1. Introduction
All cellular organisms, including humans, have multiple highly conserved DNA damage repair and tolerance pathways to contend with different DNA lesions formed spontaneously or upon treatment with DNA damaging agents, including radio- and chemo-therapeutics [
1]. For example, bulky and/or helix-distorting lesions are repaired by nucleotide excision repair (NER). On the other hand, non-bulky and non-helix-distorting base lesions, as well as single-strand breaks (SSBs), are repaired by base excision repair (BER). Double strand breaks (DSBs) can be repaired by homologous recombination repair (HRR), which is generally error-free, and nonhomologous end-joining (NHEJ), which generally creates insertion/deletion at the damaged sites. DNA lesions can also be tolerated by the so-called translesion synthesis (TLS), catalyzed by specialized and generally error-prone DNA polymerases. Activating the DNA damage checkpoint (DDC), which temporarily arrests the cell cycle before DNA lesions are repaired, is also an important mechanism for the cell to contend with DNA damage [
2]. By maintaining genome stability, most genes involved in DNA damage repair, tolerance and DDC serve as
bona fide cancer suppressors.
Synthetic lethality (SL), where the functional loss of two genes together, but not alone, results in cell death, is a conceptually new strategy for cancer therapies [
3]. The first successful SL treatment strategy is using PARP inhibitors (PARPis) to treat tumors with functional loss of BRCA1 or BRCA2, two cancer suppressors involved in the HRR of DSBs. PARPis prevent PARP1 and PARP2 from repairing SSBs, leading to stalled and collapsed DNA replication forks. Subsequently, SSBs are converted to DSBs that HRR-deficient cells cannot repair effectively, leading to cell death. Recently, PARPis have been shown to be effective for killing PCa cells with functional loss of BRCA1, BRCA2, or other proteins directly or indirectly involved in HRR and moderately extend survival of mCRPC patients having functional loss [4-6].
Cisplatin (CPT) reacts with DNA, producing single purine adducts and intra- and inter-strand crosslinks (ICLs) [7-10]. The single purine adducts and intra-strand crosslinks can be repaired by NER or tolerated by TLS. The repair of inter-strand crosslinks (ICLs) is more complicated, requiring the participation of Fanconi anemia (FA) proteins and subsets of proteins involved in NER, TLS, HRR, NHEJ and BER (
Figure 1B) [11,12]. Therefore, deficiency in any of the DNA damage repair, tolerance and DDC mechanisms may cause CPT sensitivity, and combined deficiencies of two or more of these mechanisms will synergistically enhance the sensitivity.
CPT has been most frequently used for adjuvant or neoadjuvant treatment of most solid tumors[
13]. Impressively, CPT treatment is highly efficient against testicular germ cell cancer, leading to a durable complete remission in > 80% of patients [
14]. While CPT-based therapy has not been the treatment of choice for prostate cancer (PCa), partly due to significant renal toxicity (which can be faithfully recapitulated in mice [
15]), recent studies indicated that lower dosing in combination with inhibitors of DNA damage repair could be quite effective [
10]. However, as multiple DNA damage repair, tolerance and DDC mechanisms are implicated in CPT resistance (
Figure 1) [8, 16], the genes that can be targeted to improve CPT-based PCa therapies remain largely unexplored.
CPT is highly effective against several forms of cancer, most notably testicular tumors, and it is also commonly used to treat breast, ovarian, bladder, lung, and head and neck cancer. However, PCa is resistant to CPT chemotherapy due to poor targeting and the development of resistance. Some strategies for targeting CPT by piggybacking carrier nanoparticles onto PSMA [
17]
, as well as recent clinical studies, have indicated that lower dosing in combination with DNA damage repair inhibitors (or for cases with defective DNA repair genes) can be quite effective [18, 19]. However, as multiple DNA damage repair, tolerance and DDC mechanisms are implicated in CPT resistance [8, 16], it is not obvious which genes must be targeted to improve CPT-based therapies for most PCa cases.
TLK1 is a serine/threonine kinase [
20] frequently upregulated in PCa cells upon anti-androgen therapies [21, 22]. By phosphorylating its substrates, TLK1 plays a role in resistance to several DNA-damaging agents, including CPT [23-26]. Our recent work showed that TLK1 specifically catalyzes the phosphorylation of the HRR proteins RAD54L and RAD54B [
27], which at least in part explains the role of TLK1 in CPT resistance, as together, these two paralogs carry out some fundamental functions in HRR along with RAD51 [
28]. Since the processing of ICLs typically results in the formation of DSB intermediates that require the HRR pathway to complete lesions repair, it is expected that the combination of CPT with inhibitors of HRR will result in synthetic lethality. In this work, we tested the effect of a specific inhibitor of TLK1, J54, in combination with CPT as a therapeutic strategy for androgen-insensitive PCa cells.
