Submitted:
13 October 2023
Posted:
13 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
2.1. Isolation and Identification of Strain SNC087
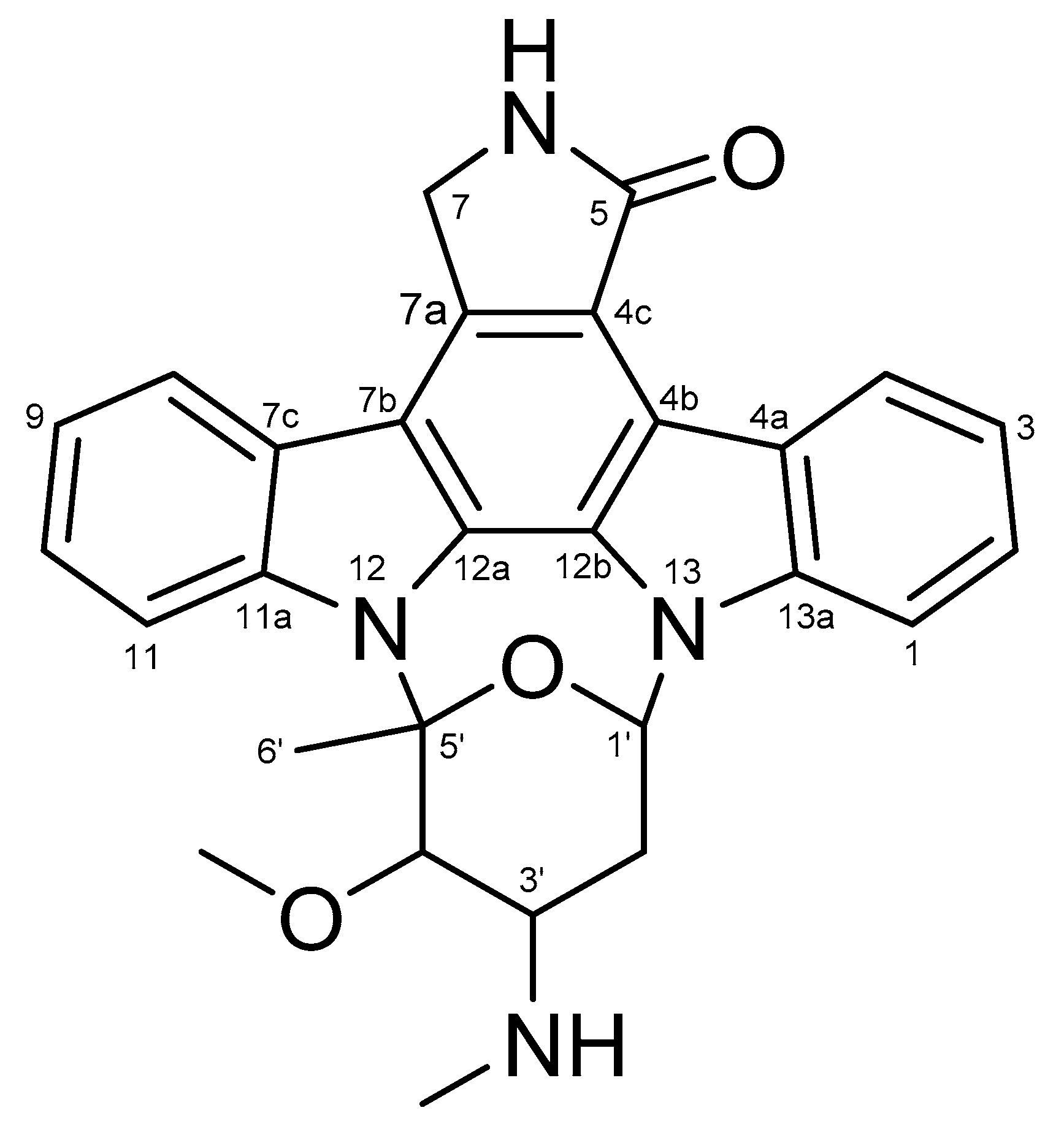
2.2. Structural Elucidation of STA
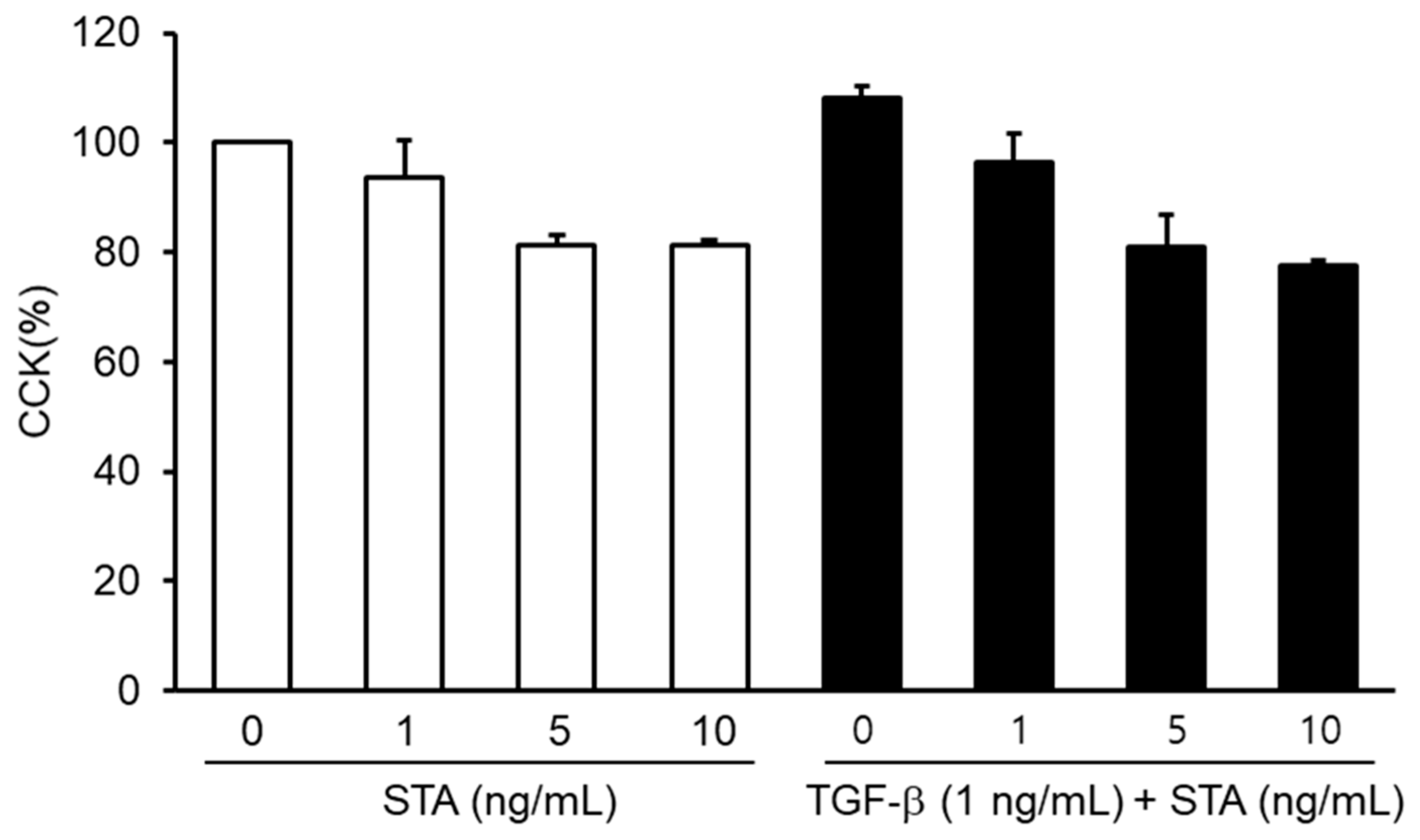
2.3. Toxicity of STA in Nasal Polyp-Derived Fibroblasts (NPDFs)
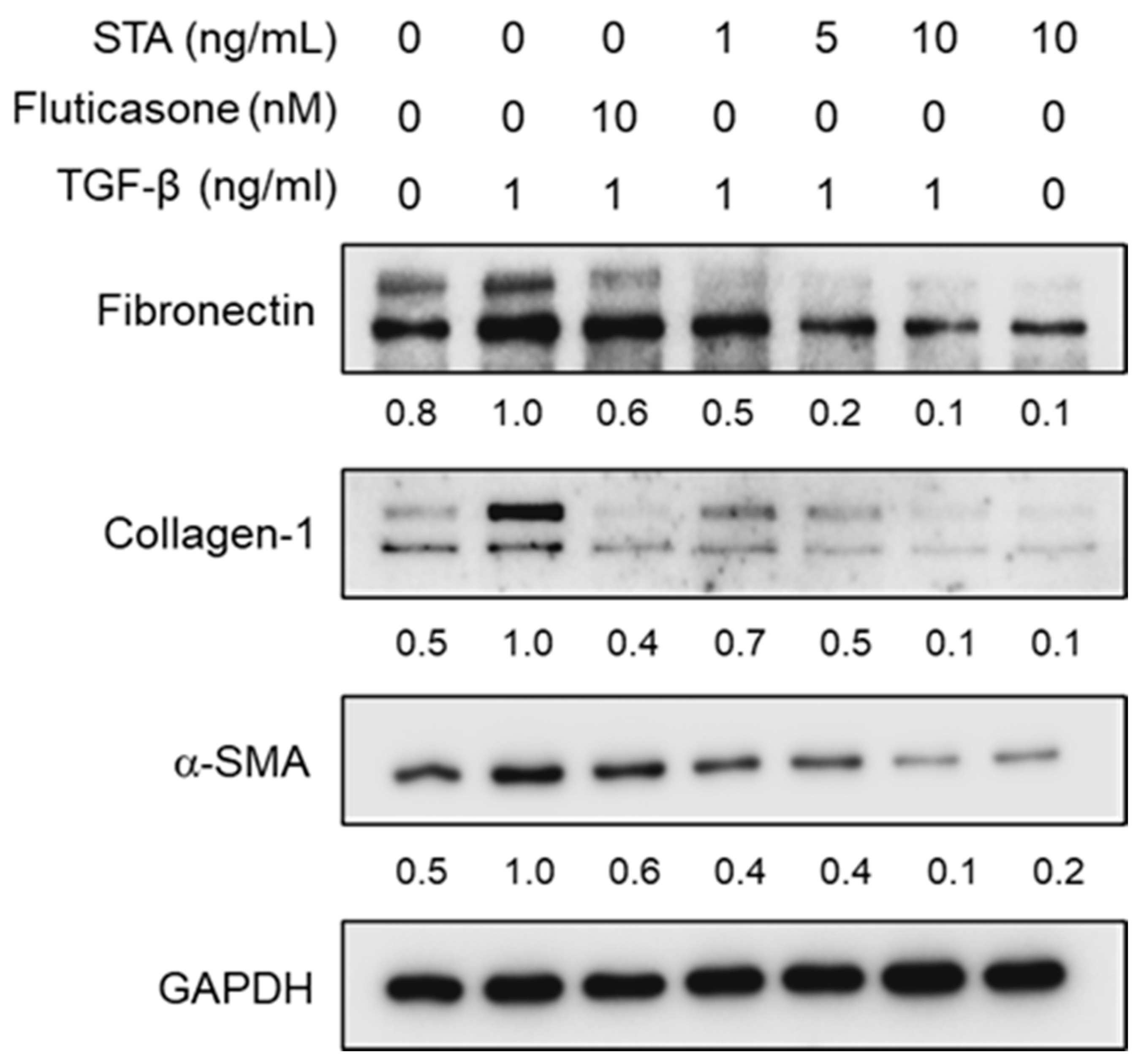
2.4. Effect of STA on the Expression of α-SMA, Col-1, and Fibronectin in TGF-β1-Activated NPDFs
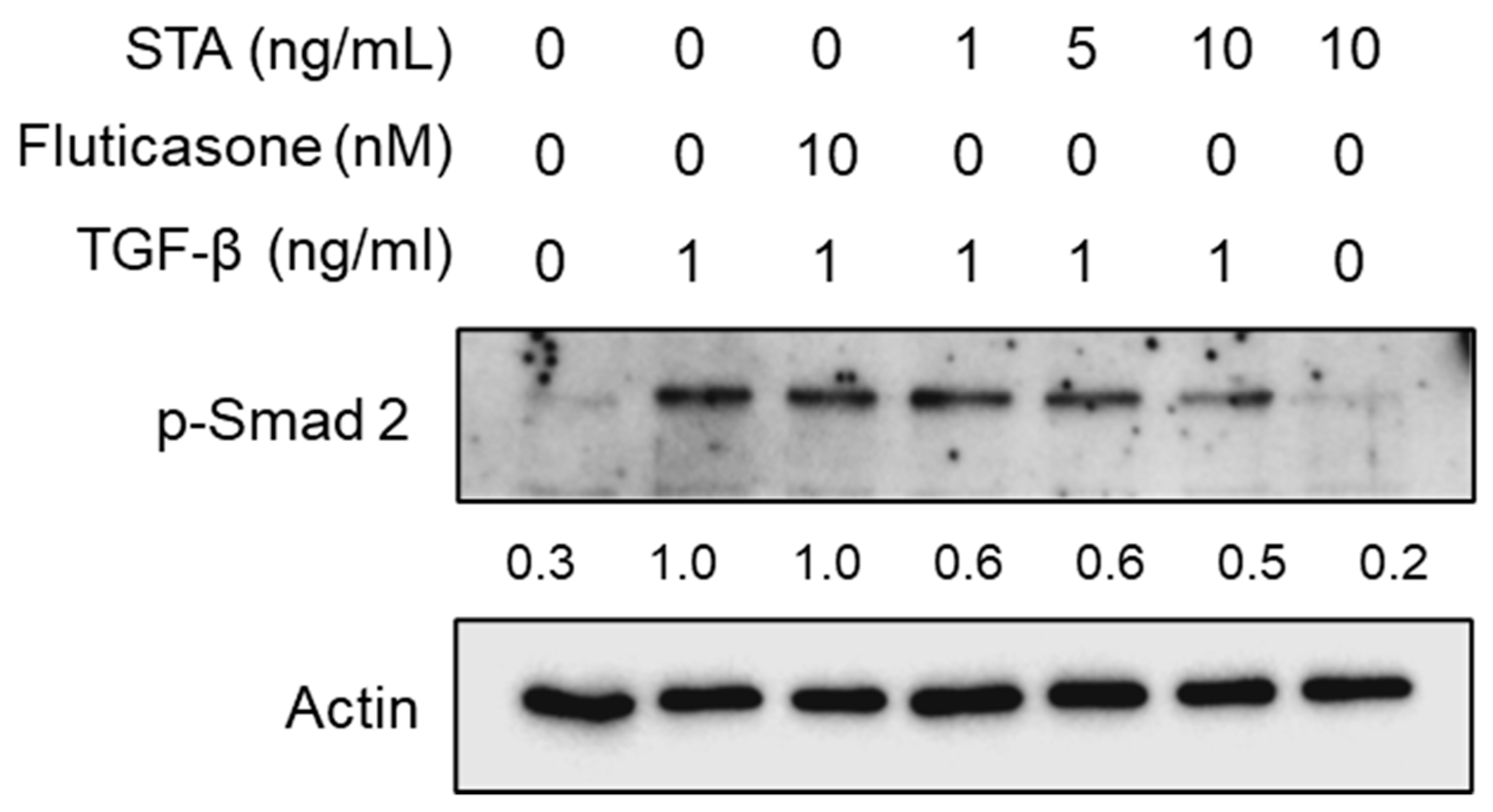
2.5. Regulatory Mechanisms of the Fibrosis Inhibition by STA in TGF-β1-Activated NPDFs
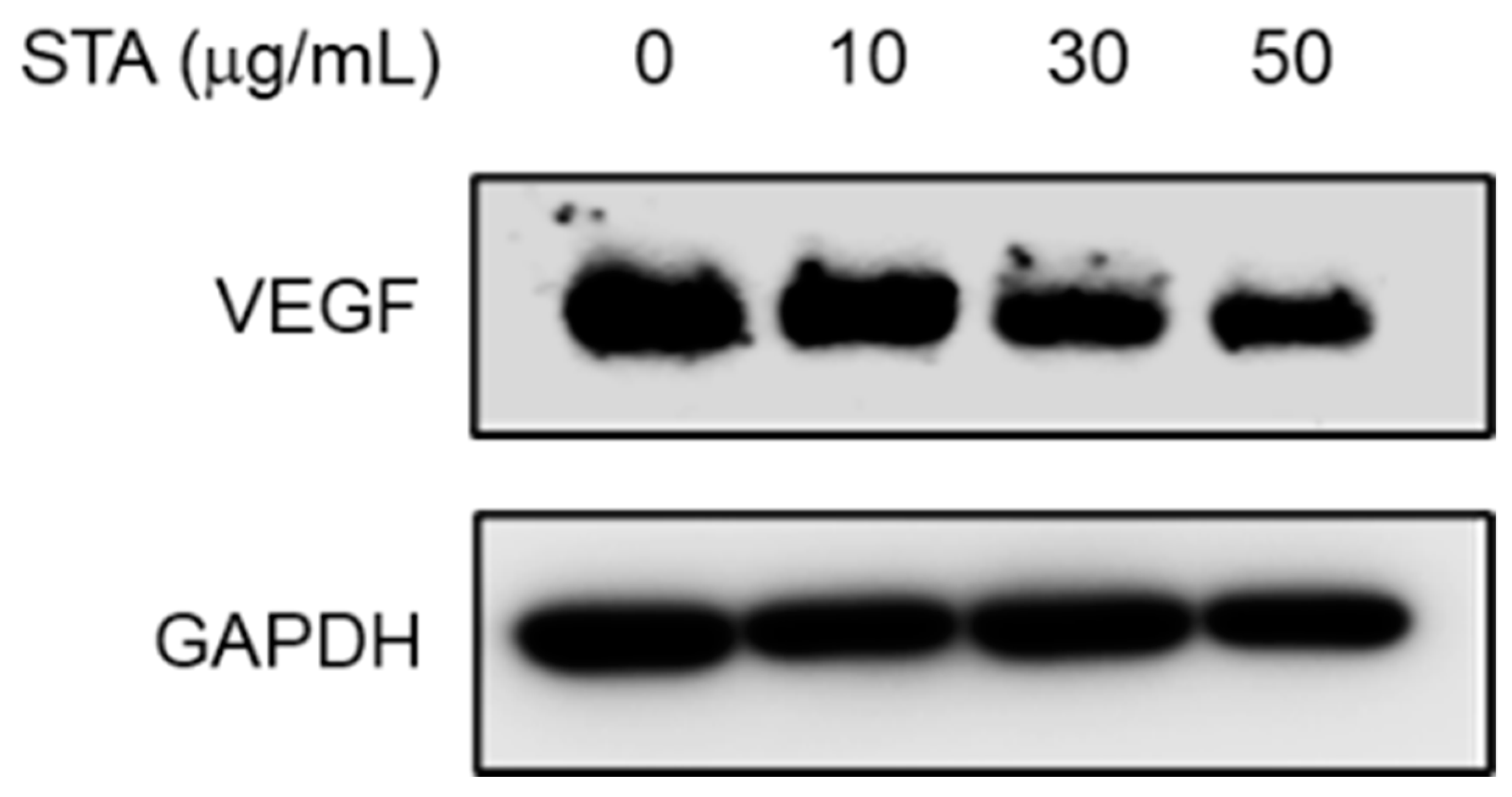
2.6. Measurement of Expression Inhibition Efficacy of VEGF (Vascular Endothelial Growth Factor) using NPs (Ex Vivo)
3. Materials and Methods
3.1. Chemical and Reagents
3.2. Strains and Culture Conditions
3.3. Isolation and Purification of STA from the SNC087 Strain
3.4. Cell Culture Method
3.5. Cytotoxicity
3.6. Western Blot Analysis
3.7. Ex Vivo Experiments
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jonathan, R.N.; Kim, W.A. A review of nasal polyposis. Ther. Clin. Risk Manag. 2008, 4, 507–512. [Google Scholar]
