [116]. |
| 2 | Folic Acid (Folate) | Targeted drug delivery and imaging in cancer diagnosis. | [117]. |
| 3 | Aptamer AS1411 | Diagnostic and therapeutic applications in leukemia and other cancer types. | [118]. |
| 4 | Herceptin (Trastuzumab) | Detection of HER2-positive breast cancer for personalized medicine | [119]. |
| 5 | Anti-PSMA Antibodies | Prostate-specific membrane antigen (PSMA) targeting in prostate cancer diagnosis. | [120]. |
| 6 | Anti-CEA Antibodies | Carcinoembryonic antigen (CEA) targeting in colorectal cancer diagnosis. | [121]. |
| 7 | Anti-HER2 Antibodies | Human epidermal growth factor receptor 2 (HER2) detection in breast and gastric cancers. | [122]. |
| 8 | Anti-EGFR Antibodies | Epidermal growth factor receptor (EGFR) targeting in various cancers. | [123]. |
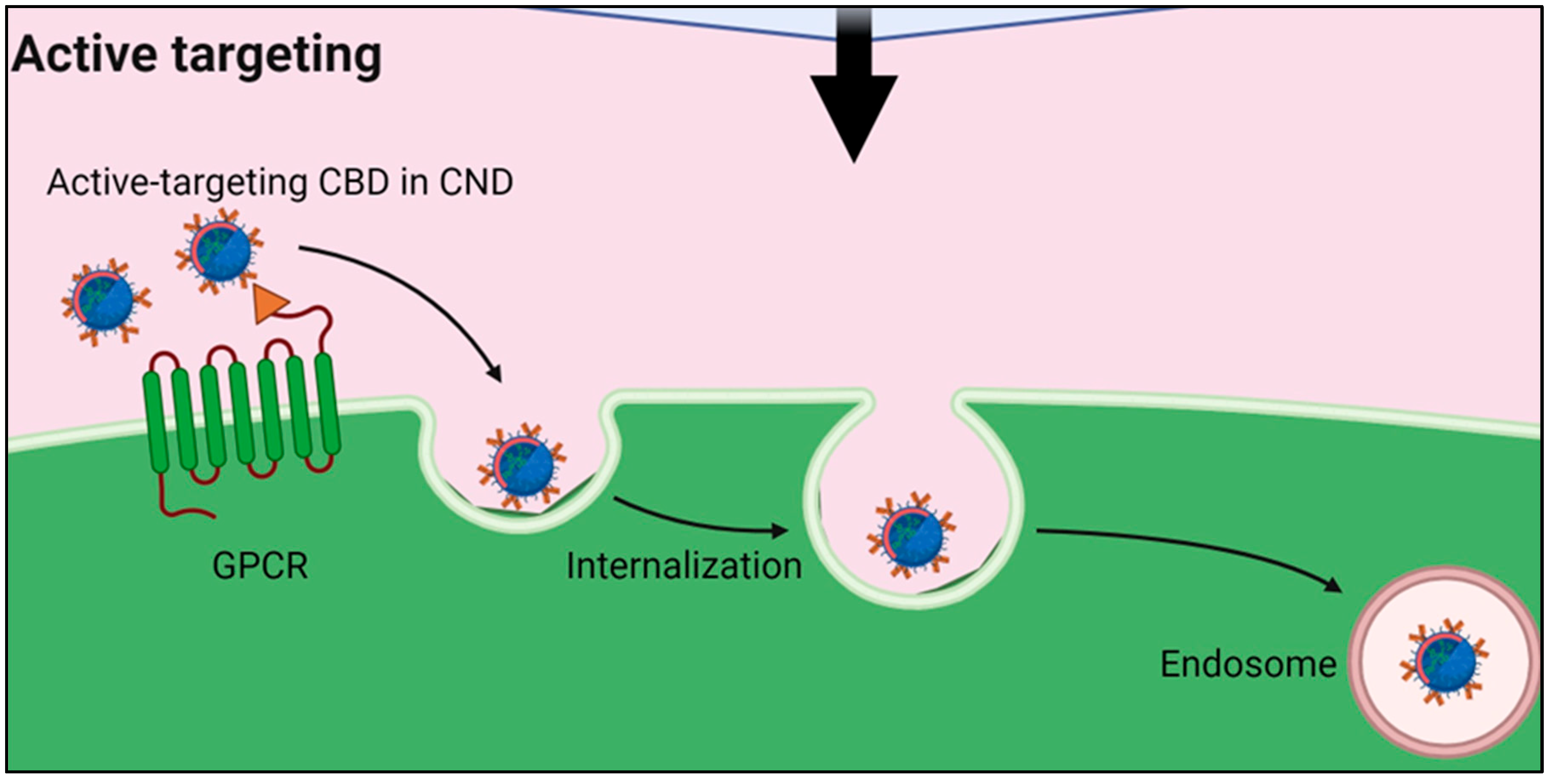
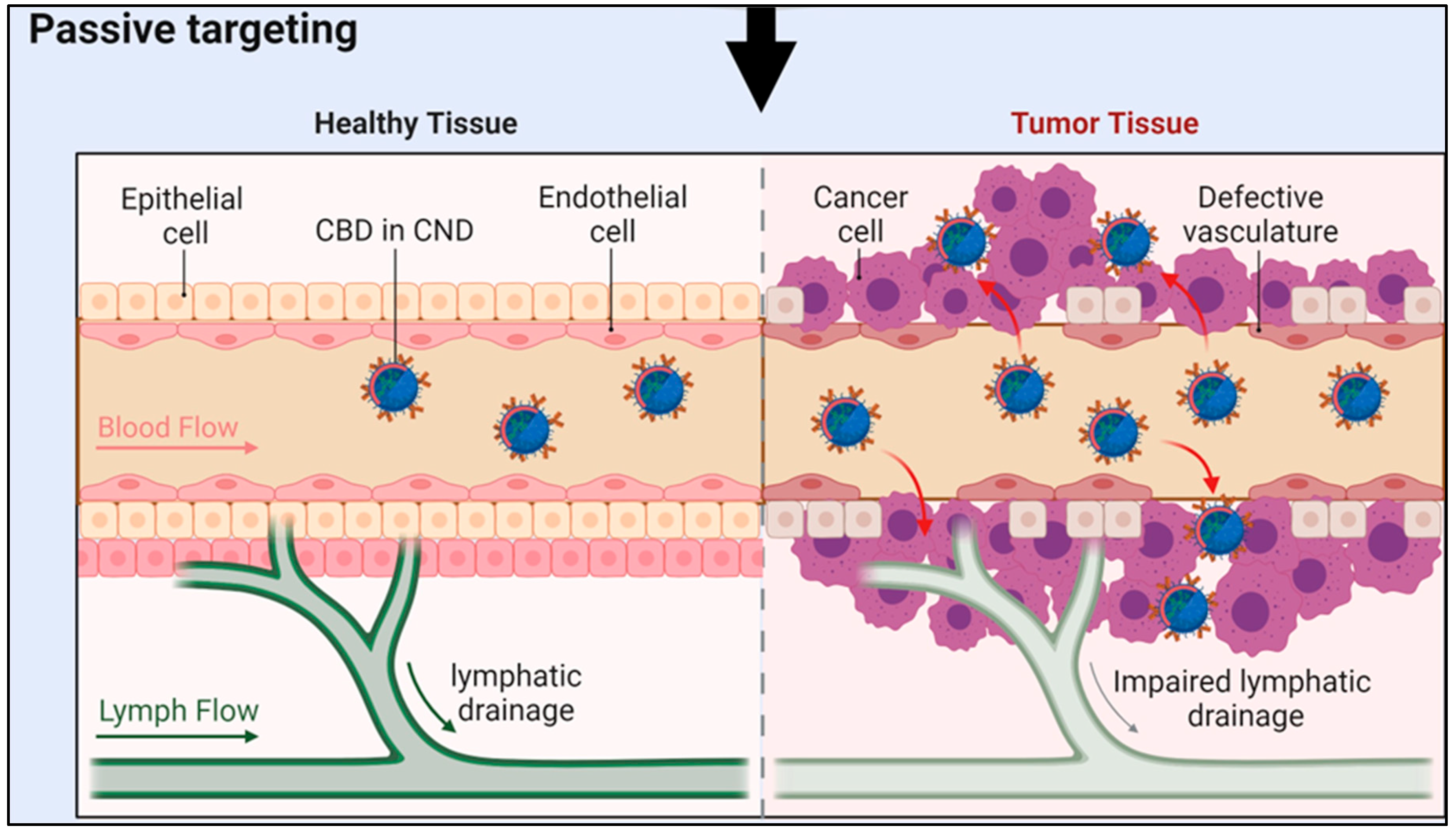
Comparative Analysis for Drug Loading Methods
Drug Loading Efficiency
The loading efficiency is calculated as follows,
Challenges and Potential Solutions
Stability and Long-term Storage
Safety and Toxicity Concerns
Regulatory Aspects
Conclusion
References
- Qader, I.B. and Prasad, K., Recent developments on ionic liquids and deep eutectic solvents for drug delivery applications. Pharmaceutical Research 2022, 39, pp.2367–2377. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R. , Mehta, P., Shankar, K.R., Rajora, M.A., Mishra, Y.K., Mostafavi, E. and Kaushik, A., Nanotechnology-assisted metered-dose inhalers (MDIs) for high-performance pulmonary drug delivery applications. Pharmaceutical research 2022, 39, pp.2831–2855. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, I. , Ayariga, J.A., Xu, J., Adebanjo, A., Robertson, B.K., Samuel-Foo, M. and Ajayi, O.S., CBD resistant Salmonella strains are susceptible to epsilon 34 phage tailspike protein. Frontiers in Medicine, 2023, 10, p–1075698. [Google Scholar] [CrossRef] [PubMed]
- Palrasu, M. , Wright, L., Patel, M., Leech, L., Branch, S., Harrelson, S. and Khan, S., Perspectives on Challenges in Cannabis Drug Delivery Systems: Where Are We? Medical Cannabis and Cannabinoids, 2022, 5, pp.102–119. [Google Scholar] [CrossRef] [PubMed]
- Mocci, F. , de Villiers Engelbrecht, L., Olla, C., Cappai, A., Casula, M.F., Melis, C., Stagi, L., Laaksonen, A. and Carbonaro, C.M., Carbon nanodots from an in silico perspective. Chemical Reviews, 2022, 122, pp.13709–13799. [Google Scholar] [CrossRef] [PubMed]
- Vora, L.K., Gholap, A.D., Jetha, K., Thakur, R.R.S., Solanki, H.K. and Chavda, V.P., 2023. Artificial Intelligence in Pharmaceutical Technology and Drug Delivery Design. Pharmaceutics, 15(7), p.1916.
