Submitted:
22 August 2023
Posted:
23 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
Hypothesis
2. Materials and methods
Statistics
3. Results
4. Discussion
4.1. Limitations of our study
4.2. Strength of our study
Author Contributions
Conflicts of Interest
References
- Ziats CA, Patterson WG, Friez M. Syndromic Autism Revisited: Review of the Literature and Lessons Learned. Pediatr Neurol. 2021, 114:21-25. [CrossRef]
- Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z,. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. MMWR Surveill Summ. 2018, 67:1-23. [CrossRef]
- Rapin I, Tuchman RF. Autism: definition, neurobiology, screening, diagnosis. Pediatr Clin North Am. 2008, 55:1129-46, . [CrossRef]
- Kočovská E, Fernell E, Billstedt E, Minnis H, Gillberg C. Vitamin D and autism: clinical review. Res Dev Disabil. 2012, 33:1541-50. [CrossRef]
- Rylaarsdam L, Guemez-Gamboa A. Genetic Causes and Modifiers of Autism Spectrum Disorder. Front Cell Neurosci. 2019, 20;13:385. [CrossRef]
- Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet. 2008, 9:341-55. [CrossRef]
- Finegold SM, Downes J, Summanen PH. Microbiology of regressive autism. Anaerobe.2012, 18:260-2. [CrossRef]
- Finegold SM. (2011) State of the art; microbiology in health and disease. Intestinal bacterial flora in autism. Anaerobe. 2011, 17:367-8. [CrossRef]
- Finegold SM. Therapy and epidemiology of autism--clostridial spores as key elements. Med Hypotheses, 2008, 70:508-11. [CrossRef]
- Pequegnat B, Sagermann M, Valliani M, Toh M, Chow H, Allen-Vercoe E, et al. A vaccine and diagnostic target for Clostridium bolteae, an autism-associated bacterium. Vaccine. 2013, 31:2787-90. [CrossRef]
- Finegold SM. Desulfovibrio species are potentially important in regressive autism. Med Hypotheses, 2011, 77:270-4. [CrossRef]
- Heberling CA, Dhurjati PS, Sasser M. Hypothesis for a systems connectivity model of Autism Spectrum Disorder pathogenesis: links to gut bacteria, oxidative stress, and intestinal permeability. Med Hypotheses. 2013 80:264-70. doi:10.1016/j.mehy.2012.11.044.
- Zafeiriou DI, Ververi A, Vargiami E. Childhood autism and associated comorbidities. Brain Dev. 2007, 29:257-72. [CrossRef]
