Submitted:
08 October 2023
Posted:
09 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Strains and plant
2.2. Gene cloning of Thfb6
2.3. Expression and purification of Thfb6 protein
2.4. Hypersensitive response assays
2.5. Analysis of callose deposition
2.6. Detection of phenolic and lignin content
2.7. Bioassay for Thfb6-induced disease resistance in tobacco TMV resistance assays
2.8. Determination of peroxidase, phenylalanine ammonia-lyase, and polyphenol oxidase activities
2.9. RT-qPCR analysis of defense-related genes induced by Thfb6
2.10. Statistical analysis
3. Results
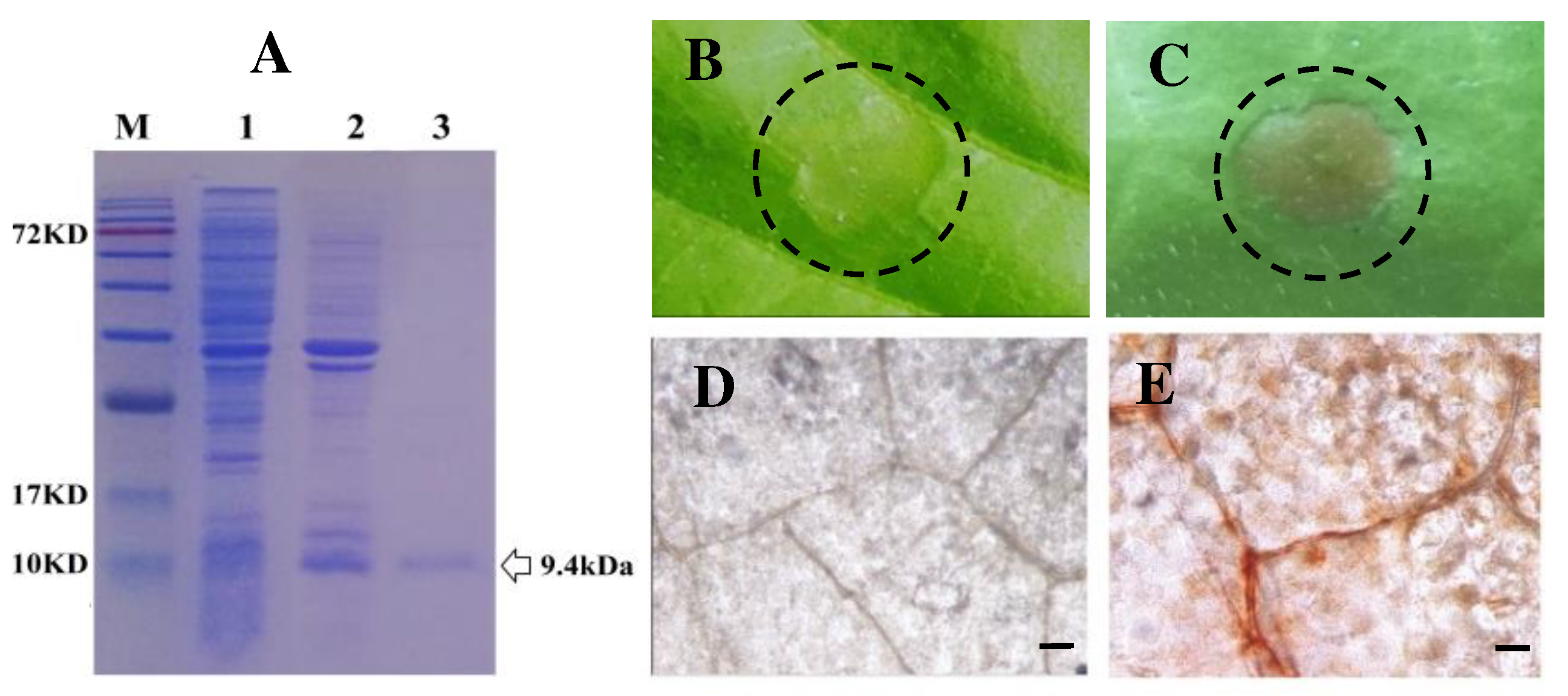
3.1. cDNA cloning of Thfb6 and expression in E. coli
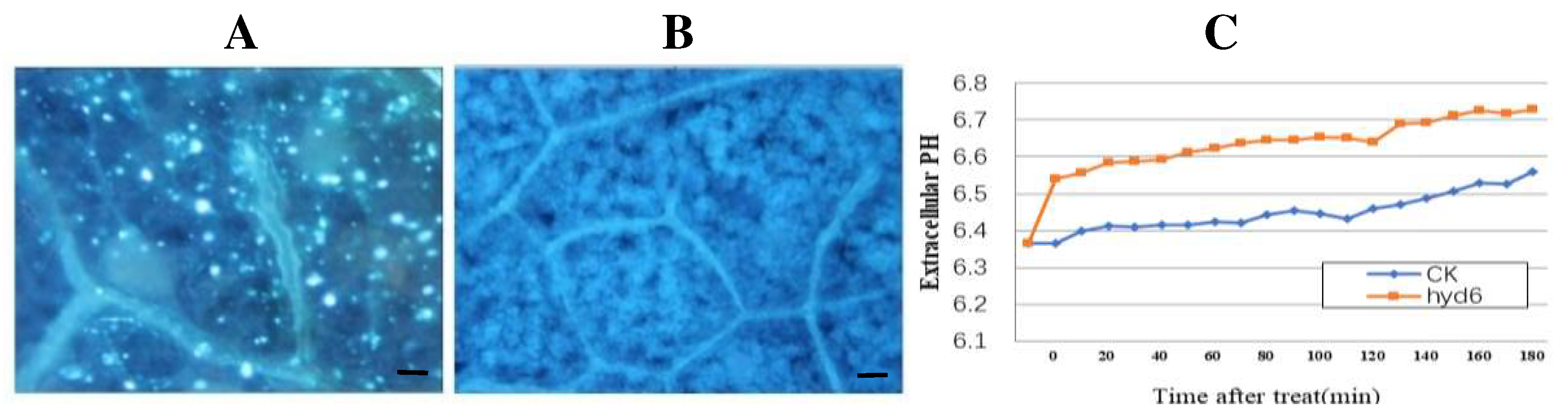
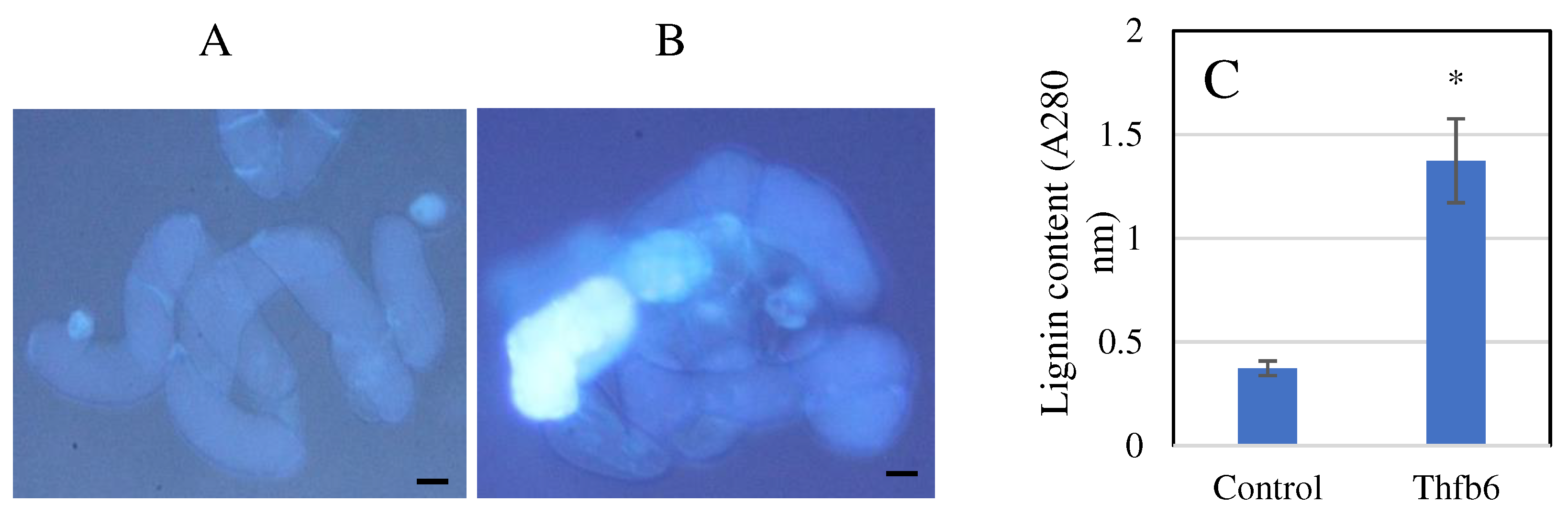
3.2. Thfb6 triggers defense responses in tobacco
3.3. Induction of TMV resistance in tobacco by Thfb6
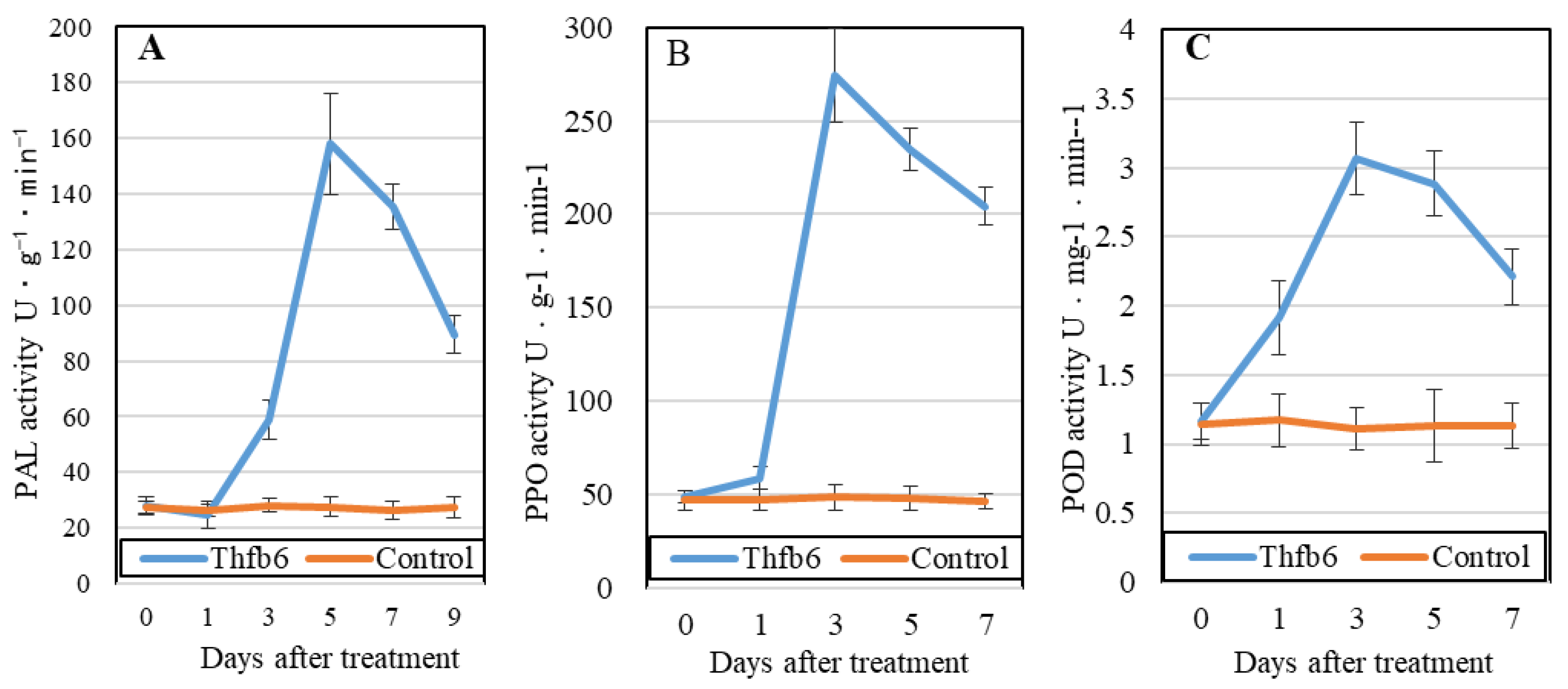
3.4. Thfb6 increased the activities of defensive enzymes PAL, POD and PPO in tobacco
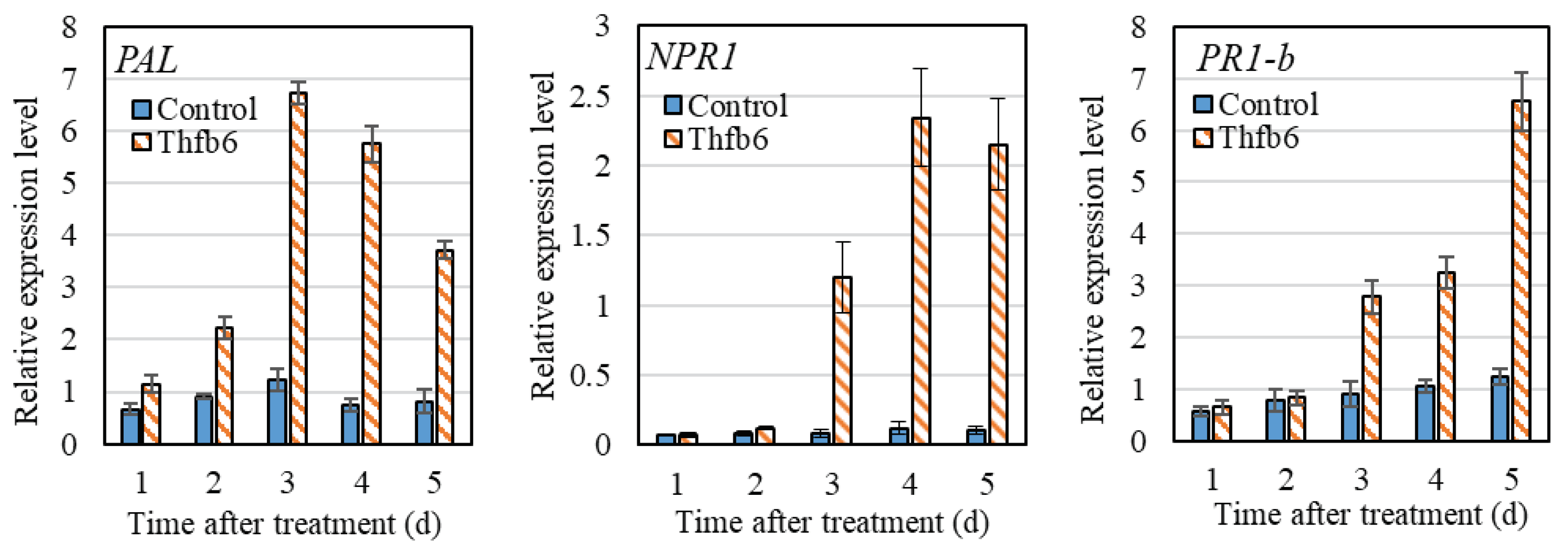
3.5. Expression of resistance-related genes induced by Thfb6
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Harman, G. E. Overview of mechanisms and uses of Trichoderma spp. Phytopathology 2006, 96, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P. K.; Horwitz, B. A.; Kenerley, C. M. Secondary metabolism in Trichoderma a genomic perspective. Microbiology 2012, 158, 35–45. [Google Scholar] [CrossRef]
- Mishra, D. S.; Prajapati, C. R.; Gupta, A. K.; Sharma, S. D. Relative bio-efficacy and shelf-life of mycelial fragments, conidia and chlamydospores of Trichoderma harzianum. Int. J. Plant Res. 2012, 25, 225–232. [Google Scholar]
- Zhang, H.; Ji, S.; Guo, R.; Zhou, C.; Wang, Y.; Fan, H.; Liu, Z. Hydrophobin HFBII-4 from Trichoderma asperellum induces antifungal resistance in poplar. Braz. J. Microbiol. 2019, 50, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, C. A.; Kloepper, J. W. Plant growth promotion by Bacillus amyloliquefaciens FZB45 depend on inoculum rate and P-related soil properties. Biol. Fert. Soils 2010, 46, 835–844. [Google Scholar] [CrossRef]