2. Results
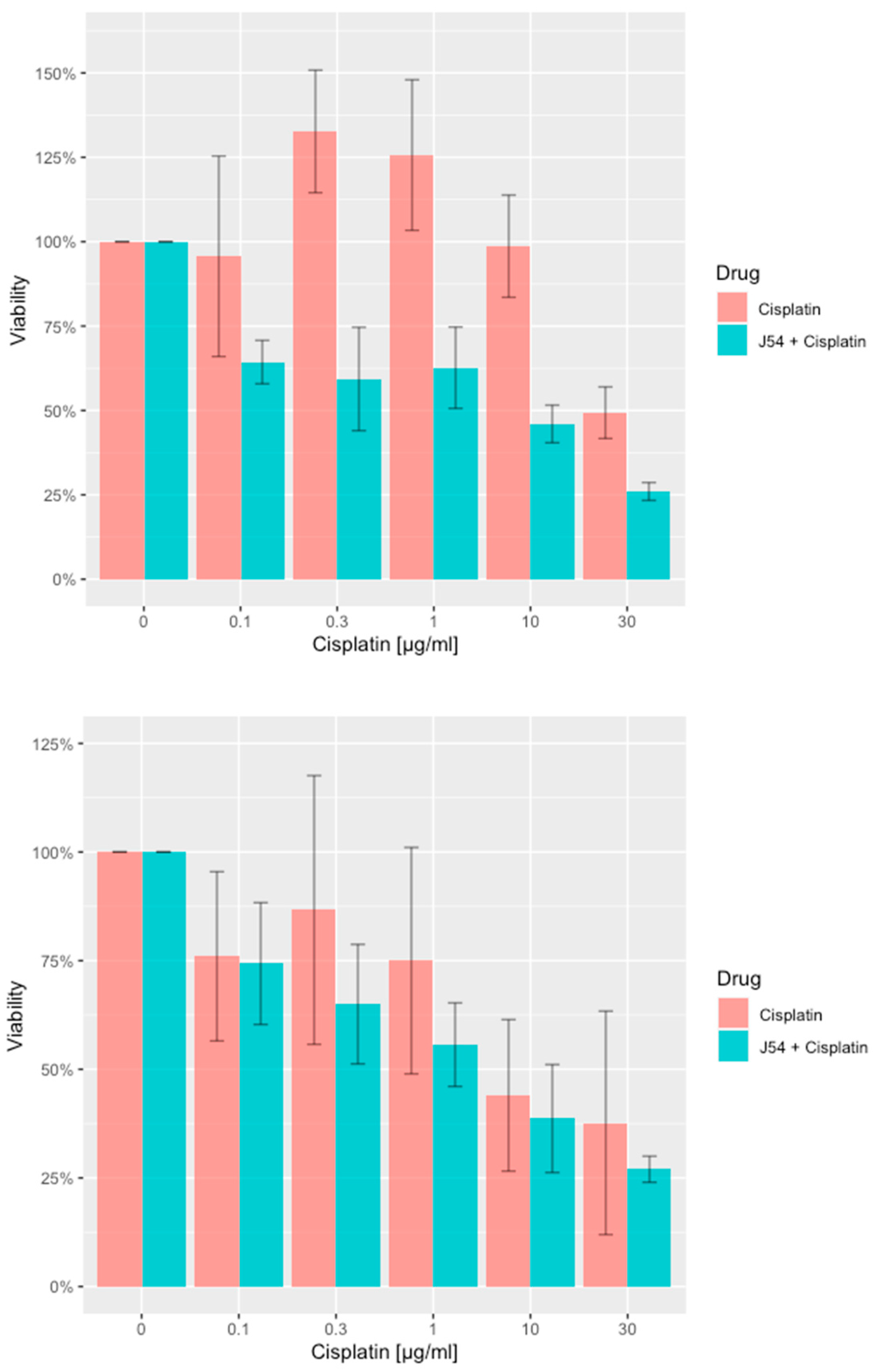
Combining CPT with J54 enhances its growth inhibitory potential on CRPC cells. For our work, we selected two CRPC cell lines that utilize different mechanisms of androgen-insensitive growth (PC-3 and C4-2B), which are known to be rather resistant to CPT. In
Figure 1, we show the viability results, using MTT, of the two cell lines culture over 48h with cisplatin alone or in combination with the TLK inhibitor, J54. It is noticeable that at up to a concentration of 0.3 μg/ml (already quite high for the therapeutic index), both cell lines were quite insensitive to CPT, and in fact, C4-2B grew even slightly better than untreated cells (possibly an effect of the induction of DNA repair enzymes by CPT that may help in the complex process of DNA replication). As expected, both cell lines display progressive growth inhibition at higher CPT concentrations. The situation, however, was quite different when the experiment was carried out in combination with 5 μM J54. In this case, both cell lines displayed a much stronger, dose-dependent reduction in viability, and the peculiar growth stimulation observed at low doses of CPT for C4-2B cells was eliminated. As previously reported, J54 alone (0 CPT) had no growth inhibitory effect on the cells [
29].
