I. Introduction
COVID-19 mainly affects the lungs, yet it also contributes to cardiac conditions (1). The inflammatory response of the body to COVID-19 is quite strong; inflammation is the major cause of myocardial injury (2). The plaque buildup in the arteries is usually harmless but can inflate, causing blood thrombi, myocardial infarction (MI), and strokes. There are several potential causes of short-term or long-term heart tissue damage.
A lack of Oxygen. Inflammation and fluid accumulation in the lungs' air sacs brought on by the virus reduce the amount of Oxygen that may enter the bloodstream. When the heart needs to work harder to pump blood, it poses a risk, especially for patients with cardiac disease. Overuse can lead to heart failure (HF), and hypoxia can kill cells and destroy tissue in the heart and other organs.
Cardiac inflammation is known as myocarditis. Like other viruses, such as the flu, the coronavirus can infect the heart muscle directly and cause harm. Indirectly, the body's immunological reaction can cause injury and inflammation to the heart.
In addition to affecting the inner surfaces of veins and arteries, coronavirus infection also causes inflamed blood vessels, small vessel damage, and blood clots, all of which contribute to decreased blood flow to the heart and other organs. Endothelial cells, which line the blood vessels, are affected by the illness known as severe COVID-19.
Cardiomyopathy (CM), which is a heart condition caused by stress, impairs the heart's ability to effectively pump blood and can be triggered by a viral infection. Thus, due to a viral infection, the body releases an excess of chemicals known as catecholamines, which can temporarily halt the heart. When the illness clears up, the heart no longer has something to worry about.
Long-term CV outcomes of COVID-19 patients were sought in a study including over 11 million participants and published in the journal Nature Medicine in February 2022. (1). The risk of CV illness following COVID-19 infection persisted for at least a year after infection, suggesting that the virus's effects last longer than the typical incubation period of two weeks. After 30 days of infection, the risk of CV disorders such as stroke, cardiac disease, pericarditis, myocarditis, HF, and blood thrombi was higher in COVID-19-infected patients, regardless of whether they were hospitalized. Those with COVID-19 were 63% more likely to experience cardiac problems within a year of infection. 45 extra CV incidents were found for every 1,000 persons who tested positive. Some investigators have recently found damage mechanisms to the CV and cerebrovascular systems (2). The utilization of state-of-the-art single-cell R.N.A. sequencing has revealed the mechanism of COVID-19 invasion of myocardial tissue, indicating the prospect of utilizing modern cell and molecular biological research techniques in exploring the complicated involvement of COVID-19 in CVD and other comorbidities (3). According to reports, 26% to 60% of individuals hospitalized due to COVID-19 infection have myocardial involvement detected with CV magnetic resonance (CMR). (4–6).
The long-term CV manifestations of SARS-COV-2 (COVID-19) infection remain unknown (3–7). Moreover, clinical factors associated with COVID-19-associated pathophysiology and underlying cellular molecular biological mechanisms are poorly understood (2–6). Therefore, this study aims to provide an updated account of clinical, cellular, and molecular factors in cardiovascular diseases associated with COVID-19. A thorough analysis of the latest published literature was carried out for this purpose.
2. Myocardial Injury and COVID-19
Damage or injury to the heart muscle is known as myocardial injury. It can result from MI, surgery, trauma, viral infections, or drug toxicity. Myocardial injuries can range from mild to severe and have significant health implications. CV biomarkers are commonly used to diagnose the myocardial injury. These biomarkers are troponin, creatine kinase-MB (CK-MB), and myoglobin, released by damaged heart muscle cells (8). MI of type I (T1MI) is caused by an acute atherosclerotic plaque rupture.
In contrast, type 2 (T2MI) MI results from myocardial oxygen demand and supply mismatches without an acute thrombotic event (9). Initial definitions of T2MI were included in the universal definition of MI (U.D.M.I.). This term identifies individuals with elevated cardiac troponin (cTn) levels that are not attributed to an ischemic etiology (9). A global task force of cardiology experts published The Fourth UDMI in 2018. It provides updated and standardized criteria to diagnose MI, defined as ischemia-induced myocardial cell death.
The Fourth Universal Definition of MI includes five types of MI (9,10):
1. Type 1 MI: Spontaneous MI resulting from a primary coronary event, such as plaque rupture or erosion, leading to prolonged ischemia.
2. Type 2 MI: MI resulting from an imbalance between myocardial oxygen supply and demand without a primary coronary event and occurring in severe anemia, hypotension, or tachyarrhythmias.
3. Type 3 MI: MI resulting from sudden cardiac death, with symptoms consistent with MI but without diagnostic E.C.G. changes or biomarker elevation.
4. Type 4a MI: MI associated with percutaneous coronary intervention (PCI), where biomarker elevation occurs following PCI.
5. Type 4b MI: MI associated with stent thrombosis, where biomarker elevation occurs associated with angiographic evidence of stent thrombosis.
COVID-19 is a viral respiratory infection caused by the SARS-CoV-2 virus. Since the virus was initially discovered in Wuhan, China, in December 2019, a global pandemic has evolved. Cough, fever, and shortness of breath are the most prevalent COVID-19 symptoms. However, patients also experience fatigue, muscle aches, a sore throat, and a loss of taste or smell. Furthermore, COVID-19 infection can cause severe respiratory disease, pneumonia, acute respiratory distress syndrome (ARDS), and other life-threatening complications may occur (11,12).
Generally, mild to severe infection may occur without any medical intervention. Some individuals, however, will develop serious conditions that necessitate medical attention, particularly those older and with existing illnesses, such as CVD, diabetes, chronic respiratory disease, or cancer. The above-mentioned complications associated with COVID-19 infection can also lead to being seriously ill or leading to death at any age (13,14,15). The heart may be affected in some patients, regardless of whether they previously had a CV diagnosis.
COVID-19 has been linked to myocardial injury, which can lead to serious cardiovascular complications. Despite the fact that the mechanism of this association is unknown, it is believed to be caused by direct viral invasion of the heart muscle as well as indirect effects such as cytokine release and hypoxia. SARS-CoV-2 penetrates cells through angiotensin-converting enzyme 2 (ACE2), which is a membrane protein that functions as a counterbalance to the adverse effects of the renin–angiotensin– aldosterone system (R.A.A.S.) through converting angiotensin II (Ang II) to Ang-(1–7) and interacting with the ACE2 receptor through the spike (S) protein
(16). The exact mechanism by which SARS-CoV-2 causes myocardial injury remains unclear, but cells expressing high ACE2 levels are more susceptible to SARS-CoV-2 invasion and subsequent organ injury, including cardiomyocytes (C.M.s)
(17). Clinical observations revealed that increased myocardial biomarker levels unrelated to obstructive coronary artery disease (CAD), mostly diagnosed as myocardial injury, occurred in 7.2–40.9% of patients with COVID-19
(18). Numerous publications have reported a high prevalence of myocardial injury in COVID-19 patients. According to a 2020 research published in J.A.M.A. Cardiology, approximately one-third of hospitalized COVID-19 patients had high troponin levels, indicating myocardial injury.
(19).
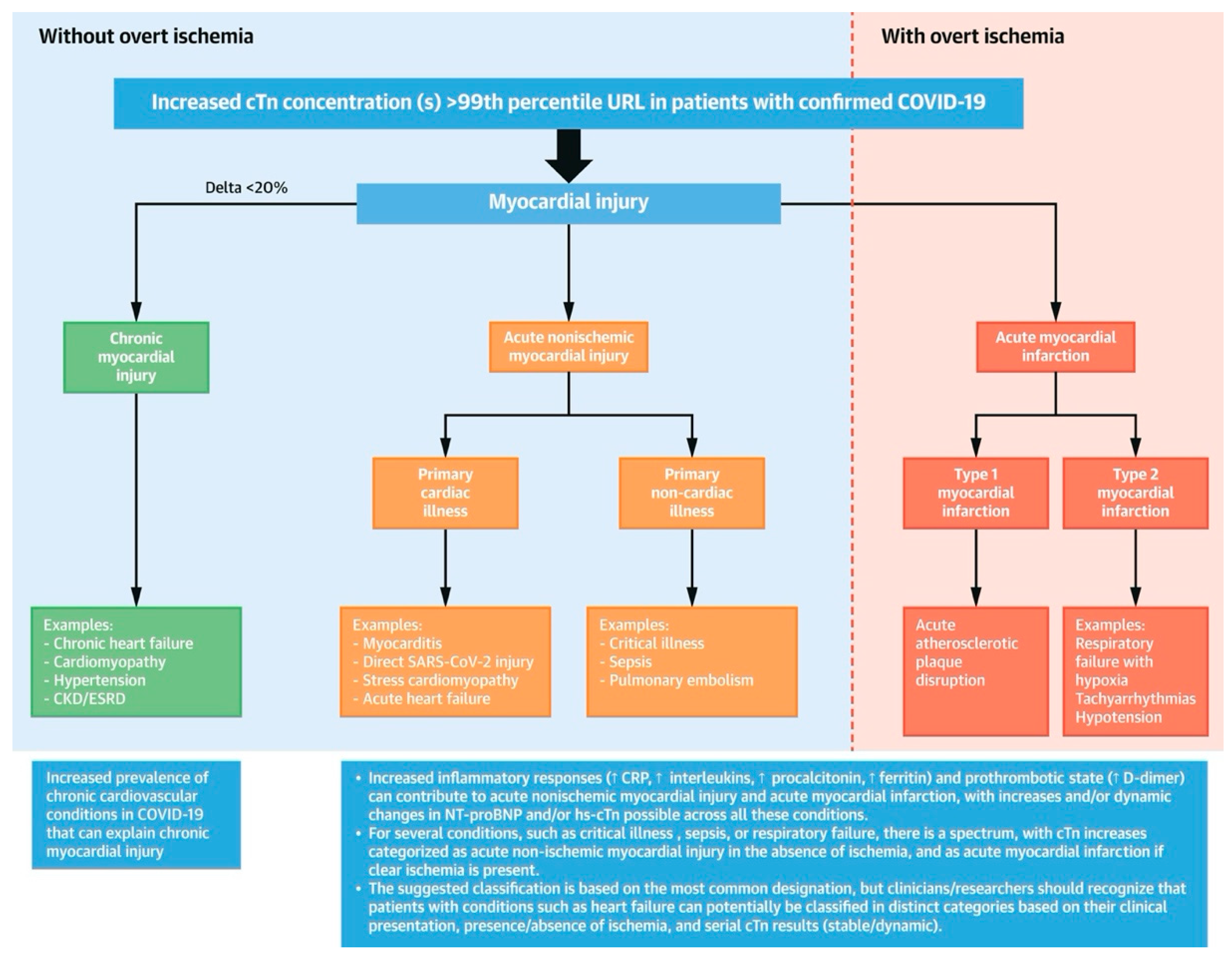
Figure 1 depicts the Classification of Myocardial Injury in COVID-19
(20).
