Submitted:
06 September 2023
Posted:
07 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
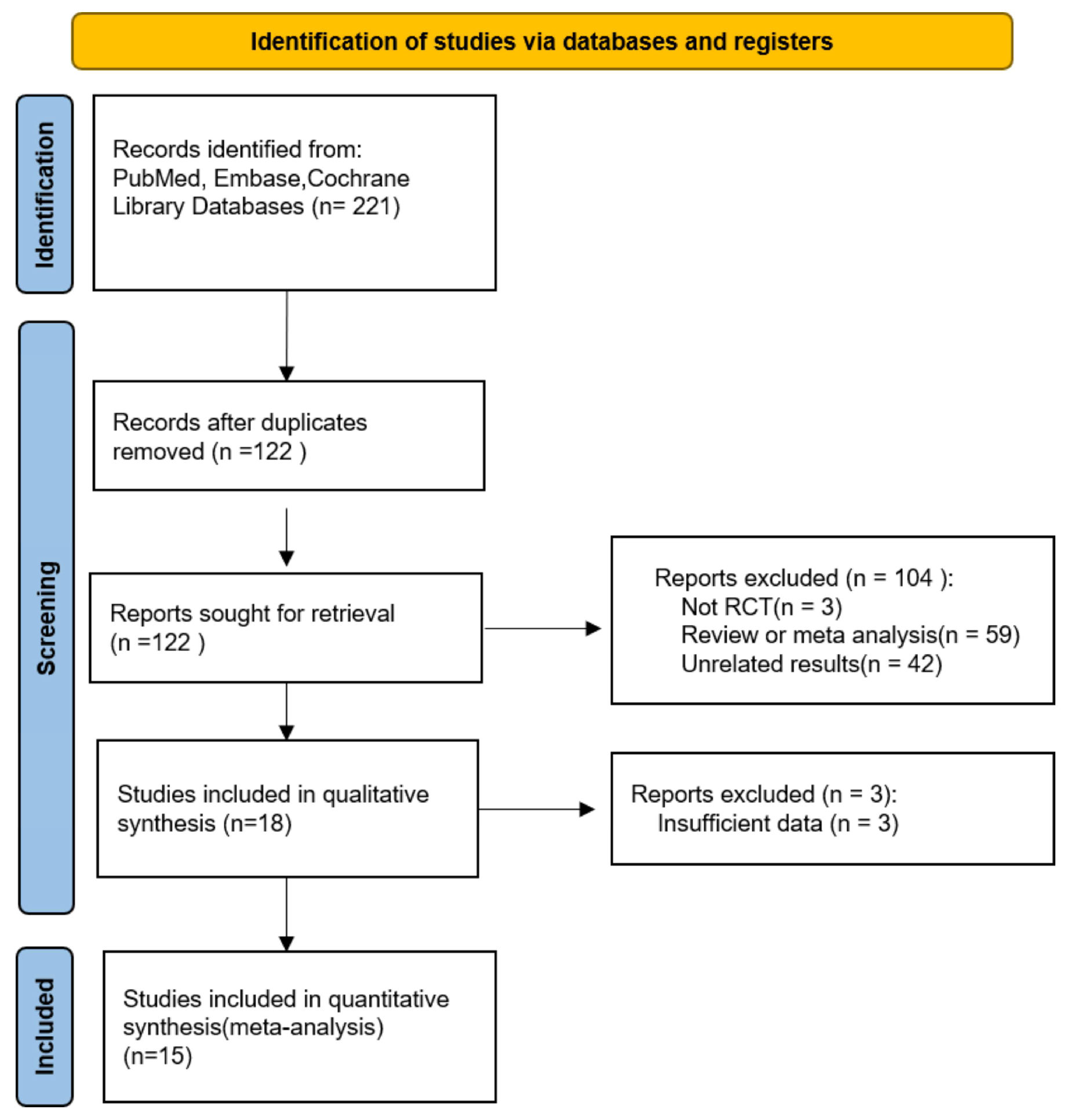
2.1. Literature Search
2.2. Inclusion Criteria And Exclusion Criteria
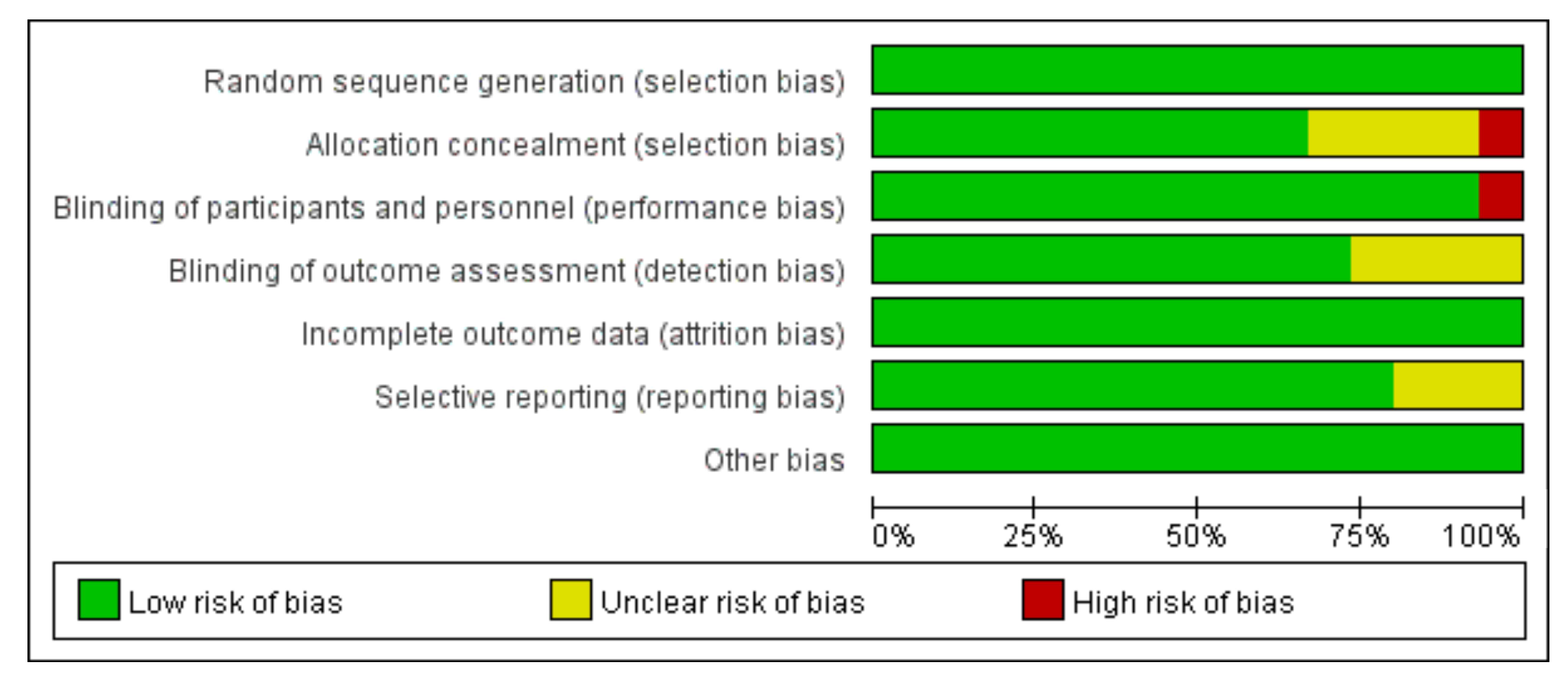
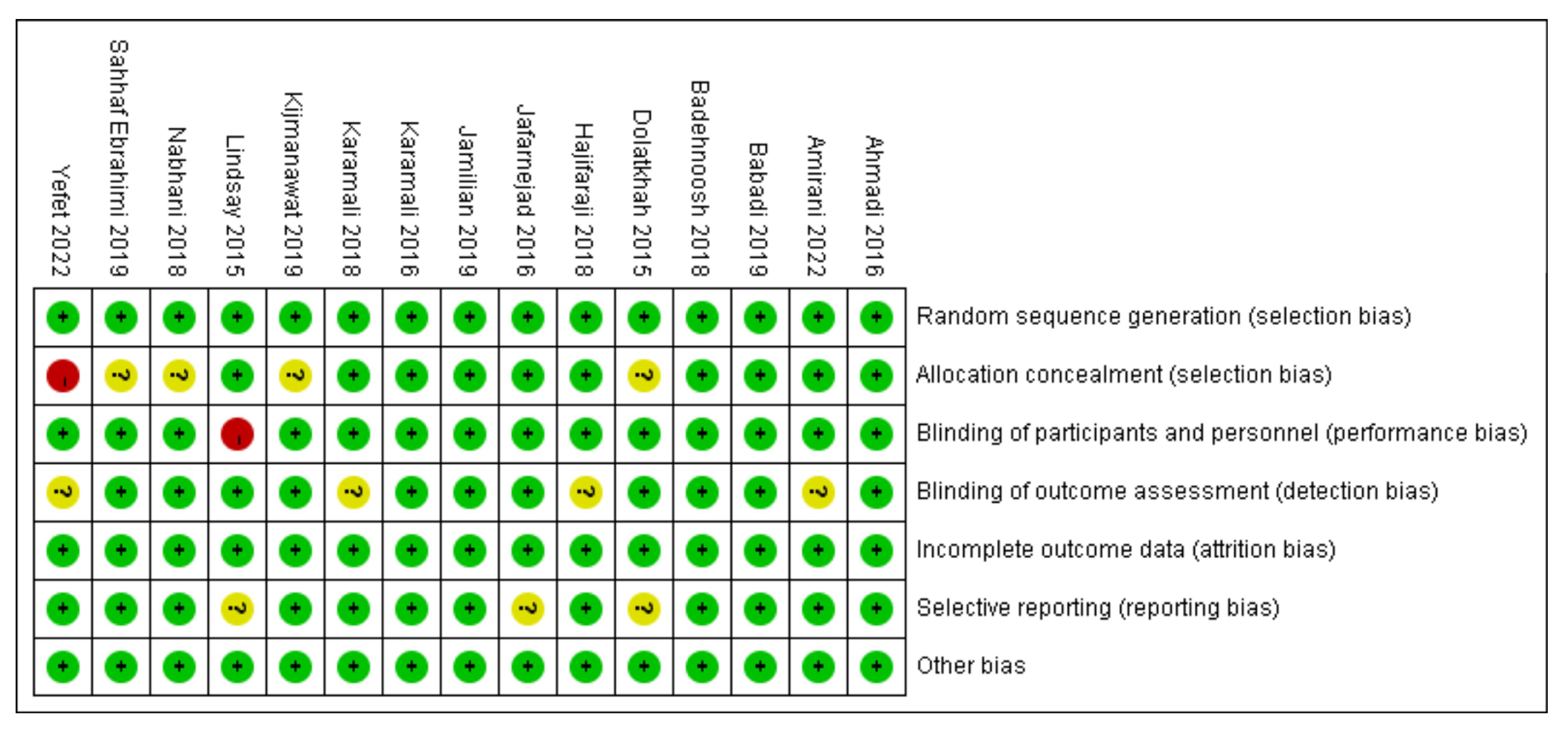
2.3. Risk of Bias Assessment
2.4. Data Extraction
2.5. Quality Assessment
2.6. Statistical Analysis
3. Results
3.1. Study Selection and Characteristics
3.2. Glycemic outcomes
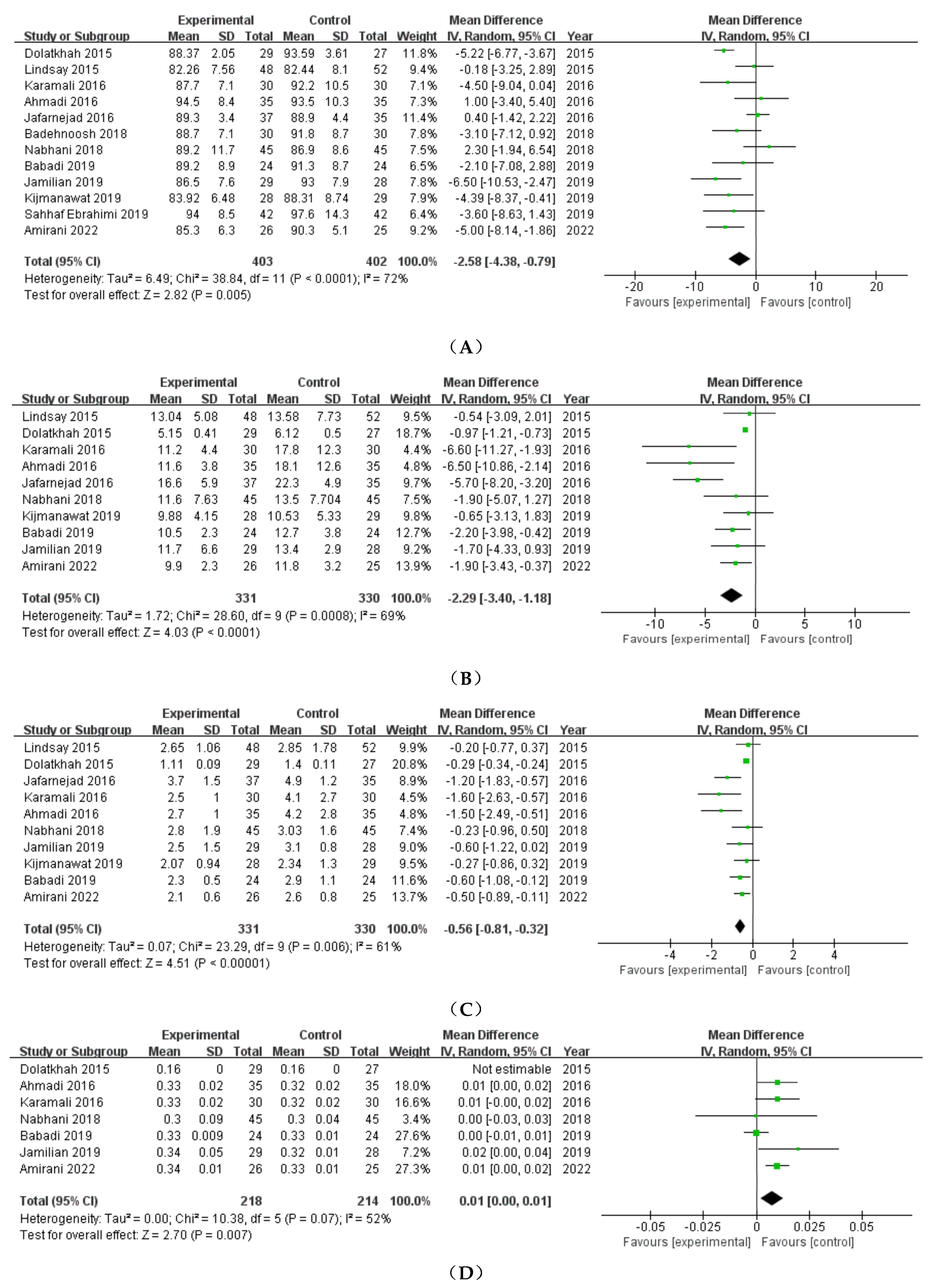
3.2.1. FBS
3.2.2. FSI
3.2.3. HOMA-IR
3.2.4. QUICKI
3.3. Maternal outcomes
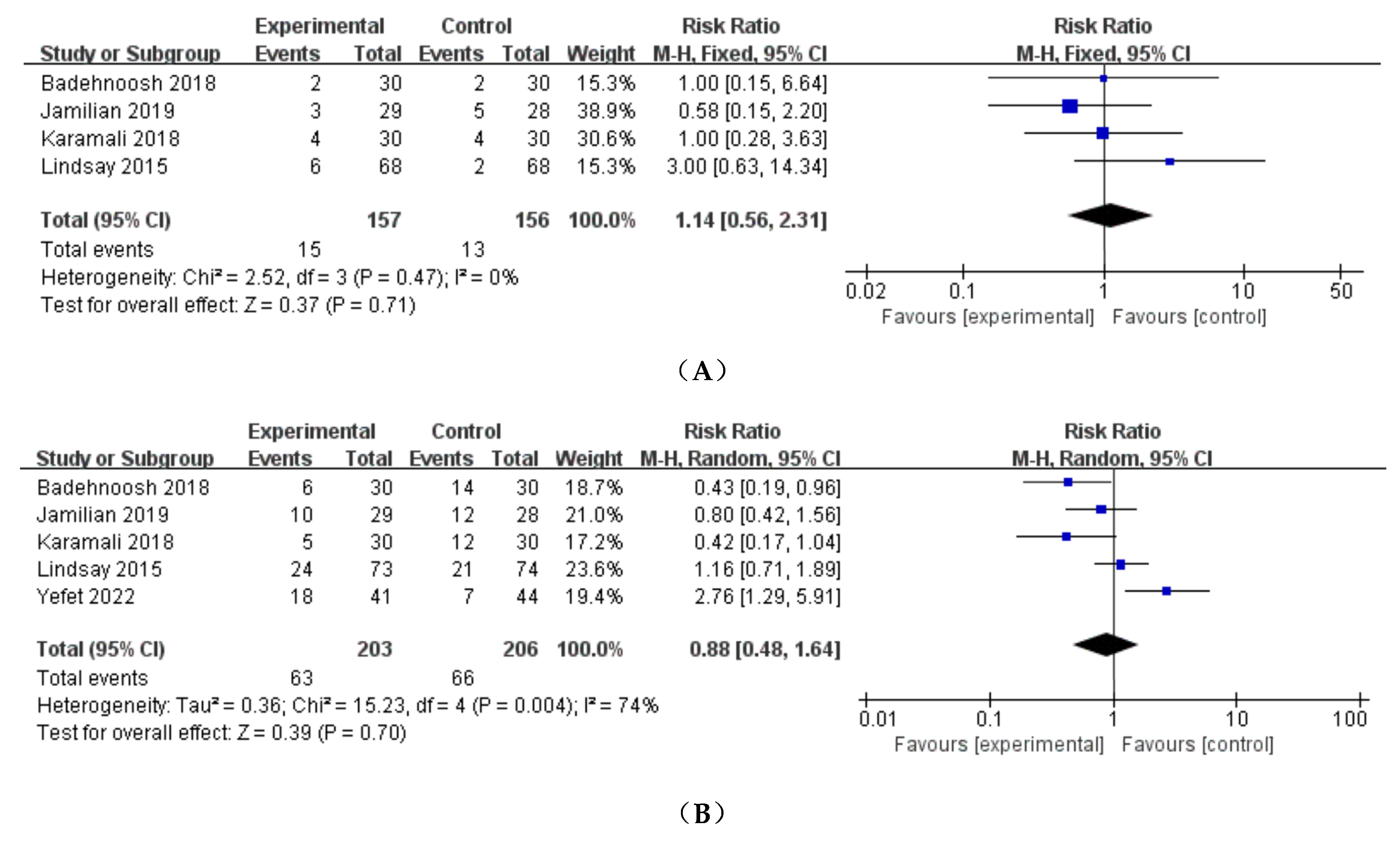
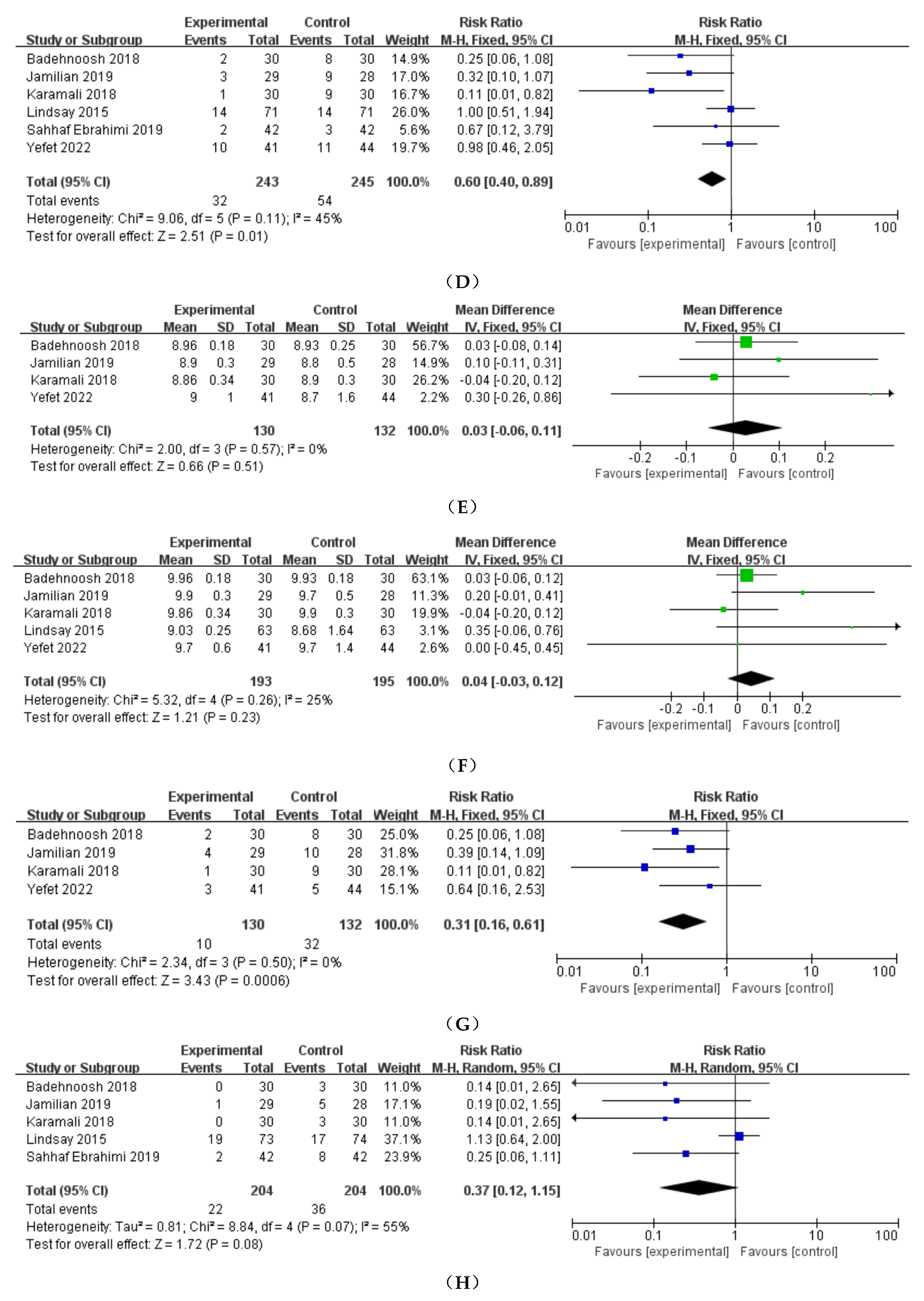
3.3.1. Preeclampsia
3.3.2. Cesarean delivery
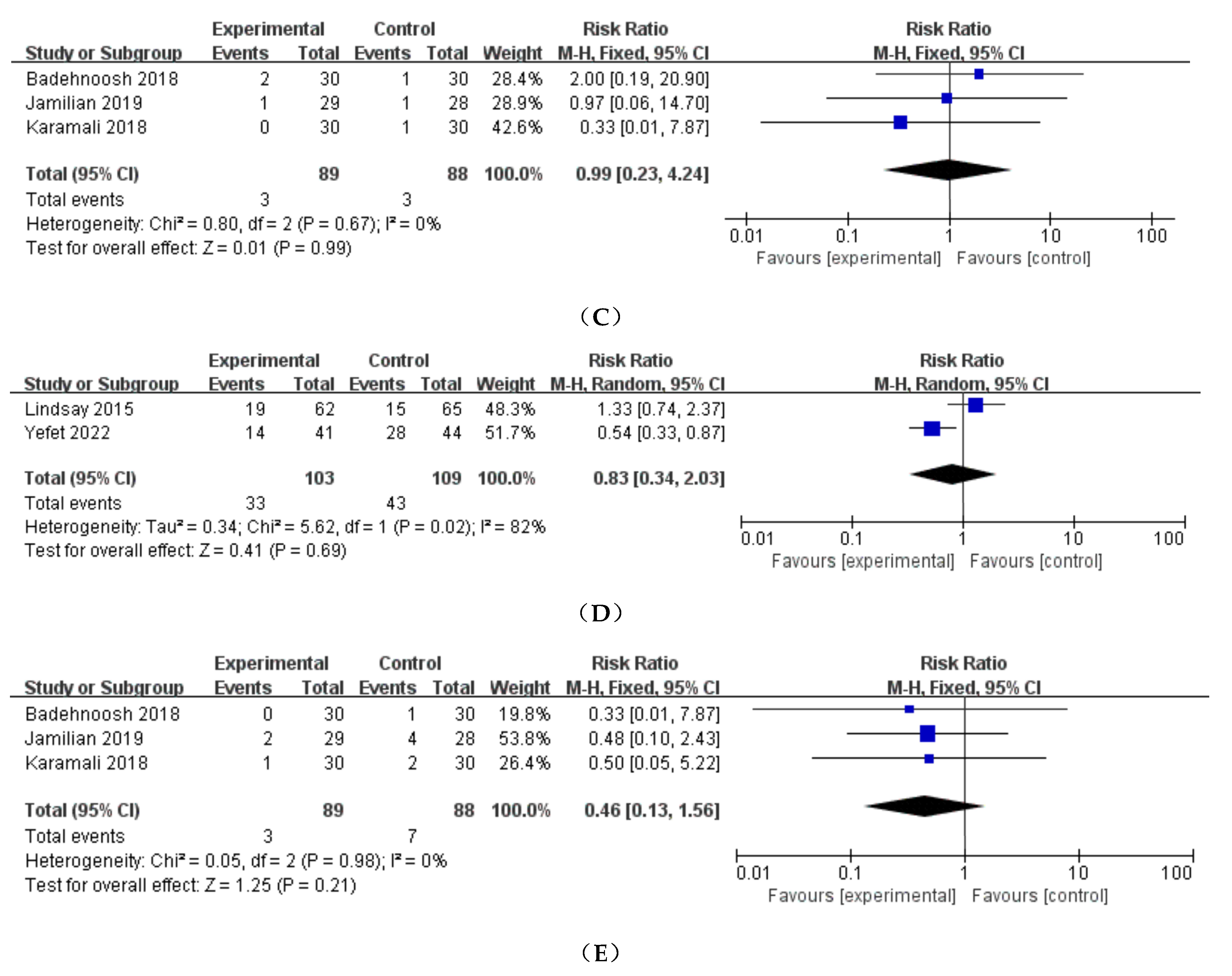
3.3.3. Preterm delivery
3.3.4. Induction of labor
3.3.5. Polyhydramnios
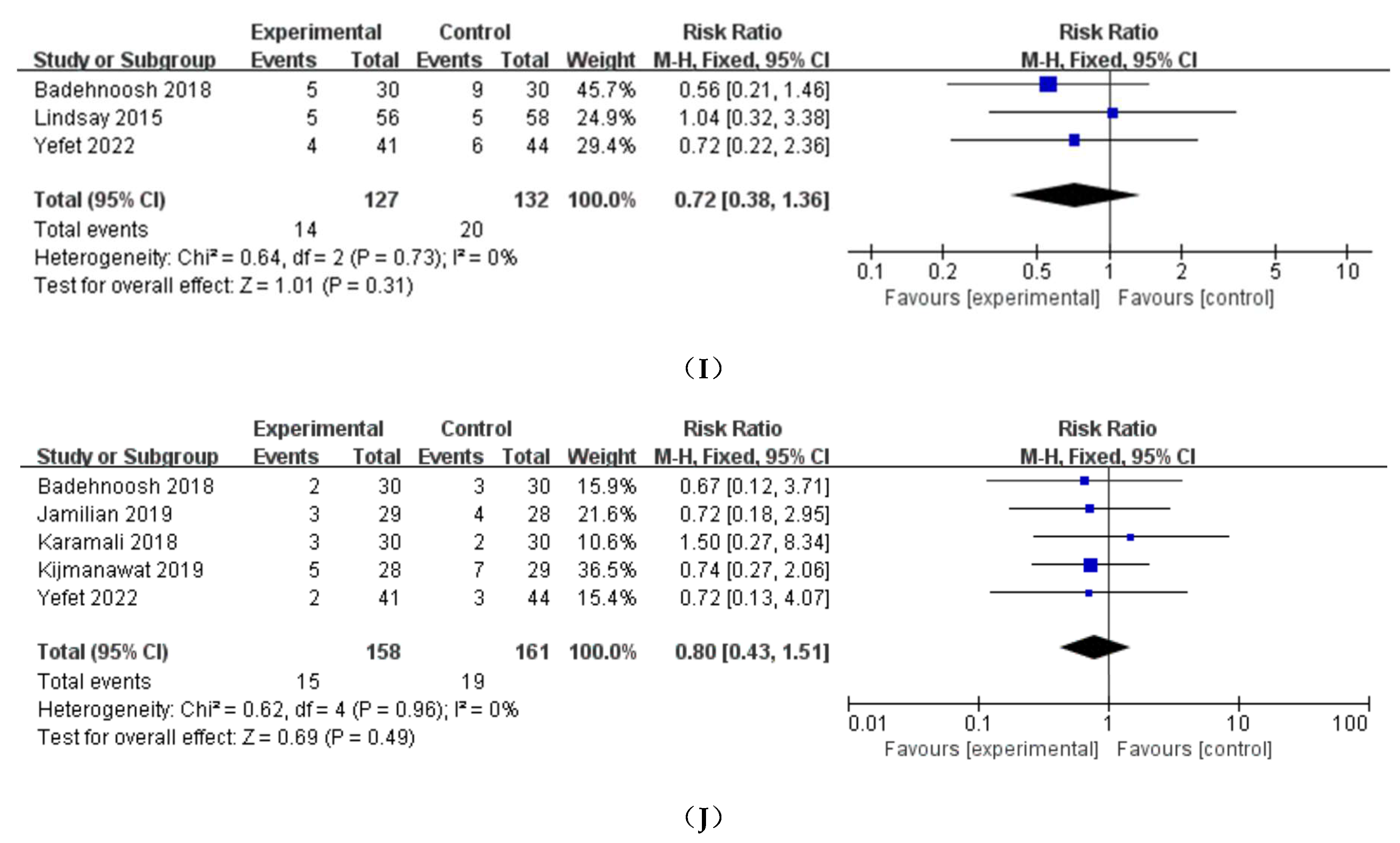
3.4. Neonatal outcomes
3.4.1. Birthweight、Neonatal length and head circumference
3.4.2. NICU
3.4.3. Apgar score
3.4.4. Hyperbilirubinemia
3.4.5. Other neonatal outcomes
3.5. Subgroup Analysis
3.6. Assessment of study quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szmuilowicz, E.D.; Josefson, J.L.; Metzger, B.E. Gestational Diabetes Mellitus. Endocrinol. Metab. Clin. N. Am. 2019, 48, 479–493. [Google Scholar]
- Lowe, W.L., Jr.; Scholtens, D.M.; Lowe, L.P.; Kuang, A.; Nodzenski, M.; Talbot, O.; Catalano, P.M.; Linder, B.; Brickman, W.J.; Clayton, P., et al.; et al. Association of Gestational Diabetes With Maternal Disorders of Glucose Metabolism and Childhood Adiposity. JAMA 2018, 320, 1005–1016. [Google Scholar] [PubMed]
- Alfadhli, E.M. Gestational diabetes mellitus. Saudi Med. J. 2015, 36, 399–406. [Google Scholar] [PubMed]
- Brown, J.; Grzeskowiak, L.; Williamson, K.; Downie, M.R.; Crowther, C.A. Insulin for the treatment of women with gestational diabetes. Cochrane Database Syst. Rev. 2017, 11, Cd012037. [Google Scholar]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar]
