Submitted:
27 September 2023
Posted:
28 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Expression and Purification of Vaccine Candidate Proteins and Preparation of Whole-Cell Lysate from Virulent C. perfringens MLG_7820
2.2. Bacterial Strains, Media, Culture Conditions, and Inoculum Preparation
2.3. Birds Housing, Feeding, Experimental NE Disease Model, and Cecal Content Collection
2.4. Vaccination of Broiler Chickens
2.5. DNA Extraction from Cecal Samples
2.6. 16S rRNA Gene Amplification, Amplicon Sequencing, and Bioinformatics Analysis
2.7. Measurement of Serum Antibody Levels by ELISA
2.8. Statistical Analysis
3. Results
3.1. Expression and Purification of Vaccine Candidate Proteins and Preparation of Whole-Cell Lysate of Virulent C. Perfringens MLG_7820
| Groups | Size (KDa) | Location | VaxiJen Score |
|---|---|---|---|
| P153 | 5.6 | Extracellular/Cytoplasmic membrane | 0.69 |
| P264 | 9.2 | Extracellular/Cytoplasmic | 1.47 |
| P509 | 13 | Extracellular | 0.91 |
| P537 | 21 | Cytoplasmic | 0.21 |
| P561 | 17 | Extracellular | 0.64 |
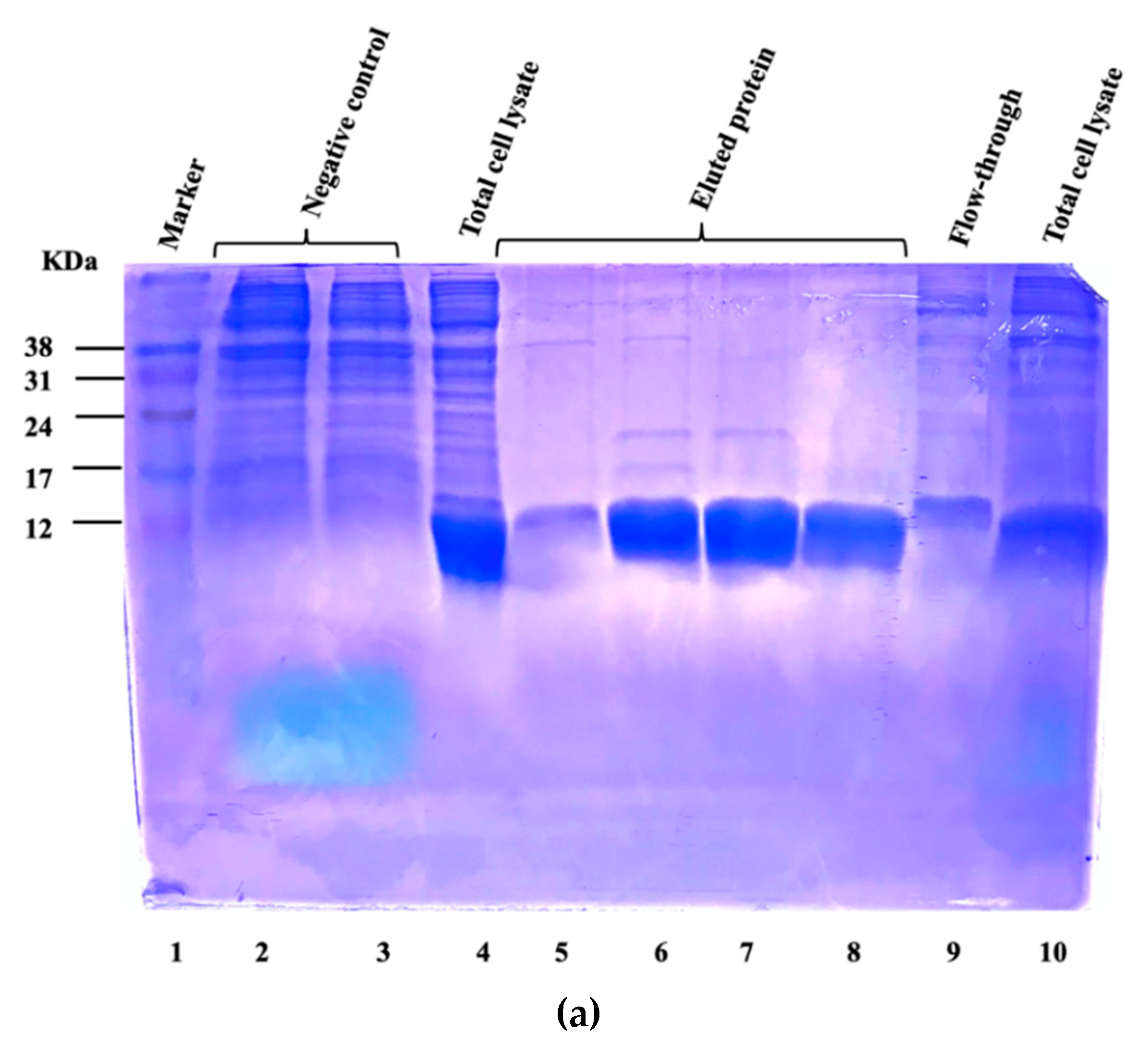
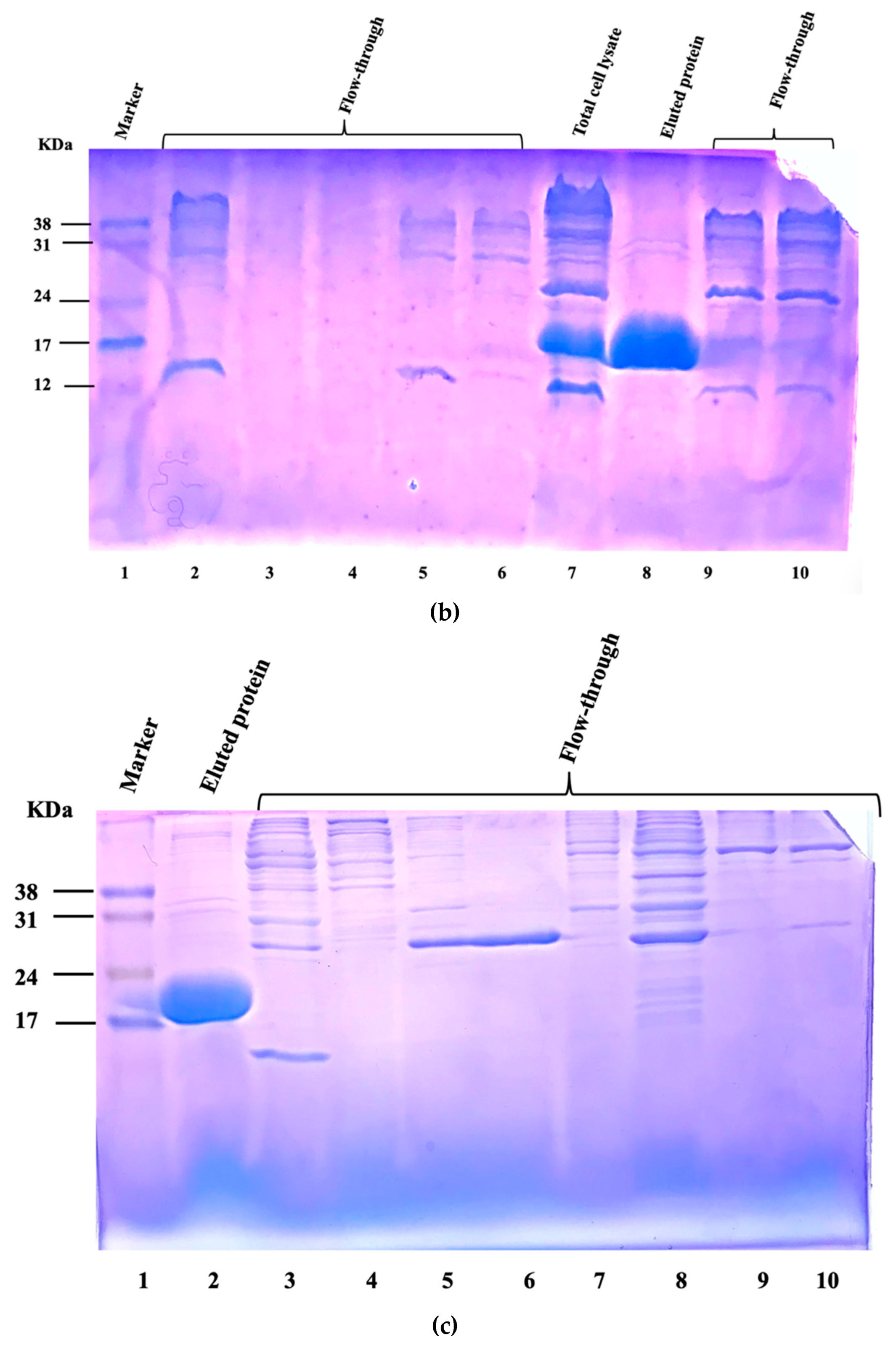
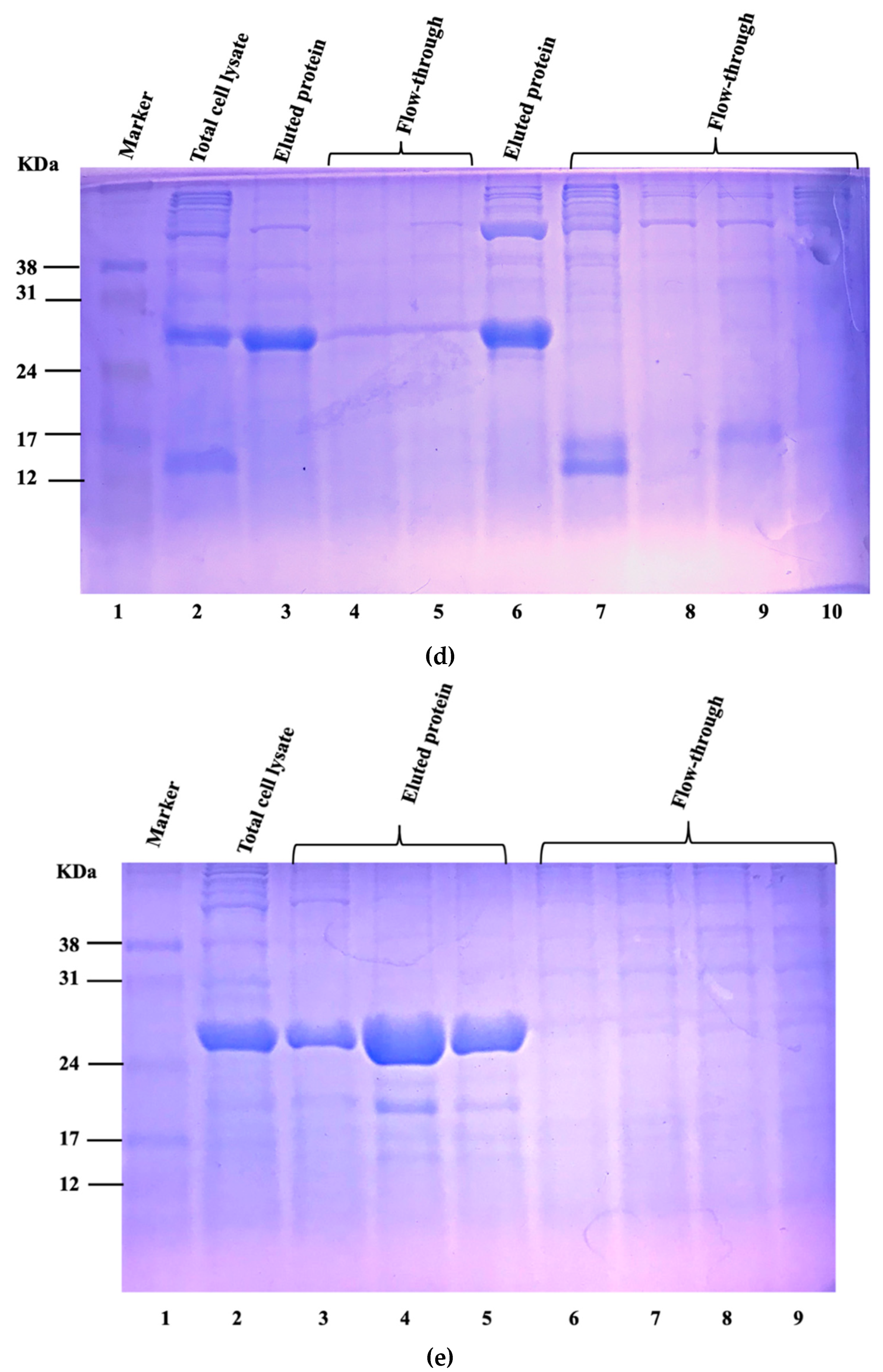
3.2. Bacterial Strains, Media, Culture Conditions, and Inoculum Preparation
3.3. NE Lesion Scoring and Average Body Weight Gain of Broiler Chickens
| Group | No. of birds | Lesion scores | Average lesion score (±SE) | Average weight (±SE) | ||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||||
| P153 | 13 | 8 | 4 | 1 | 0 | 0 | 0.46 (0.18) | 2066.77(40.6) |
| P264 | 14 | 9 | 2 | 3 | 0 | 0 | 0.57 (0.23) | 1952.50 (40.3) |
| P509 | 14 | 10 | 2 | 1 | 1 | 0 | 0.50 (0.25) | 1946.79 (52.2) |
| P537 | 13 | 6 | 3 | 4 | 0 | 0 | 0.85 (0.25) | 2087.69 (65.1) |
| P561 | 14 | 13 | 1 | 0 | 0 | 0 | 0.07 (0.07) | 1900.71 (41.1) |
| Bacitracin | 8 | 8 | 0 | 0 | 0 | 0 | 0.00 (0.00) | 2790.00 (61.5) |