- Jana, P. and Dev, A., 2022. Carbon quantum dots: A promising nanocarrier for bioimaging and drug delivery in cancer. Materials Today Communications, p.104068.
- Chauhan, D.S., Quraishi, M.A. and Verma, C., 2022. Carbon nanodots: recent advances in synthesis and applications. Carbon Letters, 32(7), pp.1603-1629.
- Huang, S., Song, Y., Zhang, J.R., Chen, X. and Zhu, J.J., 2023. Antibacterial Carbon Dots-Based Composites. Small, p.2207385.
- Zhang, W., Chen, J., Gu, J., Bartoli, M., Domena, J.B., Zhou, Y., Ferreira, B.C., Cilingir, E.K., McGee, C.M., Sampson, R. and Arduino, C., 2023. Nano-carrier for gene delivery and bioimaging based on pentaetheylenehexamine modified carbon dots. Journal of colloid and interface science, 639, pp.180-192.
- Kumar, V.B., Sher, I., Rencus-Lazar, S., Rotenstreich, Y. and Gazit, E., 2023. Functional carbon quantum dots for ocular imaging and therapeutic applications. Small, 19(7), p.2205754.
- Sonju, J.J., Dahal, A. and Jois, S.D., 2022. Liposome Nanocarriers for Peptide Drug Delivery. In Peptide Therapeutics: Fundamentals of Design, Development, and Delivery (pp. 203-235). Cham: Springer International Publishing.
- Qi, J., Zhang, R., Liu, X., Liu, Y., Zhang, Q., Cheng, H., Li, R., Wang, L., Wu, X. and Li, B., 2023. Carbon Dots as Advanced Drug-Delivery Nanoplatforms for Antiinflammatory, Antibacterial, and Anticancer Applications: A Review. ACS Applied Nano Materials.
- Farooq, A., Sabah, S., Dhou, S., Alsawaftah, N. and Husseini, G., 2022. Exogenous contrast agents in photoacoustic imaging: an in vivo review for tumor imaging. Nanomaterials, 12(3), p.393.
- Mou, C., Wang, X., Liu, Y., Xie, Z. and Zheng, M., 2023. A robust carbon dot-based antibacterial CDs-PVA film as a wound dressing for antibiosis and wound healing. Journal of Materials Chemistry B, 11(9), pp.1940-1947.
- Wang, Z.X., Wang, Z. and Wu, F.G., 2022. Carbon dots as drug delivery vehicles for antimicrobial applications: A minireview. ChemMedChem, 17(13), p.e202200003.
- Pagano, C., Savarese, B., Coppola, L., Navarra, G., Avilia, G., Laezza, C. and Bifulco, M., 2023. Cannabinoids in the modulation of oxidative signaling. International Journal of Molecular Sciences, 24(3), p.2513.
- Chafiq, M., Chaouiki, A. and Ko, Y.G., 2023. Advances in COFs for Energy Storage Devices: Harnessing the Potential of Covalent Organic Framework Materials. Energy Storage Materials, p.103014.
- Gildea, L., Ayariga, J.A., Ajayi, O.S., Xu, J., Villafane, R. and Samuel-Foo, M., 2022. Cannabis sativa CBD Extract Shows Promising Antibacterial Activity against Salmonella typhimurium and S. newington. Molecules, 27(9), p.2669.
- Jugl, S., Sajdeya, R., Morris, E.J., Goodin, A.J. and Brown, J.D., 2021. Much ado about dosing: the needs and challenges of defining a standardized cannabis unit. Medical cannabis and cannabinoids, 4(2), pp.121-124.
- Ramalho, Í.M.D.M., Pereira, D.T., Galvão, G.B.L., Freire, D.T., Amaral-Machado, L., Alencar, É.D.N. and Egito, E.S.T.D., 2021. Current trends on cannabidiol delivery systems: where are we and where are we going?. Expert Opinion on Drug Delivery, 18(11), pp.1577-1587.
- Kosović, E., Sýkora, D. and Kuchař, M., 2021. Stability study of cannabidiol in the form of solid powder and sunflower oil solution. Pharmaceutics, 13(3), p.412.
- Yoo, H.W., Moon, J.H., Jo, S.J., Lee, Y.N., Park, J.K., Lee, T.H., Cha, S.W., Cho, Y.D., Park, S.H., Park, S.I. and Jeong, S., 2021. A novel electrocautery-enhanced delivery system for one-step endoscopic ultrasound-guided drainage of the gallbladder and bile duct using a lumen-apposing metal stent: a feasibility study. Endoscopy, 53(09), pp.922-926.
- Wu, A., March, L., Zheng, X., Huang, J., Wang, X., Zhao, J., Blyth, F.M., Smith, E., Buchbinder, R. and Hoy, D., 2020. Global low back pain prevalence and years lived with disability from 1990 to 2017: estimates from the Global Burden of Disease Study 2017. Annals of translational medicine, 8(6).
- Peres, M.A., Macpherson, L.M., Weyant, R.J., Daly, B., Venturelli, R., Mathur, M.R., Listl, S., Celeste, R.K., Guarnizo-Herreño, C.C., Kearns, C. and Benzian, H., 2019. Oral diseases: a global public health challenge. The Lancet, 394(10194), pp.249-260.
- Atalay, S., Jarocka-Karpowicz, I. and Skrzydlewska, E., 2019. Antioxidative and anti-inflammatory properties of cannabidiol. Antioxidants, 9(1), p.21.
- Lewandowska, A., Rudzki, G., Lewandowski, T., Próchnicki, M., Rudzki, S., Laskowska, B. and Brudniak, J., 2020. Quality of life of cancer patients treated with chemotherapy. International journal of environmental research and public health, 17(19), p.6938.
- Kim, J., Choi, J.Y., Seo, J. and Choi, I.S., 2021. Neuroprotective effect of cannabidiol against hydrogen peroxide in hippocampal neuron culture. Cannabis and Cannabinoid Research, 6(1), pp.40-47.
- Tambe, S.M., Mali, S., Amin, P.D. and Oliveira, M., 2023. Neuroprotective potential of cannabidiol: Molecular mechanisms and clinical implications. Journal of Integrative Medicine.
- Aychman, M.M., Goldman, D.L. and Kaplan, J.S., 2023. Cannabidiol's neuroprotective properties and potential treatment of traumatic brain injuries. Frontiers in neurology, 14, p.1087011.
