Submitted:
08 September 2023
Posted:
11 September 2023
You are already at the latest version
Abstract
Keywords:
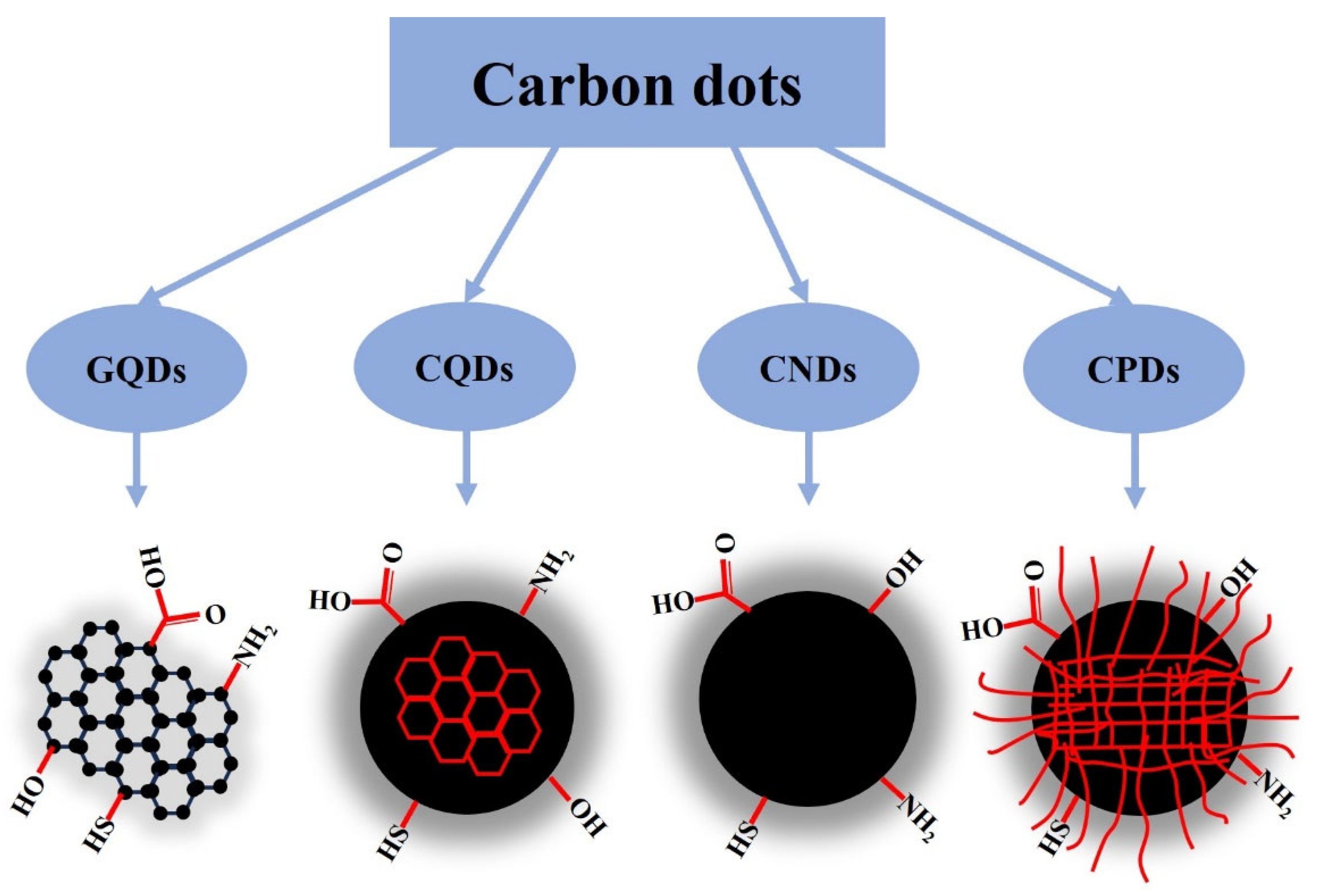
1. Introduction
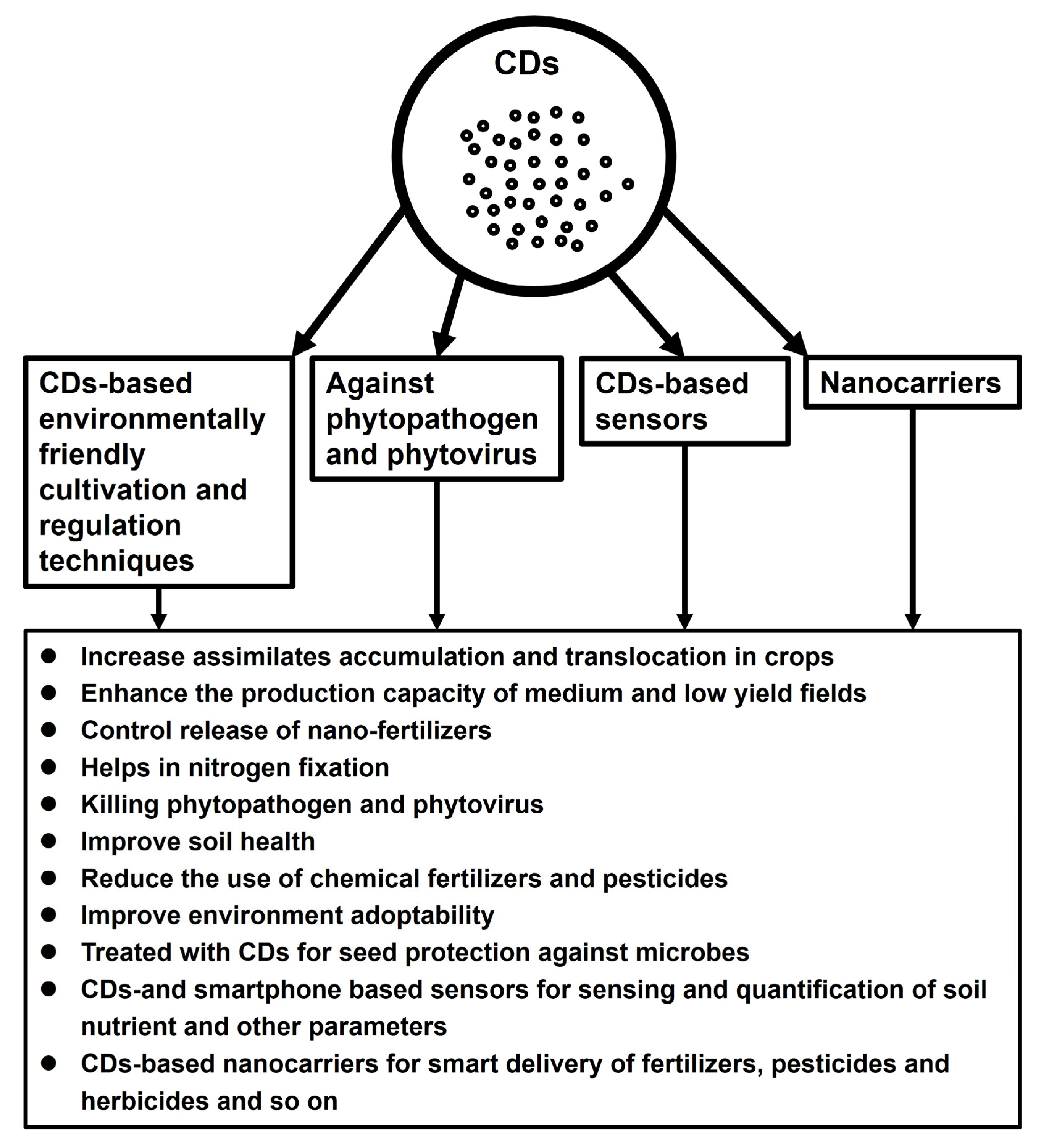
2. Applications of Carbon Dots in Crop Production
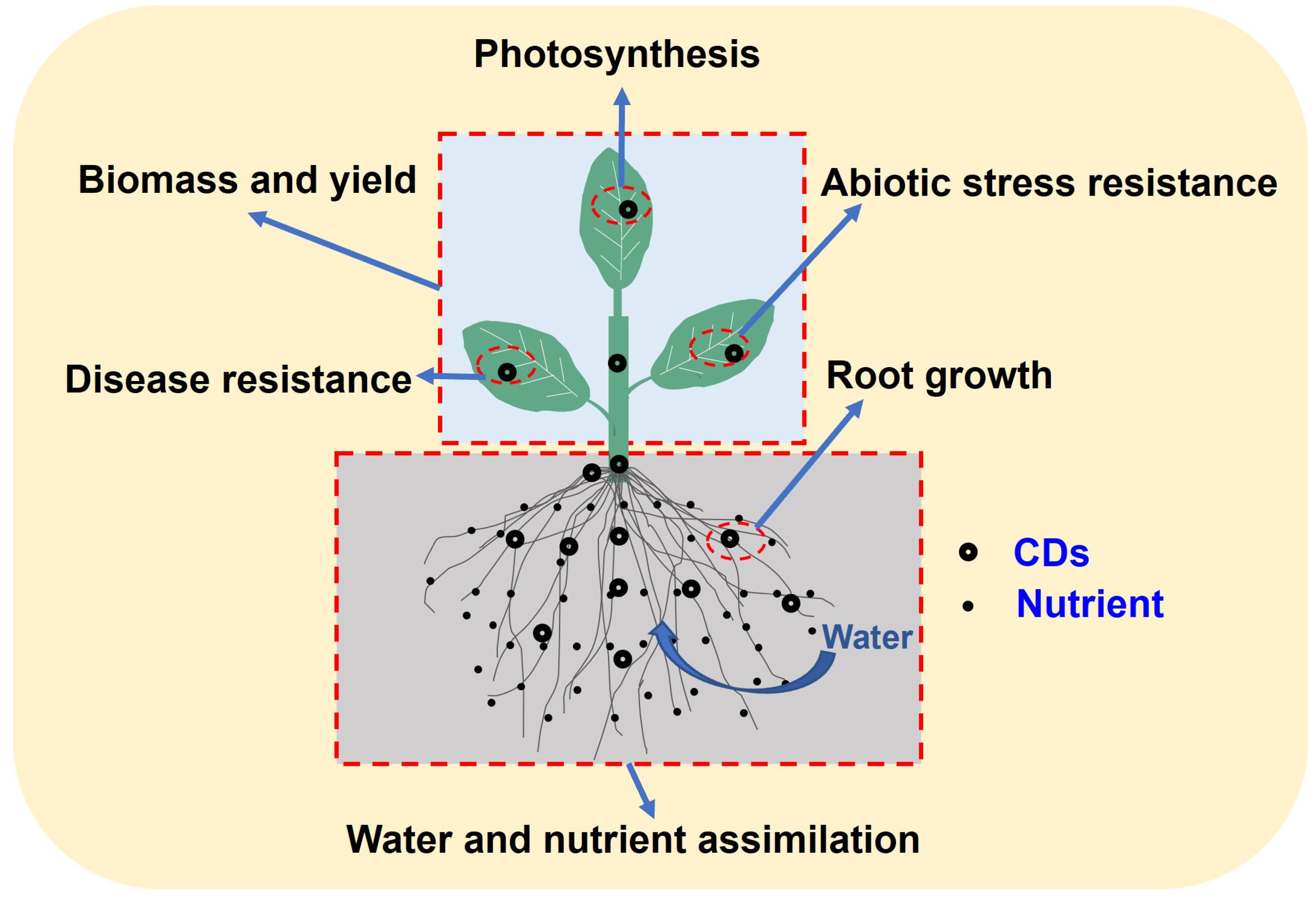
3. The Physiological Role of Carbon Dots and Their Impact on Crop Growth and Development
3.1. The Pathways of Carbon Dots Uptake by Crops and Their Accumulation and Transport Characteristics within Plants
3.2. Carbon Dots Enhance Crop Photosynthesis
3.3. Improving Crop Quality with Carbon Dots
3.4. Carbon Dots Promote Seed Germination, Increase Water and Nutrient Absorption
3.5. Carbon Dots Improve Crop Resistance to Abiotic Stress and Disease Resistance
3.6. The Protective Effect of Carbon Dots on Agricultural Ecological Environment
3.7. The Role of Carbon Dots as Biosensors in Agriculture
4. Futuristic Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Li, Y.; Xu, X.; Wu, Y.; Zhuang, J.; Zhang, X.; Zhang, H.; Lei, B.; Hu, C.; Liu, Y. A review on the effects of carbon dots in plant systems. Materials Chemistry Frontiers. 2020, 4, 437–448. [Google Scholar] [CrossRef]
- Dasgupta, N.; Ranjan, S.; Ramalingam, C. Applications of nanotechnology in agriculture and water quality management. Environmental Chemistry Letters. 2017, 15, 591–605. [Google Scholar] [CrossRef]
- Xu, Z.P. Material Nanotechnology Is Sustaining Modern Agriculture. ACS Agricultural Science & Technology. 2022, 2, 232–239. [Google Scholar] [CrossRef]
- He, X.; Deng, H.; Hwang, H.M. The current application of nanotechnology in food and agriculture. Journal of Food and Drug Analysis. 2019, 27, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Maholiya, A.; Ranjan, P.; Khan, R.; Murali, S.; Nainwal, R.C.; Chauhan, P.S.; Sathish, N.; Chaurasia, J.P.; Srivastava, A.K. An insight into the role of carbon dots in the agriculture system: a review. Environmental Science: Nano. 2023, 10, 959–995. [Google Scholar] [CrossRef]
- Xia, C.; Zhu, S.; Feng, T.; Yang, M.; Yang, B. Evolution and Synthesis of Carbon Dots: From Carbon Dots to Carbonized Polymer Dots. Advanced Science. 2019, 6, 1901316. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Arpita; Kumar, S.; Kumar, P.; Kataria, N.; Bhankar, V.; Kumar, K.; Kumar, R.; Hsieh, C.-T.; Khoo, K.S. Assessment of biomass-derived carbon dots as highly sensitive and selective templates for the sensing of hazardous ions. Nanoscale 2023. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Rani, N.; Kumar, S.; Kumar, P.; Mohan, B.; Pallavi; Bhankar, V.; Kataria, N.; Kumar, R.; Kumar, K. Assessing the biomass-based carbon dots and their composites for photocatalytic treatment of wastewater. Journal of Cleaner Production 2023, 413, 137474. [Google Scholar] [CrossRef]
- Shen, J.; Zhu, Y.; Yang, X.; Li, C. Graphene quantum dots: emergent nanolights for bioimaging, sensors, catalysis and photovoltaic devices. Chemical Communications. 2012, 48, 3686. [Google Scholar] [CrossRef] [PubMed]
- Hoang, V.C.; Dave, K.; Gomes, V.G. Carbon quantum dot-based composites for energy storage and electrocatalysis: Mechanism, applications and future prospects. Nano Energy. 2019, 66, 104093. [Google Scholar] [CrossRef]
