1. Introduction
Wheat bran peel (WBP) and brewers’ spent grain (BSG) are the main by-products of wheat milling and beer manufacturing industries, respectively, most of them commonly used as livestock feed. The world average annual production amounts of WBP and BSG are estimated at around 90 million tons and 39 million tons and 39 million tons respectively [
1]. WBP is a by-product, dumped as a result of wheat processing for flour production[
2,
3]. BSG is one of the most abundant by-products the beer fermentation process, during which the unresolved residues after brewing must be filtered and discarded. Due to the low economic value of by-products, a recent study has paid attention to converting them to novel food products with higher commercial value and greater health benefits[
4].
WBP contains 5.9-6.8% of lipid, 15-20% of protein, 11-23% of starch, and 43-53% of dietary fiber, while BSG contains 5.8-6.0% of lipid, 15.3-16.2% of protein, 6.4-7.0% of moist and 46% of dietary fiber [
4,
5]. The major insoluble fiber WBP consists of lignin (5-20%), cellulose (16-30%), arabinoxylan (38-55%) and other nonstarch polysaccharides. Researcher have shown particular interest in the production of xylooligosaccharides (XOS) from xylan, which can selectively stimulate the growth of probiotic bifidogenic and lactic acid bacteria residing in the human gut[
6]. XOS are defined as oligomers composed of 2-10 xylose residues and selectively fermented. They stimulate the expansion of the limited number of species of gut microbiota, conferring health benefits to the host, are produced by hydrolysis of arabinoxylan [
7]. There are three different methods to extracting XOS: (1) enzymatic hydrolysis of the xylan-containing source; (2) isolation of xylan from suitable materials by chemical fractionation, with further enzymatic hydrolysis to supply XOS; and (3) hydrolytic degradation of xylan via steam or diluted solutions of mineral acids and alkaline[
8,
9].
Before enzymatic hydrolysis, plant materials are needed for any pretreatment, such as grinding, soaking a suitable solution, and microwave irradiation. Microwave-assisted extraction is an alternative method, of pretreatment especially the case of material extraction, because the microwaves interact with free water molecules the system, which ends up releasing active compounds from the plant part. WBPs are treated with microwave irradiation to release XOS[
10].
The significance of this research article lies in its development of a methodology for low-cost processing and purification of biologically active XOS from raw materials in to recycle a huge amount of waste in Mongolia. This study aimed to obtain XOS from industrial by-products WBP and BSG through microwave-assisted enzymatic hydrolysis.
2. Materials and methods
2.1. Materials
We conducted this survey by laboratory experimental design, in which all experiments were repeated three times, and the average values and the numerical values with standard deviations of the three measurements were expressed. During the experiment, two types of samples were gathered. The first was wheat bran peels (unprocessed waste), which is the principal waste that is discarded during the processing of wheat bran in the flour mill. The other sample was taken from a sample of brewery’s spent grain (chemically treated debris) that was thrown after brewing in a brewery, and laboratory experiments were conducted to separate XOS from arabinoxylan-rich hemicellulose-rich organic waste. WBP were purchased from the local mill company Altantaria Co., Ltd., and dried BSG was obtained from local beverage company, APU Co., Ltd. The raw materials were washed with distilled water and dried at 45ºC in an oven for two days. Dried WBP and BSG were ground in an electromotive mill and sieved through a 45-mesh sieve. The resulting fine powder was stored at room temperature for further experiments. Commercial xylanase (≥2500 units/g, recombinant, expressed in Aspergillus oryzae) was purchased from Sigma-Aldrich Co., (USA), and D-xylose, xylobiose, xylotriose, and xylotetraose were purchased from Biosynth Co., Ltd. (UK) as standards.
