Submitted:
13 October 2023
Posted:
16 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Epidemiology and risk factors
1.2. Pathophysiology
1.3. Disease Mechanisms
1.4. Clinical Manifestations
2. Quinones as a privileged structure for the development of new derivatives for PD
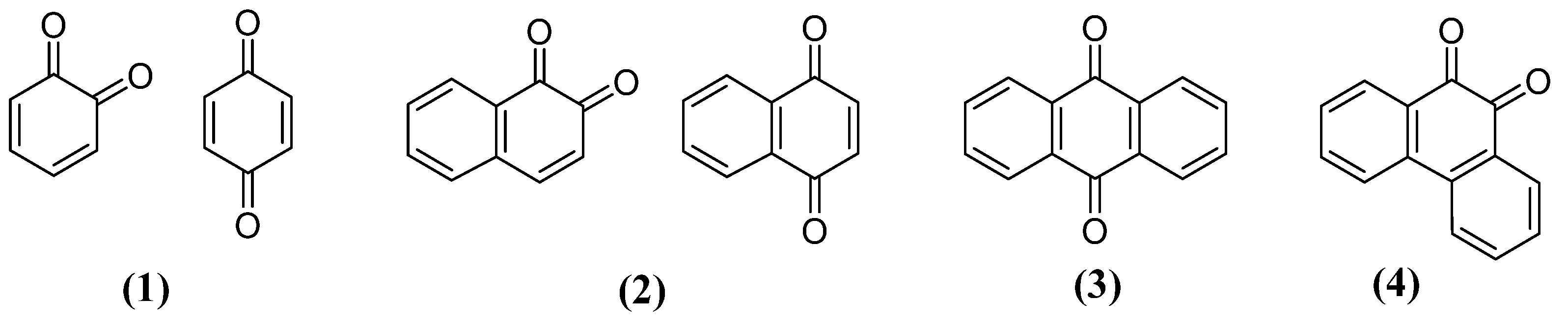
3. Naphthoquinones
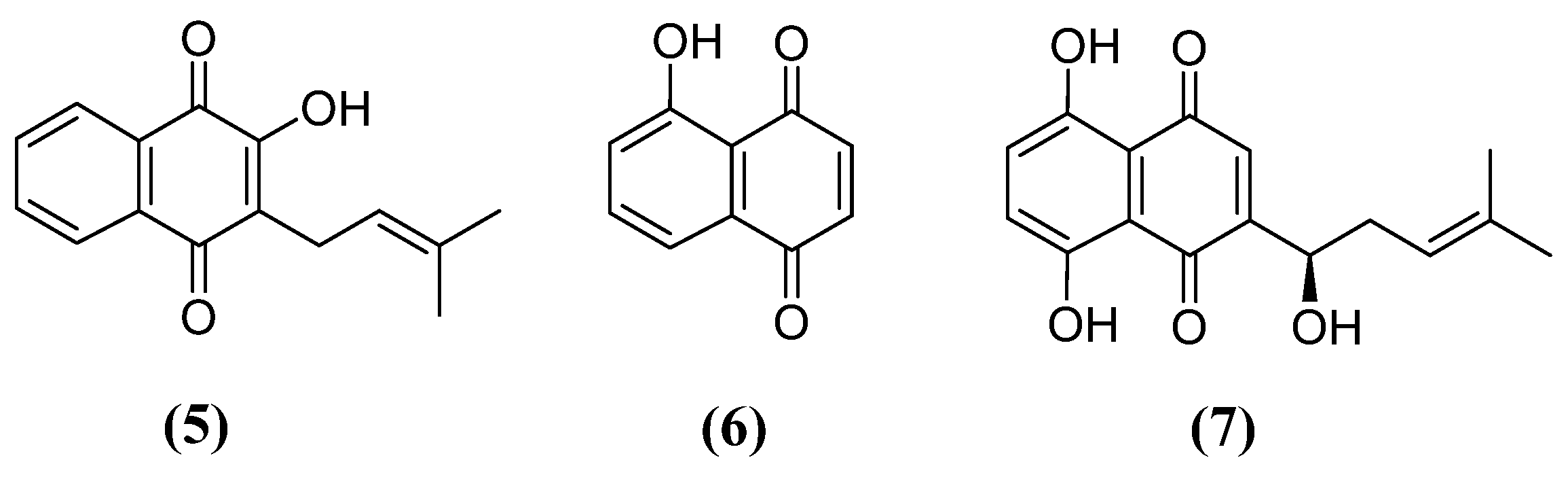
3.1. Neuroprotective effect of naphthoquinones
3.2. Naphthoquinones with activity against PD
4. Conclusions
References
- a) Parkinson J. An Essay on the Shaking Palsy. London: Sherwood, Neely and Jones, 1817. b) Kempster, P.A.; Hurwitz, B.; Lees, A.J. A new look at James Parkinson's Essay on the Shaking Palsy. Neurology 2007, 69, 482–485. doi:10.1212/01.wnl.0000266639.50620.d1 c) Goetz C.G. The history of Parkinson's disease: early clinical descriptions and neurological therapies. Cold Spring Harb. Perspective Med. 2011, 1, a008862. doi: 10.1101/cshperspec.a008862. [CrossRef]
- a) GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. doi:10.1016/S1474-4422(18)30499-X b) Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloem, B.R. The Emerging Evidence of the Parkinson Pandemic. J Parkinsons Dis. 2018, 8, S3-S8. doi:10.3233/JPD-181474. c) Deuschl, G.; Beghi, E.; Fazekas, F.; Varga, T.; Christoforidi, K.A.; Sipido, E.; Bassetti, C.L.; Vos, T.; Feigin, V.L. The burden of neurological diseases in Europe: an analysis for the Global Burden of Disease Study 2017. Lancet Public Health 2020, 5, 551-567. doi:10.1016/S2468-2667(20)30190-0. [CrossRef]
- Pringsheim, T.; Jette, N.; Frolkis, A; Steeves, T.D. The prevalence of Parkinson's disease: a systematic review and meta-analysis. Move Discord. 2014, 13, 1583-1590. [CrossRef]
- a) Bjornestad, A.; Forsaa, E.B.; Pedersen, K.F.; Tysnes, O.B.; Larsen, J.P.; Alves, G. Risk and course of motor complications in a population-based incident Parkinson's disease cohort. Park. Relat. Disord. 2016, 22, 48-53. doi:10.1016/j.parkreldis.2015.11.007. b) Picillo, M., Palladino, R.; Moccia, M.; Erro, R.; Amboni, M.; Vitale, C.; Barone, P.; Pellecchia, M.T. Gender and non motor fluctuations in Parkinson's disease: A prospective study. Park. Relat. Disord. 2016, 27, 89-92. doi:10.1016/j.parkreldis.2016.04.001. [CrossRef]
- Nicoletti, A.; Vasta, R.; Mostile, G.; Nicoletti, G.; Arabia, G.; Iliceto, G.; Lamberti, P.; Marconi R.; Morgante, L.; Barone P.; Quattrone, A.; Zappia, M. Gender effect on non-motor symptoms in Parkinson's disease: are men more at risk? Park. Relat. Disord. 2017, 35, 69-74. [CrossRef]
- a) Fullard, M.E.; Thibault, D.P.; Hill, A.; Fox, J.; Bhatti, D.E.; Burack, M.A.; Dahodwala, N.; Haberfeld, E.; Kern, D.S.; Klepitskava, O.S.; Urrea-Mendoza, E.; Myers, P.; Nutt, J.; Rafferty, M.R.; Schwalb, J.M.; Shulman, L.M.; Willis, A.W. Parkinson Study Group Healthcare Outcomes and Disparities Working Group. Utilization of rehabilitation therapy services in Parkinson disease in the United States. Neurology 2017, 89, 1162-1169. doi:10.1212/WNL.0000000000004355. b) Fullard, M.E.; Thibault, D.P.; Todaro, V.; Foster, S.; Katz, L.; Morgan, R.; Kern, D.S.; Schwalb, J.M.; Urrea-Mendoza, E.; Dahodwala, N.; Shulman, L.; Willis, A.W. Sex disparities in health and health care utilization after Parkinson diagnosis: Rethinking PD associated disability. Park. Relat. Disord. 2018, 48, 45-50. doi:10.1016/j.parkreldis.2017.12.012. [CrossRef]
- Breckenridge, C.B.; Berry, C.; Chang, E.T.; Sielken, R.L.; Mandel, J.S. Association between Parkinson's Disease and Cigarette Smoking, Rural Living, Well-Water Consumption, Farming and Pesticide Use: Systematic Review and Meta-Analysis. PLoS One 2016, 11, e0151841. [CrossRef]
- a) Langston, J.W.; Ballard, P.; Tetrud, J.W.; Irwin, I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science 1983, 219, 979-980. doi:10.1126/science.6823561. b) Angibaud, G.; Gaultier, C.; Rascol, O. Atypical parkinsonism and Annonaceae consumption in New Caledonia. Mov. Disord. 2004, 19, 603-604. doi:10.1002/mds.20104. c) Höglinger, G.U.; Michel, P.P.; Champy, P.; Feger, J.; Hirsch, E.C.; Ruberg, M.; Lannuzel, A. Experimental evidence for a toxic etiology of tropical parkinsonism. Mov. Disord. 2005, 20, 118-119. doi:10.1002/mds.20300. [CrossRef]
- Mittal, S.; Bjørnevik, K.; Im, D.S.; Flierl, A.; Dong, X.; Locascio, J.J.; Abo, K.M.; Long, E.; Jin, M.; Xu, B.; Xiang, Y.K.; Rochet, J.C.; Engeland, A.; Rizzu, P.; Heutink, P.; Bartels, T.; Selkoe, D.J.; Caldarone, B.J.; Glicksman, M.A.; Khurana, V.; Schule, B.; Park, D.S.; Riise, T.; Scherzer, C.R. β2-Adrenoreceptor is a regulator of the α-synuclein gene driving risk of Parkinson's disease. Science. 2017, 357, 891-898. [CrossRef]
- Palacios, N.; Gao X.; McCullough, M.L.; Schwarzschild, M.A.; Shah, R.; Gapstur S.; Ascherio, A. Caffeine and risk of Parkinson's disease in a large cohort of men and women. Mov. Disord. 2012, 27, 1276-1282. [CrossRef]
- Bai, S.; Song, Y.; Huang, X.; Peng, L.; Jia, J.; Liu, Y.; Lu, H.; Statin Use and the Risk of Parkinson's Disease: An Updated Meta-Analysis. PLoS One 2016, 11, e0152564. [CrossRef]
- Gudala, K.; Kanukula, R.; Bansal, D. Reduced Risk of Parkinson's Disease in Users of Calcium Channel Blockers: A Meta-Analysis. Int. J. Chronic Dis. 2015, 2015, 697404. [CrossRef]
- a) Gagne, J.J.; Power, M.C. Anti-inflammatory drugs and risk of Parkinson disease: a meta-analysis. Neurology. 2010, 74, 995-1002. doi:10.1212/WNL.0b013e3181d5a4a3. b) Becker, C.; Jick, S.S.; Meier, C.R. NSAID use and risk of Parkinson disease: a population-based case-control study. Eur. J. Neurol. 2011, 18, 1336-1342. doi:10.1111/j.1468-1331.2011.03399.x. [CrossRef]
- Gasser, T. Genetics of Parkinson's disease. Curr. Opin. Neurol. 2005, 18, 363-369. [CrossRef]
- Wakabayashi, K.; Tanji, K.; Odagiri, S.; Miki, Y.; Mori, F.; Takahashi, H. The Lewy body in Parkinson's disease and related neurodegenerative disorders. Mol. Neurobiol. 2013, 47, 495-508. [CrossRef]
- Hsu, L.J.; Sagara, Y.; Arroyo, A.; Rockenstein, E.; Sisk, A.; Mallory, M.; Wong, J.; Takenouchi, T.; Hashimoto, M.; Masliah, E. α-Synuclein promotes mitochondrial deficit and oxidative stress. Am. J. Clin. Pathol. 2000, 157, 401-410. [CrossRef]
- Snyder, H.; Mensah, K.; Theisler, C.; Lee, J.; Matouschek, A.; Wolozin, B. Aggregated and monomeric α-synuclein bind to the S6' proteasomal protein and inhibit proteasomal function. J. Biol. Chem. 2003, 278, 11753-11759. [CrossRef]
- Danzer, K.M.; Haasen, D.; Karow, A.R.; Moussaud, S.; Habeck, M.; Giese, A.; Kretzschmar, H.; Hengerer, B.; Kostka, M. Different species of alpha-synuclein oligomers induce calcium influx and seeding. J. Neurosci. 2007, 27, 9220-9232. [CrossRef]
- Alim, M.A.; Ma, Q.L.; Takeda, K.; Aizawa, T.; Matsubara, M.; Nakamura, M.; Asada, A.; Saito, T.; Kaji, H.; Yoshii, M.; Hisanaga, S.; Uéda, K. Demonstration of a role for α-synuclein as a functional microtubule-associated protein. J. Alzheimer’s Dis. 2004, 6, 435-442. [CrossRef]
- Scott, D.A.; Tabarean, I.; Tang, Y.; Cartier, A.; Masliah, E.; Roy, S. A pathologic cascade leading to synaptic dysfunction in alpha-synuclein-induced neurodegeneration. J. Neurosci. 2010, 30, 8083-8095. [CrossRef]
- Marsden, C.D. Parkinson's disease. Lancet 1990, 335, 948-952. [CrossRef]
- Braak, H.; Del-Tredici, K.; Rüb, U.; de Vos, R.A.; Jansen-Steur, E.N.; Braak, E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol. Aging 2003, 24, 197-211. [CrossRef]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; Chesselet, M.F.; Keshavarzian, A.; Shannon, K.M.; Krajmalnik-Brown, R.; Wittung-Stafshede, P.; Knight, R.; Mazmanian, S.K. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson's Disease. Cell 2016, 167, 1469-1480. e12. [CrossRef]
- a) Polymeropoulos, M.H.; Lavedan, C.; Leroy, E.; Ide, S.E.; Dehejia, A.; Dutra, A.; Pike, B.; Root, H.; Rubenstein, J.; Boyer, R.; Stenroos, E.S.; Chandrasekharappa, S.; Athanassiadou, A.; Papapetropoulos, T.; Johnson, W.G.; Lazzarini, A.M.; Duvoisin, R.C.; Di-Iorio, G.; Golbe, L.I.; Nussbaum, R.L. Mutation in the α-synuclein gene identified in families with Parkinson's disease. Science. 1997, 276, 2045-207. doi:10.1126/science.276.5321.2045. b) Trinh, J.; Zeldenrust, F.M.J.; Huang, J.; Kasten, M.; Schaake, S.; Petkovic, S.; Madoev, H.; Grünewald, A.; Almuammar, S.; König, I.R.; Lill, C.M.; Lohmann, K.; Klein, C.; Marras, C. Genotype-phenotype relations for the Parkinson's disease genes SNCA, LRRK2, VPS35: MDSGene systematic review. Mov. Disord. 2018, 33, 1857-1870. doi:10.1002/mds.27527. [CrossRef]
- Schapira, A.H.; Cooper, J.M.; Dexter, D.; Jenner, P.; Clark, J.B.; Marsden, C.D. Mitochondrial complex I deficiency in Parkinson's disease. Lancet 1989, 1, 1269. [CrossRef]
