Submitted:
16 August 2023
Posted:
18 August 2023
You are already at the latest version
Abstract
Keywords:
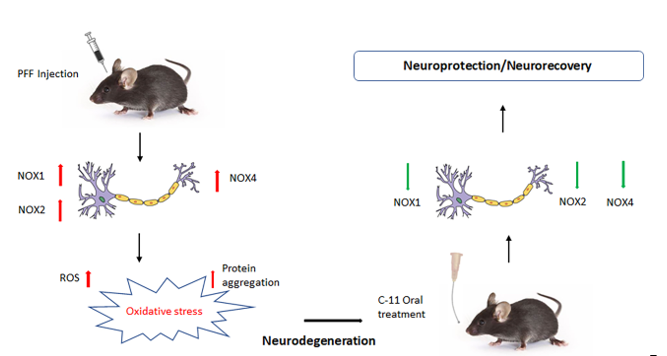
1. Introduction
2. Results
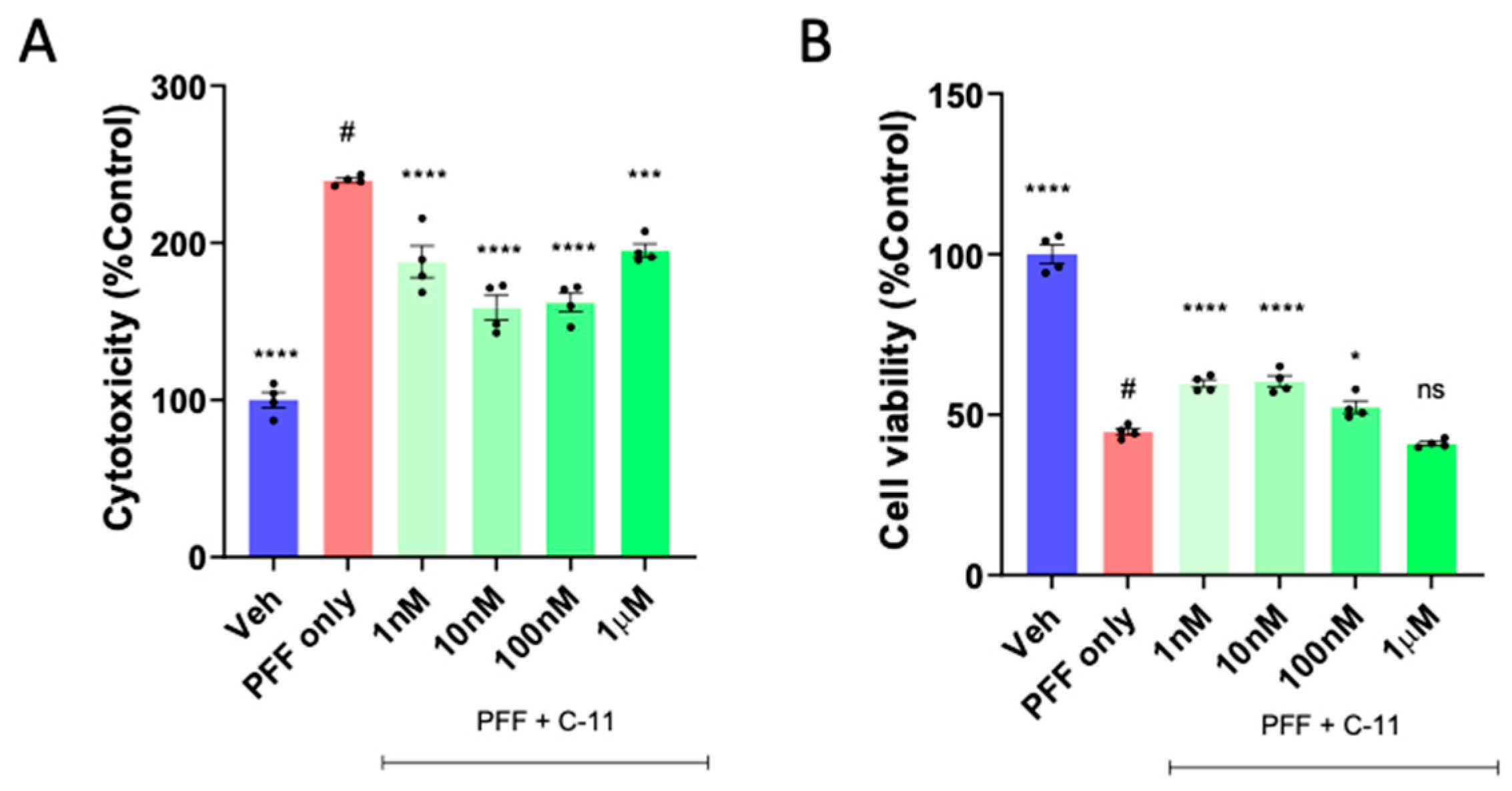
2.1. A novel NOX inhibitor treatment decreases cytotoxicity and increases cell viability in PFF treated N27P dopaminergic cells
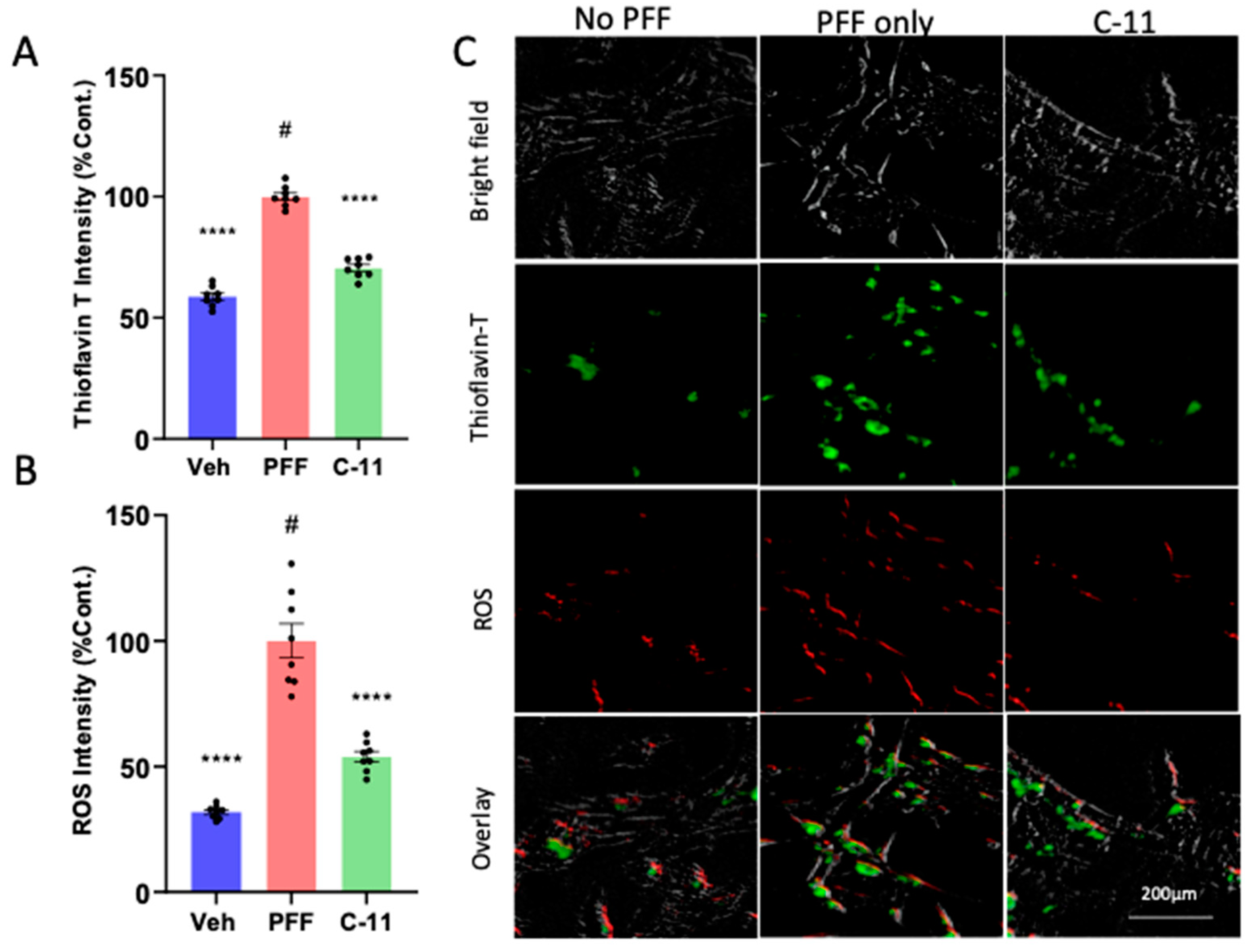
2.2. The C-11 treatment decreases protein aggregation and ROS generation induced by PFF
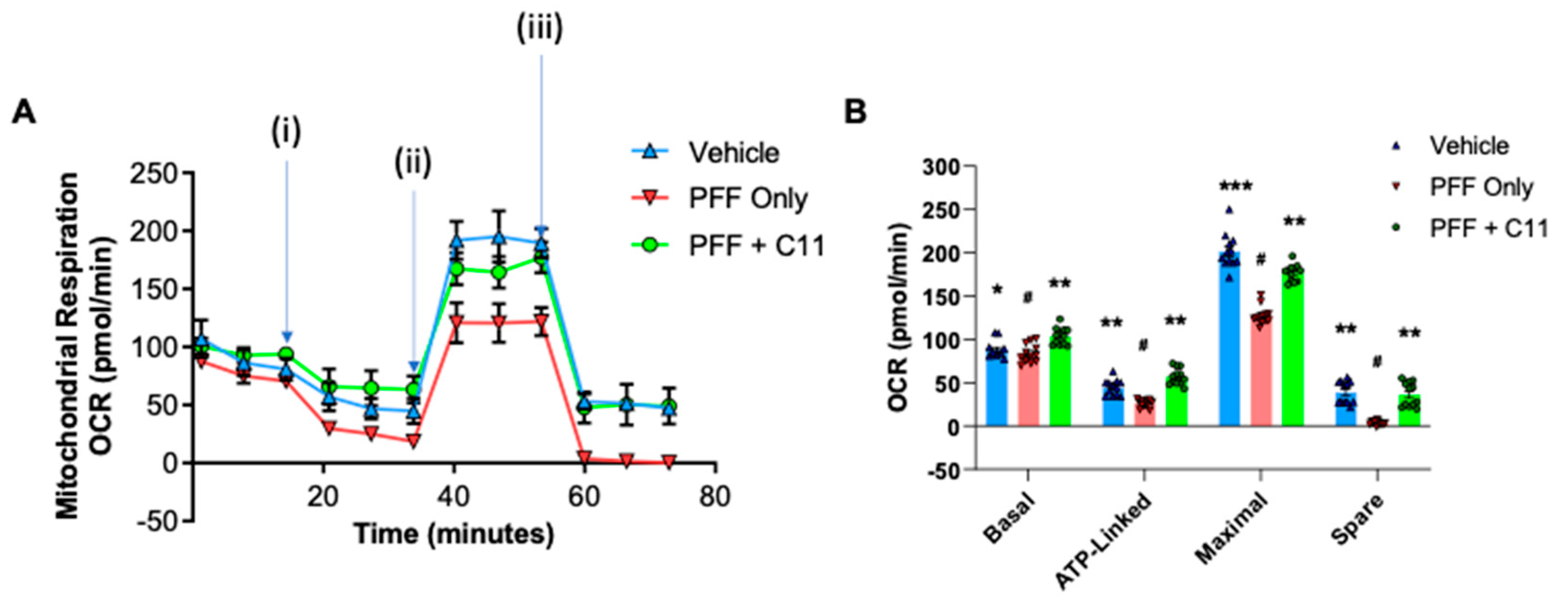
2.3. The C-11 treatment prevents PFF-induced mitochondrial dysfunctions in N27 cells
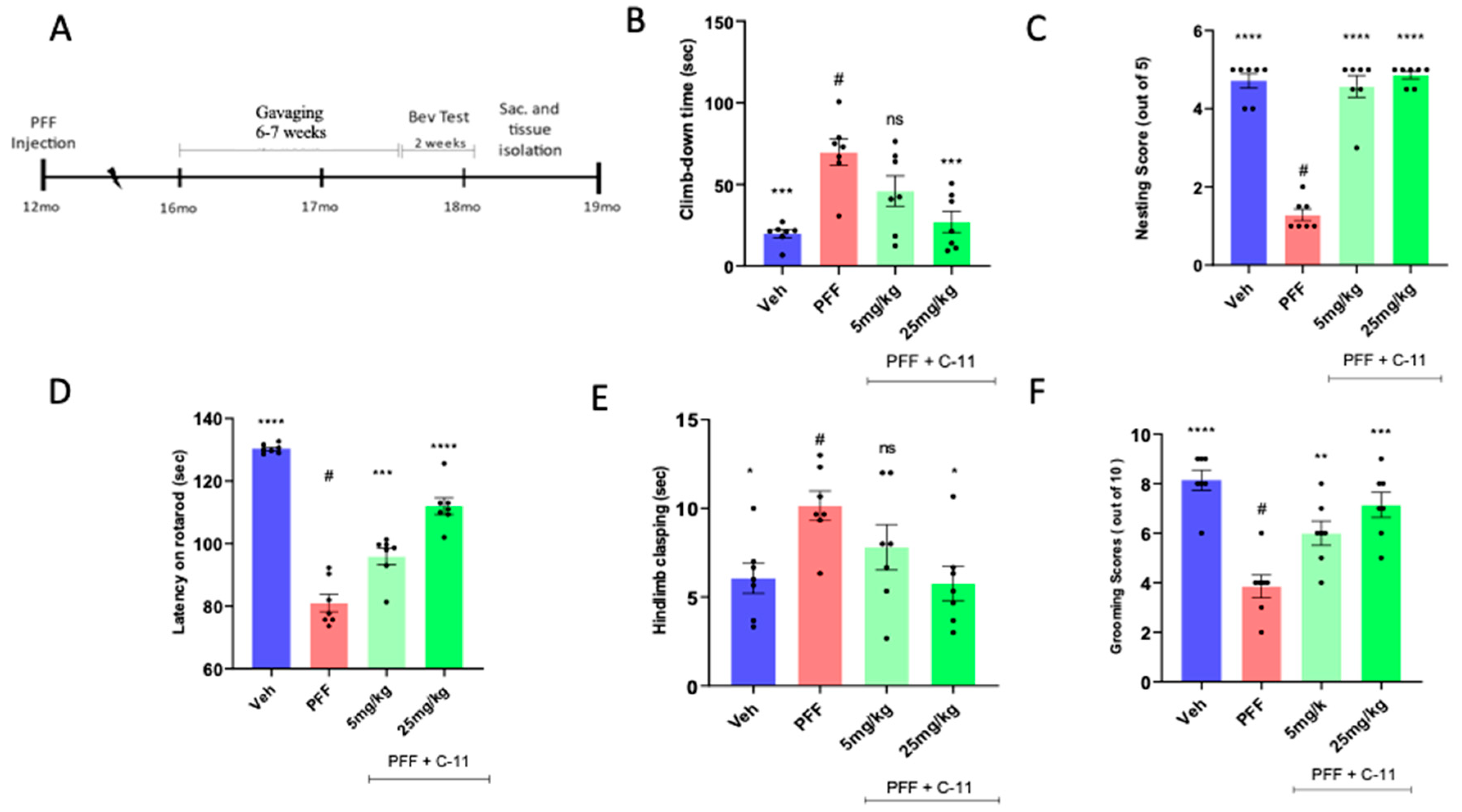
2.4. In behavioral analyses, the oral C-11 treatment reduces motor deficits in PFF injected mice
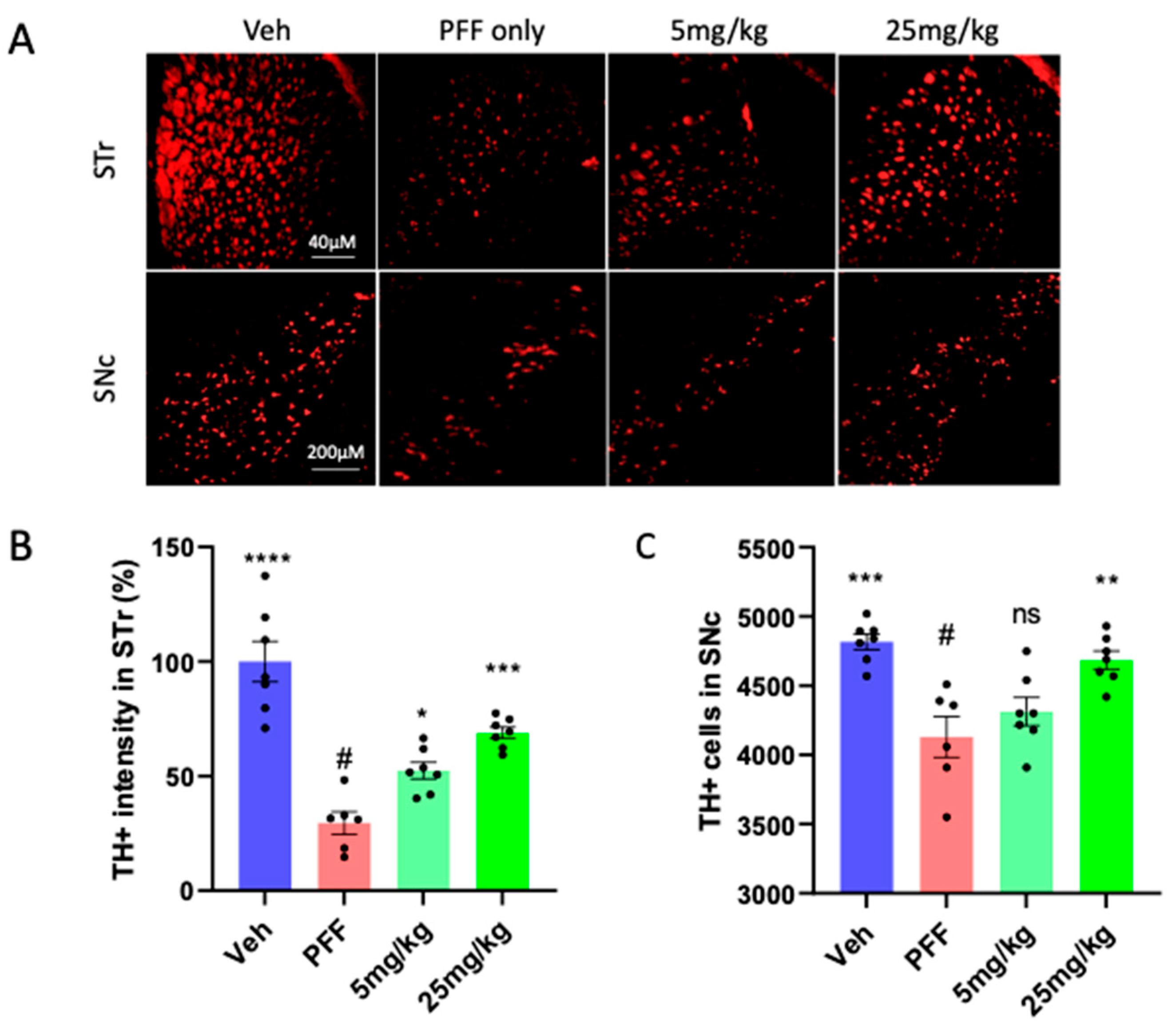
2.5. The neuronal loss in the STr and SNc induced by PFF was prevented or partially reversed by the C-11 treatment
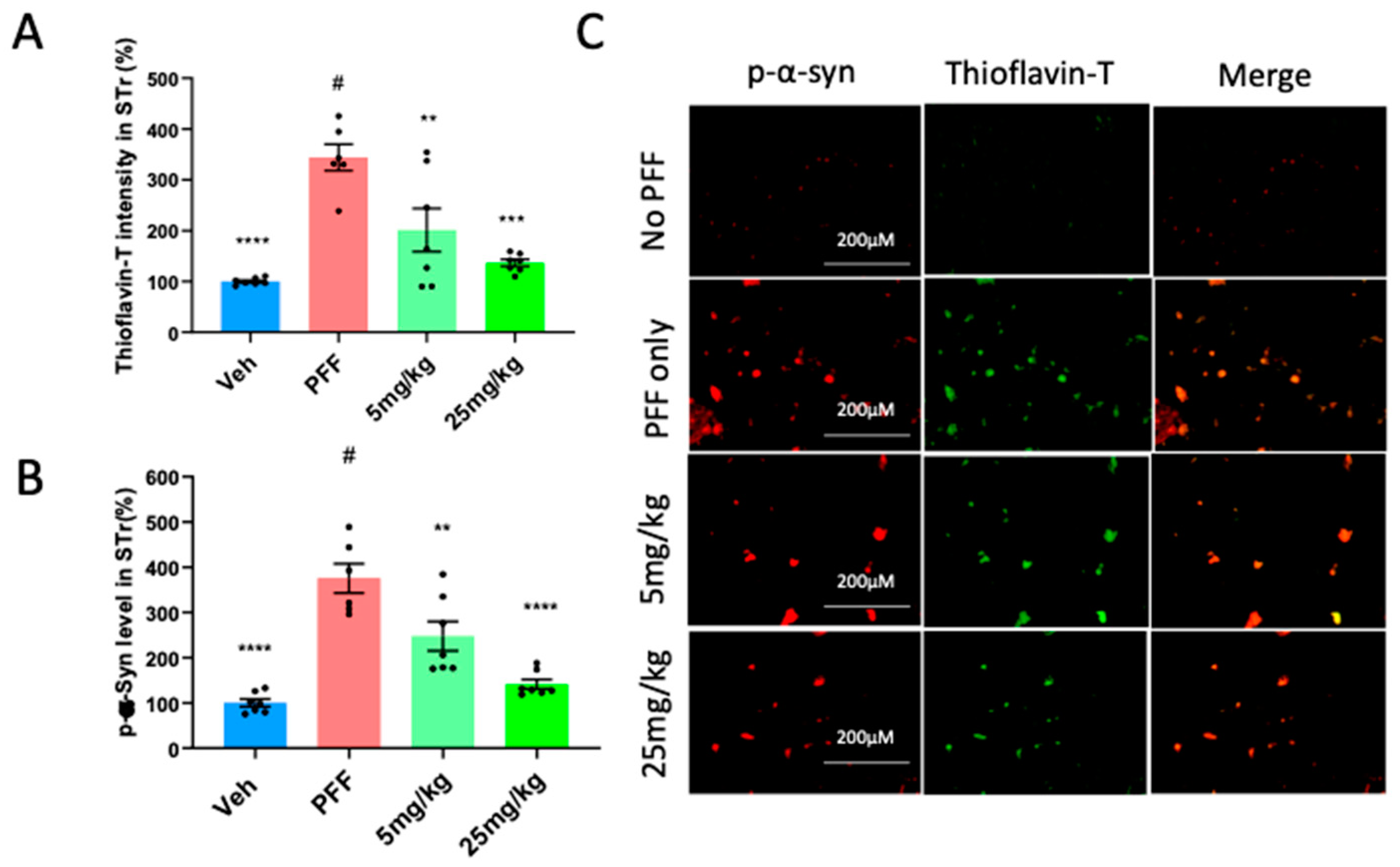
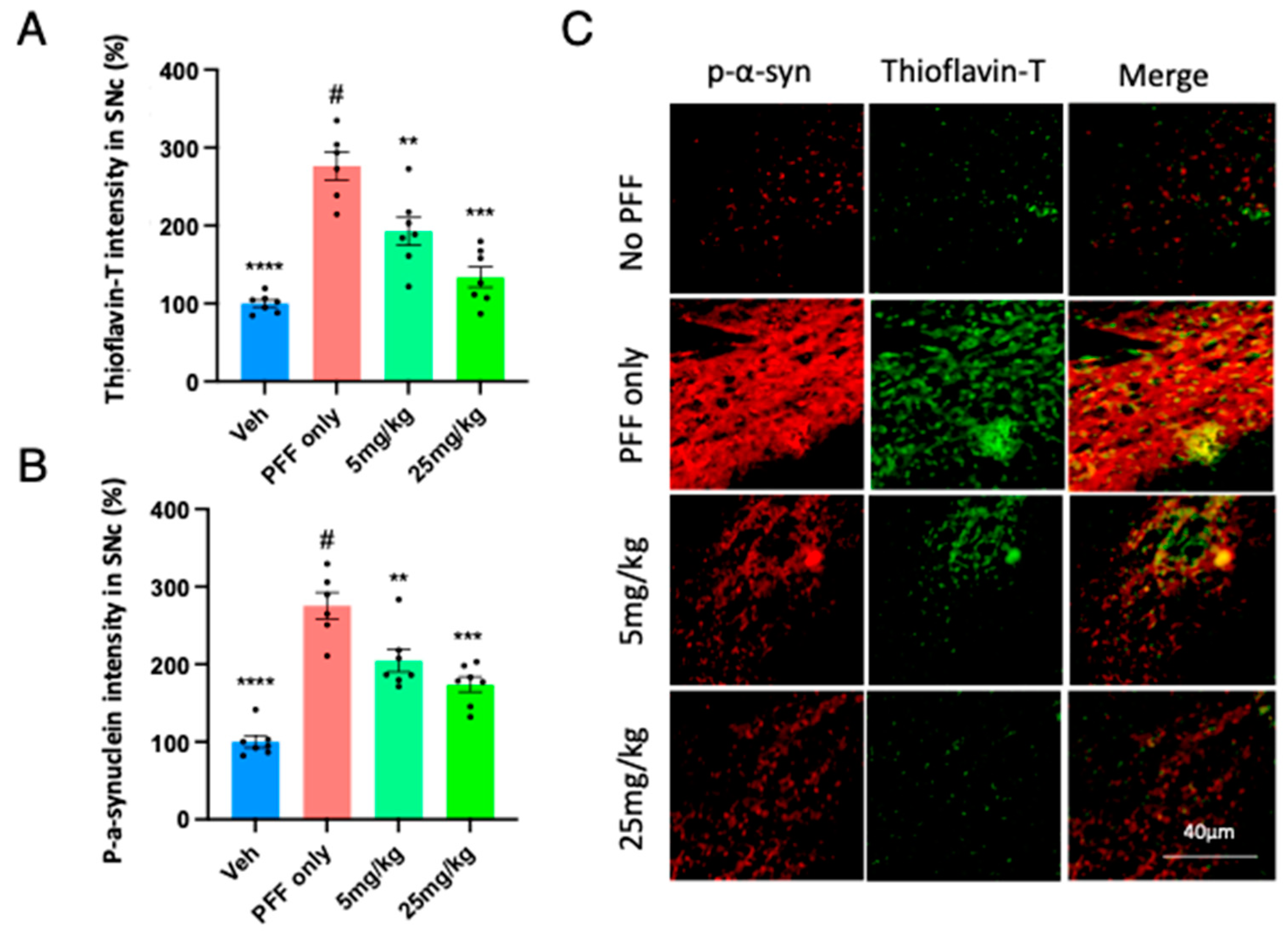
2.6. The C-11 redces PFF-induced protein aggregates in Thioflavin-T stain & phospho-α-syn in the Striatum (STr) and Substantia Nigra compacta (SNc)
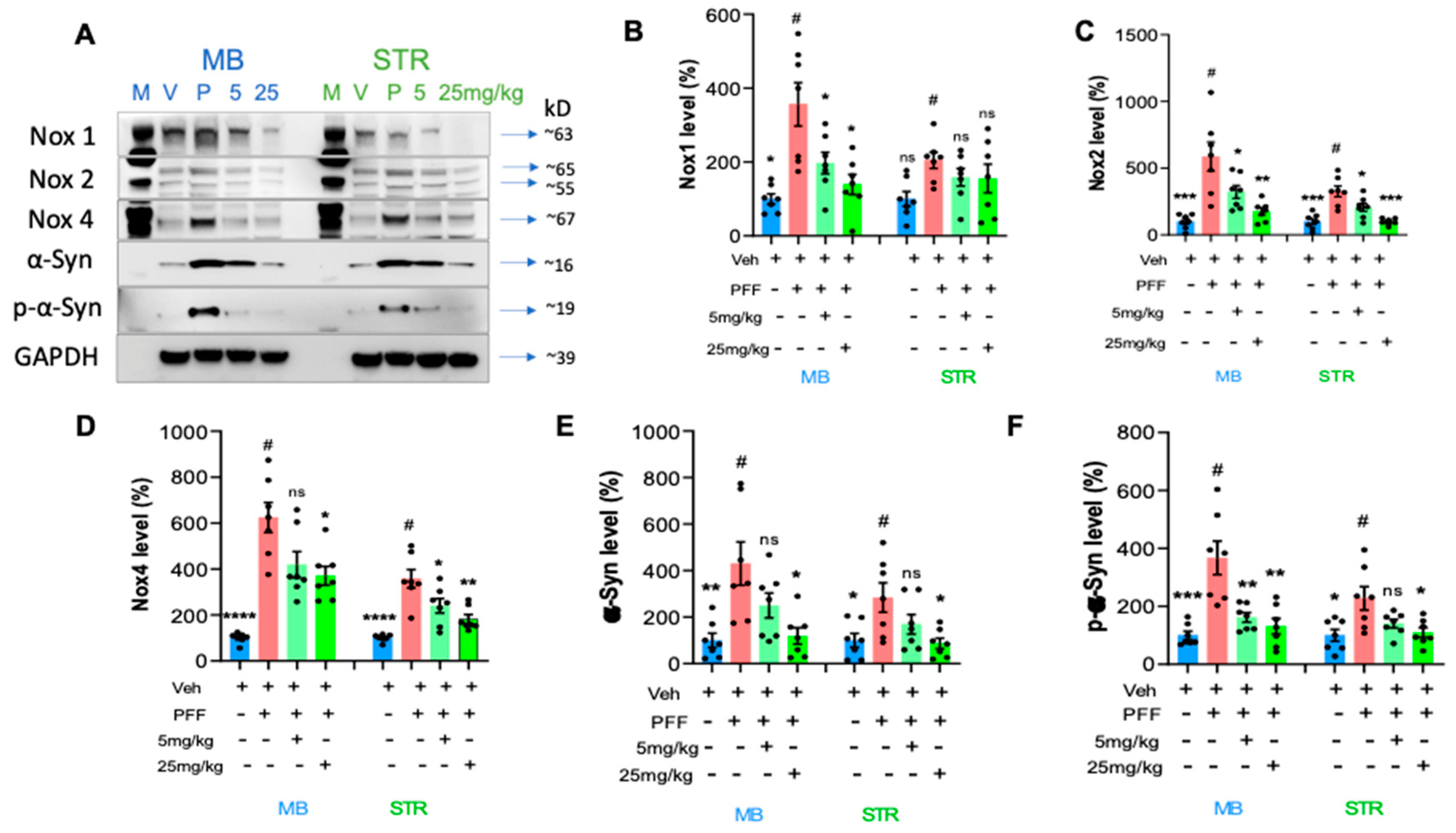
2.7. The levels of phospho-α-synuclein, NOX-1, 2, & 4 in the STr and the ventral midbrain were reduced by the treatment in Western blot analysis
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Cell culture and treatment
4.3. Cell viability Assay
4.4. Cytotoxicity assay
4.5. ROS levels and Thioflavin-T assessment
4.6. Oxygen consumption rate (OCR) measurements using Agilent Seahorse XF analyzer
4.7. Stereotaxic α-synuclein PFF injection and brain isolation
4.8. Gavaging schedule
4.9. Tissue Isolation
4.10. Behavioral Assays:
4.10.1. Hindlimb Clasping
4.10.2. Rotarod
4.10.3. Pole Test
4.10.4. Nesting
