Submitted:
19 September 2023
Posted:
20 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
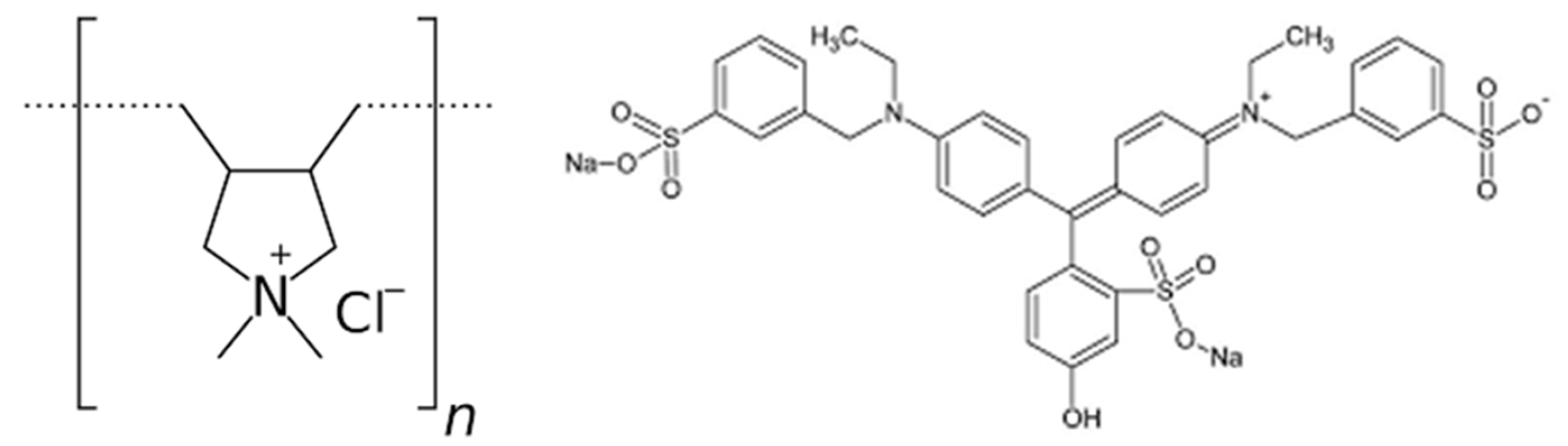
2.1. Materials
2.2. Complexation/calibration experiments description
2.3. Implementation in water with commercial PD
2.4. Influence of pH
2.5. Quantification of poly-DADMAC in coagulation water treatment samples
3. Results
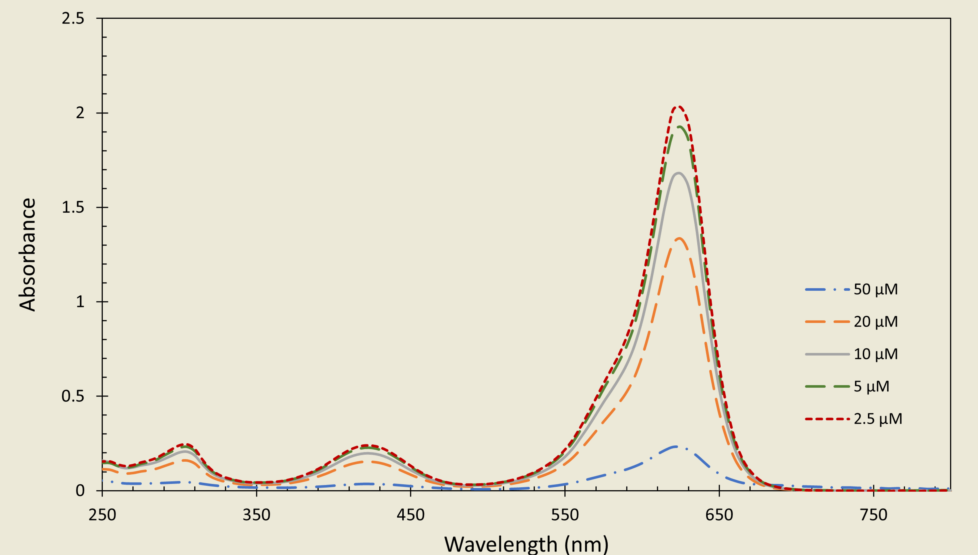
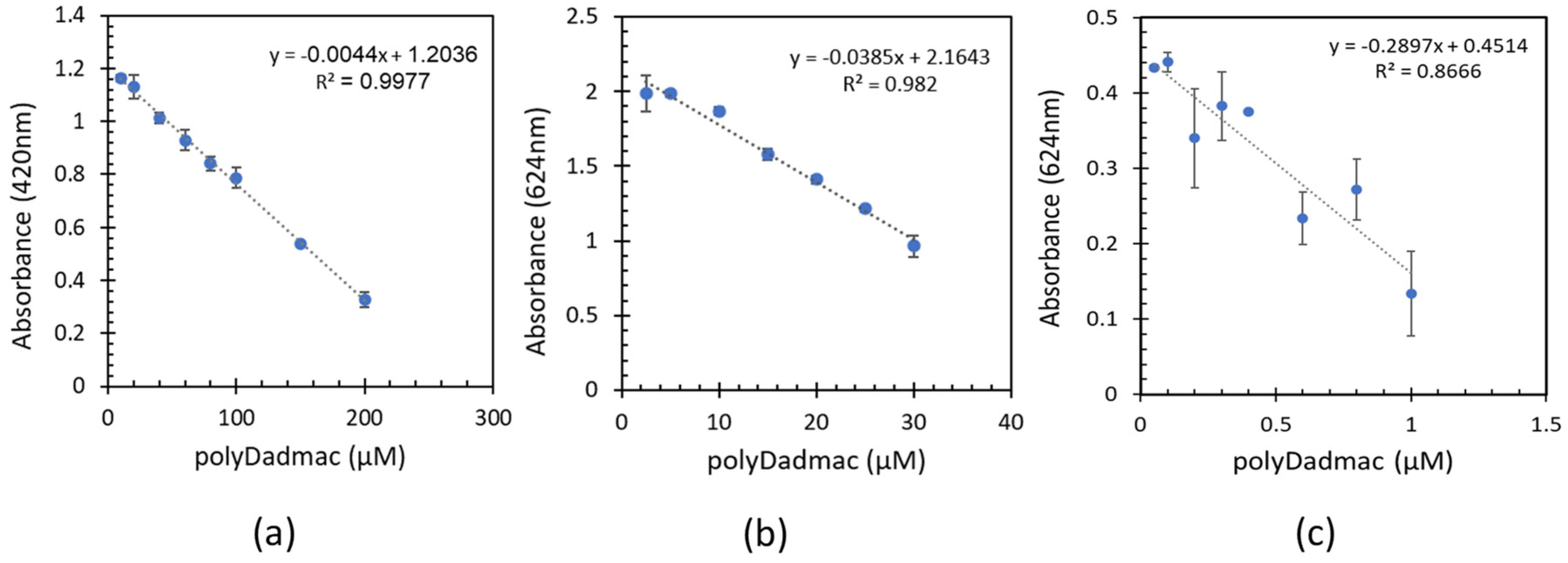
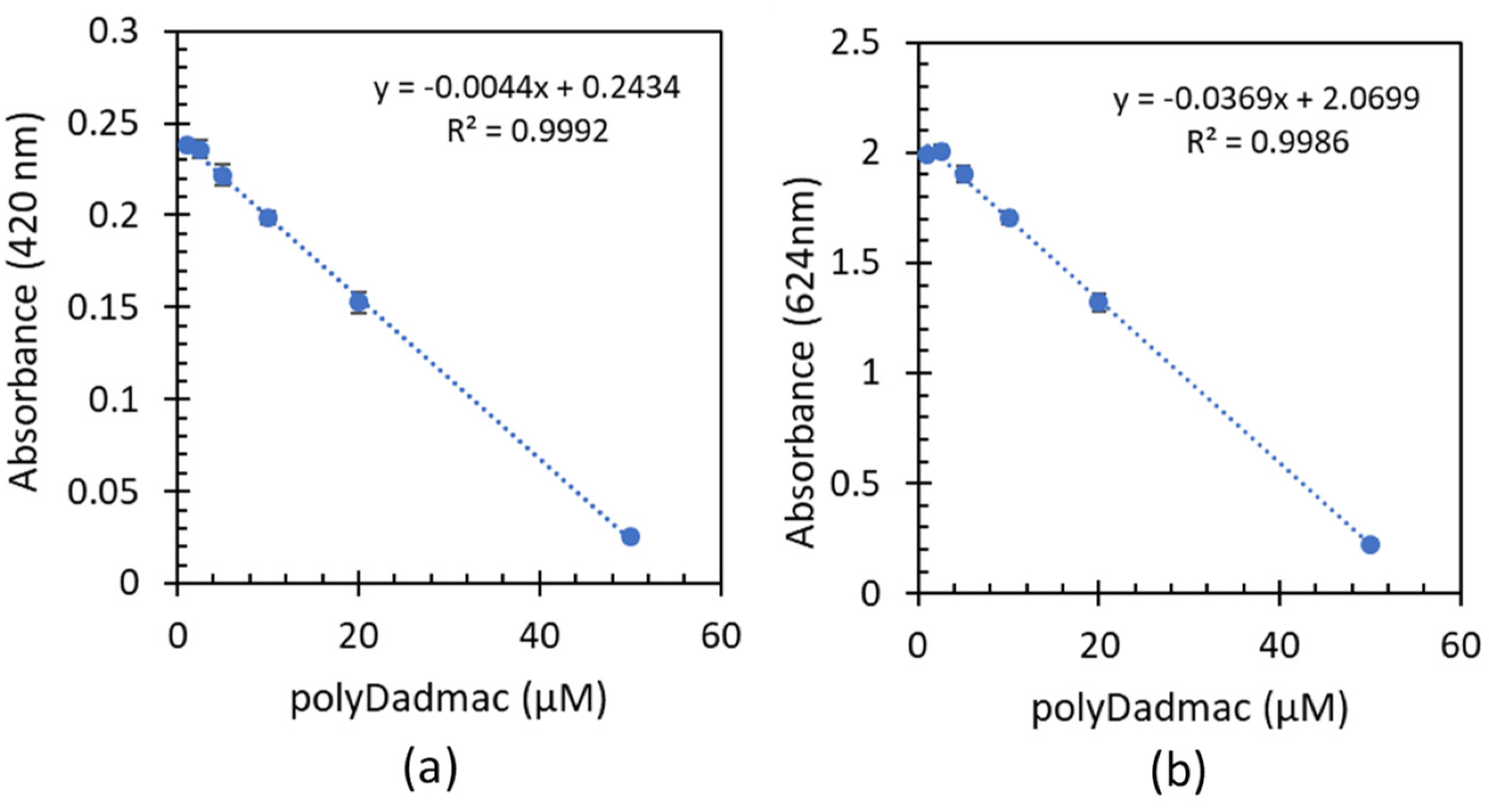
3.1. Quantification of analytical poly-DADMAC concentrations
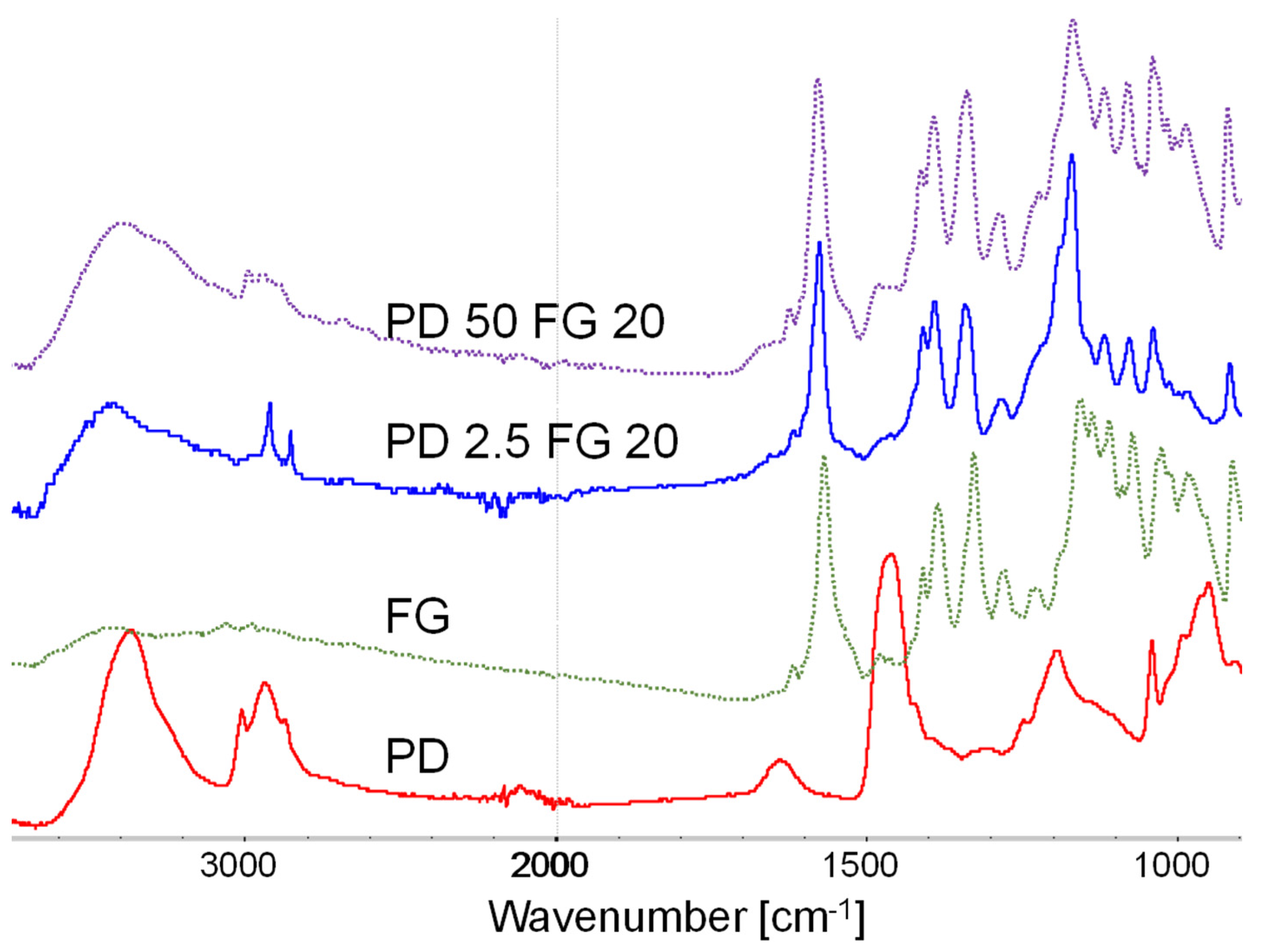
3.2. FTIR measurements of PD-FG complexes
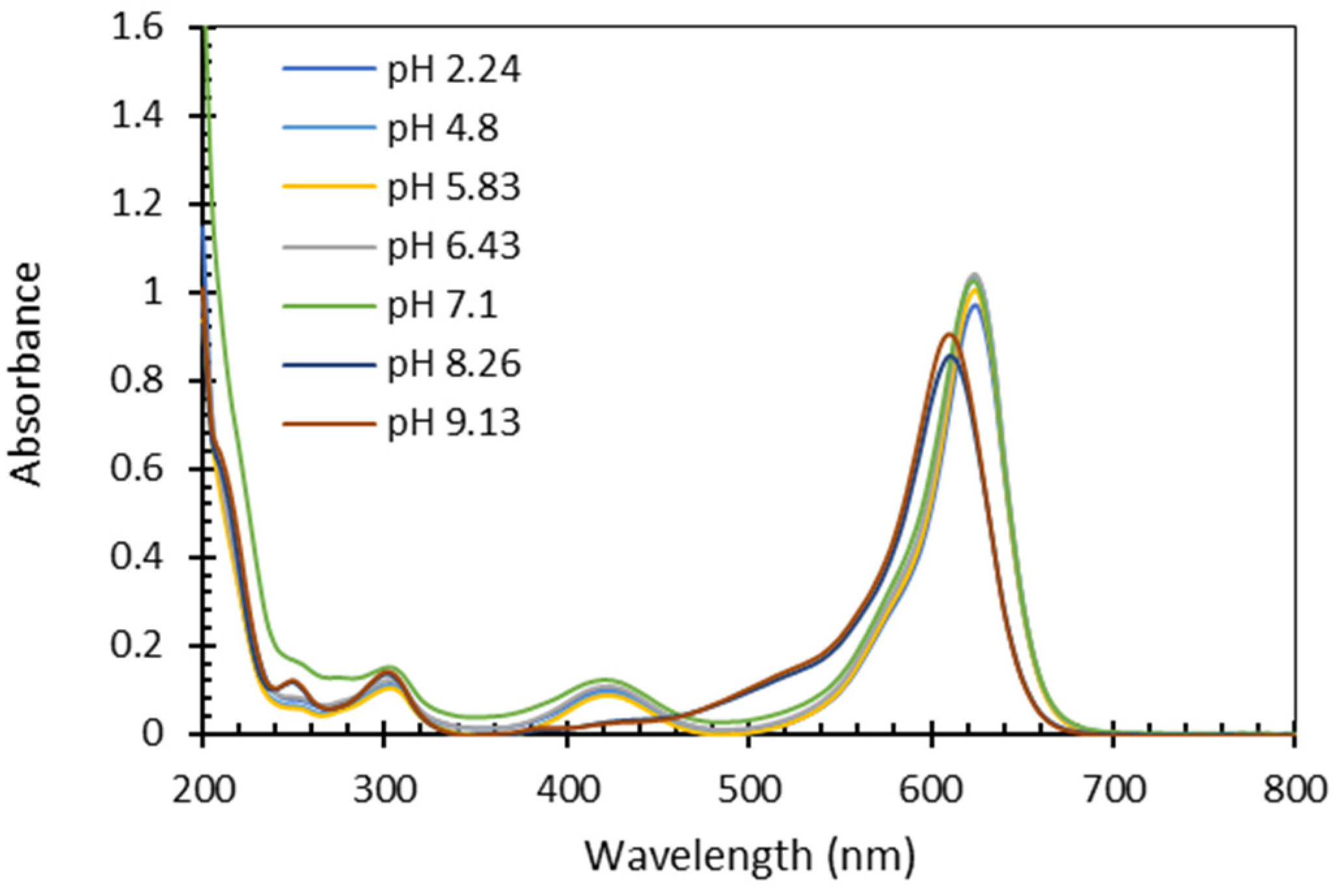
3.3. Influence of pH
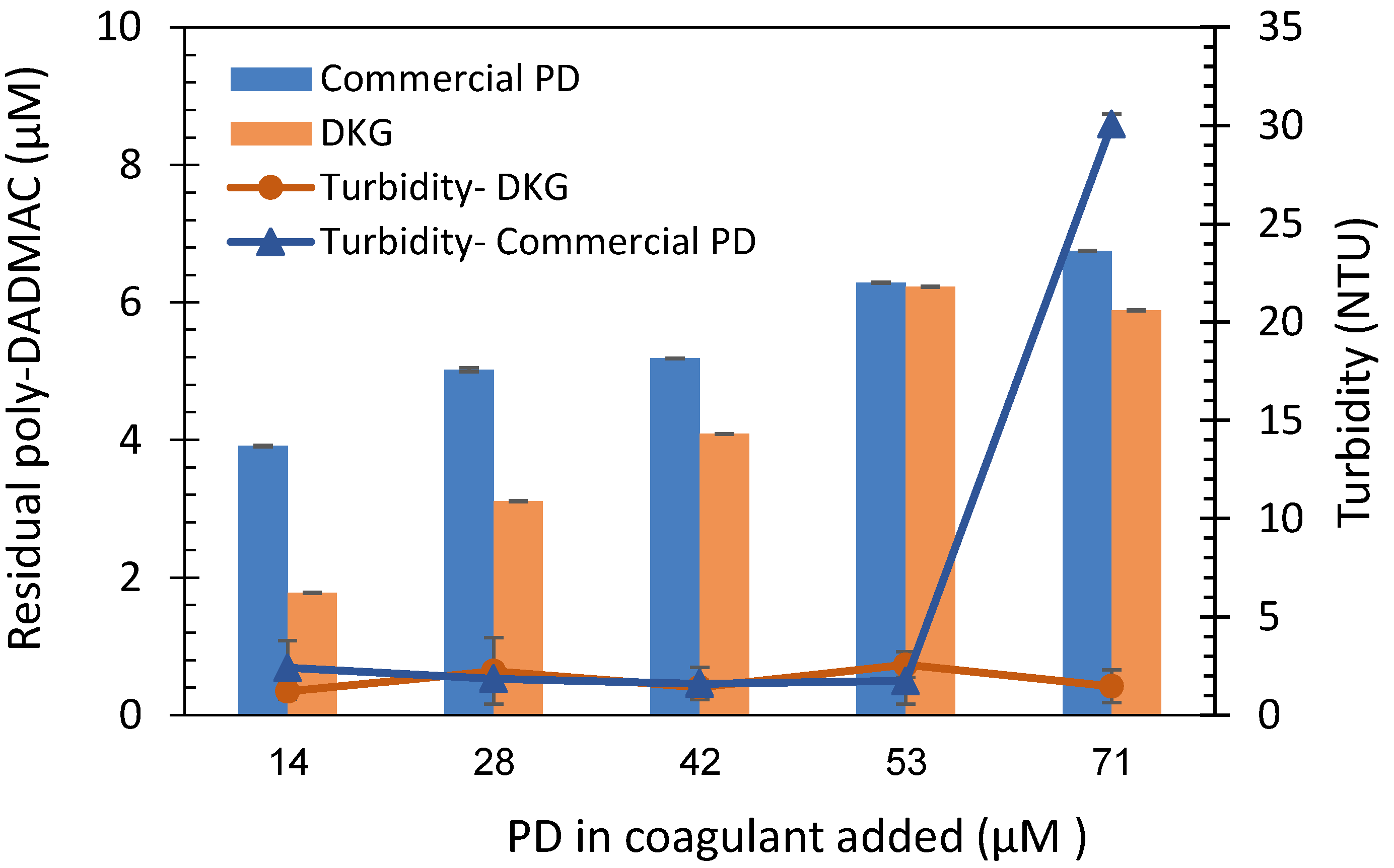
3.4. Quantification of commercial poly-DADMAC
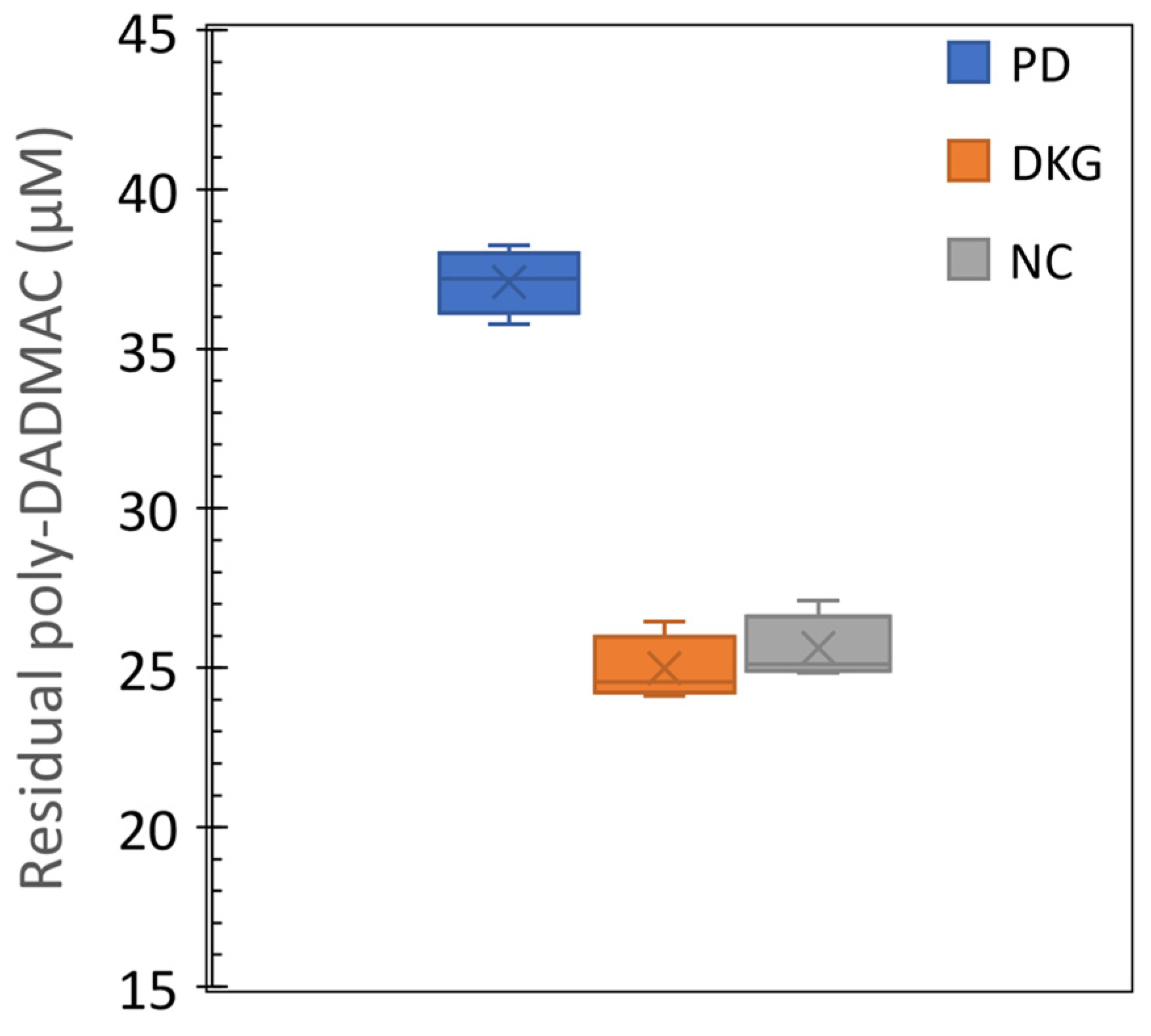
3.5. Quantification of poly-DADMAC in coagulation treatment samples
4. Conclusions
- In this study, a colorimetric quantification method for poly-DADMAC through complexation with fast green dye was developed and evaluated.
- The method exhibited high sensitivity with a detection limit of 0.02 µM (0.0032 mg L-1), meeting regulatory demands according to the world standard limitations.