- Liu, M.L.; Chen, B.B.; Li, C.M.; Huang, C.Z. Carbon dots: synthesis, formation mechanism, fluorescence origin and sensing applications. Green Chemistry. 2019, 21, 449–471. [Google Scholar] [CrossRef]
- Yan, Y.; Gong, J.; Chen, J.; Zeng, Z.; Huang, W.; Pu, K.; Liu, J.; Chen, P. Recent Advances on Graphene Quantum Dots: From Chemistry and Physics to Applications. Advanced Materials. 2019, 31, e1808283. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Geng, Y.; Li, D.; Yao, H.; Huo, Z.; Li, Y.; Zhang, K.; Zhu, S.; Wei, H.; Xu, W.; Jiang, J.; Yang, B. Deep Red Emissive Carbonized Polymer Dots with Unprecedented Narrow Full Width at Half Maximum. Adv Mater. 2020, 32, e1906641. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Wang, Y.K.; Sharma, G.; Dong, Y.; Zheng, X.; Li, P.; Johnston, A.; Bappi, G.; Fan, J.Z.; Kung, H.; Chen, B.; Saidaminov, M.L.; Singh, K.; Voznyy, O.; Bakr, O.M.; Lu, Z.H.; Sargent, E.H. Bright high-colour-purity deep-blue carbon dot light-emitting diodes via efficient edge amination. Nature Photonics. 2020, 14, 171–176. [Google Scholar] [CrossRef]
- Zhang, Z.; Yi, G.; Li, P.; Zhang, X.; Fan, H.; Zhang, Y.; Wang, X.; Zhang, C. A minireview on doped carbon dots for photocatalytic and electrocatalytic applications. Nanoscale. 2020, 12, 13899–13906. [Google Scholar] [CrossRef]
- Tuerhong, M.; Xu, Y.; Yin, X.-B. Review on Carbon Dots and Their Applications. Chinese Journal of Analytical Chemistry. 2017, 45, 139–150. [Google Scholar] [CrossRef]
- Xu, X.Y.; Ray, R.; Gu, Y.L.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. Journal of the American Chemical Society. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Sun, Y.P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.F.; Luo, P.; Yang, H.; Kose, M.E.; Chen, B.; Veca, M.; Xie, S.Y. Quantum-sized carbon dots for bright and colorful photoluminescence. Journal of the American Chemical Society. 2006, 128, 7756–7757. [Google Scholar] [CrossRef]
- Zhu, Z.J.; Wang, H.; Yan, B.; Zheng, H.; Jiang, Y.; Miranda, O.R.; Rotello, V.M.; Xing, B.; Vachet, R.W. Effect of surface charge on the uptake and distribution of gold nanoparticles in four plant species. Environmental Science & Technology. 2012, 46, 12391–12398. [Google Scholar] [CrossRef]
- Li, H.; Huang, J.; Lu, F.; Liu, Y.; Song, Y.; Sun, Y.; Zhong, J.; Huang, H.; Wang, Y.; Li, S.; Lifshitz, Y.; Lee, S.T.; Kang, Z. Impacts of Carbon Dots on Rice Plants: Boosting the Growth and Improving the Disease Resistance. ACS Applied Bio Materials. 2018, 1, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Wang, X.; Lu, Q.; Liu, X.; Wang, L. A biocompatible fluorescent ink based on water-soluble luminescent carbon nanodots. Angew Chem Int Ed Engl. 2012, 51, 12215–12218. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pan, X.; Xu, X.; Wu, Y.; Zhuang, J.; Zhang, X.; Zhang, H.; Lei, B.; Hu, C.; Liu, Y. Carbon dots as light converter for plant photosynthesis: Augmenting light coverage and quantum yield effect. J Hazard Mater. 2021, 410, 124534. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.L.; Zulkifli, N.A.; Zaman, A.S.K.; Jusoh, M.B.; Yaapar, M.N.; Rashid, S.A. Impact of photoluminescent carbon quantum dots on photosynthesis efficiency of rice and corn crops. Plant Physiology and Biochemistry. 2021, 162, 737–751. [Google Scholar] [CrossRef]