4.10.5. Grooming
4.11. Immunohistochemistry (IHC)
4.12. Immunoblot analysis
4.13. Statistical analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parkinson, J. An essay on the shaking palsy 1817. J Neuropsychiatry Clin Neurosci. (2002) 14:223–36; discussion 222. https://doi.org/10.1176/jnp.14.2.223. [CrossRef]
- Dias, V.; Junn E., Mouradian, M.M. The role of oxidative stress in Parkinson's disease. J Parkinson’s Dis. 2013;3(4):461-91. https://doi.org/10.3233/JPD-130230. PMID: 24252804; PMCID: PMC4135313. [CrossRef]
- Angelova, P.R.; Abramov, A.Y. Role of Mitochondrial ROS in the Brain: From Physiology to Neurodegeneration. FEBS Letters 2018, 592, 692–702. https://doi.org/10.1002/1873-3468.12964. [CrossRef]
- Risiglione, P.; Zinghirino F., Di Rosa M.C., Magrì A., Messina A. Alpha-Synuclein and Mitochondrial Dysfunction in Parkinson’s Disease: The Emerging Role of VDAC. Biomolecules. 2021 May 11;11(5):718. https://doi.org/10.3390/biom11050718. PMID: 34064816. [CrossRef] [PubMed]
- Payne B.A.I., Chinnery P.F. Mitochondrial dysfunction in aging: Much progress but many unresolved questions. Biochim. Et Biophys. Acta. 2015; 1847:1347–1353. https://doi.org/10.1016/j.bbabio.2015.05.022.]. [CrossRef]
- Li, W.G.; Miller, F.J., Zhang, H.J., Spitz, D.R., Oberley, L.W., Weintraub, N.L. H2O2-Induced O⨪2Production by a Non- Phagocytic NAD(P)H Oxidase Causes Oxidant Injury. Journal of Biological Chemistry 2001, 276, 29251–29256. https://doi.org/10.1074/jbc.M102124200. [CrossRef]
- Jenner, P.; Olanow, C.W. The Pathogenesis of Cell Death in Parkinson’s Disease. Neurology 2006, 66, S24–S36. https://doi.org/10.1212/WNL.66.10_suppl_4. S24. [CrossRef]
