Submitted:
18 August 2023
Posted:
18 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
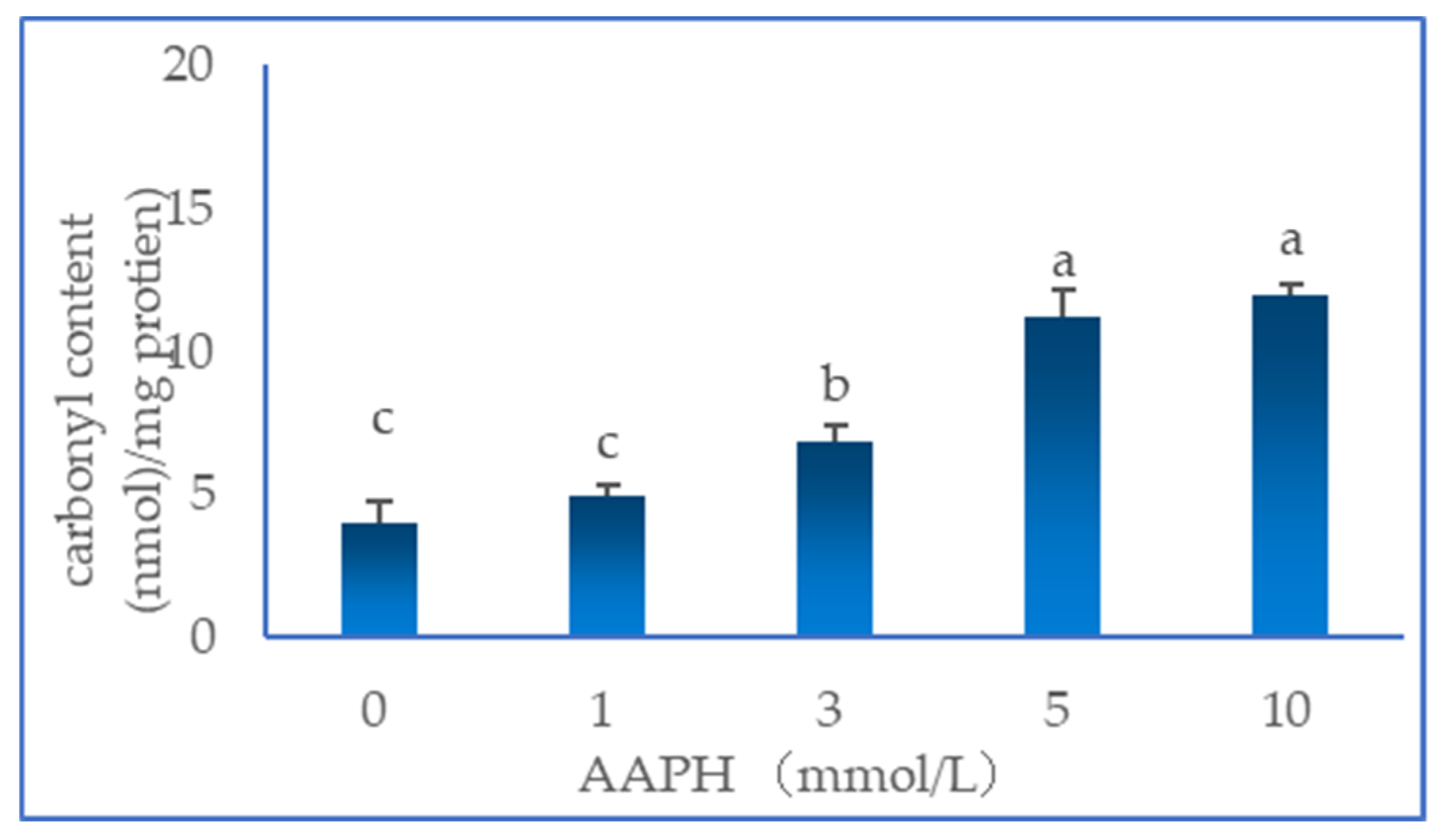
3.1. Total Carbonyl Content
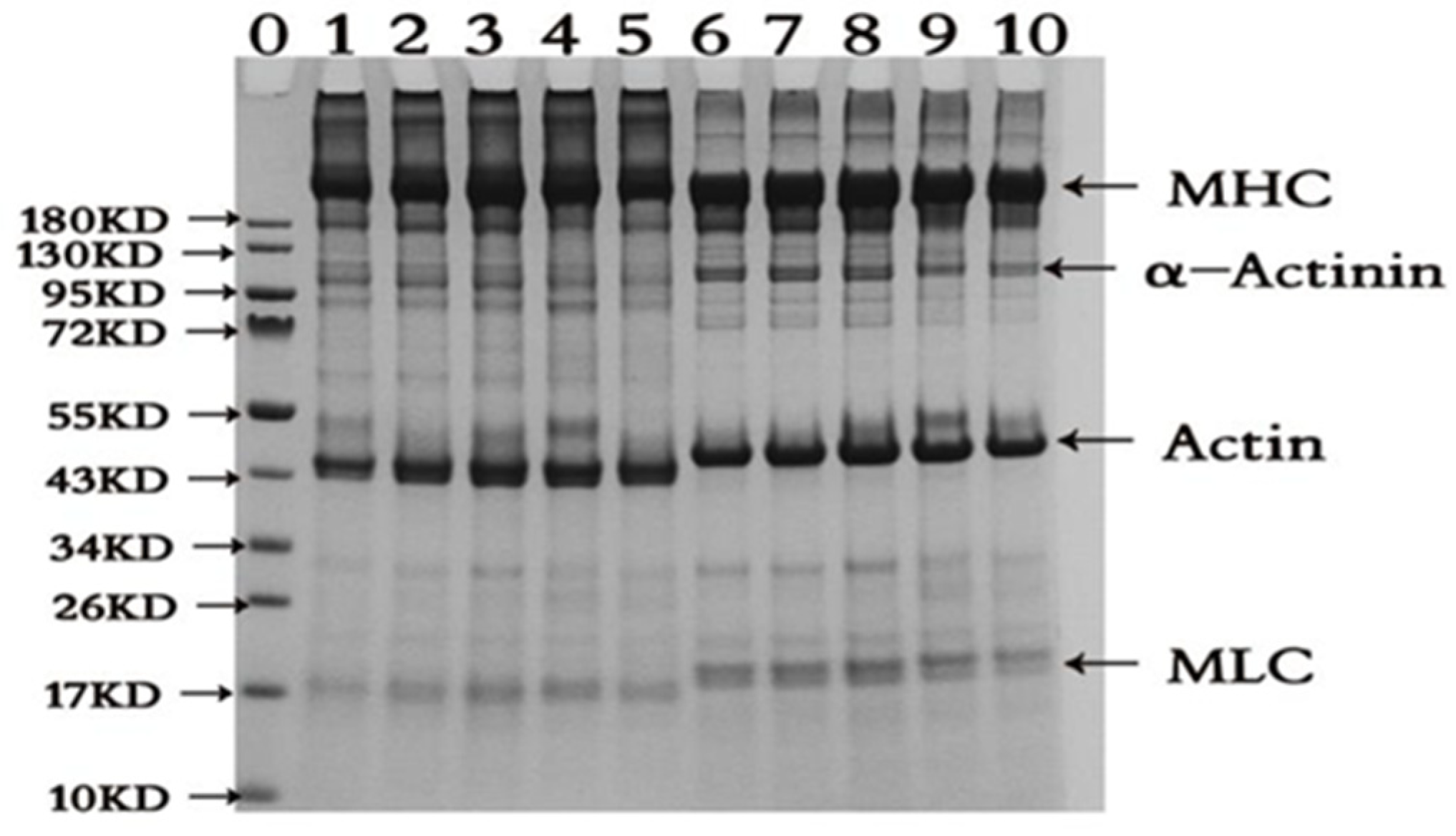
3.2. SDS-PAGE Pattern
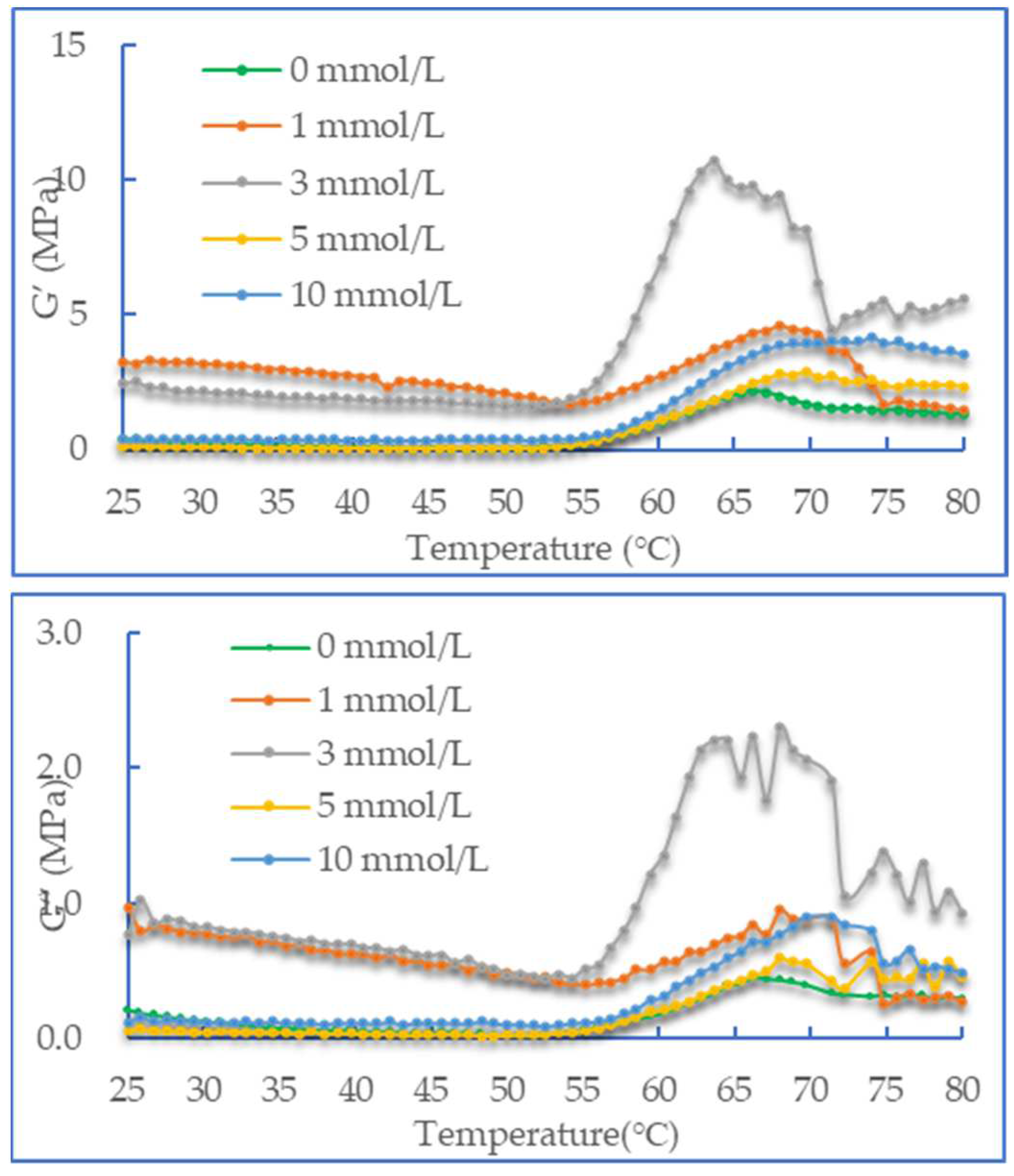
3.3. Dynamic Rheological Properties
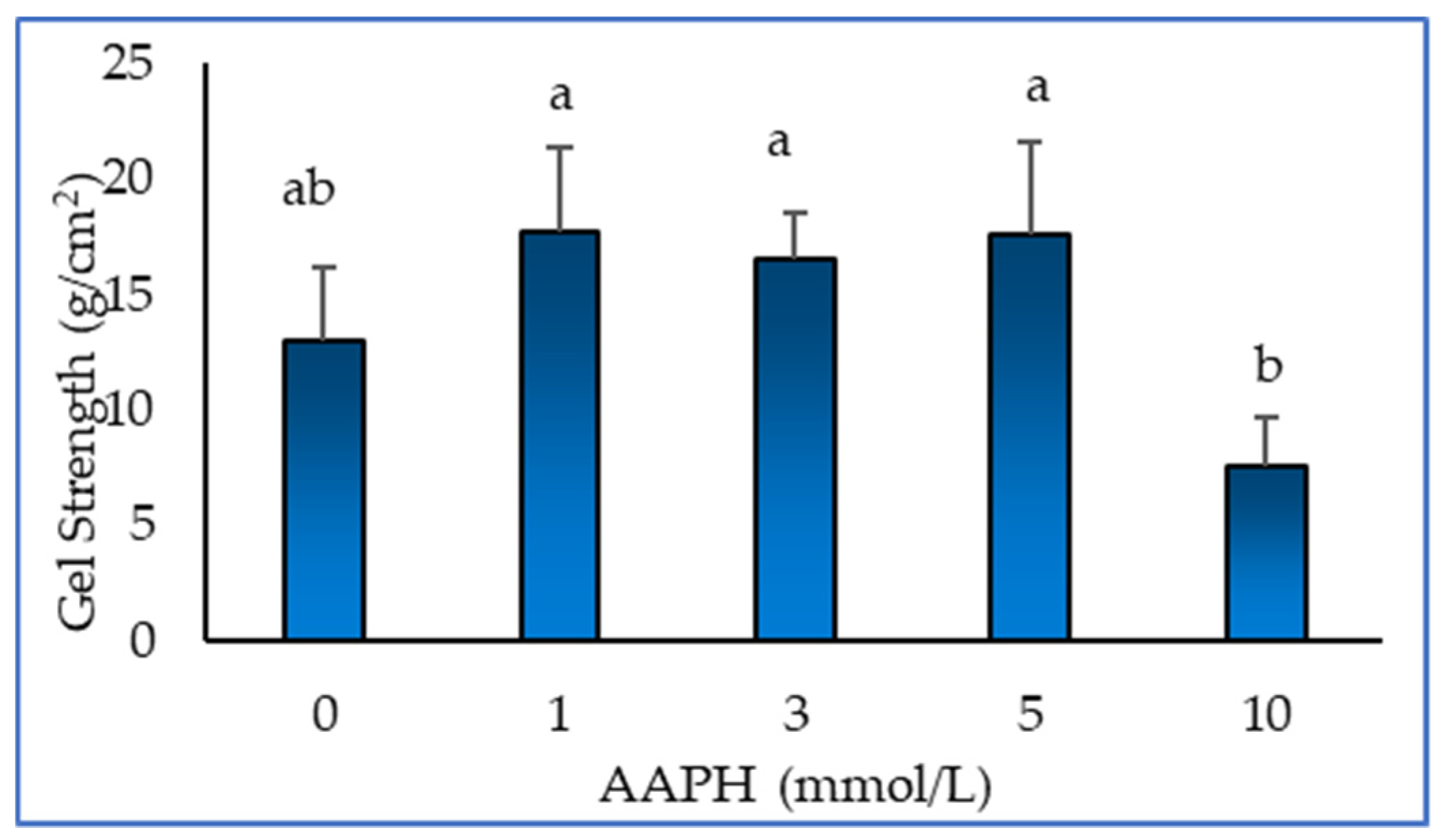
3.4. Gel strength
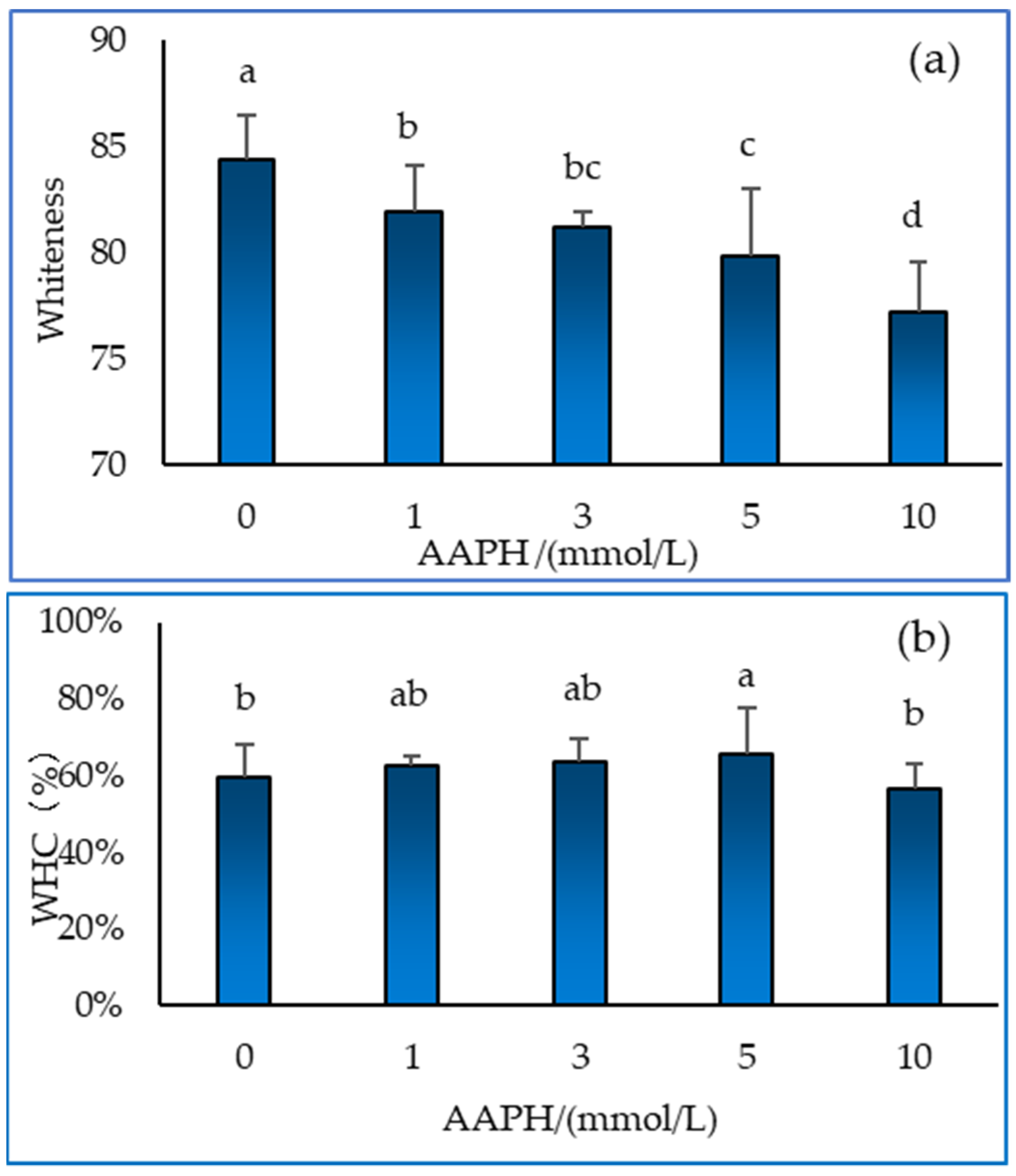
3.5. Whiteness and water holding capacity of gel
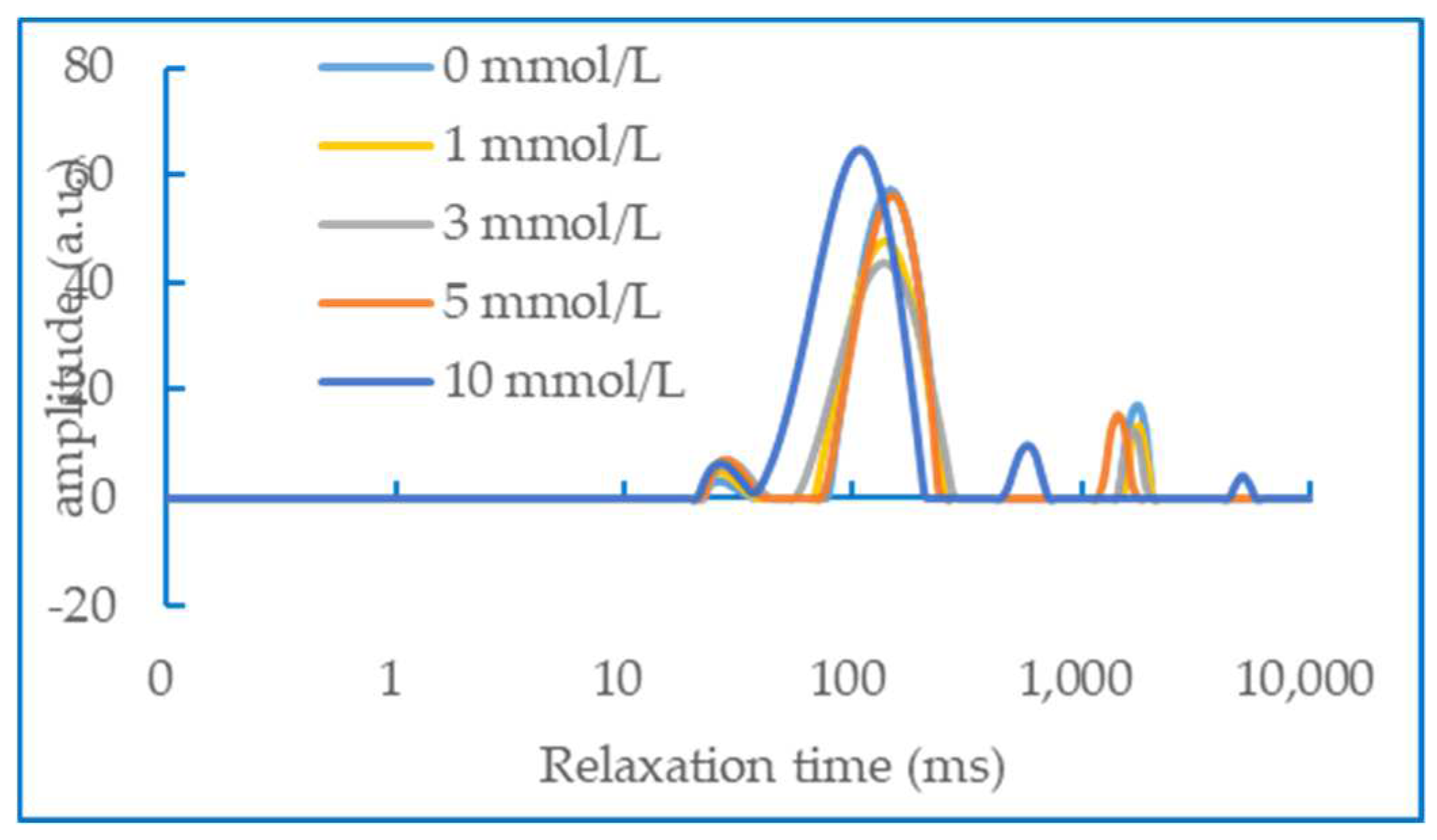
3.6. Water status in gel
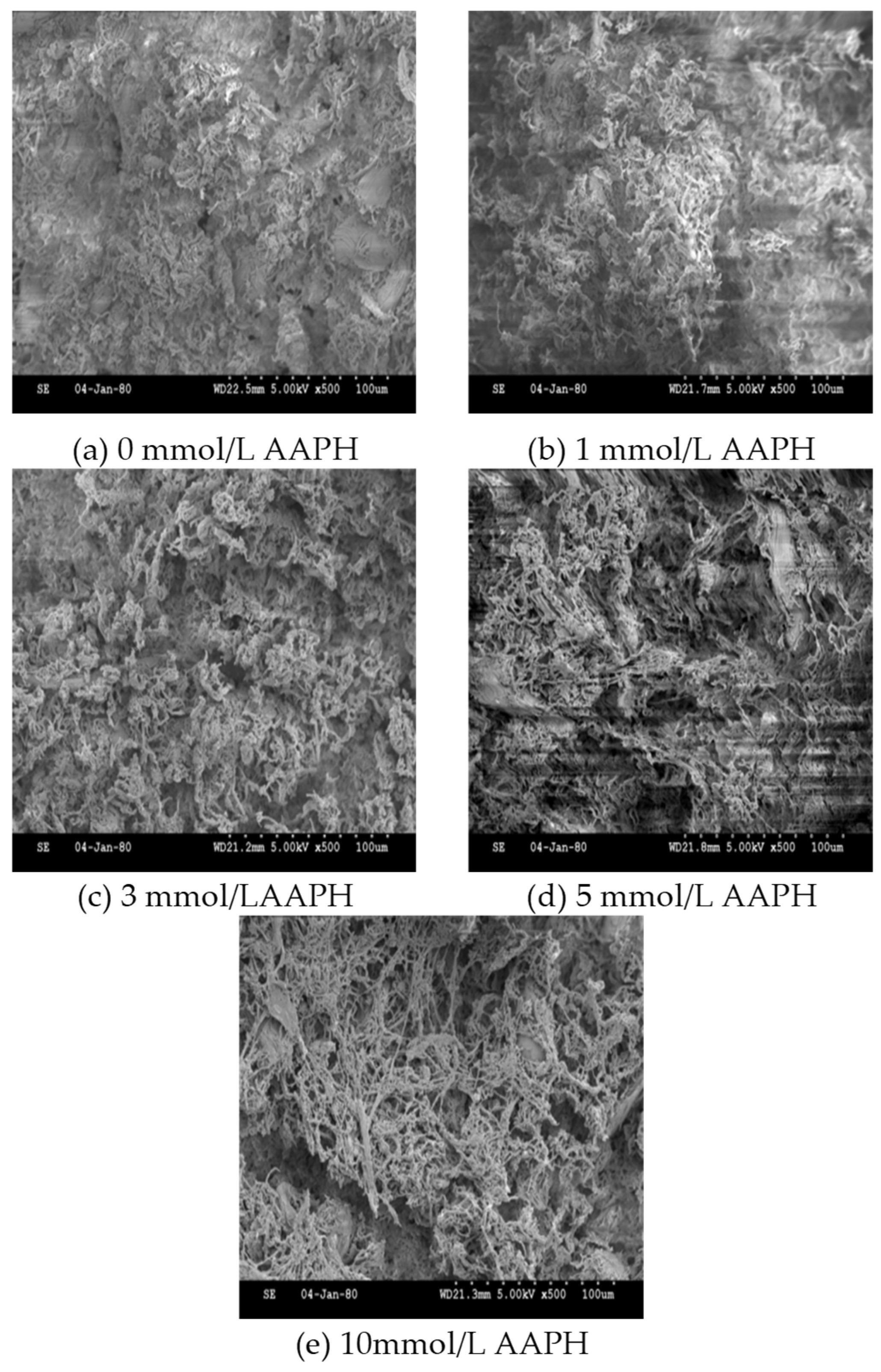
3.7. Gel microstructure
3. Materials and Methods
3.1. Sample Preparation and Reagents
3.2. Extraction of Duck Myofibrillar Proteins (DMPs)
3.3. Oxidation of Duck Myofibrillar Proteins
3.4. Carbonyl Content
3.5. Sodium Dodecyl Sulfonate Polyacrylamide Gel Electrophoresis Analysis (SDS-PAGE)
3.6. Dynamic Rheological Test
3.7. Gel Preparation
3.8. Gel Strength Measurement
3.9. Gel Whiteness and Water Holding Capacity
3.10. Water Status in Gel
3.11. Gel Microstructure
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Johns, A.M.; Birkinshaw, L.H.; Ledward, D.A. Catalysts of lipid oxidation in meat-products. Meat Science 1989, 25, 209-220. https://doi.org/10.1016/0309-1740(89)90073-9. [CrossRef]
- Dominguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.G.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8. https://doi.org/10.3390/antiox8100429. [CrossRef]
- Sohaib, M.; Anjum, F.M.; Sahar, A.; Arshad, M.S.; Rahman, U.U.; Imran, A.; Hussain, S. Antioxidant proteins and peptides to enhance the oxidative stability of meat and meat products: A comprehensive review. Int. J. Food Prop. 2017, 20, 2581-2593. https://doi.org/10.1080/10942912.2016.1246456. [CrossRef]