- Li, Y.; Xu, X.; Lei, B.; Zhuang, J.; Zhang, X.; Hu, C.; Cui, J.; Liu, Y. Magnesium-nitrogen co-doped carbon dots enhance plant growth through multifunctional regulation in photosynthesis. Chemical Engineering Journal. 2021, 422, 130114. [Google Scholar] [CrossRef]
- Tripathi, S.; Sarkar, S. Influence of water soluble carbon dots on the growth of wheat plant. Applied Nanoscience. 2014, 5, 609–616. [Google Scholar] [CrossRef]
- Wang, C.; Yang, H.; Chen, F.; Yue, L.; Wang, Z.; Xing, B. Nitrogen-doped carbon dots increased light conversion and electron supply to improve the corn photosystem and yield. Environmental Science & Technology. 2021, 55, 12317–12325. [Google Scholar] [CrossRef]
- Milenković, I.; Borišev, M.; Zhou, Y.; Spasić, S.Z.; Leblanc, R.M.; Radotić, K. Photosynthesis Enhancement in Maize via Nontoxic Orange Carbon Dots. Journal of Agricultural and Food Chemistry. 2021, 69, 5446–5451. [Google Scholar] [CrossRef]
- Yang, H. Mechanism of nitrogen-doped carbon dots on photosynthesis and drought tolerance of maize. Master dissertation, Jiangnan University, Wuxi, 2022. [Google Scholar]
- Yan, X.; Wang, J.; Li, D.; Feng, J.; Xu, Q.; Chen, H.; Han, R. Response of primary root to nitrogen-doped carbon dots in Arabidopsis thaliana: alterations in auxin level and cell division activity. Environmental Science: Nano. 2021, 8, 1352–1363. [Google Scholar] [CrossRef]
- Chen, J.; Dou, R.; Yang, Z.; Wang, X.; Miao, C.; Gao, X.; Wang, L. The effect and fate of water-soluble carbon nanodots in Maize (Zea mays L. ). Nanotoxicology. 2016, 10, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, M.; Song, Y.; Li, H.; Huang, H.; Shao, M.; Liu, Y.; Kang, Z. Carbon dots promote the growth and photosynthesis of mung bean sprouts. Carbon. 2018, 136, 94–102. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, L.; Wang, H.; Song, Y.; Liu, Y.; Li, H.; Shao, M.; Huang, H.; Kang, Z. One-step hydrothermal synthesis of chiral carbon dots and their effects on mung bean plant growth. Nanoscale. 2018, 10, 12734–12742. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zheng, Y.; Zhang, H.; Liu, Z.; Su, W.; Chen, S.; Liu, Y.; Zhuang, J.; Lei, B. Phytotoxicity, Uptake, and Translocation of Fluorescent Carbon Dots in Mung Bean Plants. ACS Applied Materials & Interfaces. 2016, 8, 19939–19945. [Google Scholar] [CrossRef]
- Jiang, F.; Chen, D.; Li, R.; Wang, Y.; Zhang, G.; Li, S.; Zheng, J.; Huang, N.; Gu, Y.; Wang, C.; Shu, C. Eco-friendly synthesis of size-controllable amine-functionalized graphene quantum dots with antimycoplasma properties. Nanoscale. 2013, 5, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Meng, Q.; Wang, L.; Zhang, J.; Song, Y.; Jin, H.; Zhang, K.; Sun, H.; Wang, H.; Yang, B. Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew Chem Int Ed Engl. 2013, 52, 3953–3957. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Sahu, S.; Anilkumar, P.; Bunker, C.E.; Xu, J.; Fernando, K.A.; Wang, P.; Guliants, E.A.; Tackett K.N., 2nd; Sun, Y.P. Carbon nanoparticles as visible-light photocatalysts for efficient CO2 conversion and beyond. J Am Chem Soc. 2011, 133, 4754–4757. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Chandra, S.; Patra, P.; Pramanik, P.; Goswami, A. Novel fluorescent matrix embedded carbon quantum dots for the production of stable gold and silver hydrosols. Journal of Materials Chemistry. 2011, 21, 17638. [Google Scholar] [CrossRef]
