Submitted:
01 September 2023
Posted:
04 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. H2 Regulates Oxidative Stress
2.1. History and Progress of Research on Medical Applications of H2
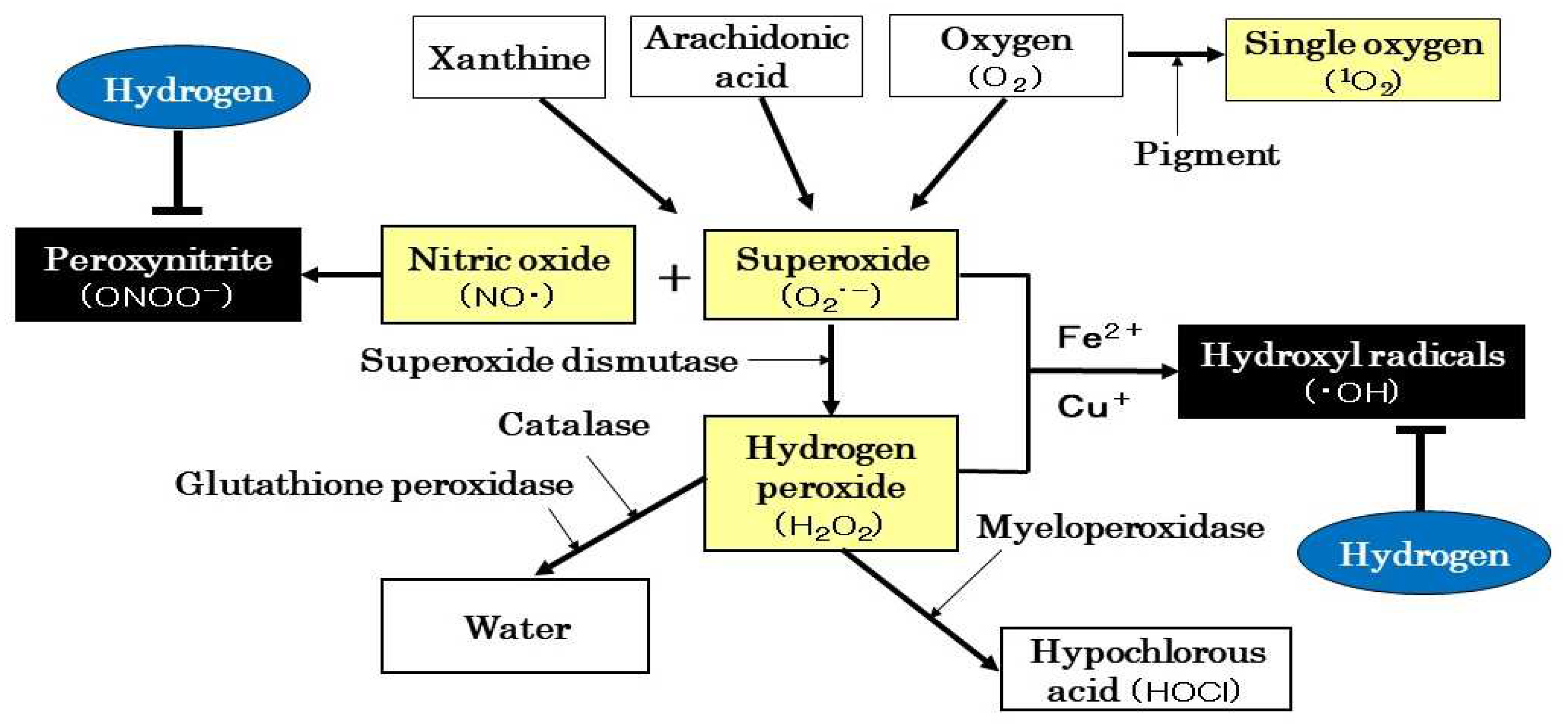
2.2. ROS Production and Scavenging Systems
2.3. “.Beneficial” and “Detrimental” Effects of ROS
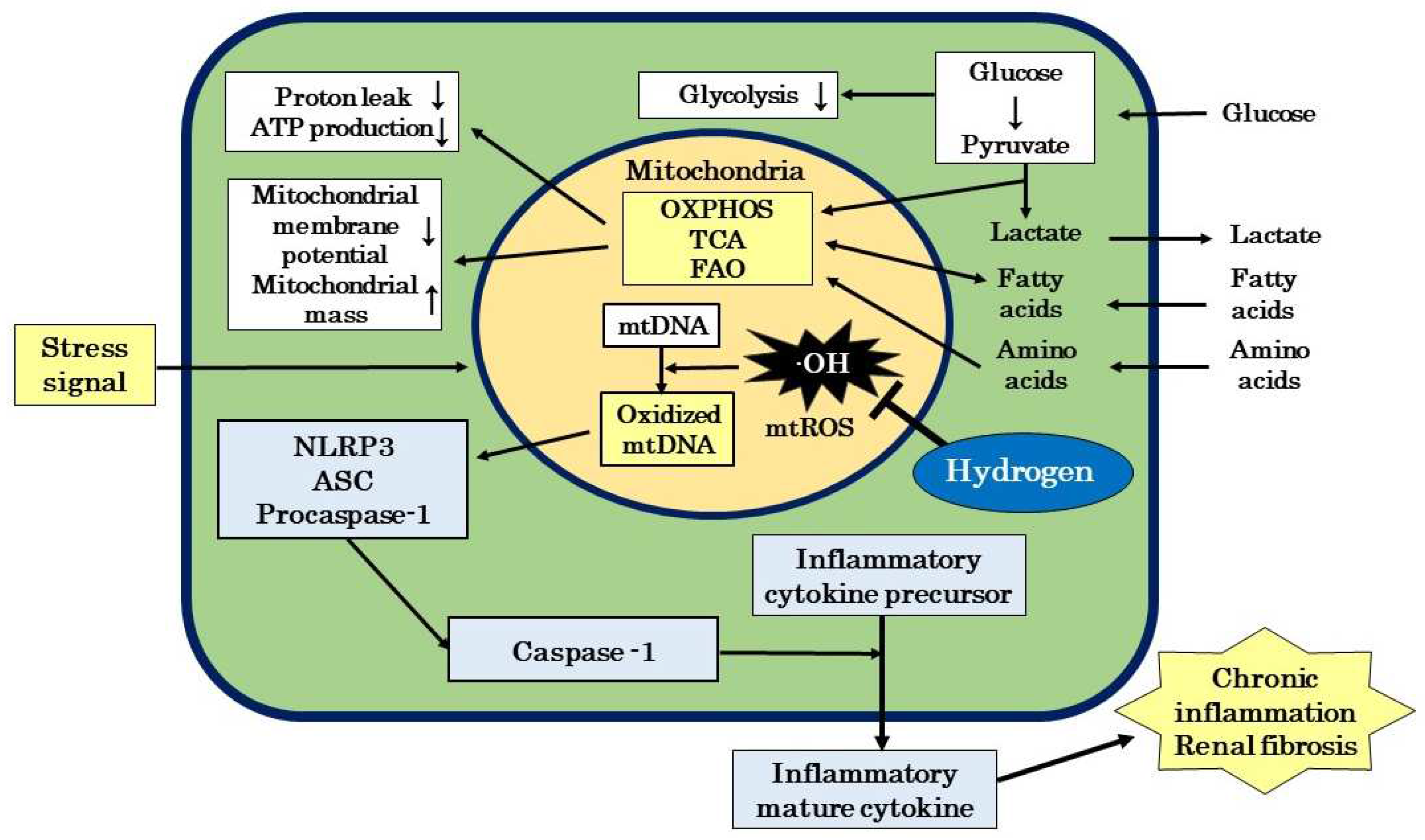
3. Mitochondrial Involvement in Renal Disease
3.1. Mitochondrial Structure and Function
3.2. Role of ROS in Renal Disease
4. Effects of H2 on Various Renal Diseases
4.1. Effects on Animal Disease Models
4.1.1. I/R Injury
4.1.2. Transplantation
4.1.3. CKD
4.1.4. Drug-Induced Renal Injury
4.1.5. Renal Stones
4.1.6. Renal Fibrosis
4.1.7. Sepsis-Related AKI
4.1.8. Others
4.2. Effects on Human Diseases
4.2.1. PD
4.2.2. HD
