1. Introduction
Despite increasing evidence supports the overt dynamic adaptation capacity of autoantibodies (AAbs) in relation to immunological activation, attention is mainly focused on the pathological AAb formation and the subsequent potential adverse consequences [
1,
2,
3,
4,
5,
6]. The other subset; also cited as non-pathological or natural autoantibodies (nAAbs) is much less studied. Although remain usually quiet, these fundamental participants of the immunological network feature some very important, yet unrecognized physiological functions [
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27].
According to current knowledge, the term 'natural antibodies' (nAAbs) refers to immunoglobulin molecules preexistent prior to antigen stimulation, originating mainly from B1-B and marginal zone B cells. Despite their recognized significance in the innate immune defense as well as in the removal of altered cells and debris, their restricted immunological capacities are also reflected in their underappreciated significance in scientific research. These antibodies have moderate affinity, are typically poly-reactive, and their levels are physiological and thought to be relatively constant throughout life [
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27]. However, it has been described earlier that pathogen-associated environmental triggers, as well as the host biome can have a substantial impact on the makeup of the nAAb repertoire [
28,
29,
30]. Today, it is also acknowledged that enhanced vaccination strategies combine primary and secondary vaccine components to achieve optimal bioavailability and bioactivity of target substances while exhibiting a sufficiently broad spectrum of immune stimulation [
31]. Consequently, we suppose that although primarily aimed at disease prevention, vaccination may also have an unintended impact on the natural antibody repertoire [
29]. In other words, we hypothesized that upon a competent antigenic trigger, nAAbs display a moderate level of dynamic adaptability, also detectable at the level of antibody titers. Regarding this theory, evidence had been previously reported [
32,
33,
34,
35]. Therefore, we have taken the immunoserological approach of addressing the scientific question whether there is a quantifiable difference in the adaptation capacity of the nAAb pool in response to an aged antigenic trigger (like the historic MMR vaccine or childhood infection) versus a relatively recent stimulation (provided by anti-SARS-CoV-2 vaccines).
Accordingly, we determined our research objectives as follows:
Humoral immunity; vaccine efficacy studies I. Evaluating IgG antibody titers elicited by the historical measles, mumps, and rubella (MMR) vaccines (or the relevant viral pathogens), similarly to our former seroepidemiological reports [
32,
36,
37]. Delineating gaps of humoral immunity and defining potentially susceptible age groups
Looking for potential connections between nAAb levels (anti-citrate synthase) and persistent antibody titers after a decades-old antigenic trigger; Is there an association between the aged, aforetime elicited anti-viral (MMR) antibody levels and the nAAbs?
Humoral immunity; vaccine efficacy studies II. Evaluating IgG antibody titers elicited by the contemporary COVID-19 vaccines.
Looking for potential connections between nAAb levels (anti-citrate synthase) and a latter antigenic trigger; Is there an association between the relatively recent anti-SARS-CoV-2 IgG antigen-induced antibodies and the nAAbs?
2. Results
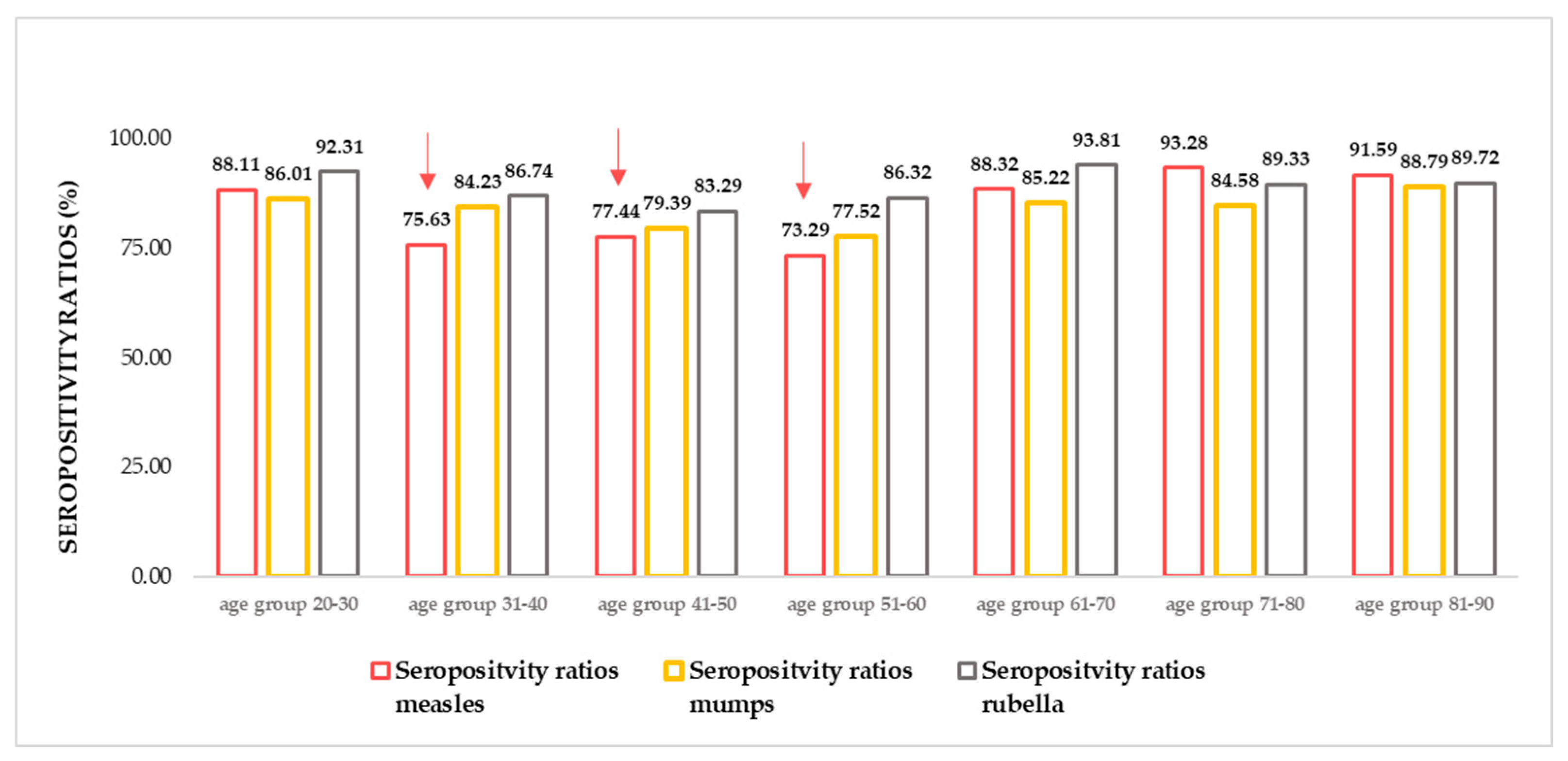
2.1. Relative differences in anti-MMR seropositivity ratios by age groups
In accordance with previous findings [
37,
38,
39,
40], in the recently tested Croatian samples insufficiencies have been found in the anti-MMR (and especially anti-measles) humoral protection. The seropositivity ratio calculated based on circulating IgG antibody titers (number of positive samples / number of all samples * 100) was the lowest in age groups 31-40 years, 41- 50 years, and 51-60 years. The herein illustrated findings can be considered suboptimal, as far as humoral antibody titers are considered ‘correlates of protection’ [
41,
42,
43,
44,
45]. To maintain stable anti-measles herd immunity, at least 95% of immunization coverage (in coexistence with adequate responsiveness) would be required [
46,
47,
48,
49].
Figure 1.
Measles, mumps, rubella (MMR) seropositivity ratios. N total =1739, n measles = 1431, n mumps =1438, n rubella =1533. (For detailed age group numbers, please see Supplementary Table S1) The lowest seropositivity ratios were detectable in the age groups 31-40, 41- 50, 51-60 (highlighted with red arrows.) Herd immunity threshold (HIT) values; HIT measles = 92–95%, HIT Mumps = 85–90%, HIT Rubella = 83–86%.
Figure 1.