- Chandra, S.; Pradhan, S.; Mitra, S.; Patra, P.; Bhattacharya, A.; Pramanik, P.; Goswami, A. High throughput electron transfer from carbon dots to chloroplast: a rationale of enhanced photosynthesis. Nanoscale. 2014, 6, 3647–3655. [Google Scholar] [CrossRef]
- Paul, M. Photosynthesis. Plastid biology, energy conversion and carbon assimilation. Annals of Botany. 2013, 111, ix. [Google Scholar]
- Li, H.; Huang, J.; Liu, Y.; Lu, F.; Zhong, J.; Wang, Y.; Li, S.; Lifshitz, Y.; Lee, S.-T.; Kang, Z. Enhanced RuBisCO activity and promoted dicotyledons growth with degradable carbon dots. Nano Research. 2019, 12, 1585–1593. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, S.; Yang, F.; Wang, G.; Jing, X.; Wang, X.; You, C. New strategy of light quality regulation with leaf-spraying fluorescent coatings for enhancing photosynthesis efficiency. RSC Advances. 2021, 11, 26620–26628. [Google Scholar] [CrossRef]
- Huang, C.; Xia, T.; Niu, J.; Yang, Y.; Lin, S.; Wang, X.; Yang, G.; Mao, L.; Xing, B. Transformation of 14C-labeled graphene to 14CO2 in the shoots of a rice plant. Angew Chem Int Ed Engl. 2018, 57, 9759–9763. [Google Scholar] [CrossRef]
- A/P Chowmasundaram, Y.; Tan, T.L.; Nulit, R.; Jusoh, M.; Rashid, S.A. Recent developments, applications and challenges for carbon quantum dots as a photosynthesis enhancer in agriculture. RSC Advances. 2023, 13, 25093–25117. [Google Scholar] [CrossRef]
- Tan, J.; Zhao, S.; Chen, J.; Pan, X.; Li, C.; Liu, Y.; Wu, C.; Li, W.; Zheng, M. Preparation of nitrogen-doped carbon dots and their enhancement on lettuce yield and quality. Journal of Materials Chemistry B. 2023, 11, 3113–3123. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Hao, R.; Tian, X.; Wang, X.; Ma, G.; Tang, H. Effects of adding nono-carbon in slow-released fertilizer on grain yield and nitrogen use efficiency of super hybrid rice. Hybrid Rice. 2010, 25, 86–90. [Google Scholar]
- Farshbaf, M.; Davaran, S.; Rahimi, F.; Annabi, N.; Salehi, R.; Akbarzadeh, A. Carbon quantum dots: recent progresses on synthesis, surface modification and applications. Artificial Cells Nanomedicine and Biotechnology. 2018, 46, 1331–1348. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, Y.; Yang, H.; Liu, X.; Wang, C. Study on the mechanism of carbon dots promoting crop seed germination and growth. J Anhui Agric. Sci. 2021, 49, 1–5. [Google Scholar]
- Zheng, Y.; Xie, G.; Zhang, X.; Chen, Z.; Cai, Y.; Yu, W.; Liu, H.; Shan, J.; Li, R.; Liu, Y.; Lei, B. Bioimaging Application and Growth-Promoting Behavior of Carbon Dots from Pollen on Hydroponically Cultivated Rome Lettuce. ACS Omega. 2017, 2, 3958–3965. [Google Scholar] [CrossRef]
- Hu, J.; Jia, W.; Yu, X.; Yan, C.; White, J.C.; Liu, J.; Shen, G.; Tao, S.; Wang, X. Carbon dots improve the nutritional quality of coriander (Coriandrum sativum L.) by promoting photosynthesis and nutrient uptake. Environmental Science: Nano. 2022, 9, 1651–1661. [Google Scholar] [CrossRef]
- An, Z.; Zhang, H.; Li, W.; Yang, X.; Kang, Y.; Zheng, M.; Lei, B. Large-Scale Preparation of Peanut-Bran-Derived Carbon Dots and Their Promoting Effect on Italian Lettuce. ACS Agricultural Science & Technology. 2021, 2, 215–221. [Google Scholar] [CrossRef]
