Submitted:
10 August 2023
Posted:
14 August 2023
You are already at the latest version
Abstract
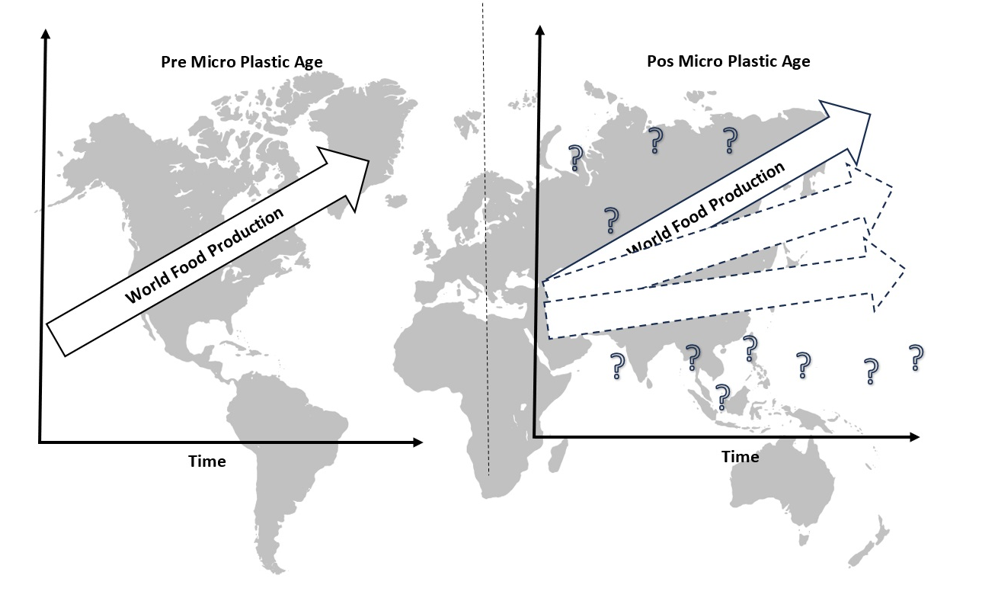
Keywords:
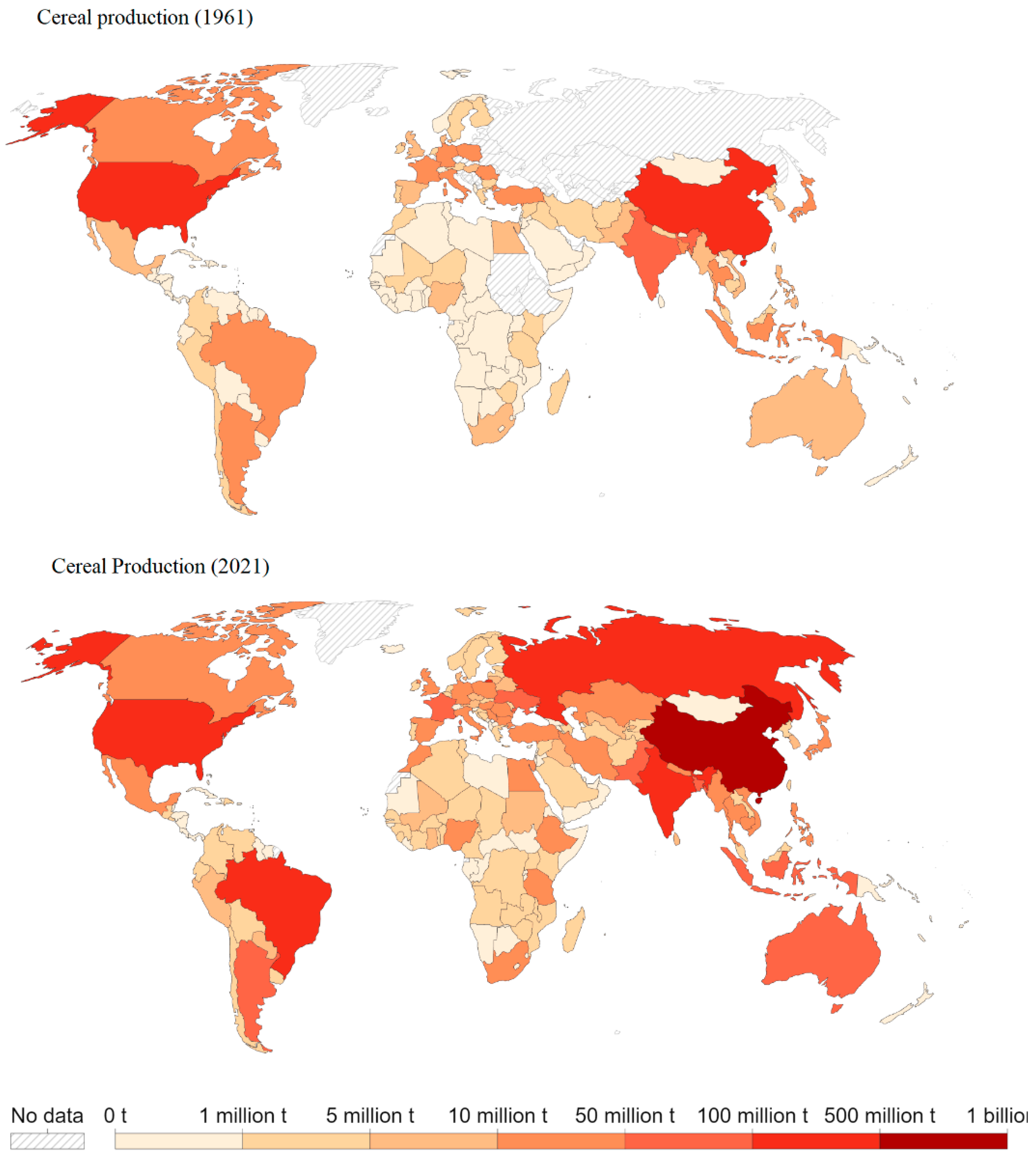
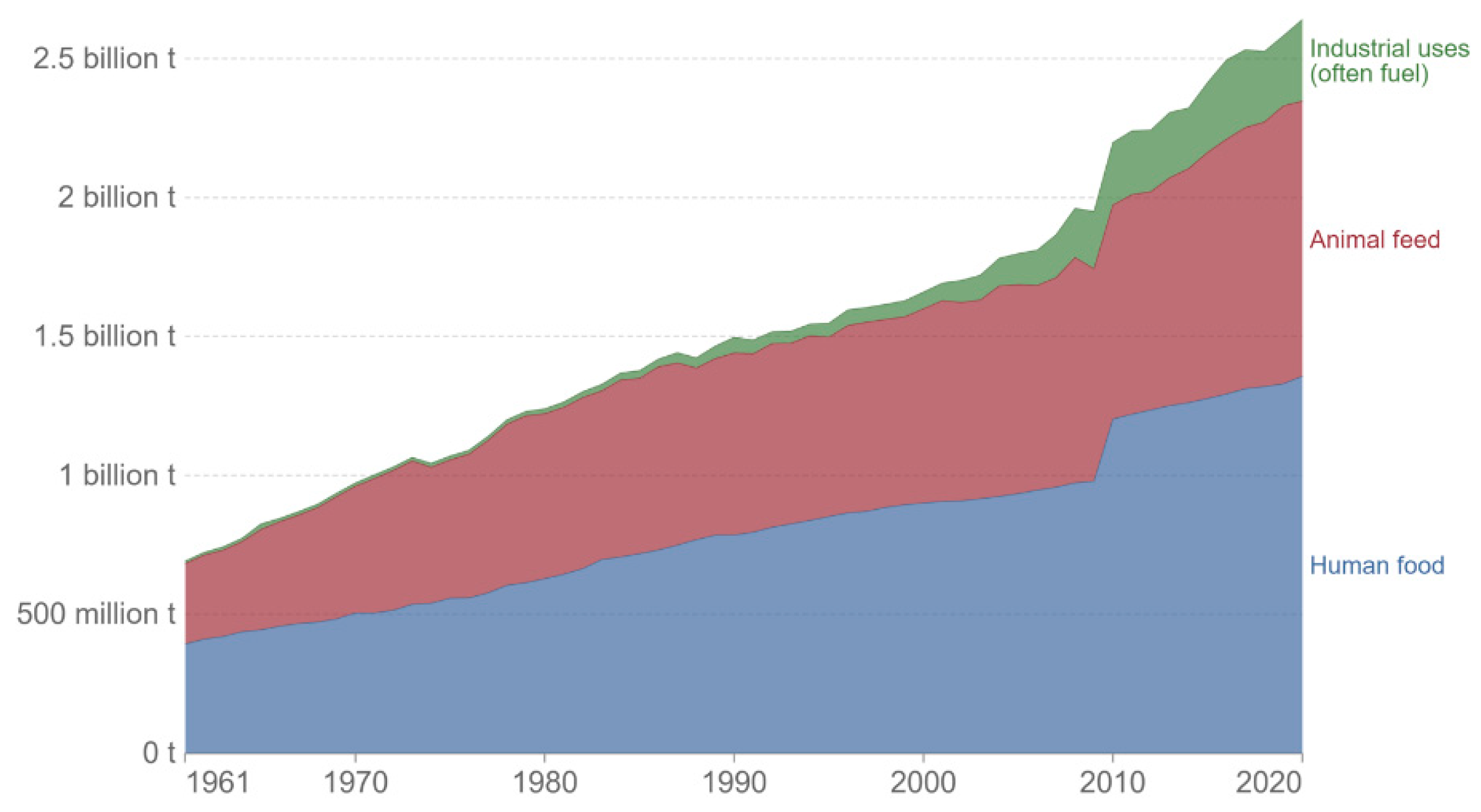
1. Introduction
2. Potential Impact of Microplastics on Agriculture
2.1. Direct Toxic Effects on Plants
2.2. Alteration of Soil Characteristics
2.3. Intoxication of Soil and its Microbial Communities
2.4. Intoxication of pollinating agents
2.5. Toxicity of Microplastics on Interstitial Organisms
3. Potential Impact of Microplastic on Animal Protein Production
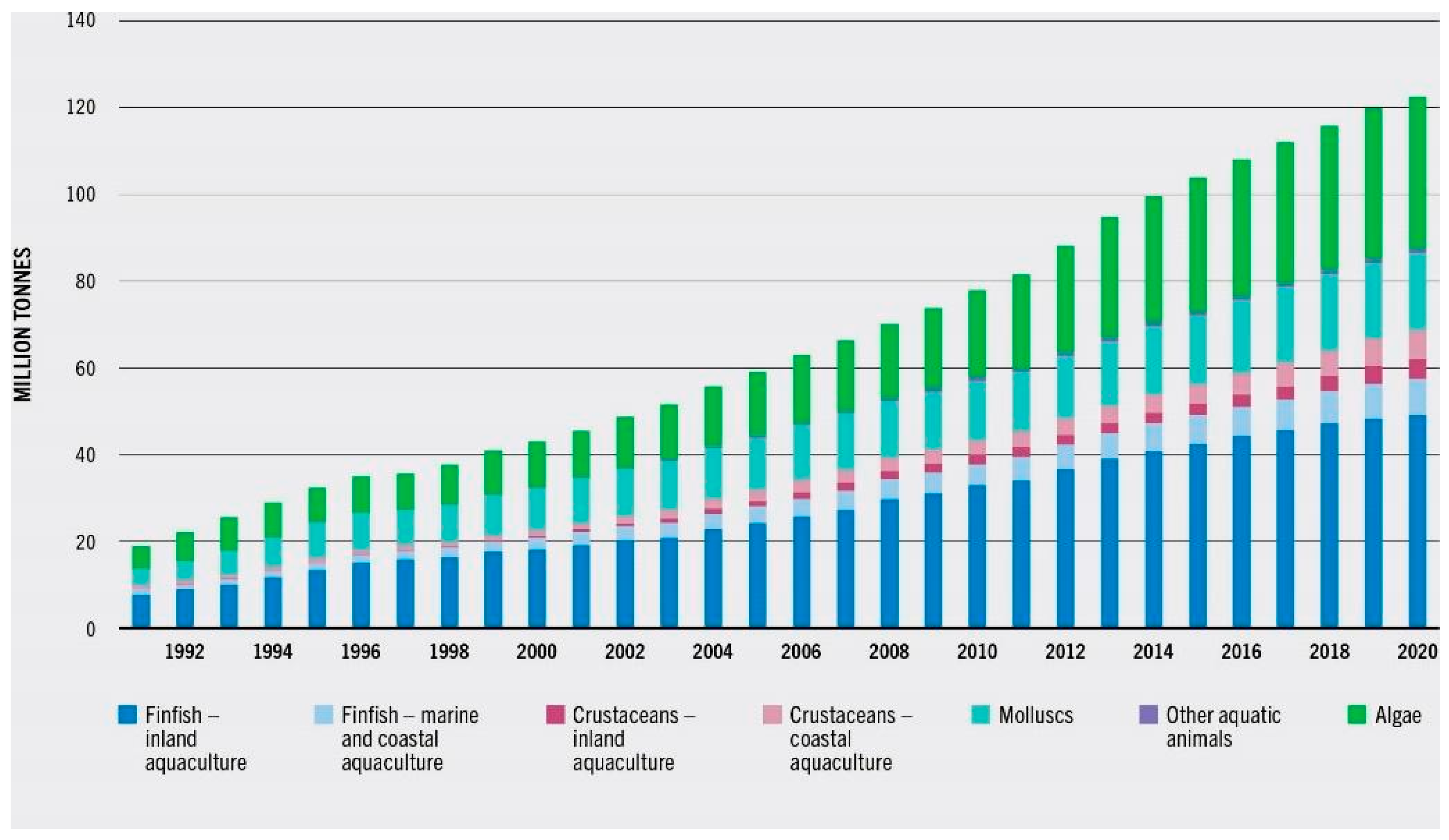
4. Potential Impact of Microplastics on Aquaculture
5. Conclusions
Author Contributions
Acknowledgments
References
- Crawford, C.B.; Quinn, B. Plastic Production, Waste and Legislation. In: Crawford, C.B. and Quinn, B., Eds., Microplastic Pollutants, Elsevier Science, Amsterdam. 2017, 1, 39-56. [CrossRef]
- Hahladakis, J. N.; Velis, C. A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling, J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.G. A Brief History of Marine Litter Research. In: Bergmann, M., Gutow, L., Klages, M. (eds) Marine Anthropogenic Litter. Springer, Cham. 2015. [CrossRef]
- Anderson, J.C.; Park, B.J.; Palace, V.P. Microplastics in aquatic environments: Implications for Canadian ecosystems. Environ Pollut. 2016, 1, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Hammer, J.; Kraak, M.H.; Parsons, J.R. , Plastics in the marine environment: the dark side of a modern gift. Reviews of Environmental Contamination and Toxicology. Springer New York. 2012, 1–44. [Google Scholar] [CrossRef]
- Andrady, A.L. Persistence of plastic litter in the oceans. In Marine anthropogenic litter. Edited by M. Bergmann, L. Gutow, and M. Klages. Springer. 2015, 57–72.
- Crawford, C.B. , and Quinn, B. 2016. Microplastic pollutants. Elsevier.
- GESAMP. Sources, fate and effects of microplastics in the marine environment: part two of a global assessment. IMO, London. 2016.