Measles, mumps, rubella (MMR) seropositivity ratios. N total =1739, n measles = 1431, n mumps =1438, n rubella =1533. (For detailed age group numbers, please see Supplementary Table S1) The lowest seropositivity ratios were detectable in the age groups 31-40, 41- 50, 51-60 (highlighted with red arrows.) Herd immunity threshold (HIT) values; HIT measles = 92–95%, HIT Mumps = 85–90%, HIT Rubella = 83–86%.
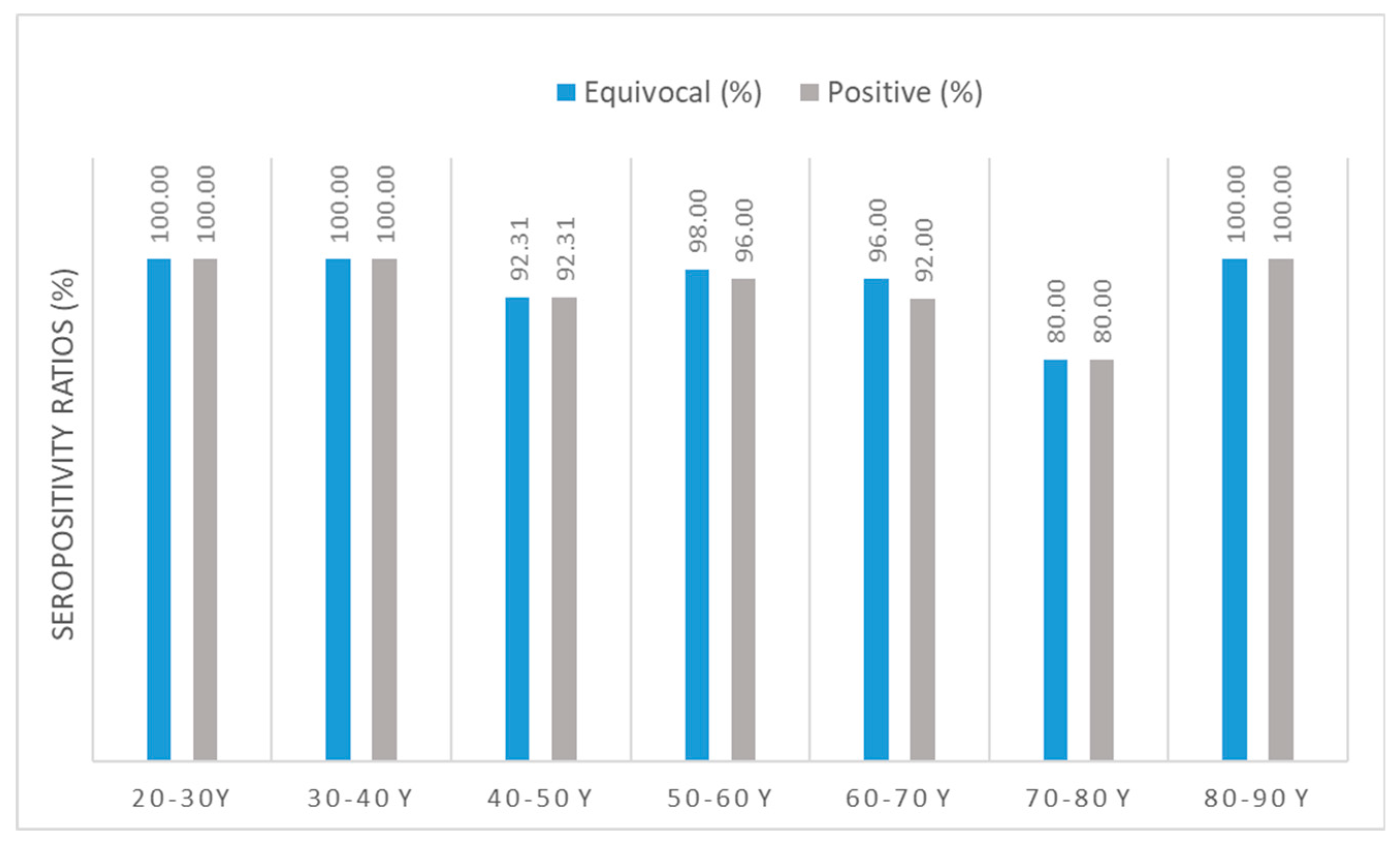
2.2. Relative differences in anti-SARS-CoV-2 specific seropositivity ratios by age groups
In terms of anti-SARS-CoV-2 IgG seropositivity ratios (without differentiation between vaccines), the lowest ratio (number of positive samples / number of all samples * 100) was found in the age group of 70- to 80-year-old individuals. All clusters had sufficiently high [
50] seropositivity ratios ≥80%.
Figure 2.
Anti-SARS-CoV-2 IgG seropositivity ratios. N total =237, only vaccinated individuals. Sample numbers: n mRNA= 170, n Adenoviral vector= 25, n mRNA + adenoviral vector= 42. Sample numbers according to age groups: n 21-30 y = 21, n 31-40 y= 30, n 41-50 y= 26, n 51-60 y=50, n 61-70 y= 50, n 71-80 y= 30, n 81-90 y= 22. (For detailed seropositive sample numbers per age group, please see Supplementary Table S2) Blue bars show results calculated using the cut-off value of the equivocal range suggested by the manufacturer; antibody titers ≥ 8 RU/mL; grey bars show results calculated using the cut-off value of the positive range suggested by the manufacturer; antibody titers ≥ 11 RU/mL have been considered ‘seropositive’.
Figure 2.
Anti-SARS-CoV-2 IgG seropositivity ratios. N total =237, only vaccinated individuals. Sample numbers: n mRNA= 170, n Adenoviral vector= 25, n mRNA + adenoviral vector= 42. Sample numbers according to age groups: n 21-30 y = 21, n 31-40 y= 30, n 41-50 y= 26, n 51-60 y=50, n 61-70 y= 50, n 71-80 y= 30, n 81-90 y= 22. (For detailed seropositive sample numbers per age group, please see Supplementary Table S2) Blue bars show results calculated using the cut-off value of the equivocal range suggested by the manufacturer; antibody titers ≥ 8 RU/mL; grey bars show results calculated using the cut-off value of the positive range suggested by the manufacturer; antibody titers ≥ 11 RU/mL have been considered ‘seropositive’.
