Submitted:
10 September 2023
Posted:
12 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
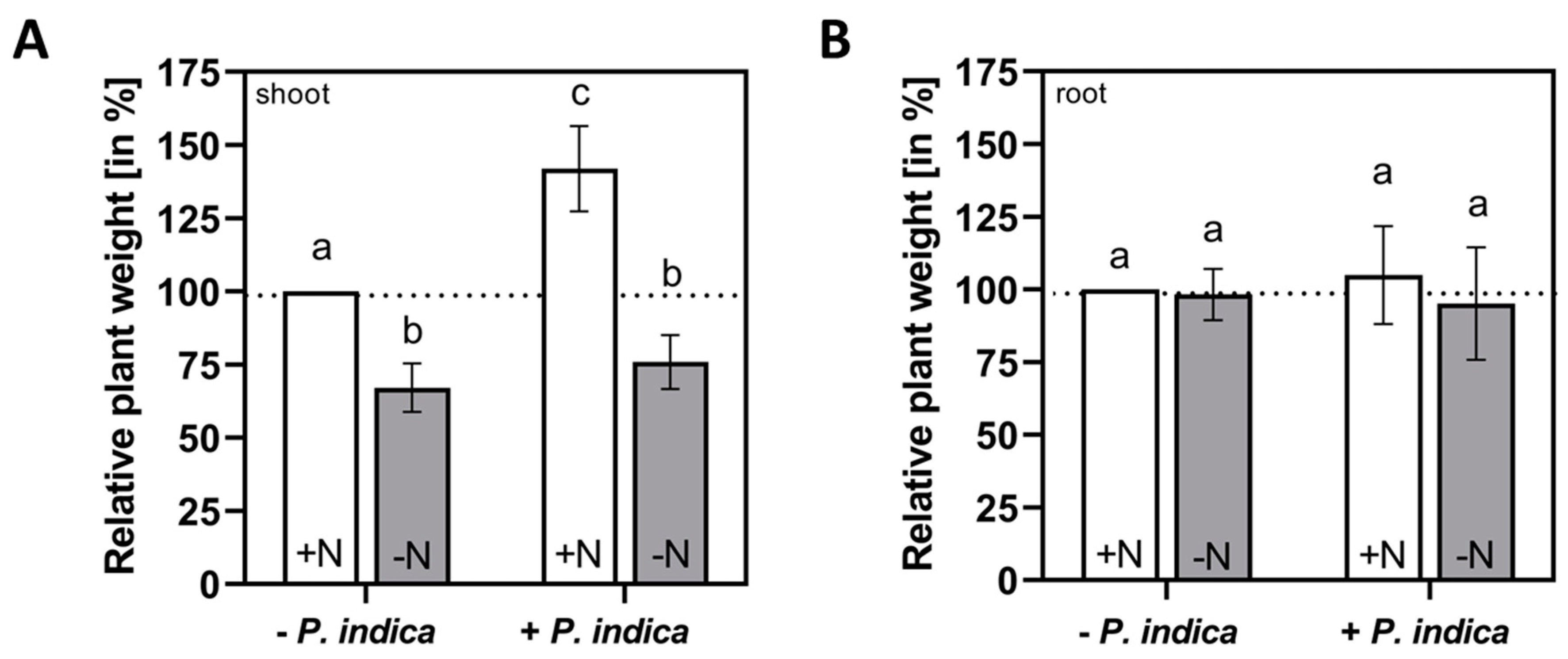
2.1. Shoot growth promotion by P. indica requires external N supply
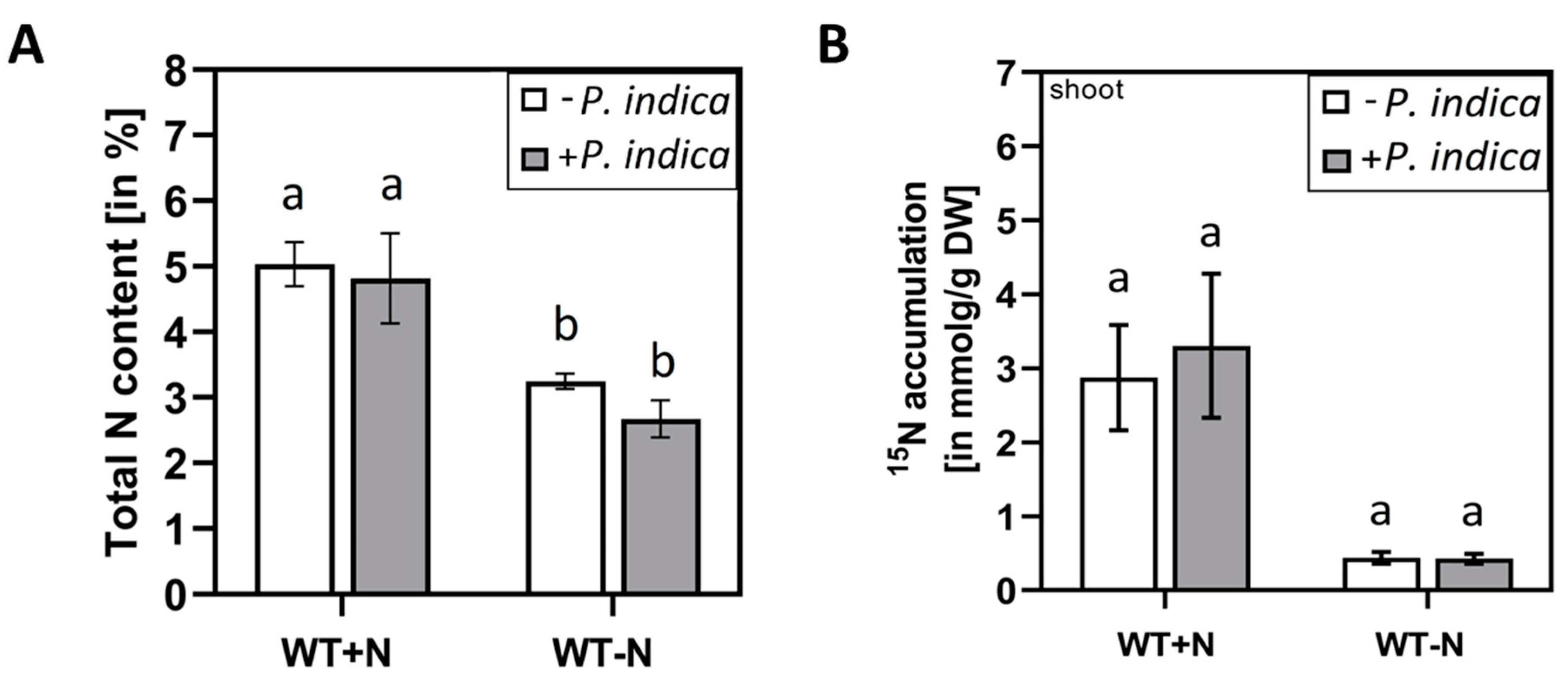
2.2. P. indica colonisation did not change the total N content in the shoots and transfer of 15N from the medium to the shoots
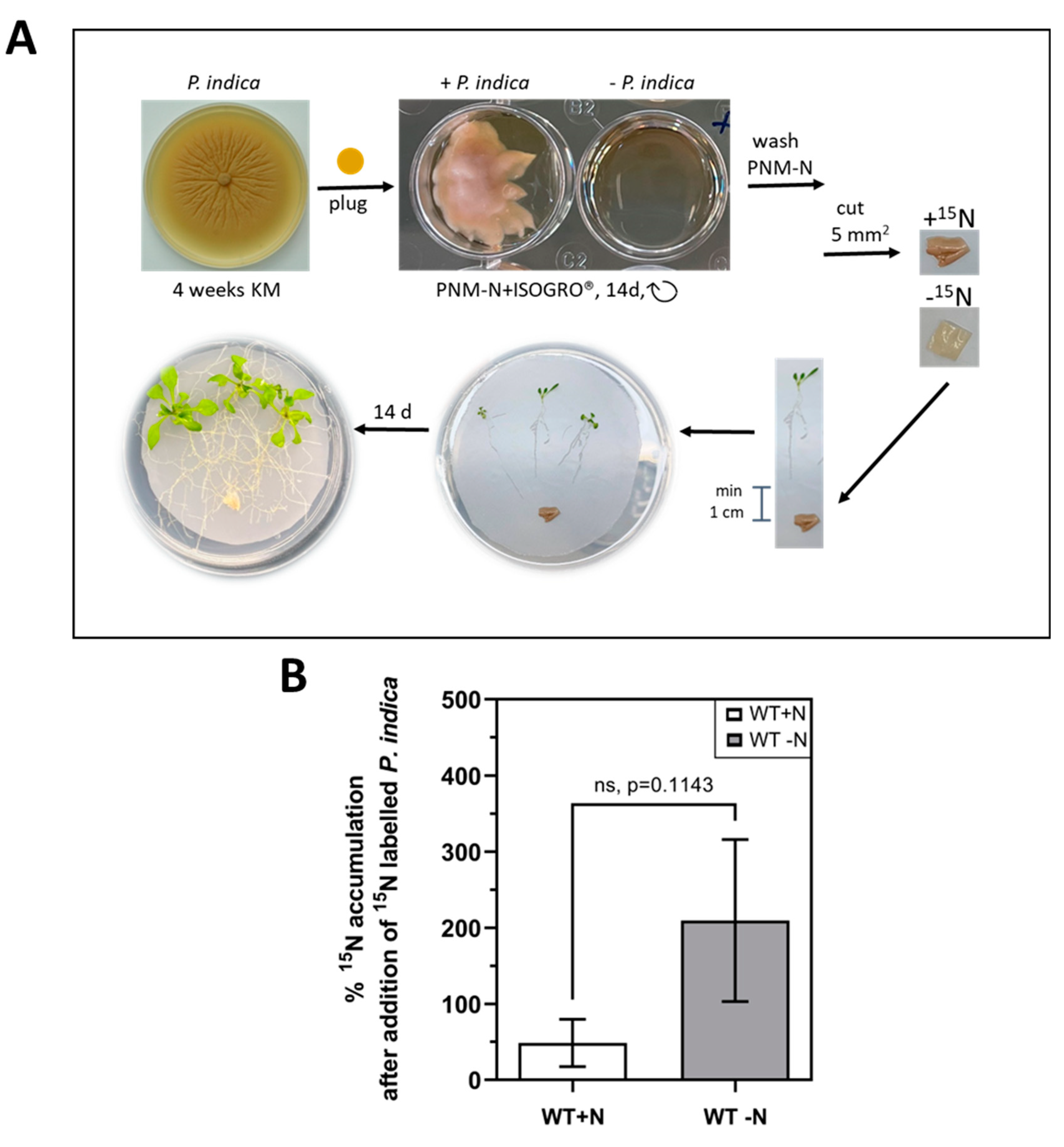
2.3. 15N label is transferred from P. indica to the host under N-limiting conditions
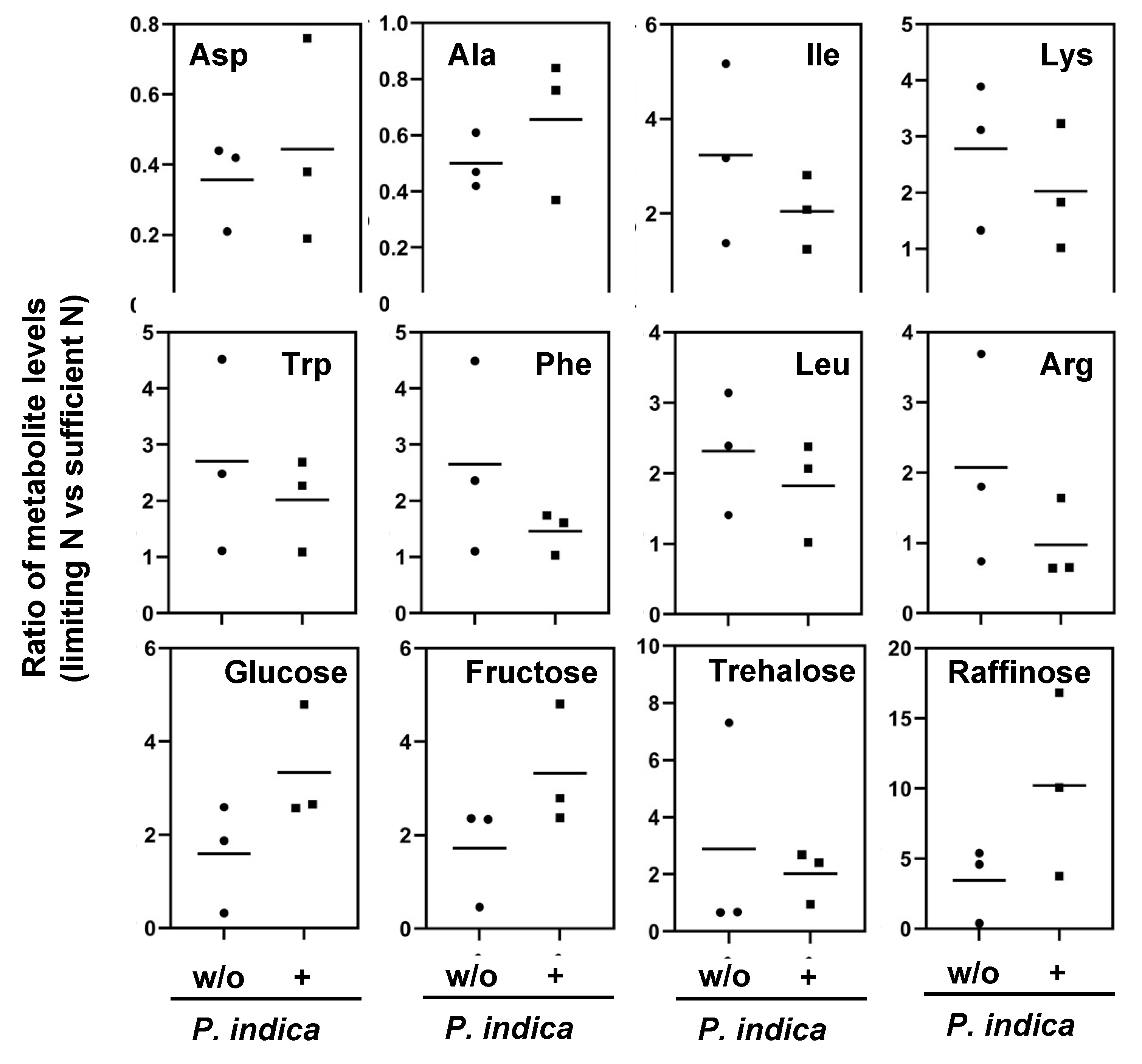
2.4. Reprogramming of the metabolite profiles to N limitation conditions is alleviated by P. indica
2.5. P. indica stimulates expression of specific host´s transporter genes under N limitation
3. Discussion
4. Materials and Methods
4.1. Plant and fungus material, corresponding growth conditions
4.2. Plant-fungus co-cultures and determination of growth promotion
4.3. 15N Labeling experiments in the medium
4.4. 15N fungus labeling experiments
4.5. Isolation and clean-up of RNA
4.6. RNAseq and data analysis
4.7. Analysis of fungal colonization by qPCR
4.8. Determination of total nitrogen and 15N enrichment
4.9. Metabolomic analysis
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, K.; Yang, J.; Yu, N.; Luo, L.; Wang, E. Biological nitrogen fixation in cereal crops: Progress, strategies, and perspectives. Plant Commun. 2023, 4(2),100499. Epub 2022 Nov 28. PMID: 36447432; PMCID: PMC10030364. [CrossRef]