- Webb SJ, Mourad PD. Prenatal Ultrasonography and the Incidence of Autism Spectrum Disorder. JAMA Pediatr. 2018, 172:319-320. [CrossRef]
- Richmond BJ. Hypothesis: conjugate vaccines may predispose children to autism spectrum disorders. Med Hypotheses. 2011, 77:940-7. [CrossRef]
- Steinman G, Mankuta D. Insulin-like growth factor and the etiology of autism. Med Hypotheses. 2013, 80:475-80. [CrossRef]
- Odent M. (2010) Autism and anorexia nervosa: Two facets of the same disease? Med Hypotheses. 75(1):79-81. [CrossRef]
- Herbert MR, Sage C. Autism and EMF? Plausibility of a pathophysiological link part II. Pathophysiology. 2013, 20:211-34. [CrossRef]
- Good P. Evidence the U.S. autism epidemic initiated by acetaminophen (Tylenol) is aggravated by oral antibiotic amoxicillin/clavulanate (Augmentin) and now exponentially by herbicide glyphosate (Roundup). Clin Nutr ESPEN. 2018, 23:171-183. [CrossRef]
- Marchezan J. Editorial: Autism Spectrum Disorder and Autoimmune Diseases: A Pathway in Common? J Am Acad Child Adolesc Psychiatry. 2019, 58:481-483. [CrossRef]
- Gottfried C, Bambini-Junior V, Francis F, Riesgo R, Savino W. The Impact of Neuroimmune Alterations in Autism Spectrum Disorder. Front Psychiatry, 2015, 6:121. [CrossRef]
- Mead J, Ashwood P. Evidence supporting an altered immune response in ASD. Immunol Lett. 2015, 163:49-55. [CrossRef]
- Wing L. The autistic spectrum. Lancet. 1997, 350:1761-6. [CrossRef]
- Caronna EB, Milunsky JM, Tager-Flusberg H. Autism spectrum disorders: clinical and research frontiers. Arch Dis Child. 2008, 93:518-23. [CrossRef]
- Phillips KL, Schieve LA, Visser S, Boulet S, Sharma AJ, Kogan MD, et al Prevalence and impact of unhealthy weight in a national sample of US adolescents with autism and other learning and behavioral disabilities. Matern Child Health J. 2014, 18:1964-75. [CrossRef]
- Sharon G, Cruz NJ, Kang DW, Gandal MJ, Wang B, Kim YM, et al Human Gut Microbiota from Autism Spectrum Disorder Promote Behavioral Symptoms in Mice. Cell. 2019, 30:1600-1618.e17. [CrossRef]
- Nagaraju K, Sudeep KS, Kurhekar MP. A cellular automaton model to find the risk of developing autism through gut-mediated effects. Comput Biol Med. 2019, 110:207-217. [CrossRef]
