Submitted:
22 September 2023
Posted:
26 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Basic Equations
2.1. Johnson-Mehl-Avrami-Kolmogorov (JMAK) – Equation
2.2. Description of the Rates of Nucleation and Growth and the Kinetics of Relaxation
2.3. Possible Extensions
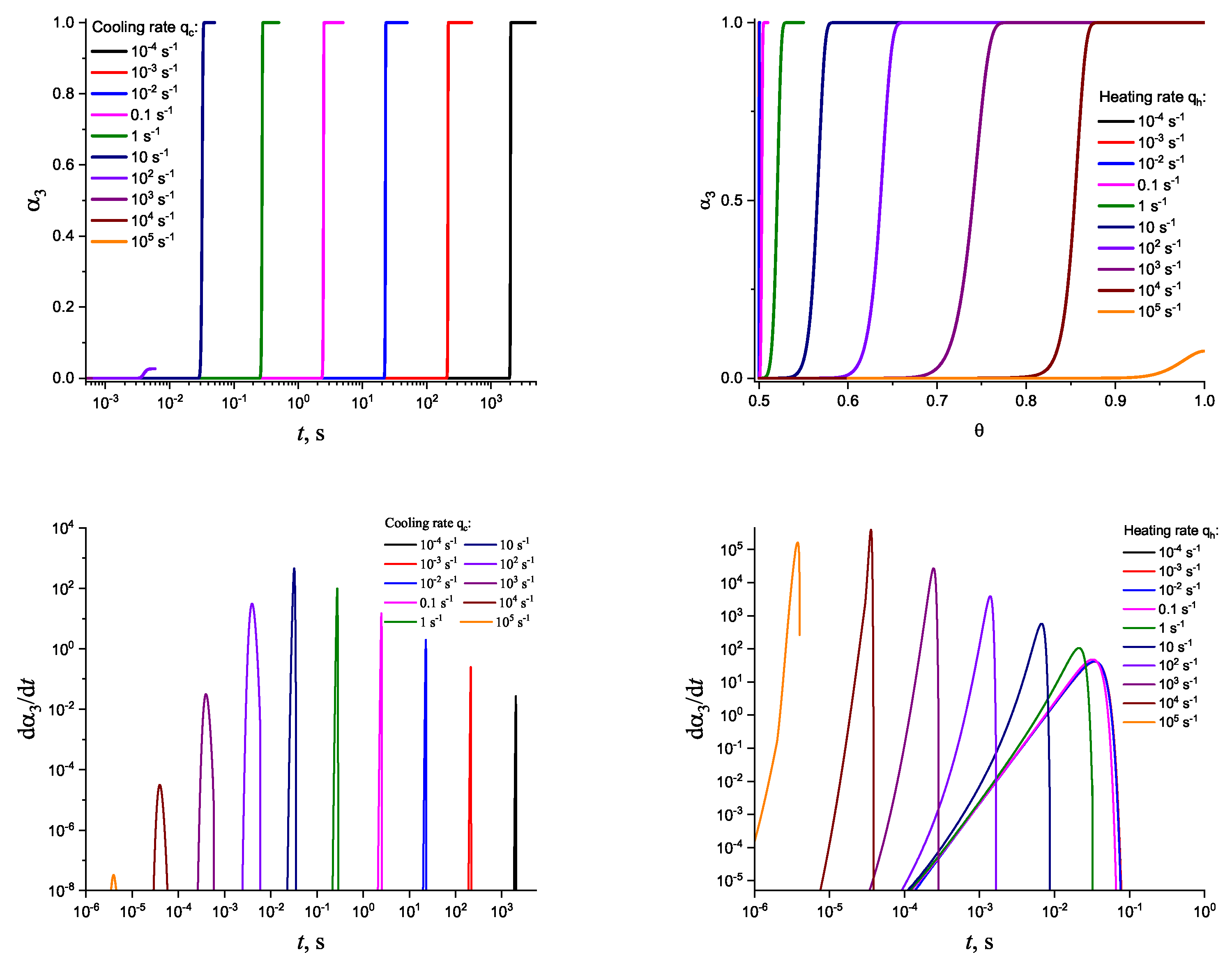
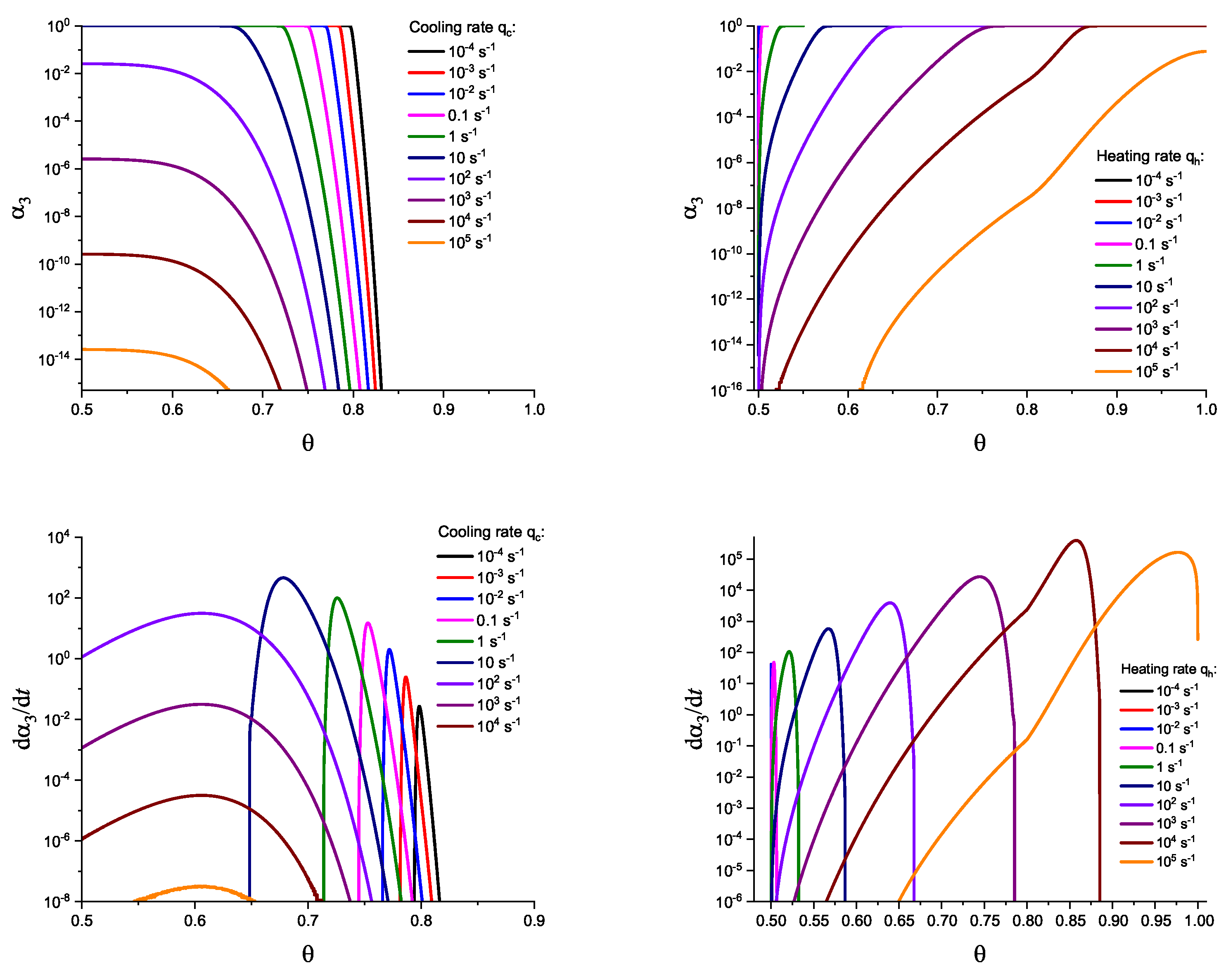
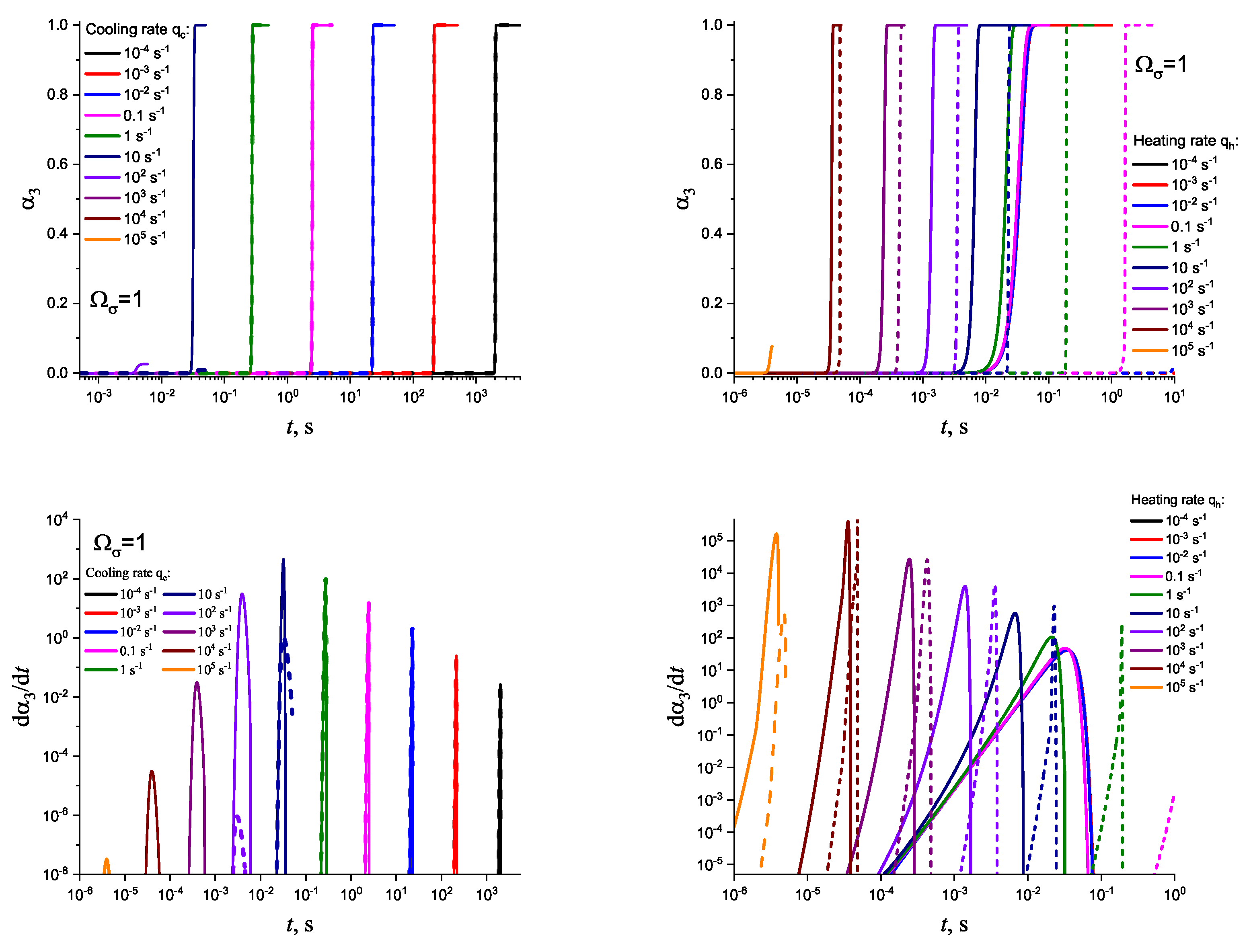
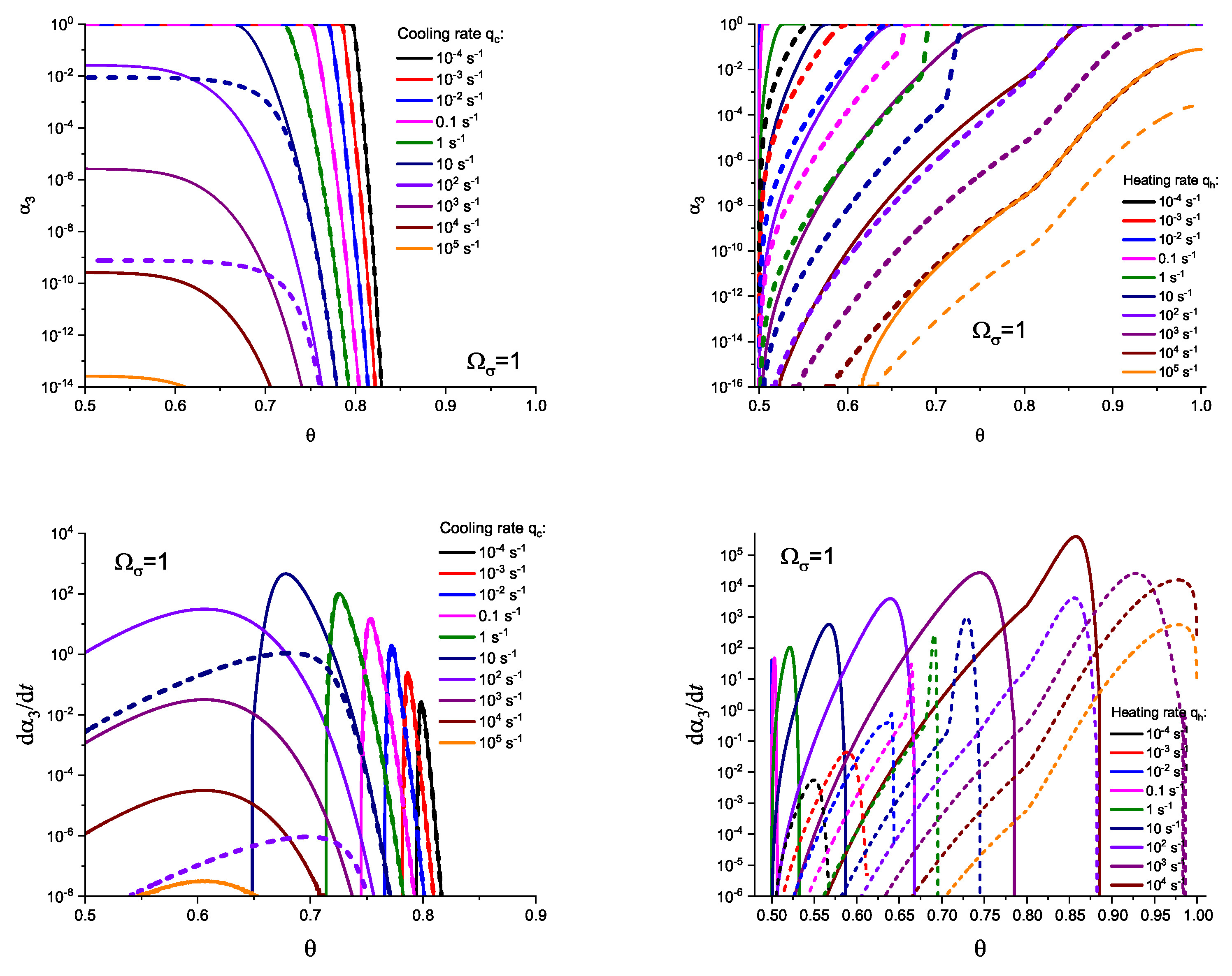
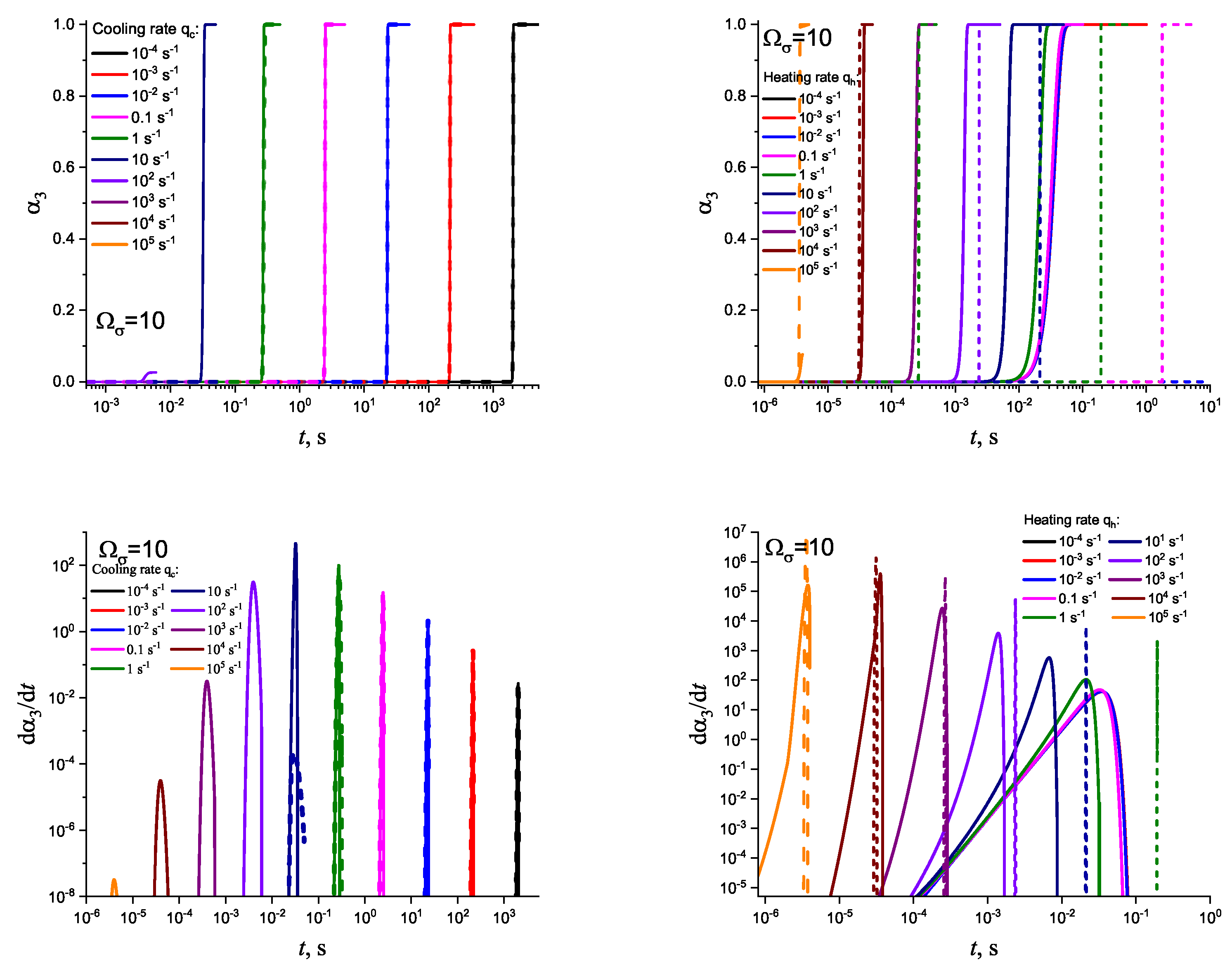
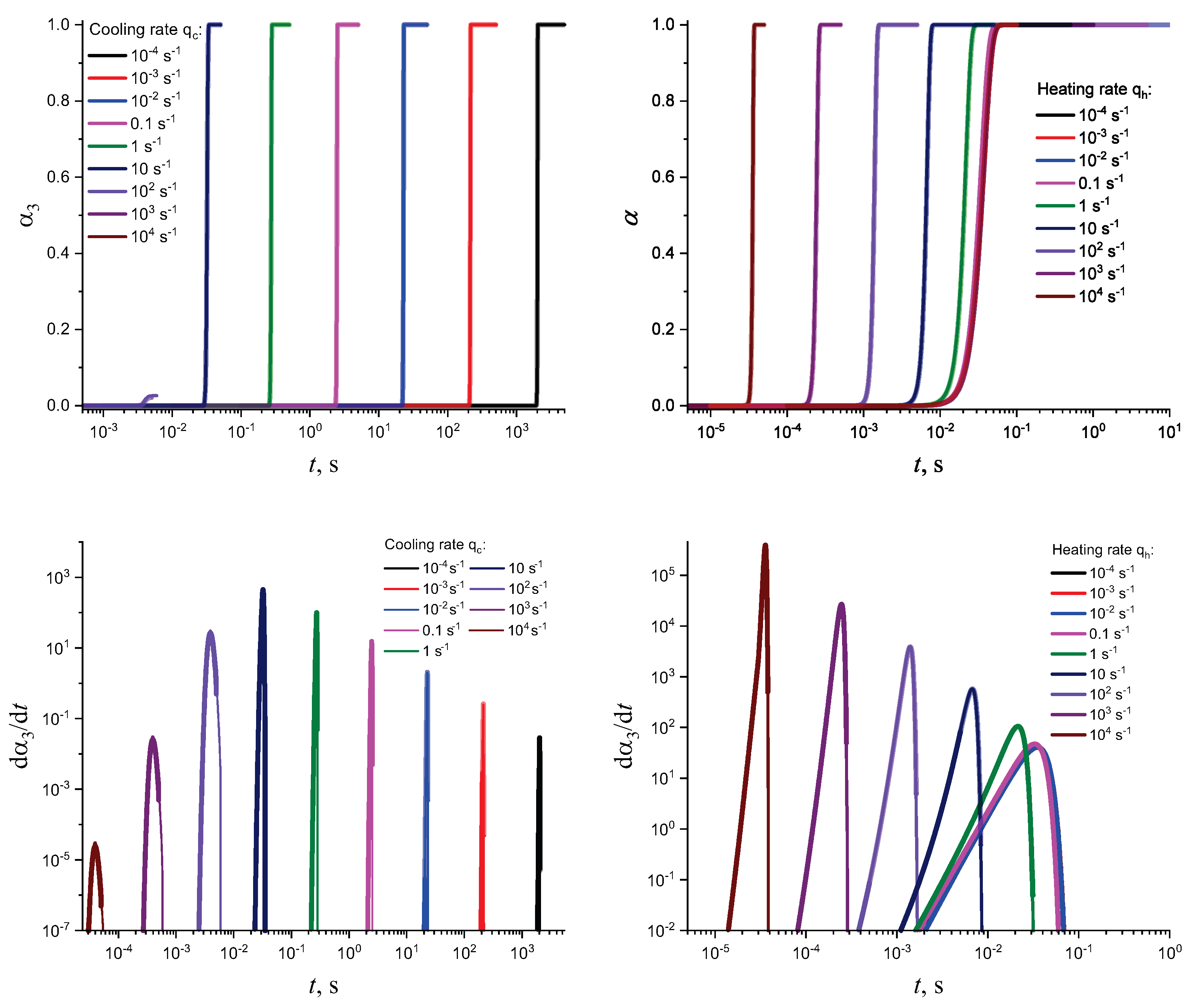
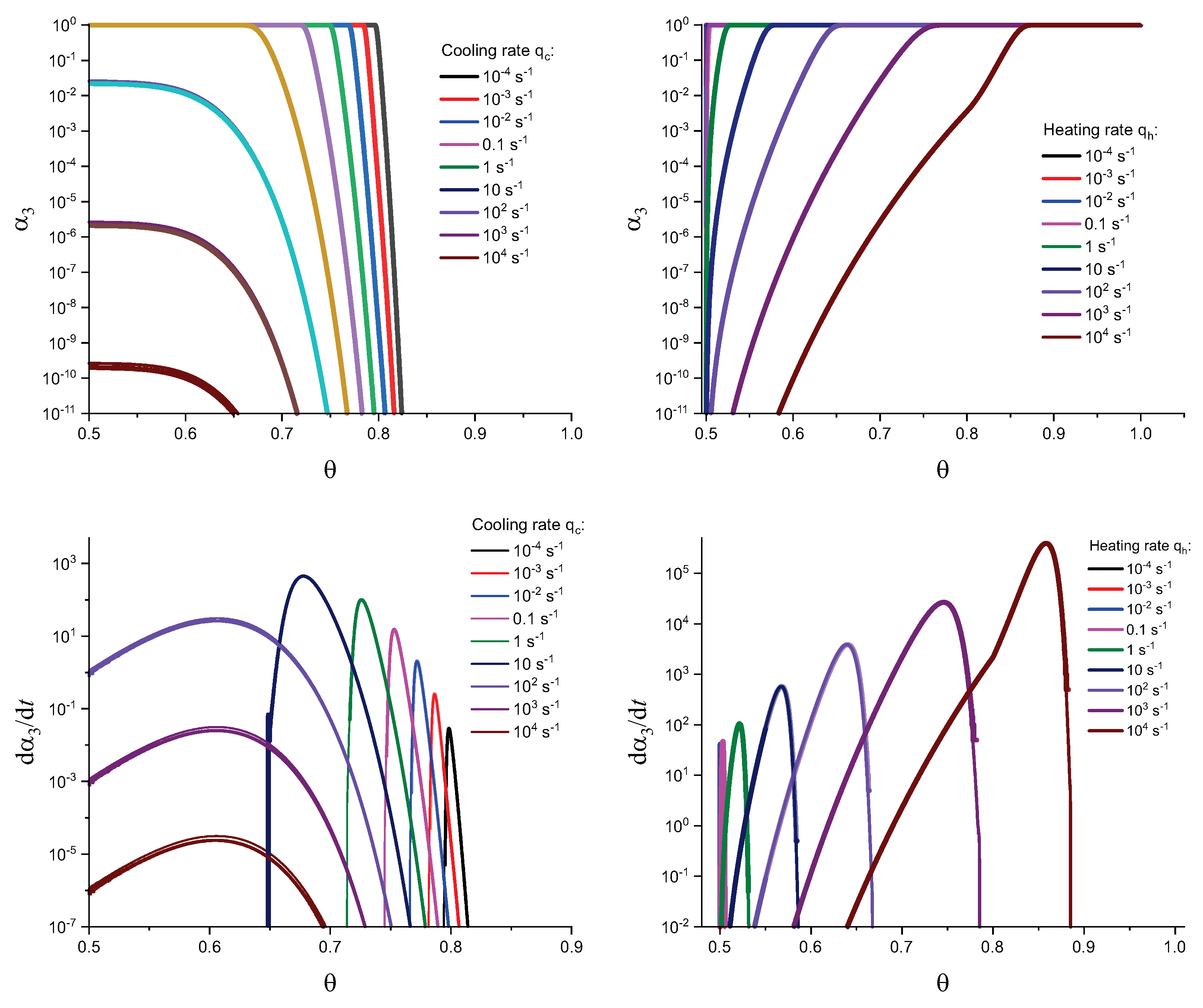
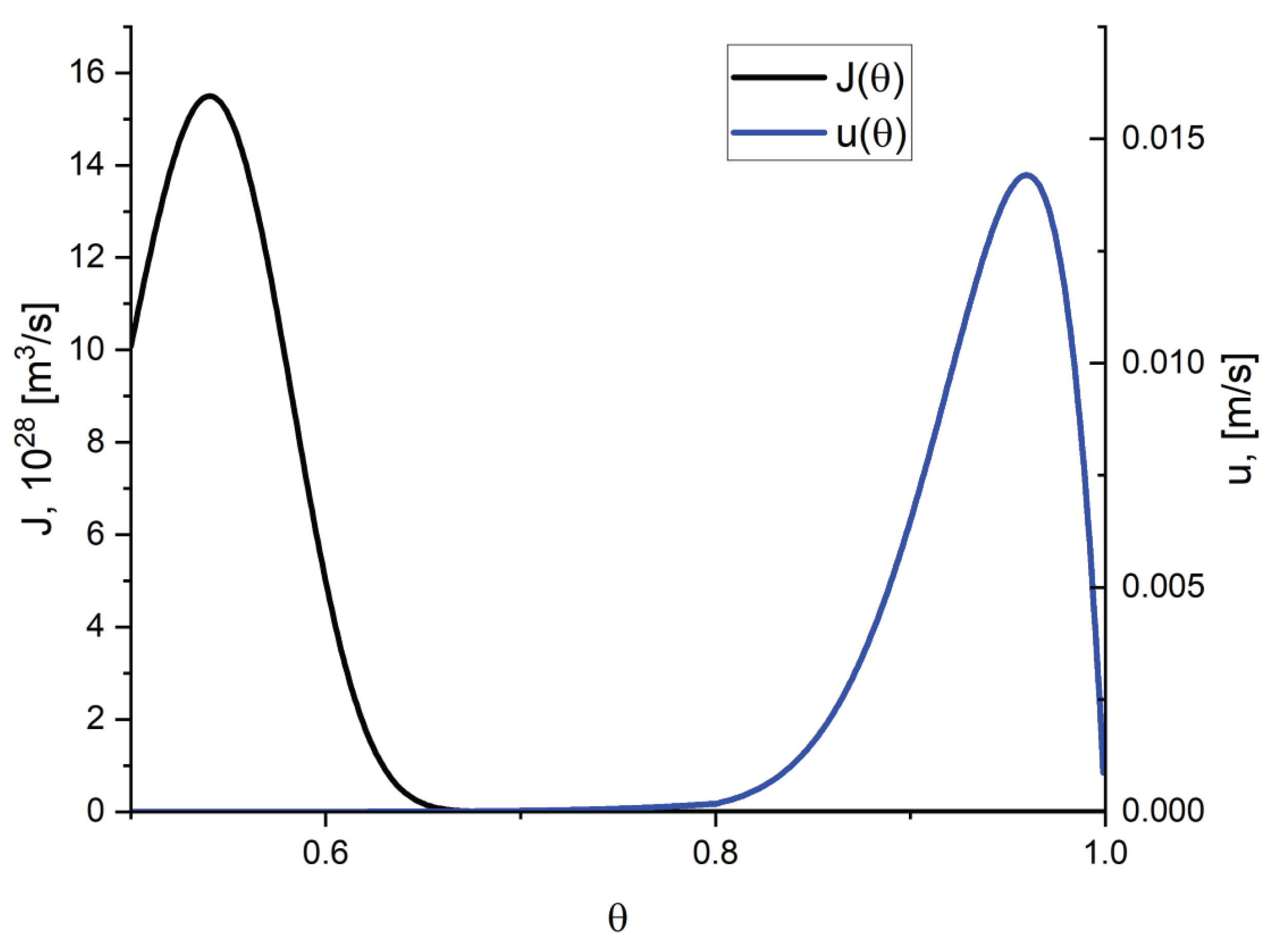
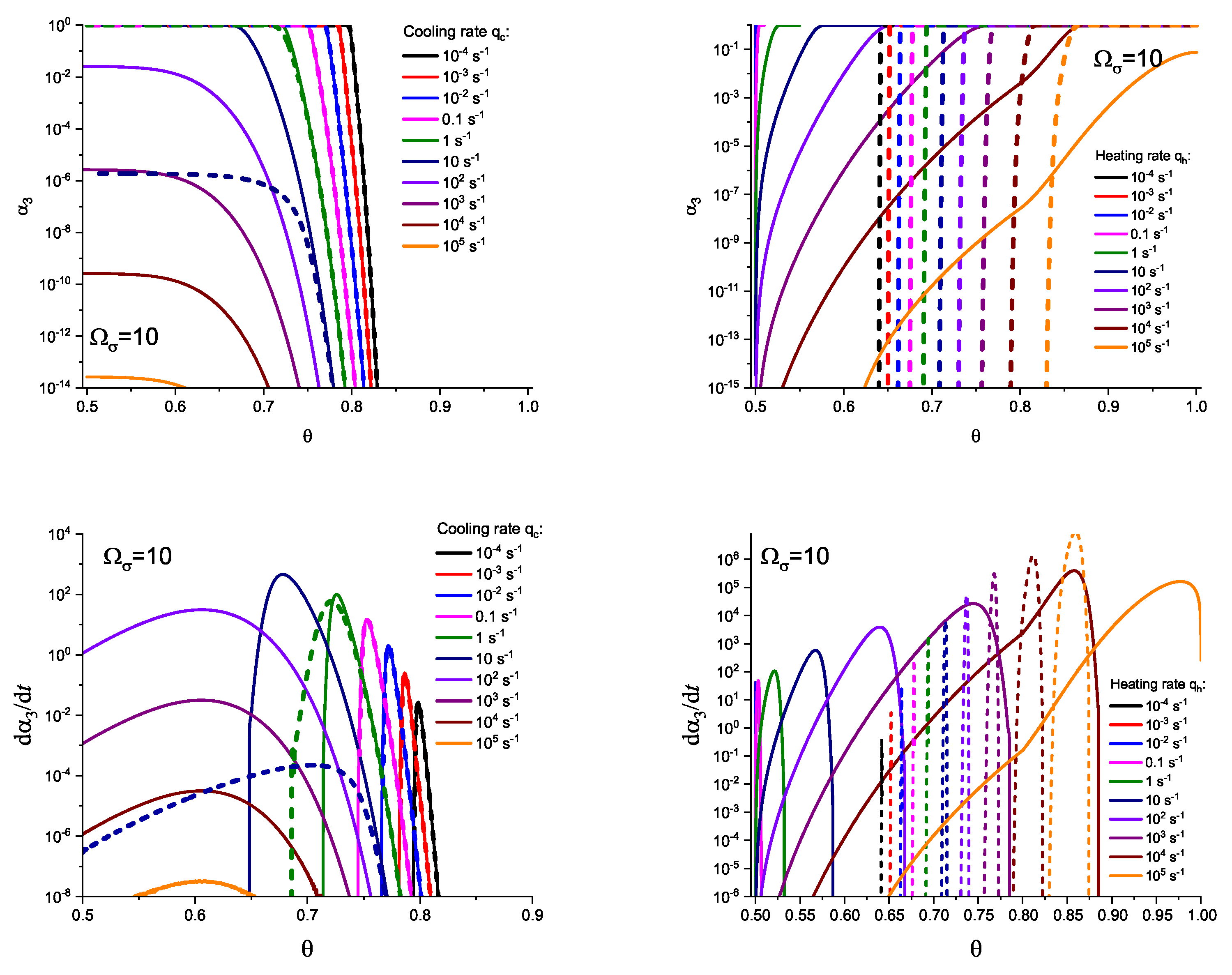
3. Results of Numerical Computations
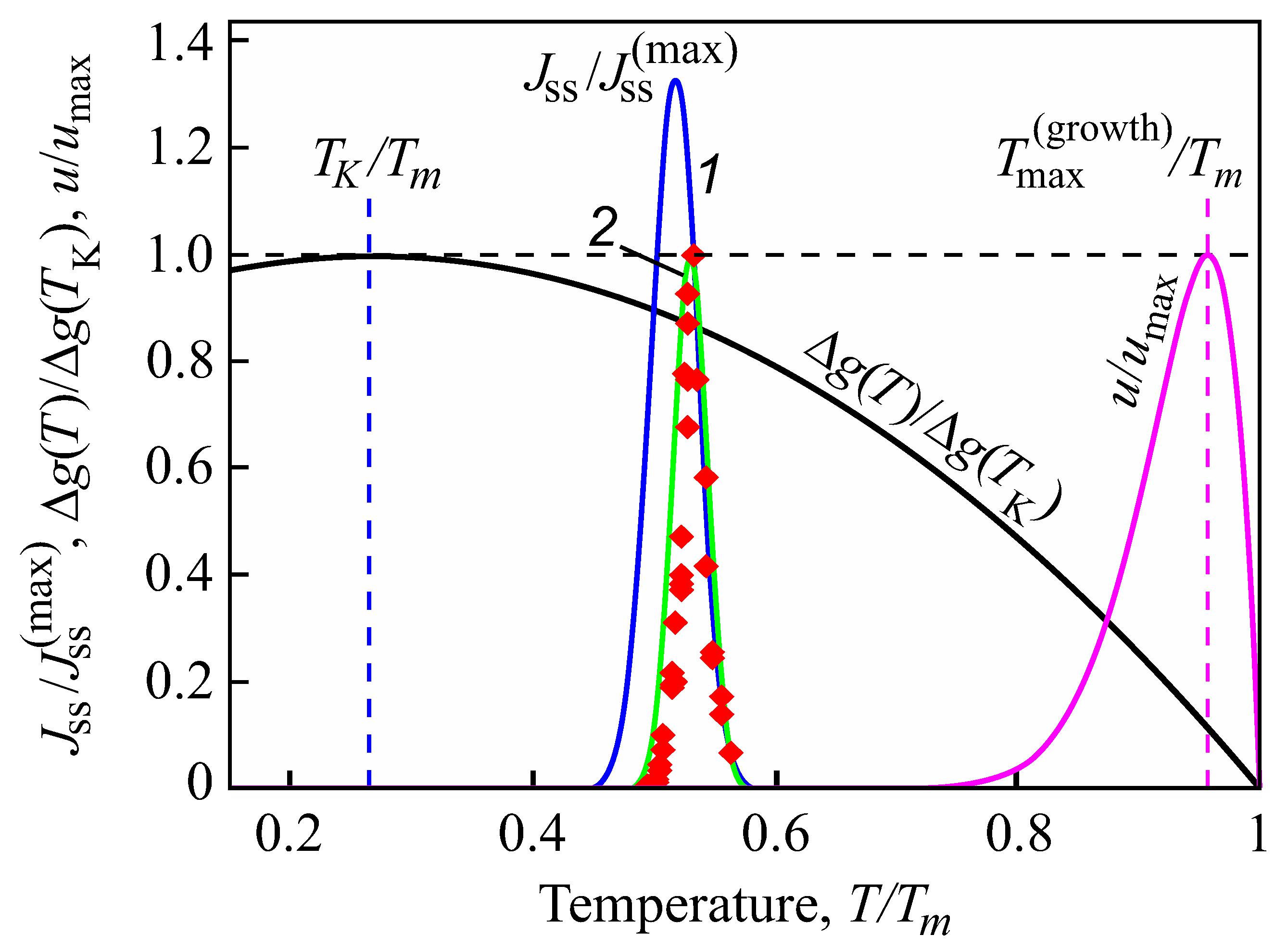
4. Theoretical Analysis
4.1. Some General Considerations
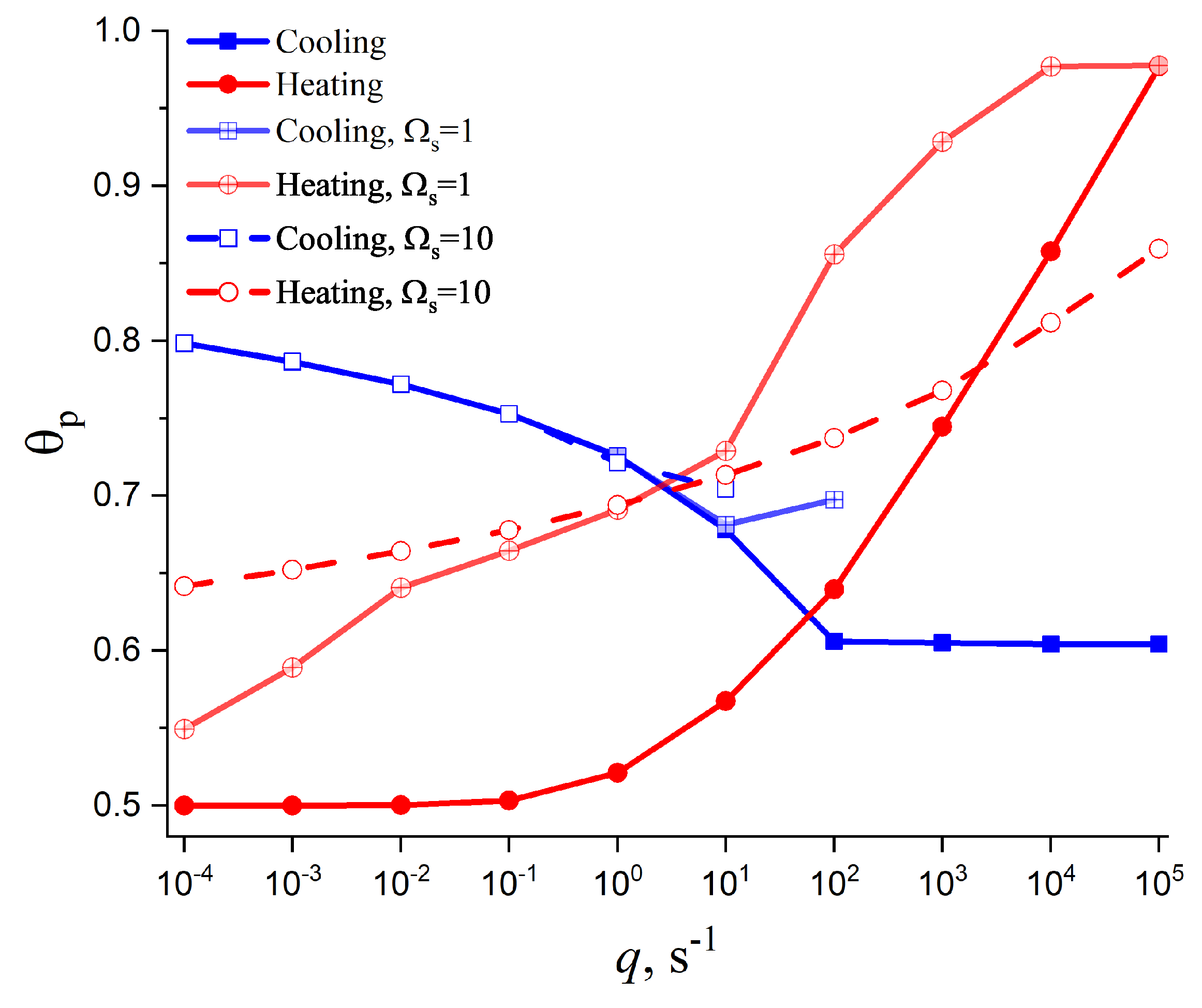
4.2. Cold Crystallization Peak Temperature in Heating as a Function of the Heating Rate: Homogeneous Nucleation
4.3. Crystallization Peak Temperature in Cooling as a Function of the Cooling Rate: Heterogeneous Nucleation
5. Summary of Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
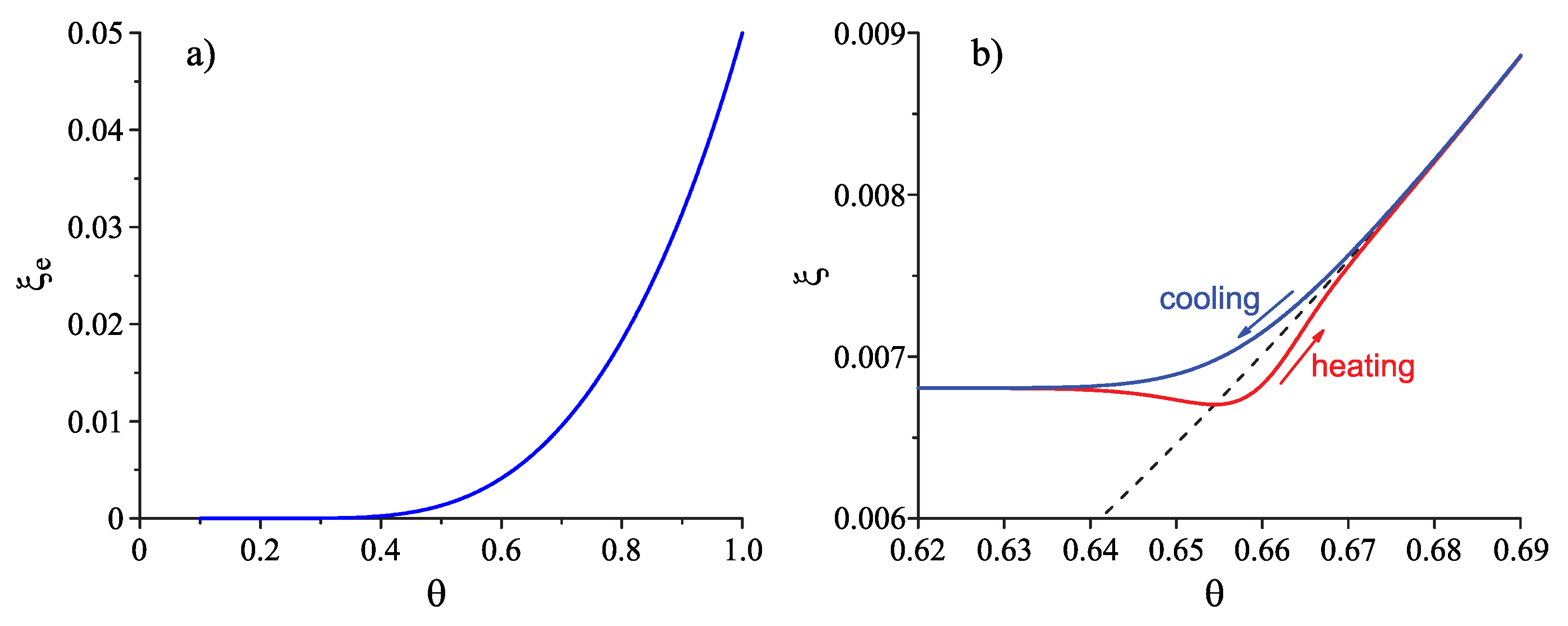
Appendix A. Characteristic times of relaxation and crystallization: Some general considerations
Appendix B. Application of the lattice–hole model in the computations and some possible generalization of this model
References