5. Mechanism of Action of H2 on Renal Disease
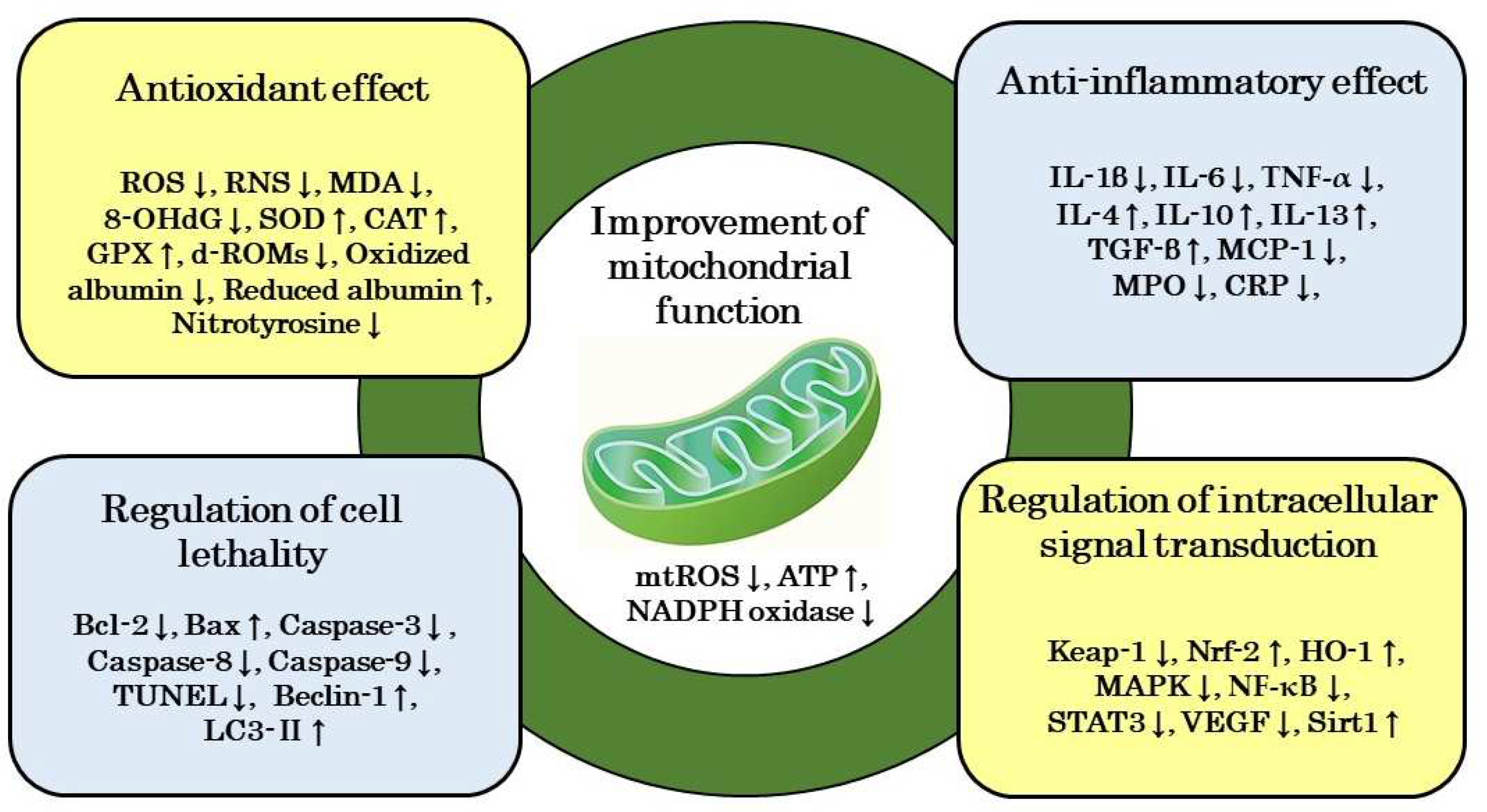
5.1. Improvement of Mitochondrial Function
5.2. Antioxidant Effects
5.3. Anti-Inflammatory Effects
5.4. Regulation of Cell Lethality
5.5. Regulatory Effects of Signal Transduction
6. Therapeutic Potential of H2 for DKD
6.1. Development Status of DKD Therapeutics
6.2. Therapeutic Potential of H2 in the Etiology of DKD
6.3. Prospects for H2 as a Therapeutic Substance for DKD
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koye, D.N.; Magliano, D.J.; Nelson, R.G.; Pavkov, M.E. The global epidemiology of diabetes and kidney disease. Adv. Chronic Kidney Dis. 2018, 25, 121–132. [CrossRef]
- Bonner, R.; Albajrami, O.; Hudspeth, J.; Upadhyay, J. Diabetic kidney disease. Prim. Care 2020, 47, 645 –649.
