Submitted:
22 September 2023
Posted:
25 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Taxane in HNSCC clinical application
2.1. Locally advanced HNSCC
2.2. Recurrent/metastatic HNSCC
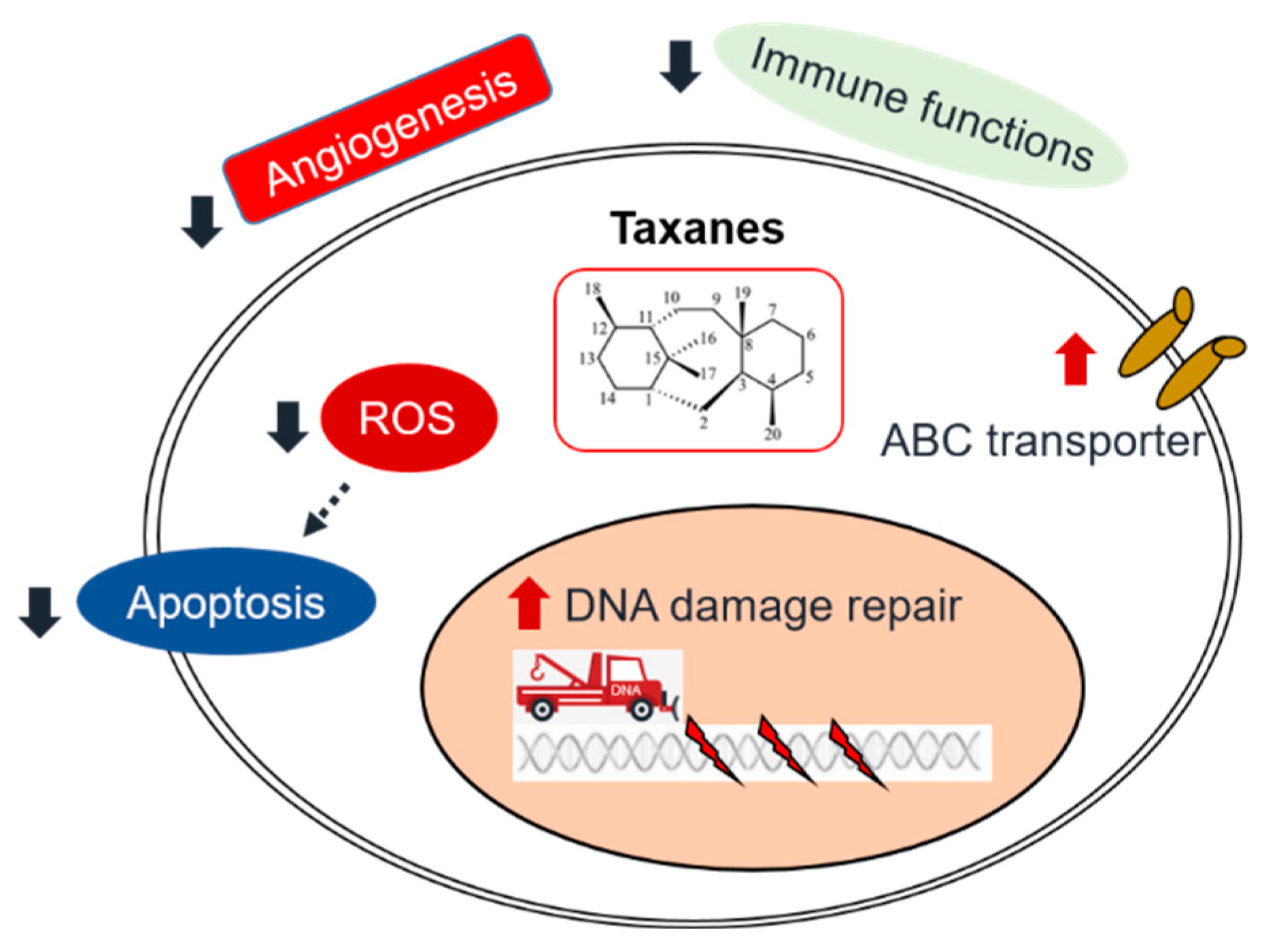
3. Molecular Mechanisms of taxane
3.1. Interfering the function of microtubules
3.2. Induction of apoptosis
3.3. DNA damage and DNA repair inhibition
4. Taxane resistance in HNSCC
4.1. Intrinsic mechanisms
4.1.1. DNA/RNA damage repair
4.1.2. Drug efflux
4.1.3. Apoptosis inhibition
4.2. Extrinsic mechanisms
4.2.1. Angiogenesis
4.2.2. The interaction of immune therapy and chemotherapy
4.2.3. Optimizing the pharmacokinetics of chemotherapy
5. Conclusion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat Rev Dis Primers. 2020, 6, 92. [Google Scholar] [CrossRef]
- Leemans, C.R.; Snijders, P.J.F.; Brakenhoff, R.H. The molecular landscape of head and neck cancer. Nat Rev Cancer. 2018, 18, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Economopoulou, P.; de Bree, R.; Kotsantis, I.; Psyrri, A. Diagnostic Tumor Markers in Head and Neck Squamous Cell Carcinoma (HNSCC) in the Clinical Setting. Front Oncol. 2019, 9, 827. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.Q.M. Head and Neck Cancer. N Engl J Med. 2020, 382, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Gau, M.; Karabajakian, A.; Reverdy, T.; Neidhardt, E.M.; Fayette, J. Induction chemotherapy in head and neck cancers: Results and controversies. Oral Oncol. 2019, 95, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, G.; Krüger, A.; Boehm, A.; Kolb, M.; Hofer, M.; Fischer, M.; Müller, S.; Purz, S.; Stumpp, P.; Sabri, O.; et al. Induction chemotherapy followed by radiotherapy for larynx preservation in advanced laryngeal and hypopharyngeal cancer: Outcome prediction after one cycle induction chemotherapy by a score based on clinical evaluation, computed tomography-based volumetry and 18F-FDG-PET/CT. Eur J Cancer. 2017, 72, 144–155. [Google Scholar] [CrossRef]
- Hsieh, C.Y.; Lein, M.Y.; Yang, S.N.; Wang, Y.C.; Lin, Y.J.; Lin, C.Y.; Hua, C.H.; Tsai, M.H.; Lin, C.C. Dose-dense TPF induction chemotherapy for locally advanced head and neck cancer: a phase II study. BMC Cancer. 2020, 20, 832. [Google Scholar] [CrossRef]
- Mosca, L.; Ilari, A.; Fazi, F.; Assaraf, Y.G.; Colotti, G. Taxanes in cancer treatment: Activity, chemoresistance and its overcoming. Drug Resist Updat. 2021, 54, 100742. [Google Scholar] [CrossRef]
- Churchill, C.D.; Klobukowski, M.; Tuszynski, J.A. Elucidating the mechanism of action of the clinically approved taxanes: a comprehensive comparison of local and allosteric effects. Chem Biol Drug Des. 2015, 86, 1253–1266. [Google Scholar] [CrossRef]
- Schiff, P.B.; Fant, J.; Horwitz, S.B. Promotion of microtubule assembly in vitro by taxol. Nature. 1979, 277, 665–667. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.; Sadeghi, S.; Tabatabaeian, H. Battling Chemoresistance in Cancer: Root Causes and Strategies to Uproot Them. Int J Mol Sci. 2021, 22, 9451. [Google Scholar] [CrossRef] [PubMed]