| Mix | 12 | 10 | 0 | 1 | 0 | 1 | 0.50 (0.36) | 2043.00 (37.9) |
| MLG_7820 | 9 | 2 | 3 | 4 | 0 | 0 | 1.22 (0.28) | 2023.11 (54.9) |
| Quil-A | 6 | 5 | 1 | 0 | 0 | 0 | 0.17 (0.17) | 1925.00 (77.5) |
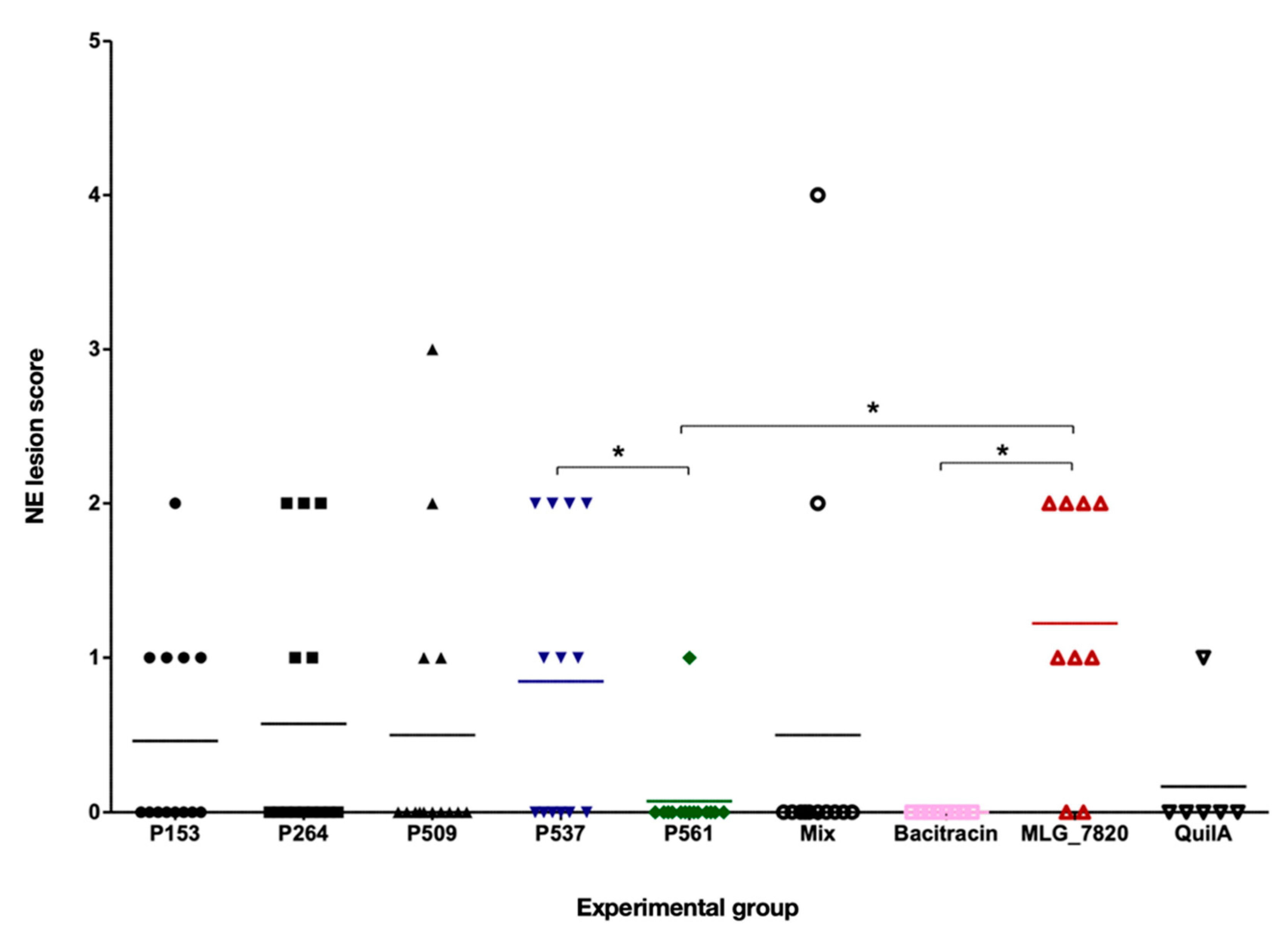
3.4. Measurement of Serum Antibody Levels by ELISA
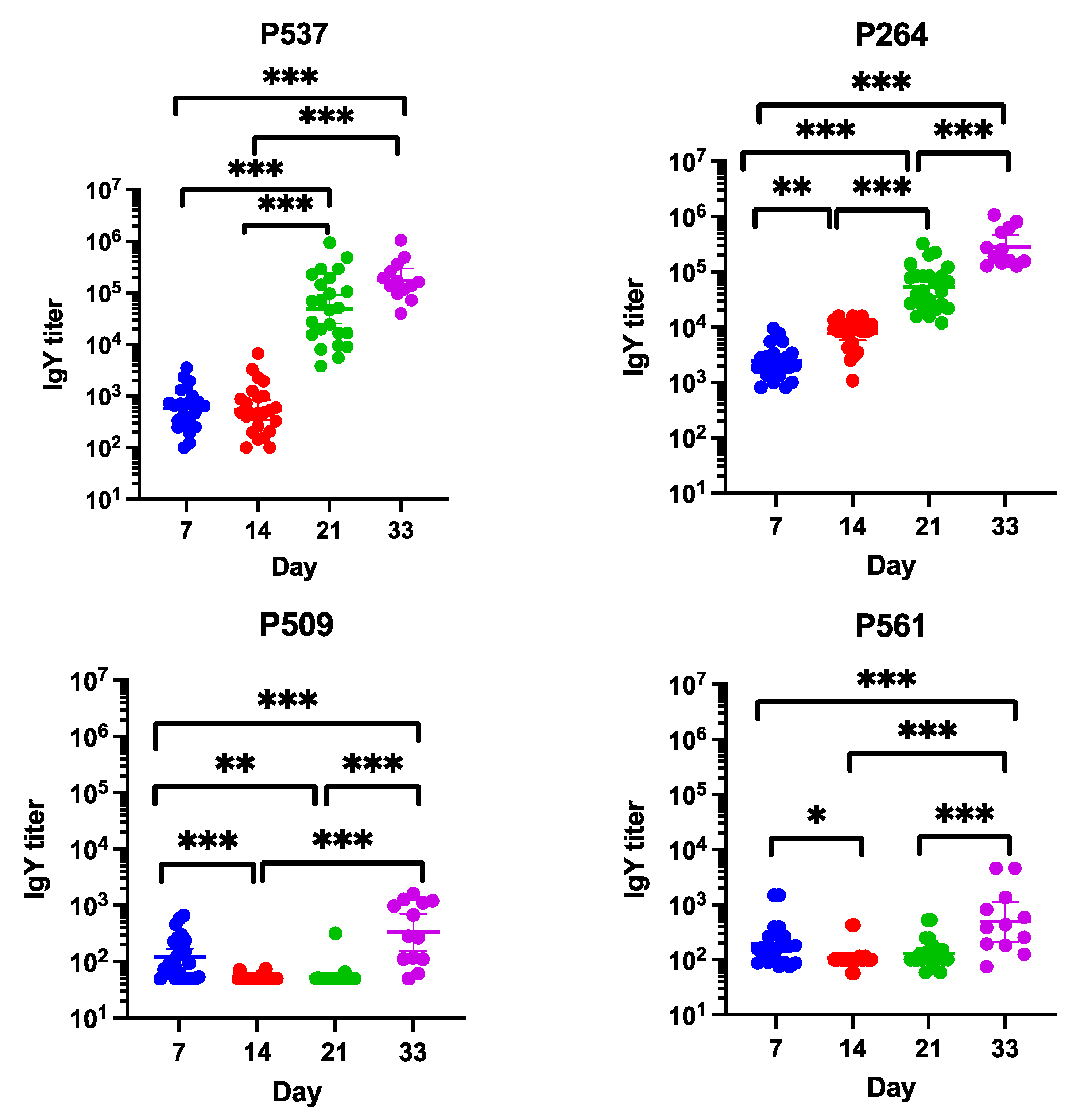
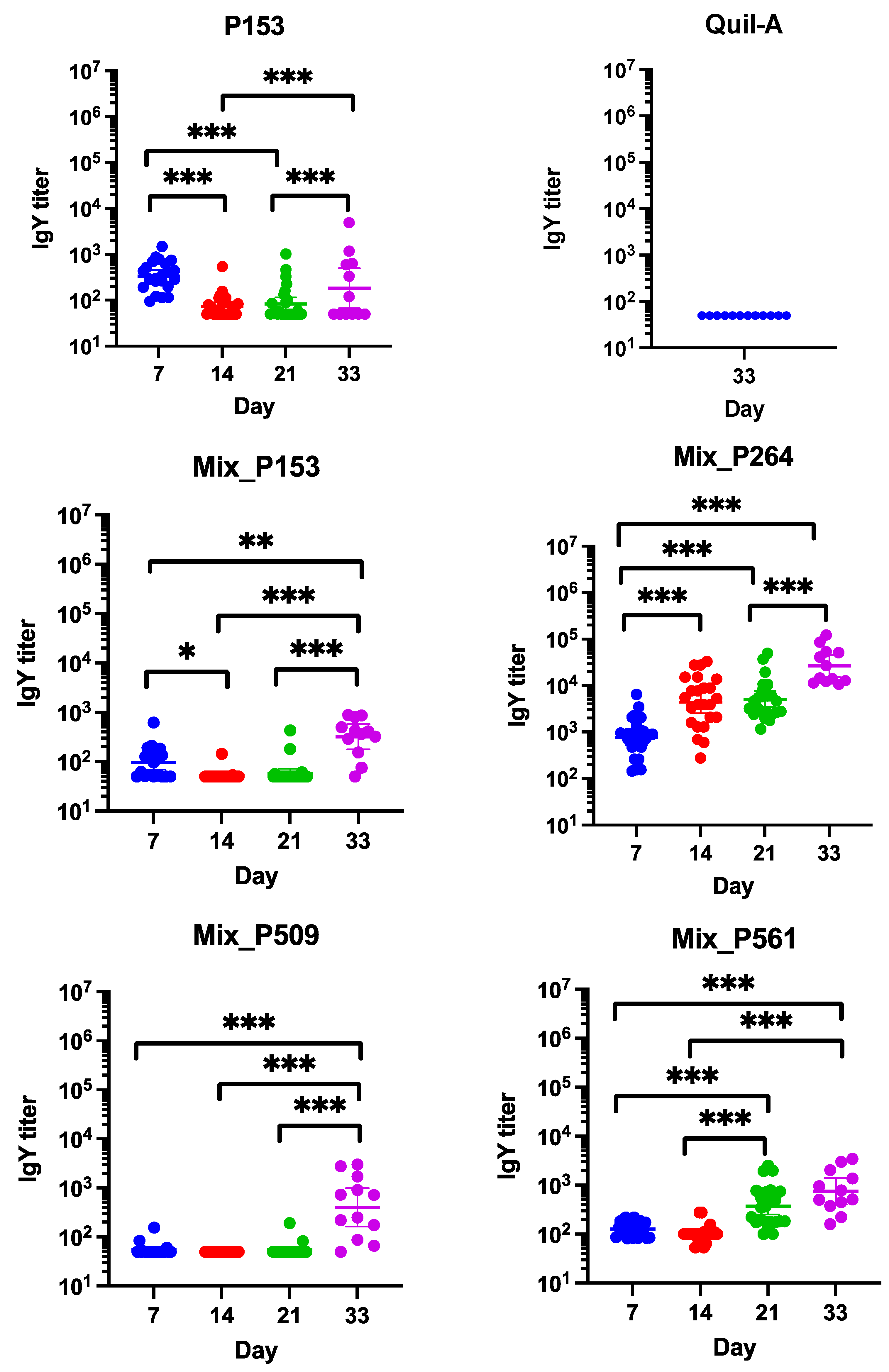
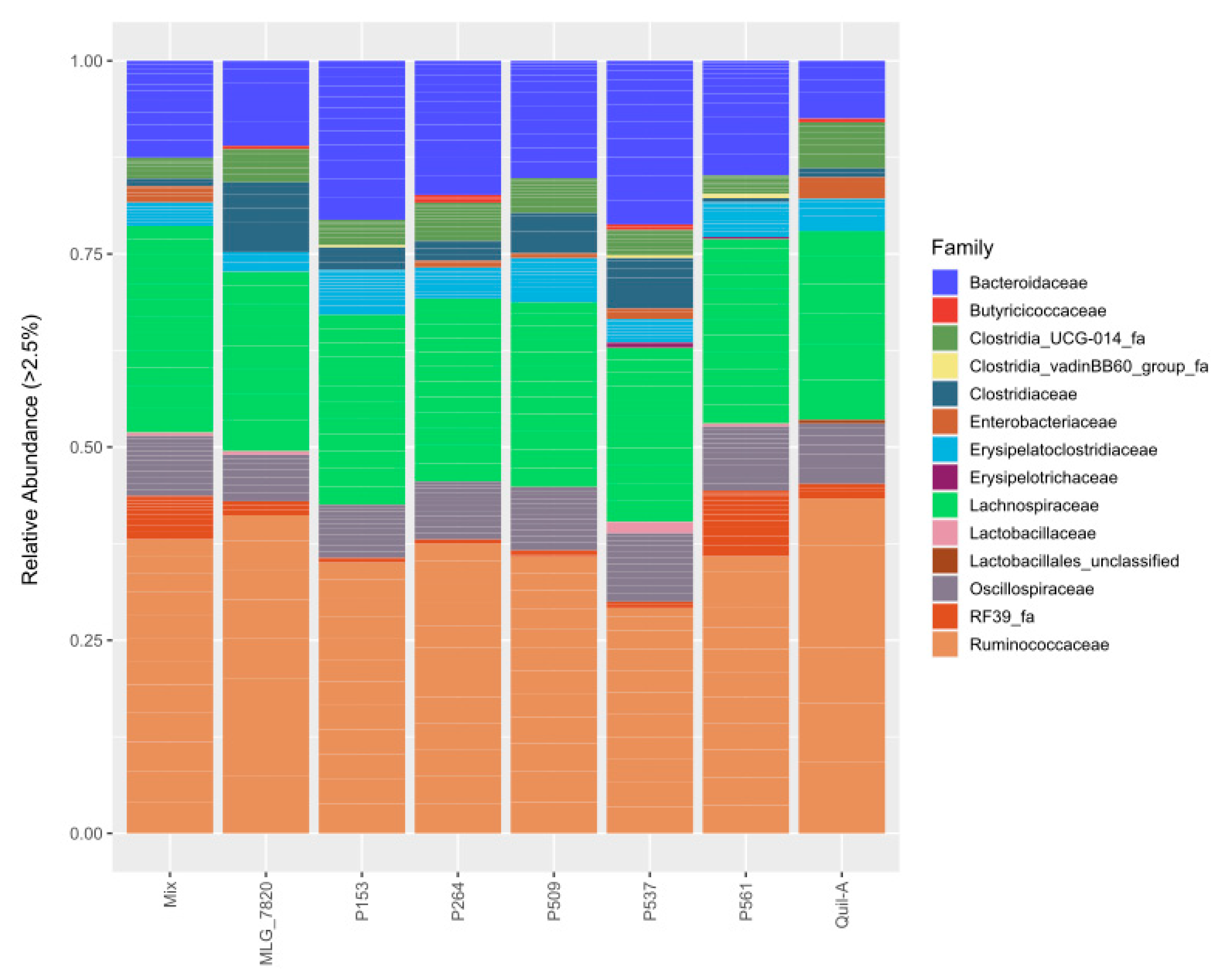
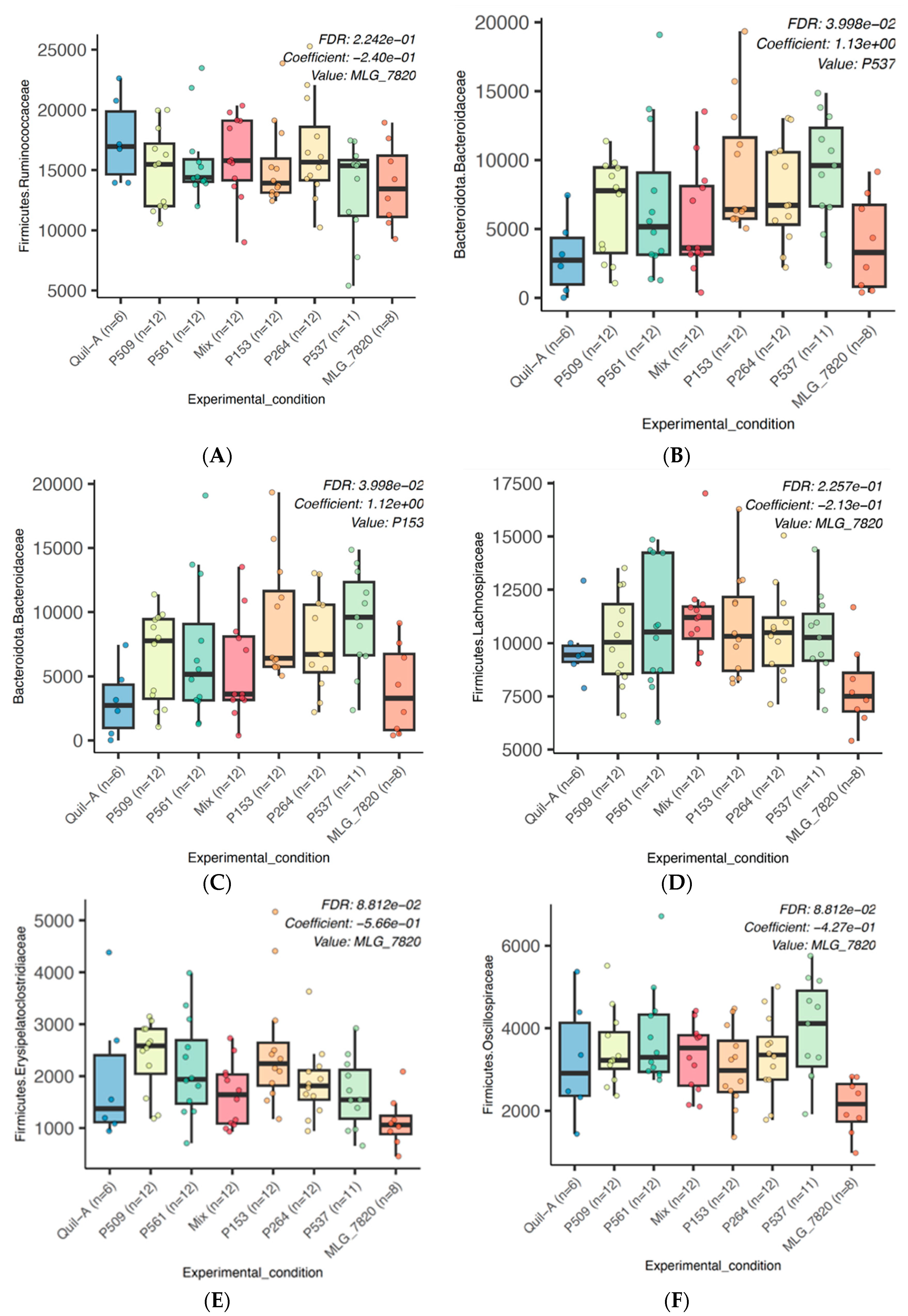
3.5. 16S rRNA Gene Amplicon Metagenomic Sequencing
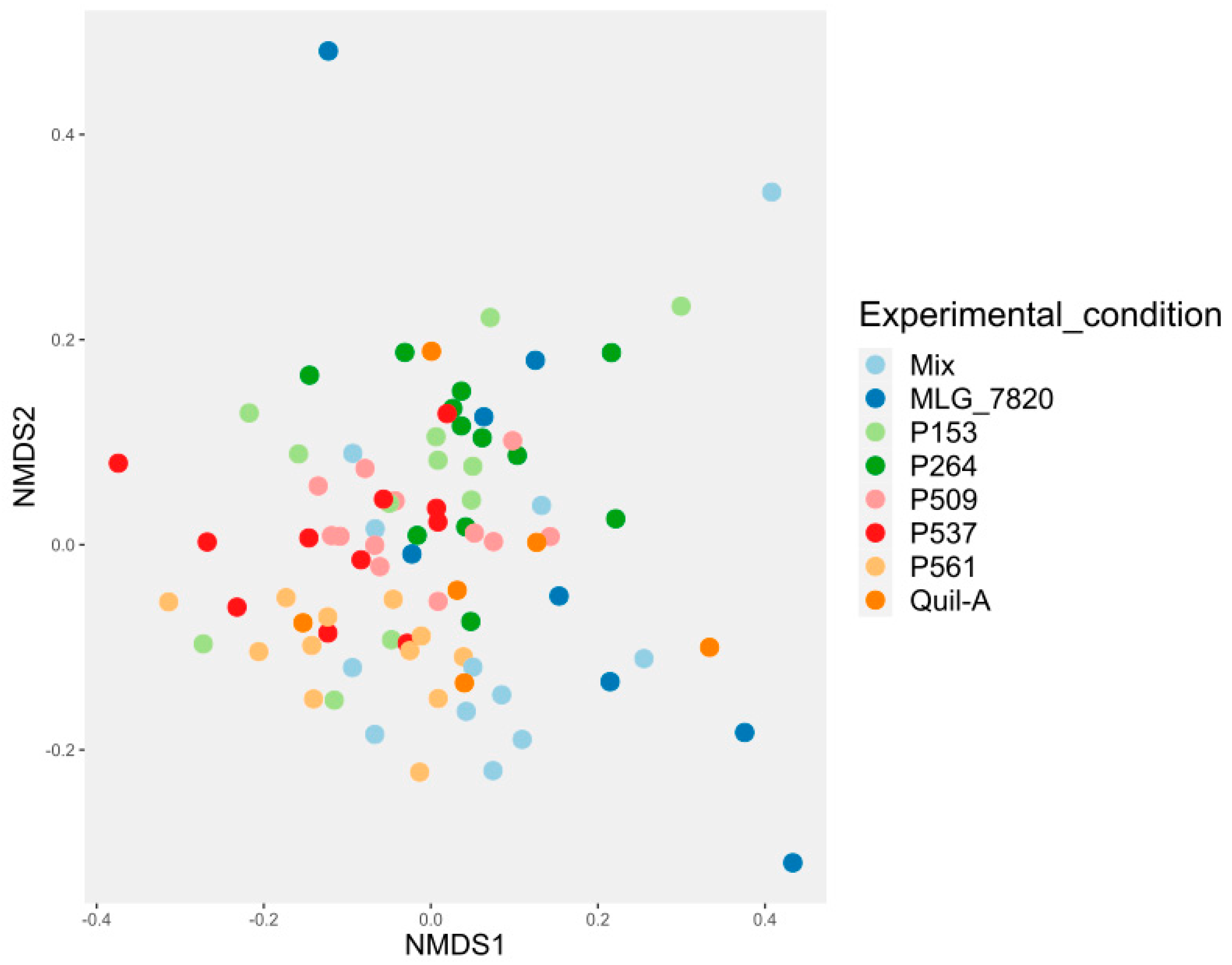
3.6. Alpha and Beta Diversities
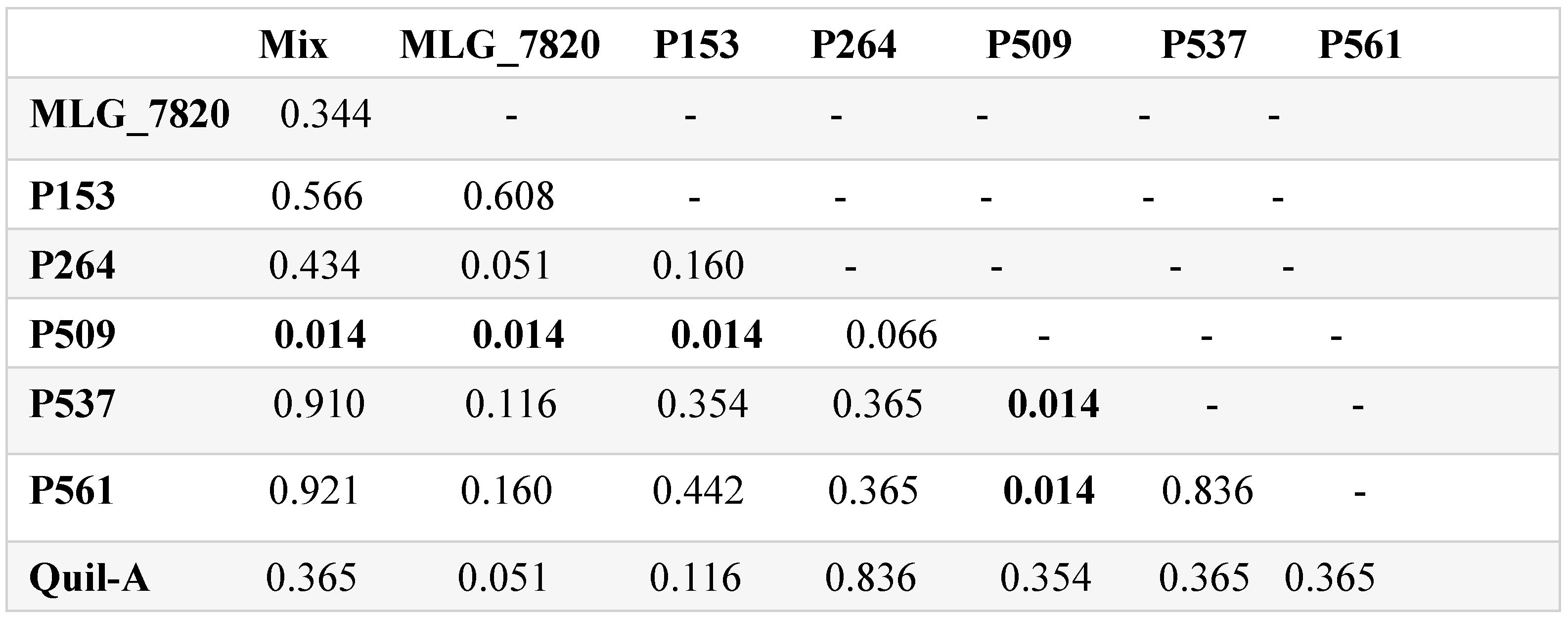
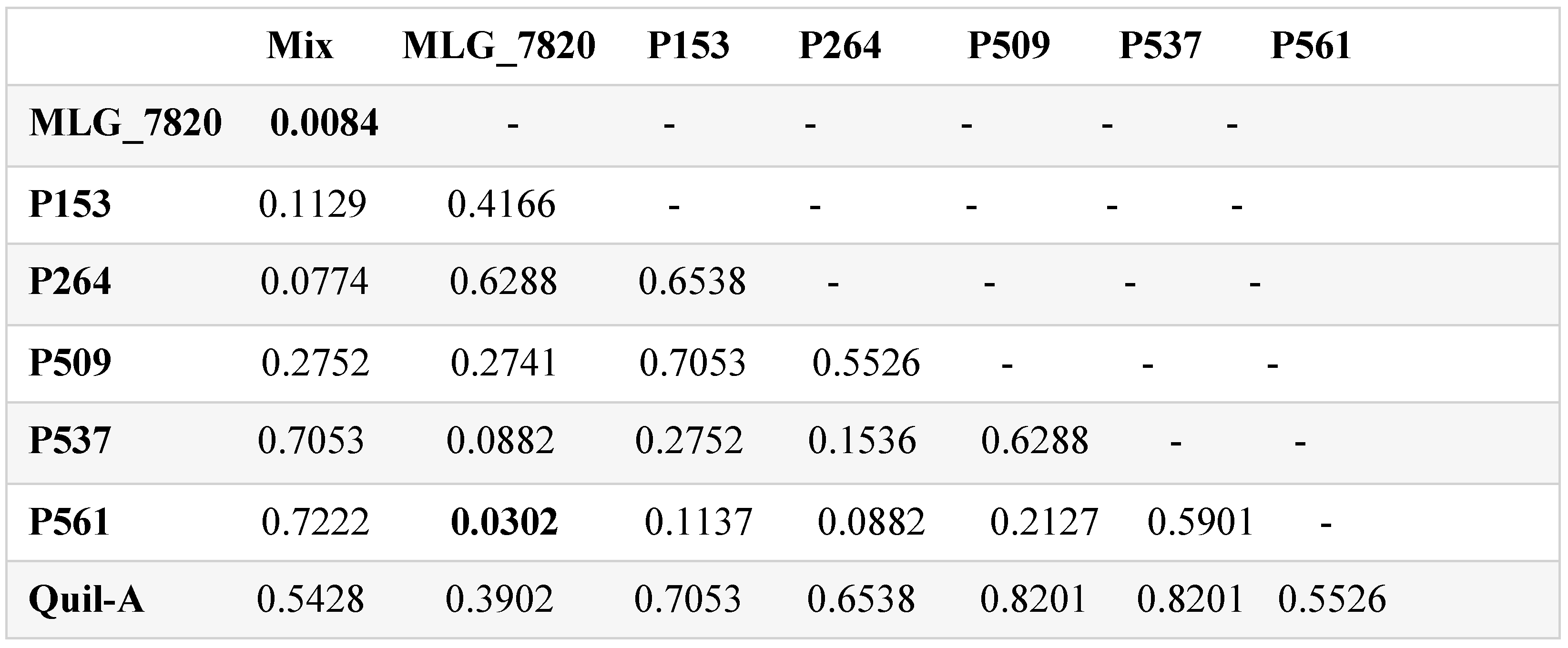
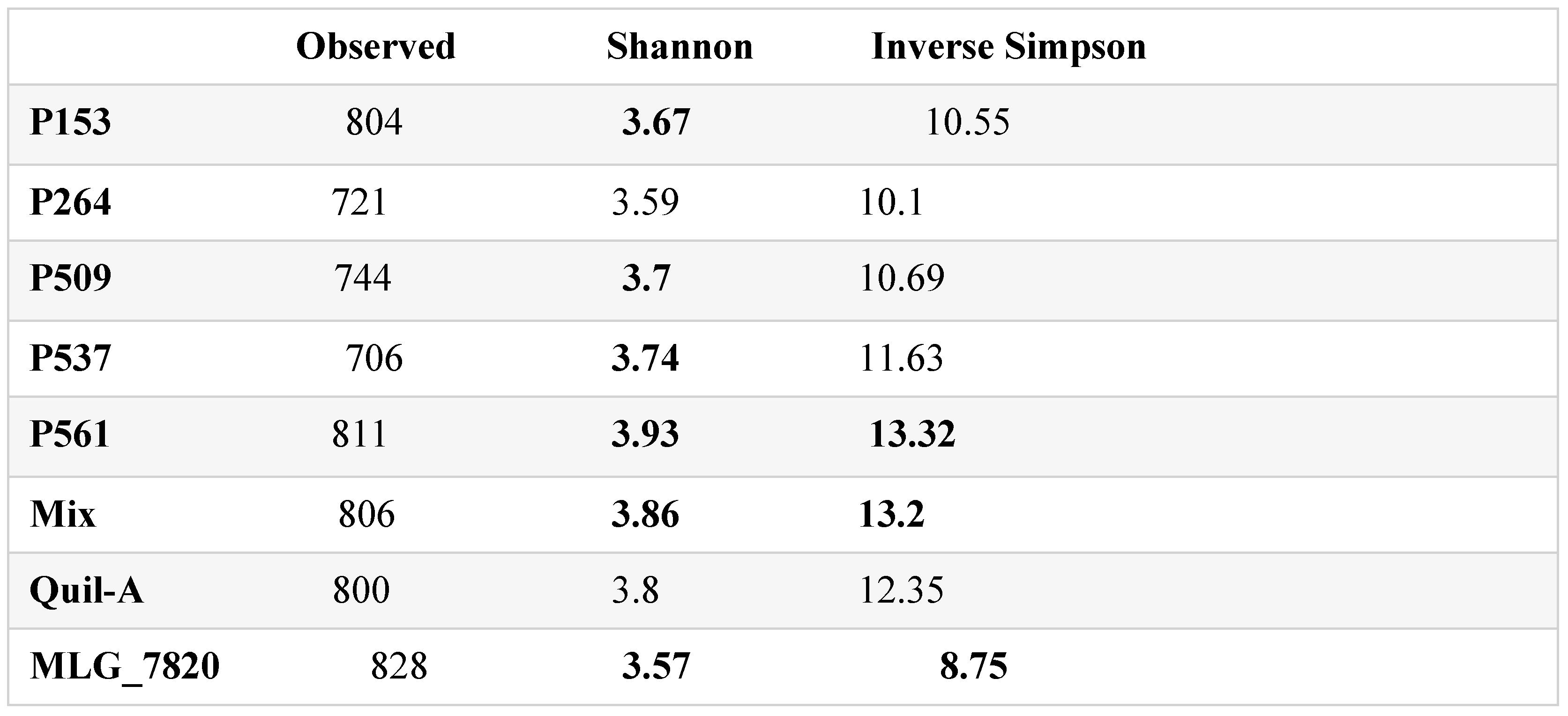
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diaz Carrasco, J.M.; Redondo, L.M.; Redondo, E.A.; Dominguez, J.E.; Chacana, A.P.; Fernandez Miyakawa, M.E. Use of plant extracts as an effective manner to control Clostridium perfringens induced necrotic enteritis in poultry. Biomed Res. Int. 2016, 2016, 3278359. [Google Scholar] [CrossRef]
- Scott, K.P.; Gratz, S.W.; Sheridan, P.O.; Flint, H.J.; Duncan, S.H. The influence of diet on the gut microbiota. Pharmacol. Res. 2013, 69, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Round, J.L.; Mazmanian, S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Lacey, J.A.; Keyburn, A.L.; Ford, M.E.; Portela, R.W.; Johanesen, P.A.; Lyras, D.; Moore, R.J. Conjugation-mediated horizontal gene transfer of Clostridium perfringens plasmids in the chicken gastrointestinal tract results in the formation of new virulent strains. AEM 2017, 83, e01814-17. [Google Scholar] [CrossRef]
- Fathima, S.; Hakeem, W.G.A.; Shanmugasundaram, R.; Selvaraj, R.K. Necrotic Enteritis in Broiler Chickens: A Review on the Pathogen, Pathogenesis, and Prevention. Microorganisms 2022, 10, 1958. [Google Scholar] [CrossRef] [PubMed]