- IDF Diabetes Atlas 2021. Available online: https://diabetesatlas.org/atlas/tenth-edition/ (accessed on 19 June 2023).
- Jha, V.; Garcia-Garcia, G.; Iseki, K.; Naicker, S.; Plattner, B.; Saran, R.; Wang A.Y.M.; Yang, C.W. Chronic kidney disease: global dimension and perspectives. Lancet 2013, 382, 260–272. [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katura, K.I.; Katayama, Y.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [CrossRef]
- Nogueira, J.E.; Passaglia, P.; Mota, C.M.D.; Santos, B.M., Batalhão, M.E.; Carnio, E.C.; Branco, L.G.S. Molecular hydrogen reduces acute exercise-induced inflammatory and oxidative stress status. Free Radic. Biol. Med. 2018, 129, 186–193. [CrossRef]
- Wang, D.; Wang, L.; Zhang, Y.; Zhao, Y.; Chen, G. Hydrogen gas inhibits lung cancer progression through targeting SMC3. Biomed. Pharmacother. 2018, 104, 788–797. [CrossRef]
- Hirano, S.i.; Ichikawa, Y.; Sato, B.; Takefuji, Y.; Satoh, F. Molecular hydrogen as a potential clinically applicable radioprotective agent. Int. J. Mol. Sci. 2021, 22, 4566. [CrossRef]
- Kawamura, T.; Wakabayashi, N.; Shigemura, N.; Huang, C.S.; Masutani, K.; Tanaka, Y, Nota, K, Peng, X.; Takahashi, T.; Billiar, T.R.; et al. Hydrogen gas reduces hyperoxic lung injury via the Nrf2 pathway in vivo. Am. J. Physiol. Lung. Cell Mol. Physiol. 2013, 304, L646–656. [CrossRef]
- Ohta, S. Molecular hydrogen as a novel antioxidant: Overview of the advantages of hydrogen for medical applications. Methods Enzymol. 2015, 555, 289–317.
- Jin, Z.; Zhao, P.; Gong, W.; Ding, W.; He, Q. Fe-porphyrin: a redox-related biosensor of hydrogen molecule. Nano Research 2023, 16, 2020–2025. [CrossRef]
- Wei, P. Z.; Szeto, C.C. Mitochondrial dysfunction in diabetic kidney disease. Clin. Chim. Acta. 2019, 496, 108–116. [CrossRef]
- Su, S.; Ma, Z.; Wu, H.; Xu, Z.; Yi, H. Oxidative stress as a culprit in diabetic kidney disease. Life Sci. 2023, 322, 121661. [CrossRef]
- Tanase, D.M.; Gosav E.M.; Anton, M.I.; Floria, M.; Isac, P.N.S.; Hurjui, L.L.; Tarniceriu, C.C.; Costea, C.F.; Ciocoiu, M.; Rezus, C. Oxidative stress and NRF2/Keap1/ARE pathway in Diabetic kidney disease (DKD): new perspective. Biomolecules 2022, 12, 1227.
- Galvan, D.L.; Mise, K.; Danesh, F.R. Mitochondrial regulation of diabetic kidney disease. Front. Med. 2021, 8, 745279. [CrossRef]
- Kanda, H.; Yamawaki, K. Bardoxolone methyl: drug development for diabetic kidney disease. Clin. Exp. Nephrol. 2020, 24, 857–864. [CrossRef]
- Liu, H.; Sridhar, V.S.; Boulet, J.; Dharia, A.; Khan, A.; Lawier, P.R.; Cherney, D.Z.I. Cardiorenal protection with Sglt2 inhibitors in patients with diabetes mellitus: from biomarkers to clinical outcomes in heart failure and diabetic kidney disease. Metab. Clin. Exp. 2022, 126, 154918. [CrossRef]
- Yang, S.; Lin, C.; Zhuo, X.; Wang, J.; Rao, S.; Xu, W.; Cheng, Y.; Yang, L. Glucagon-like peptide-1 alleviates diabetic kidney disease through activation of autophagy by regulating Amp-activated protein kinase-mammalian target of rapamycin pathway. Am. J. Phys. Endocrinol. Metab. 2020, 319, E1019–E1030. [CrossRef]
- Murphy, M.P.; Smith, R.A. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 629–656. [CrossRef]
- Suzuki, T.; Yamaguchi, H.; Kikusato, M. Hashizume, O.; Nagatoshi, S.; Matsuo, A.; Sato, T.; Kudo, T.; Matsuhashi, T.; et al. Mitochonic acid 5 binds mitochondria and ameliorates renal tubular and cardiac myocyte damage. J. Am. Soc. Nephrol. 2016, 27, 1925–1932.
- Shingu, C.; Koga, H.; Hagiwara, S.; Matsumoto, S.; Goto, K.; Yokoi, I.; Noguchi, T. Hydrogen-rich saline solution attenuates renal ischemia-reperfusion injury. J. Anesth. 2010, 24, 569–574. [CrossRef]
- Wang, F.; Yu, G.; Liu, S.Y.; Li, J.B.; Wang, J.F.; Bo, L.L.; Qian, L.R.; Sun, X.J.; Deng, X.M. Hydrogen-rich saline protects against renal ischemia/reperfusion injury in rats. J. Surg. Res. 2011, 167, e339–344. [CrossRef]
- Li, J.; Hong, Z.; Liu, H.; Zhou, J.; Cui, L.; Yuan, S.; Chu, X.; Yu, P. Hydrogen-rich saline promotes the recovery of renal function after ischemia/reperfusion injury in rats via anti-apoptosis and anti-inflammation. Front. Pharmacol. 2016, 7, 106. [CrossRef]
- Chen, J.; Zhang, H.; Hu, J.; Gu, Y.; Shen, Z.; Xu, L.; Jia, X.; Zhang, X.; Ding, X. Hydrogen-rich saline alleviates kidney fibrosis following AKI and retains Klotho expression. Front. Pharmacol. 2017, 8, 499. [CrossRef]
- Xu, X.; He, X.; Liu, J.; Qin, J.; Ye, J.; Fan, M. Protective effects of hydrogen-rich saline against renal ischemia-reperfusion injury by increased expression of heme oxygenase-1 in aged rats. Int. J. Clin. Exp. Pathol. 2019, 12, 1488–1496.