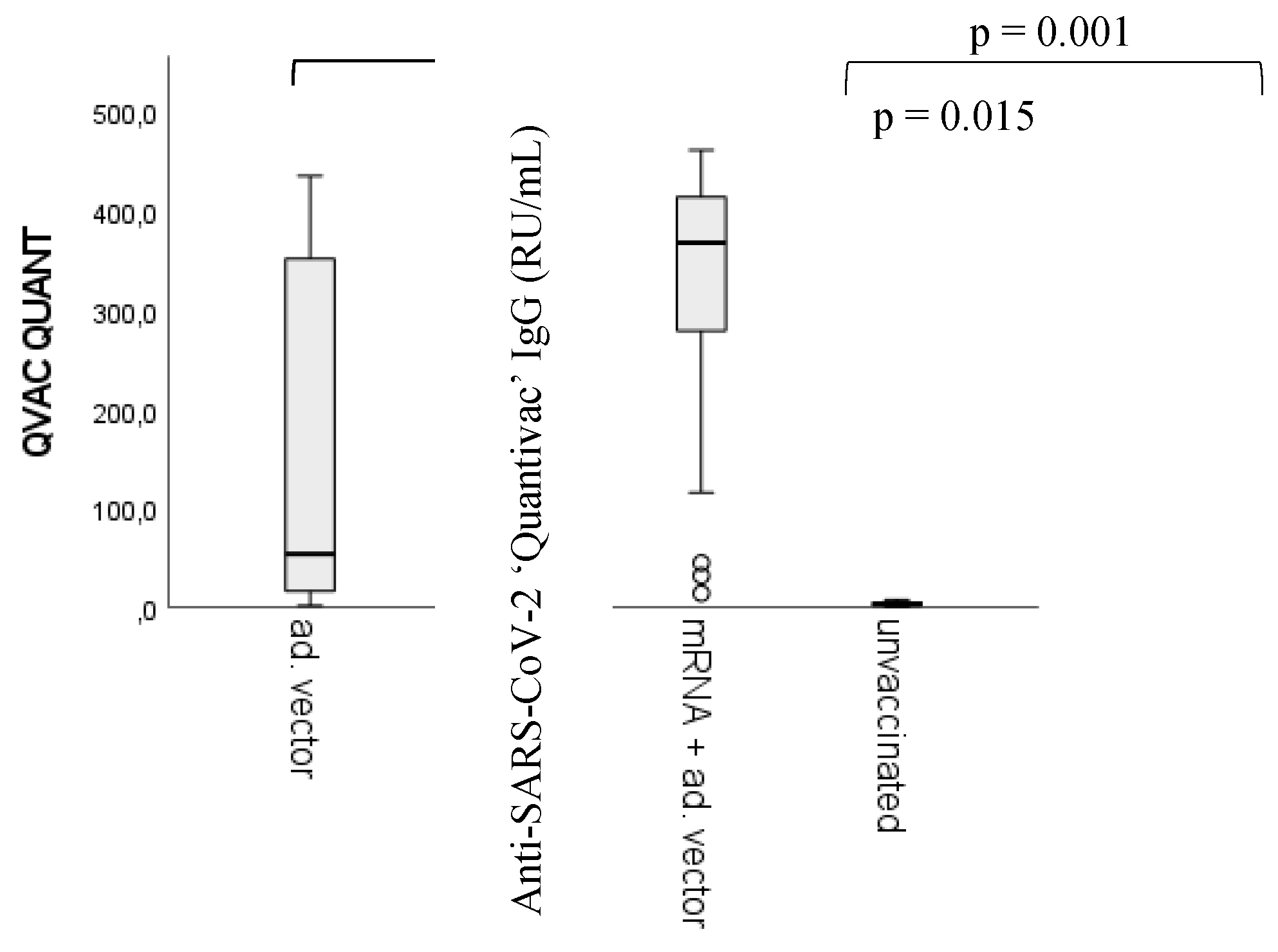
2.3. Differences in vaccine response by anti-SARS-CoV-2 vaccines
We would like to emphasize that the primary target of this article is not the ranking between vaccine types or different vaccination regimens based on their capacity to evoke humoral immune response relative to the correlates of protection [
41,
42,
43,
44,
45]. Nevertheless, for the comparison between the potential ‘side product’ nAAbs and viral antigen-triggered anti-SARS-CoV-2-specific ‘target’ antibodies, the measurement of anti-SARS-CoV-2 IgG titers was fundamental. We found significant differences between the unvaccinated (control) group and all the other groups (p < 0.001) (markers are not shown). Statistically significant differences have been found between the homologous adenoviral vector recipients and the heterologous vaccine regimen (mRNA/adenoviral vector vaccines) (p = 0.001), as well as between the mRNA and the adenoviral vector vaccine groups (p = 0.015).
Regarding ‘post-vaccination time’ (i.e., the number of days passed between sample taking and the last registered immunization episode) versus anti-viral antibody titers, statistically significant inverse correlation was found only in the heterologous (mRNA/adenoviral vector vaccines) group; post-vaccination time - vaccine induced anti-SARS-CoV-2 IgG titers; spearman's rho correlation coefficient <0.001.
Figure 3.
Differences in anti-SARS-CoV-2 IgG quantitative antibody titers between vaccination groups. Sample numbers: n unvaccinated= 93, n mRNA= 170, n Adenoviral vector= 25, n mRNA + adenoviral vector= 42. n total =330. statistically significant differences have been found between the unvaccinated (control) group and all the other groups (p < 0.001), between the homologous adenoviral vector and the heterologous (mRNA/adenoviral vector vaccines) vaccination groups (p = 0.001), and between the mRNA and the adenoviral vector vaccine groups (p = 0.015). For detailed sample numbers, please see
Table 2 in the Materials and Methods section.
Figure 3.
Differences in anti-SARS-CoV-2 IgG quantitative antibody titers between vaccination groups. Sample numbers: n unvaccinated= 93, n mRNA= 170, n Adenoviral vector= 25, n mRNA + adenoviral vector= 42. n total =330. statistically significant differences have been found between the unvaccinated (control) group and all the other groups (p < 0.001), between the homologous adenoviral vector and the heterologous (mRNA/adenoviral vector vaccines) vaccination groups (p = 0.001), and between the mRNA and the adenoviral vector vaccine groups (p = 0.015). For detailed sample numbers, please see
Table 2 in the Materials and Methods section.
2.4. Differences in nAAb (anti-CS) IgG levels between vaccination groups
When investigating the differences in nAAb (anti-CS) levels between different vaccination groups and the unvaccinated controls (
Figure 4 a), we found statistically significant differences in terms of IgG isotype nAAbs. Between the unvaccinated group and the adenoviral vector vaccine recipients (p = 0.032), the unvaccinated group and the heterologous vaccine regimen recipients (mRNA/adenoviral vector vaccines) (p = 0.002), as well as between the mRNA vaccine recipients and the heterologous group (p = 0.018). Interestingly, no statistical difference was detectable between the mRNA vaccine recipients and the unvaccinated individuals considering the nAAb (anti-CS Ig) levels.
Focusing on connections between anti-CS IgG levels and anti-viral qualitative (positive, negative) data (
Figure 4 b), we found that in the case of anti-SARS-CoV-2 IgG seropositivity (titer ≥11 RU/mL), also the nAAb levels proved to be significantly higher (p = 0.019).
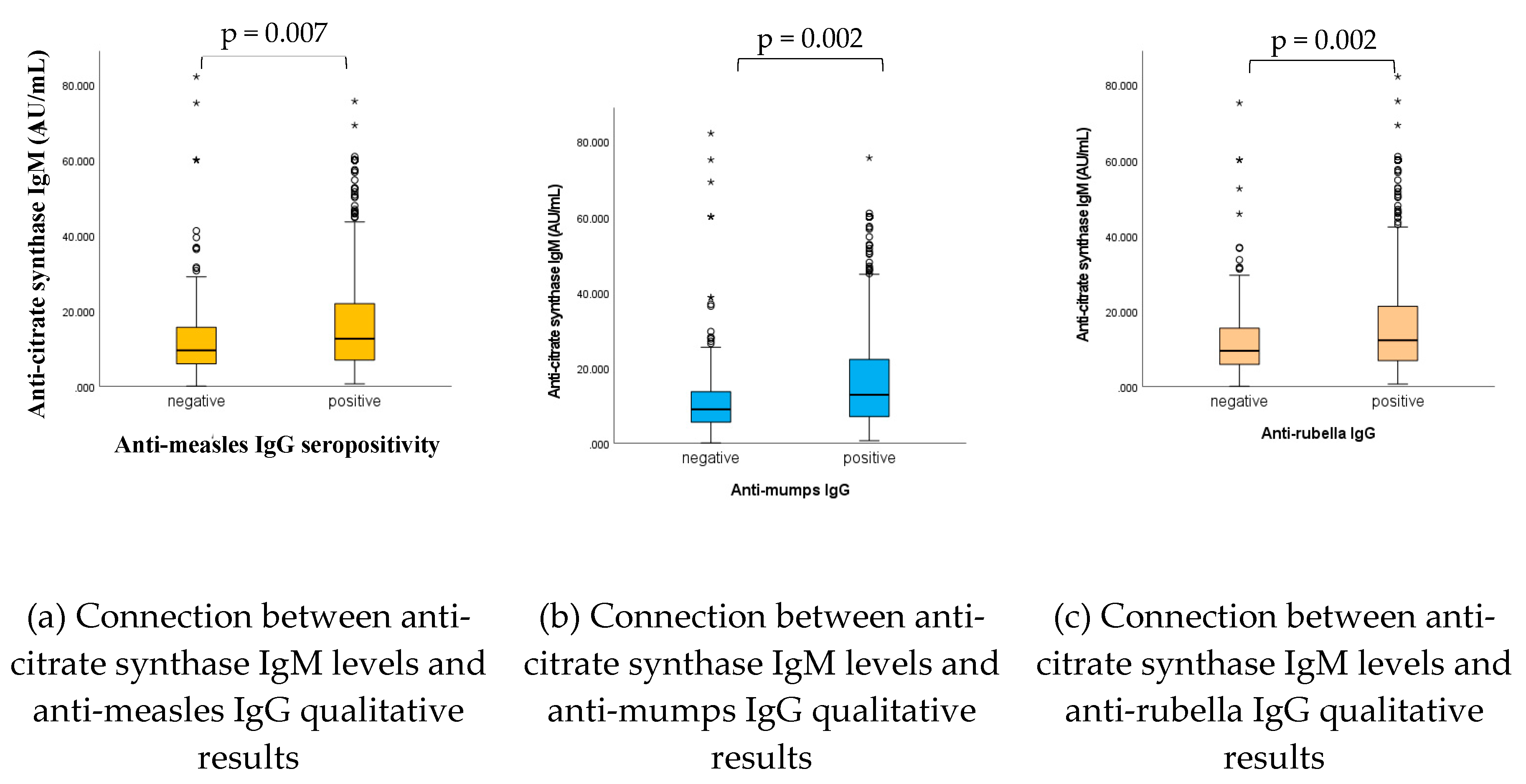
2.5. Connection between nAAb (anti-CS) IgM levels and anti-viral (MMR) humoral IgG levels
As shown in
Figure 5, and in accordance with previous findings [
32,
33,
35], statistically significant connections have been found between anti-CS IgM levels and anti-viral (measles, mumps, rubella) IgG qualitative (positive, negative) results; in case of adequate vaccine or infection-induced humoral antibody levels also the natural antibody IgG levels proved to be significantly higher (p= 0.007, p= 0.002, p= 0.002, for measles, mumps and rubella, respectively).
