1. Introduction
Tsetseflies (Diptera, Glossinidae,
Glossina) are blood-sucking cyclical vectors of protozoan trypanosomes that cause sleeping sickness in humans (HAT) and nagana (AAT) in domestic animals [
1,
2]. Tsetseflies inhabit only sub-Saharan Africa [
3] from Kalahari to Namibian deserts in the Southern part and from Sahara to Somali desert in the Northern part [
1]. About 33 species and subspecies of these arthropods have been identified so far [
4] and sub-divided into three subgenera namely Austenina (Fusca group), Nemorhina (Palpalis group), and Glossina (Morsitans group) [
5]. The savannah tsetseflies;
Glossina morsitans, G. pallidipes and G. swynnertoni are the most dominant species in East African region including Tanzania [
6]. There are also species that are occurring with limited distribution include
Glossina brevipalpis,
Glossina longipennis, Glossina fuscipes martinii and
G. fuscipes fuscipes [
7,
8,
9]. Of all identified species, only 6-10 species have public health and veterinary significance [
1,
4,
9]. Examples of those species include
G. pallidipes, G. brevipalpis, G. m. morsistans and G. swynnertoni [
10,
11]. The major vectors of Human African Trypanosomiasis (HAT) in East Africa include;
G. pallidipes and
G. swynnertoni [
8,
13].
Like other animals with learning ability, insects can also learn and adjust their intrinsic and extrinsic behaviours accordingly [
13,
14,
15,
16,
17]. Such learning ability helps insects to locate and assess the quality of resources such as food, breeding sites and mates [
14]. For instance,
Anopheles arabiensis mosquitoes and
Lutzomyia whitmani sandflies can return to the site and host where they were originally collected [
14,
18,
19]. Similarly,
Glossina species can return to same host on second blood meal whenever the feeding interval is within two days [
16]. Such behaviours are sometimes referred to as host fidelity and site fidelity behaviours.
The feeding behaviours of tsetseflies are genetically determined [
20,
21]. They are mostly opportunistic feeders, however in the absence of preferred host, they adapt to feeding on available host(s) [
21]. Their choice to specific host is influenced by multiple factors such as shape of host, colour of host, odour emanation, size of host, and host availability [
21,
22]. Understanding these and other behaviours is critical in designing and implementing surveillance and control strategies [
17,
23,
24]. In depth knowledge of host choice and feeding behaviours of tsetseflies, could be important as it may influence vectors’ parasites transmission [
14] thus facilitating the formation of new strategies to minimize vectors-host contacts especially in settings where human, wildlife and livestock interact. Similarly, such knowledge may be useful during monitoring and evaluation of tsetse surveillance and control programs [
22]. Despite such urge and adverse impact in public health, the behaviours of most hematophagous insects including tsetseflies are understudied. Therefore, in this study we investigated the host choice and feeding behaviours of
Glossina morsitans, one of the most predominant tsetse vector species in Tanzania and elsewhere in Africa.
2. Materials and Methods
2.1. Tsetseflies rearing
A colony of
Glossina morsitans was established using pupa from the Tsetse and Vector Control Centre- Tanga, Tanzania. The pupa was transferred into large fine-meshed emergence cages and rearing cages, and thereof maintained under ambient conditions at 25±2°C, 70±2% RH, and 12 h photo-phase within an insectary at the Institute of Pest Management (IPM), Sokoine University of Agriculture, Tanzania. Emerging flies were sorted by sex, and transferred to separate cages; either 25 flies (7.5 × 5 × 4 cm) or 40 flies (13.5 × 8 × 4.5 cm). Virgin female flies were mated with 6 to 8 day-old virgin males in separate cages at 1:3 male to female ratios [
25,
26]. While in the insectary, different cohorts of adult tsetse, consisting of at least 80-100 tsetse flies, were respectively blood-fed on a guinea pig (
Cavia porcellus), rodent (
Cricetomys gambianus), rabbit (
Oryctolagus cuniculus), and squirrel (
Paraxerus ochraceus). The blood-feeding process was done for 3 hours on Monday, Wednesday, and Friday every week from 11:00 am to 2:00 pm for five weeks.
