Submitted:
02 August 2023
Posted:
03 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
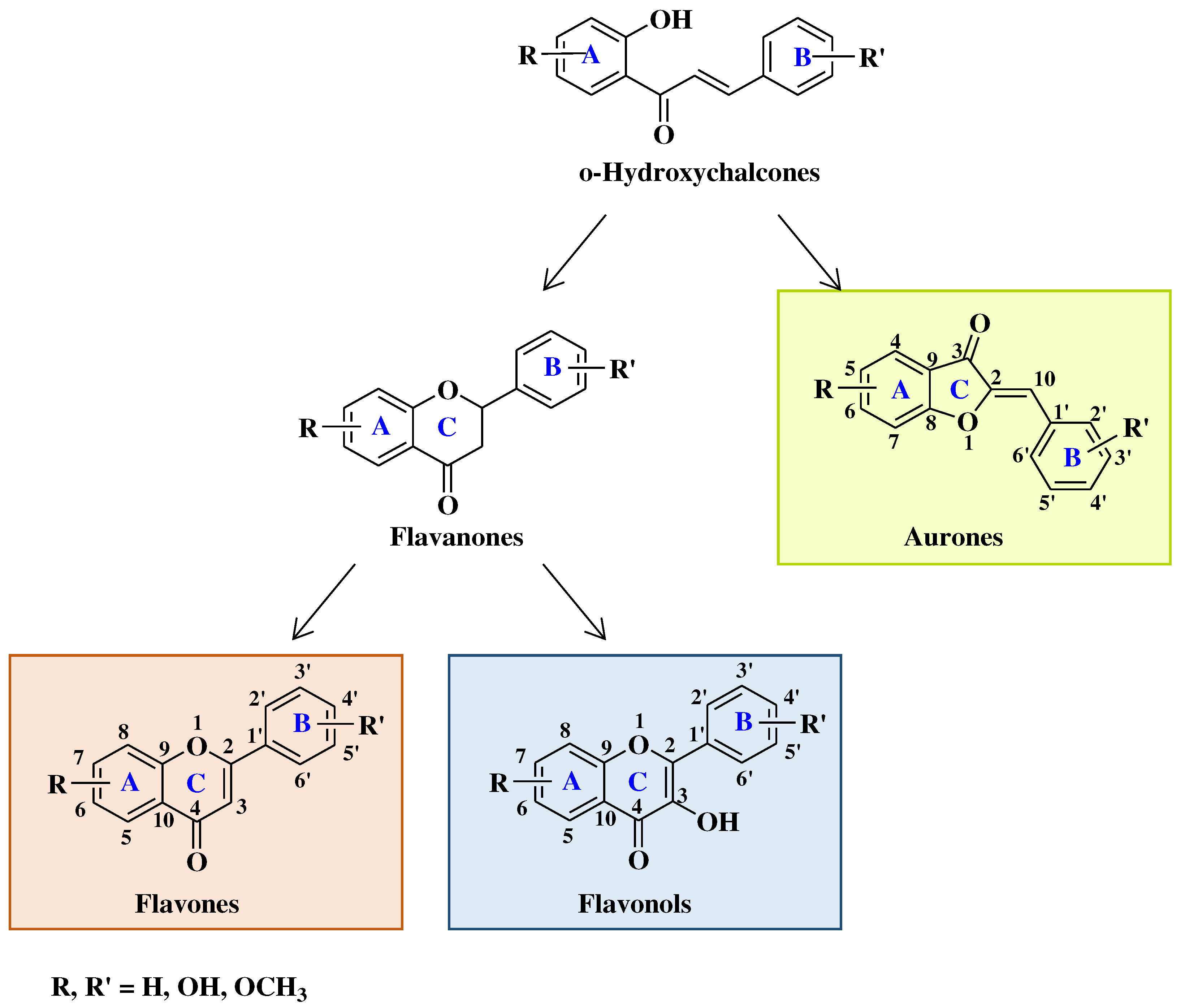
2. Biological activity of flavones, flavonols and aurones
2.1. Anticancer activity
| Entry | Chemical structure | Cancer cell lines against the tested compounds present cytotoxic activity | Ref. |
|---|---|---|---|
| 1 | 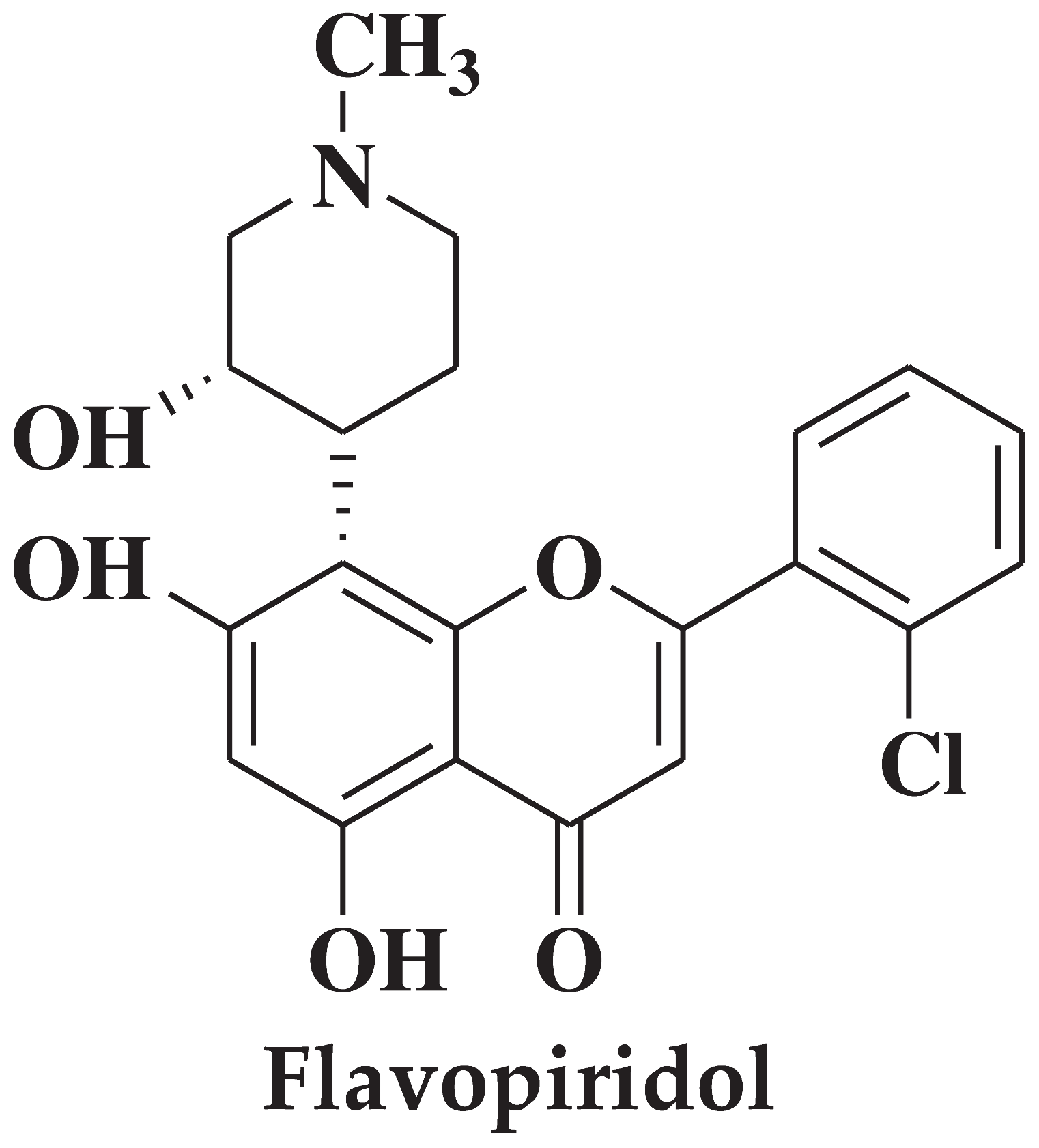 |
- acute myeloid leukemia cells | [28] |
| 2 | 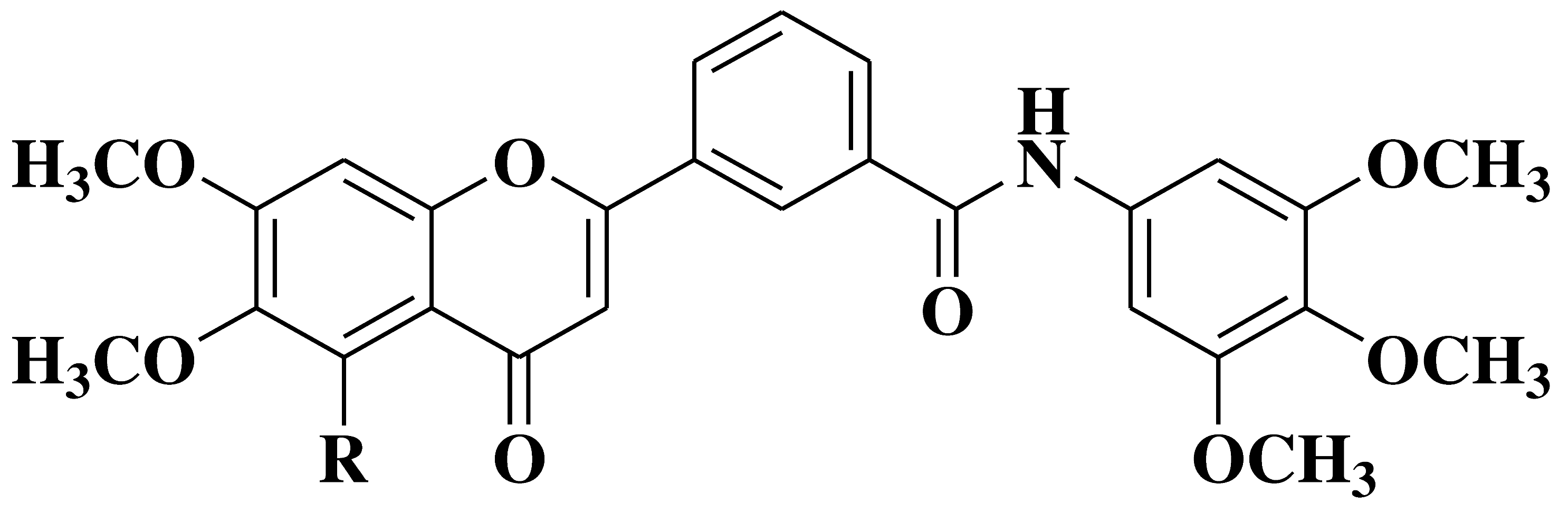 |
R = OCH3 - cell lines of hematologic cancers RPMI8226, CCRFCEM, HL60(TB), K562, MOLT4, SR - non-small-cell lung cancer (NSCLC) A549, HOP62, HOP92, H226, H23, H460, H522 - breast cancer cells MCF7, HS578T, BT549, MDAMB468 |
[17] |
|
R = OH - ovarian cancer cell lines OVCAR3, OVCAR8, ADRRES - breast cancer cells MCF7, HS578T, BT549, MDAMB468 | |||
| 3 | 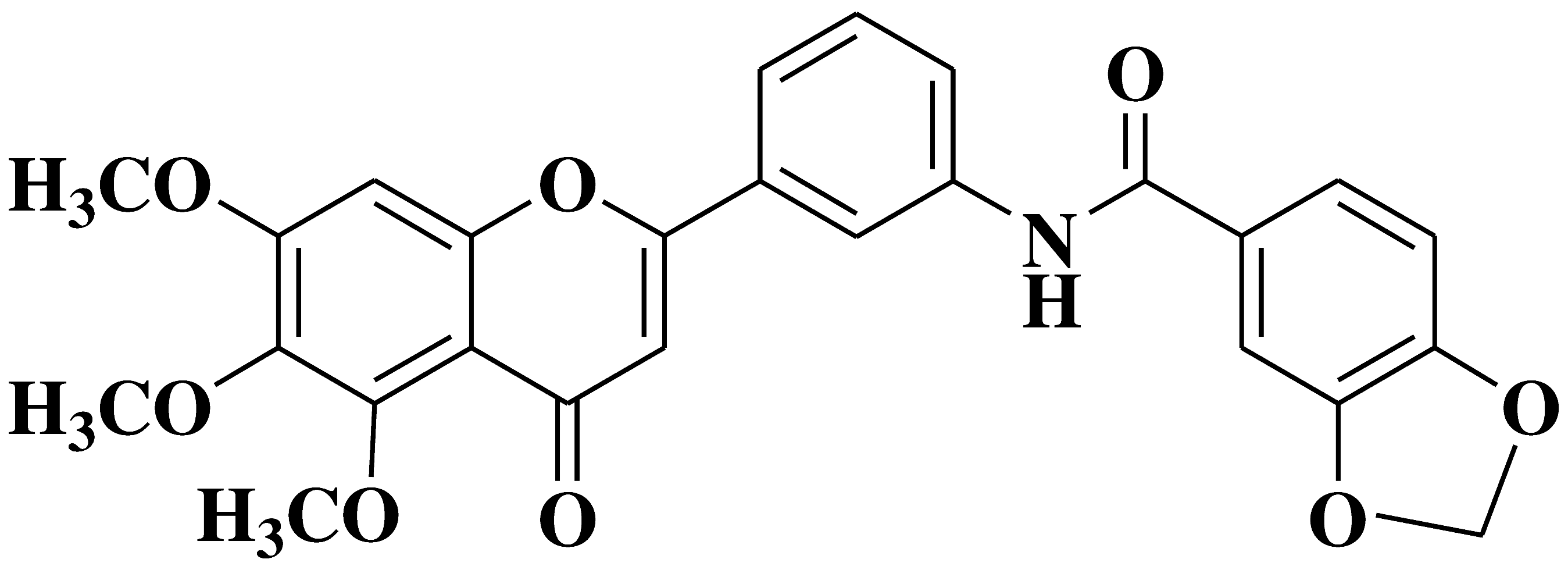 |
- cell lines of hematologic cancers RPMI8226, CCRFCEM, HL60(TB), K562, MOLT4, SR | [17] |
| - non-small-cell lung cancer cell lines (NSCLC) HOP92, H322M, H460, H522 | |||
| 4 | 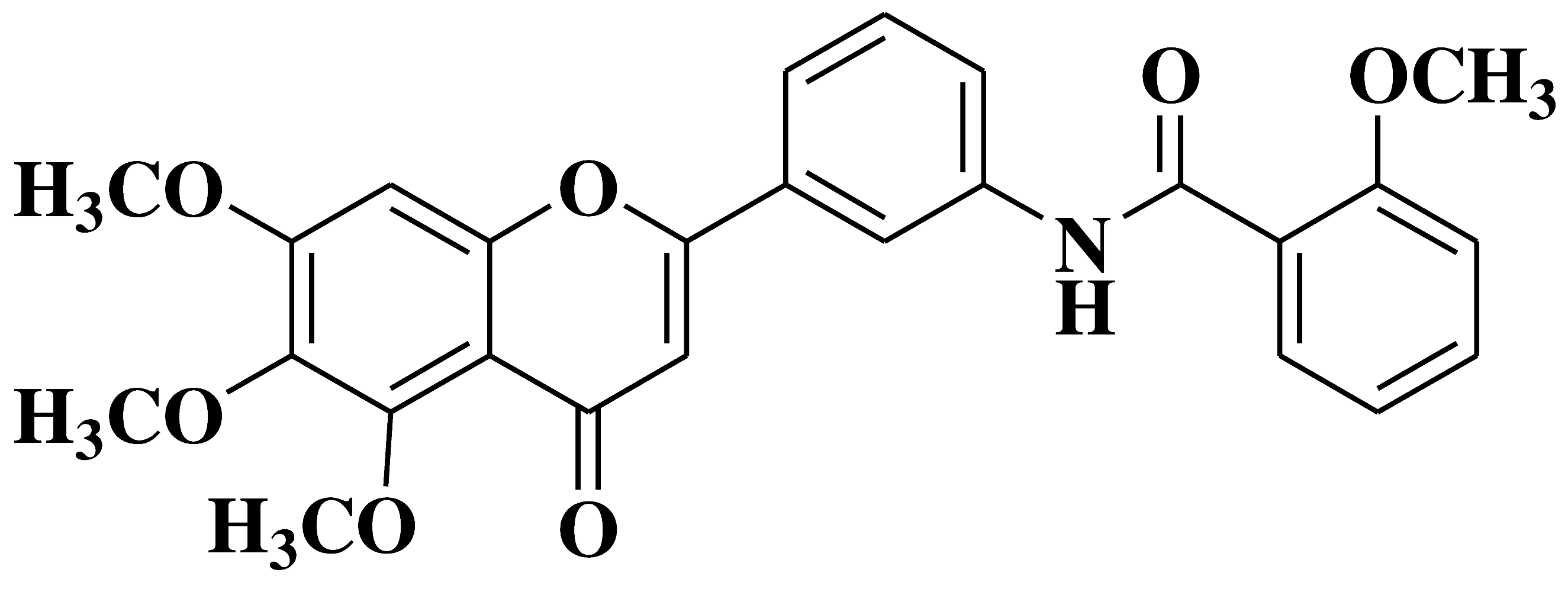 |
- cell lines of hematologic cancers RPMI8226, CCRFCEM, HL60(TB), K562, MOLT4 | [17] |
| - non-small-cell lung cancer cell lines (NSCLC) A549, HOP92 H322M, H522 | |||
| 5 | 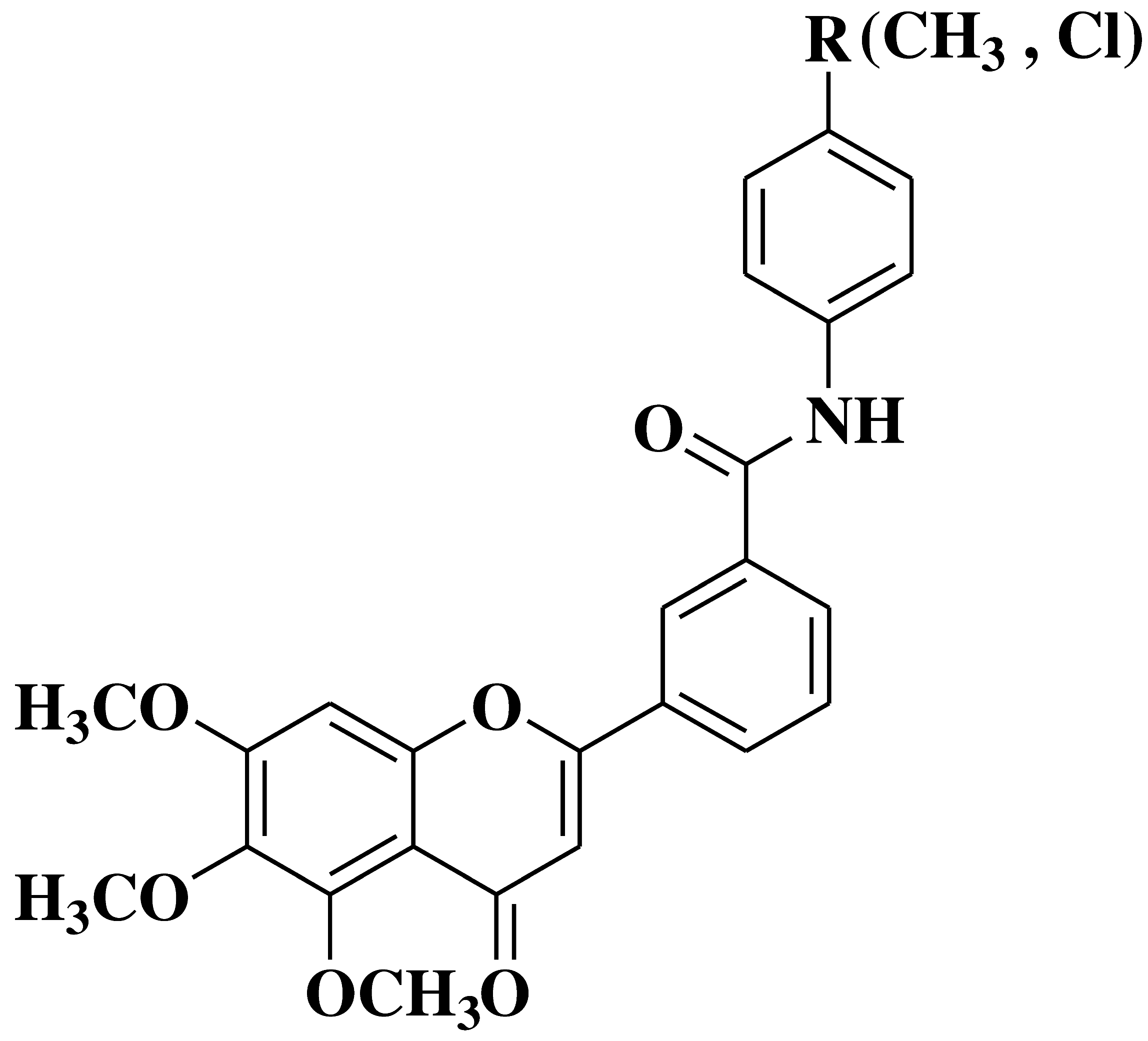 |
- large spectra antitumour activity: melanoma, hematologic, renal, colon, lung, brain, ovarian, breast and prostate cancer cell lines |
[18] |
| 6 | 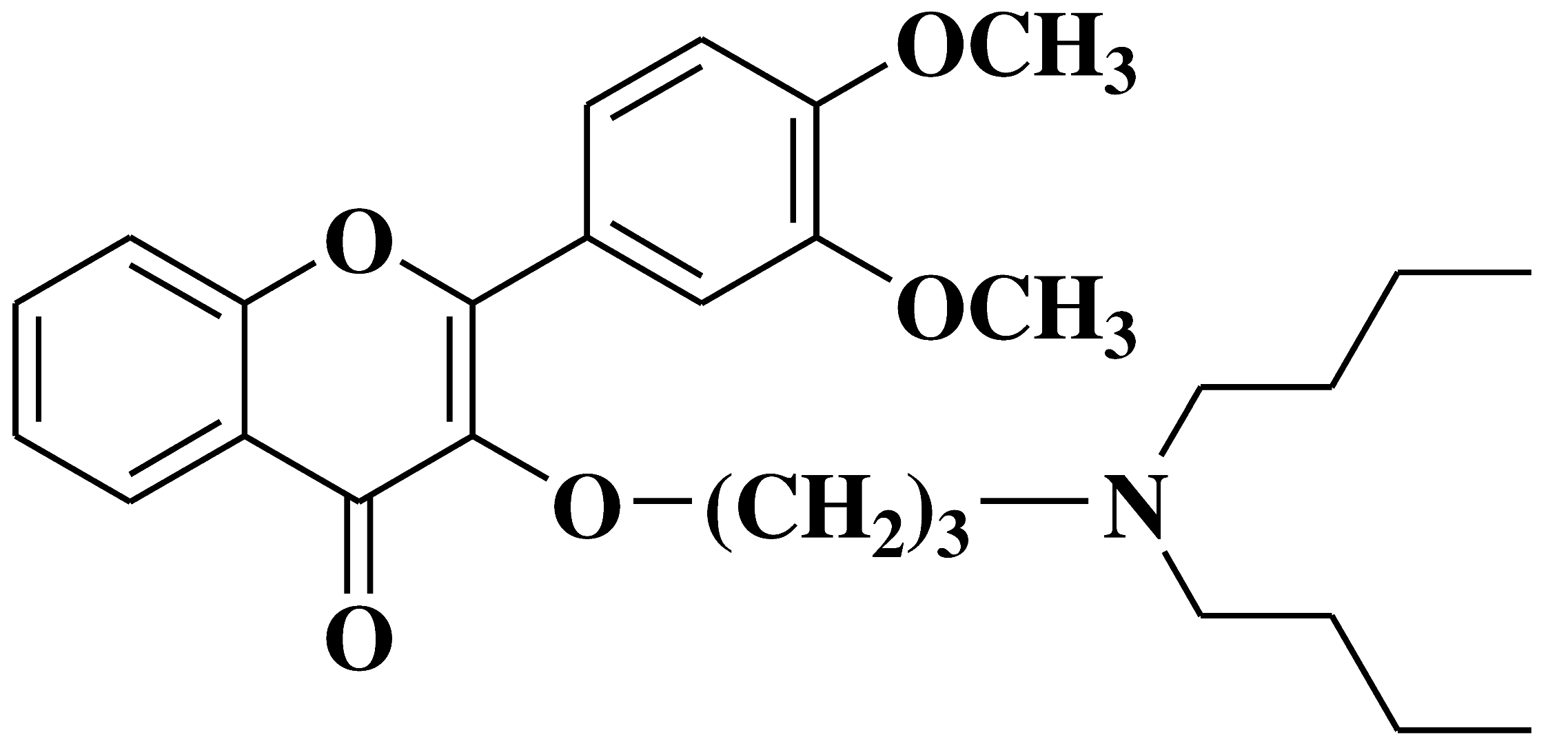 |
- androgen-sensitive (LNCaP) - androgen-insensitive (PC-3 and DU145) prostate cancer cell lines |
[19] |
| 7 | 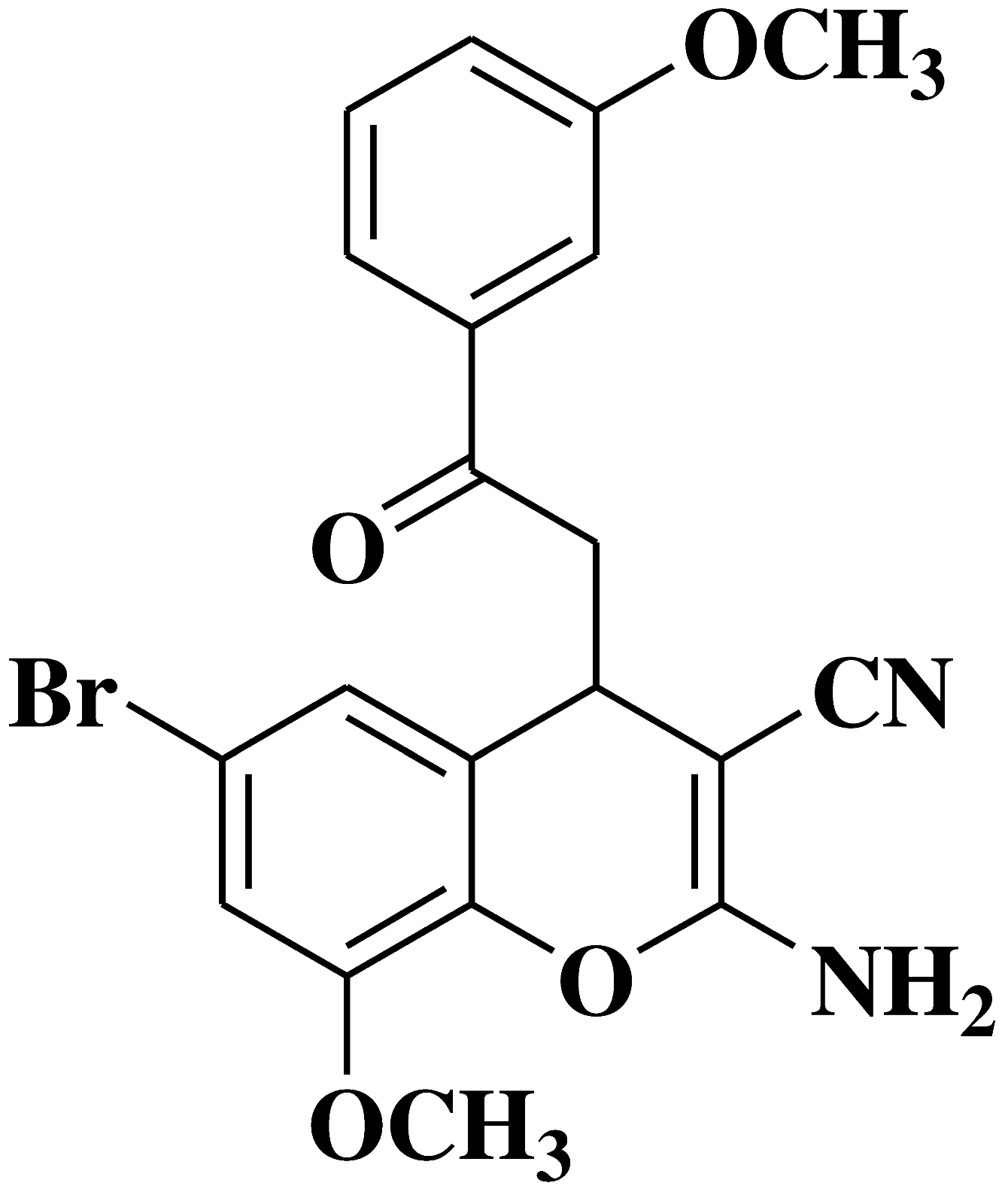 |
- breast cancer cell lines MCF-7, Hs578T, with tumor selectivity compared to non-cancer cell lines MCF-10A | [9] |
| 8 | 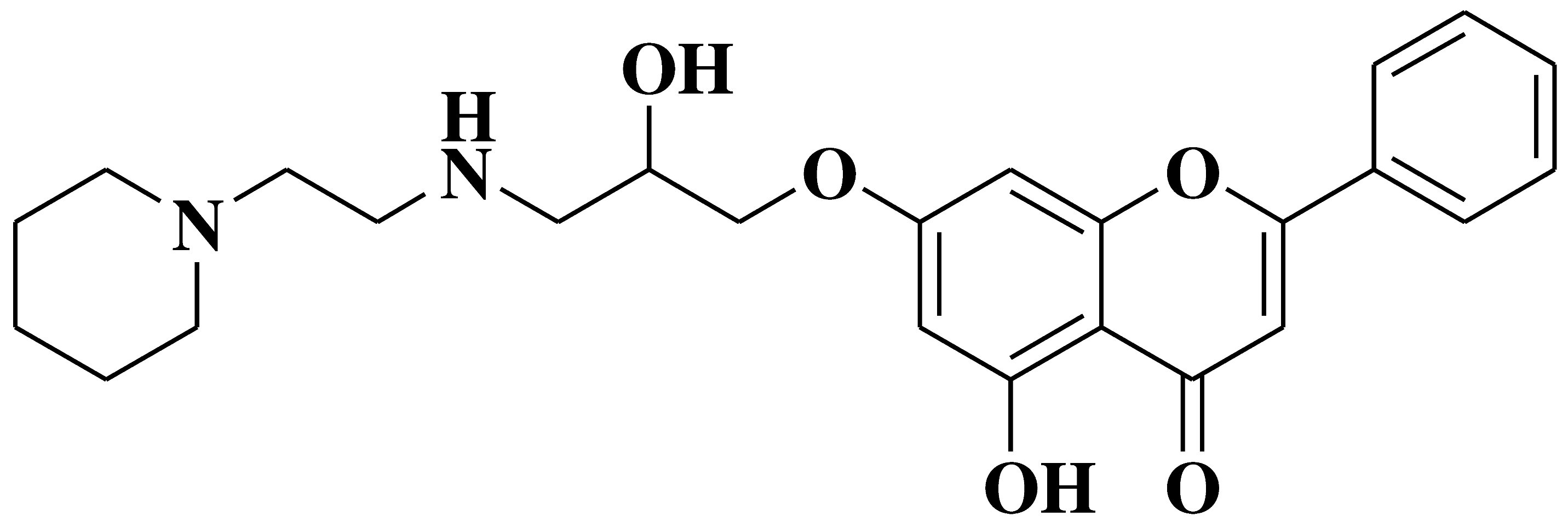 |
- AR negative castration-resistant prostate cancer cell line (CRPC) as topoisomerase II catalytic inhibitor and by intercalating and binding to the DNA minor groove - sensitizes AR-positive CRPC cells to enzalutamide and taxanes |
[29] |
| 9 | 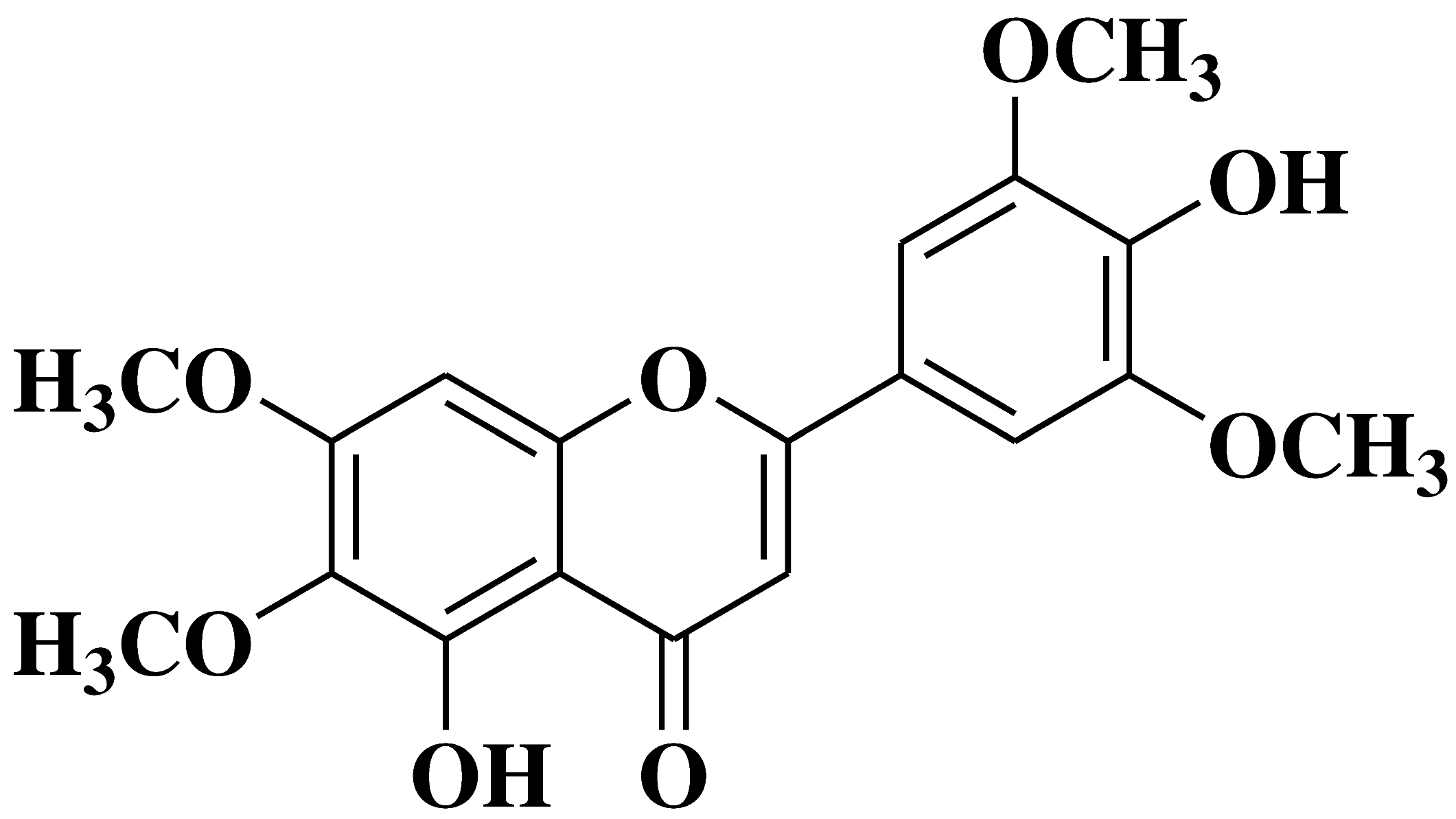 |
- human pancreas adenocarcinoma ascites metastasis Aspc-1 cancer cell lines | [30] |
| 10 | 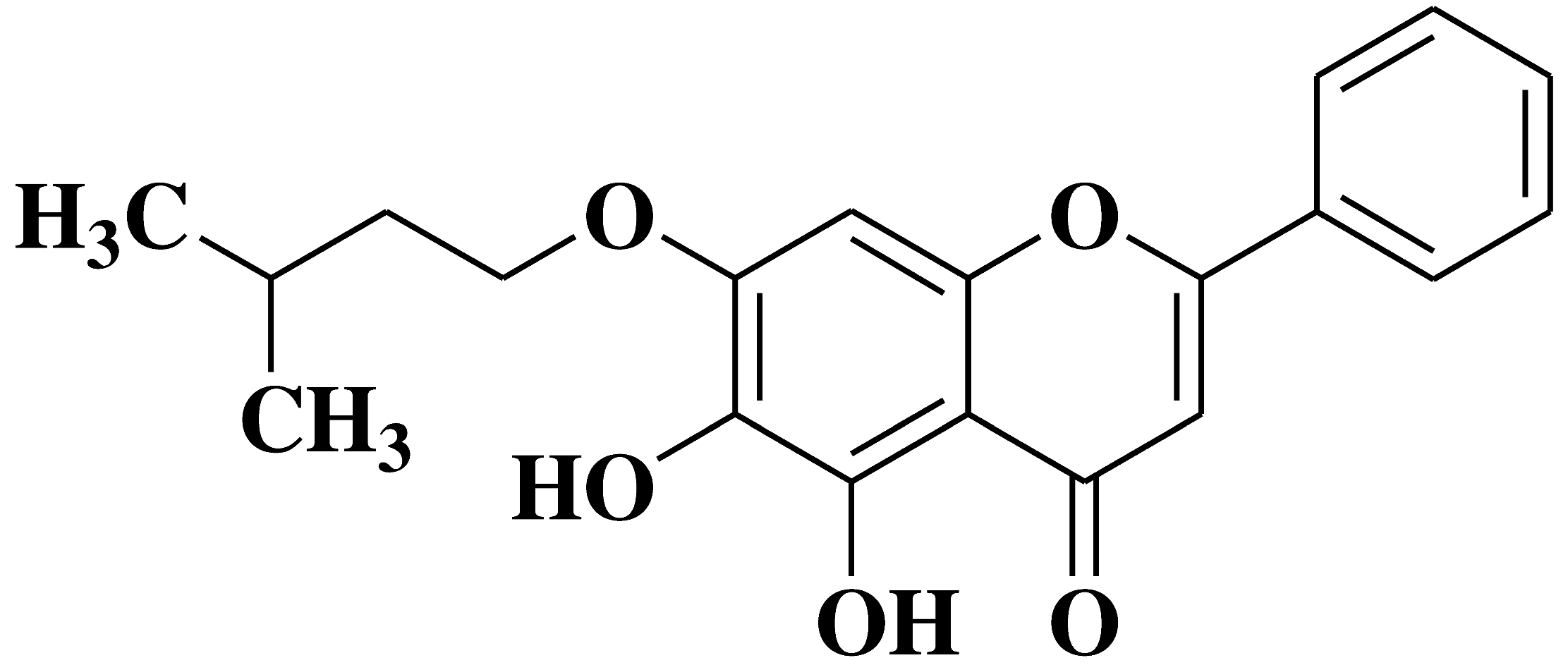 |
- MCF-7 breast cancer cells and yeasts expressing human caspase-7 | [11] |
| 11 | 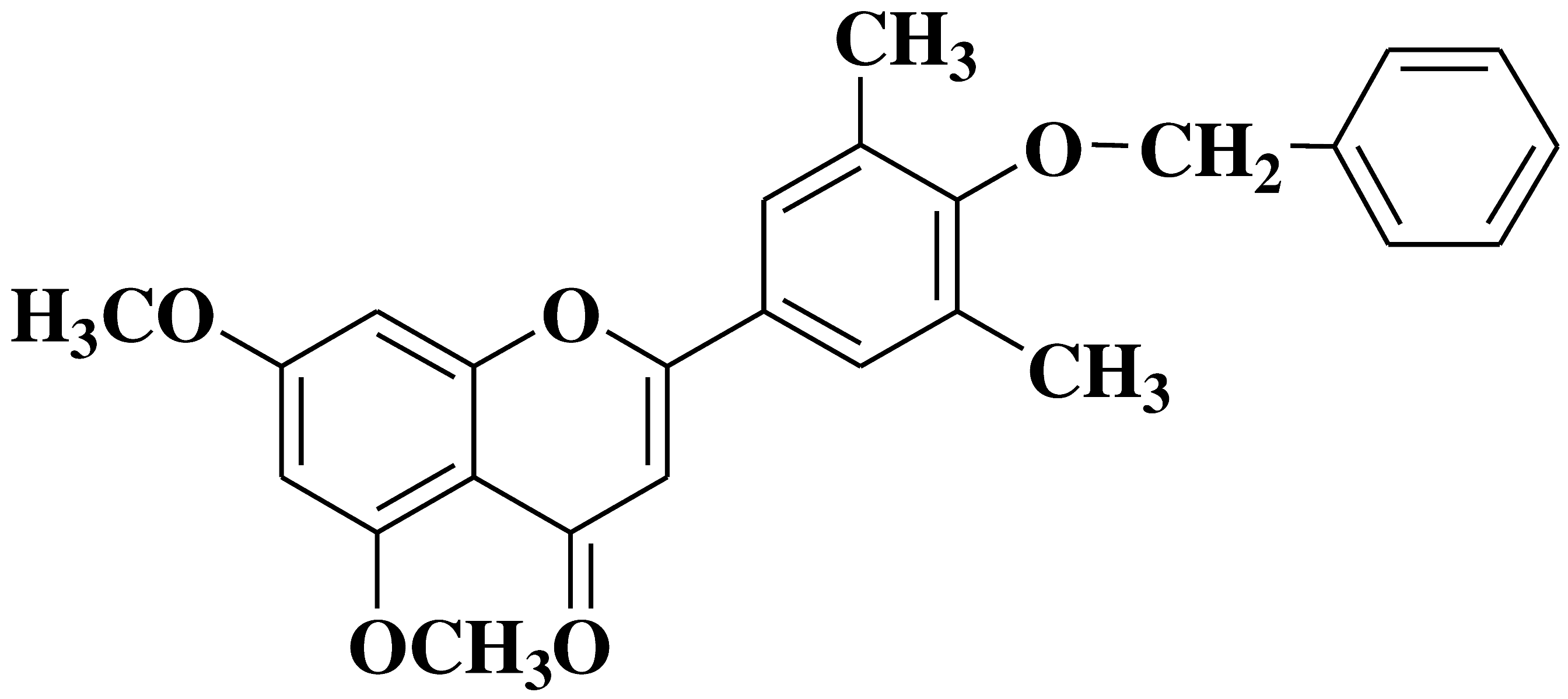 |
- human erythroleukemia cell line HEL prostate cancer cell line PC3 |
[31] |
| 12 | 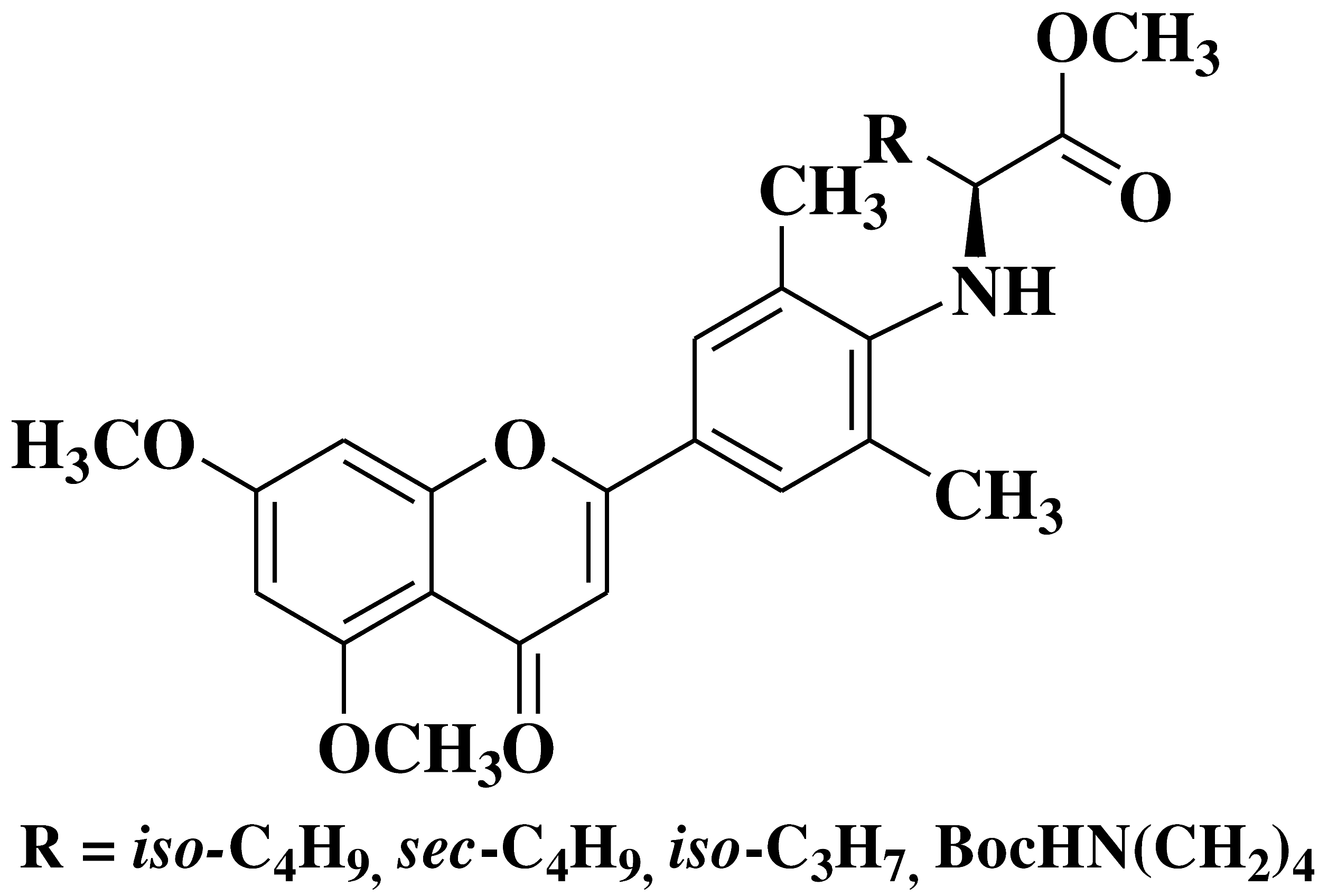 |
- human erythroleukemia cell line HEL prostate cancer cell line PC3 |
[31] |
| 13 | 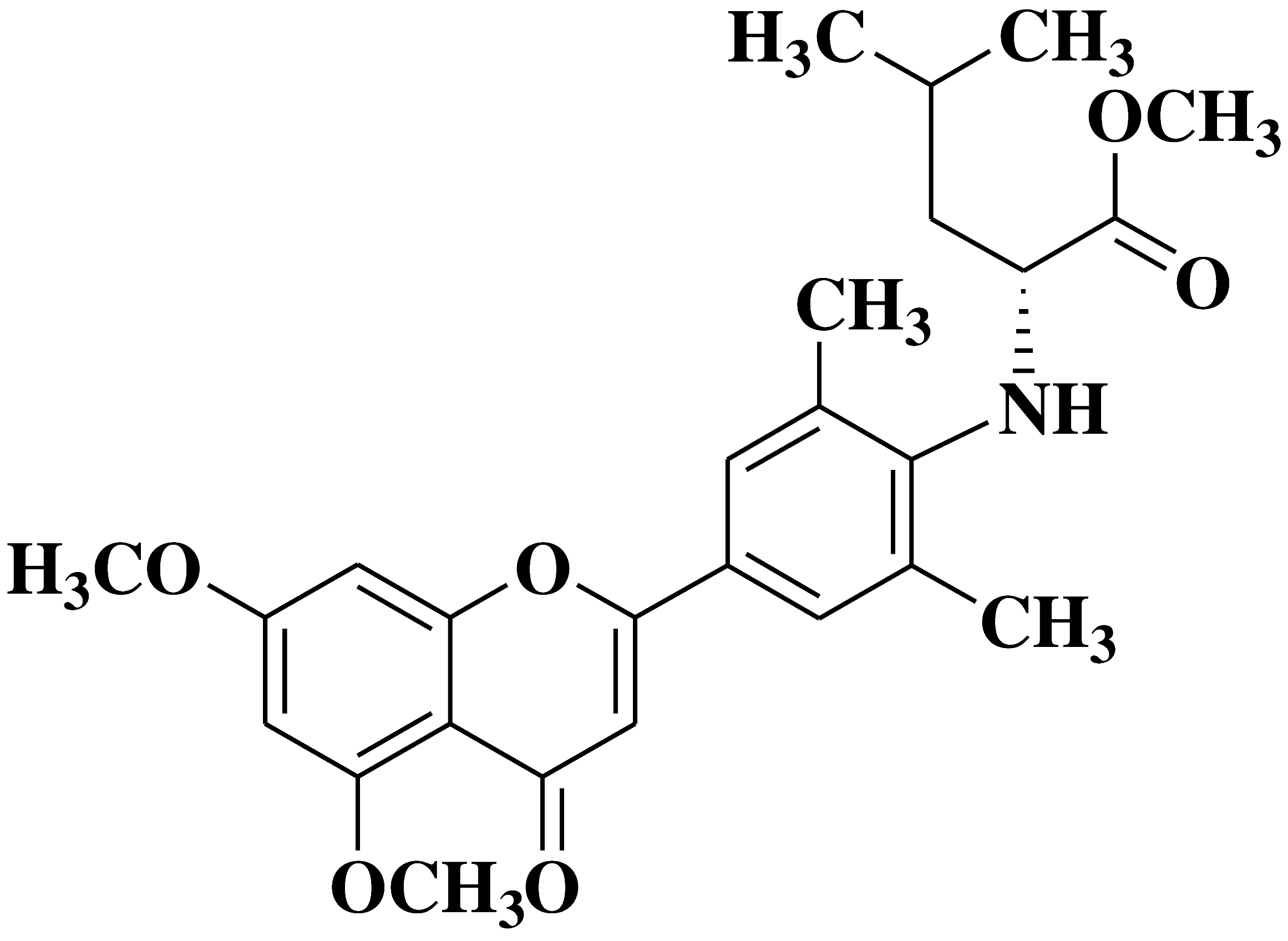 |
- human erythroleukemia cell line HEL prostate cancer cell line PC3 |
[31] |
| 14 | 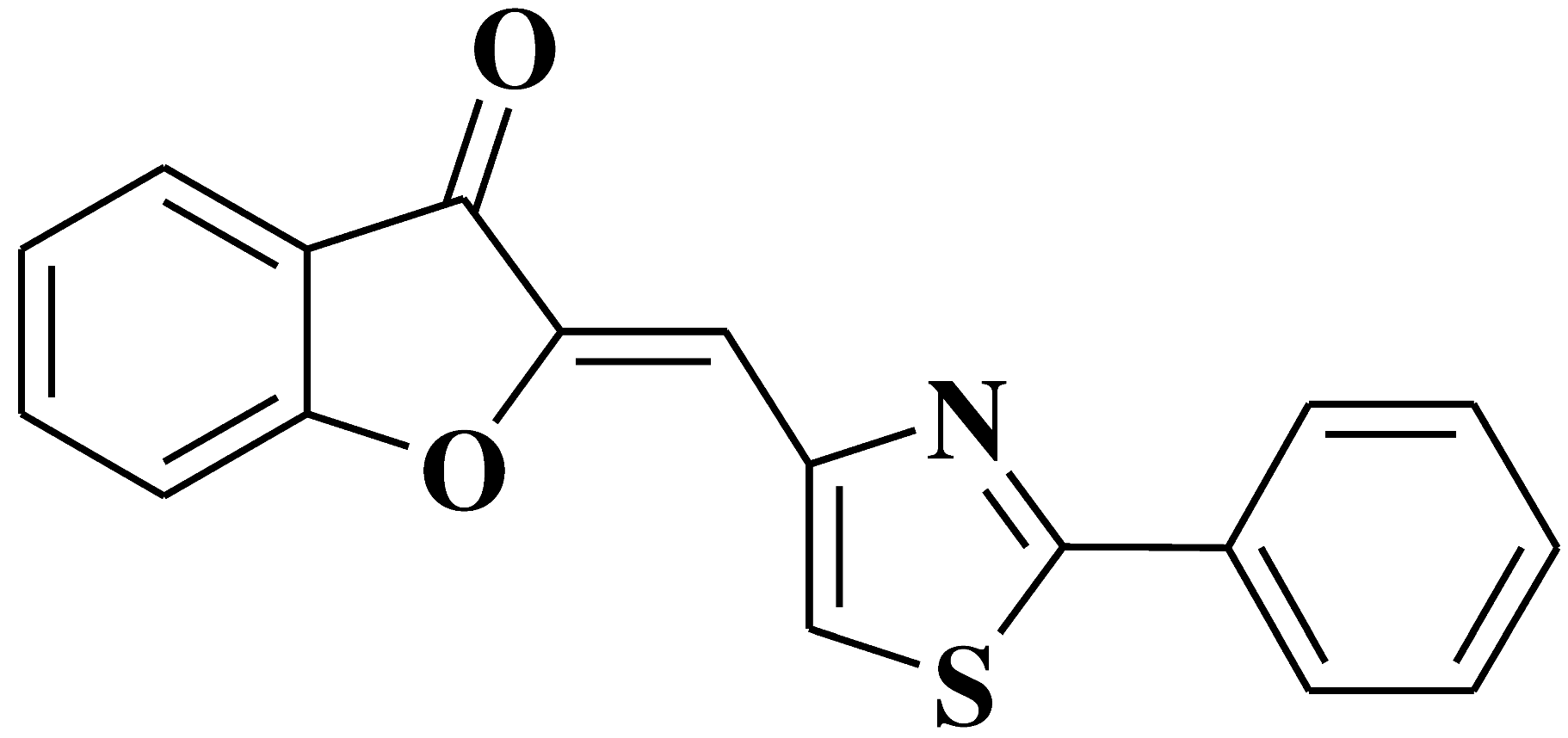 |
- leukemia cell line, doxorubicin-resistant phenotype CEM/ADR5000 | [27] |
| 15 | 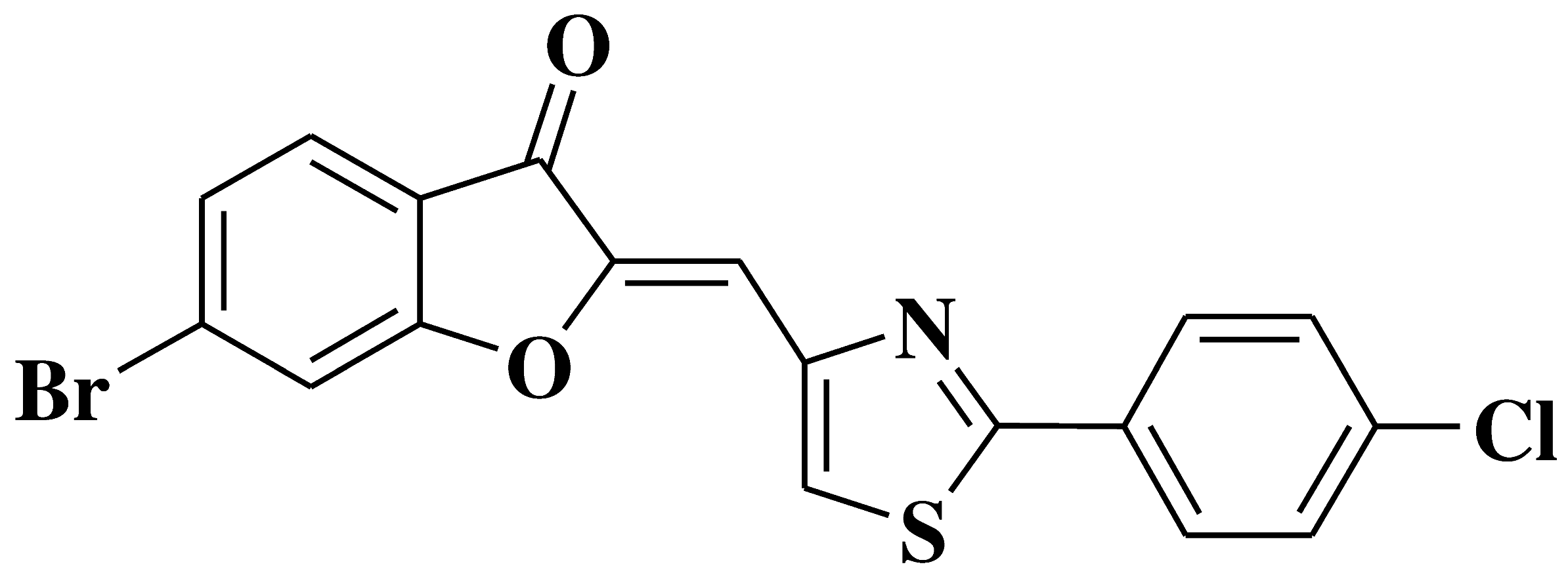 |
- breast adenocarcinoma cell line, resistant phenotype MDA-MB231/BCRP | [27] |
| 16 |
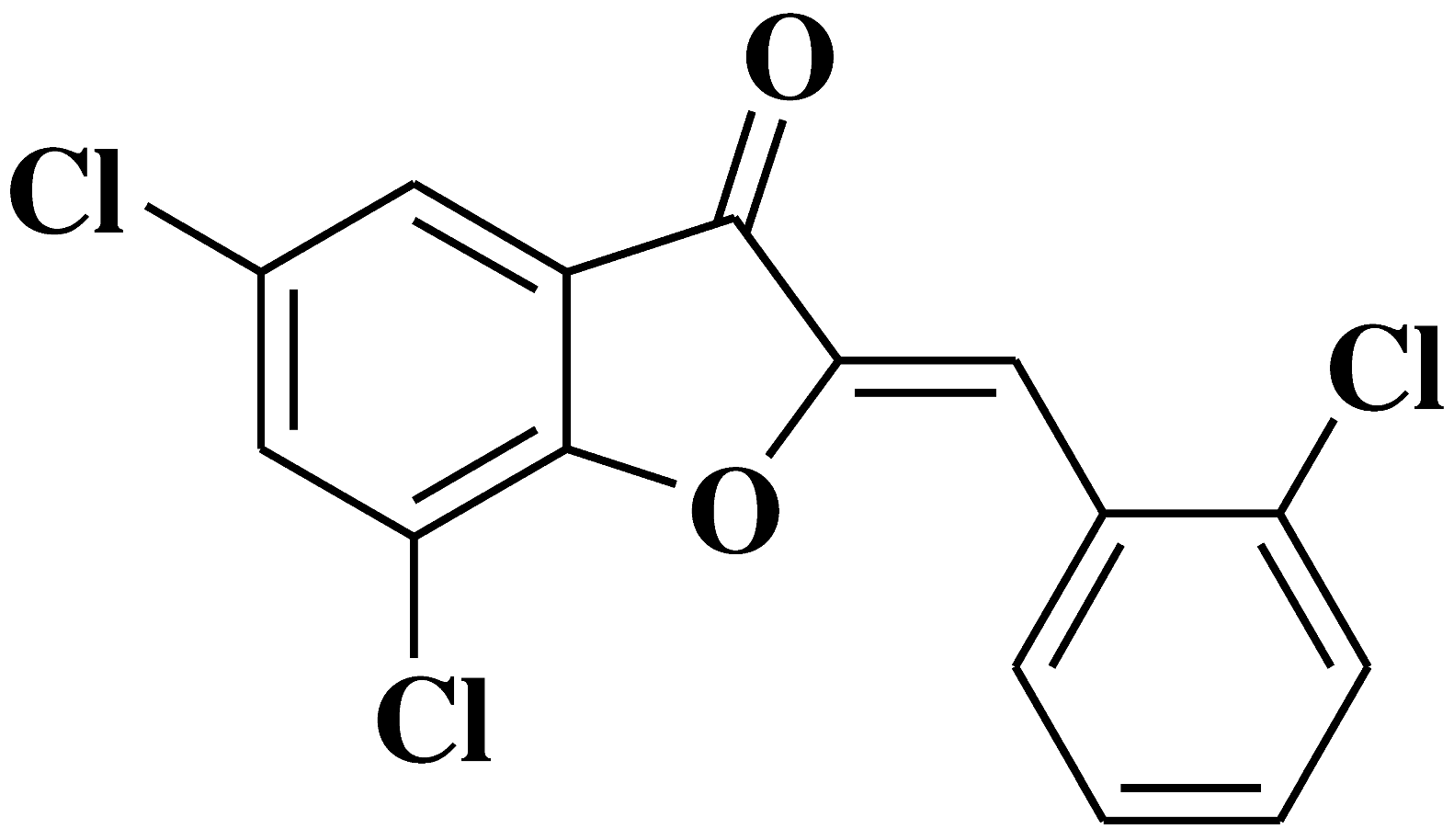 |
- human colorectal cancer cell line HCT 116 - human chronic myelogenous leukemia cell line K562 - hormone-dependent breast cancer cell line MCF-7 |
[32] |
| 17 | 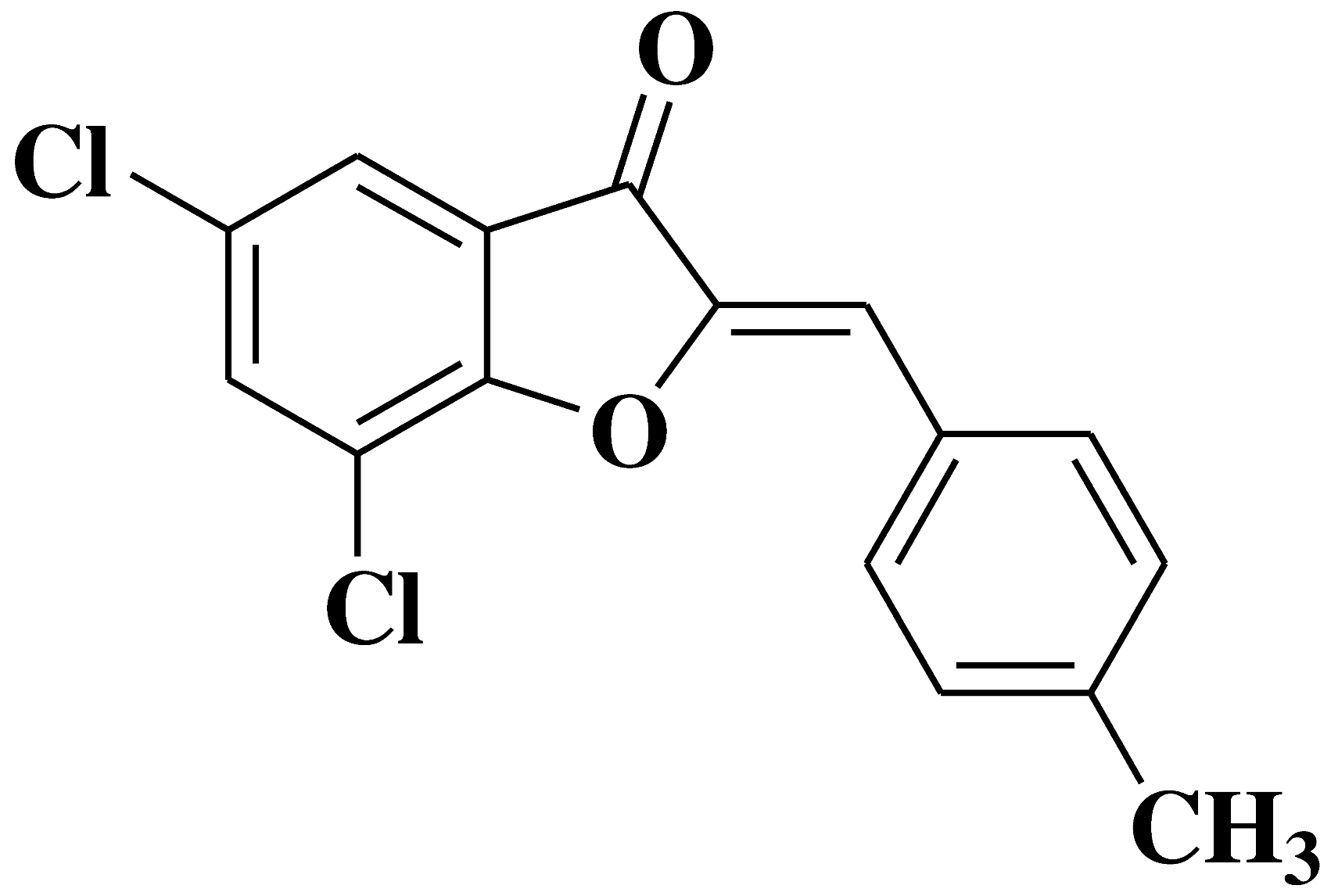 |
- human chronic myelogenous leukemia cell line K562 | [32] |
| 18 | 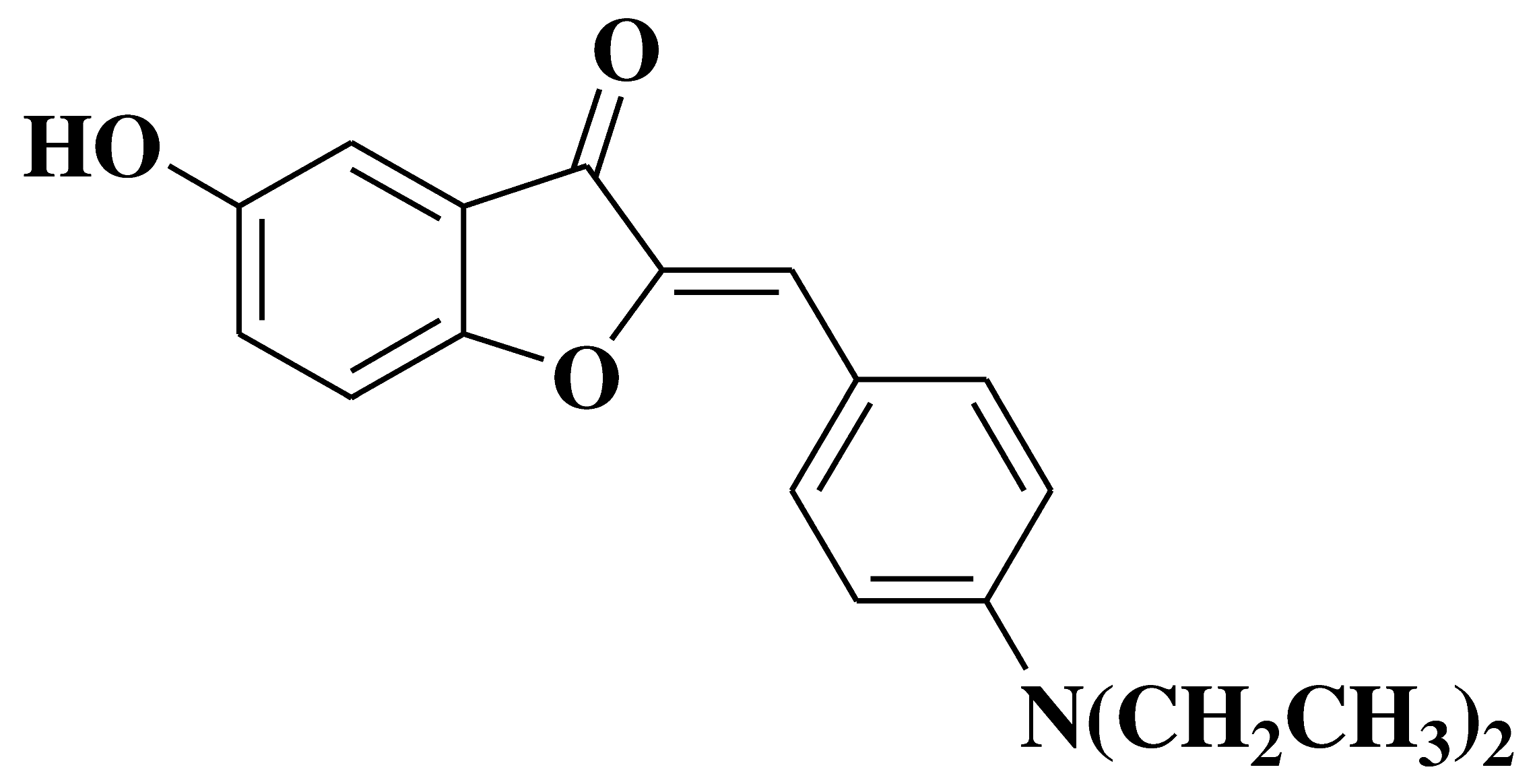 |
- inhibition of in vitro angiogenesis of HUVEC (human umbilical vein endothelial cells) proliferation, motility and tube formation - anti-proliferative and anti-invasive activities against A549 (non-small cell lung cancer cell line) and MCF-7 (breast cancer cell line) |
[33] |
| 19 | 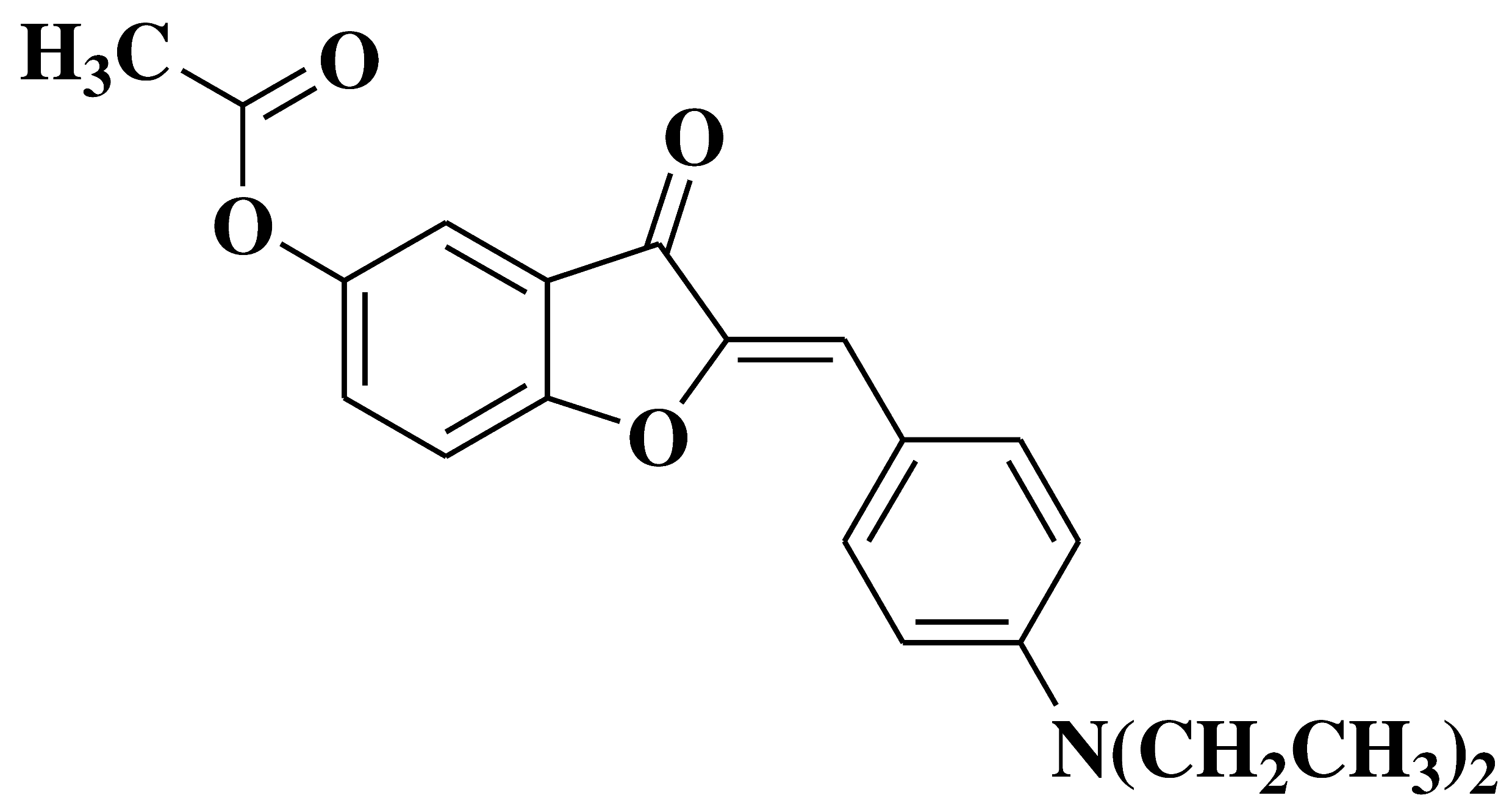 |
- inhibition of in vitro angiogenesis of HUVEC (human umbilical vein endothelial cells) proliferation, motility and tube formation - anti-proliferative and anti-invasive activities against A549 (non-small cell lung cancer cell line) and MCF-7 (breast cancer cell line) |
[33] |
| 20 | 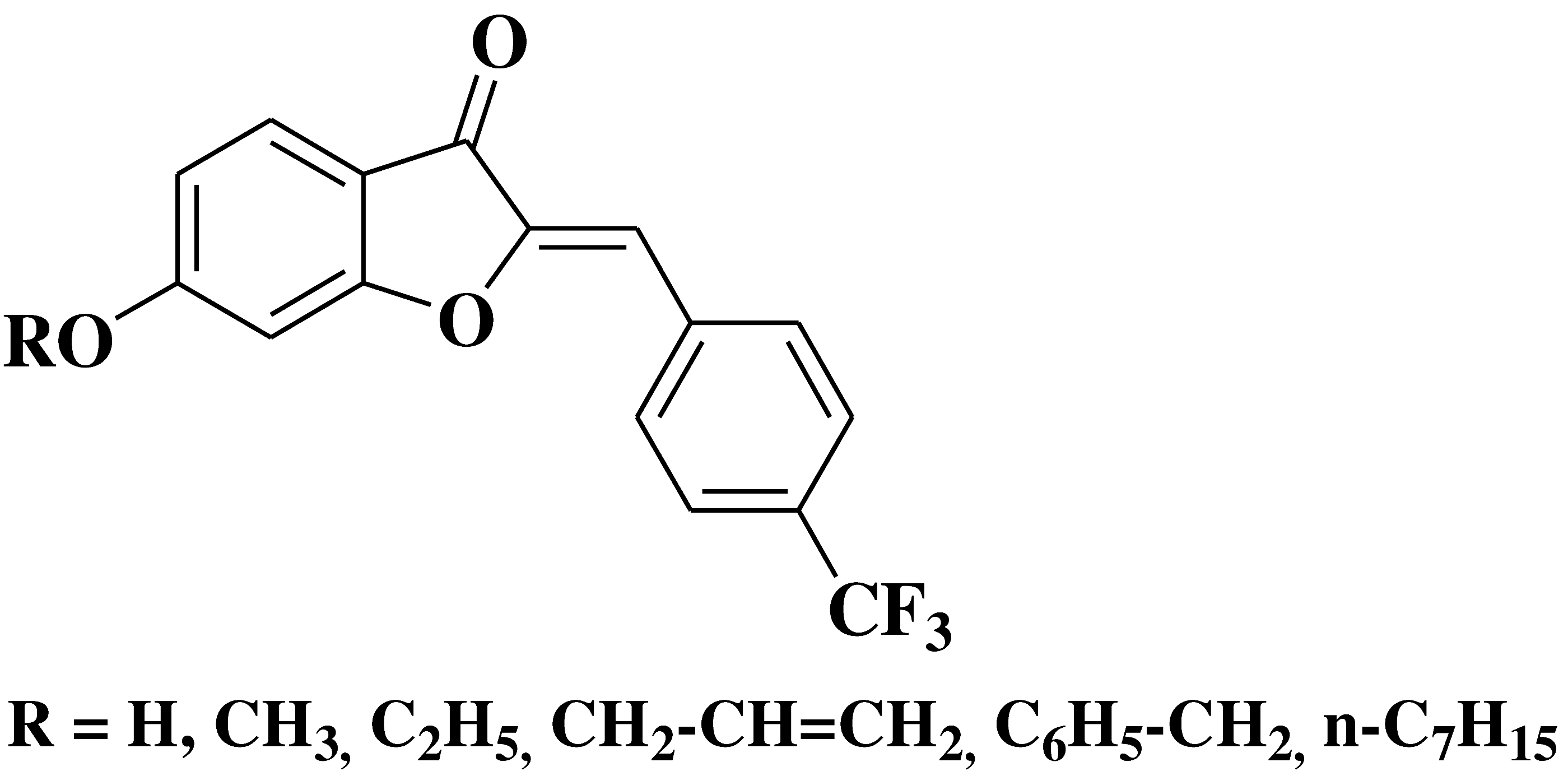 |
- leucocythemia cell line HL-60 - colorectal adenocarcinoma cell line HT-29 |
[34] |
| 21 | 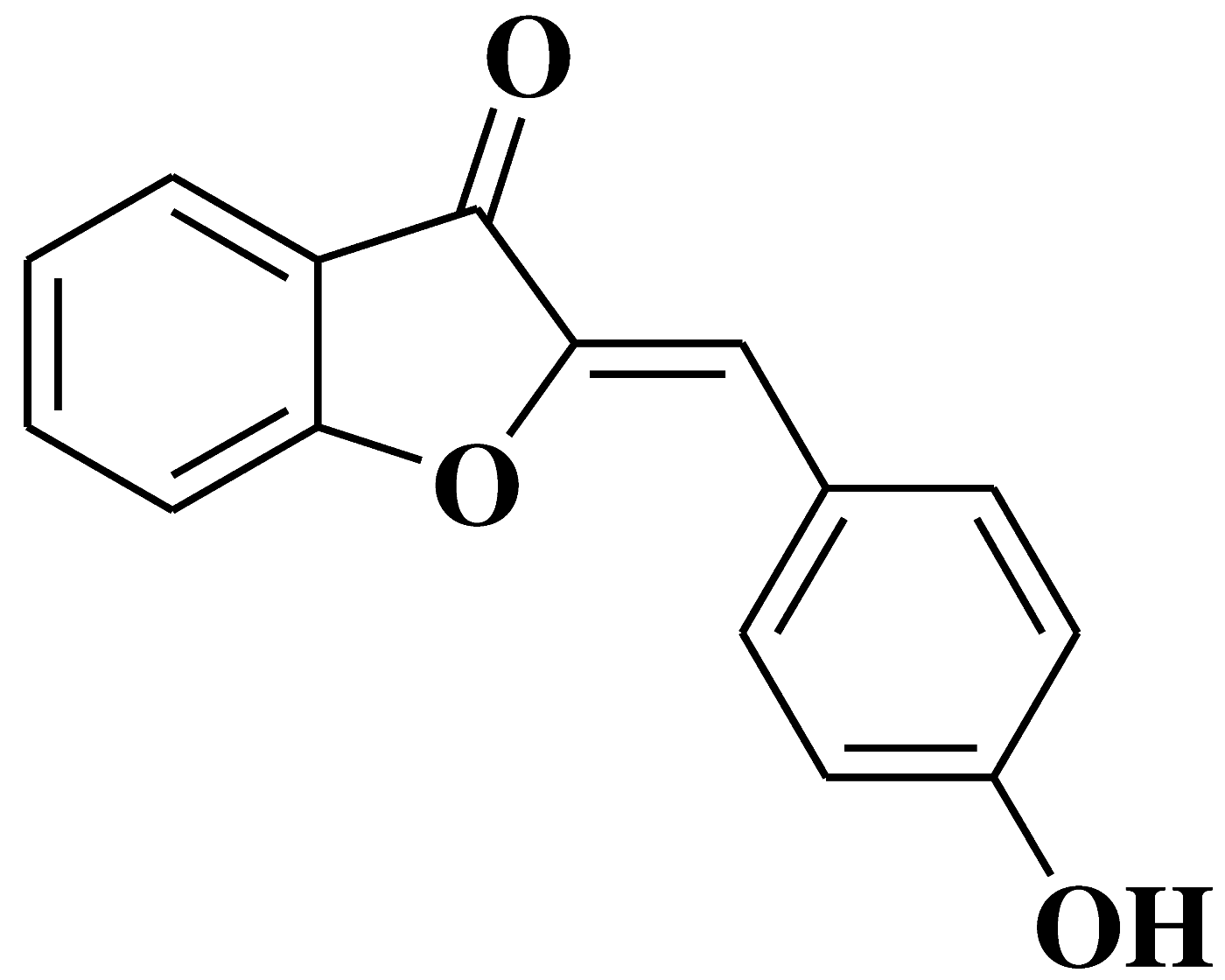 |
- human oral squamous carcinoma cell lines Ca9-22 (derived from gingival tissue), HSC-2 and HSC-4 (derived from tongue), with tumor-specificity in comparison to oral normal cells | [35] |
| 22 | 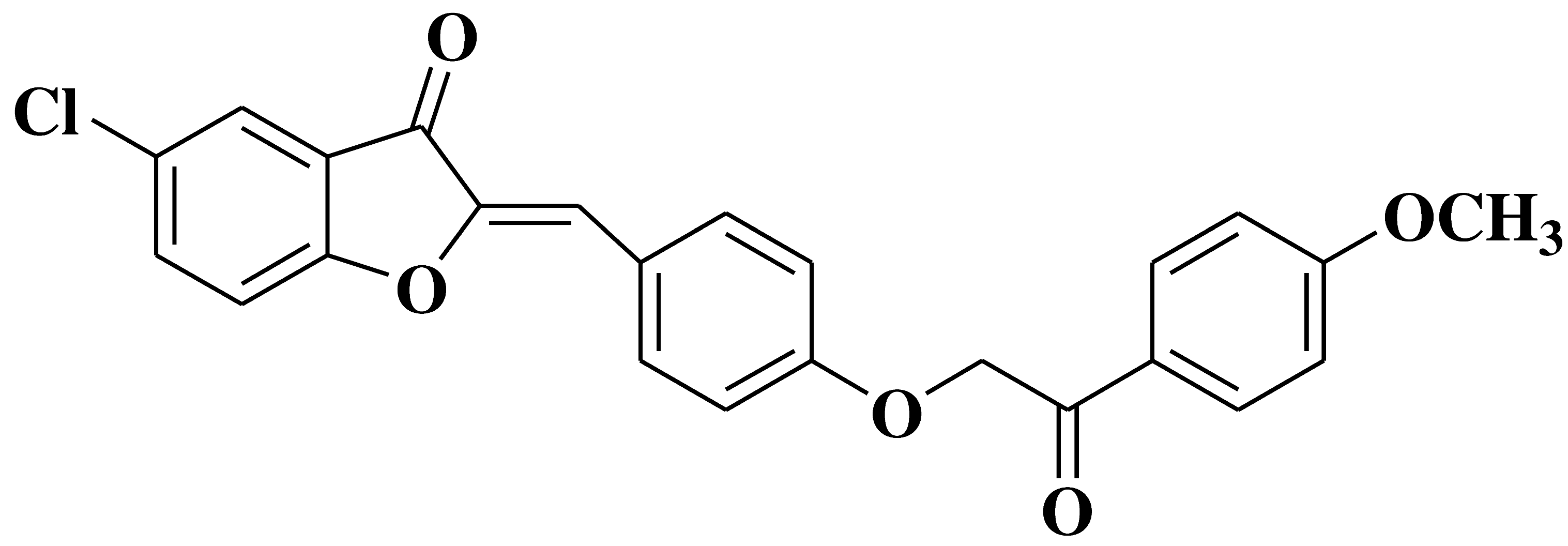 |
- leukemia cell lines MOLT-4 and SR | [36] |
| 23 | 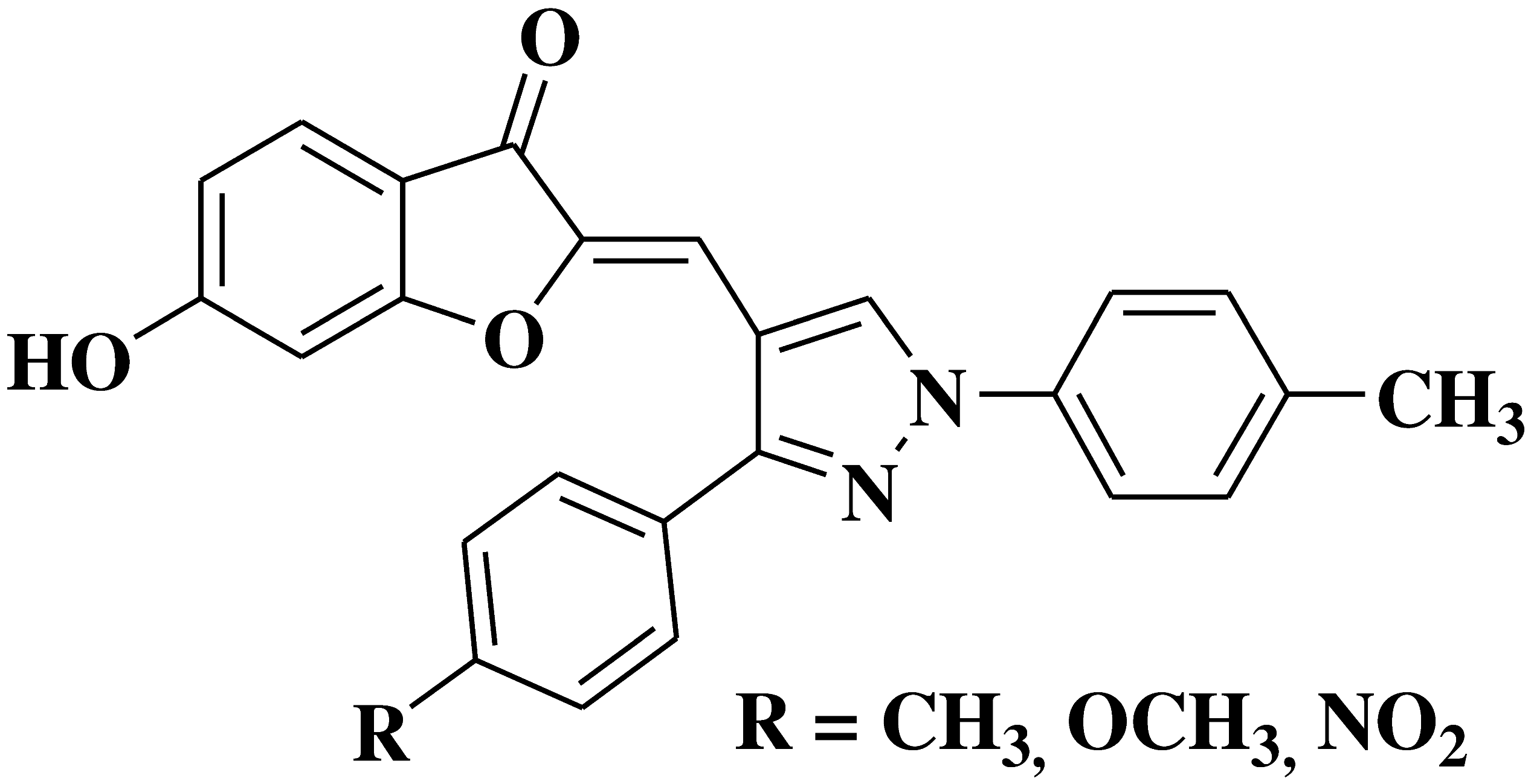 |
- gastric cancer cell line CRL-1739 | [37] |
| 24 | 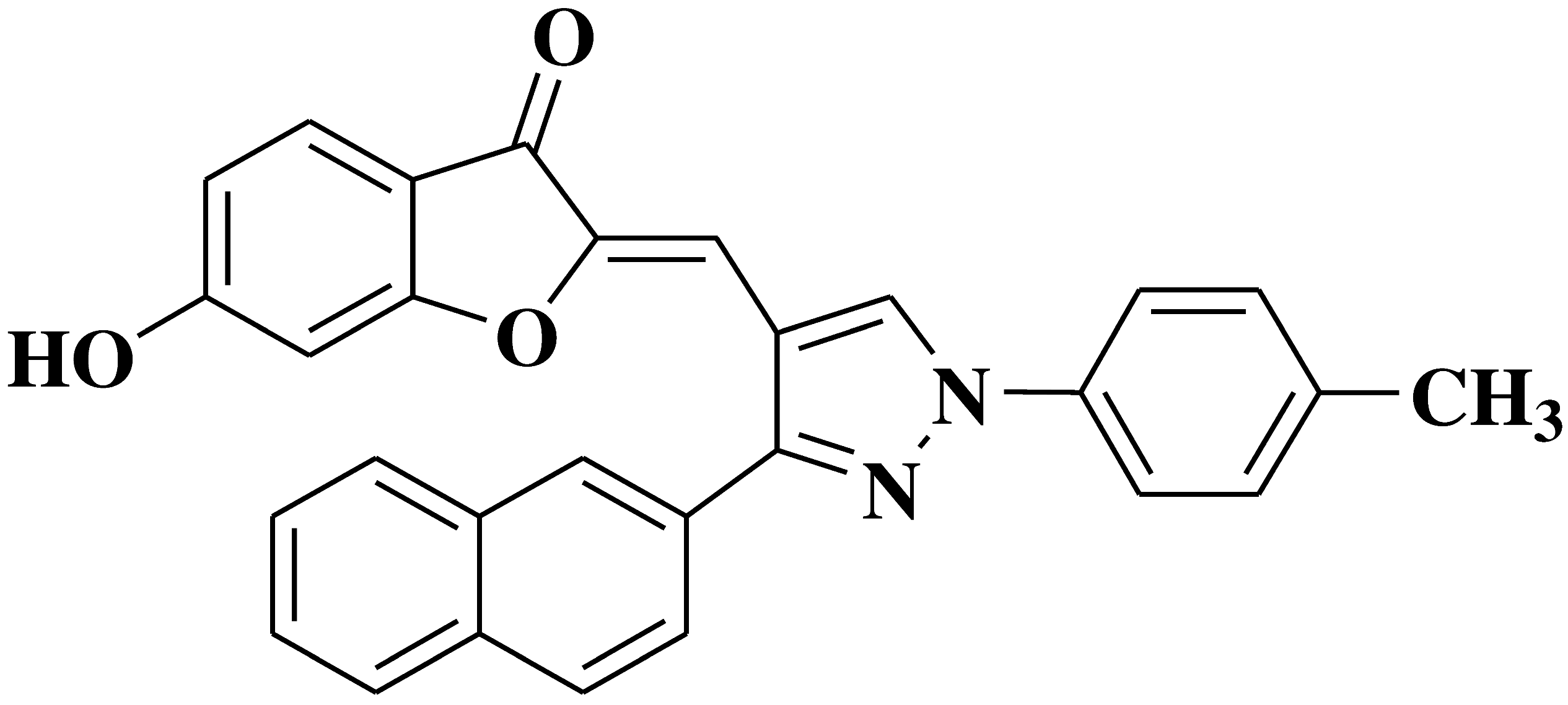 |
- gastric cancer cell line CRL-1739 | [37] |
2.2. Antibacterial and antifungal activity
| Entry | Chemical structure | Cancer cell lines against the tested compounds present cytotoxic activity | Ref. |
|---|---|---|---|
| 1 | 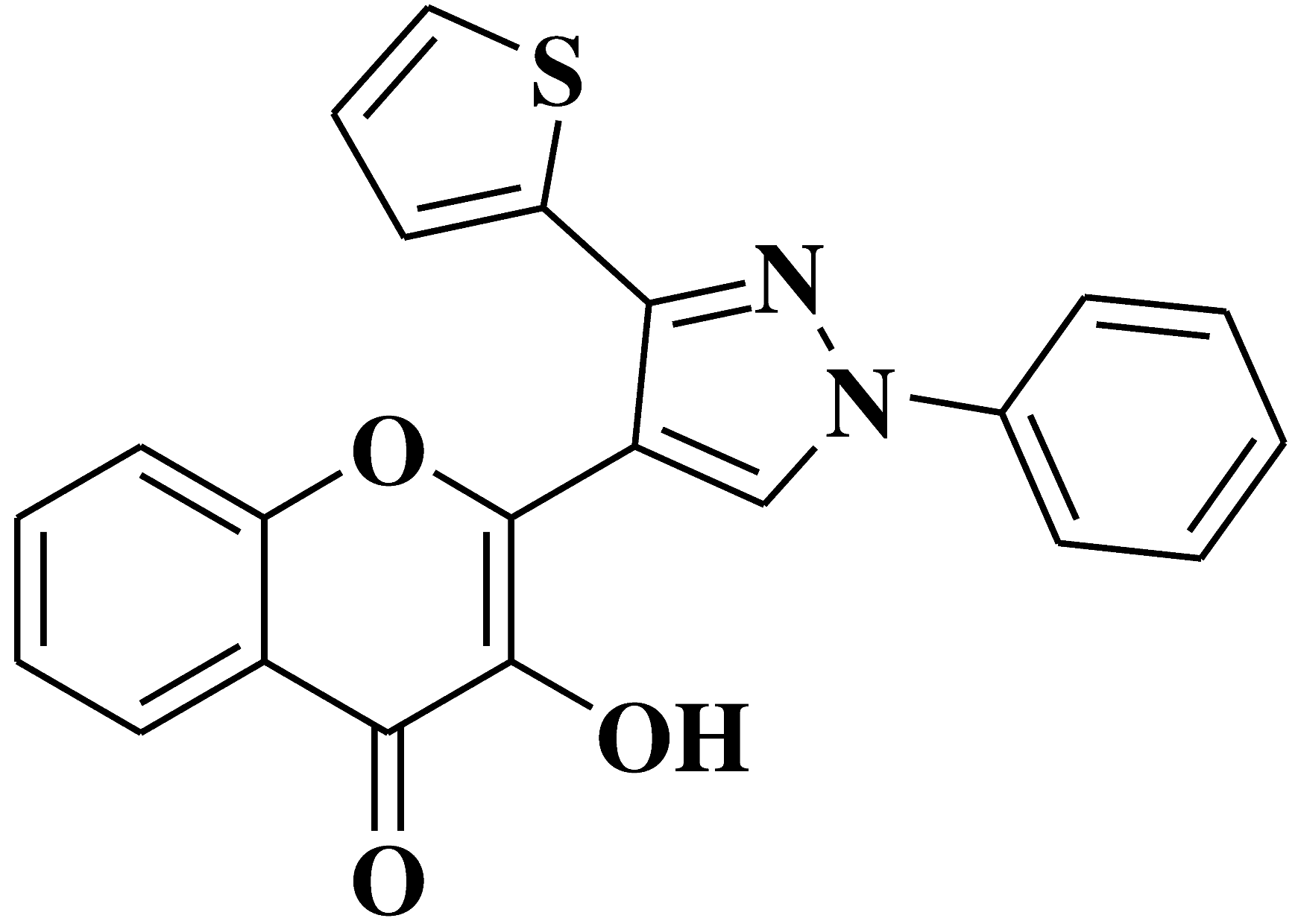 |
Antifungal activity: Aspergillus niger Penicillium italicum Fusarium oxysporum |
[38] |
| 2 | 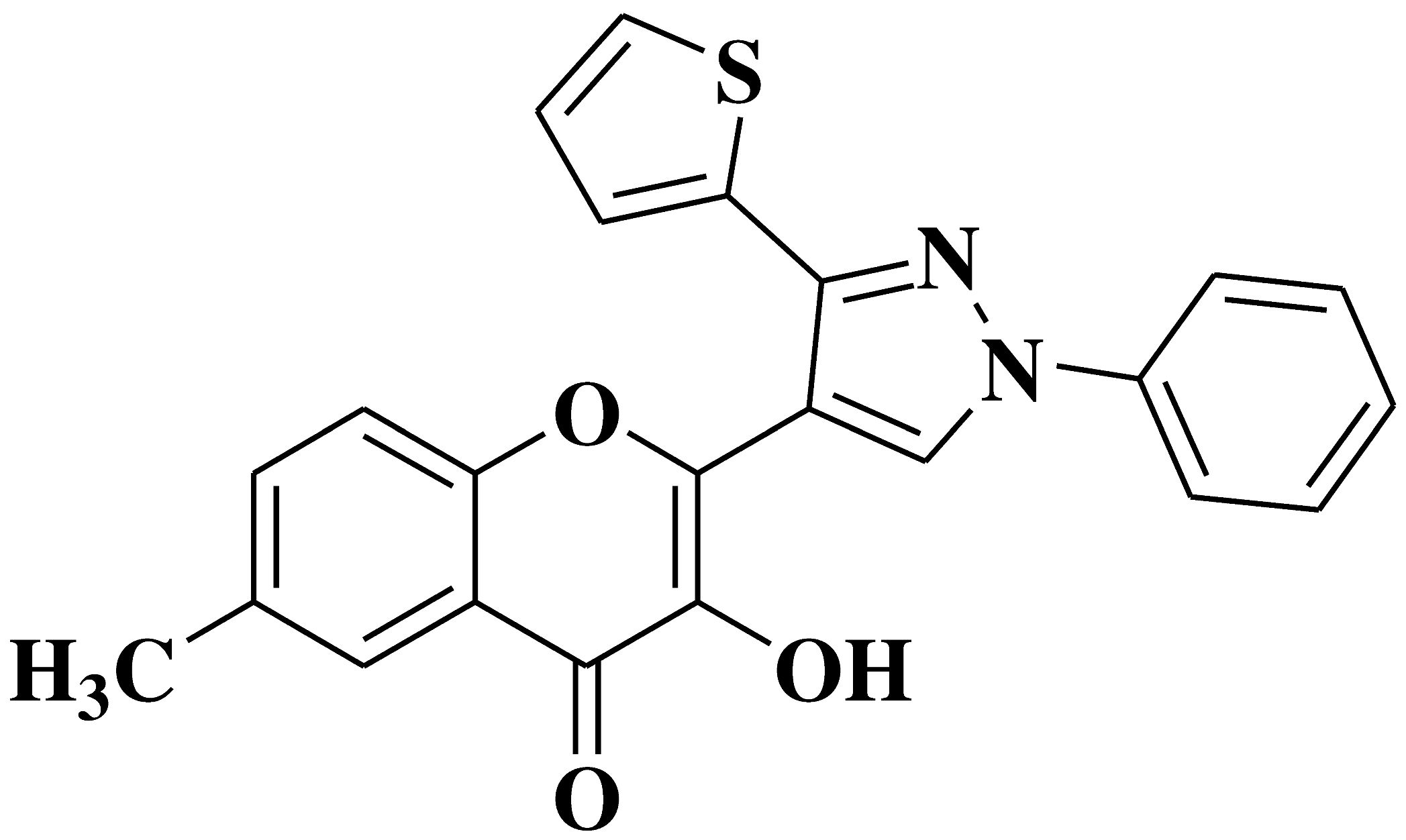 |
Antibacterial activity: Staphylococcus aureus Pseudomonas aeruginosa Escherichia coli |
[38] |
| 3 | 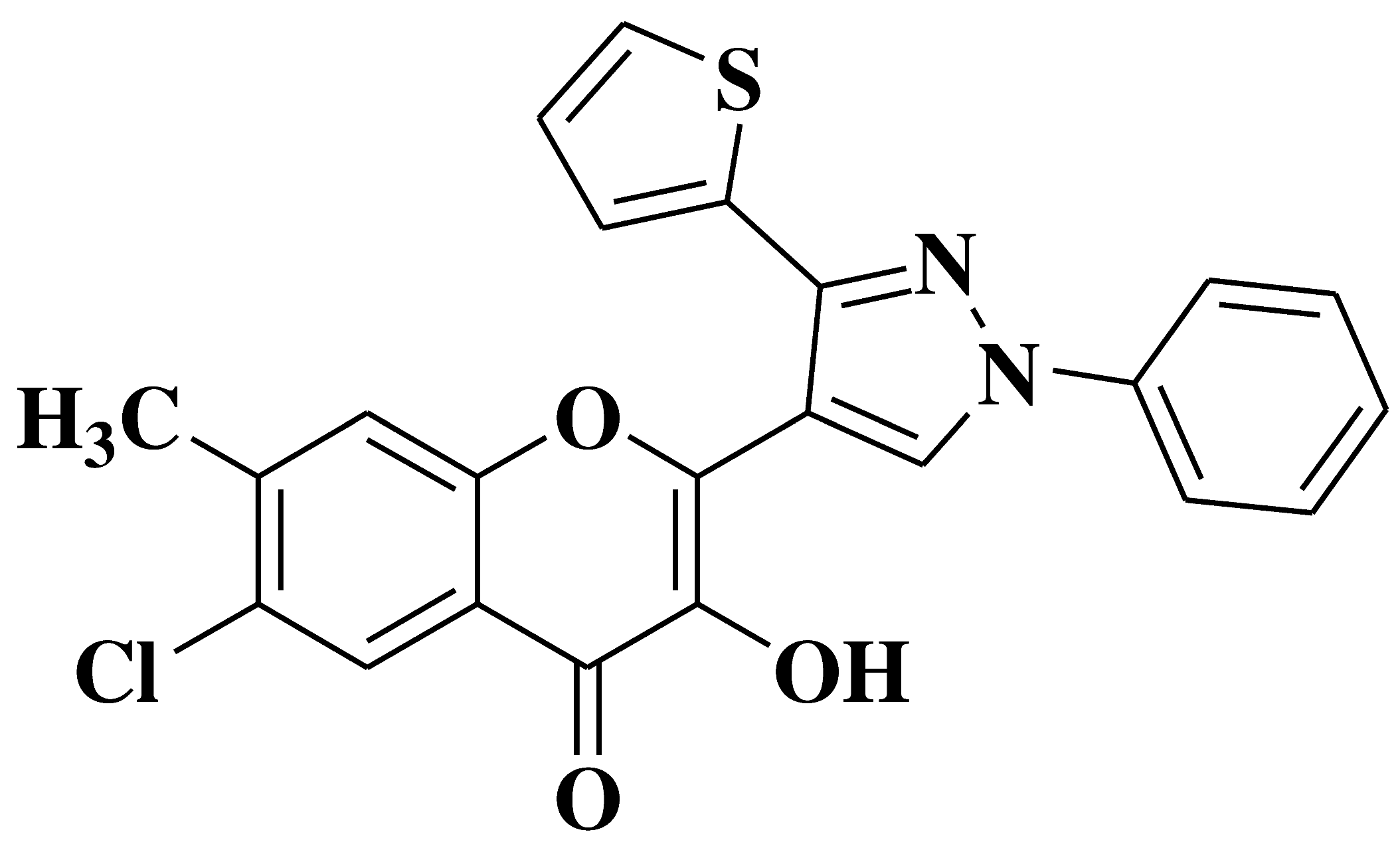 |
Antibacterial activity: Staphylococcus aureus Bacillus subtilis Escherichia coli Antifungal activity: Aspergillus niger Penicillium italicum Fusarium oxysporum |
[38] |
| 4 | 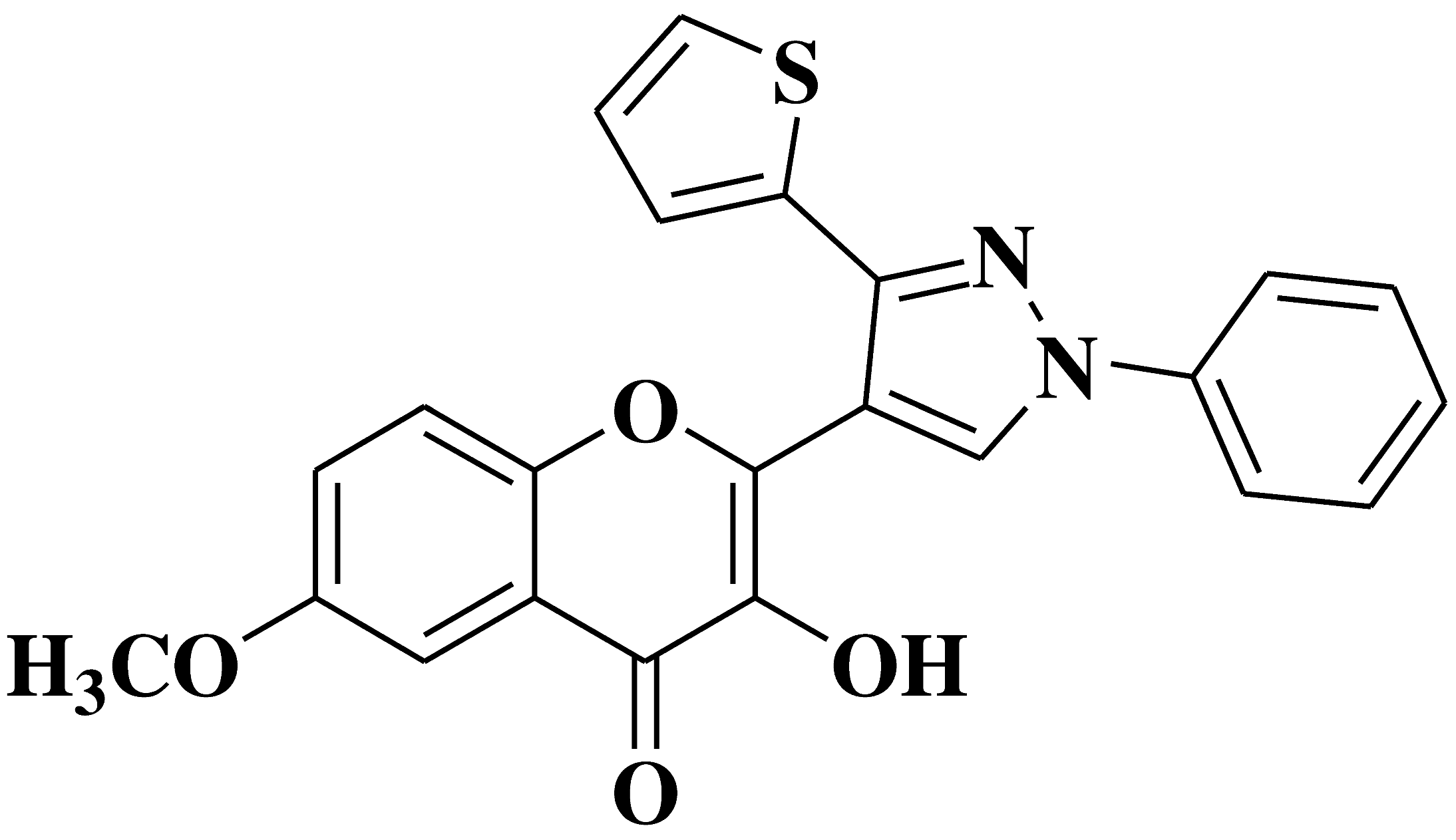 |
Antibacterial activity: Staphylococcus aureus Bacillus subtilis Escherichia coli Antifungal activity: Aspergillus niger Penicillium italicum Fusarium oxysporum |
[38] |
