Submitted:
29 August 2023
Posted:
31 August 2023
You are already at the latest version
Abstract
Keywords:
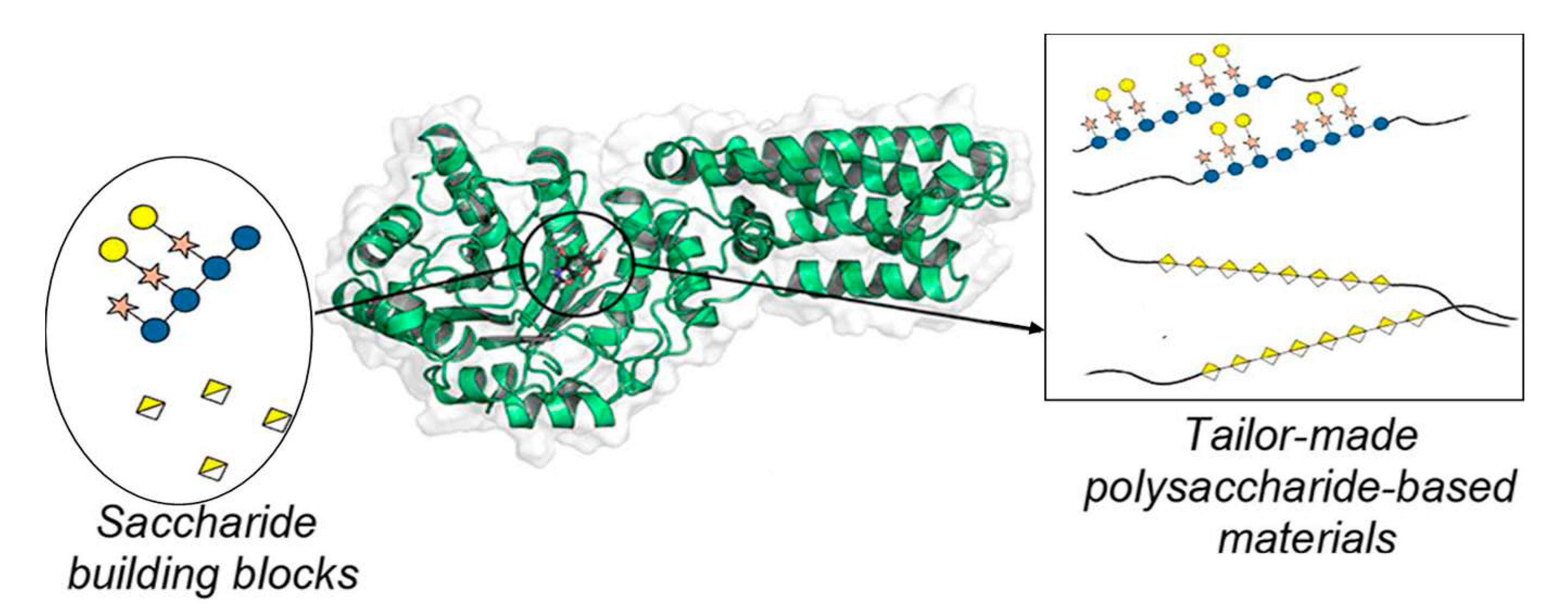
1. Introduction
- (i)
- Practical access to the corresponding substrates (and catalyst).
- (ii)
- Ability of the catalyst to act on a wide range of substrates.
- (iii)
- Efficiency of glycosidic bond formation.
2. Enzymatic Glycosylation of phenolic compounds
2.1. Glycosyltransferase
2.1.1. Leloir glycosyltransferase
2.1.2. Non-Leloir glycosyltransferase
2.1.3. C-glycosyltransferases
2.2. Glycosidases
2.2.1. Microbial glycosylation
3. Enzymatic glycosylation of other alcohols
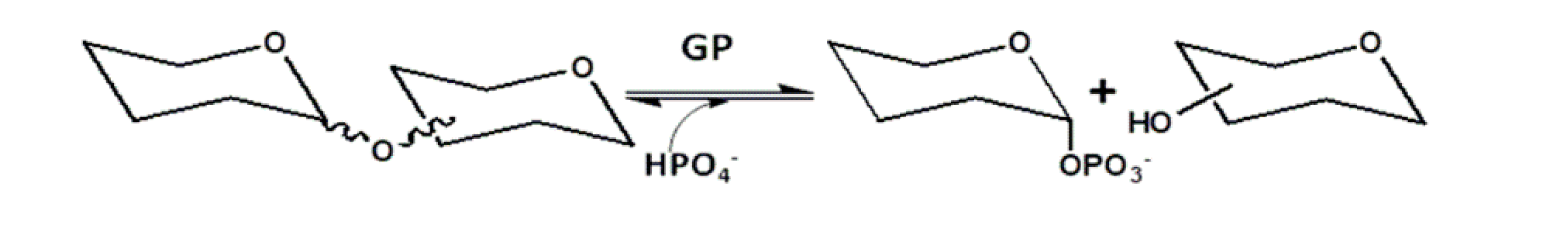
3.1. Glycan phosphorylases (GP)
4. Enzymatic synthetic glycosylation of oligosaccharides
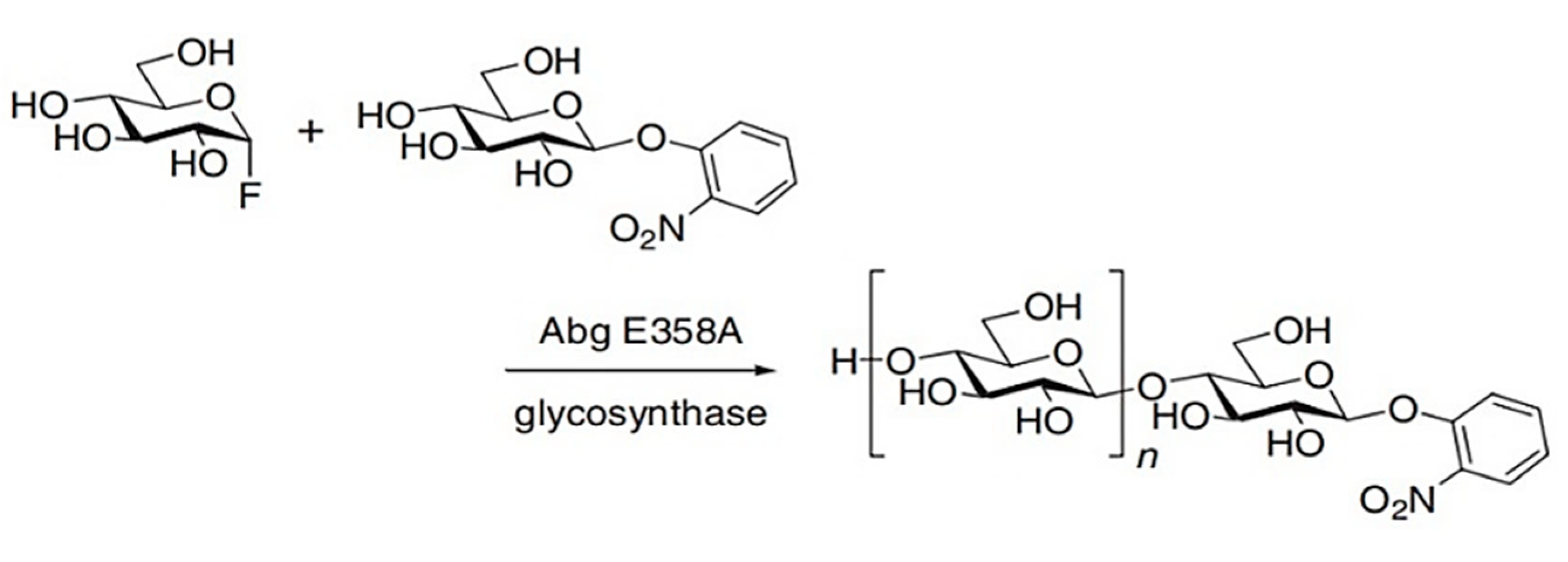
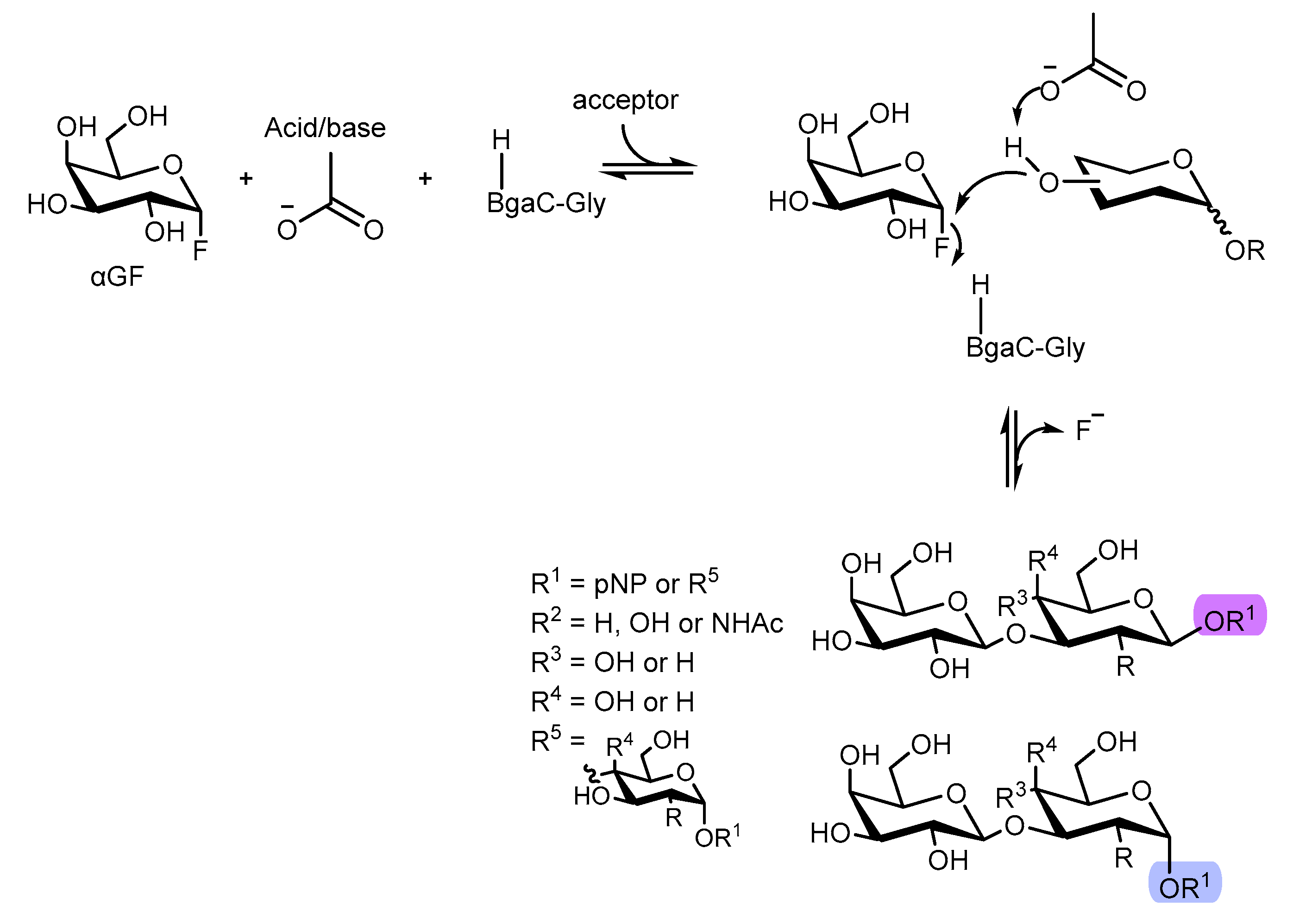
4.1. Glucosynthases
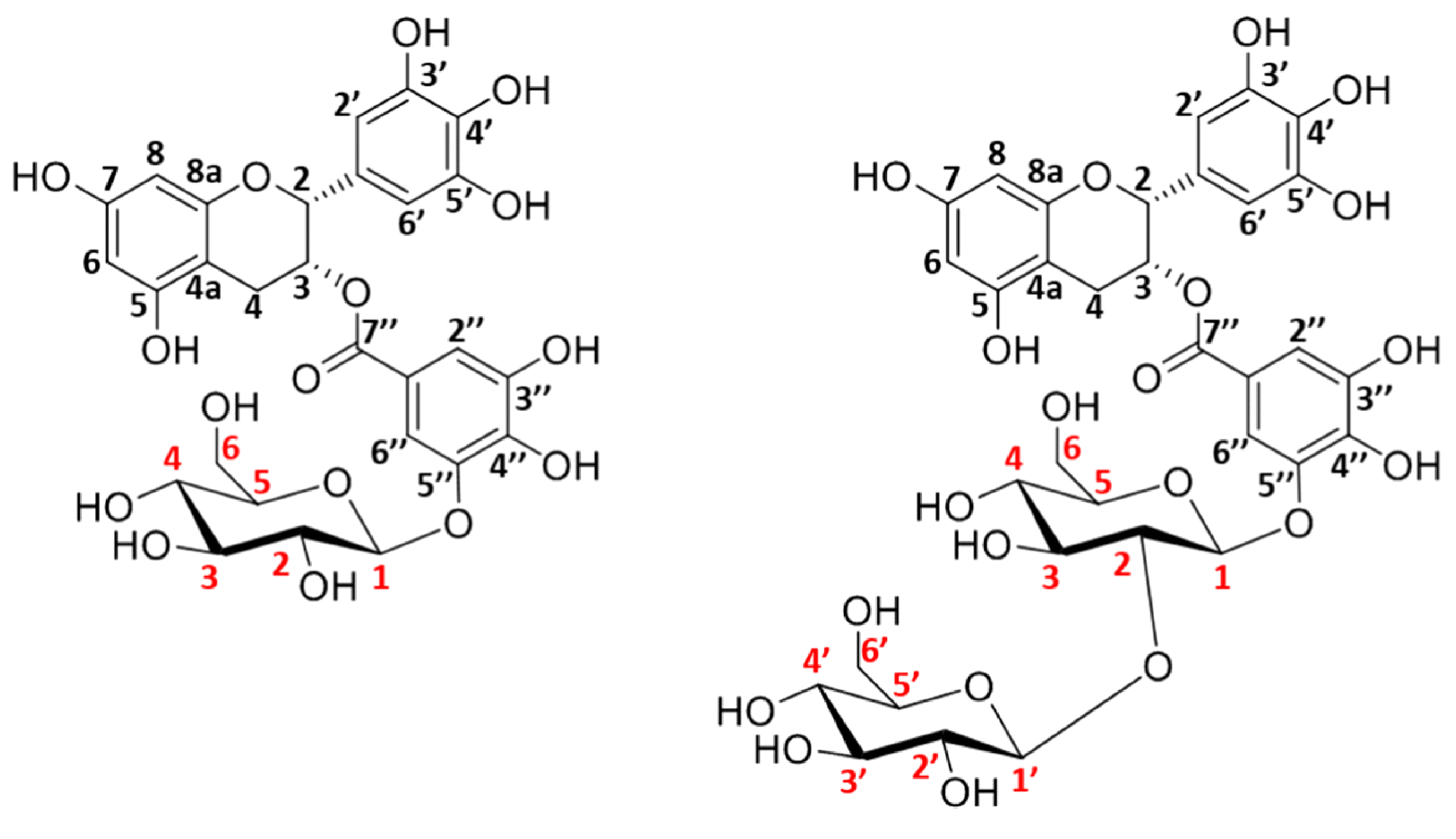
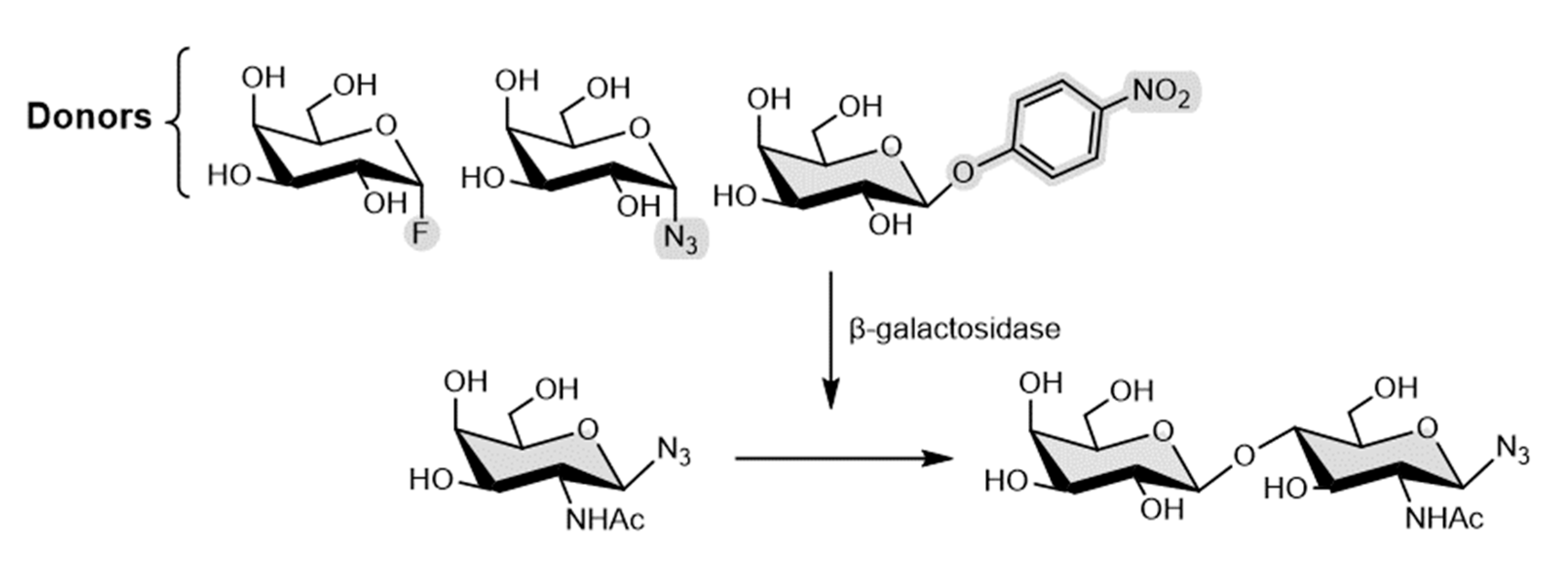
4.2. Galactosynthases
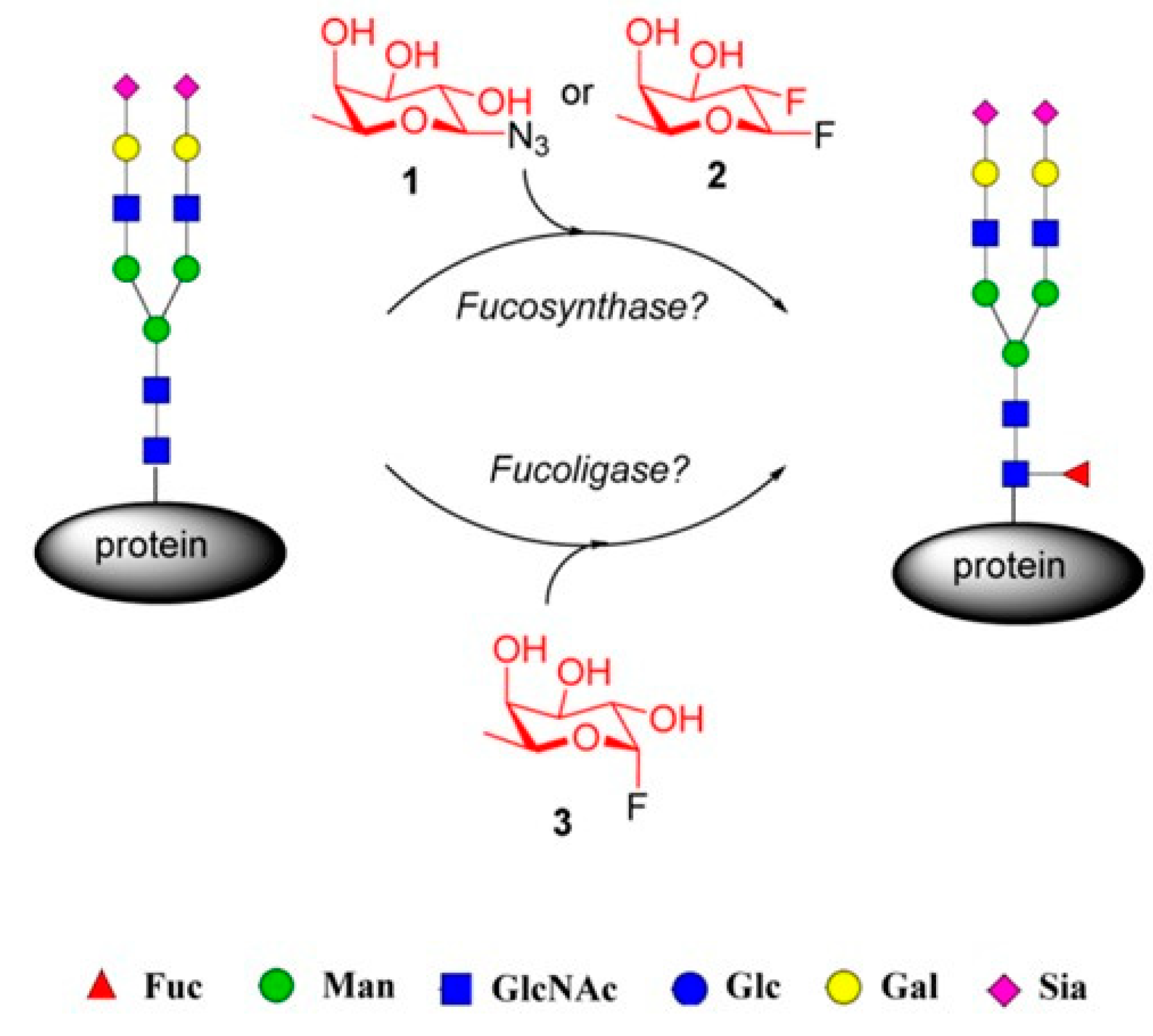
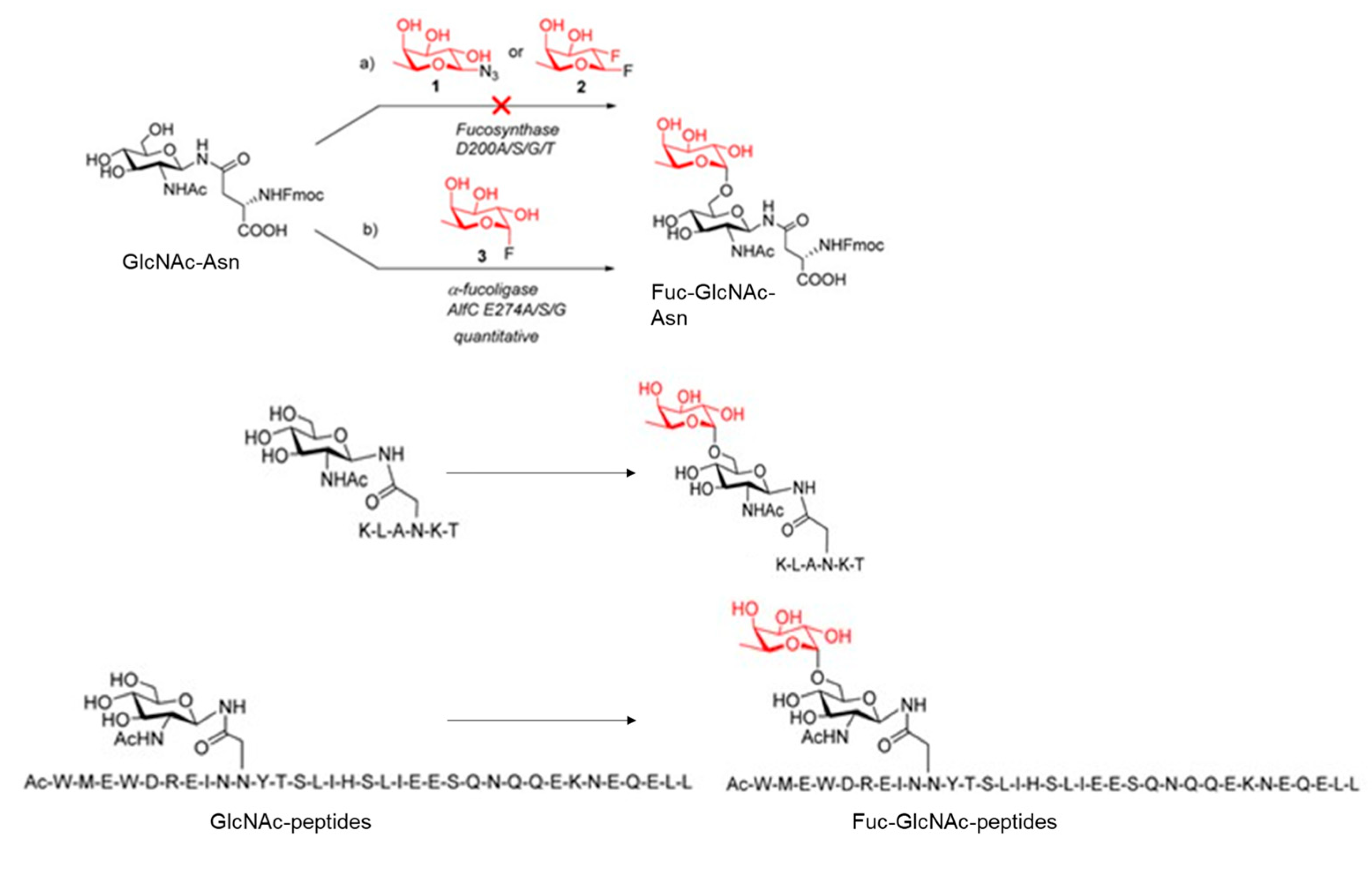
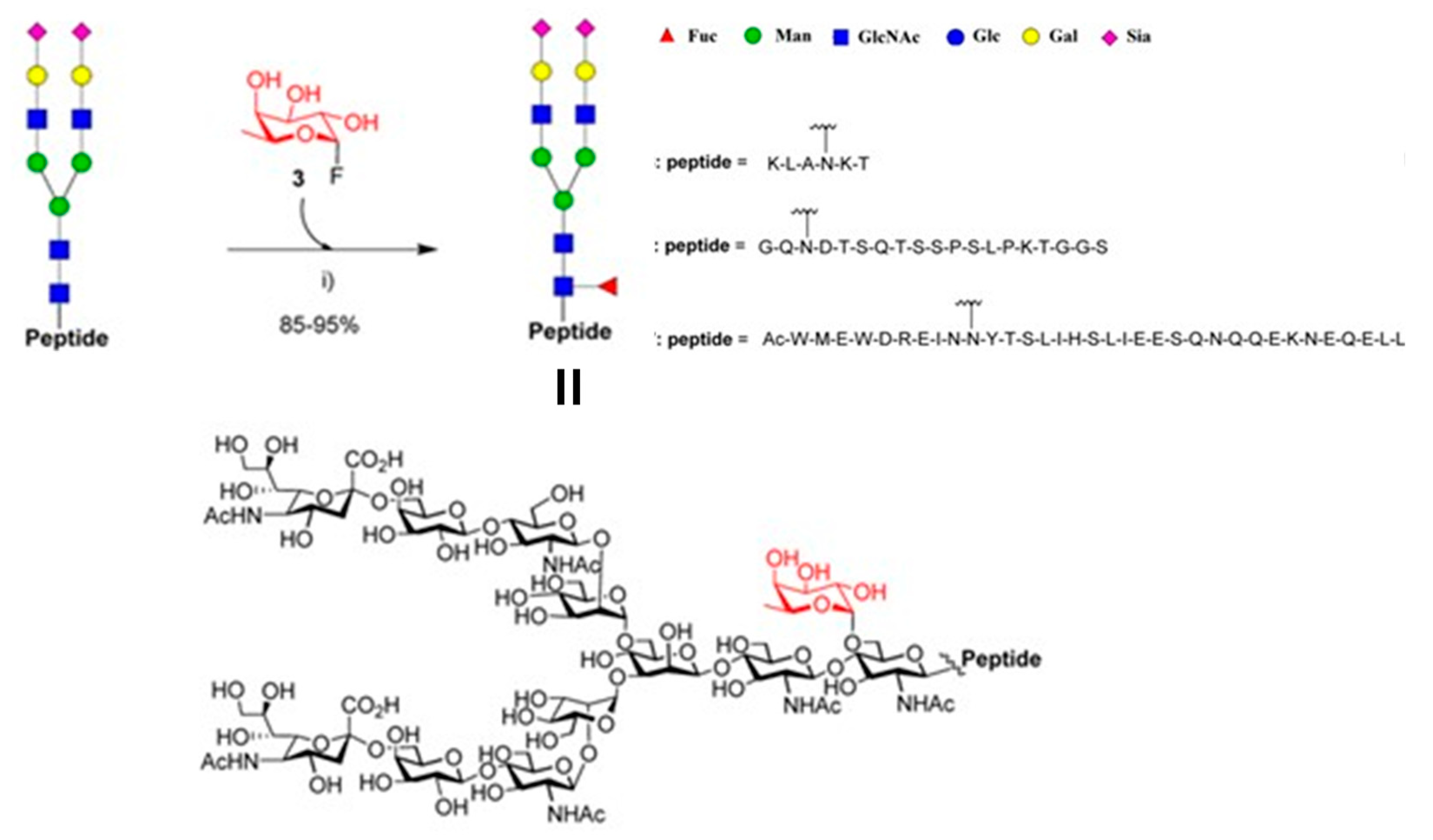
4.3. Fucosynthases
4.4. Chitinases
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Gantt, R. W.; Pauline Peltier-Pain, P; Thorson, J. S. Enzymatic methods for glyco(diversification/randomization) of drugs and small molecules. Nat. Prod. Rep. 2011, 28, 1811. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Phillips, G. N. Jr.; Thorson, J. S. The structural biology of enzymes involved in natural product glycosylation. Nat Prod Rep. 2012, 29, 1201–1237. [Google Scholar] [CrossRef] [PubMed]
- Song, M. C.; Kim, E.; Ban, Y. H.; Yoo, Y. J.; Kim, E. J.; Park, S. R.; Pandey, R. P.; Sohng, J. K.; Yoon, Y. J. Achievements and impacts of glycosylation reactions involved in natural product biosynthesis in prokaryotes. Appl Microbiol Biotechnol 2013, 97, 5691–5704. [Google Scholar] [CrossRef] [PubMed]
- D. J. Newman, D. J.; Cragg, G. M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef]
- Butler, M. S. Natural products to drugs: natural product-derived compounds in clinical trials. Nat. Prod. Rep. 2008, 25, 475–516. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Nguyen, T. T. H.; Kim, N. M.; Moon, Y.-H.; Ha, J.-M.; Park, N.; Lee, D.-G.; Hwang, K.-H.; Park, J.-S.; Kim, D. Functional properties of novel epigallocatechin gallate glucosides synthesized by using dextransucrase from Leuconostoc mesenteroides B-1299CB4. J. Agricul. Food Chem 2016, 64, 9203–9213. [Google Scholar] [CrossRef]
