1. Introduction
Hemorrhage of the gastrointestinal (GI) tract is a major disease; more than 500,000 patients are hospitalized in the United States, and the readmission rate is the highest. Bleeding from the upper GI (UGI) tract, including the esophagus, stomach, and duodenum, accounts for approximately 40% of the bleeding from the entire GI tract [
1]. Apart from hemorrhage of the esophageal or gastric varices, non-variceal UGI bleeding (NVUGIB) continues to increase owing to the aging population and increased use of nonsteroidal anti-inflammatory drugs (NSAID) and antiplatelet agents [
2]. Antiplatelet agents, such as low-dose aspirin (LDA) or the more potent clopidogrel, P2Y12 inhibitors, cilostazol, and sarpogrelate, are commonly used drugs that are effective in the prevention of acute coronary syndrome (ACS), peripheral vascular disease, and cerebrovascular accident (CVA) [
3]. Dual antiplatelet therapy (DAPT) is the mainstay of management of acute vascular events and maintenance therapy after percutaneous intervention [
4]. However, long-term use of antiplatelet agents or DAPT is significantly associated with major hemorrhage in the GI tract, which may lead to re–hospitalization or mortality after acute vascular events [
5].
Previous studies have shown that the incidence of 30-day NVUGIB among patients who underwent percutaneous coronary intervention (PCI) for ACS was 1.2-1.3% [
6,
7], and a recent nationwide study in the US showed that the 11-month incidence of NVUGIB after PCI for the management of acute myocardial infarction was 1.6%, and 11-month mortality was significantly associated with the development of NVUGIB [
8]. Several clinical features, such as old age, anemia, thrombocytopenia, renal insufficiency, malignancy, or prior history of bleeding, are known to be high bleeding risk factors among patients undergoing PCI and receiving DAPT [
9]. However, the re-bleeding rate and its risk factors in patients with a history of GIB during DAPT are rarely known. Moreover, when the bleeding focus was limited to the UGI tract, data regarding re-bleeding from NVUGIB have not yet been reported.
Therefore, in this study, we investigated the re-bleeding rate and risk factors of NVUGIB in patients with a history of NVUBIG during DAPT. We also determined the all-cause and re-bleeding related mortality rates in this population group.
2. Materials and Methods
Patients
We retrospectively enrolled patients who were receiving DAPT as a regular medication and were admitted for the treatment of NVUGIB between 2006 and 2020 in a tertiary hospital. All patients received DAPT with confirmed NVUGIB; one was aspirin, and the other antiplatelet agents were clopidogrel, cilostazole, or sarpogrelate (
Table 1). Patients taking concurrent anticoagulants such as warfarin or direct oral anticoagulants (DOAC) were excluded. Medication use was evaluated by reviewing the medical records and drug charts. All enrolled patients underwent UGI endoscopy due to 1) symptoms of UGI bleeding, such as hematemesis, melena, or black tarry stool, or 2) a decrease in serum hemoglobin (Hb) greater than 2 g/dL from baseline. NVUGIB was defined as 1) current active bleeding or 2) fresh bloody material with a definite bleeding focus in the UGI tract. All index NVUGIB events were stabilized using endoscopic or conservative management. Patients who had symptoms, UGI bleeding, or significant anemia but did not undergo UGI endoscopy or who were confirmed to have bleeding from the lower GI tract by colonoscopy were excluded.
Parameters
We collected data regarding major comorbidities (heart failure, ischemic heart disease, diabetes, chronic kidney disease [CKD], liver cirrhosis, malignancy, cerebrovascular attack, hypertension), Charlson comorbidity index, which is a validated and highly-predictive index for mortality [
10], previous history of UGI bleeding, a combination of other drugs such as nonsteroidal anti-inflammatory drugs (NSAIDs) or steroid, location, and cause of bleeding, vital sign (heart rate and blood pressure), laboratory findings (Hb, platelet count, international normalized ratio [INR]), renal function, scoring systems of UGI bleeding (Rockall score, Glasgow-Blatchford score [GBS]), treatment modalities, status of
Helicobacter pylori (
H. pylori), length of hospital stay and resumption of antiplatelet agents (
Table 1 and
Table 2). This study was approved by the Institutional Review Board of Korea University, Guro Hospital (IRB number:2022GR0318).