- Salas-Marina, M. A.; Silva-Flores, M. A.; Uresti-Rivera, E. E.; Castro-Longoria, E.; Herrera-Estrella, A.; Casas-Flore, S. Colonization of Arabidopsis roots by Trichoderma atroviride promotes growth and enhances systemic disease resistance through jasmonic acid/ethylene and salicylic acid pathways. Eur. J. Plant Pathol. 2011, 131, 15–26. [Google Scholar] [CrossRef]
- Cai, F.; Yu, G.; Wang, P.; Wei, Z.; Fu, L.; Shen, Q.; Chen, W. Harzianolide, a novel plant growth regulator and systemic resistance elicitor from Trichoderma harzianum. Plant Physiol. Bioch. 2013, 73, 106–113. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H. A.; Macías-Rodríguez, L.; Del-Val, E.; Larsen, J. Ecological functions of Trichoderma spp. and their secondary metabolites in the rhizosphere: interactions with plants. FEMS Microbiol. Ecol. 2016, 92, fiw036–fiw036. [Google Scholar] [CrossRef]
- Wessels, J. G. H. Hydrophobins: proteins that change the nature of the fungal surface. Adv. Microb. Physiol. 1997, 38, 1–45. [Google Scholar]
- Zhang, S.; Xia, Y.X.; Kim, B.; Keyhani, N. O. Two Hydrophobins are involved in fungal spore coat rodlet layer assembly and each play distinct roles in surface interactions, development and pathogenesis in the entomopathogenic fungus, Beauveria bassiana. Mol. Microbiol. 2011, 80, 811–826. [Google Scholar] [CrossRef]
- Whiteford, J. R.; Lacroix, H.; Talbot, N. J.; Spanu, P. D. Stage-specific cellular localisation of two hydrophobins during plant infection by the pathogenic fungus Cladosporium fulvum. Fungal Genet. Biol. 2004, 41, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Han, A. B. W.; Karin, S. Applications of hydrophobins: current state and perspectives. Appl. Microbiol. Biot. 2015, 99, 1587–1597. [Google Scholar]
