Submitted:
28 July 2023
Posted:
31 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Source of biowastes
2.2. Inoculum
2.3. Experimental design
2.3.1. Specific phototrophic activity (SPA) tests
2.3.2. Semicontinuous photobioreactors
2.4. Analytical methods
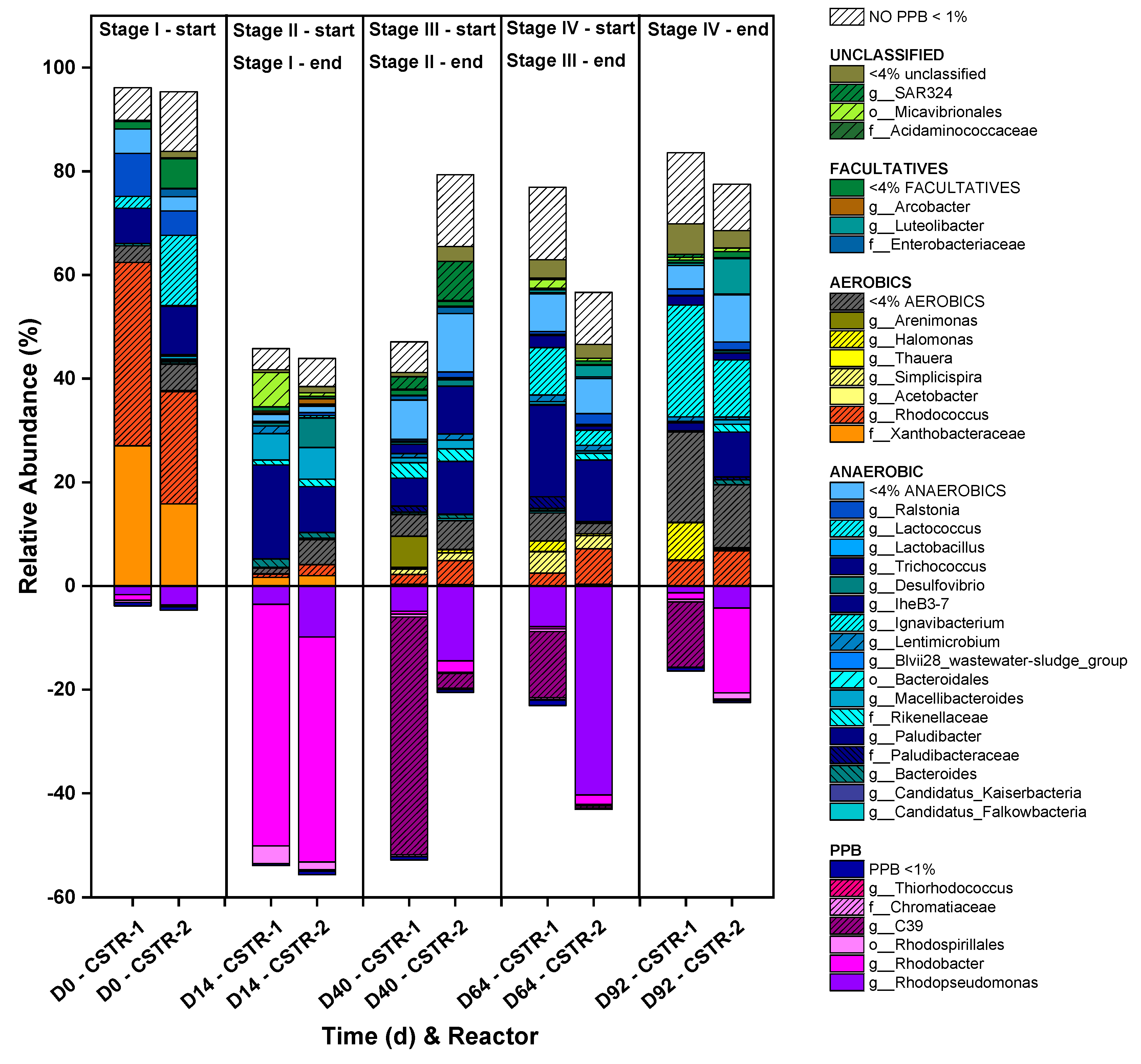
2.5. Microbial community analysis
3. Results and Discussion
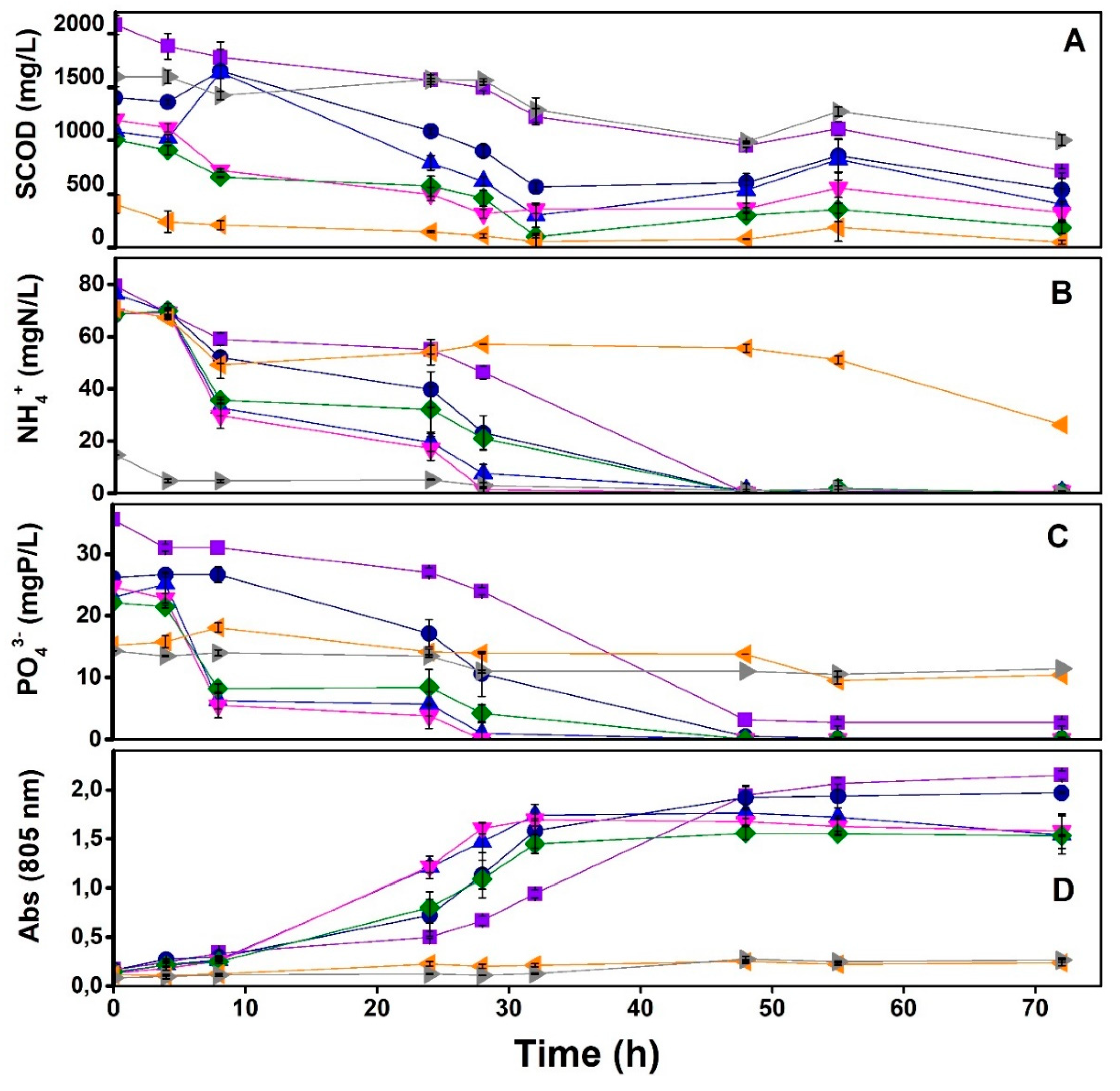
3.1. Specific Phototrophic Assay (SPA)
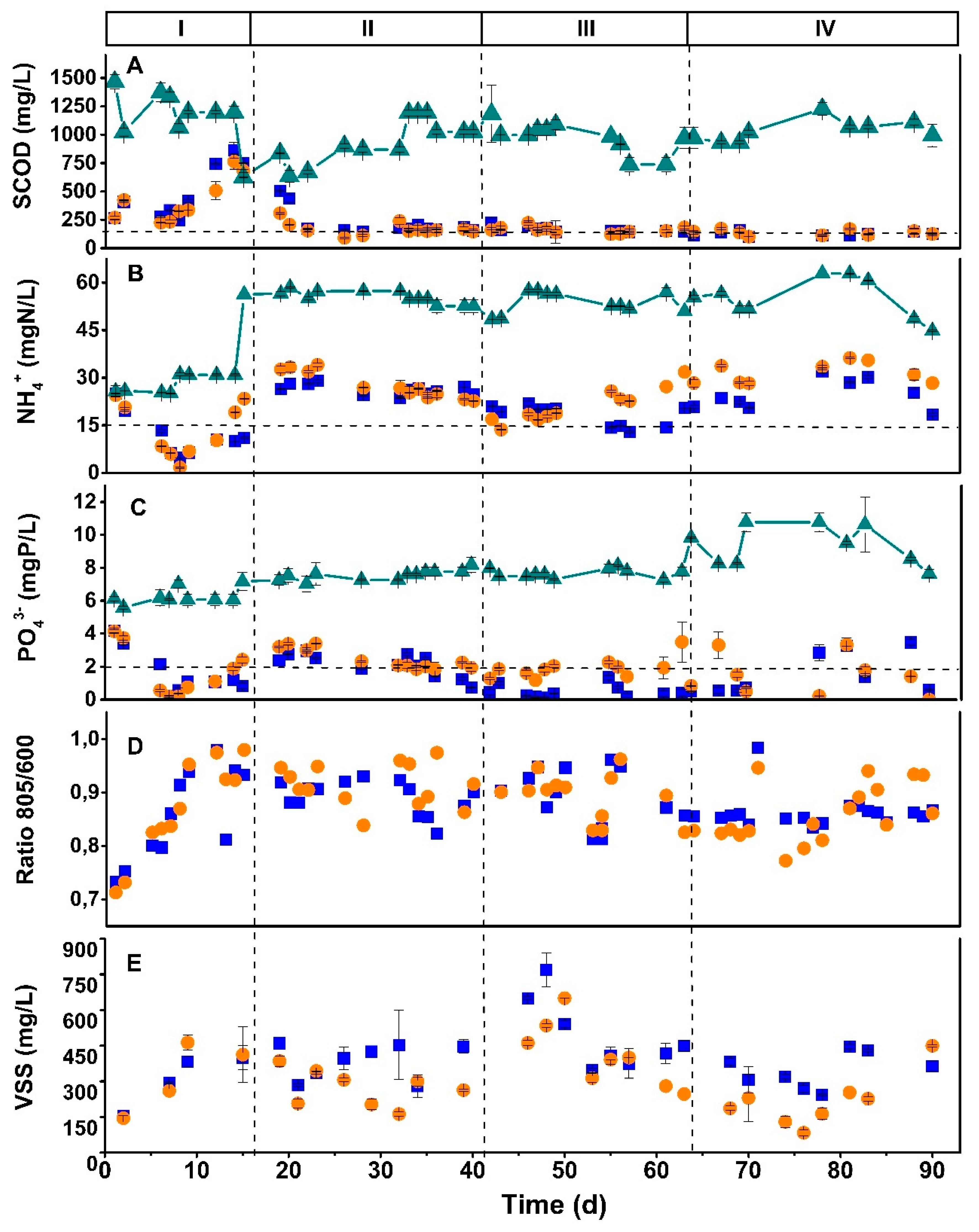
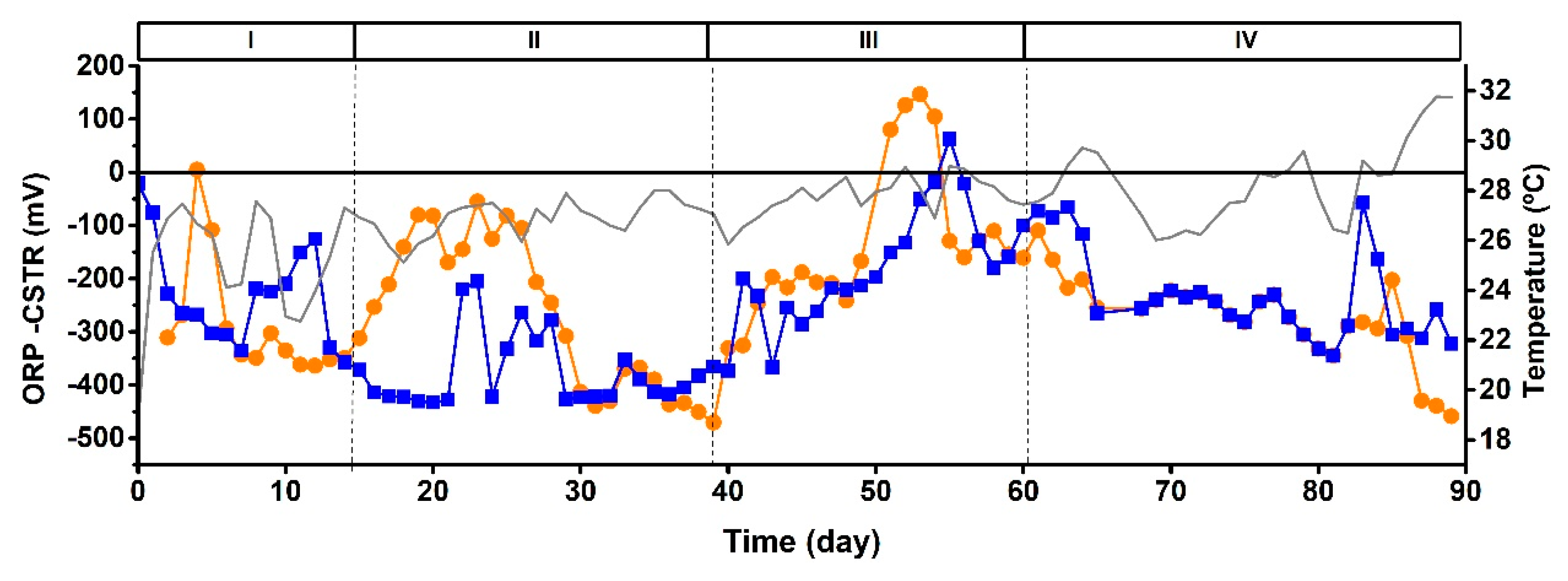
3.2. Semicontinuous operation using CSTR to evaluate the anaerobic and aerobic conditions: ORP influence on the phototrophic mixed culture