- Cardinal, J.S.; Zhan, J.; Wang, Y.; Sugimoto, R.; Tsung, A.; McCurry, K.R.; Billiar, T.R.; Nakao, A. Oral hydrogen water prevents chronic allograft nephropathy in rats. Kidney Int. 2010, 77, 101–109. [CrossRef]
- Abe, T.; Li, X.K.; Yazawa, K.; Hatayama, N.; Xie, L.; Sato, B.; Kakuta, Y.; Tsutahara K.; Okumi, M.; Tsuda, H.; et al. Hydrogen-rich University of Wisconsin solution attenuates renal cold ischemia-reperfusion injury. Transplantation 2012, 94, 14–21. [CrossRef]
- Du, H.; Sheng, M.; Wu, L.; Zhang, Y.; Shi, D.; Weng, Y.; Xu, R.; Yu, W. Hydrogen-rich saline attenuates acute kidney injury after liver transplantation via activating p53-mediated autophagy. Transplantation 2016, 100, 563–570. [CrossRef]
- Zhu, W.J.; Nakayama, M.; Mori, T.; Nakayama, K.; Katoh, J.; Murata, Y.; Sato, T.; Kabayama, S.; Ito, S. Intake of water with high levels of dissolved hydrogen (H2) suppresses ischemia-induced cardio-renal injury in Dahl salt-sensitive rats. Nephrol. Dial. Transplant. 2011, 26, 2112–2118. [CrossRef]
- Zhu, W.J.; Nakayama, M.; Mori, T.; Hao, K.; Terawaki, H.; Katoh, J.; Kabayama, S.; Ito. S. Amelioration of cardio-renal injury with aging in dahl salt-sensitive rats by H2-enriched electrolyzed water. Med. Gas. Res. 2013, 3, 26. [CrossRef]
- Xin, H.G.; Zhang, B.B.; Wu, Z.Q.; Hang, X.F.; Xu, W.S.; Ni, W.; Zhang, R.Q.; Miao, X.H. Consumption of hydrogen-rich water alleviates renal injury in spontaneous hypertensive rats. Mol. Cell Biochem. 2014, 392, 117–124. [CrossRef]
- Nakashima-Kamimura, N.; Mori, T.; Ohsawa, I.; Asoh, S.; Ohta. S. Molecular hydrogen alleviates nephrotoxicity induced by an anti-cancer drug cisplatin without compromising anti-tumor activity in mice. Cancer Chemother. Pharmacol. 2009, 64, 753–761. [CrossRef]
- Li, F.Y.; Zhu, S.X.; Wang, Z.P.; Wang, H.; Zhao, Y.; Chen, G.P. Consumption of hydrogen-rich water protects against ferric nitrilotriacetate-induced nephrotoxicity and early tumor promotional events in rats. Food Chem. Toxicol. 2013, 61, 248–254. [CrossRef]
- Lu, Y.; Li, C.F.; Ping, N.N.; Sun, Y.Y.; Wang, Z.; Zhao, G.X.; Yuan, S.H.; Zibrila, A.I.; Soong, L.; Liu, J.J. Hydrogen-rich water alleviates cyclosporine A-induced nephrotoxicity via the Keap1/Nrf2 signaling pathway. J. Biochem. Mol. Toxicol. 2020, 34, e22467.
- Peng, Z.; Chen, W.; Wang, L.; Ye, Z.; Gao, S.; Sun, X.; Guo, Z. Inhalation of hydrogen gas ameliorates glyoxylate-induced calcium oxalate deposition and renal oxidative stress in mice. Int. J. Clin. Exp. Pathol. 2015, 8, 2680–2689.
- Xu, B.; Zhang, Y.B.; Li, Z.Z.; Yang, M.W.; Wang, S.; Jiang, D.P. Hydrogen-rich saline ameliorates renal injury induced by unilateral ureteral obstruction in rats. Int. Immunopharmacol. 2013, 17, 447–452. [CrossRef]
- Xing, Z.; Pan, W.; Zhang, J.; Xu, X.; Zhang, X.; He, X.; Fan, M. Hydrogen rich water attenuates renal injury and fibrosis by regulation transforming growth factor-β induced Sirt1. Biol. Pharm. Bull. 2017, 40, 610–615. [CrossRef]
- Mizutani, A.; Endo, A.; A.; Saito, M.; Hara, T.; Nakagawa, M.; Sakuraya, K.; Murano, Y.; Nishizaki, N.; Hirano, D.; Fujinaga,S.; et al. Hydrogen-rich water reduced oxidative stress and renal fibrosis in rats with unilateral ureteral obstruction. Pediatr. Res. 2022, 91, 1695–1702.
- Liu, W.; Dong, X.S.; Sun, Y.Q.; Liu, Z. A novel fluid resuscitation protocol: provide more protection on acute kidney injury during septic shock in rats. Int. J. Clin. Exp. Med. 2014,15, 919–926.
- Yao, W.; Guo, A.; Han, X.; Wu, S.; Chen, C.; Luo, C.; Li, H.; Li, S.; Hei, Z. Aerosol inhalation of a hydrogen-rich solution restored septic renal function. Aging (Albany NY) 2019, 11, 12097–12113. [CrossRef]
- Guo, S.X.; Fang, Q.; You, C.G.; Jin, Y.Y.; Wang, X.G.; Hu, X.L.; Han, C.M. Effects of hydrogen-rich saline on early acute kidney injury in severely burned rats by suppressing oxidative stress induced apoptosis and inflammation. J. Transl. Med. 2015, 13, 183. [CrossRef]
- Shi, Q.; Liao, K.S.; Zhao, K.L.; Wang, W.X.; Zuo, T.; Deng, W.H.; Chen, C.; Yu, J.; Guo, W.Y.; He, X.B.; et al. Hydrogen-rich saline attenuates acute renal injury in sodium taurocholate-induced severe acute pancreatitis by inhibiting ROS and NF-κB pathway. Mediators Inflamm. 2015, 2015, 685043.