- Choi, D.H.; Cristóvão, A.C., Guhathakurta, S., Lee, J., Joh, T.H., Beal, M.F., Kim, Y.-S. NADPH Oxidase 1-Mediated Oxidative Stress Leads to Dopamine Neuron Death in Parkinson’s Disease. Antioxidants & redox signaling 2012, 16, 1033–1045. https://doi.org/10.1089/ars.2011.3960. [CrossRef]
- Abbott, A. Levodopa: the story so far. Nature 466, S6–S7 (2010). https://doi.org/10.1038/466S6a. [CrossRef]
- Bedard, K.; Krause, K.H. The NOX Family of ROS-Generating NADPH Oxidases: Physiology and Pathophysiology. Physiological reviews 2007, 87, 245–313. https://doi.org/10.1152/physrev.00044.2005. [CrossRef]
- Bánfi, B.; Clark, R.A., Steger, K., Krause, K.-H. Two Novel Proteins Activate Superoxide Generation by the NADPH Oxidase NOX1. Journal of Biological Chemistry 2003, 278, 3510–3513. https://doi.org/10.1074/jbc.C200613200. [CrossRef]
- Marden, J.J.; Harraz, M.M., Williams, A.J., Nelson, K., Luo, M., Paulson, H., Engelhardt, J.F. Redox Modifier Genes in Amyotrophic Lateral Sclerosis in Mice. The Journal of clinical investigation 2007, 117, 2913–2919. https://doi.org/10.1172/JCI31265. [CrossRef]
- Chan, P.H. Mitochondria and Neuronal Death/Survival Signaling Pathways in Cerebral Ischemia. Neurochemical Research 2004, 29, 1943–1949. https://doi.org/10.1007/s11064-004-6869-x. [CrossRef]
- Head, E. Oxidative Damage and Cognitive Dysfunction: Antioxidant Treatments to Promote Healthy Brain Aging. Neurochemical Research 2009, 34, 670–678. https://doi.org/10.1007/s11064-008-9808-4. [CrossRef]
- Cooney, S.J.; Bermudez-Sabogal, S.L., Byrnes, K.R. Cellular and Temporal Expression of NADPH Oxidase (NOX) Isotypes after Brain Injury. Journal of Neuroinflammation 2013, 10, 917. https://doi.org/10.1186/1742-2094-10-155. [CrossRef]
- Verma, D.K.; Seo, B.A., Ghosh, A., Ma, S.X., Hernandez-Quijada, K., Andersen, J.K., Ko, H.S., Kim, Y.H. Alpha-Synuclein Preformed Fibrils Induce Cellular Senescence in Parkinson’s Disease Models. Cells 2021, 10. https://doi.org/10.3390/cells10071694. [CrossRef]