- a) Kasten, M.; Hartmann, C.; Hampf, J.; Schaake, S.; Westenberger, A.; Vollstedt, E.J.; Black, A; Domingo, A.; Vulinovic, F.; Dulovic, M.; Zorn, I.; Madoev, H.; Zehnle, H.; Lembeck, C.M.; Schawe, L.; Reginold, J.; Huang, J.; König, I.R.; Bertram, L.; Marras, C.; Lohmann, K.; Lill, C.M.; Klein, C. Genotype-Phenotype Relations for the Parkinson's Disease Genes Parkin, PINK1, DJ1: MDSGene Systematic Review. Mov. Disord. 2018, 33, 730-741. doi:10.1002/mds.27352. b) Valente, E.M;.; Abou-Sleiman, P.M.; Caputo, V.; Muqit, M.M.; Harvey, K.; Gispert, S.; Ali, Z.; Del-Turco, D.; Bentivoglio, A.R.; Healy, D.G.; Albanese, A.; Nussbaum, R.; González-Maldonado, R.; Deller, T.; Salvi, S.; Cortelli, P.; Gilks, W.P.; Latchman, D.S.; Harvey, R.J.; Dallapiccola, B.; Auburger, G.; Wood, N.W. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science 2004, 304, 1158-60. doi:10.1126/science.1096284. c) Kitada, T.; Asakawa, S.; Hattori, N.; Matsumine, H.; Yamamura, Y.; Minoshima, S.; Yokochi, M.; Mizuno, Y.; Shimizu, N. Mutations in the parkin gene causes autosomal recessive juvenile parkinsonism. Nature 1998, 392, 605-608. doi:10.1038/33416. [CrossRef]
- Bonifati, V.; Rizzu, P.; van Baren, M.J.; Schaap, O.; Breedveld, G.J.; Krieger, E.; Dekker, M.C.; Squitieri, F.; Ibanez, P.; Joosse, M.; van Dongen, J.W.; Vanacore, N.; van Swieten, J.C.; Brice, A.; Meco, G.; van Duijn, C.M.; Oostra, B.A.; Heutink, P. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003, 299, 256-259. [CrossRef]
- Bender, A.; Krishnan, K.J.; Morris, C.M.; Taylor, G.A.; Reeve, A.K.; Perry, R.H.; Jaros, E.; Hersheson, J.S.; Betts, J.; Klopstock, T.; Taylor, R.W.; Turnbull, D.M. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat. Genet. 2006, 38, 515-517. [CrossRef]
- Gan-Or, Z.; Dion, P.A.; Rouleau, G.A. Genetic perspective on the role of the autophagy-lysosome pathway in Parkinson disease. Autophagy 2015, 11, 1443-1457. [CrossRef]
- a) Funayama, M.; Hasegawa, K.; Kowa, H.; Saito, M.; Tsuji, S.; Obata, F. A new locus for Parkinson's disease (PARK8) maps to chromosome 12p11.2-q13.1. Ann. Neurol. 2002, 51, 296-301. doi:10.1002/ana.10113. b) Yue, Z.; Yang, X.W. Dangerous duet: LRRK2 and α-synuclein jam at CMA. Nat. Neurosci. 2013, 16, 375-377. doi:10.1038/nn.3361. [CrossRef]
- Balestrino, R.; Schapira, A.H.V. Glucocerebrosidase and Parkinson Disease: Molecular, Clinical, and Therapeutic Implications. Neuroscientist 2018, 24, 540-559. doi:10.1177/1073858417748875. b) Ryan, E.; Seehra, G.; Sharma, P.; Sidransky, E. GBA1-associated parkinsonism: new insights and therapeutic opportunities. Curr. Opin. Neurol. 2019, 32, 589-596. doi:10.1097/WCO.0000000000000715. [CrossRef]
- Quadri, M.; Mandemakers, W.; Grochowska, M.M.; Masius, R.; Geut, H.; Fabrizio, E.; Breedveld, G.J.; Kuipers, D.; Minneboo, M.; Vergouw, L.J.M.; Carreras-Mascaro, A.; Yonova-Doing, E.; Simons, E.; Zhao, T.; Di Fonzo, A.B.; Chang, H.C.; Parchi, P.; Melis, M.; Correia-Guedes, L.; Criscuolo, C.; Thomas, A.; Brouwer, R.W.W.; Heijsman, D.; Ingrassia, A.M.T; Calandra-Buonaura, G.; Rood, J.P.; Capellari, S.; Rozemuller, A.J.; Sarchioto, M.; Fen, C.H.; Vanacore, N.; Olgiati, S.; Wu-Chou, Y.H.; Yeh, T.H.; Boon, A.J.W.; Hoogers, S.E.; Ghazvini, M.; IJpma, A.S.; van IJcken, W.F.J.; Onofrj, M.; Barone, P.; Nicholl, D.J.; Puschmann, A.; De Mari, M.; Kievit, A.J.; Barbosa, E.; De Michele, G.; Majoor-Krakauer, D.; van Swieten, J.C.; de Jong, F.J.; Ferreira, J.J.; Cossu, G.; Lu, C.S.; Meco, G.; Cortelli, P.; van de Berg, W.D.J.; Bonifati, V. International Parkinsonism Genetics Network. LRP10 genetic variants in familial Parkinson's disease and dementia with Lewy bodies: a genome-wide linkage and sequencing study. Lancet Neurol. 2018, 17, 597-608. doi:10.1016/S1474-4422(18)30179-0. Erratum in: Lancet Neurol. 2020, 19, e2. b) Williams, E.T.; Chen, X.; Moore, D.J. VPS35, the Retromer Complex and Parkinson's Disease. J Parkinsons Dis. 2017, 7, 219-233. doi:10.3233/JPD-161020. [CrossRef]
- Calì, T.; Ottolini, D.; Brini, M. Mitochondria, calcium, and endoplasmic reticulum stress in Parkinson's disease. Biofactors 2011, 37, 228-240. [CrossRef]
- Spielman, L.J.; Gibson, D.L.; Klegeris, A. Unhealthy gut, unhealthy brain: The role of the intestinal microbiota in neurodegenerative diseases. Neurochem. Int. 2018, 120, 149-163. [CrossRef]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson's disease. Lancet 2021, 397, 2284-2303. [CrossRef]
- a) Fereshtehnejad, S.M.; Postuma, R.B. Subtypes of Parkinson's Disease: What Do They Tell Us About Disease Progression? Curr. Neurol. Neurosci. Rep. 2017, 17, 34. doi:10.1007/s11910-017-0738-x. b) Stebbins, G.T.; Goetz, C.G.; Burn, D.J.; Jankovic, J.; Khoo, T.K.; Tilley, B.C. How to identify dominant tremor and postural instability/gait difficulty groups with the movement disorder society unified Parkinson's disease rating scale: comparison with the unified Parkinson's disease rating scale. Mov. Disord. 2013, 28, 668-670. doi:10.1002/mds.25383. [CrossRef]
- a) Schapira, A.H.V.; Chaudhuri, K.R.; Jenner, P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 435-450. doi:10.1038/nrn.2017.62. Erratum in: Nat. Rev. Neurosci. 2017, 18, 509. b) Balestrino, R.; Martinez-Martin, P. Neuropsychiatric symptoms, behavioral disorders, and quality of life in Parkinson's disease. J. Neurol. Sci. 2017, 373, 173-178. doi:10.1016/j.jns.2016.12.060. [CrossRef]
- Postuma, R.B.; Aarsland, D.; Barone, P.; Burn, D.J.; Hawkes, C.H.; Oertel, W.; Ziemssen, T. Identifying prodromal Parkinson's disease: pre-motor disorders in Parkinson's disease. Mov. Disord. 2012, 27, 617-626. [CrossRef]
- Sousa, E.T.; Lopes, W.A.; Andrade, J.B. Fontes, formação, reatividade e determinação de quinonas na atmosfera. Quim. Nova 2016, 39, 486-495. [CrossRef]
- Futuro, D.O.; Ferreira, P.G.; NicolettiI, C.D.; Borba-Santos, L.P.; da Silva, F.C.; Rozental, S.; Ferreira, V.F. The Antifungal Activity of Naphthoquinones: An Integrative Review. An. Acad. Bras. Cienc. 2018, 90, 1187-1214. [CrossRef]
- Aminin, D.; Polonik, S. 1,4-Naphthoquinones: Some Biological Properties and Application. Chem. Pharm. Bull. 2020, 68, 46-57. [CrossRef]
- da Silva, M.N.; Ferreira, V.F.; de Souza, M.C.B.V. Um panorama atual da química e da farmacologia de naftoquinonas, com ênfase na beta-lapachona e derivados. Quim. Nova, 2003, 26, 407–416. [CrossRef]
- Ramos-Peralta, L.; López-López, L.I.; Silva-Belmares, S.Y.; Zugasti-Cruz, A.; Rodríguez-Herrera, R.; Aguilar-González, C.N. Naphthoquinone: Bioactivity and Green Synthesis. In The Battle Against Microbial Pathogens: Basic Science, Technological Advances and Educational Programs (A. Mendez-Vilas, Ed.) 2015, 542-550.
- Nicolaides, D.N.; Gautam, D.R.; Litinas, K.E.; Litina, D.J.H.; Fylaktakidou, K.C. Synthesis and evaluation of the antioxidant and antiinflammatory activities of some benzo[1]khellactone derivatives and analogues. Eur. J. Med. Chem. 2004, 39, 323-332. [CrossRef]
- Carneiro, P.F.; Pinto, M.C.R.F.; Marra, R.K.F.; Silva, F.C.; Resende, J.A.L.C.; Rocha e Silva, L.F.; Alves, H.G.; Barbosa, G.S.; Vasconcellos, M.C.; Lima, E.S.; Pohlit, A.M.; Ferreira, V.F. Synthesis and antimalarial activity of quinones and structurally-related oxirane derivatives. Eur. J. Med. Chem. 2016, 108, 134-140. [CrossRef]
- Lamberti, M.J.; Rumie, V.N.B.; Silva, F.C.; Ferreira, V.F.; Rivarola, V.A. Synergistic enhancement of antitumor effect of β-Lapachone by photodynamic induction of quinone oxidoreductase (NQO1). Phytomedicine, 2013, 20, 1007-1012. [CrossRef]
- Pinto, C.N.; Dantas, A.P.; de Moura, K.C.G.; Emery, F.S.; Polequevitch, P.F.; Pinto, M.C.F.R.; de Castro, S.L.; Pinto, A.V. Chemical reactivity studies with naphthoquinones from tabebuia with antitrypanosomal efficacy. Arzneim. Forsc. Drug. Res. 2000, 50, 1120-1128. [CrossRef]
- Ferreira, M.P.; Cardoso, M.F.C.; da Silva, F.C.; Ferreira, V.F.; Lima, E.S.; Souza, B.V.J. Antifungal activity of synthetic naphthoquinones against dermatophytes and opportunistic fungi: preliminary mechanism-of-action tests. Ann. Clin. Microbiol. Antimicrob. 2014, 13, 26-32. [CrossRef]
- Ravichandiran, P.; Sheet, S.; Premnath, D.; Kim, A.R.; Yoo, D.J. 1,4-naphthoquinone analogues: potent antibacterial agents and mode of action evaluation. Molecules 2019, 24,1437. [CrossRef]
- Ferraz, P.A.L.; de Abreu, F.C.; Pinto, A.V.; Glezer, V.; Tonholo, J. Goulart, M.O.F.J. Electrochemical aspects of the reduction of biologically active 2- hydroxy-3-alkyl-1,4-naphthoquinones. Electroanal. Chem. 2001, 507, 275. [CrossRef]
- Rover Júnior, L.; Höehr, N.F.; Vellasco, E.A.P.; Kubota, L.T. Sistema antioxidante envolvendo o ciclo metabólico da glutationa associado a métodos eletroanalíticos na avaliação do estresse oxidativo. Quim. Nova 2001, 24, 112. [CrossRef]
- Menchinskaya, E.; Chingizova, E.; Pislyagin, E.; Likhatskaya, G.; Sabutski, Y.; Pelageev, D.; Polonik, S.; Aminin, D. Neuroprotective Effect of 1,4-Naphthoquinones in an In Vitro Model of Paraquat and 6-OHDA-Induced Neurotoxicity. Int. J. Mol. Sci. 2021, 22, 9933.. [CrossRef]
- Hussain, H.; Krohn, K.; Ahmad, V.U.; Miana, G.A.; Green, I.R. Lapachol: An overview. Arkivoc, 2007, 2, 145. [CrossRef]
- Thakur, A. Juglone: A therapeutic phytochemical from Juglans regia L. J. Med. Plants Res. 2011, 5, 5324-5330.