- Yinnon, H.; Uhlmann, D.R. Applications of thermoanalytical techniques to the study of crystallization kinetics in glass-forming liquids, Part 1: Theory. J. Non-Crystalline Solids 1983, 54, 253–275. [Google Scholar] [CrossRef]
- Schick, C.; Mathot, V. (Eds.) Fast Scanning Calorimetry; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Vyazovkin, S.; Sbirrazzuoli, N. Review: Nonisothermal Crystallization Kinetics by DSC: Practical Overview. Processes 2023, 11, 1438. [Google Scholar] [CrossRef]
- Skripov, V.P.; Koverda, V.P. Spontaneous Crystallization of Undercooled Liquids; Nauka: Moscow, Russia, 1984 (in Russian).
- Gutzow, I.S.; Schmelzer, J.W.P. The Vitreous State: Thermodynamics, Structure, Rheology, and Crystallization; Springer: Berlin/Heidelberg, Germany, 1995. [Google Scholar]
- Gutzow, I.S.; Schmelzer, J.W.P. The Vitreous State: Thermodynamics, Structure, Rheology, and Crystallization; 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Henderson, D.W. Thermal analysis of non-isothermal crystallization kinetics in glass-forming liquids. J. Non-Crystalline Solids 1979, 30, 301–315. [Google Scholar]
- Schmelzer, J.W.P.; Schmelzer, J., Jr. Kinetics of Nucleation at Increasing Supersaturation. J. Colloid Interface Sci. 1999, 215, 345–355. [Google Scholar] [PubMed]
- Kashchiev, D.; Borissova, A.; Hammond, R.B.; Roberts, K.J. Effect of cooling rate on the critical undercooling for crystallization. J. Cryst. Growth 2010, 312, 698–704. [Google Scholar]