- Gantt, R. W.; Peltier-Pain, P.; Thorson, J. S. Enzymatic methods for glyco(diversification/randomization) of drugs and small molecules. Nat. Prod. Rep. 2011, 28, 1811. [Google Scholar] [CrossRef]
- Lyu, J.; Zhang, J.; Zhu, J.; Chen, S.; Han, T.; Zhang, Y.; Gao, R.; Xie, G.; Guo, Z. Molecular dynamics simulation guided distal mutation of Thermotoga naphthophila β-glucosidase for significantly enhanced synthesis of galactooligosaccharides and expanded product scope. Inter.J. Biol.l Macromol. 2022, 210, 21–32. [Google Scholar] [CrossRef]
- Paliya, B. S.; Sharma, V. K.; Tuohy, M. G.; Singh, H. B.; Koffas, N.; Benhida, R.; Tiwari, B. K.; Kalaskar, D. M.; Singh, B. N.; Gupta, V. K. Bacterial glycobiotechnology: A biosynthetic route for the production of biopharmaceutical glycans. Biotechnol. Adv.s, 2023; 67, 108180. [Google Scholar]
- Xu, L.; Qi, T.; Xu, L.; Lu, L.; Xiao, M. Recent progress in the enzymatic glycosylation of phenolic compounds. Journal of Carbohydrate Chemistry 2016, 35, 1–23. [Google Scholar] [CrossRef]
- Saranraj, P.; Behera, S.S.; Ray, R.C. ; Traditional foods from tropical root and tuber crops: innovations and challenges, Innovations in traditional foods. Elsevier 2019, 159–191. [Google Scholar]
- Sun, Q.; Heilmann, J.; König, B. Natural phenolic metabolites with anti-angiogenic properties–a review from the chemical point of view. Beilstein journal of organic chemistry 2015, 11, 249–264. [Google Scholar] [CrossRef]
- Orhan, D. D.; Özçelik, B.; Özgen, S.; Ergun, F. Antibacterial, antifungal, and antiviral activities of some flavonoids. Microbiological research 2010, 165, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Taamalli, A.; Arráez-Román, D.; Zarrouk, M.; Segura-Carretero, A.; Fernández-Gutiérrez, A. The Occurrence and Bioactivity of Polyphenols in Tunisian Olive Products and by-Products: A Review. Journal of food science 2012, 77, R83–R92. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Nguyen, T. T. H.; Kim, N. M.; Moon, Y.-H.; Ha, J.-M.; Park, N.; Lee, D.-G.; Hwang, K.-H.; Park, J.-S.; Kim, D. Functional properties of novel epigallocatechin gallate glucosides synthesized by using dextransucrase from Leuconostoc mesenteroides B-1299CB4, Journal of agricultural and food chemistry 2016, 64, 9203–9213.