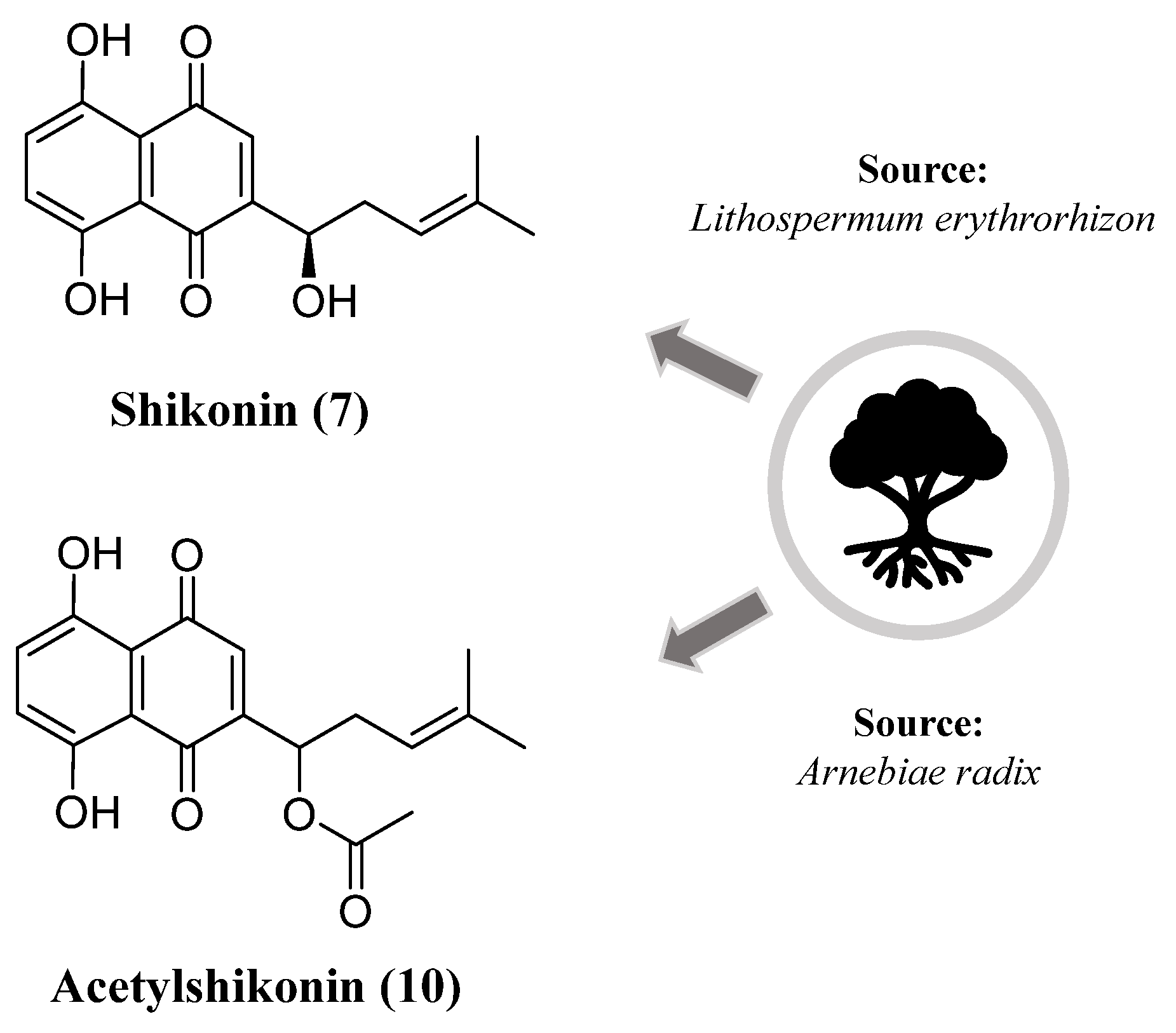
- Andújar, I.; Ríos, J.L.; Giner, R.M.; Recio, M.C. Pharmacological Properties of Shikonin - A Review of Literature since 2002. Planta Med. 2013, 79, 1685-1697. [CrossRef]
- Rodriguez-Rodriguez, A.; Egea-Guerrero, J.; Murillo-Cabezas, F.; Carrillo-Vico, A. Oxidative Stress in Traumatic Brain Injury. Curr. Med. Chem. 2014, 21, 1201-1211. [CrossRef]
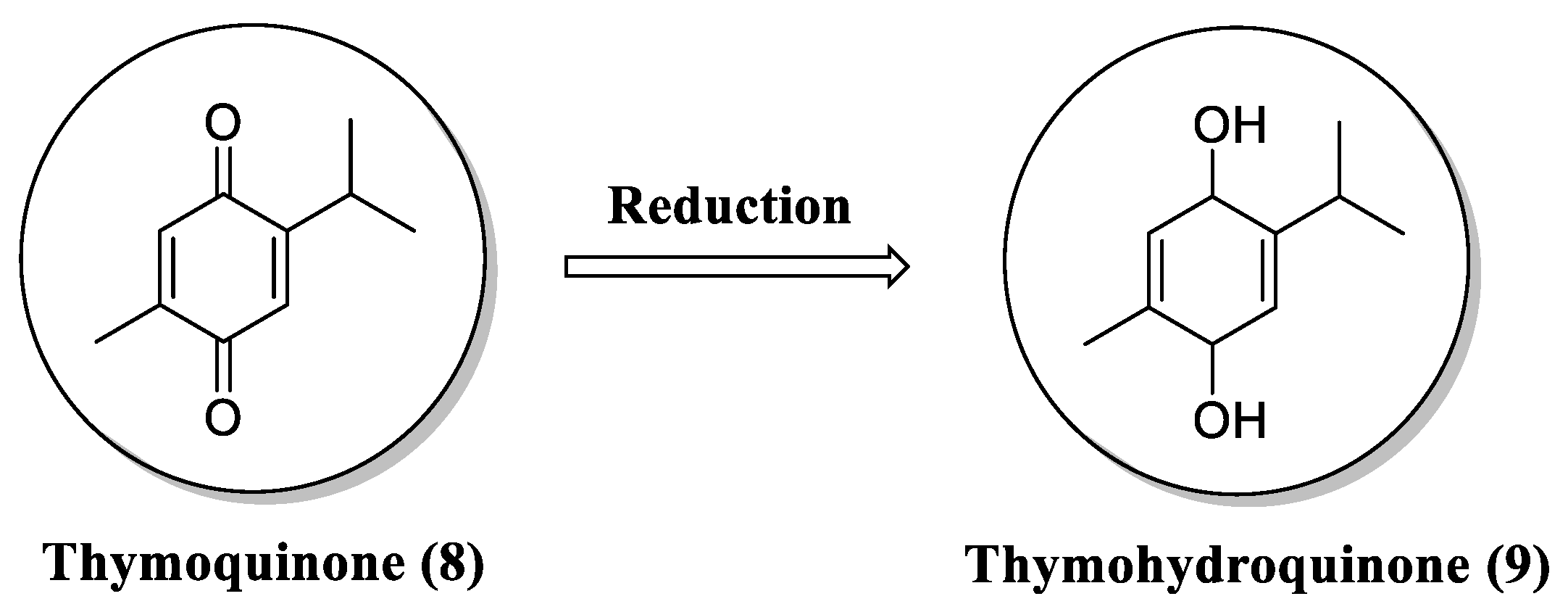
- Staniek, K.; Gille, L. Is thymoquinone an antioxidant? BMC Pharmacol. 2010, 10. [CrossRef]
- Al-Majed, A.A.; Al-Omar, F.A.; Nagi, M.N. Neuroprotective effects of thymoquinone against transient forebrain ischemia in the rat hippocampus. Eur. J. Pharmacol. 2006, 543, 40-47. [CrossRef]
- Kanter, M. Protective effects of thymoquinone on the neuronal injury in frontal cortex after chronic toluene exposure. J. Mol. Histol. 2011, 42, 39-46. [CrossRef]
- Radad, K.; Hassanein, K.; Al-Shraim, M.; Moldzio, R.; Rausch, W.D. Thymoquinone ameliorates lead-induced brain damage in Sprague Dawley rats. Exp. Toxicol. Pathol. 2014, 66, 13-17. [CrossRef]
- Kassab, R.B.; El-Hennamy, R.E. The role of thymoquinone as a potent antioxidant in ameliorating the neurotoxic effect of sodium arsenate in female rat. Egypt. J. Basic Appl. Sci. 2017, 4, 160-167. [CrossRef]
- Wang, Z.; Liu, T.; Gan, L.; Wang, T.; Yuan, X.; Zhang, B.; Chen, H.; Zheng Qiusheng, Q. Shikonin protects mouse brain against cerebral ischemia/reperfusion injury through its antioxidant activity. Eur. J. Pharmacol. 2010, 643, 211-217. [CrossRef]
- Wang, Y.; Pan, W.L.; Liang, W.C.; Law, W.K.; Tsz-Ming Ip, D.; Ng, T.B.; Miu-Yee Waye, M.; Chi-Cheong Wan, D. Acetylshikonin, a novel ache inhibitor, inhibits apoptosis via upregulation of heme oxygenase-1 expression in sh-sy5y cells. Evidence-based Complement. Altern. Med. 2013, 2013. [CrossRef]
- Vomhof-DeKrey, E.E.; Picklo, M.J. The Nrf2-antioxidant response element pathway: A target for regulating energy metabolism. J. Nutr. Biochem. 2012, 23, 1201-1206. [CrossRef]
- Son, T.G.; Camandola, S.; Arumugam, T. V.; Cutler, R.G.; Telljohann, R.S.; Mughal, M.R.; Moore, T.A.; Luo, W.; Yu, Q.S.; Johnson, D.A.; et al. Plumbagin, a novel Nrf2/ARE activator, protects against cerebral ischemia. J. Neurochem. 2010, 112, 1316-1326. [CrossRef]
- Yuan, J.H.; Pan, F.; Chen, J.; Chen, C.E.; Xie, D.P.; Jiang, X.Z.; Guo, S.J.; Zhou, J. Neuroprotection by plumbagin involves BDNF-TrkB-PI3K/Akt and ERK1/2/JNK pathways in isoflurane-induced neonatal rats. J. Pharm. Pharmacol. 2017, 69, 896-906. [CrossRef]
- Messeha, S.S.; Zarmouh, N.O.; Mendonca, P.; Kolta, M.G.; Soliman, K.F.A. The attenuating effects of plumbagin on pro-inflammatory cytokine expression in LPS-activated BV-2 microglial cells. J. Neuroimmunol. 2017, 313, 129-137. [CrossRef]
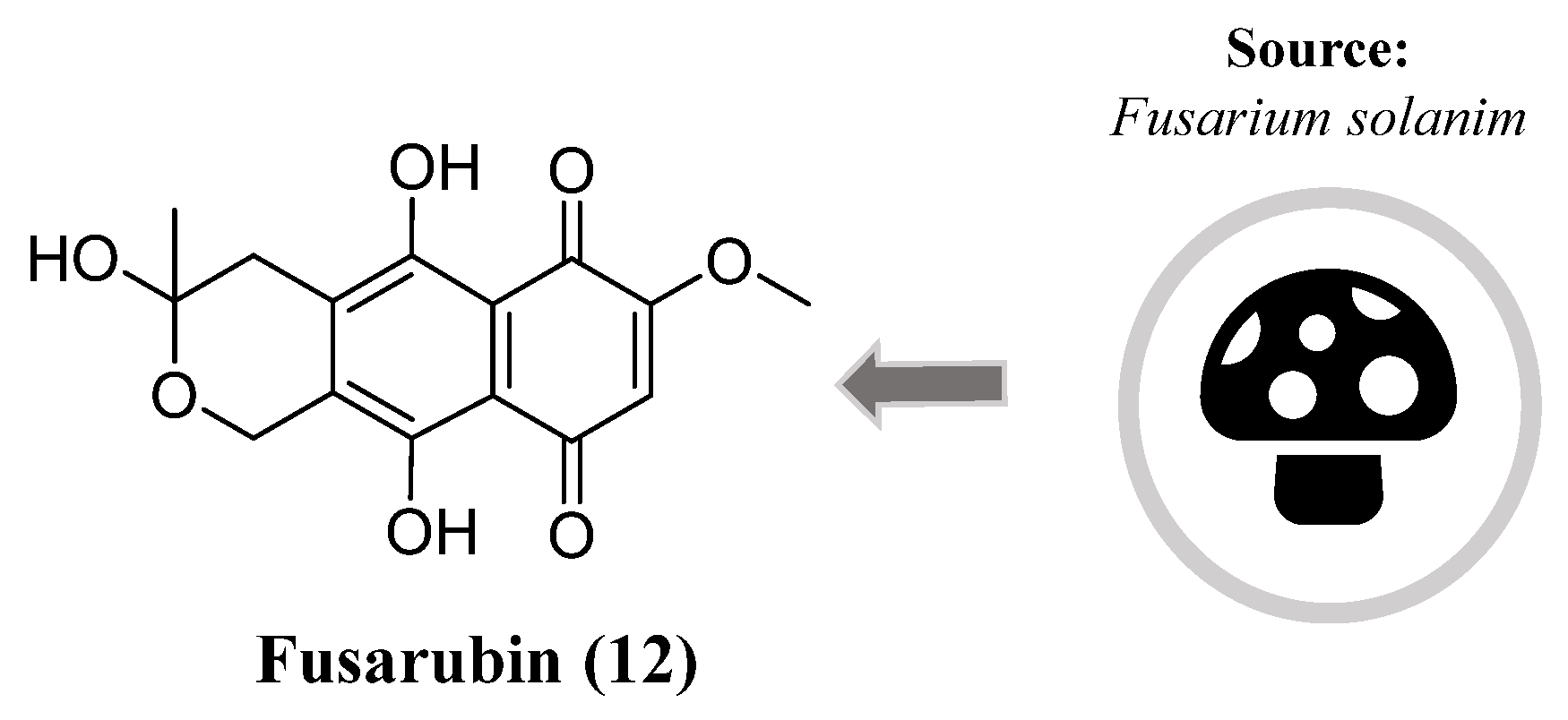
- Choi, H.G.; Song, J.H.; Park, M.; Kim, S.; Kim, C.E.; Kang, K.S.; Shim, S.H. Neuroprotective γ-pyrones from fusarium solani js-0169: Cell-based identification of active compounds and an informatics approach to predict the mechanism of action. Biomolecules 2020, 10. [CrossRef]
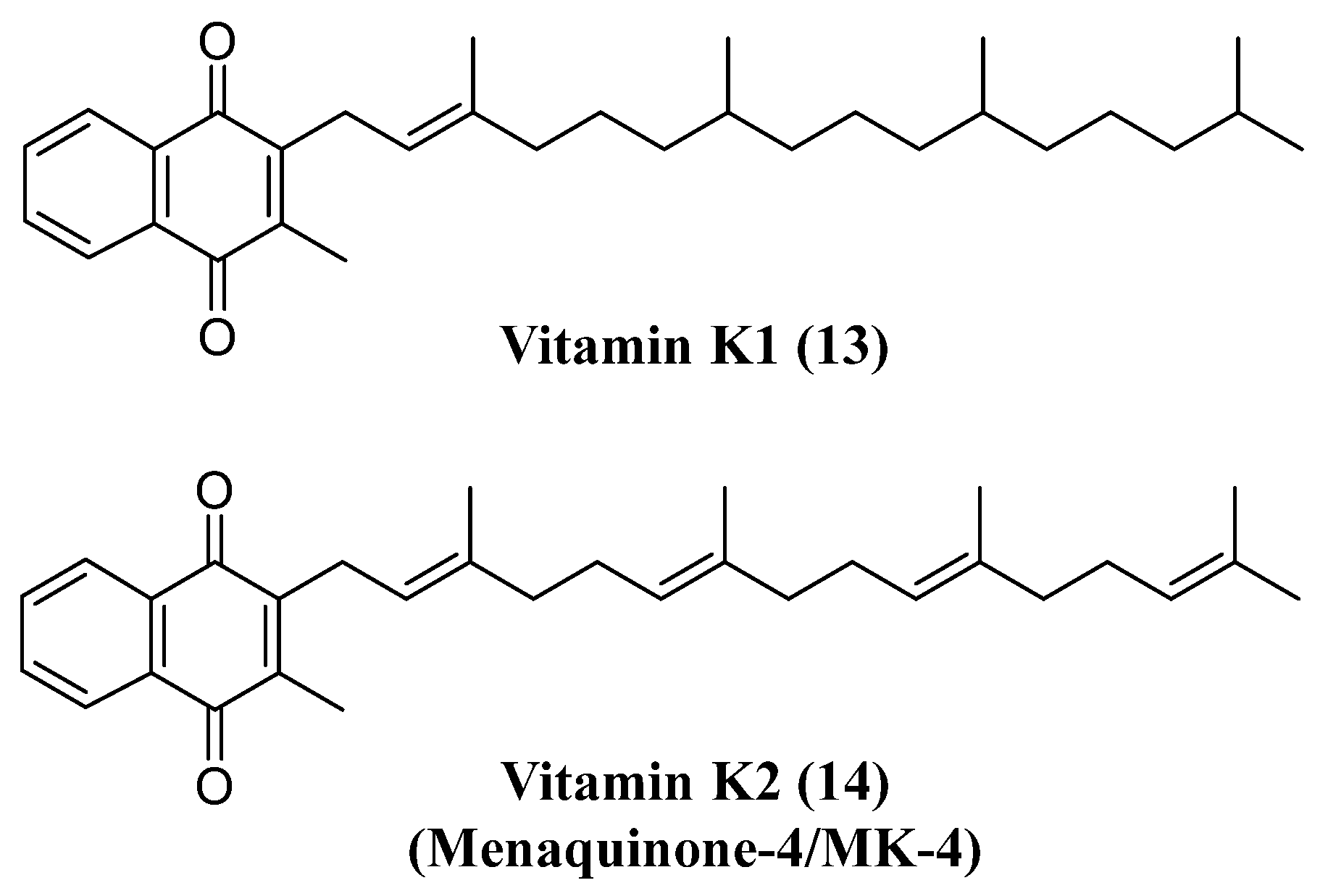
- Thijssen, H.H.W.; Drittij-Reijnders, M.J. Vitamin K distribution in rat tissues: dietary phylloquinone is a source of tissue menaquinone-4. Br. J. Nutr. 1994, 72, 415-425. [CrossRef]
- Moghadam, B.F.; Fereidoni, M. Neuroprotective effect of menaquinone-4 (MK-4) on transient global cerebral ischemia/ reperfusion injury in rat. PLoS One 2020, 15. [CrossRef]