2.2. Experimental setup
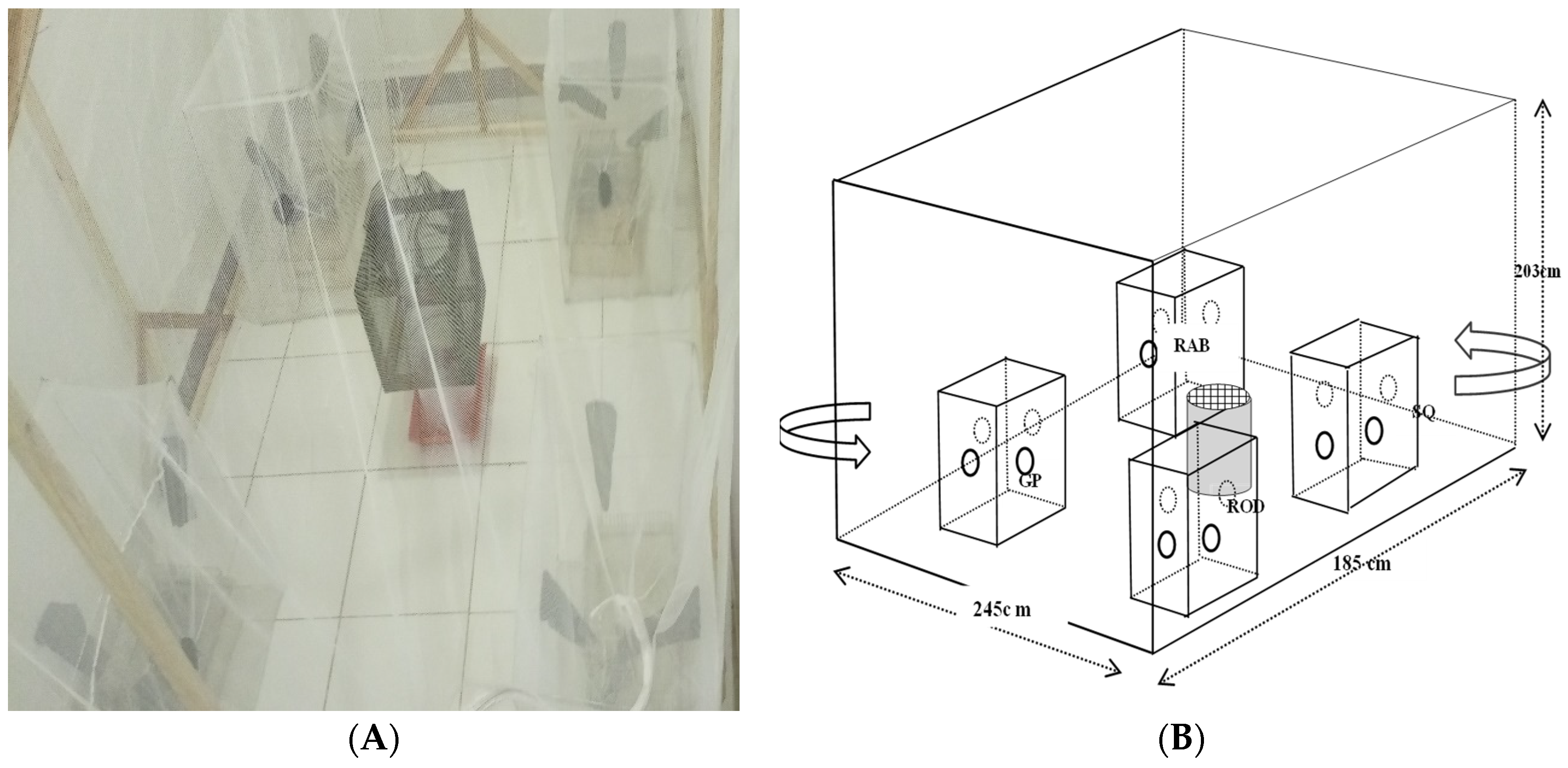
Large semi-field cage made of inert mosquito nets (size: 245 cm x 185 cm x 203 cm) was constructed and placed inside a room (size: 336 cm x 195cm x 308cm) with ambient conditions similar to those of the rearing insectary. Then, four small screen cages of same size (size: 62cm x 42cm x 62cm) were fixed one in each of the four sides of the semi-field cage (
Figure 1). The host screen cages were made of metal bars and improvised with four openings, one on each of their four sides, through which the host seeking tsetse would access hosts. The openings were tapered such that tsetseflies visiting the large cages were unable to leave. The large semi-field and screen cages were regularly checked for intactness to prevent experimental tsetse flies from escaping to the outside.