- Méndez-Líter, J.A.; Tundidor, I.; Nieto-Domínguez, M.; de Toro, B. F.; González Santana, A.; de Eugenio, L.I.; Prieto, A.; Asensio, J.L.; Sánchez, C.; Martínez, M.J. Transglycosylation products generated by Talaromyces amestolkiae GH3 β-glucosidases: Effect of hydroxytyrosol, vanillin and its glucosides on breast cancer cells. Microbial cell factories 2019, 18, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Li, B.; Qiao, M.; Li, J.; Xu, H.; Zhang, L.; Zhang, X. Advances on the in vivo and in vitro glycosylations of flavonoids. Applied Microbiology and Biotechnology 2020, 104, 6587–6600. [Google Scholar] [CrossRef]
- Schmid, J.; Heider, D.; Wendel, N. J.; Sperl, N.; Sieber, V. Bacterial glycosyltransferases: challenges and opportunities of a highly diverse enzyme class toward tailoring natural products. Frontiers in microbiology 2016, 7, 182. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, P.; Cui, Y.; Li, K.; Qiao, X.; Zhang, Y. T.; Li, S. M.; Cox, R. J.; Wu, B.; Ye, M. Regio-and Stereospecific O-Glycosylation of Phenolic Compounds Catalyzed by a Fungal Glycosyltransferase from Mucor hiemalis. Advanced Synthesis & Catalysis 2017, 359, 995–1006. [Google Scholar]
- Chang, S.K.; Jiang, Y.; Yang, B. An update of prenylated phenolics: Food sources, chemistry and health benefits. Trends in Food Science & Technology 2021, 108, 197–213. [Google Scholar]
- Xie, K.; Dou, X.; Chen, R.; Chen, D.; Fang, C.; Xiao, Z.; Dai, J. Two novel fungal phenolic UDP glycosyltransferases from Absidia coerulea and Rhizopus japonicus. Applied and environmental microbiology 2017, 83, e03103–e03116. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Z.; Lei, H.; Chen, R.; Wang, X.; Peng, Y.; Dai, J. Neuroprotective glucosides of magnolol and honokiol from microbial-specific glycosylation. Tetrahedron 2014, 70, 8244–8251. [Google Scholar] [CrossRef]
- Li, B.; Chang, S.; Jin, D.; Zhang, S.; Chen, T.; Pan, X.; Fan, B.; Lv, K.; He, X. Ca2+ assisted glycosylation of phenolic compounds by phenolic-UDP-glycosyltransferase from Bacillus subtilis PI18. International journal of biological macromolecules 2019, 135, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Tang, W.; Barton, C. D.; Price, O. M.; Mortensen, M. W.; Phillips, A.; Wald, B.; Hulme, S. E.; Stanley, L. P.; Hevel, J. A highly versatile fungal glucosyltransferase for specific production of quercetin-7-O-β-d-glucoside and quercetin-3-O-β-d-glucoside in different hosts. Applied microbiology and biotechnology 2021, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Rha, C.-S.; Choi, J.-M.; Jung, Y. S.; Kim, E.-R.; Ko, M. J.; Seo, D.-H.; Kim, D.-O.; Park, C.