- Sakaue, M.; Mori, N.; Okazaki, M.; Kadowaki, E.; Kaneko, T.; Hemmi, N.; Sekiguchi, H.; Maki, T.; Ozawa, A.; Hara, S.; et al. Vitamin K has the potential to protect neurons from methylmercury-induced cell death In Vitro. J. Neurosci. Res. 2011, 89, 1052-1058. [CrossRef]
- Huang, S.H.; Fang, S.T.; Chen, Y.C. Molecular mechanism of vitamin k2 protection against amyloid-β-induced cytotoxicity. Biomolecules 2021, 11, 1-22. [CrossRef]
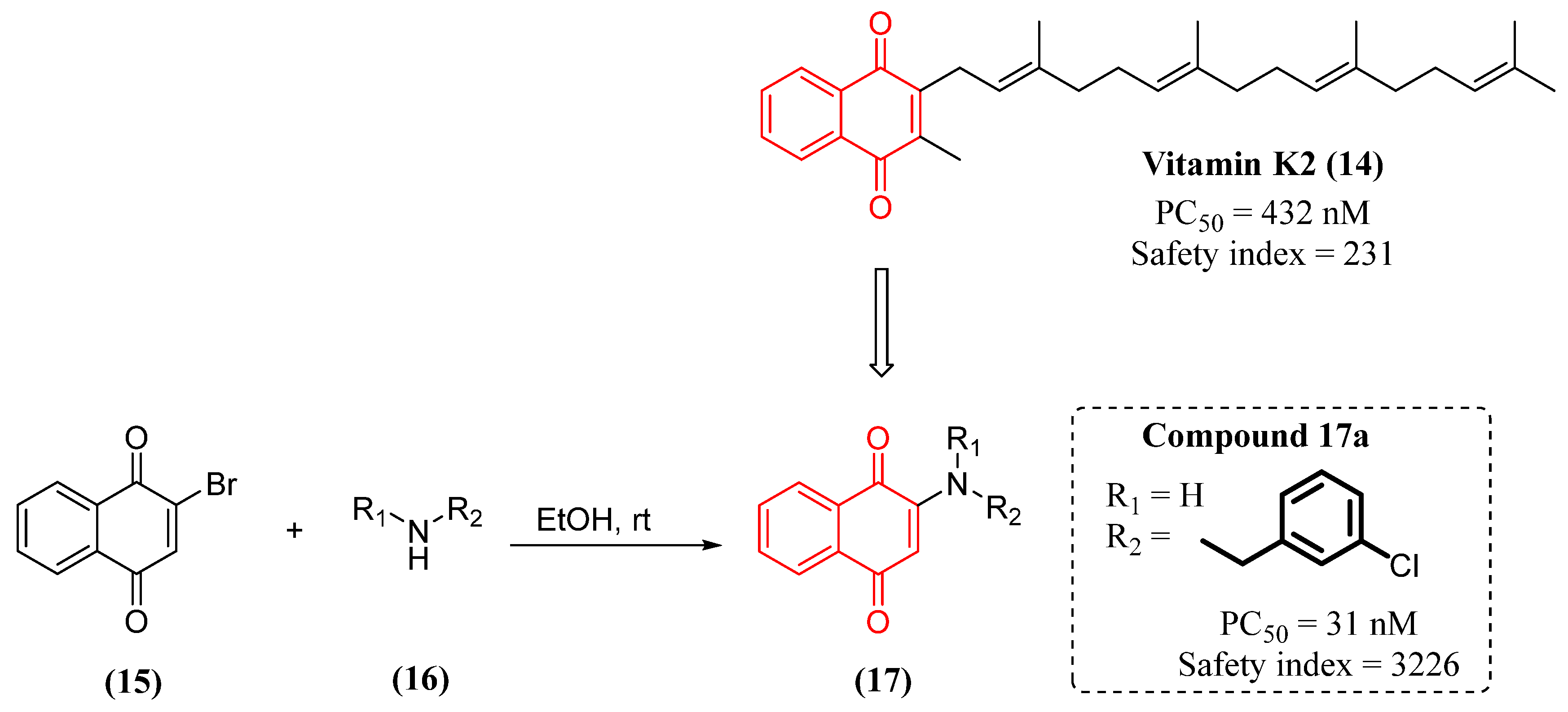
- Josey, B.J.; Inks, E.S.; Wen, X.; Chou, C.J. Structure-activity relationship study of vitamin K derivatives yields highly potent neuroprotective agents. J. Med. Chem. 2013, 56, 1007-1022. [CrossRef]
- Nepovimova, E.; Uliassi, E.; Korabecny, J.; Peña-Altamira, L.E.; Samez, S.; Pesaresi, A.; Garcia, G.E.; Bartolini, M.; Andrisano, V.; Bergamini, C.; et al. Multitarget drug design strategy: Quinone-tacrine hybrids designed to block amyloid-β aggregation and to exert anticholinesterase and antioxidant effects. J. Med. Chem. 2014, 57, 8576-8589. [CrossRef]
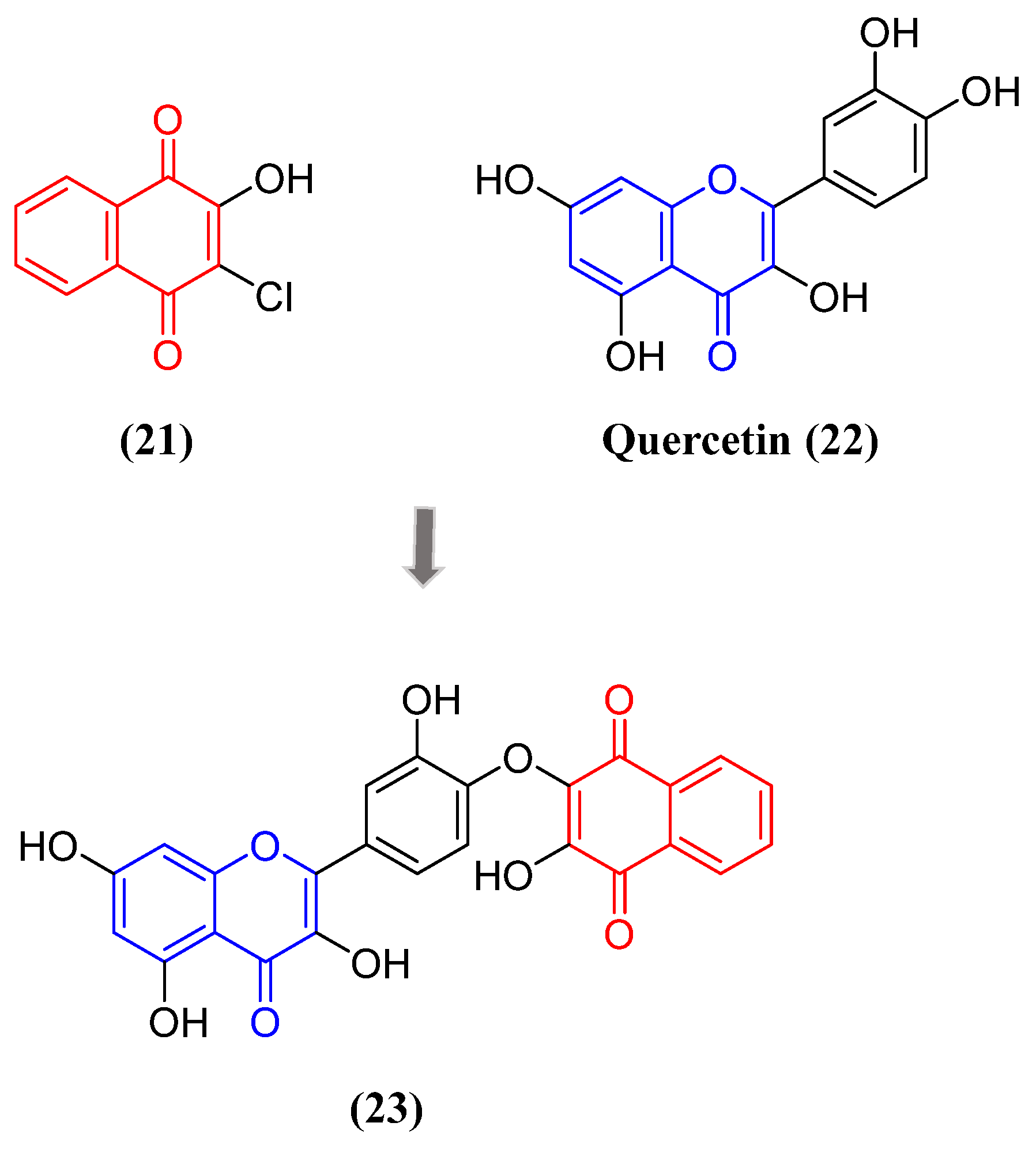
- Škandík, M.; Mrvová, N.; Bezek, Š.; Račková, L. Semisynthetic quercetin-quinone mitigates BV-2 microglia activation through modulation of Nrf2 pathway. Free Radic. Biol. Med. 2020, 152. [CrossRef]
- Chen, W.W.; Zhang, X.; Huang, W.J. Role of neuroinflammation in neurodegenerative diseases (Review). Mol. Med. Rep. 2016, 13, 3391-3396. [CrossRef]
- Inoue, K. Microglial activation by purines and pyrimidines. Glia. 2002, 40, 156-163. [CrossRef]
- a) Takenouchi, T.; Sekiyama, K.; Sekigawa, A.; Fujita, M.; Waragai, M.; Sugama, S.; Iwamaru, Y.; Kitani, H.; Hashimoto, M. P2X7 Receptor Signaling Pathway as a Therapeutic Target for Neurodegenerative Diseases. Arch. Immunol. Ther. Exp. 2010, 58, 91-96. DOI: 10.1007/s00005-010-0069-y b) Calzaferri, F.; Ruiz-Ruiz, C.; de Diego, A.M.G.; de Pascual, R.; Méndez-López, I.; Cano-Abad, M.F.; Maneu, V.; de los Ríos, C.; Gandía, L.; García, A.G. The purinergic P2X7 receptor as a potential drug target to combat neuroinflammation in neurodegenerative diseases. Med. Res. Rev. 2020, 40, 2427-2465. Doi: 10.1002/med.21710. [CrossRef]
- Pislyagin, E.; Kozlovskiy, S.; Menchinskaya, E.; Chingizova, E.; Likhatskaya, G.; Gorpenchenko, T.; Sabutski, Y.; Polonik, S.; Aminin, D. Synthetic 1,4-Naphthoquinones inhibit P2X7 receptors in murine neuroblastoma cells. Bioorganic Med. Chem. 2021, 31. [CrossRef]
- Li, X.; Li, W.; Liu, G.; Shen, X.; Tang, Y. Association between cigarette smoking and Parkinson's disease: A meta-analysis. Arch. Gerontol. Geriatr. 2015, 61, 510-516. [CrossRef]
- Mappin-Kasirer, B.; Pan, H.; Lewington, S.; Kizza, J.; Gray, R.; Clarke, R.; Peto, R. Tobacco smoking and the risk of Parkinson disease - A 65-year follow-up of 30,000 male British doctors. Neurology 2020, 94, e2132-e2138. [CrossRef]
- Castagnoli, K.; Petzer, J.B.; Steyn, S.J.; van der Schyf, C.J.; Castagnoli Jr., N. Inhibition of human MAO-A and MAO-B by a compound isolated from flue-cured tobacco leaves and its neuroprotective properties in the MPTP mouse model of neurodegeneration. Inflammopharmacology 2003, 11, 183-188. [CrossRef]
- Sari, Y.; Khalil, A. Monoamine Oxidase Inhibitors Extracted from Tobacco Smoke as Neuroprotective Factors for Potential Treatment of Parkinson’s Disease. CNS Neurol. Disord. Drug Targets 2015, 14, 777-785. [CrossRef]
- Marti, J.S.; Kettler, R.; Da Prada, M.; Richards, J.G. Molecular neuroanatomy of MAO-A and MAO-B. J. Neural Transm. Suppl. 1990, 32, 49-53. [CrossRef]
- Hauser, D.N.; Hastings, T.G. Mitochondrial dysfunction and oxidative stress in Parkinson's disease and monogenic parkinsonism. Neurobiol. Dis. 2013, 51, 35-42. [CrossRef]