3. Discussion
Due to the evident burden of the COVID-19 pandemic on public health institutions, aggravated by the ongoing European refugee crisis, epidemiological concerns are re-emerging regarding MMR vaccination effectiveness and population immunity levels [
39,
51,
52]. Therefore, we updated our previous data regarding potentially inadequate humoral immunity levels in terms of anti-MMR IgG titers. In accordance with previous reports [
33,
37,
38,
40], present findings illustrate that potentially susceptible age groups might be present (also) in the Croatian population (
Figure 1). As far as serum antibody concentrations are relative to correlates of protection (46–50), this result underlines the critical significance of constant monitoring [
39].
Regarding anti-SARS-CoV-2-specific immunity, in the age group weighed comparison, all clusters performed sufficiently well, with seropositivity ratios ≥80%. In the context of population immunity, these findings are within an acceptable range, since the herd immunity threshold value for SARS-CoV-2 variants of concern (B.1.1.7 ‘Alpha’) is usually cited around 80%, while for newer variants (B.1.617.2 ‘Delta’) it may be higher [
50].
Considering the analysis by vaccination groups, our results are consistent with data from previous studies [
4]; anti-SARS-CoV-2 antibody levels were lower after homologous adenoviral vector or mRNA vaccination compared to the heterologous vector/mRNA vaccine regimen recipients.
The above described seroepidemiological analysis served as a cornerstone for understanding the dynamic interaction between nAAbs (anti-citrate synthase) and viral antigen-elicited (measles, mumps, rubella, SARS-CoV-2), promptly inducible antibodies. The main idea behind the current immunoserological study refers back to animal experiments; it has been described that exposure of laboratory rats to 'wild-like' conditions can partially reconstitute the nAAb repertoire [
28,
29,
30]. This practice of exposing laboratory animals to foreign antigens in order to manipulate their immune functions mimics the human medical practice of vaccination [
28,
29,
30]. The empirical evidence provided by human immunization experience regarding nonspecific effects (NSEs) of vaccines, also likely to be associated with the ‘by-product’ nAAbs [
53,
54,
55,
56], is no novelty either.
Numerous conflicting accounts exist in the scientific literature about anti-SARS-CoV-2 vaccine-triggered hyperstimulation of the immune system; some of these sustain that there is an elevated risk of vaccine-associated pathological auto-antibody formation [
1,
2,
3], others state that COVID-19 vaccines do not significantly foster the appearance of pathological autoantibodies commonly linked to the most prevalent autoimmune conditions [
4]. In contrast, various publications favor the idea that natural infection is the prominent inducer of autoantibody formation [
2,
5,
6]. Interestingly, the association between vaccination (or infection) and the non-pathological (natural) autoantibodies is much less defined [
8,
9,
10,
11].
This alternative approach of investigating associations between ‘off-target’
, non-pathological nAAbs and viral antigen-triggered ‘target’ antibodies led to the realization that the nAAb pool is prone to display a certain adaptability in response to pathogenic triggers. Interestingly, the nAAb anti-citrate synthase IgM was in statistically relevant positive connection with the persisting, decades-old anti-MMR antibodies, while the IgG isotype of the same anti-citrate synthase nAAb showed significant alignment with the recently acquired anti-SARS-CoV-2 specific (IgG) seropositivity of the vaccinees. Moreover, it seems that the heterologous vaccine regimen (mRNA/adenoviral vector vaccines) induced the top antiviral IgG levels, associated with the highest nAAb formation. At the same time, our results suggest that the homologous regimen of mRNA vaccines did not induce an elevated nAAb formation; no statistically significant difference has been found compared to unvaccinated controls.
4. Materials and Methods
4.1. Human serum samples
For measles, mumps and rubella antigen-induced (MMR vaccine or natural infection) humoral antibody measurements, we evaluated a total of 1739 serum samples (
Table 1) received from the Scientific Centre for Excellence for Personalized Health Care, Josip Juraj Strossmayer University of Osijek. These specimens were anonymous residual sera with known age and COVID-19 vaccination history (
Table 1).
Of this serum bank, we selected a sample multitude representative for each age group, with the inclusion criterion of at least one documented anti-SARS-CoV-2 vaccination within one year. (Due to limited research resources and high material purchase costs, not all the serum banks could be screened for anti-SARS-CoV-2 IgG.) Thus, 237 samples belonging to vaccinated individuals and 93 unvaccinated sera were selected (n total= 330) (
Table 2) for evaluation.
Table 2.
Age group based subdivision of samples used for anti-SARS-CoV-2 IgG and anti-citrate synthase IgG/M screening.
Table 2.
Age group based subdivision of samples used for anti-SARS-CoV-2 IgG and anti-citrate synthase IgG/M screening.
| Age group |
Number of vaccinated samples |
Total number of vaccinated samples
(vaccinated + unvaccinated) |
| 11-20 y |
8 |
8 |
| 21-30 y |
21 |
21 |
| 31-40 y |
30 |
50 |
| 41-50 y |
26 |
47 |
| 51-60 y |
50 |
67 |
| 61-70 y |
50 |
61 |
| 71-80 y |
30 |
49 |
| 81-90 y |
22 |
27 |
| TOTAL |
237 |
330 |
Vaccine regimen based subdivisions and post-vaccination times are represented in
Figure 6 and
Table 3.
For the investigation of potential connections between nAAb levels (anti-citrate synthase IgG, IgM) and immunization-induced humoral antibody titers, we performed anti-citrate synthase (CS) IgG, IgM measurements (Ethical license: 5726-PTE 2015-Pécs, Hungary, 5726/8216-PTE 2020-Pécs, Hungary, 035-01/19-01/14; 381-19-18-Osijek, Croatia) using the same serum bank.
4.2. Citrate Synthase (CS) IgG and IgM in-house ELISA Assays
As nAAbs, we used anti-citrate synthase (CS) antibodies; hence, CS is a pacemaker enzyme in the Krebs cycle and commonly used as a quantitative marker enzyme for the content of intact mitochondria [
57,
58]. As proven by scientific literature [
16,
32,
33,
34,
35,
59,
60,
61,
62,
63], CS-specific autoantibodies can be considered a prominent example of nAAbs.