- Klimes, A.; Dobinson, K. F. A hydrophobin gene, vdh1, is involved in microsclerotial development and spore viability in the plant pathogen Verticillium dahliae. Fungal Genet. Biol. 2006, 43, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Ahn, I. P.; Rho, H. S.; Lee, Y. H. Mph1, a Magnaporthe grisea hydrophobin gene, is required for fungal development and plant colonization. Mol. Microbiol. 2005, 57, 1224–1237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yan, J.; Chen, Y. The progress of hydrophobin-based drug delivery system. China Biotechnology 2023, 43, 15–25. [Google Scholar]
- Xia, Y.; Talbot, N. J. Investigating the cell biology of plant infection by the rice blast fungus Magnaporthe oryzae. Curr. Opin. Microbiol. 2016, 34, 147–153. [Google Scholar]
- Jie, D.; Jie, M.; Pei, H.; Ying, T.; Yao, L.; Xiliang, J.; Mei, L. A Gα3 subunit Thga3 positively regulates conidiation, mycoparasitism, chitinase activity and hydrophobicity of Trichoderma harzianum. AMB Express 2020, 10, 221. [Google Scholar]
- Jie, M.; Lirong, W.; Xiliang, J.; Beilei, W.; Mei, L. Functions of the C2H2 transcription factor gene thmea1 in Trichoderma harzianum under copper stress based on transcriptome analysis. BioMed. Res. Int. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Yang, X.; Li, M.; Zhang, L.; Pang, L.; Sun, q.; Jiang, X. Transcriptiome analysis of Trichoderma harzianum Th-33 in chlamydospore formation. Chinese J. Biol. Control 2015, 31, 85–89. [Google Scholar]
- Xinhong, P.; Beilei, W.; Shuaihu, Z.; Mei, L.; Xiliang, J. Transcriptome dynamics underlying chlamydospore formation in Trichoderma virens GV29-8. Front. Microbiol. 2021, 12, 654855. [Google Scholar]
