Submitted:
06 November 2023
Posted:
09 November 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Economic and Public Health Implications from Salmonella
Salmonella Infections in Poultry
Mode of Salmonella Transmission
The Use of Antibiotics for Prevention and Treatment of Salmonellosis in Poultry
Probiotics and Their Roles for Poultry Health
Probiotics Mechanisms to Prevent and Control Salmonellosis
- (1)
- Competition for nutrients: Probiotics could sequester essential nutrients resulting in invading pathogens could not colonizing. Probiotics like E. coli Nissle 1917 can diminish Salmonella Typhimurium’s colonization in intestines by competing for iron, a crucial but limited nutrient necessary for Salmonella Typhimurium’s growth.52
- (2)
- Production of antimicrobial conditions and compounds: Lactic acid bacteria can produce antimicrobial substances, e.g., lactic acid, hydrogen peroxide, and bacteriocins.53 Production of organic acid may lower pH which cause an unfavorable environment for pathogen colonization.51,54,55
- (3)
- Blocking of adhesion sites: When probiotics, for example Lactobacilli, are ingested, they adhere to intestinal mucosa, competing for binding sites. Therefore, less binding sites are available pathogens which make pathogens leave the body soon before they can colonize.56
- (4)
- Immunomodulation: Probiotics can stimulate both adaptive (specific) and innate (nonspecific) immunity. When they colonized in the gut, they activate lymphocytes and mature the humoral immune mechanisms, especially the circulation of IgA and IgM secreting cells.56
Challenges and Limitations of the Usage of Probiotics in Poultry Production
Acknowledgments
References
- Steinmuller N, Demma L, Bender JB, Eidson M, Angulo FJ. Outbreaks of enteric disease associated with animal contact: not just a foodborne problem anymore. Clin Infect Dis Off Publ Infect Dis Soc Am. 2006;43(12):1596-1602. [CrossRef]
- Sørum H, Sunde M. Resistance to antibiotics in the normal flora of animals. Vet Res. 2001;32(3-4):227-241. [CrossRef]
- Muaz K, Riaz M, Akhtar S, Park S, Ismail A. Antibiotic Residues in Chicken Meat: Global Prevalence, Threats, and Decontamination Strategies: A Review. J Food Prot. 2018;81(4):619-627. [CrossRef]
- Alloui MN, Szczurek W, Świątkiewicz S. The Usefulness of Prebiotics and Probiotics in Modern Poultry Nutrition: a Review / Przydatność prebiotyków i probiotyków w nowoczesnym żywieniu drobiu – przegląd. Ann Anim Sci. 2013;13(1):17-32. [CrossRef]
- Ashraf R, Shah N. Antibiotic resistance of probiotic organisms and safety of probiotic dairy products. Int Food Res J. 2011;18:837-853.
- Food and Drug Administration, HHS. Prevention of Salmonella enteritidis in shell eggs during production, storage, and transportation. Final rule. Fed Regist. 2009;74(130):33029-33101.
- Pijnacker R, Dallman TJ, Tijsma ASL, et al. An international outbreak of Salmonella enterica serotype Enteritidis linked to eggs from Poland: a microbiological and epidemiological study. Lancet Infect Dis. 2019;19(7):778-786. [CrossRef]
- Popa GL, Papa MI. Salmonella spp. infection - a continuous threat worldwide. Germs. 2021;11(1):88-96. [CrossRef]
- Chaves Hernández AJ. Poultry and Avian Diseases. In: Van Alfen NK, ed. Encyclopedia of Agriculture and Food Systems. Academic Press; 2014:504-520. [CrossRef]
- Löfström C, Hansen T, Maurischat S, Malorny B. Salmonella: Salmonellosis. In: Caballero B, Finglas PM, Toldrá F, eds. Encyclopedia of Food and Health. Academic Press; 2016:701-705. [CrossRef]
- Saif YM. Diseases of Poultry. 12th ed. Blackwell publ; 2008.