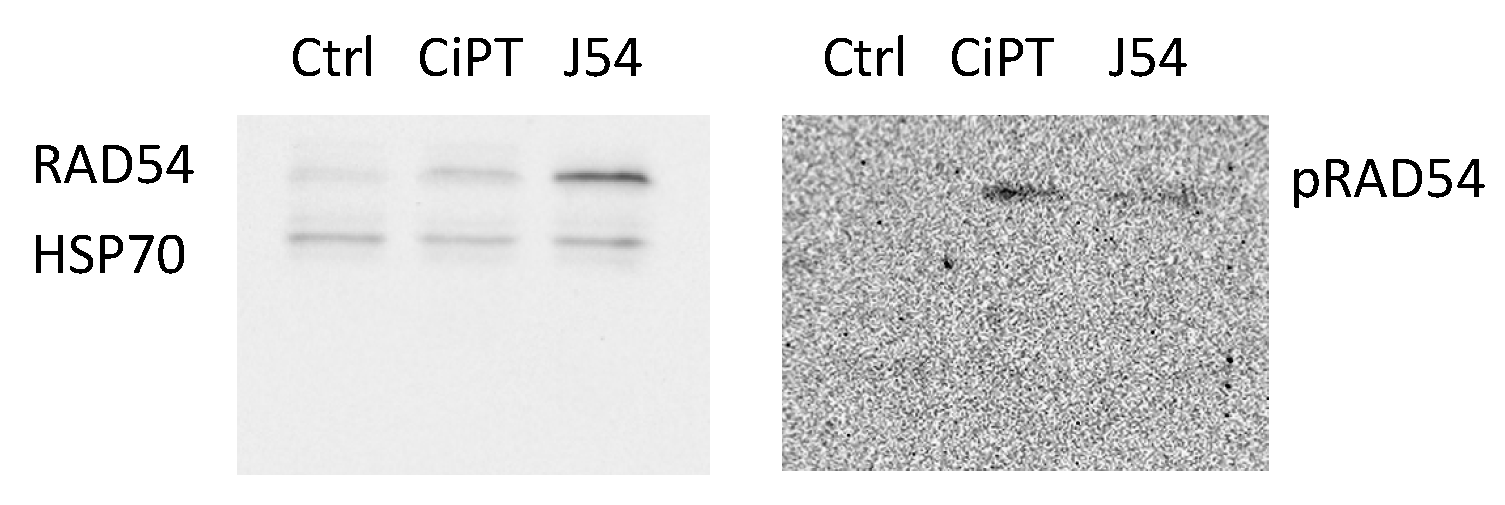
CPT treatment induces pRA54-T700. The fundamental hypothesis of our work is that the repair process of ICL lesions induced by CPT results in the formation of transient DSBs that require repair via HRR. Hence, interfering with the mechanism of HRR by preventing the critical phosphorylation of RAD54-T700, mediated specifically by TLK1, will enhance the toxic effects of CPT.
We have recently generated a highly specific pRAD54-T700 antiserum that can specifically monitor the activity of TLK1 during HRR [
27]. However, this antiserum has not been tested yet on DNA-damaging agents other than IR. We now observed that CPT is a potent inducer of RAD54 phosphorylation, while the TLK1 inhibitor J54 partly suppresses this – J54 alone reduces baseline pRAD54-T700 without DNA damage [
27]. Furthermore, it appears that CPT leads to a modest accumulation of total RAD54 (
Figure 2). Since this was also observed for the recovery period after IR [
27], it is tempting to speculate that phosphorylation of RAD54-T700 is either a general phenomenon that occurs with other DNA damaging agents, or (more likely) that ICLs getting converted to DSBs are partly repaired via canonical HRR requiring pRAD54-T700 modification.