- Hwang, C.S.; Park, S.C.; Cho, H.-J.; Park, D.-J.; Yoon, J.-H.; Kim, C.-H. Eosinophil extracellular trap formation is closely associated with disease severity in chronic rhinosinusitis regardless of nasal polyp status. Sci. Rep. 2019, 9, 8061. [Google Scholar] [CrossRef]
- Rizzi, A.; Gammeri, L.; Cordiano, R.; Valentini, M.; Centrone, M.; Marrone, S.; Inchingolo, R.; Lohmeyer, F.M.; Cavaliere, C.; Ria, F.; et al. strategies to prevent the recurrence of nasal polys after surgical treatment: An update and in vitro study on growth inhibition of fibroblasts. J. Clin. Med. 2023, 12, 2841. [Google Scholar] [CrossRef] [PubMed]
- Badia, L.; Lund, V. Topical corticosteroids in nasal polyposis. Drugs 2001, 61, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Taw, M.B.; Nguyen, C.T.; Wang, M.B. Complementary and integrative treatments. Otolaryngol. Clin. N. Am. 2013, 46, 345–366. [Google Scholar] [CrossRef]
- Nakagawa, T.; Yamane, H.; Nakai, Y.; Shigeta, T.; Takashima, T.; Takeda, Z. Comparative assessment of cell proliferation and accumulation of extracellular matrix in nasal polyps. Acta Otolaryngol. 1998, 538, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Ravikanth, M.; Soujanya, P.; Manjunath, K.; Saraswathi, T.R.; Ramachandran, C.R. Heterogenecity of fibroblasts. J. Oral Maxillofac. Pathol. 2011, 15, 247–250. [Google Scholar] [CrossRef]