- Wan, J.; Ma, J. Efficacy of dietary supplements targeting gut microbiota in the prevention and treatment of gestational diabetes mellitus. Front Microbiol 2022, 13, 927883. [Google Scholar]
- Pellonperä, O.; Mokkala, K.; Houttu, N.; Vahlberg, T.; Koivuniemi, E.; Tertti, K.; Rönnemaa, T.; Laitinen, K. Efficacy of Fish Oil and/or Probiotic Intervention on the Incidence of Gestational Diabetes Mellitus in an At-Risk Group of Overweight and Obese Women: a Randomized, Placebo-Controlled, Double-Blind Clinical Trial. Diabetes Care 2019, 42, 1009–1017. [Google Scholar] [PubMed]
- Badehnoosh, B.; Karamali, M.; Zarrati, M.; Jamilian, M.; Bahmani, F.; Tajabadi-Ebrahimi, M.; Jafari, P.; Rahmani, E.; Asemi, Z. The effects of probiotic supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in gestational diabetes. J. Matern. Fetal Neonatal Med. 2018, 31, 1128–1136. [Google Scholar]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials revisited. Contemp. Clin. Trials 2015, 45, 139–145. [Google Scholar] [PubMed]
- Dolatkhah, N.; Hajifaraji, M.; Abbasalizadeh, F.; Aghamohammadzadeh, N.; Mehrabi, Y.; Abbasi, M.M. Is there a value for probiotic supplements in gestational diabetes mellitus? A randomized clinical trial. J. Health Popul. Nutr. 2015, 33, 25. [Google Scholar]
- Lindsay, K.L.; Brennan, L.; Kennelly, M.A.; Maguire, O.C.; Smith, T.; Curran, S.; Coffey, M.; Foley, M.E.; Hatunic, M.; Shanahan, F.; et al. Impact of probiotics in women with gestational diabetes mellitus on metabolic health: a randomized controlled trial. Am. J. Obstet. Gynecol. 2015, 212, 496.e491-411. [Google Scholar]
- Ahmadi, S.; Jamilian, M.; Tajabadi-Ebrahimi, M.; Jafari, P.; Asemi, Z. The effects of synbiotic supplementation on markers of insulin metabolism and lipid profiles in gestational diabetes: a randomised, double-blind, placebo-controlled trial. Br. J. Nutr. 2016, 116, 1394–1401. [Google Scholar] [PubMed]
- Jafarnejad, S.; Saremi, S.; Jafarnejad, F.; Arab, A. Effects of a Multispecies Probiotic Mixture on Glycemic Control and Inflammatory Status in Women with Gestational Diabetes: A Randomized Controlled Clinical Trial. J Nutr Metab 2016, 2016, 5190846. [Google Scholar]