- Yang, B.; Abyzov, A.S.; Zhuravlev, E.; Gao, Y.; Schmelzer, J.W.P.; Schick, C. Size and rate dependence of crystal nucleation in single tin drops by fast scanning calorimetry. J. Chem. Phys. 2013, 138, 054501. [Google Scholar]
- Yang, B.; Perepezko, J.H.; Schmelzer, J.W.P.; Gao, Y.; Schick, C. Dependence of crystal nucleation on prior liquid overheating by differential fast scanning calorimeter. J. Chem. Phys. 2014, 140, 104513. [Google Scholar] [PubMed]
- Tanaka, K.K.; Kimura, Y. Theoretical Analysis of Crystallization by Homogeneous Nucleation of Water Droplets. Phys. Chem. Chem. Phys. 2019, 21, 2410–2418. [Google Scholar] [CrossRef]
- Deubener, J.; Schmelzer, J.W.P. Statistical Approach to Crystal Nucleation in Glass-forming Liquids. Entropy 2021, 23, 246/1–28. [Google Scholar] [CrossRef]
- Todorova, S.; Gutzow, I.; Schmelzer, J.W.P. Kinetics of nucleation at increasing or decreasing supersaturation. In Nucleation Theory and Applications; Schmelzer, J.W.P., Röpke, G., Priezzhev, V.B., Eds.; Workshop Proceedings 2000–2002, Joint Institute for Nuclear Research Publishing Department: Dubna, Russia, 2002; pp. 215–233. [Google Scholar]
- Sestak, J.; Berggren, G. Study of the kinetics of the mechanism of solid–state reactions at increasing temperatures. Thermochimica Acta 1971, 3, 1–12. [Google Scholar] [CrossRef]