2.3. Assessing the host choice and feeding success of offspring whose mothers were fed on different host species
Using a 4 by 4 Latin Square design, four host species (1-guinea pig, 1-rodent, 1- rabbit, and 1-squirrel) were placed in each of the four screen cages inside the large semi-field cage (
Figure 1). In each replicate, four cohorts of offspring (20 tsetse flies each) obtained from mothers, for
Glossina morsitans species, blood-fed on the different hosts and labelled with different colour of fluorescent powder, were released simultaneously at the centre of the large semi-field cage. The released tsetseflies were starved for 72 hours to maximize their physiological demand for blood and urge for host seeking. After release, the tsetseflies were left to forage for 24 hours and recaptured independently from all the four screen cages and elsewhere in the semi-field system using aspirators. Recaptured tsetseflies were identified and categorized as fed, unfed, live, or dead and the location collected (hosts’ screen cage). This experiment was repeated four times; and in each time host was randomly rotated across the four cages. To assess the feeding success of released flies, the proportion of live or dead engorged flies obtained in host choice experiment were compared. By observing the abdomen, engorged recaptured flies were sorted out, counted and recorded.
2.4. Determination of Haemoglobin (Hb) concentrations and total blood protein of blood samples from the experimental host species
Haemoglobin concentration and total blood protein were assessed in all hosts before they were deployed in the experiment. Prior to blood samples collection, hosts were anaesthetized shortly using diethyl ether for about 2 minutes. Using micro-capillary tubes, blood samples (2 mL per host) were drawn from the retro-orbital sinus and transferred into two well labelled EDTA K3 2.5 mL tubes. These samples were shipped to the laboratory for analysis inside a cool box with recyclable ice.
Haemoglobin concentration (Hb) was determined using Cyanomethemoglobin method. The blood samples were gently mixed before taking 0.02ml of the sample using pipette. Excess blood on the pipette surface was wiped using clean tissue paper. The individual samples were then transferred into test tubes containing 5 ml Drabkin’s reagents. The tubes containing samples were stoppered then gently mixed, and then left for 10 minutes for maximum colour development. The samples were then poured into the cuvette, where, absorbance at 540 nm versus a reagent blank were compared [
27]. Haemoglobin concentration (mg/ml) and percentage (%) were obtained and recorded accordingly.
Biuret method was used to determine total blood protein for all collected blood samples following Erba Total Protein protocol. This method involves the formation of blue-violet ion complex resulting from the reaction between peptide bonds of protein and copper II ions in alkaline solution.
2.5. Data analysis
The data were entered, cleaned and organized in Microsoft Excel 2010 prior to statistical analysis. The variation in total number of flies that entered screen cages and the proportion of flies returned to the same host were analysed using a Generalized Linear Mixed Models (GLMMs) in R statistical software version 4.2.2. Hetero-scedasticity and non-normal distribution of count data was confirmed using Bartlett’s test and Shapiro-Wilk test respectively. Hence, negative binomial distribution (glmer.nb function of the lme4 package) was used to account for over-dispersion of the data. An initial model fixed the number of flies entered screen cages as dependent factor predicted by fixed factors; Hb concentration, total blood protein, screen cage, blood meal sources used to maintain parents, average temperature and relative humidity. Cage position was set as random effect in all models. Insignificant fixed predictors were removed from the model until the lower Akaike Information Criterion (AIC) was achieved.
2.6. Ethical approval
Ethical clearance for conducting this particular study were obtained from Sokoine University of Agriculture Research and Publication Committee (reference number SUA/DRRTC/R/186/18) and the Tanzanian Commission for Science and Technology (reference number: 2022-735-NA-2022-082).
3. Results
3.1. Choice of adult tsetsefly, Glossina morsitans, on different host species
A total of 320 adult
G.morsitans were released for the host choice experiment where 292 (91.25% of released) were recovered and 28 (8.75% of released flies) un-recovered. Out of the recovered adult tsetse, 213 (72.95%) were attracted to different hosts: Rodent (n= 80, 27.4%), Rabbit (n= 59, 20.21%), Guinea pig (n= 49, 16.78%), and squirrel (n=25, 8.56%). The remaining flies (n=51, 17.47% of recovered flies) were collected in the large semi-field cage (
Table 01). The number of flies attracted to individual hosts varied significantly regardless of the position (χ²
4= 33.685, p= 0.0001). Rodent attracted the highest number of flies (p=0.006) followed by Rabbit (p=0.331), Guinea pig (p=0.057) and squirrel (p=0.005) (
Table 1). Nevertheless, the difference in number of flies attracted to Guinea pig and Rodent (p= 0.001), Rabbit and rodent (p= 0.003), Guinea pig and squirrel (p=0.002), Rabbit and Squirrel (p<0.001), and Rodent and Squirrel (p<0.001) was statistically significant.