- Bao, Y.; Ertbjerg, P.; Estevez, M.; Yuan, L.; Gao, R. Freezing of meat and aquatic food: Underlying mechanisms and implications on protein oxidation. Compr. Rev. Food. Sci. Food Saf. 2021, 20, 5548-5569. https://doi.org/10.1111/1541-4337.12841. [CrossRef]
- Zhang, L.T.; Li, Q.; Bao, Y.L.; Tan, Y.Q.; Lametsch, R.; Hong, H.; Luo, Y.K. Recent advances on characterization of protein oxidation in aquatic products: A comprehensive review. Critical Reviews in Food Science and Nutrition. https://doi.org/10.1080/10408398.2022.2117788. [CrossRef]
- Bao, Y.L.; Ertbjerg, P. Effects of protein oxidation on the texture and water-holding of meat: a review. Critical Reviews in Food Science and Nutrition 2019, 59, 3564-3578. https://doi.org/10.1080/10408398.2018.1498444. [CrossRef]
- Liu, Z.; Xiong, Y.L.; Chen, J. Protein Oxidation Enhances Hydration but Suppresses Water-Holding Capacity in Porcine Longissimus Muscle. Journal of Agricultural and Food Chemistry 2010, 58, 10697-10704. https://doi.org/10.1021/jf102043k. [CrossRef]
- Zhu, X.; Shi, X.; Liu, S.; Gu, Y.; Liu, J.; Fu, Q.; Wang, R. Physicochemical properties and gel-forming ability changes of duck myofibrillar protein induced by hydroxyl radical oxidizing systems. Frontiers in Nutrition 2022, 9. https://doi.org/10.3389/fnut.2022.1029116. [CrossRef]
- Zhu, X.; Ma, Z.; Zhang, X.; Huang, X.; Liu, J.; Zhuang, X. Effect of Malondialdehyde-Induced Oxidation Modification on Physicochemical Changes and Gel Characteristics of Duck Myofibrillar Proteins. Gels 2022, 8. https://doi.org/10.3390/gels8100633. [CrossRef]