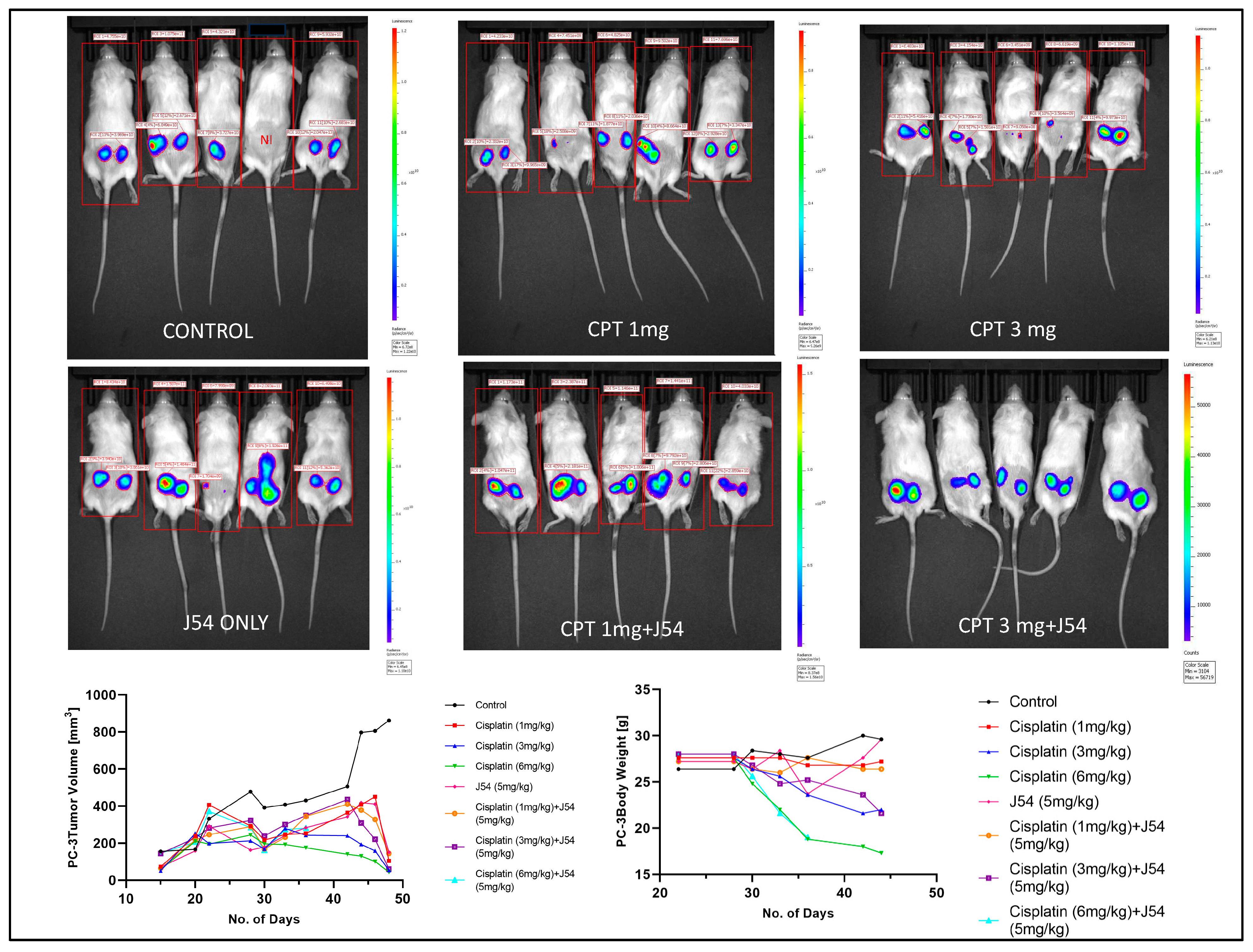
The combination of CPT+J54 results in dose-dependent regression of tumor xenografts. To establish if adding J54 to CPT has a therapeutic effect from a synthetic lethal combination with a genotoxic agent, we investigated the effect on the growth of a subcutaneous PC-3-Luc tumor model in NOD-SCID mice. After inoculating 0.5M cells on both dorsal flanks, the tumors were allowed to form to a size of ~150 mm3, after which the treatment started. We used a fixed dose of 5 mg/kg J54 and three dosages of CPT (0, 1, 3, and 6 mg/kg).
The progression or regression of the tumors was followed in each mouse with calipers, and the average tumor size over time is plotted in
Figure 3, bottom left panel. Notably, all the treatments were at least cytostatic compared to controls, while the combination treatments with 1 mg/kg and 3 mg/kg CPT after 40 days resulted in an actual regression of the tumors. When the experiment was concluded, and the tumors recovered from the group treated with 3 mg/kg CPT+J54, the residual tumors were hardly visible. The 6 mg/kg CPT (+/- J54) dose was also highly effective at cytoreduction, but the treatment could be continued for only six cycles (bi-weekly) before the toxicity was such that required euthanasia of the mice before the conclusion of the experiment, and even before the visualization with the IVIS.