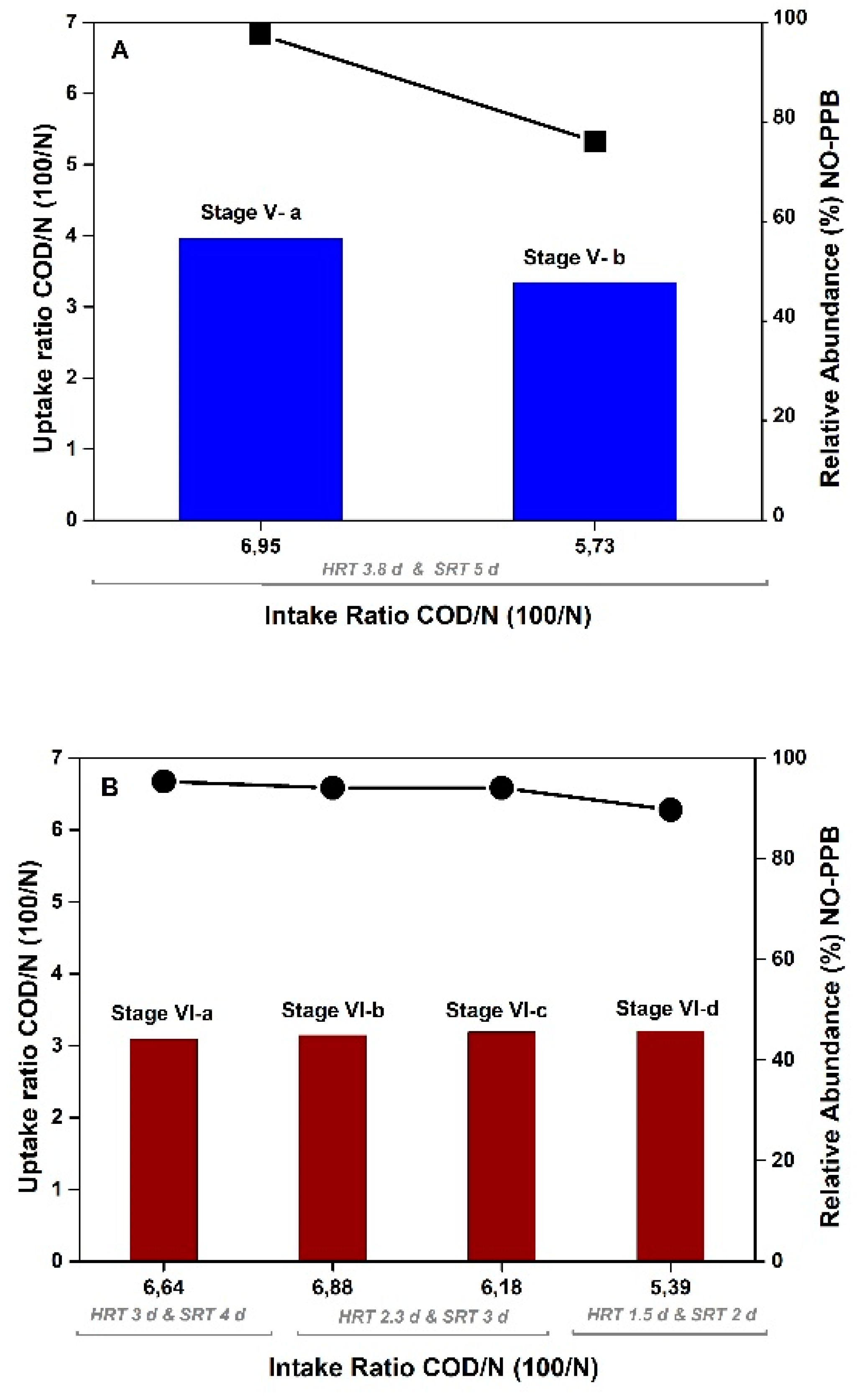
3.3. Semicontinuous operation using MBR to evaluate the SRT and HRT parameters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- “Agua y saneamiento - Desarrollo Sostenible.” https://www.un.org/sustainabledevelopment/es/water-and-sanitation/ (accessed Oct. 24, 2022).
- “EUR-Lex - 52020DC0098 - ES - EUR-Lex.” https://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1583933814386&uri=COM:2020:98:FIN (accessed Oct. 24, 2022).
- L. Rizzo et al., "Best available technologies and treatment trains to address current challenges in urban wastewater reuse for irrigation of crops in EU countries," Science of The Total Environment, vol. 710, p. 136312, Mar. 2020. [CrossRef]
- A. Poustie, Y. Yang, P. Verburg, K. Pagilla, and D. Hanigan, "Reclaimed wastewater as a viable water source for agricultural irrigation: A review of food crop growth inhibition and promotion in the context of environmental change," Science of The Total Environment, vol. 739, p. 139756, Oct. 2020. [CrossRef]
- G. Deviller, L. Lundy, and D. Fatta-Kassinos, "Recommendations to derive quality standards for chemical pollutants in reclaimed water intended for reuse in agricultural irrigation," Chemosphere, vol. 240, p. 124911, Feb. 2020. [CrossRef]
- ilje pikaar et al., Resource Recovery from Water Principles and Application.