- Karamali, M.; Dadkhah, F.; Sadrkhanlou, M.; Jamilian, M.; Ahmadi, S.; Tajabadi-Ebrahimi, M.; Jafari, P.; Asemi, Z. Effects of probiotic supplementation on glycaemic control and lipid profiles in gestational diabetes: a randomized, double-blind, placebo-controlled trial. Diabetes Metab. 2016, 42, 234–241. [Google Scholar]
- Hajifaraji, M.; Jahanjou, F.; Abbasalizadeh, F.; Aghamohammadzadeh, N.; Abbasi, M.M.; Dolatkhah, N. Effect of probiotic supplements in women with gestational diabetes mellitus on inflammation and oxidative stress biomarkers: a randomized clinical trial. Asia Pac. J. Clin. Nutr. 2018, 27, 581–591. [Google Scholar]
- Karamali, M.; Nasiri, N.; Taghavi Shavazi, N.; Jamilian, M.; Bahmani, F.; Tajabadi-Ebrahimi, M.; Asemi, Z. The Effects of Synbiotic Supplementation on Pregnancy Outcomes in Gestational Diabetes. Probiotics and antimicrobial proteins 2018, 10, 496–503. [Google Scholar]
- Nabhani, Z.; Hezaveh, S.J.G.; Razmpoosh, E.; Asghari-Jafarabadi, M.; Gargari, B.P. The effects of synbiotic supplementation on insulin resistance/sensitivity, lipid profile and total antioxidant capacity in women with gestational diabetes mellitus: a randomized double blind placebo controlled clinical trial. Diabetes Res. Clin. Pract. 2018, 138, 149–157. [Google Scholar]
- Babadi, M.; Khorshidi, A.; Aghadavood, E.; Samimi, M.; Kavossian, E.; Bahmani, F.; Mafi, A.; Shafabakhsh, R.; Satari, M.; Asemi, Z. The Effects of Probiotic Supplementation on Genetic and Metabolic Profiles in Patients with Gestational Diabetes Mellitus: a Randomized, Double-Blind, Placebo-Controlled Trial. Probiotics and antimicrobial proteins 2019, 11, 1227–1235. [Google Scholar] [PubMed]
- Jamilian, M.; Amirani, E.; Asemi, Z. The effects of vitamin D and probiotic co-supplementation on glucose homeostasis, inflammation, oxidative stress and pregnancy outcomes in gestational diabetes: a randomized, double-blind, placebo-controlled trial. Clinical nutrition (Edinburgh, Scotland) 2019, 38, 2098–2105. [Google Scholar]