1.2. Biochemical analysis
The WBP was protein content determined using the Kjeldahl method [
11], and fat content was measured by weight, extracted the Soxhlet method[
12]. Moisture content was at 105 °C in the drying oven for 2h, and mineral content was determined when the samples were burned in the incinerator at 650 °C for four hours. The total carbohydrate in the oligosaccharides was estimated by means of the phenol-sulfuric acid method[
13]. Briefly, a 50 L sample of WBP was placed in a test tube; phenol (5%,0.3 ml) and concentrated sulfuric acid (1.8 ml) were added and mixed thoroughly to increase the amount of the sample to 500 L, and the mixture was cooled down to room temperature for 15 min, and the absorbance of the solution was measured 490 nm. Sugar content was calculated by referring to the graph using D-xylose (5 µg/50 ml) as a standard.
1.2. Microwave-assisted enzymatic hydrolysis
Xylooligosaccharide production was conducted using by repeated microwave-assisted enzymatic hydrolysis according to the previously described protocol[
10]. WBP and BSG powders (50 g) were soaked in distilled water (100 mL) and then mixed in a 0.6 L heat-resistant and microwave-safe pyrex flask (Guandong, ROC). The powders were then treated with microwave radiation in the convenient microwave oven (Galanz RMW 1480, China) at 200 ºC for 5 min and cooled to room temperature, after which distilled, water was added to make 10% slurry. After microwave irradiation, the slurry was cooled to room temperature, and distilled water was added to make an H2O to slurry solution ratio of 10:1 (v/w). Xylanase (≥2500 units/g, recombinant, expressed in
Aspergillus oryzae; Sigma-Aldrich) was added at rates of 0.062 g /100 g substrate, 0.125 g/ 100 g substrate, 0.25 g/ 100 g substrate, 0.5 g/ 100 g substrate, 0.75 g /100 g substrate and 1 g / 100 g substrate, and mixed for 30 min at 50°C with continuous mild stirring. The enzymatic reactions were carried out in a 55ºC water bath with an orbital shaker, set to 200 rpm, for 24 hours [
10]. To evaluate the hydrolysis, reducing sugars in the supernatant were determined with dinitrosalicylic acid DNS. After immersing the mixture in boiling water for 5 min, the enzymatic reaction was stopped and the mixture centrifuged at 9,000 rpm for 10 min, and reducing sugars were estimated with DNS by using D-xylose as a standard[
14].
1.2. Isolation of xylooligosaccharides
The supernatant of the enzymatic reaction mixture was recovered by centrifugation at 9000 rpm for 10 min. XOS was purified using the activated carbon adsorption method. Briefly, activated carbon powder was added to the supernatant at the final concentration of 10% (w/w) and incubated at room temperature on a shaker at 200 rpm for 30 minutes. Final mixtures were filtered twice through a 0.45µm mixed cellulose ester filter (SciLab, Korea) and washed with double distilled water. Oligosaccharides absorbed onto activated carbons were eluted with 50% ethanol and freeze-dried.
1.2. Scanning electron microscopy
The samples were dried and ground to a powder, fixed to an aluminum stub, and sputter coated with gold to improve the conductivity of the materials, scanned by electron microscopy (Hitachi TM-1000 Tokyo, Japan).
