Submitted:
26 July 2023
Posted:
28 July 2023
You are already at the latest version
Abstract
Keywords:
INTRODUCTION
MATERIAL AND METHOD
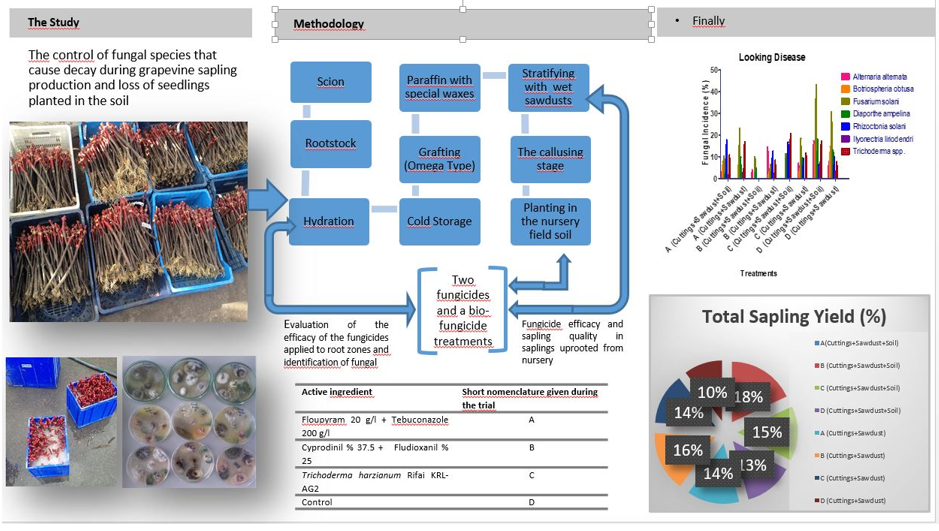
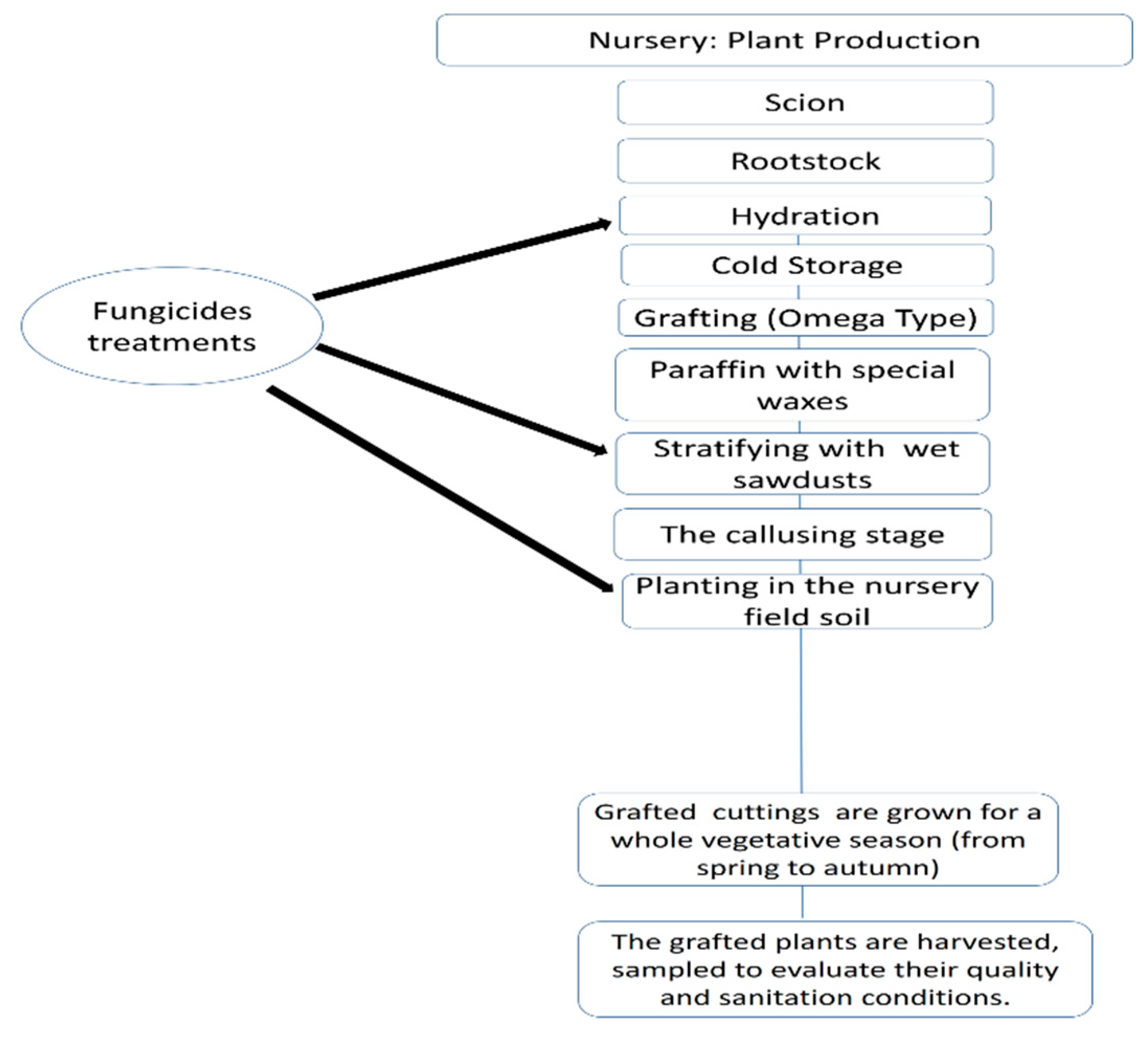
Plant Material
Fungicides Applications in the production of grapevine planting material
Evaluation of the Efficacy of the Fungicides Applied to Root Zones
Identification of Fungal Pathogens
Statistical analysis
RESULTS
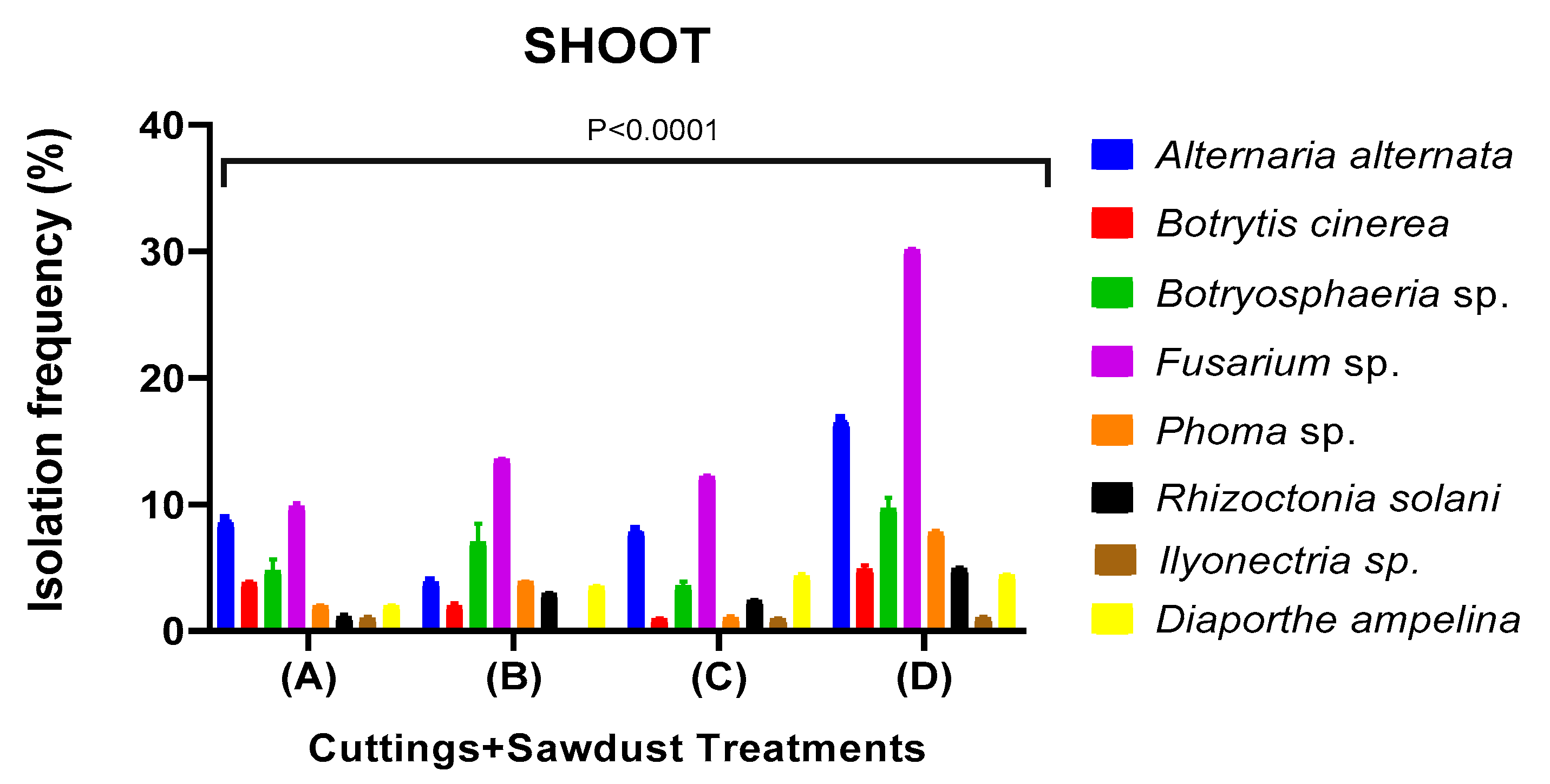
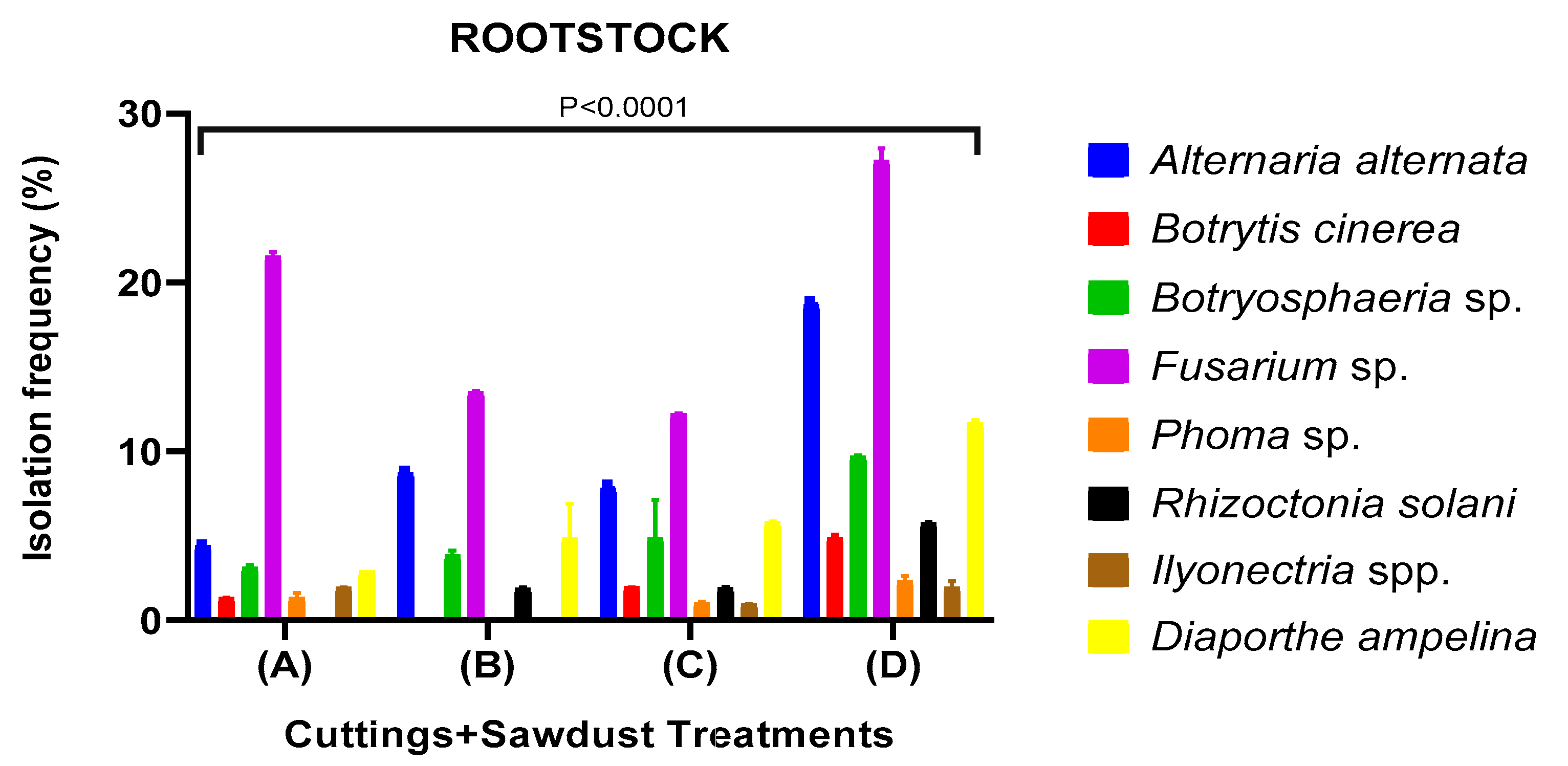
Identification of Fungi that Cause Rot During Callus Development
Morphological and molecular identification of fungal pathogens
| Species | Isolate Code | Host | Origin | GenBank ITS Accession No |
|---|---|---|---|---|
| Phoma exigua var. exigua | MBAE77 | Vitis vinifera | Manisa | OQ392363 |
| Fusarium solani | MBAE236 | V. vinifera | Manisa | OQ392364 |