- Mdlalose, L.; Chimuka, L. Mitigation Approaches to Prevent Microplastics Effects in the Aquatic Env ironment: Exploration of Microbeads from Personal Care and Cosmetic Products. Int. J. Environ. Res. 2022, 16, 84. [Google Scholar] [CrossRef]
- Bakadia, B.M.; Zhong, A.; Li, X.; Boni, B.O.O.; Ahmed, A.A.Q.; Souho, T.; Zheng, R.; Shi, Z.; Shi, D.; Lamboni, L.; Yang, G. Biodegradable and injectable poly(vinyl alcohol) microspheres in silk sericin-based hydrogel for the controlled release of antimicrobials: application to deep full-thickness burn wound healing. Adv. Compos. Hybrid. Mater. 2022, 5, 2847–2872. [Google Scholar] [CrossRef]
- Qi, F.; He, L.; Cui, L.; Wang, W.; Siddique K. H., M.; Li, S. Smart Antibacterial Food Packaging Based on MIL-53 (Fe) Functionalized Polylactic Acid Film for pH-Responsive Controlled Release. J. Polym. Environ. 2023. [Google Scholar] [CrossRef]
- Gaylarde, C. C.; Baptista Neto, J. A.; Fonseca, E. M. Paint fragments as polluting microplastics: a brief review. Mar. Pollut. Bull., 2021, 162, 9. [Google Scholar] [CrossRef]
- Gaylarde, C. C.; Baptista-Neto, J. A.; Fonseca, E. M. da. Plastic microfibre pollution: how important is clothes’ laundering? Heliyon 2021, 7, 07105. [Google Scholar] [CrossRef]
- Sundt, P.; Schulze, P.-E.; Syversen, F. Sources of Microplastic-pollution to the Marine Environment. Report no M-321/2015 Mepex Consult, Asker, Norway. 2014.
- Essel, R.; Engel, L.; Carus, M.; Ahrens, R. Sources of microplastics relevant to marine protection in Germany. Texte. 2015, 64. [Google Scholar]
- Lassen, C.; Hansen, S.F.; Magnusson, K.; Hartmann, N.B.; Jensen, P. R.; Nielsen, T.G. , Brinch, A. Microplastics: Occurrence, Effects and Sources of Releases to the Environment in Denmark. Danish Environmental Protection Agency, Copenhagen K. 2015. Available online: http://mst.dk/service/publikationer/publikationsarkiv/2015/nov/rapport-om-mikroplast.
- Magnusson, K.; Noren, F. Screening of Microplastic Particles in and Downstream a Wastewater Treatment Plant. IVL Swedish Environmental Research Institute, Sweden. 2014.