-S. High-efficiency enzymatic production of α-isoquercitrin glucosides by amylosucrase from Deinococcus geothermalis. Enzyme and microbial technology 2019, 120, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Rha, C.-S.; Jung, Y.S.; Seo, D.-H.; Kim, D.-O.; Park, C.-S. Site-specific α-glycosylation of hydroxyflavones and hydroxyflavanones by amylosucrase from Deinococcus geothermalis. Enzyme and microbial technology 2019, 129, 109361. [Google Scholar] [CrossRef]
- Gonzalez-Alfonso, J.L.; Leemans, L.; Poveda, A.; Jimenez-Barbero, J.s.; Ballesteros, A. O.; Plou, F. J. Efficient α-glucosylation of epigallocatechin gallate catalyzed by cyclodextrin glucanotransferase from Thermoanaerobacter species. Journal of agricultural and food chemistry 2018, 66, 7402–7408. [Google Scholar] [CrossRef]
- González-Alfonso, J.L.; Míguez, N.; Padilla, J.D.; Leemans, L.; Poveda, A.; Jiménez-Barbero, J.; Ballesteros, A. O.; Sandoval, G.; Plou, F.J. Optimization of regioselective α-glucosylation of hesperetin catalyzed by cyclodextrin glucanotransferase. Molecules 2018, 23, 2885. [Google Scholar] [CrossRef]
- Méndez-Líter, J. A.; Nieto-Domínguez, M.; de Toro, B. F.; Santana, A. G.; Prieto, A.; Asensio, J. L.; de Eugenio, L. I.; Martínez, M. J. A glucotolerant β-glucosidase from the fungus Talaromyces amestolkiae and its conversion into a glycosynthase for glycosylation of phenolic compounds. Microbial cell factories 2020, 19, 1–13. [Google Scholar] [CrossRef]
- Yang, B.; Liu, H.; Yang, J.; Gupta, V. K.; Jiang, Y. New insights on bioactivities and biosynthesis of flavonoid glycosides. Trends in food science & technology, 2018; 79, 116–124. [Google Scholar]
- He, J. B.; Zhao, P.; Hu, Z. M.; Liu, S.; Kuang, Y.; Zhang, M.; Li, B.; Yun, C. H.; Qiao, X.; Ye, M. Molecular and Structural Characterization of a Promiscuous C-Glycosyltransferase from Trollius chinensis. Angewandte Chemie 2019, 131, 11637–11644. [Google Scholar] [CrossRef]
- Pei, J.; Sun, Q.; Gu, N.; Zhao, L.; Fang, X.; Tang, F.; Cao, F. Production of isoorientin and isovitexin from luteolin and apigenin using coupled catalysis of glycosyltransferase and sucrose synthase. Applied biochemistry and biotechnology 2020, 190, 601–615. [Google Scholar] [CrossRef]
- J. Pei, Q. Sun, L. Zhao, H. Shi, F. Tang, F. Cao, Efficient biotransformation of luteolin to isoorientin through adjusting induction strategy, controlling acetic acid, and increasing UDP-glucose supply in Escherichia coli, Journal of agricultural and food chemistry, 67 (2018) 331-340.
