1. Introduction
The awareness about global climate change has notably increased with the increased environmental pollution. Global effort is in share to push the environmental protection, economic viability, and social quality pillars of sustainability in various fields. Analytical chemistry plays a dual role in sustainability. On the one hand, it can aid in monitoring soil, air, and water pollutants. On the other hand, it depends on using many (hazardous) chemicals, solvents, and reagents. In 1998, Paul Anastas and John Warner introduced green analytical chemistry (GAC) to reduce the hazardous impact on the environment and the operator [
1]. Conducting environmentally benign chemical analysis and thus greening the analytical laboratories is still far from being achieved. Improving analytical chemistry’s impact on the environment and humans still demand further effort towards better chemical and pharmaceutical sustainability. Millions of analytical tests are conducted on a routine daily basis in pharmaceutical and chemical laboratories worldwide. Chromatographic analysis is a big consumer of non-green solvents. Their ecological impact is high, utilizing high energy resources and producing tons of environmentally hazardous waste [
2]. So far, reversed-phase high-performance liquid chromatography (RP-HPLC) is the most widely utilized analytical tool. Nowadays, there is an exponential increasing trend of applying green analytical chemistry. For example, analytical chemistry regulatory bodies increased sustainability awareness to ensure operators' safety and environmental preservation. This should consider the steps starting from sample collection to waste treatment [
3].
The Green Chemistry principle of 12 was formulated by Galuszka et al. to consider different aspects that can make a method greener [
4]. Four assessment tools, namely Eco-Scale [
5], National Environmental method (NEMI) [
6,
7,
8], Green Analytical Procedure Index (GAPI) [
9], and Analytical GREEnness Metric Approach and Software (AGREE) [
10] were published and used to evaluate the greenness of analytical methods. AGREE is the most useful, as being the quantitative and more representative assessment of the greenness of analytical procedures. It is open-source software that considers the 12 principles of GAC [
10]
White analytical chemistry (WAC) was introduced with a more holistic view to avoid increasing greenness at the expense of method performance and functionality. Thus, it is a more global assessment that considers additional criteria. A balance between the greenness and usefulness of the analytical method should be achieved by considering analytical efficiency and practical and economic aspects, as can be represented by white analytical chemistry. This balance remains a challenge in many cases. Analysts should green the analytical methods without compromising the quality and keep the method useful for its intended application [
11].
The RGB12 tool is a good assessment tool to evaluate the whiteness of the analytical method. It is based on analytical efficiency (R), ecological (G), and practical economic efficiency (B), where R and B are not captured by any of the available green assessment tools. Analytical efficiency is represented by validation parameters such as limit of detection (LOD), accuracy, and precision, while practical and economic efficiency (B) express productivity from both aspects. White was then obtained by mixing the three colors, red, green, and blue, to show a score depending on the saturation degree of each color. A free access Excel sheet is available to calculate the RGB12 [
12]. The RGB model includes the 12 WAC principles, each involving four principles. In practice, R and B should have priority over G to have a method sufficient for the intended application. However, the 12 principles may have different weights and impacts in practice. Coherency and synergy of analytical performance ecological and practical aspects give white analytical chemistry. Thus, in analytical chemistry, the aiming for sustainable development is, in principle aiming for the white method.
Various actions can be taken based on the principles of GAC and WAC to make the method greener and whiter. At present, there are several approaches for greening the analytical method via organic solvent replacement, including the use of superheated water [
13,
14], ethanol [
15,
16,
17,
18,
19], propylene carbonate [
20,
21,
22], acetone [
23,
24], and micellar [
25,
26,
27,
28,
29]. These greener solvents have advantages and limitations when applied as mobile phase components in chromatographic applications. Replacing classical mobile phase solvents with eco-friendly, environmentally benign solvents remains an excellent factor in making analytical methods greener and, thus, possibly whiter [
30].
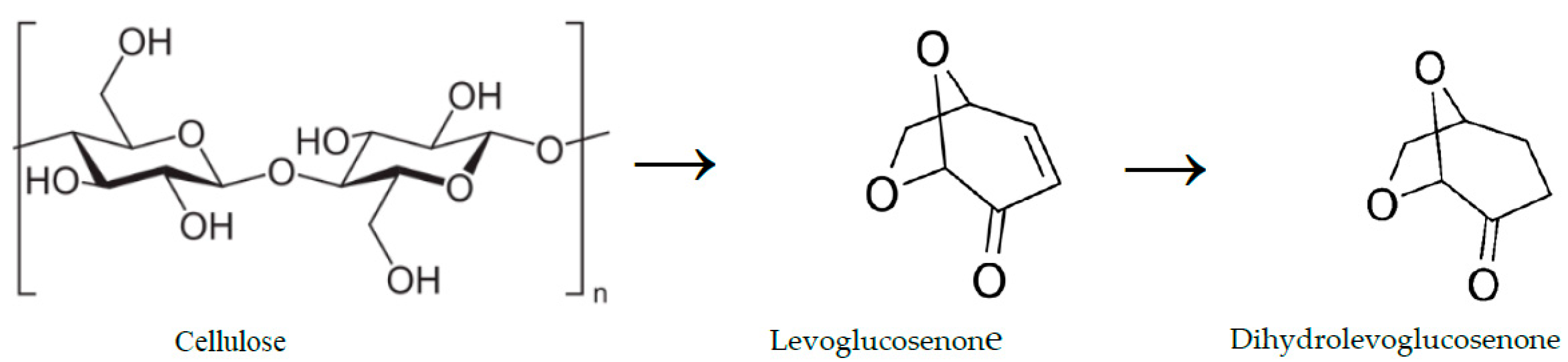
Dihydrolevoglucsenone (C
6H
8O
3), also known as Cyrene
TM, is a bicyclic ketone containing an acetal functional group that is produced in two steps from cellulose biomass through levoglucosenone as presented in
Figure 1. Cyrene is biodegradable, non-toxic, and non-mutagenic, in addition to other physicochemical properties of Cyrene, making it a green polar aprotic alternative solvent replacing toxic organic solvents such as dimethyl formamide (DMF), N-methyl pyrrolidone (NMP), N, N-dimethylacetamide (DMAc), and dimethyl sulfoxide (DMSO) in peptide chemistry, material chemistry and organic synthesis [
31,
32,
33,
34,
35].
Metronidazole (1-β-hydroxyethyl-2-methyl-5-nitroimidazole), (MET) is a nitroimidazole antibiotic used in clinical practice since 1959. Metronidazole is active against a wide range of anaerobic bacteria and protozoans [
36,
37,
38]. Moxifloxacin (1-cyclopropyl-7-(2,8-diazo bicyclo [4.3.0.] nonane)-6-fluoro-8-methoxy-1,4-dihydro-4-oxo-3-quinolone carboxylic acid), (MOX) is a fluoroquinolone antibacterial with a methoxy group in the C-8 position. Moxifloxacin has antibacterial activity against a broad spectrum of Gram-positive and Gram-negative organisms [
39,
40]. The structural formulae of metronidazole and moxifloxacin are shown in
Figure 2. Several RP-HPLC methods were reported for the assay of metronidazole and moxifloxacin alone or in combination with other drugs using either acetonitrile or methanol in the mobile phase [
41,
42,
43,
44,
45,
46,
47]. The simultaneous HPLC determination of metronidazole and moxifloxacin developed by El-Yazbi et al. [
48] using acetonitrile was considered as a reference method for further comparison.
