Submitted:
19 September 2023
Posted:
21 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Ab-Initio Simulation Details
3. Results and discussion
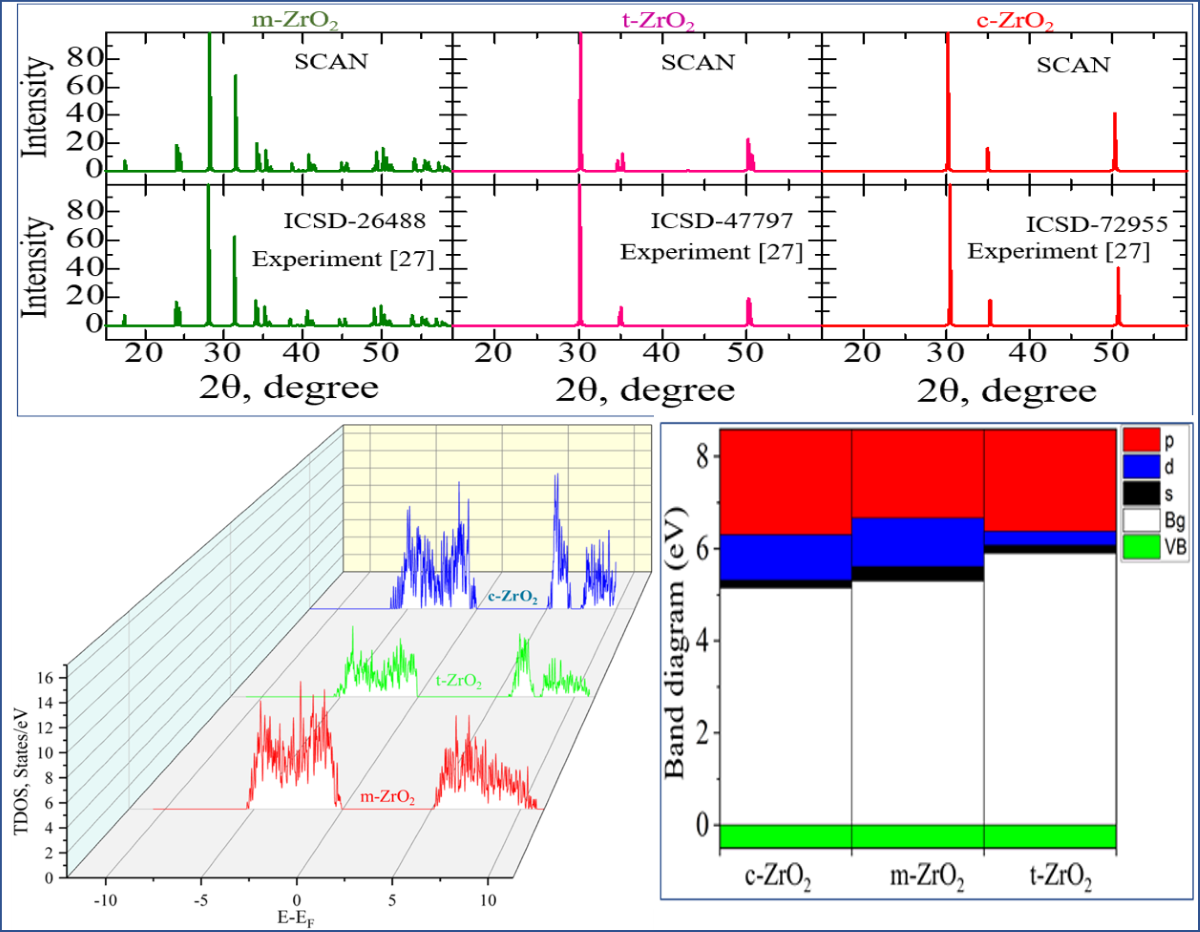
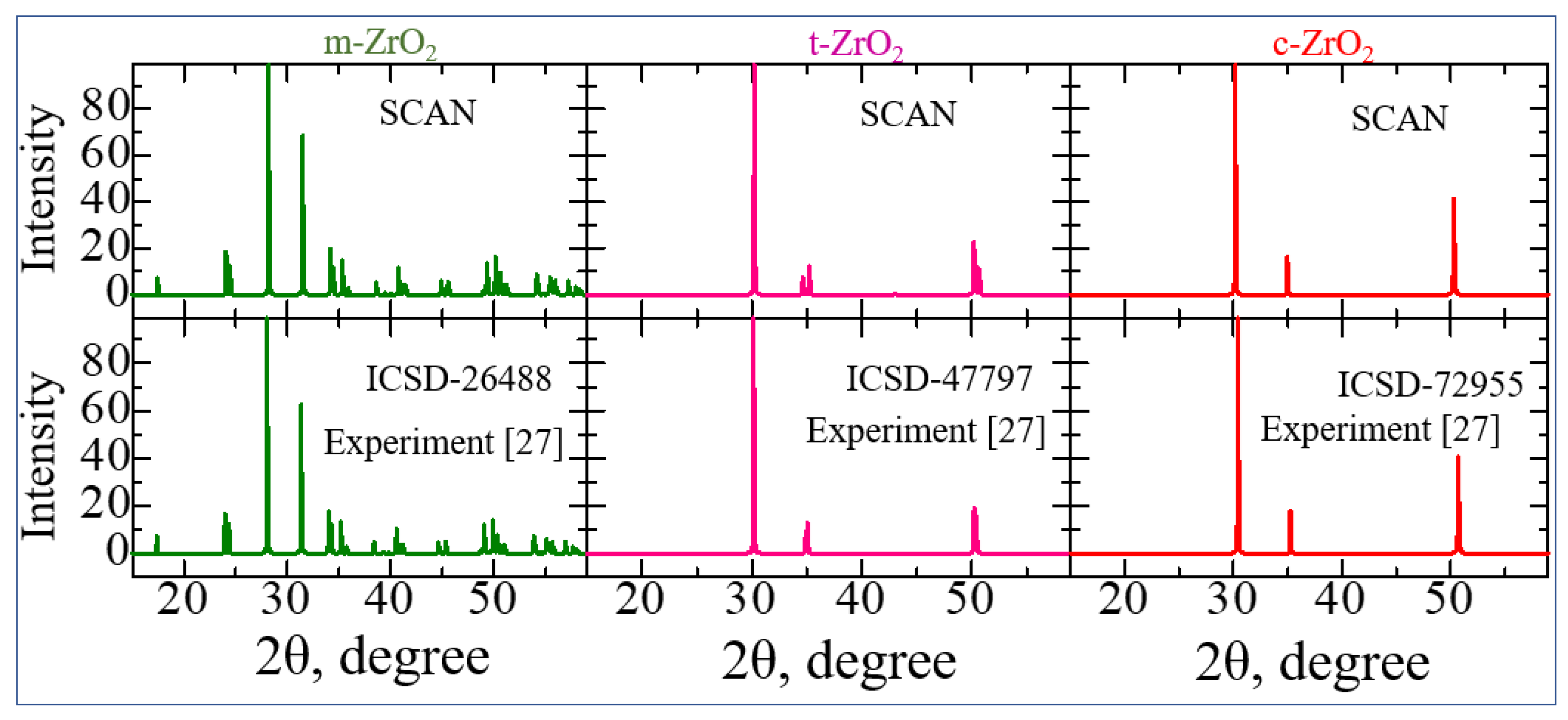
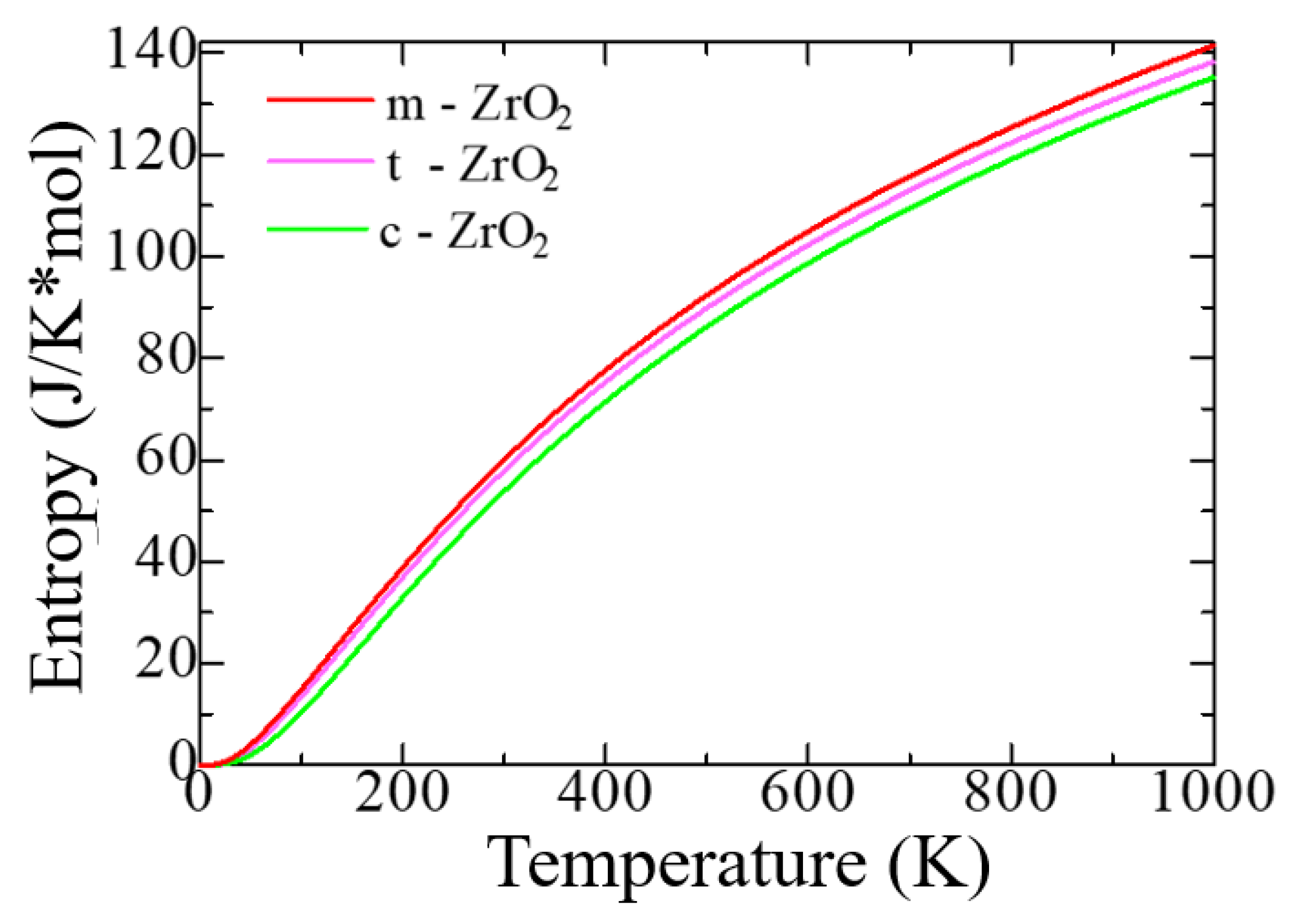
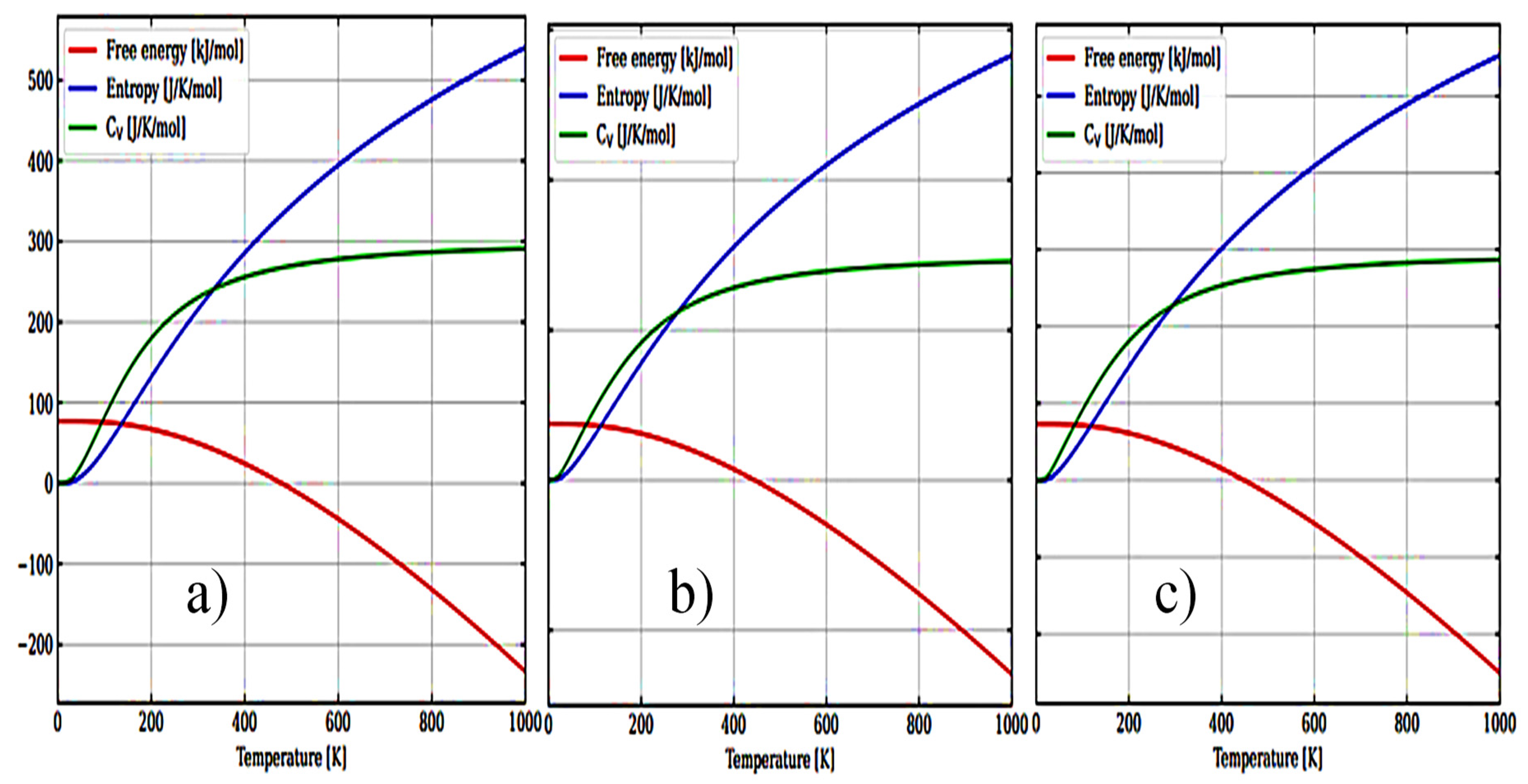
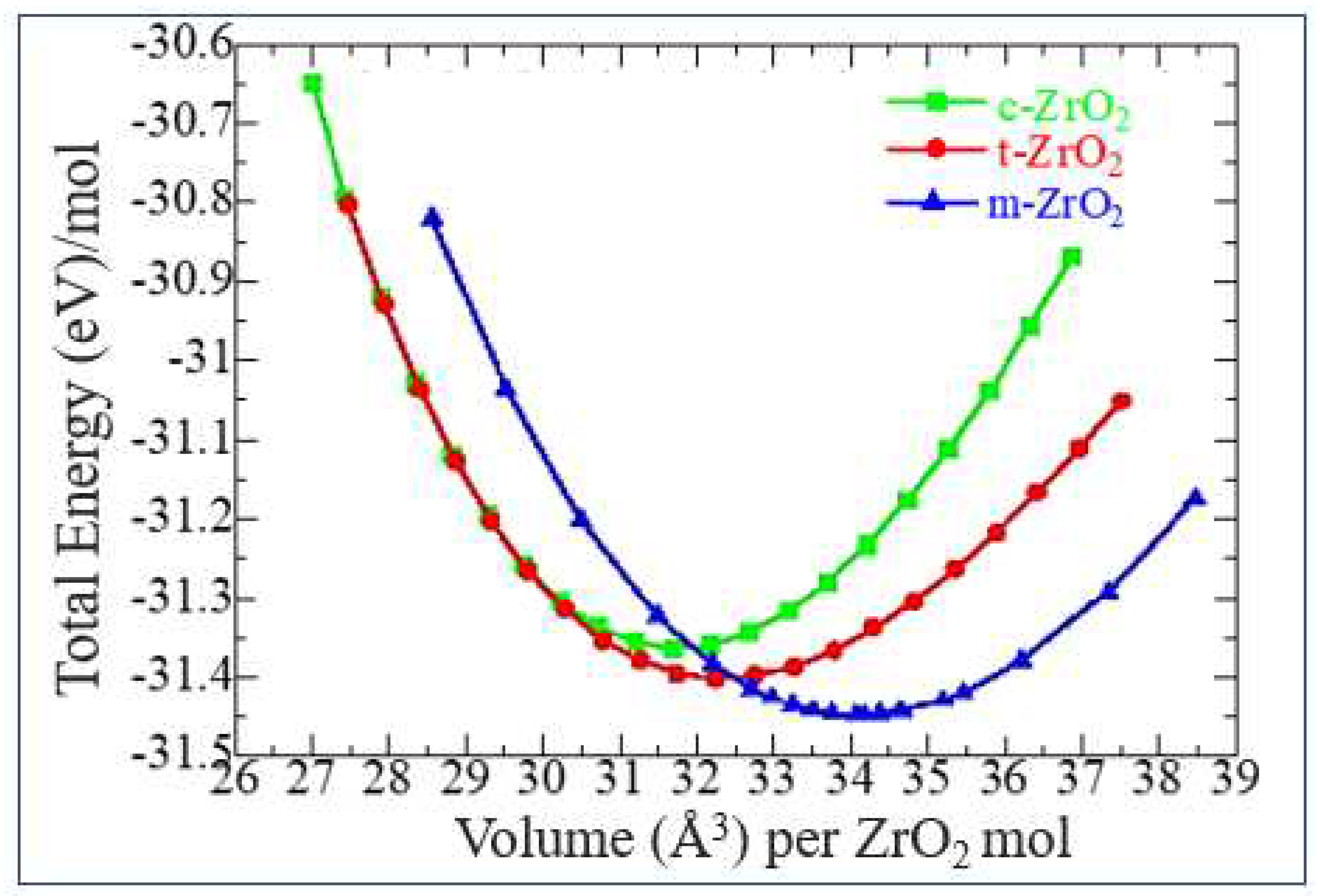
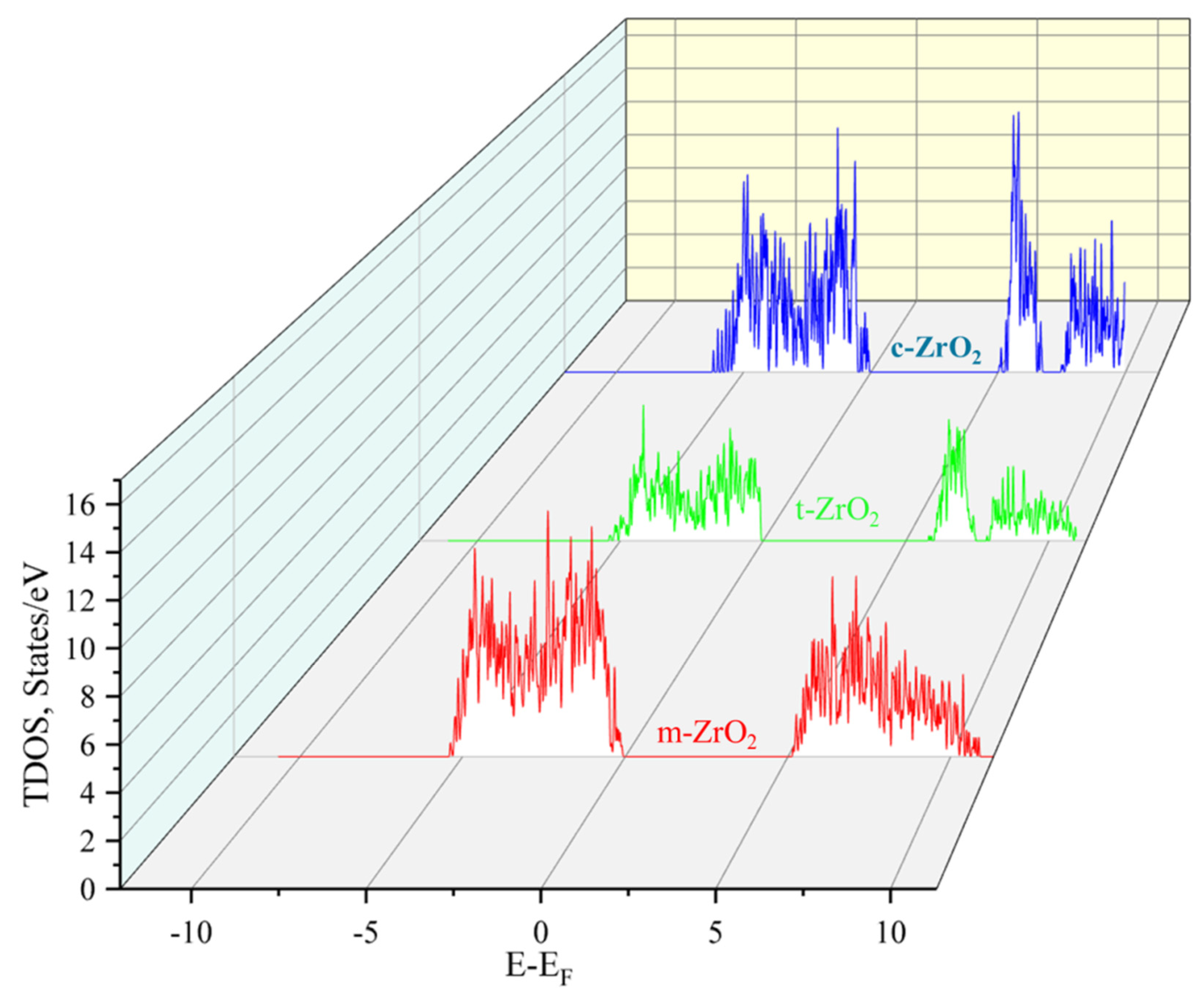
3.1. Structural, electronic and phonon properties of ZrO2
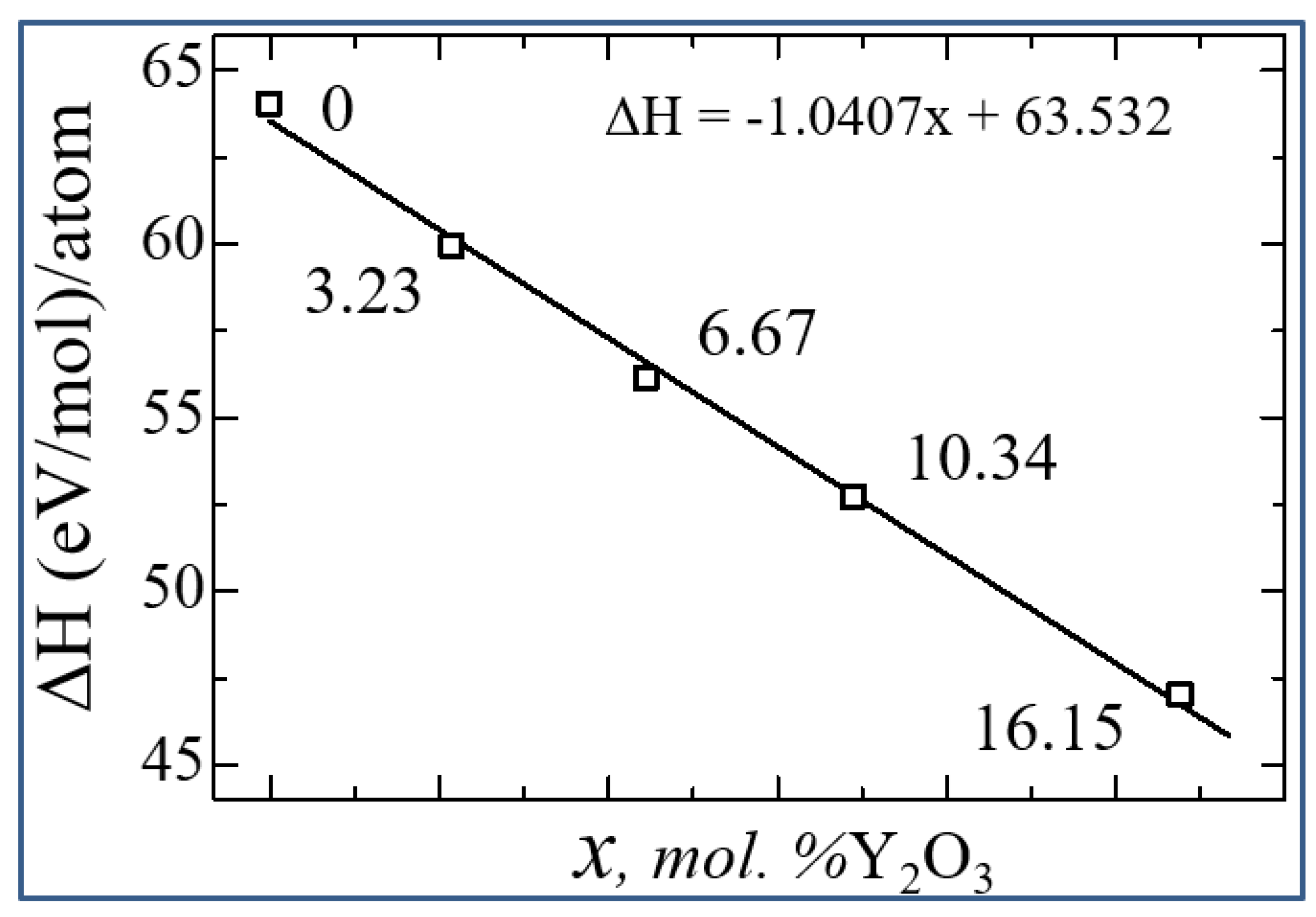
3.2. Stabilization of m-ZrO2 and electronic properties of YSZ
4. Conclusion
Funding
References
- Chu, S.; Cui, Y.; Liu, N. The path towards sustainable energy. Nat. Mater. 2016, 16, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Xiao, M.; Zou, G.; Liu, L.; Duley, W.W.; Zhou, Y.N. Self-Powered Wearable Electronics Based on Moisture Enabled Electricity Generation. Adv. Mater. 2018, 30, 1705925. [Google Scholar] [CrossRef]
- Shao, C.; Ji, B.; Xu, T.; Gao, J.; Gao, X.; Xiao, Y.; Zhao, Y.; Chen, N.; Jiang, L.; Qu, L. Large-Scale Production of Flexible, High-Voltage Hydroelectric Films Based on Solid Oxides. ACS Appl. Mater. Interfaces 2019, 11, 30927–30935. [Google Scholar] [CrossRef] [PubMed]
- Yashima, M.; Ohtake, K.; Arashi, H.; Kakihana, M.; Yoshimura, M. Determination of cubic-tetragonal phase boundary in Zr1− XYX O2− X/2 solid solutions by Raman spectroscopy. Journal of applied physics 1993, 74, 7603–7605. [Google Scholar] [CrossRef]
- Yashima, M.; Sasaki, S.; Kakihana, M.; Yamaguchi, Y.A.S.U.O.; Arashi, H.A.R.U.O.; Yoshimura, M.A.S.A.H.I.R.O. Oxygen-induced structural change of the tetragonal phase around the tetragonal–cubic phase boundary in ZrO2–YO1. 5 solid solutions. Acta Crystallographica Section B: Structural Science 1994, 50, 663–672. [Google Scholar] [CrossRef]