| 5 | 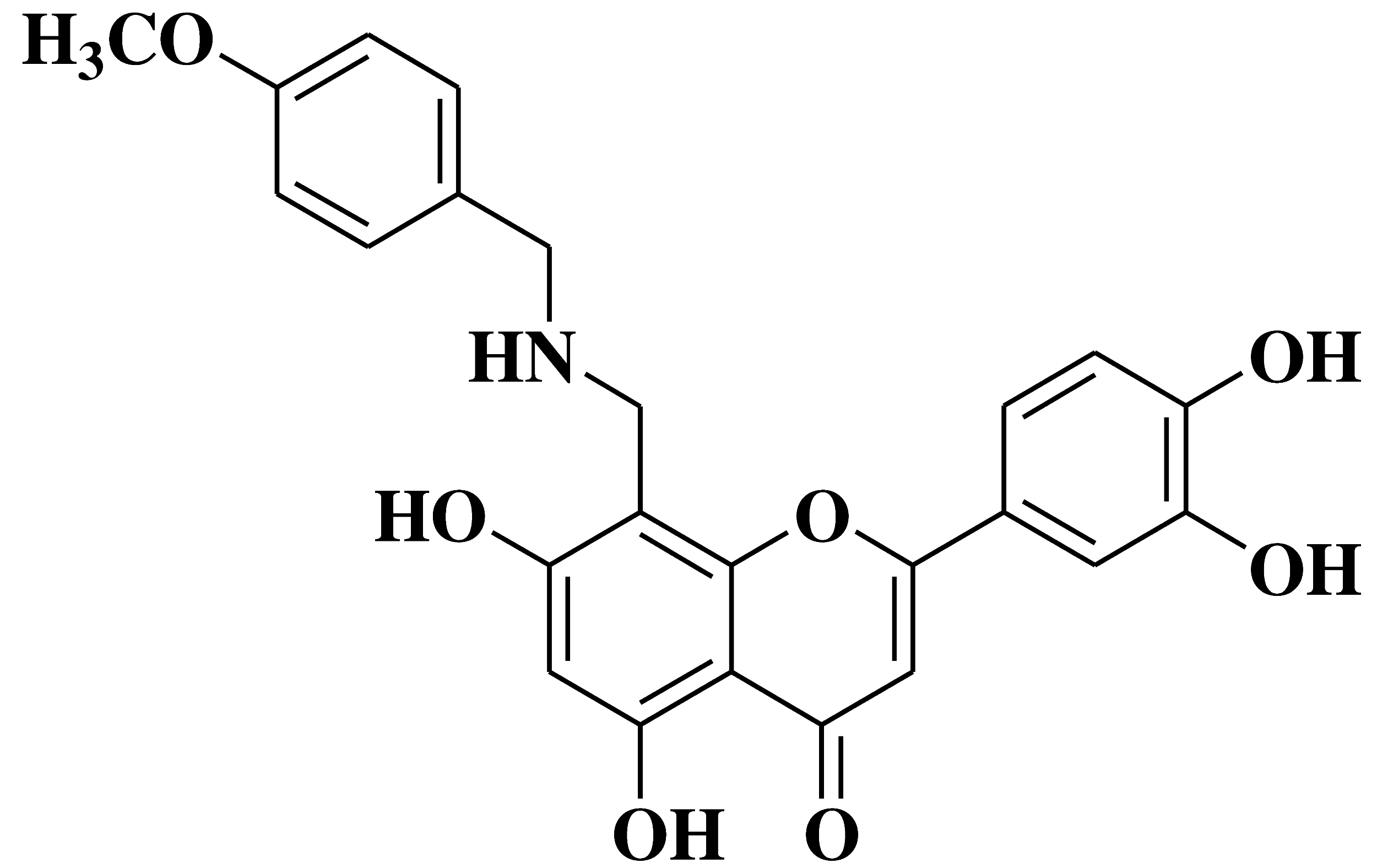 |
Antibacterial activity: Staphylococcus aureus Escherichia coli Salmonella gallinarum |
[39] |
| 6 | 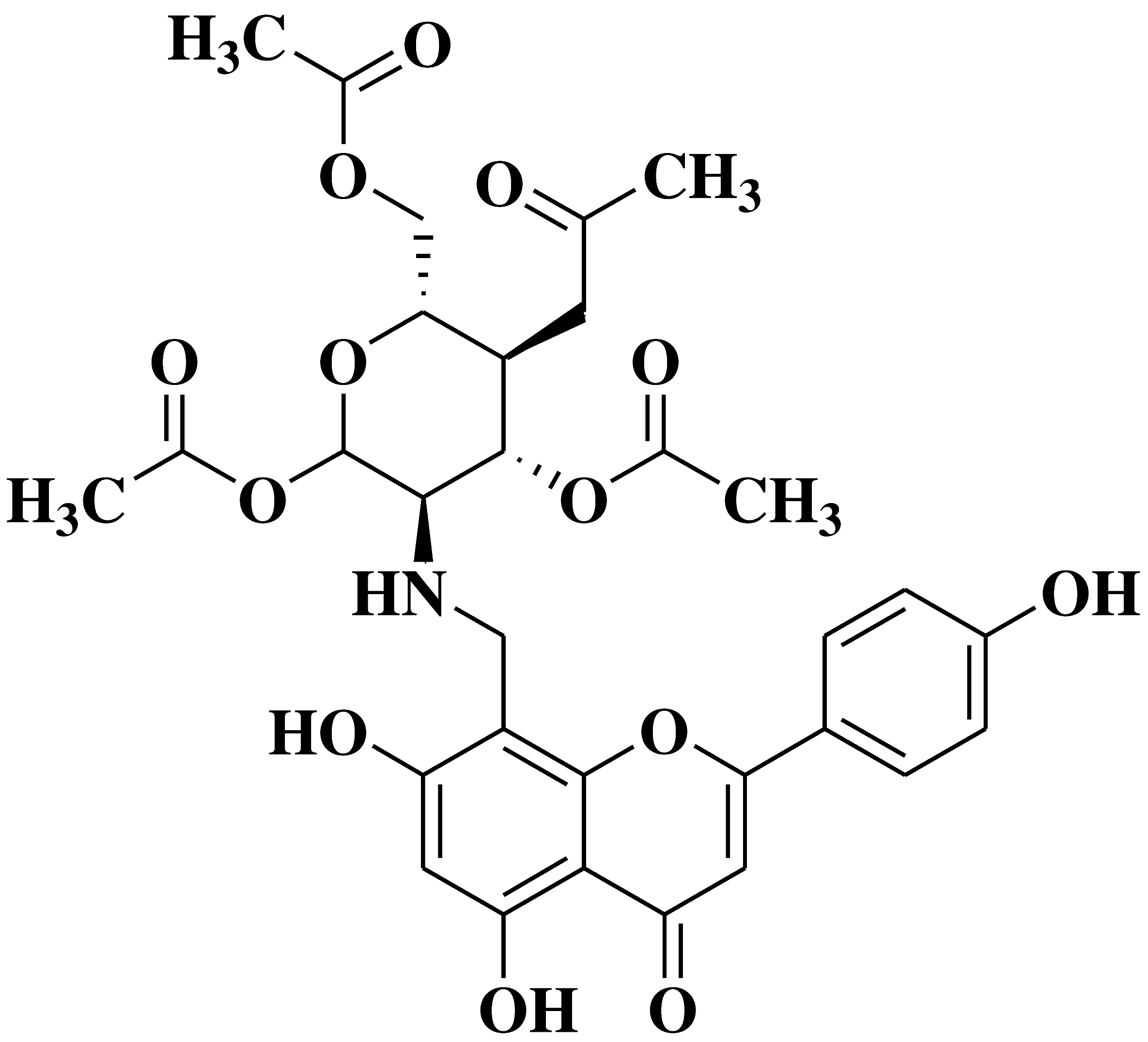 |
Antibacterial activity: Staphylococcus aureus Escherichia coli Salmonella gallinarum Listeria monocytogenes |
[39] |
| 7 | 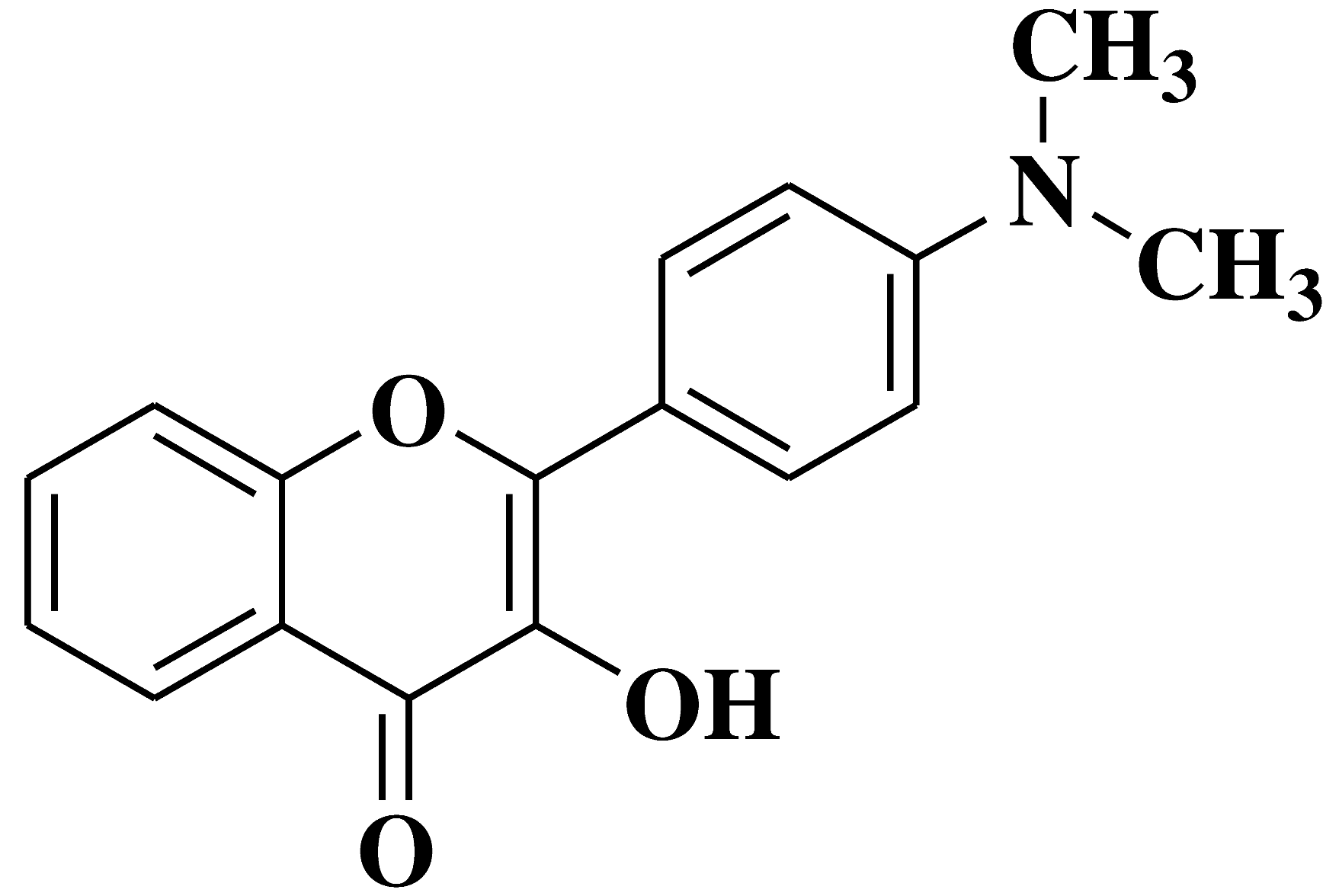 |
Antifungal activity: Acremonium strictum Penicillium expansum Aspergillus flavus |
[40] |
| 8 | 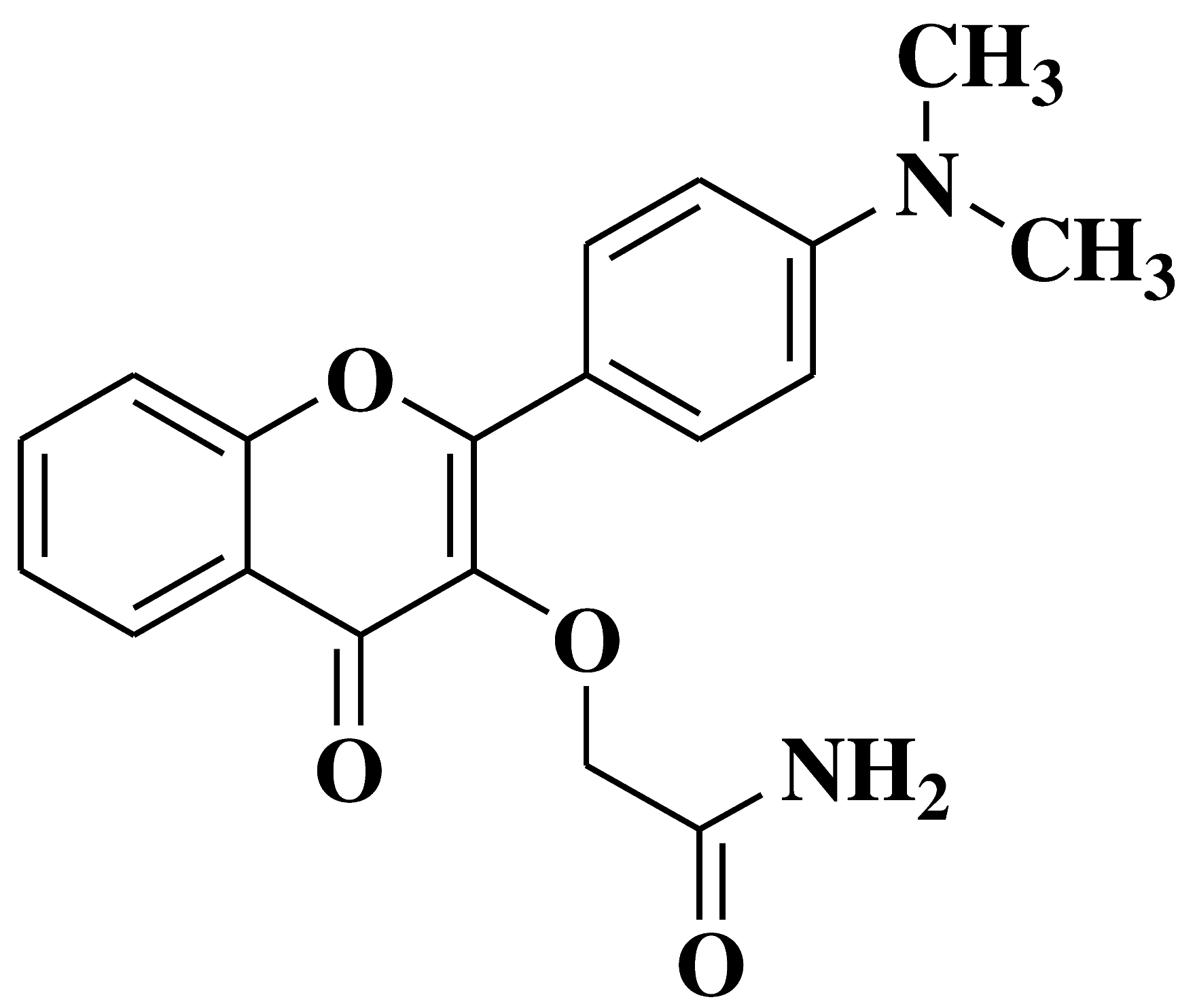 |
Antifungal activity: Acremonium strictum Penicillium expansum Aspergillus flavus (moderate inhibition) |
[40] |
| 9 | 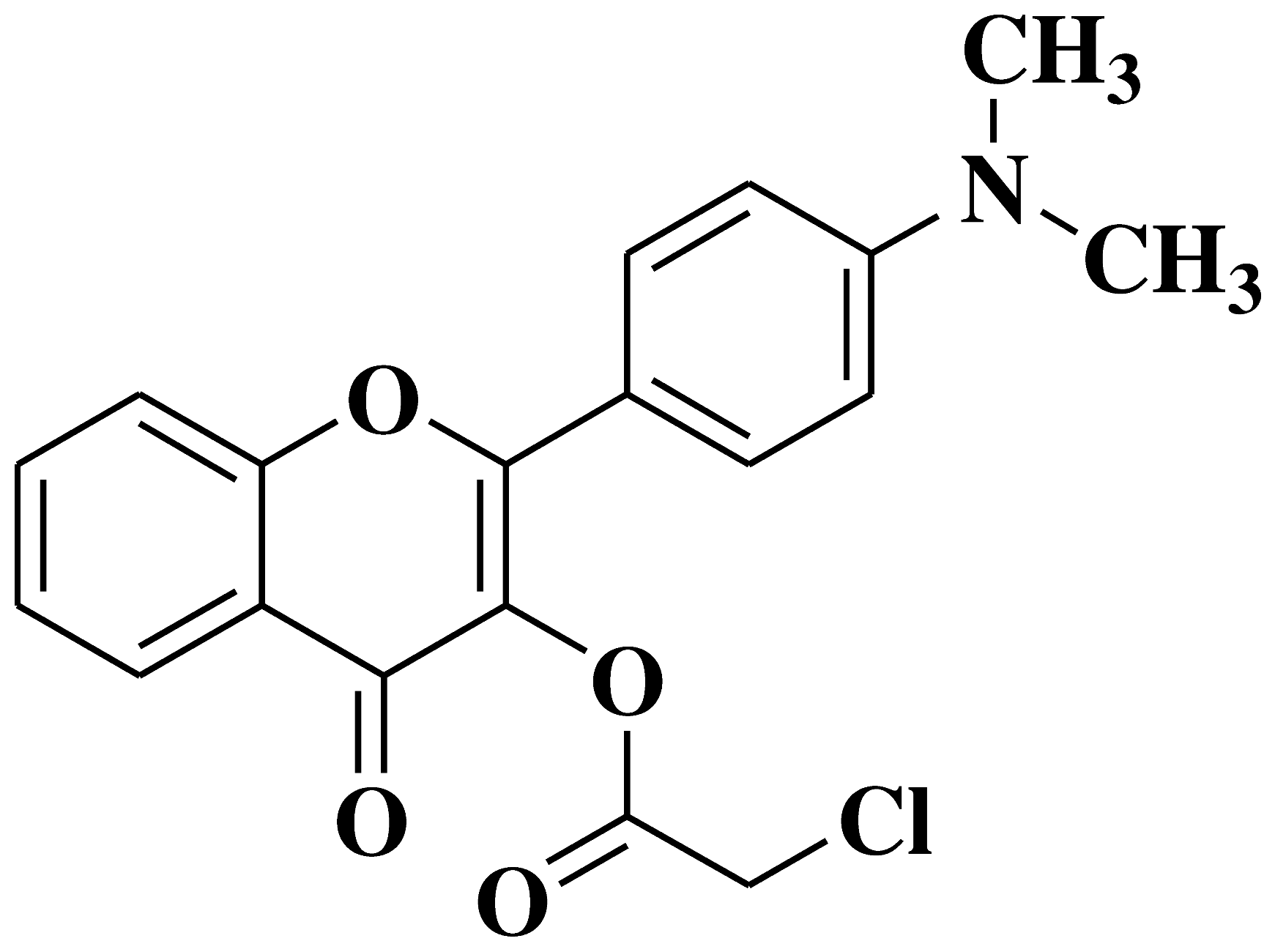 |
Antifungal activity: Acremonium strictum |
[40] |
| 10 | 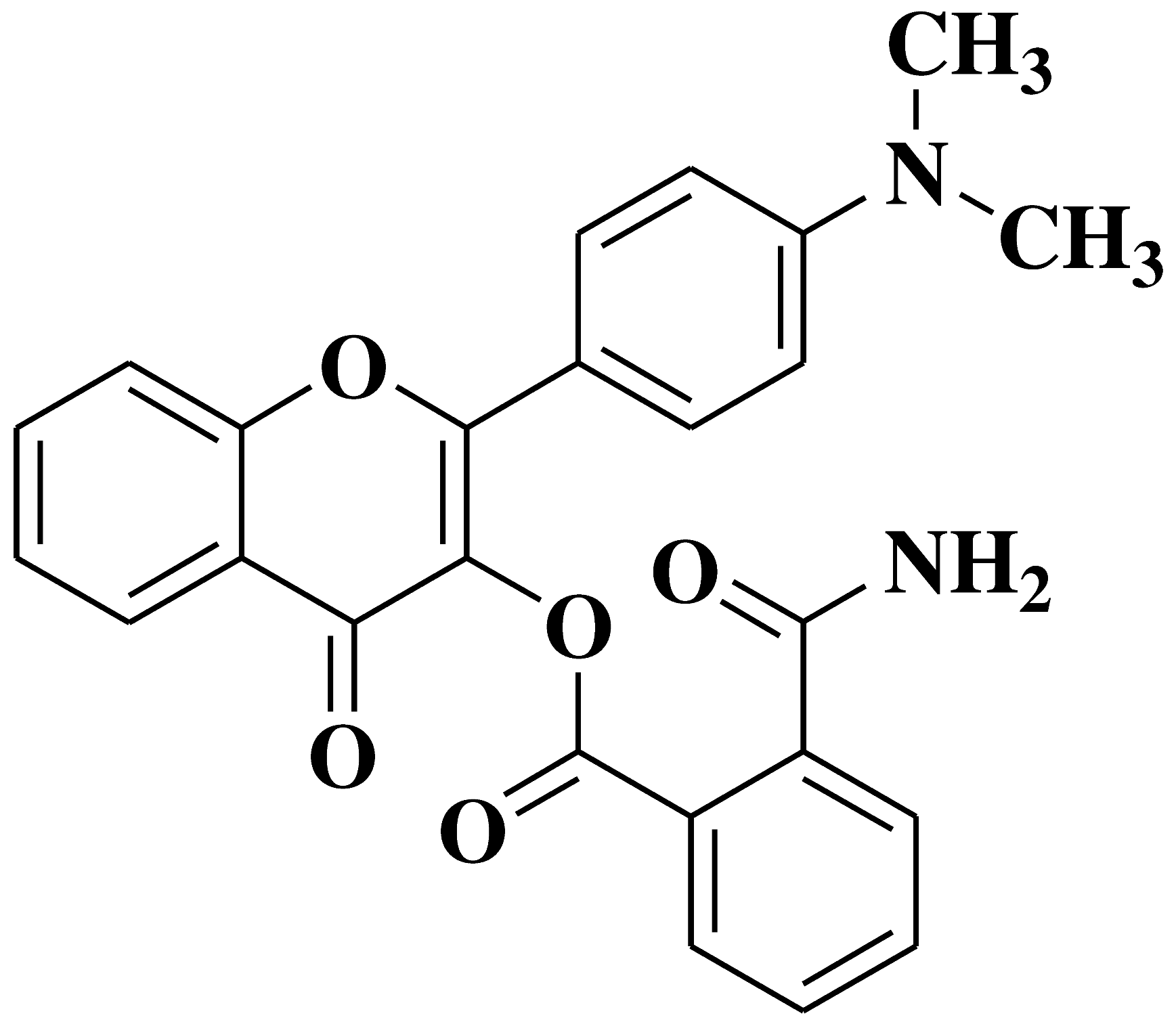 |
Antifungal activity: Acremonium strictum |
[40] |
| 11 | 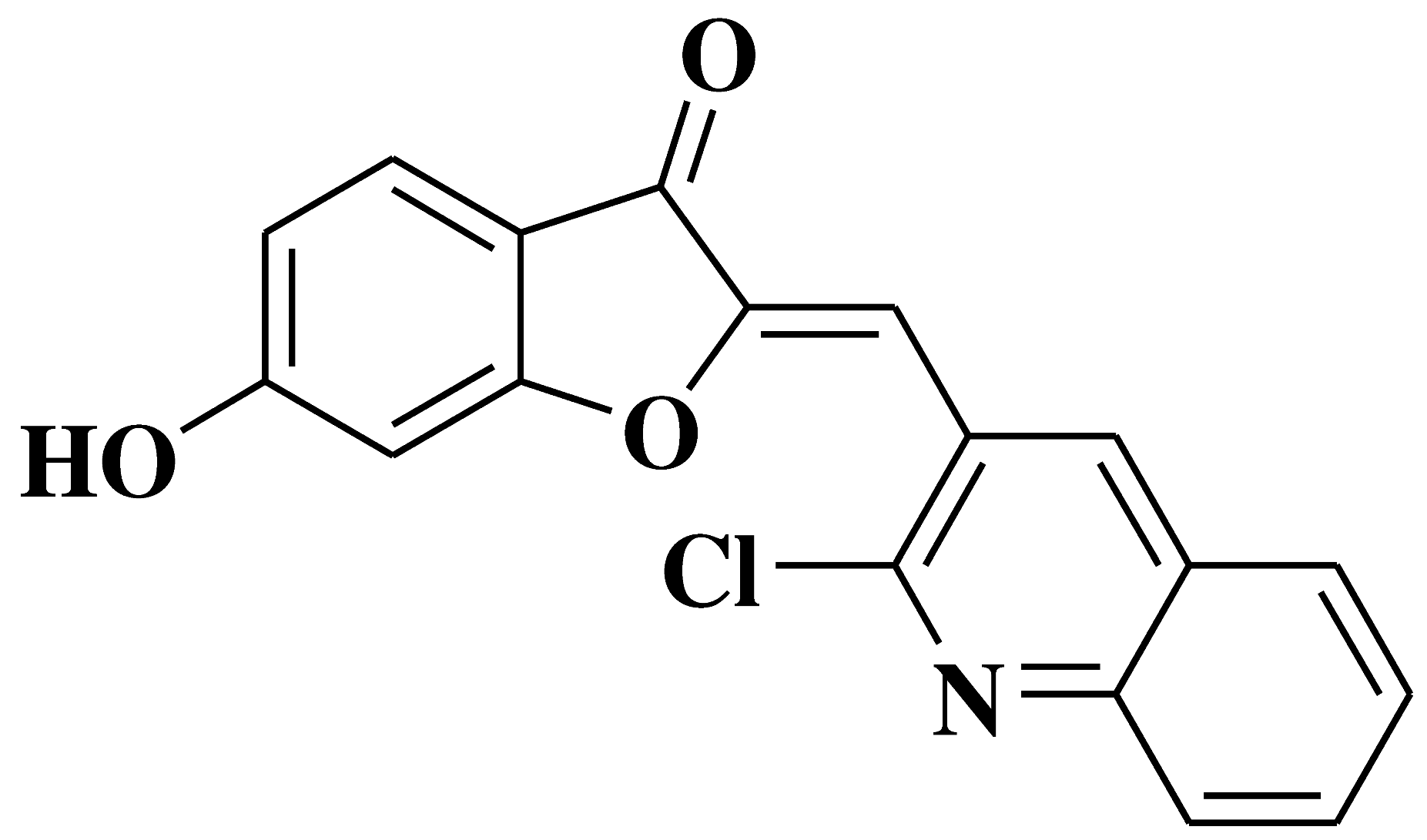 |
Antibacterial activity: Staphylococcus aureus Bacillus subtilis Antifungal activity: Mycobacterium smegmatis Fusarium oxysporum |
[41] |
| 12 | 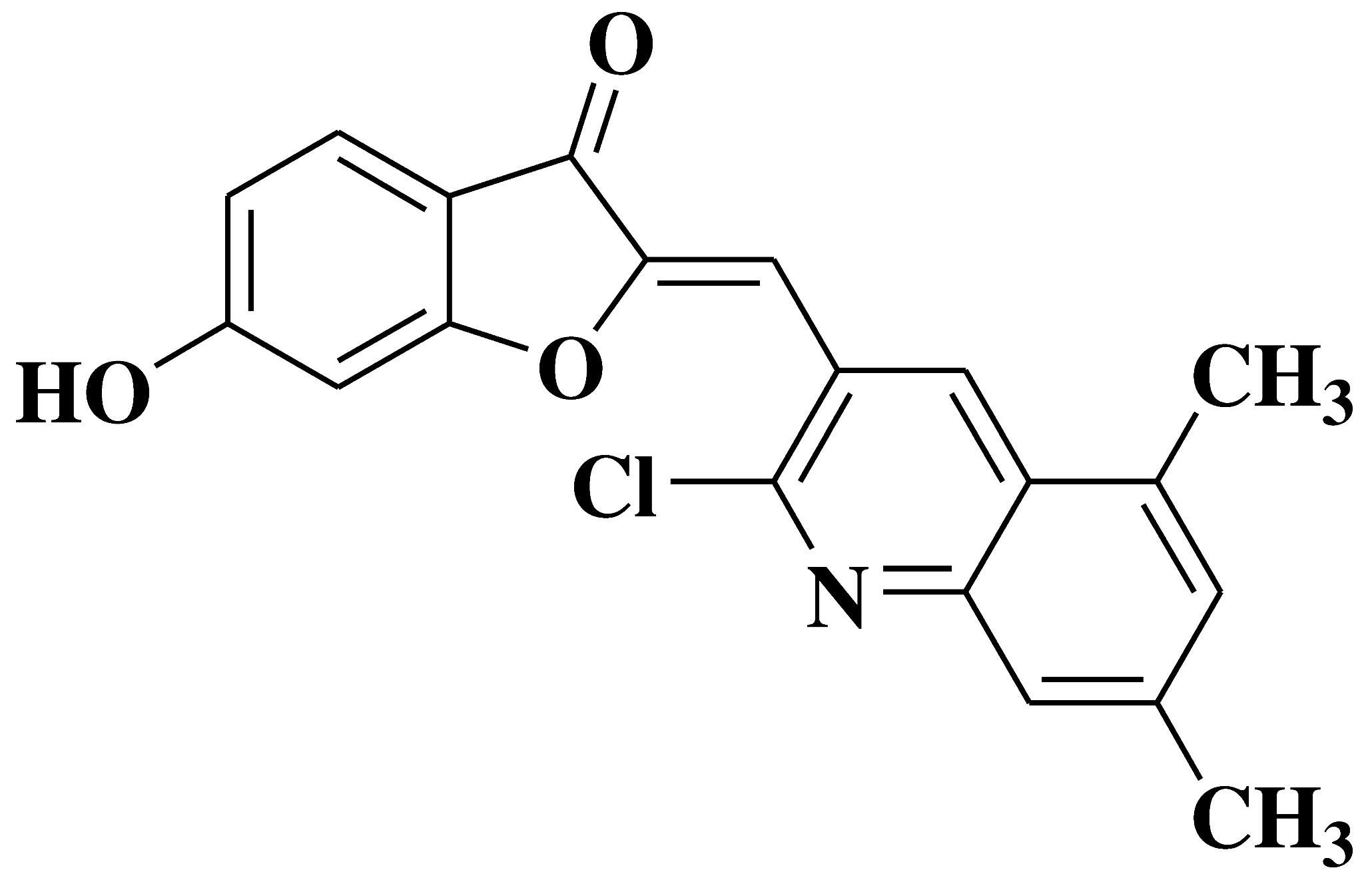 |
Antibacterial and antibiofilm activity: Staphylococcus aureus Bacillus subtilis Antifungal activity: Mycobacterium smegmatis Fusarium oxysporum Candida albicans |
[41] |
| 13 | 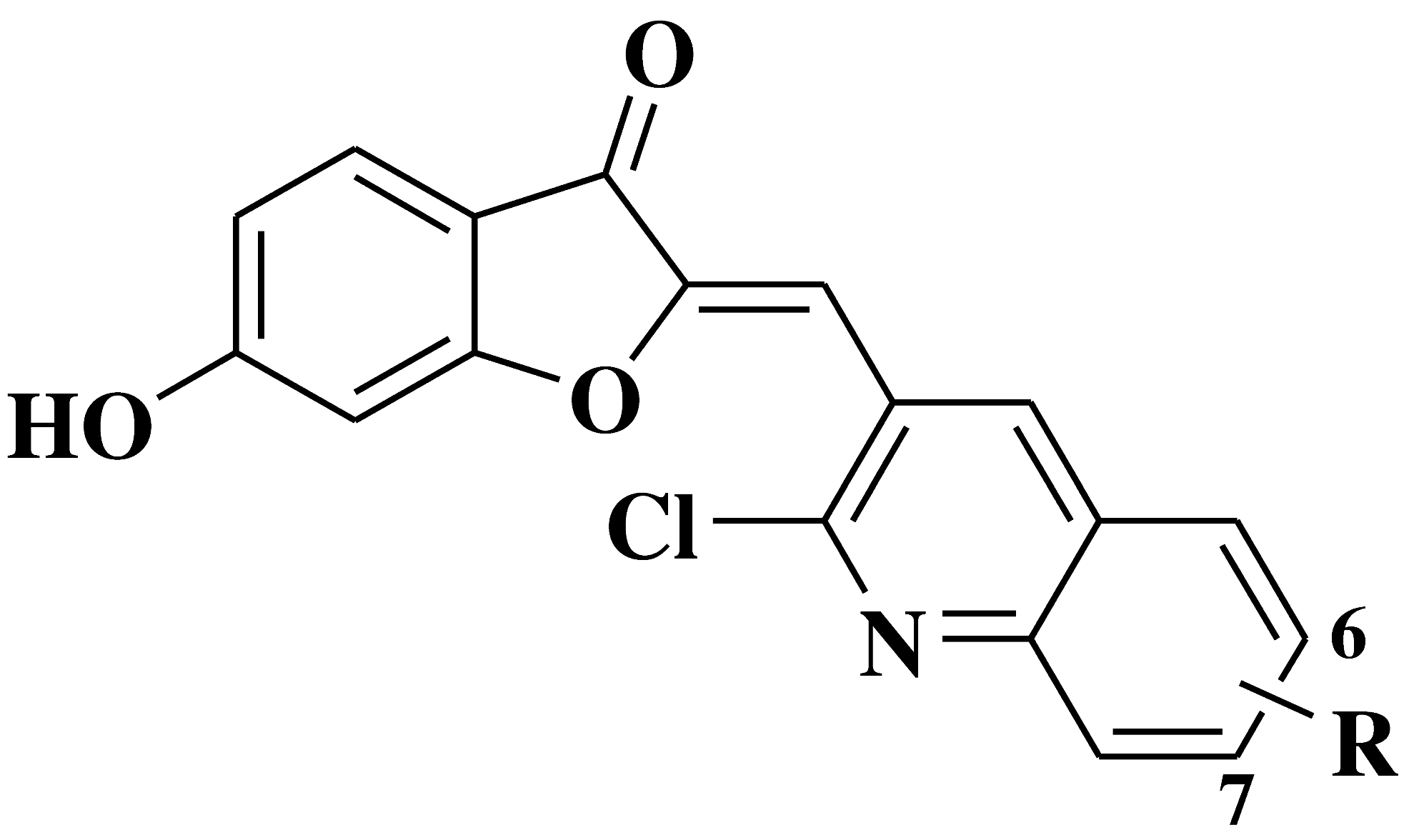 |
Antibacterial (R=6-OCH3, 7-Cl) and anti-biofilm (R=6-OCH3) activity: Staphylococcus aureus Bacillus subtilis Klebsiella pneumoniae Antifungal activity (R=7-Cl): Candida albicans |
[41] |
| 14 | 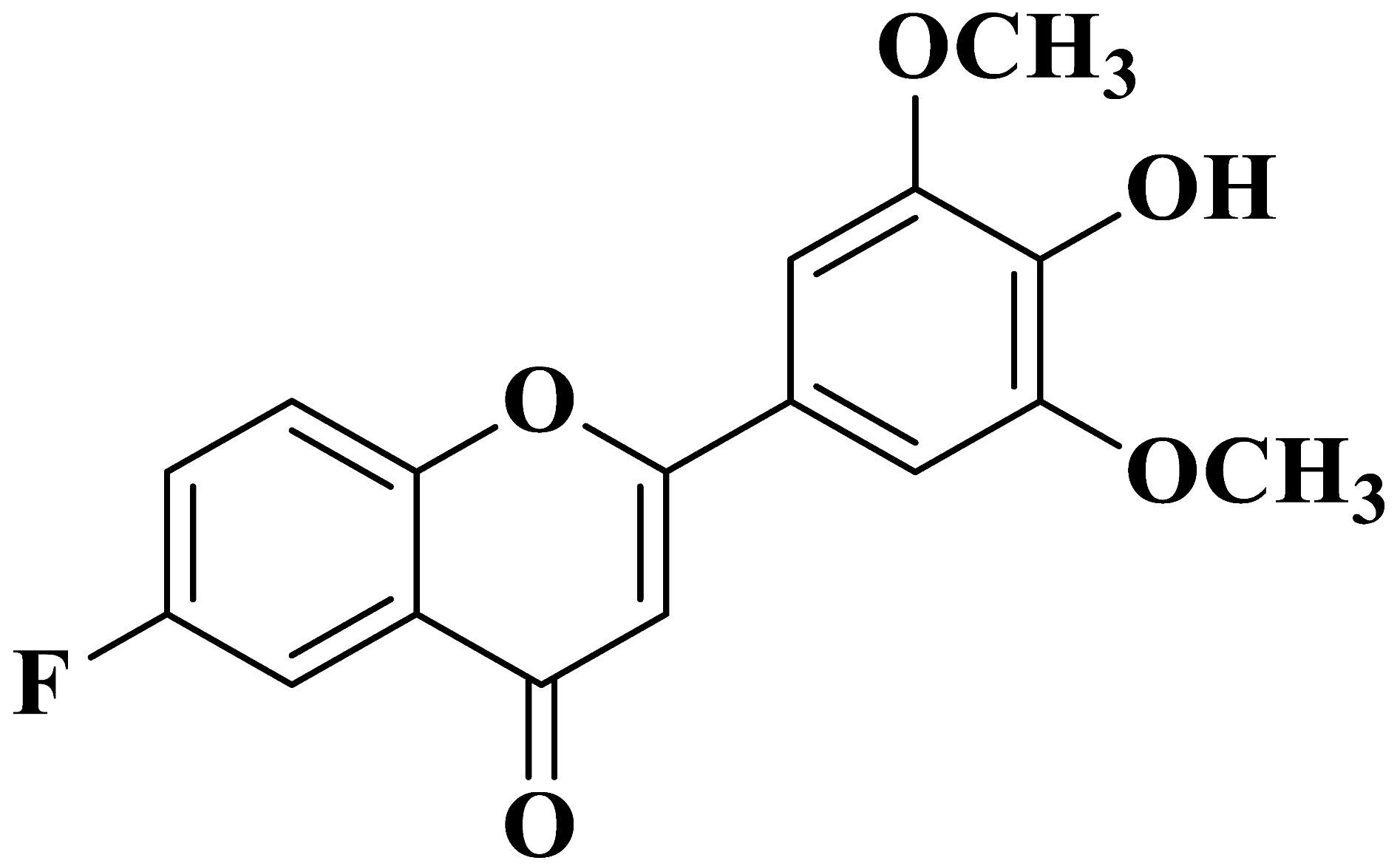 |
Antiviral activity against human cytomegalovirus | [42] |
| 15 | 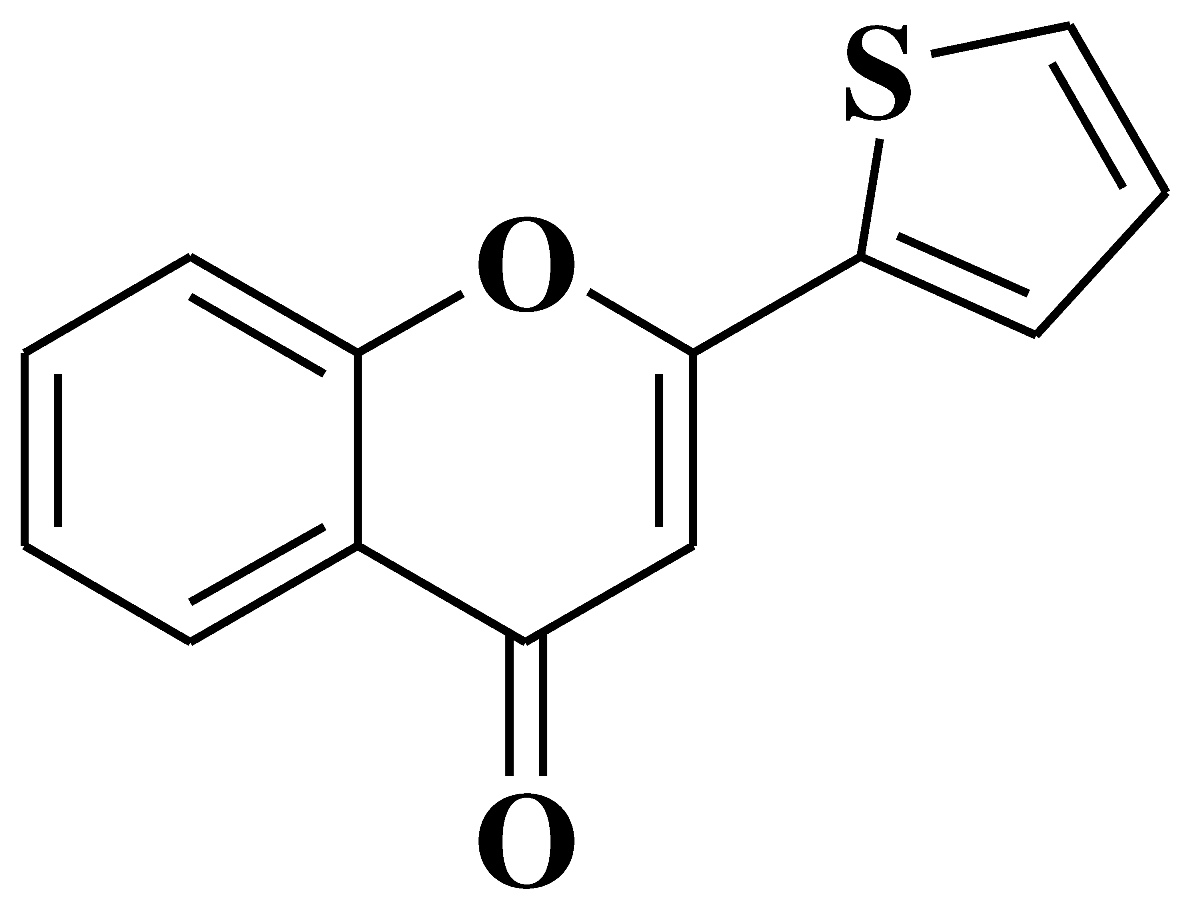 |
Antiviral activity against Chikungunya Virus |
[43] |
| 16 | 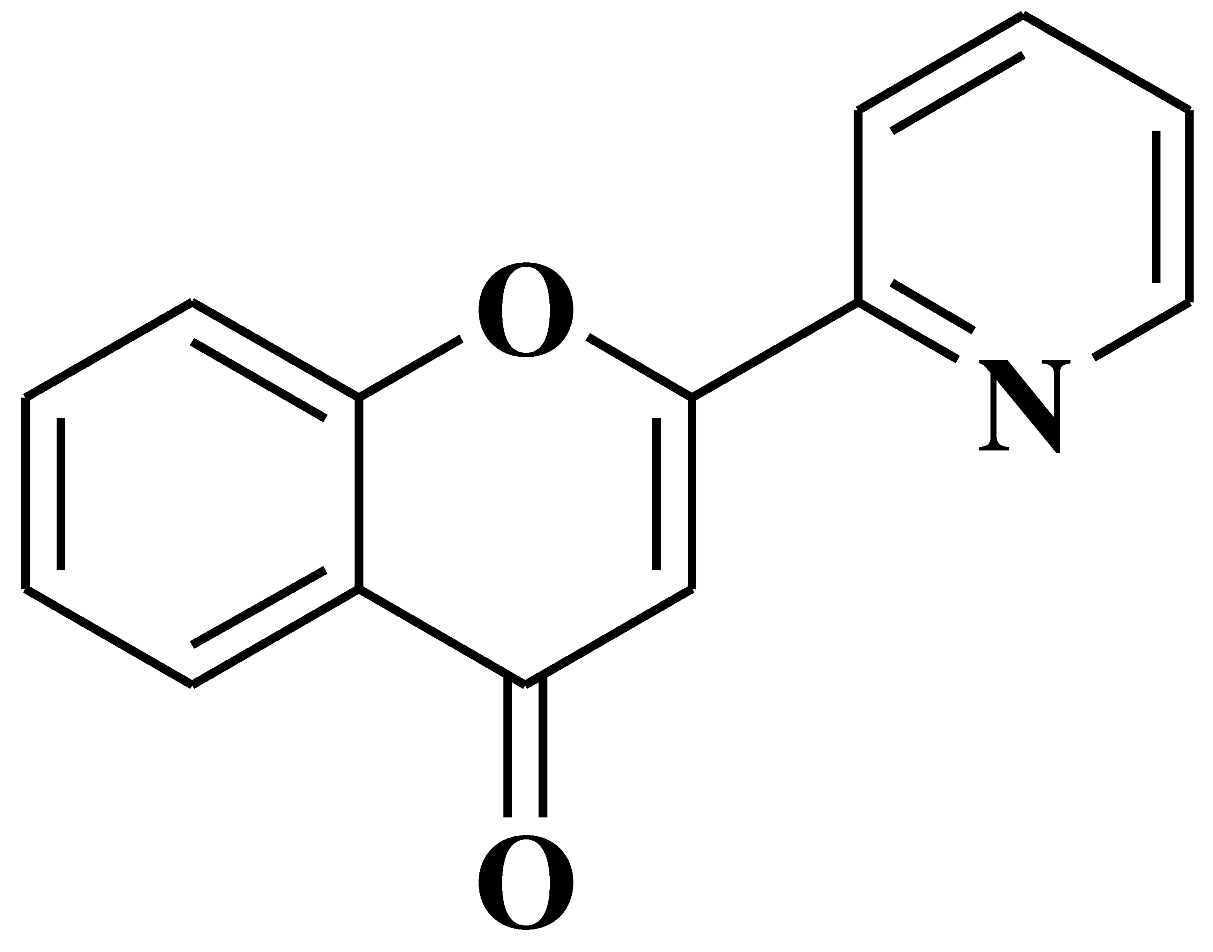 |
Antiviral activity against Chikungunya Virus |
[43] |
2.3. Antiviral activity
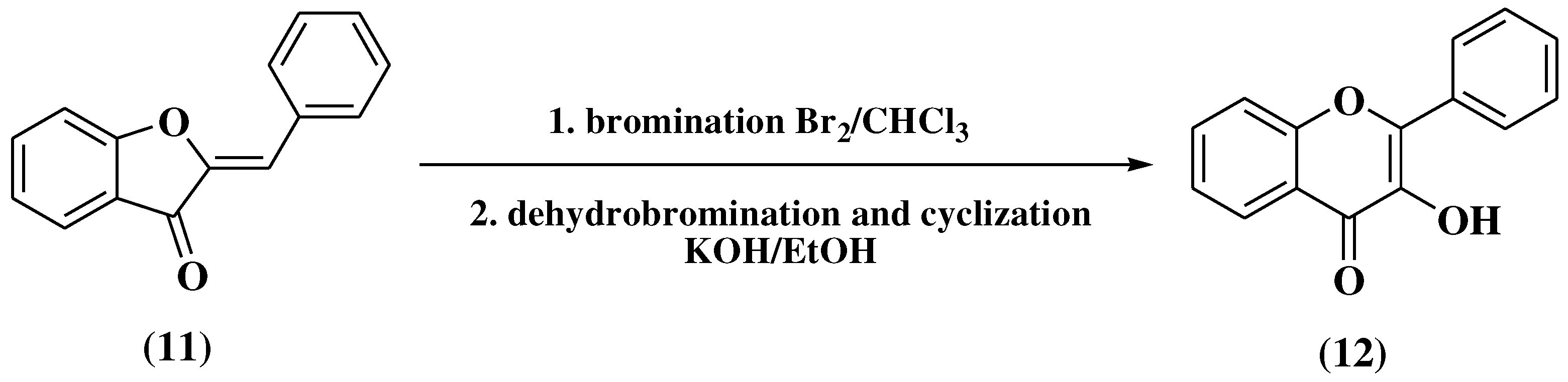
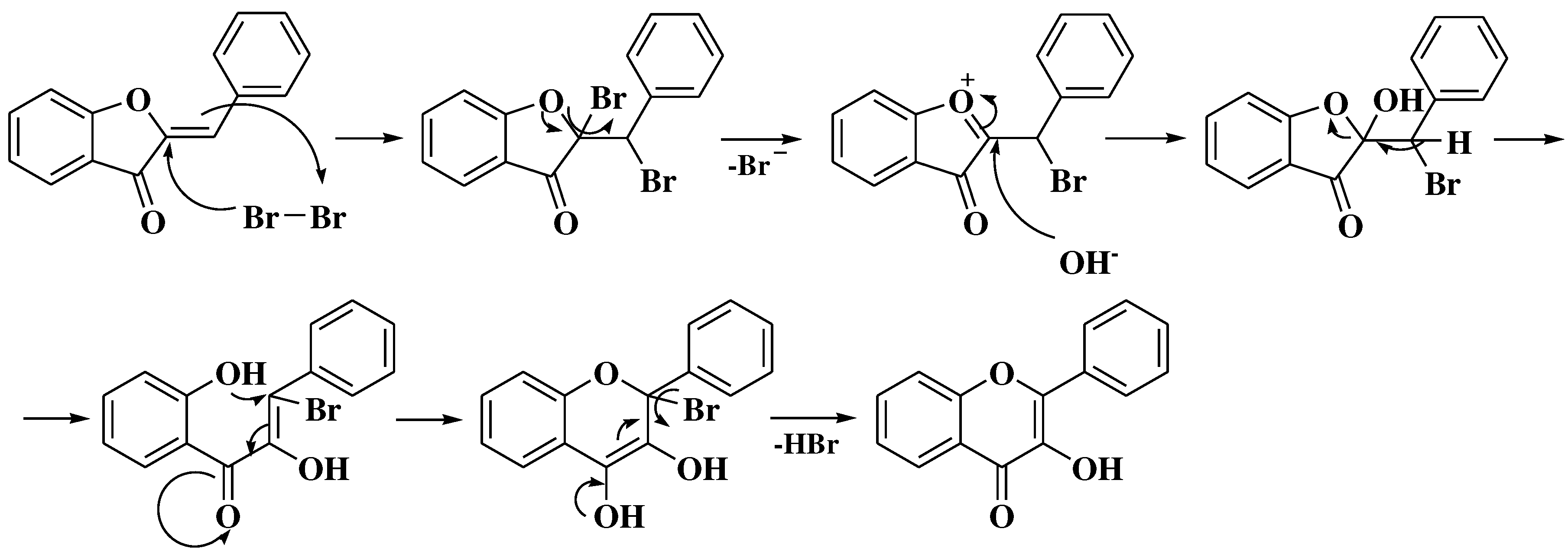
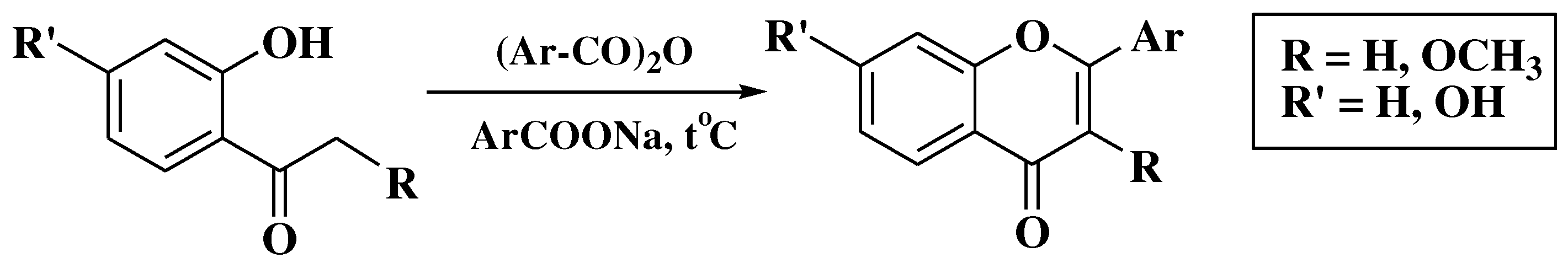
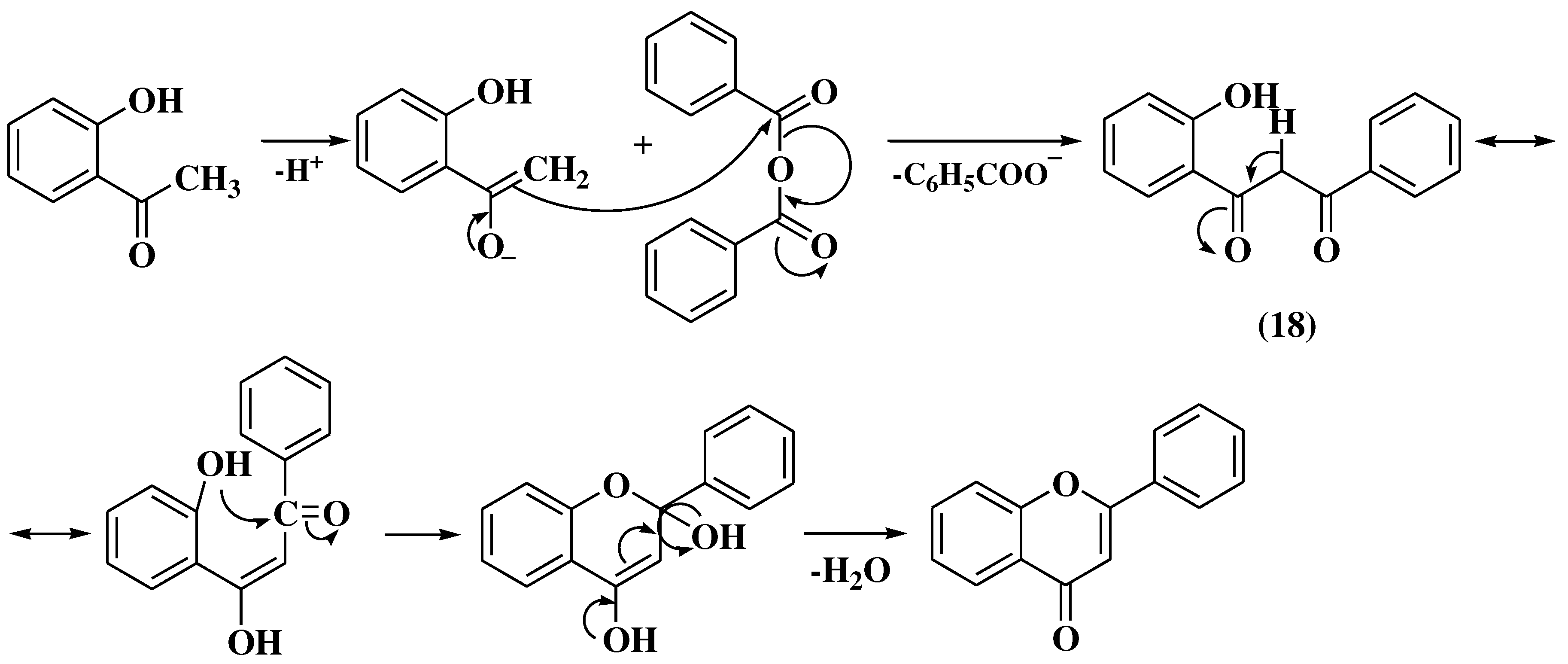
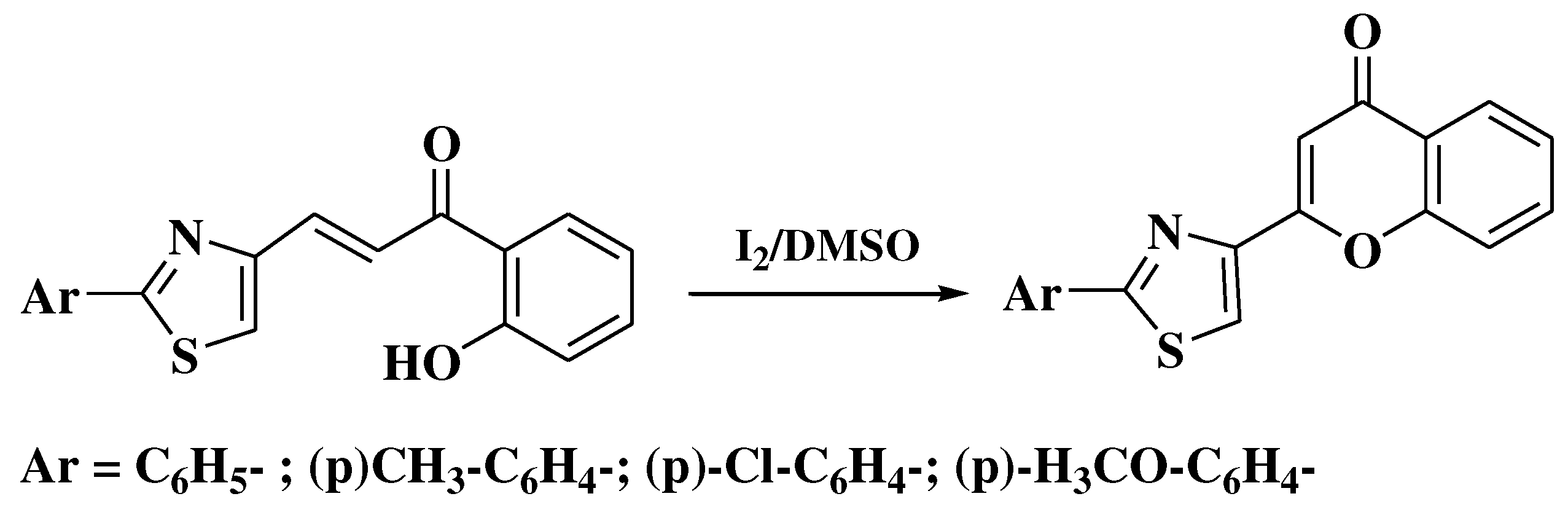
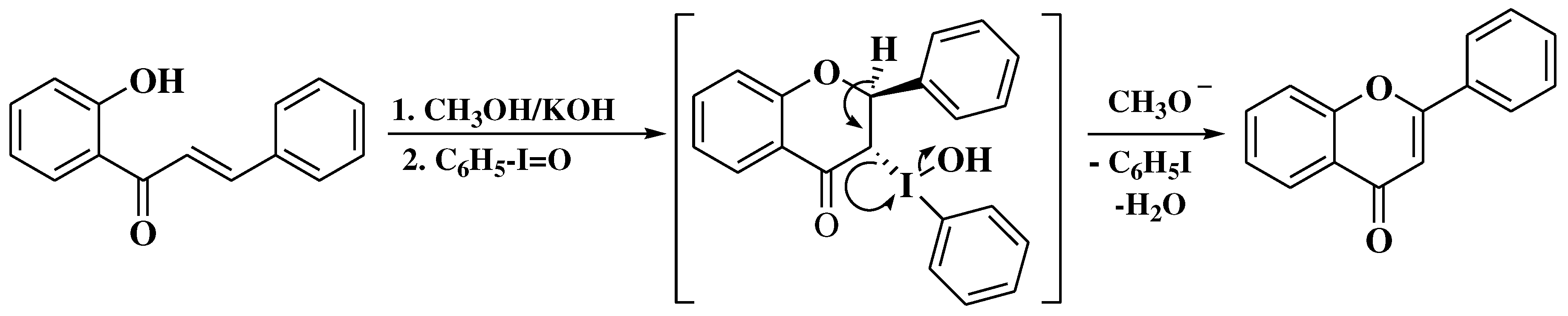
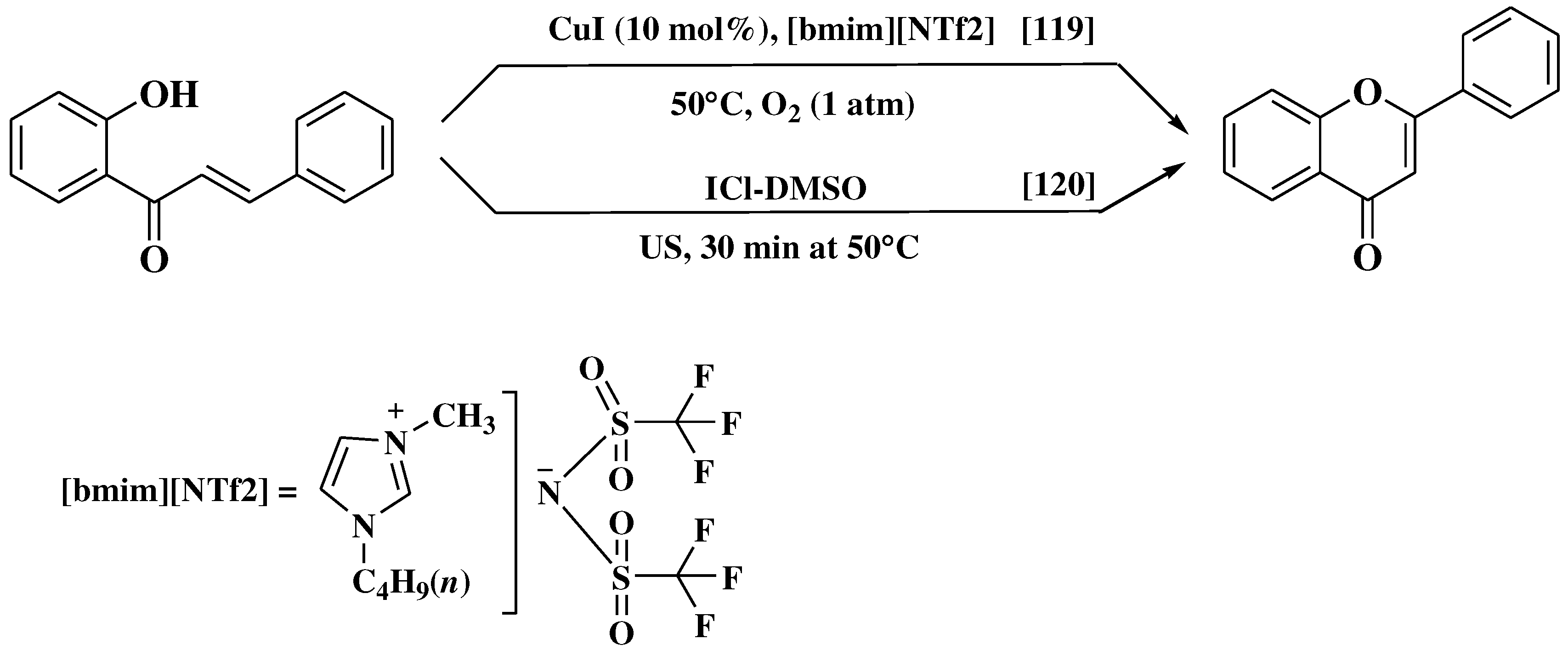
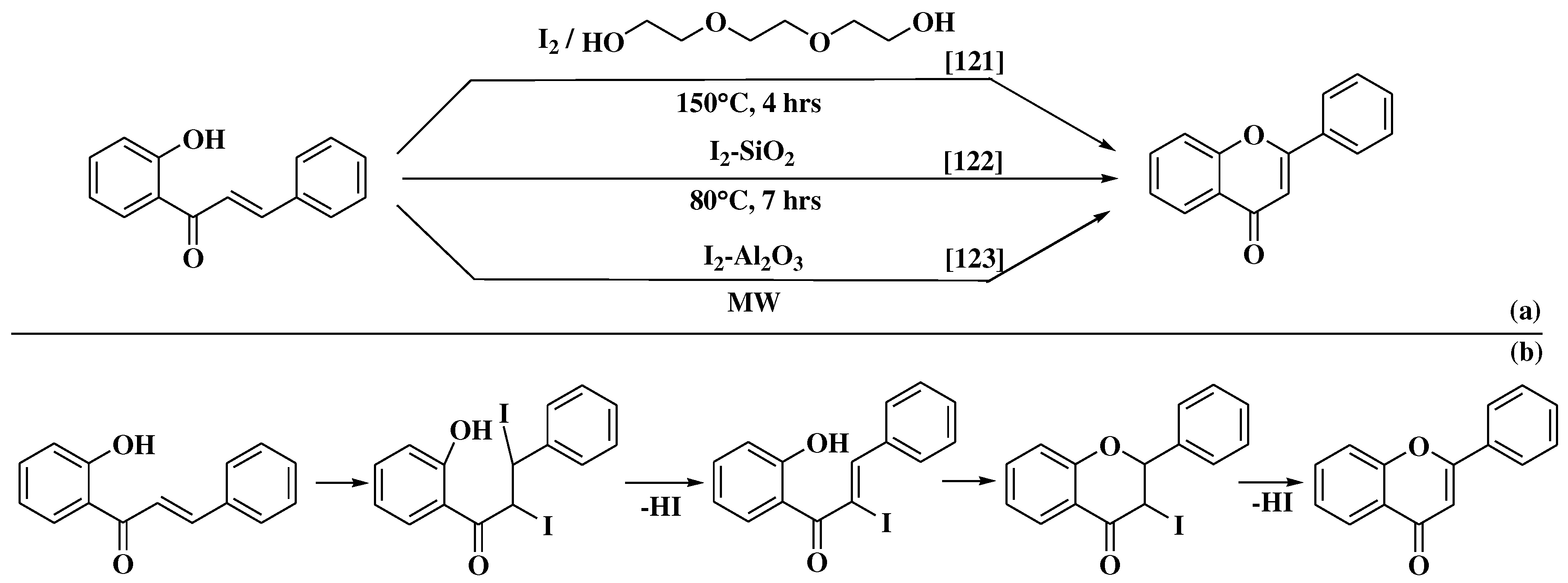
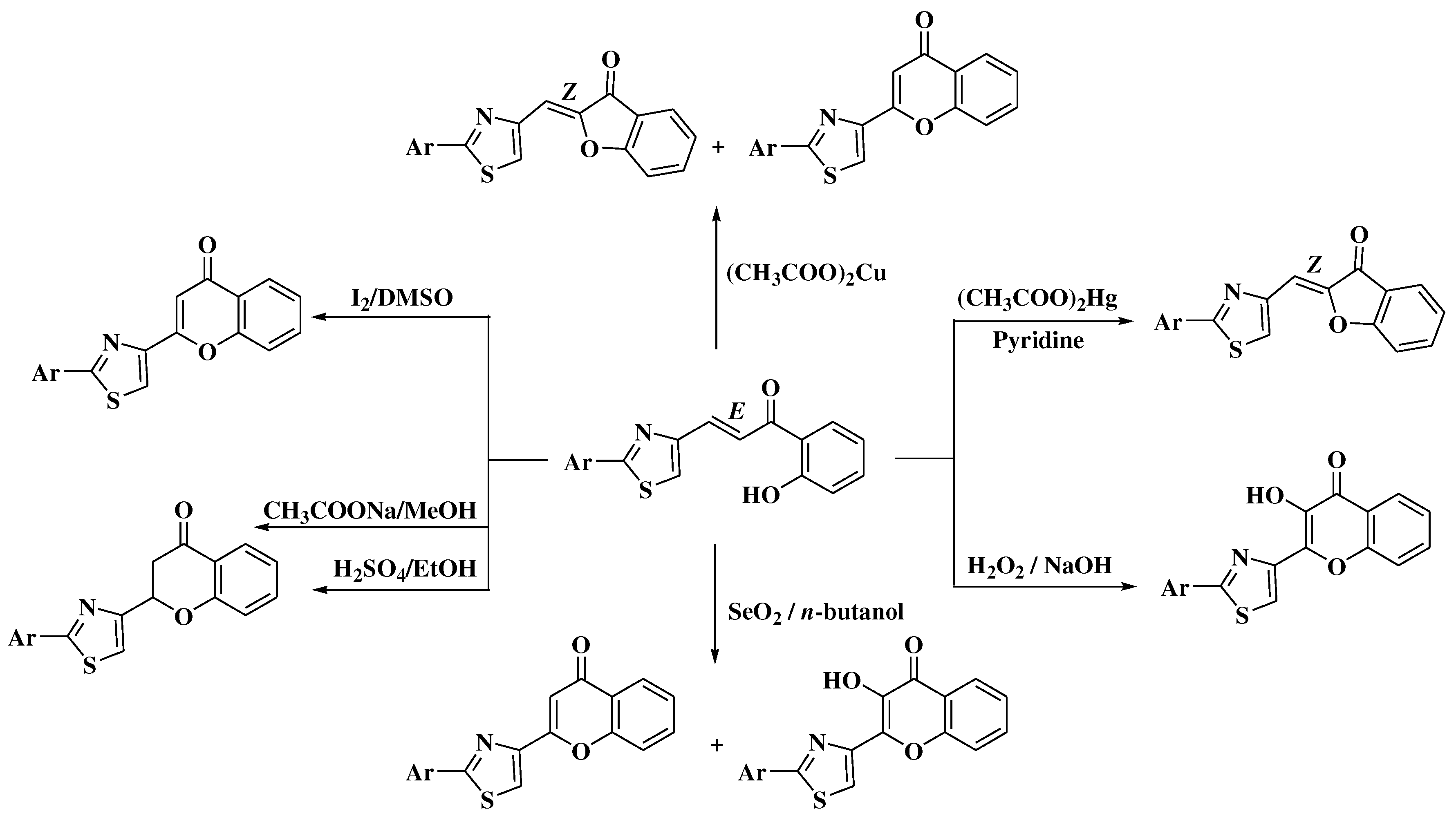
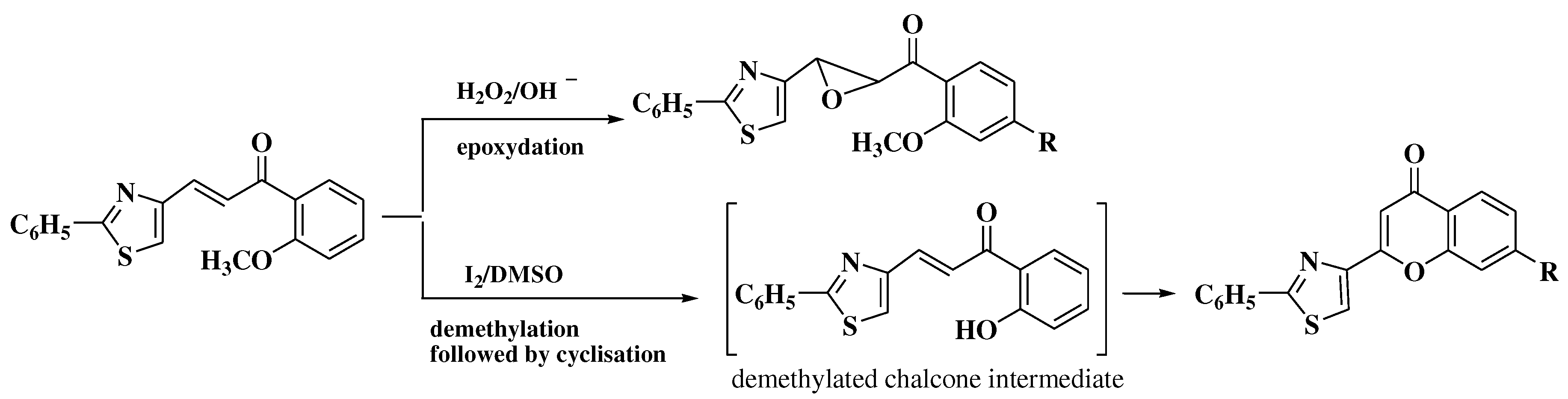
3. Chemical synthesis of flavones
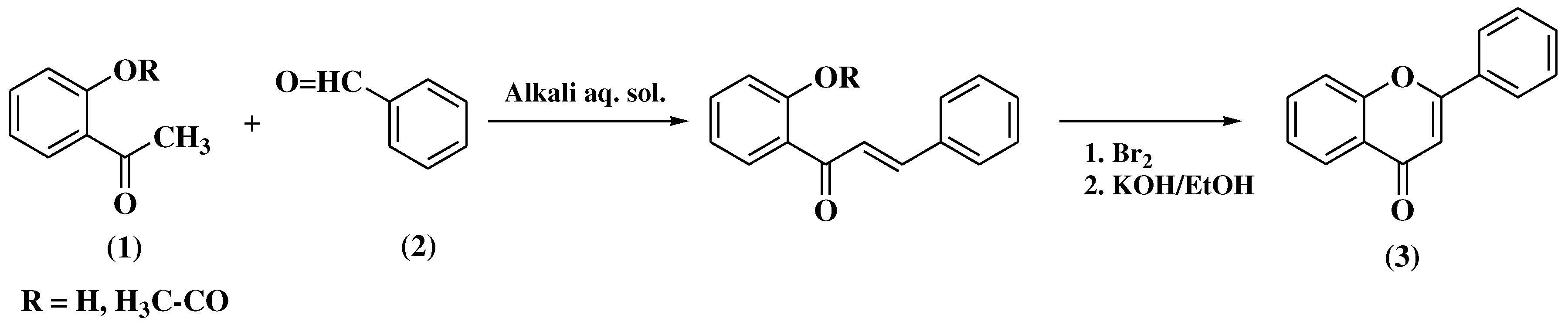
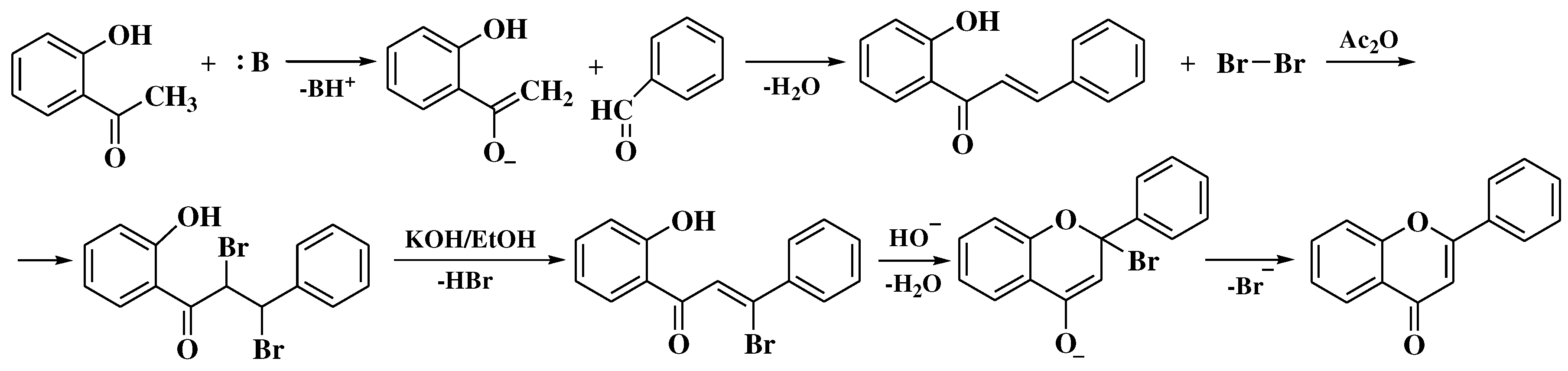
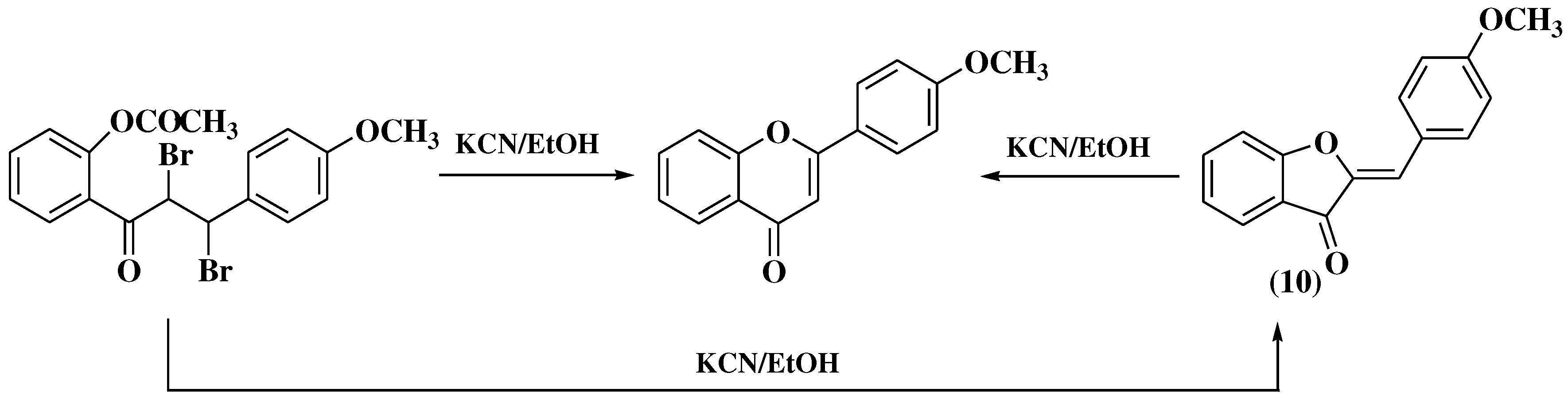
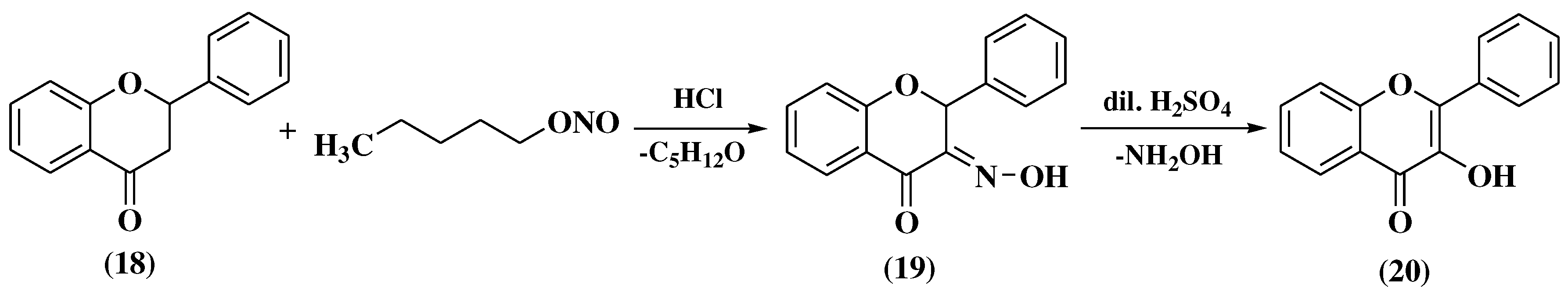
3.1. Von Kostanecki method
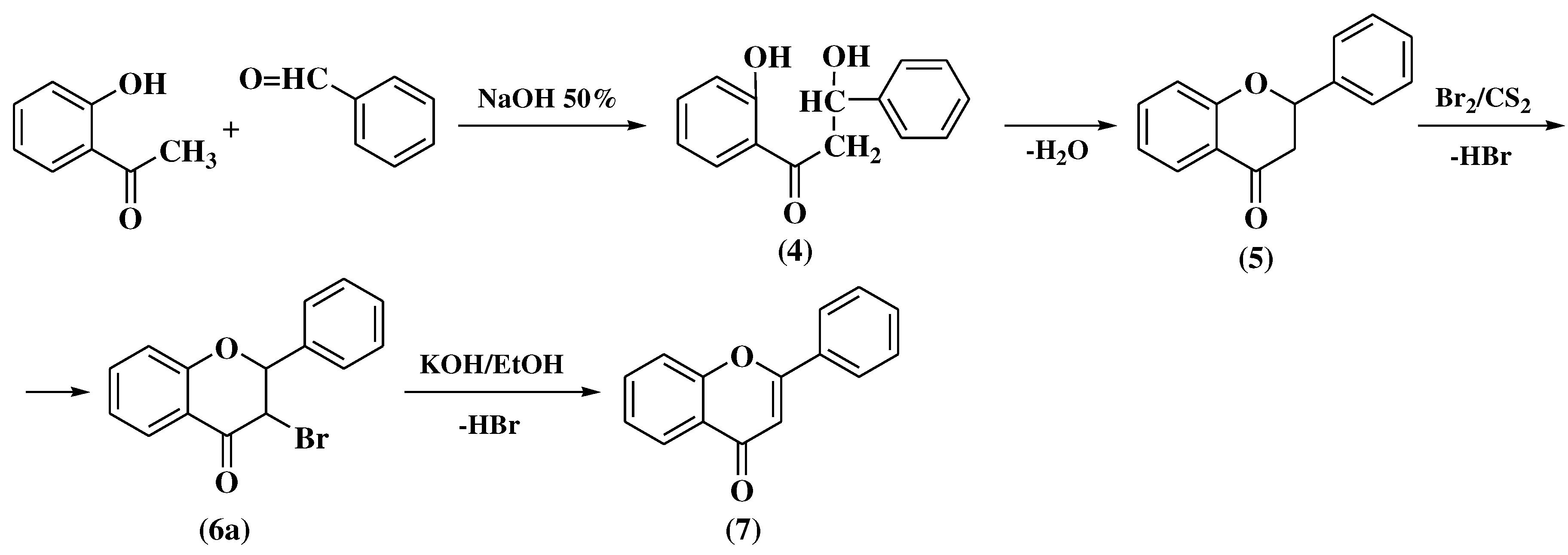
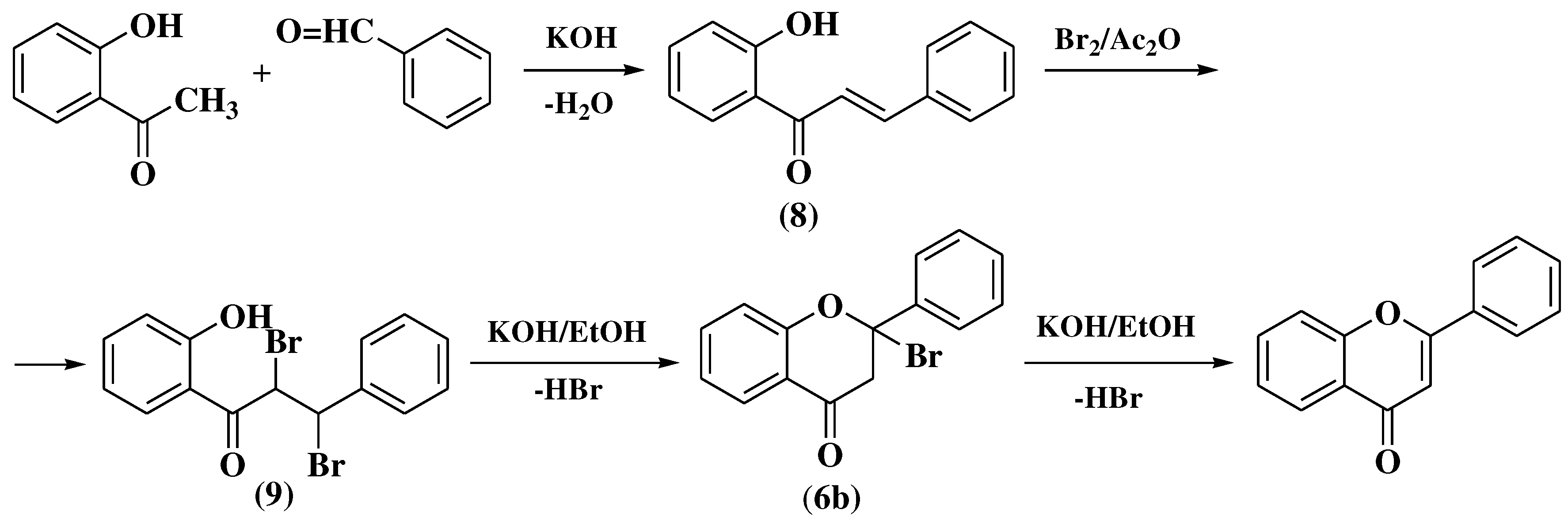
3.2. Von Auwers-Müller method
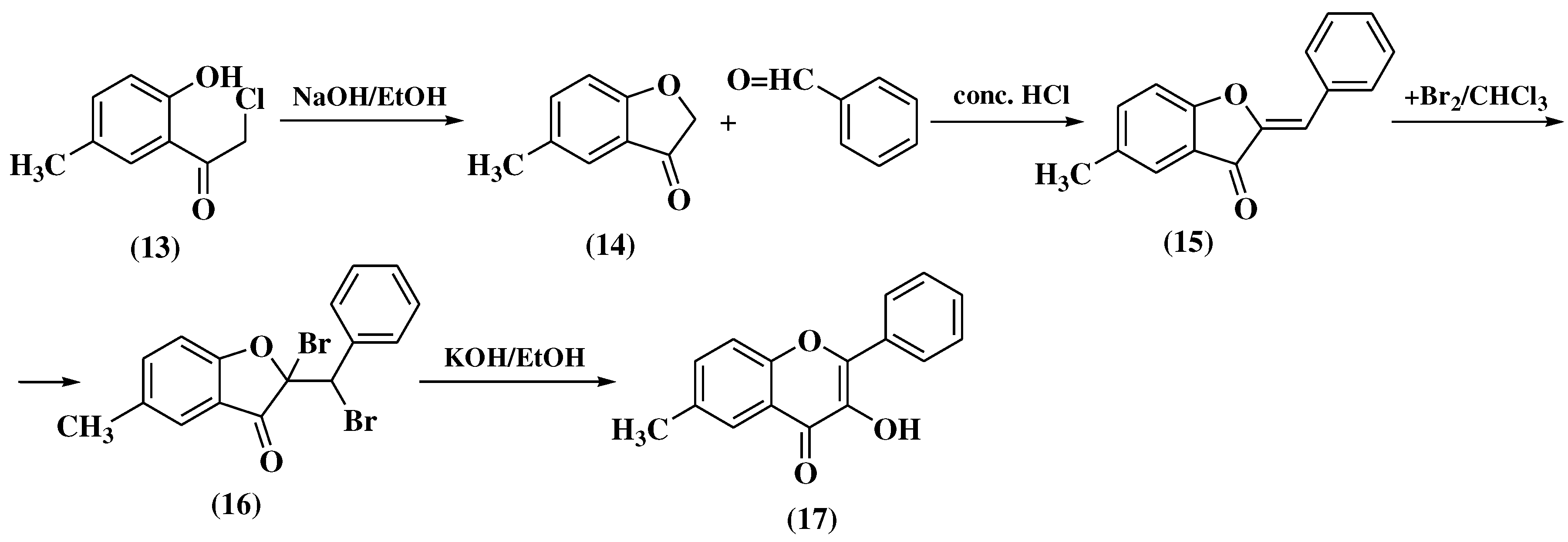
3.3. Allan-Robinson method
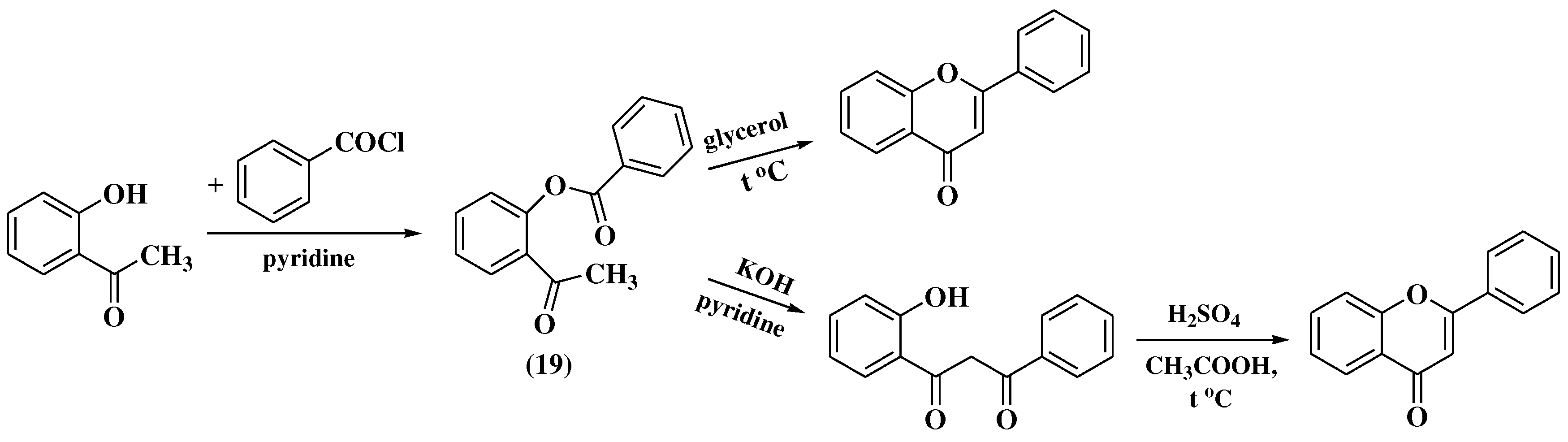
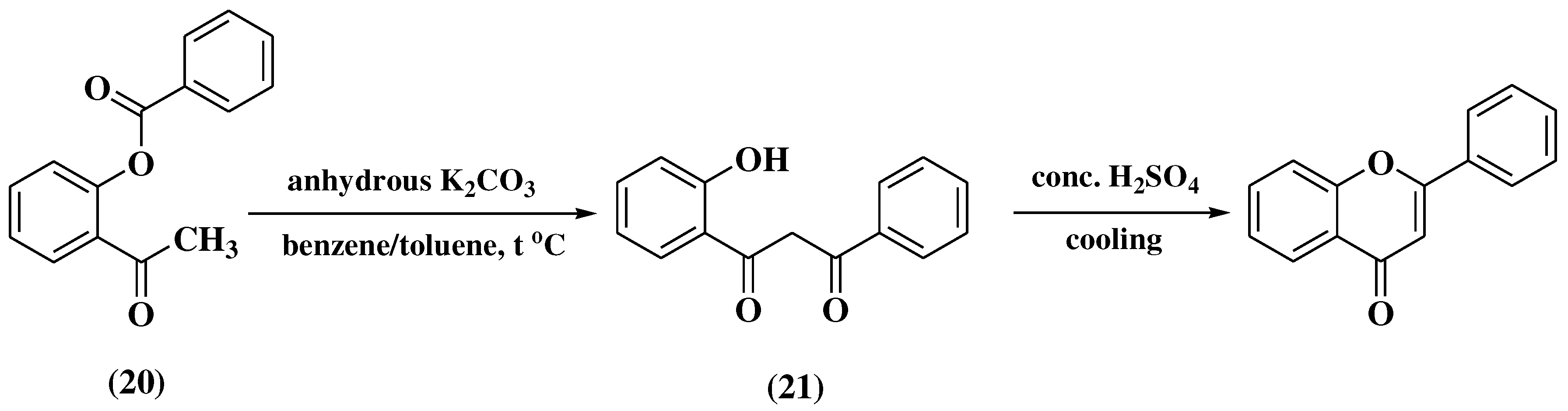
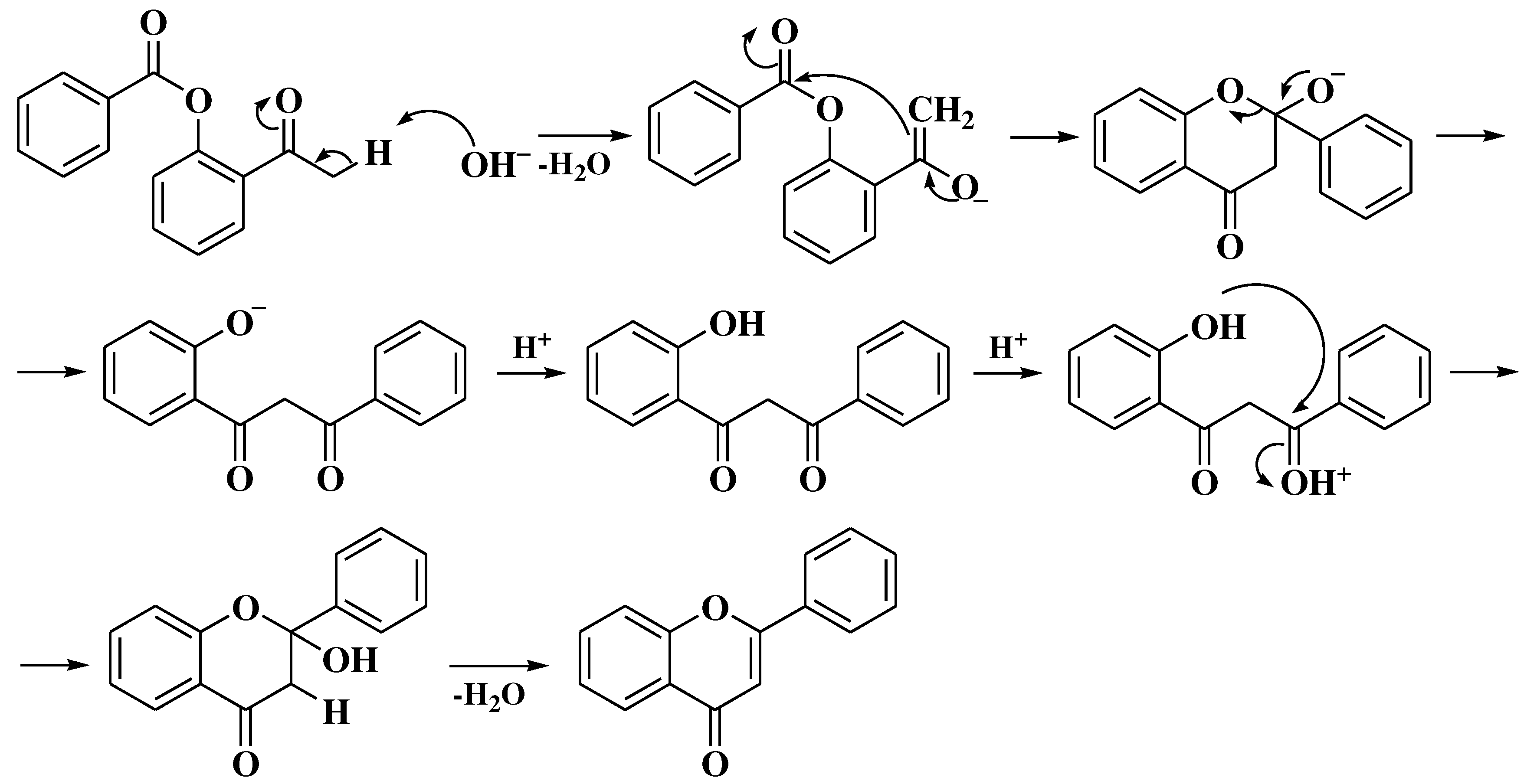
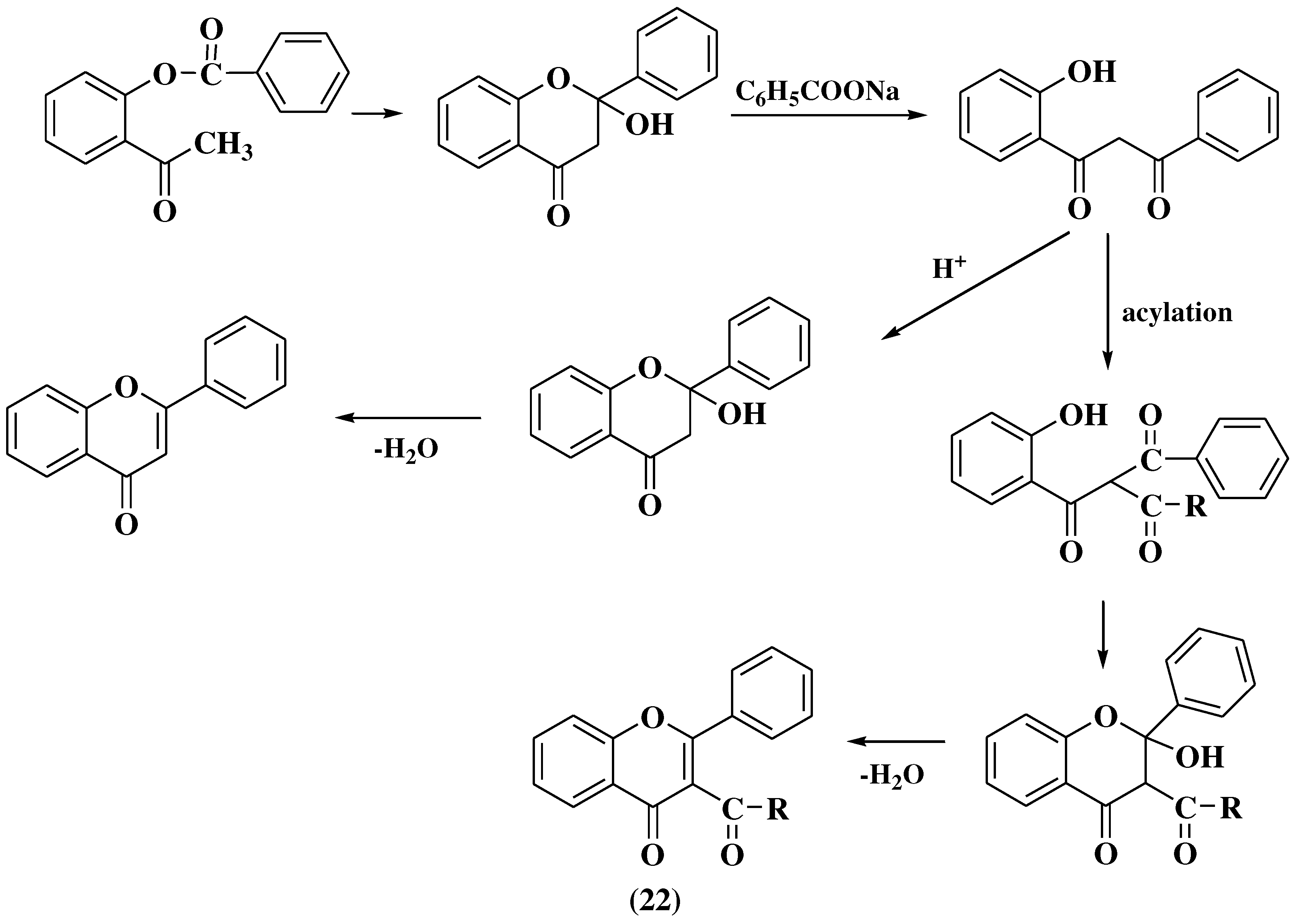
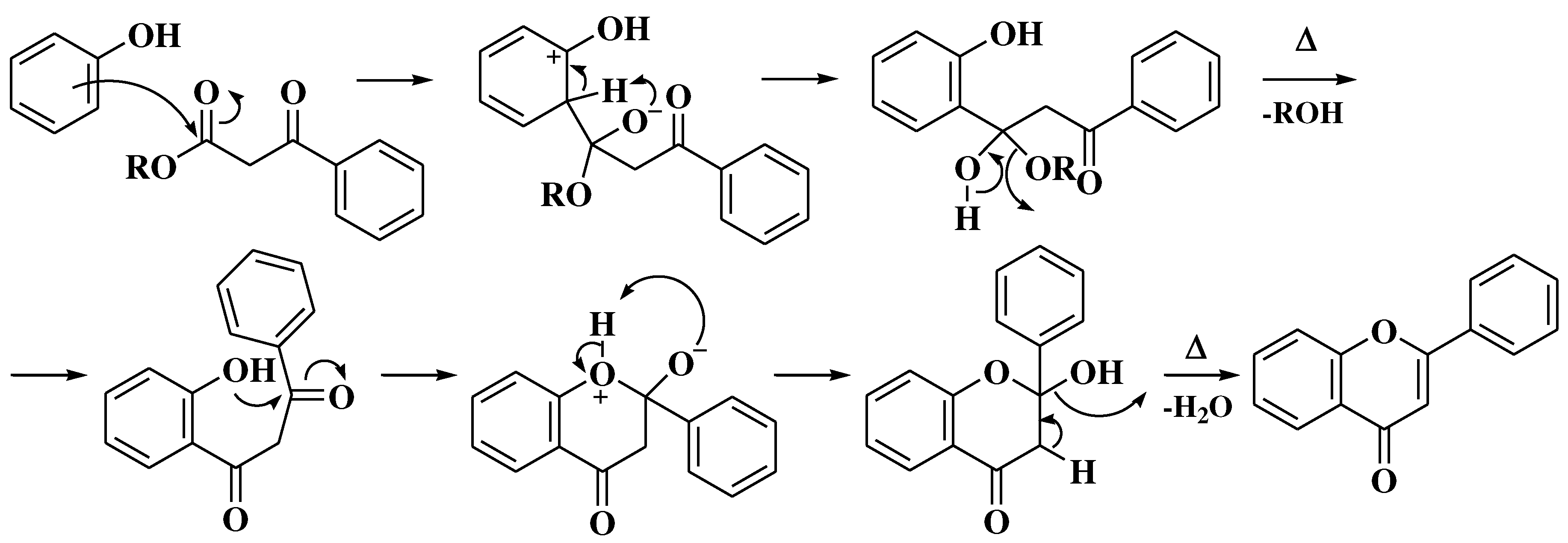
3.4. Baker-Verkataraman method
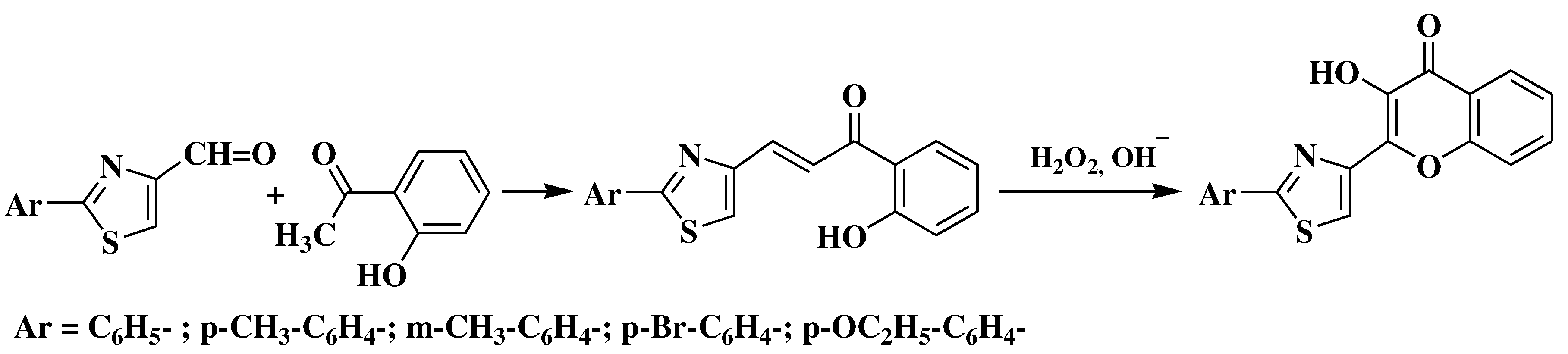
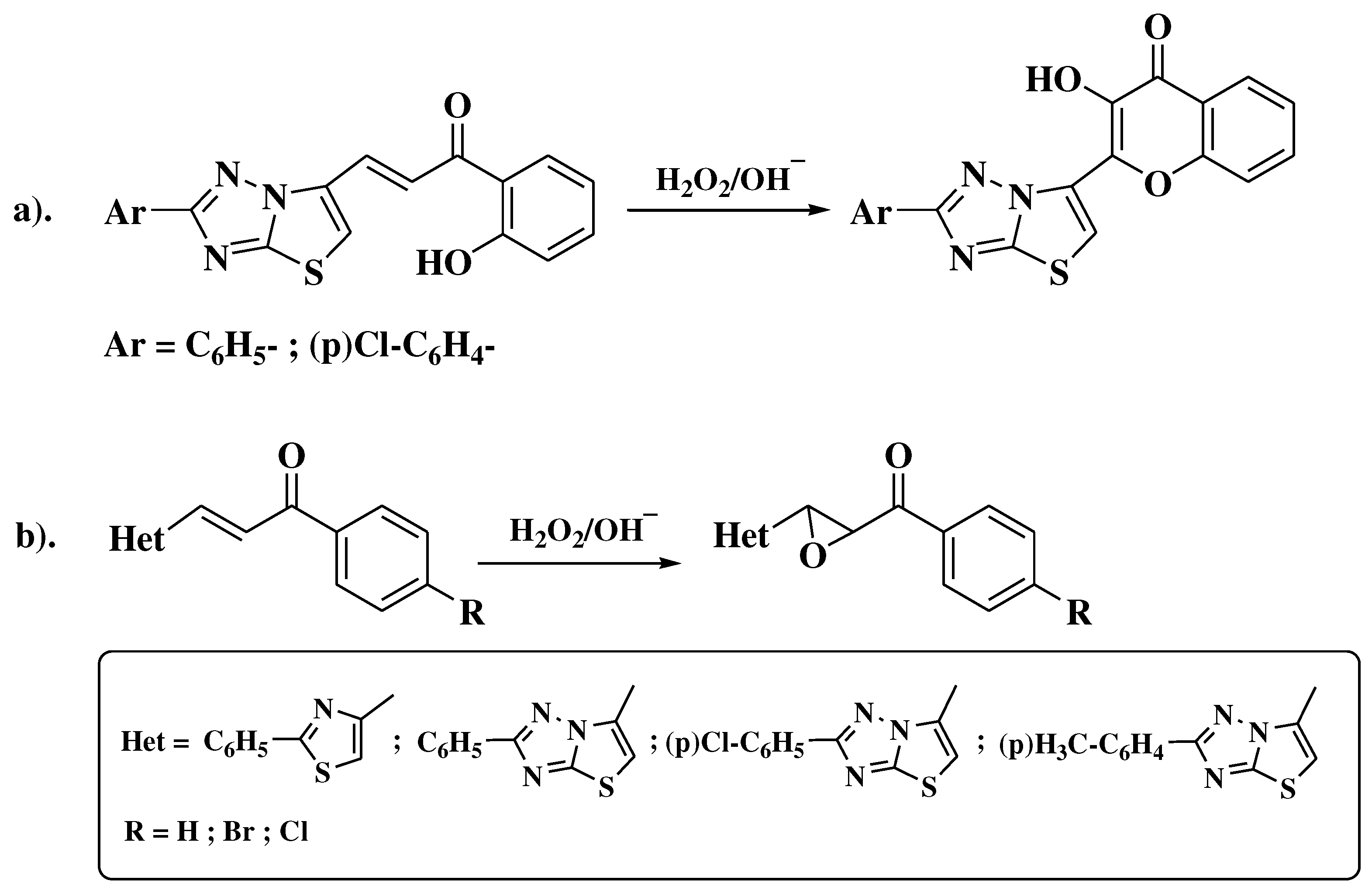
3.5. Algar-Flynn-Oyamada method
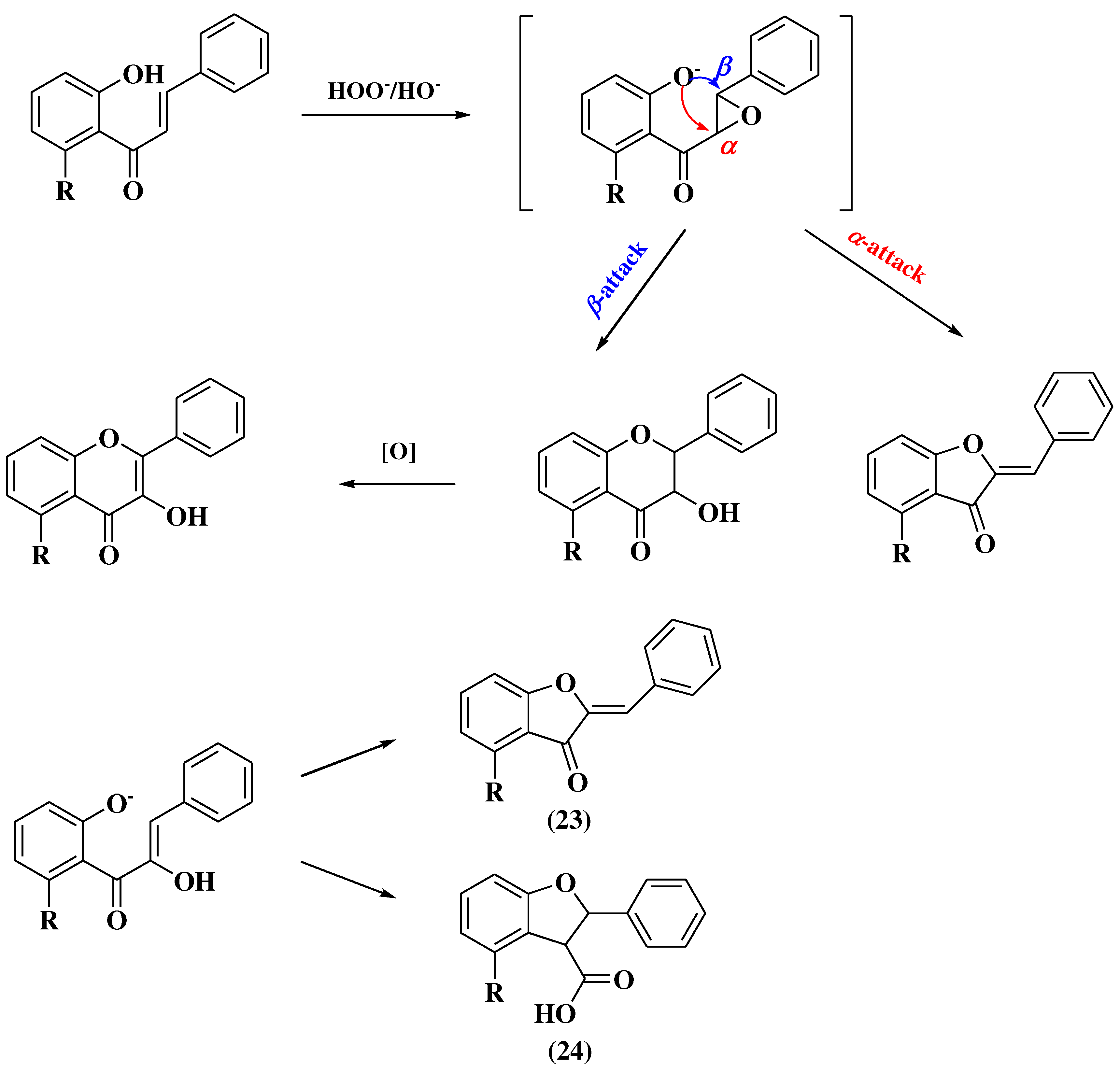
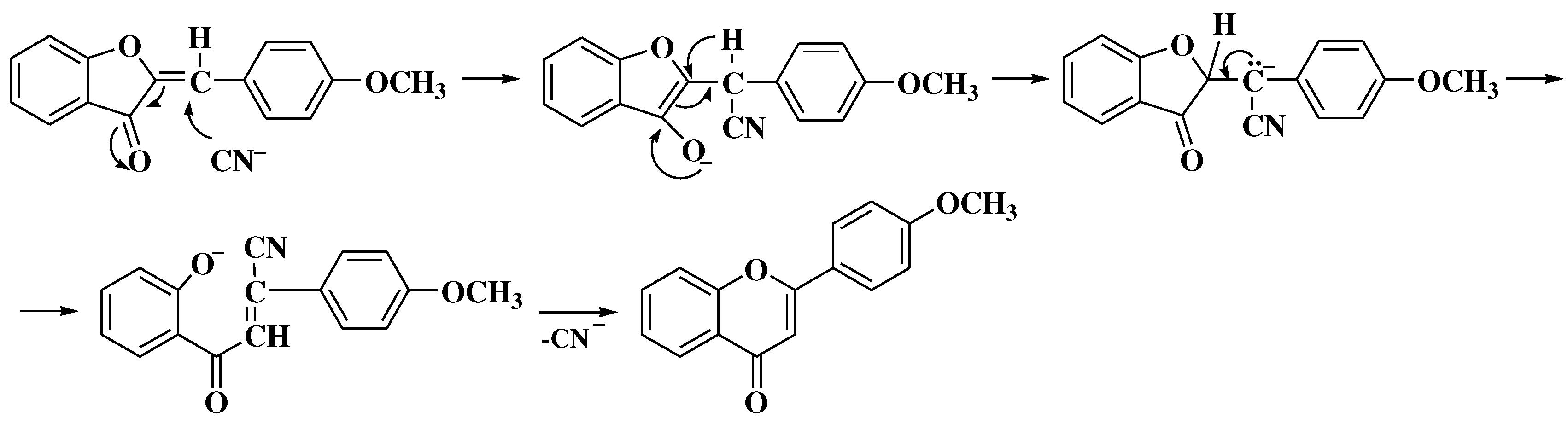
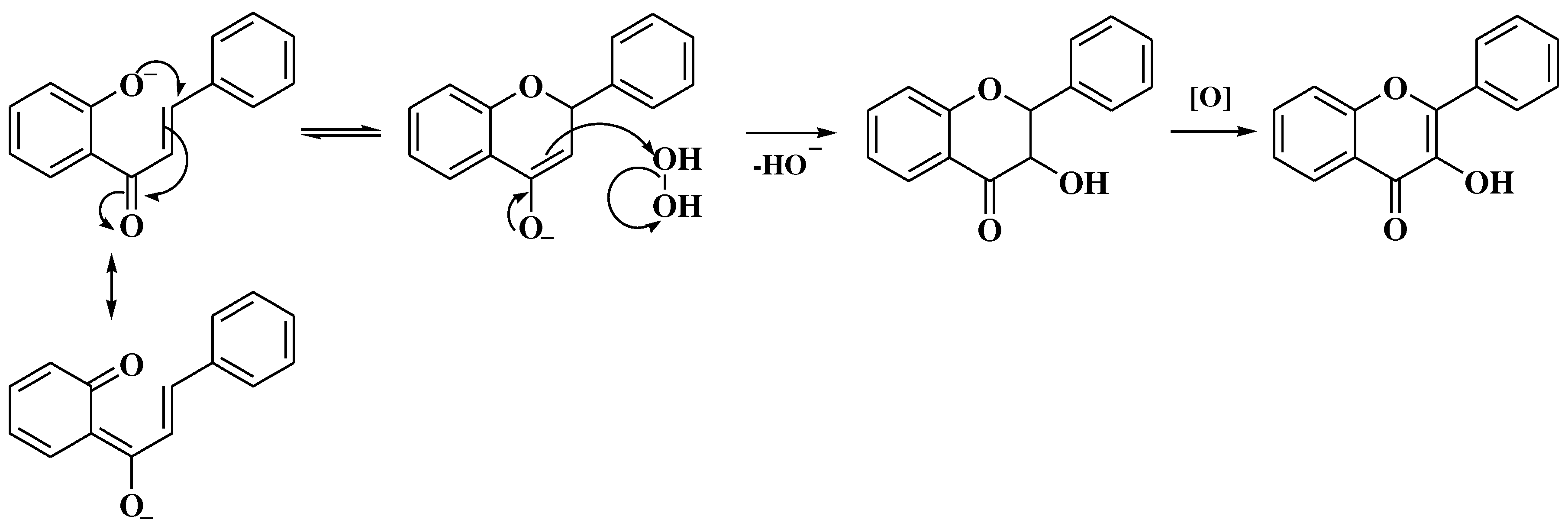
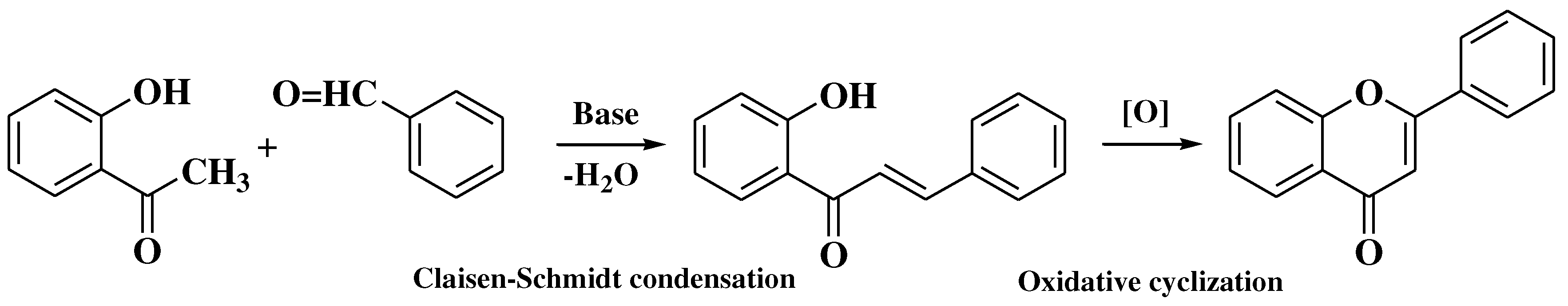
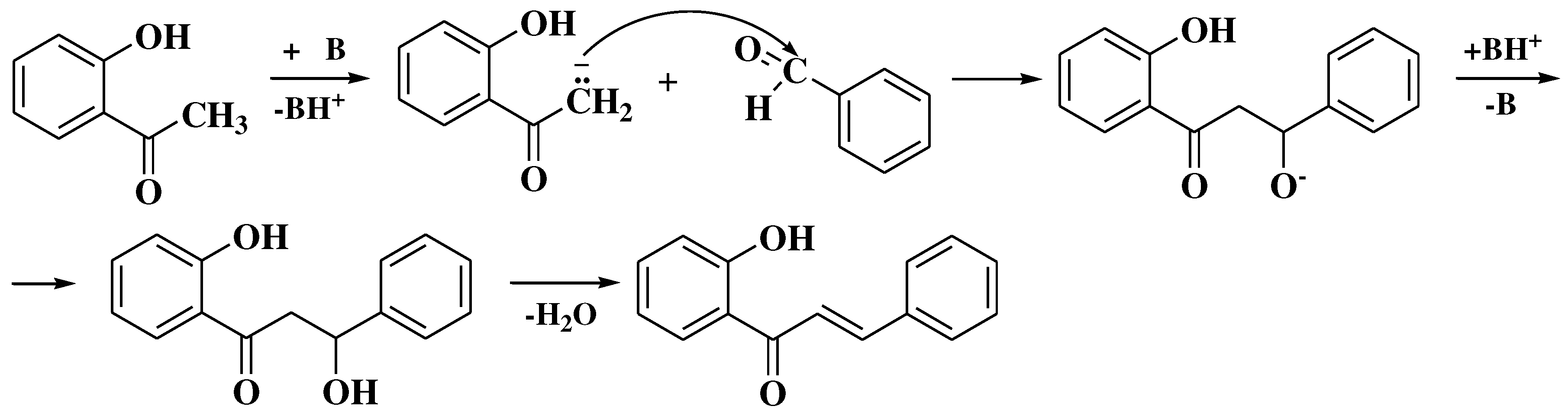
3.6. Claisen-Schmidt method
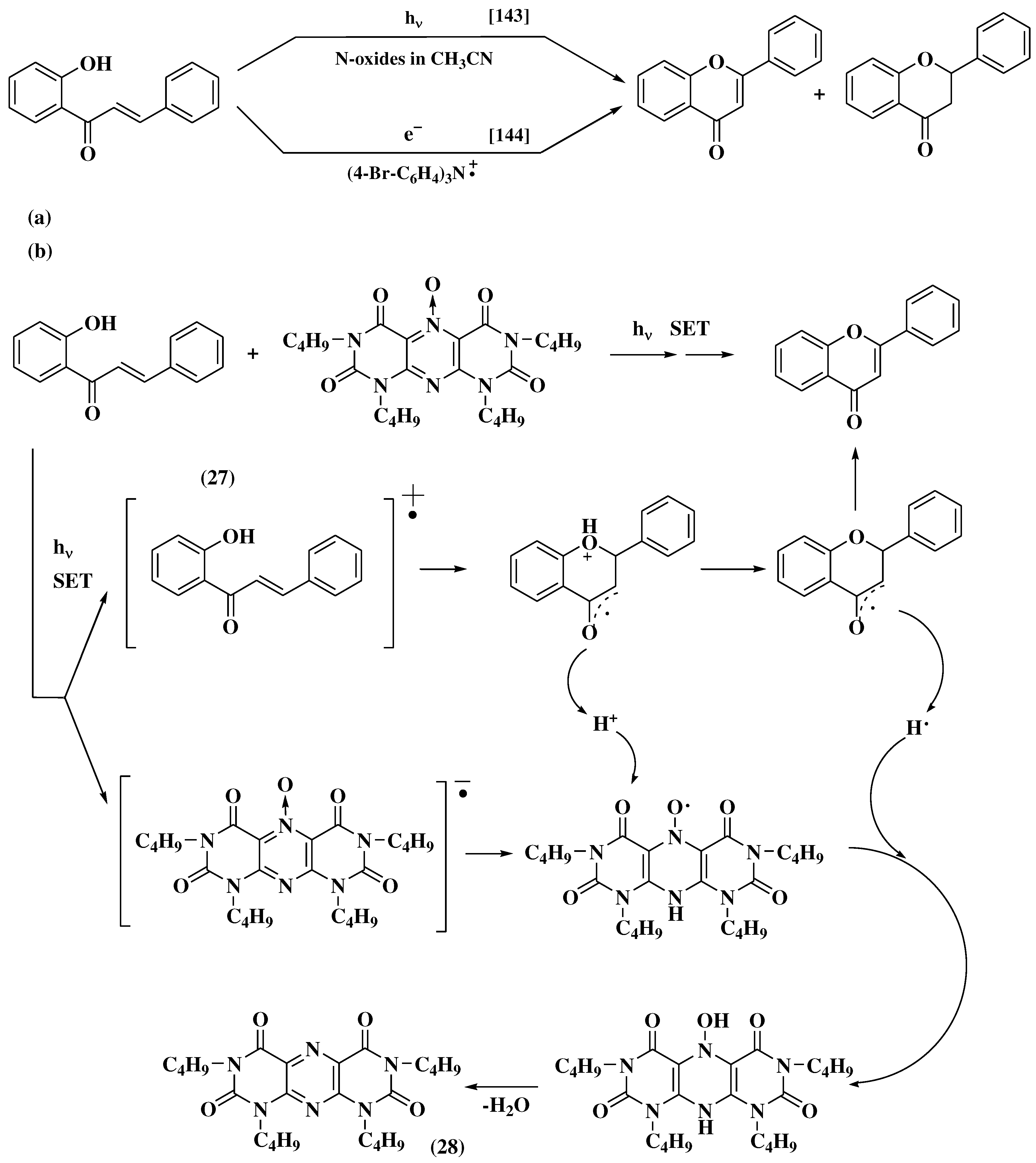
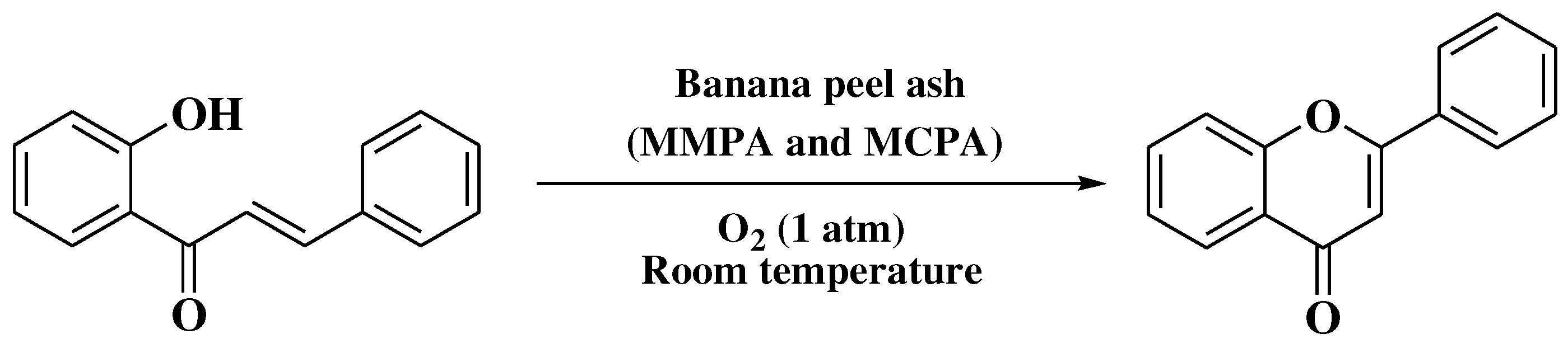
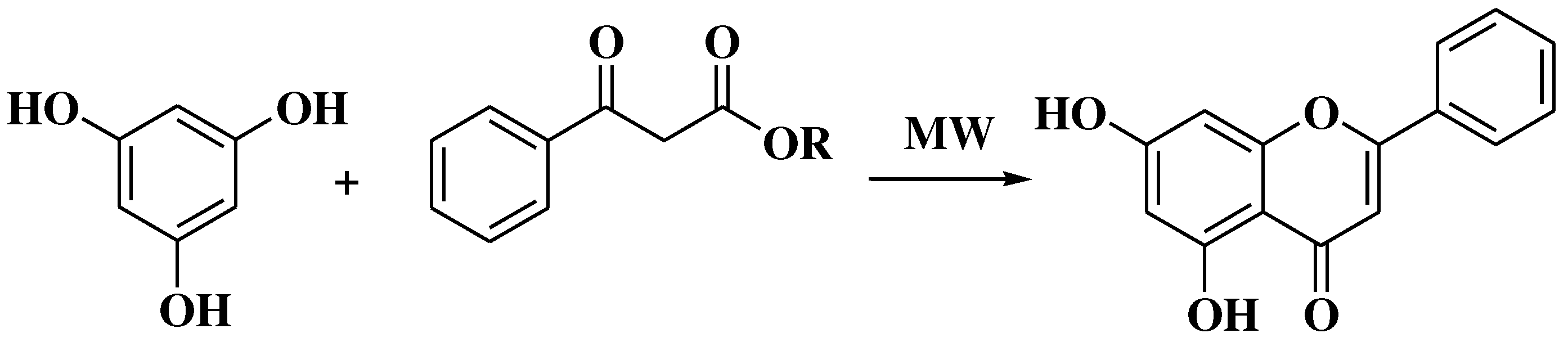
3.7. Mentzer method
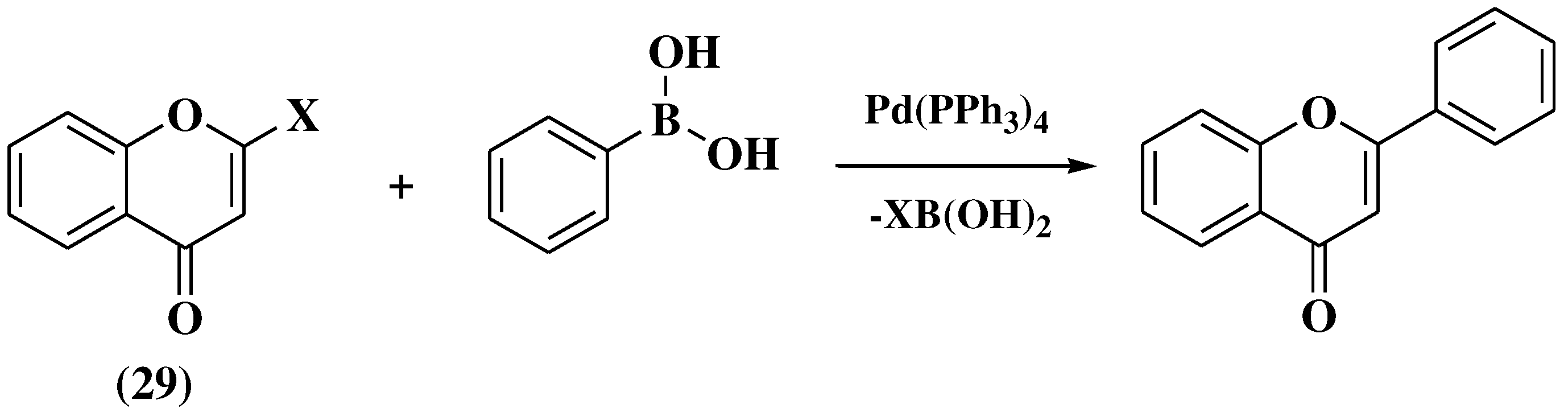
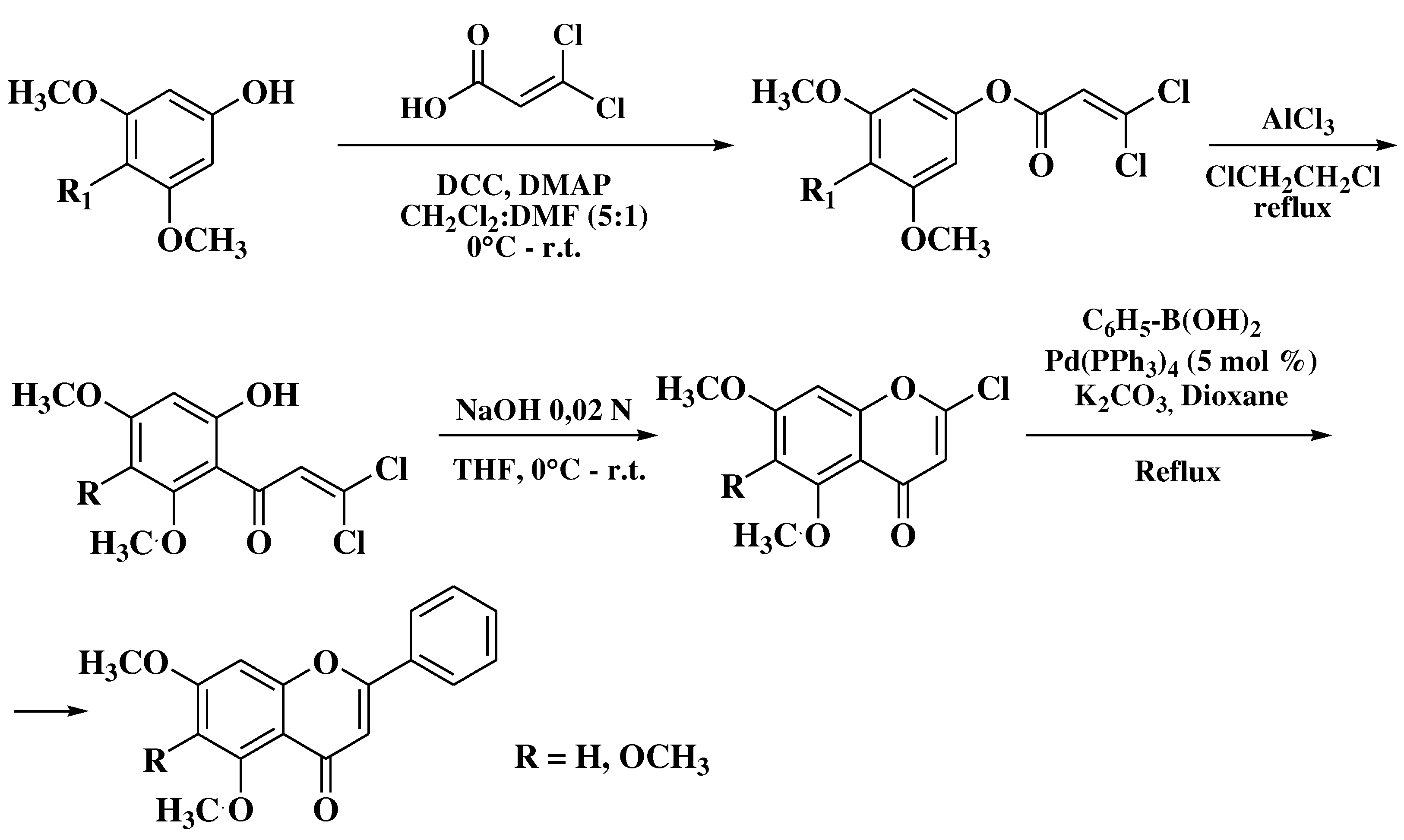
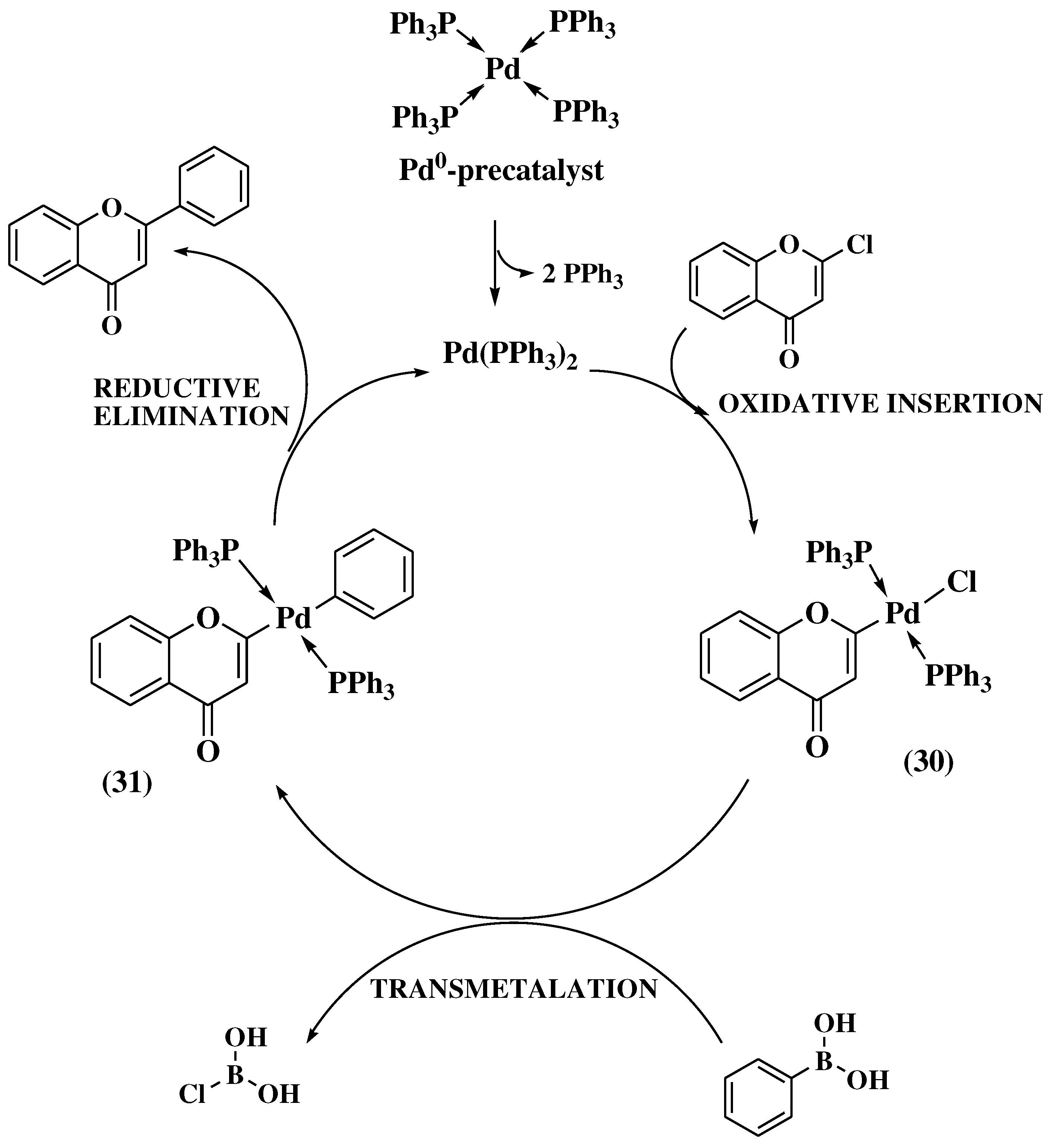
3.8. Suzuki-Miyaura method
4. Chemical synthesis of aurones
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berim, A.; Gang, D.R. Methoxylated flavones: Occurrence, importance, biosynthesis. Phytochem Rev. 2016, 15, 363–390. [Google Scholar] [CrossRef]
- Kshatriya, R.; Jejurkar, V.P.; Saha, S. In memory of Prof. Venkataraman: Recent advances in the synthetic methodologies of flavones. Tetrahedron 2018, 74, 811–833. [Google Scholar] [CrossRef]
- Tóth, S.; Szepesi, Á.; Tran-Nguyen, V.K.; Sarkadi, B.; Német, K.; Falson, P.; et al. Synthesis and anticancer cytotoxicity of azaaurones overcoming multidrug resistance. Molecules 2020, 25, 764. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.X.; Kumamoto, T. Flavonoids as protein kinase inhibitors for cancer chemoprevention: Direct binding and molecular modeling. Antioxidants Redox Signal 2010, 13, 691–719. [Google Scholar] [CrossRef]
- Polier, G.; Ding, J.; Konkimalla, B.V.; Eick, D.; Ribeiro, N.; Köhler, R.; et al. Wogonin and related natural flavones are inhibitors of CDK9 that induce apoptosis in cancer cells by transcriptional suppression of Mcl-1. Cell Death Dis. 2011, 2, e182. [Google Scholar] [CrossRef] [PubMed]
- Golub, A.G.; Bdzhola, V.G.; Ostrynska, O.V.; Kyshenia, I.V.; Sapelkin, V.M.; Prykhod’Ko, A.O.; et al. Discovery and characterization of synthetic 4′-hydroxyflavones - New CK2 inhibitors from flavone family. Bioorganic Med Chem. 2013, 21, 6681–6689. [Google Scholar] [CrossRef]
- Chao, S.W.; Su, M.Y.; Chiou, L.C.; Chen, L.C.; Chang, C.I.; Huang, W.J. Total Synthesis of Hispidulin and the Structural Basis for Its Inhibition of Proto-oncogene Kinase Pim-1. J Nat Prod. 2015, 78, 1969–1976. [Google Scholar] [CrossRef]
- Sedlacek, H.H.; Czech, J.; Naik, R.; Kaur, G.; Worland, P.; Losiewicz, M.; et al. Flavopiridol (L86 8275; NSC 649890), a new kinase inhibitor for tumor therapy. Int J Oncol. 1996, 9, 1143–1168. [Google Scholar] [CrossRef]