There is evidence of myocardial injury among COVID-19 patients, manifested by an increased troponin level. Stress cardiomyopathy, hypoxic injury, ischemic injury, and systemic inflammatory response syndrome (cytokine storm) may be contributing factors. Troponin levels are elevated in a minority of patients with characteristics suggestive of an acute coronary syndrome (A.C.S.). Patients with cardiovascular disease, high blood pressure, obesity, and diabetes have a poor prognosis. In addition, patients with myocardial injury, regardless of cause, have a poorer prognosis
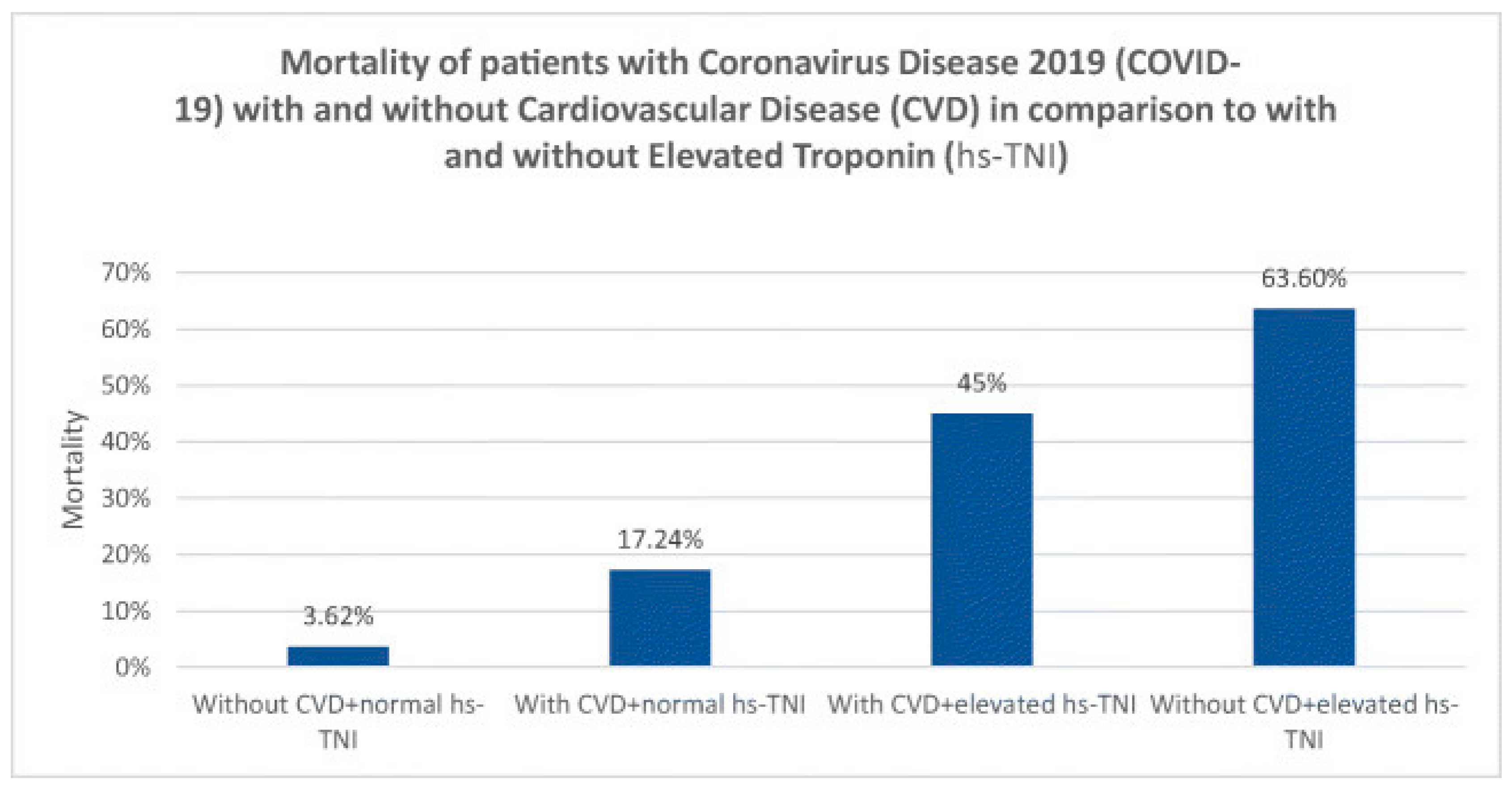
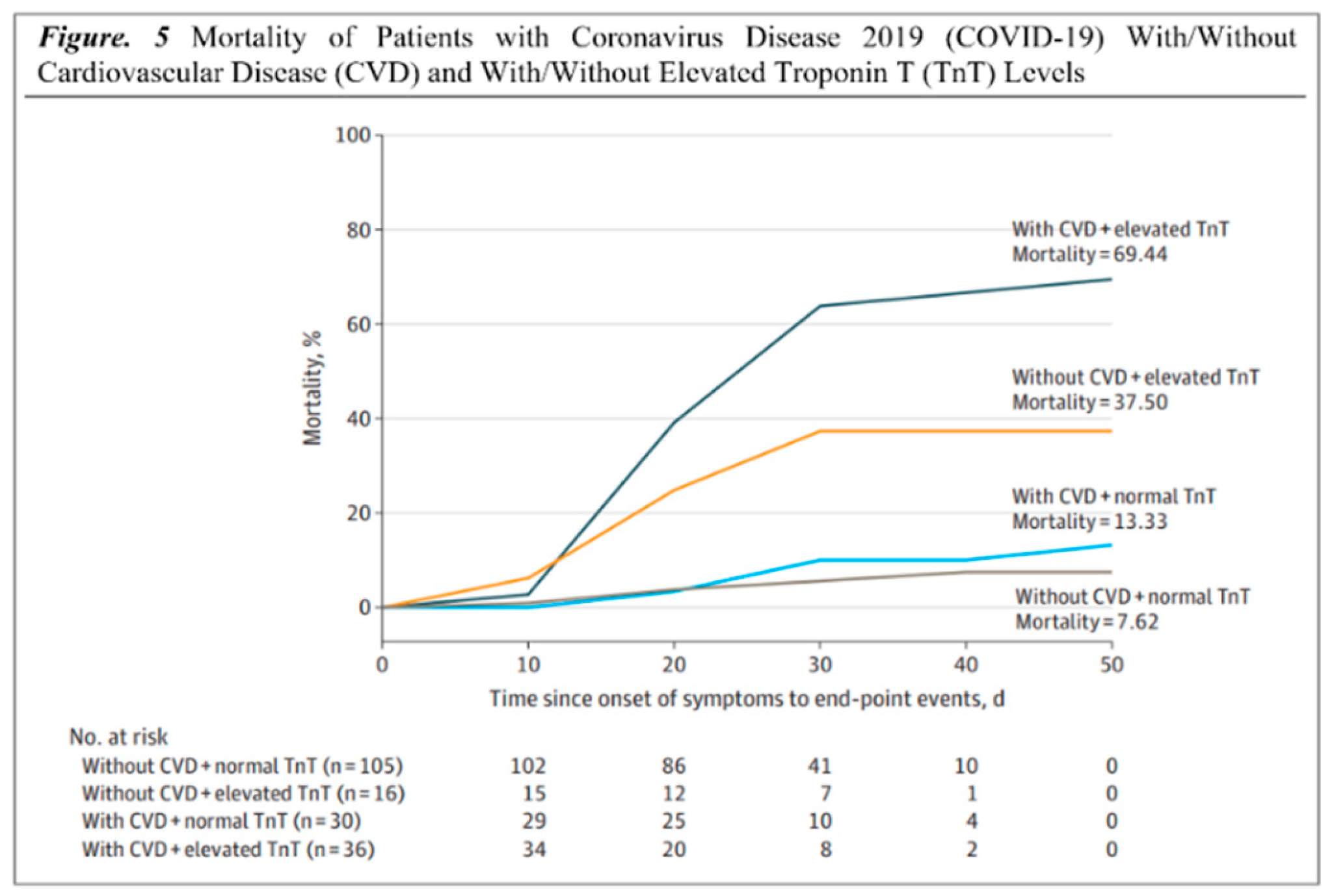
(21-26). The mortality rates of patients with Coronavirus disease 2019 (COVID-19) with and without CVD in comparison with patients with and without elevated troponin levels (hs-TNI) are shown in (
Figure 2)
(26). Cardiac troponin is routinely used to diagnose and treat ACS since it is a sensitive and specific marker of myocardial damage. Patients with COVID-19 who have elevated cardiac troponin levels are at a higher risk of mortality and morbidity. Studies have shown that COVID-19 is associated with myocardial injury and MI. As previously stated, SARS-CoV-2 infection is dependent on the ACE2 receptor, which is abundant in diverse tissues and expressed at varying levels in different organs
(27,28).
3. Mechanism of Myocardial Injury and COVID-19:
A. Cytokine Storm
Numerous studies have mentioned that COVID-19 patients with severe illness have significantly increased levels of pro-inflammatory cytokines, including interleukin IL-6, IL-1β, and tumor necrosis factor-alpha (TNF-α), in comparison to patients with mild illness, suggesting the involvement of cytokine storm in COVID-19- induced myocardial injury. A study conducted in Wuhan, China, found that elevated levels of IL-6, C-reactive protein (C.R.P.), and D-dimer were associated with adverse cardiovascular events, including myocardial injury (29).
As a result of cytokine storm, endothelial dysfunction and increased vascular permeability are induced, which eventually trigger capillary dilation, microvascular leakage, and micro-thrombi formation, resulting in myocardial injury. A recent study reported that COVID-19-induced cytokine storm upregulates the expression of ACE2 in the heart, providing an opportunity for ACE2-dependent myocardial infection (29).
The excessive inflammatory response, also known as cytokine storm, can cause collateral damage to different organs and tissues, including the heart, leading to endothelial dysfunction, increased vascular permeability, and myocardial injury. Furthermore, the cytokine storm may disrupt the myocardium's balance between oxidative stress and antioxidant defense, causing oxidative damage to cardiac tissues (30).
B. Oxygen supply-demand mismatch.
A study inspected the host vulnerability to severe COVID-19 and the establishment of a host risk score which concluded an oxygen supply-demand mismatch caused by respiratory failure was a prevalent mechanism underlying severe COVID-19 and was associated with an increased risk of unfavorable outcomes. The study highlights the importance of monitoring and managing oxygenation in patients with COVID-19 to prevent complications such as severe disease and mortality (31).
It was suggested that COVID-19 could cause Oxygen supply-demand mismatch, leading to myocardial injury through various mechanisms, including direct invasion of the virus, respiratory damage causing hypoxia, cytokine storm, and acute plaque rupture.
A direct invasion of the virus leads to damage of the heart tissue, resulting in myocardial injury. Respiratory damage caused by COVID-19 can lead to hypoxia, which increases the oxygen demand of the myocardium and can cause an oxygen supply-demand mismatch, leading to myocardial injury.
The cytokine storm caused by COVID-19 can potentially lead to myocardial injury through various pathways, including oxidative stress and inflammation. Acute plaque rupture can also cause an oxygen supply-demand mismatch, leading to myocardial injury (31).
Various studies have reported myocardial injury in COVID-19 patients, one of which observed elevated cardiac troponin levels in 36.1% of COVID-19 patients, associated with a higher mortality risk. In another study, myocardial injury, as defined by elevated cardiac troponin levels, was reported in 19.7% of COVID-19 patients, and these patients were more likely to require mechanical ventilation and have a poor prognosis (30).