The main aim of this research was to consider the potential applicability of Cyrene as a mobile phase component in simultaneous chromatographic separation and determination of metronidazole and moxifloxacin. The authors aimed to introduce an innovative RP-HPLC method involving the first-time use of Cyrene as a mobile phase component. Cyrene is investigated as a possible greener chromatographic mobile phase co-eluent together with ethanol. Compatibility of Cyrene as mobile phase component, UV-absorptivity and cut-off value, eluotropic effect, viscosity, applicability with high flow rates, effect on column performance, selectivity, and other separation aspects is studied. Furthermore, the role of stationary phase morphologies in enhancing green chemistry using monolithic silica columns and the possible application of high flow rates is also considered. Evaluation of the greenness of the developed method using AGREE metric and the whiteness using the RGB12 tool is conducted as well.
2. Results
2.1. Evaluation of Cyrene as co-Eluent in the Mobile Phase
Initially, using UV-Vis spectrophotometry, Cyrene's UV cut-off wavelength was determined to be 350 nm. Therefore, moxifloxacin and metronidazole were selected as model analytes with wide-range absorptivity below and above 350 nm to investigate Cyrene as a mobile phase component. To show the limitations and advantages of Cyrene over classical organic solvents, a comparison of properties to other selected green and non-green reversed-phase solvents has been conducted as listed in
Table 1.
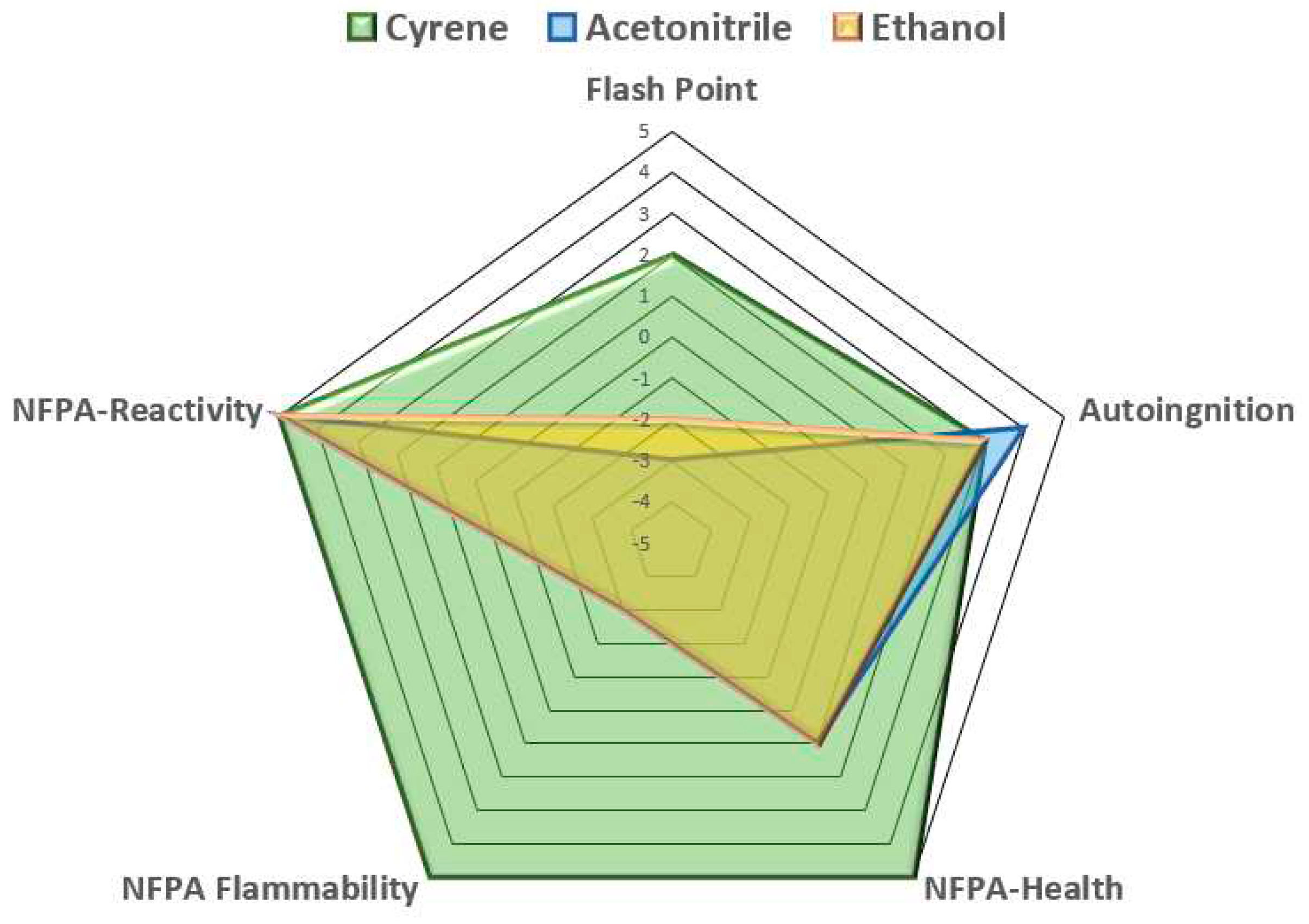
The fire safety of Cyrene was compared to that of acetonitrile and ethanol, as shown in
Table 2. A fire safety chart has also been generated to show Cyrene’s superiority as a greener solvent over ethanol, as presented in
Figure 3.
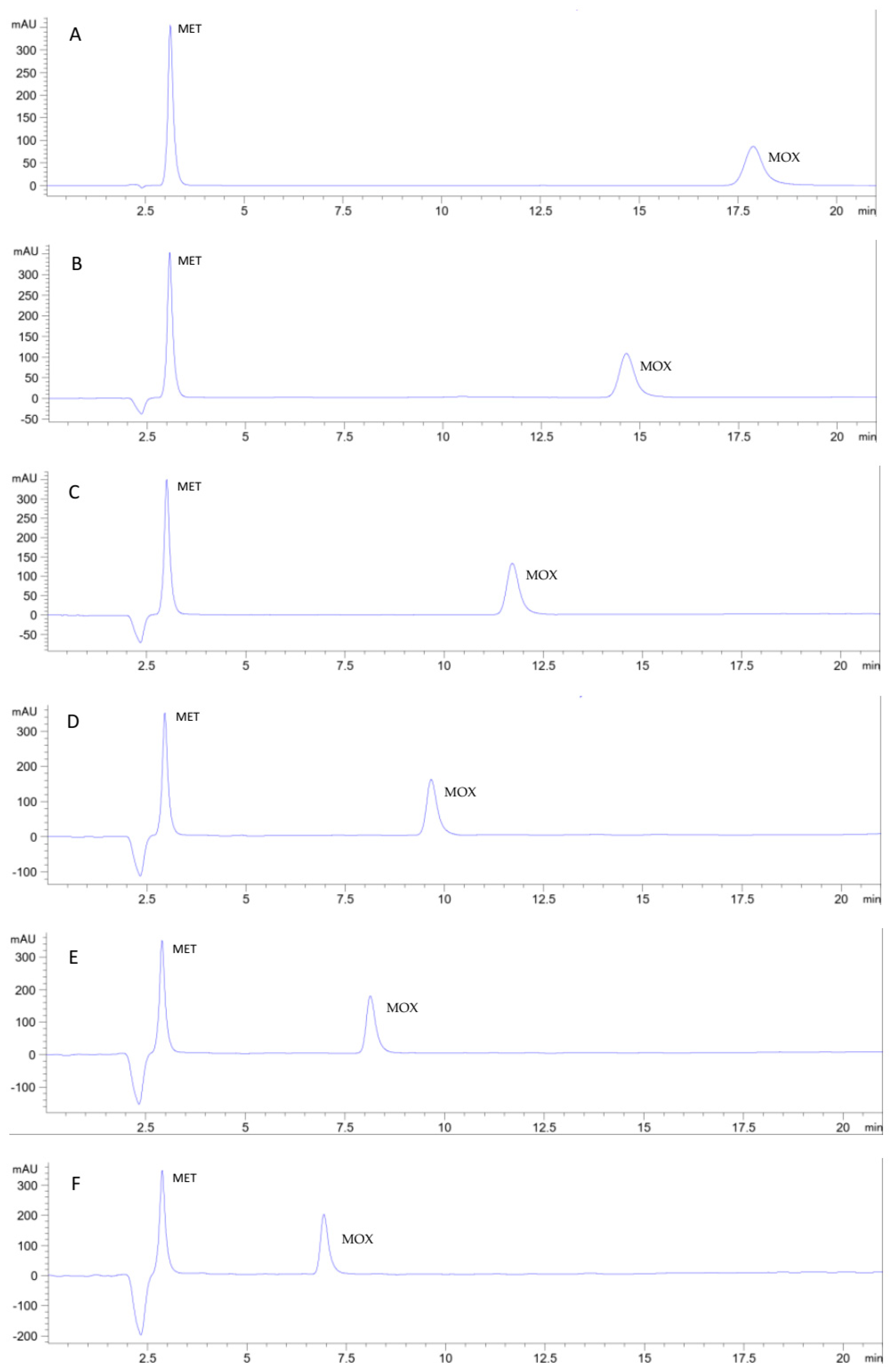
To study the potential use of Cyrene as a mobile phase component in RP-HPLC, a method using ethanol and sodium acetate buffer was first developed as a green ethanol-based reference method (
Figure 4A) in replacement of the reported acetonitrile-based non-green method [
48] for the simultaneous determination of the two analytes. Further reduction of the analysis time of the developed ethanol-based method may be possible by increasing the percentage of ethanol in the mobile phase. However, we aimed to reduce the consumption of ethanol and alternatively reduce the analysis time including the greener solvent Cyrene as co-eluent in the mobile phase.