- Dimitra, K.; Konstantinos, C. Nonisothermal Crystallization Kinetics: Studying the Validity of Different Johnson–Mehl–Avrami–Erofeev–Kolmogorov (JMAEK) Based Equations. Thermochimica Acta 2021, 704, 179030. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Analytical Chemistry 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Blaine, R.L.; Kissinger, H.E. Homer Kissinger and the Kissinger equation. Thermochimica Acta 2012, 540, 1–6. [Google Scholar] [CrossRef]
- Vyazovkin, S. Is the Kissinger Equation Applicable to the Processes that Occur on Cooling? Macromolecular Rapid Communications 2002, 23, 771–775. [Google Scholar] [CrossRef]
- Vyazovkin, S. Kissinger Method in Kinetics of Materials: Things to Beware and Be Aware of. Molecules 2020, 25, 2813. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P.; Tropin, T.V.; Fokin, V.M.; Abyzov, A.S.; Zanotto, E.D. Effects of Glass Transition and Structural Relaxation on Crystal Nucleation: Theoretical Description and Model Analysis. Entropy 2020, 22, 1098. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P.; Schick, C. Dependence of Crystallization Processes of Glass-forming Melts on Prehistory: A Theoretical Approach to a Quantitative Treatment. Physics and Chemistry of Glasses: European Journal of Glass Science and Technology 2012, B 53, 99–106. [Google Scholar]