- Rui, W.; Mao, Z.; Li, Z. The Roles of Phosphorus and Nitrogen Nutrient Transporters in the Arbuscular Mycorrhizal Symbiosis. Int. J. Mol. Sci. 2022, 23(19),11027. PMID: 36232323; PMCID: PMC9570102. [CrossRef]
- Chaudhary, P.; Agri, U.; Chaudhary, A.; Kumar, A.; Kumar, G. Endophytes and their potential in biotic stress management and crop production. Front. Microbiol. 2022, 13, 933017. PMID: 36325026; PMCID: PMC9618965. [CrossRef]
- Saleem, S.; Sekara, A.; Pokluda, R. Serendipita indica-A Review from Agricultural Point of View. Plants (Basel). 2022, 11(24), 3417. PMID: 36559533; PMCID: PMC9787873. [CrossRef]
- Del Barrio-Duque, A.; Ley, J.; Samad, A.; Antonielli, L.; Sessitsch, A.; Compant, S. Beneficial Endophytic Bacteria-Serendipita indica Interaction for Crop Enhancement and Resistance to Phytopathogens. Front. Microbiol. 2019, 10, 2888. PMID: 31921065; PMCID: PMC6930893. [CrossRef]
- Sherameti, I.; Shahollari, B.; Venus, Y.; Altschmied, L.; Varma, A.; Oelmüller, R. The endophytic fungus Piriformospora indica stimulates the expression of nitrate reductase and the starch-degrading enzyme glucan-water dikinase in tobacco and Arabidopsis roots through a homeodomain transcription factor that binds to a conserved motif in their promoters. J. Biol. Chem. 2005, 280(28), 26241-7. Epub 2005 Feb 14. PMID: 15710607. [CrossRef]
- Eliaspour, S.; Seyed Sharifi, R.; Shirkhani, A. Evaluation of interaction between Piriformospora indica, animal manure and NPK fertilizer on quantitative and qualitative yield and absorption of elements in sunflower. Food Sci. Nutr. 2020, 8(6), 2789-2797. PMID: 32566196; PMCID: PMC7300063. [CrossRef]