- Pontes, O.; Costa, M.; Santos, F.; Sampaio-Marques, B.; Dias, T.; Ludovico, P. Exploitation of new chalcones and 4H-chromenes as agents for cancer treatment. Eur J Med Chem 2018, 157, 101–114. [Google Scholar] [CrossRef]
- Monasterio, A.; Urdaci, M.; Pinchuk, I.; Moratalla, N.; Martínez, I. Flavonoids induce apoptosis in human leukemia U937 cells through caspase-and caspase-calpain-dependent pathways. Nutr Cancer 2004, 50, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Moreira, J.; Ribeiro, D.; Silva, P.M.A.; Nazareth, N.; Monteiro, M.; Palmeira, A.; et al. New alkoxy flavone derivatives targeting caspases: Synthesis and antitumor activity evaluation. Molecules 2019, 24, 129. [Google Scholar] [CrossRef]
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu Rev Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Zhu, M.; Wang, L.; Yu, S. Anti-tumor effects and associated molecular mechanisms of myricetin. Biomed Pharmacother 2019, 120, 109506. [Google Scholar] [CrossRef]
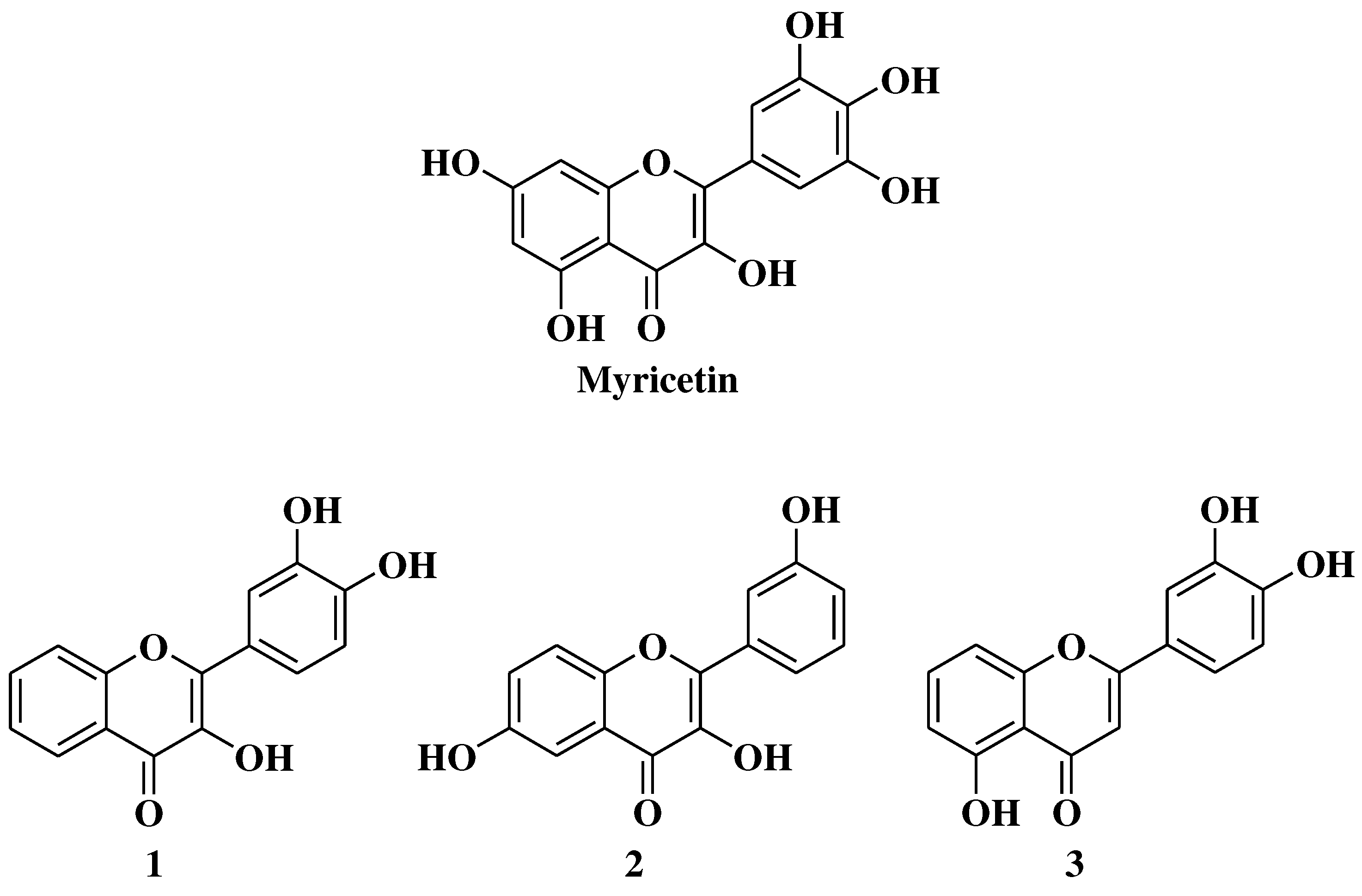
- Grigalius, I.; Petrikaite, V. Relationship between antioxidant and anticancer activity of trihydroxyflavones. Molecules 2017, 22, 2169. [Google Scholar] [CrossRef]
- Zhao, L.; Yuan, X.; Wang, J.; Feng, Y.; Ji, F.; Li, Z.; et al. A review on flavones targeting serine/threonine protein kinases for potential anticancer drugs. Bioorganic Med Chem 2019, 27, 677–685. [Google Scholar] [CrossRef]
- Chao, S.H.; Price, D.H. Flavopiridol Inactivates P-TEFb and Blocks Most RNA Polymerase II Transcription in Vivo. J Biol Chem 2001, 276, 31793–31799. [Google Scholar] [CrossRef]
- Hassan, A.H.E.; Choi, E.; Yoon, Y.M.; Lee, K.W.; Yoo, S.Y.; Cho, M.C.; et al. Natural products hybrids: 3,5,4′-Trimethoxystilbene-5,6,7-trimethoxyflavone chimeric analogs as potential cytotoxic agents against diverse human cancer cells. Eur J Med Chem 2019, 161, 559–580. [Google Scholar] [CrossRef]
- Hassan, A.H.E.; Lee, K.T.; Lee, Y.S. Flavone-based arylamides as potential anticancers: Design, synthesis and in vitro cell-based/cell-free evaluations. Eur J Med Chem 2020, 187, 111965. [Google Scholar] [CrossRef]
- Li, X.; Zhang, C.; Guo, S.; Rajaram, P.; Lee, M.; Chen, G.; et al. Structure-activity relationship and pharmacokinetic studies of 3-O-substitutedflavonols as anti-prostate cancer agents. Eur J Med Chem 2018, 157, 978–993. [Google Scholar] [CrossRef] [PubMed]
- Schoepfer, J.; Fretz, H.; Chaudhuri, B.; Muller, L.; Seeber, E.; Meijer, L.; et al. Structure-based design and synthesis of 2-benzylidene-benzofuran-3-ones as flavopiridol mimics. J Med Chem 2002, 45, 1741–1747. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshani, G.; Nayak, A.; Amrutkar, S.M.; Das, S.; Guchhait, S.K.; Kundu, C.N.; et al. Scaffold-Hopping of Aurones: 2-Arylideneimidazo[1,2-a]pyridinones as Topoisomerase IIα-Inhibiting Anticancer Agents. ACS Med Chem Lett 2016, 7, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- French, K.J.; Schrecengost, R.S.; Lee, B.D.; Zhuang, Y.; Smith, S.N.; Eberly, J.L.; et al. Discovery and evaluation of inhibitors of human sphingosine kinase. Cancer Res. 2003, 63, 5962–5969. [Google Scholar] [PubMed]
- Lawrence, N.J.; Rennison, D.; McGown, A.T.; Hadfield, J.A. The total synthesis of an aurone isolated from Uvaria hamiltonii: Aurones and flavones as anticancer agents. Bioorganic Med Chem Lett 2003, 13, 3759–3763. [Google Scholar] [CrossRef] [PubMed]
- Detsi, A.; Majdalani, M.; Kontogiorgis, C.A.; Hadjipavlou-Litina, D.; Kefalas, P. Natural and synthetic 2′-hydroxy-chalcones and aurones: Synthesis, characterization and evaluation of the antioxidant and soybean lipoxygenase inhibitory activity. Bioorganic Med Chem 2009, 17, 8073–8085. [Google Scholar] [CrossRef] [PubMed]
- Hadjeri, M.; Barbier, M.; Ronot, X.; Mariotte, A.M.; Boumendjel, A.; Boutonnat, J. Modulation of P-glycoprotein-mediated multidrug resistance by flavonoid derivatives and analogues. J Med Chem 2003, 46, 2125–2131. [Google Scholar] [CrossRef]
- Sim, H.M.; Wu, C.P.; Ambudkar, S.V.; Go, M.L. In vitro and in vivo modulation of ABCG2 by functionalized aurones and structurally related analogs. Biochem Pharmacol 2011, 82, 1562–1571. [Google Scholar] [CrossRef]
- Coman, F.M.; Mbaveng, A.T.; Marc, G.; Leonte, D.; Brém, B.; Vlase, L.; et al. Heterocycles 47. Synthesis, Characterization and Biological Evaluation of some New Thiazole Aurones as Antiproliferative Agents. Farmacia 2020, 68, 492–506. [Google Scholar] [CrossRef]
- Semenov, I.; Akyuz, C.; Roginskaya, V.; Chauhan, D.; Corey, S.J. Growth inhibition and apoptosis of myeloma cells by the CDK inhibitor flavopiridol. Leuk Res. 2002, 26, 271–280. [Google Scholar] [CrossRef]
- Jeon, K.H.; Park, S.; Shin, J.H.; Jung, A.R.; Hwang, S.Y.; Seo, S.H.; et al. Synthesis and evaluation of 7-(3-aminopropyloxy)-substituted flavone analogue as a topoisomerase IIα catalytic inhibitor and its sensitizing effect to enzalutamide in castration-resistant prostate cancer cells. Eur J Med Chem 2023, 246, 114999. [Google Scholar] [CrossRef]
- Su, L.; Li, W.; Liu, K.; Wang, Q. Synthesis and anti-proliferative activities of 5,6,7-trimethoxyflavones and their derivatives. Nat Prod Res. 2022, 36, 4070–4075. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, J.; Li, K.; Luo, J.; Yang, S.; Song, J.R.; et al. Synthesis of flavone derivatives via N-amination and evaluation of their anticancer activities. Molecules 2019, 24, 2723. [Google Scholar] [CrossRef]