- Dion, M.Z.; Wang, Y.J.; Bregante, D.; Chan, W.M.; Andersen, N.; Hilderbrand, A.; Leiske, D.; Salisbury, C.M. The Use of a 2,2 '-Azobis (2-Amidinopropane) Dihydrochloride Stress Model as an Indicator of Oxidation Susceptibility for Monoclonal Antibodies. Journal of Pharmaceutical Sciences 2018, 107, 550-558. https://doi.org/10.1016/j.xphs.2017.09.022. [CrossRef]
- Sadeghinejad, N.; Sarteshnizi, R.A.; Gavlighi, H.A.; Barzegar, M. Pistachio green hull extract as a natural antioxidant in beef patties: Effect on lipid and protein oxidation, color deterioration, and microbial stability during chilled storage. LWT-Food Sci. Technol. 2019, 102, 393-402. https://doi.org/10.1016/j.lwt.2018.12.060. [CrossRef]
- Bridi, R.; Giordano, A.; Peñailillo, M.F.; Montenegro, G. Antioxidant Effect of Extracts from Native Chilean Plants on the Lipoperoxidation and Protein Oxidation of Bovine Muscle. Molecules 2019, 24, 3264. [CrossRef]
- Zhou, F.; Zhao, M.; Zhao, H.; Sun, W.; Cui, C. Effects of oxidative modification on gel properties of isolated porcine myofibrillar protein by peroxyl radicals. Meat Science 2014, 96, 1432-1439. https://doi.org/10.1016/j.meatsci.2013.12.001. [CrossRef]
- Fuentes-Lemus, E.; Silva, E.; Barrias, P.; Aspee, A.; Escobar, E.; Lorentzen, L.G.; Carroll, L.; Leinisch, F.; Davies, M.J.; López-Alarcón, C. Aggregation of α- and β- caseins induced by peroxyl radicals involves secondary reactions of carbonyl compounds as well as di-tyrosine and di-tryptophan formation. Free Radical Biology and Medicine 2018, 124, 176-188. https://doi.org/10.1016/j.freeradbiomed.2018.06.005. [CrossRef]