- Strehmel, N.; Mönchgesang, S.; Herklotz, S.; Krüger, S.; Ziegler, J.; Scheel, D. Piriformospora indica Stimulates Root Metabolism of Arabidopsis thaliana. Int. J. Mol. Sci. 2016, 17(7),1091. PMID: 27399695; PMCID: PMC4964467. [CrossRef]
- Ghaffari, M.R.; Ghabooli, M.; Khatabi, B.; Hajirezaei, M.R.; Schweizer, P.; Salekdeh, G.H. Metabolic and transcriptional response of central metabolism affected by root endophytic fungus Piriformospora indica under salinity in barley. Plant Mol. Biol. 2016, 90(6), 699-717. Epub 2016 Mar 7. PMID: 26951140. [CrossRef]
- Lahrmann, U.; Ding, Y.; Banhara, A.; Rath, M.; Hajirezaei, M.R.; Döhlemann, S.; von Wirén, N.; Parniske, M.; Zuccaro, A. Host-related metabolic cues affect colonization strategies of a root endophyte. Proc. Natl. Acad. Sci. U S A. 2013, 110(34), 13965-70. Epub 2013 Aug 5. PMID: 23918389; PMCID: PMC3752250. [CrossRef]
- Hua, M.D.; Senthil Kumar, R.; Shyur, L.F.; Cheng, Y.B.; Tian, Z.; Oelmüller, R.; Yeh, K.W. Metabolomic compounds identified in Piriformospora indica-colonized Chinese cabbage roots delineate symbiotic functions of the interaction. Sci. Rep. 2017, 7(1), 9291. PMID: 28839213; PMCID: PMC5571224. [CrossRef]
- Bandyopadhyay, P.; Yadav, B.G.; Kumar, S.G., Kumar, R.; Kogel, K.H.; Kumar, S. Piriformospora indica and Azotobacter chroococcum Consortium Facilitates Higher Acquisition of N, P with Improved Carbon Allocation and Enhanced Plant Growth in Oryza sativa. J. Fungi (Basel). 2022, 8(5), 453. PMID: 35628709; PMCID: PMC9146537. [CrossRef]