- Kou, E.; Yao, Y.; Yang, X.; Song, S.; Li, W.; Kang, Y.; Qu, S.; Dong, R.; Pan, X.; Li, D.; Zhang, H.; Lei, B. Regulation Mechanisms of Carbon Dots in the Development of Lettuce and Tomato. ACS Sustainable Chemistry & Engineering. 2021, 9, 944–953. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, L.; Nie, X.; Man, H.; Guo, Z.; Wang, X.; Tu, J.; Jin, G.; Ci, L. Impacts of surface chemistry of functional carbon nanodots on the plant growth. Ecotoxicol Environ Saf. 2020, 206, 111220. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, L. Effects of nanocarbon mixing with nitrogen fertilizer on soil microbial community structure and nutrient content. China Rice. 2019, 25, 70–73. [Google Scholar] [CrossRef]
- Gramer, G.R.; Urano, K.; Delrot, S.; Pezzotti, M.; Shinozaki, K. Effects of abiotic stress on plants: a systems biology perspective. BMC Plant Biology. 2011, 163. [Google Scholar]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.K. Abiotic stress responses in plants. Nat Rev Genet. 2021, 23. [Google Scholar] [CrossRef] [PubMed]
- Bidinger, F.; Musgrave, R.B.; Fischer, R.A. Contribution of stored pre-anthesis assimilate to grain yield in wheat and barley. Nature. 1977, 270, 431–433. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Wang, Z.; Zhu, Q.; Liu, L. Water deficit–induced senescence and its relationship to the remobilization of pre-stored carbon in wheat during grain filling. Agronomy Journal. 2001, 93, 196–206. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Wang, Z.; Zhu, Q.; Wei, W. Remobilization of carbon reserves in response to water deficit during grain filling of rice. Field Crops Research. 2001, 71, 47–55. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant Journal. 2017, 90, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Dadhich, P.; Pal, P.; Srivas, P.K.; Bankoti, K.; Dhara, S. Carbon nanodots from date molasses: new nanolights for the in vitro scavenging of reactive oxygen species. Journal of Materials Chemistry B. 2014, 2, 6839–6847. [Google Scholar] [CrossRef]
- Zhao, S.; Lan, M.; Zhu, X.; Xue, H.; Ng, T.W.; Meng, X.; Lee, C.S.; Wang, P.; Zhang, W. Green Synthesis of Bifunctional Fluorescent Carbon Dots from Garlic for Cellular Imaging and Free Radical Scavenging. ACS Applied Materials & Interfaces. 2015, 7, 17054–17060. [Google Scholar] [CrossRef]
- Su, L.X.; Ma, X.L.; Zhao, K.K.; Shen, C.L.; Lou, Q.; Yin, D.M.; Shan, C.X. Carbon Nanodots for Enhancing the Stress Resistance of Peanut Plants. ACS Omega. 2018, 3, 17770–17777. [Google Scholar] [CrossRef]
- Wang, C.; Ji, Y.; Cao, X.; Yue, L.; Chen, F.; Li, J.; Yang, H.; Wang, Z.; Xing, B. Carbon Dots Improve Nitrogen Bioavailability to Promote the Growth and Nutritional Quality of Soybeans under Drought Stress. ACS Nano. 2022, 16, 12415–12424. [Google Scholar] [CrossRef]
- Ji, Y.; Yue, L.; Cao, X.; Chen, F.; Li, J.; Zhang, J.; Wang, C.; Wang, Z.; Xing, B. Carbon dots promoted soybean photosynthesis and amino acid biosynthesis under drought stress: Reactive oxygen species scavenging and nitrogen metabolism. Sci Total Environ. 2023, 856, 159125. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Kang, Y.; Li, H.; Huang, S.; Li, W.; Zheng, M.; Huang, R.; Lei, B.; Yang, X. Salvia miltiorrhiza Derived Carbon Dots and Their Heat Stress Tolerance of Italian Lettuce by Promoting Growth and Enhancing Antioxidant Enzyme Activity. ACS Omega. 2021, 6, 32262–32269. [Google Scholar] [CrossRef]