Cyrene was added as co-eluent to the ethanol: 0.1 M sodium acetate buffer pH 4.25 (13:87, v/v) mobile phase, and the effects on elution, detectability of the two analytes of the detected analytes, selectivity, and back pressure were monitored and reported. A successive increase in Cyrene percentage in the mobile phase with a 2% step substitution of buffer was found to increase the elution power of the mobile phase in synergy with ethanol as two organic eluents and thus reduce the total analysis time. No change in selectivity was observed. The effect on detectability was reported by monitoring the absorbance in the 200-400 nm range. The two analytes were found to be detectable at 320 nm (a value below the cut-off value of Cyrene) but with higher noise. Best detectability was obtained at 350 nm.
A Cyrene mobile phase percentage of up to 8% reduced run time while preserving a smooth baseline with good detectability. At this percentage, the increased backpressure due to the higher density of Cyrene was tolerable. A proportion of 10% Cyrene in the mobile phase resulted in an even shorter analysis time. However, the peak of metronidazole was eluted very close to the solvent peak with a relatively noisy baseline. Therefore, the mobile phase consisting of Cyrene: ethanol: 0.1 M sodium acetate buffer pH 4.25 (8:13:79, v/v/v) was selected as the best suitable mobile phase system for separating and simultaneously determining metronidazole and moxifloxacin. The developed method was validated according to ICH guidelines [
55]. Representative chromatograms for metronidazole and moxifloxacin elution using different percentages of Cyrene in the mobile phase are shown in
Figure 4.
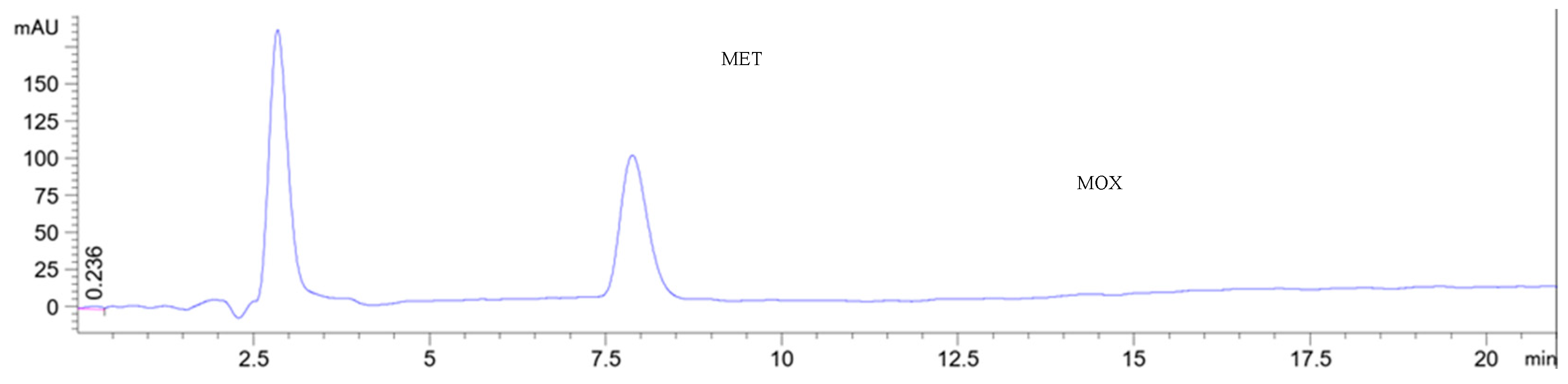
For the selected Cyrene-based RP-HPLC method, the observed negative solvent peak has been avoided by applying the mobile phase as the sample solvent, as shown in
Figure 5.
Cyrene did not negatively impact column performance when a pre- and post-run with ethanol: 0.1 M sodium acetate buffer pH 4.25 mobile phases were compared for the elution of metronidazole and moxifloxacin. No significant changes in retention times, peak areas, and theoretical plates were obtained. However, the long-term effect on column half-life needs further investigation. Cyrene has a higher density than all common green and non-green RP-HPLC solvents. However, the used mobile phases consisting of Cyrene: ethanol: sodium acetate buffer in different ratios generated acceptable backpressures as shown in
Table 3, which would work with all classical conventional columns. Using monolithic silica columns with a bimodal pore structure and high porosity makes this issue less significant.
2.2. Greenness and Whiteness Assessments of the Methods
The greenness of the three methods with the elution conditions listed in
Table 4 was evaluated and compared using the quantitative greenness assessment tool AGREE. Greenness profiles are presented in
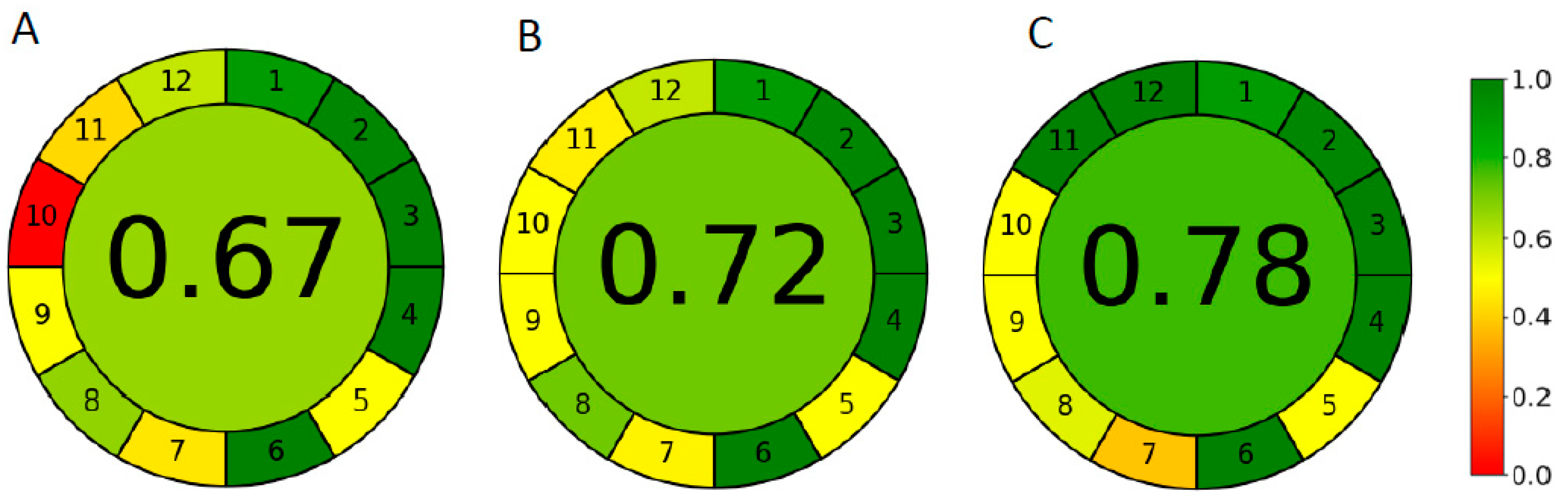
Figure 6. Results showed that the developed Cyrene/ethanol-based method has superior greenness profiles.