- Sanyal D, Douglas T, Roberts R. Salmonella infection acquired from reptilian pets. Arch Dis Child. 1997;77(4):345-346. [CrossRef]
- Sato G, Adler HE. Bacteriological and Serological Observations on Turkeys Naturally Infected with Arizona 7:1,7,8. Avian Dis. 1966;10(3):291-295. [CrossRef]
- Bäumler AJ, Hargis BM, Tsolis RM. Tracing the origins of Salmonella outbreaks. Science. 2000;287(5450):50-52. [CrossRef]
- Shivaprasad HL, Timoney JF, Morales S, Lucio B, Baker RC. Pathogenesis of Salmonella enteritidis Infection in Laying Chickens. I. Studies on Egg Transmission, Clinical Signs, Fecal Shedding, and Serologic Responses. Avian Dis. 1990;34(3):548-557. [CrossRef]
- Transmission of Salmonella between wildlife and meat-production animals in Denmark | Journal of Applied Microbiology | Oxford Academic. Available online: https://academic.oup.com/jambio/article-abstract/105/5/1558/6720211 (accessed on 5 August 2023).
- Rabsch W, Andrews HL, Kingsley RA, et al. Salmonella enterica Serotype Typhimurium and Its Host-Adapted Variants. Infect Immun. 2002;70(5):2249-2255. [CrossRef]
- OzFoodNet Working Group. Monitoring the Incidence and Causes of Diseases Potentially Transmitted by Food in Australia: Annual Report of the OzFoodNet Network, 2008. Commun Dis Intell Q Rep. 2020;33(4):389-413.
- Salmonella in poultry - SVA. Available online: https://www.sva.se/en/what-we-do/feed-safety/general-facts-about-salmonella/salmonella-in-poultry/ (accessed on 29 July 2023).
- Ramtahal MA, Amoako DG, Akebe ALK, Somboro AM, Bester LA, Essack SY. A Public Health Insight into Salmonella in Poultry in Africa: A Review of the Past Decade: 2010–2020. Microb Drug Resist. 2022;28(6):710-733. [CrossRef]
- Calenge F, Kaiser P, Vignal A, Beaumont C. Genetic control of resistance to salmonellosis and to Salmonella carrier-state in fowl: a review. Genet Sel Evol. 2010;42(1):11. [CrossRef]
- Gast RK, Holt PS. Persistence of Salmonella enteritidis from one day of age until maturity in experimentally infected layer chickens. Poult Sci. 1998;77(12):1759-1762. [CrossRef]
- Rose N, Beaudeau F, Drouin P, Toux JY, Rose V, Colin P. Risk factors for Salmonella enterica subsp. enterica contamination in French broiler-chicken flocks at the end of the rearing period. Prev Vet Med. 1999;39(4):265-277. [CrossRef]
- Henken AM, Frankena K, Goelema JO, Graat EAM, Noordhuizen JPTM. Multivariate Epidemiological Approach to Salmonellosis in Broiler Breeder Flocks. Poult Sci. 1992;71(5):838-843. [CrossRef]
- Angen Ø, Skov MN, Chriél M, Agger JF, Bisgaard M. A retrospective study on salmonella infection in Danish broiler flocks. Prev Vet Med. 1996;26(3):223-237. [CrossRef]
- Baggesen DL, Olsen JE, Bisgaard M. Plasmid profiles and phage types of Salmonella typhimurium isolated from successive flocks of chickens on three parent stock farms. Avian Pathol J WVPA. 1992;21(4):569-579. [CrossRef]
- Mares M. Current Topics in Salmonella and Salmonellosis. BoD – Books on Demand; 2017.
- Gast RK, Beard CW. Detection and Enumeration of Salmonella enteritidis in Fresh and Stored Eggs Laid by Experimentally Infected Hens. J Food Prot. 1992;55(3):152-156. [CrossRef]
- Salmonella. UNL Food. Published August 13, 2015. Available online: https://food.unl.edu/salmonella (accessed on 30 July 2023).