- Ghosh, A. A.; Verma, D.K., Cabrera, G., Ofori, K., Hernandez-Quijada, K., Kim, J.K., Chung, J.H., Moore, M., Moon, S.H., Seo, J.B., Kim, Y.H. A Novel NOX Inhibitor Treatment Attenuates Parkinson's Disease-Related Pathology in Mouse Models. Int J Mol Sci. 2022 Apr 12;23(8):4262. https://doi.org/10.3390/ijms23084262. PMID: 35457082; PMCID: PMC9030373. [CrossRef]
- Dumont, M.; Beal, M.F. Neuroprotective strategies involving ROS in Alzheimer’s disease. Free Radic Biol Med. 2011; 51:1014– 1026. [CrossRef]
- Yan, M.H.; Wang X., Zhu X. Mitochondrial defects and oxidative stress in Alzheimer disease and Parkinson disease. Free Radic Biol Med. 2011; 62:90–101. [CrossRef]
- Johnson, W.M.; Wilson-Delfosse, A.L., Mieyal J.J. Dysregulation of glutathione homeostasis in neurodegenerative diseases. Nutrients. 2012; 4:1399–1440. [CrossRef]
- Schneider, C. A.; Rasband, W. S., & Eliceiri K. W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nature Methods, 9(7), 671–675. https://doi.org/10.1038/nmeth.2089. [CrossRef]
- Bhatia, S.; Thompson, E.W., Gunter, J.H. Studying the Metabolism of Epithelial-Mesenchymal Plasticity Using the Seahorse Xfe96 Extracellular Flux Analyzer. Methods Mol Biol. 2021; 2179:327-340. https://doi.org/10.1007/978-1-0716-0779-425. PMID: 32939731. [CrossRef] [PubMed]
- Masliah, E.; Rockenstein, E., Veinbergs, I., Mallory, M., Hashimoto, M., Takeda, A., Sagara, Y., Sisk, A., Mucke L. Dopaminergic Loss, and Inclusion Body Formation in Alpha-Synuclein Mice: Implications for Neurodegenerative Disorders. Science (New York, N.Y.) 2000, 287, 1265–1269. https://doi.org/10.1126/science.287.5456.1265. [CrossRef]