- Kijmanawat, A.; Panburana, P.; Reutrakul, S.; Tangshewinsirikul, C. Effects of probiotic supplements on insulin resistance in gestational diabetes mellitus: a double-blind randomized controlled trial. Journal of diabetes investigation 2019, 10, 163–170. [Google Scholar]
- Sahhaf Ebrahimi, F.; Homayouni Rad, A.; Mosen, M.; Abbasalizadeh, F.; Tabrizi, A.; Khalili, L. Effect of L. acidophilus and B. lactis on blood glucose in women with gestational diabetes mellitus: A randomized placebo-controlled trial. Diabetol. Metab. Syndr. 2019, 11. [Google Scholar]
- Amirani, E.; Asemi, Z.; Taghizadeh, M. The effects of selenium plus probiotics supplementation on glycemic status and serum lipoproteins in patients with gestational diabetes mellitus: a randomized, double-blind, placebo-controlled trial. Clinical nutrition ESPEN 2022, 48, 56–62. [Google Scholar]
- Yefet, E.; Perlitz, Y.; Yaakov, L.S.; Magril, G.; Vitner, D.; Zipori, Y.; Weiner, E.; Alon, A.S.; Paz, Y.G.; Nezer, M.; et al. The effect of probiotics on glycemic control of women with gestational diabetes mellitus-multicenter, randomized, double blind, placebo controlled-trial. Am. J. Obstet. Gynecol. 2022, 226, S258–S259. [Google Scholar]
- McIntyre, H.D.; Catalano, P.; Zhang, C.; Desoye, G.; Mathiesen, E.R.; Damm, P. Gestational diabetes mellitus. Nat Rev Dis Primers 2019, 5, 47. [Google Scholar]
- Catalano, P.M.; Tyzbir, E.D.; Roman, N.M.; Amini, S.B.; Sims, E.A. Longitudinal changes in insulin release and insulin resistance in nonobese pregnant women. Am. J. Obstet. Gynecol. 1991, 165, 1667–1672. [Google Scholar]
- Catalano, P.M.; Tyzbir, E.D.; Wolfe, R.R.; Roman, N.M.; Amini, S.B.; Sims, E.A. Longitudinal changes in basal hepatic glucose production and suppression during insulin infusion in normal pregnant women. Am. J. Obstet. Gynecol. 1992, 167, 913–919. [Google Scholar]
- Rasmussen, L.; Poulsen, C.W.; Kampmann, U.; Smedegaard, S.B.; Ovesen, P.G.; Fuglsang, J. Diet and Healthy Lifestyle in the Management of Gestational Diabetes Mellitus. Nutrients 2020, 12. [Google Scholar]