- Dezsi, L.; Vecsei, L. Monoamine Oxidase B Inhibitors in Parkinson’s Disease. CNS Neurol. Disord. Drug Targets 2017, 16, 425-439. [CrossRef]
- Khalil, A.A.; Steyn, S.; Castagnoli Jr, N. Isolation and Characterization of a Monoamine Oxidase Inhibitor from Tobacco Leaves. Chem. Res. Toxicol. 2000, 13, 31-35, 2000. [CrossRef]
- Norris, R.K.; Sternhell, S. Long-range spin-spin coupling in 1,4-benzoquinones and some related compounds. Aust. J. Chem. 1966, 19, 617-627. [CrossRef]
- Castagnoli, K.P.; Steyn, S.J.; Petzer, J.P.; van der Schyf, C.J.; Castagnoli Jr, N. Neuroprotection in the MPTP Parkinsonian C57BL/6 Mouse Model by a Compound Isolated from Tobacco. Chem. Res. Toxicol. 2001, 14, 523-527. [CrossRef]
- Chiba, K.; Trevor, A.; Castagnoli Jr, N. Metabolism of the neurotoxic tertiary amine, MPTP, by brain monoamine oxidase. Biochem. Biophys. Res. Commun. 1984, 120, 574-578. [CrossRef]
- Cerqueira, E.C.; Netz, P.A.; Diniz, C.; do Canto, V.P.; Follmer, C. Molecular insights into human monoamine oxidase (MAO) inhibition by 1,4-naphthoquinone: Evidences for menadione (vitamin K3) acting as a competitive and reversible inhibitor of MAO. Bioorg. Med. Chem. 2011, 19, 7416-7424. [CrossRef]
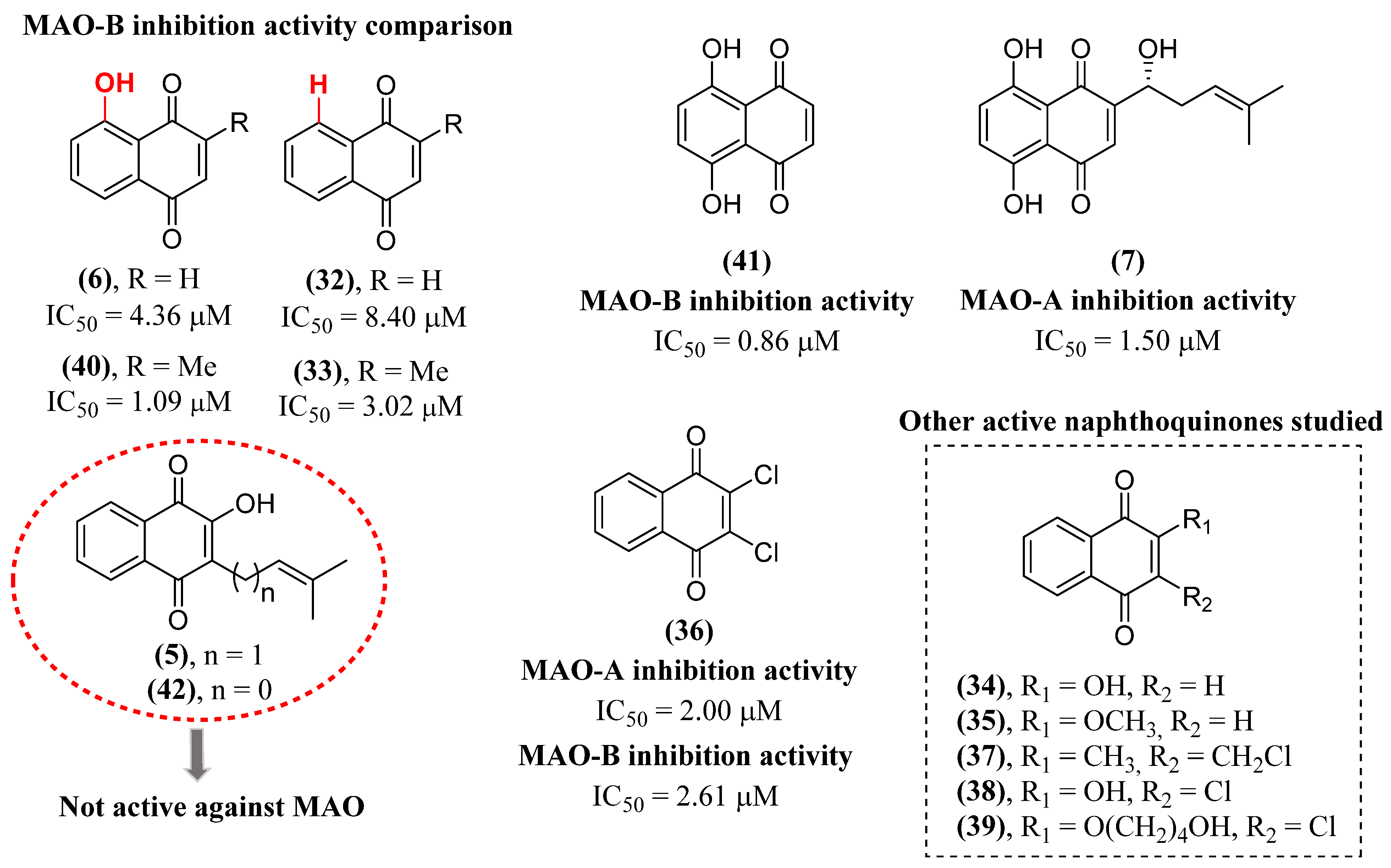
- Mostert, S.; Petzer, A.; Petzer, J.P. Evaluation of Natural and Synthetic 1,4-naphthoquinones as Inhibitors of Monoamine Oxidase. Chem. Biol.Drug Des. 2016, 87, 737-746. [CrossRef]
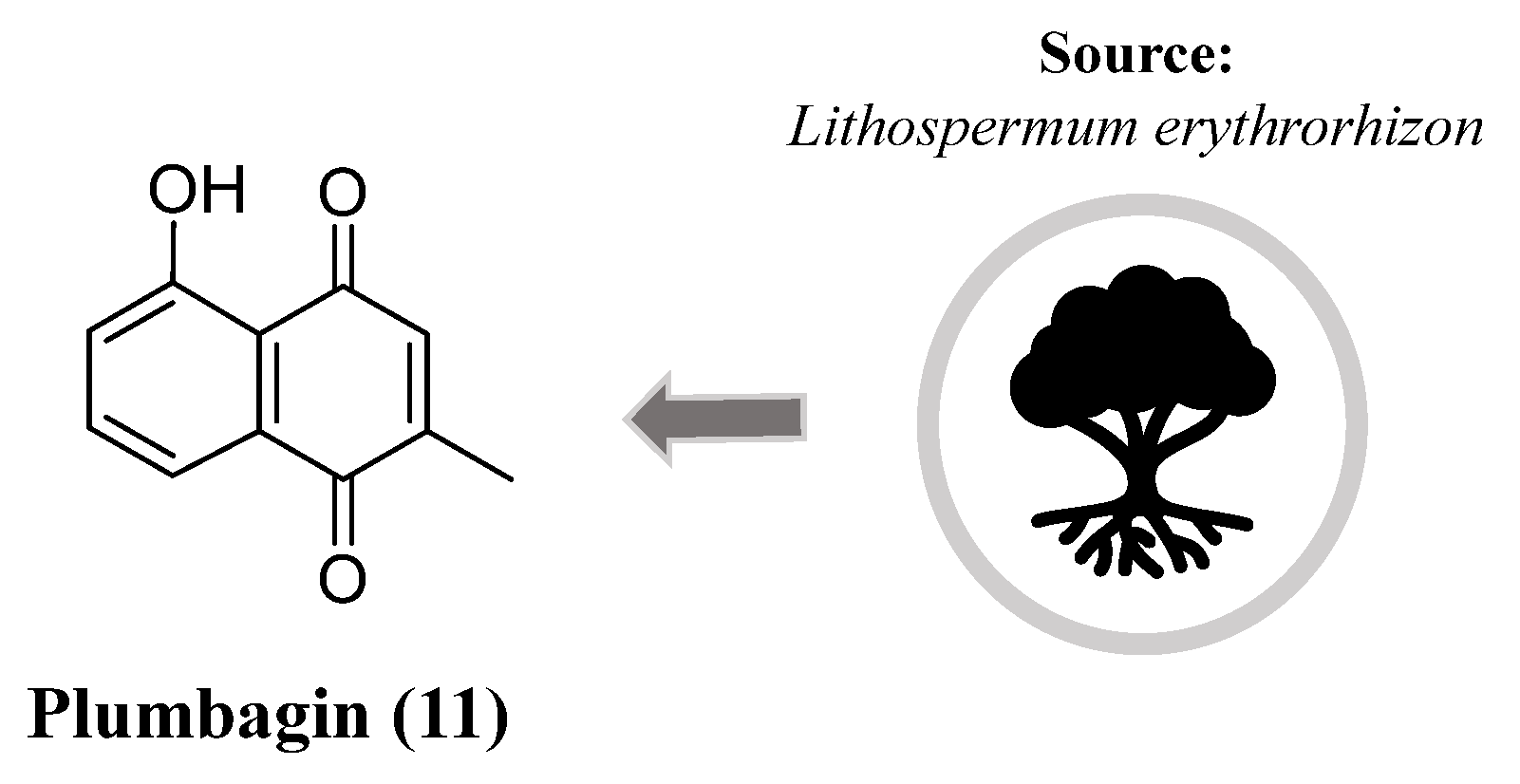
- Choi, W.H.; Hong, S.S.; Lee, S.A.; Han, X.H.; Lee, K.S.; Lee, M.K.; Hwang, B.Y.; Ro, J.S. Monoamine Oxidase Inhibitory Naphthoquinones from the Roots of Lithospermum erythrorhizon. Arch. Pharm. Res. 2005, 28, 400-404. [CrossRef]
- Lashuel, H.A.; Overk, C.R.; Oueslati, A.; Masliah, E. The many faces of α-synuclein: from structure and toxicity to therapeutic target. Nat. Rev. Neurosci. 2013, 14, 38-48. [CrossRef]
- Bengoa-Vergniory, N.; Roberts, R.F.; Wade-Martins, R.; Alegre-Abarrategui, J. Alpha-synuclein oligomers: a new hope. Acta Neuropathol. 2017, 134, 819-838. [CrossRef]
- Fields, C.F.; Bengoa-Vergniory, N.; Wade-Martins, R. Targeting Alpha-Synuclein as a Therapy for Parkinson’s Disease. Front. Mol. Neurosci. 2019, 12, 299. [CrossRef]
- Choi, B.; Choi, M.; Kim, J.; Yang, Y.; Lai, Y.; Kweon, D.; Lee, N.K.; Shin, Y. Large α-synuclein oligomers inhibit neuronal SNARE-mediated vesicle docking. Proc. Natl. Acad. Sci, USA 2013, 110, 4087-4092. [CrossRef]
- Di Maio, R.; Barrett, P.J.; Hoffman, E.K.; Barrett, C.W.; Zharikov, A.; Borah, A.; Hu, X.; McCoy, J.; Chu, C.T.; Burton, E.A.; Hastings, T.G.; Greenamyre, J.T. α-Synuclein binds to TOM20 and inhibits mitochondrial protein import in Parkinson’s disease. Sci. Transl. Med. 2016, 8, 342ra78. [CrossRef]
- Colla, E.; Jensen, P.H.; Pletnikova, O.; Troncoso, J.C.; Glabe, C.; Lee, M.K. Accumulation of Toxic α-Synuclein Oligomer within Endoplasmic Reticulum Occurs in α-Synucleinopathy In Vivo. J. Neurosci. 2012, 10, 3301-3305. [CrossRef]
- da Silva, F.L.; Cerqueira, E.C.; de Freitas, M.S.; Gonçalves, D.L.; Costa, L.T.; Follmer, C. Vitamins K interact with N-terminus α-synuclein and modulate the protein fibrillization in vitro. Exploring the interaction between quinones and α-synuclein. Neurochem. Int. 2013, 62, 113-112. [CrossRef]
- Wang, Y.; Mandelkow, E. Tau in physiology and pathology. Nat. Rev. Neurosci. 2016, 17, 22-35. [CrossRef]
- Venkatramani, A.; Mukherjee, S.; Kumari, A.; Panda, D. Shikonin impedes phase separation and aggregation of tau and protects SH-SY5Y cells from the toxic effects of tau oligomers. Int. J. Biol. Macromol. 2022, 204, 19-33. [CrossRef]