- Grégoire, V.; Lefebvre, J.L.; Licitra, L.; Felip, E. ; EHNS-ESMO-ESTRO Guidelines Working Group. Squamous cell carcinoma of the head and neck: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010, 21 Suppl 5, v184–v186. [Google Scholar] [CrossRef]
- Marur, S.; Forastiere, A.A. Head and Neck Squamous Cell Carcinoma: Update on Epidemiology, Diagnosis, and Treatment. Mayo Clin Proc. 2016, 91, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Pignon, J.P.; le Maître, A.; Maillard, E.; Bourhis, J.; MACH-NC Collaborative Group. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009, 92, 4–14. [Google Scholar] [CrossRef]
- Vermorken, J.B.; Remenar, E.; van Herpen, C.; Gorlia, T.; Mesia, R.; Degardin, M.; Stewart, J.S.; Jelic, S.; Betka, J.; Preiss, J.H.; et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007, 357, 1695–1704. [Google Scholar] [CrossRef]
- Posner, M.R.; Hershock, D.M.; Blajman, C.R.; Mickiewicz, E.; Winquist, E.; Gorbounova, V.; Tjulandin, S.; Shin, D.M.; Cullen, K.; Ervin, T.J.; et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007, 357, 1705–1715. [Google Scholar] [CrossRef]
- Hitt, R.; Grau, J.J.; López-Pousa, A.; Berrocal, A.; García-Girón, C.; Irigoyen, A.; Sastre, J.; Martínez-Trufero, J.; Brandariz Castelo, J.A.; Verger, E.; et al. A randomized phase III trial comparing induction chemotherapy followed by chemoradiotherapy versus chemoradiotherapy alone as treatment of unresectable head and neck cancer. Ann Oncol. 2014, 25, 216–225. [Google Scholar] [CrossRef]
- Cohen, E.E.; Karrison, T.G.; Kocherginsky, M.; Mueller, J.; Egan, R.; Huang, C.H.; Brockstein, B.E.; Agulnik, M.B.; Mittal, B.B.; Yunus, F.; et al. Phase III randomized trial of induction chemotherapy in patients with N2 or N3 locally advanced head and neck cancer. J Clin Oncol. 2014, 32, 2735–2743. [Google Scholar] [CrossRef]
- Ghi, M.G.; Paccagnella, A.; Ferrari, D.; Foa, P.; Alterio, D.; Codecà, C.; Nolè, F.; Verri, E.; Orecchia, R.; Morelli, F.; et al. Induction TPF followed by concomitant treatment versus concomitant treatment alone in locally advanced head and neck cancer. A phase II-III trial. Ann Oncol. 2017, 28, 2206–2212. [Google Scholar] [CrossRef]
- Haddad, R.I.; Posner, M.; Hitt, R.; Cohen, E.E.W.; Schulten, J.; Lefebvre, J.L.; Vermorken, J.B. Induction chemotherapy in locally advanced squamous cell carcinoma of the head and neck: role, controversy, and future directions. Ann Oncol. 2018, 29, 1130–1140. [Google Scholar] [CrossRef] [PubMed]