- Weston B, Fogal B, Cook D, Dhurjati P. An agent-based modeling framework for evaluating hypotheses on risks for developing autism: effects of the gut microbial environment. Med Hypotheses. 2015, 84:395-401. [CrossRef]
- Finegold SM, Dowd SE, Gontcharova V, Liu C, Henley KE, Wolcott RD, et al 3rd. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe. 2010, 16:444-53. [CrossRef]
- De Angelis M, Piccolo M, Vannini L, Siragusa S, De Giacomo A, Serrazzanetti DI, et al Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLoS One. 2013, 8:e76993. [CrossRef]
- Finegold SM, Molitoris D, Song Y, Liu C, Vaisanen ML, Bolte E, et al Gastrointestinal microflora studies in late-onset autism. Clin Infect Dis. 2002, 35(Suppl 1):S6-S16. [CrossRef]
- Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Elevated fecal short chain fatty acid and ammonia concentrations in children with autism spectrum disorder. Dig Dis Sci. 2012, 57:2096-102. [CrossRef]
- Parracho HM, Bingham MO, Gibson GR, McCartney AL. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J Med Microbiol. 2005, 54:987-991. [CrossRef]
- Andreo-Martínez P, García-Martínez N, Sánchez-Samper EP, Martínez-González AE. An approach to gut microbiota profile in children with autism spectrum disorder. Environ Microbiol Rep. 2020, 12:115-135. [CrossRef]
- S.M. Bolte ER. Autism and Clostridium tetani. Med Hypotheses. 1998, 51:133-44. [CrossRef]
- Tomova A, Husarova V, Lakatosova S, Bakos J, Vlkova B, Babinska K, et al Gastrointestinal microbiota in children with autism in Slovakia. Physiol Behav. 2015, 138:179-87. [CrossRef]
- De Angelis M, Francavilla R, Piccolo M, De Giacomo A, Gobbetti M. Autism spectrum disorders and intestinal microbiota. Gut Microbes. 2015, 6:207-13. [CrossRef]
- Persico AM, Napolioni V. Urinary p-cresol in autism spectrum disorder. Neurotoxicol Teratol. 2013, 36:82-90. [CrossRef]
- Macfabe DF. Short-chain fatty acid fermentation products of the gut microbiome: implications in autism spectrum disorders. Microb Ecol Health Dis. 2012, 24;23. [CrossRef]
- Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, et al The Microbiota-Gut-Brain Axis. Physiol Rev. 2019, 99:1877-2013. [CrossRef]
- Roullet FI, Wollaston L, Decatanzaro D, Foster JA. Behavioral and molecular changes in the mouse in response to prenatal exposure to the anti-epileptic drug valproic acid. Neuroscience. 2010, 170:514-22. [CrossRef]