- Guan, P.; Sun, Z.M.; Luo, L.F.; Zhou, J.; Yang, S.; Zhao, Y.S.; Yu, F.Y.; An, J.R.; Wang, N.; Ji, E.S. Hydrogen protects against chronic intermittent hypoxia induced renal dysfunction by promoting autophagy and alleviating apoptosis. Life Sci. 2019, 225, 46–54. [CrossRef]
- Terawaki, H.; Hayashi, Y.; Zhu, W.J.; Matsuyama, Y.; Terada, T.; Kabayama, S.; Watanabe, T.; Era, S.; Sato, B.; Nakayama, M. Transperitoneal administration of dissolved hydrogen for peritoneal dialysis patients: a novel approach to suppress oxidative stress in the peritoneal cavity. Med. Gas Res. 2013, 3, 14. [CrossRef]
- Nakayama, M.; Nakano, H.; Hamada, H.; Itami, N.; Nakazawa, R.; Ito, S. A novel bioactive haemodialysis system using dissolved dihydrogen (H2) produced by water electrolysis: a clinical trial. Nephrol. Dial. Transplant. 2010, 25, 3026–3033. [CrossRef]
- Terawaki, H.; Zhu, W.J.; Matsuyama, Y.; Terada, T.; Takahashi, Y.; Sakurai, K.; Kabayama, S.; Miyazaki, M.; Itami, N.; Nakazawa, R. Effect of a hydrogen (H2)-enriched solution on the albumin redox of hemodialysis patients. Hemodial. Int. 2014, 18, 459–466. [CrossRef]
- Sokawa, S.; Matsuura, A.; Suga, Y.; Sokawa, Y.; Kojima, T.; Nakamura, H. Reduction of oxidative stress and CRP levels in hemodialysis patients by hydrogen gas inhalation. J. Jpn. Ass. Dial. Physicians 2021, 54, 433–439. [CrossRef]
- Dole, M.; Wilson, F.R.; Fife, W.P. Hyperbaric hydrogen therapy: A possible treatment for cancer. Science 1975, 190, 152–154. [CrossRef]
- Abraini, J.H.; Gardette-Chauffour, M.C.; Martinez, E.; Rostain, J.C.; Lemaire, C. Psychophysiological reactions in humans during an open sea dive to 500 m with a hydrogen-helium-oxygen mixture. J. Appl. Physiol. 1994, 76, 1113–1118. [CrossRef]
- Gharib, B.; Hanna, S.; Abdallahi, O.M.; Lepidi, H.; Gardette, B.; De Reggi, M.; Anti-inflammatory properties of molecular hydrogen: investigation on parasite-induced liver inflammation. C. R. Acad. Sci. III 2001, 324, 719–724. [CrossRef]
- Yanagihara, T.; Arai, K.; Miyamae, K.; Sato, B.; Shudo, T.; Yamada, M.; Aoyama, M. Electrolyzed hydrogen-saturated water for drinking use elicits an antioxidative effect: a feeding test with rats. Biosci. Biotechnol. Biochem. 2005, 69, 1985–1987. [CrossRef]
- Ohta, S. Molecular hydrogen may activate the transcription factor Nrf2 to alleviate oxidative stress through the hydrogen-targeted porphyrin. Aging Pathobiol. Ther. 2023, 5, 25–32. [CrossRef]
- Hirano, S.i.; Yamamoto, H.; Ichikawa, Y.; Sato, B.; Takefuji, Y. Molecular hydrogen as a novel antitumor agent: possible mechanisms underlying gene expression. Int. J. Mol. Sci. 2021, 22, 8724. [CrossRef]
- Ohta S. Molecular hydrogen as a preventive and therapeutic medical gas: Initiation, development and potential of hydrogen medicine. Pharmacol. Ther. 2014,144, 1–11. [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Biologically relevant metal ion-dependent hydroxyl radical generation. FEBS Lett. 1992, 307, 108–112. [CrossRef]
- Setsukinai, K.I.; Urano, Y.; Kakinuma, K.; Majima, H.J.; Nagano, T. Development of novel fluorescence probes that can reliably detect reactive oxygen species and distinguish specific species. J. Biol. Chem, 2003, 278, 3170–3175. [CrossRef]
- Li, S.; Takahara, T.; Que, W.; Fujino, M.; Guo, W.Z.; Hirano, S.i.; Ye, L.P.; Li, X.K. Hydrogen-rich water protects liver injury in nonalcoholic steatohepatitis though HO-1 enhancement via IL-10 and Sirt 1 signaling. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 320, G450–463.
- Levine, A.J.; Oren, M. The first 30 years of p53: Growing ever more complex. Nat. Rev. Cancer 2009, 9, 749–758. [CrossRef]
- Ohsawa, I. Biological responses to hydrogen molecule and its preventive effects on inflammatory disease. Curr. Pharm. Des. 2021, 27, 659–666. [CrossRef]
- Klein, E.A.; Thompson, I.M.; Tangen, C.M.; Growley, J.J.; Lucia, M.S.; Goodman, P.J.; Minasian L.M.; Ford, L.G.; Parnes, H.L.; Gaziano, J.M. et al. Vitamin E and the risk of prostate cancer. The selenium and vitamin E cancer prevention trial (Select). J. Am. Med. Assoc. 2011, 306, 1549–1556.
- Chandel, N.S.; Tuveson D.A, The promise and perils of antioxidants for cancer patients. N Engl J Med, 2014, 371, 177–178. [CrossRef]
- Sayin, V.I.; Ibrahim, M.X.; Larsson, E.; Nilsson, J.A.; Lindahl, P.; Bergo, M.O. Antioxidants accelerate lung progression in mice. Sci. Transl. Med. 2014, 6, 221ra15. [CrossRef]
- DeNicola, G.M; Karreth, F.A.; Humpton, T.J.; Gopinathan, A.; Wei, C.; Frese, K.; Mangal, D.; Yu, K.H.; Yeo, C.J.; Calhoun, E.S. et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis, Nature, 2011, 475, 106–109. [CrossRef]
- Schafer, Z.T.; Grassian, A.R.; Song, L.; Jiang, Z.; Gerhart-Hines, Z.; Irie, H.Y.; Gao, S.; Puigserver, P.; Brugge, J.S. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature, 2009, 461, 109–113. [CrossRef]
- Kawai, D.; Takaki, A.; Nakatsuka, A.; Wada, J.; Tamaki, N.; Yasunaka, T.; Koike, K.; Tsuzaki, R.; Matsumoto, K.; Miyake, Y. et al. Hydrogen-rich water prevents progression of nonalcoholic steatohepatitis and accompanying hepatocarcinogenesis in mice. Hepatology. 2012, 56, 912–921. [CrossRef]
- Zerbes, R.M.; van der Klei, I.J.; Veenhuis, M.; Pfanner, M.; van der Laan, M.; Bohnert, M. Mitofilin complexes: conserved organizers of mitochondrial membrane architecture. Bio. Chem. 2012, 393, 1247.