- Zamora-Sanabria R, Alvarado AM, Zamora-Sanabria R, Alvarado AM. Preharvest Salmonella Risk Contamination and the Control Strategies. In: Current Topics in Salmonella and Salmonellosis. IntechOpen; 2017. [CrossRef]
- Mathew AG, Liamthong S, Lin J, Hong Y. Evidence of class 1 integron transfer between Escherichia coli and Salmonella spp. on livestock farms. Foodborne Pathog Dis. 2009;6(8):959-964. [CrossRef]
- Castanon JIR. History of the use of antibiotic as growth promoters in European poultry feeds. Poult Sci. 2007;86(11):2466-2471. [CrossRef]
- Salmonellosis, S. Enteritidis and S. Typhimurium infections. Available online: https://www.thepoultrysite.com/disease-guide/salmonellosis-s-enteritidis-and-s-typhimurium-infections (accessed on 2 August 2023).
- Goetting V, Lee KA, ℡l LA. Pharmacokinetics of veterinary drugs in laying hens and residues in eggs: a review of the literature. J Vet Pharmacol Ther. 2011;34(6):521-556. [CrossRef]
- Noenchat P, Direksin K, Sornplang P. The phenotypic and genotypic antimicrobial resistance patterns of Salmonella isolated from chickens and meat at poultry slaughterhouses in Japan and Thailand. Vet World. 2023;16(7):1527-1533. [CrossRef]
- Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis - The Lancet. Available online: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)02724-0/fulltext (accessed on 8 October 2023).
- Schrezenmeir J, de Vrese M. Probiotics, prebiotics, and synbiotics—approaching a definition123. Am J Clin Nutr. 2001;73(2):361s-364s. [CrossRef]
- Lilly DM, Stillwell RH. Probiotics: Growth-Promoting Factors Produced by Microorganisms. Science. 1965;147(3659):747-748. [CrossRef]
- Marteau P, Cuillerier E, Meance S, et al. Bifidobacterium animalis strain DN-173 010 shortens the colonic transit time in healthy women: a double-blind, randomized, controlled study. Aliment Pharmacol Ther. 2002;16(3):587-593. [CrossRef]
- Howarth GS, Wang H. Role of Endogenous Microbiota, Probiotics and Their Biological Products in Human Health. Nutrients. 2013;5(1):58-81. [CrossRef]
- Vimala Y, Kumar PD. Some aspects of probiotics. Indian J Microbiol. 2006;46:1-7.
- Ramlucken U, Ramchuran SO, Moonsamy G, Jansen van Rensburg C, Thantsha MS, Lalloo R. Production and stability of a multi-strain Bacillus based probiotic product for commercial use in poultry. Biotechnol Rep. 2021;29:e00575. [CrossRef]
- Kabir SML. The Role of Probiotics in the Poultry Industry. Int J Mol Sci. 2009;10(8):3531-3546. [CrossRef]
- Nurmi EV, Schneitz JE, Makela PH. Process for the production of a bacterial preparation for the prophylaxis of intestinal disturbances in poultry. Published online August 25, 1987. Available online: https://patents.google.com/patent/US4689226A/en (accessed on 28 July 2023).
- Distribution of pathogen inhibition in the Lactobacillus isolates of a commercial probiotic consortium - Chateau - 1993 - Journal of Applied Bacteriology - Wiley Online Library. Available online: https://ami-journals.onlinelibrary.wiley.com/doi/abs/10.1111/j.1365-2672.1993.tb02993.x (accessed on 28 July 2023).
- Watkins BA, Miller BF, Neil DH. In Vivo Inhibitory Effects of Lactobacillus acidophilus Against Pathogenic Escherichia coli in Gnotobiotic Chicks1. Poult Sci. 1982;61(7):1298-1308. [CrossRef]
- The Dynamics of Probiotics on Growth Performance and Immune Response in Broilers. [CrossRef]
- Peralta-Sánchez JM, Martín-Platero AM, Ariza-Romero JJ, et al. Egg Production in Poultry Farming Is Improved by Probiotic Bacteria. Front Microbiol. 2019;10. Available online: https://www.frontiersin.org/articles/10.3389/fmicb.2019.01042 (accessed on 1 August 2023).