- Hernandez, T.L.; Mande, A.; Barbour, L.A. Nutrition therapy within and beyond gestational diabetes. Diabetes Res. Clin. Pract. 2018, 145, 39–50. [Google Scholar]
- Davenport, M.H.; Ruchat, S.M.; Poitras, V.J.; Jaramillo Garcia, A.; Gray, C.E.; Barrowman, N.; Skow, R.J.; Meah, V.L.; Riske, L.; Sobierajski, F.; et al. Prenatal exercise for the prevention of gestational diabetes mellitus and hypertensive disorders of pregnancy: a systematic review and meta-analysis. Br. J. Sports Med. 2018, 52, 1367–1375. [Google Scholar]
- Allman, B.R.; McDonald, S.; May, L.; Børsheim, E. Resistance Training as a Countermeasure in Women with Gestational Diabetes Mellitus: A Review of Current Literature and Future Directions. Sports Med. 2022, 52, 2871–2888. [Google Scholar] [PubMed]
- Martín-Estal, I.; Castorena-Torres, F. Gestational Diabetes Mellitus and Energy-Dense Diet: What Is the Role of the Insulin/IGF Axis? Front Endocrinol (Lausanne) 2022, 13, 916042. [Google Scholar]
- Kamińska, K.; Stenclik, D.; Błażejewska, W.; Bogdański, P.; Moszak, M. Probiotics in the Prevention and Treatment of Gestational Diabetes Mellitus (GDM): A Review. Nutrients 2022, 14. [Google Scholar]
- Davidson, S.J.; Barrett, H.L.; Price, S.A.; Callaway, L.K.; Dekker Nitert, M. Probiotics for preventing gestational diabetes. Cochrane Database Syst. Rev. 2021, 4, Cd009951. [Google Scholar]
- Okesene-Gafa, K.A.; Moore, A.E.; Jordan, V.; McCowan, L.; Crowther, C.A. Probiotic treatment for women with gestational diabetes to improve maternal and infant health and well-being. Cochrane Database Syst. Rev. 2020, 6, Cd012970. [Google Scholar] [PubMed]
- Shahriari, A.; Karimi, E.; Shahriari, M.; Aslani, N.; Khooshideh, M.; Arab, A. The effect of probiotic supplementation on the risk of gestational diabetes mellitus among high-risk pregnant women: A parallel double-blind, randomized, placebo-controlled clinical trial. Biomed. Pharmacother. 2021, 141, 111915. [Google Scholar]