- “BOE.es - BOE-A-1998-24166 Real Decreto 2116/1998, de 2 de octubre, por el que se modifica el Real Decreto 509/1996, de 15 de marzo, de desarrollo del Real Decreto-ley 11/1995, de 28 de diciembre, por el que se establecen las normas aplicables al tratamiento de las aguas residuales urbanas.” https://www.boe.es/buscar/doc.php?id=BOE-A-1998-24166 (accessed Oct. 14, 2022).
- A. Oehmen et al., "Advances in enhanced biological phosphorus removal: From micro to macro scale," Water Res, vol. 41, no. 11, pp. 2271–2300, Jun. 2007. [CrossRef]
- D. Puyol, D. J. Batstone, T. Hülsen, S. Astals, M. Peces, and J. O. Krömer, "Resource Recovery from Wastewater by Biological Technologies: Opportunities, Challenges, and Prospects," Front Microbiol, vol. 7, no. JAN, Jan. 2016. [CrossRef]
- Y. da Du et al., "Wastewater treatment and simultaneous production of algal lipids in sequencing batch reactors containing a microalgal-bacterial consortium," Int Biodeterior Biodegradation, vol. 175, p. 105491, Nov. 2022. [CrossRef]
- A. Anbalagan, S. Schwede, C. F. Lindberg, and E. Nehrenheim, "Influence of hydraulic retention time on indigenous microalgae and activated sludge process," Water Res, vol. 91, pp. 277–284, Mar. 2016. [CrossRef]
- K. Tanavarotai et al., "Storage and reactivation of aerobic granular sludge: A review," Fuel, vol. 330, p. 125536, Dec. 2022. [CrossRef]
- Y. Hamid and A. Masłó, "Impact of Uneven Flow Wastewater Distribution on the Technological Efficiency of a Sequencing Batch Reactor," Sustainability 2022, Vol. 14, Page 2405, vol. 14, no. 4, p. 2405, Feb. 2022. [CrossRef]
- C. S. Lee, S. A. Lee, S. R. Ko, H. M. Oh, and C. Y. Ahn, "Effects of photoperiod on nutrient removal, biomass production, and algal-bacterial population dynamics in lab-scale photobioreactors treating municipal wastewater," Water Res, vol. 68, pp. 680–691, Jan. 2015. [CrossRef]
- Y. da Du et al., "Wastewater treatment and simultaneous production of algal lipids in sequencing batch reactors containing a microalgal-bacterial consortium," Int Biodeterior Biodegradation, vol. 175, p. 105491, Nov. 2022. [CrossRef]
- V. Díaz, J. C. Leyva-Díaz, M. C. Almécija, J. M. Poyatos, M. del Mar Muñío, and J. Martín-Pascual, "Microalgae bioreactor for nutrient removal and resource recovery from wastewater in the paradigm of circular economy," Bioresour Technol, vol. 363, p. 127968, Nov. 2022. [CrossRef]
- H. U. Cho, J. M. Park, and Y. M. Kim, "Enhanced microalgal biomass and lipid production from a consortium of indigenous microalgae and bacteria present in municipal wastewater under gradually mixotrophic culture conditions," Bioresour Technol, vol. 228, pp. 290–297, Mar. 2017. [CrossRef]
- V. C. F. Carvalho, M. Kessler, J. C. Fradinho, A. Oehmen, and M. A. M. Reis, "Achieving nitrogen and phosphorus removal at low C/N ratios without aeration through a novel phototrophic process," Science of The Total Environment, vol. 793, p. 148501, Nov. 2021. [CrossRef]
- K. Cao, R. Zhi, and G. Zhang, "Photosynthetic bacteria wastewater treatment with the production of value-added products: A review," Bioresour Technol, vol. 299, p. 122648, Mar. 2020. [CrossRef]
- T. Hülsen, D. J. Batstone, and J. Keller, "Phototrophic bacteria for nutrient recovery from domestic wastewater," Water Res, vol. 50, pp. 18–26, Mar. 2014. [CrossRef]
- S. Imam et al., "IRsp1095: A genome-scale reconstruction of the Rhodobacter sphaeroides metabolic network," BMC Syst Biol, vol. 5, no. 1, pp. 1–17, Jul. 2011. [CrossRef]
- J. R. Almeida, J. C. Fradinho, G. Carvalho, A. Oehmen, and M. A. M. Reis, "Dynamics of Microbial Communities in Phototrophic Polyhydroxyalkanoate Accumulating Cultures," Microorganisms, vol. 10, no. 2, p. 351, Feb. 2022. [CrossRef]
- J. R. Almeida, E. Serrano, M. Fernandez, J. C. Fradinho, A. Oehmen, and M. A. M. Reis, "Polyhydroxyalkanoates production from fermented domestic wastewater using phototrophic mixed cultures," Water Res, vol. 197, p. 117101, Jun. 2021. [CrossRef]
- L. D. Allegue, M. Ventura, J. A. Melero, and D. Puyol, "Unraveling PHA production from urban organic waste with purple phototrophic bacteria via organic overload," Renewable and Sustainable Energy Reviews, vol. 166, p. 112687, Sep. 2022. [CrossRef]
- J. C. Fradinho, M. A. M. Reis, and A. Oehmen, "Beyond feast and famine: Selecting a PHA accumulating photosynthetic mixed culture in a permanent feast regime," Water Res, vol. 105, pp. 421–428, Nov. 2016. [CrossRef]
- L. Lorini, F. di Re, M. Majone, and F. Valentino, "High rate selection of PHA accumulating mixed cultures in sequencing batch reactors with uncoupled carbon and nitrogen feeding," N Biotechnol, vol. 56, pp. 140–148, May 2020. [CrossRef]
- D. M. George, A. S. Vincent, and H. R. Mackey, "An overview of anoxygenic phototrophic bacteria and their applications in environmental biotechnology for sustainable Resource recovery," Biotechnology Reports, vol. 28, p. e00563, Dec. 2020. [CrossRef]
- A. Alloul, M. Muys, N. Hertoghs, F. M. Kerckhof, and S. E. Vlaeminck, "Cocultivating aerobic heterotrophs and purple bacteria for microbial protein in sequential photo- and chemotrophic reactors," Bioresour Technol, vol. 319, p. 124192, Jan. 2021. [CrossRef]
- T. Hülsen, K. Hsieh, Y. Lu, S. Tait, and D. J. Batstone, "Simultaneous treatment and single cell protein production from agri-industrial wastewaters using purple phototrophic bacteria or microalgae – A comparison," Bioresour Technol, vol. 254, pp. 214–223, Apr. 2018. [CrossRef]
- J. Spanoghe, K. J. Ost, W. van Beeck, P. Vermeir, S. Lebeer, and S. E. Vlaeminck, "Purple bacteria screening for photoautohydrogenotrophic food production: Are new H2-fed isolates faster and nutritionally better than photoheterotrophically obtained reference species?," N Biotechnol, vol. 72, pp. 38–47, Dec. 2022. [CrossRef]
- M. Cerruti, G. Crosset-Perrotin, M. Ananth, J. L. Rombouts, D. G. Weissbrodt, and D. Weissbrodt, "Syntrophy between fermentative and purple phototrophic bacteria for carbohydrate-based wastewater treatment," bioRxiv, p. 2021.05.13.444055, May 2021. [CrossRef]
- T. Hülsen, S. Stegman, D. J. Batstone, and G. Capson-Tojo, "Naturally illuminated photobioreactors for resource recovery from piggery and chicken-processing wastewaters utilising purple phototrophic bacteria," Water Res, vol. 214, May 2022. [CrossRef]
- T. Hülsen, E. M. Barry, Y. Lu, D. Puyol, J. Keller, and D. J. Batstone, "Domestic wastewater treatment with purple phototrophic bacteria using a novel continuous photo anaerobic membrane bioreactor," Water Res, vol. 100, pp. 486–495, Sep. 2016. [CrossRef]
- L. D. Allegue, D. Puyol, and J. A. Melero, "Food waste valorization by purple phototrophic bacteria and anaerobic digestion after thermal hydrolysis," Biomass Bioenergy, vol. 142, p. 105803, Nov. 2020. [CrossRef]
- R. Baird, A. D. Eaton, E. W. Rice, and L. Bridgewater, Standard methods for the examination of water and wastewater, 23rd ed. American Public Health Association, 2017. Accessed: Oct. 24, 2022. [Online]. Available: https://www.worldcat.org/es/title/standard-methods-for-the-examination-of-water-and-wastewater/oclc/1019687035.