- Mansotra, P.; Sharma, P.; Sharma, S. Bioaugmentation of Mesorhizobium cicer, Pseudomonas spp. and Piriformospora indica for Sustainable Chickpea Production. Physiol. Mol. Biol. Plants 2015, 21(3), 385-93. Epub 2015 Apr 16. PMID: 26261403; PMCID: PMC4524863. [CrossRef]
- Hallasgo, A.M.; Spangl, B.; Steinkellner, S.; Hage-Ahmed, K. The Fungal Endophyte Serendipita williamsii Does Not Affect Phosphorus Status but Carbon and Nitrogen Dynamics in Arbuscular Mycorrhizal Tomato Plants. J. Fungi (Basel). 2020, 6(4), 233. PMID: 33086650; PMCID: PMC7711999. [CrossRef]
- Pérez-Alonso, M.M.; Guerrero-Galán, C.; Scholz, S.S.; Kiba, T.; Sakakibara, H.; Ludwig-Müller, J.; Krapp, A.; Oelmüller, R.; Vicente-Carbajosa, J.; Pollmann, S. Harnessing symbiotic plant-fungus interactions to unleash hidden forces from extreme plant ecosystems. J. Exp. Bot. 2020, 71(13), 3865-3877. PMID: 31976537; PMCID: PMC7316966. [CrossRef]
- Krapp, A.; Berthomé, R.; Orsel, M.; Mercey-Boutet, S.; Yu, A.; Castaings, L.; Elftieh, S.; Major, H.; Renou, J.P.; Daniel-Vedele, F. Arabidopsis roots and shoots show distinct temporal adaptation patterns toward nitrogen starvation. Plant Physiol. 2011, 157(3),1255-1282. Epub 2011 Sep 7. PMID: 21900481; PMCID: PMC3252138. [CrossRef]
- Hodge, A.; Stewart, J.; Robinson, D.; Griffiths, B.S.; Fitter, A.H. Competition between roots and soil microorganisms for nutrientsfrom nitrogen-rich patches of varying complexity. J. Ecology 2000, 88: 150–164.
- Hodge, A.; Campbell, C.D.; Fitter AH. An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material. Nature 2001, 413, 297–299.