- Fokin, V.M.; Abyzov, A.S.; Yuritsyn, N.S.; Schmelzer, J.W.P.; Zanotto, E.D. Effect of structural relaxation on crystal nucleation in glasses. Acta Materialia 2021, 203, 116472/1–13. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P.; Tropin, T.V. Reply to "Comment on ’Glass Transition, Crystallization of Glass-Forming Melts, and Entropy’" by Zanotto and Mauro. Entropy 2018, 20, 704–1. [Google Scholar] [CrossRef]
- Golovchak, R.; Kozdras, A.; Balitska, V.; Shpotyuk, O. J. Step-wise kinetics of natural physical ageing in arsenic selenide glasses. J. Phys.: Condens. Matter 2012, 24, 505106/1–10. [Google Scholar] [CrossRef]
- Lijian Song; Wei Xu; Juntao Huo; Jun-Qiang Wang; Xinmin Wang; Runwei Li Two–step relaxations in metallic glasses during isothermal annealing. Intermetallics 93 2018, 93, 101–105. [CrossRef]
- Morvan, A.; Delpouve, N.; Vella, A.; Saiter–Fourcin, A. Physical aging of selenium glass: Assessing the double mechanism of equilibration and the crystallization process. Journal of Non-Crystalline Solids 2021, 570, 121013. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P.; Müller, R.; Möller, J.; Gutzow, I.S. Elastic Stresses, Stress Relaxation, and Crystallization: Theory. Physics and Chemistry of Glasses 2002, 43 C, 291–300. [Google Scholar]
- Schmelzer, J.W.P.; Müller, R.; Möller, J.; Gutzow, I.S. Theory of Nucleation in Viscoelastic Media: Application to Phase Formation in Glassforming Melts. J. Non-Crystalline Solids 2003, 315, 144–160. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P.; Potapov, O.V.; Fokin, V.M.; Müller, R.; Reinsch, S. The Effect of Elastic Stress Evolution and Relaxation on Crystal Nucleation in Lithium Disilicate Glasses. J. Non-Crystalline Solids 2004, 333, 150–160. [Google Scholar] [CrossRef]
- Abyzov, A. S; Fokin, V.M.; Rodrigues, A.M.; Zanotto, E.D.; Schmelzer, J.W.P. The effect of elastic stresses on the thermodynamic barrier for crystal nucleation. J. Non-Crystalline Solids 2016, 432, 325–333. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P.; Schick, C. General concepts of crystallization: Some recent results and possible future developments. In: Advances in Dielectrics Series; Kremer, F., Series editor: Dielectrics and Crystallization; Ezquerra, T.A.; Nogales, A.; Eds.; Springer, Nature Switzerland AG, 2020, pgs. 1–22.
- Rodrigues, L.R.; Abyzov, A.S.; Fokin, V.M.; Zanotto, E.D. Effect of structural relaxation on crystal nucleation in a sodalime–silica glass. Journal American Ceramic Society 104, 3212–3223 (2021). [CrossRef]
- Rodrigues, L.R.; Abyzov, A.S.; Fokin, V.M.; Schmelzer, J.W.P.; Zanotto, E.D. Relaxation effect on crystal nucleation in a glass unveiled by experimental, numerical, and analytical approaches. Acta Materialia 223, 117458/1–10 (2022). [CrossRef]
- Abyzov, A.S.; Fokin, V.M.; Yuritsyn, N.S.; Nascimento, M.L.F.; Schmelzer, J.W.P.; Zanotto, E.D. Crystal nucleation in a L2S-glass during aging well below Tg. J. Chem. Phys. 2023, 158, 064501/1–10. [Google Scholar] [CrossRef] [PubMed]