Furthermore, whiteness of the Cyrene-based method was evaluated and compared with the reference acetonitrile method using the RGB12 tool. The results presented in
Figure 7 show that the Cyrene-based method is regarded as a whiter analytical method mainly because of the better green and blue components without compromising the red component of the evaluation matrix.
2.3. Potential of Heated/Superheated Cyrene Chromatography vs. Heated/Superheated Water Chromatography
The best selected successfully developed Cyrene containing RP-HPLC method at ambient temperature and a flow rate of 1 mL/min for the elution of the metronidazole moxifloxacin mixture with the mobile phase Cyrene: ethanol: sodium acetate buffer pH 4.25 (8:13:79) was then tested at higher temperature 35 °C and 50 °C. Increasing the temperature from ambient 25 °C to 35 °C and then to 50 °C at the flow rate of 1 mL/min reduced the analysis time and the back pressure [
56,
57,
58,
59]. Chromatograms showing elution at high temperatures are presented in
Figure 8 A, and B. Increasing the temperature is supposed to decrease the dielectric constant and strengthen the elution power while decreasing the viscosity and, thus, the generated backpressure. Keeping in mind the higher energy consumption associated with higher column temperature will negatively impact the greenness and, thus the whiteness of the method. Conducting the chromatographic analysis at an increased flow rate of 2 mL/min and a high column temperature of 50, °C resulted in a significant reduction in the retention time with a relatively noisy baseline but an acceptable backpressure of 130 bars. A chromatogram showing elution at higher temperatures of 50 °C and a higher flow rate of 2 mL/min is presented in
Figure 8C.
3. Materials and Methods
3.1. List of Chemicals
Reference material of metronidazole was obtained from Caelo (Hilden, Germany), and moxifloxacin was obtained from Sigma-Aldrich Chemie GmbH, (Germany)
Ethanol >99.7 HPLC grade was obtained from VWR International S.A.S. (France)
glacial acetic acid for HPLC was obtained from Applichem (Darmstadt, Germany)
Cyrene™ and sodium acetate were obtained from Merck (Darmstadt, Germany) Hydrochloric acid analytical grade was obtained from Fisher Scientific (Loughborough, UK)
3.2. Buffer and Sample Preparation
Sodium acetate buffer 0.1 M pH 4.25 was prepared by adding 5.772 g of sodium acetate and 1.778 g of acetic acid to 800 ml bidistelled water. The pH was adjusted to 4.25 by adding 10N HCl, and the volume was completed to 1 L by bidistelled water. Stock solutions of 500 µg/mL of moxifloxacin and 1000 µg/mL metronidazole were made for prepration of calibrants and quality control samples .
3.3. HPLC Analysis
An Agilent 1260 (Agilent Technologies GmbH, Waldbronn, Germany) equipment with quaternary pump (G1311B), autosampler (G1329B), and diode array detector (G1315D) was used. Chromolith Performance RP-18e (10 x 4.6 mm) column (Merck, Darmstadt, Germany) was used.
4. Conclusions
In this study, Cyrene was applied for the first time as a constituent of the mobile phase in chromatography. Cyrene showed strong potential as a chromatographic organic modifier component in the mobile phase. The present study developed and validated the first to report the RP-HPLC method using Cyrene as co-eluent with ethanol in the mobile phase. Using Cyrene in the mobile phase resulted in scaling down the amount of the green ethanol and substituting it with Cyrene as a greener solvent. The two green solvents Cyrene and ethanol, were used successfully to carry out the chromatographic separation.
Cyrene has one main drawback that limits its use in chromatography. This drawback is the high cut-off value of 350 nm which covers most of the UV region and is above the maximum absorbance wavelength of most pharmaceuticals. This cut-off value is significantly higher than that of acetonitrile and methanol, and also higher than that of ethanol. Therefore, the UV absorptivity of Cyrene is the significant obstacle. Our work showed successful detection for compounds eluted using Cyrene in the mobile phase at a detection wavelength of 350 nm. Detectability with noisy baseline was also possible below the cut-off value of Cyrene at 320 nm. However, one should consider that Cyrene is not fully compatible with UV/Vis detector. It would still work well with compounds having strong UV chromophores as dyes, and aromatic hydrocarbons, making it easily possible to carry out green HPLC analysis in industries handling such substances or more suitable to environmental areas or hazardous, restricted areas, thus enhancing a benign analysis technology that might be possible in offices in future. Even though UV/Vis detection is the most common primary detection mode in HPLC, Cyrene is expected to work better without this limitation using other detection techniques like electrochemical detection. Most attractive is the try of Cyrene with MS detection as a solvent for LC-MS and LC-MS/MS to ensure that it does not pose a problem if the mobile phase contains certain Cyrene and to see its effect on ionization efficiency and general compatibility with different ion sources. The authors are currently investigating the compatibility with the MS detector. Cyrene will also be investigated as a possible co-eluent in supercritical fluid chromatography.
Cyrene shows a weak eluotropic strength and, thus, weaker elution power than ethanol in reversed-phase chromatography in agreement with the Kamlet-Taft polarity parameter value. The unique eluotropic strength of Cyrene will further investigated and defined using a larger number of compounds or model drugs with high chemical diversity. Cyrene may also be applied as a co-eluent/ modifier to fine-tune the elution and improve the separation of a complex mixture. It is worth emphasizing that because ethanol has stronger eluotropic strength than methanol and acetonitrile, a mixture of ethanol and Cyrene may produce an equivalent eluotropic strength compared to methanol or acetonitrile portions in mobile phases. This may result in easier method transfer from methanol or acetonitrile to Cyrene/ethanol and thus easier switching to greener methods. Cyrene showed a similar selectivity to other classical solvents such as acetonitrile, methanol, and the green solvent ethanol in the RP-LC. Therefore, it does not impact the elution order when substituting other classically used organic solvents in the mobile phase to make the method greener.