- Sun, Q.; Jiang, X.; Pang, L.; Wang, L.; Li, M. Functions of thga1 gene in Trichoderma harzianum based on transcriptome analysis. BioMed. Res. Int. 2016, 2016, 8329513. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yang, X.; Zeng, H.; Liu, H.; Zhou, T.; Tan, B.; Yuan, J.; Guo, L.; Qiu, D. The purification and characterization of a novel hypersensitive-like response-inducing elicitor from Verticillium dahliae that induces resistance responses in tobacco. Appl. Microbiol. Biotechnol. 2012, 93, 191–201. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Chen, S.; Wang, J. Construction of stable infectious full-length and eGFP-tagged cDNA clones of Mirabilis crinkle mosaic virus via In-Fusion cloning. Virus Res. 2020, 286, 198039–198039. [Google Scholar] [CrossRef] [PubMed]
- D’Silva, I.; Heath, M.C. Purification and characterization of two novel hypersensitive response-inducing specific elicitors produced by the cowpea rust fungus. J. Biol. Chem. 1997, 272, 3924–3927. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zeng, H.; Liu, Z.; Yang, X.; Guo, L.; Qiu, D. Mutational analysis of the Verticillium dahliae protein elicitor PevD1 identifies distinctive regions responsible for hypersensitive response and systemic acquired resistance in tobacco. Microbiol. Res. 2014, 169, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zeng, H.; Qiu, D.; Guo, L.; Yang, X.; Shi, H.; Zhou, T. Purification and characterization of a novel hypersensitive response-inducing elicitor from Magnaporthe oryzae that triggers defense response in rice. PLoS One 2012, 7, e37654. [Google Scholar] [CrossRef]
- Yu, C.; Dou, K.; Wang, S.; Wu, Q.; Ni, M.; Zhang, T.; Lu, Z.; Tang, J.; Chen, J. Elicitor hydrophobin hyd1 interacts with ubiquilin1-like to induce maize systemic resistance. J. Integr. Plant Biol. 2019, 62, 509–526. [Google Scholar] [CrossRef]
- Jensen, B. G.; Andersen, M. R.; Pedersen, M. H.; Frisvad, J. C.; Søndergaard, Ib. Hydrophobins from Aspergillus species cannot be clearly divided into two classes. BMC Res. Notes 2010, 3, 344. [Google Scholar] [CrossRef]