- Pesántez Ríos, G., Armijos Acurio, L., Jimbo Sotomayor, R., Cueva, V., Pesántez Ríos, X., Navarrete Zambrano, H., Pascual, S. and Pesántez Cuesta, G., 2022. A Pilot Study on the Use of Low Doses of CBD to Control Seizures in Rare and Severe Forms of Drug-Resistant Epilepsy. Life, 12(12), p.2065.
- Huntsman, R.J., Tang-Wai, R. and Shackelford, A.E., 2020. Cannabis for pediatric epilepsy. Journal of Clinical Neurophysiology, 37(1), pp.2-8.
- Mazurkiewicz-Bełdzińska, M. and Zawadzka, M., 2022. Use of cannabidiol in the treatment of epilepsy. Neurologia i neurochirurgia polska, 56(1), pp.14-20.
- Burt, R.K., Muraro, P.A., Farge, D., Oliveira, M.C., Snowden, J.A., Saccardi, R., Han, X., Quigley, K., Bueno, V., Frasca, D. and Fedorenko, D., 2021. New autoimmune diseases after autologous hematopoietic stem cell transplantation for multiple sclerosis. Bone Marrow Transplantation, 56(7), pp.1509-1517.
- Vodjgani, M., Salehi, Z. and Izad, M., 2020. The influence of reactive oxygen species in the immune system and pathogenesis of multiple sclerosis. Autoimmune diseases, 2020.
- Kopustinskiene, D.M., Masteikova, R., Lazauskas, R. and Bernatoniene, J., 2022. Cannabis sativa L. bioactive compounds and their protective role in oxidative stress and inflammation. Antioxidants, 11(4), p.660.
- Cetin, S., Knez, D., Gobec, S., Kos, J. and Pišlar, A., 2022. Cell models for Alzheimer’s and Parkinson’s disease: At the interface of biology and drug discovery. Biomedicine & Pharmacotherapy, 149, p.112924.
- Yousaf, M., Chang, D., Liu, Y., Liu, T. and Zhou, X., 2022. Neuroprotection of cannabidiol, its synthetic derivatives and combination preparations against microglia-mediated neuroinflammation in neurological disorders. Molecules, 27(15), p.4961.
- Maleki, S.J., Crespo, J.F. and Cabanillas, B., 2019. Anti-inflammatory effects of flavonoids. Food chemistry, 299, p.125124.
- tsiopoulos, C., Mayr, H.L. and Thomas, C.J., 2022. The anti-inflammatory effects of a Mediterranean diet: A review. Current opinion in clinical nutrition and metabolic care, 25(6), pp.415-422.
- O'Connor, C., 2022. Anti-Inflammatory Effect of CBD on Cultured Microglia.
- Harrison, S.R., Li, D., Jeffery, L.E., Raza, K. and Hewison, M., 2020. Vitamin D, autoimmune disease and rheumatoid arthritis. Calcified tissue international, 106, pp.58-75.
- Lee, M. and Chang, E.B., 2021. Inflammatory bowel diseases (IBD) and the microbiome—searching the crime scene for clues. Gastroenterology, 160(2), pp.524-537.
- Ho, A.W. and Kupper, T.S., 2019. T cells and the skin: from protective immunity to inflammatory skin disorders. Nature Reviews Immunology, 19(8), pp.490-502.
- Szántó, M., Dózsa, A., Antal, D., Szabó, K., Kemény, L. and Bai, P., 2019. Targeting the gut-skin axis—Probiotics as new tools for skin disorder management. Experimental dermatology, 28(11), pp.1210-1218.
- Brave, L., 2019. CBD the Skin, Mind and Body. J Altern Complement Integr Med, 5, p.060.
- Thakker, A.M. and Sun, D., 2021. Sustainable plant-based bioactive materials for functional printed textiles. The Journal of the Textile Institute, 112(8), pp.1324-1358.
- Itzhaki, E., Hadad, E., Moskovits, N., Stemmer, S.M. and Margel, S., 2021. Tumor-targeted fluorescent proteinoid nanocapsules encapsulating synergistic drugs for personalized cancer therapy. Pharmaceuticals, 14(7), p.648.
- O’Brien, K., 2022. Cannabidiol (CBD) in cancer management. Cancers, 14(4), p.885.
- Abrams, D.I., 2022. Cannabis, cannabinoids and cannabis-based medicines in cancer care. Integrative Cancer Therapies, 21, p.15347354221081772.
- Joca, S., Silote, G.P., Sartim, A., Sales, A., Guimarães, F. and Wegener, G., 2021. Putative effects of cannabidiol in depression and synaptic plasticity. The neuroscience of depression, pp.459-467.
- Karaźniewicz-Łada, M., Główka, A.K., Mikulska, A.A. and Główka, F.K., 2021. Pharmacokinetic drug–drug interactions among antiepileptic drugs, including CBD, drugs used to treat COVID-19 and nutrients. International Journal of Molecular Sciences, 22(17), p.9582.
- Kumric, M., Dujic, G., Vrdoljak, J., Svagusa, K., Kurir, T.T., Supe-Domic, D., Dujic, Z. and Bozic, J., 2023. CBD supplementation reduces arterial blood pressure via modulation of the sympatho-chromaffin system: A substudy from the HYPER-H21-4 trial. Biomedicine & Pharmacotherapy, 160, p.114387.
- Schubert-Bast, S. and Strzelczyk, A., 2021. Review of the treatment options for epilepsy in tuberous sclerosis complex: towards precision medicine. Therapeutic Advances in Neurological Disorders, 14, p.17562864211031100.
- Saviano, A., Raucci, F., Tallarico, M., De Caro, C., Di Martino, S., Nesci, V., Roberti, R., Iannone, L.F., Colia, A.L., Dimonte, S. and Furgiuele, A., 2021. Cannabidiol and the central nervous system: translating into clinics. Pharm. Adv, 3, p.369.
- Muresan, P., Woodhams, S., Smith, F., Taresco, V., Shah, J., Wong, M., Chapman, V., Smith, S., Hathway, G., Rahman, R. and Gershkovich, P., 2023. Evaluation of cannabidiol nanoparticles and nanoemulsion biodistribution in the central nervous system after intrathecal administration for the treatment of pain. Nanomedicine: Nanotechnology, Biology and Medicine, 49, p.102664.
- Montané, X., Bajek, A., Roszkowski, K., Montornés, J.M., Giamberini, M., Roszkowski, S., Kowalczyk, O., Garcia-Valls, R. and Tylkowski, B., 2020. Encapsulation for cancer therapy. Molecules, 25(7), p.1605.
- Demisli, S., Galani, E., Goulielmaki, M., Kyrilis, F.L., Ilić, T., Hamdi, F., Crevar, M., Kastritis, P.L., Pletsa, V., Nallet, F. and Savić, S., 2023. Encapsulation of cannabidiol in oil-in-water nanoemulsions and nanoemulsion-filled hydrogels: A structure and biological assessment study. Journal of Colloid and Interface Science, 634, pp.300-313.
- Grifoni, L., Vanti, G., Donato, R., Sacco, C. and Bilia, A.R., 2022. Promising nanocarriers to enhance solubility and bioavailability of cannabidiol for a plethora of therapeutic opportunities. Molecules, 27(18), p.6070.
- Arvapalli, D.M., Sheardy, A.T., Allado, K., Chevva, H., Yin, Z. and Wei, J., 2020. Design of curcumin loaded carbon nanodots delivery system: Enhanced bioavailability, release kinetics, and anticancer activity. ACS Applied Bio Materials, 3(12), pp.8776-8785.
- Dhamodharan, D., Byun, H.S., Shree, M.V., Veeman, D., Natrayan, L. and Stalin, B., 2022. Carbon nanodots: synthesis, mechanisms for bio-electrical applications. Journal of Industrial and Engineering Chemistry, 110, pp.68-83.
- Choi, S.A., Jeong, Y., Lee, J., Huh, Y.H., Choi, S.H., Kim, H.S., Cho, D.H., Lee, J.S., Kim, H., An, H.R. and Lee, S., 2020. Biocompatible liquid-type carbon nanodots (C-paints) as light delivery materials for cell growth and astaxanthin induction of Haematococcus pluvialis. Materials Science and Engineering: C, 109, p.110500.
- Bhattacharya, D., Kumar, V. and Packirisamy, G., 2021. Biocompatible carbon nanodots from red onion peels for anti-oxidative and bioimaging applications. Materials Express, 11(12), pp.1958-1965.
- Utterström, J., Naeimipour, S., Selegård, R. and Aili, D., 2021. Coiled coil-based therapeutics and drug delivery systems. Advanced Drug Delivery Reviews, 170, pp.26-43.