- Buckley, C.D.; Pilling, D.; Lord, J.M.; Akbar, A.N.; Scheel-Toellner, D.; Salmon, M. Fibroblasts regulate the switch from acute resolving to chronic persistent inflammation. Trends Immunol. 2001, 22, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Francesca Cialdai, F.; Chiara Risaliti, C.; Monici, M. Role of fibroblasts in wound healing and tissue remodeling on Earth and in space. Front Bioeng. Biotechnol. 2022, 10, 958381. [Google Scholar] [CrossRef] [PubMed]
- Fromm, S.; Cunningham, C.C.; Dunne, M.R.; Veale, D.J.; Fearon, U.; Wade, S.M. Enhanced angiogenic function in response to fibroblasts from psoriatic arthritis synovium compared to rheumatoid arthritis. Arthritis Res. Ther. 2019, 21, 297. [Google Scholar] [CrossRef]
- Kendall, R.T.; Feghali-Bostwick, C.A. Fibroblasts in fibrosis: Novel roles and mediators. Front. Pharmacol. 2014, 5, 123. [Google Scholar] [CrossRef]
- Baum, J.; Duffy, H.S. Fibroblasts and myofibroblasts: What are we talking about? J. Cardiovasc. Pharmacol. 2011, 57, 376–379. [Google Scholar] [CrossRef]
- Darby, I.A.; Laverdet, B.; Bonté, F.; Desmoulière, A. Fibroblasts and myofibroblasts in wound healing. Clin. Cosmet. Investig. Dermatol. 2014, 7, 301–311. [Google Scholar] [CrossRef]