- Chiang SH, Hsieh WM. Effect of direct-fed microorganisms on broiler growth performance and litter ammonia level. Asian-Australas J Anim Sci. 1995;8(2):159-162.
- Zammit VA, Park SO. Impact of the Combination of Probiotics and Digital Poultry System on Behavior, Welfare Parameters, and Growth Performance in Broiler Chicken. Microorganisms. 2023;11(9):2345. [CrossRef]
- Britton RA, Versalovic J. Probiotics and Gastrointestinal Infections. Interdiscip Perspect Infect Dis. 2009;2008:e290769. [CrossRef]
- Deriu E, Liu JZ, Pezeshki M, et al. Probiotic Bacteria Reduce Salmonella Typhimurium Intestinal Colonization by Competing for Iron. Cell Host Microbe. 2013;14(1):26-37. [CrossRef]
- Mishra C, Lambert J. Production of anti-microbial substances by probiotics. Asia Pac J Clin Nutr. 1996;5(1):20-24.
- Sherman PM, Ossa JC, Johnson-Henry K. Unraveling Mechanisms of Action of Probiotics. Nutr Clin Pract. 2009;24(1):10-14. [CrossRef]
- Abd El-Hack ME, Abdelnour SA, Taha AE, et al. Herbs as thermoregulatory agents in poultry: An overview. Sci Total Environ. 2020;703:134399. [CrossRef]
- FUNCTIONAL MECHANISMS OF PROBIOTICS | Journal of microbiology, biotechnology and food sciences. Available online: https://office2.jmbfs.org/index.php/JMBFS/article/view/8188 (accessed on 1 August 2023).
- Ruvalcaba-Gómez JM, Villagrán Z, Valdez-Alarcón JJ, et al. Non-Antibiotics Strategies to Control Salmonella Infection in Poultry. Anim Open Access J MDPI. 2022;12(1):102. [CrossRef]
- Kizerwetter-Swida M, Binek M. Protective effect of potentially probiotic Lactobacillus strain on infection with pathogenic bacteria in chickens. Pol J Vet Sci. 2009;12(1):15-20.
- El-Ghany WAA, El-Shafii SSA, Hatem ME, Dawood RE. A Trial to Prevent Salmonella Enteritidis Infection in Broiler Chickens Using Autogenous Bacterin Compared with Probiotic Preparation. J Agric Sci. 2012;4(5):p91. [CrossRef]
- Effect of a Multi-Species Probiotic on the Colonisation of Salmonella in Broilers | SpringerLink. Available online: https://link.springer.com/article/10.1007/s12602-019-09593-y (accessed on 1 August 2023).
- El-Sharkawy H, Tahoun A, Rizk AM, et al. Evaluation of Bifidobacteria and Lactobacillus Probiotics as Alternative Therapy for Salmonella typhimurium Infection in Broiler Chickens. Animals. 2020;10(6):1023. [CrossRef]
- Chaiyawan N, Taveeteptaikul P, Wannissorn B, et al. Characterization and Probiotic Properties of Bacillus Strains Isolated from Broiler. Thai J Vet Med. 2010;40(2):207-214.
- Yaqoob MU, Wang G, Wang M. An updated review on probiotics as an alternative of antibiotics in poultry — A review. Anim Biosci. 2022;35(8):1109-1120. [CrossRef]
- Krysiak K, Konkol D, Korczyński M. Overview of the Use of Probiotics in Poultry Production. Anim Open Access J MDPI. 2021;11(6):1620. [CrossRef]
- Cutlip SE, Hott JM, Buchanan NP, Rack AL, Latshaw JD, Moritz JS. The Effect of Steam-Conditioning Practices on Pellet Quality and Growing Broiler Nutritional Value. J Appl Poult Res. 2008;17(2):249-261. [CrossRef]
- Rakshit S. Microbial and Processing Criteria for Production of Probiotics: A Review. Food Technol Biotechnol. Published online January 1, 2006. Available online: https://www.academia.edu/72790604/Microbial_and_Processing_Criteria_for_Production_of_Probiotics_A_Review (accessed on 8 October 2023).