- Lazzara, C.A.; Riley, R.R., Rane, A., Andersen, J.K., Kim, Y.H. The Combination of Lithium and L-Dopa/Carbidopa Reduces MPTP-Induced Abnormal Involuntary Movements (AIMs) via Calpain-1 Inhibition in a Mouse Model: Relevance for Parkinson׳s Disease Therapy. Brain research 2015, 1622, 127–136. https://doi.org/10.1016/j.brainres.2015.06.018. [CrossRef]
- Aniszewska, A.; Bergström, J., Ingelsson, M., Ekmark-Lewén, S. Modeling Parkinson's disease-related symptoms in alpha- synuclein overexpressing mice. Brain Behav. 2022 Jul;12(7): e2628. https://doi.org/10.1002/brb3.2628. Epub 2022 Jun 1. PMID: 35652155; PMCID: PMC9304846. [CrossRef]
- Deacon, R.M. Measuring motor coordination in mice. J Vis Exp. 2013 May 29;(75): e2609. https://doi.org/10.3791/2609. PMID: 23748408; PMCID: PMC3724562. [CrossRef]
- Matsuura, K.; Kabuto, H., Makino, H., Ogawa, N. Pole test is a useful method for evaluating the mouse movement disorder caused by striatal dopamine depletion. J Neurosci Methods. 1997 Apr 25;73(1):45-8. https://doi.org/10.1016/s0165-0270(96)02211-x. PMID:9130677. [CrossRef]
- Sager, T.N., Kirchhoff, J., Mørk, A., Van Beek, J., Thirstrup, K., Didriksen, M., Lauridsen J.B. Nest building performance following MPTP toxicity in mice. Behav Brain Res. 2010 Apr 2;208(2):444-9. https://doi.org/10.1016/j.bbr.2009.12.014. Epub 2009 Dec 23. PMID: 20035793. [CrossRef] [PubMed]
- Pelosi, A.; Girault, J.A., Hervé, D. Unilateral Lesion of Dopamine Neurons Induces Grooming Asymmetry in the Mouse. PLoS One. 2015 Sep 23;10(9): e0137185. https://doi.org/10.1371/journal.pone.0137185. PMID: 26397369; PMCID: PMC4580614. [CrossRef]
- Volpicelli-Daley, L.A.; Luk, K.C., Patel, T.P., Tanik S.A., Riddle D.M., Stieber A., Meaney D.F., Trojanowski J.Q., Lee V.M.-Y. Exogenous α-Synuclein Fibrils Induce Lewy Body Pathology Leading to Synaptic Dysfunction and Neuron Death. Neuron 2011, 72, 57–71. https://doi.org/10.1016/j.neuron.2011.08.033. [CrossRef]