- Park, H.H.; Park, I.H.; Cho, J.S.; Lee, Y.M.; Lee, H.M. The effect of macrolides on myofibroblast differentiation and collagen production in nasal polyp-derived fibroblasts. Am. J. Rhinol. Allergy 2010, 24, 348–353. [Google Scholar] [CrossRef]
- Denney, L.; Byrne, A.J.; Shea, T.J.; Buckley, J.S.; Pease, J.E.; Herledan, G.M.; Walker, S.A.; Gregory, L.G.; Lloyd, C.M. Pulmonary Epithelial Cell-Derived Cytokine TGF-beta1 Is a Critical Cofactor for Enhanced Innate Lymphoid Cell Function. Immunity 2015, 43, 945–958. [Google Scholar] [CrossRef] [PubMed]
- Pohlers, D.; Brenmoehl, J.; Loffler, I.; Muller, C.K.; Leipner, C.; Schultze-Mosgau, S.; Stallmach, A.; Kinne, R.W.; Wolf, G. TGF- and fibrosis in different organs—Molecular pathway imprints. Biochim. Biophys. Acta 2009, 1792, 746–756. [Google Scholar] [CrossRef]
- Neuzillet, C.; Tijeras-Raballand, A.; Cohen, R.; Cros, J.; Faivre, S.; Raymond, E.; de Gramont, A. Targeting the TGFbeta pathway for cancer therapy. Pharmacol. Ther. 2015, 147, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Jung, M.Y.; Choudhury, M.; Leof, E.B. Transforming growth factor beta induces fibroblasts to express and release the immunomodulatory protein PD-L1 into extracellular vesicles. FASEB J. 2020, 34, 2213–2226. [Google Scholar] [CrossRef] [PubMed]