- Yano, R.; Konno, A.; Watanabe, K.; Tsukamoto, H.; Kayano, Y.; Ohnaka, H.; Goto, N.; Nakamura, T.; Masada, M. Pharmacoethnicity of docetaxel-induced severe neutropenia: integrated analysis of published phase II and III trials. Int J Clin Oncol. 2013, 18, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Okano, S.; Enokida, T.; Onoe, T.; Ota, Y.; Motegi, A.; Zenda, S.; Akimoto, T.; Tahara, M. Induction TPF chemotherapy followed by CRT with fractionated administration of cisplatin in patients with unresectable locally advanced head and neck cancer. Int J Clin Oncol. 2019, 24, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Fang, J.; Huang, Z.; Yang, Y.; Lian, M.; Liu, H.; Zhang, Y.; Ye, J.; Hui, X.; Wang, Y.; et al. A response prediction model for taxane, cisplatin, and 5-fluorouracil chemotherapy in hypopharyngeal carcinoma. Sci Rep. 2018, 8, 12675. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.; Yang, W.F.; Li, K.Y.; Su, Y.X. Systemic Therapy in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma- A Systematic Review and Meta-Analysis. Crit Rev Oncol Hematol. 2020, 153, 102984. [Google Scholar] [CrossRef]
- Guigay, J.; Aupérin, A.; Fayette, J.; Saada-Bouzid, E.; Lafond, C.; Taberna, M.; Geoffrois, L.; Martin, L.; Capitain, O.; Cupissol, D.; et al. Cetuximab, docetaxel, and cisplatin versus platinum, fluorouracil, and cetuximab as first-line treatment in patients with recurrent or metastatic head and neck squamous-cell carcinoma (GORTEC 2014-01 TPExtreme): a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2021, 22, 463–475. [Google Scholar] [CrossRef]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G Jr.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, Å.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019, 394, 1915–1928. [CrossRef]
- Dzienis, M.R.; Cundom, J.E.; Fuentes, C.S.; Hansen, A.R.; Nordlinger, M.J.; Pastor, A.V.; Oppelt, P.; Neki, A.; Gregg, R.W.; Lima, I.P.F.; et al. Pembrolizumab (pembro)+carboplatin (carbo)+paclitaxel (pacli) as first-line (1L) therapy in recurrent/metastatic (R/M) head and neck squamous cell carcinoma (HNSCC): Phase VI KEYNOTE-B10 study. Ann Oncol. 2022, 33 suppl 7, S295–S322. [Google Scholar] [CrossRef]
- Guigay, J.; Fayette, J.; Mesia, R.; Saada-Bouzid, E.; Lafond, C.; Geoffrois, L.; Martin, L.; Capitain, O.; Cupissol, D.; Castanie, H.; et al. TPExtreme randomized trial: Quality of Life (QoL) and survival according to second-line treatments in patients with recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC). J. Clin. Oncol. 2020, 38 Suppl 15, 6507. [Google Scholar] [CrossRef]
- Saleh, K.; Daste, A.; Martin, N.; Pons-Tostivint, E.; Auperin, A.; Herrera-Gomez, R.G.; Baste-Rotllan, N.; Bidault, F.; Guigay, J.; Le Tourneau, C.; et al. Response to salvage chemotherapy after progression on immune checkpoint inhibitors in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. Eur J Cancer. 2019, 121, 123–129. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gümüş, M.; Mazières, J.; Hermes, B.; Çay Şenler, F.; Csőszi, T.; Fülöp, A.; et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef]
- Fuereder, T.; Minichsdorfer, C.; Mittlboeck, M.; Wagner, C.; Heller, G.; Putz, E.M.; Oberndorfer, F.; Müllauer, L.; Aretin, M.B.; Czerny, C.; et al. Pembrolizumab plus docetaxel for the treatment of recurrent/metastatic head and neck cancer: A prospective phase I/II study. Oral Oncol. 2022, 124, 105634. [Google Scholar] [CrossRef] [PubMed]