- Prescott, J.F.; Parreira, V.R.; Gohari, I.M.; Lepp, D.; Gong, J. The pathogenesis of necrotic enteritis in chickens: what we know and what we need to know: a review. Avian Pathol. 2016, 45, 288–294. [Google Scholar] [CrossRef]
- Mot, D.; Timbermont, L.; Haesebrouck, F.; Ducatelle, R.; Van Immerseel, F. Progress and problems in vaccination against necrotic enteritis in broiler chickens. Avian Pathol. 2014, 43, 290–300. [Google Scholar] [CrossRef]
- Zekarias, B.; Mo, H.; Curtiss III, R. Recombinant attenuated Salmonella enterica serovar Typhimurium expressing the carboxy-terminal domain of alpha toxin from Clostridium perfringens induces protective responses against necrotic enteritis in chickens. CVI 2008, 15, 805–816. [Google Scholar] [CrossRef]
- Hoang, T.H.; Hong, H.A.; Clark, G.C.; Titball, R.W.; Cutting, S.M. . Recombinant Bacillus subtilis expressing the Clostridium perfringens alpha toxoid is a candidate orally delivered vaccine against necrotic enteritis. Infect. Immun. 2008, 76, 5257–5265. [Google Scholar] [CrossRef]
- Cooper, K.K.; Trinh, H.T.; Songer, J.G. Immunization with recombinant alpha toxin partially protects broiler chicks against experimental challenge with Clostridium perfringens. Vet. Microbiol. 2009, 133, 92–97. [Google Scholar] [CrossRef]
- Crouch, C.F.; Withanage, G.S.K.; de Haas, V.; Etoré, F.; Francis, M.J. Safety and efficacy of a maternal vaccine for the passive protection of broiler chicks against necrotic enteritis. Avian Pathol. 2010, 39, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Lillehoj, H.S.; Jeong, W.; Jeoung, H.Y.; An, D.J. Avian necrotic enteritis: experimental models, host immunity, pathogenesis, risk factors, and vaccine development. Poult. Sci. 2011, 90, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Heidarpanah, S.; Thibodeau, A.; Parreira, V.R.; Quessy, S.; Segura, M.; Meniaï, I.; Gottschalk, M.; Gaudreau, A.; Juette, T.; Gaucher, M.L. Immunization of broiler chickens with five newly identified surface-exposed proteins unique to Clostridium perfringens causing necrotic enteritis. Sci. Rep. 2023, 13, 5254. [Google Scholar] [CrossRef] [PubMed]