The number of offspring attracted to their parent’s blood meal source varied significantly regardless of the position (χ²
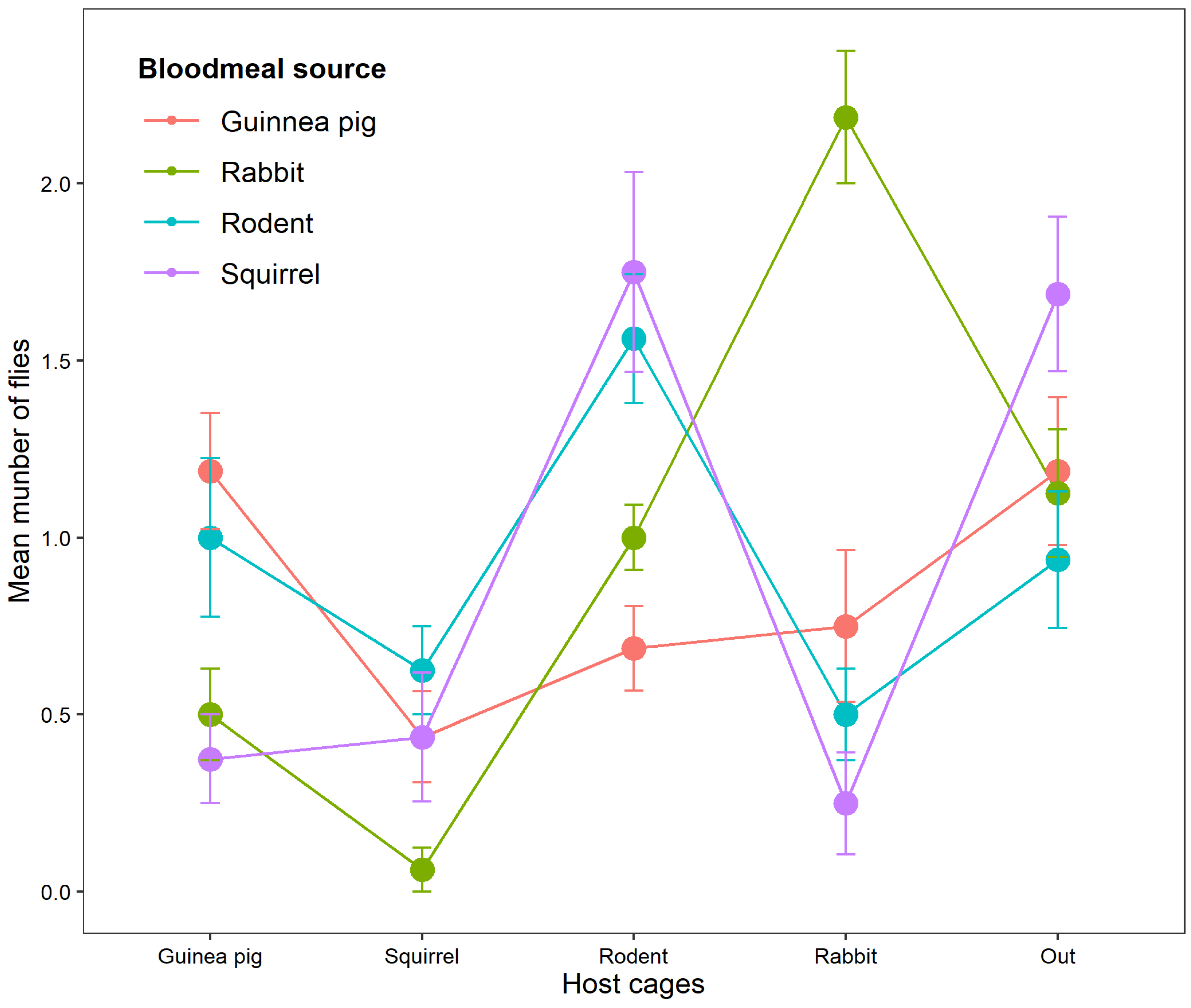
12 = 56.476, p<0.001). Furthermore, the highest number of offspring flies attracted to their parent’s bloodmeal source was observed in rabbits (n= 35, 59.32%, p<0.001), rodent (n=25, 31.25%, p=0.043) and guinea pig (n= 19, 38.78%, p=0.45). Considering the contribution of offspring from different bloodmeal source attracted to individual hosts in the host choice experiment: rodents attracted more flies from squirrel (n=28, 35%), and rodent blood (n=25, 31.25%); guinea pig attracted more of the flies from guinea pig blood (n= 19, 38.78%) and Rodent blood (n= 16, 32.65%) ; rabbits attracted more of flies from rabbit blood (n=35, 59.32%) and guinea pig (n=12, 20.34%); finally, squirrels attracted more of flies from rodent blood (n=10, 40%) (
Table 1). The distribution of mean number of flies that were attracted to parent’s bloodmeal source is shown in
Figure 2 and
Table 1. Interestingly, most of the offspring flies whose parents were fed on squirrels did not visit any host including the squirrels themselves (n= 27, 34.18% of total number flies outside the host cages) (
Figure 2). Unlike the significant variation in the number of offspring flies attracted to rabbits, guinea pigs and rodents (p<0.001), no significance variation was observed in the number of flies attracted to Squirrel (χ²
3 = 4.9624, p= 0.1746).