- Werber, J.; Wang, Y.J.; Milligan, M.; Li, X.; Ji, J.A. Analysis of 2,2 '-Azobis (2-Amidinopropane) Dihydrochloride Degradation and Hydrolysis in Aqueous Solutions. Journal of Pharmaceutical Sciences 2011, 100, 3307-3315. https://doi.org/10.1002/jps.22578. [CrossRef]
- 1Wu, W.; Zhang, C.M.; Kong, X.Z.; Hua, Y.F. Oxidative modification of soy protein by peroxyl radicals. Food Chemistry 2009, 116, 295-301. https://doi.org/10.1016/j.foodchem.2009.02.049. [CrossRef]
- Essex, D.W.; Li, M.R.; Miller, A.; Feinman, R.D. Protein disulfide isomerase and sulfhydryl-dependent pathways in platelet activation. Biochemistry 2001, 40, 6070-6075. https://doi.org/10.1021/bi002454e. [CrossRef]
- Zhang, W.; Xiao, S.; Ahn, D.U. Protein Oxidation: Basic Principles and Implications for Meat Quality. Critical Reviews in Food Science and Nutrition 2013, 53, 1191-1201. https://doi.org/10.1080/10408398.2011.577540. [CrossRef]
- Stadtman, E.R.; Levine, R.L. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 2003, 25, 207-218. https://doi.org/10.1007/s00726-003-0011-2. [CrossRef]
- Estevez, M. Protein carbonyls in meat systems: A review. Meat Science 2011, 89, 259-279. https://doi.org/10.1016/j.meatsci.2011.04.025. [CrossRef]
- Grune, T.; Jung, T.; Merker, K.; Davies, K.J.A. Decreased proteolysis caused by protein aggregates, inclusion bodies, plaques, lipofuscin, ceroid, and 'aggresomes' during oxidative stress, aging, and disease. International Journal of Biochemistry & Cell Biology 2004, 36, 2519-2530. https://doi.org/10.1016/j.biocel.2004.04.020. [CrossRef]