Study outcomes
We investigated recurrent NVUGIB and all-cause and re-bleeding related mortality in the enrolled patients. Clinically significant re-bleeding was defined as the identical criteria of index NVUGIB after 24 hours of clinical stability, which was if 1) the patients had symptoms of UGI bleeding or a decrease of serum Hb ≥ 2 g/dL from the previous level, and 2) endoscopy showed a current active bleeding or fresh bloody materials with a definite bleeding focus in UGI tract. The total number of re-bleeding episodes per patient was also counted.
Statistical analysis
SPSS 22.0 (SPSS Inc., Chicago, Ill, USA) was used for statistical analysis. Continuous data were assessed using the Student’s t-test or Mann-Whitney U test and discontinuous data were assessed using the chi-square test. Logistic rank analysis was used for multivariate analysis. The re-bleeding rate and all-cause and bleeding-related mortality rates were assessed using Kaplan-Meier survival analysis and the log-rank test. A P-value less than 0.05 was considered statistically significant.
3. Results
3.1. Patients and baseline characteristics
A total of 124 patients were hospitalized due to NVUGIB during DAPT and were enrolled in this study (mean age: 69.4 years, male: 103, 83.1%, female: 21, 16.9%). Among them, 111 patients (89.5%) took aspirin with clopidogrel, and 13 took aspirin with other antiplatelet agents such as cilostazole and sarpogrelate. More than half of the patients had hypertension (84, 67.7%) and ischemic heart disease (68, 54.8%), and the mean Charlson comorbidity index was 5.4± 2.6. Eight patients (6.5%) had a history of UGI bleeding and 16 were taking other drugs that may cause UGI injury, such as NSAIDs or steroids.
After successful hemostasis of the index NVUGIB, we analyzed the clinical course of the enrolled patients. A total of 62 re-bleeding episodes occurred during the 40.7 ± 40.8 months of follow-up, and 36 patients had at least one episode of re-bleeding (29.0%). The mean number of bleeding episodes per patient was 1.7 ± 1.3, and the mean duration from index bleeding to re-bleeding was 12.9 ± 18.0 months. Compared with the non rebleeding group, patients in the re-bleeding group were significantly older (73.3 vs. 67.8,
P=0.010) and CKD was more frequent (11/36 vs. 13/88,
P=0.043) than those in the non-re-bleeding group. However, other variables, such as sex, type of DAPT, comorbidities, Carlson comorbidity index, symptoms, previous UGI bleeding, and combination of NSAID or steroids, were not significantly different between the two groups (
Table 1).
3.2. Clinical characteristics and outcomes
Most index NVUGIB cases occurred in the stomach, followed by the duodenum and esophagus, and peptic ulcers. Endoscopic hemostasis was performed in 44 patients (35.5%), and RBC transfusion was performed in 86 patients (69.9%). The mean length of hospital stay was 8.9 ± 11.5 days, and most patients (108/115, 93.9%) restarted antiplatelet agents after clinical stabilization. Compared with the non re-bleeding group, patients in the re-bleeding group showed lower serum Hb level (7.8 vs. 9.0,
P=0.009) and a marginally higher Charlson comorbidity score (6.0 vs. 5.1,
P=0.063); however, other clinical characteristics were not significantly different between the two groups (
Table 2). Multivariate analysis showed that age was the only significant risk factor for re-bleeding (odds ratio [OR],1.050; 95% confidence interval [CI]:1.001-1.102,
P=0.047) (
Table 3).