The same assay protocol already used for previous reports [
32] has been applied. Accordingly, 96-well polystyrene plates (NUNC) were coated with CS from porcine heart (Sigma-Merck, Munich, Germany) in 0.1 M bicarbonate buffer, pH 9.6 [
16]. Following this, the saturation of nonspecific binding sites with our alternative, combined blocking buffer (0.5% polyvinyl alcohol solution combined with bovine gelatin solution, at a ratio of 2:1) at room temperature (RT) for 2 h was performed. After being washed with PBS + 0.05% Tween 20 (washing buffer; WB), sera were diluted (1:100 in WB) and incubated for 50 min at 37 °C. The secondary antibodies were incubated at 37 °C for 45 min (horseradish peroxidase-conjugated antihuman IgG and IgM, polyclonal rabbit antihuman (Agilent-Dako Santa Clara, CA, US). TMB substrate solution (Sigma-Merck, Munich, Germany) was used to visualize the HRP enzymatic reaction, and the reaction was stopped by 1 M H
2SO
4. Reading was performed at λ = 450/620 nm using the BEP2000 Advanced automated system. Results are expressed in absorbance (OD) and in quantitative (standard curve-based) results. For data comparison, results were handled as continuous, non-normally distributed integers, and the alterations of titers were considered.
4.3. Anti-SARS-CoV-2 Quantivac ELISA (IgG)
Commercial kits SARS-CoV-2 Quantivac ELISA (EI 2606-9601-10 G; EUROIMMUN Medizinische Labordiagnostika AG, Lübeck, Germany) have been applied as per manufacturer standard. The ELISA assay provides quantitative in vitro determination of human antibodies of the immunoglobulin class IgG against SARS-CoV-2 in serum. The immunoassay supports the diagnosis of SARS-CoV-2 infection; moreover, serological data obtained using this kit can be applied to collect epidemiological data as well as for antibody determination following vaccination with S1/RBD-based vaccines [
64]. Reagent wells are coated with recombinant S1 domain of the spike protein of SARS-CoV-2. In the first reaction step, diluted samples (1:101) are incubated in the first wells. In the case of positive samples, specific IgG antibodies will bind to the antigens. To detect bound antibodies, a second incubation is carried out using peroxidase enzyme-labeled anti-human IgG (enzyme conjugate), catalyzing a color reaction [
64]. For test evaluation, the standard curve from which the concentration of antibodies in the samples (expressed in relative units; RU) can be calculated is obtained by point-to-point plotting of the extinction readings measured for the 6 calibration sera. Calibration sera are in a linear correlation with the “First WHO International Standard for SARS-CoV-2”(NIBSC code 20/136), as stated in Manufacturer's Instructions for Use [
64]. Euroimmun recommends quantitative result interpretation as follows: result < 8 RU/mL: negative, 11 RU/mL > result ≥ 8 RU/mL: borderline, result ≥ 11 RU/mL: positive [
64].
4.4. Anti-measles, mumps, rubella (MMR) IgG in-house ELISA Assays
The assay protocol with the same assay execution guidelines thoroughly detailed in our previous publications [
65,
66] has been applied. Briefly; coating antigens: Bio-Rad PIP013 Measles virus, Edmonston strain (coating concentration: 2.8 µg/mL), Bio-Rad PIP014 Mumps virus, Enders strain (coating concentration: 3.0 µg/mL), Bio-Rad PIP044 Rubella virus, HPV-77 strain (coating concentration: 0.4 µg/mL). Antigens were dissolved in ELISA Coating Buffer (Bio-Rad BUF030) and applied on 96-well plates overnight at 4-6°C. Blocking was performed for ≥ 2 hours, RT, with our in-house developed, PVA-based blocking buffer. Standards: 3rd WHO International Standard for Anti-Measles (NIBSC code: 97/648), Anti-Mumps Quality Control Reagent Sample 1 (NIBSC code: 15/B664), Anti-Rubella Immunoglobulin 1st WHO International Standard Human (NIBSC code: RUBI-1-94). Human serum samples were applied in a final dilution of 1:200 after non-specific background reduction (incubation followed by centrifugation) using a matrix equalizing, mammalian protein-containing buffer (IgM Reducing Assay Diluent- Bio-Rad BUF038) diluted in washing buffer in a ratio of 2:1. Washing steps: 5-times, automated. Uniform incubation times for primary, secondary antibody binding, and substrate reaction: 3 x 20 minutes, 37°C. For the visualization of the immunological reaction, we used HRP-conjugated Dako polyclonal rabbit anti-human IgG (+ TMB). Automated assay execution, photometric reading (λ = 450/620 nm), and quantitative result calculation (4-parametric fitting) were performed using Siemens BEP 2000 Advance System.
4.5. Anti-measles, mumps, rubella commercial ELISA Assays
Commercial kits from EUROIMMUN Medizinische Labordiagnostika AG (Lübeck, Germany) have been used as a validated control parallel to in-house assay measurements. Assay execution has been performed as per manufacturer standard.
4.5.1. Anti-Measles Virus ELISA (IgG) (EI 2610-9601 G)
The commercial kit was used to provide quantitative in vitro determination for IgG class human antibodies against the measles virus in serum. The test kit contains microtiter strips, each with 8 break-off reagent wells coated with measles virus antigens (inactivated cell lysates of Vero cells infected with the ‘Edmonston’ strain of measles virus). In the first reaction step, diluted patient samples (1:101) are incubated in the wells. In the case of positive samples, specific IgG antibodies (also IgA and IgM) will bind to the antigens. To detect the bound antibodies, a second incubation is carried out using an enzyme-labeled anti-human IgG (enzyme conjugate), catalyzing a color reaction. The controls of the Anti-Measles Virus ELISA (IgG) were calibrated using the 3rd international standard serum NIBSC 97/648 (anti-measles and anti-polio virus serum, National Institute for Biological Standards and Control, Hertfordshire, England).
Quantitative evaluation: the standard curve from which the concentration of antibodies in the patient samples can be taken is obtained by point-to-point plotting of the extinction values measured for the 4 calibrators against the corresponding units (linear/linear). Euroimmun recommends quantitative result interpretation as follows: result < 200 IU/L: negative, 275 IU/L > result ≥ 200 IU/L: borderline, result ≥ 275 IU/L: positive
4.5.2. Anti-Mumps Virus ELISA (IgG) (EI 2630-9601 G)
The commercial kit was used to provide quantitative in vitro determination for IgG class human antibodies against measles virus in serum. The test kit contains microtiter strips, each with 8 break-off reagent wells coated with mumps antigens (inactivated cell lysates of Vero cells infected with the ‘Enders’ strain of mumps virus). In the first reaction step, diluted patient samples (1:101) are incubated in the wells. In the case of positive samples, specific Ig antibodies will bind to the antigens. To detect the bound antibodies, a second incubation is carried out using an enzyme-labeled anti-human IgG (enzyme conjugate) catalyzing a color reaction.
As no international reference serum exists for antibodies against the mumps virus, the calibration is performed in relative units (RU/ml).
Quantitative evaluation: the standard curve from which the concentration of antibodies in the patient samples can be taken is obtained by point-to-point plotting of the extinction readings measured for the 3 calibration sera against the corresponding units (linear/linear). Euroimmun recommends quantitative result interpretation as follows: result < 16 RU/mL: negative, 22 RU/mL > result ≥ 16 RU/mL: borderline, result ≥ 22 RU/mL: positive.
4.5.3. Anti-Rubella Virus ELISA (IgG) (EI 2590-9601 G)
The commercial kit was used to provide quantitative in vitro determination for IgG class human antibodies against measles virus in serum. The test kit contains microtiter strips, each with 8 break-off reagent wells coated with mumps antigens. (The antigen source is provided by inactivated cell lysates of Vero cells infected with the "HPV-77" strain of rubella virus.). In the first reaction step, diluted patient samples (1:101) are incubated in the wells. In the case of positive samples, specific Ig antibodies will bind to the antigens. To detect the bound antibodies, a second incubation is carried out using an enzyme-labeled anti-human IgG (enzyme conjugate) catalyzing a color reaction. Calibration is performed in international units (I) using the international reference preparation NIBSC RUBI-1-94 (Anti-Rubella Serum, 1* International Standard for Anti-Rubella Immunoglobulin, Human, National Institute for Biological Standards and Control, Hertfordshire, England).