- Bacus, S.S.; Gudkov, A.V.; Lowe, M.; Lyass, L.; Yung, Y.; Komarov, A.P.; Keyomarsi, K.; Yarden, Y.; Seger, R. Taxol-induced apoptosis depends on MAP kinase pathways (ERK and p38) and is independent of p53. Oncogene. 2001, 20, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Gurbi, B.; Brauswetter, D.; Varga, A.; Gyulavári, P.; Pénzes, K.; Murányi, J.; Zámbó, V.; Birtalan, E.; Krenács, T.; Becker, D.L.; et al. The Potential Impact of Connexin 43 Expression on Bcl-2 Protein Level and Taxane Sensitivity in Head and Neck Cancers-In Vitro Studies. Cancers. 2019, 11, 1848. [Google Scholar] [CrossRef] [PubMed]
- Selimovic, D.; Hassan, M.; Haikel, Y.; Hengge, U.R. Taxol-induced mitochondrial stress in melanoma cells is mediated by activation of c-Jun N-terminal kinase (JNK) and p38 pathways via uncoupling protein 2. Cell Signal. 2008, 20, 311–322. [Google Scholar] [CrossRef]
- Hsieh, C.Y.; Lin, C.C.; Huang, Y.W.; Chen, J.H.; Tsou, Y.A.; Chang, L.C.; Fan, C.C.; Lin, C.Y.; Chang, W.C. Macrophage secretory IL-1β promotes docetaxel resistance in head and neck squamous carcinoma via SOD2/CAT-ICAM1 signaling. JCI Insight. 2022, 7, e157285. [Google Scholar] [CrossRef]
- Hung, C.H.; Chan, S.H.; Chu, P.M.; Tsai, K.L. Docetaxel Facilitates Endothelial Dysfunction through Oxidative Stress via Modulation of Protein Kinase C Beta: The Protective Effects of Sotrastaurin. Toxicol Sci. 2015, 145, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Gollahon, L. Taxol directly induces endoplasmic reticulum-associated calcium changes that promote apoptosis in breast cancer cells. Breast J. 2011, 17, 56–70. [Google Scholar] [CrossRef]
- Pan, Z.; Avila, A.; Gollahon, L. Paclitaxel induces apoptosis in breast cancer cells through different calcium--regulating mechanisms depending on external calcium conditions. Int J Mol Sci. 2014, 15, 2672–2694. [Google Scholar] [CrossRef]
- Swanton, C.; Nicke, B.; Schuett, M.; Eklund, A.C.; Ng, C.; Li, Q.; Hardcastle, T.; Lee, A.; Roy, R.; East, P.; et al. Chromosomal instability determines taxane response. Proc Natl Acad Sci U S A. 2009, 106, 8671–8676. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.L.; Eklund, A.C.; Kohane, I.S.; Harris, L.N.; Szallasi, Z. A signature of chromosomal instability inferred from gene expression profiles predicts clinical outcome in multiple human cancers. Nat Genet. 2006, 38, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Jean, S.; Li, J.; Katsaros, D.; Wubbenhorst, B.; Maxwell, K.N.; Fishbein, L.; McLane, M.W.; Benedetto, C.; Canuto, E.M.; Mitra, N.; et al. Paclitaxel is necessary for improved survival in epithelial ovarian cancers with homologous recombination gene mutations. Oncotarget. 2016, 7, 48577–48585. [Google Scholar] [CrossRef] [PubMed]