- Coste, A.; Lefaucheur, J.P.; Wang, Q.P.; Lesprit, E.; Poron, F.; Peynegre, R.; Escudier, E. Expression of the transforming growth factor beta isoforms in inflammatory cells of nasal polyps. Arch. Otolaryngol. Head Neck Surg. 1998, 124, 1361–1366. [Google Scholar] [CrossRef]
- Omura, S.; Iwai, Y.; Hirano, A.; Nakagawa, A.; Awaya, J.; Tsuchya, H.; Takahashi, Y.; Masuma, R. A new alkaloid AM-2282 of Streptomyces origin. Taxonomy, fermentation, isolation and preliminary characterization. J. Antibiot. 1977, 30, 275–282. [Google Scholar] [CrossRef]
- Rüegg, U.T.; Burgess, G.M. Staurosporine, K-252 and UCN-01: Potent but nonspecific inhibitors of protein kinase. Trends Pharmacol. Sci. 1989, 10, 218–220. [Google Scholar] [CrossRef]
- Antonsson, A.; Persson, J.L. Induction of apoptosis by staurpsorine involves the inhibition of expression of the major cell cycle proteins at the G2/M checkpoint accompanied by alterations in Erk and Akt kinase activities. Anticancer Res. 2009, 29, 2893–2898. [Google Scholar]
- Chae, H.J.; Kang, J.S.; Byun, J.O.; Han, K.S.; Kim, D.U.; Oh, S.M.; Kim, H.M.; Chae, S.W.; Kim, H.R. Molecular mechanism of staurosporine-induced apoptosis in osteoblasts. Pharmacol. Res. 2000, 42, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.; Lin, S.; Wang, Z.; Fu, P.; Wang, C.; Zhu, W. Cytotoxic indolocarbazoles from a marine-derived Streptomyces sp. OUCMDZ-5380. Front. Microbiol. 