- Polinski, N.K.; Volpicelli-Daley, L.A., Sortwell, C.E., Luk, K.C., Cremades, N., Gottler, L.M., Froula, J., Duffy, M.F., Lee, V.M.Y., Martinez, T.N. et al. Best Practices for Generating and Using Alpha-Synuclein Pre-Formed Fibrils to Model Parkinson’s Disease in Rodents. Journal of Parkinson’s Disease 2018, 8, 303–322. https://doi.org/10.3233/JPD-171248. [CrossRef]
- Murphy, M.P.; Bayir, H., Belousov, V. et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat Metab 4, 651–662 (2022). https://doi.org/10.1038/s42255-022-00591-z. [CrossRef]
- Chung, H.K.; Ho, H.A., Pérez-Acuña, D., Lee. S.J. Modeling α-Synuclein Propagation with Preformed Fibril Injections. J Mov Disord. 2019 Sep;12(3):139-151. https://doi.org/10.14802/jmd.19046. Epub 2019 Sep 30. Erratum in: J Mov Disord. 2020 Jan;13(1):77-79. PMID: 31556259; PMCID: PMC6763716. [CrossRef]
- Sato, H.; Kato, T., Arawaka, S. The role of Ser129 phosphorylation of α-synuclein in neurodegeneration of Parkinson's disease: a review of in vivo models. Rev Neurosci. 2013;24(2):115-23. https://doi.org/10.1515/revneuro-2012-0071. PMID: 23314528. [CrossRef] [PubMed]
- Verma, D.K.; Gupta, S, Biswas J., Joshi, N., Singh, A., Gupta, P., Tiwari, S., Raju, K.S., Chaturvedi, S., Wahajuddin, M., Singh, S., New therapeutic activity of metabolic enhancer piracetam in treatment of neurodegenerative disease: Participation of caspase independent death factors, oxidative stress, inflammatory responses and apoptosis, Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, Volume 1864, Issue 6, Part A, 2018, Pages 2078-2096, ISSN 0925-4439. [CrossRef]