- Thirkell, T.J.; Cameron, D.D.; Hodge, A. Resolving the ‘nitrogen paradox’ of arbuscular mycorrhizas: fertilisation with organic matter brings considerable benefits for plant nutrition and growth. Plant, Cell Environ. 2016, 39, 1683–1690.
- Hoysted, G.A.; Field, K.J.; Sinanaj, B.; Bell, C.A.; Bidartondo, M.I.; Pressel, S. Direct nitrogen, phosphorus and carbon exchanges between Mucoromycotina 'fine root endophyte' fungi and a flowering plant in novel monoxenic cultures. New Phytol. 2023, 238(1), 70-79. Epub 2023 Feb 5. PMID: 36739554. [CrossRef]
- Breuillin-Sessoms, F.; Floss, D.S.; Karen Gomez, S.; et al. Suppression of arbuscule degeneration in Medicago truncatula phosphate transporter4 mutants is dependent on the ammonium transporter 2 family protein AMT2;3. Plant Cell 2015, 27, 1352–1366.
- Paul, E.A.; Kucey, R.M.N. Carbon flow in plant microbial associations incriminated in perinatal morbidity and mortality. Science 1981, 213, 473–474.
- Wright, D.P.; Read, D.J.; Scholes, J.D. Mycorrhizal sink strength influences whole plant carbon balance of Trifolium repens L. Plant, Cell Environ. 1998, 21, 881–891.
- Lendenmann, M.; Thonar, C.; Barnard, R.L.; et al. Symbiont identity matters: carbon and phosphorus fluxes between Medicago truncatula and different arbuscular mycorrhizal fungi. Mycorrhiza 2011, 21, 689–702.
- Cope, K.R.; Kafle, A.; Yakha, J.K.; et al. Physiological and transcriptomic response of Medicago truncatula to colonization by high- or low-benefit arbuscular mycorrhizal fungi. Mycorrhiza 2022, 32, 281–303. https://doi.org/10.1007/s00572-022-01077-2.
- Belmondo, S.; Fiorilli, V.; Pérez-Tienda, J.; Ferrol, N. Marmeisse, R., Lanfranco, L. A dipeptide transporter from the arbuscular mycorrhizal fungus Rhizophagus irregularis is upregulated in the intraradical phase. Front. Plant Science 2014, 5, 436.
- Liu, Y.; Li, T.; Zhang, C.; Zhang, W.; Deng, N.; Dirk, L.M.A.; Downie, A.B.; Zhao, T. Raffinose positively regulates maize drought tolerance by reducing leaf transpiration. Plant J. 2023, 114(1), 55-67. Epub 2023 Feb 7. PMID: 36703577. [CrossRef]
- ElSayed, A.I.; Rafudeen, M.S.; Golldack, D. Physiological aspects of raffinose family oligosaccharides in plants: protection against abiotic stress. Plant Biol. (Stuttg). 2014,16(1), 1-8. Epub 2013 Aug 12. PMID: 23937337. [CrossRef]
- Gu, L.; Jiang, T.; Zhang, C.; Li, X.; Wang, C.; Zhang, Y.; Li, T.; Dirk, L.M.A.; Downie, A.B.; Zhao, T. Maize HSFA2 and HSBP2 antagonistically modulate raffinose biosynthesis and heat tolerance in Arabidopsis. Plant J. 2019, 100(1), 128-142. Epub 2019 Jul 12. PMID: 31180156. [CrossRef]
- Han, Q.; Qi, J.; Hao, G.; Zhang, C.; Wang, C.; Dirk, L.M.A.; Downie, A.B.; Zhao, T. ZmDREB1A Regulates RAFFINOSE SYNTHASE Controlling Raffinose Accumulation and Plant Chilling Stress Tolerance in Maize. Plant Cell Physiol. 2020, 61(2), 331-341. PMID: 31638155. [CrossRef]
- Li, C.H.; Tien, H.J.; Wen, M.F.; Yen, H.E. Myo-inositol transport and metabolism participate in salt tolerance of halophyte ice plant seedlings. Physiol. Plant. 2021, 172(3), 1619-1629. Epub 2021 Mar 5. PMID: 33511710. [CrossRef]
- Yang, J.; Ling, C.; Liu, Y.; Zhang, H.; Hussain, Q.; Lyu, S.; Wang, S.; Liu, Y. Genome-Wide Expression Profiling Analysis of Kiwifruit GolS and RFS Genes and Identification of AcRFS4 Function in Raffinose Accumulation. Int. J. Mol. Sci. 2022, 23(16), 8836. PMID: 36012101; PMCID: PMC9408211. [CrossRef]
- Wang, S.; Chen, A.; Xie, K.; Yang, X.; Luo, Z.; Chen, J.; Zeng, D.; Ren, Y.; Yang, C.; Wang, L.; Feng, H.; López-Arredondo, D.L.; Herrera-Estrella, L.R.; Xu, G. Functional analysis of the OsNPF4.5 nitrate transporter reveals a conserved mycorrhizal pathway of nitrogen acquisition in plants. Proc Natl Acad Sci U S A. 2020, 117(28), 16649-16659. Epub 2020 Jun 25. PMID: 32586957; PMCID: PMC7368293. [CrossRef]
- Kiba, T., Feria-Bourrellier, A.B.; Lafouge, F.; Lezhneva, L.; Boutet-Mercey, S.; Orsel, M.; Bréhaut, V.; Miller, A.; Daniel-Vedele, F.; Sakakibara, H.; Krapp, A. The Arabidopsis nitrate transporter NRT2.4 plays a double role in roots and shoots of nitrogen-starved plants. Plant Cell 2012, 24(1), 245-58. Epub 2012 Jan 6. PMID: 22227893; PMCID: PMC3289576. [CrossRef]