3. Results
3.1. Compositions of Wheat Bran Peel (WBP) and Brewers’ Spent Grain (BSG)
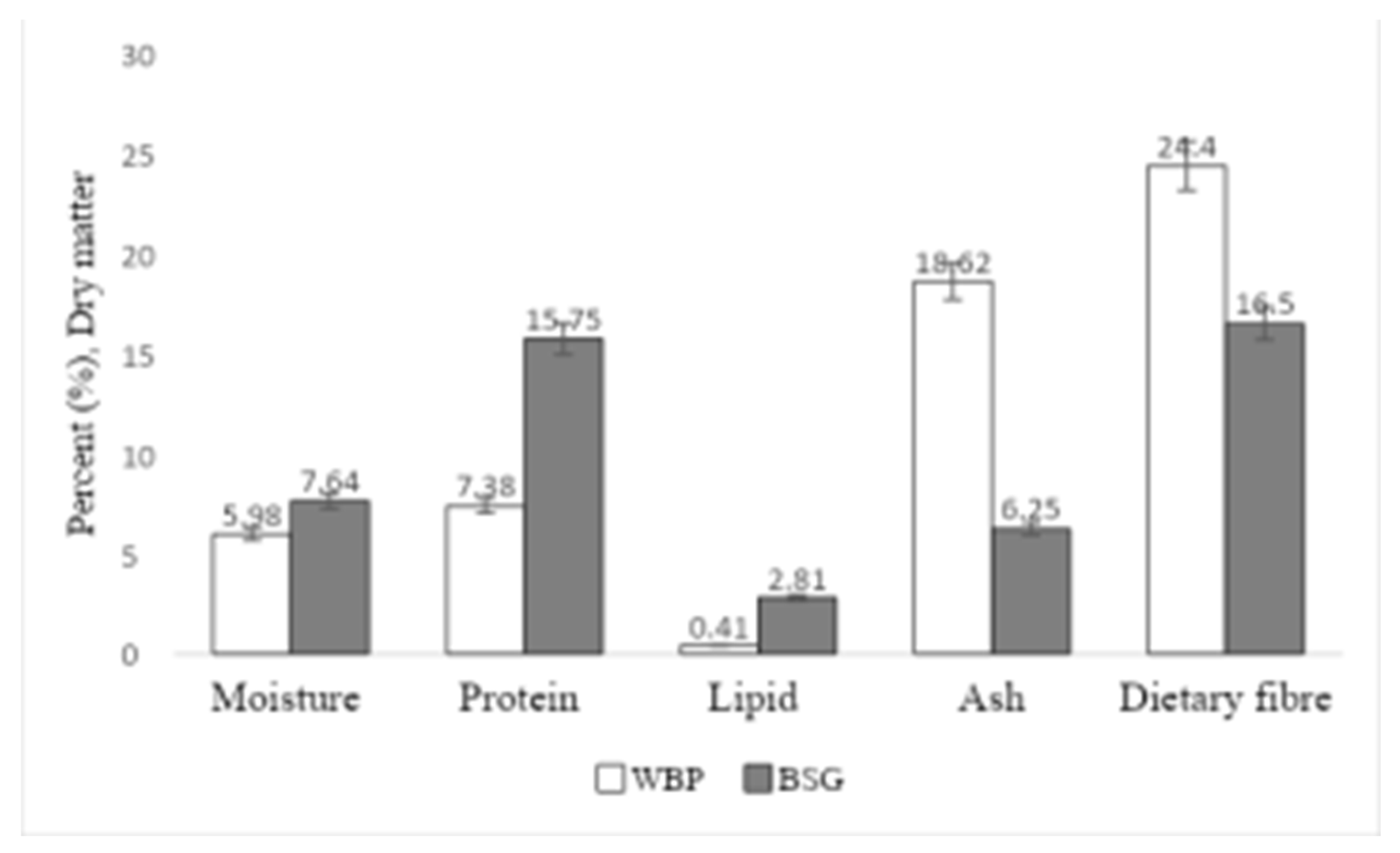
As shown in
Figure 1, the compositions of WBP and BSG were measured and compared. The result
indicates that the dietary fiber content was higher in WBP than in BSG, but protein and lipid were higher in BSG.
1.2. Microwave pretreatment of WBP and BSG increases reducing sugar yield with enzymatic hydrolysis
For the successful production of xylooligosaccharides from WBP and BSG, hydrolyzing enzyme-(xylanase) must effectively reach the xylan through a cellulose and lignin barriers. An effect of microwave pretreatment on enzymatic hydrolysis was demonstrated, as it increased the effectiveness of enzymatic hydrolysis [
15,
16]. In this study, we attempted to apply microwave pretreatment to the xylanase action of WBP and BSG. XOS production from microwave-pretreated WBP and BSG with xylanase was characterized by in increased reducing sugars.