- Hofmann, K.; Hamm, R. Sulfhydryl and Disulfide Groups in Meats**Dedicated to Professor Dr. Alfons Schoberl, Hannover (Germany), a pioneer in the chemistry of organic sulfur compounds. In Advances in Food Research, Chichester, C.O., Ed.; Academic Press: 1978; Volume 24, pp. 1-111.
- Morzel, M.; Gatellier, P.; Sayd, T.; Renerre, M.; Laville, E. Chemical oxidation decreases proteolytic susceptibility of skeletal muscle myofibrillar proteins. Meat Science 2006, 73, 536-543. https://doi.org/10.1016/j.meatsci.2006.02.005. [CrossRef]
- Dorta, E.; Avila, F.; Fuentes-Lemus, E.; Fuentealba, D.; Lopez-Alarcon, C. Oxidation of myofibrillar proteins induced by peroxyl radicals: Role of oxidizable amino acids. Food Research International 2019, 126. https://doi.org/10.1016/j.foodres.2019.108580. [CrossRef]
- Relkin, P.; Fabre, M.; Guichard, E. Effect of Fat Nature and Aroma Compound Hydrophobicity on Flavor Release from Complex Food Emulsions. Journal of Agricultural and Food Chemistry 2004, 52, 6257-6263. https://doi.org/10.1021/jf049477a. [CrossRef]
- Fuentes-Lemus, E.; Silva, E.; Leinisch, F.; Dorta, E.; Lorentzen, L.G.; Davies, M.J.; López-Alarcón, C. α- and β-casein aggregation induced by riboflavin-sensitized photo-oxidation occurs via di-tyrosine cross-links and is oxygen concentration dependent. Food Chemistry 2018, 256, 119-128. https://doi.org/10.1016/j.foodchem.2018.02.090. [CrossRef]