A day before sacrificing the mice, the animals were injected with luciferin, and the actual tumor cell mass was visualized with an IVIS-Spectrum/CT machine (Perkin Elmer). While the IVIS was run unassisted with auto-acquisition, which allows for the detection of the full range of cells, from very few to very large numbers, resulting in the production of tumors images from all the animals, the details are much better appreciated in the heat-map scale (or from the individual radiance quantitations). The most remarkable result is observed in the CPT 3 mg/kg+J54 group. While PC3-Luc cell tumors are also seen in this group, the heat-map scale is drastically different: it goes from 10,000 to 50,000. In contrast, in control (for example), the scale goes from 0.2 to 1.2e1010, meaning up to 6 logs radiance units lower in the combo than in the tumors from the control group. For a more precise comparison between the control group and the 3mg/kg combo, we measured the average Flux of all the tumors in each group. For the control, the average Flux was 4.61e10, while for the combo, it was 31604. Another interesting observation was that J54 alone affected tumor growth in some animals, for example, very noticeable in the 3d mouse from the left. Although the growth curve measured with calipers is displayed as the average of all the tumors in a group, we did notice an overall partial cytostatic effect with J54 alone, which became an actual tumor regression toward the late part of the experiment. Another important observation is that J54 not only had no apparent toxic effect and did not affect the mice’’s weight and water/food consumption but showed a partial improvement of the visual health conditions of the mice treated with the higher 3 mg/kg CPT dose. Rather than losing weight, a possible sign of cachexia from tumor burden and treatment toxicity, the animals in the combo groups showed stable weight and appeared to be more active and frequently foraging.
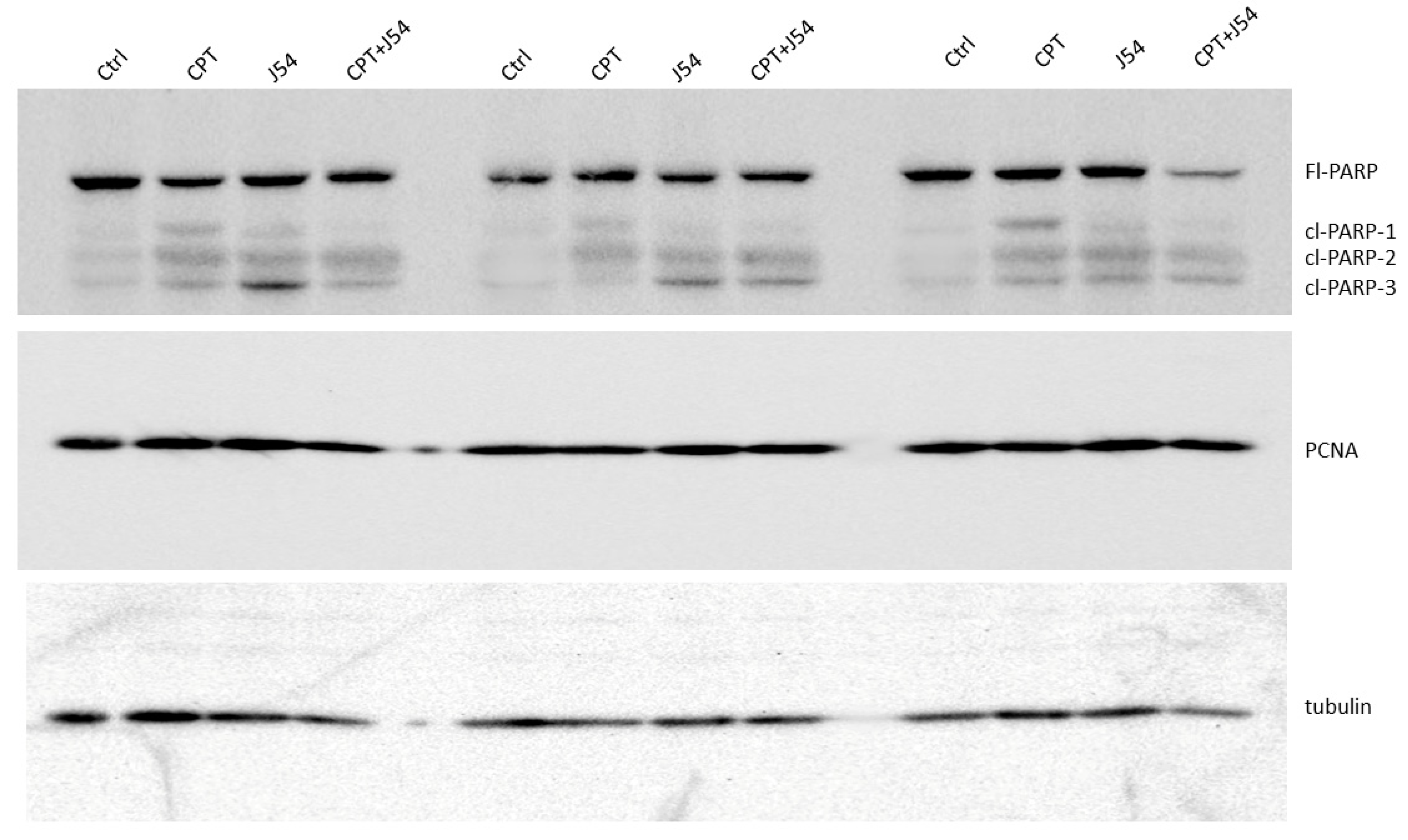
The strong tumor regression in mice treated with CPT+J54 is hallmarked by apoptosis. To establish more firmly the mechanism of tumor regression (or at least cytostasis) in the mice treated with CPT, J54, or combination, we focused on the group of mice treated with the 3 mg/kg CPT, where the effects were more evident, but the toxicity was tolerable. Immediately after necropsy, all the tumors were weighed, and one from three random mice in each group (from the 3 mg/kg dose) were prepared for western blot analysis of PARP/cleaved PARP (for determination of the intratumoral extent of apoptosis) and PCNA (for determination of the fraction of still proliferating cells), as illustrated in
Figure 4. It is immediately clear that all the mice treated with CPT alone displayed reduced level of full-length (Fl) PARP and an array of cleavage products, with an abundant ~90 kDa product (cl-PARP1) that is considered its classic apoptotic signature [
30]; but smaller processed product were also generated, as indicated in
Figure 4. In the CPT+J54 combination treatment, and most prominently in the right-most tumor, Fl-PARP was strongly reduced, and there is a prominent formation of cl-PARP2 and cl-PARP-3 and entirely bypassing cl-PARP1 suggesting much more advanced stage of apoptosis and loss of PARP integrity (PARP degradation is mediated by a caspase cascade [
31]). It should be noted that J54 treatment alone also resulted in some cleaved PARP products, consistent with our previous report that its related compound (Thioridazine also acting as an inhibitor of TLK1), while not toxic for PC-3 cells in vitro, resulted in some tumor regression with distinctive apoptotic signatures when administered in xenografts due to the partly hypoxic tumor micro-environment that tends to generate DNA lesions and activation of the DDR [