- Chen, H.Y.; Chen, Y.N.; Wang, H.Y.; Liu, Z.T.; Frommer, W.B.; Ho, C.H. Feedback inhibition of AMT1 NH4+-transporters mediated by CIPK15 kinase. BMC Biol. 2020 18(1), 196. PMID: 33317525; PMCID: PMC7737296. [CrossRef]
- Li, J.Y.; Fu, Y.L.; Pike, S.M.; Bao, J.; Tian, W.; Zhang, Y.; Chen, C.Z.; Zhang, Y.; Li, H.M., Huang, J.; Li, L.G.; Schroeder, J.I.; Gassmann, W.; Gong, J.M. The Arabidopsis nitrate transporter NRT1.8 functions in nitrate removal from the xylem sap and mediates cadmium tolerance. Plant Cell 2010, 22(5),1633-46. Epub 2010 May 25. PMID: 20501909; PMCID: PMC2899866. [CrossRef]
- Dechorgnat, J.; Patrit, O.; Krapp, A.; Fagard, M.; Daniel-Vedele, F. Characterization of the Nrt2.6 gene in Arabidopsis thaliana: a link with plant response to biotic and abiotic stress. PLoS One 2012;7(8):e42491. Epub 2012 Aug 7. PMID: 22880003; PMCID: PMC3413667. [CrossRef]
- Wang, Y.Y.; Tsay, Y.F. Arabidopsis nitrate transporter NRT1.9 is important in phloem nitrate transport. Plant Cell 2011, 23(5), 1945-57. Epub 2011 May 13. PMID: 21571952; PMCID: PMC3123939. [CrossRef]
- Lu, Y.T.; Liu, D.F.; Wen, T.T.; Fang, Z.J.; Chen, S.Y.; Li, H.; Gong, J.M. Vacuolar nitrate efflux requires multiple functional redundant nitrate transporter in Arabidopsis thaliana. Front Plant Sci. 2022, 13, 926809. PMID: 35937356; PMCID: PMC9355642. [CrossRef]
- Wu, X.; Liu, T.; Zhang, Y.; Duan, F.; Neuhäuser, B.; Ludewig, U.; Schulze, W.X.: Yuan, L. Ammonium and nitrate regulate NH4+ uptake activity of Arabidopsis ammonium transporter AtAMT1;3 via phosphorylation at multiple C-terminal sites. J. Exp. Bot. 2019, 70(18), 4919-4930. PMID: 31087098; PMCID: PMC6760267. [CrossRef]
- Yuan, L.; Loqué, D.; Kojima, S.; Rauch, S.; Ishiyama, K.; Inoue, E.; Takahashi, H.; von Wirén, N. The organization of high-affinity ammonium uptake in Arabidopsis roots depends on the spatial arrangement and biochemical properties of AMT1-type transporters. Plant Cell 2007, 19(8), 2636-52. Epub 2007 Aug 10. PMID: 17693533; PMCID: PMC2002620. [CrossRef]
- Yuan, L.; Graff, L.; Loqué, D.; Kojima, S.; Tsuchiya, Y.N.; Takahashi, H.; von Wirén, N. AtAMT1;4, a pollen-specific high-affinity ammonium transporter of the plasma membrane in Arabidopsis. Plant Cell Physiol. 2009, 50(1),13-25. Epub 2008 Dec 10. PMID: 19073648; PMCID: PMC2638712. [CrossRef]
- Zanin, L.; Tomasi, N.; Wirdnam, C.; Meier, S.; Komarova, N.Y.; Mimmo, T.; Cesco, S.; Rentsch, D.; Pinton. R. Isolation and functional characterization of a high affinity urea transporter from roots of Zea mays. BMC Plant Biol. 2014, 14, 222. PMID: 25168432; PMCID: PMC416055. [CrossRef]
- Wang, W.H.; Köhler, B.; Cao, F.Q.; Liu, G.W.; Gong, Y.Y.; Sheng, S.; Song, Q.C.; Cheng, X.Y.; Garnett, T.; Okamoto, M.; Qin, R.; Mueller-Roeber, B.; Tester, M.; Liu, L.H. Rice DUR3 mediates high-affinity urea transport and plays an effective role in improvement of urea acquisition and utilization when expressed in Arabidopsis. New Phytol. 2012, 193(2), 432-44. Epub 2011 Oct 19. PMID: 22010949. [CrossRef]
- Yu, S.; Pratelli, R.; Denbow, C.; Pilot, G. Suppressor mutations in the Glutamine Dumper1 protein dissociate disturbance in amino acid transport from other characteristics of the Gdu1D phenotype. Front Plant Sci. 2015, 6, 593. PMID: 26300894; PMCID: PMC4523740. [CrossRef]
- Hirner, A.; Ladwig, F.; Stransky, H.; Okumoto, S.; Keinath, M.; Harms, A.; Frommer, W.B.; Koch, W. Arabidopsis LHT1 is a high-affinity transporter for cellular amino acid uptake in both root epidermis and leaf mesophyll. Plant Cell 2006, 18(8),1931-46. Epub 2006 Jun 30. PMID: 16816136; PMCID: PMC1533986. [CrossRef]
- Havé, M.; Marmagne, A.; Chardon, F.; Masclaux-Daubresse, C. Nitrogen remobilization during leaf senescence: lessons from Arabidopsis to crops. J. Exp. Bot. 2017, 68(10), 2513-2529. PMID: 27707774. [CrossRef]
- Choi, J.; Eom, S.; Shin, K.; Lee, R.A.; Choi, S.; Lee, J.H.; Lee, S.; Soh, M.S. Identification of Lysine Histidine Transporter 2 as an 1-Aminocyclopropane Carboxylic Acid Transporter in Arabidopsis thaliana by Transgenic Complementation Approach. Front. Plant Sci. 2019, 10:1092. PMID: 31572413; PMCID: PMC6749071. [CrossRef]
- Fujiwara, T.; Hirai, M.Y.; Chino, M.; Komeda, Y.; Naito, S. Effects of Sulfur Nutrition on Expression of the Soybean Seed Storage Protein Genes in Transgenic Petunia. Plant Physiol. 1992, 99 (1), 263-268.