| Fusarium solani | MBAE237 | V. vinifera | Manisa | OQ392365 |
| Fusarium solani | MBAE259 | V. vinifera | Manisa | OQ392370 |
| Diaporthe ampelina | MBAE239 | V. vinifera | Manisa | OQ392366 |
| Rhizoctonia solani | MBAE241 | V. vinifera | Manisa | OQ392367 |
| Botryosphaeria dothidea | MBAE242 | V. vinifera | Manisa | OQ392368 |
| Ilyonectria liriodendri | MBAE322 | V. vinifera | Manisa | OQ392371 |
| Alternaria alternata | MBAE329 | V. vinifera | Manisa | OQ392372 |
| Trichoderma gamsii | MBAE374 | V. vinifera | Manisa | OQ359568 |
| Trichoderma afroharzianum | MBAE377NS | V. vinifera | Manisa | ON819609 |
Fungicide Efficacy and Sapling Quality in Saplings Uprooted from Nursery
| Looking Disease | Fungal İncidence % | ||||||
|---|---|---|---|---|---|---|---|
| Treatment | Alternaria alternata | Botryospheria obtusa | Fusarium solani | Diaporthe ampelina | Rhizoctonia solani | Ilyonectria liriodendri | Trichoderma spp. |
| A(Cuttings+Sawdust+Soil) | 3,125 ab | 6,51 bc | 9,08 c | 1,92 a | 15,625 d | 1,46 a | 9,56 c |
| A(Cuttings+Sawdust) | 0,00 a | 5,08 ab | 15,31 b | 6,25 ab | 0,00 a | 3,125 a | 15,54 b |
| B(Cuttings+Sawdust+Soil) | 3,14 b | 0,00 a | 9,16 c | 3,13 b | 0,00 a | 0,00 a | 0,00 a |
| B(Cuttings+Sawdust) | 12,53 c | 0,00 a | 4,79 ab | 3,25 a | 9,46 bc | 0,00 a | 6,25 ab |
| C(Cuttings+Sawdust+Soil) | 0,00 a | 0,00 a | 10,88 b | 9,43 b | 15,625 c | 0,00 a | 18,28 c |
| C(Cuttings+Sawdust) | 6,25 ab | 3,16 ab | 14,88 c | 9,38 bc | 6,25 ab | 0,00 a | 10,63 bc |
| D(Cuttings+Sawdust+Soil) | 15,68 a | 15,63 a | 36,86 b | 12,52 a | 6,25 a | 6,25 a | 15,65 a |
| D(Cuttings+Sawdust) | 6,25 a | 12,25 a | 25,99 b | 12,50 a | 6,50 a | 3,13 a | 6,25 ab |
| Looking Healthy | Fungal Incidence % | ||||||
|---|---|---|---|---|---|---|---|