C. Stress cardiomyopathy
Stress cardiomyopathy, which is also termed Takotsubo cardiomyopathy, is a reversible left ventricular (LV) dysfunction due to transient wall motion abnormalities in the absence of significant obstructive coronary artery disease (CAD). The presence of stress cardiomyopathy among COVID-19-infected patients has been reported, and it may represent one of the disease's most significant complications.
The exact pathophysiology of stress cardiomyopathy in patients infected with COVID-19 has yet to be fully understood. However, it has been hypothesized that COVID-19 infection can cause an oxygen supply and demand mismatch, resulting in myocardial injury and stress cardiomyopathy. As a consequence of COVID-19 infection, hypoxemia may occur, resulting in a decrease in oxygen delivery to the myocardium; this may result in myocardial injury manifested as stress cardiomyopathy (32)(33).
Several studies have reported cases of stress cardiomyopathy in patients infected with COVID-19. In a study of 100 patients with COVID-19, stress cardiomyopathy was identified in five patients, suggesting a prevalence of 5%. Another study reported a prevalence of 7.8% of stress cardiomyopathy in COVID-19 patients with severe disease (32). Moreover, several reports suggest that COVID-19 infection can cause stress cardiomyopathy, characterized by left ventricular dysfunction and acute myocardial infarction clinical symptoms. The exact mechanism by which COVID-19 infection causes stress cardiomyopathy is vague. However, a few proposed hypotheses include catecholamine surge, direct myocardial injury, and microvascular dysfunction.
In a study that comprised 416 hospitalized patients with COVID-19, 7.2% were diagnosed with stress cardiomyopathy. A higher rate of in-hospital mortality and mechanical ventilation was observed among patients with stress cardiomyopathy (34). Although stress cardiomyopathy is reversible, it can cause significant morbidity and mortality in some patients. Therefore, it is essential to recognize the potential association between COVID-19 infection and stress cardiomyopathy. higher incidence of in-hospital mortality and mechanical ventilation
D. Hypoxic injury
Several studies have reported elevated levels of inflammatory markers, such as interleukin-6 (IL 6), in COVID-19 patients, which suggests an underlying inflammatory process. It has been proposed that systemic inflammation may contribute to myocardial injury by releasing inflammatory mediators. These mediators, such as IL-6, can cause endothelial dysfunction and microvascular thrombosis, decreasing oxygen supply to the myocardium (34).
In a study of 416 hospitalized patients with COVID-19, higher levels of IL-6 were observed in those with myocardial injury compared to those without. The study concluded that inflammatory mediators, such as IL-6, may contribute to myocardial injury in COVID-19 patients (19). Moreover, other increased inflammatory markers including ferritin and C-reactive protein (C.R.P.) have been observed in 19 patients with myocardial injury, which is an indication of a possible role of systemic inflammation in the development of myocardial injury in COVID-19 (29). In another study, patients with severe COVID-19 were found to have elevated levels of troponin, a biomarker of myocardial injury, which correlated with the levels of inflammatory markers such as IL-6 and C.R.P. (19).
The precise mechanism of inflammatory mediator-induced hypoxic injury in COVID-19 patients is unidentified, but systemic inflammation is thought to play a role. Identifying and managing inflammatory mediators may help improve the prognosis of COVID-19 patients with myocardial injury.
E. Endothelial Dysfunction
Endothelial dysfunction and its associated effect on microvascular and epicardial vessels can contribute to many pathological conditions, such as inflammation and thrombosis. In COVID-19 patients, endothelial damage in the vascular wall can lead to a pro-thrombotic environment and micro-thrombi formation, contributing to myocardial injury (35).
According to recent research, the SARS-CoV-2 virus can infect endothelial cells, causing endothelial dysfunction (E.D.), capillary dilation, and increased vascular permeability. Furthermore, as the inflammatory response is triggered, raising cytokine levels such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-alpha) can lead to endothelial dysfunction (35).
A study on inflammatory markers and endothelial dysfunction in COVID-19 patients showed that elevated levels of IL-6, C.R.P., and D-dimer were associated with increased inflammatory activity and endothelial dysfunction, indicating a correlation between COVID-19 severity and endothelial injury. Another study found that damage to epicardial vessels due to endothelial dysfunction could lead to cardiac remodeling and subsequent myocardial injury (36). In addition, a survey on COVID-19 patients with acute respiratory distress syndrome (ARDS) found a significant correlation between pulmonary dysfunction and myocardial injury, indicating a possible involvement of microvascular dysfunction in COVID-19-induced myocardial injury (37).
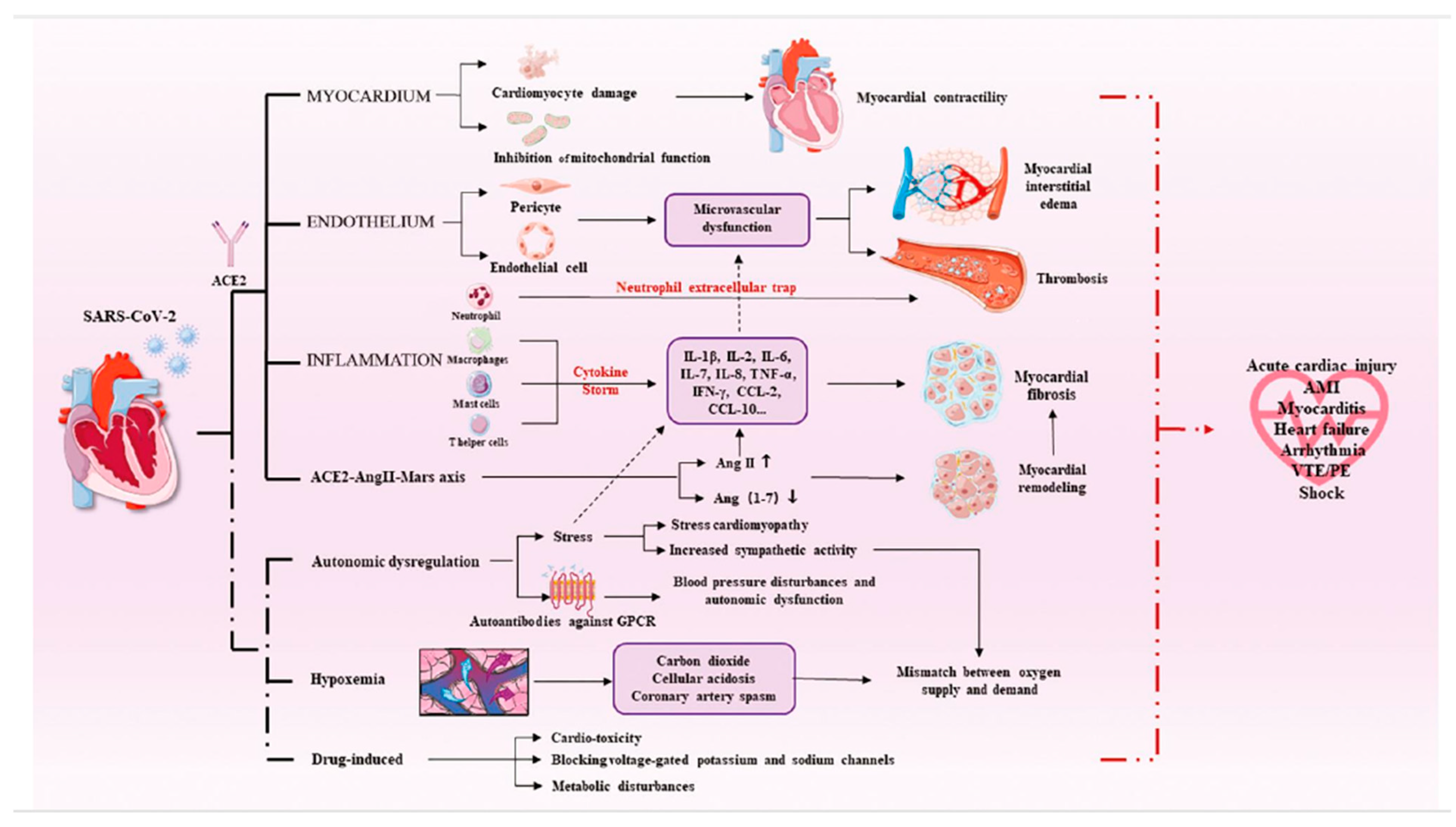
To develop effective preventative and therapeutic techniques, it is critical to understand the influence of COVID-19 on endothelial dysfunction and its association with myocardial injury. Early detection of endothelial dysfunction may aid in the prevention of myocardial injury development in COVID-19 patients. More research is needed to determine the particular mechanism of myocardial injury (
Figure 3)
(16).
4. Clinical Manifestations Of Myocardial Injury
A. Signs and symptoms
The symptoms of myocardial infarction (MI) include chest pain that radiates from the left arm to the neck, shortness of breath, diaphoresis, gastrointestinal symptoms (such as nausea and vomiting), an abnormal heart rate, anxiety, fatigue, weakness, stress, and depression (37). It has been reported that there will be low blood pressure, an elevated heart rate, a high respiratory rate, signs of low cardiac output, and a third heart sound when examining patients with myocardial infarction (38).
MI can be classified by typical symptoms in patients with chest, arm, or jaw pain in the form of dull, heavy, tight, pressure, ache, squeezing, crushing, or gripping in nature. Also, it can be classified as atypical symptoms in patients with epigastric back pain if it is present as burning, stabbing, or indigestion-like. It has been reported, however, that pain radiation (to the right arm, left arm, neck, jaw, and back) and associated symptoms (nausea, vomiting, sweating, dyspnea, and palpitations) are clinical manifestations. (39).
Myocardial ischemia symptoms include various combinations of chest, arm, jaw, or epigastric discomfort that occurs during rest or exercise. Acute myocardial infarction usually causes discomfort for at least 20 minutes. This discomfort is typically diffuse, not localized, not positional, and not exacerbated by movement, and it may be associated with dyspnea, excessive sweating, nausea, or syncope. These symptoms are not diagnostic for myocardial ischemia and may be misdiagnosed as gastrointestinal, neurological, pulmonary, or musculoskeletal disorders. MI symptoms can be atypical or even absent (8).
Diagnosing myocardial injury might be challenging if associated with coronavirus disease 2019 (COVID-19), especially in patients with advanced disease. Patients with myocardial injury associated with COVID-19 usually present with an atypical clinical presentation, so it is hard to diagnose. Myocardial injury can present in patients with no previous CVD history, even if the patient is not complaining of chest pain (40).
B. Investigations:
Cardiac biomarkers, ECG, echocardiography, and CMR are valuable noninvasive investigations for assessing CV involvement and complications in COVID-19 patients (41). Precautions should be taken to minimize the risk of contagion during these procedures.
Cardiac biomarkers like troponin, C.R.P., D-dimer, and NT-pro BNP are important for diagnosing and predicting the prognosis of COVID-19. Elevated biomarkers indicate a poor prognosis and increased risk of CV complications (42). COVID-19-related CVD can be categorized as primary, caused by direct viral injury, or secondary, resulting from an exaggerated inflammatory response (43).