- Nelson, K.M., Bisson, J., Singh, G., Graham, J.G., Chen, S.N., Friesen, J.B., Dahlin, J.L., Niemitz, M., Walters, M.A. and Pauli, G.F., 2020. The essential medicinal chemistry of cannabidiol (CBD). Journal of medicinal chemistry, 63(21), pp.12137-12155.
- Bruno, F., Sciortino, A., Buscarino, G., Soriano, M.L., Ríos, Á., Cannas, M., Gelardi, F., Messina, F. and Agnello, S., 2021. A comparative study of top-down and bottom-up carbon nanodots and their interaction with mercury ions. Nanomaterials, 11(5), p.1265.
- Banger, A., Gautam, S., Jadoun, S., Jangid, N.K., Srivastava, A., Pulidindi, I.N., Dwivedi, J. and Srivastava, M., 2023. Synthetic Methods and Applications of Carbon Nanodots. Catalysts, 13(5), p.858.
- Li, Q., Li, X. and Zhao, C., 2020. Strategies to obtain encapsulation and controlled release of small hydrophilic molecules. Frontiers in bioengineering and biotechnology, 8, p.437.
- Sood, A., Gupta, A. and Agrawal, G., 2021. Recent advances in polysaccharides based biomaterials for drug delivery and tissue engineering applications. Carbohydrate Polymer Technologies and Applications, 2, p.100067.
- Sun, T., Dasgupta, A., Zhao, Z., Nurunnabi, M. and Mitragotri, S., 2020. Physical triggering strategies for drug delivery. Advanced drug delivery reviews, 158, pp.36-62.
- Rizzo, F. and Kehr, N.S., 2021. Recent advances in injectable hydrogels for controlled and local drug delivery. Advanced healthcare materials, 10(1), p.2001341.
- Wagle, S.R., Kovacevic, B., Walker, D., Ionescu, C.M., Shah, U., Stojanovic, G., Kojic, S., Mooranian, A. and Al-Salami, H., 2020. Alginate-based drug oral targeting using bio-micro/nano encapsulation technologies. Expert Opinion on Drug Delivery, 17(10), pp.1361-1376.
- Agarwal, A., Majumder, S. and Sankapal, B.R., 2022. Carbon nanotube-functionalized surface-assisted growth of cobalt phosphate nanodots: a highly stable and bendable all-solid-state symmetric supercapacitor. Energy & Fuels, 36(11), pp.5953-5964.
- Yang, T., Zhan, L. and Huang, C.Z., 2020. Recent insights into functionalized electrospun nanofibrous films for chemo-/bio-sensors. TrAC Trends in Analytical Chemistry, 124, p.115813.
- Li, Y., Zhang, J., Chen, Q., Xia, X. and Chen, M., 2021. Emerging of heterostructure materials in energy storage: a review. Advanced Materials, 33(27), p.2100855.
- Cortés, F.B., Zapata, K., Rojano, B.A., Carrasco-Marín, F., Gallego, J., Hernández, M.A. and Franco, C.A., 2019. Dual-purpose materials based on carbon xerogel microspheres (CXMS) for delayed release of cannabidiol (cbd) and subsequent aflatoxin removal. Molecules, 24(18), p.3398.
- Li, H., Yang, T.X., Zhao, Q.S., Hou, S.B., Tian, R.R. and Zhao, B., 2023. Comparative study of encapsulated cannabidiol ternary solid dispersions prepared by different techniques: The application of a novel technique jet milling. Food Research International, 168, p.112783.
- Δεμισλή, Σ.Κ., 2023. Development of nanocarriers for the encapsulation of cannabinoids and other bioactive compounds.
- Grifoni, L., Vanti, G., Donato, R., Sacco, C. and Bilia, A.R., 2022. Promising nanocarriers to enhance solubility and bioavailability of cannabidiol for a plethora of therapeutic opportunities. Molecules, 27(18), p.6070.
- Gheorghita, R., Anchidin-Norocel, L., Filip, R., Dimian, M. and Covasa, M., 2021. Applications of biopolymers for drugs and probiotics delivery. Polymers, 13(16), p.2729.
- Millar, S.A., Maguire, R.F., Yates, A.S. and O’Sullivan, S.E., 2020. Towards better delivery of cannabidiol (CBD). Pharmaceuticals, 13(9), p.219.
- Sushma, A.A., 2021. Coherent Biomaterials: A Virus Self-assembly Based Approach. Indiana University.
- Wang, Z., Zhang, P., Yin, C., Li, Y., Liao, Z., Yang, C., Liu, H., Wang, W., Fan, C., Sun, D. and Cheng, L., 2023. Antibiotic-Derived Carbon-Nanodot-Decorated Hydrogel for Reactive Oxygen Species-Enhanced Anti-Infection Through Biofilm Damage. Advanced Functional Materials, p.2300341.
- Bankoti, K., Rameshbabu, A.P., Datta, S., Roy, M., Goswami, P., Roy, S., Das, A.K., Ghosh, S.K. and Dhara, S., 2020. Carbon nanodot decorated acellular dermal matrix hydrogel augments chronic wound closure. Journal of Materials Chemistry B, 8(40), pp.9277-9294.
- Erikson, K.M., El-Khouri, K., Petric, R., Tang, C., Chen, J., Vasquez, D.E.C., Fordahl, S.C. and Jia, Z., 2023. Carbon Nanodots Attenuate Lipid Peroxidation in the LDL Receptor Knockout Mouse Brain. Antioxidants, 12(5), p.1081.
- Gutiérrez-Gálvez, L., García-Mendiola, T., Gutiérrez-Sánchez, C., Guerrero-Esteban, T., García-Diego, C., Buendía, I., García-Bermejo, M.L., Pariente, F. and Lorenzo, E., 2021. Carbon nanodot–based electrogenerated chemiluminescence biosensor for miRNA-21 detection. Microchimica Acta, 188, pp.1-12.
- Bahrampour Juybari, K., Rizwan, K., Faramarz, S., Sadeghi, A., Amirkhosravi, A., Hadi Nematollahi, M. and Mehrabani, M., 2023. Carbon Quantum Dots as Multi-Purpose Nanomaterial in Stem Cell Therapy. Chemistry & Biodiversity, 20(4), p.e202200721.
- Smith, R., Strandberg, O., Leuzy, A., Betthauser, T.J., Johnson, S.C., Pereira, J.B. and Hansson, O., 2021. Sex differences in off-target binding using tau positron emission tomography. NeuroImage: Clinical, 31, p.102708.
- Zhou, Y., Chen, X., Cao, J. and Gao, H., 2020. Overcoming the biological barriers in the tumor microenvironment for improving drug delivery and efficacy. Journal of Materials Chemistry B, 8(31), pp.6765-6781.
- Wang, W.T., Han, C., Sun, Y.M., Chen, T.Q. and Chen, Y.Q., 2019. Noncoding RNAs in cancer therapy resistance and targeted drug development. Journal of hematology & oncology, 12(1), pp.1-15.
- Aytar Çelik, P., Erdogan-Gover, K., Barut, D., Enuh, B.M., Amasya, G., Sengel-Türk, C.T., Derkus, B. and Çabuk, A., 2023. Bacterial Membrane Vesicles as Smart Drug Delivery and Carrier Systems: A New Nanosystems Tool for Current Anticancer and Antimicrobial Therapy. Pharmaceutics, 15(4), p.1052.
- Pillai, S., Kwan, J.C., Yaziji, F., Yu, H. and Tran, S.D., 2023. Mapping the Potential of Microfluidics in Early Diagnosis and Personalized Treatment of Head and Neck Cancers. Cancers, 15(15), p.3894.
- Koganemaru, S. and Shitara, K., 2021. Antibody–drug conjugates to treat gastric cancer. Expert Opinion on Biological Therapy, 21(7), pp.923-930.
- Bezombes, C. and Pérez-Galán, P., 2021. Immunotherapies in non-Hodgkin’s lymphoma. Cancers, 13(14), p.3625.
- Hoetzel, J. and Suess, B., 2022. Structural changes in aptamers are essential for synthetic riboswitch engineering. Journal of Molecular Biology, p.167631.
- Zheng, L., Zhang, Q., Zhang, Y., Qiu, L. and Tan, W., 2022. Aptamer-based cell recognition and detection. Current Analytical Chemistry, 18(6), pp.612-621.
- Yamada, Y., Onda, T., Wada, Y., Hamada, K., Kikkawa, Y. and Nomizu, M., 2023. Structure–Activity Relationships of RGD-Containing Peptides in Integrin αvβ5-Mediated Cell Adhesion. ACS omega, 8(5), pp.4687-4693.