- Quantification experiments of analytical PD concentrations including low, medium, and high ranges demonstrated a linear correlation between the absorbance and PD concentrations.
- The influence of pH on the quantification process was tested, revealing that the absorbance spectrum of FG remains unchanged within the pH range of 2 to 7, exhibiting an hypsochromic and hyperchromic shift at higher pH. Therefore, an acidification step (or accurate pH measurements) is essential before quantification to ensure the FG spectrum falls within the required range.
- The quantification method’s feasibility was demonstrated in coagulation experiments, estimating remaining PD concentrations in treated water samples.
- The method’s feasibility in estimating PD concentrations under actual coagulation conditions highlights its potential for efficient monitoring and control of coagulation processes. It offers a rapid, cost-effective, and sensitive tool for accurate PD concentration measurement. The colorimetric PD quantification holds promising potential for advancing water treatment systems and quality control.
- Further research may concentrate on upscaling the application of the quantification method for large-scale water treatment systems, as well as refining its adaptation and addressing limitations. This may include examining factors such as temperature, reaction time, and ionic strength.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation list
- PD poly-dadmac (Poly-diallyl-dimethylammonium chloride)
- FG Fast-green
- LOD limit of detection
- DKG kaolinite-PD nanocomposites
- NC sepiolite- PD nanocomposites
- PCD particle charge detector
- NTU nephelometric turbidity unit
- TSS total suspended solids
References
- Amuda, O.S.; Amoo, I.A. Coagulation/flocculation process and sludge conditioning in beverage industrial wastewater treatment. J. Hazard. Mater. 2007, 141, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Dautzenberg, H.; Görnitz, E.; Jaeger, W. Synthesis and characterization of poly(diallyldimethylammonium chloride) in a broad range of molecular weight. Macromol. Chem. Phys. 1998, 199, 1561–1571. [Google Scholar] [CrossRef]
- Becker, N.S. .; Bennett, D..; Bolto, B..; Dixon, D..; Eldridge, R..; Le, N..; Rye, C.. Detection of polyelectrolytes at trace levels in water by fluorescent tagging. React. Funct. Polym. 2004, 60, 183–193. [Google Scholar] [CrossRef]