Electrocardiogram (E.C.G.) changes, including arrhythmias, conduction defects, and S.T. segment and T-wave abnormalities, are common in COVID-19 patients (44-46). These changes can resemble those seen in acute myocardial infarction (A.M.I.) and myocarditis, making diagnosis challenging (47-50). Cardiac arrhythmias, such as ventricular tachycardia and fibrillation, are more prevalent in critically ill COVID-19 patients (51).
Echocardiography is recommended for assessing cardiac structure and function in COVID-19 patients (52). However, precautions should be taken to minimize the risk of contamination (53, 54). Transthoracic echocardiography (TTE) and stress echocardiography are preferred, while transesophageal echocardiography (TEE) carries a higher contamination risk (54, 55). Bedside echocardiography can help diagnose myocarditis in suspected COVID-19 cases (56).
Cardiovascular Magnetic Resonance (CMR) is the gold standard for evaluating myocardial structure and function (57). Portable C.M.R. devices are recommended for convenience and easier cleaning in COVID-19 settings.
A coronary angiogram is valuable for detecting and assessing CAD, identifying structural anomalies, and measuring hemodynamic parameters (58). COVID-19-associated coagulopathy has revealed the presence of intracoronary thrombus (I.C.T.) in individuals undergoing angiograms for acute coronary syndromes, as coagulation problems are common in COVID-19 affecting venous and arterial Circulation (59, 60). Liori et al. reported a case of a 39-year-old male with COVID-19 presenting with acute retrosternal chest pain and S.T. segment elevation in leads V4-V6 on the E.C.G. Thrombus aspiration restored flow in the left anterior descending coronary artery (LAD) as confirmed by coronary angiography (60).
Piccolo and Esposito emphasized the higher mortality risk in patients with concomitant coronary artery disease and COVID-19, suggesting avoiding invasive angiography with revascularization (61). Xie et al. found that invasive coronary angiography (I.C.A.) in stable CAD patients was associated with a higher risk of major adverse cardiovascular events, all-cause mortality, and severe procedure-related complications compared to computed tomography coronary angiography (C.T.C.A.) (62).
COVID-19 and myocardial infarction patients may require a prolonged hospital stay and multiple anticoagulants for severe symptoms (63). Increased availability of C.T.C.A. was associated with a significantly shorter length of stay for patients with chest pain without increased adverse outcomes (64). Panjer et al. demonstrated good diagnostic accuracy of dynamic cadmium-zinc-telluride single-photon emission tomography (CZT-SPECT) in coronary artery disease (65).
5. Cardiac Complications of COVID-19:
A. Myocarditis:
One of the cardiac consequences in individuals infected with SARSCoV-2 and its accompanying systemic inflammation is myocarditis. Alike to viral infections like COVID-19 with or without myocarditis, patients with myocarditis frequently present with chest discomfort, exhaustion, and dyspnea; however, some patients also report symptoms, including myalgia, diarrhea, nausea, vomiting, and headaches (66). Due to early studies lacking proper diagnostic tools, the prevalence of myocarditis among COVID-19 patients remains unknown. Yet, myocarditis is claimed to have been a factor in up to 7% of COVID-19-related deaths (67).
B. Arrhythmia:
Cardiac arrhythmias were recognized as one of the potential complications in COVID-19 patients. Arrhythmia was observed to occur in 7.3% of the 137 patients in one observational study on the clinical characteristics of COVID-19 patients in Hubei, China (68). In addition, Wang et al. noted that 44.4% of COVID-19 patients transferred to an intensive care unit had an arrhythmia (21). After acute COVID-19 infection recovery, researchers have reported a rise in arrhythmic problems, and the long-term frequency is still under study. According to recent studies, atrial arrhythmias and bradyarrhythmia, in particular, had an incidence of 13% and 12.8%, respectively, in the acute setting of COVID-19. Furthermore, ventricular arrhythmia, the less common variant, was recorded in 5.9% of patients, whereas the prevalence of atrioventricular block was 8.6% (69, 70). There are a few pathophysiological mechanisms of cardiac arrhythmias: 1- direct injury to cardiomyocytes altering the electrical conduction; 2- infection of the pericardium causing massive edema; 3- ischemia from microvascular disease; 4- re-entrant arrhythmias due to myocardial fibrosis or scars; and 5- proinflammatory cytokines predisposing to arrhythmogenicity (71, 72).
C. Hypotension:
Hypotension following COVID-19 is a serious complication, even if it may occur in fewer hypertensive individuals. It is crucial because frail elderly patients with hypertension have a greater mortality risk and are more likely to develop cognitive fragility
(73). In spite of the fact that hypotension may occur in fewer hypertensive patients, it is nevertheless a serious complication as frail elderly patients with hypertension are at greater risk of mortality and are more likely to suffer cognitive impairment as a result
(74). A recent case report was done by Koudelka M. et al. for five elderly patients known to be hypertensive and on medications
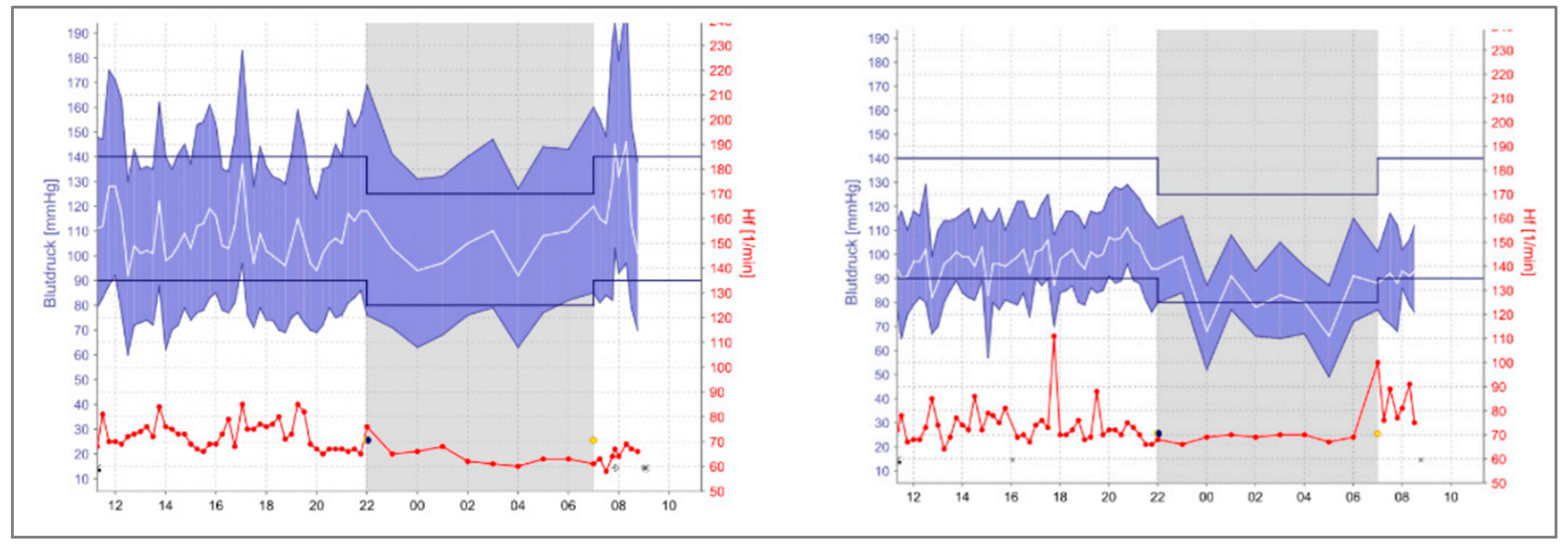
(73). All patients included in the study have similar essential clinical characteristics; their age range was 65–85 years, and two of five were men. Following the COVID-19 infection, their B.P. was checked in the office, and all patients had well-controlled blood pressure. However, the patients were monitored by A.B.P.M. at home, where they detected multiple episodes of hypotension. (
Figure 4) shows the A.B.P.M. home readings for one of the subjects in the study before and after COVID-19 infection, indicating the need for medication adjustments
(73). All five patients showed the same pattern of this phenomenon. Regardless of factors that could contribute to lowering blood pressure, it is crucial to keep monitoring the patient, not only with office measurements. Since it is still unknown how B.P. readings change in these people, further studies and research will answer these questions.
D. Sudden cardiac death:
With COVID-19 infection, sudden cardiac death (S.C.D.) has been one of the worrying concerns
(75, 76). The available data reveals a probable connection despite lacking evidence for a direct causal link between cardiac death and COVID-19. Both community and hospital settings have shown increased S.C.D. incidence
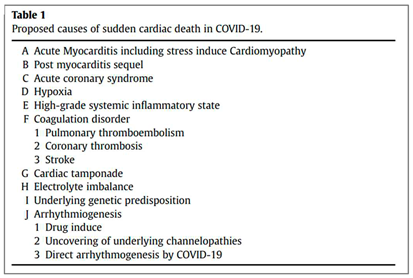
(77). The mechanism of S.C.D. may involve many factors (
Table 1). The most prevalent mechanism involved still needs to be determined due to a lack of evidence. According to a study conducted by Yang C et al which included 187 patients, 37.50% (6 of 16) had elevated TnT levels without underlying CVD, while 69.44% (25 of 36) had elevated TnT levels with underlying CVD. Conversely, 7.62% (8 of 105) of patients with normal TnT levels died in the hospital; therefore, patients with underlying CVD and high TnT levels had the highest mortality (69.44%) and shortest survival times. (
Figure 5)
(78).
6. Long-term cardiovascular outcomes of COVID-19:
Numerous investigations revealed that COVID-19 survivors had a significant risk of cardiovascular disease and ongoing symptoms. According to a report by Al-Aly Z from June 2021, the cardiovascular system's pulmonary and extrapulmonary organs are all impacted by SARS-CoV-2 post-acute sequelae (79).
Nearly a third of hospitalized patients following acute COVID-19 were readmitted, and more than one in ten passed away after discharge (80). COVID-19 patients had substantially higher rates of respiratory illness, diabetes, and cardiovascular disease (CVD), with 770 diagnoses per 1000 person-years. Those under 70 and members of ethnic minorities were more likely to see increased risk.
Additionally, a study conducted by Huang C et al. enrolled 1733 COVID-19 patients; the study discovered that the primary issues faced by COVID-19 survivors included exhaustion or weakness of the muscles, trouble sleeping, and anxiety or sadness. Abnormal chest imaging symptoms and more severely reduced pulmonary diffusion capabilities were observed in patients who were more critically unwell throughout their hospital stay (81). Furthermore, 143 individuals were examined for COVID-19 symptoms by Carfì A et al.; just 18 patients had no COVID-19-related symptoms. 44.1% of patients had a worse quality of life, and many reported joint discomfort, chest pain, fatigue, and dyspnoea (82).