- Feng, J.; Xiong, Y.L. Interaction and Functionality of Mixed Myofibrillar and Enzyme-hydrolyzed Soy Proteins. Journal of Food Science 2003, 68, 803-809. https://doi.org/10.1111/j.1365-2621.2003.tb08246.x. [CrossRef]
- Zhu, Z.; Yang, J.; Zhou, X.; Khan, I.A.; Bassey, A.P.; Huang, M. Comparison of two kinds of peroxyl radical pretreatment at chicken myofibrillar proteins glycation on the formation of N-epsilon-carboxymethyllysine and N-epsilon-carboxyethyllysine. Food Chemistry 2021, 353. https://doi.org/10.1016/j.foodchem.2021.129487. [CrossRef]
- Xiong, Y.L.; Blanchard, S.P.; Ooizumi, T.; Ma, Y.Y. Hydroxyl Radical and Ferryl-Generating Systems Promote Gel Network Formation of Myofibrillar Protein. Journal of Food Science 2010, 75, C215-C221. https://doi.org/10.1111/j.1750-3841.2009.01511.x. [CrossRef]
- Utrera, M.; Estevez, M. Oxidation of Myofibrillar Proteins and Impaired Functionality: Underlying Mechanisms of the Carbonylation Pathway. Journal of Agricultural and Food Chemistry 2012, 60, 8002-8011. https://doi.org/10.1021/jf302111j. [CrossRef]
- Hwang, J.-S.; Lai, K.-M.; Hsu, K.-C. Changes in textural and rheological properties of gels from tilapia muscle proteins induced by high pressure and setting. Food Chemistry 2007, 104, 746-753. https://doi.org/10.1016/j.foodchem.2006.11.075. [CrossRef]
- Lund, M.N.; Heinonen, M.; Baron, C.P.; Estevez, M. Protein oxidation in muscle foods: A review. Molecular Nutrition & Food Research 2011, 55, 83-95. https://doi.org/10.1002/mnfr.201000453. [CrossRef]
- Han, M.; Wang, P.; Xu, X.; Zhou, G. Low-field NMR study of heat-induced gelation of pork myofibrillar proteins and its relationship with microstructural characteristics. Food Research International 2014, 62, 1175-1182. https://doi.org/10.1016/j.foodres.2014.05.062. [CrossRef]