The eluotropic strength of Cyrene is still stronger than water and increases at higher column temperature and eventually at higher mobile phase temperature if a mobile phase oven or mobile phase water bath as a mobile phase preheater is applied using the conventional HPLC system. Increasing the column temperature from ambient to 50 °C without approaching the maximum allowed monolithic silica column temperature of 60 °C would enhance the elution power of Cyrene by decreasing the dielectric constant and viscosity, which also decreases the generated backpressure. Thus, a promising application of Cyrene in chromatography may be a kind of what could be called “Heated Cyrene Chromatography” (with or without water as other mobile phase constituents) inconsistent with what is known as superheated water chromatography. The limitations of facing insoluble hydrophobic compounds when applying heated water chromatography does not apply in heated Cyrene or heated Cyrene water chromatography because of the good solving power of Cyrene. It is worth noting that high-temperature chromatography would be more successful using temperature-resistant stationary phase columns such as polystyrene divinyl benzene and zirconia particles with polybutadiene. Our initial investigations showed improved elution strength and lower generated backpressure, indicating a potentially successful promising elution principle. This may even be more successful for normal phase chromatography for eluting polar analytes. The higher used temperature and thus energy and the need for additional equipment to heat the mobile phase will negatively impact GAC and WAC.
Moreover, Cyrene has a higher viscosity than other classical mobile phase organic solvents and limited solubility with water and aqueous buffer at high concentrations. The high backpressure might result in greater wear on instrumentation. However, practical experiments showed that the generated backpressure with the low needed percentage of Cyrene in the mobile phase of less than 10% is below 100 bar despite higher viscosity, which will be affordable even with classical conventional columns. It is worth mentioning that Cyrene could also perform better with ultra-high-performance liquid chromatographic equipment where pressure tolerance on instrumentation is higher. However, the higher viscosity of Cyrene and, thus, the higher developed backpressure would not be a problem when using monolithic silica columns as a stationary phase because of the high permeability and lower developed backpressure. No significant change in monolithic silica column performance was noted when regenerating ethanol/ buffer methods before and after the use of Cyrene on a monolithic silica column, and good precision has been reported. However, the long-term effect of Cyrene on column life is under investigation.
Cyrene is also greening the sample by substituting other toxic or less green organic sample solvents if required to solubilize not fully water-soluble analytes. In large-scale industries, this may also reduce risk hazards. Using Cyrene as a sample solvent component instead of, e.g., methanol enhances the greenness and, thus, the whiteness of the analytical method. It is worth noting that Cyrene has shown good capacity to solubilize organic compounds. From the economic aspect of sustainability, Cyrene is cheaper than HPLC-grade acetonitrile and much cheaper than HPLC-grade ethanol. It is not toxic as both methanol and acetonitrile or limited toxic as ethanol and not flammable as the three other mentioned solvents but rather an eco-friendly bio-based biodegradable solvent that would even reduce and avoid costs required for waste treatment. Using Cyrene as a green benign biobased, and biodegradable solvent also reduce expensive and time-consuming waste treatment, thus potentially decrease the cost of analysis. Therefore, rendering the developed method more cost-effective from an economic standpoint with green eluents and green effluents. Cyrene has a high boiling point and flashpoint temperature, thus reducing the fire possibilities in laboratories and enhancing the analytical method's greenness by reducing the use of flammable ethanol and substituting it with non-flammable. Compared to the reported non-green acetonitrile and the developed ethanol-based green method, the developed Cyrene-based method showed superior greenness and whiteness scores. The boiling point affects pump performance and safety. Thus, higher boiling solvents such as Cyrene, which has a boiling point of 227 °C, are usually preferred.
Results showed the potential for Cyrene to replace hazardous non-green organic solvents in combination with ethanol and possibly alone if high temperature is also considered or other detection modes are applied. Method accuracy and precision were maintained, while the method efficiency was not compromised. Results showed similar redness of the acetonitrile and Cyrene based methods, and the obtained LOQ is still appropriate and sufficient. Therefore, the main added effect on the RGB12 tool is the significant increase in the greenness and blueness aspects due to Cyrene’s use. Even though the LOD and LOQ are somewhat lower, giving a good score is fair because they are sufficient for the intended application. The critical factor remains the sufficiency of the method to fit its intended purpose. Thus, a good whiteness percentage was achieved.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: title; Table S1: title; Video S1: title.
Author Contributions
Conceptualization, S.E.; methodology, S.E.; software, M.P.; validation, S.E., and K.A.; formal analysis, S.E. and K.A.; investigation, S.E. and K.A.; resources, S.E. and M.P.X.; data curation, S.E. and K.A.; writing—original draft preparation, S.E: and K.A.; writing—review and editing, S.E:, K.A. and M.P.; visualization, S.E. and K,A,; supervision, S.E:; project administration, S.E: and M.P.; All authors have read and agreed to the published version of the manuscript
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available from the corresponding author upon reasonable request.
Acknowledgments
Prof. Dr. Sami El Deeb is an Alexander von Humboldt Research Fellow. Merck KGaA, Darmstadt, Germany for kindly providing the monolithic silica HPLC columns and other research materials.
Conflicts of Interest
The authors declare no conflict of interest
References
- Anastas, P. Green Chemistry. Front. (Boulder) 1998, 640, 850. [Google Scholar]
- Płotka-Wasylka, J.; Namieśnik, J. Green Chemistry and Sustainable Technology Green Analytical Chemistry.
- Tobiszewski, M.; Mechlińska, A.; Namieśnik, J. Green Analytical Chemistry—Theory and Practice. Chem. Soc. Rev. 2010, 39, 2869. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 Principles of Green Analytical Chemistry and the SIGNIFICANCE Mnemonic of Green Analytical Practices. TrAC Trends Anal. Chem. 2013, 50, 78–84. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.M.; Konieczka, P.; Namieśnik, J. Analytical Eco-Scale for Assessing the Greenness of Analytical Procedures. TrAC Trends Anal. Chem. 2012, 37, 61–72. [Google Scholar] [CrossRef]
- National Environmental Methods Index, Www.Nemi.Gov.