- Yashima, M.; Kakihana, M.; Yoshimura, M. Metastable-stable phase diagrams in the zirconia-containing systems utilized in solid-oxide fuel cell application. Solid State Ionics 1996, 86, 1131–1149. [Google Scholar] [CrossRef]
- Yashima, M.; Ohtake, K.; Kakihana, M.; Arashi, H.; Yoshimura, M. Determination of tetragonal-cubic phase boundary of Zr1− XRXO2− X2 (R= Nd, Sm, Y, Er and Yb) by Raman scattering. Journal of Physics and Chemistry of Solids 1996, 57, 17–24. [Google Scholar] [CrossRef]
- Yashima, M.; Ishizawa, N.; Yoshimura, M. High-Temperature X-ray Study of the Cubic-Tetragonal Diffusionless Phase Transition in the ZrO2─ ErO1. 5 System: I, Phase Change between Two Forms of a Tetragonal Phase, t′-ZrO2 and t ″-ZrO2, in the Compositionally Homogeneous 14 mol% ErO1. 5-ZrO2. Journal of the American Ceramic Society 1993, 76, 641–648. [Google Scholar] [CrossRef]
- Leger, J.M.; Tomaszewski, P.E.; Atouf, A.; Pereira, A.S. Pressure-induced structural phase transitions in zirconia under high pressure. Phys. Rev. B 1993, 47, 14075–14083. [Google Scholar] [CrossRef]
- Liu, L.G. New high pressure phases of ZrO2 and HfO2. Journal of Physics and Chemistry of Solids 1980, 41, 331–334. [Google Scholar] [CrossRef]
- Yashima, M.; Mitsuhashi, T.; Takashina, H.; Kakihana, M.; Ikegami, T.; Yoshimura, M. Tetragonal—monoclinic phase transition enthalpy and temperature of ZrO2-CeO2 solid solutions. Journal of the American Ceramic Society 1995, 78, 2225–2228. [Google Scholar] [CrossRef]