Nineteen of the 124 patients (15.3%) died during the follow-up period, and re-bleeding was the main cause of death in seven patients (36.8%). Univariate analysis showed that age (76.2 vs. 68.1,
P=0.001), underlying malignancy (63.2 vs. 31.4%,
P=0.008), and rebleeding episode (47.4 vs. 25.7%,
P=0.056) were significantly or marginally different between the dead and undead patients (Supplementary
Table 1). Multivariate analysis showed that age (OR,1.096; 95% CI:1.020-1.178,
P=0.013) and underlying malignancy (OR,3.646; 95% CI:1.103-12.053,
P=0.024) were significant risk factors for all-cause mortality (
Table 4). Similarly, when comparing the re-bleeding-related dead and undead patients, age (79.6 vs. 68.7,
P=0.013), underlying malignancy (71.4 vs. 31.4%,
P=0.030), and re-bleeding episodes (71.4 vs. 25.7,
P=0.010) were significantly different between the two groups (Supplementary
Table 2). However. Multivariate analysis showed that only age was a significant risk factor for re-bleeding-related mortality (OR,1.187; 95% CI:1.032-1.364,
P=0.016) (
Table 5).
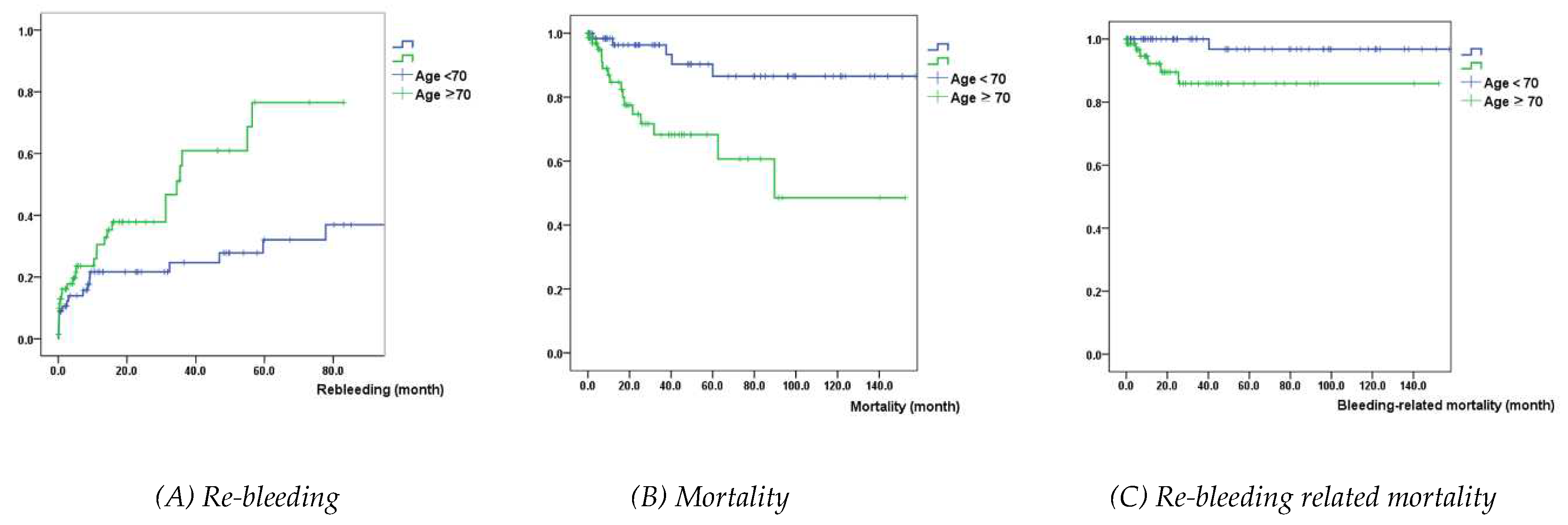
Kaplan-Meier analysis showed that patients with age > 70 years had a significantly higher probability of re-bleeding, mortality, and re-bleeding-related mortality, compared with those with age ≤ 70 years (
P=0.008, <0.001, 0.009, respectively) (
Figure 1).
4. Discussion
We found that 29.0% of the patients with a history of NVUGIB during DAPT had at least one episode of re-bleeding. Among the 19 patients who died during the follow-up period after stabilization of the index NVUGIB event, 7 (36.8%) had re-bleeding-related mortality. To the best of our knowledge, this is the first study to report the re-bleeding rate in patients with a history of NVUBIG during DAPT. The strength of our study is that all NVUBIG events were confirmed by endoscopic examination and did not rely solely on laboratory findings such as anemia or patient symptoms such as melena or hematemesis.