According to a study conducted in May 2021 by Daugherty SE, 14% of adults 65 and older had at least one new clinical sequela following the acute phase of SARS-CoV-2 infection, which required medical attention. This was 4.95% higher than the comparison group from 2020
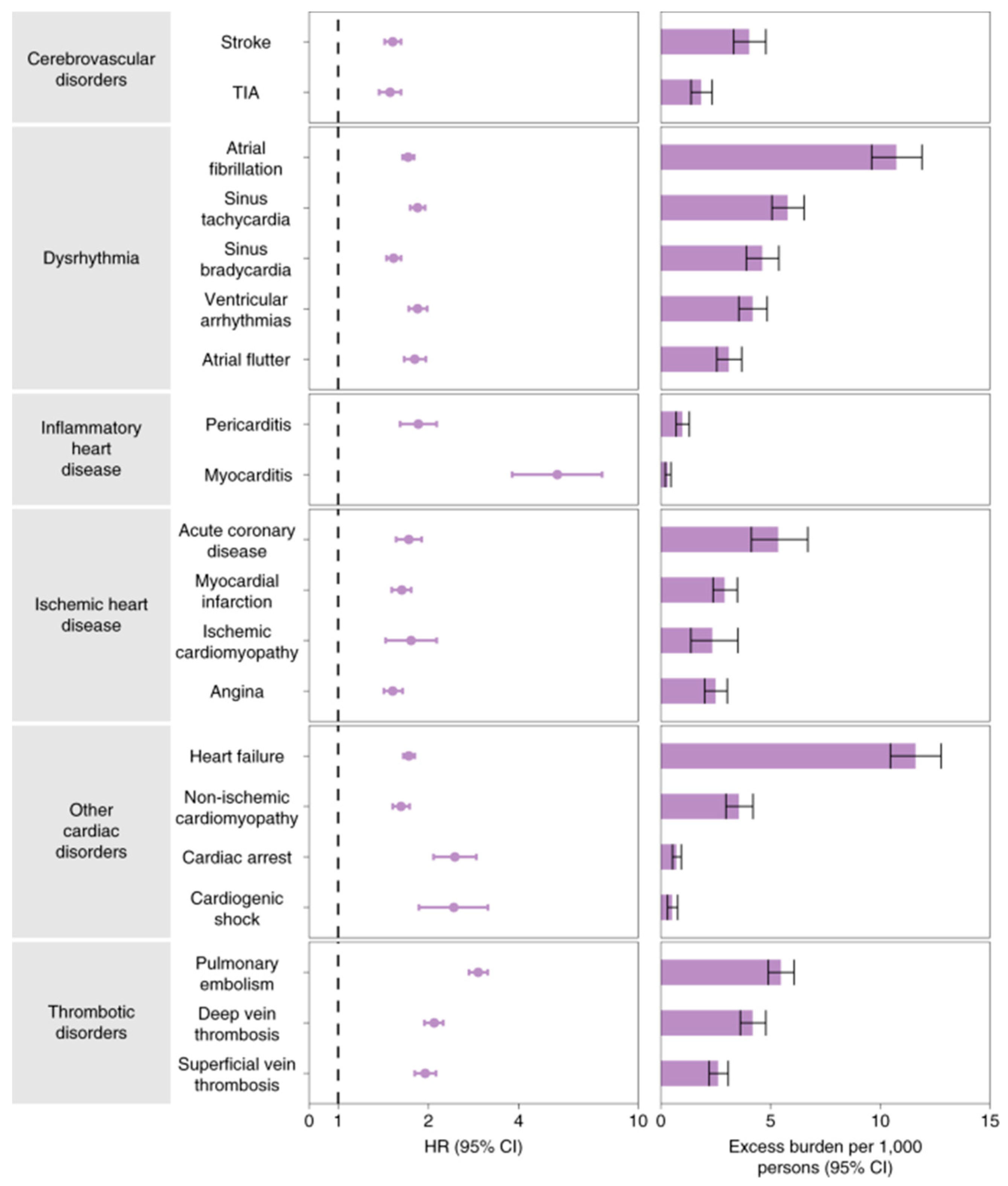
(83). The conditions included chronic respiratory failure, cardiac arrhythmia, hypercoagulability, encephalopathy, peripheral neuropathy, amnesia, diabetes, abnormal liver test results, myocarditis, anxiety, and fatigue. On the other hand, according to a study that included 5,859,411 VHA users who were not infected with COVID-19 and 153,760 US veterans, those who had the virus had higher incidence burdens and risk factors over 12 months as shown in
Figure 6 (84).
The sympathetic nervous system can be overstimulated by COVID-19, which can also cause a cytokine storm, hypercoagulopathy, and inflammation. Even after recovering from COVID-19, these pathways may cause permanent harm to the cardiovascular or respiratory systems. Cardiovascular or cerebrovascular disease among COVID-19 survivors is anticipated to rise due to these permanent consequences, such as congestive heart failure or diminished lung function.
The National Healthcare Databases (V.H.A.) of the U.S. Department of Veterans Affairs (V.H.A.) provide evidence that hospitalized and non-hospitalized survivors of acute COVID-19 have a high risk of CVD and a high 1-year disease burden. Based on data from 48 healthcare organizations (HCOs) in the U.S. Collaborative Network (84).
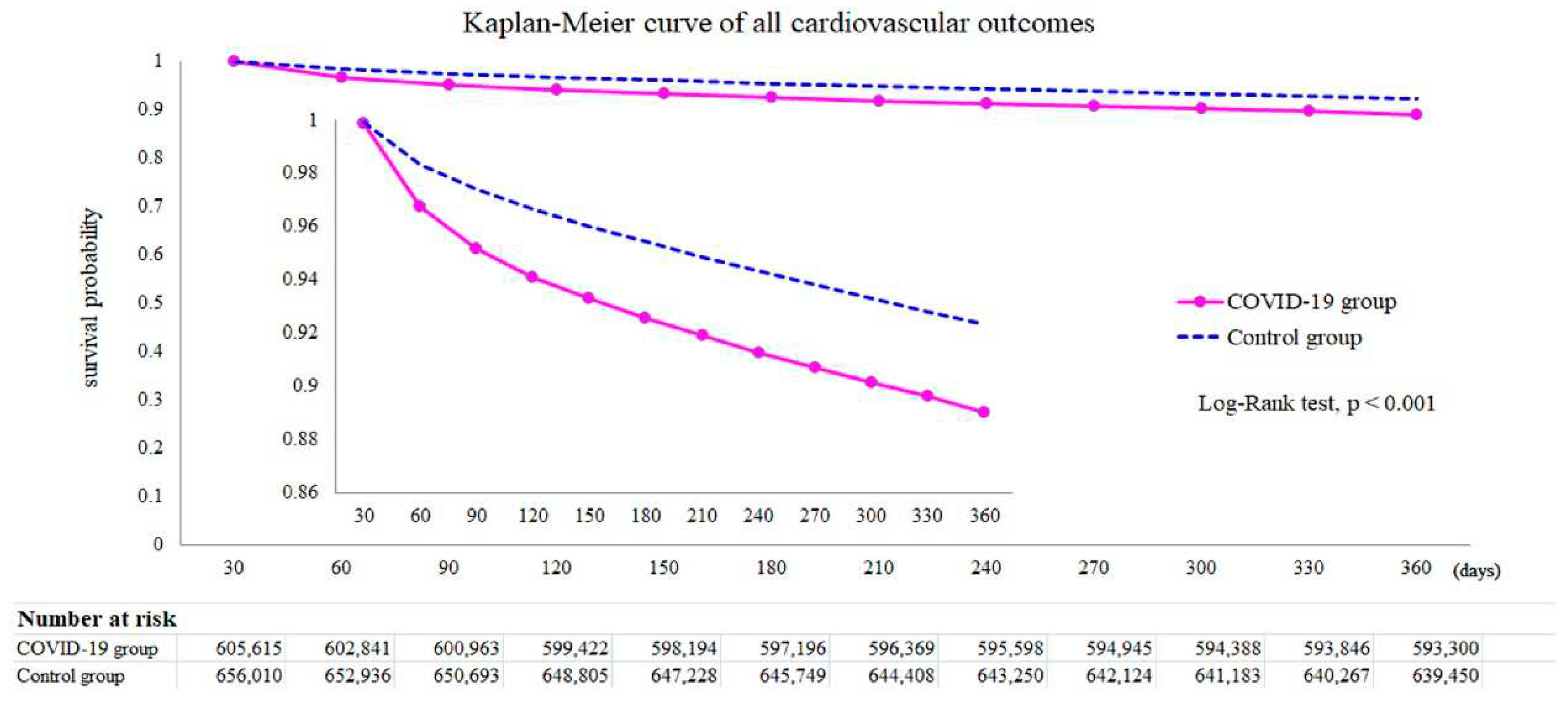
After all, it was found that dysrhythmias, pericardial or inflammatory heart disease, and cerebrovascular disorders were among the CVD cases more common in COVID-19 than in current controls. Furthermore, based on the Kaplan-Meier curve in
Figure 7, the mortality rate in the COVID-19 group was superior to that in the control group. Among individuals not hospitalized during the acute phase, ischemic heart disease, atrial fibrillation and flutter, tachycardia, myocardial infarction, pulmonary embolism, and thromboembolic disorders present significant risks and limits, with a graded increase across the severity range
(85).
TriNetX Research Network reported the COVID-19 survivors were discovered to have higher odds of acquiring these cardiovascular problems than the controls after a 12-month follow-up (Wang W, Long-term cardiovascular outcomes in COVID-19 survivors among non-vaccinated population: A retrospective cohort study from the TriNetX US collaborative networks. , 2022 N.O.V.) as described below.
Higher risks of cerebrovascular complications, such as stroke (H.R. = 1.52 [1.43-1.62]) and T.I.A. (H.R. = 1.503 [1.353-1.670]) were observed in the COVID-19 survivors (85). Atrial fibrillation and flutter (H.R. = 2.407 [2.296-2.523]), tachycardia (H.R. = 1.682 [1.626-1.740]), bradycardia (H.R. = 1.599 [1.521-1.681]), and ventricular arrhythmias (H.R. = 1.600 [1.535-1.668]) were also associated with elevated risks. In particular, myocarditis (H.R. = 4.406 [2.890-6.716]) and pericarditis (H.R. = 1.621 [1.452-1.810]) were more common among COVID-19 survivors. Ischemic heart disease (I.H.D.), including angina (H.R. = 1.707 [1.545-1.885]), acute coronary syndrome (H.R. = 2.048 [1.752-2.393]), myocardial infarction (H.R. = 1.979 [1.831-2.138]), and ischemic cardiomyopathy (H.R. = 2.811 [2.477-3.190]). The mortality rate in the COVID-19 group was higher than in the control group (H.R. = 1.604 [1.510–1.703]).
7. Long-Term Cardiovascular Results with Covid-19 Pearls:
1. The care of the post-COVID syndrome necessitates comprehensive methods instead of organ- or disease-specific ones.
2. For those under the age of 70 and members of ethnic minority groups, the risk rise was more pronounced.
3. The primary target population for long-term recovery intervention is those with significantly reduced pulmonary diffusion capabilities and aberrant chest imaging symptoms.
4. A significant percentage mentioned joint pain, chest pain, fatigue, and dyspnoea.
5. Cerebrovascular disorders, dysrhythmias, pericardial or heart inflammation, ischemic heart disease, atrial fibrillation and flutter, tachycardia, myocardial infarction, pulmonary embolism, and thromboembolic diseases were the CVDs that were documented.
6. The higher chance of event aftereffects should be considered when organizing healthcare.
7. These results imply that cardiovascular health and illness should be part of care pathways, even for individuals who do not need to be hospitalized.