- Du, Y.; Jin, Z.; Huang, P. Thermodynamic Assessment of the ZrO2─ YO1. 5 System. Journal of the American Ceramic Society 1991, 74, 1569–1577. [Google Scholar] [CrossRef]
- Yashima, M.; Hirose, T.; Katano, S.; Suzuki, Y.; Kakihana, M.; Yoshimura, M. Structural changes of ZrO 2-CeO 2 solid solutions around the monoclinic-tetragonal phase boundary. Physical Review B 1995, 51, 8018. [Google Scholar] [CrossRef] [PubMed]
- Clearfield, A. Crystalline Hydrous Zirconia. Inorg. Chem. 1964, 3, 146–148. [Google Scholar] [CrossRef]
- Doroshkevich, A.; Nabiev, A.; Shylo, A.; Pawlukojć, A.; Doroshkevich, V.; Glazunova, V.; Zelenyak, T.; Doroshkevich, N.; Rahmonov, K.; Khamzin, E.; et al. Frequency modulation of the Raman spectrum at the interface DNA - ZrO2 nanoparticles. Egypt. J. Chem. 2019, 62, 13–20. [Google Scholar] [CrossRef]
- Kvist, A. Physics of Electrolytes; Hladik, J., Ed.; Academic Press: London, 1972; Vol. 1, p. 319. [Google Scholar]
- Lughi, V.; Sergo, V. Low temperature degradation -aging- of zirconia: A critical review of the relevant aspects in dentistry. Dent. Mater. 2010, 26, 807–820. [Google Scholar] [CrossRef]
- Kobayashi, K.; Kuwajima, H.; Masaki, T. Phase change and mechanical properties of ZrO2-Y2O3 solid electrolyte after ageing. Solid State Ionics 1981, 3, 489–493. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Sun, J.; Ruzsinszky, A.; Perdew, J.P. Strongly Constrained and Appropriately Normed Semilocal Density Functional. Phys. Rev. Lett. 2015, 115, 036402. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Howard, C.J.; Hill, R.J.; Reichert, B.E. Structures of ZrO2 polymorphs at room temperature by high-resolution neutron powder diffraction. Acta Crystallogr. Sect. B Struct. Sci. 1988, 44, 116–120. [Google Scholar] [CrossRef]