Quantitative evaluation: the standard curve from which the concentration of antibodies in the patient samples can be taken is obtained by point-to-point plotting of the extinction values measured for the 4 calibrators against the corresponding units (linear/linear). Euroimmun recommends quantitative result interpretation as follows: result < 16 RU/mL: negative, 8 IU/mL > result ≥ 11 IU/mL: borderline, result ≥ 11 IU/mL: positive.
4.6. Statistical evaluation
For statistical evaluation (IBM SPSS), the Mann-Whitney U test was selected (α=0.05). Natural autoantibody (nAAb) levels have been treated as ordinal, non-normally distributed variables, while immunization-induced qualitative (positive, negative) results as grouping parameters. Simple bar chart based seropositivity evaluations were represented using MS Excel.
5. Conclusions
Growing evidence supports the connection between immunization and the associated dynamic change of the nAAb repertoire [
8,
9,
10,
11,
32,
33,
34,
35,
62]. Despite acting as covert contributors to the proper balance of the immune system [
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27], upon immune activation, nAAbs seem to feature limitedly dynamic adaptation capacities. Although their plasticity lags far behind that of the B2-B cell derived ‘target’ antibodies, there synergistic behavior is increasingly supported [
8,
9,
10,
11,
32,
33,
34,
35,
62]. This observation, however, raises another question; their potential feedback mechanisms and their ensuing silent influence on the effectiveness of immunization. In a broader context, we propose the possible role of nAAbs in the individual variability of vaccine responsiveness as a focus of further investigations, as well as the amendment of the underrated contribution of nAAbs to the complexity of the immunological network.
Author Contributions
Dávid Szinger: conceptualization, formal analysis, investigation, data curation, writing—original draft preparation, Tímea Berki: conceptualization, writing—review and editing, supervision, funding acquisition, Péter Németh: conceptualization, project administration, investigation, Szabina Erdő-Bonyár: methodology, validation, formal analysis, Diána Simon: writing—review and editing, Ines Drenjančević: supervision, resources, project administration, Senka Samardzic: supervision, resources, validation, data curation, Marija Zelić: methodology, data curation, validation, Magdalena Sikora: methodology, data curation, validation, Arlen Požgain: methodology, data curation, validation, Katalin Böröcz: conceptualization, formal analysis, investigation, data curation, writing—original draft preparation, supervision.
Funding
“Project TKP-2021-EGA-10 and TKP-2021-EGA-13 grants have been implemented with the support provided from the National Research, Development and Innovation Fund of Hungary, financed under the TKP-2021-EGA funding scheme.”
Informed Consent Statement
Patient consent was waived due to use of anonymous samples
Data Availability Statement
research data and investigation results are available upon request.
Acknowledgments
We would like to say special thanks to all contributors of Teaching Institute For Public Health of The Osijek-Baranja County for the elaborate sample collection. We would like to say special thanks to Euroimmun Hungary (Immunotrin) for the prompt shipping of ELISA kits and to EUROIMMUN Medizinische Labordiagnostika AG for the detailed, professional and user friendly manufacturer’s instructions.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AAb |
Autoantibody |
| AU |
Arbitrary unit (in-house elisas) |
| CS |
Citrate synthase |
| ELISA |
Enzyme-linked immunosorbent assay |
| HIT |
Herd immunity threshold |
| IgG |
Immunoglobulin G isotype |
| IgM |
Immunoglobulin M isotype |
| IU |
International units |
| MMR |
Measles, mumps, rubella |
| mRNA |
Messenger ribonucleic acid |
| n |
Number of samples |
| nAAb |
Natural autoantibody |
| OD |
Optical density |
| PVA |
Polyvinyl alchol |
| RBD |
Receptor binding domain |
| RT |
Room temperature |
| RU |
Relative Unit (Euroimmun ELISAs); quantitative measurement entity in linear correlation with the “First WHO International Standard for SARS-cov-2” |
| S1 |
S1 Subunit of the SARS-cov-2 Spike Protein |
| SARS-CoV-2 |
Severe acute respiratory syndrome coronavirus 2 |
| WB |
Washing buffer |
| y |
Years of age |
References
- Chen, Y.; Xu, Z.; Wang, P.; Li, X.M.; Shuai, Z.W.; Ye, D.Q.; Pan, H.F. New-Onset Autoimmune Phenomena Post-COVID-19 Vaccination. Immunology 2022, 165, 386–401. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Li, X.; Yang, D.; Chan, S.C.W.; Zhou, J.; Wan, E.Y.F.; Chui, C.S.L.; Lai, F.T.T.; Wong, C.K.H.; Chan, E.W.Y.; et al. Risk of Autoimmune Diseases Following COVID-19 and the Potential Protective Effect from Vaccination: A Population-Based Cohort Study. eClinicalMedicine 2023, 63, 102154. [Google Scholar] [CrossRef]
- Sacchi, M. .; Pelazza, C.; Bertolotti, M.; Agatea, L.; De Gaspari, P.; Tamiazzo, S.; Ielo, D.; Stobbione, P.; Grappiolo, M.; Bolgeo, T.; et al. The Onset of de Novo Autoantibodies in Healthcare Workers after MRNA Based Anti-SARS-CoV-2 Vaccines: A Single Centre Prospective Follow-up Study. Autoimmunity 2023, 56. [Google Scholar] [CrossRef]
- Thurm, C.; Reinhold, A.; Borucki, K.; Kahlfuss, S.; Feist, E.; Schreiber, J.; Reinhold, D.; Schraven, B. Homologous and Heterologous Anti-COVID-19 Vaccination Does Not Induce New-Onset Formation of Autoantibodies Typically Accompanying Lupus Erythematodes, Rheumatoid Arthritis, Celiac Disease and Antiphospholipid Syndrome. Vaccines 2022, 10. [Google Scholar] [CrossRef]
- Dobrowolska, K.; Zarębska-Michaluk, D.; Poniedziałek, B.; Jaroszewicz, J.; Flisiak, R.; Rzymski, P. Overview of Autoantibodies in COVID-19 Convalescents. J. Med. Virol. 2023, 95. [Google Scholar] [CrossRef]
- Taeschler, P.; Cervia, C.; Zurbuchen, Y.; Hasler, S.; Pou, C.; Tan, Z.; Adamo, S.; Raeber, M.E.; Bächli, E.; Rudiger, A.; et al. Autoantibodies in COVID-19 Correlate with Antiviral Humoral Responses and Distinct Immune Signatures. Allergy Eur. J. Allergy Clin. Immunol. 2022, 77, 2415–2430. [Google Scholar] [CrossRef]
- Fukushima, K.; Tsujino, K.; Futami, S.; Kida, H. Natural Autoantibodies in Chronic Pulmonary Diseases. Int. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef]
- Chen, C.; Amelia, A.; Ashdown, G.W.; Mueller, I.; Coussens, A.K.; Eriksson, E.M. Risk Surveillance and Mitigation: Autoantibodies as Triggers and Inhibitors of Severe Reactions to SARS-CoV-2 Infection. Mol. Med. 2021, 27. [Google Scholar] [CrossRef]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Béziat, V.; et al. Autoantibodies against Type I IFNs in Patients with Life-Threatening COVID-19. Science (80-. ). 2020, 370. [Google Scholar] [CrossRef]