- Zheng Y, Bek MK, Prince NZ, Peralta Marzal LN, Garssen J, Perez Pardo P, Kraneveld AD. The Role of Bacterial-Derived Aromatic Amino Acids Metabolites Relevant in Autism Spectrum Disorders: A Comprehensive Review. Front Neurosci. 2021, 15:738220. [CrossRef]
- Atladóttir HÓ, Henriksen TB, Schendel DE, Parner ET. Autism after infection, febrile episodes, and antibiotic use during pregnancy: an exploratory study. Pediatrics. 2012 130(6):e1447-54. [CrossRef]
- Niehus, R., and Lord, C. Early medical history of children with autism spectrum disorders. J. Dev. Behav. Pediatr. 2006, 2(Suppl.), S120–S127. [CrossRef]
- Davidovitch M, Slobodin O, Weisskopf MG, Rotem RS. Age-Specific Time Trends in Incidence Rates of Autism Spectrum Disorder Following Adaptation of DSM-5 and Other ASD-Related Regulatory Changes in Israel. Autism Res. 2020, 13:1893-1901. [CrossRef]
- Sandler RH, Finegold SM, Bolte ER, Buchanan CP, Maxwell AP, Väisänen ML, et al Short-term benefit from oral vancomycin treatment of regressive-onset autism. J Child Neurol. 2000, 15:429-35. [CrossRef]
- Żebrowska P, Łaczmańska I, Łaczmański Ł. Future Directions in Reducing Gastrointestinal Disorders in Children With ASD Using Fecal Microbiota Transplantation. Front Cell Infect Microbiol. 2021, 11:630052. [CrossRef]
- Abuaish S, Al-Otaibi NM, Aabed K, Abujamel TS, Alzahrani SA, Alotaibi SM, et al The Efficacy of Fecal Transplantation and Bifidobacterium Supplementation in Ameliorating Propionic Acid-Induced Behavioral and Biochemical Autistic Features in Juvenile Male Rats. J Mol Neurosci. 2022, 72:372-381. [CrossRef]
- Johnson D, Letchumanan V, Thurairajasingam S, Lee LH. A Revolutionizing Approach to Autism Spectrum Disorder Using the Microbiome. Nutrients. 2020, 12:1983. [CrossRef]
- Mueller NT, Bakacs E, Combellick J, Grigoryan Z, Dominguez-Bello MG. The infant microbiome development: mom matters. Trends Mol Med. 2015, 21:109-17. [CrossRef]
- Steinman G. The putative etiology and prevention of autism. Prog Mol Biol Transl Sci. 2020, 173:1-34. [CrossRef]
- Hamad AF, Alessi-Severini S, Mahmud SM, Brownell M, Kuo IF. Prenatal antibiotics exposure and the risk of autism spectrum disorders: A population-based cohort study. PLoS One. 2019, 14:e0221921. [CrossRef]