- Elhadi, A.A.; Osman, H.; Iqbal, M.A.; Rajeswari, S.K.; Ahamed, M.B.K.; Abdul Majid, A.M.S.; et al. Synthesis and structural elucidation of two new series of aurone derivatives as potent inhibitors against the proliferation of human cancer cells. Med Chem Res. 2015, 24, 3504–3515. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, L.; Liu, Y.; Chen, S.; Cheng, H.; Lu, X.; et al. Design, synthesis and discovery of 5-hydroxyaurone derivatives as growth inhibitors against HUVEC and some cancer cell lines. Eur J Med Chem. 2010, 45, 5950–5957. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wang, H.; Liu, Y.M.; Yao, X.; Tong, M.; Wang, Y.H.; et al. Synthesis, Characterization, and Anticancer Effect of Trifluoromethylated Aurone Derivatives. J Heterocycl Chem. 2014, 6, 1098–1107. [Google Scholar] [CrossRef]
- Uesawa, Y.; Sakagami, H.; Ikezoe, N.; Takao, K.; Kagaya, H.; Sugita, Y. Quantitative structure-cytotoxicity relationship of aurones. Anticancer Res. 2017, 37, 6169–6176. [Google Scholar] [CrossRef]
- Demirayak, S.; Yurttas, L.; Gundogdu-Karaburun, N.; Karaburun, A.C.; Kayagil, I. Synthesis and anti-cancer activity evaluation of new aurone derivatives. J Enzyme Inhib Med Chem. 2015, 30, 816–825. [Google Scholar] [CrossRef]
- Lathwal, E.; Kumar, S.; Kumar Sahoo, P.; Ghosh, S.; Mahata, S.; Nasare, V.D.; et al. Synthesis, cytotoxic evaluation and structure activity relationship of pyrazole hybrid aurones on gastric cancer (AGS) cell lines. Results Chem. 2022, 4, 100590. [Google Scholar] [CrossRef]
- Ashok, D.; Kifah, M.A.; Lakshmi, B.V.; Sarasija, M.; Adam, S. Microwave-assisted one-pot synthesis of some new flavonols by modified Algar–Flynn–Oyamada reaction and their antimicrobial activity. Chem Heterocycl Compd. 2016, 52, 172–176. [Google Scholar] [CrossRef]
- Lv, X.H.; Liu, H.; Ren, Z.L.; Wang, W.; Tang, F.; Cao, H.Q. Design, synthesis and biological evaluation of novel flavone Mannich base derivatives as potential antibacterial agents. Mol Divers. 2019, 23, 299–306. [Google Scholar] [CrossRef]
- Khdera, H.A.; Saad, S.Y.; Moustapha, A.; Kandil, F. Synthesis of new flavonoid derivatives based on 3-hydroxy-4′-dimethylamino flavone and study the activity of some of them as antifungal. Heliyon 2022, 8. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Lathwal, E.; Saroha, B.; Kumar, S.; Kumar, S.; Chauhan, N.S.; et al. Synthesis and Biological Evaluation of Quinoline-Based Novel Aurones. ChemistrySelect 2020, 5, 3539–3543. [Google Scholar] [CrossRef]
- Fujimoto, K.J.; Nema, D.; Ninomiya, M.; Koketsu, M.; Sadanari, H.; Takemoto, M.; et al. An in silico-designed flavone derivative, 6-fluoro-4′-hydroxy-3′,5′-dimetoxyflavone, has a greater anti-human cytomegalovirus effect than ganciclovir in infected cells. Antiviral Res. 2018, 154, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Badavath, V.N.; Jadav, S.; Pastorino, B.; de Lamballerie, X.; Sinha, N.B.; Jayaprakash, V. Synthesis and Antiviral Activity of 2-aryl-4H-chromen-4-one Derivatives Against Chikungunya Virus. Lett Drug Des Discov. 2016, 13, 1019–1024. [Google Scholar] [CrossRef]
- Zemplén, G.; Bognár, R. Umwandlung des Hesperetins in Diosmetin, des Hesperidins in Diosmin und des Isosakuranetins in Acacetin. Berichte der Dtsch Chem Gesellschaft 1943, 76, 452–457. [Google Scholar] [CrossRef]
- Kostanecki, S.V.; Levi, R.; Tambor, J. Synthese des 2-Oxyflavons. Berichte der Dtsch Chem Gesellschaft. 1899, 32, 326–332. [Google Scholar] [CrossRef]
- Donnelly, J.A.; Acton, J.P.; Donnelly, D.J.; Philbin, E.M. Steric and Electronic Effects in the Emilewiczvon Kostanecki Cyclization of Chalcone Dihalides. Proc R Ir Acad B. 1983, 83B, 49–56. [Google Scholar]
- Bate-Smith, E.C.; Geissman, T.A. Benzalcoumaranones. Nature 1951, 167, 688–688. [Google Scholar] [CrossRef]
- Narasimhachari, N.; Seshadri, T.R. A new synthesis of flavones. Proc Indian Acad Sci - Sect A. 1949, 30, 151–162. [Google Scholar] [CrossRef]
- Donnelly, D.J.; Donnelly, J.A.; Murphy, J.J.; Philbin, E.M.; Wheeler, T.S. Steric effects on the cyclisation of chalcone dibromides. Chem Commun. 1966, 12, 351–352. [Google Scholar] [CrossRef]
- Hutchins, W.A.; Wheeler, T.S. 17. Chalkones: A new synthesis of chrysin, apigenin, and luteolin. J Chem Soc. 1939, 83, 91. [Google Scholar] [CrossRef]
- Fitzgerald, D.M.; O’Sullivan, J.F.; Philbin, E.M.; Wheeler, T.S. Ring expansion of 2-benzylidenecoumaran-3-ones—A synthesis of flavones. J Chem Soc. 1955, 860, 860–862. [Google Scholar] [CrossRef]
- Donnelly, J.A.; Doran, H.J. Chalcone dihalides-VII. The course of the cyclization of 2′-hydroxy-6′-methoxyl derivatives. Tetrahedron 1975, 31, 1565–1569. [Google Scholar] [CrossRef]
- Donnelly, J.A.; Doran, H.J. Chalcone dihalides-VI. Generalisation of their use in the synthesis of naturally occurring flavones. Tetrahedron Lett. 1974, 15, 4083–4084. [Google Scholar] [CrossRef]
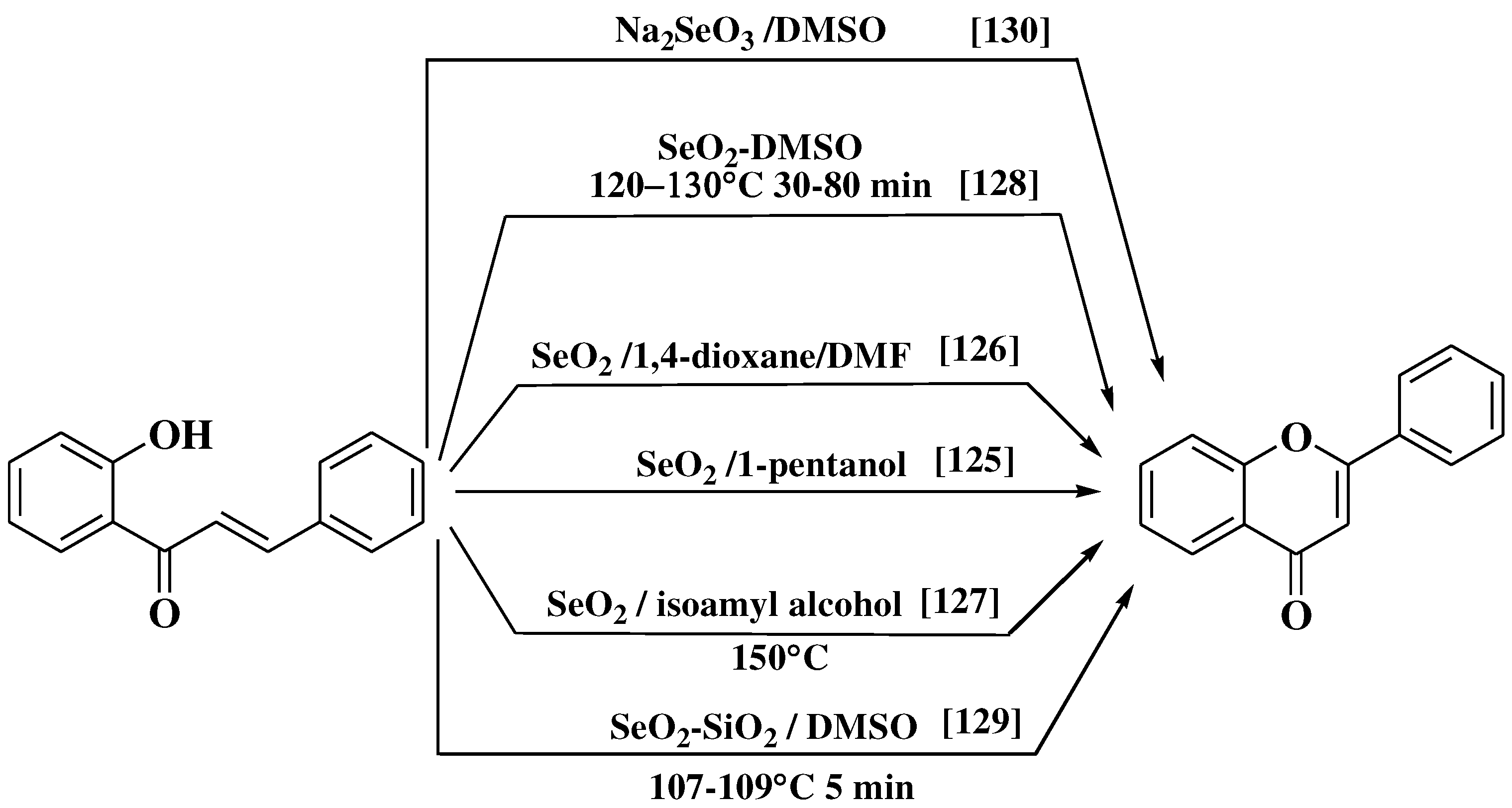
- Mahal, H.S.; Rai, H.S.; Venkataraman, K. Synthetical experiments in the chromone group. Part XVI. Chalkones and flavanones and their oxidation to flavones by means of selenium dioxide. J Chem Soc. 1935, 866, 866–868. [Google Scholar] [CrossRef]
- Kostanecki, V.S.; Szabranski, W. Synthese des Flavonols. Berichte der Dtsch Chem Gesellschaft 1904, 37, 2819–2820. [Google Scholar] [CrossRef]
- Auwers, K.; Müller, K. Umwandlung von Benzal-cumaranonen in Flavonole. Berichte der Dtsch Chem Gesellschaft 1908, 41, 4233–4241. [Google Scholar] [CrossRef]
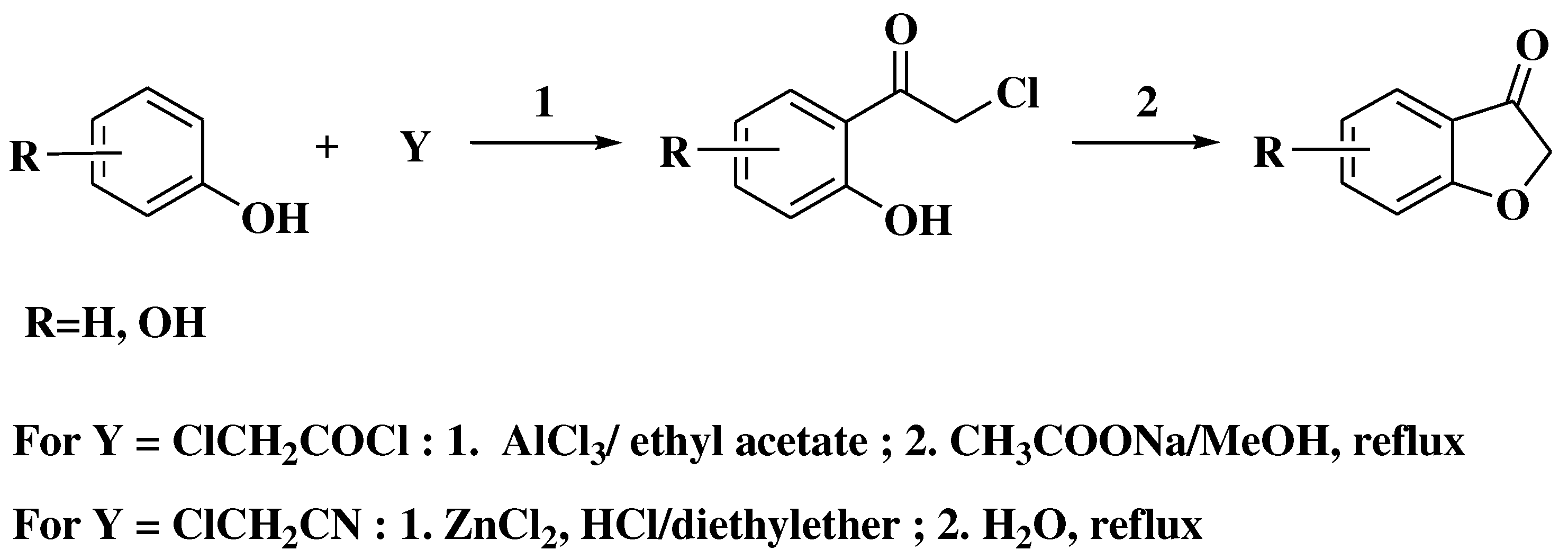
- Stoermer, R.; Bartsch, F. Synthesen des Cumaranons (Ketocumarans) und seiner Homologen aus Phenoxyessigsäuren. Berichte der Dtsch Chem Gesellschaft 1900, 33, 3175–3181. [Google Scholar] [CrossRef]