3.2. Feeding success of tsetseflies attracted to different hosts
Of the flies attracted to different hosts, only 39 flies (18.31%, alive=6, dead =33) successively bloodfed on hosts. The number of flies that were attracted and successfully fed varied across the different hosts (χ²
4=49.478, p<0.001): Guinea pigs (n=10, 25.64%), Rodents (n=23, 58.97%), Rabbits (n=6, 15.38%). None of the flies attracted to squirrels were bloodfed. Most of the flies that were attracted and successively fed on rodent (n=13, 56.52%) originates from parents maintained on blood from squirrels (
Table 2).
3.3. Haemoglobin concentration (Hb) and total plasma protein in different hosts
Of all hosts used in the choice experiment, squirrels had the highest Hb concentration (Mean: 19.32 ± 0.51 g/dl) while rabbits had the least (Mean: 14.515 ± 0.05 g/dl). Furthermore, rodents had the highest total plasma protein (Mean: 75.17 ± 0.497 g/dl) and squirrels had the least among all (Mean: 7.756 ± 0.028 g/dl) (
Table 3). There was statistically significant difference in both mean Hb concentration (χ²
3= 155.24, p<0.001) and total blood protein (χ²
3= 302.91, p<0.001) between host. The number of flies attracted to specific hosts insignificantly correlated with neither host’s haemoglobin concentration (r (1) =-0.03, p= 0.5368) nor the total plasma protein (r (1) = 0.05, p= 0.3431). Furthermore, the number of bloodfed flies, positively correlated with host’s haemoglobin concentration (Hb) (r (1) = 0.17, p= 0.002) and insignificantly correlated with the total plasma protein (r (1) = 0.04, p = 0.478).
4. Discussion
This study assessed the variation of the host choice and feeding success behaviours of Glossina morsitans siblings whose parents were maintained from guinea pig, rabbit, rodent, and squirrel.
The results show the variation in the proportion of attracted tsetseflies across individual hosts. Rodent attracted the highest proportion of released flies (27.4%) followed by Rabbit (20.21%), Guinea pig (16.78%) and squirrel (8.56%). This can be explained by the variation in the level of hosts’ attractiveness to the flies. Host’s cues such as odour emanated from hosts’ body triggers flies searching behaviours while hosts shape, colour and size determines their choice to specific host [
21,
28]. It is likely that rodents and rabbits had relatively larger body size than Squirrel and Guinea pig, hence influenced their level of attractiveness to the flies. Similar finding was also reported in other studies [
29,
30], where hosts with larger body size attracted relatively larger proportion of flies than hosts with small body size. Example, most tsetseflies were attracted to cattle and donkey compared to monitor lizard, goat and sheep. Moreover, rodents had buff-grey pelage and Rabbits had black pelage while Squirrel had dull yellowish-brown pelage and Guinea pig had yellow-white pelage. Considering tsetseflies’ preference to black/dark colours [
31,
32,
33,
34], it is possible that, deployed hosts colours influenced the flies’ host choice. Example, one of these studies reported higher proportion of flies being attracted to hosts with relatively dark pelage than those with yellow pelage/colour [
32].
Likewise, rabbits attracted the highest proportion (59.32%) of flies originating from parents that were maintained on rabbits followed by rodents which attracted more of the flies (31.25%) whose parents bloodfed on rodents. This can be attributed to hosts’ differential attractiveness to released flies. Similar studies done elsewhere which deployed teneral flies, reported similar results where hosts with larger body size (cow) attracted more flies than that with small body size (lizards) [
15,
30]. The study done on mosquitoes by reported the evidence that host choice to mosquitoes can be explained by physiological/behavioural conditioning rather than genetic variability [
35]. This may be true in case of deployed tsetseflies where feeding of the flies on experimental hosts, was done in only one generation. Hence, there could be less chance for parents’ behaviour to be inherited by their siblings if at all existed.
Interestingly, about 34% of the flies whose parents bloodfed of squirrels remained in large semi-field cage (did not visit any screen cage that contained hosts). This can be explained by the variation of the flies’ physical fitness and their ability to detect hosts. Several studies have reported the influence of the blood quality on physiology and biology of flies [
36,
37]. These studies reported the impact of bloodmeal sources on mosquitoes’ feeding rates, survivorship, and fecundity [
36], as well as the variation in feeding activities and reproductive capacity and efficiency [
37]. Since hosts’ haematological properties influences blood nutrition [
38] and is known to vary across species [
39], this could have influenced flies physical fitness. Squirrels had the least total plasma protein and largest Haemoglobin (Hb) concentration among all (
Table 3). Most of these offspring which remained in large cage were originating from parents that were maintained on squirrels. Future studies may perhaps focus on assessing the influence of hosts’ haematological parameters on tsetse siblings’ behaviours.