- D. Puyol, T. Hülsen, B. Padrino, D. J. Batstone, F. Martinez, and J. A. Melero, "Exploring the inhibition boundaries of mixed cultures of purple phototrophic bacteria for wastewater treatment in anaerobic conditions," Water Res, vol. 183, p. 116057, Sep. 2020. [CrossRef]
- V. Montiel-Corona and G. Buitrón, "Polyhydroxyalkanoates from organic waste streams using purple non-sulfur bacteria," Bioresour Technol, vol. 323, p. 124610, Mar. 2021. [CrossRef]
- C. Nairn et al., "Alkalinity, and Not the Oxidation State of the Organic Substrate, Is the Key Factor in Domestic Wastewater Treatment by Mixed Cultures of Purple Phototrophic Bacteria," Resources 2020, Vol. 9, Page 88, vol. 9, no. 7, p. 88, Jul. 2020. [CrossRef]
- G. Capson-Tojo et al., "Purple phototrophic bacteria for resource recovery: Challenges and opportunities," Biotechnol Adv, vol. 43, p. 107567, Nov. 2020. [CrossRef]
- G. Capson-Tojo, S. Lin, D. J. Batstone, and T. Hülsen, "Purple phototrophic bacteria are outcompeted by aerobic heterotrophs in the presence of oxygen," Water Res, vol. 194, p. 116941, Apr. 2021. [CrossRef]
- M. Cerruti, H. T. Ouboter, V. Chasna, M. C. M. van Loosdrecht, C. Picioreanu, and D. G. Weissbrodt, "Effects of light / dark diel cycles on the photoorganoheterotrophic metabolism of Rhodopseudomonas palustris for differential electron allocation to PHAs and H2," bioRxiv, p. 2020.08.19.258533, Aug. 2020. [CrossRef]
- "The r/K selection theory and its application in biological wastewater treatment processes - ScienceDirect." [Online]. Available: https://www.sciencedirect.com/science/article/pii/S0048969722009287?ref=pdf_download&fr=RR-2&rr=74b94c6b4bec69bf#f0020.
- M. K. H. Winkler, P. Boets, B. Hahne, P. Goethals, and E. I. P. Volcke, "Effect of the dilution rate on microbial competition: R-strategist can win over kstrategist at low substrate concentration," PLoS One, vol. 12, no. 3, Mar. 2017. [CrossRef]
- T. Hülsen, K. Hsieh, and D. J. Batstone, "Saline wastewater treatment with purple phototrophic bacteria," Water Res, vol. 160, pp. 259–267, Sep. 2019. [CrossRef]
- C. A. Sepúlveda-Muñoz, I. de Godos, and R. Muñoz, "Wastewater Treatment Using Photosynthetic Microorganisms," Symmetry (Basel), vol. 15, no. 2, p. 525, Feb. 2023. [CrossRef]
- P. Dalaei, D. Ho, G. Nakhla, and D. Santoro, "Low temperature nutrient removal from municipal wastewater by purple phototrophic bacteria (PPB)," Bioresour Technol, vol. 288, Sep. 2019. [CrossRef]
- J. Heider and G. Fuchs, "Thauera ," in Bergey's Manual of Systematics of Archaea and Bacteria, Wiley, 2015, pp. 1–11. [CrossRef]
- A. Alloul, N. Blansaer, P. Cabecas Segura, R. Wattiez, S. E. Vlaeminck, and B. Leroy, "Dehazing redox homeostasis to foster purple bacteria biotechnology," Trends Biotechnol, vol. 41, no. 1, pp. 106–119, Jan. 2023. [CrossRef]
- M. T. Madrigan, J. M. Martinko, K. S. Bender, D. H. Buckley, and D. A. Stahl, Brock Biología de los Microorganismos 14a Edición.