| Treatment | Alternaria alternata | Botryospheria obtusa | Fusarium solani | Diaporthe ampelina | Rhizoctonia solani | Ilyonectria liriodendri | Trichoderma spp. |
| A(Cuttings+Sawdust+Soil) | 0,00 a | 0,00 a | 0,00 a | 0,00 a | 0,00 a | 0,00 a | 3,125 b |
| A(Cuttings+Sawdust) | 0,00 a | 0,00 a | 6,50 b | 0,00 a | 0,00 a | 0,00 a | 10,75 b |
| B(Cuttings+Sawdust+Soil) | 0,00 a | 0,00 a | 6,50 b | 0,00 a | 0,00 a | 0,00 a | 8,67 b |
| B(Cuttings+Sawdust) | 0,00 a | 0,00 a | 3,75 a | 0,00 a | 0,00 a | 0,00 a | 18,13 c |
| C(Cuttings+Sawdust+Soil) | 0,00 a | 0,00 a | 6,25 b | 3,25 b | 0,00 a | 0,00 a | 56,25 d |
| C(Cuttings+Sawdust) | 15,63 c | 12,25 c | 12,65 c | 6,25 b | 0,00 a | 0,00 a | 34,36 c |
| D(Cuttings+Sawdust+Soil) | 3,25 a | 12,50 b | 31,25 c | 6,17 ab | 0,00 a | 0,00 a | 31,25 c |
| D(Cuttings+Sawdust) | 3,13 ab | 8,49 c | 18,68 d | 6,25 bc | 0,00 a | 0,00 a | 28,79 d |