- Xu, S.; Ma, J.; Ji, R.; Pan, K.; Miao, A.J. Microplastics in aquatic environments: Occurrence, accumulation, and biological effects. Sci. Total. Environ. 2020, 10, 134699. [Google Scholar] [CrossRef]
- De Ruijter, V.N.; Redondo-Hasselerharm, P.E.; Gouin, T.; Koelmans, A.A. Quality criteria for microplastic effect studies in the context of risk assessment: a critical review. Environ. Sci. Technol. 2020, 54, 11692–11705. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Wan, Z.; Luo, T.; Fu, Z.; Jin, Y. Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice. Sci. Total Environ. 2018, 631, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Lu, L.; Tu, W.; Luo, T.; Fu, Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci. Total Environ. 2019, 649, 308–317. [Google Scholar] [CrossRef]
- Desforges, J.P.; Galbraith, M.; Ross, P.S. Ingestion of microplastics by zooplankton in the Northeast Pacific Ocean. Arch. Environ. Contam. Toxicol. 2015, 69. [Google Scholar] [CrossRef]
- Boerger, C. M.; Lattin, G. L.; Moore, S. L.; Moore, C. J. Plastic Ingestion by Planktivorous Fishes in the North Pacific Central Gyre. Mar. Pollut. Bull. 2010, 60(12), 2275–2278. [Google Scholar] [CrossRef]
- Avery-Gomm, S.; O'Hara, P.D.; Kleine, L.; Bowes, V.; Wilson, L.K.; Barry, K.L. , Northern fulmars as biological monitors of trends of plastic pollution in the eastern North Pacific. Mar. Pollut. Bull. 2012, 64. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, M.C.; Titmus, A.J.; Ford, M. Scales of spatial heterogeneity of plastic marine debris in the northeast Pacific Ocean. PLoS One. 2013, 8. [Google Scholar] [CrossRef]
- Frias, J.P.G.L.; Otero, V.; Sobral, P. Evidence of microplastics in samples of zooplankton from Portuguese coastal waters. Mar. Environ. Res. 2014, 95, 89–95. [Google Scholar] [CrossRef]
- Kvale, K.; Hunt, C. , James, A.; Koeve, W. Regionally disparate ecological responses to microplastic slowing of faecal pellets yields coherent carbon cycle response. Front. in Mar. Sci. 2023, 10, 1111838. [Google Scholar] [CrossRef]
- Botterell, L. R.; Beaumont, N.; Dorrington, T.; Steinke, M.; Thompson, R. C.; Lindeque, P. K. L. Bioavailability and effects of microplastics on marine zooplankton: a review. Environ. Pollut. 2019, 245, 98–110. [Google Scholar] [CrossRef]
- Fanzo, J.; Bellows, A.L.; Spiker, M. L.; Thorne-Lyman, A. L.; Bloem, M. W. The importance of food systems and the environment for nutrition. The American Journal of Clinical Nutrition 2021, 113, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, M.; Vollrath, D. The effect of agricultural technology on the speed of development. World Development 2018, 109. [Google Scholar] [CrossRef]
- Sharma, P.; Singh, S.P.; Iqbal, H.M.N.; Parra-Saldivar, R.; Varjani, S.; Tong, Y.W. Genetic modifications associated with sustainability aspects for sustainable developments. Bioengineered. 2022, 13(4), 9508–9520. [Google Scholar] [CrossRef] [PubMed]
- Msangi, S.; Enahoro, D.; Herrero, M.; Magnan, N.; Havlik, P.; Notenbaert, A.; Nelgen, S. Integrating livestock feeds and production systems into agricultural multi-market models: The example of Impact. Food Policy. 2014, 49(2), 365–377. [Google Scholar] [CrossRef]
- Dwivedi, S.L.; Lammerts van Bueren, E.T.; Ceccarelli, S.; Grando, S.; Upadhyaya, H.D.; Ortiz, R. Diversifying Food Systems in the Pursuit of Sustainable Food Production and Healthy Diets. Trends Plant Sci. 2017, 22(10), 842–856. [Google Scholar] [CrossRef]
- Ritchie, H.; Rosado, P.; Roser, M. - "Agricultural Production". 2023. Published online at OurWorldInData.org. Available online: https://ourworldindata.org/agricultural-production.
- Pradeepkiran, J. A. Aquaculture role in global food security with nutritional value: a review. Transl. Anim. Sci. 2019, 3(2), 903–910. [Google Scholar] [CrossRef]
- OECD/FAO. OECD-FAO Agricultural Outlook 2021-2030, OECD Publishing, Paris. [CrossRef]
- Neves, C. V.; Gaylarde, C. C.; Baptista Neto, J. A.; Vieira, K. S.; Pierri, B.; Waite, C.C.C.; Scott, D.C.; da Fonseca, E.M. The transfer and resulting negative effects of nano- and micro-plastics through the aquatic trophic web—A discreet threat to human health. Water Biology and Security 2022, 1, 100080. [Google Scholar] [CrossRef]
- FAO. FAOSTAT Online Database; FAO: Rome, Italy, 2020. [Google Scholar]
- Wang, F.; Wang, Q.; Adams, C. A.; Sun, Y.; Zhang, S. Effects of Microplastics on Soil Properties: Current Knowledge and Future Perspectives. J. Hazard. Mater. 2022, 424, 127531. [Google Scholar] [CrossRef]
- Hartmann, G. F.; Ricachenevsky, F. K.; Silveira, N. M.; Pita-Barbosa, A. Phytotoxic Effects of Plastic Pollution in Crops: what Is the Size of the Problem? Environ. Pollut. 2022, 292, 118420. [Google Scholar] [CrossRef]
- Okeke, E. S.; Okoye, C. O.; Atakpa, E. O.; Ita, R. E.; Nyaruaba, R.; Mgbechidinma, C. L.; Akan, O. D. Microplastics in Agroecosystems-Impacts on Ecosystem Functions and Food Chain. Resour. Conservation Recycling 2022, 177, 105961. [Google Scholar] [CrossRef]
- Ren, X.; Yin, S.; Wang, L.; Tang, J. Microplastics in Plant-Microbes-Soil System: A Review on Recent Studies. Sci. Total Environ. 2022, 816, 151523. [Google Scholar] [CrossRef] [PubMed]
- Horton, A. A.; Walton, A.; Spurgeon, D. J.; Lahive, E.; Svendsen, C. Microplastics in Freshwater and Terrestrial Environments: Evaluating the Current Understanding to Identify the Knowledge Gaps and Future Research Priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A. P.; Ferreira, T.; Dordio, A. V.; Carvalho, A. J. P.; Faria, J. M. What Do We Know About the Effects of Microplastics on Soil? . Microplastics in the Ecosphere: Air, Water, Soil, and Food. 2023, 1, 271–304. [Google Scholar]
- Neto, J.A.B.; Gaylarde, C.C.; de Carvalho, D.G.; Lourenço, M.F.; da Fonseca, E.M. Occurrence of microplastics derived from tyres in bottom sediments of Guanabara Bay, Brazil: a form of pollution that is neglected or difficult to detect? Water Emerging Contaminants & Nanoplastics. 2023, 2, 10. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Kang, S.; Wang, Z.; Wu, C. Microplastics in soil: A review on methods, occurrence, sources, and potential risk. Sci. Total Environ. 2021, 780, 146546. [Google Scholar] [CrossRef]
- Radford, F.; Horton, A.; Hudson, M.; Shaw, P.; Williams, I. Agricultural soils and microplastics: Are biosolids the problem? Front. Soil Sci. 2023, 2. [Google Scholar] [CrossRef]
- Horton, A.A.; Svendsen, C.; Williams, R.J.; Spurgeon, D.J.; Lahive, E. Large microplastic particles in sediments of tributaries of the River Thames, UK - abundance, sources and methods for effective quantification. Mar. Pollut. Bull. 2017, 114(1), 218–226. [Google Scholar] [CrossRef]
- Chae, Y.; An, Y.-J. Current Research Trends on Plastic Pollution and Ecological Impacts on the Soil Ecosystem: A Review. Environ. Pollut. 2018, 240, 387–395. [Google Scholar] [CrossRef]
- He, P.; Chen, L.; Shao, L.; Zhang, H.; Lü, F. Municipal Solid Waste (MSW) Landfill: A Source of Microplastics? -Evidence of Microplastics in Landfill Leachate. Water Res. 2019, 159, 38–45. [Google Scholar] [CrossRef]
- Lu, X.; Vogt, R. D.; Li, H.; Han, S.; Mo, X.; Zhang, Y.; Ullah, S.; Chen, C.; Han, X.; Li, H.; Seip, H. M. China’s Ineffective Plastic Solution to Haze. Science. 2019, 364, 1145. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, J.; Zhang, H.; Shi, H.; Fei, Y.; Huang, S. , Tong, Y.; Wen, D., Luo; Barceló, D. Microplastics in Agricultural Soils on the Coastal plain of Hangzhou Bay, east China: Multiple Sources Other Than Plastic Mulching Film. J. Hazard. Mater. 2020, 388, 121814. [Google Scholar] [CrossRef]