2022, 13, 957473. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Zhou, P.; Liu, Y.; Liu, F.; Yi, X.; Liu, S.; Holtappels, G.; Bachert, C.; Zhang, N. The development of nasal polyp disease involves early nasal mucosal inflammation and remodelling. PLoS ONE 2013, 8, e82373. [Google Scholar] [CrossRef]
- Park, S.K.; Jin, Y.D.; Park, Y.K.; Yeon, S.H.; Xu, J.; Han, R.N.; Rha, K.S.; Kim, Y.M. IL-25-induced activation of nasal fibroblast and its association with the remodeling of chronic rhinosinusitis with nasal polyposis. PLoS ONE 2017, 12, e0181806. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Choi, G.; Yim, M.-J.; Lee, J.M.; Yoo, J.S.; Park, W.S.; Park, S.K.; Park, S.; Seo, S.-K.; Kim, T.-G.; et al. Effect of phlorotannins on myofibroblast differentiation and ECM protein expression in transforming growth factor β1-induced nasal polyp-derived fibroblasts. Int. J. Mol. Med. 2018, 42, 2213–2220. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Lee, D.-S.; Park, S.K.; Choi, J.S.; Jung, W.-K.; Park, W.S.; Choi, I.-W. Fucoxanthin Inhibits Myofibroblast Differentiation and Extracellular Matrix Production in Nasal Polyp-Derived Fibroblasts via Modulation of Smad-Dependent and Smad-Independent Signaling Pathways. Mar. Drugs 2018, 16, 323. [Google Scholar] [CrossRef]