Despites higher proportion of attracted flies on rabbit and rodents, only 28.75% (23 flies) and 10.17% (6 flies) of attracted flies fed successfully on these hosts respectively. This finding can be explained by the variation in level of hosts’ defensive behaviour which affects flies’ feeding success [
40,
41,
42,
43]. It is possible that, the hosts deployed in this study varied on the level of their defensive behaviours hence affected flies’ feeding success. This finding agree with the study which reported reduced feeding success of tsetseflies due to hosts’ defensive behaviour [
44]. And, the study which reported relatively higher feeding rate of
G.pallidipes on adult cattle compared to the young cattle due to variation on the level of their defensive behaviours [
45]. In addition to above factor, nature of the hosts’ furs could have also affected flies feeding success. Rabbit’s body, unlike that for rodent was covered with long dense furs which could have minimized the surface area available for the attracted flies to feed on. Finally, the taste system determines attracted flies’ biting decision [
24]. The difference in chemical signatures which results from host’s dermal secretion or metabolism of microbiota could have influenced flies’ biting decision. But, since these factors were not assessed in this study, we lack evidences to confirm their influence on observed behaviours. Future studies can be done to assess the influence of host chemical signatures on the behaviours of tsetsefly siblings using similar hosts species in Tanzania.
5. Conclusions
This study reports the varied proportion of tsetseflies’ siblings attracted and successfully bloodfed on different hosts that were used to maintain their parents. It was hard to confirm the presence of the inherited behaviours of the parents to their siblings, however only hosts related factors explained the observed variations in deployed tsetseflies siblings. Future studies need to be done to assess the same behaviours using more species of tsetseflies and small mammals which on the other hand could be alternative blood hosts of these flies in absence of their preferred hosts. Resulting findings will inform tsetse control programs on the possible ways of altering offspring choice and feeding behaviours as a way of controlling African Trypanosomiasis.
Author Contributions
Conceptualization, F.E.M, J.L, E.M, and L.L.M.; methodology, F.E.M, E.M and L.L.M.; formal analysis, F.E.M and L.L.M.; writing—original draft preparation, F.E.M.; writing—review and editing, F.E.M, E.M, and L.L.M.; visualization, F.E.M.; supervision, E.M and L.L.M. All authors have read and agreed to the published version of the manuscript.
Funding
The financial support for conducting this study was obtained from “The Centre of Excellence for Innovative Rodent Pest Management and Biosensor Technology Development (ACE II IRPM-BTD)”.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The authors sincerely appreciate the advice and support from staffs of the Institute of Pest Management (IPM) and Tanzania Veterinary Laboratory Agency (TVLA) Tanga.
Conflicts of Interest
The authors declare no conflict of interest.
References
- M. J. B. Vreysen, M. T. Seck, B. Sall, and J. Bouyer, “Tsetse flies: Their biology and control using area-wide integrated pest management approaches,” J. Invertebr. Pathol., vol. 112, no. SUPPL.1, pp. S15–S25, 2013. [CrossRef]
- R. S. Gashururu et al., “An update on the distribution of Glossina (tsetse flies) at the wildlife-human-livestock interface of Akagera National Park, Rwanda,” Parasites and Vectors, vol. 14, no. 1, pp. 1–13, 2021. [CrossRef]
- World Health Organization, “Control and surveillance of human African trypanosomiasis.,” World Health Organ. Tech. Rep. Ser., no. 984, pp. 1–237, 2013.
- R. H. Gooding and E. S. Krafsur, “Tsetse genetics: Contributions to biology, systematics, and control of tsetse flies,” Annu. Rev. Entomol., vol. 50, no. 69, pp. 101–123, 2005. [CrossRef]
- G. Cecchi, R. C. Mattioli, J. Slingenbergh, and S. De La Rocque, “Land cover and tsetse fly distributions in sub-Saharan Africa,” Med. Vet. Entomol., vol. 22, no. 4, pp. 364–373, 2008. [CrossRef]
- B. Bouteille, “Human African Trypanosomiasis,” vol. 2, pp. 618–624, 2014. [CrossRef]
- S. K. Moloo, “Distribution of Glossina species in Africa,” Acta Trop., vol. 42, no. 3, pp. 275–281, 1985.