- Vega, F.M., Colmenero-Repiso, A., Gómez-Muñoz, M.A., Rodríguez-Prieto, I., Aguilar-Morante, D., Ramírez, G., Márquez, C., Cabello, R. and Pardal, R., 2019. CD44-high neural crest stem-like cells are associated with tumour aggressiveness and poor survival in neuroblastoma tumours. EBioMedicine, 49, pp.82-95.
- Scaranti, M., Cojocaru, E., Banerjee, S. and Banerji, U., 2020. Exploiting the folate receptor α in oncology. Nature reviews clinical oncology, 17(6), pp.349-359.
- MacDonald, E., Forrester, A., Valades-Cruz, C.A., Madsen, T.D., Hetmanski, J., Dransart, E., Ng, Y., Godbole, R., Akhil Shp, A., Leconte, L. and Chambon, V., 2023. Growth factor-induced desialylation for the fast control of endocytosis. bioRxiv, pp.2023-09.
- Kushner, B.H., Modak, S., Kramer, K., Basu, E.M., Iglesias-Cardenas, F., Roberts, S.S. and Cheung, N.K.V., 2023. Immunotherapy with anti-GD2 monoclonal antibody in infants with high-risk neuroblastoma. International Journal of Cancer, 152(2), pp.259-266.
- Liu, D., Zhao, J. and Song, Y., 2019. Engineering switchable and programmable universal CARs for CAR T therapy. Journal of Hematology & Oncology, 12, pp.1-9.
- Anand, V., Khandelwal, M., Appunni, S., Gupta, N., Seth, A., Singh, P., Mathur, S. and Sharma, A., 2019. CD44 splice variant (CD44v3) promotes progression of urothelial carcinoma of bladder through Akt/ERK/STAT3 pathways: novel therapeutic approach. Journal of cancer research and clinical oncology, 145, pp.2649-2661.
- Llovet, J.M., Castet, F., Heikenwalder, M., Maini, M.K., Mazzaferro, V., Pinato, D.J., Pikarsky, E., Zhu, A.X. and Finn, R.S., 2022. Immunotherapies for hepatocellular carcinoma. Nature reviews Clinical oncology, 19(3), pp.151-172.
- Ponsiglione, A., Ascione, R., Nappi, C., Imbriaco, M., Klain, M., Cuocolo, R., Cuocolo, A. and Petretta, M., 2021. Cardiac hybrid imaging: novel tracers for novel targets. Journal of Geriatric Cardiology: JGC, 18(9), p.748.
- Teo, M.Y., Rathkopf, D.E. and Kantoff, P., 2019. Treatment of advanced prostate cancer. Annual review of medicine, 70, pp.479-499.
- Hajebrahimi, Z. and Salavatifar, M., 2022. CD44 expression changes and increased apoptosis in MCF-7 cell line of breast cancer in simulated microgravity condition. Pars Journal of Medical Sciences, 17(3), pp.26-34.
- Gutiérrez-Gálvez, L., García-Mendiola, T., Gutiérrez-Sánchez, C., Guerrero-Esteban, T., García-Diego, C., Buendía, I., García-Bermejo, M.L., Pariente, F. and Lorenzo, E., 2021. Carbon nanodot–based electrogenerated chemiluminescence biosensor for miRNA-21 detection. Microchimica Acta, 188, pp.1-12.
- Kakodkar, S., Dhawal, P. and Kadam, J., 2023. Applications of Nanomaterials in Medicine: Current Status and Future Scope. In Novel Technologies in Biosystems, Biomedical & Drug Delivery (pp. 71-103). Singapore: Springer Nature Singapore.
- Mehata, A.K., Viswanadh, M.K., Solomon, V.R. and Muthu, M.S., 2022. Radionanotheranostics for breast cancer diagnosis and therapy: Recent advances and future opportunities. Targeted Nanomedicine for Breast Cancer Therapy, pp.465-508.
- Afzal, S., Hassan, M., Ullah, S., Abbas, H., Tawakkal, F. and Khan, M.A., 2022. Breast cancer; discovery of novel diagnostic biomarkers, drug resistance, and therapeutic implications. Frontiers in molecular biosciences, 9, p.783450.
- Musa, N., Banerjee, S., Singh, N.B. and Usman, U.L., 2023. Carbon nanomaterials and their applications. Emerging Nanomaterials and Their Impact on Society in the 21st Century, 135, pp.40-71.
- Mortezagholi, S., Mahmoudi, A.R., Shojaeian, S., Vafaei, S., Soltanghoraei, H., Bayat, A.A., Shokri, F., Ghods, R. and Zarnani, A.H., 2023. Discovery of a novel marker for human granulocytes and tissue macrophages: RTL1 revisited. Cell and Tissue Research, pp.1-12.
- Rosch, J.C., Balikov, D.A., Gong, F. and Lippmann, E.S., 2020. A systematic evolution of ligands by exponential enrichment workflow with consolidated counterselection to efficiently isolate high-affinity aptamers. Engineering Reports, 2(1), p.e12089.
- Pillay, T.S. and Muyldermans, S., 2021. Application of single-domain antibodies (“nanobodies”) to laboratory diagnosis. Ann Lab Med, 41(6), pp.549-558.
- Li, X., Liu, X. and Liu, X., 2021. Self-assembly of colloidal inorganic nanocrystals: nanoscale forces, emergent properties and applications. Chemical Society Reviews, 50(3), pp.2074-2101.
- Jiao, Y., Sun, H., Jia, Y., Liu, Y., Gao, Y., Xian, M., Shuang, S. and Dong, C., 2019. Functionalized fluorescent carbon nanoparticles for sensitively targeted folate-receptor-positive cancer cells. Microchemical Journal, 146, pp.464-470.
- Fong, J.F.Y., Ng, Y.H. and Ng, S.M., 2019. Recent advances in carbon dots for bioanalysis and the future perspectives. Carbon Nanomaterials for Bioimaging, Bioanalysis, and Therapy, pp.203-264.
- Aswathya, R.G. and Kumarb, D.S., 2022. Optical Nanoprobes for Diagnosis. Bionanotechnology in Cancer: Diagnosis and Therapy, p.195.
- Zhi, D., Yang, T., Yang, J., Fu, S. and Zhang, S., 2020. Targeting strategies for superparamagnetic iron oxide nanoparticles in cancer therapy. Acta biomaterialia, 102, pp.13-34.
- Okyem, S., Awotunde, O., Ogunlusi, T., Riley, M.B. and Driskell, J.D., 2021. High-affinity points of interaction on antibody allow synthesis of stable and highly functional antibody–gold nanoparticle conjugates. Bioconjugate Chemistry, 32(8), pp.1753-1762.
- Sargazi, S., Simge, E.R., Mobashar, A., Gelen, S.S., Rahdar, A., Ebrahimi, N., Hosseinikhah, S.M., Bilal, M. and Kyzas, G.Z., 2022. Aptamer-conjugated carbon-based nanomaterials for cancer and bacteria theranostics: A review. Chemico-Biological Interactions, 361, p.109964.
- Li, Q., Xiong, Y., Ji, C. and Yan, Z., 2019. The application of nanotechnology in the codelivery of active constituents of plants and chemotherapeutics for overcoming physiological barriers during antitumor treatment. BioMed Research International, 2019.
- Mitchell, M.J., Billingsley, M.M., Haley, R.M., Wechsler, M.E., Peppas, N.A. and Langer, R., 2021. Engineering precision nanoparticles for drug delivery. Nature reviews drug discovery, 20(2), pp.101-124.
- Wan, T., Zhong, J., Pan, Q., Zhou, T., Ping, Y. and Liu, X., 2022. Exosome-mediated delivery of Cas9 ribonucleoprotein complexes for tissue-specific gene therapy of liver diseases. Science Advances, 8(37), p.eabp9435.
- Sahu, T., Ratre, Y.K., Chauhan, S., Bhaskar, L.V.K.S., Nair, M.P. and Verma, H.K., 2021. Nanotechnology based drug delivery system: Current strategies and emerging therapeutic potential for medical science. Journal of Drug Delivery Science and Technology, 63, p.102487.
- Alzhrani, R., Alsaab, H.O., Petrovici, A., Bhise, K., Vanamala, K., Sau, S., Krinock, M.J. and Iyer, A.K., 2020. Improving the therapeutic efficiency of noncoding RNAs in cancers using targeted drug delivery systems. Drug discovery today, 25(4), pp.718-730.
- Zhao, Z., Ukidve, A., Kim, J. and Mitragotri, S., 2020. Targeting strategies for tissue-specific drug delivery. Cell, 181(1), pp.151-167.
- Shi, P., Cheng, Z., Zhao, K., Chen, Y., Zhang, A., Gan, W. and Zhang, Y., 2023. Active targeting schemes for nano-drug delivery systems in osteosarcoma therapeutics. Journal of Nanobiotechnology, 21(1), pp.1-27.