- Sugiura, H.; Ichikawa, T.; Liu, X.; Kobayashi, T.; Wang, X.Q.; Kawasaki, S.; Togo, S.; Kamio, K.; Mao, L.; Ann, Y.; et al. N-acetyl-L-cysteine inhibits TGF-β1-induced profibrotic responses in fibroblasts. Pulm. Pharmacol. Ther. 2009, 22, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Molet, S.M.; Hamid, Q.A.; Hamilos, D.L. IL-11 and IL-17 expression in nasal polyps: Relationship to collagen deposition and suppression by intranasal fluticasone propionate. Laryngoscope 2003, 113, 1803–1812. [Google Scholar] [CrossRef]
- Sheppard, D.; Massague, J. TGF-ß signaling in health and disease. Cell 2023, 186, 4007–4037. [Google Scholar]
- Hu, K.H.; Lee, F.P.; Cheng, Y.J.; Huang, H.M. Vascular endothelial growth factor and children featuring nasal polyps. Int. J. Pediatr. Otorhinolaryngol. 2007, 71, 23–28. [Google Scholar] [CrossRef]
- Hong, T.U.; Park, S.K. The Roles of Vascular Endothelial Growth Factor, Angiostatin, and Endostatin in Nasal Polyp Development. J. Rhinol. 2022, 29, 82–87. [Google Scholar] [CrossRef]
- Bao, P.; Kodra, A.; Tomic-Canic, M.; Golinko, M.S.; Ehrlich, H.P.; Berm, H. The Role of Vascular Endothelial Growth Factor in Wound Healing. J Surg. Res. 2009, 153, 347–358. [Google Scholar] [CrossRef]