- Auwers, K.V.; Pohl, P. Über die Umwandlung von Benzalcumaranonen in Flavonole. Justus Liebig’s Ann der Chemie 1914, 405, 243–294. [Google Scholar] [CrossRef]
- v. Auwers, K. Zur Bildung von Flavonolen aus Benzal-cumaranonen. Berichte der Dtsch Chem Gesellschaft 1916, 49, 809–819. [Google Scholar] [CrossRef]
- Allan, J.; Robinson, R. CCXC.—An accessible derivative of chromonol. J Chem Soc 1924, 125, 2192–2195. [Google Scholar] [CrossRef]
- Wheeler, T.S. Flavone. Org Synth. 1952, 32, 72. [Google Scholar]
- Allan, J.; Robinson, R. CCCIX.—A new synthesis of fisetin and of quercetin. J Chem Soc. 1926, 129, 2334–2336. [Google Scholar] [CrossRef]
- Kalff, J.; Robinson, R. CCLXIV.—A synthesis of datiscetin. J Chem Soc. Trans. 1925, 127, 1968–1973. [Google Scholar] [CrossRef]
- Kalff, J.; Robinson, R. XXVIII.—A synthesis of myricetin and of a galangin monomethyl ether occurring in galanga root. J Chem Soc. Trans. 1925, 127, 181–184. [Google Scholar] [CrossRef]
- Dreyer, D.L.; Tabata, S.; Horowitz, R.M. Flavonoids of citrus—VIII. Tetrahedron 1964, 20, 2977–2983. [Google Scholar] [CrossRef]
- Robinson, R.; Shinoda, J. CCLXV.—The synthesis of certain 2-styrylchromonol derivatives. J Chem Soc. Trans. 1925, 127, 1973–1980. [Google Scholar] [CrossRef]
- Fukui, K.; Nakayama, M.; Horie, T. Synthetic Studies of the Flavone Derivatives. IX. The Syntheses of Axillarin and Its Related Compounds. Bull Chem Soc Jpn. 1969, 42, 1649–1652. [Google Scholar] [CrossRef]
- Fukui, K.; Matsumoto, T.; Nakamura, S.; Nakayama, M.; Horie, T. Synthetic Studies of the Flavone Derivatives. VII. The Synthesis of Jaceidin. Bull Chem Soc Jpn. 1968, 41, 1413–1417. [Google Scholar] [CrossRef]
- Baker, W. Molecular Rearrangement of Some o-Acyloxyacetophenones. J Chem Soc. 1933, (I), 1381–1389. [Google Scholar] [CrossRef]
- Mahal, H.S.; Venkataraman, K. A Synthesis of Flavones at Room Temperature. Curr Sci. 1933, 2, 214–215. [Google Scholar]
- Chadha, T.C.; Venkataraman, K. 256. Synthetical experiments in the chromone group. Part VIII. Derivatives of o-hydroxy-, 2:5-dihydroxy-, and 2:4:5-trihydroxy-acetophenone. J Chem Soc. 1933, 1073. [Google Scholar] [CrossRef]
- Mahal, H.S.; Venkataraman, K. 387. Synthetical experiments in the chromone group. Part XIV. The action of sodamide on 1-acyloxy-2-acetonaphthones. J Chem Soc. 1934, (I), 1767. [Google Scholar] [CrossRef]
- Ameen, D.; Snape, T.J. Mechanism and application of Baker-Venkataraman O→C acyl migration reactions. Synth. 2015, 47, 141–158. [Google Scholar] [CrossRef]
- Cramer, F.; Elschnig, G.H. Über Einschluß-verbindungen, IX. Mitteil.: Die blauen Jodverbindungen der Flavone. Chem Ber. 1956, 89, 1–12. [Google Scholar] [CrossRef]
- Ares, J.J.; Outt, P.E.; Kakodkar, S.V.; Buss, R.C.; Geiger, J.C. A Convenient Large-Scale Synthesis of 5-Methoxyflavone and Its Application to Analog Preparation. J Org Chem. 1993, 58, 7903–7905. [Google Scholar] [CrossRef]
- Jain, P.K.; Makrandi, J.K.; Grover, S.K. A Facile Baker-Venkataraman Synthesis of Flavones using Phase Transfer Catalysis. Synthesis (Stuttg). 1982, 1982, 221–222. [Google Scholar] [CrossRef]
- Saxena, S.; Makrandi, J.; Grover, S. Synthesis of 5- and/or 7-Hydroxyflavones using A Modified Phase Transfer-Catalysed Baker-Venkataraman Transformation. Synthesis (Stuttg). 1985, 1985, 697–697. [Google Scholar] [CrossRef]
- Song, G.-Y.; Ahn, B.-Z. Synthesis of dibenzoylmethanes as intermediates for flavone synthesis by a modified Baker-Venkataraman rearrangement. Arch Pharm Res. 1994, 17, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Stanek, F.; Stodulski, M. Mild and efficient organocatalytic method for the synthesis of flavones. Tetrahedron Lett. 2016, 57, 3841–3843. [Google Scholar] [CrossRef]
- Pinto, D.C.G.A.; Silva, A.M.S.; Cavaleiro, J.A.S. Baker-Venkataraman rearrangement under microwave irradiation: A new strategy for the synthesis of 3-aroyl-5-hydroxyflavones. Synlett. 2007, 12, 1897–1900. [Google Scholar] [CrossRef]
- Sarda, S.R.; Pathan, M.Y.; Paike, V.V.; Pachmase, P.R.; Jadhav, W.N.; Pawar, R.P. A facile synthesis of flavones using recyclable ionic liquid under microwave irradiation. Arkivoc 2006, 2006, 43–48. [Google Scholar] [CrossRef]
- Miyake, H.; Nishino, S.; Nishimura, A.; Sasaki, M. New synthesis of 3-bromoflavones via bromination of 1-(2-hydroxyphenyl)-3- arylpropane-1,3-dione by CuBr2, and conversion into 3-aminoflavones. Chem Lett. 2007, 36, 522–523. [Google Scholar] [CrossRef]
- Zubaidha, P.K.; Hashmi, A.M.; Bhosale, R.S. FeCl3 catalyzed dehydrative cyclisation of 1, 3 - (diaryl diketones) to flavones. Heterocycl Commun. 2005, 11, 97–100. [Google Scholar] [CrossRef]
- Kabalka, G.W.; Mereddy, A.R. Microwave-assisted synthesis of functionalized flavones and chromones. Tetrahedron Lett. 2005, 46, 6315–6317. [Google Scholar] [CrossRef]
- Nishinaga, A.; Ando, H.; Maruyama, K.; Mashino, T. A New Metal Complex Promoted System for Highly Selective Synthesis of 4 H -Chromen-4-ones (Chromones). Synthesis (Stuttg). 1992, 1992, 839–841. [Google Scholar] [CrossRef]
- Varma, R.S.; Saini, R.K.; Kumar, D. An Expeditious Synthesis of Flavones on Montmorillonite K 10 Clay with Microwaves. J Chem Res - Part S. 1998, 6, 348–349. [Google Scholar] [CrossRef]
- Hoshino, Y.; Takeno, N. A Facile Preparation of Flavones Using Nonaqueous Cation-Exchange Resin. Vol. 60. Bulletin of the Chemical Society of Japan 1987, 1919–1920. [Google Scholar] [CrossRef]