- Razali, M.A.A.; Ahmad, Z.; Ahmad, M.S.B.; Ariffin, A. Treatment of pulp and paper mill wastewater with various molecular weight of polyDADMAC induced flocculation. Chem. Eng. J. 2011, 166, 529–535. [Google Scholar] [CrossRef]
- Ariffin, A.; Razali, M.A.A.; Ahmad, Z. PolyDADMAC and polyacrylamide as a hybrid flocculation system in the treatment of pulp and paper mills waste water. Chem. Eng. J. 2012, 179, 107–111. [Google Scholar] [CrossRef]
- Hamad, M.J.A.; Chirwa, E.M.N. Forward osmosis for water recovery using polyelectrolyte PolyDADMAC and DADMAC draw solutions as a low pressure energy saving process. Desalination 2019, 453, 89–101. [Google Scholar] [CrossRef]
- Sampl, C.; Schaubeder, J.; Hirn, U.; Spirk, S. Interplay of electrolyte concentration and molecular weight of polyDADMAC on cellulose surface adsorption. Int. J. Biol. Macromol. 2023, 239, 124286. [Google Scholar] [CrossRef]
- Ramos, P.; Singh Kalra, S.; Johnson, N.W.; Khor, C.M.; Borthakur, A.; Cranmer, B.; Dooley, G.; Mohanty, S.K.; Jassby, D.; Blotevogel, J.; et al. Enhanced removal of per- and polyfluoroalkyl substances in complex matrices by polyDADMAC-coated regenerable granular activated carbon. Environ. Pollut. 2022, 294, 118603. [Google Scholar] [CrossRef]