- Verma, D.K.; Singh, D.K., Gupta. S., Gupta, P., Singh, A., Biswas, J., Singh, S. Minocycline diminishes the rotenone induced neurotoxicity and glial activation via suppression of apoptosis, nitrite levels and oxidative stress, NeuroToxicology, Volume 65, 2018, Pages 9-21, ISSN 0161-813X. [CrossRef]
- Verma, D.K.; Gupta, S., Biswas, J. et al. Metabolic Enhancer Piracetam Attenuates the Translocation of Mitochondrion-Specific Proteins of Caspase-Independent Pathway, Poly [ADP-Ribose] Polymerase 1 Up-regulation and Oxidative DNA Fragmentation. Neurotox Res 34, 198–219 (2018). [CrossRef]
- Biswas, J.; Gupta, S., Verma, D.K., Gupta, P., Singh, A., Tiwari, S., Goswami, P., Sharma, S., Singh, S. Involvement of glucose related energy crisis and endoplasmic reticulum stress: Insinuation of streptozotocin induced Alzheimer's like pathology, Cellular Signalling, Volume 42, 2018, Pages 211-226, ISSN 0898-6568. [CrossRef]
- Gupta, S.; Verma, D.K., Biswas, J., Raju, K.S., Joshi, N., Wahajuddin, M., Singh, S. The metabolic enhancer piracetam attenuates mitochondrion-specific endonuclease G translocation and oxidative DNA fragmentation. Free Radical Biology and Medicine, Volume 73, 2014, Pages 278-290, ISSN 0891-5849. [CrossRef]
- Butterfield, D.A; Boyd-Kimball, D. Mitochondrial Oxidative and Nitrosative Stress and Alzheimer Disease. Antioxidants (Basel). 2020;9(9):818. Published 2020 Sep 2. [CrossRef]
- Verma, D.K.; Joshi, N., Raju, K.S., Wahajuddin, M., Singh, R.K., Singh, S. Metabolic enhancer piracetam attenuates rotenone induced oxidative stress: a study in different rat brain regions. Acta Neurobiol. Exp., 75 (2015), pp. 399-411.
- Kim, G.H.; Kim, J.E., Rhie, S.J., Yoon, S. The Role of Oxidative Stress in Neurodegenerative Diseases. Experimental Neurobiology 2015, 24, 325–340, doi:10.5607/en.2015.24.4.325. [CrossRef]