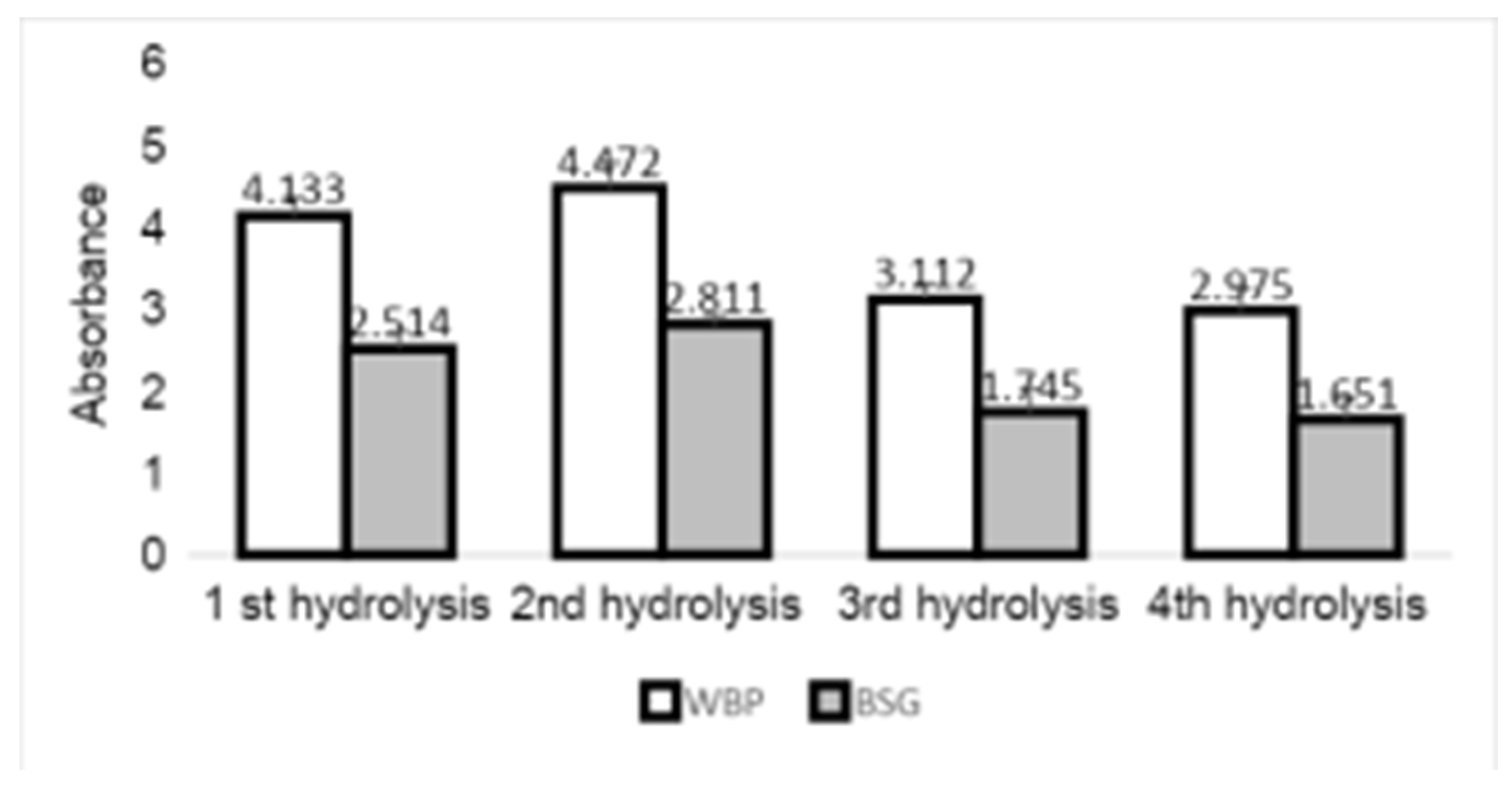
Figure 2 shows the effect of repeated microwave pretreatment on reducing sugar production. The greatest sugar production reduction in both samples occurred with the second hydrolysis, and the lowest was with the fourth hydrolysis.