- Busatto, F.F.; Viero, V.P.; Schaefer, B.T.; Saffi, J. Cell growth analysis and nucleotide excision repair modulation in breast cancer cells submitted to a protocol using doxorubicin and paclitaxel. Life Sci. 2021, 268, 118990. [Google Scholar] [CrossRef] [PubMed]
- Burcher, K.M.; Faucheux, A.T.; Lantz, J.W.; Wilson, H.L.; Abreu, A.; Salafian, K.; Patel, M.J.; Song, A.H.; Petro, R.M.; Lycan, T. Jr.; et al. Prevalence of DNA Repair Gene Mutations in Blood and Tumor Tissue and Impact on Prognosis and Treatment in HNSCC. Cancers (Basel). 2021, 13, 3118. [Google Scholar] [CrossRef]
- Cury, S.S.; Miranda, P.M.; Marchi, F.A.; Canto, L.M.D.; Chulam, T.C.; Petersen, A.H.; Aagaard, M.M.; Pinto, C.A.L.; Kowalski, L.P.; Rogatto, S.R. Germline variants in DNA repair genes are associated with young-onset head and neck cancer. Oral Oncol. 2021, 122, 105545. [Google Scholar] [CrossRef] [PubMed]
- Prochnow, S.; Wilczak, W.; Bosch, V.; Clauditz, T.S.; Muenscher, A. ERCC1, XPF and XPA-locoregional differences and prognostic value of DNA repair protein expression in patients with head and neck squamous cell carcinoma. Clin Oral Investig. 2019, 23, 3319–3329. [Google Scholar] [CrossRef]
- Psyrri, A.; Gkotzamanidou, M.; Papaxoinis, G.; Krikoni, L.; Economopoulou, P.; Kotsantis, I.; Anastasiou, M.; Souliotis, V.L. The DNA damage response network in the treatment of head and neck squamous cell carcinoma. ESMO Open. 2021, 6, 100075. [Google Scholar] [CrossRef]
- Hsieh, C.Y.; Chang, W.C.; Lin, C.C.; Chen, J.H.; Lin, C.Y.; Liu, C.H.; Lin, C.; Hung, M.C. Combination treatment of arsenic trioxide and osimertinib in recurrent and metastatic head and neck squamous cell carcinoma. Am J Cancer Res. 2022, 12, 5049–5061. [Google Scholar]
- De Vos, M.; Schreiber, V.; Dantzer, F. The diverse roles and clinical relevance of PARPs in DNA damage repair: current state of the art. Biochem Pharmacol. 2012, 84, 137–146. [Google Scholar] [CrossRef]
- Pascal, J.M. The comings and goings of PARP-1 in response to DNA damage. DNA Repair. 2018, 71, 177–182. [Google Scholar] [CrossRef]
- Moutafi, M.; Economopoulou, P.; Rimm, D.; Psyrri, A. PARP inhibitors in head and neck cancer: Molecular mechanisms, preclinical and clinical data. Oral Oncol. 2021, 117, 105292. [Google Scholar] [CrossRef] [PubMed]
- Glorieux, M.; Dok, R.; Nuyts, S. Novel DNA targeted therapies for head and neck cancers: clinical potential and biomarkers. Oncotarget. 2017, 8, 81662–81678. [Google Scholar] [CrossRef] [PubMed]
- de Haan, R.; van Werkhoven, E.; van den Heuvel, M.M.; Peulen, H.M.U.; Sonke, G.S.; Elkhuizen, P.; van den Brekel, M.W.M.; Tesselaar, M.E.T.; Vens, C.; Schellens, J.H.M.; et al. Study protocols of three parallel phase 1 trials combining radical radiotherapy with the PARP inhibitor olaparib. BMC Cancer. 2019, 19, 901. [Google Scholar] [CrossRef]
- Jelinek, M.J.; Foster, N.R.; Zoroufy, A.J.; Schwartz, G.K.; Munster, P.N.; Seiwert, T.Y.; de Souza, J.A.; Vokes, E.E. A phase I trial adding poly(ADP-ribose) polymerase inhibitor veliparib to induction carboplatin-paclitaxel in patients with head and neck squamous cell carcinoma: Alliance A091101. Oral Oncol. 2021, 114, 105171. [Google Scholar] [CrossRef]