- Collier, C.T.; Hofacre, C.L.; Payne, A.M.; Anderson, D.B.; Kaiser, P.; Mackie, R.I.; Gaskins, H.R. Coccidia-induced mucogenesis promotes the onset of necrotic enteritis by supporting Clostridium perfringens growth. Vet. Immunol. Immunopathol. 2008, 122, 104–115. [Google Scholar] [CrossRef]
- Wu, S.B.; Stanley, D.; Rodgers, N.; Swick, R.A.; Moore, R.J. Two necrotic enteritis predisposing factors, dietary fishmeal and Eimeria infection, induce large changes in the caecal microbiota of broiler chickens. Vet. Microbiol. 2014, 169, 188–197. [Google Scholar] [CrossRef]
- Jia, W.; Slominski, B.A.; Bruce, H.L.; Blank, G.; Crow, G.H.; Jones, O. Effects of diet type and enzyme addition on growth performance and gut health of broiler chickens during subclinical Clostridium perfringens challenge. Poult. Sci. 2009, 88, 132–140. [Google Scholar] [CrossRef]
- Huang, T.; Gao, B.; Chen, W.-L.; Xiang, R.; Yuan, M.; Xu, Z.; Peng, X. Temporal effects of high fishmeal diet on gut microbiota and immune response in Clostridium perfringens-challenged chickens. Front. Microbiol. 2018, 9, 2754. [Google Scholar] [CrossRef]
- Stanley, D.; Wu, S.-B.; Rodgers, N.; Swick, R.A.; Moore, R.J. Differential responses of cecal microbiota to fishmeal, Eimeria and Clostridium perfringens in a necrotic enteritis challenge model in chickens. PloS one 2014, 9, e104739. [Google Scholar] [CrossRef]
- Huang, T.; Peng, X.; Gao, B.; Qi-Lin, W.; Xiang, R.; Yuan, M.; Xu, Z. The effect of Clostridium butyricum on gut microbiota, immune response and intestinal barrier function during the development of necrotic enteritis in chickens. Front Microbiol 2019, 10, 2309. [Google Scholar] [CrossRef]