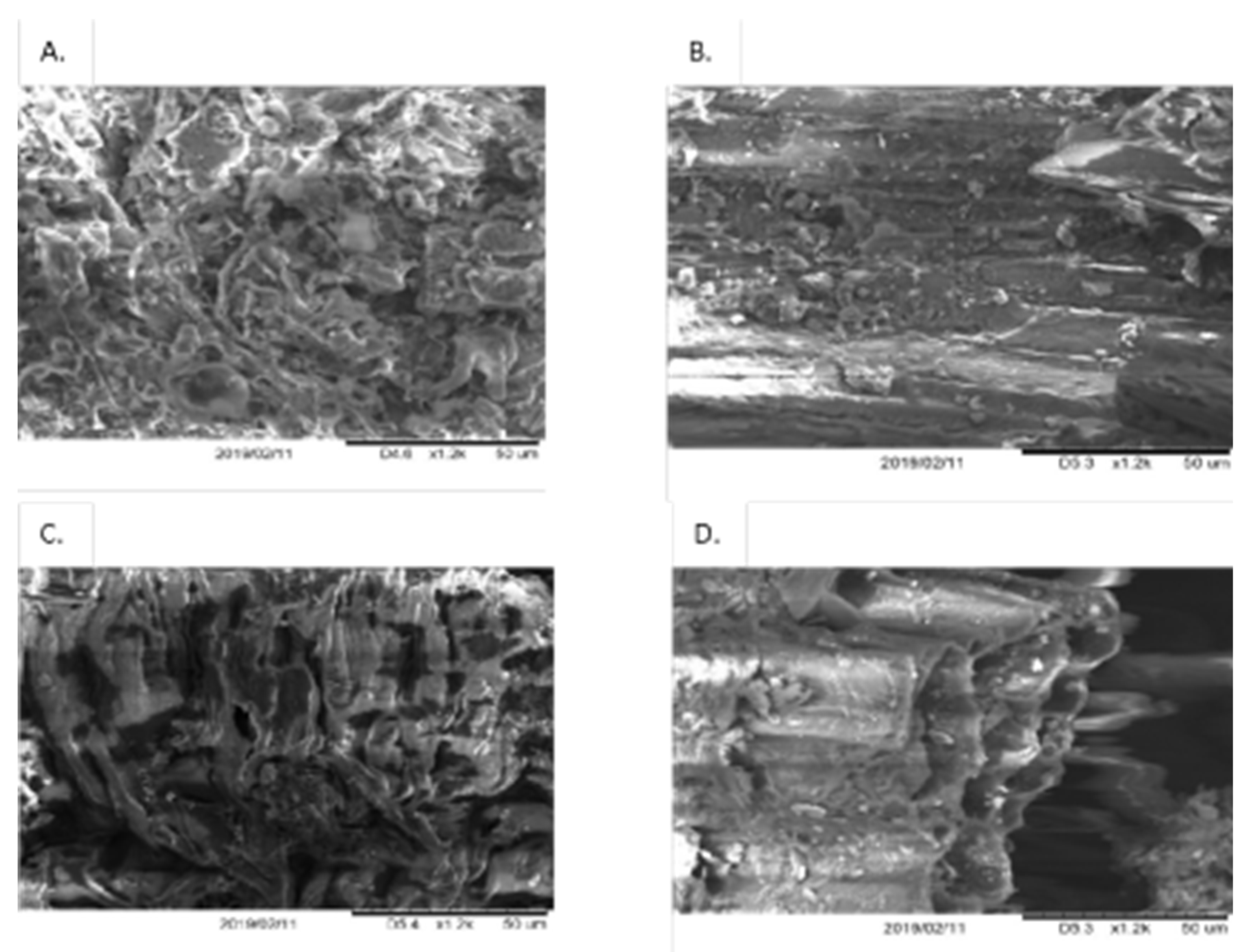
We demonstrated that the microwave pretreatment alone improved enzymatic hydrolysis; however, it appeared that a single pretreatment did not efficiently improve the hydrolysis yield. The reducing sugar assay together with scanning electron microscopy observation (
Figure 3) confirmed the effectiveness of a single pretreatment over multiple ones.