- Johnson, J.M.; Sherameti, I.; Ludwig, A.; Nongbri, P.L.; Sun, C.; Varma, A.; Oelmüller, R. Protocols for Arabidopsis thaliana and Piriformospora indica co-cultivation: a model system to study plant beneficial traits. Endocyt. Cell Res. 2011, 21, 101–113.
- Johnson, J.M.; Sherameti, I.; Nongbri, P.L.; Oelmüller, R. Standardized conditions to study beneficial and nonbeneficial traits in the Piriformospora indica/Arabidopsis thaliana interaction. In A Varma, G Kost, R Oelmüller, eds, Piriformospora indica: Sebacinales and Their Biotechnological Applications. Soil Biology, 2013, 33, 325–343.
- Pérez-Alonso, M.-M.; Guerrero-Galán, C.; González Ortega-Villaizán, A.; Ortiz-García, P.; Scholz, S.S.; Ramos, P.; et al. The calcium sensor CBL7 is required for Serendipita indica-induced growth stimulation in Arabidopsis thaliana, controlling defense against the endophyte and K+ homoeostasis in the symbiosis. Plant, Cell Environ. 2022, 45, 3367–3382. [CrossRef]
- Chen, A.; Gu, M.; Wang, S.; Chen, J.; Xu, G. Transport properties and regulatory roles of nitrogen in arbuscular mycorrhizal symbiosis. Seminars in Cell and Developmental Biology 2018, 74, 80–88.
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nature Biotechnol. 2019, 37(8), 907–915. [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30(7), 923-30.