- Han, L. H.; Xu, L.; Li, Q. L.; Lu, A. X.; Yin, J. W.; Tian, J. Y. Levels, Characteristics, and Potential Source of Micro(meso)plastic Pollution of Soil in Liaohe River basin. Environ. Sci. 2021, 42(04), 1781–1790. [Google Scholar] [CrossRef]
- Almeida, M.P.d.; Gaylarde, C.C.; Pompermayer, F.C.; Lima., L.d.S.; Delgado, J.d.F.; Scott, D.; Neves, C.V.; Vieira, K.S.; Baptista Neto, J.A.; Fonseca, E.M. The Complex Dynamics of Microplastic Migration through Different Aquatic Environments: Subsidies for a Better Understanding of Its Environmental Dispersion. Microplastics 2023, 2, 62–77. [Google Scholar] [CrossRef]
- O’Connor, D.; Pan, S.; Shen, Z.; Song, Y.; Jin, Y.; Wu, W.-M.; Hou, D. Microplastics Undergo Accelerated Vertical Migration in Sand Soil Due to Small Size and Wet-Dry Cycles. Environ. Pollut. 2019, 249, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Wang, X.; Wei, N.; Song, Z.; Li, D. Accurate quantification and transport estimation of suspended atmospheric microplastics in megacities: Implications for human health. Environ. Intern. 2019, 132, 105127. [Google Scholar] [CrossRef] [PubMed]
- Zang, H.; Zhou, J.; Marshall, M. R.; Chadwick, D. R.; Wen, Y.; Jones, D. L. Microplastics in the Agroecosystem: Are They an Emerging Threat to the Plant-Soil System? Soil Biol. Biochem. 2020, 148, 107926. [Google Scholar] [CrossRef]
- Qi, R.; Jones, D. L.; Li, Z.; Liu, Q.; Yan, C. Behavior of Microplastics and Plastic Film Residues in the Soil Environment: A Critical Review. Sci. Total Environ. 2020, 703, 134722. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, R.; Li, Z.; Yan, B. Adsorption properties and influencing factors of Cu(II) on polystyrene and polyethylene terephthalate microplastics in seawater. Sci. Total Environ 2022, 812, 152573. [Google Scholar] [CrossRef]
- Tang, K. H. D. Microplastics in agricultural soils in China: Sources, impacts and solutions. Environ. Pollut 2023, 121235. [Google Scholar] [CrossRef]
- Lima, J. Z.; Cassaro, R.; Ogura, A. P.; Vianna, M. M. G. R. A systematic review of the effects of microplastics and nanoplastics on the soil-plant system. Sustain. Prod. Consum. 2023, 38, 266–282. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Shi, Q.; Liu, Y.; Lei, H.; Chen, Y. Microplastics in arid soils: Impact of different cropping systems (Altay,Xinjiang). Environ. Pollut. 2022, 303, 119162. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lu, X.; Wang, S.; Zheng, B.; Xu, Y. Vertical migration of microplastics along soil profile under different crop root systems. Environ. Pollut. 2021, 278, 116833. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, Q.; Li, R.; Zhao, Y.; Geng, J.; Wang, G. Physiological responses of lettuce (Lactuca sativa L.) to microplastic pollution. Environ. Sci. Pollut. Res. 2020, 27, 30306–30314. [Google Scholar] [CrossRef]
- Giorgetti, L.; Spanò, C.; Muccifora, S.; Bottega, S.; Barbieri, F.; Bellani, L.; Castiglione, M.R. Exploring the Interaction between Polystyrene Nanoplastics and Allium cepa during Germination: Internalization in Root Cells, Induction of Toxicity and Oxidative Stress. Plant Physiol. Biochem. 2020, 149, 170–177. [Google Scholar] [CrossRef]
- Gong, W.; Zhang, W.; Jiang, M.; Li, S.; Liang, G.; Bu, Q.; Xu, L.; Zhu, H.; Lu, A. Species-dependent Response of Food Crops to Polystyrene Nanoplastics and Microplastics. Sci. Total Environ. 2021, 796, 148750. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Wen, X.; Huang, D.; Du, C.; Deng, R.; Zhou, Z.; Tao, J.; Li, R.; Zhou, W.; Wang, Z.; Chen, H. Interactions between Microplastics/nanoplastics and Vascular Plants. Environ. Pollut. 2021, 290, 117999. [Google Scholar] [CrossRef]
- Bosker, T.; Bouwman, L. J.; Brun, N. R.; Behrens, P.; Vijver, M. G. Microplastics accumulate on pores in seed capsule and delay germination and root growth of the terrestrial vascular plant Lepidium sativum. Chemosphere 2019, 226, 774–781. [Google Scholar] [CrossRef]
- Kováčik, J.; Babula, P.; Hedbavny, J.; Švec, P. Manganese-induced oxidative stress in two ontogenetic stages of chamomile and amelioration by nitric oxide. Plant Science. 2014, 215–216, 1–10. [Google Scholar] [CrossRef]
- Qi, Y.; Yang, X.; Pelaez, A. M.; Lwanga, E. H.; Beriot, N.; Gertsen, H.; Garbeva, P.; Geissen, V. Macro- and micro- plastics in soil-plant system: Effects of plastic mulch film residues on wheat (Triticum aestivum) growth. Sci. of The Total Environ. 2018, 645, 1048–1056. [Google Scholar] [CrossRef]
- Weert, S. van; Redondo-Hasselerharm, P. E.; Diepens, N. J.; Koelmans, A. A. Effects of nanoplastics and microplastics on the growth of sediment-rooted macrophytes, Sci. of The Total Environ. 2019, 654, 1040–1047. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, H.; Liao, Y.; Ye, Z.; Li, M.; Klobučar, G. Ecotoxicity and genotoxicity of polystyrene microplastics on higher plant Vicia faba. Environ. Pollut. 2019, 250, 831–38. [Google Scholar] [CrossRef]
- Moghaddasi, S.; Khoshgoftarmanesh, A. H.; Karimzadeh, F.; Chaney, R. Fate and effect of tire rubber ash nano-particles (RANPs) in cucumber. Ecotoxicol. Environ. Saf. 2015, 115, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Liu, W.; Meng, L.; Wu, J.; Chao, L.; Zeb, A.; Sun, Y. Foliar-applied polystyrene nanoplastics (PSNPs) reduce the growth and nutritional quality of lettuce (Lactuca sativa L.). Environ. Poll. 2021, 280, 116978. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Lei, C.; Xu, J.; Li, R. Foliar uptake and leaf-to-root translocation of nanoplastics with different coating charge in maize plants. J. Hazard. Mater. 2021, 416, 125854. [Google Scholar] [CrossRef] [PubMed]
- Azeem, I.; Adeel, M.; Ahmad, M. A.; Shakoor, N.; Jiangcuo, G. D.; Azeem, K.; Ishfaq, M.; Shakoor, A.; Ayaz, M.; Xu, M.; Rui, Y. Uptake and Accumulation of Nano/Microplastics in Plants: A Critical Review. Nanomaterials. 2021, 11(11), 2935. [Google Scholar] [CrossRef]
- Yin, L.; Wen, X.; Huang, D.; Du, C.; Zhou, Z.; Tao, J.; Li, R.; Zhou, W.; Wang, Z.; Chen, H. Interactions between microplastics/nanoplastics and vascular plants. Environ. Pollut. 2021, 290, 117999. [Google Scholar] [CrossRef]
- van der Heijden, M.G.A.; de Bruin, S., Luckerhoff; van Logtestijn, R.S.P.; Schlaeppi, K. A widespread plant-fungal-bacterial symbiosis promotes plant biodiversity, plant nutrition and seedling recruitment. ISME Journal 2016, 10, 389–399. [Google Scholar] [CrossRef]
- Rillig, M. C.; Ingraffia, R.; de Souza Machado, A. A. Microplastic Incorporation into Soil in Agroecosystems. Front. Plant Sci. 2017, 8, 1805. [Google Scholar] [CrossRef]
- de Souza Machado, A.A.; Lau, C. W.; Till, J.; Kloas, W.; Lehmann, A.; Becker, R.; Rillig, M. C. Impacts of Microplastics on the Soil Biophysical Environment. Environ. Sci. Technol. 2018, 52(17), 9656–9665. [Google Scholar] [CrossRef]
- de Souza Machado, A. A.; Lau, C. W.; Kloas, W.; Bergmann, J.; Bachelier, J. B.; Faltin, E.; Becker, R.; Görlich, A. S.; Rillig, M. C. Microplastics Can Change Soil Properties and Affect Plant Performance. Environ. Sci. Technol. 2019, 53(10), 6044–6052. [Google Scholar] [CrossRef]