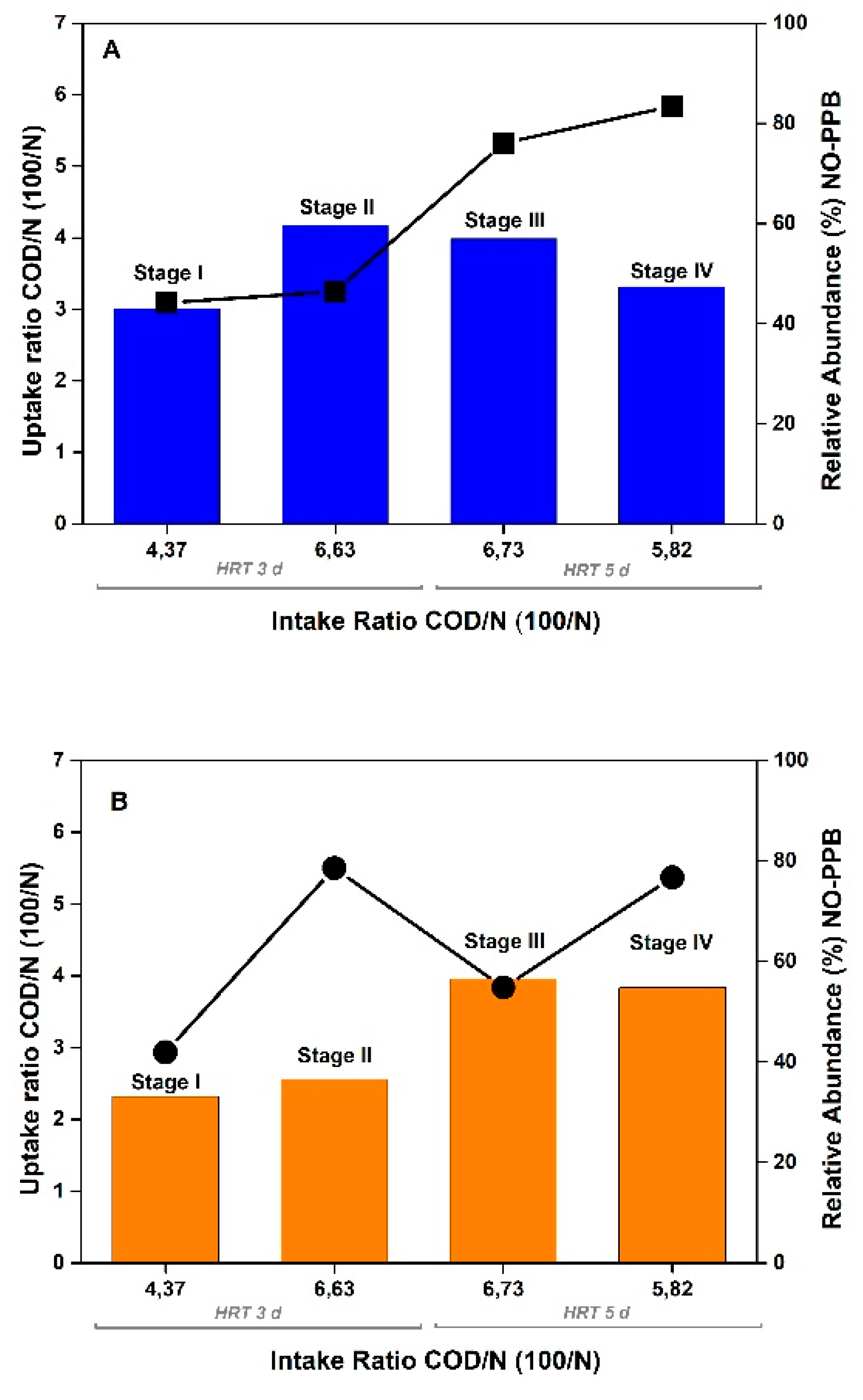
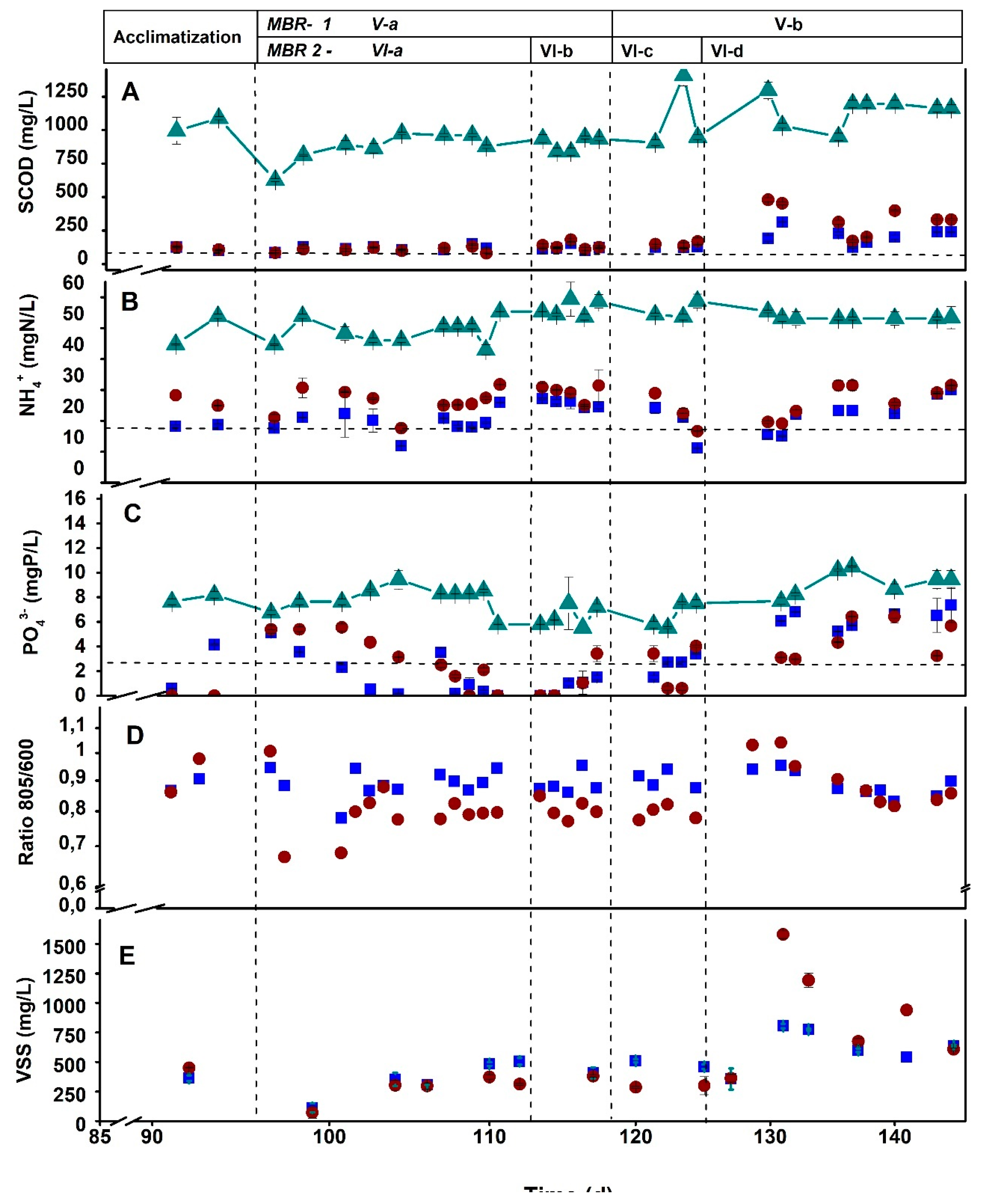
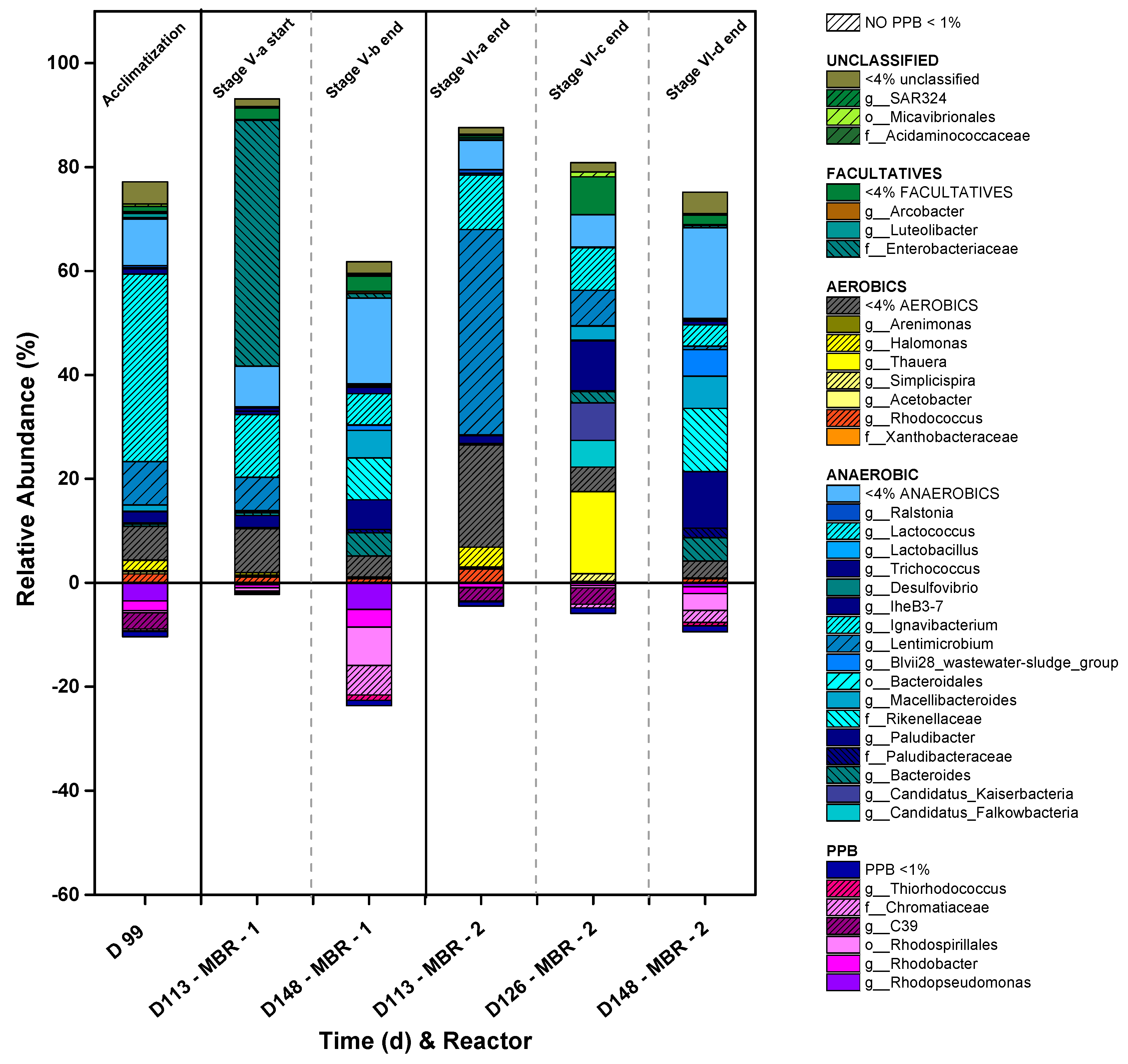
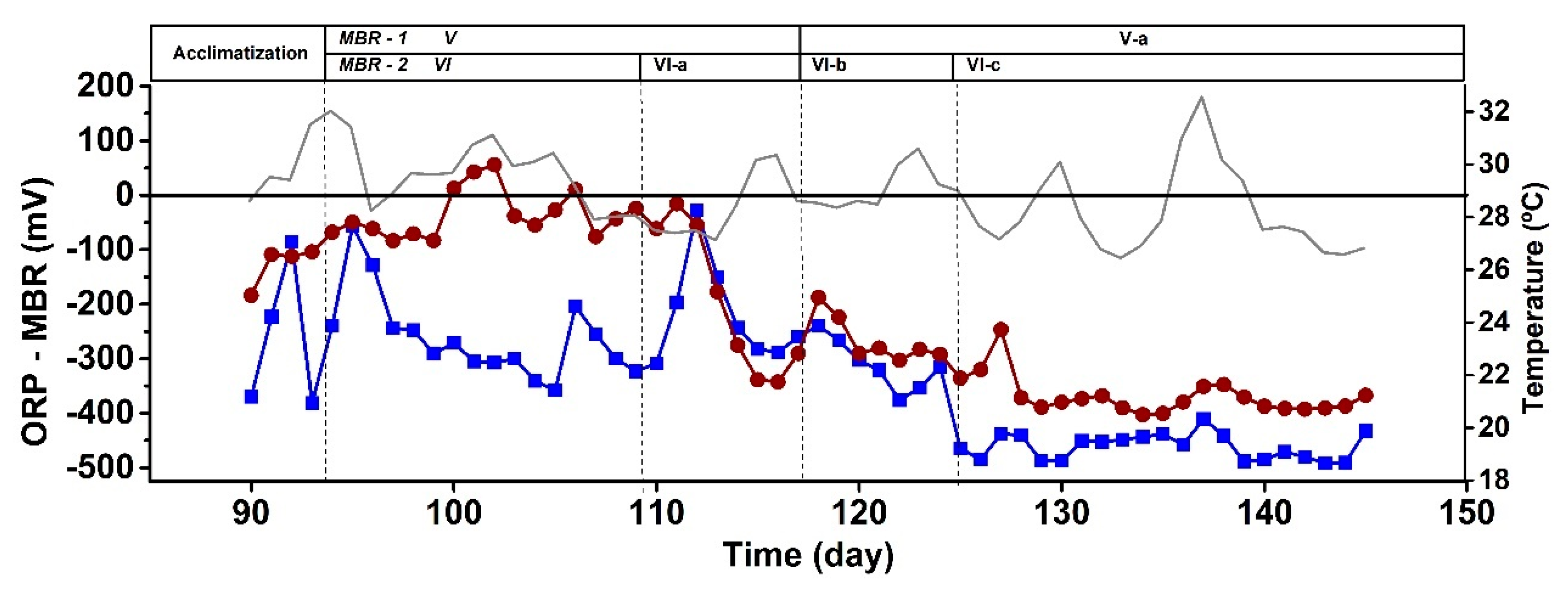
| SCOD (mg/L) |
TCOD (mg/L) |
NH4+ (mgN/L) |
PO43- (mgP/L) |
VSS (mg/L)* |
|
|---|---|---|---|---|---|
| Campaign 1: days 1-39 | |||||
| OFMSW hydrolysate (n=9) | 67000 (1000) | 78000 (3000) | 59 (1) | 75 (4) | 58 (1) |
| DWW Estiviel (n=18) | 196 (6) | 270 (8) | 48 (1) | 5 (1) | 26 (4) |
| Campaign 2: days 40-60 | |||||
| OFMSW hydrolysate (n=9) | 62000 (2000) | 78000 (3000) | 37 (2) | 132 (2) | 58 (1) |
| DWW Estiviel (n=18) | 168 (3) | 270 (8) | 54 (1) | 6 (1) | 24 (2) |
| Campaign 3: days 61-147 | |||||
| OFMSW hydrolysate (n=9) | 51000 (1000) | 57000 (300) | 85 (4) | 130 (20) | 53 (1) |
| DWW Estiviel (n=18) | 106 (3) | 500 (22) | 48 (2) | 5 (1) | 90 (15) |
| SPA source biowastes | |||||
| OFMSW hydrolysate (n=9) | 67000 (1000) | 78000 (3000) | 59 (1) | 75 (4) | 58 (1) |
| DWW La Gavia (n=4) | 155 (10) | 203 (20) | 65 (1) | 16 (1) | 69 (10) |
| Inoculum (SPA and semicontinuous photobioreactors) | |||||
| Inoculum of PPB (n=3) | 330 (10) | 800 (20) | 6 (1) | 5 (1) | 368 (3) |
| COD:N ratio | SCOD (mg/L) |
NH4+ (mgN/L) |
PO43 (mgP/L) |
VSS (mg/L) |
TOC (mg/L) |
pH |
|---|---|---|---|---|---|---|
| 100:3 | 2080 ± 90 | 62 ± 1 | 11.6 ± 0.6 | 92.0 ± 0.0 | 890.5 ± 59.4 | 6.92 ± 0.02 |