- Abelson N, Meiri G, Solomon S, Flusser H, Michaelovski A, Dinstein I, et al Association Between Antenatal Antimicrobial Therapy and Autism Spectrum Disorder-A Nested Case-Control Study. Front Psychiatry. 2021, 12:771232. PMID: 34867555. [CrossRef]
- Volkova A, Ruggles K, Schulfer A, Gao Z, Ginsberg SD, Blaser MJ. Effects of early-life penicillin exposure on the gut microbiome and frontal cortex and amygdala gene expression. Science, 2021, 24:102797. [CrossRef]
- Leclercq S, Mian FM, Stanisz AM, Bindels LB, Cambier E, Ben-Amram H, et al. Low-dose penicillin in early life induces long-term changes in murine gut microbiota, brain cytokines and behavior. Nat Commun. 2017, 8:15062. [CrossRef]
- Dhudasia MB, Flannery DD, Pfeifer MR, Puopolo KM. (2021) Updated Guidance: Prevention and Management of Perinatal Group B Streptococcus Infection. Neoreviews. 2021, (3):e177-e188. [CrossRef]
- Amangelsin Y, Semenova Y, Dadar M, Aljofan M, Bjørklund G. The Impact of Tetracycline Pollution on the Aquatic Environment and Removal Strategies. Antibiotics (Basel). 2023, 12:440. PMID: 36978308; PMCID: PMC10044355. [CrossRef]
- Lee BK, Magnusson C, Gardner RM, Blomström Å, Newschaffer CJ, Burstyn I, et al Maternal hospitalization with infection during pregnancy and risk of autism spectrum disorders. Brain Behav Immun. 2015, 44:100-5. [CrossRef]
- Brown AS, Sourander A, Hinkka-Yli-Salomäki S, McKeague IW, Sundvall J, Surcel HM. Elevated maternal C-reactive protein and autism in a national birth cohort. Mol Psychiatry. 2014, 19:259-64. [CrossRef]
- Atladóttir HO, Thorsen P, Østergaard L, Schendel DE, Lemcke S, Abdallah M, et al Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord. 2010, 40:1423-30. [CrossRef]
- Choi GB, Yim YS, Wong H, Kim S, Kim H, Kim SV, et al. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science, 2016, 26;351(6276):933-9. [CrossRef]
- Kim S, Kim H, Yim YS, Ha S, Atarashi K, Tan TG, et al (2017) Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature, 2017, 28;549(7673):528-532. [CrossRef]