- Teufer, G. The crystal structure of tetragonal ZrO2. Acta Crystallogr. 1962, 15, 1187–1187. [Google Scholar] [CrossRef]
- Martin, U.; Boysen, H.; Frey, F. Neutron powder investigation of tetragonal and cubic stabilized zirconia, TZP and CSZ, at temperatures up to 1400 K. Acta Crystallogr. Sect. B Struct. Sci. 1993, 49, 403–413. [Google Scholar] [CrossRef]
- Martin, U.; Boysen, H.; Frey, F. Neutron powder investigation of tetragonal and cubic stabilized zirconia, TZP and CSZ, at temperatures up to 1400 K. Acta Crystallogr. Sect. B Struct. Sci. 1993, 49, 403–413. [Google Scholar] [CrossRef]
- Pascal, R.; Pross, A. Stability and its manifestation in the chemical and biological worlds. Chem. Commun. 2015, 51, 16160–16165. [Google Scholar] [CrossRef]
- Teter, D.M.; Gibbs, G.V.; Boisen, M.B., Jr.; Allan, D.C.; Teter, M.P. First-principles study of several hypothetical silica framework structures. Physical Review B 1995, 52, 8064. [Google Scholar] [CrossRef]
- Heyd, J.; Scuseria, G.E.; Ernzerhof, M. Hybrid functionals based on a screened Coulomb potential. J. Chem. Phys. 2003, 118, 8207–8215. [Google Scholar] [CrossRef]
- Verma, P.; Truhlar, D.G. HLE16: A Local Kohn–Sham Gradient Approximation with Good Performance for Semiconductor Band Gaps and Molecular Excitation Energies. J. Phys. Chem. Lett. 2017, 8, 380–387. [Google Scholar] [CrossRef]