| Treatments | Total Sapling Yield (%) | I. Length Grafted Vine Sapling Yield (%) | Callus development level (scale 0-4) | Shoot length (cm) |
| A(Cuttings+Sawdust+Soil) | 69.38 ab** | 40.625 ab* | 3.75 ab** | 23.60 ab* |
| B (Cuttings+Sawdust+Soil) | 78.75 a | 61.25 a | 3.80 b | 22.90 ab |
| C (Cuttings+Sawdust+Soil) | 64.38 abc | 44.25 ab | 3.60 ab | 16.40 bc |
| D (Cuttings+Sawdust+Soil) | 57.50 bc | 34. 38 b | 3.45 ab | 16.00 bc |
| A (Cuttings+Sawdust) | 61.25 abc | 43.75 ab | 3.65 ab | 21.00 ab |
| B (Cuttings+Sawdust) | 70.63 ab | 50.00 ab | 3.85 b | 20.70 ab |
| C (Cuttings+Sawdust) | 60.00 abc | 43.13 ab | 3.90 a | 28.70 a |
| D (Cuttings+Sawdust) | 45.00 c | 25.00 ab | 3.60 ab | 11.20 b |
DISCUSSION
Conclusion
Acknowledgements
Conflict of interest
Ethical approval
References
- FAOSTAT. 2021. Available online at: http://www.fao.org/faostat/en/#data/QC (accessed on 25 June 2021).