32].
We should mention that we did not carry out other confirmatory assays of apoptosis, like TUNEL/IHC, since several residual tumors, particularly in the most significant 3mg/kg CPT+J54 group, were very small, and we had to choose between preparation for WBs or PEFF sections. In contrast to the apoptotic hallmark in the tumor tissue from the treated mice, which alone could explain the obvious size regression in the CPT+J54 combination group, as well as in the other groups to a lesser extent, the proliferative capacity (measured by the PCNA expression as an indicator of the % dividing cells) was not affected in any of the 3 tumors from each group (
Figure 4). We conclude that heightened apoptotic induction, rather than inhibition of proliferation, is the key explanation for the tumor cytoreduction throughout treatment.
3. Discussion
The concept of synthetic lethality has evolved around the central tenet that by affecting two pathways, each one of which is not essential for viability, the result is engendering a non-viable outcome, either via a genetic approach or by pharmacologic targeting. Perhaps its most successful use in cancer therapy is illustrated by the application of PARPis, sometimes combined with other drugs, for managing gynecological malignancies [
33] or PCa [33-35], particularly in the context of a BRCAness cancer presentation.
In this work, we have somewhat expanded on the concept of synthetic lethality by taking advantage of the knowledge of the repair process of CPT-induced lesions (a commonly used chemotherapy) that are known to yield DSBs that are repaired via HRR, a key pathway for their accurate repair, which would otherwise result in lethality from unrepaired damage or engender combination of genomic rearrangements that in most cases would produce non-viable configurations [
36]. Specifically, we exploited our knowledge of a regulatory pathway we recently uncovered governing the activity of the key HHR protein, RAD54, by the kinase TLK1 [
27]. RAD54 performs critical multiple functions during the HRR process [
28], and both its activity and cellular localization were found to be regulated differently by TLK1 during sequential phases of the process. Therefore, our strategy was to treat two of the most common CRPC human cell lines with progressively more tolerable doses of CPT in combination with J54, a relatively new TLK1 inhibitor [29, 37]. After a relatively rapid verification, in vitro, of the proof-of-principle of this strategy by determining the viability of these cells, which are normally quite resistant to CPT [
38], when challenged in combination with a non-toxic concentration of J54, we then progressed to the more critical xenograft model. For this more critical analysis, we used two methods to follow tumor growth or regression over time: direct measurement of tumors’ diameters with calipers, and IVIS imaging at midpoint (~1 month) and endpoint (day before sacrifice) of the time course. Direct visual evidence of tumor regression for the most representative group (3 mg/kg CPT+ J54) is displayed in SI-Figure 1, which is best appreciated when viewed in comparison with the endpoint image. Of particular importance is again the radiance scale that at treatment midpoint was 1.89e8 (min) to 3.64e9 (max), showing that the tumors were up to 5 logs more emissive (larger number of cells) than at the endpoint shrinkage stage. But note that even at midpoint, the radiance was already ~1 log lower than that of the control group.