- Kourounakis, A.P.; Assimopoulou, A.N.; Papageorgiou, V.P.; Gavalas, A.; Kourounakis, P.N. Alkannin and shikonin: effect on free radical processes and on inflammation - a preliminary pharmacochemical investigation. Arch. Pharm. (Weinheim) 2002, 335, 262-266. [CrossRef]
- Esmaeilzadeh, E.; Gardaneh, M.; Gharib, E.; Sabouni, F. Shikonin Protects Dopaminergic Cell Line PC12 Against 6-Hydroxydopamine-Mediated Neurotoxicity Via Both Glutathione-Dependent and Independent Pathways and by Inhibiting Apoptosis. Neurochem. Res. 2013, 38, 1590-1604. [CrossRef]
- Li, J.; Mo, C.; Guo, Y.; Zhang, B.; Feng, X.; Si, Q.; Wu, X.; Zhao, Z.; Gong, L.; He, D.; Shao, J. et al. Roles of peptidyl-prolyl isomerase Pin1 in disease pathogenesis. Theranostics 2021, 11, 3348-3358. [CrossRef]
- Ryo, A.; Togo, T.; Nakai, T.; Hirai, A.; Nishi, M.; Yamaguchi, A.; Suzuki, K.; Hirayasu, Y.; Kobayashi, H.; Perrem, K.; Liou, Y; Aoki, I. Prolyl-isomerase Pin1 Accumulates in Lewy Bodies of Parkinson Disease and Facilitates Formation of α-Synuclein Inclusions. J. Biol. Chem. 2006, 281, 4117-4125. [CrossRef]
- Tang, Y.T.; Li, Y.; Chu, P.; Ma, X.D.; Tang, Z.Y.; Sun, Z.L. Molecular biological mechanism of action in cancer therapies: Juglone and its derivatives, the future of development. Biomed. Pharmacother. 2022, 148, 112785. [CrossRef]
- Hennig, L.; Christner, C.; Kipping, M.; Schelbert, B.; Rücknagel, K.P.; Grabley, S.; Küllertz, G.; Fischer, G.A. Selective Inactivation of Parvulin-Like Peptidyl-Prolyl cis/trans Isomerases by Juglone. Biochemistry 1998, 37, 5953-5960. [CrossRef]
- Ghosh, A.; Saminathan, H.; Kanthasamy, A.; Anantharam, V.; Jin, H.; Sondarva, G.; Harischandra, D.S.; Qian, Z.; Rana, A.; Kanthasamy, A.G. The Peptidyl-prolyl Isomerase Pin1 Up-regulation and Proapoptotic Function in Dopaminergic Neurons: Relevance to the Pathogenesis of Parkinson Disease. J. Biol. Chem. 2013, 288, 21955-21971. [CrossRef]
- Choi, S.Y.; Son, T.G.; Park, H.R.; Jang, Y.J.; Oh, S.B.; Jin, B.; Lee, J. Naphthazarin Has a Protective Effect on the 1-Methyl-4-Phenyl-1,2,3,4-Tetrahydropyridine-Induced Parkinson’s Disease Model. J. of Neurosci. Res. 2012, 90, 1842-1849. [CrossRef]
- Park, J.; Leem, Y.; Park, J.; Kim, D.; Kim, H. Neuroprotective Effect of β-Lapachone in MPTP-Induced Parkinson’s Disease Mouse Model: Involvement of Astroglial p-AMPK/Nrf2/HO-1 Signaling Pathways. Biomol. Ther. (Seoul) 2019, 27, 178-184. [CrossRef]
- Ryu, Y.; Park, H.; Go, J.; Lee, I.; Choi, Y.; Lee, C.; Kim, K. β-Lapachone ameliorates L-DOPA-induced dyskinesia in a 6-OHDA-induced mouse model of Parkinson's disease. Mol. Med. Rep. 2021, 23, 217. [CrossRef]
- Saleem, U.; Gull, Z.; Saleem, A.; Shah, M.A.; Akhtar, M.F.; Anwar, F.; Ahmad, B.; Panichayupakaranant, P. Appraisal of anti-Parkinson activity of rhinacanthin-C in haloperidol-induced parkinsonism in mice: A mechanistic approach. J. Food Biochem. 2021, 45, e13677. [CrossRef]
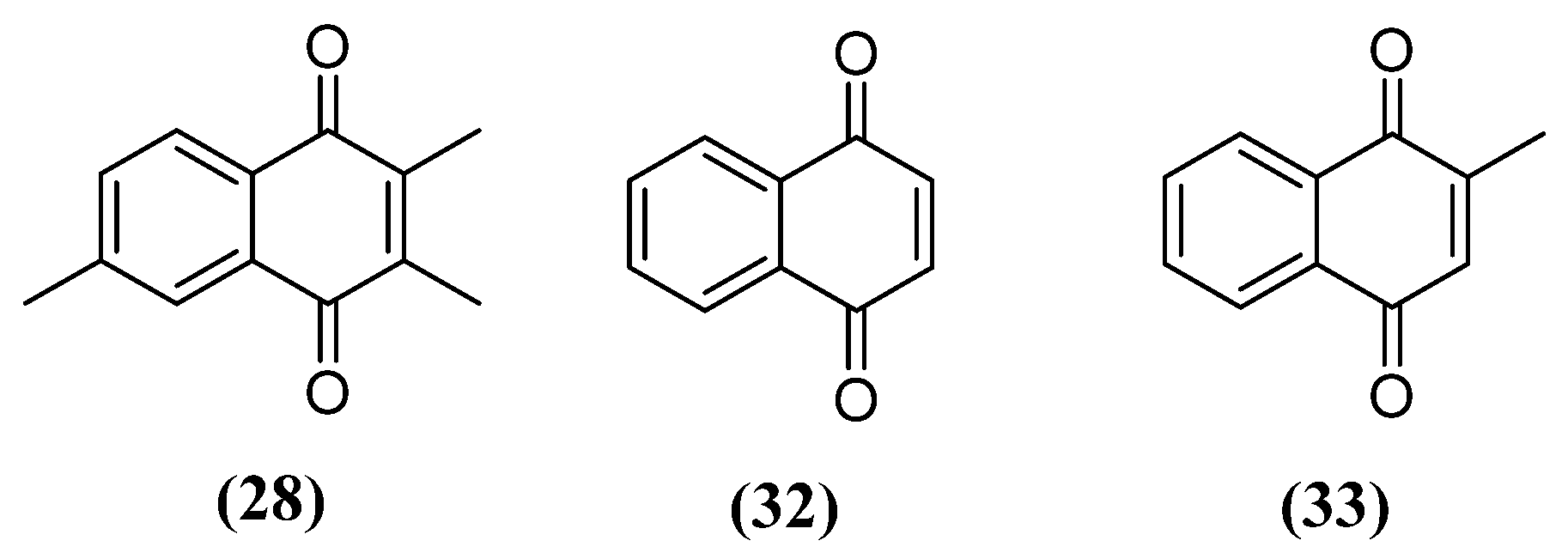
- Cerqueira, E.C.; Netz, P.A.; do Canto, V.P.; Pinto, A.C.; Follmer, C. Beyond Topoisomerase Inhibition: Antitumor 1,4-Naphthoquinones as Potential Inhibitors of Human Monoamine Oxidase. Chem. Biol. Drug Des. 2014, 84, 401-410. [CrossRef]
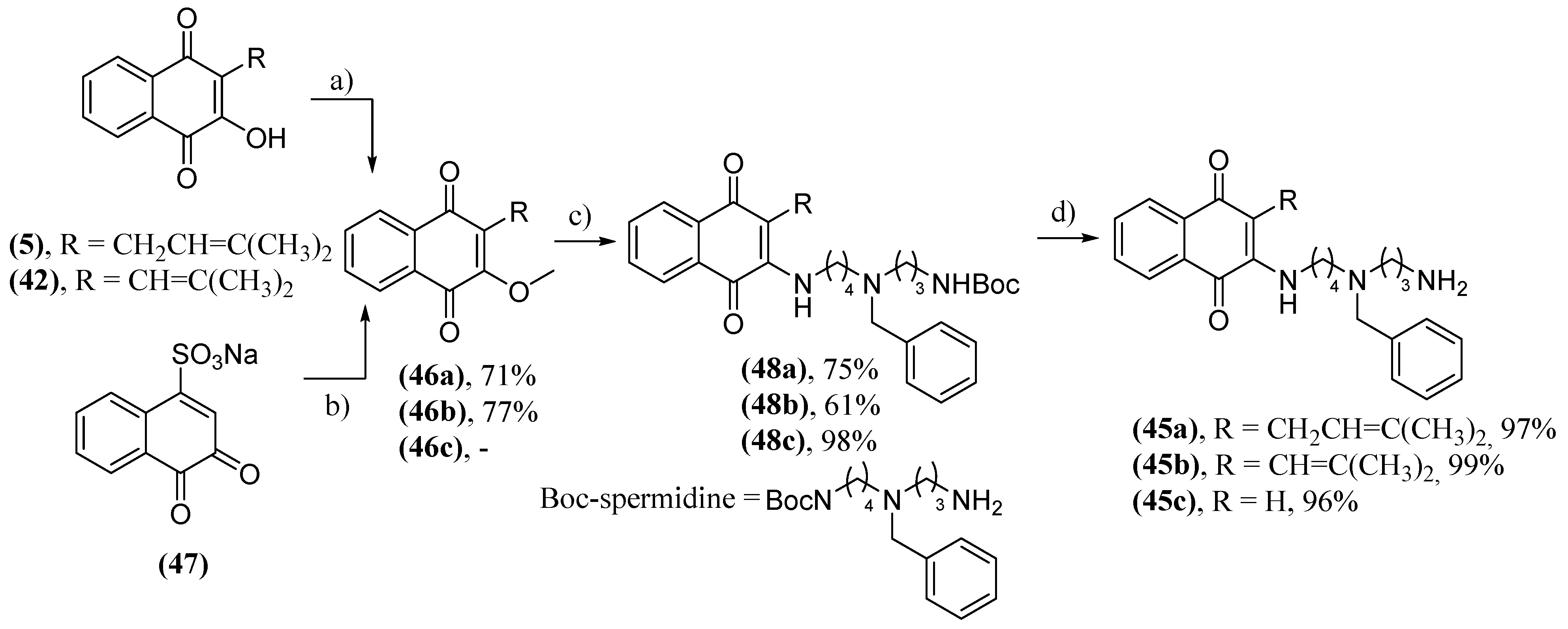
- Cunha, A.S.; Lima, E.L.S.; Pinto, A.C.; Esteves-Souza, A.; Echevarria, A.; Camara, C.A.; Vargas, M.D.; Torres, J.C. Synthesis of Novel Naphthoquinone-Spermidine Conjugates and their Effects on DNA-Topoisomerases I and II-α. J. Braz. Chem. Soc. 2006, 17, 439-442. [CrossRef]
- Fiezer, L.F.; Martin, E.L. 2-Hydroxy-1,4-naphthoquinone. Org. Synth. 1941, 21, 56. [CrossRef]