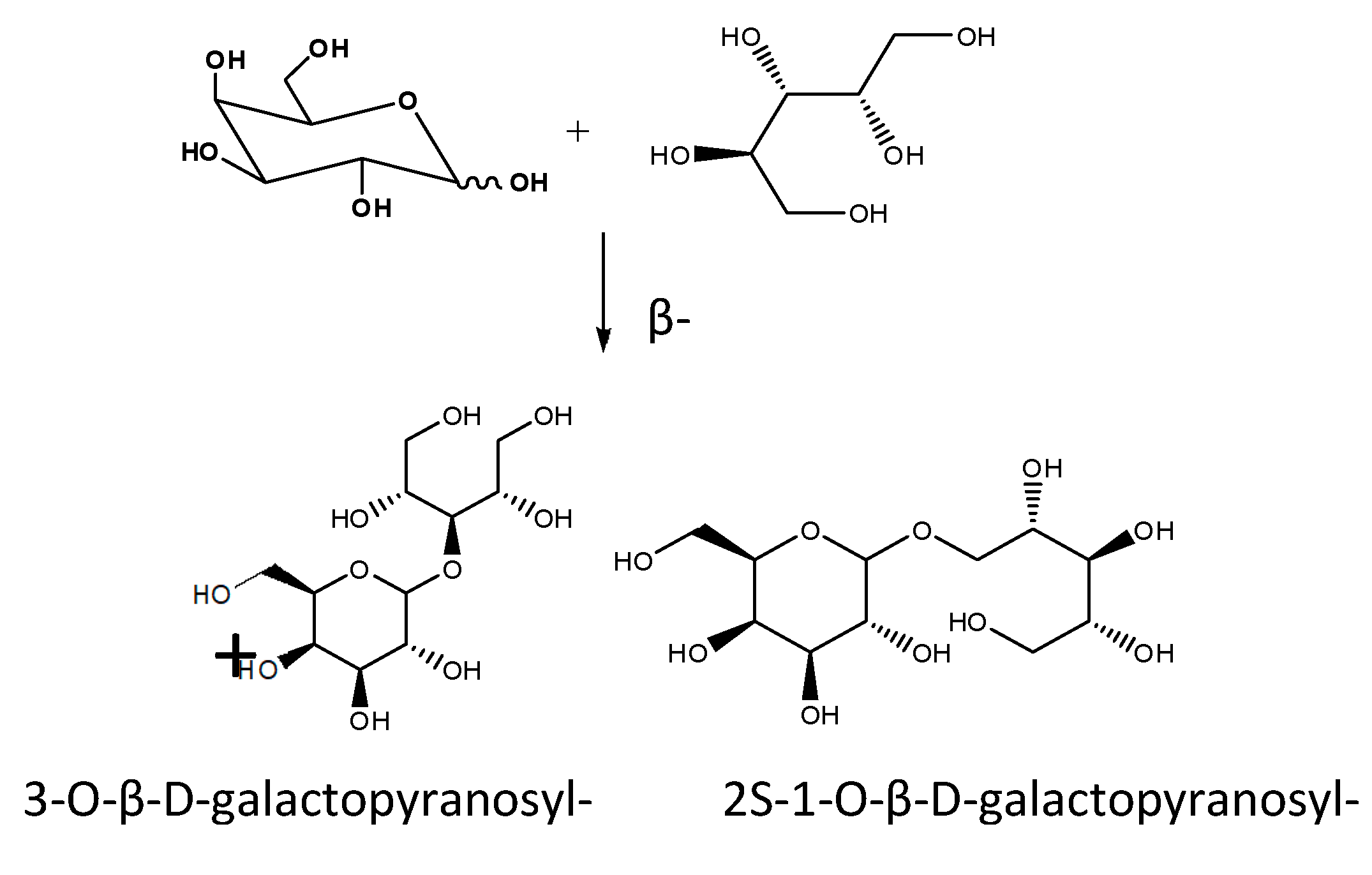
- Carević, M.; Veličković, D.; Stojanović, M.; Milosavić, N.; Rogniaux, H.; Ropartz, D.; Bezbradica, D. Insight in the regioselective enzymatic transgalactosylation of salicin catalyzed by β-galactosidase from Aspergillus oryzae. Process Biochemistry 2015, 50, 782–788. [Google Scholar] [CrossRef]
- Murguiondo, C.; Mestre, A.; Méndez-Líter, J. A.; Nieto-Domínguez, M.; de Eugenio, L. I.; Molina-Gutiérrez, M.; Martínez, M. J.; Prieto, A. Enzymatic glycosylation of bioactive acceptors catalyzed by an immobilized fungal β-xylosidase and its multi-glycoligase variant. International Journal of Biological Macromolecules 2021, 167, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Alfonso, J.L.; Ubiparip, Z.; Jimenez-Ortega, E.; Poveda, A.; Alonso, C.; Coderch, L.; Jimenez-Barbero, J.; Sanz-Aparicio, J.; Ballesteros, A. O.; Desmet, T. Enzymatic Synthesis of Phloretin α-Glucosides Using a Sucrose Phosphorylase Mutant and its Effect on Solubility, Antioxidant Properties and Skin Absorption. Advanced Synthesis & Catalysis, 2021. [Google Scholar]
- Sordon, S.; Popłoński, J.; Huszcza, E. Microbial glycosylation of flavonoids, Polish journal of microbiology 2016, 65, 7. 65.