Aspirin has antiplatelet activity through the inhibition of the cyclooxygenase (COX) enzyme and inhibits COX-1 predominantly than COX-2 by 8–10 times, which can cause reduction of mucosal blood flow, inhibition of production of mucus or bicarbonate, inhibition of platelet aggregation, and eventually leading to mucosal injury and hemorrhage of the UGI tract [
11]. Furthermore, the combination of aspirin with other antiplatelet agents has a synergistic effect on hemorrhage from the UGI [
7,
12,
13]. Thus, physicians who prescribe DAPT should be cautious about the occurrence of UGI bleeding and its prognosis, focusing not only on cardiovascular events but also on recurrent GI bleeding. However, previous studies did not show the long-term clinical course after the index NVUGIB by DAPT [
12], or only focused on post-NVUGIB cardiovascular events and did not demonstrate data regarding recurrent UGI bleeding [
7,
13].
Several scoring systems have been developed for patients with NVUBIG, including the Rockall score (RS), Glasgow-Blatchford score (GBS), and AIMS65 score, which have been validated as useful for predicting the risk of re-bleeding or mortality [
14,
15]. However, our study did not demonstrate the significance of RS or GBS in predicting re-bleeding, mortality, or re-bleeding-related mortality. As no study has shown the effectiveness of RS or GBS among patients with NVUGIB treated with DAPT, it is unclear whether both systems are useful for this subpopulation. One possibility is that our study enrolled a small number of patients (124) to reach statistical significance. Our data support the hypothesis that RS and GBS were not significantly higher in the re-bleeding group than in the non re-bleeding group (6.0 vs. 5.9,
P=0.337; 10.7 vs. 9.3,
P=0.116) (
Table 2).
Our study showed that only age was a significant risk factor for re-bleeding, all-cause, and re-bleeding related mortality. Kaplan-Meier graphs showed that patients aged > 70 years had a significant probability of re-bleeding, death, and re-bleeding related death compared to patients aged < 70 years. These findings suggest that age is a simple and reliable prognostic factor in patients with NVUGIB during DAPT. Previous studies reported that age is a significant risk factor for GI hemorrhage and all-cause or cardiac mortality in patients receiving DAPT [
7,
12,
13,
16,
17]. Physicians need to be alert to re-bleeding and the associated mortality if a patient taking DAPT is hospitalized for NVUGIB. It was also interesting that re-bleeding episodes were marginally associated with re-bleeding related mortality, even though it did not reach statistical significance (OR,6.161, 95%; CI:0.957-39.660,
P=0.056), which might have been caused by the small sample size. Recurrent bleeding episodes may be a sign of associated mortality, and physicians should be aware of this.
To reduce the UGI complications associated with DAPT, the concomitant use of acid inhibitors such as proton pump inhibitors (PPIs) is recommended [
13]. However, it is unclear whether this strategy is effective in preventing re-bleeding in patients with NVUGIB treated with DAPT. In our study, most patients were administered concomitant acid inhibitors, including PPIs, after the onset of the index NVUGIB; however, we could not find any significance of the post-NVUGIB use of acid inhibitors for the prevention of recurrent bleeding. A nationwide Japanese study showed a 6-month incidence of UGI bleeding (3.1-4.2%) among patients who were taking multiple antithrombotic agents, and vonoprazan, a potassium-competitive acid blocker (P-CAB), which is a more potent acid inhibitor, was non-inferior to conventional PPIs for the prevention of 6-month UGI bleeding [
18]. Recently, several other P-CABs have shown clinical efficacy in the management of gastroesophageal reflux disorders [
19,
20] and peptic ulcer disease [
21]. P-CABs administered during DAPT are expected to show non-inferior or even superior effectiveness in preventing index or recurrent NVUGIB. Further studies are required to elucidate this issue in the near future.
This study had several limitations. First, this was a retrospective single-center study with a relatively small sample size. Thus, the significance of several factors, such as RS, GBS, CKD, underlying malignancy, or Charlson comorbidity score, could be underestimated compared with the real clinical importance. Second, the impairment of other systemic functions after the index NVUGIB, such as cardiac, renal, or pulmonary function, was not estimated, which could also significantly affect the prognosis of the patients. However, we found that more than one-third of the mortality was associated with recurrent bleeding, even after clinical stabilization of the NVUGIB index during DAPT.