- Adepu, S. and Ramakrishna, S., 2021. Controlled drug delivery systems: current status and future directions. Molecules, 26(19), p.5905.
- Qu, J., Zhang, X., Gu, R., Gu, Z., Lu, X., Zhang, Z., Yang, H. and Jing, S., 2023. pH-controlled construction of 2D and 3D micro-nano hybrid carbon architectures with fluorescence/phosphorescence dual-mode emission for white-emitting diodes. Carbon, 214, p.118355.
- Reva, Y., Jana, B., Langford, D., Kinzelmann, M., Bo, Y., Schol, P.R., Scharl, T., Zhao, X., Crisp, R.W., Drewello, T. and Clark, T., 2023. Understanding the Visible Absorption of Electron Accepting and Donating CNDs. Small, p.2207238.
- Mathew, A.A., Varghese, M. and Balachandran, M., 2023. Biosafety and Toxicity Evaluation of Carbon Nanomaterials. In Carbon Nanostructures in Biomedical Applications (pp. 363-398). Cham: Springer International Publishing.
- Rayamajhi, S., Nguyen, T.D.T., Marasini, R. and Aryal, S., 2019. Macrophage-derived exosome-mimetic hybrid vesicles for tumor targeted drug delivery. Acta biomaterialia, 94, pp.482-494.
- Guerrero-Esteban, T., Gutierrez-Sanchez, C., Garcia-Mendiola, T., Revenga-Parra, M., Pariente, F. and Lorenzo, E., 2021. Bifunctional carbon nanodots for highly sensitive HER2 determination based on electrochemiluminescence. Sensors and Actuators B: Chemical, 343, p.130096.
- Yu, M., Yuan, W., Li, D., Schwendeman, A. and Schwendeman, S.P., 2019. Predicting drug release kinetics from nanocarriers inside dialysis bags. Journal of Controlled Release, 315, pp.23-30.
- Royuela, S., Almarza, J., Mancheño, M.J., Pérez-Flores, J.C., Michel, E.G., Ramos, M.M., Zamora, F., Ocón, P. and Segura, J.L., 2019. Synergistic effect of covalent bonding and physical encapsulation of sulfur in the pores of a microporous COF to improve cycling performance in Li-S batteries. Chemistry–A European Journal, 25(53), pp.12394-12404.
- Wulandari, I.O., Sulistyarti, H., Safitri, A., Santjojo, D.J.H. and Sabarudin, A., 2019. Development of synthesis method of magnetic nanoparticles modified by oleic acid and chitosan as a candidate for drug delivery agent. Journal of Applied Pharmaceutical Science, 9(7), pp.001-011.
- Wang, J., Chen, D. and Ho, E.A., 2021. Challenges in the development and establishment of exosome-based drug delivery systems. Journal of Controlled Release, 329, pp.894-906.
- Khandaker, S., Chowdhury, M.F., Awual, M.R., Islam, A. and Kuba, T., 2021. Efficient cesium encapsulation from contaminated water by cellulosic biomass based activated wood charcoal. Chemosphere, 262, p.127801.
- Chen, C. and Pan, Z., 2021. Cannabidiol and terpenes from hemp–ingredients for future foods and processing technologies. Journal of Future Foods, 1(2), pp.113-127.