- Ilter, E.; Uzun, I. The importance and drawbacks of grapevine nursery in Turkey. Turkey 1st Nursery Symposium (26-28 October 1987), Ankara, Türkiye, 1991, p.133.
- Ilter, E.; Uzun, I. The importance and drawbacks of grapevine nursery in Turkey. Turkey 1st Nursery Symposium (26-28 October 1987), Ankara, Türkiye, 1991, p.133.
- Sen, A.; Yağcı, A. Effects of Different Rooting Sites on Sapling Yield and Quality in Grapevine Sapling Production. Fruit Science 2016, 3, 22–28. [Google Scholar]
- Cangi, R.; Yanar, Y.; Yılmaz, Y.D. Effects of Brining and Picking Time on The Degradation of Pesticide Residue in Grapevine Leaves. Turkish Journal of Agriculture - Food Science and Technology 2019, 7, 1773–1779. [Google Scholar] [CrossRef]
- Halleen, F.; Crous, P.W.; Petrini, O. Fungi associated with healthy grapevine cuttings in nurseries, with special reference to pathogens involved in the decline of young vines. Australas. Plant Pathol. 2003, 32, 47–52. [Google Scholar] [CrossRef]
- Lade, S.B.; Štraus, D.; Oliva, J. Variation in Fungal Community in Grapevine (Vitis vinifera) Nursery Stock Depends on Nursery, Variety and Rootstock. J. Fungi 2022, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.F.; Pires, M.C.; Soares, W.R.O.; Rezende, D.V.; Blum, L.E.B. Soil-Borne Plant Pathogens Associated to Decline of Grapevine Grown in Greenhouse. J Plant Physiol Pathol. 2014, 2, 1. [Google Scholar] [CrossRef]
- Machowicz-Stefaniak, Z.; Krol, E. Characterization of Phoma negriana thun. A new species from grapevine canes. Acta Mycol. 2006. [Google Scholar]
- Król, E. Fungi Inhabiting Decaying Grapevine (Vitis spp.) Cuttings. Journal of Plant Protection Research 2006, 46, 353–358. [Google Scholar]
- Koycu, N.D.; Ozer, C.; Coskuntuna, A.; Ozer, N. The Control of Fungal Diseases on Vine Grafts During Callus Formation. 12th Congress of the Mediterranean Phytopathological Union, Rhodes Island, Greece, June 11-15. 2006; pp. 475–477.
- Becker, H.; Hiller, M.H. Hygiene in modern bench grafting. American Journal of Enology and Viticulture 1977, 28, 113–118. [Google Scholar] [CrossRef]
- Akman, İ.; Ilgın, C. The effect of layering material used in germination on seedling yield and quality in grafted grapevine sapling production. Viticulture Research Ins., Manisa, Turkey, Publication No: 52, 1993.