- Bhuyan, C.; Konwar, A.; Bora, P.; Rajguru, P.; Hazarika, S. Cellulose nanofiber-poly(ethylene terephthalate) nanocomposite membrane from waste materials for treatment of petroleum industry wastewater. J. Hazard. Mater. 2023, 442, 129955. [Google Scholar] [CrossRef]
- Gogoi, M.; Goswami, R.; Borah, A.; Sarmah, H.; Rajguru, P.; Hazarika, S. Amide functionalized DWCNT nanocomposite membranes for chiral separation of the racemic DOPA. Sep. Purif. Technol. 2021, 279, 119704. [Google Scholar] [CrossRef]
- Gabelich, C.J.; Ishida, K.P.; Bold, R.M. Testing of water treatment copolymers for compatibility with polyamide reverse osmosis membranes. Environ. Prog. 2005, 24, 410–416. [Google Scholar] [CrossRef]
- Wang, S.; Liu, C.; Li, Q. Fouling of microfiltration membranes by organic polymer coagulants and flocculants: Controlling factors and mechanisms. Water Res. 2011, 45, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Tang, H.; Shen, Y.; Liu, G.; Shi, W. Comparative analysis of membrane fouling mechanisms induced by colloidal polymer: Effects of sodium and calcium ions. J. Colloid Interface Sci. 2022, 608, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Ngo, H.H.; Li, J. A mini-review on membrane fouling. Bioresour. Technol. 2012, 122, 27–34. [Google Scholar] [CrossRef]