- Wu, Y.; Zhen, W.; Geng, Y.; Wang, Z.; Guo, Y. Pretreatment with probiotic Enterococcus faecium NCIMB 11181 ameliorates necrotic enteritis-induced intestinal barrier injury in broiler chickens. Sci. Rep. 2019, 9, 1–17. [Google Scholar] [CrossRef]
- Bortoluzzi, C.; Vieira, B.S.; Hofacre, C.; Applegate, T.J. Effect of different challenge models to induce necrotic enteritis on the growth performance and intestinal microbiota of broiler chickens. Poult. Sci. 2019, 98, 2800–2812. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liu, J.; Wang, X.; Robinson, K.; Whitmore, M.A.; Stewart, S.; Zhao, J.; Zhang, G. Identification of an intestinal microbiota signature associated with the severity of necrotic enteritis. Front Microbiol 2021, 12, 703693. [Google Scholar] [CrossRef] [PubMed]
- Latorre, J.D.; Adhikari, B.; Park, S.H.; Teague, K.D.; Graham, L.E.; Mahaffey, B.D.; Baxter, M.F.; Hernandez-Velasco, X.; Kwon, Y.M.; Ricke, S.C.; et al. Evaluation of the epithelial barrier function and ileal microbiome in an established necrotic enteritis challenge model in broiler chickens. Front. vet. sci 2018, 5, 199. [Google Scholar] [CrossRef] [PubMed]
- Gyles, C.L. Relevance in pathogenesis research. Vet. Microbiol. 2011, 153, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Rood, J.I.; Keyburn, A.L.; Moore, R.J. NetB and necrotic enteritis: the hole movable story. Avian Pathol. 2016, 45, 295–301. [Google Scholar] [CrossRef]
- Cheung, J.K.; Keyburn, A.L.; Keyburn, A.L.; Keyburn, A.L.; Carter, G.P.; Lanckriet, A.; Van Immerseel, F.; Moore, R.J.; Rood, J.I.; Rood, J.I. The VirSR two-component signal transduction system regulates NetB toxin production in Clostridium perfringens. Infect. Immun. 2010, 78, 3064–3072. [Google Scholar] [CrossRef]
- Ohtani, K.; Shimizu, T. Regulation of toxin gene expression in Clostridium perfringens. Res. Microbiol. 2015, 166, 280–289. [Google Scholar] [CrossRef]