- Abyzov, A.S.; Fokin, V.M.; Yuritsyn, N.S.; Nascimento, M.L.F.; Zanotto, E.D. Structure relaxation of a silicate glass revealed by crystal nucleation, present special issue (in preparation).
- Schmelzer, J.W.P.; Tropin, T.V. Theory of Crystal Nucleation of Glass-forming Liquids: Some New Developments. Int. J. Appl. Glass Sci. 2022, 13, 171–198. [Google Scholar] [CrossRef]
- Tammann, G. Der Glaszustand (English: The Vitreous State); Leopold Voss Verlag: Leipzig, Germany, 1933. [Google Scholar]
- Schmelzer, J.W.P.; Abyzov, A.S.; Baidakov, V.G. Time of formation of the first supercritical nucleus, time-lag, and the steady-state nucleation rate. Int. J. Appl. Glass Sci. 2017, 8, 48–60. [Google Scholar] [CrossRef]
- Kashchiev, D. Nucleation at existing cluster size distributions. Surf. Sci. 1969, 18, 389–397. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P.; Abyzov, A.S.; Fokin, V.M.; Schick, C. Kauzmann paradox and the crystallization of glass-forming melts. J. Non-Cryst. Solids 2018, 501, 21–35. [Google Scholar] [CrossRef]
- Gutzow, I.S.; Kashchiev, D. The kinetics of overall crystallization of undercooled melts in terms of the non-steady state theory of nucleation. In: Advances in Nucleation and Crystallization in Glasses; Hench, L.L.; S. Freiman, S.W., Editors: American Ceramic Society, Columbus, Ohio, 1971, pgs. 116–122.