Of interest are the observations we made about the effect of J54 on the weight and overall health appearance of the mice throughout the experiment, with the exclusion of the 6 mg/kg CPT two groups for which the toxicity was excessive after 6 doses. Starting from the obvious observation that the mice treated with 3 mg/kg CPT progressively lost weight, whereas those that also received J54 did not and appeared healthier and more active, we noted some peculiar differences in the adipose tissue. Whereas the mice treated with 3 mg/kg CPT alone showed either cytostatic or modest cytoreduction of the tumors. However, without an obvious infiltration of adipose tissue, the mice that also received J54 displayed a noticeable replacement of the shrunk tumor space with an overgrowth of adipocytes. We suggest that this may be due to the combination of two factors: 1) There is already well-described literature on the interplay between cancer cells and locally adjacent adipocytes, which secrete cross-stimulatory adipokines and cytokines [
39]. 2) It is well known that several phenothiazine (PTH) antipsychotics (as well as newer atypical ones) cause accumulation of white fat tissue and some weight gain as an unfortunate side-effect [40, 41]. Although J54 is structurally a PTH, it was designed to have a minimal binding activity to the D2R, explaining its low antidopaminergic properties [
29]. Such activity is held to be the main mechanism responsible for adipogenesis stimulation. However, other mechanisms have been proposed, such as
increased expression of sterol regulatory element-binding protein (SREBP) and very low-density lipoprotein (VLDL) genes (rev. in [
41]) which could be alternative explanations for the effect of J54 on the localized adipogenesis and overall maintenance of body fat/weight in the face of mice treated with CPT alone. These progressively lost some weight and became visibly thin during treatment, likely from the combination of tumor burden and toxicity from CPT, for which it is well-known that cachexia is a major issue during the terminal phases of cancer progression.
Overall, we would like to conclude that the use of J54 to achieve pharmacologic synthetic lethality with CPT (one of the most widely used chemotherapeutic agents) has shown promising results in one model of PCa xenograft, whereby the synthetic targeting of HRR after the generation of DNA adducts and ICLs was shown to improve the efficacy of the therapy in tumor regression greatly. An unexpected benefit was better general health and weight maintenance in the most effective dose combination group. While we do not yet know if there are other more subtle side-effects for J54 that, in the long run, may make its potential clinical use unattainable, so far and at least in mice, we never lost an animal nor have we seen altered behaviors in those treated with a dose of up to 20 mg/kg. Of course, our recommendation for the best use of J54 is still before progression to CRPC in order to bypass the cell cycle checkpoint induced by anti-androgens in androgen-sensitive cells and enforce the mitotically-induced apoptosis that engenders global ablation of PCa cells rather than inducing quiescence, that is the expectation from the current standard of care [29, 37].
4. Materials and Methods
4.1. Cell Viability Assay
Cell viability was evaluated using the MTT assay, which measures the reduction of 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) by mitochondrial enzymes in live cells. We seeded 20,000 PC-3 and C4-2B cells into 100 μL of medium in 96-well plates and allowed them to adhere for 24 hours. Then, we replaced the media with a fresh medium containing various cisplatin and/or J54 concentrations and incubated for an additional 24 hours. Finally, we added MTT reagent to each well and incubated it for 35 minutes before measuring the absorbance intensity at 490 nm using a spectrophotometer.
4.2. Animal Studies
All animals used in this study received humane care based on the recommendations set by the American Veterinary Medical Association, and the Institutional Animal Care and Use Committee of LSU Health Sciences Center at Shreveport approved all the test protocols. Immune-deficient NOD SCID mice (Charles River) were used in this research to host human PCa PC-3 tumors. 0.5*10
6 human PCa PC-3-Luc cells suspended in matrigel were grafted subcutaneously into the two lower back flanks of the NOD SCID mice. After the tumor sizes of ~150 mm
3 were established, the tumor-bearing mice were randomized into eight treatment groups. Mice were treated with vehicle control (PBS), TLK1-RAD54 axis inhibitor J54 (5 mg/kg), and different dosages of CPT (1, 3, and 6 mg/kg) with or without J54. Intraperitoneal (IP) injections of J54 (dissolved in 200 sterile saline with 10% Polysorbate-80 – PS-80) were given bi-weekly. The dose for J54 was based on our previous work [
29]. CPT dissolved in 0.9% Sterile-filtered NaCl Solution (Saline) was administered individually at different dosages- (1, 3, and 6 mg/kg) in 100 ul volume by IP injection twice a week. Tumor sizes were measured every other day by using a caliper. The inhibitor treatment lasted 28 days for about nine bi-weekly drug cycles. The tumor-bearing mice were monitored every other day and euthanized if there was an apparent loss in body weight (≥ 20%), abdominal palpitation due to the development of prostate tumors or cancer metastasis, poor body condition, or mice were too sick and unable to reach food and water. The mice were sacrificed at the end of the experiment by CO2 asphyxiation, and the tumors were excised for the tissue western blots.