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq. 2. Genome Biology 2014, 15(12), 550. [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Royal Statistical Soc.:Series B. 1995, 57(1), 289–300. [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45.
- Bütehorn, B.; Rhody, D.; Franken, P. Isolation and characterisation of Pitef1 encoding the translation elongation factor EF-1α of the root endophyte Piriformospora indica. Plant Biol. 2000, 2(6), 687–692. [CrossRef]
- Scholz, S. S.; Vadassery, J.; Heyer, M.; Reichelt, M.; Bender, K. W.; Snedden, W. A.; Boland, W.; Mithöfer, A., Mutation of the Arabidopsis Calmodulin-Like Protein CML37 Deregulates the Jasmonate Pathway and Enhances Susceptibility to Herbivory. Mol Plant, 2014, 7(12), 1712-1726. [CrossRef]
- Forzani, C.; Duarte, G.T.; Van Leene, J.; Clément, G.; Huguet, S.; Paysant-Le-Roux, C.; Mercier, R.; De Jaeger, G.; Leprince, A.-S.; Meyer, C.. Mutations of the AtYAK1 Kinase Suppress TOR Deficiency in Arabidopsis. Cell Rep. 2019, 27, 3696-3708.e5.
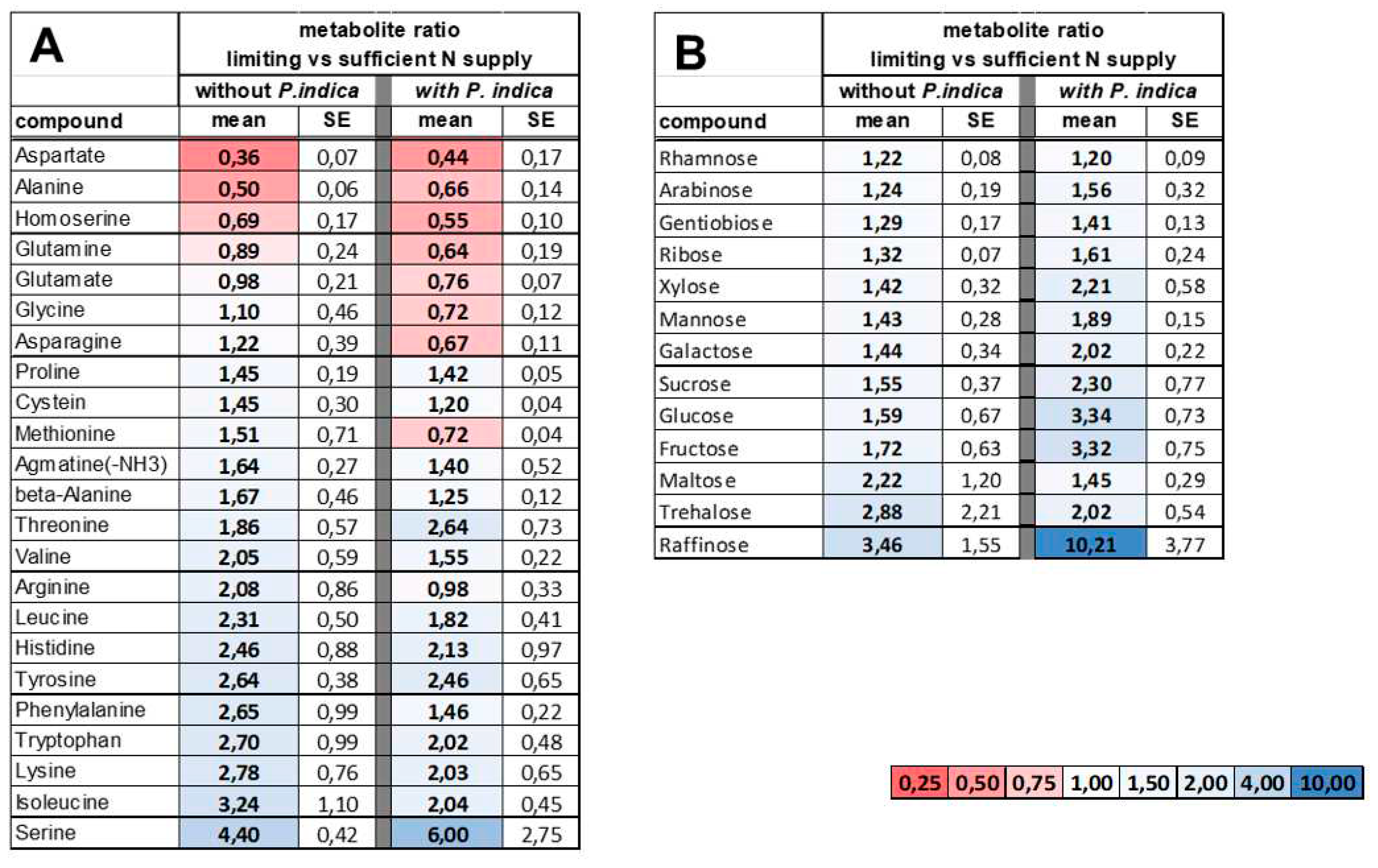 |
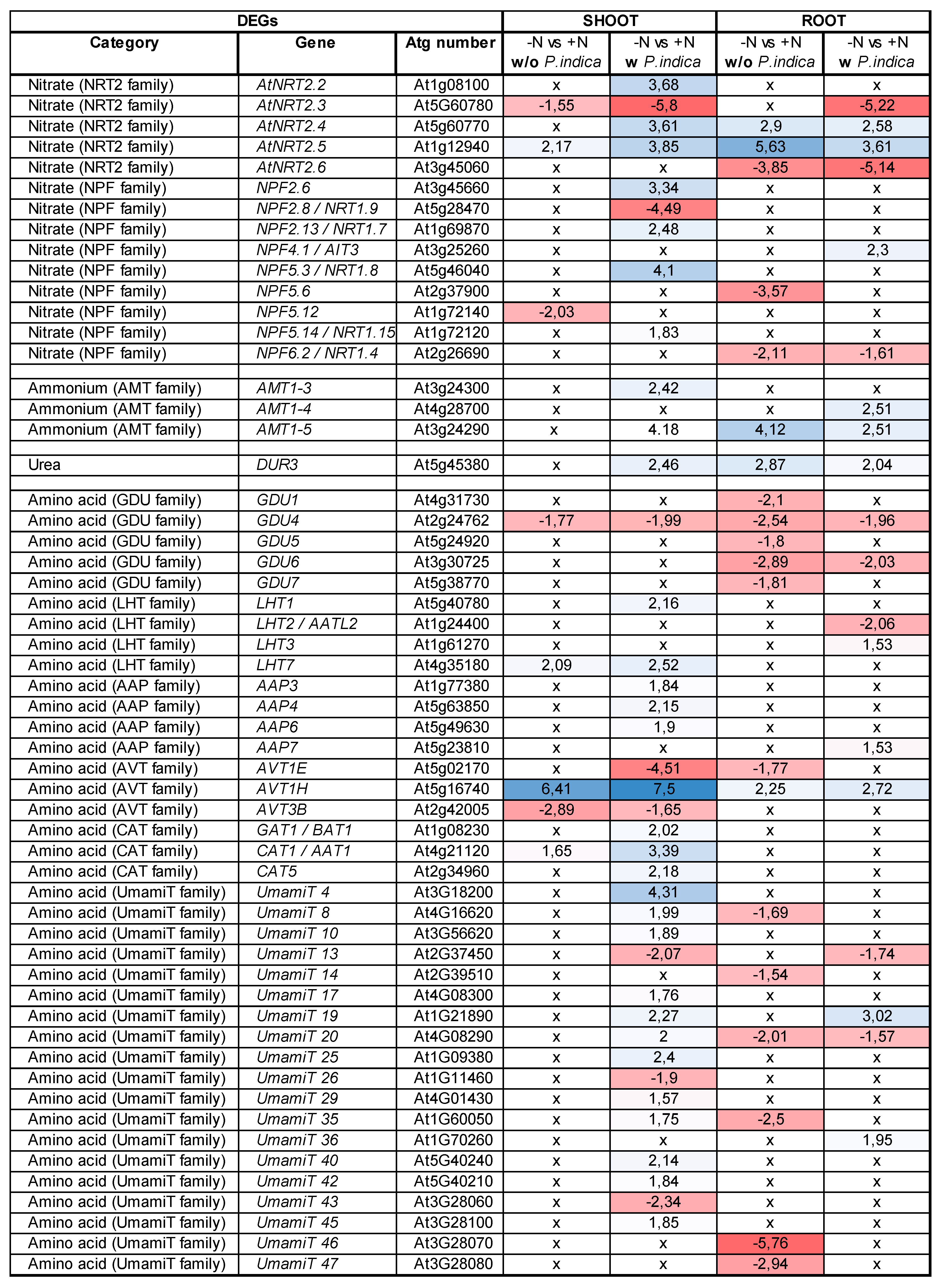 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