- Sordon, S.; Popłoński, J.; Tronina, T.; Huszcza, E. Microbial glycosylation of daidzein, genistein and biochanin A: Two new glucosides of biochanin A. Molecules 2017, 22, 81. [Google Scholar] [CrossRef]
- Sordon, S.; Popłoński, J.; Tronina, T.; Huszcza, E. Regioselective O-glycosylation of flavonoids by fungi Beauveria bassiana, Absidia coerulea and Absidia glauca. Bioorganic chemistry 2019, 93, 102750. [Google Scholar] [CrossRef] [PubMed]
- Dymarska, M.; Janeczko, T.; Kostrzewa-Susłow, E. Biotransformations of flavones and an isoflavone (daidzein) in cultures of entomopathogenic filamentous fungi. Molecules 2018, 23, 1356. [Google Scholar] [CrossRef]
- Dymarska, M.; Janeczko, T.; Kostrzewa-Susłow, E. Glycosylation of 3-hydroxyflavone, 3-methoxyflavone, quercetin and baicalein in fungal cultures of the genus Isaria. Molecules, 2018, 23, 2477. [Google Scholar] [CrossRef] [PubMed]
- Rosado, E.; Delgado-Fernandez, P.; de las Rivas, B.; Muñoz, R.; Moreno, J.; Corzo, N.; Mateo. C. Production of β-Galactosyl Xylitol Derivatives Using Heterogeneous Catalysts of LacA β-Galactosidase From Lactobacillus plantarum WCFS1. Molecules 2022, 27, 1235. [Google Scholar] [CrossRef]
- Cheng, K. L.; Bradely, T.; Budman, D. R. Novel microtubule-targeting agents- the epothilones. Biologics 2008, 2, 789–811. [Google Scholar]
- Hofle, G.; Reichenbach, H. Epothilone, a myxobacterial metabolite with promising antitumor activity. In: Cragg GM, Kingston DGI, Newman DJ (eds). Anticancer agents from natural products. CRC Press, 2005; 413–450. [Google Scholar]
- Hardt, I.H.; Steinmetz, H.; Gerth, K.; Sasse, F.; Reichenbach, H.; Hofle, G. New natural epothilones from Sorangium cellulosum, strains So ce 90/B2 and So ce90/D13:isolation, structure elucidation, and SAR studies. J Nat Prod 2001, 64, 847–856. [Google Scholar] [CrossRef]
- Lee, F. Y.; Borzilleri, R.; Fairchild, C. R.; Kim, S. H.; Long, B. H.; Reventos-Suarez, C.; Vite, G. D.; Rose, W. C.; Kramer, R. A. BMS-247550: a novel epothilone analog with a mode of action similar to paclitaxel but possessing superior antitumor efficacy. Clin Cancer Res 2001, 7, 1429–1437. [Google Scholar]
- Thomas, E.; Tabemero, J.; Fornier, M.; Conte, P.; Fumoleau, P.; Liuch, A.; Vahdat, L. T.; Bunnell, C. A.; Burris, H. A.; Viens, P.; Baselqa, J.; Rivera, E.; Guameri, V.; Poulart, V.; Klimovsky, J.; Lebwohl, D.; Martin, M. Phase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, in patients with taxane-resistant metastatic breast cancer. J Clin Oncol 2007, 25, 3399–3406. [Google Scholar] [CrossRef]
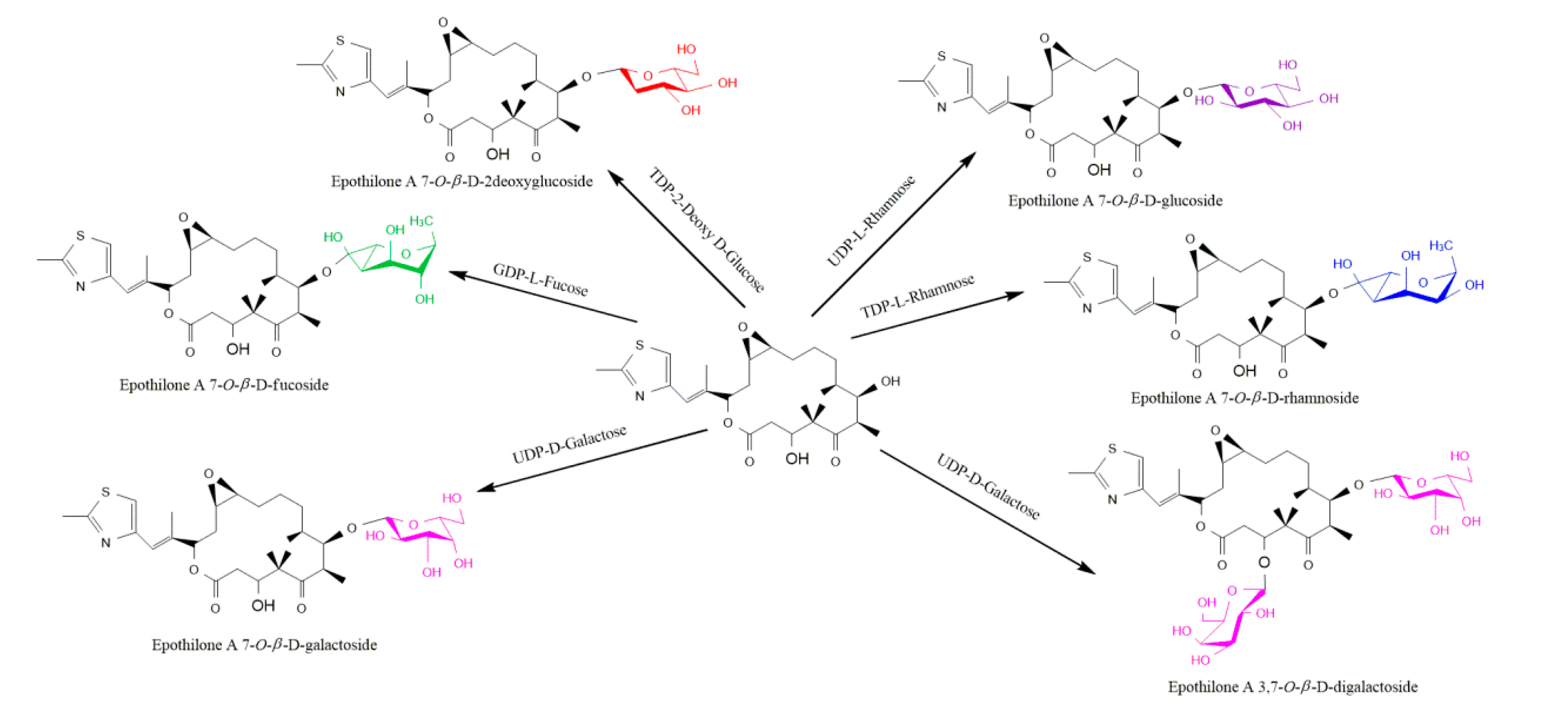
- Parajuli, P.; Pandey, R. P.; Koirala, N. et al. Enzymatic synthesis of epothilone A glycosides. AMB Expr 2014, 4, 31. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, E. C.; Field, R. A. Enzymatic synthesis using glycoside phosphorylases. Carbohydr. Res. 2015, 403, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Puchart, V. Glycoside phosphorylases: Structure, catalytic properties and biotechnological potential. Biotechnol. Adv. 2015, 33, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Nakai, H.; Kitaoka, M.; Svensson, B.; Ohtsubo, K.I. Recent development of phosphorylases possessing large potential for oligosaccharide synthesis. Curr. Opin. Chem. Biol. 2013, 17, 301–309. [Google Scholar] [CrossRef]
- Pergolizzia, G.; Kuhaudomlarpa, S.; Kalitaa, E.; Fielda, R. A. Glycan Phosphorylases in Multi-Enzyme Synthetic Processes. Protein & Peptide Letters 2017, 24, 696–709. [Google Scholar]
- Wildberger, P.; Pfeiffer, M.; Brecker, L.; Nidetzky, B. Diastereoselective synthesis of glycosyl phosphates by using a phosphorylase-phosphatase combination catalyst. Angew. Chem. Int. Ed. 2015, 54, 15867–15871. [Google Scholar] [CrossRef]
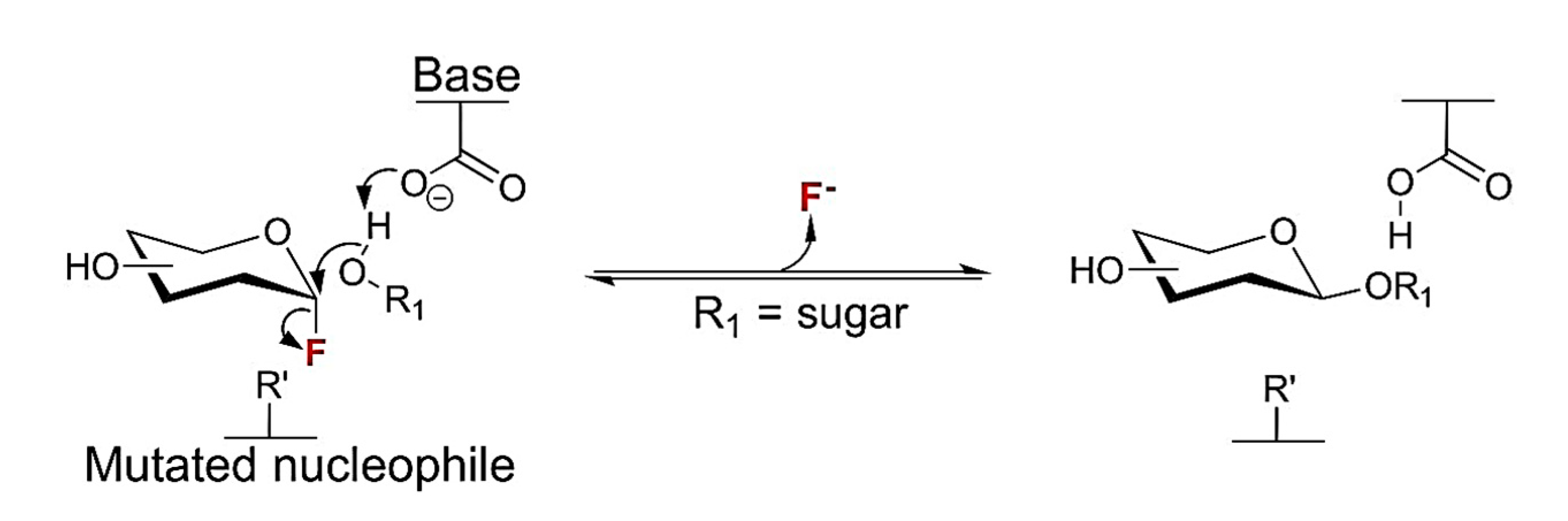
- Hancock, S. M.; Vaughan, M. D.; Withers, S. G. Engineering of glycosidases and glycosyltransferases. Current Opinion in Chemical Biology. 2006, 10, 509–519. [Google Scholar] [CrossRef]
- Schmölzer, K.; Weingarten, M.; Baldenius, K.; Nidetzky, B. Glycosynthase Principle Transformed into Biocatalytic Process Technology: Lacto N triose II Production with Engineered exo-Hexosaminidase. ACS Catal. 2019, 9, 5503–5514. [Google Scholar] [CrossRef]
- Bulmer, G. S.; de Andrade, P.; Field, R. A.; van Munster, J. M. Recent advances in enzymatic synthesis of β-glucan and cellulose. Carbohydrate Research. 2021, 508, 108411. [Google Scholar]
- Mészáros, Z.; Nekvasilová, P.; Bojarová, P.; Kren, V.; Fernández Slámová, K. Advanced glycosidases as ingenious biosynthetic instruments. Biotechnology Advances. 2021, 49, 107733. [Google Scholar] [CrossRef]
- Williams, S. J.; Withers, S. G. Glycosynthases: Mutant Glycosidases for Glycoside Synthesis. Australian Journal of Chemistry. 2002, 55, 3–12. [Google Scholar]
- Méndez-Líter, J.A.; Nieto-Domínguez, M.; de Toro, B. et al. A glucotolerant β-glucosidase from the fungus Talaromyces amestolkiae and its conversion into a glycosynthase for glycosylation of phenolic compounds. Microb Cell Fact. 2020, 19, 127. [Google Scholar] [CrossRef] [PubMed]
- Hrmova, M.; Imai, T.; Rutten, S. J.; Fairweather, J. K.; Pelosi, L.; Bulone, V.; Driguez, H.; Fincher, G. B. Mutated Barley (1,3)-β-d -Glucan Endohydrolases Synthesize Crystalline (1,3)-β-d -Glucans*. Journal of Biological Chemistry. 2002, 277, 30102–30111. [Google Scholar] [CrossRef] [PubMed]
- Pérez, X.; Faijes, M.; Planas, A. Artificial Mixed-Linked β-Glucans Produced by Glycosynthase-Catalyzed Polymerization: Tuning Morphology and Degree of Polymerization. Biomacromolecules. 2011, 12, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Smith, P. J.; Ortiz-Soto, M. E.; Roth, C.; Barnes, W. J.; Seibel, J.; Urbanowicz, B. R.; Pfrengle, F. Enzymatic Synthesis of Artificial Polysaccharides. ACS Sustainable Chem. Eng. 2020, 8, 11853–11871. [Google Scholar] [CrossRef]
- E. Steensma, W. G. J. Hol, Protein Sci. 1996, 5, 1184–1188;
- R. Bukowski, L. M. Morris, R. J. Woods, T. Weimar, Eur. J. Org. Chem. 2001, 2697 – 2705.