8. A concerted, long-term, worldwide response plan will be necessary to meet the difficulties of COVID-19.
8. Genetics of COVID19-induced heart complications:
Molecular biology and genetics play a role in every physiological function or pathological condition. Many recent reports have been published regarding the molecular genetic basis of cardiovascular complications in persons infected with COVID-19.
In addition to adversely affecting the respiratory system, the COVID-19 pandemic also adversely affects the cardiovascular system (86). Cardiovascular disorders can be caused by atypical gene expression in vascular endothelial cells and cardiomyocytes, which are crucial in regulating heart function. Numerous genes were found to be differentially expressed in cardiomyocytes and vascular endothelial cells of COVID-19-infected heart patients as reported by Xu et al., including MALAT1, CD36, LARGE1, RYR2, PLCG2 (in cardiomyocytes), MALAT1, ID1, ID3, MT-CO1 and EGFL7 (in vascular endothelial cells) (87). These genes regulate many vital functions in these cardiac cells (88-96, 97-103). The discovery of these novel contributors of COVID-19 mediated cardiac pathophysiology may help identify novel therapeutic targets. Luo et al. recalled HSP90AA1, HSPA9, and SRSF1 regulated by Mir-16-5p and KLF9 transcription factors as hub genes involved in the co-pathogenesis of ischemic cardiomyopathy (I.C.M.) and COVID-19, and they demonstrated that the co-pathogenesis of I.C.M. and COVID-19 may be associated to angiogenesis (104). It was hypothesized that vindesine and ON-01910 may act as possible therapeutic agents. The results of their research will add to a more in-depth knowledge of the correlation between I.C.M. and COVID-19. In a similar study carried out by Qi et al., V.E.G.F.A., FOXO1, CXCR4, and SMAD4 were found to be upregulated hub genes in COVID19-induced cardiomyopathy, whereas downregulated hub genes included K.R.A.S. and T.X.N. (105). Liu et al. have successfully identified some hub genes in ischemic stroke induced by COVID-19.
The identified gene list included IL1R2 (interleukin 1 receptor type 2), NCR3 (natural cytotoxicity triggering receptor 3), OLR1 (oxidized low-density lipoprotein receptor 1), IL18R1 (interleukin 18 receptor 1), and JAK2 (Janus kinase 2). Out of these identified hub genes, JAK2 is a potential biomarker and could be used to develop novel therapeutics. (106).
Some recent reports about the role of specific genes and micro-RNAs in COVID-19-inducing heart failure (H.F.). Gao et al. reported upregulation of OAS1, OAS2, OAS3, and O.A.S.L. genes in cardiomyocytes from heart failure patients infected with SARS-CoV-2 infection (107). These genes belong to the OAS-gene family, contributing to antiviral and innate immune responses (108). The authors found differential expression of micro-RNAs associated with these genes, including hsa-miR-15a-3p, hsa-miR-23a-5p, hsa-miR-26b-5p, hsa-miR-186-3p, hsa-miR-4433a-3p, hsa-miR-548aq-5p, hsa-miR-548d-5p, hsa-miR-576-5p, hsa-miR-580-3p, and hsa-miR-6850-5p (107). Furthermore, Estradiol was identified to be the common molecule that regulates the four O.A.S. genes. Estradiol's ability to treat severe heart failure is mediated by the classical estrogen receptor beta (E.R.) in the heart (109). In pulmonary hypertension, estradiol protects right ventricular function via BMPR2 and apelin, according to Frump et al.. (110). These results demonstrate that Estradiol has a cardioprotective effect, even on the COVID-19 heart, and therefore can help treat patients infected with COVID-19 suffering from heart failure and other cardiac diseases COVID-19.
New insights into the effect of the COVID-19 genes on cardiac stem cells have revealed some fascinating findings. Liu et al. have reported that COVID-19 genes (Nsp6, Nsp8, and M) severely damage human pluripotent stem cell-derived cardiomyocytes, emphasizing the importance of ATP homeostasis in cardiomyocyte mortality and functional abnormalities caused by these SARS-CoV-2 genes (111). They found that hESC-CMs, overexpression of SARS-CoV-2 viral genes promoted cell death/apoptosis-associated gene expression and upregulated Nsp6, Nsp8, and M hESC-CM proteins caused cardiac fibrosis, arrhythmia, inflammation, and heart failure. These findings show that Nsp6, Nsp8, and M may reduce cellular ATP generation and trigger apoptosis in hPSC-CMs. They reported that FDA-approved antiparasitic and antiemetic drugs ivermectin and meclizine can alleviate this effect. Despite its main application in treating parasite diseases, ivermectin has been shown to preserve mitochondrial ATP in C.M.s by upregulating Cox6a2 transcription in HL-1 CMs (112). However, meclizine promotes CM glycolysis (113), which increases ATP production, reduces ATP depletion, and protects mitochondrial function (114-115). The abovementioned findings suggest that ivermectin and meclizine may boost ATP levels to reduce SARS-CoV-2-induced cell death in hPSC-CMs, revealing that ivermectin and meclizine can help treat cardiac patients infected with COVID-19. Similarly, Wu et al in very recent findings have elucidated the molecular biomarkers and genetic pathogenesis of COVID-19-induced ischemic heart failure (116).
Moreover, all these recent findings show how deep insights into COVID-19-induced cardiac diseases using state-of-the-art molecular and cell biological techniques can help unravel its pathogenesis and help find novel potential therapeutic agents for patient-tailored treatment of COVID-19-associated cardiovascular diseases that is a major focus of personalized medicine in 21st century (86,116,117).
Conclusions
Ultimately, the relationship between COVID-19 and CV risk is complex and multifaceted. While COVID-19 primarily affects the respiratory system, it can also significantly affect CV health. Emerging evidence suggests that COVID-19 survivors may experience long-term CV effects. These include persistent myocardial inflammation, myocardial fibrosis, cardiac dysfunction, and an increased risk of future CV events. Moreover, recent reports show the involvement of a large number of genomic alterations, microRNAs, and novel viral as well as host proteins in different types of cardiocytes, which has helped identify some novel drug targets to treat COVID-19-related cardiovascular complications. Long-term follow-up studies are ongoing and demanded to understand these underlying effects better, to identify novel molecular biomarkers of different cardiovascular diseases, and to find novel therapeutic agents for personalized treatment of resulting clinical complications.
Funding
The study was approved by the Institutional Review Board (IRB) of King Abdullah International Medical Research Center (KAIMRC) (NRA22A/012/05) which serves as the Ethical Review Board of the participating centers.
Conflicts of Interest
The authors have declared that no competing interests exist.
References
- Xie, Y., Xu, E., Bowe, B. et al. Long-term cardiovascular outcomes of COVID-19. Nat Med, 2022; 28, 583–590. [CrossRef]
- Terzic CM, Medina-Inojosa BJ. Cardiovascular Complications of Coronavirus Disease-2019. Phys Med Rehabil Clin N Am. 2023, 34, 551–561. [CrossRef]
- Chen, C., Wang, J., Liu, YM. et al. Single-cell analysis of adult human heart across healthy and cardiovascular disease patients reveals the cellular landscape underlying SARS-CoV-2 invasion of myocardial tissue through ACE2. J Transl Med, 2023; 358.
- Ferreira, V.M., Plein, S., Wong, T.C. et al. Cardiovascular magnetic resonance for evaluation of cardiac involvement in COVID-19: recommendations by the Society for Cardiovascular Magnetic Resonance. J Cardiovasc Magn Reson, 2023; 25, 21.
- Palmisano, A., Vignale, D., Bruno, E., et al., A. Cardiac magnetic resonance findings in acute and post-acute COVID-19 patients with suspected myocarditis. Journal of clinical ultrasound. Journal of clinical ultrasound : J.C.U, 2023; 51, 613–621.
- Yugar-Toledo, J. C., Yugar, L. B. T., Sedenho-Prado, L. G., Schreiber, R., & Moreno, H. Pathophysiological effects of SARS-CoV-2 infection on the cardiovascular system and its clinical manifestations-a mini review. Frontiers in cardiovascular medicine 2023, 10, 1162837 ().
- Al-Kindi S, Zidar DA. COVID-lateral damage: cardiovascular manifestations of SARS-CoV-2 infection. Transl Res. 2022, 241, 25–40. [CrossRef]
- Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. Circulation. 2007, 116, 2634–2653. [CrossRef]
- Thygesen K, Alpert JS, Jaffe AS, et al. Fourth Universal Definition of Myocardial Infarction (2018) [published correction appears in Circulation. Circulation. 2018, 138, e618–e651. [CrossRef]
- Altaweel M, AlMukhaylid S, AlAnazi F, AlRammadan A, Altuwaim I. Type II myocardial infarction: What do we know? Int J Innov Res Med Sci. 2022, 7, 298–300. [CrossRef]
- Centers for Disease Control and Prevention. COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/index.html. (accessed on 22 July 2023).
- World Health Organization. Coronavirus disease (COVID-19) pandemic. Accessed Jul 22, 2023. https://www.who.int/emergencies/disease/novel-coronavirus-2019.