- Li, Y.; Tang, Z.; Pan, Z.; Wang, R.; Wang, X.; Zhao, P.; Liu, M.; Zhu, Y.; Liu, C.; Wang, W.; Liang, Q.; Gao, J.; Yu, Y.; Li, Z.; Lei, B.; Sun, J. Calcium-Mobilizing Properties of Salvia miltiorrhiza-Derived Carbon Dots Confer Enhanced Environmental Adaptability in Plants. ACS Nano. 2022, 16, 4357–4370. [Google Scholar] [CrossRef] [PubMed]
- Gohari, G.; Panahirad, S.; Sadeghi, M.; Akbari, A.; Zareei, E.; Zahedi, S.M.; Bahrami, M.K.; Fotopoulos, V. Putrescine-functionalized carbon quantum dot (put-CQD) nanoparticles effectively prime grapevine (Vitis vinifera cv. 'Sultana') against salt stress. BMC Plant Biol. 2021, 21, 120. [Google Scholar] [CrossRef]
- Li, H.; Huang, J.; Song, Y.; Zhang, M.; Wang, H.; Lu, F.; Huang, H.; Liu, Y.; Dai, X.; Gu, Z.; Yang, Z.; Zhou, R.; Kang, Z. Degradable Carbon Dots with Broad-Spectrum Antibacterial Activity. ACS Applied Materials & Interfaces. 2018, 10, 26936–26946. [Google Scholar] [CrossRef]
- Luo, X.; Cao, X.; Wang, C.; Yue, L.; Chen, X.; Yang, H.; Le, X.; Zhao, X.; Wu, F.; Wang, Z.; Xing, B. Nitrogen-doped carbon dots alleviate the damage from tomato bacterial wilt syndrome: systemic acquired resistance activation and reactive oxygen species scavenging. Environmental Science: Nano. 2021, 8, 3806–3819. [Google Scholar] [CrossRef]
- Cruz, A.; Gallareta-Olivares, G.; Rivas-Sanchez, A.; González-González, R.B.; Ahmed, I.; Parra-Saldívar, R.; Iqbal, H.M.N. Recent Advances in Carbon Dots Based Biocatalysts for Degrading Organic Pollutants. Current Pollution Reports. 2022, 8, 384–394. [Google Scholar] [CrossRef]
- Thara, R.; Korah, B.K.; Mathew, S.; John, B.K.; Mathew, B. Dual mode detection and sunlight-driven photocatalytic degradation of tetracycline with tailor-made N-doped carbon dots. Environ Res. 2023, 216, 114450. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Guo, H.; Wang, S.; Li, J.; Wang, Y.; Xing, B. Carbon dots alleviate the toxicity of cadmium ions (Cd2+) toward wheat seedlings. Environmental Science: Nano. 2019, 6, 1493–1506. [Google Scholar] [CrossRef]
- Dučić, T.; Milenkovic, I.; Mutavdzic, D.; Nikolic, M.; de Yuso, M.V.M.; Vucinic, Z.; Algarra, M.; Radotic, K. Estimation of carbon dots amelioration of copper toxicity in maize studied by synchrotron radiation-FTIR. Colloids and Surfaces B: Biointerfaces. 2021, 204, 111828. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y. Effect of carbon quantum dots on dissimilatory iron reduction and cadmium transformation in soil and its microbial mechanism. Master dissertation, Guangdong University of Technology, Guangzhou, 2022. [Google Scholar]
- Kumar, S.; Yadav, S.; Kataria, N.; Chauhan, A.K.; Joshi, S.; Gupta, R.; Kumar, P.; Chong, J.W.R.; Khoo, K.S.; Show, P.L. Recent Advancement in Nanotechnology for the Treatment of Pharmaceutical Wastewater: Sources, Toxicity, and Remediation Technology. Current Pollution Reports. 2023, 9, 110–142. [Google Scholar] [CrossRef]
- Rani, N.; Singh, P.; Kumar, S.; Kumar, P.; Bhankar, V.; Kumar, K. Plant-mediated synthesis of nanoparticles and their applications: A review. Materials Research Bulletin. 2023, 163, 112233. [Google Scholar] [CrossRef]
- Tafreshi, F.; Fatahi, Z.; Ghasemi, S.F.; Taherian, A.; Esfandiari, N. Ultrasensitive fluorescent detection of pesticides in real sample by using green carbon dots. PLoS One. 2020, 15, e0230646. [Google Scholar] [CrossRef]