- O’Connor R, Moloney GM, Fulling C, O’Riordan KJ, Fitzgerald P, Bastiaanssen TFS, et al. Maternal antibiotic administration during a critical developmental window has enduring neurobehavioural effects in offspring mice. Behav Brain Res. 2021, 404:113156. [CrossRef]
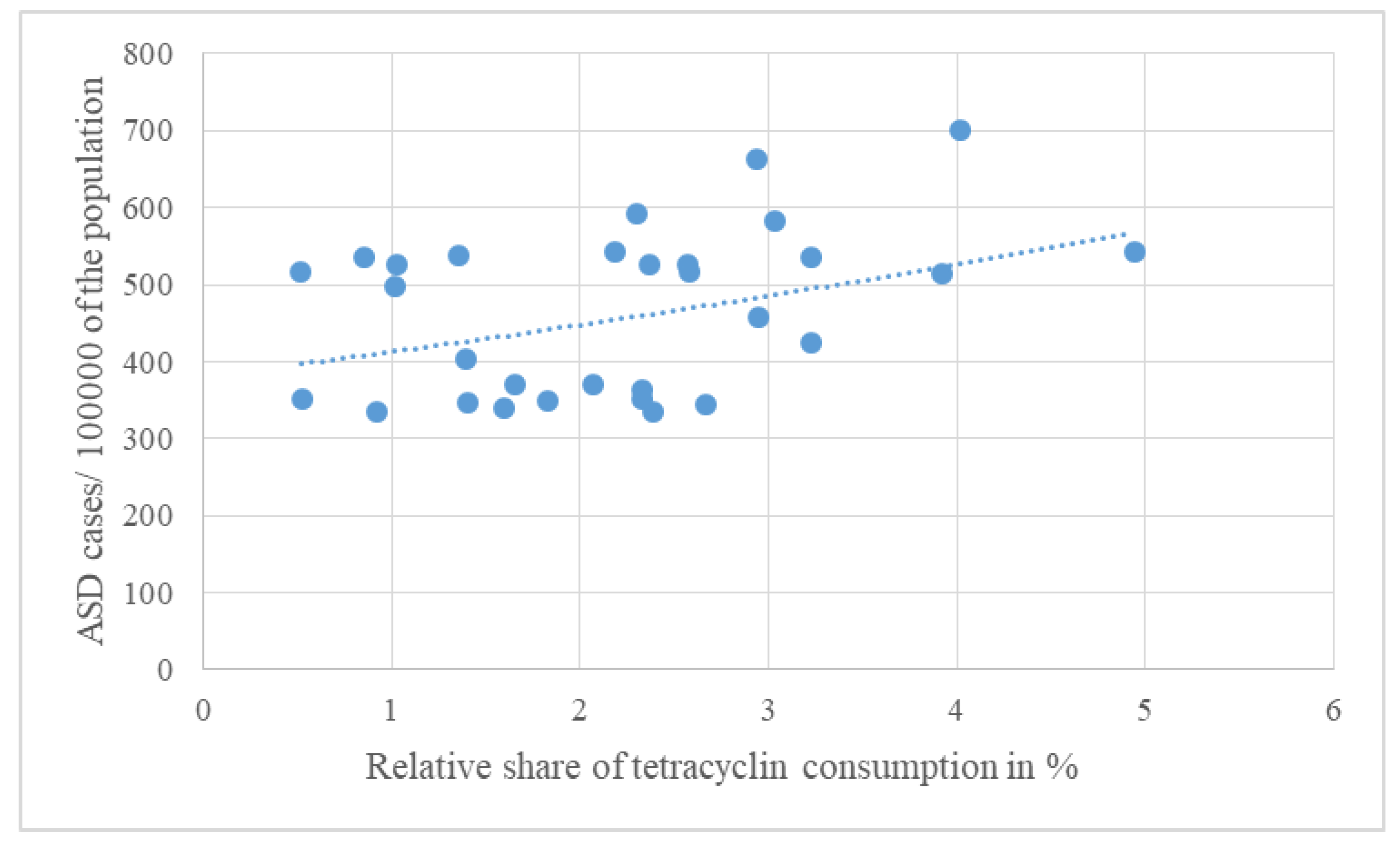
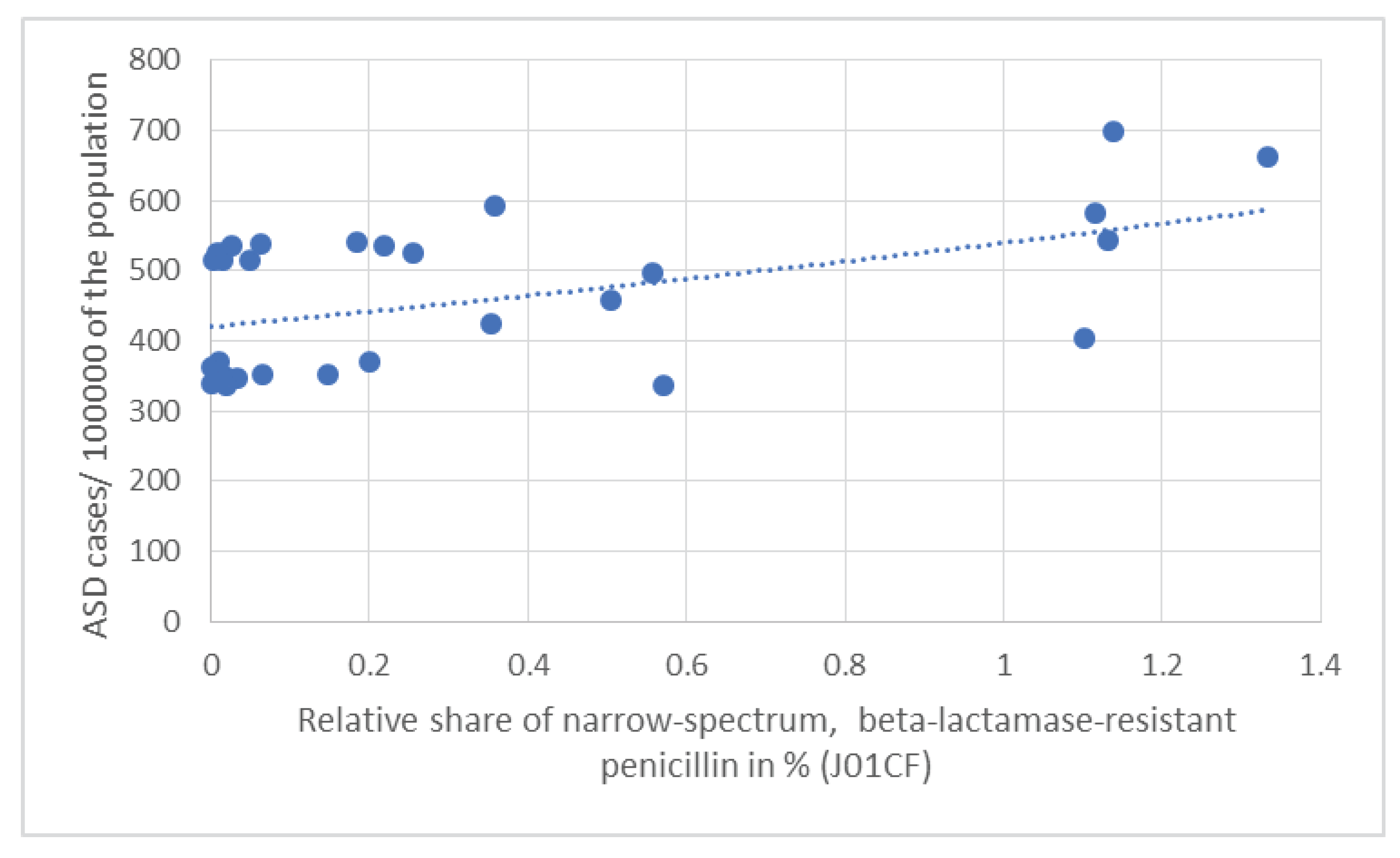
| Average antibiotic consumption 1997-2020 | J01 (DID) | J01A% | J01C% | J01CA% | J01CE% | J01CF% | J01CR% | J01D% | J01F% | J01M% | People living with ASD 2023/100000 population | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Countries | ||||||||||||
| UK | 15.294 | 26.259 | 38.257 | 21.296 | 4.878 | 7.447 | 4.799 | 3.570 | 17.177 | 3.492 | 700.070 | |
| Sweden | 13.370 | 21.960 | 47.771 | 7.831 | 28.579 | 9.970 | 1.361 | 2.094 | 5.460 | 6.171 | 661.850 | |
| Netherlands | 9.218 | 24.995 | 32.046 | 13.734 | 3.873 | 3.884 | 10.545 | 0.966 | 15.003 | 9.069 | 591.540 | |
| Ireland | 18.256 | 16.608 | 45.021 | 15.425 | 5.094 | 6.113 | 18.482 | 8.600 | 18.695 | 4.700 | 583.690 | |
| Iceland | 19.148 | 25.804 | 48.146 | 17.715 | 12.774 | 5.907 | 11.657 | 2.961 | 8.147 | 4.162 | 543.420 | |
| Luxembourg | 22.410 | 9.768 | 35.582 | 13.788 | 0.393 | 0.826 | 20.607 | 18.237 | 17.840 | 10.768 | 541.660 | |
| Malta | 18.465 | 7.344 | 33.929 | 2.908 | 0.498 | 0.336 | 30.176 | 21.847 | 20.422 | 11.373 | 537.950 | |
| Cyprus | 27.338 | 11.800 | 34.582 | 11.405 | 0.369 | 0.095 | 22.803 | 21.666 | 11.435 | 16.911 | 535.350 | |
| Spain | 17.697 | 4.826 | 51.794 | 20.405 | 0.571 | 1.232 | 29.011 | 11.804 | 14.341 | 13.330 | 535.140 | |
| Belgium | 21.427 | 11.051 | 40.720 | 17.716 | 0.425 | 1.195 | 21.366 | 11.140 | 14.958 | 10.295 | 526.130 | |
| Austria | 11.683 | 8.816 | 36.386 | 7.010 | 8.097 | 0.068 | 21.176 | 13.396 | 26.329 | 11.221 | 526.020 | |
| Germany | 12.610 | 20.412 | 27.113 | 16.122 | 8.438 | 0.111 | 2.395 | 15.131 | 18.184 | 9.556 | 525.310 | |
| Italy | 21.524 | 2.407 | 42.241 | 16.303 | 0.060 | 0.070 | 25.832 | 13.171 | 21.957 | 14.277 | 516.090 | |
| Greece | 30.474 | 8.470 | 28.152 | 13.415 | 1.447 | 0.013 | 13.185 | 23.571 | 26.337 | 8.814 | 515.700 | |
| Finland | 16.157 | 24.293 | 28.217 | 14.539 | 9.371 | 0.297 | 4.023 | 12.960 | 9.123 | 4.945 | 514.680 | |
| Portugal | 18.094 | 5.610 | 43.307 | 11.087 | 0.155 | 3.078 | 29.004 | 12.756 | 17.569 | 13.795 | 496.610 | |