- Dong, Y.; Gao, M.; Qiu, W.; Song, Z. Effect of microplastics and arsenic on nutrients and microorganisms in rice rhizosphere soil, Ecotoxicology and Environmental Safety 2021, 211, 111899. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, X.; Liu, G.; Liang, C.; Xue, S.; Chen, H.; Ritsema C., J.; Geissen, V. Response of Soil Dissolved Organic Matter to Microplastic Addition in Chinese Loess Soil. Chemosphere. 2017, 185, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, N. B.; Rist, S.; Bodin, J.; Jensen, L. H.; Schmidt, S. N.; Mayer, P.; Meibom, A.; Baun, A. Microplastics as Vectors for Environmental Contaminants: Exploring Sorption, Desorption, and Transfer to Biota. Integr. Environ. Assess. Manag. 2017, 13(3), 488–493. [Google Scholar] [CrossRef] [PubMed]
- Awet, T. T.; Kohl, Y.; Meier, F.; Straskraba, S.; Grün, A.-L.; Ruf, T.; et al. Effects of Polystyrene Nanoparticles on the Microbiota and Functional Diversity of Enzymes in Soil. Environ. Sci. Eur. 2018, 30(1), 11. [Google Scholar] [CrossRef]
- Fei, Y.; Huang, S.; Zhang, H.; Tong, Y.; Wen, D.; Xia, X.; Wang, H.; Luo, Y.; Barcel, D. Response of Soil Enzyme Activities and Bacterial Communities to the Accumulation of Microplastics in an Acid Cropped Soil. Sci. Total Environ. 2020, 707, 135634. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Fan, P.; Hou, J.; Dang, Q.; Cui, D.; Xi, B.; Tan, W. Inhibitory Effect of Microplastics on Soil Extracellular Enzymatic Activities by Changing Soil Properties and Direct Adsorption: An Investigation at the Aggregate-Fraction Level. Environ. Pollut. 2020, 267, 115544. [Google Scholar] [CrossRef]
- Guo, Q. Q.; Xiao, M. R.; Ma, Y.; Niu, H.; Zhang, G. S. Polyester Microfiber and Natural Organic Matter Impact Microbial Communities, Carbon-Degraded Enzymes, and Carbon Accumulation in a Clayey Soil. J. Hazard. Mater. 2021, 405, 124701. [Google Scholar] [CrossRef]
- Burns, R. G.; DeForest, J. L.; Marxsen, J.; Sinsabaugh, R. L.; Stromberger, M. E.; Wallenstein, M. D.; Weintraub, M. N.; Zoppini, A. Soil Enzymes in a Changing Environment: Current Knowledge and Future Directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Bandick, A. K.; Dick, R. P. Field Management Effects on Soil Enzyme Activities. Soil Biol. Biochem. 1999, 31(11), 1471–1479. [Google Scholar] [CrossRef]
- Hou, J.; Xu, X.; Yu, H.; Xi, B.; Tan, W. Comparing the Long-Term Responses of Soil Microbial Structures and Diversities to Polyethylene Microplastics in Different Aggregate Fractions. Environ. Int. 2021, 149, 106398. [Google Scholar] [CrossRef]
- Zhang, Z.; Peng, W.; Duan, C.; Zhu, X.; Wu, H.; Zhang, X.; Fang, L. Microplastics Pollution from Different Plastic Mulching Years Accentuate Soil Microbial Nutrient Limitations. Gondwana Res. 2021, 28. [Google Scholar] [CrossRef]
- de Almeida, M.P.; Gaylarde, C.C.; Baptista Neto, J.A.; Delgado, J.F.; Lima, L.S.; Neves, C.V.; Pompermayer, L.L.O.; Vieira, K.; da Fonseca, E.M. The prevalence of microplastics on the earth and resulting increased imbalances in biogeochemical cycling. Water Emerg. Contam. Nanoplastics 2023, 2, 7. [Google Scholar] [CrossRef]
- Chang, J.; Havlík, P.; Leclère, D.; Vries, W. de; Valin, H.; Deppermann, A.; Hasegawa, T.; Obersteiner, M. Reconciling regional nitrogen boundaries with global food security. Nat. Food. 2021, 2, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Cluzard, M.; Kazmiruk, T.; Kazmiruk, V.; Bendell, L. Intertidal Concentrations of Microplastics and Their Influence on Ammonium Cycling as Related to the Shellfish Industry. Archives of environmental contamination and toxicology. 2015, 69. [Google Scholar] [CrossRef] [PubMed]
- Seeley, M.E.; Song, B.; Passie, R.; Hale, R.C. Microplastics affect sedimentary microbial communities and nitrogen cycling. Nat. Commun. 2020, 11, 2372. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, L.; Mei, Q.; Dong, B.; Dai, X.; Ding, G.; Zeng, E. Y. Microplastics in sewage sludge from the wastewater treatment plants in China. Wat. Res. 2018, 142, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, S.A.M.; Elshafiey, E.H.; Shetaia, A.A.; El-Wahed, A.A.A.; Algethami, A.F.; Musharraf, S.G.; AlAjmi, M.F.; Zhao, C.; Masry, S.H.D.; Abdel-Daim, M.M.; et al. Overview of Bee Pollination and Its Economic Value for Crop Production. Insects. 2021, 12, 688. [Google Scholar] [CrossRef]
- Gill, R.J.; Baldock, K.C.; Brown, M.J.; Cresswell, J.E.; Dicks, L.V.; Fountain, M.T.; Garratt, M.P.; Gough, L.A.; Heard, M.S.; Holland, J. M.; Ollerton, J.; Graham, N.; Tang, S. C. Q.; Vanbergen, A. J.; Vogler, A. P.; Woodward, G.; Arce, A. N.; Boatman, N. D.; Brand-Hardy, R.; Breeze, T. D.; Green, M.; Hartfield, C. M.; O'Connor, R. S.; Osborne, J. L.; Phillips, J.; Sutton, P. B.; Potts, S. G. Protecting an Ecosystem Service: Approaches to Understanding and Mitigating Threats to Wild Insect Pollinators. Adv. Ecol. Res 2016, 54, 135–206. [Google Scholar] [CrossRef]
- Hristov, P.; Neov, B.; Shumkova, R.; Palova, N. Significance of apoidea as main pollinators. Ecological and economic impact and implications for human nutrition. Diversity. 2020, 12, 280. [Google Scholar] [CrossRef]
- Gallai, N.; Salles, J.-M.; Settele, J.; Vaissière, B.E. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 2009, 68, 810–821. [Google Scholar] [CrossRef]
- Geeraert, L.; Aerts, R.; Berecha, G.; Daba, G.; De Fruyt, N.; D’Hollander, J.; Helsen, K.; Stynen, H.; Honnay, O. Effects of landscape composition on bee communities and coffee pollination in Coffea arabica production forests in southwestern Ethiopia. Agric. Ecosyst. Environ. 2020, 288, 106706–106717. [Google Scholar] [CrossRef]
- Luo, D.; Silva, D.P.; De Marco Júnior, P.; Pimenta, M.; Caldas, M.M. Model approaches to estimate spatial distribution of bee species richness and soybean production in the Brazilian Cerrado during 2000 to 2015. Sci. Total Environ. 2020, 737, 139674. [Google Scholar] [CrossRef] [PubMed]
- Calderone, N.W. Insect pollinated crops, insect pollinators and US agriculture: Trend analysis of aggregate data for the period 1992–2009. PLoS ONE. 2012, 7, 37235. [Google Scholar] [CrossRef] [PubMed]
- Moritz, R.F.A.; de Miranda, J.; Fries, I.; Le Conte, Y.; Neumann, P.; Paxton, R.J. Research strategies to improve honeybee health in Europe. Apidologie. 2010, 41, 227–242. [Google Scholar] [CrossRef]
- Al Naggar, Y.; Codling, G.; Giesy, J.P.; Safer, A. Beekeeping and the Need for Pollination from an Agricultural Perspective in Egypt. Bee World. 2018, 95, 107–112. [Google Scholar] [CrossRef]
- Le Conte, Y.; Ellis, M.; Ritter, W. Varroa mites and honeybee health: Can Varroa explain part of the colony losses? Apidologie. 2010, 41, 353–363. [Google Scholar] [CrossRef]
- Staveley, J.P.; Law, S.A.; Fairbrother, A.; Menzie, C.A. A Causal Analysis of Observed Declines in Managed Honey Bees (Apis mellifera). Hum. Ecol. Risk Assess. 2014, 20(2), 566–591. [Google Scholar] [CrossRef]
- Jacques, A.; Laurent, M.; Ribière-Chabert, M.; Saussac, M.; Bougeard, S.; Budge, G.E.; Hendrikx, P.; Chauzat, M.-P. A pan-European epidemiological study reveals honey bee colony survival depends on beekeeper education and disease control. PLoS ONE. 2017, 12, 0172591. [Google Scholar] [CrossRef]
- Kulhanek, K.; Steinhauer, N.; Rennich, K.; Caron, D.M.; Sagili, R.R.; Pettis, J.S.; Ellis, J.D.; Wilson, M.E.; Wilkes, J.T.; Tarpy, D.R.; Tarpy, D. R.; Icon, R. R.; Lee, K.; Rangel, J.; van Engelsdorp, D. A national survey of managed honeybee 2015–2016 annual colony losses in the USA. J. Apic. Res. 2017, 56, 328–340. [Google Scholar] [CrossRef]