- Li, Y.; Li, X.; Wang, J.-z.; Zhang, C.-h.; Sun, H.-m.; Wang, C.-q.; Xie, X.-l. Effects of Oxidation on Water Distribution and Physicochemical Properties of Porcine Myofibrillar Protein Gel. Food Biophysics 2014, 9, 169-178. https://doi.org/10.1007/s11483-013-9329-9. [CrossRef]
- Wang, Z.; He, Z.; Gan, X.; Li, H. Effect of peroxyl radicals on the structure and gel properties of isolated rabbit meat myofibrillar proteins. International Journal of Food Science and Technology 2018, 53, 2687-2696. https://doi.org/10.1111/ijfs.13878. [CrossRef]
- Zhu, X.S.; Ruusunen, M.; Gusella, M.; Zhou, G.H.; Puolanne, E. High post-mortem temperature combined with rapid glycolysis induces phosphorylase denaturation and produces pale and exudative characteristics in broiler Pectoralis major muscles. Meat Science 2011, 89, 181-188. https://doi.org/10.1016/j.meatsci.2011.04.015. [CrossRef]
- Soglia, F.; Petracci, M.; Ertbjerg, P. Novel DNPH-based method for determination of protein carbonylation in muscle and meat. Food Chemistry 2016, 197, 670-675. https://doi.org/10.1016/j.foodchem.2015.11.038. [CrossRef]
- Jia, N.; Zhang, F.X.; Liu, Q.; Wang, L.T.; Lin, S.W.; Liu, D.Y. The beneficial effects of rutin on myofibrillar protein gel properties and related changes in protein conformation. Food Chemistry 2019, 301. https://doi.org/10.1016/j.foodchem.2019.125206. [CrossRef]