- Ghosh, S.; Gul, A.R.; Park, C.Y.; Xu, P.; Baek, S.H.; Bhamore, J.R.; Kim, M.W.; Lee, M.; Kailasa, S.K.; Park, T.J. Green synthesis of carbon dots from Calotropis procera leaves for trace level identification of isoprothiolane. Microchemical Journal. 2021, 167, 106272. [Google Scholar] [CrossRef]
- Ansari, L.; Hallaj, S.; Hallaj, T.; Amjadi, M. Doped-carbon dots: Recent advances in their biosensing, bioimaging and therapy applications. Colloids and Surfaces B: Biointerfaces. 2021, 203, 111743. [Google Scholar] [CrossRef]
- Li, X.; Fu, Y.; Zhao, S.; Xiao, J.; Lan, M.; Wang, B.; Zhang, K.; Song, X.; Zeng, L. Metal ions-doped carbon dots: Synthesis, properties, and applications. Chemical Engineering Journal. 2022, 430, 133101. [Google Scholar] [CrossRef]
- Yahyai, I.; Al-Lawati, H.A.J.; Hassanzadeh, J. A paper-based chemiluminescence detection device based on S, N-doped carbon quantum dots for the selective and highly sensitive recognition of bendiocarb. Analytical Methods. 2021, 13, 3461–3470. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Wei, J.; Zhao, Y.; Sun, Y.; Liang, H.; Wang, S.; Li, P. Fluorescent and visual detection of methyl-paraoxon by using boron-and nitrogen-doped carbon dots. Microchemical Journal. 2020, 154, 104547. [Google Scholar] [CrossRef]
- Wang, C.Y.; Sun, X.F.; Wang, M.; Wang, J.B.; Ding, Q.F. Chinese cropland quality and its temporal and spatial changes due to urbanization in 2000 -2015. J. Resour. Ecol. 2019, 10, 174–183. [Google Scholar] [CrossRef]
- Fu, J.; Huang, Z.; Wang, Z.; Yang, J.; Zhang, J. Pre-anthesis non-structural carbohydrate reserve in the stem enhances the sink strength of inferior spikelets during grain filling of rice. Field Crops Research. 2011, 123, 170–182. [Google Scholar] [CrossRef]
- Yang, J. Study on grain filling characteristics and physiological basis of inter-subspecific hybrid rice. Doctoral Dissertation, China Agricultural University, Beijing, 1996. [Google Scholar]
- Yang, J.; Zhang, J.; Wang, W. Hormonal changes in the grains of rice subjected to water stress during grain filling. Plant Physiology. 2001, 127, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, J.; Wang, Z.; Zhu, Q. Activities of starch hydrolytic enzymes and sucrose-phosphate synthase in the stems of rice subjected to water stress during grain filling. Journal of Experimental Botany. 2001, 52, 2169–2179. [Google Scholar] [CrossRef]
- Fu, J.; Xu, Y.; Chen, L.; Yuan, L.; Wang, Z.; Yang, J. Changes in enzyme activities involved in starch synthesis and hormone concentrations in superior and inferior spikelets and their association with grain filling of super Rice. Rice Science. 2013, 20, 120–128. [Google Scholar] [CrossRef]
- Braun, D.M.; Wang, L.; Ruan, Y.L. Understanding and manipulating sucrose phloem loading, unloading, metabolism, and signalling to enhance crop yield and food security. Journal of Experimental Botany. 2014, 65, 1713–1735. [Google Scholar] [CrossRef]
- Todorenko, D.A.; Gvozdev, D.A.; Tsoraev, G.V.; Baizhumanov, A.A.; Lukashev, E.P.; Matorin, D.N. The effect of carbon nanodots and graphene quantum dots on the green microalga Scenedesmus quadricauda. Journal of Applied Phycology. 2023. [CrossRef]