- Scherzer-Attali, R.; Convertino, M.; Pellarin, R.; Gazit, E.; Segal, D.; Caflisc, A. Methylations of Tryptophan-Modified Naphthoquinone Affect Its Inhibitory Potential toward Aβ Aggregation. J. Phys. Chem. B 2013, 117, 1780-1789. [CrossRef]
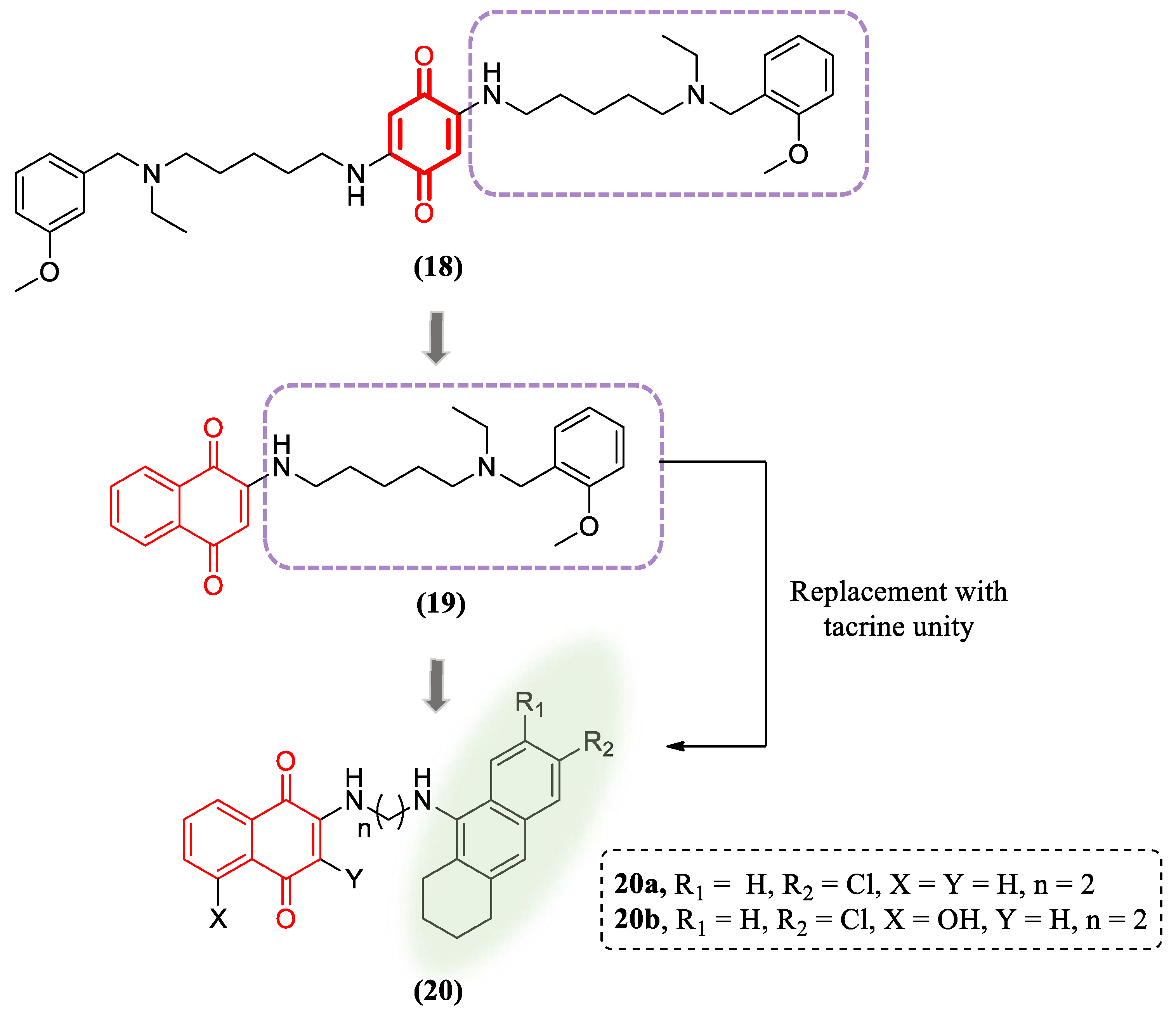
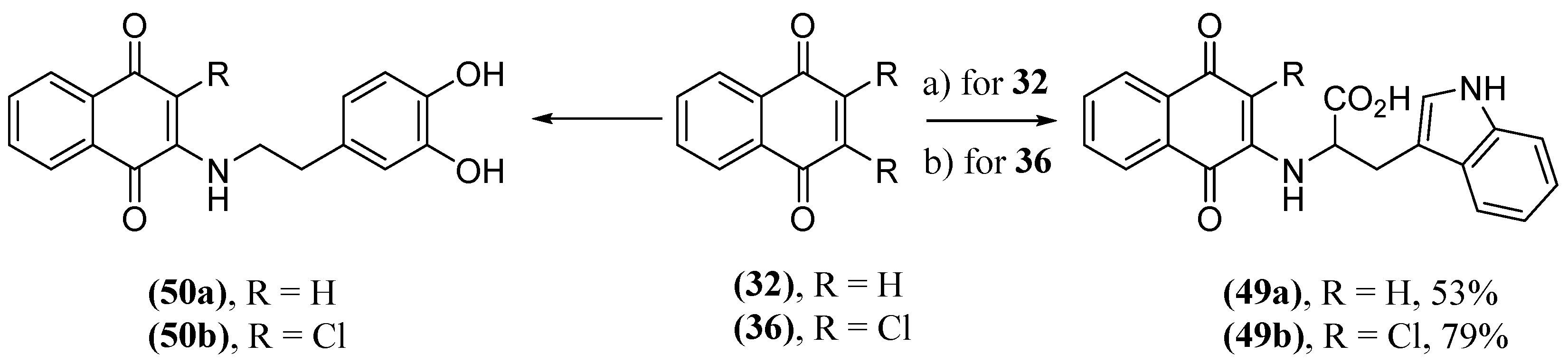
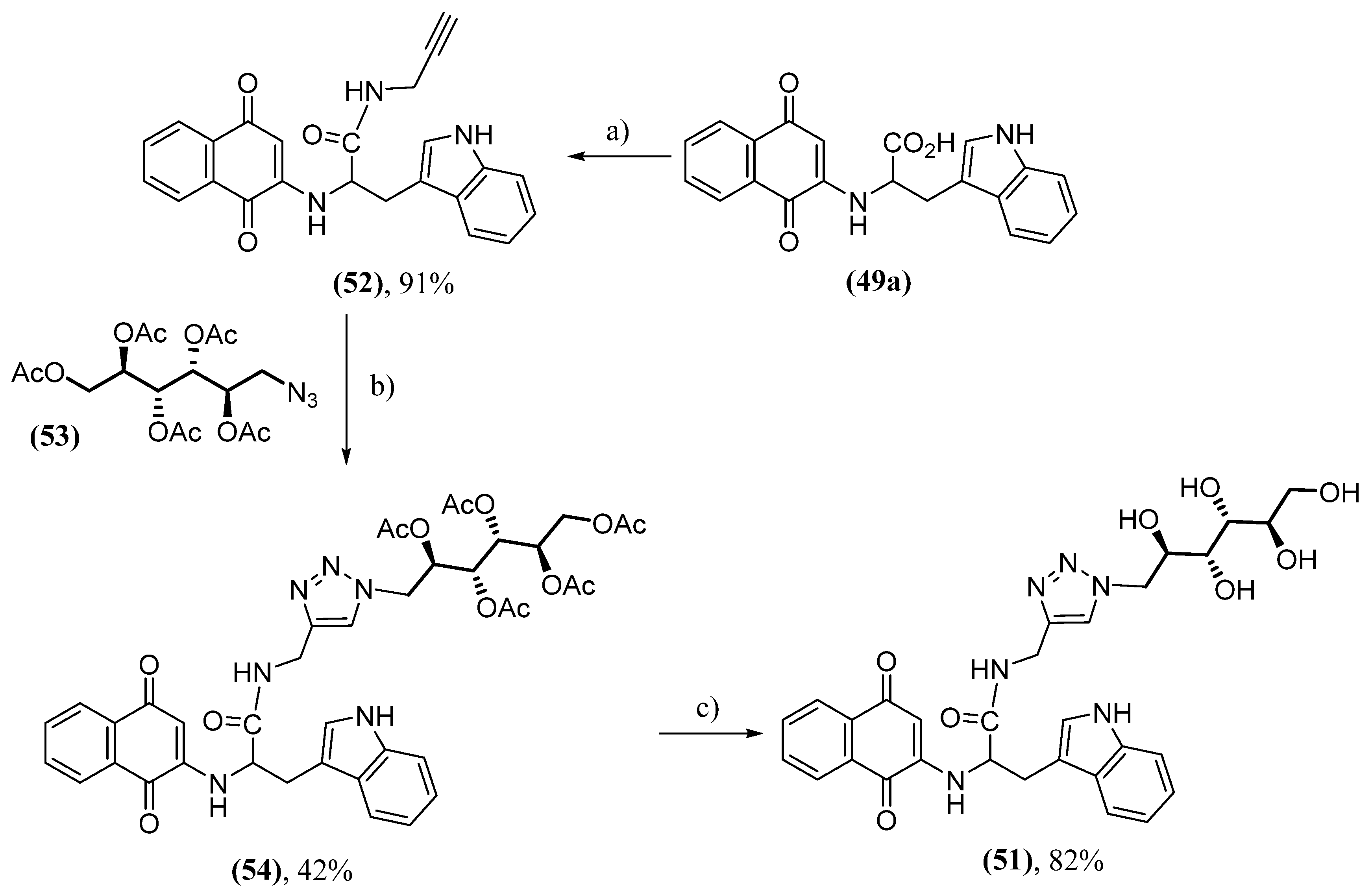
- Paul, A.; Viswanathan, G.K.; Mahapatra, S.; Balboni, G.; Pacifico, S.; Gazit, E.; Segal, D. Antagonistic Activity of Naphthoquinone-Based Hybrids toward Amyloids Associated with Alzheimer’s Disease and Type-2 Diabetes. ACS Chem. Neurosci. 2019, 10, 3510-3520. [CrossRef]
- Scherzer-Attali, R.; Shaltiel-Karyo, R.; Adalist, Y. H.; Segal, D.; Gazit, E. Generic inhibition of amyloidogenic proteins by two naphthoquinone–tryptophan hybrid molecules. Proteins 2012, 80, 1962-1973. [CrossRef]
- Paul, A.; Huber, A.; Rand, D.; Gosselet, F.; Cooper, I.; Gazit, E.; Segal, D. Naphthoquinone–Dopamine Hybrids Inhibit α-Synuclein Aggregation, Disrupt Preformed Fibrils, and Attenuate AggregateInduced Toxicity. Chem. Eur. J. 2020, 26, 16486-16496. [CrossRef]
- Shrestha-Dawadi, P.D.; Bittner, S.; Fridkin, M.; Rahimipour, S. On the synthesis of naphthoquinonyl heterocyclic amino acids. Synthesis 1996, 12, 1468-1472. [CrossRef]
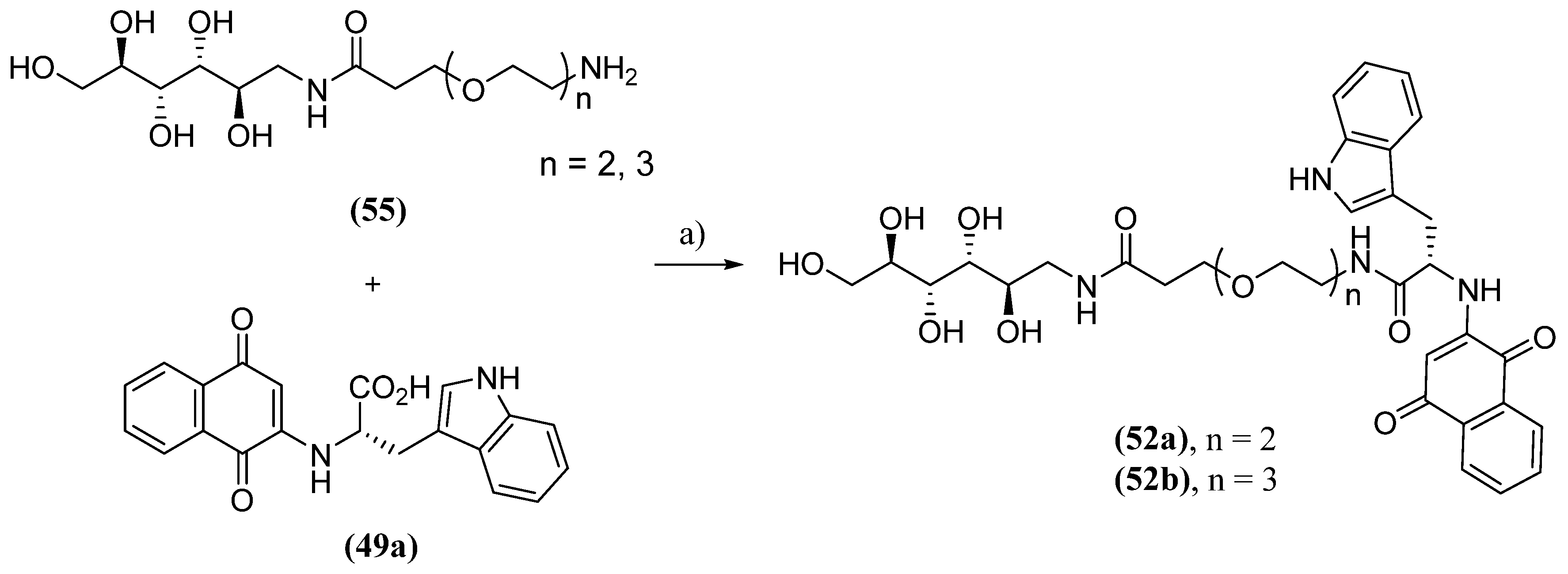
- Paul, A.; Zhang, B.; Mohapatra, S.; Li, G.; Li, Y.; Gazit, E.; Segal, D. Novel Mannitol-Based Small Molecules for Inhibiting Aggregation of α-Synuclein Amyloids in Parkinson’s Disease. Front. Mol. Biosci. 2019, 6, 16. [CrossRef]
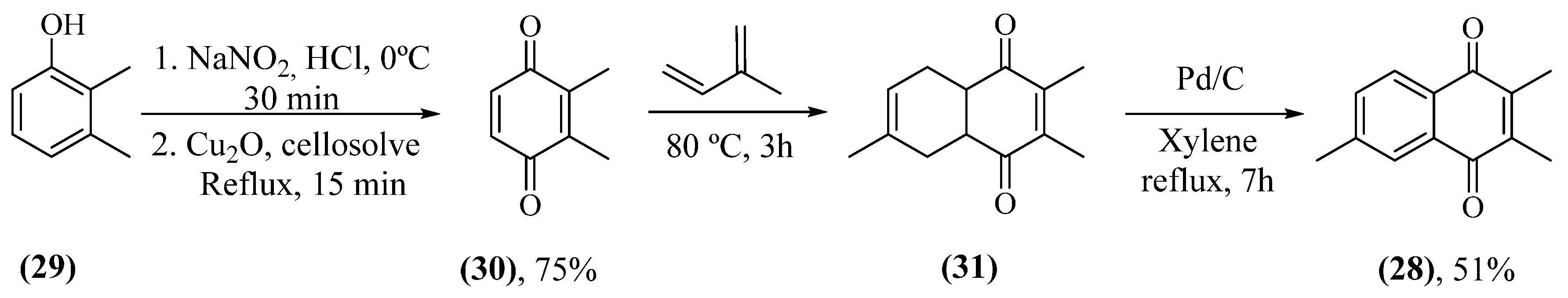
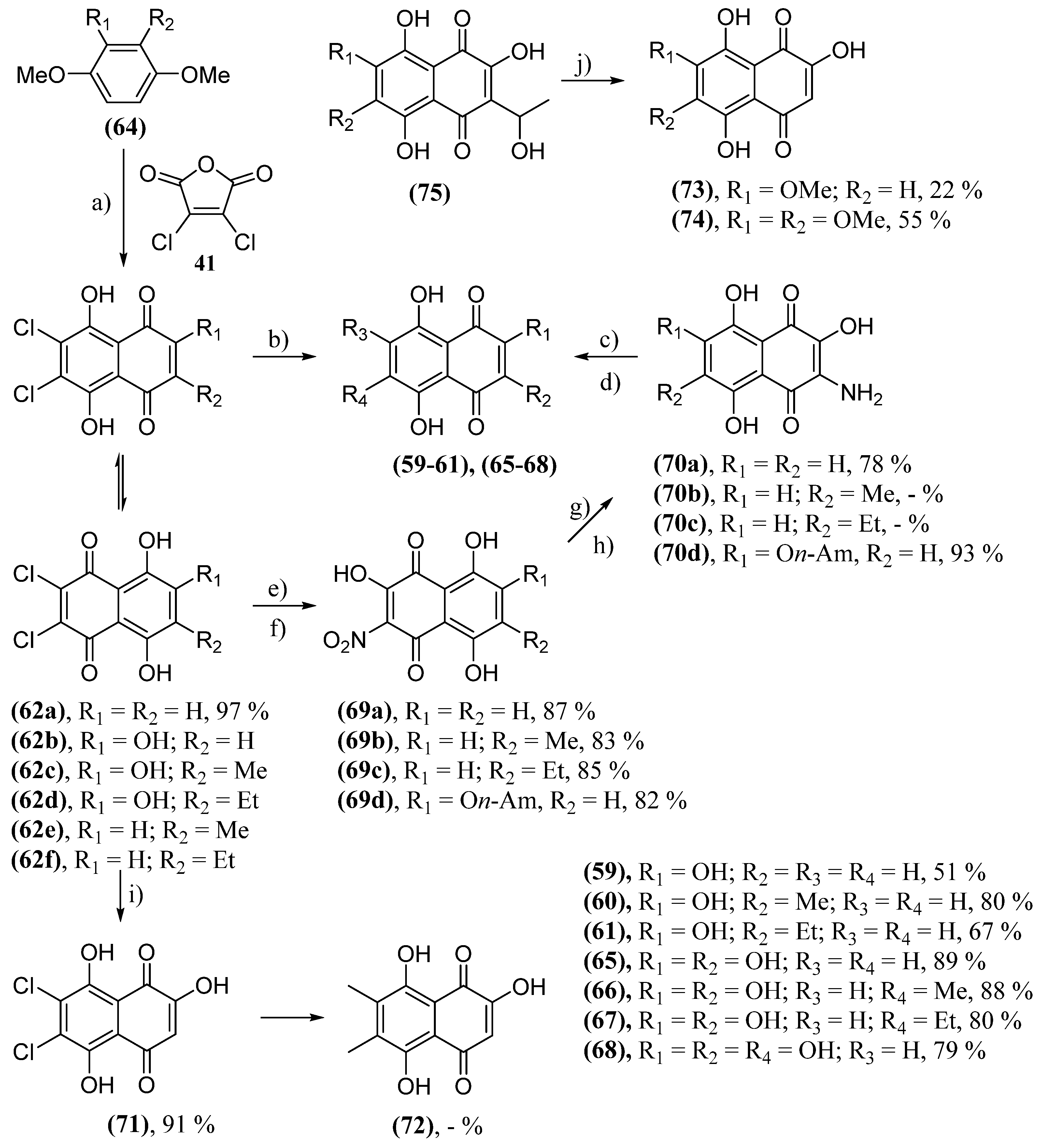
- Anufriev, V.P.; Malinovskaya, G.V.; Novikov, V.L.; Balanyova, N.N.; Polonik, S.G. The Reductive Dehalogenation Of Halosubstituted Naphthazarinsand Quinizarins As A Simple Route To Parent Compounds. Synth. Commun. 1998, 28, 2149-2157. [CrossRef]
- Huot, R.; Brassard, P. Friedel-Crafts Condensations with Maleic Anhydrides. III. The Synthesis of Polyhydroxylated Naphthoquinones. Can. J. Chem. 1974, 52, 838-842. [CrossRef]
- Polonik, N.S.; Polonik, S.G.; Denisenko, V.A.; Moiseenko, O.P. Reaction of Dichloronaphthazarins with Sodium Nitrite as a Route to Natural Pigments Echinamines A and B and Related Aminonaphthazarins. Synthesis 2011, 2011, 3350-3358. [CrossRef]
- Polonik, N.S.; Polonik, S.G. DMSO-mediated transformation of 3-amino-2-hydroxynaphthazarins to natural 2,3-dihydroxynaphthazarins and related compounds. Tetrahedron Lett. 2016, 57, 3303-3306. [CrossRef]
- Sabutskii, Y.E.; Denisenko, V.A.; Polonik, S.G. The Acid-Catalyzed 2-O-Alkylation of Substituted 2-Hydroxy-1,4-naphthoquinones by Alcohols: Versatile Preparative Synthesis of Spinochrome D and Its 6-Alkoxy Derivatives. Synthesis 2018, 50, 3738-3748. [CrossRef]
- Anufriev, V.P.; Novikov, V.L.; Maximov, O.B.; Elyakov, G.B.; Levitsky, D.O.; Lebedev, A.V.; Sadretdinov, S.M.; Shvilkin, A.V.; Afonskaya, N.I.; Ruda, M.Y.; Cherpachenko, N.M. Synthesis Of Some Hydroxynaphthazarins And Their Cardioprotective Effects Under Ischemia-Reperfusion In Vivo. Bioorg. Med. Chem. Lett. 1998, 8, 587-592. [CrossRef]
- Pelageev, D.N.; Panchenko, M.N.; Pokhilo, N.D.; Anufriev, V. F. Synthesis of 2,2'-(ethane-1,1-diyl)bis(3,5,6,7,8-pentahydroxy-1,4-naphthoquinone), a metabolite of the sea urchins Spatangus purpureus, Strongylocentrotus intermedius, and S. droebachiensis. Russ. Chem. Bull. 2010, 59, 1472-1476. [CrossRef]
- Polonik S.G.; Tolkach A.M.; Uvarova N.I. Glycosidation of echinochrome and related hydroxynaphthazarins by orthoester method. Russ. J. Org. Chem. 1994, 30, 248-253.