- Lepp, D.; Zhou, Y.; Zhou, Y.; Ojha, S.; Gohari, I.M.; Carere, J.; Yang, C.; Prescott, J.F.; Gong, J. Clostridium perfringens produces an adhesive pilus required for the pathogenesis of necrotic enteritis in poultry. J. Bacteriol. 2021, 203. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. The ARRIVE guidelines animal research: reporting in vivo experiments. PLoS Biol 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Kulkarni, R.R.; Parreira, V.R.; Sharif, S.; Prescott, J.F. Immunization of broiler chickens against Clostridium perfringens-induced necrotic enteritis. CVI 2007, 14, 1070–1077. [Google Scholar] [CrossRef]
- Turcotte, C.; Thibodeau, A.; Quessy, S.; Topp, E.; Beauchamp, G.; Fravalo, P.; Archambault, M.; Gaucher, M.L. Impacts of short-term antibiotic withdrawal and long-term judicious antibiotic use on resistance gene abundance and cecal microbiota composition on commercial broiler chicken farms in Québec. Front. vet. sci 2020, 7, 1067. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. AEM 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Larivière-Gauthier, G.; Thibodeau, A.; Letellier, A.; Yergeau, E.; Fravalo, P. Reduction of Salmonella shedding by sows during gestation in relation to its fecal microbiome. Front Microbiol 2017, 8, 2219. [Google Scholar] [CrossRef] [PubMed]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: a versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- Braley, C.; Gaucher, M.L.; Fravalo, P.; Shedleur-Bourguignon, F.; Longpré, J.; Thibodeau, A. Slight Temperature Deviation during a 56-Day Storage Period Does Not Affect the Microbiota of Fresh Vacuum-Packed Pork Loins. Foods 2023, 12, 1695. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PloS one 2013, 8, e61217. [Google Scholar] [CrossRef]
- Oksanen, J. Vegan: community ecology package. 2010. Available online: http://CRAN. R-project. org/package= vegan.