- Wang, E.Y.; Mao, T.; Klein, J.; Dai, Y.; Huck, J.D.; Liu, F.; Zheng, N.S.; Zhou, T.; Israelow, B.; Wong, P.; et al. Diverse Functional Autoantibodies in Patients with COVID-19. medRxiv 2021. [Google Scholar] [CrossRef]
- Jeannet, R.; Descazeaud, A.; Daix, T.; Pauthier, H.; Pascal, V.; Hantz, S.; Cam, S. Le; Francois, B.; Feuillard, J.; Lafarge, X. De Novo Natural Anti-M Alloantibody Emergence in Severe Coronavirus Disease 2019. J. Infect. Public Health 2022, 15, 1455–1458. [Google Scholar] [CrossRef] [PubMed]
- Holodick, N.E.; Rodríguez-Zhurbenko, N.; Hernández, A.M. Defining Natural Antibodies. Front. Immunol. 2017, 8, 2–9. [Google Scholar] [CrossRef]
- Baumgarth, N.; Herman, O.C.; Jager, G.C.; Brown, L.E.; Herzenberg, L.A.; Chen, J. B-1 and b-2 Cell-Derived Immunoglobulin m Antibodies Are Nonredundant Components of the Protective Response to Influenza Virus Infection. J. Exp. Med. 2000, 192, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Grönwall, C.; Vas, J.; Silverman, G.J. Protective Roles of Natural IgM Antibodies. Front. Immunol. 2012, 3, 66. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, A.F.; Flores-Langarica, A.; Bobat, S.; Medina, C.C.D.; Cook, C.N.L.; Ross, E.A.; Lopez-Macias, C.; Henderson, I.R. B1b Cells Recognize Protective Antigens after Natural Infection and Vaccination. Front. Immunol. 2014, 5. [Google Scholar]
- Czömpöly, T.; Olasz, K.; Simon, D.; Nyárády, Z.; Pálinkás, L.; Czirják, L.; Berki, T.; Németh, P. A Possible New Bridge between Innate and Adaptive Immunity: Are the Anti-Mitochondrial Citrate Synthase Autoantibodies Components of the Natural Antibody Network? Mol. Immunol. 2006, 43, 1761–1768. [Google Scholar] [CrossRef] [PubMed]
- Baumgarth, N. B-1 Cell Heterogeneity and the Regulation of Natural and Antigen-Induced IgM Production. Front. Immunol. 2016, 7. [Google Scholar]
- Panda, S.; Zhang, J.; Tan, N.S.; Ho, B.; Ding, J.L. Natural IgG Antibodies Provide Innate Protection against Ficolin-Opsonized Bacteria. EMBO J. 2013, 32, 2905–2919. [Google Scholar] [CrossRef]
- Lacroix-Desmazes, S.; Mouthon, L.; Coutinho, A.; Kazatchkine, M.D. Analysis of the Natural Human IgG Antibody Repertoire: Life-Long Stability of Reactivities towards Self Antigens Contrasts with Age-Dependent Diversification of Reactivities against Bacterial Antigens. Eur. J. Immunol. 1995, 25, 2598–2604. [Google Scholar] [CrossRef]
- Boyden, S. V. Natural Antibodies and the Immune Response. Adv. Immunol. 1966, 5, 1–28. [Google Scholar] [CrossRef]
- Kaveri, S. V.; Silverman, G.J.; Bayry, J. Natural IgM in Immune Equilibrium and Harnessing Their Therapeutic Potential. J. Immunol. 2012, 188, 939–945. [Google Scholar] [CrossRef]
- Merbl, Y.; Zucker-Toledano, M.; Quintana, F.J.; Cohen, I.R. Newborn Humans Manifest Autoantibodies to Defined Self Molecules Detected by Antigen Microarray Informatics. J. Clin. Invest. 2007, 117, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Maddur, M.S.; Lacroix-Desmazes, S.; Dimitrov, J.D.; Kazatchkine, M.D.; Bayry, J.; Kaveri, S. V Natural Antibodies: From First-Line Defense Against Pathogens to Perpetual Immune Homeostasis. 2016. [CrossRef]
- Amital, H.; Shoenfeld, Y. NATURAL AUTOANTIBODIES, HERALDING, PROTECTING AND INDUCING AUTOIMMUNITY. Autoantibodies 2007, 7–12. [Google Scholar] [CrossRef]
- Kearney, J.F.; Patel, P.; Stefanov, E.K.; King, R.G. Natural Antibody Repertoires: Development and Functional Role in Inhibiting Allergic Airway Disease. Annu. Rev. Immunol. 2015, 33, 475–504. [Google Scholar] [CrossRef] [PubMed]
- Avrameas, S.; Alexopoulos, H.; Moutsopoulos, H.M. Natural Autoantibodies: An Undersugn Hero of the Immune System and Autoimmune Disorders-A Point of View. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Reyneveld, G.Ij.; Savelkoul, H.F.J.; Parmentier, H.K. Current Understanding of Natural Antibodies and Exploring the Possibilities of Modulation Using Veterinary Models. A Review. Front. Immunol. 2020, 11, 2139. [Google Scholar] [CrossRef]
- Devalapalli, A.P.; Lesher, A.; Shieh, K.; Solow, J.S.; Everett, M.L.; Edala, A.S.; Whitt, P.; Long, R.R.; Newton, N.; Parker, W. Increased Levels of IgE and Autoreactive, Polyreactive IgG in Wild Rodents: Implications for the Hygiene Hypothesis. Scand. J. Immunol. 2006, 64, 125–136. [Google Scholar] [CrossRef]
- Beinart, D.; Ren, D.; Pi, C.; Poulton, S.; Holzknecht, Z.E.; Swanson, C.; Parker, W. Immunization Enhances the Natural Antibody Repertoire. EXCLI J. 2017, 16, 1018–1030. [Google Scholar] [CrossRef]
- Pi, C.; Allott, E.H.; Ren, D.; Poulton, S.; Ryan Lee, S.Y.; Perkins, S.; Everett, M. Lou; Holzknecht, Z.E.; Lin, S.S.; Parker, W. Increased Biodiversity in the Environment Improves the Humoral Response of Rats. PLoS One 2015, 10. [Google Scholar] [CrossRef]
- Zeng, C.; Zhang, C.; Walker, P.G.; Dong, Y. Formulation and Delivery Technologies for MRNA Vaccines. Curr. Top. Microbiol. Immunol. 2022, 440, 71. [Google Scholar] [CrossRef]
- Böröcz, K.; Kinyó, Á.; Simon, D.; Erdő-Bonyár, S.; Németh, P.; Berki, T. Complexity of the Immune Response Elicited by Different COVID-19 Vaccines, in the Light of Natural Autoantibodies and Immunomodulatory Therapies. Int. J. Mol. Sci. 2023, 24. [Google Scholar] [CrossRef]
- Simon, D.; Balogh, P.; Erdő-Bonyár, S.; Böröcz, K.; Minier, T.; Czirják, L.; Berki, T. Increased Frequency of Activated Switched Memory B Cells and Its Association With the Presence of Pulmonary Fibrosis in Diffuse Cutaneous Systemic Sclerosis Patients. Front. Immunol. 2021, 12, 1–8. [Google Scholar] [CrossRef]
- Hayden, Z.; Erdő-Bonyár, S.; Bóné, B.; Balázs, N.; Bodó, K.; Illes, Z.; Berki, T.; Simon, D. Toll-Like Receptor Homolog CD180 Expression Is Diminished on Natural Autoantibody-Producing B Cells of Patients with Autoimmune CNS Disorders. J. Immunol. Res. 2021, 2021. [Google Scholar] [CrossRef]
- Böröcz, K.; Simon, D.; Erdő-Bonyár, S.; Kovács, K.T.; Tuba, É.; Czirják, L.; Németh, P.; Berki, T. Relationship between Natural and Infection-Induced Antibodies in Systemic Autoimmune Diseases (SAD): SLE, SSc and RA. Clin. Exp. Immunol. 2020. [Google Scholar] [CrossRef]
- Böröcz, K.; Markovics, Á.; Zsuzsanna, C.; Joseph, N.; Timea, B.; Németh, P. Imported Infections versus Herd Immunity Gaps ; a Didactic Demonstration of Compartment Models through the Example of a Minor Measles Outbreak In. Southeast. Eur. Med. J. 2021, 5, 1–16. [Google Scholar]
- Böröcz, K.; Samardžić, S.; Drenjančević, I.; Markovics, Á.; Berki, T.; Németh, P. Dynamic Features of Herd Immunity: Similarities in Age-Specific Anti-Measles Seroprevalence Data between Two Countries of Different Epidemiological History. J. Clin. Med. 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Borcˇic´1, B.; Borcˇic´1, B.; Mazˇuran, R.M.; Kaic´1, B.K. Immunity to Measles in the Croatian Population. Infect. Dis. (Auckl).