- Zhuang, X.; Wang, L.; Jiang, X.; Chen, Y.; Zhou, G. Insight into the mechanism of myofibrillar protein gel influenced by konjac glucomannan: Moisture stability and phase separation behavior. Food Chemistry 2021, 339, 127941. https://doi.org/10.1016/j.foodchem.2020.127941. [CrossRef]
- Xia, T.; Xu, Y.; Zhang, Y.; Xu, L.; Kong, Y.; Song, S.; Huang, M.; Bai, Y.; Luan, Y.; Han, M.; et al. Effect of oxidation on the process of thermal gelation of chicken breast myofibrillar protein. Food Chemistry 2022, 384, 132368. https://doi.org/10.1016/j.foodchem.2022.132368. [CrossRef]
- Salvador, P.; Toldra, M.; Saguer, E.; Carretero, C.; Pares, D. Microstructure-function relationships of heat-induced gels of porcine haemoglobin. Food Hydrocolloids 2009, 23, 1654-1659. https://doi.org/10.1016/j.foodhyd.2008.12.003. [CrossRef]
- Zhu, X.; Zhang, J.; Liu, S.; Gu, Y.; Yu, X.; Gao, F.; Wang, R. Relationship between Molecular Structure and Heat-Induced Gel Properties of Duck Myofibrillar Proteins Affected by the Addition of Pea Protein Isolate. Foods 2022, 11, 1040, https://doi.org/10.3390/foods11071040. [CrossRef]
- Xia, T.L.; Cao, Y.Y.; Chen, X.; Zhang, Y.L.; Xue, X.W.; Han, M.Y.; Li, L.; Zhou, G.H.; Xu, X.L. Effects of chicken myofibrillar protein concentration on protein oxidation and water holding capacity of its heat-induced gels. Journal of Food Measurement and Characterization 2018, 12, 2302-2312. https://doi.org/10.1007/s11694-018-9847-8. [CrossRef]
| AAPH concentraton (mmol/L) | 0 | 1 | 3 | 5 | 10 |
|---|---|---|---|---|---|
| DMPs (mL) | 6 | ||||
| AAPH Stock solution (mL) | 0 | 0.2 | 0.6 | 1 | 2 |
| PBS (mL) | 4 | 3.8 | 3.4 | 3 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