- Mallick, H.; Mallick, H.; Rahnavard, A.; McIver, L.J.; McIver, L.J.; Ma, S.; Ma, S.; Zhang, Y.; Zhang, Y.; Nguyen, L.H. Multivariable association discovery in population-scale meta-omics studies. PLoS Comput. Biol. 2021, 17, e1009442. [Google Scholar] [CrossRef]
- Timbermont, L.; Haesebrouck, F.; Ducatelle, R.; Van Immerseel, F. Necrotic enteritis in broilers: an updated review on the pathogenesis. Avian Pathol. 2011, 40, 341–347. [Google Scholar] [CrossRef]
- Tsiouris, V. , Poultry management: a useful tool for the control of necrotic enteritis in poultry. Avian Pathol. 2016, 45, 323–325. [Google Scholar] [CrossRef]
- Lacey, J.A.; Stanley, D.; Keyburn, A.L.; Ford, M.E.; Chen, H.; Johanesen, P.A.; Lyras, D.; Moore, R.J. Clostridium perfringens-mediated necrotic enteritis is not influenced by the pre-existing microbiota but is promoted by large changes in the post-challenge microbiota. Vet. Microbiol. 2018, 227, 119–126. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Elbestawy, A.R.; Nahed, A.; Saad, A.M.; Salem, H.M.; El-Tahan, A.M.; Khafaga, A.F.; Taha, A.E.; AbuQamar, S.F.; et al. Necrotic enteritis in broiler chickens: disease characteristics and prevention using organic antibiotic alternatives–a comprehensive review. Poult. Sci. 2022, 101, 101590. [Google Scholar] [CrossRef] [PubMed]
- M'Sadeq, S.A.; Wu, S.-B.; Swick, R.A.; Choct, M. Towards the control of necrotic enteritis in broiler chickens with in-feed antibiotics phasing-out worldwide. Anim Nutr 2015, 1, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P.J.; Guo, Q.; Waag, D.M.; Donnenberg, M.S. The type IV pilin of Burkholderia mallei is highly immunogenic but fails to protect against lethal aerosol challenge in a murine model. Infect. Immun. 2007, 75, 3027–3032. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lux, R.; Pelling, A.E.; Gimzewski, J.K.; Shi, W. Analysis of type IV pilus and its associated motility in Myxococcus xanthus using an antibody reactive with native pilin and pili. Microbiology 2005, 151, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Lepp, D.; Ojha, S.; Gohari, I.M.; Chakravarty, B.; Prescott, J.F.; Gong, J. Immunization with subunits of a novel pilus produced by virulent Clostridium perfringens strains confers partial protection against necrotic enteritis in chickens. Vet. Microbiol. 2019, 230, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Giltner, C.L.; Nguyen, Y.; Burrows, L.L. Type IV pilin proteins: versatile molecular modules. Microbiol. Mol. Biol. Rev. 2012, 76, 740–772. [Google Scholar] [CrossRef] [PubMed]
- Ohkuri, T.; Nagatomo, S.; Oda, K.; So, T.; Imoto, T.; Ueda, T. A protein’s conformational stability is an immunologically dominant factor: evidence that free-energy barriers for protein unfolding limit the immunogenicity of foreign proteins. The J Immunol 2010, 185, 4199–4205. [Google Scholar] [CrossRef]
- Craig, L.; Taylor, R.K.; Pique, M.E.; Adair, B.D.; Arvai, A.S.; Singh, M.; Lloyd, S.J.; Shin, D.S.; Getzoff, E.D.; Yeager, M. Type IV pilin structure and assembly: X-ray and EM analyses of Vibrio cholerae toxin-coregulated pilus and Pseudomonas aeruginosa PAK pilin. Mol. Cell 2003, 11, 1139–1150. [Google Scholar] [CrossRef]
- Fernandes da Costa, S.P.; Mot, D.; Geeraerts, S.; Bokori-Brown, M.; Van Immerseel, F.; Titball, R.W. Variable protection against experimental broiler necrotic enteritis after immunization with the C-terminal fragment of Clostridium perfringens alpha-toxin and a non-toxic NetB variant. Avian Pathol. 2016, 45, 381–388. [Google Scholar] [CrossRef]
- Mot, D.; Timbermont, L.; Delezie, E.; Haesebrouck, F.; Ducatelle, R.; Van Immerseel, F. Day-of-hatch vaccination is not protective against necrotic enteritis in broiler chickens. Avian Pathol. 2013, 42, 179–184. [Google Scholar] [CrossRef]
- Fernandes da Costa, S.P.; Mot, D.; Bokori-Brown, M.; Savva, C.G.; Basak, A.K.; Van Immerseel, F.; Titball, R.W. Protection against avian necrotic enteritis after immunisation with NetB genetic or formaldehyde toxoids. Vaccine 2013, 31, 4003–4008. [Google Scholar] [CrossRef] [PubMed]
- Keyburn, A.L.; Portela, R.W.; Sproat, K.; Ford, M.E.; Bannam, T.L.; Yan, X.; Rood, J.I.; Moore, R.J. Vaccination with recombinant NetB toxin partially protects broiler chickens from necrotic enteritis. Vet. Res. 2013, 44, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.G.L.; Wilde, S.; Tafoya, A.M.; Horsman, J.; Yousif, M.; Diamos, A.G.; Roland, K.L.; Mason, H.S. Evaluation of a toxoid fusion protein vaccine produced in plants to protect poultry against necrotic enteritis. PeerJ 2019, 7, e6600. [Google Scholar] [CrossRef] [PubMed]
- Shojadoost, B.; Vince, A.R.; Prescott, J.F. The successful experimental induction of necrotic enteritis in chickens by Clostridium perfringens: a critical review. Vet. Res. 2012, 43, 1–12. [Google Scholar] [CrossRef]
- Prescott, J.F.; Smyth, J.A.; Shojadoost, B.; Vince, A.R. Experimental reproduction of necrotic enteritis in chickens: a review. Avian Pathol. 2016, 45, 317–322. [Google Scholar] [CrossRef]
- Keyburn, A.L.; Boyce, J.D.; Vaz, P.K.; Bannam, T.L.; Ford, M.E.; Parker, D.; Di Rubbo, A.; Rood, J.I.; Moore, R.J. NetB, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens. PLoS Pathog. 2008, 4, e26. [Google Scholar] [CrossRef]
- Dierick, E.; Ducatelle, R.; Van Immerseel, F.; Goossens, E. Research Note: The administration schedule of coccidia is a major determinant in broiler necrotic enteritis models. Poult. Sci. 2021, 100, 100806. [Google Scholar] [CrossRef]
- Proctor, A.; Phillips, G.J. Differential effects of bacitracin methylene disalicylate (BMD) on the distal colon and cecal microbiota of young broiler chickens. Front. vet. sci. 2019, 6, 114. [Google Scholar] [CrossRef]
- Carrasco, J.M.D.; Redondo, E.A.; Viso, N.D.P.; Redondo, L.M.; Farber, M.D.; Miyakawa, M.E.F. Tannins and bacitracin differentially modulate gut microbiota of broiler chickens. Biomed Res. Int. 2018. [Google Scholar] [CrossRef]
- Shang, Y.; Kumar, S.; Oakley, B.B.; Kim, W.K. Chicken gut microbiota: importance and detection technology. Front. vet. sci. 2018, 5, 254. [Google Scholar] [CrossRef]