5. Conclusions
Some patients with NVUGIB during DAPT may experience recurrent UGI bleeding, and age is a simple and reliable factor for the prediction of re-bleeding and all-cause and bleeding-related deaths during follow-up. Further large-scale prospective studies are expected to show the prognosis of patients with NVUGIB taking DAPT, especially focusing on recurrent GI complications and related mortality.
Supplementary Materials
Supplementary
Table 1. Baseline and Clinical Characteristics according to All-Cause Mortality, Supplementary
Table 2. Baseline and Clinical Characteristics according to Rebleeding-Related Mortality.
Author Contributions
Study design and concepts: AYY and JMK. Endoscopic procedure performance: AYY, JMK, PJJ, BJL, SHK, and WSK. AYY, JMK, and WSK collected and analyzed the data. Manuscript writing: AYY and JMK. Advice on study design and writing of the manuscript: LBJ, PJJ, and CHJ. Reading of articles and final approval: All authors.
Funding
This research is financially supported by Korea University Grant (Grant no. K2321281).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Korea University Guro hospital approved the protocol (2022GR0318).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are not publicly available because of our institutional guidelines.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Peery, A.F.; Crockett, S.D.; Murphy, C.C.; Lund, J.L.; Dellon, E.S.; Williams, J.L.; Jensen, E.T.; Shaheen, N.J.; Barritt, A.S.; Lieber, S.R.; et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: update 2018. Gastroenterol 2019, 156, 254–272. [Google Scholar] [CrossRef]
- Tielleman, T.; Bujanda, D.; Cryer, B. Epidemiology and risk factors for upper gastrointestinal bleeding. Gastrointest Endosc Clin N Am 2015, 25, 415–428. [Google Scholar] [CrossRef]
- Mele, F.; Gendarini, C.; Pantoni, L. The use of dual antiplatelet therapy for ischemic cerebrovascular events. Neurol Sci 2023, 44, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Levine, G.N.; Jeong, Y.H.; Goto, S.; Anderson, J.L.; Huo, Y.; Mega, J.L.; Taubert, K.; Smith, S.C. Expert consensus document: World Heart Federation expert consensus statement on antiplatelet therapy in East Asian patients with ACS or undergoing PCI. Nat Rev Cardiol 2014, 11, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.J.; Lau, J.Y.; Ching, J.Y.; Wu, J.C.; Lee, Y.T.; Chiu, P.W.; Leung, V.K.; Wong, V.W.; Chan, F.K. Continuation of low-dose aspirin therapy in peptic ulcer bleeding: a randomized trial. Ann Intern Med 2010, 152, 1–9. [Google Scholar] [CrossRef]
- Kinnaird, T.D.; Stabile, E.; Mintz, G.S.; Lee, C.W.; Canos, D.A.; Gevorkian, N.; Pinnow, E.E.; Kent, K.M.; Pichard, A.D.; Satler, L.F.; et al. Incidence, predictors, and prognostic implications of bleeding and blood transfusion following percutaneous coronary interventions. Am J Cardiol 2003, 92, 930–935. [Google Scholar] [CrossRef]
- Nikolsky, E.; Mehran, R.; Stone, G.W. Gastrointestinal bleeding in percutaneous coronary intervention and acute coronary syndromes. Am J Cardiol 2009, 104, 22C–29C. [Google Scholar] [CrossRef]
- Bilal, M.; Samuel, R.; Khalil, M.K.; Singh, S.; Parupudi, S.; Abougergi, M.S. Nonvariceal upper GI hemorrhage after percutaneous coronary intervention for acute myocardial infarction: a national analysis over 11 months. Gastrointest Endosc 2020, 92, 65–74. [Google Scholar] [CrossRef]