| 100:4 | 1400 ± 40 | 53 ±1 | 8.5 ± 0.4 | 100 ± 10 | 587.2 ± 39.1 | 6.91 ± 0.02 |
| 100:5 | 1080 ± 50 | 59 ± 2 | 8 ± 2 | 104 ± 5 | 554.8 ± 37.0 | 6.9 ± 0.02 |
| 100:6 | 1190 ± 50 | 53 ± 1 | 8.0 ± 0.7 | 92 ± 5 | 478.8 ± 31.9 | 7.03 ± 0.02 |
| 100:7 | 1010 ± 60 | 53 ± 3 | 7.2 ± 0.2 | 88 ± 5 | 401.3 ± 26.8 | 7.04 ± 0.02 |
| DWW (control) | 400 ± 90 | 54.8 ± 0.6 | 5.0 ± 0.9 | 86 ± 8 | 54.4 ± 3.6 | 7.55 ± 0.02 |
| LF (control) | 1600 ± 90 | 11.4 ± 0.6 | 4.7 ± 0.1 | 41 ± 2 | 716.8 ± 47.8 | 4.46 ± 0.02 |
| CSTR mode | MBR mode | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reactor | CSTR-1 and CSTR-2 | MBR-1 | MBR-2 | |||||||||||
| Stage | I | II | III | IV | V-a | V-b | VI-a | VI-b | VI-c | VI-d | ||||
| Period (d) | 01-15 | 15-40 | 40-62 | 62-96 | 96-118 | 118-145 | 96-111 | 111-118 | 118-125 | 125-145 | ||||
| Time (d) | 15 | 25 | 22 | 34 | 22 | 27 | 15 | 7 | 7 | 20 | ||||
| HRT (d) | 3 | 3 | 5 | 5 | 3.8 | 3.8 | 3 | 2.3 | 2.3 | 1.5 | ||||
| SRT (d) | 3 | 3 | 5 | 5 | 5 | 5 | 4 | 3 | 3 | 2 | ||||
| COD:N | 100:4.4±1.7 | 100: 6.6±0.9 | 100:6.7±0.7 | 100:5.8±0.6 | 100:6.9±0.6 | 100:5.8±0.6 | 100:6.6±0.8 | 100:6.9±0.7 | 100:6.2±1.4 | 100:5.4±0.6 | ||||
| OLR (g COD / L·d) | 0.41 (0.05) | 0.30 (0.07) | 0.21 (0.04) | 0.21 (0.05) | 0.23 (0.03) | 0.30 (0.04) | 0.29 (0.05) | 0.39 (0.02) | 0.40 (0.01) | 0.76 (0.09) | ||||
| SLR (g COD / gVSS·d)* | 1.6 (0.6) | 1.6 (0.7) |