- Asgerov, E.B.; Beskrovnyy, A.I.; Doroshkevich, N.V.; Mita, C.; Mardare, D.M.; Chicea, D.; Lazar, M.D.; Tatarinova, A.A.; Lyubchyk, S.I.; Lyubchyk, S.B.; Lyubchyk, A.I.; Doroshkevich, A.S. Reversible Martensitic Phase Transition in Yttrium-Stabilized ZrO2 Nanopowders by Adsorption of Water. Nanomaterials 2022, 12, 435. [Google Scholar] [CrossRef] [PubMed]
- Nematov, D.D.; Kholmurodov, K.T.; Husenzoda, M.A.; Lyubchyk, A.; Burhonzoda, A.S. Molecular Adsorption of H2O on TiO2 and TiO2:Y Surfaces. J. Human, Earth, Futur. 2022, 3, 213–222. [Google Scholar] [CrossRef]
- Nematov, D. Influence of Iodine Doping on the Structural and Electronic Properties of CsSnBr3. International Journal of Applied Physics 2022, 7, 36–47. [Google Scholar]
- Nematov, D.; Kholmurodov, K.; Yuldasheva, D.; Rakhmonov, K.; Khojakhonov, I. Ab-initio Study of Structural and Electronic Properties of Perovskite Nanocrystals of the CsSn[Br1−xIx]3 Family. HighTech and Innovation Journal 2022, 3, 140–150. [Google Scholar] [CrossRef]
- Davlatshoevich, N.D. Investigation Optical Properties of the Orthorhombic System CsSnBr3-xIx: Application for Solar Cells and Optoelectronic Devices. J. Human, Earth, Futur. 2021, 2, 404–411. [Google Scholar] [CrossRef]
- Davlatshoevich, N.D.; Ashur, K.; Saidali, B.A.; Kholmirzo, Kh.; Lyubchyk, A.; Ibrahim, M. Investigation of structural and optoelectronic properties of N-doped hexagonal phases of TiO2 (TiO2-xNx) nanoparticles with DFT realization: Optimization of the band gap and optical properties for visible-light absorption and photovoltaic applications. Biointerface Research in Applied Chemistry 2022, 12, 3836–3848. [Google Scholar]