| Crop | Source of CDs | Treated part of plant | Experimental methods | Effecet of CDs on plnat | Mechanism of action | Reference | ||
|---|---|---|---|---|---|---|---|---|
| Rice | Electrochemical etching using graphite rods, CDs are about 5 mm | Seed | Soke in MS medium supplemented with CDs of 0.56 mg/mL, and expose for 10 days | Enhance disease-resistance ability and grain yield | Increase the thionin gene expression; CDs degrade to formplant-hormone analogues and CO2; RuBisCO activity increase by 42% | [20] | ||
| Rice | Microwave pyrolysis using citric acid and ethanolamine, CDs are 3-4 nm | Leave | Spray with 300 μg/mL CDs three times a week with 5 mL/pot | Increase shoot length, dry weights of shoot and root | Increases the electron transport rate and photo-synthetic efficiency of photosystem II by 29.81% and 29.88%; increased the chlorophyll content and RuBisCO carboxylase activity by 64.53% and 23.39% | [22] | ||
| Rice | Microwave pyrolysis using biochar, CDs are 1-4 nm | Leave | Spray with 150 mg/mL CDs twice a month until the end of heading | Increase plant height and grain weight by 4.8% and 5.1% | Increase CO2 assimilate rate by 56% and 56% | [23] | ||
| Rice | Hydrothermal using citric acid, ethanolamine, and magnesium hydrate | Leave | Spray with 50, 100, and 300 μg/mL CDs with a dosage of 5 mL/pot and exposure for 16 days | Increased the height and fresh biomass by 22.34% and 70.60% | Up-regulated the gene expressions of enzymes related to chlorophyll by 15.26–115.02%; increasing chlorophyll a and chlorophyll b contents by 14.39% and 26.54%; increased the RuBisCO activity plants by 46.62% | [24] | ||
| Wheat | Carbon soot of mustard oil lamp, CDs are 20-100 nm | Seed | Soake in CDs aqueous solution for 3-4 days | Increase root and shoot length | Regulate the movement of water and ions | [25] | ||
| Maize | Thermo-polymerization of melamine and ethylenediamine tetraacetic acid, CDs are 2.5 nm | Leave | Spray 5 mL per plant with 1, 5, 10, 50 mg/L CDs and expose for 7 days | Increase yield and 1000-grain weight by 24.5% and 15.0% | Increased light conversion efficiency by 121.00%; increased the chlorophyll content by 15.41%; increase relative gene expression of psbA by 22.30 fold, ATPase activity by 41.44%, and NADPH production by 110.31% | [26] | ||
| Maize | Microwave pyrolysis using biochar, CDs are 1-4 nm | Leave | Spray with 150 mg/mL twice a month until the end of heading | Increase plant height and ear weight by 20.9% and 39.6% | Increase CO2 assimilate rate and stomatal conductance by 16% and 18% | [23] | ||
| Maize | Ultrasonication using citric acid and o-phenylenediamine | Root; leave | Hydroponic medium cultivation and sparying on leaves with 1, 5, and 10 mg/L CDs, and expose for 14 days | Increase photosynthetic parameters | Increase photosynthetic pigments | [27] | ||
| Maize | Hydrothermal using citric acid and ethylenediamine | Leave | spray 5 mL per plant using 5 mg/L CDs solutions, and expose for 33 days | Increase fresh weight of shoots and roots by 62.1% and 50.6%, and dry weight of shoots androots by 29.2% and 37.5% | Increase net photosynthes by 122.9%; increase root exudates of succinic acid (14.5 folds), pyruvic acid (10.0 folds), and betaine (11.8 folds), and relative abundance of microbial community by 122.6%-344.4% | [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