- Rychlik, I. Composition and Function of Chicken Gut Microbiota. Animals 2020, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Ji, Z.; Shen, Z.; Xue, Y.; Zhang, B.; Yu, D.; Liu, T.; Luo, D.; Xing, G.; Tang, J.; et al. Increase dietary fiber intake ameliorates cecal morphology and drives cecal species-specific of short-chain fatty acids in white Pekin ducks. Front Microbiol 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Antonissen, G.; Eeckhaut, V.; Van Driessche, K.; Onrust, L.; Haesebrouck, F.; Ducatelle, R.; Moore, R.J.; Van Immerseel, F. Microbial shifts associated with necrotic enteritis. Avian Pathol. 2016, 45, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Elce, A.; Amato, F.; Zarrilli, F.; Calignano, A.; Troncone, R.; Castaldo, G.; Berni Canani, R. Butyrate modulating effects on pro-inflammatory pathways in human intestinal epithelial cells. Benef. Microbes 2017, 8, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Huynh, U.T.D.; Zastrow, M.L. Metallobiology of Lactobacillaceae in the gut microbiome. J. Inorg. Biochem. 2022, 112023. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yan, C.; Liu, T.; Xu, C.; Wen, K.; Liu, L.; Zhao, M.; Jun, Z.; Geng, T.; Gong, D. Research Note: Increase of bad bacteria and decrease of good bacteria in the gut of layers with vs. without hepatic steatosis. Poult. Sci. 2020, 99, 5074–5078. [Google Scholar] [CrossRef]
- Memon, F.U.; Yang, Y.; Zhang, G.; Leghari, I.H.; Lv, F.; Wang, Y.; Laghari, F.; Khushk, F.A.; Si, H. Chicken Gut Microbiota Responses to Dietary Bacillus subtilis Probiotic in the Presence and Absence of Eimeria Infection. Microorganisms 2022, 10, 1548. [Google Scholar] [CrossRef]
- Xu, S.; Lin, Y.; Zeng, D.; Zhou, M.; Zeng, Y.; Wang, H.; Zhou, Y.; Zhu, H.; Pan, K.; Jing, B. Bacillus licheniformis normalize the ileum microbiota of chickens infected with necrotic enteritis. Sci. Rep. 2018, 8, 1744. [Google Scholar] [CrossRef]
- Brenner, D.J.; Farmer, J.J. Bergey’s Manual of Systematics of Archaea and Bacteria; Enterobacteriaceae; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2015; pp. 1–24. [Google Scholar]
- Bortoluzzi, C.; Vieira, B.S.; Lumpkins, B.; Mathis, G.F.; King, W.D.; Graugnard, D.E.; Dawson, K.A.; Applegate, T.J. Can dietary zinc diminish the impact of necrotic enteritis on growth performance of broiler chickens by modulating the intestinal immune-system and microbiota? Poult. Sci. 2019, 98, 3181–3193. [Google Scholar] [CrossRef]
- Abdelli, N.; Pérez, J.F.; Vilarrasa, E.; Cabeza Luna, I.; Melo-Durán, D.; D'Angelo, M.; Solà-Oriol, D. Targeted-release organic acids and essential oils improve performance and digestive function in broilers under a necrotic enteritis challenge. Animals 2020, 10, 259. [Google Scholar] [CrossRef]
| Groups | No. of birds per group | Days of vaccination |
|---|---|---|
| P153 | 28 | 7,14,21 |
| P264 | 28 | 7,14,21 |
| P509 | 28 | 7,14,21 |
| P537 | 28 | 7,14,21 |
| P561 | 28 | 7,14,21 |
| Mix | 28 | 7,14,21 |
| Quil-A | 6 | 7,14,21 |
| Bacitracin | 8 | |
| MLG_7820 | 9 | 7,14,21 |
| P153 | P264 | P509 | P537 | P561 | Bacitracin | Mix | MLG_7820 | |
|---|---|---|---|---|---|---|---|---|
| P264 | 0.928 | - | - | - | - | - | - | - |
| P509 | 0.802 | 0.784 | - | - | - | - | - | - |
| P537 | 0.488 | 0.531 | 0.441 | - | - | - | - | - |
| P561 | 0.213 | 0.216 | 0.273 | 0.082 | - | - | - | - |
| Bacitracin | 0.213 | 0.216 | 0.262 | 0.142 | 0.615 | - | - | - |
| Mix | 0.531 | 0.533 | 0.678 | 0.273 | 0.533 | 0.478 | - | - |
| MLG_7820 | 0.176 | 0.220 | 0.184 | 0.493 | 0.031 | 0.060 | 0.150 | - |
| Quil-A | 0.493 | 0.493 | 0.622 | 0.262 | 0.641 | 0.492 | 0.943 | 0.142 |
| Compared groups | Estimate | ±SE | Z-ratio | p-value adj. |
|---|---|---|---|---|
| P153 - P264 | 114.33 | 65.90 | 1.734 | 0.226 |
| P153 - P509 | 120.05 | 65.90 | 1.821 | 0.208 |
| P153 - P537 | -20.92 | 67.10 | -0.312 | 0.855 |
| P153 - P561 | 166.12 | 65.90 | 2.520 | 0.062 |
| P153 - Bacitracine | -723.11 | 77.20 | -9.367 | < 0.001 |
| P153 - Mix | 23.66 | 68.50 | 0.345 | 0.855 |
| P153 - MLG_7820 | 43.74 | 74.40 | 0.588 | 0.767 |
| P153 - Quil-A | 142.20 | 85.00 | 1.672 | 0.235 |
| P264 - P509 | 5.71 | 64.70 | 0.088 | 0.965 |
| P264 - P537 | -135.26 | 65.90 | -2.052 | 0.148 |
| P264 - P561 | 51.79 | 64.70 | 0.801 | 0.632 |
| P264 - Bacitracine | -837.44 | 76.20 | -10.995 | < 0.001 |
| P264 - Mix | -90.67 | 67.40 | -1.346 | 0.322 |
| P264 - MLG_7820 | -70.59 | 73.30 | -0.962 | 0.532 |
| P264 - Quil-A | 27.87 | 84.10 | 0.331 | 0.855 |
| P509 - P537 | -140.97 | 65.90 | -2.138 | 0.140 |
| P509 - P561 | 46.07 | 64.70 | 0.713 | 0.692 |
| P509 - Bacitracine | -843.16 | 76.20 | -11.070 | < 0.001 |
| P509 - Mix | -96.38 | 67.40 | -1.431 | 0.296 |
| P509 - MLG_7820 | -76.30 | 73.30 | -1.040 | 0.487 |
| P509 - Quil-A | 22.15 | 84.10 | 0.263 | 0.855 |
| P537 - P561 | 187.04 | 65.90 | 2.837 | 0.028 |
| P537 - Bacitracine | -702.19 | 77.20 | -9.096 | < 0.001 |
| P537 - Mix | 44.59 | 68.50 | 0.651 | 0.729 |
| P537 - MLG_7820 | 64.67 | 74.40 | 0.869 | 0.591 |
| P537 - Quil-A | 163.12 | 85.00 | 1.918 | 0.177 |
| P561 - Bacitracine | -889.23 | 76.20 | -11.675 | < 0.001 |
| P561 - Mix | -142.45 | 67.40 | -2.114 | 0.140 |
| P561 - MLG_7820 | -122.37 | 73.30 | -1.668 | 0.235 |
| P561 - Quil-A | -23.92 | 84.10 | -0.284 | 0.855 |
| Bacitracine - Mix | 746.78 | 78.40 | 9.529 | < 0.001 |
| Bacitracine - MLG_7820 | 766.86 | 83.20 | 9.214 | < 0.001 |
| Bacitracine - Quil-A | 865.31 | 92.70 | 9.335 | < 0.001 |
| Mix - MLG_7820 | 20.08 | 75.60 | 0.266 | 0.855 |
| Mix - Quil-A | 118.54 | 86.10 | 1.377 | 0.315 |
| MLG_7820 - Quil-A | 98.46 | 90.60 | 1.087 | 0.466 |
| Comparison | p-value |
| P509 - P561 | 0.004 |
| P509 - Mix | 0.031 |
| P509 - P153 | 0.046 |
| P509 - P264 | 0.007 |
| P509 - P537 | 0.024 |
| P509 - MLG_7820 | 0.077 |
| P509 - Quil-A | 0.06 |
| P561 - Mix | 0.064 |
| P561 - P153 | 0.001 |
| P561 - P264 | 0.001 |
| P561 - P537 | 0.005 |
| P561 - MLG_7820 | 0.002 |
| P561 – Quil-A | 0.017 |
| Mix - P153 | 0.007 |
| Mix - P264 | 0.006 |
| Mix - P537 | 0.008 |
| Mix - MLG_7820 | 0.046 |
| Mix - Quil-A | 0.241 |
| P153 - P264 | 0.06 |
| P153 – P537 | 0.09 |
| P153 - MLG_7820 | 0.019 |
| P153 - Quil-A | 0.009 |
| P264 – P537 | 0.002 |
| P264 - MLG_7820 | 0.053 |
| P264 - Quil-A | 0.015 |
| P537 - MLG_7820 | 0.009 |
| P537 - Quil-A | 0.005 |
| MLG_7820 - Quil-A | 0.325 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