- Nematov, D.; Burhonzoda, A.; Khusenov, M. First Principles Analysis of Crystal Structure, Electronic and Optical Properties of CsSnI3–xBrx Perovskite for Photoelectric Applications. J. Surf. Invest. 2021, 15, 532–533. [Google Scholar] [CrossRef]
- Nematov, D.D. Kholmurodov, Kh.T.; Aliona, S.; Faizulloev, K.; Gnatovskaya, V. A DFT Study of Structure, Electronic and Optical Properties of Se-Doped Kesterite Cu2ZnSnS4 (CZTSSe). Letters in Applied NanoBioScience 2022, 12, 67. [Google Scholar]
- Nematov, D.; Makhsudov, B.; Kholmurodov, Kh.; Yarov, M. Optimization Optoelectronic Properties ZnxCd1-xTe System for Solar Cell Application: Theoretical and Experimental Study. Biointerface Research in Applied Chemistry 2023, 13, 90. [Google Scholar]
- Nematov, D.; Burhonzoda, A.; Khusenov, M.; Kholmurodov, K.; Doroshkevych, A.; Doroshkevych, N.; Zelenyak, T.; Majumder, S.; Ibrahim, M. Molecular Dynamics Simulations of the DNA Radiation Damage and Conformation Behavior on a Zirconium Dioxide Surface. Egypt. J. Chem. The First International Conference on Molecular Modeling and Spectroscopy 19-22 February, 2019. 2019, 62, 149–161. [Google Scholar] [CrossRef]
- Nematov, D.D.; Burhonzoda, A.S.; Khusenov, M.A.; Kholmurodov, K.T.; Ibrahim, M.A. The Quantum-Chemistry Calculations of Electronic Structure of Boron Nitride Nanocrystals with Density Functional Theory Realization. Egypt. J. Chem. The First International Conference on Molecular Modeling and Spectroscopy 19-22 February, 2019. 2019, 62, 21–27. [Google Scholar] [CrossRef]
- Nizomov, Z.; Asozoda, M.; Nematov, D. Characteristics of Nanoparticles in Aqueous Solutions of Acetates and Sulfates of Single and Doubly Charged Cations. Arab. J. Sci. Eng. 2022, 47, 1–7. [Google Scholar] [CrossRef]