- Méndez, E.; Rodriguez, C.P.; Kao, M.C.; Raju, S.; Diab, A.; Harbison, R.A.; Konnick, E.Q.; Mugundu, G.M.; Santana-Davila, R.; Martins, R.; et al. A Phase I Clinical Trial of AZD1775 in Combination with Neoadjuvant Weekly Docetaxel and Cisplatin before Definitive Therapy in Head and Neck Squamous Cell Carcinoma. Clin Cancer Res. 2018, 24, 2740–2748. [Google Scholar] [CrossRef] [PubMed]
- Portugal, J.; Mansilla, S.; Bataller, M. Mechanisms of drug-induced mitotic catastrophe in cancer cells. Curr Pharm Des. 2010, 16, 69–78. [Google Scholar] [CrossRef]
- Kao, M.; Green, C.; Sidorova, J.; Méndez, E. Strategies for Targeted Therapy in Head and Neck Squamous Cell Carcinoma Using WEE1 Inhibitor AZD1775. JAMA Otolaryngol Head Neck Surg. 2017, 143, 631–633. [Google Scholar] [CrossRef]
- Yang, Z.; Liao, J.; Lapidus, R.G.; Fan, X.; Mehra, R.; Cullen, K.J.; Dan, H. Targeting Wee1 kinase to suppress proliferation and survival of cisplatin-resistant head and neck squamous cell carcinoma. Cancer Chemother Pharmacol. 2022, 89, 469–478. [Google Scholar] [CrossRef]
- Li, D.W.; Gao, S.; Shen, B.; Dong, P. Effect of apoptotic and proliferative indices, P-glycoprotein and survivin expression on prognosis in laryngeal squamous cell carcinoma. Med Oncol. 2011, 28 Suppl 1, S333–S3440. [Google Scholar] [CrossRef]
- Zhigang, H.; Qi, Z.; Jugao, F.; Xiaohong, C.; Wei, Z.; Hong, W.; Hu, H.; Na, M.; Zheng, Y.; Demin, H. Reverse multidrug resistance in laryngeal cancer cells by knockdown MDR1 gene expression. J Otolaryngol Head Neck Surg. 2009, 38, 440–448. [Google Scholar]
- Li, L.; Jiang, A.C.; Dong, P.; Wan, Y.; Yu, Z.W. The characteristics of Hep-2 cell with multiple drug resistance induced by Taxol. Otolaryngol Head Neck Surg. 2007, 137, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Dechsupa, N.; Khamto, N.; Chawapun, P.; Siriphong, S.; Innuan, P.; Suwan, A.; Luangsuep, T.; Photilimthana, N.; Maita, W.; Thanacharttanatchaya, R.; et al. Pentagalloyl Glucose-Targeted Inhibition of P-Glycoprotein and Re-Sensitization of Multidrug-Resistant Leukemic Cells (K562/ADR) to Doxorubicin: In Silico and Functional Studies. Pharmaceuticals 2023, 16, 1192. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.H. Crosstalk between Microtubule Stabilizing Agents and Prostate Cancer. Cancers. 2023, 15, 3308. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Patil, V.; Noronha, V.; Dhumal, S.; Pande, N.; Chandrasekharan, A.; Turkar, S.; Dsouza, H.; Shrirangwar, S.; Mahajan, A.; et al. Results of a phase II randomized controlled clinical trial comparing efficacy of Cabazitaxel versus Docetaxel as second line or above therapy in recurrent head and neck cancer. Oral Oncol. 2017, 75, 54–60. [Google Scholar] [CrossRef]