- Roger, A.G.; Muñoz-Gómez, S.A.; Kamikawa R. The origin and diversification of mitochondria. Curr. Biol. 2017, 27, R1177–R1192. [CrossRef]
- Gorman, G.S.; Chinnery, P.F.; DiMauro, S, Hirano, M.; Koga, Y.; McFarlamd, R.; Suomalainen, A.; Thorburn, D.R.; Zeviani, M.; Turnbull, D.M. Mitochondrial diseases. Nat. Rev. Dis. Primers 2016, 2, 16080.
- Emma, F.; Montini, G.; Parikh, S.M.; Salviati, L. Mitochondrial dysfunction in inherited renal disease and acute kidney injury. Nat. Rev. Nephrol. 2016, 12, 267. [CrossRef]
- Schapira, A.H. Mitochondrial disorders. Curr. Opin. Neurol. 2000, 13, 527–532.
- Rahman, J.; Rahman, S. Mitochondrial medicine in the omics era. Lancet, 2018, 391, 2560. [CrossRef]
- Guerrero-Molina, M.P.; Morales–Conejo, M.; Delmiro, A.; Morán, M.; Domínguez-González, C.; Arranz-Canales, E.; Ramos-González, A.; Arenas, J.; Martín, M.A.; de la Aleja, J.G. High-dose oral glutamine supplementation reduces elevated glutamate levels in cerebrospinal fluid in patients with mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes syndrome. Eur. J. Neurol. 2023, 30, 538–547.
- Archer, S.L. Mitochondrial dynamics--mitochondrial fission and fusion in human diseases. N. Engl. J. Med. 2013, 369, 2236. [CrossRef]
- Bhargava, P.; Schnellmann, R.G. Mitochondria energetics in the kidney. Nat. Rev. Nephrol. 2017, 13, 629–646.
- Abe, Y.; Sakairi, T.; Kajiyama, H. Shrivastav, S.; Beeson, C. Kopp, J.B. Bioenergetic characterization of mouse podocytes. Am. J. Physiol. Cell Physiol. 2010, 299, C464–476. [CrossRef]
- Ozawa, S.; Ueda, S.; Imamura, H.; Mori, K.; Asanuma, K.; Yanagita, M.; Nakagawa, T. Glycolysis, but not mitochondria, responsible for intracellular ATP distribution in cortical area of podocytes. Sci. Rep. 2015, 5, 18575. [CrossRef]
- Imasawa, T.; Rossignol, R. Podocytes energy metabolism and glomerular disease. Int. J. Biochem. Cell Biol. 2013, 45, 2109–2118. [CrossRef]
- Fink, B.D.; Herlein, J.A.; O'Malley, Y.; Sivitz, W.I. Endothelial cell and platelet bioenergetics: effect of glucose and nutrient composition. PLoS One 2012, 7, e39430. [CrossRef]
- Czajka, A.; Malik, A.N. Hyperglycemia induced damage to mitochondria respiration in renal mesangial and tubular cells: Implication for diabetic nephropathy. Redox. Biol. 2016, 10, 100–107.
- Dugan, L.L.; You, Y.H.; Ali, S.S.; Diamond-Stanic, M.; Miyamoto, S.; DeCleves, A.E.: Andreyev, A.; Quach, T.; Ly, S.; Shekhtman, G.; et al. AMPK dysregulation promotes diabetes-related reduction of superoxide and mitochondrial function. J. Clin. Invest. 2013, 123, 4888–4899. [CrossRef]
- Forbes, J.M.; Thorburn, D.R.; Mitochondrial dysfunction in diabetic kidney disease. Nat. Rev. Nephrol. 2018, 14, 291–312. [CrossRef]
- Nishikawa, T.; Edelstein, D.; Du, X.L.; Yamagishi, S.; Matsumura, T.; Kaneda, Y.; Yorek, M.A.; Beebe, D.; Oates, P.J.; Hammes, H.P.; et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000, 404, 787–790. [CrossRef]
- Brownlee, M. The Pathobiology of diabetic complications: a unifying mechanism. Diabetes 2005, 54, 1615–1625.
- Sagoo, M.K.; Gnudi, L. Diabetic nephropathy: Is there a role for oxidative stress? Free Radic. Biol. Med. 2018, 116, 50–63.
- Zhan, M.; Brooks, C.; Liu, F.; Sun, L.; Dong, Z. Mitochondrial dynamics: regulatory mechanisms and emerging role in renal pathophysiology. Kidney Int. 2013, 83, 568–581. [CrossRef]
- Sharma, K. Mitochondrial hormesis and diabetic complications. Diabetes 2015, 64, 663–672. [CrossRef]
- Coughlan, M.T.; Sharma, K. Challenging the dogma of mitochondrial reactive oxygen species overproduction in diabetic kidney disease. Kidney Int. 2016, 90, 272–279. [CrossRef]
- Hong, D.S.; Kurzrock, R.; Supko, J.G.; He, X.; Naing, A.; Wheler, J.; Lawrence, D.; Eder, J.P.; Meyer, C.J.; Ferguson, D.A.; et al. A phase I first-in-human trial of bardoxolone methyl in patients advanced solid tumors and lymphomas. Clin. Cancer Res. 2012, 18, 3396–3406. [CrossRef]
- Nangaku, M.; Takama, H.; Ichikawa, K.; Mukai, K.; Kojima, M.; Suzuki, Y.; Watada, H.; Wada, T.; Ueki, K.; Narita, I.; et al. Randomized, double-blind, placebo-controlled phase 3 study of bardoxolone methyl in patients with diabetic kidney disease: design and baseline characteristics of Ayame study. Nephrol. Dial. Transplant. 2023, 38, 1204–1216. [CrossRef]
- A phase III double-blind placebo-controlled trial of bardoxolone methyl (AYAME trial) in Japan. News releases from Kyowa Kirin Co. Ltd. Available online: https://www.kyowakirin.co.jp/pressroom/news_releases/2023/20230510_01.html (accessed on 19 July 2023).