- Anastas, P.T.; Zimmerman, J.B. Peer Reviewed: Design Through the 12 Principles of Green Engineering. Env. Sci. Technol. 2003, 37, 94A–101A. [Google Scholar] [CrossRef]
- Ballester-Caudet, A.; Campíns-Falcó, P.; Pérez, B.; Sancho, R.; Lorente, M.; Sastre, G.; González, C. A New Tool for Evaluating and/or Selecting Analytical Methods: Summarizing the Information in a Hexagon. TrAC Trends Anal. Chem. 2019, 118, 538–547. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J. A New Tool for the Evaluation of the Analytical Procedure: Green Analytical Procedure Index. Talanta 2018, 181, 204–209. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE—Analytical GREEnness Metric Approach and Software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef]
- Nowak, P.M.; Wietecha-Posłuszny, R.; Pawliszyn, J. White Analytical Chemistry: An Approach to Reconcile the Principles of Green Analytical Chemistry and Functionality. TrAC Trends Anal. Chem. 2021, 138, 116223. [Google Scholar] [CrossRef]
- Hussain, C.M.; Hussain, C.G.; Keçili, R. White Analytical Chemistry Approaches for Analytical and Bioanalytical Techniques: Applications and Challenges. TrAC Trends Anal. Chem. 2023, 159, 116905. [Google Scholar] [CrossRef]
- Fields, S.M.; Ye, C.Q.; Zhang, D.D.; Branch, B.R.; Zhang, X.J.; Okafo, N. Superheated Water as Eluent in High-Temperature High-Performance Liquid Chromatographic Separations of Steroids on a Polymer-Coated Zirconia Column. J. Chromatogr. A 2001, 913, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Droux, S.; Félix, G. Green Chiral HPLC Enantiomeric Separations Using High Temperature Liquid Chromatography and Subcritical Water on Chiralcel OD and Chiralpak AD. Chirality 2011, 23, E105–E109. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, F.; Navidpour, L.; Bayat, S.; Afshar, M. Validation and Uncertainty Estimation of an Ecofriendly and Stability-Indicating HPLC Method for Determination of Diltiazem in Pharmaceutical Preparations. J. Anal. Methods Chem. 2013, 2013, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.M.; Lamie, N.T. Analytical Eco-Scale for Assessing the Greenness of a Developed RP-HPLC Method Used for Simultaneous Analysis of Combined Antihypertensive Medications. J. AOAC Int. 2016, 99, 1260–1265. [Google Scholar] [CrossRef] [PubMed]
- Spagnol, C.M.; Isaac, V.L.B.; Corrêa, M.A.; Salgado, H.R.N. Validation of HPLC–UV Assay of Caffeic Acid in Emulsions. J. Chromatogr. Sci. 2015, bmv142. [Google Scholar] [CrossRef]
- Ribeiro, R.L.V.; Bottoli, C.B.G.; Collins, K.E.; Collins, C.H. Reevaluation of Ethanol as Organic Modifier for Use in HPLS-RP Mobile Phases. J. Braz. Chem. Soc. 2004, 15, 300–306. [Google Scholar] [CrossRef]
- Welch, C.J.; Brkovic, T.; Schafer, W.; Gong, X. Performance to Burn? Re-Evaluating the Choice of Acetonitrile as the Platform Solvent for Analytical HPLC. Green. Chem. 2009, 11, 1232. [Google Scholar] [CrossRef]
- Tache, F.; Udrescu, S.; Albu, F.; Micăle, F.; Medvedovici, A. Greening Pharmaceutical Applications of Liquid Chromatography through Using Propylene Carbonate–Ethanol Mixtures Instead of Acetonitrile as Organic Modifier in the Mobile Phases. J. Pharm. Biomed. Anal. 2013, 75, 230–238. [Google Scholar] [CrossRef]
- Cheregi, M.; Albu, F.; Udrescu, Ş.; Răducanu, N.; Medvedovici, A. Greener Bioanalytical Approach for LC/MS–MS Assay of Enalapril and Enalaprilat in Human Plasma with Total Replacement of Acetonitrile throughout All Analytical Stages. J. Chromatogr. B 2013, 927, 124–132. [Google Scholar] [CrossRef]
- Dogan, A.; Basci, N.E. Green Bioanalytical and Pharmaceutical Analysis of Voriconazole and Tadalafil by HPLC. Curr. Pharm. Anal. 2017, 13. [Google Scholar] [CrossRef]
- Keppel, T.R.; Jacques, M.E.; Weis, D.D. The Use of Acetone as a Substitute for Acetonitrile in Analysis of Peptides by Liquid Chromatography/Electrospray Ionization Mass Spectrometry. Rapid Commun. Mass. Spectrom. 2010, 24, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Fritz, R.; Ruth, W.; Kragl, U. Assessment of Acetone as an Alternative to Acetonitrile in Peptide Analysis by Liquid Chromatography/Mass Spectrometry. Rapid Commun. Mass. Spectrom. 2009, 23, 2139–2145. [Google Scholar] [CrossRef]
- Hafez, H.M.; El Deeb, S.; Mahmoud Swaif, M.; Ismail Ibrahim, R.; Ali Kamil, R.; Salman Abdelwahed, A.; Ehab Ibrahim, A. Micellar Organic-Solvent Free HPLC Design of Experiment for the Determination of Ertapenem and Meropenem; Assessment Using GAPI, AGREE and Analytical Eco-Scale Models. Microchem. J. 2023, 185, 108262. [Google Scholar] [CrossRef]
- Sharaf, Y.A.; El Deeb, S.; Ibrahim, A.E.; Al-Harrasi, A.; Sayed, R.A. Two Green Micellar HPLC and Mathematically Assisted UV Spectroscopic Methods for the Simultaneous Determination of Molnupiravir and Favipiravir as a Novel Combined COVID-19 Antiviral Regimen. Molecules 2022, 27, 2330. [Google Scholar] [CrossRef] [PubMed]
- Chin-Chen, M.-L.; Rambla-Alegre, M.; Durgavanshi, A.; Bose, D.; Esteve-Romero, J. Rapid and Sensitive Determination of Nicotine in Formulations and Biological Fluid Using Micellar Liquid Chromatography with Electrochemical Detection. J. Chromatogr. B 2010, 878, 2397–2402. [Google Scholar] [CrossRef] [PubMed]
- Peris-Vicente, J.; Villareal-Traver, M.; Casas-Breva, I.; Carda-Broch, S.; Esteve-Romero, J. A Micellar Liquid Chromatography Method for the Quantification of Abacavir, Lamivudine and Raltegravir in Plasma. J. Pharm. Biomed. Anal. 2014, 98, 351–355. [Google Scholar] [CrossRef]
- Ruiz-Angel, M.J.; Peris-García, E.; García-Alvarez-Coque, M.C. Reversed-Phase Liquid Chromatography with Mixed Micellar Mobile Phases of Brij-35 and Sodium Dodecyl Sulphate: A Method for the Analysis of Basic Compounds. Green. Chem. 2015, 17, 3561–3570. [Google Scholar] [CrossRef]
- Yabré, M.; Ferey, L.; Somé, I.T.; Gaudin, K. Greening Reversed-Phase Liquid Chromatography Methods Using Alternative Solvents for Pharmaceutical Analysis. Molecules 2018, 23. [Google Scholar] [CrossRef]
- Camp, J.E. Bio-Available Solvent Cyrene: Synthesis, Derivatization, and Applications. ChemSusChem 2018, 11, 3048–3055. [Google Scholar] [CrossRef]
- Wibisono, Y.; Noviani, V.; Ramadhani, A.T.; Fitry Baruna, A.; Devianto, L.A.; Sulianto, A.A. Proceedings 2020, 4. 4.
- Stini, N.A.; Gkizis, P.L.; Kokotos, C.G. Cyrene: A Bio-Based Novel and Sustainable Solvent for Organic Synthesis. Green. Chem. 2022, 24, 6435–6449. [Google Scholar] [CrossRef]
- Fernandes, J.; Nemala, S.S.; De Bellis, G.; Capasso, A. Green Solvents for the Liquid Phase Exfoliation Production of Graphene: The Promising Case of Cyrene. Front. Chem. 2022, 10. [Google Scholar] [CrossRef]
- Citarella, A.; Amenta, A.; Passarella, D.; Micale, N. Cyrene: A Green Solvent for the Synthesis of Bioactive Molecules and Functional Biomaterials. Int. J. Mol. Sci. 2022, 23, 15960. [Google Scholar] [CrossRef]
- Therapeutic Uses of Metronidazole and Its Side Effects: An Update.
- Leitsch, D. A Review on Metronidazole: An Old Warhorse in Antimicrobial Chemotherapy. Parasitology 2019, 146, 1167–1178. [Google Scholar] [CrossRef] [PubMed]
- Freeman, C.D.; Klutman, N.E.; Lamp3, K.C. Metronidazole A Therapeutic Review and Update; 1997; Volume 54.
- Wise, R. A Review of the Clinical Pharmacology of Moxifloxacin, a New 8-Methoxyquinolone, and Its Potential Relation to Therapeutic Efficacy. 1999; Volume 17.
- Keating, G.M.; Scott, L.J.; Bantar, C.; San Martin, H.; Rios, E.; Blondeau, J. ADIS DRUG EVALUATION Moxifloxacin A Review of Its Use in the Management of Bacterial Infections; 2004; Volume 64.
- Ansari, M. Quantitation of Metronidazole in Pharmaceutical Suspension Using High Performance Liquid Chromatographic Method Solubility Enhancement View Project Spectrophotometric Determination of Amodiaquine and Sulfadoxine in Pharmaceutical Preparations View Project; 2011.