- Pérez, M.E.; Ruiz, D.M.; Autino, J.C.; Blanco, M.N.; Pizzio, L.R.; Romanelli, G.P. Mesoporous titania/tungstophosphoric acid composites: Suitable synthesis of flavones. J Porous Mater. 2013, 20, 1433–1440. [Google Scholar] [CrossRef]
- Bennardi, D.O.; Romanelli, G.P.; Autino, J.C.; Pizzio, L.R. Supported trifluoromethanesulfonic acid as catalyst in the synthesis of flavone and chromone derivatives. Appl Catal A Gen. 2007, 324, 62–68. [Google Scholar] [CrossRef]
- Jorge, L.; Autino, C. Synthesis of substituted flavones and chromones using a Wells-Dawson heteropolyacid as catalyst. Arkivoc 2008, 11, 123. [Google Scholar]
- Bennardi, D.O.; Romanelli, G.P.; Jios, J.L.; Vázquez, P.G.; Cáceres, C.V.; Autino, C. Synthesis of substituted flavones and arylchromones using ρ and si keggin heteropolyacids as catalysts. Heterocycl Commun. 2007, 13. [Google Scholar] [CrossRef]
- Kucukislamoglu, M.; Nebioglu, M.; Zengin, M.; Arslan, M.; Yayli, N. An environmentally benign synthesis of flavones from 1,3-diketones using silica gel supported NaHSO4 catalyst. J Chem Res. 2005, 9, 556–557. [Google Scholar] [CrossRef]
- Oyamada, T. A new general method for the synthesis of the derivates of flavonol. Bull Chem Soc Jpn. 1935, 10, 182–186. [Google Scholar] [CrossRef]
- Algar, J.; Flynn, J. A new method for the synthesis of flavonols. Proc R Irish Acad Sect B Biol Geol Chem Sci. 1934, 42, 1–8. [Google Scholar]
- Serdiuk, I.E.; Roshal, A.D.; Błażejowski, J. Quantum-chemical analysis of the Algar-Flynn-Oyamada reaction mechanism. Chem Heterocycl Compd. 2014, 50, 396–403. [Google Scholar] [CrossRef]
- Dean, F.M.; Podimuang, V. 737. The course of the Algar–Flynn–Oyamada (A.F.O.) reaction. J Chem Soc. 1965, 3978, 3978–3987. [Google Scholar] [CrossRef]
- Adams, C.J.; Main, L. Synthesis of 2′-hydroxychalcone epoxides. Tetrahedron 1991, 47, 4959–4978. [Google Scholar] [CrossRef]
- Bennett, M.; Burke, A.J.; Ivo O’Sullivan, W. Aspects of the Algar-Flynn-Oyamada (AFO) reaction. Tetrahedron 1996, 52, 7163–7178. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Hatua, K. Computational insight of the mechanism of Algar-Flynn-Oyamada (AFO) reaction. RSC Adv. 2014, 4, 18702–18709. [Google Scholar] [CrossRef]
- Fougerousse, A.; Gonzalez, E.; Brouillard, R. A convenient method for synthesizing 2-aryl-3-hydroxy-4-oxo-4H-1- benzopyrans or flavonols. J Org Chem. 2000, 65, 583–586. [Google Scholar] [CrossRef]
- Cummins, B.; Donnelly, D.M.X.; Eades, J.F.; Fletcher, H.; Cinnéide, F.O.; Philbin, E.M.; et al. Oxidation of chalcones (AFO reaction). Tetrahedron 1963, 19, 499–512. [Google Scholar] [CrossRef]
- Shen, X.; Zhou, Q.; Xiong, W.; Pu, W.; Zhang, W.; Zhang, G.; et al. Synthesis of 5-subsituted flavonols via the Algar-Flynn-Oyamada (AFO) reaction: The mechanistic implication. Tetrahedron 2017, 73, 4822–4829. [Google Scholar] [CrossRef]
- Nhu, D.; Hawkins, B.C.; Burns, C.J. Phase transfer catalysis extends the scope of the Algar-Flynn-Oyamada synthesis of 3-Hydroxyflavones. Aust J Chem. 2015, 68, 1102–1107. [Google Scholar] [CrossRef]
- Jain, A.C.; Gupta, S.M.; Sharma, A. Synthesis of 2,2-Dimethyl-2 H -pyran-fused Flavonols Using the Modified Algar-Flynn-Oyamada Reaction. Bulletin of the Chemical Society of Japan 1983, 56, 1267–1268. [Google Scholar] [CrossRef]
- Dharia, J.R.; Johnson, K.F.; Schlenoff, J.B. Synthesis and Characterization of Wavelength-Shifting Monomers and Polymers Based on 3-Hydroxyflavone. Macromolecules 1994, 27, 5167–5172. [Google Scholar] [CrossRef]
- Tong, J.; Liu, C.; Wang, B. Improved Synthesis of Icaritin and Total Synthesis of β-Anhydroicaritin. Chem Res Chinese Univ. 2019, 35, 616–620. [Google Scholar] [CrossRef]
- Ashraf, J.; Mughal, E.U.; Sadiq, A.; Bibi, M.; Naeem, N.; Ali, A.; et al. Exploring 3-hydroxyflavone scaffolds as mushroom tyrosinase inhibitors: Synthesis, X-ray crystallography, antimicrobial, fluorescence behaviour, structure-activity relationship and molecular modelling studies. J Biomol Struct Dyn. 2020, 39, 7107–7122. [Google Scholar] [CrossRef] [PubMed]
- Simiti, I.; Zaharia, V.; Mager, S.; Horn, M.; Koteles-Popa, T. Heterocyclen 67. Mitt.: Darstellung und charakterisierung einiger 2-(2-aryl-thiazol-4-yl)-3-hydroxy-chromone. Arch Pharm (Weinheim). 1991, 324, 913–915. [Google Scholar] [CrossRef]
- Zaharia, V.; Imre, S.; Palibroda, N. Heterocycles. Obtaining and physico-chemical characterization of some thiazolo and thiazolo [3, 2-b][1, 2, 4] triazolic hydroxy-heterochalcones. Revista de Chimie 2009, 4, 391–397. [Google Scholar]
- Enchev, V.; Mehandzhiyski, A.Y. Computational insight on the chalcone formation mechanism by the Claisen–Schmidt reaction. Int J Quantum Chem. 2017, 117, 1–8. [Google Scholar] [CrossRef]
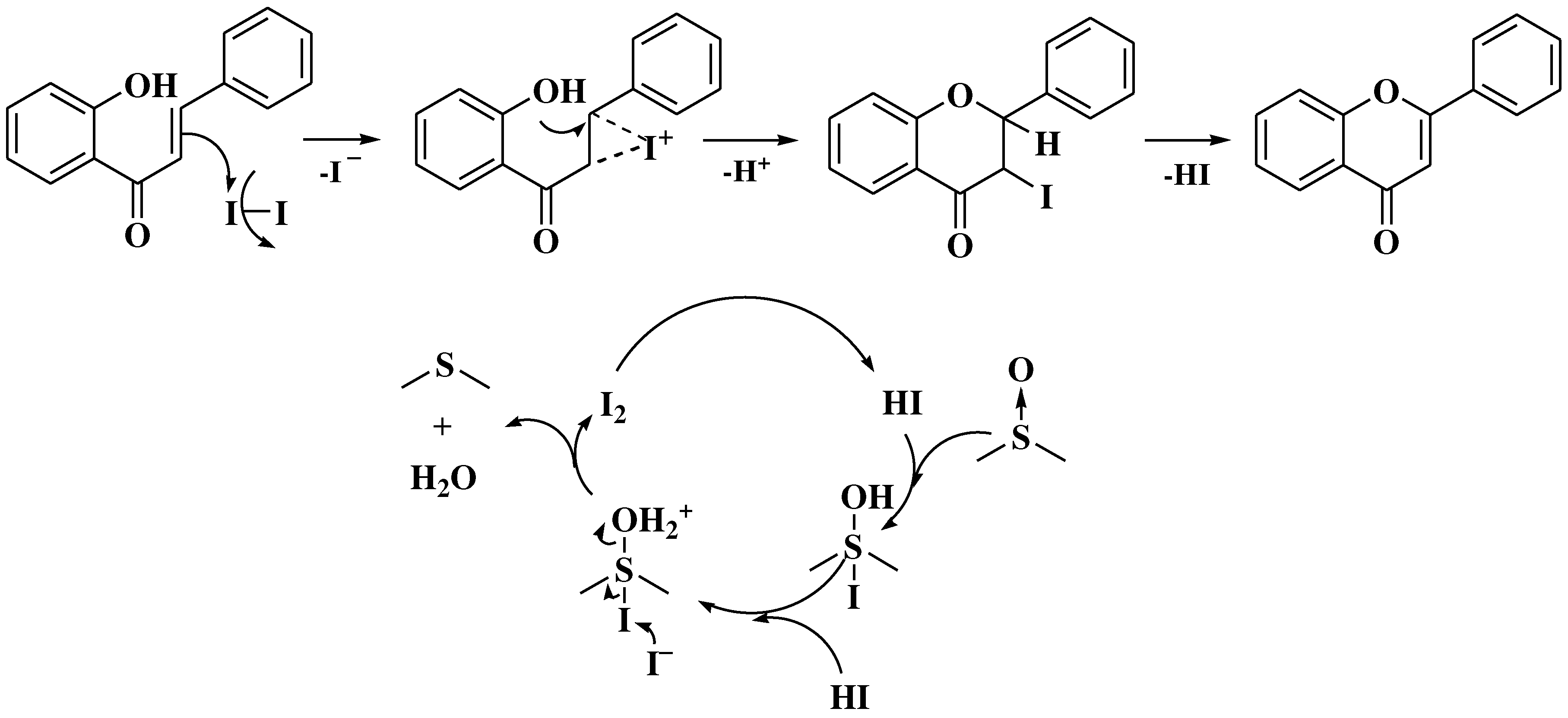
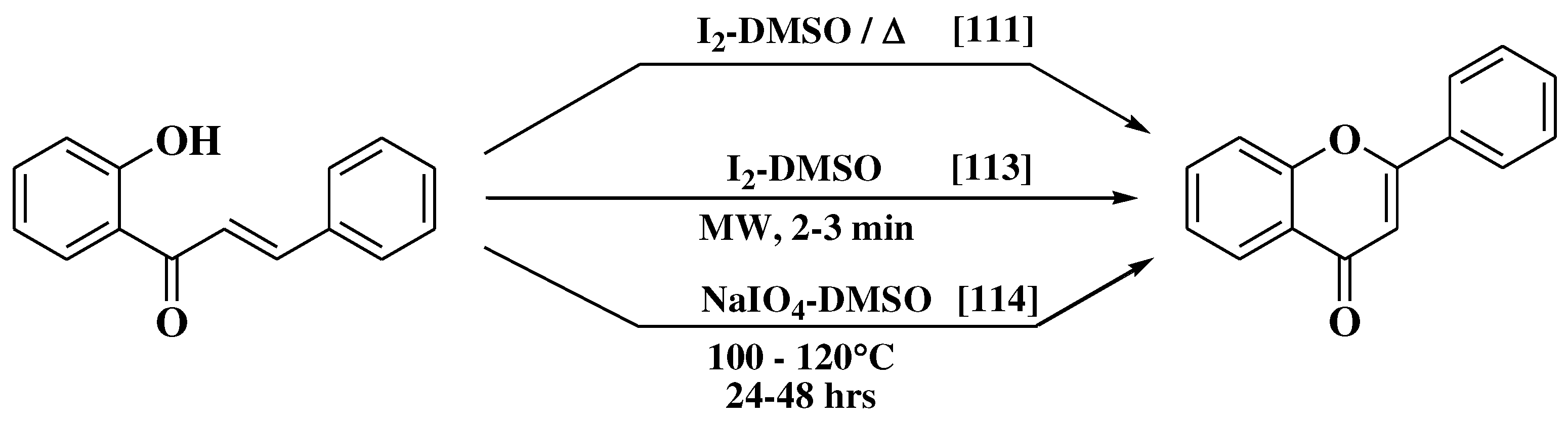
- Patonay, T.; Cavaleiro, J.A.S.; Lévai, A.; Silva, A.M.S. Dehydrogenation by iodine-dimethylsulfoxide system: A general route to susbtituted chromones and thiochromones. Heterocycl Commun. 1997, 3, 223–229. [Google Scholar] [CrossRef]
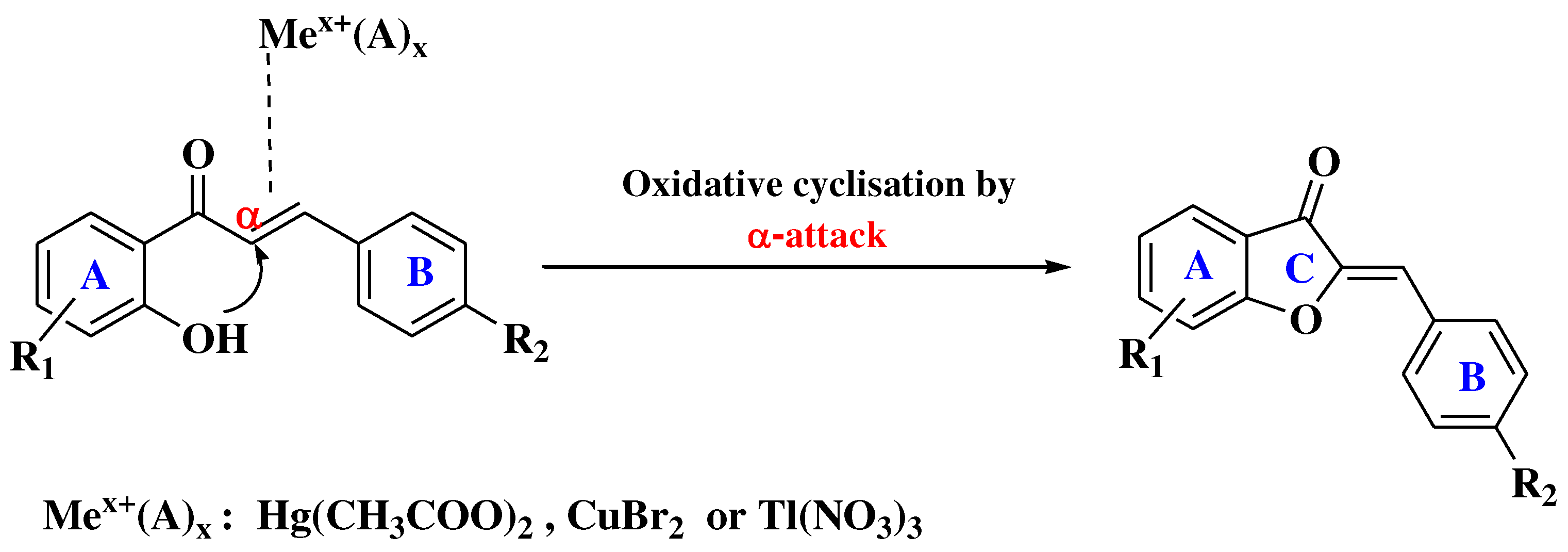
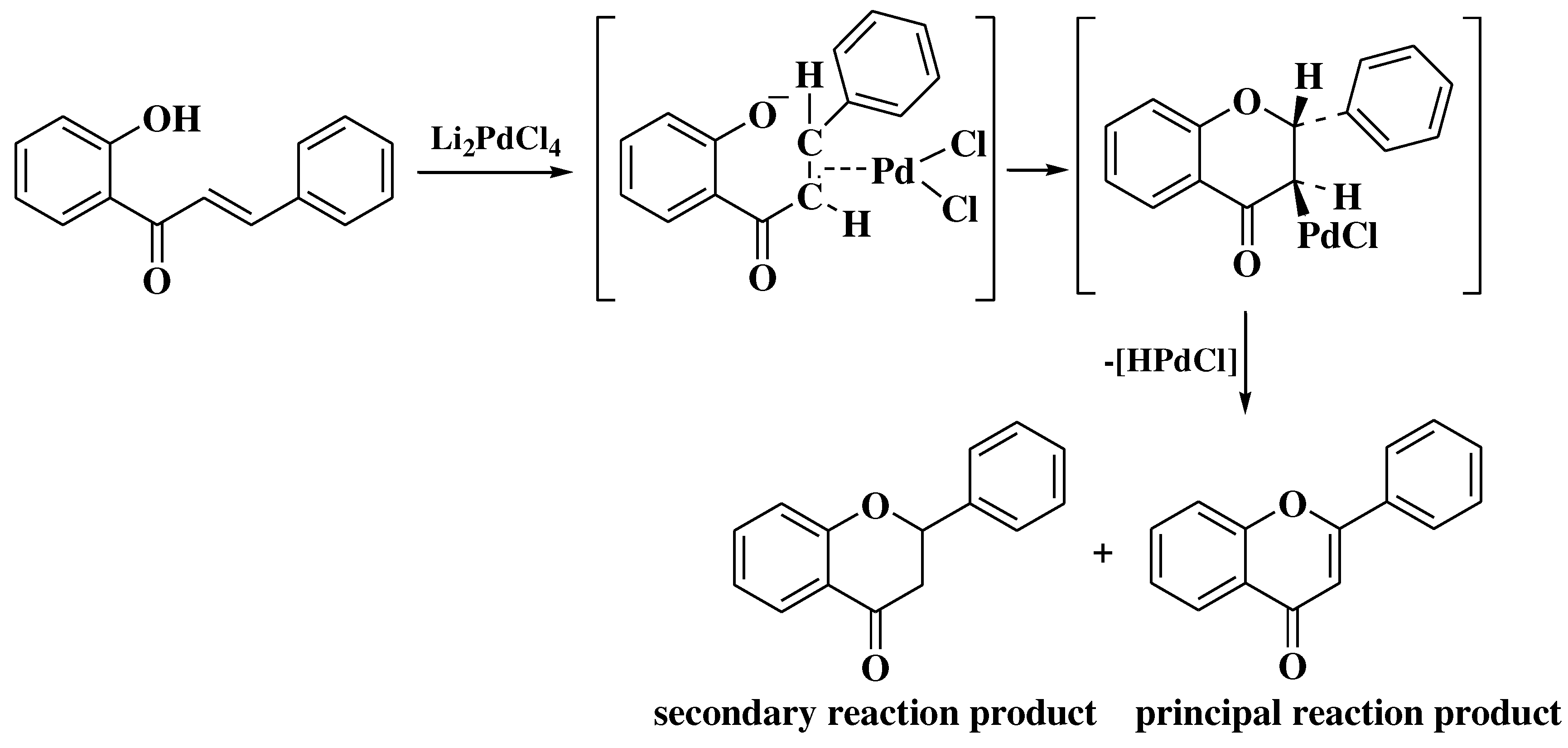
- Masesane, I.B. A comprehensive review of the oxidative cyclisation of 2’-hydroxychalcones to aurones and flavones. Int J Chem Stud. 2015, 3, 53–59. [Google Scholar]
- Menezes, M.J.; Manjrekar, S.; Pai, V.; Patre, R.E.; Tilve, S.G. A facile microwave assisted synthesis of flavones. Indian J Chem - Sect B Org Med Chem. 2009, 48, 1311–1314. [Google Scholar]