- Huang, J.; Huang, K.; Lan, T.; Xie, X.; Shen, X.; Liu, P.; Huang, H. Curcumin ameliorates diabetic nephropathy by inhibiting the activation of the SphK1-S1P signaling pathway. Mol. Cell. Endocrinol. 2013, 365, 231–240. [CrossRef]
- Shang, G.; Tang, X.; Gao, P.; Guo, F.; Liu, H.; Zhao, Z.; Chen, Q.; Jiang, T.; Zhang, N.; Li, H. Sulforaphane attenuation of experimental diabetic nephropathy involves GSK-3 Beta/Fyn/Nrf2 signaling pathway. J. Nutr. Biochem. 2015, 26, 596–606. [CrossRef]
- El-Bassossy, H.M.; Fahmy, A.; Badawy, D. Cinnamaldehyde Protects from the Hypertension Associated with Diabetes. Food Chem. Toxicol. 2011, 49, 3007–3012. [CrossRef]
- Sattarinezhad, A.; Roozbeh, J.; Shirazi Yeganeh, B.; Omrani, G.R.; Shams, M. Resveratrol reduces albuminuria in diabetic nephropathy: A randomized double-blind placebo-controlled clinical trial. Diabetes Metab. 2019, 45, 53–59. [CrossRef]
- Jiang, Z.; Tan, Z.; Meng, Z.; Li, X. Curative effects of valsartan alone or combined with alpha-lipoic acid on inflammatory cytokines and renal function in early-stage diabetic kidney disease. J. Coll. Phys. Surg. Pak. 2019, 29, 1009–1011. [CrossRef]
- Sun, J.; Zhu, H.; Wang, X.; Cao, Q.; Li, Z.; Hung, H. CoQ10 ameliorates mitochondrial dysfunction in diabetic nephropathy through mitophagy. J. Endocrinol. 2019, 240, 445-465. [CrossRef]
- Szeto, H.H. Pharmacologic approaches to improve mitochondrial function in AKI and CKD. J. Am. Soc. Nephrol. 2017, 28, 2856–2865. [CrossRef]
- Ducasa, G.M.; Mitrofanova, A.; Mallela, S.K.; Liu, X.; Molina, J.; Sloan, A.; Pedigo, C.E.; Ge, M.; Santos, J.V.; Hernandez, Y.; et al. ATP-binding cassette A1 deficiency causes cardiolipin-driven mitochondrial dysfunction in podocytes. J. Clin. Invest. 2019, 129, 3387–3400. [CrossRef]
- Matsuhashi, T.; Sato, T.; Kanno, S.I.; Suzuki, T.; Matsuo, A.; Oba, Y.; Kikusato, M.; Ogasawara, E.; Kudo, T.; Suzuki, K.; et al. Mitochonic acid 5 (MA-5) facilitates ATP synthase oligomerization and cell survival in various mitochondrial disease. EBioMedicine 2017, 20, 27–38. [CrossRef]
- Clinical trial of MA-5, a treatment for mitochondrial disease. Press releases from Tohoku University. Available online: https://www.tohoku.ac.jp/japanese/2021/12/press20211206-02-ma5.html (accessed on 24 July 2023).
- Mason, D.R.; Beck, P.L.; Muruve, D.A. Nucleotide-binding oligomerization domain-like receptors and inflammasomes in the pathogenesis of non-microbial inflammation and disease. J. Innate. Immun. 2012, 4, 16–30. [CrossRef]
- Wallet, S.M.; Puri, V.; Gibson, F.C. Linkage of infection to adverse systemic complication: Periodontal disease, toll-like receptors, and other pattern recognition systems. Vaccines 2018, 6, 21.
- Man, S.M.; Kanneganti, T.D. Regulation of inflammasome activation. Immunol. Rev. 2015, 265, 6–21.
- Elliott, E.I.; Sutterwala, F.S. Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunol. Rev. 2015, 265, 35–52. [CrossRef]
- Juliana, C.; Fernandes-Alnemri, T.; Kang, S.; Farias, A.; Qin, F.; Alnemri, E.S. Non -transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J. Biol. Chem. 2012, 287, 36617–36622. [CrossRef]
- Nishi, Y.; Satoh, M.; Nagasu, H.; Kadoya, H.; Ihoriya, C.; Kidokoro, K.; Sasaki, T.; Kashihara, N. Selective estrogen receptor modulation attenuates proteinuria-induced renal tubular damage by modulating mitochondrial oxidative status. Kidney Int. 2013, 83, 662–673. [CrossRef]
- Kadoya, H.; Satoh, M.; Sasaki, T.; Taniguchi, S.; Takahashi, M.; Kashihara, N. Aldosterone is a critical danger signal for inflammasome activation in development of renal fibrosis in mice. FASEB J. 2015, 29, 3899–3910. [CrossRef]
- Shahzad, K.; Bock, F.; Al-Dabet, M.M.; Gadi, I.; Kohli, S.; Nazir, S.; Ghosh, S.; Ranjan, S.; Wang, H.; Madhusudhan, T.; Nawroth, P.P.; Isermann, B. Caspase-1, but not caspase-3, promotes diabetic nephropathy. J. Am. Soc. Nephrol. 2016, 27, 2270–2275. [CrossRef]
- Ren, J.D.; Wu, X.B.; Jiang, R.; Hao, D.P.; Liu, Y. Molecular hydrogen inhibits lipopolysaccharide-triggered NLRP3 inflammasome activation in macrophages by targeting the mitochondrial reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 50–55. [CrossRef]
- Yang, L.; Guo, Y.; Fan, X.; Chen, Y.; Yang, B.; Liu, K.X.; Zhou, J. Amelioration of coagulation disorders and inflammation by hydrogen-rich solution reduces intestinal ischemia/reperfusion injury in rats through NF-kB/NLRP3 pathway. Mediat. Inflamm. 2020, 2020, 4359305.
- Zou, R.; Wang, M.H.; Chen, Y.; Fan, X.; Yang, B.; Du, J.; Wang, X.B.; Liu, K.X.; Zhou, J. Hydrogen-rich saline attenuates acute lung injury induced by limb ischemia/reperfusion via down-regulating chemerin and NLRP3 in rats. Shock 2018, 52, 134–141. [CrossRef]
- Chen, H.; Zhou, C.; Xie, K.; Meng, X.; Wang, Y.; Yu, Y. Hydrogen-rich saline alleviated the hyperpathia and microglia activation via autophagy mediated inflammasome inactivation in neuropathic pain rats. Neuroscience 2019, 421, 17–30. [CrossRef]
- Shao, A.;Wu, H.; Hong, Y.; Tu, S.; Sun, X.;Wu, Q.; Zhao, Q.; Zhang, J.; Sheng, J. Hydrogen-rich saline attenuated subarachnoid hemorrhage-induced early brain injury in rats by suppressing inflammatory response: Possible involvement of NF-kB pathway and NLRP3 inflammasome. Mol. Neurobiol. 2016, 53, 3462–3476.