- Mocchetti, Cell. Mol. Life Sci. 2005, 62, 2283–2294.
- Brockhausen, EMBO Rep. 2006, 7, 599 –604.
- T. Urashima, S. Asakuma, F. Leo, K. Fukuda, M. Messer, O. T. Oftedal, Adv. Nutr. 2012, 3, 473S–482S. [Google Scholar]
- ChemBioChem 2014, 15, 522 – 526.
- K. Yamamoto, Biotechnol. Lett. 2013, 35, 1733–1743. [Google Scholar]
- L.-X. Wang, W. Huang, Curr. Opin. Chem. Biol. 2009, 13, 592 –600; R. Kittl, S. G. Withers,.
- Carbohydr. Res. 2010, 345, 1272 –1279; Z. Armstrong, S. G. Withers, Biopolymers 2013, 99, 666 –674;
- X. P rez, M. Faijes, A. Planas, Biomacromolecules 2011, 12, 494–501.
- B. Cobucci-Ponzano, A. Strazzulli, M. Rossi, M. Moracci, Adv. Synth. Catal. 2011, 353, 2284–2300. [Google Scholar]
- L. F. Mackenzie, Q. Wang, R. A. J. Warren, S. G. Withers, J. Am. Chem. Soc. 1998, 120, 5583–5584. [Google Scholar]
- ChemBioChem 2014, 15, 522 – 526.
- Hovorková, M.; Dr. Kulik, N.; Konvalinková, D.; Dr. Petrásková, L.; Prof. Křen, V.; Prof. Bojarová, P. Mutagenesis of Catalytic Nucleophile of β-Galactosidase Retains Residual Hydrolytic Activity and Affords a Transgalactosidase. ChemCatChem. 2021, 13, 4532–4542. [Google Scholar] [CrossRef]
- Mészáros, Z.; Nekvasilová, P.; Bojarová, P.; Křen, V.; Slámová, K. Reprint of: Advanced glycosidases as ingenious biosynthetic instruments. Biotechnology Advances. 2021, 51, 107820. [Google Scholar] [CrossRef] [PubMed]
- The FEBS Journal ,2017, 284, 766–783.
- Dwek, R. A. A. Chem. Rev. 1996, 96, 683. [Google Scholar] [CrossRef]
- Helenius, A.; Aebi, M. Science. 2001, 291, 2364.
- Taniguchi, N.; Kizuka, Y. Adv. Cancer Res. 2015, 126, 11. [Google Scholar]
- Pinho, S. S.; Reis, C. A. Nat. Rev. Cancer 2015, 15, 540. [Google Scholar] [CrossRef]
- Chen, C. Y.; Jan, Y. H.; Juan, Y. H.; Yang, C. J.; Huang, M. S.; Yu, C. J.; Yang, P. C.; Hsiao, M.; Hsu, T. L.; Wong, C. H. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, 630. [CrossRef]
- Li, L.; Liu, Y.; Ma, C.; Qu, J.; Calderon, A. D.; Wu, B.; Wei, N.; Wang, X.; Guo, Y.; Xiao, Z.; Song, J.; Sugiarto, G.; Li, Y.; Yu, H.; Chen, X.; Wang, P. G. Chem. Sci. 2015, 6, 5652. [CrossRef]
- Brzezicka, K.; Echeverria, B.; Serna, S.; van Diepen, A.; Hokke, C. H.; Reichardt, N. C. ACS Chem. Biol. 2015, 10, 1290.
- Calderon, A. D.; Liu, Y.; Li, X.; Wang, X.; Chen, X.; Li, L.; Wang, P. G. Org. Biomol. Chem. 2016, 14, 4027.
- Tseng, T. H.; Lin, T. W.; Chen, C. Y.; Chen, C. H.; Lin, J. L.; Hsu, T. L.; Wong, C. H. J. Am. J. Am. Chem. Soc. 2017, 139, 9431. [CrossRef] [PubMed]
- Chao Li, C.; Shilei Zhu, S.; Christopher Ma, C.; Wan, L.-X. Designer α1,6-Fucosidase Mutants Enable Direct Core Fucosylation of Intact N-Glycopeptides and N-Glycoproteins. J. Am. Chem. Soc. 2017, 139, 15074–15087. [Google Scholar]
- MacKenzie, L. F.; Wang, Q.; Warren, R. A. J.; Withers, S. G. J. Am. J. Am. Chem. Soc. 1998, 120, 5583. [CrossRef]
- Armand, S.; Tomita, H.; Heyraud, A.; Gey, C.; Watanabe, T.; Henrissat, B. Stereochemical course of the hydrolysis reaction catalyzed by chitinases Al and D from Bacillus circulans WL-12. FEBS Lett. 1994, 343, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Henrissat, B.; Davies, G. Structural and sequence-based classification of glycoside hydrolases. Curr. Opin. Struct. Biol. 1997, 7, 637–644. [Google Scholar] [CrossRef] [PubMed]
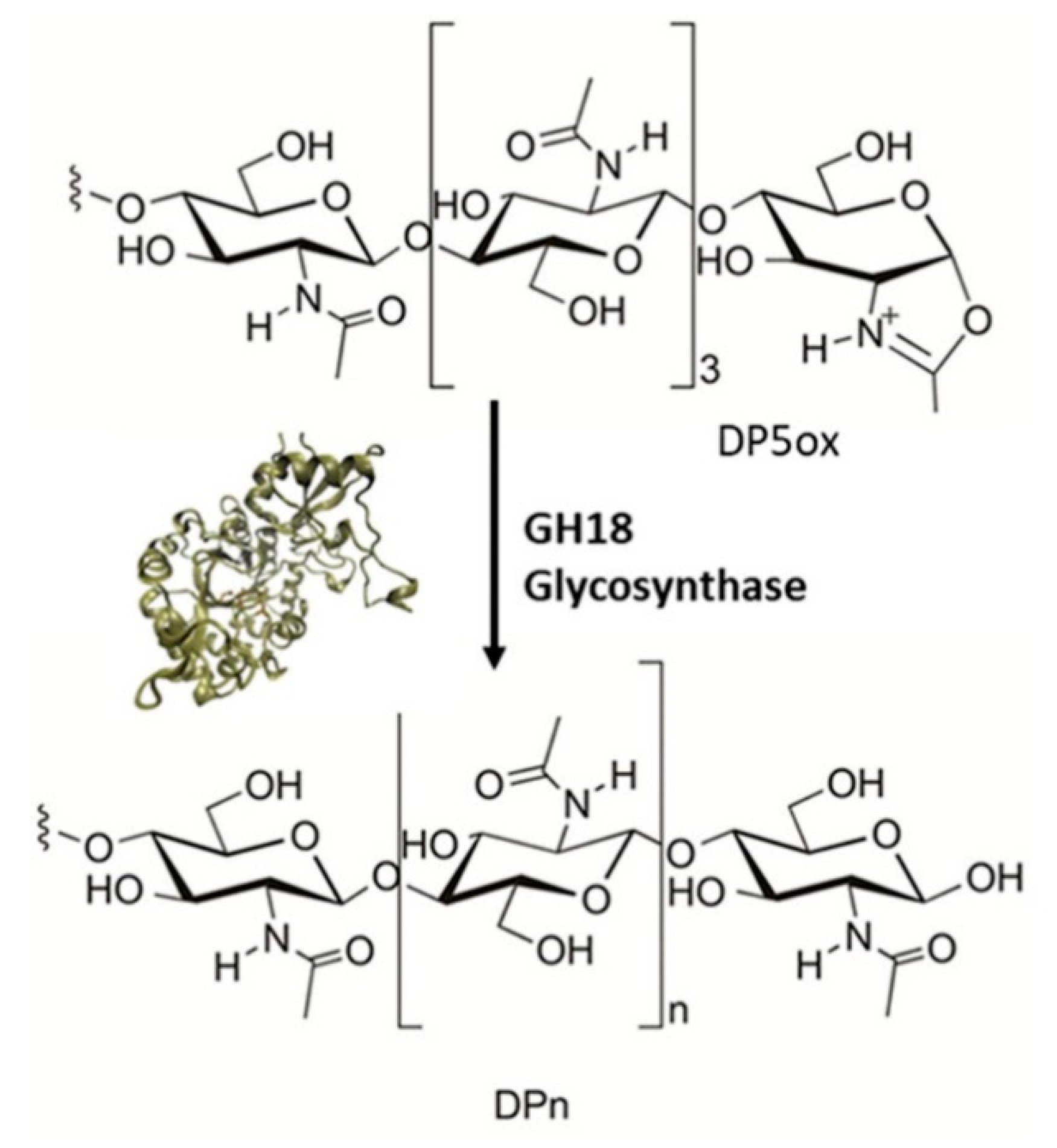
- Alsina, C.; Faijes, M.; Planas, A. Glycosynthase-type GH18 mutant chitinases at the assisting catalytic residue for polymerization of chitooligosaccharides. Carbohydrate Research 2019, 478, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Elieh Ali Komi, D.; Sharma, L.; Dela Cruz, C. S. Chitin and its effects on inflammatory and immune responses Clin. Rev. Allergy Immunol. 2018, 54, 213–223.