4.3. Western Blots
a Tissue Western Blot: Western blots were performed in three biological replicates for the tumors excised from the different treatment groups, including control (PBS), cisplatin (3mg/kg), J54 (5mg/kg) and the combination of the PC-3 grafted NOD SCID mice. The frozen tumor tissues were disrupted with the Bioruptor® Plus sonication device (Diagenode; Cat. No. B01020001), homogenized, and lyzed in the ice-cold RIPA lysis buffer system (Santa Cruz Biotechnology; Cat. No. SC-24948). The samples were clarified by centrifugation at 13,000 rpm for 20 mins in the refrigerated setting. The supernatant was collected, transferred into fresh 1.5 ml microfuge tubes, flash-frozen, and stored at -800C until further use. The total protein concentration was measured using a Pierce™ BCA protein assay kit (Thermo Scientific; Cat. No. 23225) with bovine serum albumin (BSA) as a standard control. An equal loading amount of 15 µg was calculated for each protein sample. The sample supernatant was denatured with 1X Laemmli Buffer for 10 mins at 950C and separated using 12% Mini PROTEAN TGX protein gel (BioRad; Cat. No. 4568084) at 100 volts for 120 mins. The proteins were transferred to the Immun-Blot PVDF membrane (BioRad; Cat. No. 1620177) using a Mini Trans-Blot Cell (BioRad; Cat. No. 1703930) at 100 volts for 150-180 mins on ice. The membrane was blocked with 5% Non-fat dry milk (Cell Signaling Technology; Cat. No. 9999S) in 1X Tris-buffered saline with Tween-20 (TBST) for 1 hour at room temperature. Following blocking, the membrane was washed once with 1X TBST and incubated with mouse anti-PCNA (PC10) monoclonal antibody (Santa Cruz Biotechnology; Cat. No. SC-56; 1:1000 dilution) and mouse anti-PARP-1 (F-2) monoclonal antibody (Santa Cruz Biotechnology; Cat. No. SC-8007; 1:1000 dilution) in 5% BSA in 1X TBST overnight at 40C with gentle rocking. The next day, after washing four times with 1X TBST, the membrane was incubated with horse anti-mouse antibody (Cell Signaling Technology; Cat. No. 7076S: 1:2000 dilution) labeled with horseradish peroxidase in 5% BSA in 1X TBST for 1-1.5 hours at room temperature. After incubation, the membrane was washed four times with 1X TBST, and the reactive bands were detected using Pierce™ ECL Western Blotting Substrate (Thermo Scientific; Cat. No. 32106) on ChemiDoc MP Imaging System (BioRad; Cat. No. 12003154).
b Cell Western Blot: The western blot for PC-3 cells was performed as described above but with minor modifications. Briefly, 3*106 PC-3 cells (control and drug-treated) were collected, washed twice with ice-cold PBS, and lyzed with RIPA lysis buffer system. The lysate was vortexed and centrifuged at 13,000 rpm for 10 min to remove cell debris. The total protein was estimated, and 30 μg of the cell lysate was loaded onto an SDS-PAGE gel. The separated proteins were transferred to the membrane using a wet transfer apparatus. The complete transfer was ensured by checking the membrane for uniform background staining. The membrane was then incubated in a blocking solution (e.g., 5% non-fat milk in TBST) for 1 h at room temperature to block non-specific binding sites, followed by primary antibody (custom-made anti-pRAD54 rabbit polyclonal; Thermo Scientific; Cat. No. AB1991; 1:1000) incubation in blocking solution overnight at 4°C. The next day, the membrane was washed 3X with TBST for 10 min each to remove excess primary antibody. Further, it was incubated with goat anti-rabbit HRP-conjugated secondary antibody diluted in a blocking solution for 1 h at room temperature. Next, the membrane was washed 3X with TBST for 10 min each to remove excess secondary antibody, and the bands were detected using the ECL chemiluminescent substrate.