- Alu'datt, M.H., Alrosan, M., Gammoh, S., Tranchant, C.C., Alhamad, M.N., Rababah, T., Alzoubi, H., Ghatasheh, S., Ghozlan, K. and Tan, T.C., 2022. Encapsulation-based technologies for bioactive compounds and their application in the food industry: A roadmap for food-derived functional and health-promoting ingredients. Food Bioscience, 50, p.101971.
- Elsharkasy, O.M., Nordin, J.Z., Hagey, D.W., de Jong, O.G., Schiffelers, R.M., Andaloussi, S.E. and Vader, P., 2020. Extracellular vesicles as drug delivery systems: Why and how?. Advanced drug delivery reviews, 159, pp.332-343.
- Klein, C.B., 2023. Carcinogenicity and genotoxicity of chromium. In Toxicology of Metals, Volume I (pp. 205-219). CRC Press.
- Pramanik, S., Mohanto, S., Manne, R., Rajendran, R.R., Deepak, A., Edapully, S.J., Patil, T. and Katari, O., 2021. Nanoparticle-based drug delivery system: the magic bullet for the treatment of chronic pulmonary diseases. Molecular Pharmaceutics, 18(10), pp.3671-3718.
- Germain, M., Caputo, F., Metcalfe, S., Tosi, G., Spring, K., Åslund, A.K., Pottier, A., Schiffelers, R., Ceccaldi, A. and Schmid, R., 2020. Delivering the power of nanomedicine to patients today. Journal of Controlled Release, 326, pp.164-171.
- Raijada, D., Wac, K., Greisen, E., Rantanen, J. and Genina, N., 2021. Integration of personalized drug delivery systems into digital health. Advanced drug delivery reviews, 176, p.113857.
- Gildea, L., Ayariga, J.A., Xu, J., Villafane, R., Robertson, B.K., Samuel-Foo, M. and Ajayi, O.S., 2022. Cannabis sativa CBD extract exhibits synergy with broad-spectrum antibiotics against Salmonella enterica subsp. Enterica serovar Typhimurium. Microorganisms, 10(12), p.2360.
- Gildea, L., Ayariga, J.A., Xu, J., Villafane, R., Robertson, B.K., Samuel-Foo, M. and Ajayi, O.S., 2022. Cannabis sativa CBD extract exhibits synergy with broad-spectrum antibiotics against Salmonella enterica subsp. Enterica serovar Typhimurium. Microorganisms, 10(12), p.2360.
- Ibrahim, I., Ayariga, J.A., Xu, J., Abugri, D.A., Robertson, B.K. and Ajayi, O.S., 2023. Understanding the Mechanisms of Salmonella Typhimurium Resistance to Cannabidiol. bioRxiv, pp.2023-04.
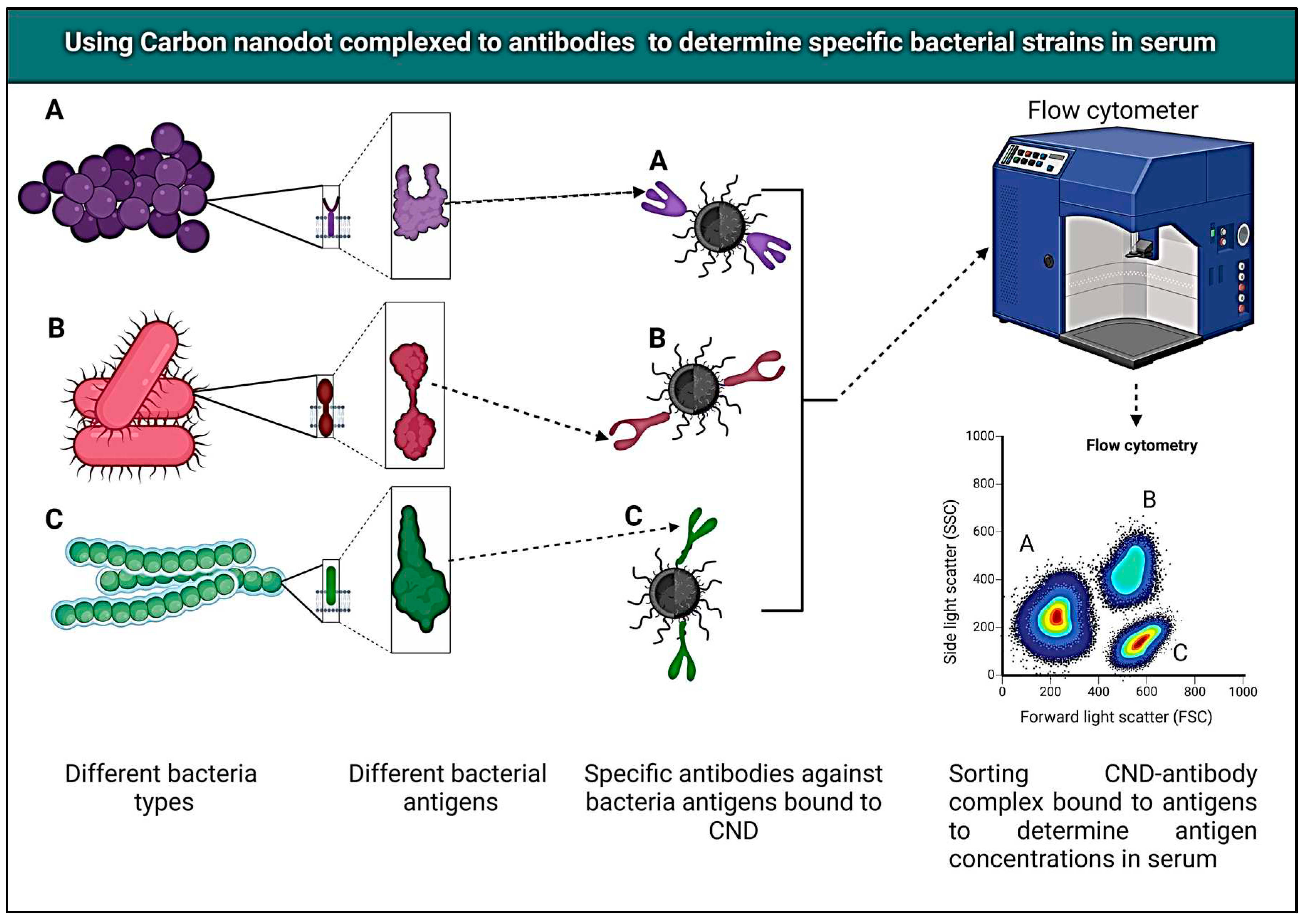
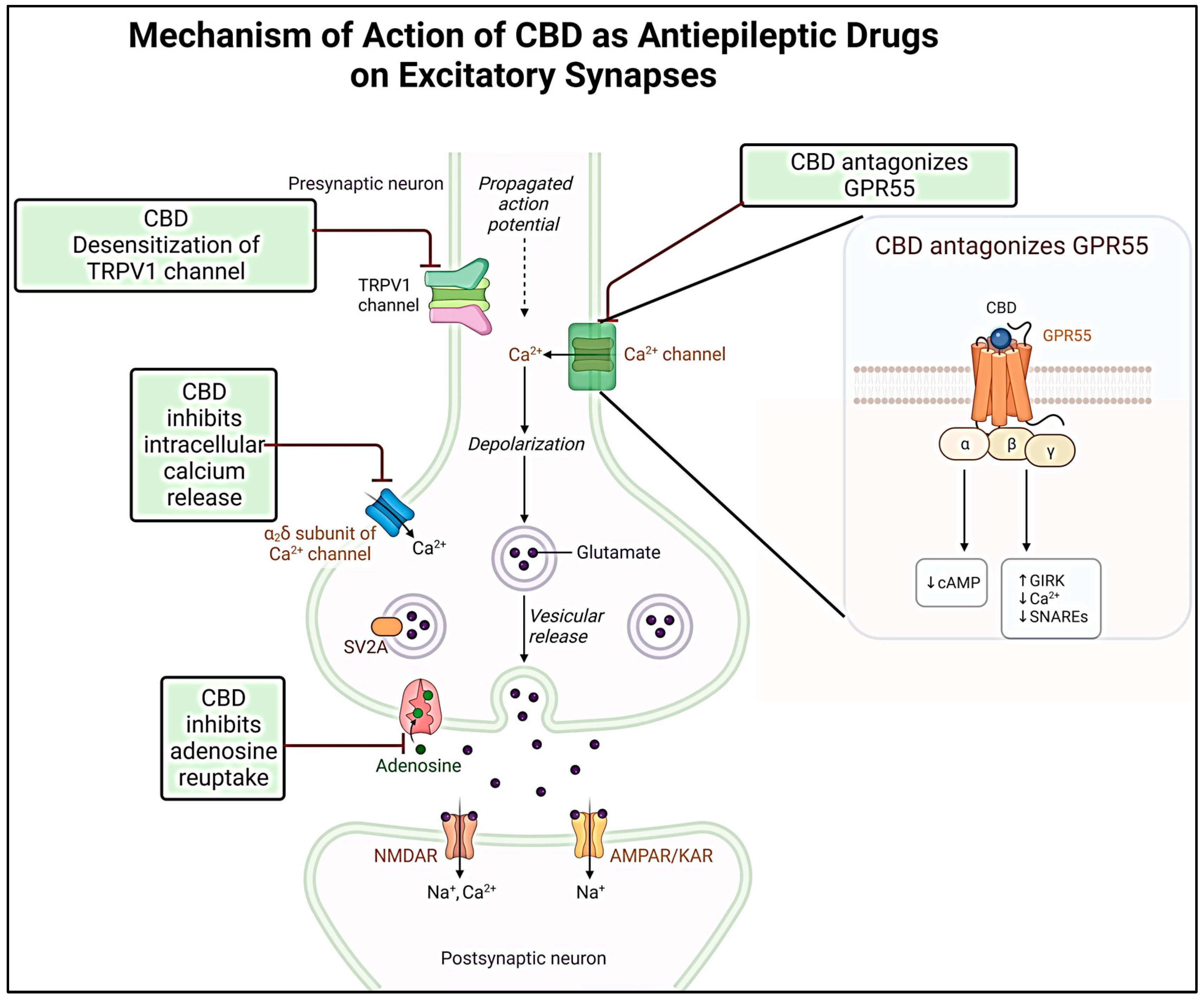
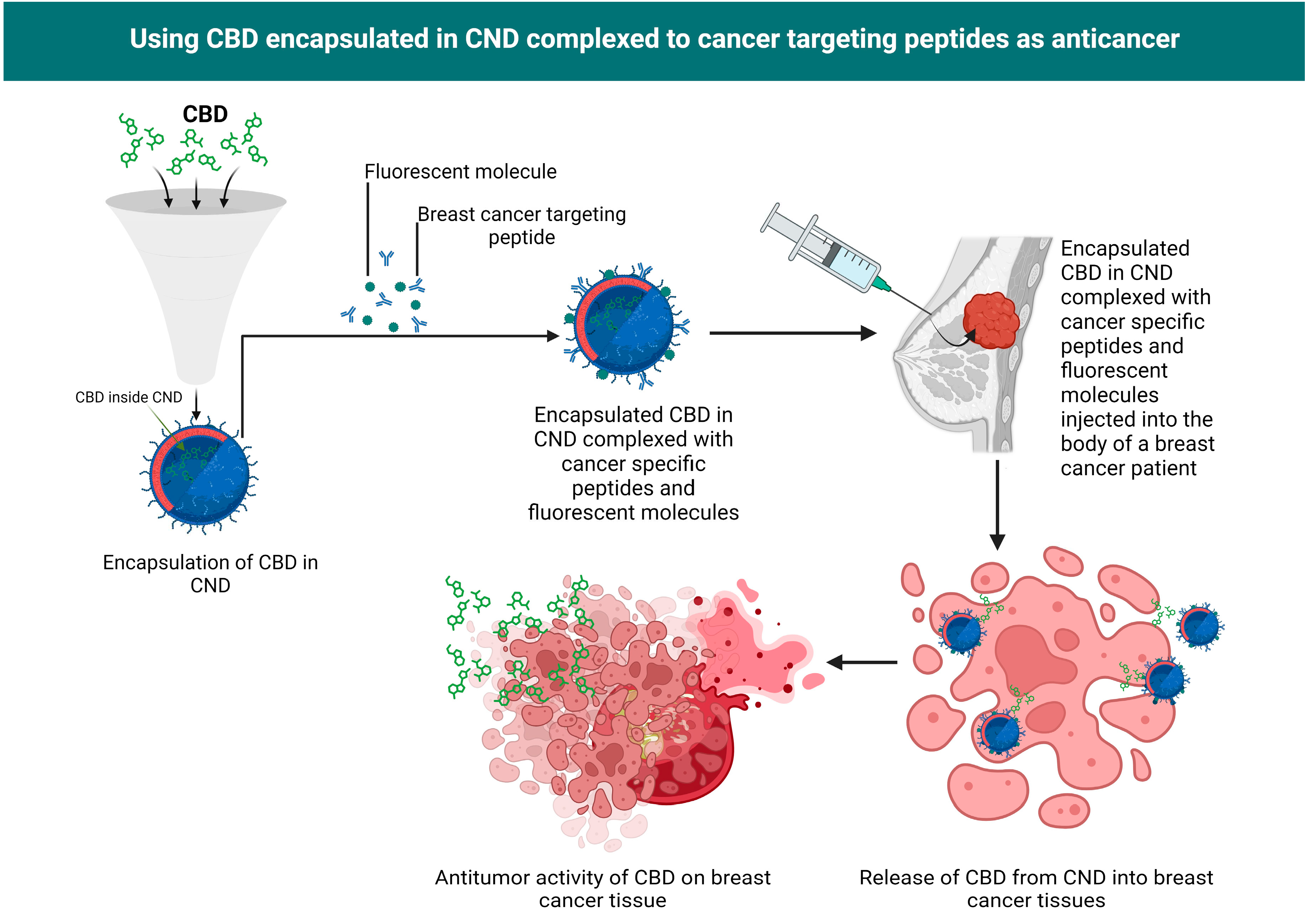
| No. | Variants | Molecular formula | Molecular Weight | Solubility | LogP Values | Structure |
|---|---|---|---|---|---|---|
| 1. | THC | C21H30O2 | 314.5 g/mol | -Highly soluble in lipid-based solvents.-Moderate solubility in ethanol (Alcohol).-Low solubility in water, propylene glycol and vegetable glycerin.-Cyclodextrin can be used to improve its solubility | 7 | 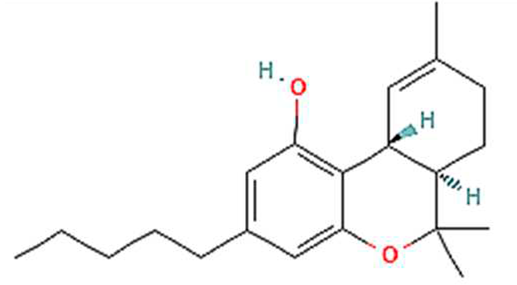 |