- Tanaka, T.; Terauchi, Y.; Yoshimi, A.; Abe, K. Aspergillus hydrophobins: Physicochemical properties, biochemical properties, and functions in solid polymer degradation. Microorganisms 2022, 10, 1498. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, L.; Liu, S.; Zhou, J.; Wu, Y.; Feng, Z.; Zhang, Y.; Zhu, H.; Wei, F.; Feng, H. Identification and functional analysis of a novel hydrophobic protein VdHP1 from Verticillium dahlia. Microbiol. Spectr. 2022, 10, e0247821–e0247821. [Google Scholar] [CrossRef]
- Moscatiello, R.; Sello, S.; Ruocco, M.; Barbulova, A.; Cortese, E.; Nigris, S.; Baldan, B.; Chiurazzi, M.; Mariani, P.; Lorito, M.; Navazio, L. The hydrophobin HYTLO1 secreted by the biocontrol fungus Trichoderma longibrachiatum triggers a NAADP-mediated calcium signaling pathway in Lotus japonicus. Int. J. Mol. Sci. 2018, 19, e2596. [Google Scholar] [CrossRef] [PubMed]
- Viterbo, A.; Chet, I. TasHyd1, a new hydrophobin gene from the biocontrol agent T. asperellum, is involved in plant root colonization. Mol. Plant Pathol. 2006, 7, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Guzmán, P.; Alemán-Duarte, M. I.; Delaye, L.; Herrera-Estrella, A.; Olmedo-Monfil, V. Identification of effector-like proteins in Trichoderma spp. and role of a hydrophobin in the plant-fungus interaction and mycoparasitism. BMC Genet. 2017, 18, 16. [Google Scholar] [CrossRef] [PubMed]
- Shoresh, M.; Yedidia, I.; Chet, I. Involvement of jasmonic acid/ethylene signaling pathway in the systemic resistance induced in cucumber by Trichoderma asperellum T203. Phytopathology 2005, 95, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Szczech, M.; Nawrocka, J.; Felczyński, K.; Małolepsza, U.; Sobolewski, J.; Kowalska, B.; Maciorowski, R.; Jas, K.; Kancelista, A. Trichoderma atroviride, TRS25 isolate reduces downy mildew and induces systemic defense responses in cucumber in field conditions. Sci. Hortic. 2017, 224, 17–26. [Google Scholar] [CrossRef]