- Drenjančević, I.; Samardžić, S.; Stupin, A.; Borocz, K.; Nemeth, P.; Berki, T. Measles Vaccination and Outbreaks in Croatia from 2001 to 2019; A Comparative Study to Other European Countries. Int. J. Environ. Res. Public Health 2022, 19. [Google Scholar] [CrossRef]
- Brzovic, M.; Juretic, K.B.; Jurcev-Savicevic, A.; Mihojevic, L.; Nonkovic, D.; Rizvan, P.; Petrovic, M.V.; Tonkic, M.; Kaic, B.; Babic-Erceg, A.; et al. Measles Cases in Split-Dalmatia County (a Croatian Tourist Region), in May–July 2019: Outbreak Report and Lessons Learnt. Eur. J. Public Health 2022, 32, 948–954. [Google Scholar] [CrossRef]
- Plotkin, S.A.; Gilbert, P.B. Nomenclature for Immune Correlates of Protection after Vaccination. Clin. Infect. Dis. 2012, 54, 1615–1617. [Google Scholar] [CrossRef]
- Plotkin, S.A. Correlates of Protection Induced by Vaccination. Clin. Vaccine Immunol. 2010, 17, 1055–1065. [Google Scholar] [CrossRef]
- 43. Correlates of Vaccine-Induced Protection: Methods and Implications Immunization, Vaccines and Biologicals.
- Plotkin, S.A. Complex Correlates of Protection after Vaccination. Clin. Infect. Dis. 2013, 56, 1458–1465. [Google Scholar] [CrossRef] [PubMed]
- Haralambieva, I.H.; Kennedy, R.B.; Ovsyannikova, I.G.; Schaid, D.J.; Poland, G.A. Current Perspectives in Assessing Humoral Immunity after Measles Vaccination. Expert Rev. Vaccines 2019, 18, 75–87. [Google Scholar] [PubMed]
- Pandey, A.; Galvani, A.P. Exacerbation of Measles Mortality by Vaccine Hesitancy Worldwide. 2023. [CrossRef]
- Plans-Rubió, P. Are the Objectives Proposed by the Who for Routine Measles Vaccination Coverage and Population Measles Immunity Sufficient to Achieve Measles Elimination from Europe? Vaccines 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Plans-Rubió, P. Low Percentages of Measles Vaccination Coverage with Two Doses of Vaccine and Low Herd Immunity Levels Explain Measles Incidence and Persistence of Measles in the European Union in 2017–2018. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1719–1729. [Google Scholar] [CrossRef] [PubMed]
- Plans-Rubió, P. Why Does Measles Persist in Europe? Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1899–1906. [Google Scholar] [CrossRef]
- Bolotin, S.; Wilson, S.; Murti, M. Achieving and Sustaining Herd Immunity to SARS-CoV-2. Cmaj 2021, 193, E1089. [Google Scholar] [CrossRef]
- Holt, E. Experts Warn over Potential for Measles in Ukraine. Lancet (London, England) 2023, 401, 719. [Google Scholar] [CrossRef]
- Loboda, A.; Smiyan, O.; Popov, S.; Petrashenko, V.; Zaitsev, I.; Redko, O.; Zahorodnii, M.; Kasyan, S. Child Health Care System in Ukraine. Turkish Arch. Pediatr. 2020, 55, S98–S104. [Google Scholar] [CrossRef]
- Benn, C.S.; Netea, M.G.; Selin, L.K.; Aaby, P. A Small Jab – a Big Effect: Nonspecific Immunomodulation by Vaccines. Trends Immunol. 2013, 34, 431–439. [Google Scholar] [CrossRef]
- Aaby, P.; Samb, B.; Simondon, F.; Seck, A.M.C.; Knudsen, K.; Whittle, H. Non-Specific Beneficial Effect of Measles Immunisation: Analysis of Mortality Studies from Developing Countries. BMJ 1995, 311, 481–485. [Google Scholar] [CrossRef]
- Aaby, P.; Benn, C.S. Developing the Concept of Beneficial Non-Specific Effect of Live Vaccines with Epidemiological Studies. Clin. Microbiol. Infect. 2019, 25, 1459–1467. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Soares-Weiser, K.; López-López, J.A.; Kakourou, A.; Chaplin, K.; Christensen, H.; Martin, N.K.; Sterne, J.A.C.; Reingold, A.L. Association of BCG, DTP, and Measles Containing Vaccines with Childhood Mortality: Systematic Review. BMJ 2016, 355, 5170. [Google Scholar] [CrossRef] [PubMed]
- Eigentler, A.; Draxl, A.; Wiethüchter, A. Laboratory Protocol : Citrate Synthase A Mitochondrial Marker Enzyme. 2012, 11, 1–11.
- Leek, B.T.; Mudaliar, S.R.D.; Henry, R.; Mathieu-Costello, O.; Richardson, R.S. Effect of Acute Exercise on Citrate Synthase Activity in Untrained and Trained Human Skeletal Muscle. Am. J. Physiol. - Regul. Integr. Comp. Physiol. 2001, 280, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Petrohai, Á.; Nagy, G.; Bősze, S.; Hudecz, F.; Zsiros, E.; Paragh, G.; Ny�r�dy, Z.; N�meth, P.; Berki, T. Detection of Citrate Synthase-Reacting Autoantibodies after Heart Transplantation: An Epitope Mapping Study. Transpl. Int. 2005. [Google Scholar] [CrossRef]
- Nyárády, Z.; Czömpöly, T.; Bosze, S.; Nagy, G.; Petrohai, Á.; Pál, J.; Hudecz, F.; Berki, T.; Németh, P. Validation of in Silico Prediction by in Vitro Immunoserological Results of Fine Epitope Mapping on Citrate Synthase Specific Autoantibodies. Mol. Immunol. 2006, 43, 830–838. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Lu, Y.P.; Yang, L.; Luo, G.H.; Song, J.; Li, Y.P. Detection of Citrate Synthase Autoantibodies in Rats with Chronic Allograft Nephropathy. Transplant. Proc. 2009, 41, 4366–4368. [Google Scholar] [CrossRef]
- Hau, L.; Tényi, T.; László, N.; Kovács, M.Á.; Erdö-Bonyár, S.; Csizmadia, Z.; Berki, T.; Simon, D.; Csábi, G. Anti-Neuronal Autoantibodies (Cell Surface and Onconeural) and Their Association With Natural Autoantibodies in Synthetic Cannabinoid-Induced Psychosis. Front. Psychiatry 2022, 13, 1–7. [Google Scholar] [CrossRef]
- Simon, D.; Gilicze, O.; Farkas, N.; Najbauer, J. Correlation of Natural Autoantibodies and Cardiovascular Disease-Related Anti-Bacterial Antibodies in Pericardial Fluid of Cardiac Surgery Patients. 2018, 55–63. [CrossRef]
- EUROIMMUN Anti-SARS-CoV-2 ELISA IgG / Anti-SARS-CoV-2 QuantiVac ELISA (IgG). Products 2020, 31–32.
- Böröcz, K.; Csizmadia, Z.; Markovics; Farkas, N. ; Najbauer, J.; Berki, T.; Németh, P. Application of a Fast and Cost-Effective “three-in-One” MMR ELISA as a Tool for Surveying Anti-MMR Humoral Immunity: The Hungarian Experience. Epidemiol. Infect. 2020, 148. [Google Scholar] [CrossRef]
- Böröcz, K.; Csizmadia, Z.; Markovics, Á.; Mészáros, V.; Farkas, K.; Telek, V.; Varga, V.; Maloba, G.O.; Bodó, K.; Najbauer, J.; et al. Development of a Robust and Standardized Immunoserological Assay for Detection of Anti-Measles IgG Antibodies in Human Sera. J. Immunol. Methods 2019, 464, 1–8. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).