- Ahmed, F. A Review on HPLC Method Development and Validation of Metronidazole Tablet A Review on HPLC Method Development and Validation of Metronidazole Tablet View Project.
- Wang, N.; Zhu, L.; Zhao, X.; Yang, W.; Sun, H. Improved HPLC Method for the Determination of Moxifloxacin in Application to a Pharmacokinetics Study in Patients with Infectious Diseases. ISRN Pharmacol. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Phani Sekhar Reddy, G.; Navyasree, K.S.; Jagadish, P.C.; Bhat, K. Analytical Method Development and Validation for HPLC-ECD Determination of Moxifloxacin in Marketed Formulations. Pharm. Chem. J. 2018, 52, 674–679. [Google Scholar] [CrossRef]
- Sankar, R.; Srinivasa Babu, P.; Ravi Sankar, P.; Durga Deepthi, K.; Srinivasa Babu, P.; Rachana, G.; Sai Geethika, A.; Bhargavi, J.; Pavan Kalyan, L.; Naga Poojitha, K.; et al. Development and Validation of a Stability-Indicating Method. for Assay. of Moxifloxacin in Oral. Pharmaceutical Dosage Forms by HPLC Controlling Hypertension: A Brief. Review View Project A Review on Step-by-Step Analytical Method. Validation View Project Development and Validation of a Stability-Indicating Method. for Assay. of Moxifloxacin in Oral. Pharmaceutical Dosage Forms by HPLC; 2019; Volume 9.
- Elkhoudary, M.M.; Abdel Salam, R.A.; Hadad, G.M. Development and Optimization of Hplc Analysis of Metronidazole, Diloxanide, Spiramycin and Cliquinol in Pharmaceutical Dosage Forms Using Experimental Design. J. Chromatogr. Sci. 2016, 54, 1701–1712. [Google Scholar] [CrossRef] [PubMed]
- Sankar, R.; Srinivasa Babu, P.; Ravi Sankar, P.; Durga Deepthi, K.; Srinivasa Babu, P.; Rachana, G.; Sai Geethika, A.; Bhargavi, J.; Pavan Kalyan, L.; Naga Poojitha, K.; et al. Development and Validation of a Stability-Indicating Method. for Assay. of Moxifloxacin in Oral. Pharmaceutical Dosage Forms by HPLC Controlling Hypertension: A Brief. Review View Project A Review on Step-by-Step Analytical Method. Validation View Project Development and Validation of a Stability-Indicating Method. for Assay. of Moxifloxacin in Oral. Pharmaceutical Dosage Forms by HPLC; 2019; Volume 9.
- El-Yazbi, A.F.; Aboukhalil, F.M.; Khamis, E.F.; Elkhatib, M.A.W.; El-Sayed, M.A.; Youssef, R.M. Simple Simultaneous Determination of Moxifloxacin and Metronidazole in Complex Biological Matrices. RSC Adv. 2022, 12, 15694–15704. [Google Scholar] [CrossRef]
- Shen, Y.; Lo, C.; Nagaraj, D.R.; Farinato, R.; Essenfeld, A.; Somasundaran, P. Development of Greenness Index as an Evaluation Tool to Assess Reagents: Evaluation Based on SDS (Safety Data Sheet) Information. Min. Eng. 2016, 94, 1–9. [Google Scholar] [CrossRef]
- Islam, T.; Islam Sarker, Md.Z.; Uddin, A.H.; Yunus, K. Bin; Prasad, R.; Mia, Md.A.R.; Ferdosh, S. Kamlet Taft Parameters: A Tool to Alternate the Usage of Hazardous Solvent in Pharmaceutical and Chemical Manufacturing/Synthesis - A Gateway towards Green Technology. Anal. Chem. Lett. 2020, 10, 550–561. [Google Scholar] [CrossRef]
- Citarella, A.; Amenta, A.; Passarella, D.; Micale, N. Cyrene: A Green Solvent for the Synthesis of Bioactive Molecules and Functional Biomaterials. Int. J. Mol. Sci. 2022, 23, 15960. [Google Scholar] [CrossRef]
- Anastas, P.T.; Zimmerman, J.B. Peer Reviewed: Design Through the 12 Principles of Green Engineering. Env. Sci. Technol. 2003, 37, 94A–101A. [Google Scholar] [CrossRef]
- Ballester-Caudet, A.; Campíns-Falcó, P.; Pérez, B.; Sancho, R.; Lorente, M.; Sastre, G.; González, C. A New Tool for Evaluating and/or Selecting Analytical Methods: Summarizing the Information in a Hexagon. TrAC Trends Anal. Chem. 2019, 118, 538–547. [Google Scholar] [CrossRef]
- Shen, Y.; Lo, C.; Nagaraj, D.R.; Farinato, R.; Essenfeld, A.; Somasundaran, P. Development of Greenness Index as an Evaluation Tool to Assess Reagents: Evaluation Based on SDS (Safety Data Sheet) Information. Min. Eng. 2016, 94, 1–9. [Google Scholar] [CrossRef]
- INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE VALIDATION OF ANALYTICAL PROCEDURES: TEXT AND METHODOLOGY Q2(R1);
- Heinisch, S.; Rocca, J.-L. Sense and Nonsense of High-Temperature Liquid Chromatography. J. Chromatogr. A 2009, 1216, 642–658. [Google Scholar] [CrossRef] [PubMed]
- Greibrokk, T.; Andersen, T. High-Temperature Liquid Chromatography. J. Chromatogr. A 2003, 1000, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, F.; Navidpour, L.; Bayat, S.; Afshar, M. Validation and Uncertainty Estimation of an Ecofriendly and Stability-Indicating HPLC Method for Determination of Diltiazem in Pharmaceutical Preparations. J. Anal. Methods Chem. 2013, 2013, 1–10. [Google Scholar] [CrossRef] [PubMed]
- McNeff, C.V.; Yan, B.; Stoll, D.R.; Henry, R.A. Practice and Theory of High Temperature Liquid Chromatography. J. Sep. Sci. 2007, 30, 1672–1685. [Google Scholar] [CrossRef]
Figure 1.
Production scheme of dihydrolevoglucosenone (Cyrene).
Figure 1.
Production scheme of dihydrolevoglucosenone (Cyrene).
Figure 2.
Structural formula of (A) metronidazole and (B) moxifloxacin.
Figure 2.
Structural formula of (A) metronidazole and (B) moxifloxacin.
Figure 3.
Spider chart generated based on the data presented in
Table 2 reveals that Cyrene significantly outperforms ethanol in fire safety.
Figure 3.
Spider chart generated based on the data presented in
Table 2 reveals that Cyrene significantly outperforms ethanol in fire safety.
Figure 4.
Chromatograms showing the separation of metronidazole and moxifloxacin on a monolithic C18 (10 x 4.6 mm) column at a flow rate of 1 mL/min and detected at 350 nm. The two drugs were eluted using different mobile phase compositions: A: ethanol: 0.1 M sodium acetate buffer pH 4.25 (13:87,v/v), B: Cyrene:ethanol: 0.1 M sodium acetate buffer pH 4.25 (2:13:85,v/v), C: Cyrene:ethanol: 0.1 M sodium acetate buffer pH 4.25 (4:13:83,v/v/v), D: Cyrene:ethanol: 0.1 M sodium acetate buffer pH 4.25 (6:13:81,v/v/v), E: Cyrene:ethanol: 0.1 M sodium acetate buffer pH 4.25 (8:13:79, v/v/v), and F: Cyrene:ethanol: 0.1 M sodium acetate buffer pH 4.25 (10:13:77, v/v/v).
Figure 4.