Figure 2 shows the effects of one and four microwave treatments on WBP and BSG.
1.2. Purification of xylooligosaccharide
After enzymatic hydrolysis, the water-soluble fraction was recovered by centrifugation. Wang et al., showed that the soluble fraction contained starch, pectin, protein, and tannin [
10], in addition to hydrolysis products. To remove these impurities, activated carbon adsorption and membrane filtration were used. The total sugar was determined by a phenol-sulfuric acid method with a D-xylose standard, total carbohydrate was 88.6% in the WBP hydrolysate and 82.4% in the BSG hydrolysate. Since BSG had high protein content, membrane dialysis was used to purify XOS from BSG samples.
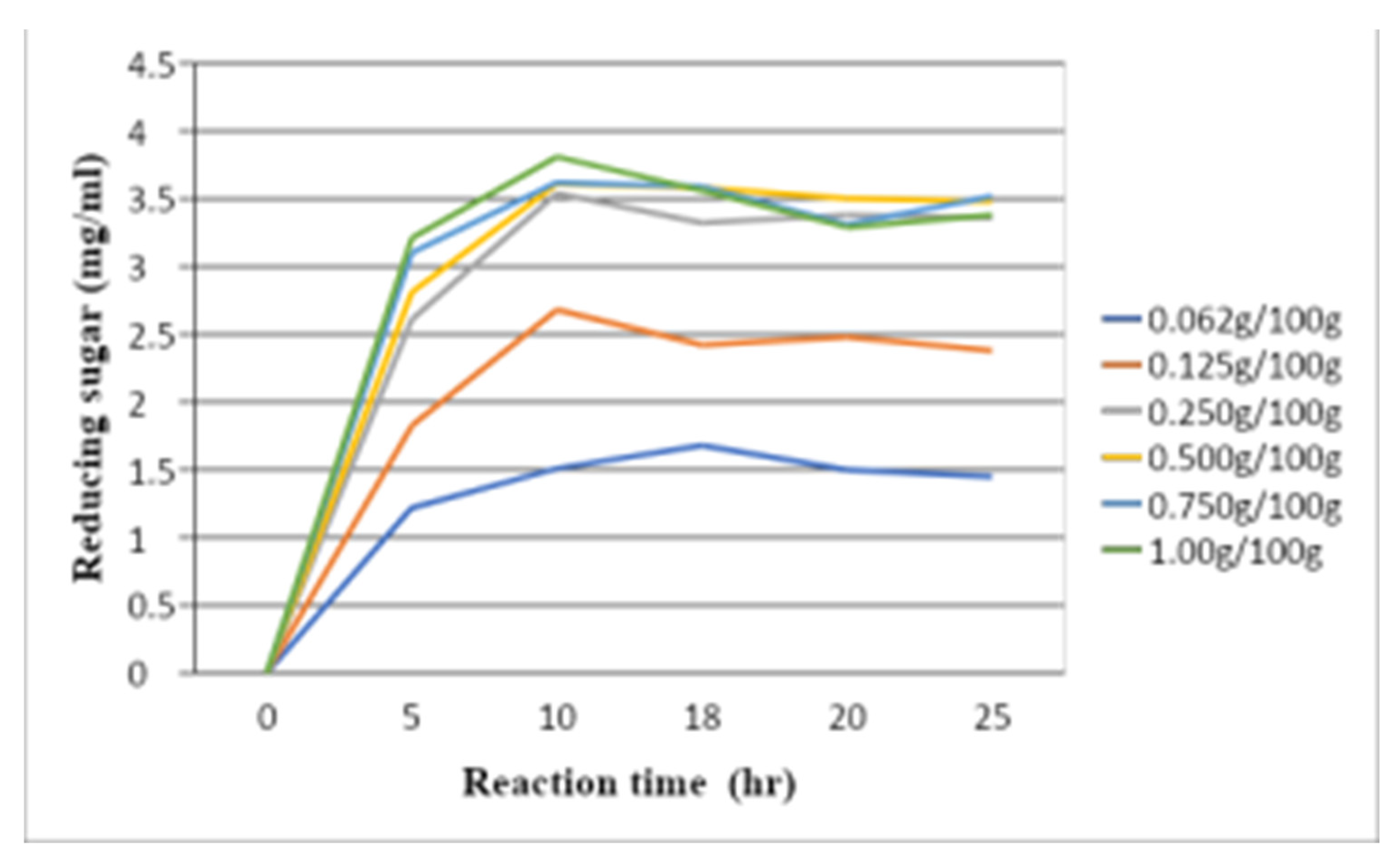
1.2. Optimization enzymatic hydrolysis of xylooligosaccharides production
Enzyme concentration plays a significant role in XOS production. The effect of hydrolysis was tested with different xylanase and substrate ratios: 0.062 g/100 g, 0.125 g/100 g, 0.25 g/100 g, 0.5 g/100 g, 0.75 g/100 g, and 1 g/100 g. The effectiveness of XOS production by microwave-assisted enzymatic hydrolysis was determined by the production with reduced sugar as shown in
Figure 5. The maximum reduced sugar production was found at 1 g/100 g enzyme-substrate ratio. There was no significant increase in XOS production when the enzyme-substrate ratio was more than 0.25 g/100 g, and incubation was longer than 12 h. In brief, optimum amount of the xylanase is 0.25 g for 100 g substrate, which means that a higher amount of the enzyme reduces sugar in an equal amount (
Figure 6).
As the hydrolysis period increased from 6 to 12 h, the production of XOS increased. The yield of reduced sugar was 3.75 mg/ml from microwave-assisted enzymatic hydrolysis in both samples. The rate of reduced sugar content declined after incubation for 12 h, owing to the decreased level of easily available hydrolysis site in xylan and decreased endoxylanase activity due to end-product inhibition. Considering product composition, the activated carbon adsorption method was used in further experiments. After following this process, it was found that 5.4 g of purified dry XOS was produced from 50 g of dry WBPs powder, and 3.6 g of purified dry XOS was produced from 50 g of dry BSG powder.