- Domanitskaya, N.; Wangari-Talbot, J.; Jacobs, J.; Peiffer, E.; Mahdaviyeh, Y.; Paulose, C.; Malofeeva, E.; Foster, K.; Cai, K.Q. Zhou, Y.; et al. Abcc10 status affects mammary tumour growth, metastasis, and docetaxel treatment response. Br J Cancer. 2014, 111, 696–707. [Google Scholar] [CrossRef]
- Liu, Y.; Li, G.; Liu, C.; Tang, Y.; Zhang, S. RSF1 regulates the proliferation and paclitaxel resistance via modulating NF-κB signaling pathway in nasopharyngeal carcinoma. J Cancer. 2017, 8, 354–362. [Google Scholar] [CrossRef]
- Kansal, V.; Kinney, B.L.C.; Uppada, S.; Saba, N.F.; Stokes, W.A.; Buchwald, Z.S.; Schmitt, N.C. The expanding role of IAP antagonists for the treatment of head and neck cancer. Cancer Med. 2023, 12, 13958–13965. [Google Scholar] [CrossRef]
- Gomez-Roca, C.; Even, C.; Le Tourneau, C.; Basté, N.; Delord, J.P.; Sarini, J.; Vergez, S.; Temam, S.; Hoffmann, C.; Rochaix, P.; et al. Exploratory window-of-opportunity trial to investigate the tumor pharmacokinetics/pharmacodynamics of the IAP antagonist Debio 1143 in patients with head and neck cancer. Clin Transl Sci. 2022, 15, 55–62. [Google Scholar] [CrossRef]
- Ariffin, A.B.; Forde, P.F.; Jahangeer, S.; Soden, D.M.; Hinchion, J. Releasing pressure in tumors: what do we know so far and where do we go from here? A review. Cancer Res. 2014, 74, 2655–2662. [Google Scholar] [CrossRef]
- Lin, Z.; Zhang, Q.; Luo, W. Angiogenesis inhibitors as therapeutic agents in cancer: Challenges and future directions. Eur J Pharmacol. 2016, 793, 76–81. [Google Scholar] [CrossRef]
- Heydar, H.; Mansouri, K.; Norooznezhad, M.; Norooznezhad, F.; Mohamadnia, A.; Bahrami, N. Bevacizumab Inhibits Angiogenic Cytokines in Head and Neck Squamous Cell Carcinoma: From Gene to the Protein. Int J Hematol Oncol Stem Cell Res. 2018, 12, 136–141. [Google Scholar] [PubMed]
- Hoang, T.; Huang, S.; Armstrong, E.; Eickhoff, J.C.; Harari, P.M. Enhancement of radiation response with bevacizumab. J Exp Clin Cancer Res. 2012, 31, 37. [Google Scholar] [CrossRef] [PubMed]
- Argiris, A.; Li, S.; Savvides, P.; Ohr, J.P.; Gilbert, J.; Levine, M.A.; Chakravarti, A.; Haigentz, M Jr. ; Saba, N.F.; Ikpeazu, C.V.; et al. Phase III Randomized Trial of Chemotherapy With or Without Bevacizumab in Patients With Recurrent or Metastatic Head and Neck Cancer. J Clin Oncol. 2019, 37, 3266–3274. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Galanopoulos, N.; Lavertu, P.; Fu, P.; Gibson, M.; Argiris, A.; Rezaee, R.; Zender, C.; Wasman, J.; Machtay, M.; et al. Phase II study of bevacizumab in combination with docetaxel and radiation in locally advanced squamous cell carcinoma of the head and neck. Head Neck. 2015, 37, 1665–1671. [Google Scholar] [CrossRef]
- Salama, J.K.; Haraf, D.J.; Stenson, K.M.; Blair, E.A.; Witt, M.E.; Williams, R.; Kunnavakkam, R.; Cohen, E.E.; Seiwert, T.; Vokes, E.E. A randomized phase II study of 5-fluorouracil, hydroxyurea, and twice-daily radiotherapy compared with bevacizumab plus 5-fluorouracil, hydroxyurea, and twice-daily radiotherapy for intermediate-stage and T4N0-1 head and neck cancers. Ann Oncol. 2011, 22, 2304–2309. [Google Scholar] [CrossRef] [PubMed]