- Danilenko, I.; Gorban, O.; Maksimchuk, P.; Viagin, O.; Malyukin, Y.; Gorban, S.; Volkova, G.; Glasunova, V.; Mendez-Medrano, M.G.; Colbeau-Justin, C.; et al. Photocatalytic activity of ZnO nanopowders: The role of production techniques in the formation of structural defects. [CrossRef]
- Catalysis Today 2019, 328, 99–104. [CrossRef]
- Danilenko, I.; Gorban, O.; Oliveira Pedro, P.M.C.Z.; Viegas, J.; Shapovalova, O.; Akhkozov, L.; Konstantinova, T.; Lyubchyk, S. Photocatalytic Composite Nanomaterial and Engineering Solution for Inactivation of Airborne Bacteria. Top. Catal. 2021, 64, 772–779. [Google Scholar] [CrossRef]
- Dilshod, N.; Kholmirzo, K.; Aliona, S.; Kahramon, F.; Viktoriya, G.; Tamerlan, K. On the Optical Properties of the Cu2ZnSn[S1−xSex]4 System in the IR Range. Trends Sci. 2023, 20, 4058–4058. [Google Scholar] [CrossRef]
- Petrov, E.G.; Shevchenko, Y.V.; Snitsarev, V.; Gorbach, V.V.; Ragulya, A.V.; Lyubchik, S. Features of superexchange nonresonant tunneling conductance in anchored molecular wires. AIP Adv. 2019, 9, 115120. [Google Scholar] [CrossRef]













| mol. %Y2O3 | Zr | Y | O | O vacancy | System |
| 0 | 32 | 0 | 64 | 0 | Zr32O64 |
| 3.23 | 30 | 2 | 63 | 1 | Zr30Y2O63 |
| 6.67 | 28 | 4 | 62 | 2 | Zr28Y4O62 |
| 10.35 | 26 | 6 | 61 | 3 | Zr26Y6O61 |
| 16.15 | 22 | 10 | 59 | 5 | Zr22Y10O59 |
| Lattice constants | This work | Exp. | ||
|---|---|---|---|---|
| GGA | SCAN | |||
| m-ZrO2 [P2_1/c] | a (Å) | 5.191 | 5.115 | 5.0950 [24] |
| b (Å) | 5.245 | 5.239 | 5.2116 [24] | |
| c (Å) | 5.202 | 5.304 | 5.3173 [24] | |
| β◦ | 99.639 | 99.110 | 99.230 [24] | |