- Hirshoren, N.; Neuman, T.; Gross, M.; Eliashar, R. Angiogenesis in chronic rhinosinusitis with nasal polyps and in antrochoanal polyps. Inflamm. Res. 2011, 60, 321–327. [Google Scholar] [CrossRef]
- Yim, M.J.; Lee, J.M.; Ko, S.C.; Kim, H.S.; Kim, J.Y.; Park, S.K.; Lee, D.S.; Choi, I.-W. Antifibrosis Efficacy of Apo-9-Fucoxanthinone-Contained Sargassum horneri Ethanol Extract on Nasal Polyp: An In Vitro and Ex Vivo Organ Culture Assay. Curr. Issues Mol. Biol. 2022, 44, 5815–5826. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Lee, C.M.; Park, S.K.; Yim, M.J.; Lee, J.M.; Choi, G.; Yoo, J.S.; Jung, W.K.; Park, S.; Seo, S.K.; et al. Anti-inhibitory potential of an ethanolic extract of Distromium decumbens on pro-inflammatory cytokine production in Pseudomonas aeruginosa lipopolysaccharide-stimulated nasal polyp-derived fibroblasts. Int. J. Mol. Med. 2017, 40, 1950–1956. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Lee, W.J.; Yang, Y.I. Organ culture at the air-liquid interface maintains structural and functional integrities of inflammatory and fibrovascular cells of nasal polyps. Am. J. Rhinol. 2007, 21, 402–407. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