- Korkutal, I.; Kaygusuz, G.; Bayram, S. ; “Different effect of scion types on callusing in bench grafting”. African Journal of Biotechno. 2011, 10, 15123–15129. [Google Scholar] [CrossRef]
- Rego, C.; L. Farropas; T. Nascimento; Cabral, A.; Oliveira, H. Black foot of grapevine, sensitivity of Cylindrocarpon destructans to fungicides. Phytopathologia Mediterr. 2006, 45, 93–100. [Google Scholar]
- Koike, S.T.; Subbarao, K.V.; Verkley, G.J.M.; Fogle, D.; O’Neill, T.M. Phoma basal rot of romaine lettuce in California caused by Phoma exigua: Occurrence, characterization, and control. Plant Dis. 2006, 90, 1268–1275. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, S.; Goltapeh, E.M.; Shams-Bakhsh, M. , Safaie, N.; Chaichi, M. Genetic and Phenotypic Diversity among Botrytis cinerea Isolates in Iran. Journal Phytopath. 2009, 157, 474–482. [Google Scholar] [CrossRef]
- Úrbez-Torres, J.R.; Peduto, F.; Smith, R.; Gubler, W. Phomopsis dieback: A grapevine trunk disease caused by Phomopsis viticola in California. Plant Dis. 2013, 97, 1571–1579. [Google Scholar] [CrossRef]
- Al-Fadhal, F.A.; AL-Abedy, A.N.; Alkhafije, D.A. Isolation and molecular identification of Rhizoctonia solani and Fusarium solani isolated from cucumber (Cucumis sativus L.) and their control feasibility by Pseudomonas fluorescens and Bacillus subtilis. Egypt J. Biol. Pest Control. 2019, 29, 47. [Google Scholar] [CrossRef]
- Cenis, J.L. Rapid extraction of fungal DNA for PCR amplification. Nucl. Acids Res. 1992, 20, 2380. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; S-Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications. Innis MA, Gel-fand DH, Sninsky JJ, White TJ, eds. Academic Press, Inc., New York. 1990; pp. 315–322.
- Gramaje, D.; Úrbez-Torres, J.R.; Sosnowski, M.R. Managing Grapevine Trunk Diseases With Respect to Etiology and Epidemiology: Current Strategies and Future Prospects. Plant Disease 2018, 102, 12–39. [Google Scholar] [CrossRef] [PubMed]
- Davari, M.; Hajieghrari, B. Phoma negriana, a new invasive pathogen for Moghan's vineyards in Iran. African Journal of Biotech. 2008, 7, 788–79. [Google Scholar]
- Yıldız, F.; Gürsoy, Z.Y. Biological Control Studies Against Fungal Agents Occurring in Grafted Grapevine Cuttings. Turkey 3rd Biological Control Congress, January 25-28, Izmir, Turkey. 1994; pp. 265–268.
- Omer, A.D.; Granett, J.; Wakeman, R.J. Pathogenicity of Fusarium oxysporum on Different Vitis Rootstocks. J. Phytopathol. 1999, 147, 433–436. [Google Scholar] [CrossRef]
- Van Coller, G.J.; Lamprecht, S.C.; Denman, S.; Crous, P.W. Fusarium species associated with roots and crowns of nursery grapevines in the Western Cape province. 40 st Congress of the Southern African Society for Plant Pathology, Cathederal Peak, South Africa, 2004.
- Pearson, C.R., Goheen, A.C. Compendium of Grapevine Diseases. Eds.; St Paul, MN, USA, APS Press, 1988.