- Callaway, L.K.; McIntyre, H.D.; Barrett, H.L.; Foxcroft, K.; Tremellen, A.; Lingwood, B.E.; Tobin, J.M.; Wilkinson, S.; Kothari, A.; Morrison, M.; et al. Probiotics for the Prevention of Gestational Diabetes Mellitus in Overweight and Obese Women: Findings From the SPRING Double-Blind Randomized Controlled Trial. Diabetes Care 2019, 42, 364–371. [Google Scholar]
- Yefet, E.; Bar, L.; Izhaki, I.; Iskander, R.; Massalha, M.; Younis, J.S.; Nachum, Z. Effects of Probiotics on Glycemic Control and Metabolic Parameters in Gestational Diabetes Mellitus: Systematic Review and Meta-Analysis. Nutrients 2023, 15. [Google Scholar]
- Hasain, Z.; Che Roos, N.A.; Rahmat, F.; Mustapa, M.; Raja Ali, R.A.; Mokhtar, N.M. Diet and Pre-Intervention Washout Modifies the Effects of Probiotics on Gestational Diabetes Mellitus: A Comprehensive Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2021, 13. [Google Scholar]
- Zhou, L.; Ding, C.; Wu, J.; Chen, X.; Ng, D.M.; Wang, H.; Zhang, Y.; Shi, N. Probiotics and synbiotics show clinical efficacy in treating gestational diabetes mellitus: A meta-analysis. Prim. Care Diabetes 2021, 15, 937–947. [Google Scholar] [PubMed]
- Chu, X.; Yan, P.; Zhang, N.; Feng, L.; Li, X.; Wang, Y.; Yang, K. Probiotics for preventing gestational diabetes mellitus in overweight or obese pregnant women: A systematic review and meta-analysis. Clin Nutr ESPEN 2022, 50, 84–92. [Google Scholar] [PubMed]

| First authors | Year | Country | Probiotic Intervention | Control Intervention |
Probiotic Dose | Probiotic group(N) |
Control group(N) |
Duration (weeks) |
|---|---|---|---|---|---|---|---|---|
| Dolatkhah | 2015 | Turkey |
Lactobacillus acidophilus LA-5, Bifidobacterium BB-12, Streptococcus thermophilus STY-31 and Lactobacillus delbrueckii bulgaricus LBY-27 |
placebo | 4 biocap>4 × 109 CFU | 29 | 27 | 8 |
| Lindsay | 2015 | Ireland | Lactobacillus salivarius UCC118 | placebo | 1×109 CFU/g | 48 | 52 | 8 |
| Karamali | 2016 | Iran |
L. acidophilus, L. casei and B. bifidum strains |
placebo | 2 × 109 CFU/g each | 30 | 30 | 6 |