- Uzun, H.; Kim, D.; Karanfil, T. Removal of wastewater and polymer derived N-nitrosodimethylamine precursors with integrated use of chlorine and chlorine dioxide. Chemosphere 2019, 216, 224–233. [Google Scholar] [CrossRef]
- Park, S.H.; Padhye, L.P.; Wang, P.; Cho, M.; Kim, J.-H.; Huang, C.-H. N-nitrosodimethylamine (NDMA) formation potential of amine-based water treatment polymers: Effects of in situ chloramination, breakpoint chlorination, and pre-oxidation. J. Hazard. Mater. 2015, 282, 133–140. [Google Scholar] [CrossRef]
- Park, S.H.; Wei, S.; Mizaikoff, B.; Taylor, A.E.; Favero, C.; Huang, C.H. Degradation of amine-based water treatment polymers during chloramination as N-nitrosodimethylamine (NDMA) precursors. Environ. Sci. Technol. 2009, 43, 1360–1366. [Google Scholar] [CrossRef]
- Huang, C.H.; Padhye, L.P.; Park, S.H. PolyDADMAC and dimethylamine as precursors of N-nitrosodimethylamine during ozonation. Water Qual. Technol. Conf. Expo. 2011 2011, 2304–2315. [Google Scholar]
- Atkinson, A.J.; Fischer, N.; Donovan, S.; Bartlett, J.; Alrehaili, O.; Sinha, S.; Kommineni, S.; Herckes, P.; Westerhoff, P. Purification and removal of the low molecular weight fraction of polyDADMAC reduces N -nitrosodimethylamine formation during water treatment. Environ. Sci. Water Res. Technol. 2020, 6, 2492–2498. [Google Scholar] [CrossRef]
- T Manickum Occurrence, Fate and Preliminary Environmental Risk Assessment of Residual Poly-Diallyldimethyl Ammonium Chloride, and Some Disinfection By-Products, in Treated (Potable), and Environmental, Waters in the Umgeni Water Catchment in Kwazulu-Natal. SM J. Public Heal. Epidemiol. 2017, 3.
- Al Momani, F.A.; Örmeci, B. Measurement of polyacrylamide polymers in water and wastewater using an in-line UV-vis spectrophotometer. J. Environ. Chem. Eng. 2014, 2, 765–772. [Google Scholar] [CrossRef]
- Di Gaudio, F.; Barreca, S.; Orecchio, S. Diallyldimethylammonium Chloride (DADMAC) in Water Treated with Poly-Diallyldimethylammonium Chloride (PDADMAC) by Reversed-Phase Ion-Pair Chromatography—Electrospray Ionization Mass Spectrometry. Separations 2023, 10, 311. [Google Scholar] [CrossRef]
- John, W. Synthesis, properties and analysis of polydadmac for water purification. 2008.