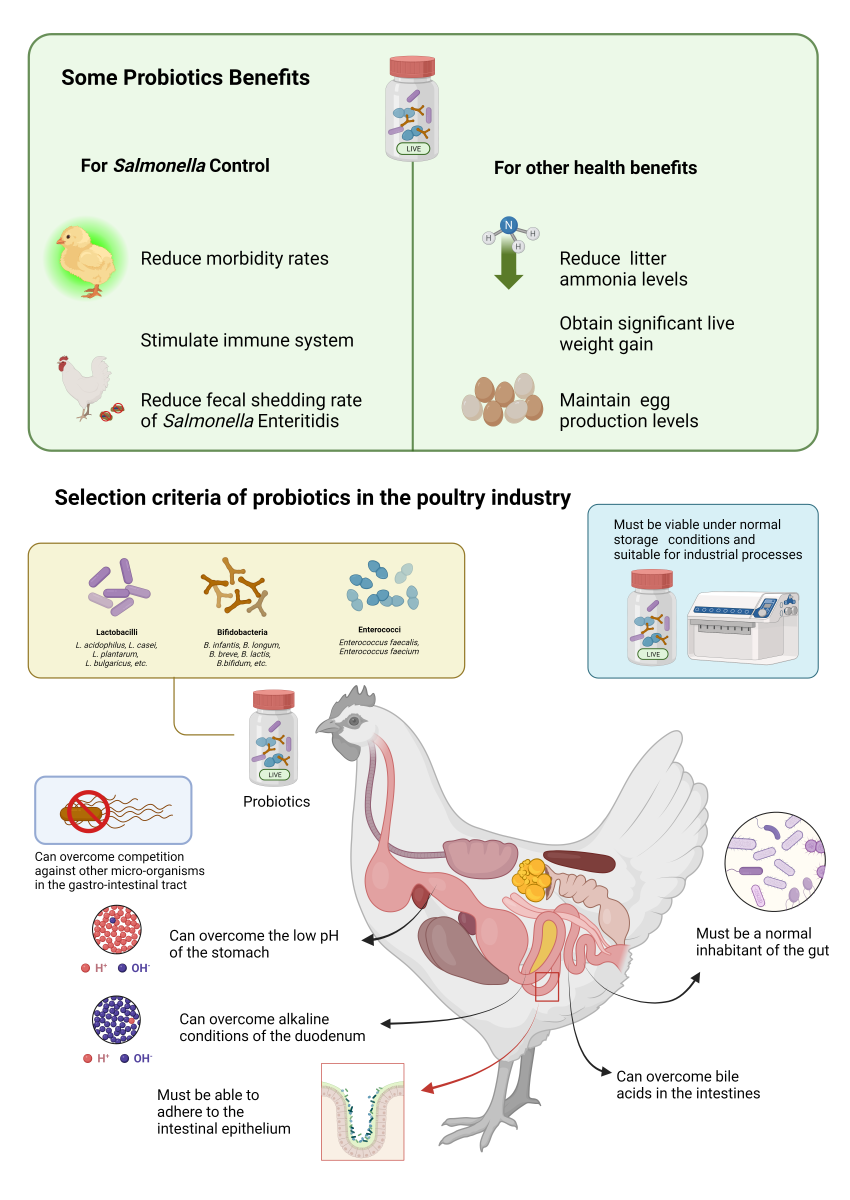
| Properties of microorganisms |
|---|
| Generally Recognized as Safe (GRAS) |
| Bile, hydrochloric acid and pancreatic juice resistance |
| Anti-carcinogenic properties |
| Stimulate immune system |
| Intestinal permeability reduction |
| Lactic acid production |
| Resistance to acidic conditions of the stomach |
| Resistance to alkaline conditions of the duodenum |
| Properties of microorganisms |
|---|
| Must be a normal inhabitant of the gut |
| Must be able to adhere to the intestinal epithelium |
| Can overcome the low pH of the stomach |
| Can overcome the presence of bile acids in the intestines |
| Can overcome competition against other micro-organisms in the gastro-intestinal tract |
| Must be viable under normal storage conditions and suitable for industrial processes |
| Must be cost effective to use for farm animals |
| Probiotic strains | Categories of chicks | Starting age for administration | Administration | Benefits | Ref. |
|---|---|---|---|---|---|
| Lactobacillus acidophilus | Gnotobiotic chicks | 2 days old | Inoculation 108-109 organism/ml. |
Decreased mortality from 100% to 0% when challenged with pathogenic Escherichia coli. | 46 |
| Protexin® Boost Lactobacillus plantarum, Lactobacillus bulgaricus, Lactobacillus acidophilus, Lactobacillus rhamnosus, Bifidobacterium bifidum, Streptococcus thermophilus, Enterococcus faecium, Aspergillus oryzae, and Candida pintolopessi |
Broiler chicks | 1 day old | Added to drinking water 2gm of Protexin® Boost/10 liters water | Significant live weight gain, high carcass yield, high breast and leg weight and high antibody production |
47 |
| Enterococcus faecalis UGRA10 | Laying hens | 16 weeks old | Fodder diet with the bacterium E. faecalis UGRA10 108 CFU/g of fodder | Maintained egg production levels | 48 |
|
Lactobacillus acidophilus, Bacillus subtilis, Streptococcus faecium |
Broilers | 1 day old | Added to diet varied from 0-1 g probiotic2/kg feed for the first 3 wks and 0-0.5 g probiotic2/kg feed for wk 4 to wk 6 | Reduced litter ammonia levels | 49 |