- Costa, F.; Montalto, C.; Branca, M.; Hong, S.J.; Watanabe, H.; Franzone, A.; Vranckx, P.; Hahn, J.Y.; Gwon, H.C.; Feres, F.; et al. Dual antiplatelet therapy duration after percutaneous coronary intervention in high bleeding risk: a meta-analysis of randomized trials. Eur Heart J 2023, 44, 954–968. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J Clin Epidemiol 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
- Sostres, C.; Lanas, A. Gastrointestinal effects of aspirin. Nat Rev Gastroenterol Hepatol 2011, 8, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Chin, M.W.; Yong, G.; Bulsara, M.K.; Rankin, J.; Forbes, G.M. Predictive and protective factors associated with upper gastrointestinal bleeding after percutaneous coronary intervention: a case-control study. Am J Gastroenterol 2007, 102, 2411–2416. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.E.; Brodie, B.; Dixon, S.; Marsalese, D.; Brewington, S.; O’Neill, W.W.; Grines, L.L.; Grines, C.L. Incidence and prognostic impact of gastrointestinal bleeding after percutaneous coronary intervention for acute myocardial infarction. Am J Cardiol 2005, 96, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.; Ouejiaraphant, C.; Akarapatima, K.; Rattanasupa, A.; Prachayakul, V. Prospective comparison of the AIMS65 score, Glasgow-Blatchford score, and Rockall score for predicting clinical outcomes in patients with variceal and nonvariceal upper gastrointestinal bleeding. Clin Endosc 2021, 54, 211–221. [Google Scholar] [CrossRef]
- Choe, J.W.; Kim, S.Y.; Hyun, J.J.; Jung, S.W.; Jung, Y.K.; Koo, J.S.; Yim, H.J.; Lee, S.W. Is the AIMS 65 score useful in Prepdicting clinical outcomes in Korean patients with variceal and nonvariceal upper gastrointestinal bleeding? Gut Liver 2017, 11, 813–820. [Google Scholar] [CrossRef]
- Gupta, K.; Khan, A.; Kumar, M.; Sawalha, K.; Abozenah, M.; Singhania, R. Readmissions rates after myocardial infarction for gastrointestinal bleeding: A national perspective. Dig Dis Sci 2021, 66, 751–759. [Google Scholar] [CrossRef]
- Aoki, T.; Nagata, N.; Niikura, R.; Shimbo, T.; Tanaka, S.; Sekine, K.; Kishida, Y.; Watanabe, K.; Sakurai, T.; Yokoi, C.; et al. Recurrence and mortality among patients hospitalized for acute lower gastrointestinal bleeding. Clin Gastroenterol Hepatol 2015, 13, 488–494. [Google Scholar] [CrossRef]
- Tsujita, K.; Deguchi, H.; Uda, A.; Sugano, K. Upper gastrointestinal bleeding in Japanese patients with ischemic heart disease receiving vonoprazan or a proton pump inhibitor with multiple antithrombotic agents: A nationwide database study. J Cardiol 2020, 76, 51–57. [Google Scholar] [CrossRef]
- Lee, K.J.; Son, B.K.; Kim, G.H.; Jung, H.K.; Jung, H.Y.; Chung, I.K.; Sung, I.K.; Kim, J.I.; Kim, J.H.; Lee, J.S.; et al. Randomised phase 3 trial: tegoprazan, a novel potassium-competitive acid blocker, vs. esomeprazole in patients with erosive oesophagitis. Aliment Pharmacol Ther 2019, 49, 864–872. [Google Scholar] [CrossRef]
- Lee, K.N.; Lee, O.Y.; Chun, H.J.; Kim, J.I.; Kim, S.K.; Lee, S.W.; Park, K.S.; Lee, K.L.; Choi, S.C.; Jang, J.Y.; et al. Randomized controlled trial to evaluate the efficacy and safety of fexuprazan compared with esomeprazole in erosive esophagitis. World J Gastroenterol 2022, 28, 6294–6309. [Google Scholar] [CrossRef]
- Cho, Y.K.; Choi, M.G.; Choi, S.C.; Lee, K.M.; Kim, T.O.; Park, S.H.; Moon, J.S.; Lim, Y.J.; Kang, D.H.; Cheon, G.J.; et al. Randomised clinical trial: tegoprazan, a novel potassium-competitive acid blocker, or lansoprazole in the treatment of gastric ulcer. Aliment Pharmacol Ther 2020, 52, 789–797. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).