- Mwangi, I.W.; Ngila, J.C.; Ndungu, P.; Msagati, T.A.M. Method Development for the Determination of Diallyldimethylammonium Chloride at Trace Levels by Epoxidation Process. Water, Air, Soil Pollut. 2013, 224, 1638. [Google Scholar] [CrossRef] [PubMed]
- Mwangi, I.W.; Catherine Ngila, J.; Ndungu, P.; Msagati, T. Preconcentration and spectrophotometric determination of polyDADMAC in treated water by in situ co-precipitation with naphthalene. Phys. Chem. Earth, Parts A/B/C 2014, 72–75, 54–60. [Google Scholar] [CrossRef]
- Mwangi, I.; Ngila, J.; Ndungu, P. A new spectrophotometric method for determination of residual polydiallyldimethylammonium chloride flocculant in treated water based on a diazotization-coupled ion pair. Water SA 2012, 38, 707–714. [Google Scholar] [CrossRef]
- Gumbi, B.; Ngila, J.C.; Ndungu, P.G. Gold nanoparticles for the quantification of very low levels of poly-diallyldimethylammonium chloride in river water. Anal. Methods 2014, 6, 6963. [Google Scholar] [CrossRef]
- Manickum, T.; John, W.; Toolsee, N.; Rajagopaul, R. Preliminary Performance Evaluation of the Gold Nanoparticle Method for Quantification of Residual Poly-(Diallyldimethyl Ammonium Chloride) in Treated Waters in the Umgeni Water Catchment, Kwazulu-Natal (South Africa). Hydrol. Curr. Res. 2015, 06. [Google Scholar]
- Magubane, S.E.; Ntlhoro, S.; Sabela, M.; Kanchi, S.; Mlambo, M.; Onwubu, S.C.; Mdluli, P.S.; Inamuddin; Asiri, A. M. Novel on-site residual screening of poly-diallyldimethylammonium chloride in treated potable water using gold nanoparticle based lovibond color filters. J. Taiwan Inst. Chem. Eng. 2019, 101, 159–166. [Google Scholar] [CrossRef]
- Majam, S.; Thompson, P.A. Polyelectrolyte determination in drinking water. Water SA 2006, 32, 705–707. [Google Scholar] [CrossRef]
- Thilak Kumar, R.; Umamaheswari, S. FTIR, FTR and UV-Vis analysis of carbamazepine. Res. J. Pharm. Biol. Chem. Sci. 2011, 2, 685–693. [Google Scholar]
- Mendelovits, A.; Prat, T.; Gonen, Y.; Rytwo, G. Improved colorimetric determination of chitosan concentrations by dye binding. Appl. Spectrosc. 2012, 66, 979–982. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, N.; Murugadoss, G.; Mohamed, A.A.A.; Kumar, M.R.; Peera, S.G.; Sakthivel, P. A Novel Nanocomposite Based on Triazine Based Covalent Organic Polymer Blended with Porous g-C3N4 for Photo Catalytic Dye Degradation of Rose Bengal and Fast Green. Molecules 2022, 27. [Google Scholar] [CrossRef] [PubMed]
- Rytwo, G.; Lavi, R.; Rytwo, Y.; Monchase, H.; Dultz, S.; König, T.N. Clarification of olive mill and winery wastewater by means of clay-polymer nanocomposites. Sci. Total Environ. 2013, 442, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Rytwo, G. The Use of Clay-Polymer Nanocomposites in Wastewater Pretreatment. Sci. World J. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Rytwo, G. Hybrid Clay-Polymer Nanocomposites for the Clarification of Water and Effluents. Recent Pat. Nanotechnol. 2017, 11, 181–193. [Google Scholar] [CrossRef]
- Rytwo, G. US20190152813 Method for production of potable water 2019.
- Sukenik, A.; Beardall, J.; Hadas, O. Photosynthetic characterization of developing and mature akinetes of Aphanizomenon ovalisporum (Cyanoprokaryota). J. Phycol. 2007, 43, 780–788. [Google Scholar] [CrossRef]
- Rytwo, G.; Lavi, R.; König, T.N.; Avidan, L. Direct Relationship Between Electrokinetic Surface-charge Measurement of Effluents and Coagulant Type and Dose. Colloids Interface Sci. Commun. 2014, 1, 27–30. [Google Scholar] [CrossRef]
- Kozak, M.; Domka, L. Adsorption of the quaternary ammonium salts on montmorillonite. J. Phys. Chem. Solids 2004, 65, 441–445. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of infrared spectra, a practical approach. In Encyclopaedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2000; pp. 10815–10837. [Google Scholar]
- Rytwo, G.; Malka, H. A pilot plant for the treatment of cowshed effluents. Water Irrig. 2013, 530, 6–9. [Google Scholar]
- Inbar, Y. New Standards for Treated Wastewater Reuse in Israel. In Proceedings of the Wastewater Reuse--Risk Assessment, Decision-Making and Environmental Security; Zaidi, M.K., Ed. Springer Netherlands: Dordrecht, 2007; pp. 291–296. [Google Scholar]
- Zeng, T.; Li, R.J.; Mitch, W.A. Structural Modifications to Quaternary Ammonium Polymer Coagulants to Inhibit N-Nitrosamine Formation. Environ. Sci. Technol. 2016, 50, 4778–4787. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