| Norway | 15.046 | 19.567 | 40.635 | 12.754 | 24.465 | 3.356 | 0.060 | 0.957 | 10.415 | 3.110 | 457.900 | |
| France | 24.478 | 13.171 | 46.666 | 27.796 | 0.735 | 1.446 | 16.615 | 11.512 | 16.697 | 7.856 | 425.410 | |
| Denmark | 14.068 | 9.937 | 62.425 | 19.420 | 32.521 | 7.840 | 2.687 | 0.213 | 14.437 | 3.234 | 403.840 | |
| Estonia | 10.947 | 18.928 | 33.808 | 22.125 | 2.412 | 0.091 | 9.199 | 8.148 | 17.786 | 7.582 | 369.490 | |
| Lithuania | 16.714 | 9.908 | 52.315 | 30.531 | 13.312 | 1.191 | 7.341 | 7.784 | 10.452 | 5.881 | 369.320 | |
| Latvia | 10.653 | 21.862 | 37.924 | 25.505 | 0.901 | 0.019 | 11.508 | 5.107 | 13.470 | 9.218 | 362.530 | |
| Slovenia | 12.873 | 4.109 | 55.543 | 16.686 | 15.397 | 1.150 | 22.380 | 4.350 | 19.125 | 9.827 | 351.370 | |
| Czech Rep. | 14.685 | 15.887 | 37.719 | 9.227 | 12.782 | 0.443 | 15.131 | 10.555 | 20.620 | 7.014 | 350.850 | |
| Slovakia | 21.098 | 8.660 | 39.241 | 10.579 | 13.366 | 0.081 | 15.253 | 17.082 | 21.955 | 8.731 | 349.180 | |
| Croatia | 17.911 | 7.839 | 42.326 | 13.774 | 5.622 | 0.190 | 22.746 | 17.710 | 14.745 | 8.352 | 346.790 | |
| Poland | 18.773 | 14.228 | 32.952 | 19.459 | 2.243 | 0.117 | 11.144 | 12.332 | 17.823 | 6.797 | 345.130 | |
| Hungary | 14.711 | 10.842 | 35.817 | 9.972 | 4.310 | 0.000 | 21.508 | 14.696 | 20.760 | 12.528 | 340.310 | |
| Romania | 22.897 | 4.044 | 46.840 | 17.745 | 3.035 | 2.494 | 23.925 | 18.697 | 12.517 | 12.901 | 335.890 | |
| Bulgaria | 17.828 | 13.383 | 36.740 | 22.655 | 5.346 | 0.107 | 8.750 | 14.988 | 14.522 | 11.168 | 335.580 | |
| Pearson R | 0.039 | 0.373 | -0.146 | -0.278 | -0.032 | 0.524 | -0.078 | -0.157 | -0.121 | -0.089 | ||
| Pearson p | 0.839 | 0.043 | 0.442 | 0.137 | 0.865 | 0.003 | 0.682 | 0.408 | 0.523 | 0.640 | ||
| OR | 1.077 | 1.312 | 1.131 | 0.808 | 0.725 | 3.240 | 0.892 | 1.073 | 1.063 | 1.296 | ||
| CI95% | 0.922 - 1.259 | 0.995 - 1.791 | 0.934 - 1.395 | 0.649 - 0.957 | 0.543 - 0.885 | 1.710 - 8.852 | 0.747 - 1.029 | 0.859 - 1.354 | 0.868 - 1.308 | 0.954 - 1.811 | ||
| p | 0.348 | 0.065 | 0.221 | 0.028 | 0.009 | 0.004 | 0.154 | 0.534 | 0.554 | 0.107 | ||
| Kruskal-Wallis p | 0.679 | 0.342 | 0.606 | 0.668 | 0.278 | 0.032 | 0.481 | 0.733 | 0.447 | 0.903 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