| Jafarnejad | 2016 | Iran | VSL#3 (Streptococcus thermophilus, Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus paracasei, and Lactobacillus delbrueckii subsp. bulgaricus) |
placebo | 112.5 × 109 CFU | 37 | 35 | 8 |
| Ahmadi | 2016 | Iran | Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum plus 0.8 g inulin | placebo | 2 × 109 CFU/g each | 35 | 35 | 6 |
| Nabhani | 2018 | Iran |
Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus fermentum, Lactobacillus gasseri |
placebo |
L. acidophilus(5 × 1010 CFU/g), L. plantarum (1.5 × 1010 CFU/g), L. fermentum(7 × 109 CFU/g), L. gasseri(2 × 1010 CFU/g) |
45 | 45 | 6 |
| Badehnoosh | 2018 | Iran | Lactobacillus acidophilus, Lactobacillus casei and Bifidobacterium bifidum | placebo | 2 × 109 CFU/g each | 30 | 30 | 6 |
| Karamali | 2018 | Iran | Lactobacillus acidophilus, Lactobacillus casei and Bifidobacterium bifidum strains plus 800 mg inulin | placebo | 2 × 109 CFU/g each | 30 | 30 | 6 |
| Hajifaraji | 2018 | Iran | L.acidophilus LA-5, Bifidobacterium BB-12, Streptococcus thermophilus STY-31 and Lactobacillus delbrueckii bulgaricus LBY-27 plus dextrose anhydrous filler and magnesium stearate lubricant | placebo | 4 biocap >4×109 CFU | 29 | 27 | 8 |
| Babadi | 2019 | Iran |
Lactobacillus acidophilus, Lactobacillus casei,Bifidobacterium bifidum, and Lactobacillus fermentum |
placebo | 2 × 109 CFU/g each | 24 | 24 | 6 |
| SahhafEbrahimi | 2019 | Iran | Probiotic yoghurt containing Lactobacillusacidophilus and Bifidobacterium lactis | placebo | 300 g/day of probiotic yoghurt(contained 106 CFU Lactobacillus acidophilus and Bifidobacterium lactis | 42 | 42 | 8 |
| Kijmanawat | 2019 | Thailand |
Lactobacillus acidophilus and Bifidobacterium bifidum |
placebo | 1 × 109 CFU/g each | 28 | 29 | 4 |
| Jamilian | 2019 | Iran |
Lactobacillus acidophilus, Bifidobacterium bifidum, Lactobacillus reuteri, and Lactobacillus fermentum |
placebo | 8 × 109 CFU/day | 29 | 28 | 6 |
| Amirani | 2022 | Iran |
Lactobacillus acidophilus, Bifidobacterium bifidum, Bifidobacterium lactis Bifidobacterium longum Additionally , selenium |
placebo | 2 × 109 CFU/day each | 26 | 25 | 6 |