- Infanger, D.W.; Sharma, R. V., Davisson, R.L. NADPH Oxidases of the Brain: Distribution, Regulation, and Function. Antioxidants & redox signaling 8, 1583–1596. https://doi.org/10.1089/ars.2006.8.1583. [CrossRef]
- Rey, F.E.; Cifuentes, M.E., Kiarash, A., Quinn, M.T., Pagano, P.J. Novel Competitive Inhibitor of NAD(P)H Oxidase Assembly Attenuates Vascular O2 − and Systolic Blood Pressure in Mice. Circulation Research 2001, 89, 408–414. https://doi.org/10.1161/hh1701.096037. [CrossRef]
- Shuhui Guo & Xiuping Chen. The human Nox4: gene, structure, physiological function and pathological significance, Journal of Drug Targeting, 23:10, (2015) 888-896. https://doi.org/10.3109/1061186X.2015.1036276. [CrossRef]
- Heger, L.M; Wise, R.M., Hees, J.T., Harbauer, A.B., Burbulla, L.F. Mitochondrial Phenotypes in Parkinson's Diseases-A Focus on Human iPSC-Derived Dopaminergic Neurons. Cells. 2021 Dec 7;10(12):3436. https://doi.org/10.3390/cells10123436. PMID: 34943944; PMCID: PMC8699816]. [CrossRef]
- Jin, H., Kanthasamy, A., Ghosh, A., Anantharam, V., Kalyanaraman, B., Kanthasamy, A.G. Mitochondria-targeted antioxidants for treatment of Parkinson's disease: preclinical and clinical outcomes. Biochim Biophys Acta. 2014 Aug;1842(8):1282-94. https://doi.org/10.1016/j.bbadis.2013.09.007. Epub 2013 Sep 20. PMID: 24060637; PMCID: PMC3961561. [CrossRef]
- Muhammad, M.H.; Allam, M.M. Resveratrol and/or exercise training counteract aging-associated decline of physical endurance in aged mice; targeting mitochondrial biogenesis and function. J Physiol Sci. 2018 Sep;68(5):681-688. https://doi.org/10.1007/s12576-017-0582-4. Epub 2017 Dec 11. PMID: 29230719. [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