- Stefania, G.; Roberta, P.; Stefano, C. Selected isolates of Trichoderma gamsii induce different pathways of systemic resistance in maize upon Fusarium verticillioides challenge. Microbiol. Res. 2020, 233, 126406. [Google Scholar]
- Wang, K. D.; Borrego, E. J.; Kenerley, C. M.; Kolomiets, M. V. Oxylipins other than jasmonic acid are xylem-resident signals regulating systemic resistance induced by Trichoderma virens in Maize. Plant Cell 2020, 32, 166–185. [Google Scholar] [CrossRef]
- Martínez-Medina, A.; Fernández, I.; Sánchez-Guzmán, M. J.; Jung, S. C.; Pascual, J. A.; Pozo, M. J. Deciphering the hormonal signaling network behind the systemic resistance induced by Trichoderma harzianum in tomato. Front. Plant Sci. 2013, 4, 206. [Google Scholar] [CrossRef]
- Mathys, J.; De, Cremer, K. ; Timmermans, P.; Van, Kerckhove, S.; Lievens, B.; Vanhaecke, M.; Cammue, B. P. A.; De, Coninck, B. Genome-wide characterization of ISR induced in Arabidopsis thaliana by Trichoderma hamatum T382 against Botrytis cinerea infection. Front. Plant Sci. 2012, 3, 108. [Google Scholar] [CrossRef]
- Ojalvo, I.; Rokem, J. S.; Navon, G.; Goldberg, I. 31P NMR study of elicitor treated Ohaeeolus vulgaris cell suspension culture. Plant Physiol. 1987, 85, 716–719. [Google Scholar] [CrossRef]
- Pachten A, Barz W. Elicitor-stimulated oxidative burst and extracellular pH changes in protoplast suspensions prepared from cultured chickpea (Cicer arietinum L.) cells. J. Plant Physiol. 1999, 155, 795–797. [Google Scholar] [CrossRef]
- Bóka, K.; Orbán, N. New aspect of H2O2 signaling. Plant Signal Behav. 2007, 2, 498–500. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.; Wang, Z.; Wei, C.; Amo, A.; Ahmed, B.; Yang, X.; Zhang, X. Phenylpropanoid pathway engineering: an emerging approach towards plant defense. Pathogens 2020, 9, 312. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhu, L.; Tu, L.; Liu, L.; Yuan, D.; Jin, L.; Long, L.; Zhang, X. Lignin metabolism has a central role in the resistance of cotton to the wilt fungus Verticillium dahliae as revealed by RNA-Seq dependent transcriptional analysis and histochemistry. J. Exp. Bot. 2011, 62, 5607–5621. [Google Scholar] [CrossRef]
- Huang, J.; Gu, M.; Lai, Z.; Fan, B.; Shi, K.; Zhou, Y.H.; Yu, J.Q.; Chen, Z. Functional analysis of the Arabidopsis PAL gene family in plant growth, development, and response to environmental stress. Plant Physiol. 2010, 153, 1526–1538. [Google Scholar] [CrossRef]
- Marjamaa, K.; Kukkola, E. M.; Fagerstedt, K. V. The role of xylem class III peroxidases in lignification. J. Exp. Bot. 2009, 60, 367–376. [Google Scholar] [CrossRef]
- Chappell, J. Biochemistry and molecular biology of the isoprenoid biosynthetic pathway in plants. Annu. Rev. Plant Physiol. Mol. Biol. 1995, 46, 521–547. [Google Scholar] [CrossRef]
| Gene | Sequences 5′–3′ | Tm | GenBank accession no. |
|---|---|---|---|
| ß-Actin | Forward:TGTTATGGTTGGTATGGGTC Reverse: TCAGTAAGTAGCACAGGATG |
56°C | AB158612.1 |
| PAL | Forward:ACTCAGTGAACGACAACCC Reverse: TCAGATTAGATGGCAACCC |
56°C | AB289452.1 |
| PR-1b | Forward:TTTATGACGGCTGCTAAGG Reverse: CCTATGACAT TACCTGGAGG |
57°C | X12486.1 |
| NPR1 | Forward:CTGTATCTCTTGCTATGGCG Reverse: AATCTACTGTTGTCCTCTGT |
56°C | DQ837218.1 |
| dtp-TMV | Number of lesions | Diameter of lesions (mm) | ||||
|---|---|---|---|---|---|---|
| Control | Thfb6 | Inhibition (%) | Control | Thfb6 | Inhibition (%) | |
| 1 | 10.30 ± 2.10 | 7.10 ± 1.36 | 31.07 | 0.84 ± 0.10 | 0.66 ± 0.13 | 21.56* |
| 3 | 23.60 ± 4.12 | 15.10 ± 2.92 | 36.02** | 2.11 ± 0.15 | 1.23 ± 0.09 | 41.57** |
| 5 | 50.60 ± 5.72 | 36.83 ± 3.83 | 27.21* | 3.91 ± 0.23 | 2.30 ± 0.14 | 41.23** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