- Al Naggar, Y.; Baer, B. Consequences of a short time exposure to a sublethal dose of Flupyradifurone (Sivanto) pesticide early in life on survival and immunity in the honeybee (Apis mellifera). Sci. Rep. 2019, 9, 19753. [Google Scholar] [CrossRef]
- Al Naggar, Y.; Paxton, R.J. Mode of transmission determines the virulence of black queen cell virus in adult honeybees, posing a future threat to bees and apiculture. Viruses. 2020, 12, 535. [Google Scholar] [CrossRef] [PubMed]
- Gray, A.; Brodschneider, R.; Adjlane, N.; Ballis, A.; Brusbardis, V.; Charrière, J.-D.; Chlebo, R.F.; Coffey, M.; Cornelissen, B.; Amaro da Costa, C.; Ballis, A.; Brusbardis, V.J.-D.C.; Chlebo, R.; Coffey, M. F.; Cornelissen, B.; da Costa, C. A.; Csáki, T.; Dahle, B.; Danihlík, J.; Dražić, M. M.; Evans, G.; Fedoriak, M.; Forsythe, I.; de Graaf, D.; Gregorc, A.; Johannesen, J.; Kauko, L.; Kristiansen, P. Martikkala, M.; Martín-Hernández, R.; Medina-Flores, C. A.; Mutinelli, F.; Patalano, S.; Petrov, P.; Raudmets, A.; Ryzhikov, V. A.; Simon-Delso, N.; Stevanovic, J.; Topolska, G.; Uzunov, A.; Vejsnaes, F.; Williams, A.; Zammit-Mangion, M.; Soroke, V. Loss rates of honeybee colonies during winter 2017/18 in 36 countries participating in the COLOSS survey, including effects of forage sources. J. Apic. Res. 2019, 58, 479–485. [Google Scholar] [CrossRef]
- Neov, B.; Georgieva, A.; Shumkova, R.; Radoslavov, G.; Hristov, P. Biotic and Abiotic Factors Associated with Colonies Mortalities of Managed Honey Bee (Apis mellifera). Diversity. 2019, 11, 237. [Google Scholar] [CrossRef]
- Nechaume Moncharmont, F.-X.; Decourtye, A.; Hennequet-Hantier, C.; Pons, O.; Pham-Delègue, M.-H. Statistical analysis of honeybee survival after chronic exposure to insecticides. Environ. Toxicol. Chem. 2003, 22, 3088. [Google Scholar] [CrossRef]
- Gill, R.J.; Ramos-Rodriguez, O.; Raine, N.E. Combined pesticide exposure severely affects individual- and colony-level traits in bees. Nature. 2012, 491, 105–108. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Goulson, D.; Nicholls, E.; Botias, C.; Rotheray, E.L. Bee declines are driven by combined stress from parasites, pesticides, and lack of flowers. Science. 2015, 347, 1255957. [Google Scholar] [CrossRef]
- Manley, R.; Boots, M.; Wilfert, L. REVIEW: Emerging viral disease risk to pollinating insects: Ecological, evolutionary and anthropogenic factors. J. Appl. Ecol. 2015, 52, 331–340. [Google Scholar] [CrossRef]
- Diaz-Basantes, M.F.; Conesa, J.A.; Fullana, A. Microplastics in Honey, Beer, Milk and Refreshments in Ecuador as Emerging Contaminants. Sustainability, 2020, 12, 5514. [Google Scholar] [CrossRef]
- Edo, C.; Fernández-Alba, A.R.; Vejsnæs, F.; van der Steen, J.J.M.; Fernández-Piñas, F.; Rosal, R. Honeybees as active samplers for microplastics. Sci. Total Environ. 2021, 767, 144481. [Google Scholar] [CrossRef]
- Alma, A. M.; de Groot, G. S.; Buteler, M. Microplastics incorporated by honeybees from food are transferred to honey, wax and larvae. Environ. Pollut 2023, 121078. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Chen, H.; Lin, Z.-G.; Niu, Q.-S.; Wang, Z.; Gao, F.-C.; Ji, T. Carbendazim exposure during the larval stage suppresses major royal jelly protein expression in nurse bees (Apis mellifera). Chemosphere. 2021, 266, 129011. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Jiang, X.; Zhao, H.; Yang, S.; Gao, J.; Wu, Y.; Diao, Q.; Hou, C. Microplastic Polystyrene Ingestion Promotes the Susceptibility of Honeybee to Viral Infection. Environ Sci Technol. 2021, 55(17), 11680–11692. [Google Scholar] [CrossRef] [PubMed]
- Al Naggar, Y.; Sayes, C.M.; Collom, C.; Ayorinde, T.; Qi, S.; El-Seedi, H.R.; Paxton, R.J.; Wang, K. Chronic Exposure to Polystyrene Microplastic Fragments Has No Effect on Honeybee Survival, but Reduces Feeding Rate and Body Weight. Toxics. 2023, 11(2), 100. [Google Scholar] [CrossRef]
- Balzani, P.; Galeotti, G.; Scheggi, S.; Masoni, A.; Santini, G.; Baracchi, D. Acute and chronic ingestion of polyethylene (PE) microplastics has mild effects on honeybee health and cognition. Environ. Pollut. 2022, 119318. [Google Scholar] [CrossRef]
- Shah, S.; Ilyas, M.; Li, R.; Yang, J.; Yang, F. L. Microplastics and Nanoplastics Effects on Plant–Pollinator Interaction and Pollination Biology. Environ. Sci. & Tech. 2023, 57, 6415–6424. [Google Scholar]
- Kim, S.W.; An, Y.J. Soil microplastics inhibit the movement of springtail species. Environ. Int. 2019, 126, 699–706. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Goodhead, R.; Moger, J.; Galloway, T.S. Microplastic ingestion by zooplankton. Environ. Sci. Technol. 2013, 47, 6646–6655. [Google Scholar] [CrossRef]
- Wu, M.; Yang, C.; Du, C.; Liu, H. Microplastics in waters and soils: Occurrence, analytical methods and ecotoxicological effects. Ecotoxicol. Environ. Saf. 2020, 1, 202–110910. [Google Scholar] [CrossRef]
- Wang, J.; Coffin, S.; Sun, C.; Schlenk, D.; Gan, J. Negligible effects of micro- plastics on animal fitness and HOC bioaccumulation in earthworm Eisenia fetida in soil. Environ. Pollut. 2019, 249, 776–784. [Google Scholar] [CrossRef]
- Cui, G.; Lü, F.; Hu, T.; Zhang, H.; Shao, L.; He, P. Vermicomposting leads to more abundant microplastics in the municipal excess sludge. Chemosphere. 2022, 307, 136042. [Google Scholar] [CrossRef] [PubMed]
- Huerta Lwanga, E.; Gertsen, H.; Gooren, H.; Peters, P.; Salanki, T.; van der Ploeg, M.; Besseling, E.; Koelmans, A.A.; Geissen, V. Microplastics in the terrestrial ecosystem: implications for lumbricus terrestris (Oligochaeta, lumbricidae) Sci. Total Environ. 2016, 50, 2685–2691. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.; Zhu, D.; Qiao, M. Effects of polyethylene microplastics on the gut microbial community, reproduction and avoidance behaviors of the soil springtail, Folsomia candida. Environ. Pollut. 2019, 247, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Liu, M.; Song, Y.; Lu, S.; Hu, J.; Cao, C.; Xie, B.; Shi, H.; He, D. Polystyrene (nano)microplastics cause size-dependent neurotoxicity, oxidative damage and other adverse effects in Caenorhabditis elegans. Environ. Sci. J. Integr. Environ. Res.: Nano. 2018, 5, 2009–2020. [Google Scholar] [CrossRef]
- Song, Y.; Cao, C.; Qiu, R.; Hu, J.; Liu, M.; Lu, S.; Shi, H.; Raley-Susman, K. M.; He, D. Uptake and adverse effects of polyethylene terephthalate microplastics fibers on terrestrial snails (Achatina fulica) after soil exposure. Environ. Pollut. 2019, 250, 447–455. [Google Scholar] [CrossRef]
- Hüffer, T.; Metzelder, F.; Sigmund, G.; Slawek, S.; Schmidt, T.C.; Hofmann, T. Polyethylene microplastics influence the transport of organic contaminants in soil. Sci. Total Environ. 2019, 657, 242–247. [Google Scholar] [CrossRef]
- Wang, J.D.; Peng, J.P.; Tan, Z.; Gao, Y.F.; Zhan, Z.W.; Chen, Q.Q.; Cai, L.Q. Microplastics in the surface sediments from the Beijiang River littoral zone: composition, abundance, surface textures and interaction with heavy metals. Chemosphere. 2017, 171, 248–258. [Google Scholar] [CrossRef]
- Hodson, M.E.; Duffus-Hodson, C.A.; Clark, A.; Prendergast-Miller, M.T.; Thorpe, K.L. Plastic bag derived-microplastics as a vector for metal exposure in terrestrial invertebrates. Environ. Sci. Technol. 2017, 51, 4714–4721. [Google Scholar] [CrossRef]
- FAO. The state of world fisheries and aquaculture 2018 - meeting the sustainable development goals. Rome: Licence: CC BY-NC-SA 3.0 IGO.