-
World Health Organization. (n.d.). Coronavirus. World Health Organization. https://www.who.int/health-topics/coronavirus#tab=tab_1
.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [published correction appears in Lancet. 2020 Jan 30;:]. Lancet. 2020, 395, 497–506. [CrossRef]
- Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020, 395, 507–513. [CrossRef]
- Bing Yu, Yalin Wu and Xiaosu Song et al. Possible Mechanisms of SARS-CoV2-Mediated Myocardial Injury. CVIA, 2023; 8. [CrossRef]
- Beyerstedt S, Casaro EB, Rangel ÉB. COVID-19: angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur J Clin Microbiol Infect Dis. 2021, 40, 905–919. [CrossRef]
- Imazio M, Klingel K, Kindermann I, et al. COVID-19 pandemic and troponin: indirect myocardial injury, myocardial inflammation, or myocarditis? Heart. 2020, 106, 1127–1131. [CrossRef]
- Shi S, Qin M, Shen B, et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. J.A.M.A. Cardiol. 2020, 5, 802–810. [CrossRef]
- Sandoval Y, Januzzi JL Jr, Jaffe AS. Cardiac Troponin for Assessment of Myocardial Injury in COVID-19: J.A.C.C. Review Topic of the Week. J Am Coll Cardiol. 2020, 76, 1244–1258. [CrossRef]
- Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China [published correction appears in J.A.M.A. 2021 Mar 16;325(11):1113]. J.A.M.A. 2020, 323, 1061–1069. [CrossRef]
- Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential Effects of Coronaviruses on the Cardiovascular System: A Review. J.A.M.A. Cardiol. 2020, 5, 831–840. [CrossRef]
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study [published correction appears in Lancet. 2020 Mar 28;395(10229):1038] [published correction appears in Lancet. Lancet. 2020, 395, 1054–1062. [CrossRef]
- Li B, Yang J, Zhao F, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020, 109, 531–538. [CrossRef]
- Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study [published correction appears in B.M.J. 2020 Mar 31;368:m1295]. B.M.J. 2020, 368, m1091 Published 2020 Mar 26. [CrossRef]
- Al-Wahaibi K, Al-Wahshi Y, Mohamed Elfadil O. Myocardial Injury Is Associated with Higher Morbidity and Mortality in Patients with 2019 Novel Coronavirus Disease (COVID-19). SN Compr Clin Med. 2020, 2, 2514–2520. [CrossRef]
- hang X, Yu J, Pan LY, Jiang HY. ACEI/ARB use and risk of infection or severity or mortality of COVID-19: A systematic review and meta-analysis. Pharmacol Res. 2020, 158, 104927. [CrossRef]
- Ma J, Shi X, Yu J, et al. Association of ACEi/A.R.B. Use and Clinical Outcomes of COVID-19 Patients With Hypertension. Front Cardiovasc Med. 2021, 8, 577398. [CrossRef]
- Guo L, Ren L, Yang S, et al. Profiling Early Humoral Response to Diagnose Novel Coronavirus Disease (COVID-19). Clin Infect Dis. 2020, 71, 778–785. [CrossRef] [PubMed]
- Giustino G, Pinney SP, Lala A, et al. Coronavirus and Cardiovascular Disease, Myocardial Injury, and Arrhythmia: J.A.C.C. Focus Seminar. J Am Coll Cardiol. 2020, 76, 2011–2023. [CrossRef] [PubMed]
- Shi Y, Yu X, Zhao H, Wang H, Zhao R, Sheng J. Host susceptibility to severe COVID-19 and establishment of a host risk score: findings of 487 cases outside Wuha. Crit Care. 2020, 24, 108. [CrossRef] [PubMed]
- Guglin M, Ballut K, Ilonze O, Jones M, Rao R. Clinical variants of myocardial involvement in COVID-19-positive patients: a cumulative experience of 2020. Heart Fail Rev. 2022, 27, 1341–1353. [Google Scholar] [CrossRef] [PubMed]
- Amin HZ, Amin LZ, Pradipta A. Takotsubo Cardiomyopathy: A Brief Review. J Med Life. 2020, 13, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19) [published correction appears in J.A.M.A. Cardiol. 2020 Jul 1;5(7):848]. J.A.M.A. Cardiol. 2020, 5, 811–818. [CrossRef] [PubMed]
- Pelle MC, Zaffina I, Lucà S, et al. Endothelial Dysfunction in COVID-19: Potential Mechanisms and Possible Therapeutic Options. Life (Basel). 2022, 12, 1605 Published 2022 Oct 14. [CrossRef]
- Trachtenberg BH, Hare JM. Inflammatory Cardiomyopathic Syndromes. Circ Res. 2017, 121, 803–818. [CrossRef] [PubMed]
- Lu L, Liu M, Sun R, Zheng Y, Zhang P. Myocardial Infarction: Symptoms and Treatments. Cell Biochem Biophys. 2015, 72, 865–867. [CrossRef]
- Azevedo RB, Botelho BG, Hollanda JVG, et al. Covid-19 and the cardiovascular system: a comprehensive review. J Hum Hypertens. 2021, 35, 4–11. [CrossRef]
- Ferry AV, Anand A, Strachan FE, et al. Presenting Symptoms in Men and Women Diagnosed With Myocardial Infarction Using Sex-Specific Criteria. J Am Heart Assoc. 2019, 8, e012307. [CrossRef]
- Del Prete A, Conway F, Della Rocca DG, et al. COVID-19, Acute Myocardial Injury, and Infarction. Card Electrophysiol Clin. 2022, 14, 29–39. [CrossRef]
- Hothi SS, Jiang J, Steeds RP, Moody WE. Utility of Noninvasive Cardiac Imaging Assessment in Coronavirus Disease 2019. Front Cardiovasc Med. 2021, 8, 663864 Published 2021 May 21. [CrossRef]
- Dawson D, Dominic P, Sheth A, Modi M. Prognostic value of Cardiac Biomarkers in COVID-19 Infection: A Meta-analysis. Preprint. Res Sq 2020, rs.3, rs-34729. [CrossRef]
- Badawi A, Ryoo S.G. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int J Infect Dis. 2016, 49, 129–133. [CrossRef]
- Inciardi RM, Lupi L, Zaccone G, et al. Cardiac Involvement in a Patient With Coronavirus Disease 2019 (COVID-19). J.A.M.A. Cardiol. 2020, 5, 819–824. [CrossRef]
- Chang D, Saleh M, Gabriels J, et al. Inpatient Use of Ambulatory Telemetry Monitors for COVID-19 Patients Treated With Hydroxychloroquine and/or Azithromycin. J Am Coll Cardiol. 2020, 75, 2992–2993. [CrossRef]
- Ramireddy A, Chugh H, Reinier K, et al. Experience With Hydroxychloroquine and Azithromycin in the Coronavirus Disease 2019 Pandemic: Implications for Q.T. Interval Monitoring. J Am Heart Assoc. 2020, 9, e017144. [CrossRef]
- Popescu BA, Andrade MJ, Badano LP, et al. European Association of Echocardiography recommendations for training, competence, and quality improvement in echocardiography. Eur J Echocardiogr. 2009, 10, 893–905. [CrossRef]
- Cameli M, Pastore MC, Soliman Aboumarie H, et al. The usefulness of echocardiography to detect cardiac involvement in COVID-19 patients. Echocardiography. 2020, 37, 1278–1286. [CrossRef]
- Skulstad H, Cosyns B, Popescu BA, et al. COVID-19 pandemic and cardiac imaging: E.A.C.V.I. recommendations on precautions, indications, prioritization, and protection for patients and healthcare personnel. Eur Heart J Cardiovasc Imaging. 2020, 21, 592–598. [CrossRef] [PubMed]
- Kirkpatrick JN, Mitchell C, Taub C, Kort S, Hung J, Swaminathan M. A.S.E. Statement on Protection of Patients and Echocardiography Service Providers During the 2019 Novel Coronavirus Outbreak: Endorsed by the American College of Cardiology. J Am Soc Echocardiogr. 2020, 33, 648–653. [CrossRef] [PubMed]
- COVID-19 clinical guidance for the cardiovascular care team. Acc.org. Accessed August 19, 2023. https://www.acc.org/~/media/665AFA1E710B4B3293138D14BE8D1213.pdf. 19 August.
- Allen BD, Wong TC, Bucciarelli-Ducci C, et al. Society for Cardiovascular Magnetic Resonance (S.C.M.R.) guidance for re-activation of cardiovascular magnetic resonance practice after peak phase of the COVID-19 pandemic. J Cardiovasc Magn Reson. 2020, 22, 58. [CrossRef] [PubMed]
- Richardson P, McKenna W, Bristow M, et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation. 1996, 93, 841–842. [CrossRef]
- Yilmaz A, Klingel K, Kandolf R, Sechtem U. Imaging in inflammatory heart disease: from the past to current clinical practice. Hellenic J Cardiol 2009, 50, 449–460.
- Lee SP, Im HJ, Kang S, et al. Noninvasive Imaging of Myocardial Inflammation in Myocarditis using 68Ga-tagged Mannosylated Human Serum Albumin Positron Emission Tomography. Theranostics. 2017, 7, 413–427. [CrossRef]
- Cau R, Bassareo P, Saba L. Cardiac Involvement in COVID-19-Assessment with Echocardiography and Cardiac Magnetic Resonance Imaging. SN Compr Clin Med. 2020, 2, 845–851. [CrossRef] [PubMed]
- Han Y, Chen T, Bryant J, et al. Society for Cardiovascular Magnetic Resonance (S.C.M.R.) guidance for the practice of cardiovascular magnetic resonance during the COVID-19 pandemic. J Cardiovasc Magn Reson. 2020, 22, 26. [CrossRef]
- Tavakol M, Ashraf S, Brener SJ. Risks and complications of coronary angiography: a comprehensive review. Glob J Health Sci. 2012, 4, 65–93. [CrossRef]
- Coy KM, Maryniak A, Blankespoor T, Stys A. Approach to high intracoronary thrombus burden in the era of COVID-19. BMJ Case Rep. 2021, 14, e246223. [CrossRef]
- Liori S, Pappas C, Rallidis L. ST-elevation myocardial infarction in a 39-year-old patient with "normal" coronary arteries as a thrombotic complication of COVID-19. J Cardiol Cases. 2022, 25, 335–337. [CrossRef]
- Piccolo R, Esposito G. Percutaneous coronary intervention in patients with COVID-19 and acute coronary syndrome: What if the old normal became the new normal? Catheter Cardiovasc Interv. 2021, 97, 199–200. [CrossRef] [PubMed]
- Xie Q, Zhou L, Li Y, et al. Comparison of prognosis between coronary computed tomography angiography versus invasive coronary angiography for stable coronary artery disease: a systematic review and meta-analysis. Front Cardiovasc Med. 2023, 10, 1010536. [CrossRef]
- Inam F, Singh PR, Khalid F, Javed A, Shah AR. Acute Coronary Syndrome and COVID-19: A Case Report of Refractory Hypercoagulability. Cureus. 2021, 13, e13675. [CrossRef] [PubMed]
- Cronin M, Wheen P, Armstrong R, et al. C.T. coronary angiography and COVID-19: inpatient use in acute chest pain service. Open Heart. 2021, 8, e001548. [CrossRef]
- Panjer M, Dobrolinska M, Wagenaar NRL, Slart RHJA. Diagnostic accuracy of dynamic CZT-SPECT in coronary artery disease. A systematic review and meta-analysis. J Nucl Cardiol. 2022, 29, 1686–1697. [CrossRef] [PubMed]
- Guo T, Fan Y, Chen M, et al. Cardiovascular Implications of Fatal Outcomes of Patients with Coronavirus Disease 2019 (COVID-19). J.A.M.A. Cardiol. 2020, 5, 811–818. [CrossRef]
- Driggin E, Madhavan MV, Bikdeli B, et al. Cardiovascular Considerations for Patients, Health Care Workers, and Health Systems During the COVID-19 Pandemic. J Am Coll Cardiol. 2020, 75, 2352–2371. [CrossRef]
- Liu K, Fang YY, Deng Y, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl). 2020, 133, 1025–1031. [CrossRef]