- Slezov, V.V. Kinetics of First–Order Phase Transitions; WILEY–VCH: Berlin–Weinheim, 2009. [Google Scholar]
- Möller, J.; Schmelzer, J.W.P.; Avramov, I. Kinetics of Segregation and Crystallization with Stress Development and Stress Relaxation. physica status solidi 1996, b 196, 49–62. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P.; Zanotto, E.D.; Avramov, I.; Fokin, V.M. Stress Development and Stress Relaxation During Crystal Growth in Glass-Forming Melts. J. Non–Crystalline Solids 2006, 352, 434–443. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P.; Boltachev, G.S.; Baidakov, V.G. Classical and Generalized Gibbs’ Approaches and the Work of Critical Cluster Formation in Nucleation Theory. J. Chem. Phys. 2006, 124, 194503. [Google Scholar] [CrossRef]
- Abyzov, A.S.; Schmelzer, J.W.P.; Fokin, V.M.; Zanotto, E.D. Crystallization of supercooled liquids: Self–consistency correction of the steady-state nucleation rate. Entropy 2020, 22, 558/1-28. [Google Scholar] [CrossRef]
- Ostwald, W. Studien über die Bildung und Umwandlung fester Körper. 1. Abhandlung: Übersättigung und Überkaltung (Engl: Studies on the formation and transformation of solid bodies. 1. Analysis: Supersaturation and undercooling). Zeitschrift für Physikalische Chemie 1897, 22, 289–330. [Google Scholar] [CrossRef]
- Shijie Xu; Zhongbi Hou; Xiaoyu Chuai; Yanfei Wang Overview of Secondary Nucleation: From Fundamentals to Application. Ind. Eng. Chem. Res. 2020, 59, 18335–18356. [CrossRef]
- Bin Yang; Yulai Gao; Changdong Zou; Qijie Zhai; Abyzov, A. S; Zhuravlev, E.; Schmelzer, J.W.P.; Schick, C. Cooling rate dependence of undercooling of pure Sn single drop by fast scanning calorimetry. Appl. Phys. A 2011, 104, 189–196. [Google Scholar] [CrossRef]
- Bin Yang; Abyzov, A. S.; Zhuravlev, E.; Yulai Gao; Schmelzer, J.W.P.; Schick, C. Size Dependent Nucleation of Single Tin Particles by Differential Fast Scanning Calorimetry. J. Chem. Phys. 2013, 138, 054501/1-5. [Google Scholar] [CrossRef]
- Chen, B.; Torkelson, J.M. Development of rigid amorphous fraction in cold–crystallized syndiotactic polystyrene films confined near the nanoscale: Novel analysis via ellipsometry. J. Polym. Sci. 2022, 60, 1631. [Google Scholar] [CrossRef]
- Kauzmann, W. The Nature of the Glassy State and the Behavior of Liquids at Low Temperatures. Chem. Rev. 1948, 43, 219–256. [Google Scholar] [CrossRef]
- Zanotto, E. D; Mauro, J.C. The glassy state of matter: Its definition and ultimate fate. J. Non-Crystalline Solids 2017, 471, 490–495. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P.; Tropin, T.V. Glass transition, crystallization of glass-forming melts, and entropy, Entropy 2018, 20, 103/1–32. [CrossRef]
- Tipeev, A.O.; Schmelzer, J.W.P.; Zanotto, E.D. Comment on: "Spinodal limits of supercooled liquid Al deduced from configuration heredity of crystal clusters". Computational Materials Science 207, 111316 (2022). submitted for publication.
- Uhlmann, D.R. A kinetic treatment of glass formation. Journal of Non–Crystalline Solids 1972, 7, 337–348. [Google Scholar] [CrossRef]
- Zhuravlev, E.; Schmelzer, J.W.P.; Abyzov, A.S.; Fokin, V.M.; Androsch, R.; Schick, C. Experimental test of Tammann’s nuclei development approach in crystallization of macromolecules. Crystal Growth & Design 2015, 15, 786–798. [Google Scholar] [CrossRef]
- Andrianov, R. A; Androsch, R.; Rui Zhang; Mukhametzyanov, T.A.; Abyzov, A.S.; Schmelzer, J.W.P.; Schick, C. Growth and dissolution of crystal nuclei in poly(L-lactic acid) (PLLA) in Tammann’s development method. Polymer 2020, 196, 122453. [Google Scholar] [CrossRef]
- Andrianov, R. A; Schmelzer, J.W.P.; Androsch, R.; Mukhametzyanov, T.A.; Schick, C. Radial growth rate of near–critical crystal nuclei in poly(L–lactic acid) (PLLA) in Tammann’s two–stage development method. J. Chem. Phys. 2023, 158, 054504. [Google Scholar] [CrossRef] [PubMed]
- Rui Zhang; Katalee Jariyavidyanon; Mengxue Du; Zhuravlev, E. ; Schick, C.; Androsch, R. Nucleation and crystallization kinetics of polyamide 12 investigated by fast scanning calorimetry. J. Polymer Science 2022, 60, 842–855. [Google Scholar] [CrossRef]
- Mukhametzyanov, T.; Schmelzer, J.W.P.; Yarko, E.; Abdullin, A.; Ziganshin, M.; Sedov, I.; Schick, C. Crystal Nucleation and Growth in Cross–Linked Poly(ε–caprolactone) (PCL). Polymers 2021, 13, 3617/1–21. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P.; Abyzov, A.S.; Fokin, V.M. Crystallization of glass: What we know, what we need to know. Int. J. Appl. Glass Sci. 2016, 7, 253–261. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P.; Abyzov, A.S.; Fokin, V.M.; Schick, C.; Zanotto, E.D. Crystallization in glass-forming liquids: Maxima of nucleation, growth, and overall crystallization rates. J. Non-Cryst. Solids 2015, 429, 24–32. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P.; Abyzov, A.S. Pressure-induced crystallization of liquids: Maxima of nucleation, growth, and overall crystallization rates. Int. J. Appl. Glass Sci. 2018, 9, 198–207. [Google Scholar] [CrossRef]
- Andreev, N.S.; Mazurin, O.V.; Porai-Koshits, E.A.; Roscova, G.P.; Filipovich, V.N. Liquid Phase Separation in Glasses; Nauka: Moscow, Russia, 1974 (in Russian).
- Mazurin, O.V.; Porai–Koshits, E.A.; Andreev, N.S. Phase separation in glass; North–Holland Publishers: Amsterdam & New York, 1984.
- Davis, M.J. Effect of the Growth Treatment on Two-Stage Nucleation Experiments. J. Amer. Ceramic Society 2001, 84, 492–96. [Google Scholar] [CrossRef]
- Davis, M.J.; Ihinger, P.D. Effects of thermal history on crystal nucleation in silicate melt: Numerical simulations. J. Geophys. Research 2002, 107, 2284/1–20. [Google Scholar] [CrossRef]
- Gutzow, I.S.; Schmelzer, J.W.P.; Todorova, S. Frozen–In Fluctuations, Immiscibility and Crystallization in Oxide Melts and the Structural and Thermodynamic Nature of Glasses. Phys. Chem. Glasses: Eur. J. Glass Science and Technology 2008, 49, 136–148. [Google Scholar]
- Schmelzer, J.W.P.; Gutzow, I.S. Glasses and the Glass Transition; WILEY–VCH: Berlin–Weinheim, Germany, 2011. [Google Scholar]
- Simon, F. On the range of stability of the fluid state. Transactions Faraday Society 1937, 33, 65–73. [Google Scholar] [CrossRef]
- Bernal, J.D. An attempt at a molecular theory of liquid structure. Transactions Faraday Society 1937, 33, 27–40. [Google Scholar] [CrossRef]
- Frenkel, Ya.I. The Kinetic Theory of Liquids; Oxford University Press: Oxford, United Kingdom, 1946. [Google Scholar]
- Skripov, V.P.; Baidakov, V.G. Absence of a spinodal in undercooled liquids. Teplofizika Vysokikh Temperatur 1972, 10, 1226–1230. [Google Scholar]
- Skripov, V.P.; Koverda, V.P. Spontaneous Crystallization of Undercooled Liquids; Nauka: Moscow, Russia, 1984 (in Russian).
- Skripov, V.P.; Faizullin, M.Z. Crystal–Liquid–Gas Phase Transitions and Thermodynamic Similarity; Wiley–VCH: Weinheim, Germany, 2006. [Google Scholar]
- Landau, L.D. On the theory of phase transitions. Zhurn. Eksper. Teor. Fiz. 1937, 7, 19–32. [Google Scholar] [CrossRef]
- Landau, L.D.; Lifshitz, E.M. Statistical Physics; Academy of Sciences Publishing House: Berlin, Germany, 1987. [Google Scholar]
- Gibbs, J.W. The Collected Works, vol. 1, Thermodynamics; Longmans & Green: New York – London – Toronto, 1928. [Google Scholar]
- Kelton, K.F.; Greer, A.L. Nucleation in Condensed Matter: Applications in Materials and Biology; Pergamon–Press: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Tammann, G. Über die Abhängigkeit der Zahl der Kerne, welche sich in verschiedenen unterkühlten Flüssigkeiten bilden, von der Temperatur (Engl: On the dependence of the number of nuclei, forming in different undercooled liquids, on temperature). Z. Phys. Chem. 1898, 25, 441–479. [Google Scholar] [CrossRef]
- Turnbull, D. Under What Conditions can a Glass be Formed? Contemporary Physics 1969, 10, 473–488. [Google Scholar] [CrossRef]
- Angell, C.A.; MacFarlane, D.R.; Oguni, M. The Kauzmann Paradox, Metastable Liquids, and Glasses: A Summary. In: Dynamic Aspects of Structural Change in Liquids and Glasses. Eds.: C. A. Angell and M. Goldstein, Annals New York Academy of Sciences 1986, 484, 241–247. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