- Li, L.; Abouelezz, K.; Gou, Z.; Lin, X.; Wang, Y.; Fan, Q.; Cheng, Z.; Ding, F.; Jiang, S.; Jiang, Z. Optimization of dietary zinc requirement for broiler breeder hens of Chinese yellow-feathered chicken. Animals (Basel). 2019, 9. [Google Scholar] [CrossRef]
- Chen, F.; Jiang, Z.; Jiang, S.; Li, L.; Lin, X.; Gou, Z.; Fan, Q. Dietary vitamin a supplementation improved reproductive performance by regulating ovarian expression of hormone receptors, caspase-3 and fas in broiler breeders. Poult. Sci. 2016, 95, 30–40. [Google Scholar] [CrossRef]
- Beriot, N.; Peek, J.; Zornoza, R.; Geissen, V.; Lwanga, E.H. Low density-microplastics detected in sheep faeces and soil: A case study from the intensive vegetable farming in Southeast Spain. Science of the Total Environment 2021, 755, 142653. [Google Scholar] [CrossRef] [PubMed]
- Cammack, K.M.; Austin, K.J.; Lamberson, W.R.; Conant, G.C.; Cunningham, H.C. Tiny but mighty: the role of the rumen microbes in livestock production. J. Anim. Sci. 2018, 6, 752–770. [Google Scholar] [CrossRef] [PubMed]
- Jami, E.; A. Israel; A. Kotser; I. Mizrahi. Exploring the bovine rumen bacterial community from birth to adulthood. ISME J. 2013, 7, 1069–1079. [Google Scholar] [CrossRef]
- Flint, H. J.; Bayer, E. A. Plant cell wall breakdown by anaerobic microorganisms from the mammalian digestive tract. Ann. N.Y. Acad. Sci. 2008, 1125, 280–288. [Google Scholar] [CrossRef]
- Baldwin, R. L. Digestion and metabolism of ruminants. BioScience. 1984, 34, 244–249. [Google Scholar] [CrossRef]
- Church, D. C. The ruminant animal: digestive physiology and nutrition. Englewood Cliffs, NJ: Prentice-Hall, Inc. 1988.
- Russell, J. B.; Rychlik, J. L. Factors that alter rumen microbial ecology. Science. 2001, 292, 1119–1122. [Google Scholar] [CrossRef] [PubMed]
- Millen, D.D.; Arrigoni, M. D. B. , Lauritano Pacheco, R.D., eds. 2016. Rumenology. Cham, Switzerland: Springer International Publishing. [CrossRef]
- Ross, E. M.; Moate, P. J.; Bath, C. R.; Davidson, S. E.; Sawbridge, T. I.; Guthridge, K. M.; Cocks, B. G.; Hayes, B. J. High throughput whole rumen metagenome profiling using untargeted massively parallel sequencing. BMC Genet. 2012, 13, 53. [Google Scholar] [CrossRef]
- Priyanka, M.; Dey, S. Ruminal impaction due to plastic materials-an increasing threat to ruminants and its impact on human health in developing countries. Vet. World. 2018, 11(9), 1307. [Google Scholar] [CrossRef]
- Mahadappa, P.; Krishnaswamy, N.; Karunanidhi, M.; Bhanuprakash, A.G.; Bindhuja, B.V.; Dey, S. Effect of plastic foreign body impaction on rumen function and heavy metal concentrations in various body fluids and tissues of buffaloes. Ecotoxicol. Environ. Saf. 2020, 189, 109972. [Google Scholar] [CrossRef]
- Beriot, N.; Peek, J.; Zornoza, R.; Geissen, V.; Lwanga, E.H. Low density-microplastics detected in sheep faeces and soil: a case study from the intensive vegetable farming in Southeast Spain. Sci. Total Environ. 2021, 755, 142653. [Google Scholar] [CrossRef]
- Tourinho, P.S.; Kočí, V.; Loureiro, S.; van Gestel, C.A.M. Partitioning of chemical contaminants to microplastics: sorption mechanisms, environmental distribution and effects on toxicity and bioaccumulation. Environ. Pollut. 2019, 252, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Jaskulak, M.; Zorena, K. Migration of Microplastic-Bound Contaminants to Soil and Their Effects. In Microplastics in the Ecosphere. 2023. (eds M. Vithanage and M.N.V. Prasad). [CrossRef]
- Wang, X. F.; Xu, Y.; Qin, M. Y.; Zhao, Z.; Fan, X. F.; Li, Q. B. Insight into the effects of Cu2+ ions and CuO species in Cu-SSZ-13 catalysts for selective catalytic reduction of NO by NH3. J. Colloid Interface Sci. 2022, 622, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Duan, P.; Tong, L.; Zhang, W. Influence of polystyrene microplastics on the volatilization, photodegradation and photoinduced toxicity of anthracene and pyrene in freshwater and artificial seawater. Sci. Total Environ. 2022, 819, 152049. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Hassan, I.; Peng, Y. T.; Huo, S. L.; Ling, L. Behaviors and influencing factors of the heavy metal’s adsorption onto microplastics: A review. J. Clean. Prod. 2021, 319, 128777. [Google Scholar] [CrossRef]
- Lavers, J.L. A review of concurrent threats to Flesh-footed Shearwaters (Puffinus carneipes): words of warning from a top marine predator in decline. ICES J. Mar. Sci. 2014, 72, 10–1093. [Google Scholar]
- Lavers, J.L.; Hutton, I.; Bond, A.L. Clinical pathology of plastic ingestion in marine birds and relationships with blood chemistry. Environ. Sci. and Tech. 2019, 53, 9224–9231. [Google Scholar] [CrossRef]
- Patangia, D.V.; Anthony Ryan, C.; Dempsey, E.; Paul Ross, R.; Stanton, C. Impact of antibiotics on the human microbiome and consequences for host health. Microbiologyopen. 2022, 11(1), 1260. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J Gastroenterol. 2015, 21(29), 8787–803. [Google Scholar] [CrossRef]
- Kong, A.; Zhang, C.; Cao, Y.; Cao, Q.; Liu, F.; Yang, Y.; Tong, Z.; Rehman, M.U.; Wang, X.; Huang, S. The fungicide thiram perturbs gut microbiota community and causes lipid metabolism disorder in chickens. Ecotoxicol. Environ. Saf. 2020, 206, 111400. [Google Scholar] [CrossRef]
- Li, A.; Wang, Y.; Fakhar-e-Alam Kulyar, M.; Iqbal, M.; Lai, R.; Zhu, H.; Li., K. Environmental microplastics exposure decreases antioxidant ability, perturbs gut microbial homeostasis and metabolism in chicken. Sci. of The Total Environ. 2023, 856, 159089. [Google Scholar] [CrossRef]
- Tidwell, J. H.; Bright, L. A. Freshwater aquaculture. In Encyclopedia of ecology (second edition). 2019, 1, 91–96. [Google Scholar] [CrossRef]
- Iheanacho, S.C.; Ikwo, T.N.; Igweze, N.; Chukwuidha, C.; Ogueji, E.O.; Onyeneke, R. Effect of different dietary inclusion levels of melon seed (Citrullus lanatus) peel on growth, hematology and histology of Oroechromis niloticus juvenile. Turkish Journal of Fisheries and Aquatic Sciences. 2018, 18, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Ogunji, O.; Iheanacho, S.C.; Mgbabu, C.C.; Amaechi, N.C.; Evulobi, O.C. Housefly maggot meal as a potent bioresource for fish feed to facilitate early gonadal development in Clarias gariepinus (Burchell, 1822). Sustainability 2021, 13, 921. [Google Scholar] [CrossRef]
- Lusher, A.; Welden, N.; Sobral, P.; Cole, M. Sampling, isolating and identifying microplastics ingested by fish and invertebrates. Anal. Methods. 2017, 9, 1346–1360. [Google Scholar] [CrossRef]
- Devriese, L.I.; Van der Meulen, M.D.; Maes, T.; Bekaert, K.; Paul-Pont, I.; Frère, L.; Robbens, J.; Vethaak, A.D. Microplastic contamination in brown shrimp (Crangon crangon, Linnaeus 1758) from coastal waters of the Southern North Sea and Channel area. Mar. Poll. Bull. 2015, 98, 179–187. [Google Scholar] [CrossRef]
- Van Cauwenberghe, L.; Claessens, M.; Vandegehuchte, M.B.; Janssen, C.R. Microplastics are taken up by mussels (Mytilus edulis) and lugworms (Arenicola marina) living in natural habitats. Environ. Poll. 2015, 199, 10–17. [Google Scholar] [CrossRef]
- Teng, J.; Wang, Q.; Ran, W.; Wu, D.; Liu, Y.; Sun, S.; Liu, H.; Cao, R.; Zhao, J. Microplastic in cultured oysters from different coastal areas of China. Sci. of the Tot. Environ. 2019, 653, 1282–1292. [Google Scholar] [CrossRef]
- Cheung, L.T.O.; Lui, C.Y.; Fok, L. Microplastic Contamination of Wild and Captive Flathead Grey Mullet (Mugil cephalus). Inter. J. of Environ. Res. and Public Health. 2018, 15(4), 597. [Google Scholar] [CrossRef]
- Savoca, M.S.; McInturf, A.G.; Hazen, E.L. Plastic ingestion by marine fish is widespread and increasing. Glob. Change Biol. 2021, 27, 2188–2199. [Google Scholar] [CrossRef]
- Bhuyan, Md. S. Effects of microplastics on fish and in human health. Front. Environ. Sci. 2022, 10, 250. [Google Scholar] [CrossRef]
- Siddiquia, S.A.; Khanc, S. , Tariqd, T.; Sameend, A.; Nawaze, A.; Walayath, N.; Oboturovai, N.P.; Ambartsumovi, T.G.; Nagdaliani, A.A. Potential risk assessment and toxicological impacts of nano/micro-plastics on human health through food products. Nano/micro-Plastics Toxicity on Food Quality and Food Safety. 2023, 103, 361. [Google Scholar] [CrossRef]
- Marco, D.G.; Conti, G.O.; Giannetto, A.; Cappello, T.; Galati, M.; Iaria, C.; Pulvirenti, E.; Capparucci, F.; Mauceri, A.; Ferrante, M.; Maisano, M. Embryotoxicity of polystyrene microplastics in zebrafish Daniorerio. Environ. Res. 2022, 208, 112552. [Google Scholar] [CrossRef]
- Jakubowska, M.; Białowąs, M.; Stankevičiūtė, M.; Chomiczewska, A.; Pažusienė, J.; Jonko-Sobuś, K.; Hallmann, A.; Urban-Malinga, B. Effects of chronic exposure to microplastics of different polymer types on early life stages of sea trout Salmo trutta. Sci. Total Environ. 2020, 740, 139922. [Google Scholar] [CrossRef]
- Pradit, S.; Noppradit, P.; Jitkaew, P.; Sengloyluan, K.; Yucharoen, M.; Suwanno, P.; Tanrattanakul, V.; Sornplang, K.; Nitiratsuwan, T. Microplastic Accumulation in Catfish and Its Effects on Fish Eggs from Songkhla Lagoon, Thailand. Journal of Marine Science and Engineering. 2023, 11(4), 723. [Google Scholar] [CrossRef]
- Angsupanich, S.; Somsak, S.; Phrommoon, J. Stomach contents of the catfishes Osteogeneiosus militaris (Linnaeus, 1758) and Arius maculatus (Thunberg, 1792) in the Songkhla lake. Warasan Songkhla Nakharin (Sakha Witthayasat lae Technology). 2005. Available online: https://agris.fao.org/agris-search/search.do?recordID=TH2008001875 (accessed on 25 February 2023).