|
Bacillus subtillus, Streptomyces galilaeus, and Sphingobacteriaceae |
Broilers | 1 day old | 3.5 × 108 CFU/g of each strain | Improved growth performance and behavioral welfare | 50 |
| Categories of chicks | Age | Probiotics | Administration | Challengedpathogen | Results | Ref. |
|---|---|---|---|---|---|---|
| Broiler | Lactobacillus salivarius strain 3d (isolated from chicken feces) | Orally 108 CFU / 100fl of Phosphate Buffered Saline. one day before with selected pathogenic bacteria |
Salmonella Enteritidis, Clostridium perfringens and Campylobacter jejuni. |
Lower salmonella in caecal content after infection compared to control group and no Salmonella detection after 7 days. | 58 | |
| Broiler chicks | 1 day old |
Lactobacillus acidophilus, Enterococcus faecium, Lactobacillus plantarum and Lactobacillus casei |
Added in drinking water for 5 consecutive days in a dose of 1gm/4 liter of the drinking water |
Salmonella Enteritidis | Significantly lower morbidity rates, fecal shedding rate of Salmonella Enteritidis, and re-isolation rate of Salmonella Enteritidis from different organs | 59 |
| Broiler chickens | newly hatched | Lactobacillus crispatus, Lactobacillus salivarius, Lactobacillus gallinarum, Lactobacillus johnsonii, Enterococcus faecalis and Bacillus amyloliquefaciens. | Added in feed 2.0 × 1010 to 8.9 × 1010 CFU per kg feed | Salmonella Enteritidis A9 | Reduced Salmonella Enteritidis A9 in ceca: detected 95% of broilers on day 14 and 55% on day 28. Stimulated immune system |
60 |
| Broiler chicks | 1 day old | Lacticaseibacillus casei, Bifidobacterium breve, Bifidobacterium longum and Bifidobacterium infantis. | Oral inoculation 2 × 109 CFU from each probiotic bacterium |
Salmonella typhimurium | Prevention of the detrimental effects of acquired Salmonella infection by B. breve, L. casei and B. infantis, An ability to bind to intestinal cells in vitro, Reduction of Salmonella typhimurium recovery from the cecal tonsils in vivo. | 61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