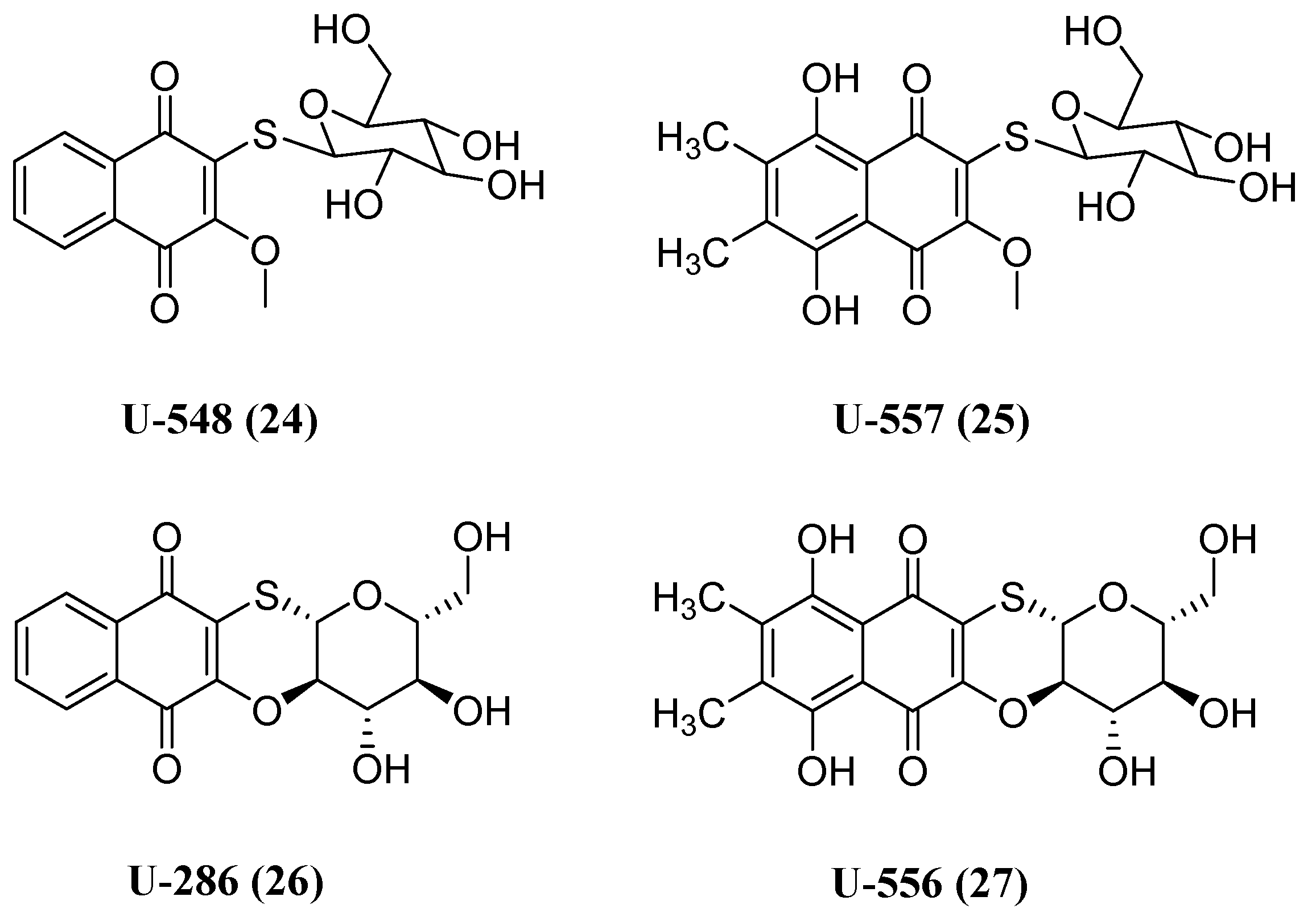
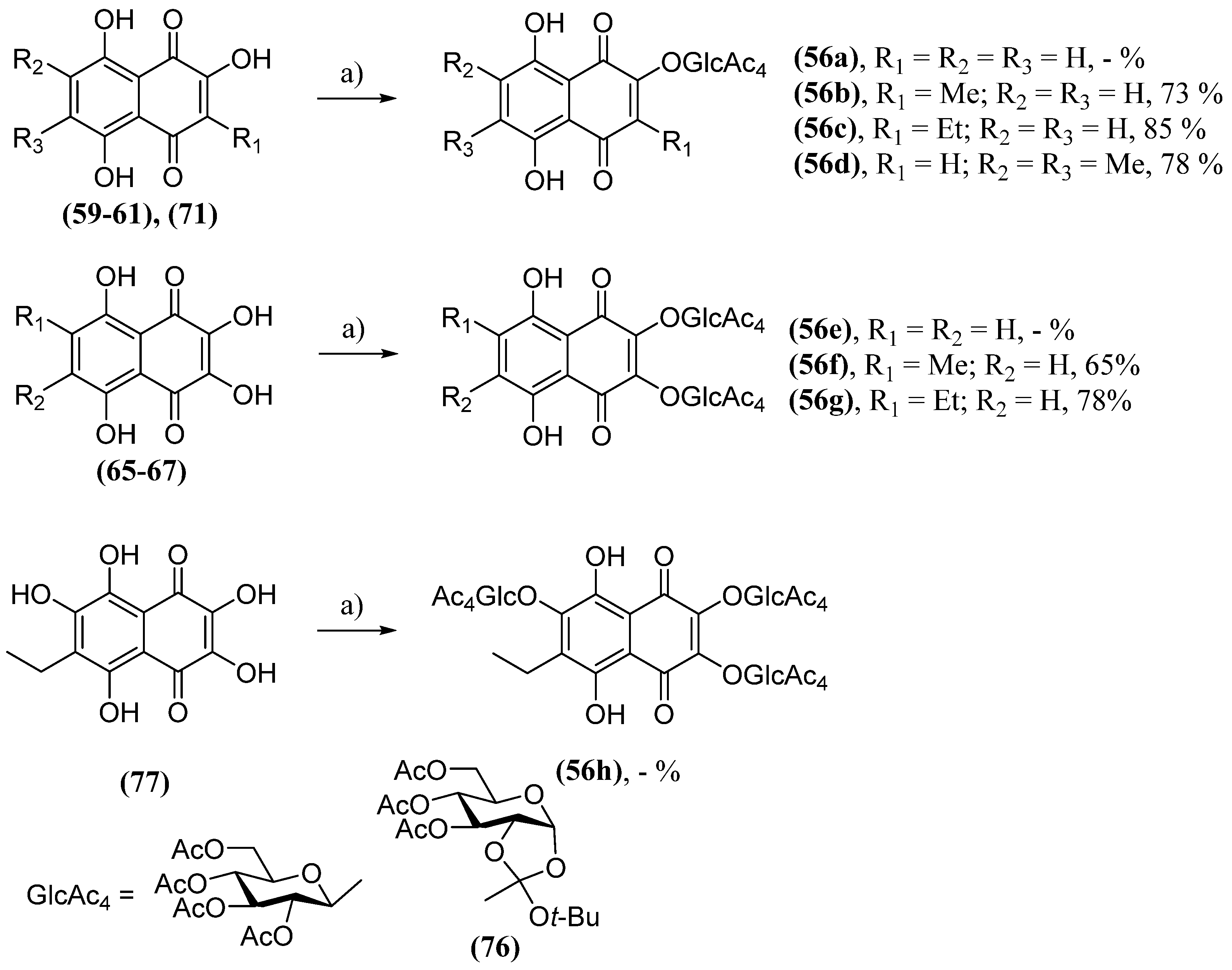
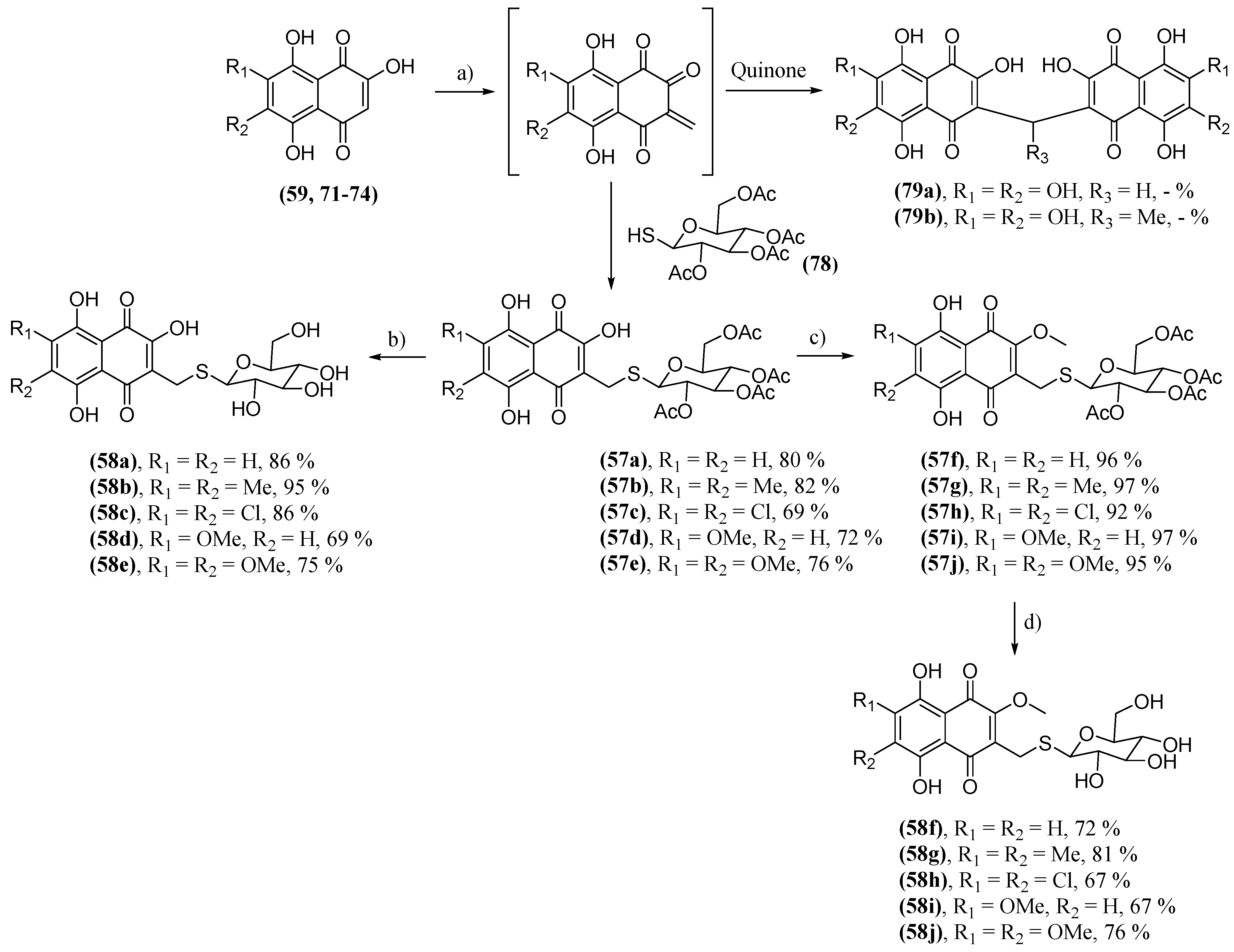
- Polonik, S.; Likhatskaya, G.; Sabutski, Y.; Pelageev, D.; Denisenko, V.; Pislyagin, E.; Chingizova, E.; Menchinskaya, E.; Aminin, D. Synthesis, Cytotoxic Activity Evaluation and Quantitative Structure-Activity Analysis of Substituted 5,8-Dihydroxy-1,4-naphthoquinones and Their O- and S-Glycoside Derivatives Tested against Neuro-2a Cancer Cells. Mar. Drugs 2020, 18, 602. [CrossRef]
- Lebedev, A. V.; Ivanova, M. V.; Ruuge, E. K. How do calcium ions induce free radical oxidation of hydroxy-1,4-naphthoquinone? Ca2+ stabilizes the naphthosemiquinone anion-radical of echinochrome A. Arch. Biochem. Biophys. 2003, 413, 191-198. [CrossRef]
- Ekimova, I.V.; Plaksina, D.V.; Pastukhov, Y.F.; Lapshina, K.V.; Lazarev, V.F.; Mikhaylova, E.R.; Polonik, S.G.; Pani, B.; 5, Margulis, B.A.; Guzhova, I.V.; Nudler, E. New HSF1 inducer as a therapeutic agent in a rodent model of Parkinson's disease. Exp. Neurol. 2018, 306, 199-208. [CrossRef]
- Belan, D.V.; Polonik, S.G.; Ekimova, I.V. Assessment of the Efficacy of Preventive Therapy with Chaperone Inducer U133 in a Model of the Preclinical Stage of Parkinson’s Disease in Elderly Rats. Neurosci. Behav. Physiol. 2021, 51, 673-680. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