Chromatograms showing the separation of metronidazole and moxifloxacin on a monolithic C18 (10 x 4.6 mm) column at a flow rate of 1 mL/min and detected at 350 nm. The two drugs were eluted using different mobile phase compositions: A: ethanol: 0.1 M sodium acetate buffer pH 4.25 (13:87,v/v), B: Cyrene:ethanol: 0.1 M sodium acetate buffer pH 4.25 (2:13:85,v/v), C: Cyrene:ethanol: 0.1 M sodium acetate buffer pH 4.25 (4:13:83,v/v/v), D: Cyrene:ethanol: 0.1 M sodium acetate buffer pH 4.25 (6:13:81,v/v/v), E: Cyrene:ethanol: 0.1 M sodium acetate buffer pH 4.25 (8:13:79, v/v/v), and F: Cyrene:ethanol: 0.1 M sodium acetate buffer pH 4.25 (10:13:77, v/v/v).
Figure 5.
Chromatograms showing the separation of metronidazole and moxifloxacin on a monolithic C18 (10 x 4.6 mm) column at a flow rate of 1 mL/min and detected at 350 nm. The two drugs were eluted using Cyrene: ethanol: 0.1 M sodium acetate buffer pH 4.25 (8:13:79, v/v/v), which was also used as a sample solvent.
Figure 5.
Chromatograms showing the separation of metronidazole and moxifloxacin on a monolithic C18 (10 x 4.6 mm) column at a flow rate of 1 mL/min and detected at 350 nm. The two drugs were eluted using Cyrene: ethanol: 0.1 M sodium acetate buffer pH 4.25 (8:13:79, v/v/v), which was also used as a sample solvent.
Figure 6.
Greenness evaluation of (A) the reported acetonitrile-based non-green method, (B) the developed green ethanol-based reference method, and (C) the developed greener Cyrene/ethanol-based method using AGREE tool.
Figure 6.
Greenness evaluation of (A) the reported acetonitrile-based non-green method, (B) the developed green ethanol-based reference method, and (C) the developed greener Cyrene/ethanol-based method using AGREE tool.
Figure 7.
Evaluation of the whiteness of the reference ACN and the developed Cyrene: EtOH methods according to the RGB12 tool.
Figure 7.
Evaluation of the whiteness of the reference ACN and the developed Cyrene: EtOH methods according to the RGB12 tool.
Figure 8.
Chromatograms show separation of metronidazole and moxifloxacin on a monolithic C18 (10 x 4.6 mm) column. The two drugs were eluted using Cyrene: ethanol: 0.1 M sodium acetate buffer pH 4.25 (8:13:79, v/v/v), which was also used as a sample solvent. The two drugs were detected at 350 nm. (A) column temperature 35 °C and flow rate 1 mL/min, (B) column temperature 50 °C and flow rate 1 mL/min, (C) column temperature 50 °C and flow rate 2 mL/min.
Figure 8.
Chromatograms show separation of metronidazole and moxifloxacin on a monolithic C18 (10 x 4.6 mm) column. The two drugs were eluted using Cyrene: ethanol: 0.1 M sodium acetate buffer pH 4.25 (8:13:79, v/v/v), which was also used as a sample solvent. The two drugs were detected at 350 nm. (A) column temperature 35 °C and flow rate 1 mL/min, (B) column temperature 50 °C and flow rate 1 mL/min, (C) column temperature 50 °C and flow rate 2 mL/min.
Table 1.
Physicochemical properties of Cyrene versus selected green and non-green RP-HPLC solvents*.
Table 1.
Physicochemical properties of Cyrene versus selected green and non-green RP-HPLC solvents*.
| Solvent |
UV Cut-off value (nm) |
Water solubility |
Density (g/cm3) at 20 °C |
Polarity Parameter Kamlet-Taft π⁕ |
Partition coefficient n-octanol/water (log value) |
Boiling Point
°C |
Flash Point
°C at 1.013 hPa (c.c.) |
| Acetonitrile |
190 |
miscible in any proportion |
0,7822 |
0,75 |
-0,34 |
82 |
2 |
| Methanol |
205 |
1000 g/l at 20 °C - completely miscible |
0,7913 |
0,61 |
-0,77 |
64,7 |
12 |
| Ethanol |
210 |
≥1000 g /l at 20 °C |
0,81 |
0,54 |
-0,31 |
78 |
9,7 |
| Water |
190 |
Not applied |
0,9982 |
1,2 |
Not applied |
100 |
Not applied |
| Propylene carbonate |
220 |
175 g/l at 25 °C |
1,2047 |
0.9 |
-0,41 |
240 |
132 |
| Acetone |
330 |
miscible in any proportion |
0,79 |
0.71 |
-0,23 |
56,05 |
-18 |
| Cyrene |
350 |
ca.52,6 g/l at 20 °C |
1,25 |
0,93 |
-1,52 |
227 |
108 |
Table 2.
Fire safety criteria for acetonitrile, ethanol and Cyrene*.
Table 2.
Fire safety criteria for acetonitrile, ethanol and Cyrene*.
| Solvent Evaluation Criteria |
|
Flash Point |
Autoignition |
NFPA Health |
NFPA Flammability |
NFPA Reactivity |
| Acetonitrile |
Value or Rating |
2 °C |
524 °C |
2 |
3 |
0 |
| Score |
- 3 |
4 |
1 |
-3 |
5 |
| Ethanol |
Value or Rating |
12 °C |
455 °C |
2 |
3 |
0 |
| Score |
-2 |
3 |
1 |
-3 |
5 |
| Cyrene |
Value or Rating |
108 °C |
296 °C |
0 |
0 |
0 |
| Score |
2 |
3 |
5 |
5 |
5 |
Table 3.
Generated backpressures for the used mobile phase compositions on a monolithic C18 (10 x 4.6 mm) column .
Table 3.
Generated backpressures for the used mobile phase compositions on a monolithic C18 (10 x 4.6 mm) column .
| Mobile phase composition |
Generated backpressure in bar |
| Ethanol: 0.1 M sodium acetate buffer pH 4.25 (13:87, v/v) |
71 |
| Cyrene: 0.1 M ethanol: sodium acetate buffer pH 4.25 (2:13:85, v/v/v) |
75 |
| Cyrene: 0.1 M ethanol: sodium acetate buffer pH 4.25 (4:13:83, v/v/v) |
80 |
| Cyrene: 0.1 M ethanol: sodium acetate buffer pH 4.25 (6:13:81, v/v/v) |
85 |
| Cyrene:ethanol: 0.1 M sodium acetate buffer pH 4.25 (8:13:79, v/v/v) |
91 |
| Cyrene:ethanol: 0.1 M sodium acetate buffer pH 4.25 (10:13:77, v/v/v) |
98 |
Table 4.
Elution conditions of the reported and developed methods for the simultaneous determination of metronidazole and moxifloxacin.
Table 4.
Elution conditions of the reported and developed methods for the simultaneous determination of metronidazole and moxifloxacin.
| Method |
Elution Conditions |
| A |
Reported acetonitrile-based non-green method |
HPLC-DAD using RP-C18 column. Isocratic elution using CAN and phosphate buffer (30:70, v/v) as mobile phase on a Zorbax Eclipse Plus C18 column |
| B |
Developed green ethanol-based reference method |
HPLC-DAD using monolithic C18 column. Isocratic elution using ethanol and 0.1 M sodium acetate buffer pH 4.25 (13:87, v/v) as mobile phase |
| C |
Develop a greener Cyrene/ethanol-based method. |
HPLC-DAD using monolithic C18 column. Isocratic elution using Cyrene: ethanol and0.1 M sodium acetate buffer pH 4.25 (8:13:79, v/v/v) as mobile phase |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).