4. Discussion
BSG is formed by the processing of malt (cereal grain) and the separation of wort. It accounts for about 85% of the solid by-product in the brewery. According to the survey of the State Statistics Committee of Mongolia, 82,415.2 L, 91,246 L, and 91,975 L of beer were produced in 2017, 2018, and 2019, respectively, showing an increase each year[
18,
18,
18](“Хүнс хөдөө аж ахуй, хөнгөн үйлдвэрийн яамны статик мэдээ “, 2017)[
18,
18].
During the WB processing seven type of waste in generated in Altan taria, and in this study, the outermost peels and dust, called black dust were used which were not even used to feed livestock. Therefore, ash was determined to be relatively high in WBP in this study. In BSG, protein and lipid compositions were significantly higher than in WBPs. In Mongolia, are the first to combine the predetermined methods and test a method for purifying prebiotic oligosaccharides from waste source materials of two major food industries in Mongolia. The practical significance of this research is that it demonstrates how to process biologically effective oligosaccharides using a low-cost, isolation method from by-products from two large food industries.
According to our findings, the chemical composition of BSG was 16.2% protein, 5.8% fat, and 3.8% mineral in the study conducted by Solongo (2018) in Mongolia, where the amounts of these constituents contained in BSG vary by country, as does the composition of the raw materials. in addition to the weather conditions and geographical features of the country. In Mongolia exhibits fewer proteins and fats in BSG than other countries, but more minerals. In Mongolia, however, no studies have been conducted to determine the chemical composition of WBP [
19].