- A. I. I. Malele et al., “Glossina dynamics in and around the sleeping sickness endemic Serengeti ecosystem of northwestern Tanzania Glossina dynamics in and around the sleeping sickness endemic Serengeti ecosystem of northwestern Tanzania,” vol. 32, no. 2, pp. 263–268, 2007. [CrossRef]
- E. J. Kweka et al., “Major Disease Vectors in Tanzania : Distribution , Major Disease Vectors in Tanzania : Distribution , Control and Challenges Control and Challenges”. [CrossRef]
- Malele et al., “The use of specific and generic primers to identify trypanosome infections of wild tsetse flies in Tanzania by PCR,” Infect. Genet. Evol., vol. 3, no. 4, pp. 271–279, 2003. [CrossRef]
- I. Malele et al., “Multiple Trypanosoma infections are common amongst Glossina species in the new farming areas of Rufiji district , Tanzania,” pp. 1–8, 2011. [CrossRef]
- E. E. Makhulu et al., “Tsetse blood-meal sources, endosymbionts and trypanosome-associations in the Maasai mara national reserve, a wildlife-human-livestock interface,” PLoS Negl. Trop. Dis., vol. 15, no. 1, pp. 1–18, 2021. [CrossRef]
- J. Jaenike, “Host specialization in phytophagous insects,” Annu. Rev. Ecol. Syst., vol. 21, no. 1, pp. 243–273, 1990. [CrossRef]
- J. Mccall and D. W. Kelly, “Learning and memory in disease vectors,” vol. 18, no. 10, pp. 429–433, 2002. [CrossRef]
- J. Bouyer, D. Cuisance, S. Messad, and P. Guerin, “Learning affects host preference in tsetse flies,” Rev. d’levage M‚decine V‚t‚rinaire des Pays Trop., vol. 58, no. 1/2, p. ’27-29, 2005, [Online]. Available: z:%5C6608.pdf.
- J. Bouyer, M. Pruvot, Z. Bengaly, P. M. Guerin, and R. Lancelot, “Learning influences host choice in tsetse,” Biol. Lett., vol. 3, no. 2, pp. 113–117, 2007. [CrossRef]
- C. Vinauger, C. Lahondère, A. Cohuet, C. R. Lazzari, and J. A. Riffell, “Learning and Memory in Disease Vector Insects,” Trends Parasitol., vol. 32, no. 10, pp. 761–771, 2016. [CrossRef]
- P. J. McCall, F. W. Mosha, K. J. Njunwa, and K. Sherlock, “Evidence for memorized site-fidelity in Anopheles arabiensis,” Trans. R. Soc. Trop. Med. Hyg., vol. 95, no. 6, pp. 587–590, 2001. [CrossRef]
- D. H. Campbell-Lendrum, S. P. Brandão-Filho, P. D. Ready, and C. R. Davies, “Host and/or site loyalty of Lutzomyia whitmani (Diptera: Psychodidae) in Brazil,” Med. Vet. Entomol., vol. 13, no. 2, pp. 209–211, 1999. [CrossRef]
- B. WEITZ, “The feeding habits of Glossina.,” Bull. World Health Organ., vol. 28, pp. 711–729, 1963.
- J. A. Onyiah, “Mechanism of host selection by tsetse flies,” Int. J. Trop. Insect Sci., vol. 1, no. 01, pp. 31–34, 1980. [CrossRef]
- P. H. Clausen, I. Adeyemi, B. Bauer, M. Breloeer, F. Salchow, and C. Staak, “Host preferences of tsetse (Diptera: Glossinidae) based on bloodmeal identifications,” Med. Vet. Entomol., vol. 12, no. 2, pp. 169–180, 1998. [CrossRef]
- P. J. McCall and D. W. Kelly, “Learning and memory in disease vectors,” Trends Parasitol., vol. 18, no. 10, pp. 429–433, 2002. [CrossRef]
- R. B. Barrozo, “Food recognition in hematophagous insects,” Curr. Opin. Insect Sci., vol. 34, pp. 55–60, 2019. [CrossRef]
- K. Camara, K. Ilboudo, E. W. Salou, and G. Gimonneau, “Evaluation of different blood-feeding frequencies on Glossina palpalis gambiensis performance in a mass-rearing insectary,” Parasites and Vectors, vol. 14, no. 1, pp. 1–8, 2021. [CrossRef]
- E. Opiyo, A. G. Parker, and A. H. Mohammed, “Standard operating procedures for mass rearing tsetse flies,” Vienna, Austria: FAO/IAEA, p. 239, 2006.