- Yoon, H. J.; Moon, M. E.; Park, H. S.; Im, S. Y.; Kim, Y. H. Chitosan oligosaccharide (COS) inhibits LPS-induced inflammatory effects in RAW 264.7 macrophage cells. Biochem. Biophys. Res. Commun. 2007, 358, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Yousef, M.; Pichyangkura, R.; Soodvilai, S.; Chatsudthipong, V.; Muanprasat, C. Chitosan oligosaccharide as potential therapy of inflammatory bowel disease: therapeutic efficacy and possible mechanisms of action. Pharmacol. Res. 2012, 66, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Chung, M. J.; Park, J. K.; Park, Y. II. Anti-inflammatory effects of low-molecular weight chitosan oligosaccharides in IgE-antigen complex-stimulated RBL-2H3 cells and asthma model mice. Int. Immunopharmacol. 2012, 12, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.-T.; Chen, M.-H.; Chan, H.-Y.; Jeng, J.-H.; Wang, Y.-J. Inhibitory effects of chitooligosaccharides on tumor growth and metastasis. Food Chem. Toxicol. 2009, 47 () 1864–1871.
- Park, J. K.; Chung, M. J.; Choi, H. N.; Park, Y. II. Effects of the molecular weight and the degree of deacetylation of chitosan oligosaccharides on antitumor activity. Int. J. Mol. Sci. 2011, 12, 266–277.
- Hadwiger, L. A. Plant science review: multiple effects of chitosan on plant systems: solid science or hype. Plant Sci. 2013, 208, 42–49.
- Katiyar, D.; Hemantaranjan, A.; Singh, B.; Bhanu, A. N. A future perspective in crop protection: chitosan and its oligosaccharides. Adv Plants Agric Res 2014, 1, 4–11. [Google Scholar]
- Azuma, K.; Ifuku, S.; Osaki, T.; Okamoto, Y.; Minami, S. Preparation and biomedical applications of chitin and chitosan nanofibers. J. Biomed. Nanotechnol. 2014, 10, 2891–2920. [Google Scholar] [CrossRef]
- Ravi Kumar, M. N. A review of chitin and chitosan applications. React. Funct Polym. 2000, 46, 1–27. [Google Scholar]
- Philibert, T.; Lee, B. H.; Fabien, N. Current status and new perspectives on chitin and chitosan as functional biopolymers. Appl. Biochem. Biotechnol. 2017, 181, 1314–1337. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Xia, W.; Liu, P.; Zhang, J.; Chen, J. Biological activities of chitosan and chitooligosaccharides. Food Hydrocolloids 2011, 25, 170–179. [Google Scholar] [CrossRef]
- Martinez, E. A.; Boer, H.; Koivula, A.; Samain, E.; Driguez, H.; Armand, S.; Cottaz, S. Engineering chitinases for the synthesis of chitin oligosaccharides: catalytic aminoacid mutations convert the GH-18 family glycoside hydrolases into transglycosylases. J. Mol. Catal. B Enzym. 2012, 74, 89–96. [Google Scholar] [CrossRef]
- Alsina, C.; Sancho-Vaello, E.; Aranda-Martínez, A.; Faijes, M.; Planas, A. Auxiliary active site mutations enhance the glycosynthase activity of a GH18 chitinase for polymerization of chitooligosaccharides. Carbohydrate Polymers 2021, 252, 117121. [Google Scholar] [CrossRef] [PubMed]
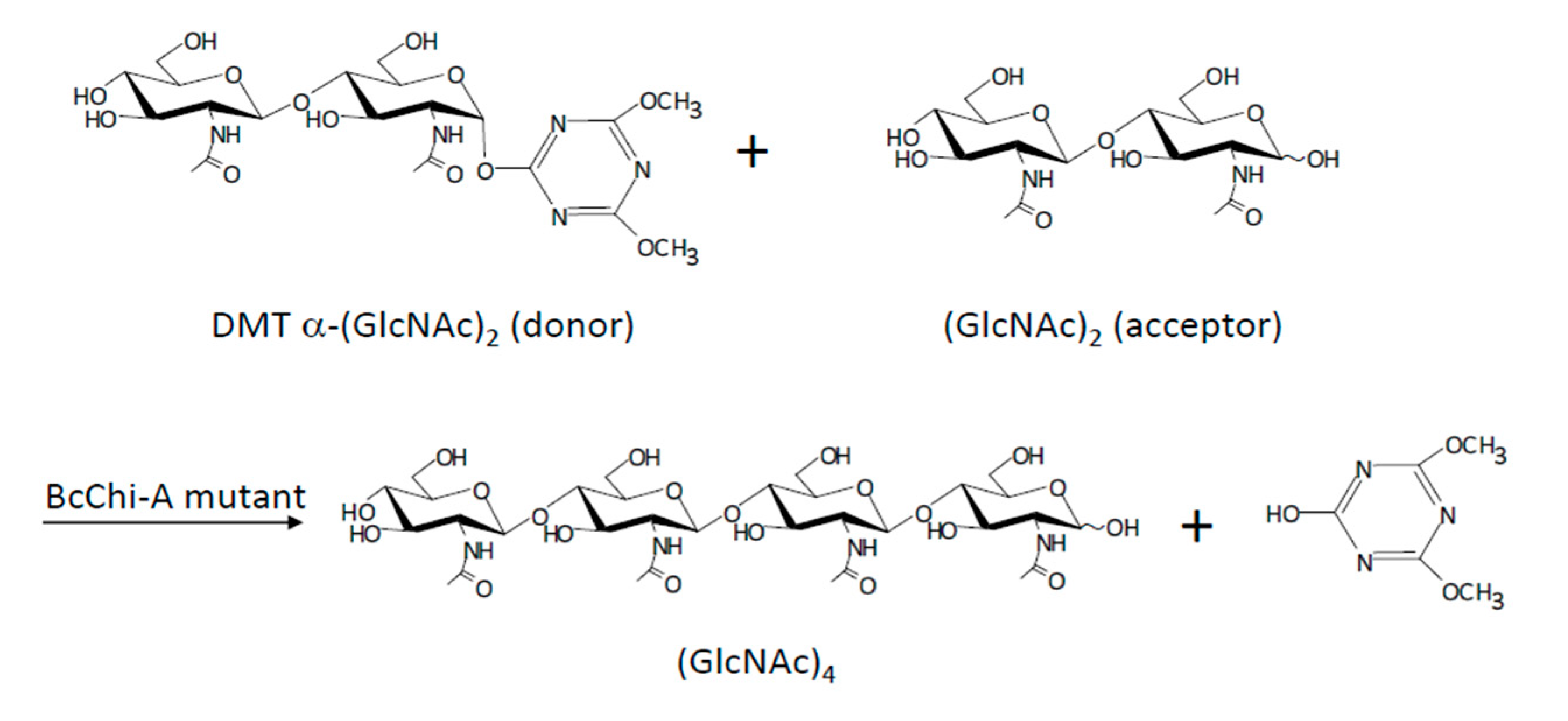
- Ohnuma, T.; Tanaka, T.; Urasaki, A.; Dozen, S.; Fukamizo, T. A novel method for chemo-enzymatic synthesis of chitin oligosaccharide catalyzed by the mutant of inverting family GH19 chitinase using 4,6-dimethoxy-1,3,5-triazin-2-yl α-chitobioside as a glycosyl donor. J. Biochem. 2019, 165, 497–503. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