- Hans, N.; Grover, S.K. An efficient conversion of 2’-hydroxychalcones to flavones. Synth Commun. 1993, 23, 1021–1023. [Google Scholar] [CrossRef]
- Wang, Z. (Ed.) Mentzer Pyrone Synthesis. In Compr Org Name React Reagents; John Wiley & Sons, Inc.: New York, NY, USA, 2010; pp. 1901–1904. [Google Scholar]
- Constantinescu, T.; Leonte, D.; Bencze, L.C.; Vlase, L.; Imre, S.; Hanganu, D.; et al. Heterocycles 43. Synthesis, characterization and antioxidant activity of some thiazole hydroxychalcones and their flavonoidic derivatives. Farmacia 2018, 66, 663–673. [Google Scholar] [CrossRef]
- Litkei, G.; Gulácsi, K.; Antus, S.; Blaskó, G. Cyclodehydrogenation of 2′-hydroxychalcones with hypervalent iodine reagent: A new synthesis of flavones. Liebigs Ann. 1995, 1995, 1711–1715. [Google Scholar] [CrossRef]
- Gulácsi, K.; Litkei, G.; Antus, S.; Gunda, T.E. A short and facile synthetic route to prenylated flavones. Cyclodehydrogenation of prenylated 2’-hydroxychalcones by a hypervalent iodine reagent. Tetrahedron 1998, 54, 13867–13876. [Google Scholar] [CrossRef]
- Du, Z.; Ng, H.; Zhang, K.; Zeng, H.; Wang, J. Ionic liquid mediated Cu-catalyzed cascade oxa-Michael-oxidation: Efficient synthesis of flavones under mild reaction conditions. Org Biomol Chem 2011, 9, 6930–6933. [Google Scholar] [CrossRef] [PubMed]
- Lahyani, A.; Trabelsi, M. Ultrasonic-assisted synthesis of flavones by oxidative cyclization of 2′-hydroxychalcones using iodine monochloride. Ultrason Sonochem. 2016, 31, 626–630. [Google Scholar] [CrossRef]
- Miyake, H.; Takizawa, E.; Sasaki, M. Syntheses of flavones via the iodine-mediated oxidative cyclization of 1,3-diphenylprop-2-en-1-ones. Bull Chem Soc Jpn 2003, 76, 835–836. [Google Scholar] [CrossRef]
- Rajesh Babu, K.; Vijaya Kumar, K.; Vijaya, M.; Madhavarao, V. A novel solid supported synthesis of flavones. Int J Pharm Technol 2012, 4, 3943–3950. [Google Scholar]
- Sarda, S.R.; Jadhav, W.N.; Pawar, R.P. I2-Al2O3: A suitable heterogeneous catalyst for the synthesis of flavones under microwave irradiation. Int J ChemTech Res 2009, 1, 539–543. [Google Scholar]
- Kulkarni, P.S.; Kondhare, D.D.; Varala, R.; Zubaidha, P.K. Cyclization of 2’-hydroxychalcones to flavones using ammonium iodide as an iodine source - An eco-friendly approach. J Serbian Chem Soc 2013, 78, 909–916. [Google Scholar] [CrossRef]
- Lee, H.H.; Tan, C.H. 496. Syntheses of flavones from Lindera lucida. J Chem Soc 1965, 2743. [Google Scholar] [CrossRef]
- Zwaagstra, M.E.; Timmerman, H.; Van de Stolpe, A.C.; De Kanter, F.J.J.; Tamura, M.; Wada, Y.; et al. Synthesis and structure - Activity relationships of carboxyflavones as structurally rigid CysLT1 (LTD4) receptor antagonists. J Med Chem 1998, 41, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- Akama, T.; Shida, Y.; Sugaya, T.; Ishida, H.; Gomi, K.; Kasai, M. Novel 5-aminoflavone derivatives as specific antitumor agents in breast cancer. J Med Chem 1996, 39, 3461–3469. [Google Scholar] [CrossRef]
- Makrandi, J.K.; Seema, S. ChemInform Abstract: An Efficient Procedure for Cyclization of 2′-Hydroxychalcones into Flavones. ChemInform 1990, 21, 181. [Google Scholar] [CrossRef]
- Gupta, M.; Paul, S.; Gupta, R.; Loupy, A. A rapid method for the cyclization of 2′-hydroxychalcones into flavones. Org Prep Proced Int 2000, 32, 280–283. [Google Scholar] [CrossRef]
- Lamba, M.; Makrandi, J.K. Sodium selenite-dimethylsulfoxide: A highly efficient reagent for dehydrogenation. J Chem Res 2008, 4, 225–226. [Google Scholar] [CrossRef]
- Kasahara, A.; Izumi, T.; Ooshima, M. A New Method of Preparing Flavones. Bull Chem Soc Jpn 1974, 47, 2526–2528. [Google Scholar] [CrossRef]
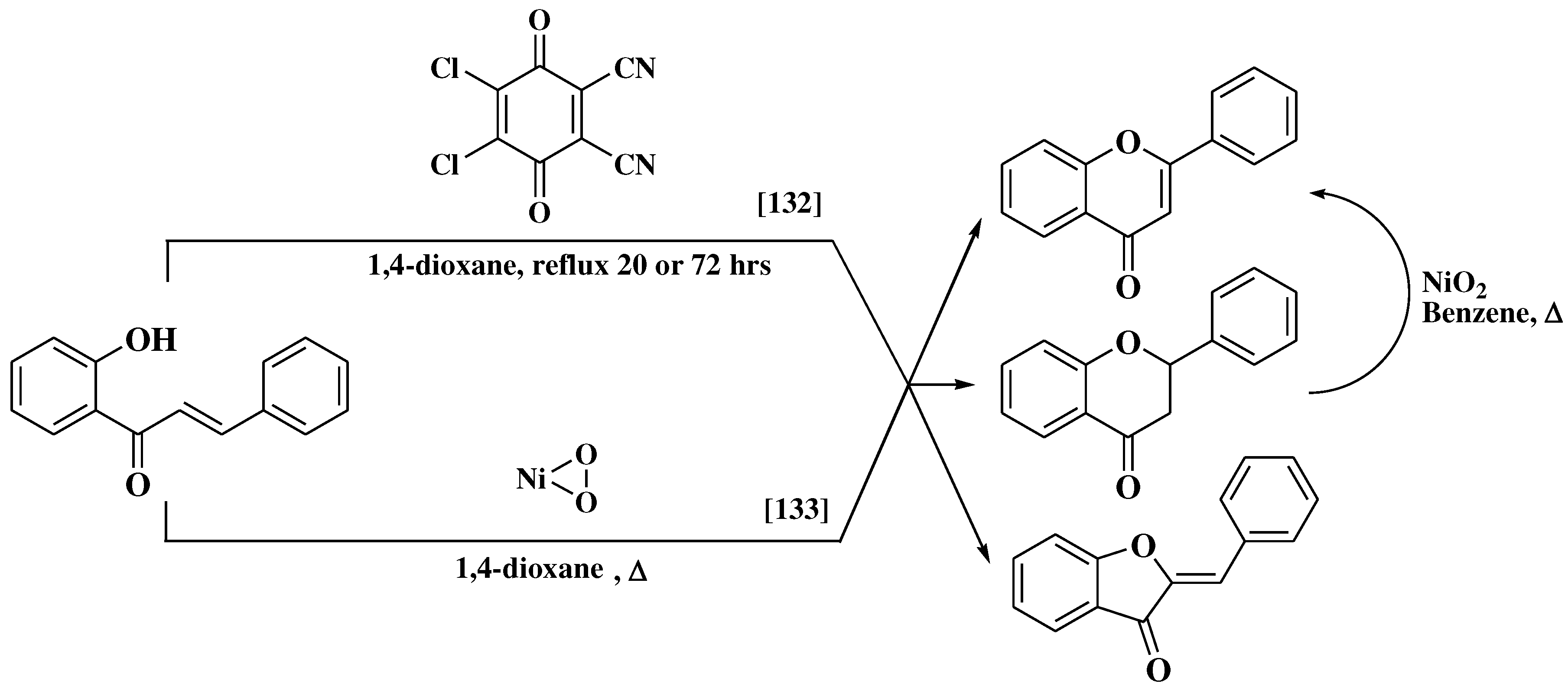
- Lmafuku, K.; Honda, M.; Mcomie, J.F. Cyclodehydrogenation of 2′-Hydroxychalcones with DDQ: A Simple Route for Flavones and Aurones. Synthesis (Stuttg) 1987, 1987, 199–201. [Google Scholar] [CrossRef]
- Mallik, U.K.; Saha, M.M.; Mallik, A.K. ChemInform Abstract: Cyclodehydrogenation of 2′-Hydroxychalcones and Dehydrogenation of Flavanones Using Nickel Peroxide. Abstract 182. ChemInform 1990, 21, 116. [Google Scholar] [CrossRef]
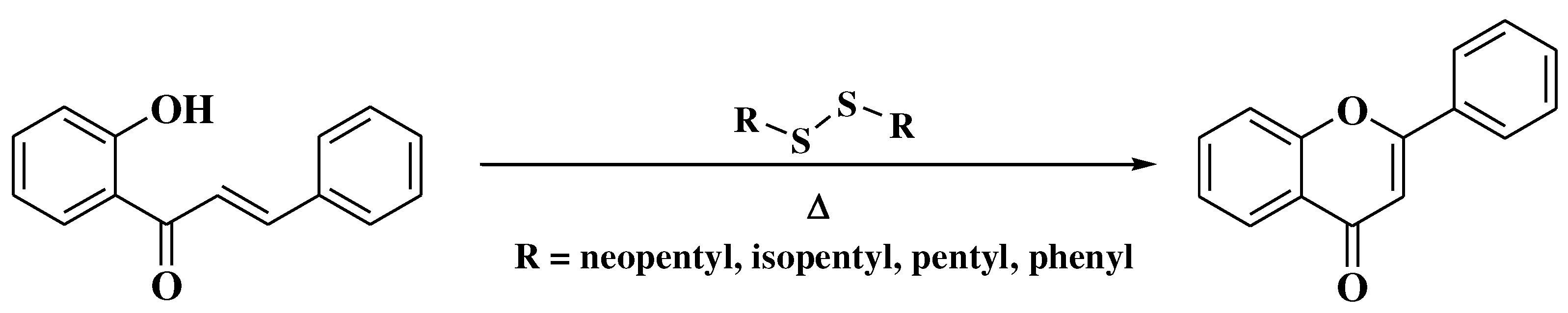
- Hoshino, Y.; Oohinata, T.; Takeno, N. The Direct Preparation of Flavones from 2′-Hydroxychalcones Using Disulfides. Bull Chem Soc Jpn 1986, 59, 2351–2352. [Google Scholar] [CrossRef]
- Hemanth Kumar, K.; Perumal, P.T. A novel one-pot oxidative cyclization of 2′-amino and 2′-hydroxychalcones employing FeCl3•6H2O-methanol. Synthesis of 4-alkoxy-2-aryl-quinolines and flavones. Tetrahedron 2007, 63, 9531–9535. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, Y.; Xu, K.; Tan, G. Silica-gel-supported Ce(SO4)2•4H2O-mediated cyclization of 2′-amino and 2′-hydroxychalcones under solvent-free conditions. Synth Commun 2017, 47, 1–9. [Google Scholar] [CrossRef]
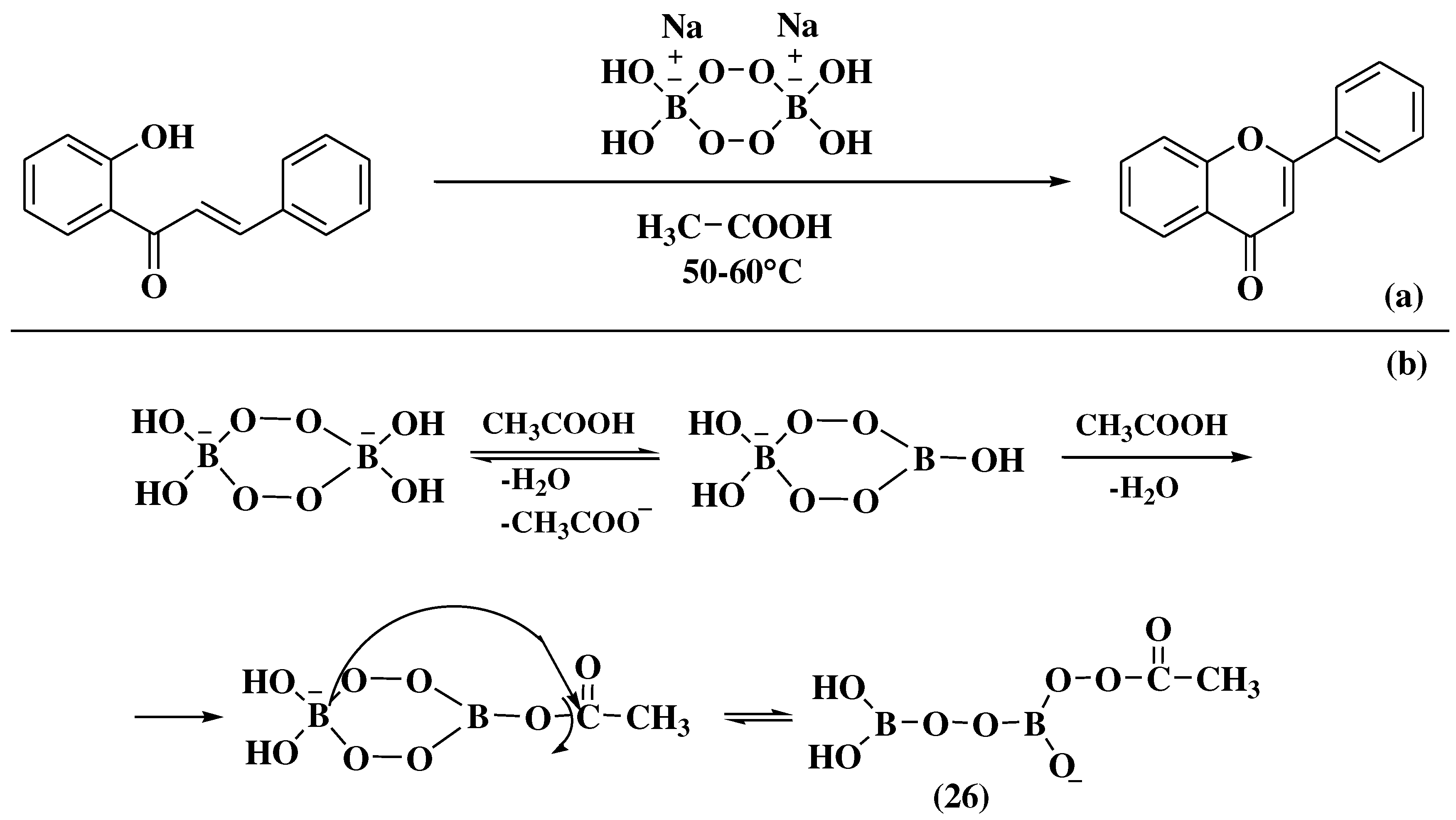
- Ganguly, N.C.; Chandra, S.; Barik, S.K. Sodium perborate tetrahydrate-mediated transformations of 2′-hydroxychalcones to flavanones, flavones, and 3′,5′-diiodoflavone under mild, environmentally friendly conditions. Synth Commun 2013, 43, 1351–1361. [Google Scholar] [CrossRef]
- McKillop, A.; Kemp, D. Further functional group oxidations using sodium perborate. Tetrahedron 1989, 45, 3299–3306. [Google Scholar] [CrossRef]