- L. P. Posner, H. N. Erb, and R. D. Gleed, “The HemoCue® for point-of-care hemoglobin measurement and packed cell volume estimation in cats,” J. Vet. Emerg. Crit. Care, vol. 15, no. 1, pp. 22–25, 2005. [CrossRef]
- Y. P. Nagagi, R. S. Silayo, and E. J. Kweka, “Advancements in bait technology to control Glossina swynnertoni Austen, the species of limited distribution in Kenya and Tanzania border: A review,” J. Vector Borne Dis., vol. 54, no. 1, pp. 16–24, 2017.
- W. P. Boyt, P. K. I. Mackenzie, and R. D. Pilson, “The relative attractiveness of donkeys , cattle , sheep and goats to Glossina morsitans morsitans Westwood and G . pallidipes Austen ( Diptera : Glos- sinidae ) in a middle-veld area of Rhodesia,” no. November 1971, pp. 497–500, 1978. [CrossRef]
- J. Bouyer and D. C. S. M. P. M. Guerin, “Learning Affects Host Preference in Tsetse Flies,” pp. 27–29, 2007. [CrossRef]
- C.H. Green, “The use of two-coloured screens for catching Glossina palpalis palpalis,” Bull. Entomol. Res., vol. 79, pp. 81–93, 1989. [CrossRef]
- S. J. TORR, “The host-orientated behaviour of tsetse flies (Glossina): the interaction of visual and olfactory stimuli,” Physiol. Entomol., vol. 14, no. 3, pp. 325–340, 1989. [CrossRef]
- C. H. Green, “The effects of odours and target colour on landing responses of Glossina morsitans morsitans and G. pallidipes (Diptera: Glossinidae),” Bull. Entomol. Res., vol. 83, no. 4, pp. 553–562, 1993. [CrossRef]
- G. Gibson and S. J. Torr, “Visual and olfactory responses of haematophagous Diptera to host stimuli,” Med. Vet. Entomol., vol. 13, no. 1, pp. 2–23, 1999. [CrossRef]
- C. Mwandawiro, M. Boots, N. Tuna, and W. Suwonkerd, “Transactionsoftheroyalsocietyoftropicalmedicineandhygiene(2000)94,238-242,” Trop. Med., pp. 238–242, 2000. [CrossRef]
- S. Phasomkusolsil et al., “Maintenance of mosquito vectors : effects of blood source on feeding , survival , fecundity , and egg hatching rates,” no. June, pp. 38–45, 2013. [CrossRef]
- H. S. Al-rashidi, K. M. Alghamdi, W. M. Al-otaibi, H. M. Al-solami, and J. A. Mahyoub, “Saudi Journal of Biological Sciences Effects of blood meal sources on the biological characteristics of Aedes aegypti and Culex pipiens ( Diptera : Culicidae ),” Saudi J. Biol. Sci., vol. 29, no. 12, p. 103448, 2022. [CrossRef]
- S. A. Khan et al., “Human blood type influences the host-seeking behavior and fecundity of the Asian malaria vector Anopheles stephensi,” Sci. Rep., vol. 11, no. 1, pp. 1–12, 2021. [CrossRef]
- C. M. Hawkey, P. M. Bennett, S. C. Gascoyne, M. G. Hart, and J. K. Kirkwood, “Erythrocyte size, number and haemoglobin content in vertebrates,” Br. J. Haematol., vol. 77, no. 3, pp. 392–397, 1991. [CrossRef]
- E. D. Walker and J. D. Edman, “The influence of host defensive behavior on mosquito (diptera: culicidae) biting persistence,” J. Med. Entomol., vol. 22, no. 4, pp. 370–372, 1985. [CrossRef]
- S. J. Torr, P. J. Wilson, S. Schofield, T. N. C. Mangwiro, S. Akber, and B. N. White, “Application of DNA markers to identify the individual-specific hosts of tsetse feeding on cattle,” Med. Vet. Entomol., vol. 15, no. 1, pp. 78–86, 2001. [CrossRef]
- N. Lyimo and H. M. Ferguson, “Ecological and evolutionary determinants of host species choice in mosquito vectors,” Trends Parasitol., vol. 25, no. 4, pp. 189–196, 2009. [CrossRef]
- W. Takken et al., “Larval nutrition differentially affects adult fitness and Plasmodium development in the malaria vectors Anopheles gambiae and Anopheles stephensi,” Parasites and Vectors, vol. 6, no. 1, 2013. [CrossRef]
- S. Schofield and S. J. Torr, “A comparison of the feeding behaviour of tsetse and stable flies,” Med. Vet. Entomol., vol. 16, no. 2, pp. 177–185, 2002. [CrossRef]
- S. J. Torr and T. N. C. Mangwiro, “Interactions between cattle and biting flies: Effects on the feeding rate of tsetse,” Med. Vet. Entomol., vol. 14, no. 4, pp. 400–409, 2000. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).