- Romeo, T.; Pietro, B.; Pedà, C.; Consoli, P.; Andaloro, F.; Fossi, M. C. First Evidence of Presence of Plastic Debris in Stomach of Large Pelagic Fish in the Mediterranean Sea. Mar. Pollut. Bull. 2015, 95(1), 358–361. [Google Scholar] [CrossRef]
- Kaleem, O.; Sabi, S. Overview of aquaculture systems in Egypt and Nigeria, prospects, potentials, and constraints. Aquaculture and Fisheries. 2020, 6, 535–547. [Google Scholar] [CrossRef]
- Hanachi, P.; Karbalaei, S.; Walker, T.R.; Cole, M.; Hosseini, S. V. Abundance and properties of microplastics found in commercial fish meal and cultured common carp (Cyprinus carpio). Environ. Sci. Pollut. Res. 2019, 26, 23777–23787. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: a review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Pironti, C.; Ricciardi, M.; Motta, O.; Miele, Y.; Proto, A.; Montano, L. Microplastics in the Environment: Intake through the Food Web, Human Exposure and Toxicological Effects. Toxics. 2021, 9(9), 224. [Google Scholar] [CrossRef]
- Barnes, D. K.; Galgani, F.; Thompson, R. C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philosophical Transactions of the Royal Society B. 2009, 364, 1985–1998. [Google Scholar] [CrossRef]
- Rochman, C. M. , Lewison, R. L., Eriksen, M., Allen, H., Cook, A. M. Polybrominated diphenyl ethers (PBDEs) in fish tissue may be an indicator of plastic contamination in marine habitats. Sci. Total Environ. 2014, 476, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Tian, J. Y.; Xu, R.; Zhang, Z. Y.; Yang, G. P.; Wang, X. D.; Chen, R. Effects of microplastics exposure on ingestion, fecundity, development, and dimethylsulfide production in Tigriopus japonicus (Harpacticoida, copepod). Environ. Pollut. 2020, 267, 115429. [Google Scholar] [CrossRef] [PubMed]
- Iheanacho, S. C.; Odo, G. E. Dietary exposure to polyvinyl chloride microparticles induced oxidative stress and hepatic damage in Clarias gariepinus (Burchell, 1822). Environ. Science and Pollut. Res. 2020, 27(17), 21159–21173. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.; Andreou, D.; Green, I. D.; Britton, J. R. Microplastics in freshwater fishes: Occurrence, impacts and future perspectives. Fish and Fisheries. 2021, 22(3), 467–488. [Google Scholar] [CrossRef]
- Farrell, P.; Nelson, K. Trophic level transfer of microplastic: Mytilus edulis (L.) to Carcinus maenas (L.). Environ. Pollut. 2013, 177, 1–3. [Google Scholar] [CrossRef]
- Kühn, E. L.; Bravo, J. A.; Franeker, V. Deleterious effects of litter on marine life, Marine Anthropogenic. Litter. Springer International Publishing. Chamber. 2015. [Google Scholar] [CrossRef]
- Seltenrich, N. New link in the food chain? Marine plastic pollution and seafood safety. Environmental Health Perspectives. 2015, 123, 34–41. [Google Scholar] [CrossRef]
- Sharma, S.; Chatterjee, S. Microplastic pollution, a threat to marine ecosystem and human health: A short review. ESPR. 2017, 24, 21530–21530. [Google Scholar] [CrossRef]
- Ferrando, J. S. The effect of microplastics on commercially value aquaculture species: A review. 2021, 48. Universitat Politécnica De Valencia. Available online: http://hdl.handle.net/10251/174972.
- Gesamp. In P. J. Kershaw (Ed.), Sources, fate and effects of microplastics in the marine environment: A global assessment. International Maritime Organization. 2015.
- Savoca, S.; Matanović, K.; D'Angelo, G.; Vetri, V.; Anselmo, S.; Bottari, T.; Mancuso, M.; Kužir, S.; Spanò, N.; Capillo, G.; Di Paola, D. Ingestion of plastic and non-plastic microfibers by farmed gilthead sea bream (Sparus aurata) and common carp (Cyprinus carpio) at different life stages. Science of the Total Environment. 2021, 782, 146851. [Google Scholar] [CrossRef]
- Talvitie, J.; Heinonen, M.; Paakkonen, J. P.; Vahtera, E.; Mikola, A.; Setala, O.; Vahala, R. Do wastewater treatment plants act as a potential point source of microplastics? Preliminary study in the coastal Gulf of Finland, baltic sea Water Sci. and Tech. 2015, 1495–1505. [Google Scholar] [CrossRef]
- Wright, S. L.; Rowe, D.; Thompson, R. C.; Galloway, T. S. Microplastic ingestion decreases energy reserves in marine worms. Curr. Biol. 2013, 23, 1031–1033. [Google Scholar] [CrossRef]
- Jovanovic, B.; Gokdag, K.; Guven, O.; Emre, Y.; Whitely, E. M.; Kideys, A. E. Virgin microplastics are not causing imminent harm to fish after dietary exposure. Mar. Pollut. Bull. 2018, 130, 123–131. [Google Scholar] [CrossRef]
- Kim, J.; Poirier, D. G.; Helm, P. A.; Bayoumi, M.; Rochman, C. M. No evidence of spherical microplastics (10-300 μm) translocation in adult rainbow trout (Oncorhynchus mykiss) after a two-week dietary exposure. PLoS One. 2020, 15(9), 0239128. [Google Scholar] [CrossRef] [PubMed]
- Hamed, M.; Soliman, H. A. M.; Badrey, A. E. A.; Osman, A. G. M. Microplastics induced histopathological lesions in some tissues of tilapia (Oreochromis niloticus) early juveniles. Tissue and Cell. 2021, 71. [Google Scholar] [CrossRef]
- Halsband, C.; Galloway, T. S. The impact of polystyrene microplastics on feeding, function and fecundity in the marine copepod Calanus helgolandicus. Environ. Science and Tech. 2015, 49, 1130–1137. [Google Scholar]
- Lyu, W.; Chen, Q.; Cheng, L.; Zhou, W. Microplastics in aquaculture systems and their transfer in the food chain. Microplastics in Terrestrial Environments. The Handbook of Environmental Chemistry. 2020, 95. Springer, Cham. [Google Scholar] [CrossRef]
- Thodesen, J.; Grisdale-Helland, B.; Helland, S. J.; Gjerde, B. Feed intake, growth and feed utilization of offspring from wild and selected Atlantic salmon Salmo salar. Aquaculture. 1999, 180, 237–246. [Google Scholar] [CrossRef]
- Rikardsen, A.; Sandring, S. Diet and size-selective feeding by escaped hatchery rainbow trout Oncorhynchus mykiss (Walbaum). J. Mar. Sci. 2006, 63, 460–465. [Google Scholar] [CrossRef]
- Skilbrei, O. T. The importance of escaped farmed rainbow trout (Oncorhynchus mykiss) as a vector for the salmon louse (Lepeophtheirus salmonis) depends on the hydrological conditions in the fjord. Hydrobiologia. 2012, 686, 287–297. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