- Zhuang, K.; Zuo, Y.C.; Scherchan, P.; Wang, J.K.; Yan, X.X.; Liu, F. Hydrogen inhalation attenuates oxidative stress related endothelial cells injury after subarachnoid hemorrhage in rats. Front. Neurosci. 2020, 13, 1441. [CrossRef]
- Hirano, S.i.; Ichikawa, Y.; Sato, B.; Yamamoto, H.; Takefuji, Y.; Satoh, F. Potential therapeutic applications of hydrogen in chronic inflammatory diseases: possible inhibiting role on mitochondrial stress. Int. J. Mol. Sci. 2021, 22, 2549. [CrossRef]
- Jiao, Y.; Yu, Y.; Li, B.; Gu, X.; Xie, K.; Wang, G.; Yu, Y. Protective effects of hydrogen-rich saline against experimental diabetic peripheral neuropathy via activation of the mitochondrial ATP-sensitive potassium channel channels in rats. Mol. Med. Rep. 2020, 21, 282–290. [CrossRef]
- Hirano, S.i.; Ichikawa, Y.; Sato, B.; Takefuji, Y.; Satoh, F. Molecular hydrogen as a medical gas for the treatment of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): possible efficacy based on a literature review. Front. Neurosci. 2022, 841310.
- Hirano, S.i.; Ichikawa, Y.; Sato, B.; Takefuji, Y.; Satoh, F. Successful treatment of myalgic encephalomyelitis/chronic fatigue syndrome using the hydrogen gas: a case report in four patients. Med Gas Res, 2024, 14, in press.
- Hirano, S.i.; Ichikawa, Y.; Sato, B.; Satoh, F.; Takefuji, Y. Hydrogen is promising for medical applications. Clean. Technol. 2020, 2, 529–541. [CrossRef]
- Liu, C.; Kurokawa, R.; Fujino, M.; Hirano, S.i.; Sato, B.; Li, X.K. Estimation of the hydrogen concentration in rat tissue using an airtight tube following the administration of hydrogen via various routes. Sci. Rep. 2014, 4, 5485. [CrossRef]
| Species | Type of H2 | Effects of H2 | Ref. | |
| Diseases | Changes in biomarkers | |||
| Rats | HRS | AKI | Swelling of Mt.↓, BUN↓, Cr↓, 8-OHdG↓ | [21] |
| Rats | HRS | I/R injury | BUN↓, Cr↓, MDA↓, 8-OHdG↓, TNF-α↓, IL-1β↓, IL-6↓, MPO↓, SOD↑, CAT↑ | [22] |
| Rats | HRS | I/R injury | Tissue injury↓, BUN↓, Cr↓, Bcl-2↓, Caspase-3, -8, and -9↓, IL-6↓, TNF-α↓, Bax↑ | [23] |
| Mice | HRS | AKI | Tissue injury↓, BUN↓, Cr↓, Klotho↑, Beclin-1↑, LC3- II↑ | [24] |
| Rats | HRS | I/R injury | BUN↓, Cr↓, MDA↓, 8-OHdG↓, HO-1↑, SOD↑ | [25] |
| Rats | HRW | Renal Transplantation | Overall survival↑, BUN↓, Cr↓, Urinary protein↓, MDA↓, TNF-α↓, IL-6↓, MAPK↓ | [26] |
| Rats | HRUW | Renal Transplantation | Overall survival↑, MDA↓, 8-OHdG↓, TUNEL-stained cells↓, ED-1-positive cells↓, Cr↓, Urinary protein↓ | [27] |
| Rats | HRW | AKI | BUN↓, Cr↓, MDA↓, SOD↑, Caspase-3↓, Cytochrome C↓, Beclin-1↑, LC3- II↑ | [28] |
| Rats | EW | CKD | MCP-1↓, Methylglyoxal↓, BUN↓, Nitrotyrosine staining↓ | [29] |
| Rats | EW | CKD | Age-related histological changes↓, Albuminuria↓, Cardiac remodeling↓, MDA↓, Nitrotyrosine staining↓ | [30] |
| Rats | HRW | CKD | BUN↓, Cr↓, ROS↓, SOD↑, GPX↑, CAT↑, NADPH oxidase↓, TNF-α↓, IL-6↓, IL-1β↓ | [31] |
| Mice | HRW/H2 gas | Cisplatin-induced injury | Histological injury ↓, BUN↓, Cr↓ | [32] |
| Rats | HRW | Fe-NTA-induced injury | Cr↓, BUN↓, MDA↓, ONOO−↓, NADPH oxidase↓, CAT↑, mtROS↓, NF-κB↓, IL-6↓, MCP-1↓, VEGF↓, STAT3↓ | [33] |
| Rats | HRW | Cyclosporin A-induced injury | ROS↓, MDA↓, Keap1↓, Nrf-2↑, HO-1↑ | [34] |
| Mice | H2 gas | Renal stones | MDA↓, 8-OHdG↓, SOD↑, GSH↑, CAT↑, MCP-1↓, IL-10↑ | [35] |
| Rats | HRS | Renal fibrosis | Injury score↓, Apoptosis index↓, Stromal fibrosis↓, MDA↓, SOD↑ | [36] |
| Mice | HRW | Renal fibrosis | Cr↓, BUN↓, Fibrosis↓, EMT↓, Sirt1↑ | [37] |
| Rats | HRW | Renal fibrosis | Fibrosis↓, TGF-β1-positive cells↓, Klotho↑ | [38] |
| Rats | H2 gas | Sepsis-related AKI | BUN↓, Cr↓, MDA↓, TNF-α↓, IL-6↓ | [39] |
| Mice | HRS | Sepsis-related AKI | IL-4 ↑, IL-13↑, IL-10↑, TGF-β↑ | [40] |
| Rats | HRS | Burn-induced AKI | BUN↓, Cr↓, Tubular apoptosis↓, Inflammation↓, MAPK↓, NF-κB↓ | [41] |
| Rats | HRS | AKI | NF-κB↓, ROS↓ | [42] |
| Rats | H2 gas | Hypoxia-induced injury | Renal function↑, Histological damage↓, Oxidative stress↓, Apoptosis↓, MAPK↓ | [43] |
| Humans | HED | PD | Reduced albumin↑, Oxidized albumin↓ | [44] |
| Humans | HED | HD | SBP↓, MCP-1↓, MPO↓ | [45] |
| Humans | HED | HD | Oxidized albumin↓ | [46] |
| Humans | H2 gas | HD | d-ROMs↓, CRP↓ | [47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