0.8 (0.3) | 1.0 (0.3) |
0.40 (0.09) | 0.49 (0.15) | 0.6 (0.2) | 1.0 (0.3) | 0.8 (0.5) |
0.6 (0.2) |
1.0 (0.1) |
1.17 (0.07) | 1.2 (0.3) | 1.2 (0.6) |
| Inlet parameters (mg/L) | ||||||||||||||
| SCOD | 1200 (40) | 970 (20) | 1010 (40) | 1100 (40) | 890 (20) | 1100 (30) | 890 (20) | 870 (30) | 910 (20) | 1210 (30) | ||||
| NH4+-N | 32 (1) | 56 (1) | 54 (1) | 55 (1) | 51 (1) | 55 (2) | 50 (1) | 54 (2) | 57 (2) | 54 (2) | ||||
| PO43--P | 6 (1) | 7 (1) | 8 (1) | 9 (1) | 8 (1) | 8 (1) | 8 (1) | 7 (1) | 7 (1) | 9 (1) | ||||
| Ratio test | COD:N: P intake | Removal (%) | pH |
Y X/S (mgVSS/mgCOD) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| COD | N | P | COD | N-NH4+ | P-PO43- | Initial | Final | ||
| 100:3 | 100 | 5.1 | 0.9 | 64.1 | 99.4 | 91.9 | 6.9 ± 0.1 | 6.9 ± 0.1 | 0.48 ± 0.01 |
| 100:4 | 100 | 7.4 | 1.2 | 58.6 | 98.8 | 100.0 | 6.9 ± 0.1 | 7.0 ± 0.1 | 0.68 ± 0.10 |
| 100:5 | 100 | 10.9 | 1.4 | 59.6 | 99.1 | 100.0 | 6.9 ± 0.1 | 6.9 ± 0.1 | 0.71 ± 0.03 |
| 100:6 | 100 | 6.4 | 1.0 | 74.2 | 98.8 | 100.0 | 7.0 ± 0.1 | 6.8 ± 0.0 | 0.46 ± 0.06 |
| 100:7 | 100 | 6.8 | 0.9 | 84.5 | 99.9 | 100.0 | 7.0 ± 0.1 | 6.9 ± 0.1 | 0.27 ± 0.09 |
| DWW | 100 | 10.9 | 0.7 | 86.8 | 62.9 | 41.7 | 7.5 ± 0.1 | 7.8 ± 0.1 | 0.26 ± 0.05 |
| LF | 100 | 1.4 | 0.3 | 30.6 | 52.4 | 28.7 | 4.5 ± 0.1 | 4.8 ± 0.1 | 0.21 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