| CBD | C21H30O2 | 314.5 g/mol | -Highly soluble in lipid-based solvents.-Moderate solubility in ethanol (Alcohol).-Low solubility in water, propylene glycol and vegetable glycerin.-Cyclodextrin can be used to improve its solubility | 6.5 | 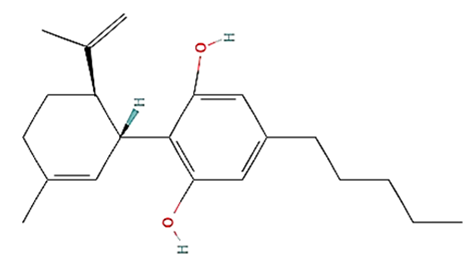 |
|
| 2. | CBG | C21H32O2 | 316.5 g/mol | -Highly soluble in lipid-based solvents.-Moderate solubility in ethanol (Alcohol).-Low solubility in water, propylene glycol and vegetable glycerin.-Cyclodextrin can be used to improve its solubility | 7.4 | 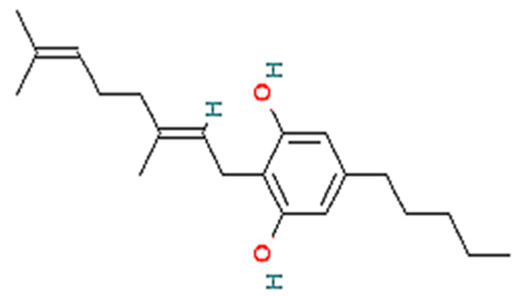 |
| 3. | CBC | C21H30O2 | 314.5 g/mol | -Highly soluble in lipid-based solvents.-Moderate solubility in ethanol (Alcohol).-Low solubility in water, propylene glycol and vegetable glycerin.-Cyclodextrin can be used to improve its solubility | 6.9 | 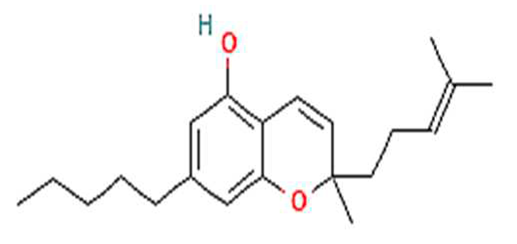 |
| 4. | CBN | C21H26O2 | 310.4 g/mol | -Highly soluble in lipid-based solvents.-Moderate solubility in ethanol (Alcohol).-Low solubility in water, propylene glycol and vegetable glycerin.-Cyclodextrin can be used to improve its solubility | 6.1 | 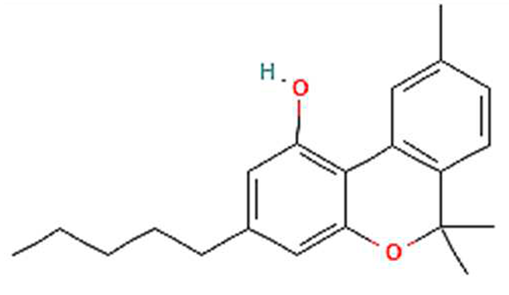 |
| 5. | THCA | C22H30O4 | 358.5 g/mol | -Highly soluble in lipid-based solvents.-Moderate solubility in ethanol (Alcohol).-Low solubility in water, propylene glycol and vegetable glycerin.-Often converted to delta-THC through decarboxylation. | 7 | 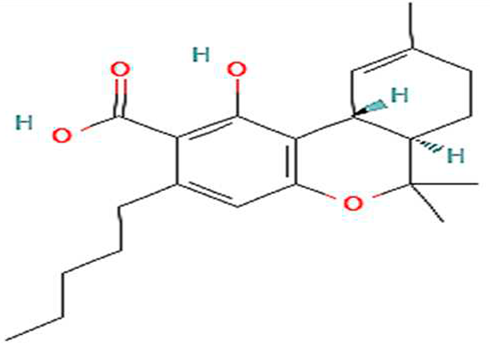 |
| 6. | CBDA | C22H30O4 | 358.5 g/mol | -Highly soluble in lipid-based solvents.-Moderate solubility in ethanol (Alcohol).-Low solubility in water, propylene glycol and vegetable glycerin.-Often converted to CBD through decarboxylation. | 6.6 | 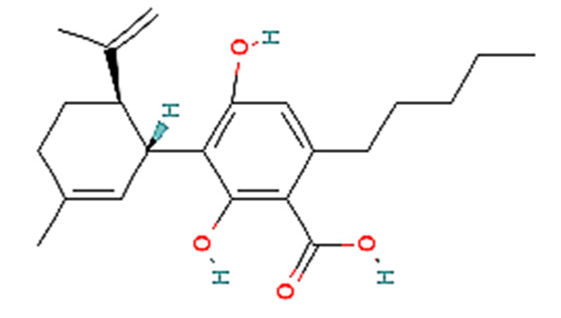 |
| 7. | CBGA | C22H32O4 | 360.5 g/mol | -Highly soluble in lipid-based solvents.-Moderate solubility in ethanol (Alcohol).-Low solubility in water, propylene glycol and vegetable glycerin.-Often converted to CBG, THC or CBD through decarboxylation. | 7.5 | 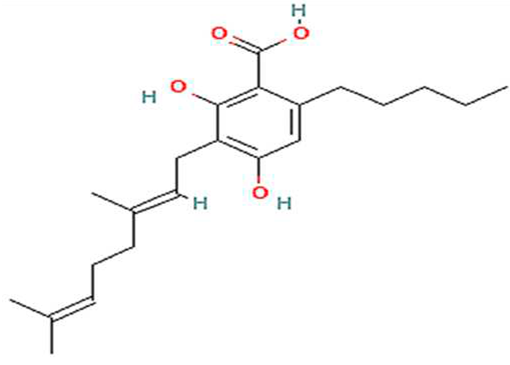 |
| 8. | Delta-10-THC | C21H30O2 | 314.5 g/mol | -High solubility in lipid-based solvents (likely).-Moderate solubility in ethanol (Alcohol).-Very low solubility in water, propylene glycol and vegetable glycerin. | 6 | 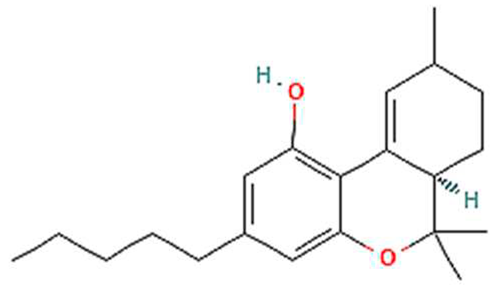 |
| 9. | Delta-8-THC | C21H30O2 | 314.5 g/mol | -High solubility in lipid-based solvents.-Moderate solubility in ethanol (Alcohol).-Very low solubility in water, propylene glycol and vegetable glycerin. | 5.7 | 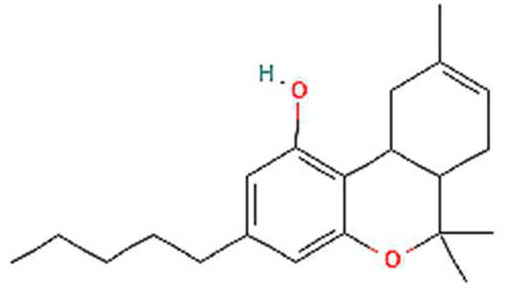 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