- Ahmed, N.; Ali, H.; Van Lier, J.E. Silica gel supported InBr3 and InCl3: New catalysts for the facile and rapid oxidation of 2′-hydroxychalcones and flavanones to their corresponding flavones under solvent free conditions. Tetrahedron Lett 2005, 46, 253–256. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, D. Oxidative cyclisation of 2′-hydroxychalcones using sodium tellurite: Synthesis of flavones. Orient J Chem 2011, 27, 761–763. [Google Scholar]
- Zambare, A.S.; Sangshetti, J.N.; Kokare, N.D.; Shinde, D.B. Development of mild and efficient method for synthesis of substituted flavones using oxalic acid catalyst. Chinese Chem Lett 2009, 20, 171–174. [Google Scholar] [CrossRef]
- Matsushima, R.; Kageyama, H. Photochemical cyclization of 2′-hydroxychalcones. J Chem Soc, Perkin Trans II 1985, 53, 743–748. [Google Scholar] [CrossRef]
- Maki, Y.; Shimada, K.; Sako, M.; Hirota, K. Photo-oxidative cyclisation of 2’-hydroxychalcones leading to flavones induced by heterocycle-oxides: High efficiency of pybimido[54-]pteridine -oxide for the photochemical dehydrogenation. Tetrahedron 1988, 44, 3187–3194. [Google Scholar] [CrossRef]
- Saničanin, Ẑ.; Tabaković, I. Electrochemical transformations of 2-hydroxychalcones into flavanoids. Tetrahedron Lett 1986, 27, 407–408. [Google Scholar] [CrossRef]
- Tamuli, K.J.; Sahoo, R.K.; Bordoloi, M. A Biocatalytic Green Alternative to Existing Hazardous Reaction Medium: Synthesis of Chalcone and Flavone Derivatives via Claisen-Schmidt Reaction at Room Temperature. New J Chem 2020, 44, 20956–20965. [Google Scholar] [CrossRef]
- Nana, F.; Kuete, V.; Zaharia, V.; Ngameni, B.; Sandjo, L.P. Synthesis of Functionalized 1-Aryl-3-phenylthiazolylpropanoids and Their Potential as Anticancer Agents. ChemistrySelect 2020, 5, 7675–7678. [Google Scholar] [CrossRef]
- Von Pechmann, H.; Duisberg, C. Ueber die Verbindungen der Phenole mit Acetessigäther. Berichte der Dtsch Chem Gesellschaft 1883, 16, 2119–2128. [Google Scholar] [CrossRef]
- Mentzer, C.; Molho, D.; Vercier, P. Sur un nouveau mode de condensation desters beta-cetoniques et de phenols en chromones. Comptes rendus 1951, 232, 1488–1490. [Google Scholar]
- Seijas, J.A.; Vázquez-Tato, M.P.; Carballido-Reboredo, R. Solvent-Free Synthesis of Functionalized Flavones under Microwave Irradiation. J Org Chem 2005, 70, 2855–2858. [Google Scholar] [CrossRef]
- Selepe, M.A.; Van Heerden, F.R. Application of the Suzuki-Miyaura reaction in the synthesis of flavonoids. Molecules 2013, 18, 4739–4765. [Google Scholar] [CrossRef] [PubMed]
- Kraus, G.A.; Gupta, V. Divergent approach to flavones and aurones via dihaloacrylic acids. Unexpected dependence on the halogen atom. Org Lett 2010, 12, 5278–5280. [Google Scholar] [CrossRef]
- Corbet, J.P.; Mignani, G. Selected patented cross-coupling reaction technologies. Chem Rev 2006, 106, 2651–2710. [Google Scholar] [CrossRef]
- Agrawal, N.N.; Soni, P.A. A new process for the synthesis of aurones by using mercury (II) acetate in pyridine and cupric bromide in dimethyl sulfoxide. Indian J Chem - Sect B Org Med Chem 2006, 45, 1301–1303. [Google Scholar] [CrossRef]
- Ameta, K.L.; Rathore, N.S.; Kumar, B.; Malaga, M.E.S.; Manuela Verastegui, P.; Gilman, R.H.; Verma, B.L. Synthesis and Trypanocidal Evaluation of Some Novel 2-(Substituted Benzylidene)-5,7-Dibromo-6-Hydroxy-1-Benzofuran-3(2H)-Ones. Int J Org Chem 2012, 02, 295–301. [Google Scholar] [CrossRef]
- Thakkar, K.; Cushman, M. A Novel Oxidative Cyclization of 2′-Hydroxychalcones to 4,5-Dialkoxyaurones by Thallium(III) Nitrate. J Org Chem 1995, 60, 6499–6510. [Google Scholar] [CrossRef]
- Khan, M.S.Y.; Mueed, M.A. Scope of mercuric acetate oxidation of chalcones and the antibacterial activity of resulting aurones. Ind J Chem 2004, 43B, 1794–1797. [Google Scholar] [CrossRef]
- Thanigaimalai, P.; Yang, H.M.; Sharma, V.K.; Kim, Y.; Jung, S.H. The scope of thallium nitrate oxidative cyclization of chalcones; synthesis and evaluation of isoflavone and aurone analogs for their inhibitory activity against interleukin-5. Bioorganic Med Chem 2010, 18, 4441–4445. [Google Scholar] [CrossRef]
- Meguellati, A.; Ahmed-Belkacem, A.; Yi, W.; Haudecoeur, R.; Crouillère, M.; Brillet, R.; et al. B-ring modified aurones as promising allosteric inhibitors of hepatitis C virus RNA-dependent RNA polymerase. Eur J Med Chem 2014, 80, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qiang, X.; Luo, L.; Yang, X.; Xiao, G.; Liu, Q.; et al. Aurone Mannich base derivatives as promising multifunctional agents with acetylcholinesterase inhibition, anti-β-amyloid aggragation and neuroprotective properties for the treatment of Alzheimer’s disease. Eur J Med Chem 2017, 126, 762–775. [Google Scholar] [CrossRef]
- Sutton, C.L.; Taylor, Z.E.; Farone, M.B.; Handy, S.T. Antifungal activity of substituted aurones. Bioorganic Med Chem Lett 2017, 27, 901–903. [Google Scholar] [CrossRef]
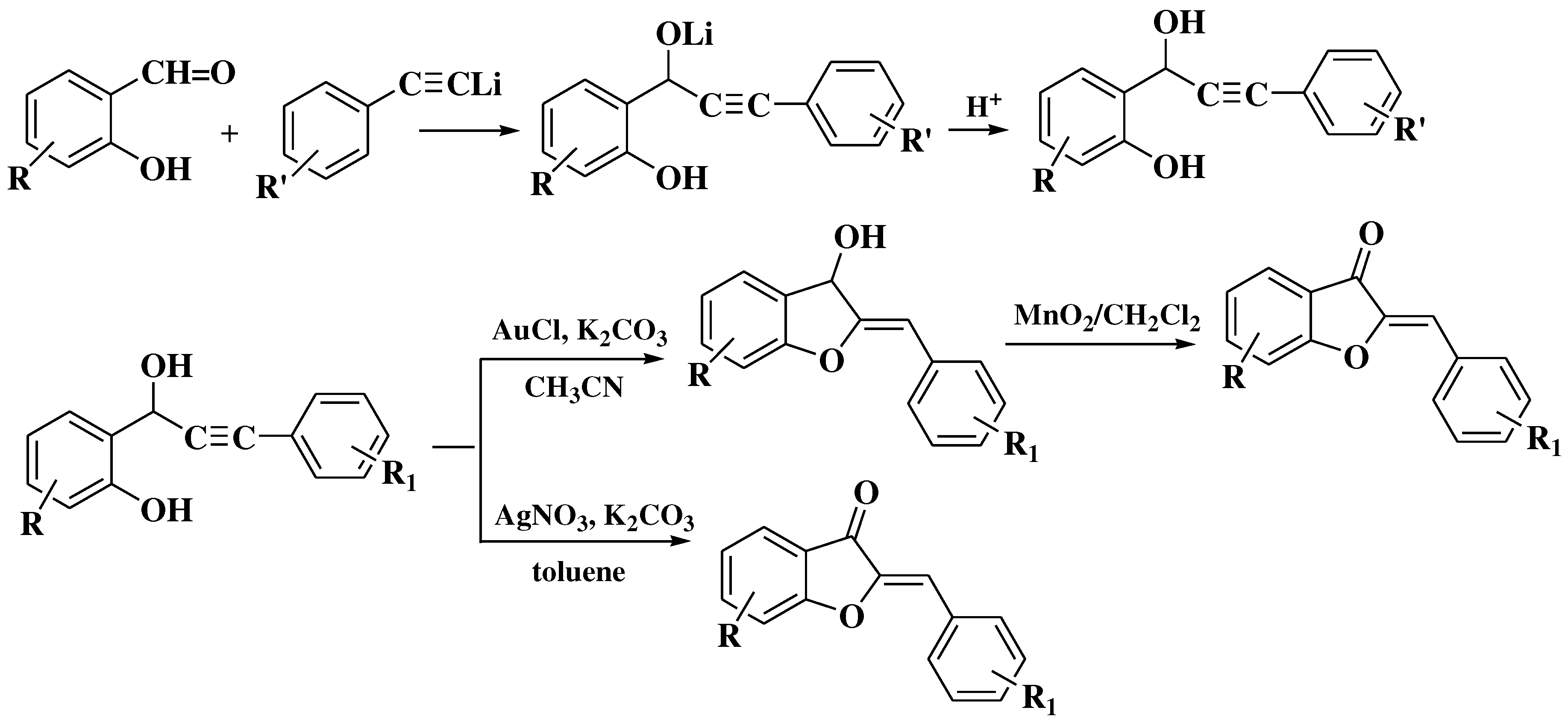
- Harkat, H.; Blanc, A.; Weibel, J.-M.; Pale, P. Versatile and Expeditious Synthesis of Aurones via Au I -Catalyzed Cyclization. J Org Chem 2008, 73, 1620–1623. [Google Scholar] [CrossRef]
- Li, S.; Jin, F.; Viji, M.; Jo, H.; Sim, J.; Kim, H.S.; et al. A novel cyclization/oxidation strategy for a two-step synthesis of (Z)-aurone. Tetrahedron Lett 2017, 58, 1417–1420. [Google Scholar] [CrossRef]
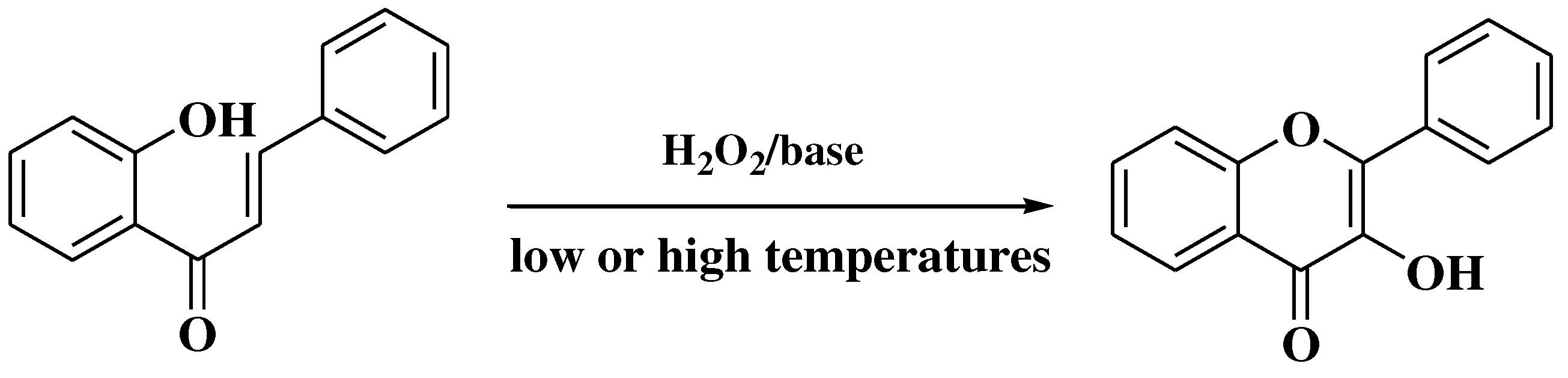
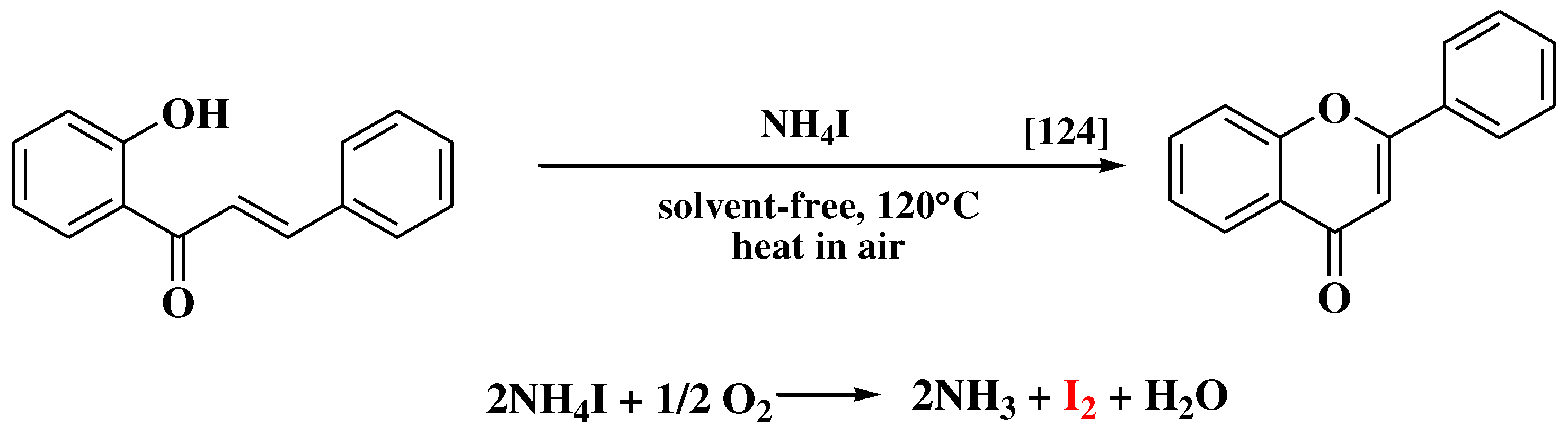
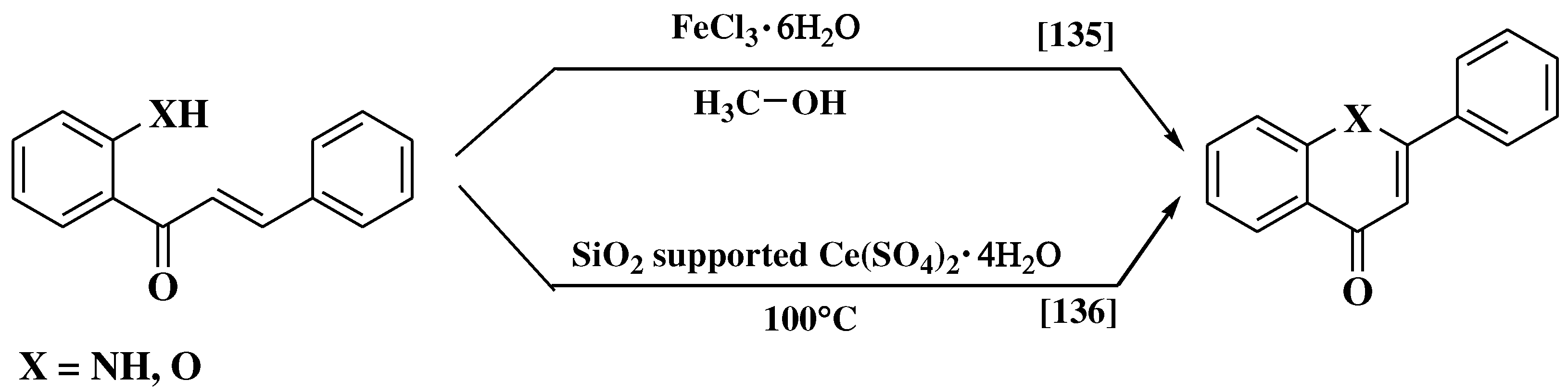
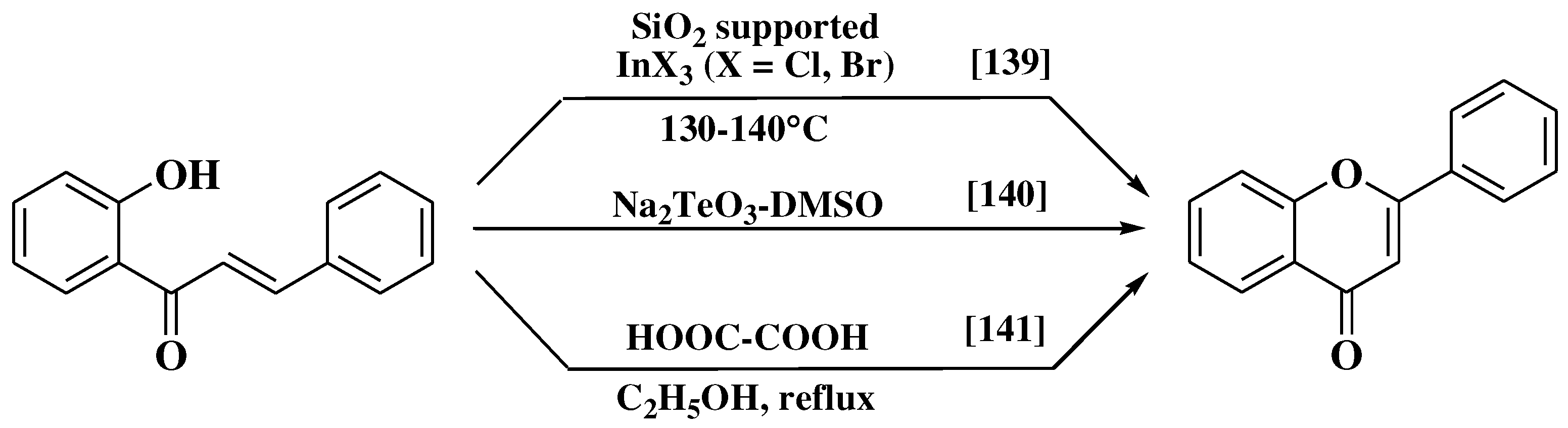
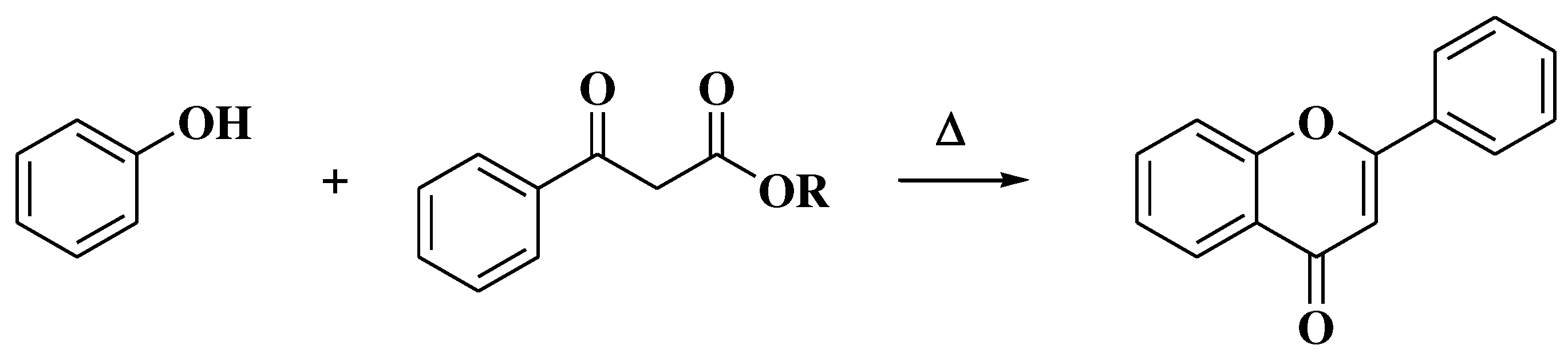
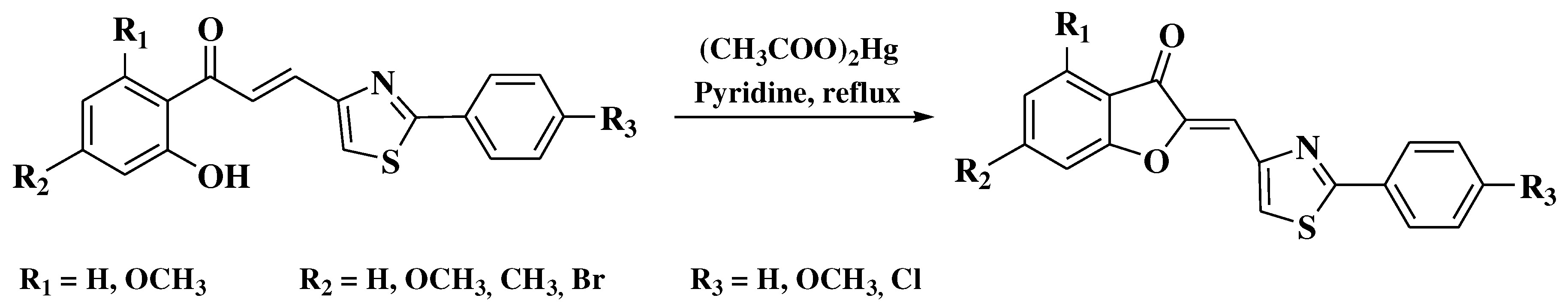
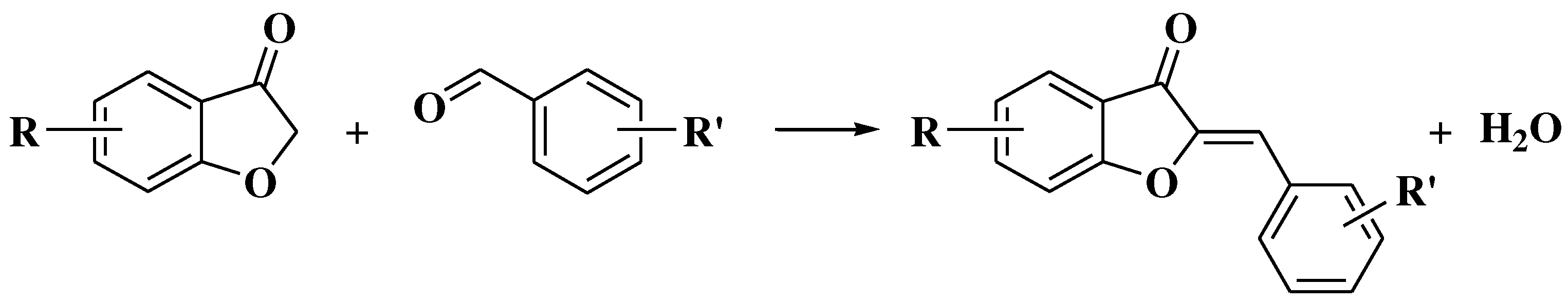
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).