| V (Å3) | 144.410 | 139.400 | 140.88 [24] | |
| t-ZrO2 [P4_2/nmc] | a=b (Å) | 3.593 | 3.622 | 3.64 [25] |
| с (Å) | 5.193 | 5.275 | 5.27 [25] | |
| c/a | 1.445 | 1.456 | 1.45 [25] | |
| V (Å3) | 67.05 | 69.214 | 69.83 [25] | |
| dz | 0.012 | 0.013 | 0.046 [25] | |
| c-ZrO2 [Fm-3m] | a=b=c (Å) | 5.075 | 5.12 | 5.129 [26,27] |
| V(Å3) | 130.709 | 134.06 | 134.9 [26,27] | |
| System | Energy | ΔE |
|---|---|---|
| m-ZrO2 | -28.7947 | 0 |
| t-ZrO2 | -28.6885 | 0.106 |
| c-ZrO2 | -28.5865 | 0.201 |
| System | This work | Experiment [30] | ||
|---|---|---|---|---|
| GGA | SCAN | HSE06 | ||
| m-ZrO2 | 3.9 | 3.8 | 5.288 | 5.78 |
| t-ZrO2 | 4.42 | 4.37 | 5.898 | 5.83 |
| c-ZrO2 | 4.03 | 3.93 | 5.140 | 6.10 |
| System | Lattice parameters | Phase | |||||
|---|---|---|---|---|---|---|---|
| a (Å) | b (Å) | c (Å) | α (◦) | β (◦) | γ (◦) | ||
| 0 | 10.23 | 10.478 | 10.608 | 90 | 99.64 | 90.00 | m - YSZ |
| 3.23 mol. %Y2O3 | 10.274 | 10.524 | 10.536 | 90.21 | 98.84 | 89.94 | m - YSZ |
| 6.67 mol. %Y2O3 | 10.512 | 10.544 | 10.603 | 89.90 | 90.12 | 89.62 | t - YSZ |
| 10.35 mol. %Y2O3 | 10.529 | 10.541 | 10.546 | 89.98 | 90.09 | 90.08 | t - YSZ |
| 16.15 mol. %Y2O3 | 10.540 | 10.541 | 10.543 | 90.08 | 90.00 | 90.02 | c - YSZ |
| System | ΔН | Еf | Еdf |
|---|---|---|---|
| 0 | 64.02917222 | -4.747216667 | 0 |
| 3.23 mol. %Y2O3 | 59.91124404 | -4.848422632 | -1.874577368 |
| 6.67 mol. %Y2O3 | 56.13271879 | -4.967857447 | -3.739875532 |
| 10.35 mol. %Y2O3 | 52.7041267 | -5.106527419 | -5.596013441 |
| 16.15 mol. %Y2O3 | 47.00229139 | -5.384704945 | -9.220196154 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