- Scheper, R.W.A.; Whisson, D.L.; Scott, E.S. Revised disease cycles of the two types of Phomopsis on grapevine. Grapegrower and Winemaker 1997, 9, 41–44. [Google Scholar]
- Mostert, L.; Denman, S.; Crous, P.W. In vitro screening of fungicides against Phomopsis viticola and Diaporthe perjuncta. Enol. Vitic. 2000, 21, 62–65. [Google Scholar] [CrossRef]
- Berlanas, C.; Ojeda, S.; Lopez-Manzanares, B.; Andres-Sodupe, M.; Bujanda, R.; Mart´ınez-Diz, M.P.; D´ıaz-Losada, E.; Gramaje, G. Occurrence and Diversity of Black-Foot Disease Fungi in Symptomless Grapevine Nursery Stock in Spain. Plant Dis. 2020, 104, 94–10. [Google Scholar] [CrossRef] [PubMed]
- Petit, E.; Barriault, E.; Baumgartner, K.; Wilcox, W.F.; Rolshausen, P.E. Cylindrocarpon species associated with black-foot of grapevine in northeastern United States and southeastern Canada. Am. J. Enol. Vitic. 2011, 62, 177–183. [Google Scholar] [CrossRef]
- Pitt, W.M.; Sosnowski, M.R.; Huang, R.; Qiu, Y.; Steel, C.C.; Savocchia, S. Evaluation of fungicides for the management of Botryosphaeria canker of grapevines. Plant Dis. 2012, 96, 1303–1308. [Google Scholar] [CrossRef] [PubMed]
- Di Marco, S.; Mazzullo, A.; Calzarano, F.; Cesari, A. The control of esca: status and perspectives. Phytopathol. Mediterr. 2000, 39, 232–240. [Google Scholar] [CrossRef]
- Diaz, G.A.; Latorre, B.A. “Efficacy of paste and liquid fungicide formulations to protect pruningwounds against pathogens associated with grapevine trunk diseases in Chile”. Crop Protec. 2013, 46, 106–112. [Google Scholar] [CrossRef]
- Delen, N. Fungicides. Nobel press. Ankara, Turkey. 2008, pp. 318.
- Mondello, V.; Songy, A.; Battiston, E.; Pinto, C.; Coppin, C.; Trotel-Aziz, P.; Clément, C.; Mugnai, L.; Fontaine, F. Grapevine Trunk Diseases: A Review of Fifteen Years of Trials for Their Control with Chemicals and Biocontrol Agents. Plant Dis. 2018, 102, 1189–1217. [Google Scholar] [CrossRef]
- Rego, C.; Nascimento, T.; Cabral, A.; Silva, Mj.; Oliveira, H. Control of grapevine wood fungi in commercial nurseries. Phytopathol. Mediterr. 2009, 48, 128–135. [Google Scholar]
- Yıldız, M.; Tosun, N. Molecular characterization of black foot disease pathogens in grapevine nurseries and evaluation of some fungicides for control of the most virulent isolates. Trakya Univ. J. Nat. Sci. 2022, 23, 95–111. [Google Scholar] [CrossRef]
- Fourie, P.H.; Halleen, F.; Van Der Vyver, J.; Schreuder, W. Effect of Trichoderma treatments on the occurrence of decline pathogens in the roots and rootstocks of nursery grapevines. Phytopathology Mediterr. 2001, 40, 473–478. [Google Scholar]
- Di Marco, S.; F. Osti, Applications ofTrichodermato prevent Phaeomoniella chlamydospora infections inorganic nurseries. Phytopathol. Mediterr. 2007, 46, 73–83. [Google Scholar] [CrossRef]
- Bavaresco, L.; Lovisolo, C. Effect of grafting on grape-vine chlorosis and hydraulic conductivity. Vitis. 2000, 39, 89–92. [Google Scholar]
- Gramaje, D.; Di Marco, S. Identifying practices likely to have impacts on grapevine trunkdisease infections: a European nursery survey. Phytopathol. Mediterr. 2015, 54, 313–324. [Google Scholar]
| Active ingredient | Short nomenclature given during the trial | Trade name/Company | Product rate (recommended dose) (100 L water) |
|---|---|---|---|
| Floupyram 20 g/l + Tebuconazole 200 g/l | A | Luna Experience SC 400/Bayer | 25 ml |
| Cyprodinil % 37.5 + Fludioxanil % 25 | B | Switch 62.5/WG Syngenta | 50 g |
| Trichoderma harzianum Rifai KRL-AG2 | C | T-22 Planter Box/ Bioglobal | 50 g |
| Control | D | Water | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