- Elser, C.; Siu, L.L.; Winquist, E.; Agulnik, M.; Pond, G.R.; Chin, S.F.; Francis, P.; Cheiken, R.; Elting, J.; McNabola, A.; et al. Phase II trial of sorafenib in patients with recurrent or metastatic squamous cell carcinoma of the head and neck or nasopharyngeal carcinoma. J Clin Oncol. 2007, 25, 3766–3773. [Google Scholar] [CrossRef]
- Cooney, M.M.; Tserng, K.Y.; Makar, V.; McPeak, R.J.; Ingalls, S.T.; Dowlati, A.; Overmoyer, B.; McCrae, K.; Ksenich, P.; Lavertu, P.; et al. A phase IB clinical and pharmacokinetic study of the angiogenesis inhibitor SU5416 and paclitaxel in recurrent or metastatic carcinoma of the head and neck. Cancer Chemother Pharmacol. 2005, 55, 295–300. [Google Scholar] [CrossRef]
- Limaye, S.; Riley, S.; Zhao, S.; O'Neill, A.; Posner, M.; Adkins, D.; Jaffa, Z.; Clark, J.; Haddad, R. A randomized phase II study of docetaxel with or without vandetanib in recurrent or metastatic squamous cell carcinoma of head and neck (SCCHN). Oral Oncol. 2013, 49, 835–841. [Google Scholar] [CrossRef]
- Seidel, J.A.; Otsuka, A.; Kabashima, K. Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations. Front Oncol. 2018, 8, 86. [Google Scholar] [CrossRef]
- Sakai, A.; Ebisumoto, K.; Iijima, H.; Yamauchi, M.; Teramura, T.; Yamazaki, A.; Watanabe, T.; Inagi, T.; Maki, D.; Okami, K. Chemotherapy following immune checkpoint inhibitors in recurrent or metastatic head and neck squamous cell carcinoma: clinical effectiveness and influence of inflammatory and nutritional factors. Discov Oncol. 2023, 14, 158. [Google Scholar] [CrossRef]
- Reinisch, M.; Ataseven, B.; Kümmel, S. Neoadjuvant Dose-Dense and Dose-Intensified Chemotherapy in Breast Cancer - Review of the Literature. Breast Care. 2016, 11, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Lyman, G.H.; Barron, R.L.; Natoli, J.L.; Miller, R.M. Systematic review of efficacy of dose-dense versus non-dose-dense chemotherapy in breast cancer, non-Hodgkin lymphoma, and non-small cell lung cancer. Crit Rev Oncol Hematol. 2012, 81, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Fayette, J.; Fontaine-Delaruelle, C.; Ambrun, A.; Daveau, C.; Poupart, M.; Ramade, A.; Zrounba, P.; Neidhardt, E.M.; Péron, J.; Diallo, A.; et al. Neoadjuvant modified TPF (docetaxel, cisplatin, fluorouracil) for patients unfit to standard TPF in locally advanced head and neck squamous cell carcinoma: a study of 48 patients. Oncotarget. 2016, 7, 37297–37304. [Google Scholar] [CrossRef] [PubMed]
- Anantharamu, S.; Jacob, L.A.; Dasappa, L.; Babu, M.C.S.; Lokesh, K.N.; Rudresha, A.H.; Lakkavalli Krishnappa, R.; Saldanha, S.C. A prospective comparative study on biweekly docetaxel, cisplatin, 5-fluorouracil, leucovorin (TPFL) versus triweekly TPF as an induction chemotherapy in locally advanced squamous cell carcinoma of head and neck. Ann Oncol. 2022, 33, S850. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