In Coelho et al., BSG was prepared using a water-based extract, where the supernatant was collected, precipitated with 70% ethanol, centrifuged, freeze-dried, constituting and heated at 180°C for 2 min. Then and the supernatant was collected again, constituting a substance that has been centrifuged and freeze-dried. The precipitate was collected again, a 0.1M KOH solution was prepared, and the supernatant was collected at 180°C for 2 min before being centrifuged with acetic acid and freeze-dried. The samples were centrifuged with 70% ethanol before being freeze-dried, a method that produced XOS with a 62% purity. In addition, as the microwave temperature increased from 140 to 210˚C, XOS production increased. Further, the microwave-assisted extraction enzymatic hydrolysis procedure produced 89% purity. This research, which requires a product with a higher purity by adding xylanase enzyme and absorbing activated carbon using the enzyme hydrolysis method with physical treatment at 200°C for 3 min, which a product with an elevated degree of purity [
20].
Aachary et al., XOS were derived by hydrolyzing corn with the enzyme xylanase (Aspergillus oryzae MTCC 5154) , and enzyme hydrolysis was conducted at 50°C for 24–36 h, after which it was boiled for 15 min to terminate the hydrolysis. The amount of XOS produced decreased when enzyme hydrolysis was conducted for a maximum of 24 h, and the xylanase (Aspergillus oryzae MTCC 5154) enzyme increased the production of XOS more so than the xylanase enzyme. xylanase units/g (Sigma-Aldrich Germany, X2753)) experienced enzymatic hydrolysis at 55°C for 24 h. In addition, it was placed in a water involvement at 96°C for 5 min to stop enzymatic hydrolysis. The current research uses a different sample, but the same enzyme and time for enzymatic hydrolysis as our study [
7].
The microenvironment of an enzyme-catalyzed reaction was exposed to microwave irradiation (Ha, Mai, An, and Koo, 2011). The microstructure of the original bran samples and the bran samples after the fourth microwave-assisted application of enzymatic hydrolysis was visualized using scanning electron microscopy. During the purification phase, the high molecular components were separated, and some XOS, such as X5 and X6, were larger than X4. Due to the lack of a standard, these oligosaccharides are shown as >X4. From wheat bran, most of the HPLC chromatograms of XOS compositions were X2, X3, and X4, with minor amounts of >X4 [
21].
Both XOS samples purified from WBPs and BSG samples showed structures similar to the standard one, which represent the stretching of O-H, C-H stretching of carbohydrate, C-O, C-C-C, and C-OH bonds in hemicellulose, and the β -glycoside bond, suggesting the presence of the xylan group in the XOS mix, reported earlier by Liang et al.[
21] . As it has been revealed earlier that the band at 896.73 cm
−1 represents the presence of glycosidic bonds between the sugar molecules [
19], the graph shown in
Figure 4 could be related to the previous study result. The spectrum was similar to that shown by Liang et al. in their FTIR study of the XOS mix obtained from Bian et al. in their FTIR study of XOS from sugarcane bagasse [
22,
23].
The XOS produced by the activated carbon adsorption method consisted primarily of X2 and X3, with a minor amount of X4. These results indicate that the adsorption technique with activated carbon was superior to the precipitation method with ethanol. In further experiments, activated carbon adsorption was used due to the product composition.
Wang et al. XOS from wheat bran using microwave-assisted enzymatic hydrolysis. Thus, 3.2 grams of XOS were extracted from 50 grams of the sample the microwave alone does not positively affect production, but it increases the amount produced when combined with the enzyme hydrolysis method, where four instances of enzyme hydrolysis and 24 h for per instance was found effective. Over 24 h of enzymatic hydrolysis, 5.4 g of XOS was extracted from 50 g of the WBP sample, and 3.6 g of purified dry XOS was produced from 50 g of dry BSG powder[
10].