- Satterfield BA, Bhatt DL, Gersh BJ. Publisher Correction: Cardiac involvement in the long-term implications of COVID-19. Nat Rev Cardiol. 2022, 19, 342. [CrossRef]
- Lavelle MP, Desai AD, Wan EY. Arrhythmias in the COVID-19 patient. Heart Rhythm O2. 2022, 3, 8–14. [CrossRef] [PubMed]
- Peretto G, Sala S, Rizzo S, et al. Arrhythmias in myocarditis: State of the art. Heart Rhythm. 2019, 16, 793–801. [CrossRef]
- Chen L, Li X, Chen M, Feng Y, Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2 [published correction appears in Cardiovasc Res. 2020 Oct 1;116(12):1994]. Cardiovasc Res. 2020, 116, 1097–1100. [CrossRef] [PubMed]
- Koudelka M, Sovová E. COVID-19 Causing Hypotension in Frail Geriatric Hypertensive Patients? Medicina (Kaunas). 2021, 57, 633. [CrossRef] [PubMed]
- Forte G, De Pascalis V, Favieri F, Casagrande M. Effects of Blood Pressure on Cognitive Performance: A Systematic Review. J Clin Med. 2019, 9, 34. [CrossRef]
- Shirazi S, Mami S, Mohtadi N, et al. Sudden cardiac death in COVID-19 patients, a report of three cases. Future Cardiol. 2021, 17, 113–118. [CrossRef]
- Beri A, Kotak K. Cardiac injury, arrhythmia, and sudden death in a COVID-19 patient. HeartRhythm Case Rep. 2020, 6, 367–369. [CrossRef]
- Yadav R, Bansal R, Budakoty S, Barwad P. COVID-19 and sudden cardiac death: A new potential risk. Indian Heart J. 2020, 72, 333–336. [CrossRef] [PubMed]
- Yang C, Jin Z. An Acute Respiratory Infection Runs Into the Most Common Noncommunicable Epidemic—COVID-19 and Cardiovascular Diseases. J.A.M.A. Cardiol. 2020, 5, 743–744. [CrossRef]
- Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021, 594, 259–264. [CrossRef]
- Ayoubkhani D, Khunti K, Nafilyan V, et al. Post-covid syndrome in individuals admitted to hospital with covid-19: retrospective cohort study. B.M.J. 2021, 372, n693. [CrossRef]
- Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021, 397, 220–232. [CrossRef] [PubMed]
- Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent Symptoms in Patients After Acute COVID-19. J.A.M.A. 2020, 324, 603–605. [CrossRef] [PubMed]
- Daugherty SE, Guo Y, Heath K, et al. Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: retrospective cohort study. B.M.J. 2021, 373, n1098 Published 2021 May 19. [CrossRef]
- Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022, 28, 583–590. [CrossRef] [PubMed]
- Wang W, Wang CY, Wang SI, Wei JC. Long-term cardiovascular outcomes in COVID-19 survivors among non-vaccinated population: A retrospective cohort study from the TriNetX US collaborative networks [published correction appears in EClinical Medicine. E Clinical Medicine 2023, 59, 101968. [CrossRef]
- Wang W, Yang J, Kang P, Bai J, Feng X, Huang L, Zhang Y, Wu Y, Tang B, Wang H, Jiang J, Li M, Zhao B, Yang X. Direct Infection of SARS-CoV-2 in Human iPSC-Derived 3D Cardiac Organoids Recapitulates COVID-19 Myocarditis. Virol Sin 2023, S1995-820X(23)00113-X. [CrossRef] [PubMed]
- Xu Y, Ma Q, Ren J, Chen L, Guo W, Feng K, Zeng Z, Huang T, Cai Y. Using Machine Learning Methods in Identifying Genes Associated with COVID-19 in Cardiomyocytes and Cardiac Vascular Endothelial Cells. Life (Basel). 2023, 13, 1011. [CrossRef]
- Zhang M., Gu H., Xu W., Zhou X. Down-regulation of lncrna malat1 reduces cardiomyocyte apoptosis and improves left ventricular function in diabetic rats. Int. J. Cardiol. 2016, 203, 214–216. [CrossRef]
- Hu H., Wu J., Yu X., Zhou J., Yu H., Ma L. Long non-coding rna malat1 enhances the apoptosis of cardiomyocytes through autophagy inhibition by regulating tsc2-mtor signaling. Biol. Res. 2019, 52, 58. [CrossRef]
- Puthanveetil P., Chen S., Feng B., Gautam A., Chakrabarti S. Long non-coding rna malat1 regulates hyperglycaemia induced inflammatory process in the endothelial cells. J. Cell. Mol. Med. 2015, 19, 1418–1425. [CrossRef]
- Chen F., Li W., Zhang D., Fu Y., Yuan W., Luo G., Liu F., Luo J. Malat1 regulates hypertrophy of cardiomyocytes by modulating the mir-181a/hmgb2 pathway. Eur. J. Histochem. 2022, 66, 3426. [CrossRef] [PubMed]
- Glatz J.F.C., Wang F., Nabben M., Luiken J.J.F.P. Cd36 as a target for metabolic modulation therapy in cardiac disease. Expert Opin. Ther. Targets. 2021, 25, 393–400. [CrossRef]
- Zeng Z., Huang N., Zhang Y., Wang Y., Su Y., Zhang H., An Y. Ctcf inhibits endoplasmic reticulum stress and apoptosis in cardiomyocytes by upregulating ryr2 via inhibiting s100a1. Life Sci. 2020, 242, 117158. [CrossRef] [PubMed]
- Kato T., Yamamoto T., Nakamura Y., Nanno T., Fukui G., Sufu Y., Hamada Y., Maeda T., Nishimura S., Ishiguchi H., et al. Correction of impaired calmodulin binding to ryr2 as a novel therapy for lethal arrhythmia in the pressure-overloaded heart failure. Heart Rhythm. 2017, 14, 120–127. [CrossRef] [PubMed]
- Acimovic I., Refaat M.M., Moreau A., Salykin A., Reiken S., Sleiman Y., Souidi M., Pribyl J., Kajava A.V., Richard S., et al. Post-translational modifications and diastolic calcium leak associated to the novel ryr2-d3638a mutation lead to cpvt in patient-specific hipsc-derived cardiomyocytes. J. Clin. Med. 2018, 7, 423. [CrossRef]
- Liang X. Investigating the protective contribution of plcg2 p522r variant in microglia-mediated immune pathways in Alzheimer's disease. Alzheimer’s Dement. 2022, 18, e062813. [CrossRef]
- Cunningham T.J., Yu M.S., McKeithan W.L., Spiering S., Carrette F., Huang C.T., Bushway P.J., Tierney M., Albini S., Giacca M., et al. Id genes are essential for early heart formation. 2017, 31, 1325–1338. [CrossRef]
- Kong D., He M., Yang L., Zhou R., Yan Y.-Q., Liang Y., Teng C.-B. Mir-17 and mir-19 cooperatively promote skeletal muscle cell differentiation. Cell. Mol. Life Sci. 2019, 76, 5041–5054. [CrossRef]
- Luo Y., Wang G., Ren T., Zhang T., Chen H., Li Y., Yin X., Zhang Z., Sun Y. Screening of host genes regulated by id1 and id3 proteins during foot-and-mouth disease virus infection. Virus Res. 2021, 306, 198597. [CrossRef]
- Pattarabanjird T., Cress C., Nguyen A., Taylor A., Bekiranov S., McNamara C. A machine learning model utilizing a novel snp shows enhanced prediction of coronary artery disease severity. Genes. 2020, 11, 1446. [CrossRef] [PubMed]
- Zhong, D. , Jia-wei Z., Yan-an W. Current research progress of egfl7 in angiogenesis regulation. China J. Oral. Maxillofac. Surg. 2019, 17, 377–content content. [Google Scholar]
- Masoud, A.G. , Lin J., Azad A.K., Farhan M.A., Fischer C., Zhu L.F., Zhang H., Sis B., Kassiri Z., Moore R.B. Apelin directs endothelial cell differentiation and vascular repair following immune-mediated injury. J. Clin. Investig. 2020, 130, 94–107. [Google Scholar] [CrossRef]
- Sezer Zhmurov, C. , Timirci-Kahraman O., Amadou F.Z., Fazliogullari O., Basaran C., Catal T., Zeybek U., Bermek H. Expression of egfl7 and mirna-126-5p in symptomatic carotid artery disease. Genet. Test. Mol. Biomark. 2016, 20, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Luo S, Zhang X, Xiao X, Luo W, Yang Z, Tang S, Huang W. Exploring Potential Biomarkers and Molecular Mechanisms of Ischemic Cardiomyopathy and COVID-19 Comorbidity Based on Bioinformatics and Systems Biology. Int J Mol Sci. 2023, 24, 6511. [Google Scholar] [CrossRef] [PubMed]
- Qi P, Huang M, Zhu H. Exploring potential biomarkers and therapeutic targets of long COVID-associated inflammatory cardiomyopathy. Front Med (Lausanne). 2023, 10, 1191354. [Google Scholar] [CrossRef]
- Liu W, Han F, Wan M, Yang XZ. Integrated bioinformatics analysis identifies shared immune changes between ischemic stroke and COVID 19. Front Immunol. 2023, 14, 1102281. [Google Scholar] [CrossRef]
- Gao LJ, He ZM, Li YY, Yang RR, Yan M, Shang X, Cao JM. Role of O.A.S. gene family in COVID-19 induced heart failure. J Transl Med. 2023, 21, 212. [Google Scholar] [CrossRef]
- Boroujeni ME, Simani L, Bluyssen HAR, Samadikhah HR, Zamanlui Benisi S, Hassani S, et al. Inflammatory response leads to neuronal death in human post-mortem cerebral cortex in patients with COVID-19. A.C.S. Chem Neurosci. 2021, 12, 2143–2150. [Google Scholar] [CrossRef]
- Iorga A, Umar S, Ruffenach G, Aryan L, Li J, Sharma S, et al. Estrogen rescues heart failure through estrogen receptor Beta activation. Biol Sex Differ. 2018, 9, 48. [Google Scholar] [CrossRef]
- Frump AL, Albrecht M, Yakubov B, Breuils-Bonnet S, Nadeau V, Tremblay E, et al. 17β-Estradiol and estrogen receptor α protect right ventricular function in pulmonary hypertension via BMPR2 and apelin. J Clin Invest. 2021, 131, e129433. [Google Scholar] [CrossRef]
- Liu J, Wu S, Zhang Y, Wang C, Liu S, Wan J, Yang L. SARS-CoV-2 viral genes Nsp6, Nsp8, and M compromise cellular ATP levels to impair survival and function of human pluripotent stem cell-derived cardiomyocytes. Stem Cell Res Ther. 2023, 14, 249. [CrossRef]
- Nagai H, Satomi T, Abiru A, Miyamoto K, Nagasawa K, Maruyama M, et al. Antihypertrophic effects of small molecules that maintain mitochondrial ATP levels under hypoxia. EBioMedicine. 2017, 24, 147–158. [CrossRef] [PubMed]
- Nagai H, Satomi T, Abiru A, et al. Antihypertrophic Effects of Small Molecules that Maintain Mitochondrial ATP Levels Under Hypoxia. EBioMedicine. 2017, 24, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Gohil VM, Sheth SA, Nilsson R, et al. Nutrient-sensitized screening for drugs that shift energy metabolism from mitochondrial respiration to glycolysis. Nat Biotechnol. 2010, 28, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Zhuo M, Gorgun MF, Englander EW. Augmentation of glycolytic metabolism by meclizine is indispensable for protection of dorsal root ganglion neurons from hypoxia-induced mitochondrial compromise. Free Radic Biol Med. 2016, 99, 20–31. [Google Scholar] [CrossRef]
- Wu G, Zhou J, Ren H, Qin Y, Qian D, Hu Q, Xu P, Yu T, Ma H, Chen H, He M, Shi J. Unraveling the molecular crosstalk and immune landscape between COVID-19 infections and ischemic heart failure comorbidity: New insights into diagnostic biomarkers and therapeutic approaches. Cell Signal. 2023, 28, 110909. [CrossRef]
- Bernardo L, Lomagno A, Mauri PL, Di Silvestre D. Integration of Omics Data and Network Models to Unveil Negative Aspects of SARS-CoV-2, from Pathogenic Mechanisms to Drug Repurposing. Biology (Basel). 2023, 12, 1196. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).