| Yefet | 2022 | Israel |
Bifidobacterium bifidum, Bifido- bacterium lactis, Lactobacillus (L) acidophilus, L. paracasei, L.rhamnosus and Streptococcus thermophilus |
placebo | 2 capsules/day ( >6×109 CFU/capsule) |
41 | 44 | 2 |
| Subgroup | Studies | Participants | MD/RR(95% CI) | Heterogeneity (I2%) | p |
|---|---|---|---|---|---|
| FBS | |||||
| Iran | 9 | 592 | -2.26 (-4.35, -0.17) | 64 | 0.03 |
| Non Iran | 3 | 213 | -3.37 (-6.64, -0.10) | 76 | 0.04 |
| Duration ≥ 8weeks | 4 | 312 | -2.56 (-3.64, -1.48) | 87 | < 0.00001 |
| Duration < 8weeks | 8 | 493 | -3.10 (-4.54, -1.66) | 52 | < 0.0001 |
| FSI | |||||
| Iran | 7 | 448 | -2.81 (-3.71, -1.91) | 55 | < 0.00001 |
| Non Iran | 3 | 213 | -0.96 (-1.20, -0.72) | 0 | < 0.00001 |
| Duration ≥ 8weeks | 3 | 228 | -1.01 (-1.25, -0.77) | 85 | < 0.00001 |
| Duration < 8weeks | 7 | 433 | -2.16 (-3.05, -1.26) | 33 | < 0.00001 |
| HOMA-IR | |||||
| Iran | 7 | 448 | -0.70 (-0.93, -0.48) | 44 | < 0.00001 |
| Non Iran | 3 | 213 | -0.29 (-0.34, -0.24) | 0 | < 0.00001 |
| Duration ≥ 8weeks | 3 | 228 | -0.30 (-0.35, -0.24) | 76 | < 0.00001 |
| Duration < 8weeks | 7 | 433 | -0.58 (-0.80, -0.36) | 35 | < 0.00001 |
| QUICKI | |||||
| Iran | 6 | 376 | 0.01 (0.00, 0.01) | 52 | 0.0001 |
| Non Iran | 1 | 56 | Not estimable | Not estimable | Not estimable |
| Duration ≥ 8weeks | 1 | 56 | Not estimable | Not estimable | Not estimable |
| Duration < 8weeks | 6 | 376 | 0.01 (0.00, 0.01) | 52 | 0.0001 |
| Cesarean delivery | |||||
| Iran | 3 | 177 | 0.57 (0.36, 0.89) | 1 | 0.01 |
| Non Iran | 2 | 232 | 1.70 (0.73, 3.97) | 72 | 0.22 |
| Duration ≥ 8weeks | 1 | 147 | 1.16 (0.71, 1.89) | Not estimable | 0.55 |
| Duration < 8weeks | 4 | 262 | 1.16 (0.71, 1.89) | 79 | 0.62 |
| Macrosomia | |||||
| Iran | 4 | 261 | 0.20 (0.07, 0.56) | 0 | 0.002 |
| Non Iran | 1 | 147 | 1.13 (0.64, 2.00) | Not estimable | 0.67 |
| Duration ≥ 8weeks | 2 | 231 | 0.85 (0.51, 1.42) | 72 | 0.53 |
| Duration < 8weeks | 3 | 177 | 0.16 (0.04, 0.71) | 0 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




