Submitted:
14 July 2023
Posted:
17 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
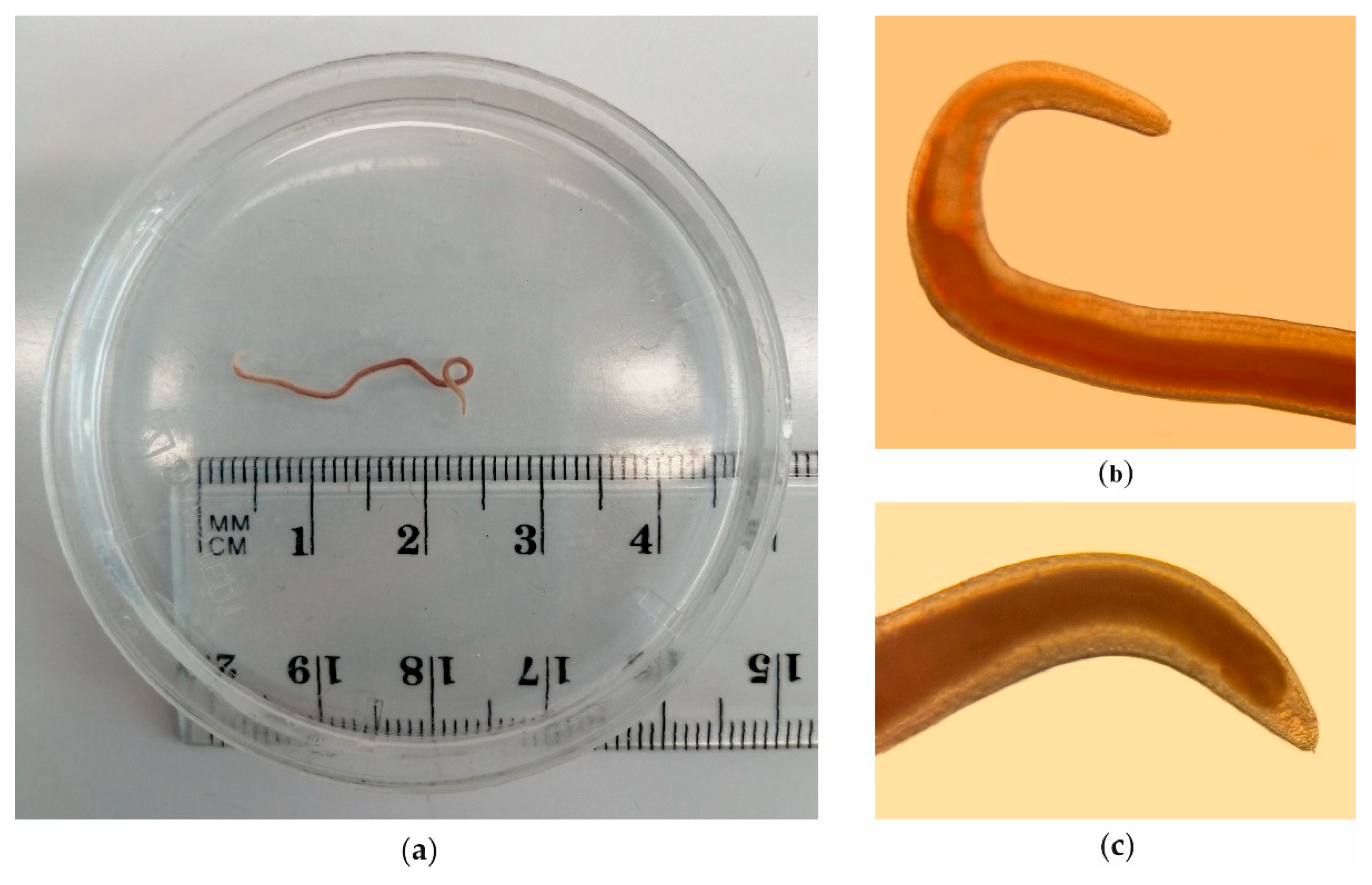
2.1. Sampling of Salmon and Parasitological Inspection
2.2. Identification of Nematode
3. Results
3.1. Salmon
3.2. Molecular Identification
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture; Food and Agriculture Organisation of the United Nations: Italy, 2014; p. 223. [Google Scholar]
- Deardorff, T.L.; Overstreet, R.M. Seafood-Transmitted Zoonoses in the United States: The Fishes, the Dishes, and the Worms. In Microbiology of Marine Food Products; Ward, D.R., Hackney, C., Eds.; Springer: Boston, MA, 1991. [Google Scholar] [CrossRef]
- Shamsi, S. Seafood-borne parasitic diseases in Australia: How much do we know about them? Microbiol. Aust. 2016, 37, 27–29. [Google Scholar] [CrossRef]
- Han, B.A.; Kramer, A.M.; Drake, J.M. Global patterns of zoonotic disease in mammals. Trends Parasitol. 2016, 32, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Grabda, J. The dynamics of the nematode larvae, Anisakis simplex (Rud.) invasion in the South Western Baltic herring (Clupea harengus L.). Acta Ichthyol. Piscat. 1974, 4, 3–21. [Google Scholar] [CrossRef]
- Podolska, M.; Horbowy, J. Infection of Baltic herring (Clupea harengus membras) with Anisakis simplex larvae, 1992–1999: a statistical analysis using generalized linear models. ICES J. Mar. Sci., 2003, 60, 85–93. [Google Scholar] [CrossRef]
- Lang, T.; Damm, U.; Weber, W.; Neudecker, T.; Kühlmorgen-Hille, G. Infestation of herring (Clupea harengus L.) with Anisakis sp. larvae in the western Baltic. Archiv für Fischereiwissenschaft 1990, 40(1/2), 101–117. [Google Scholar]
- Mehrdana, F.; Bahlool, Q.Z.M.; Skov, J.; Marana, M.H.; Sindberg, D.; Mundeling, M.; Overgaard, B.C.; Korbut R.; Strøm, S.B.; Kania, P.W.; Buchmann, K. Occurrence of zoonotic nematodes Pseudoterranova decipiens, Contracaecum osculatum and Anisakis simplex in cod (Gadus morhua) from the Baltic Sea. Vet. Parasitol. 2014, 205, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Køie, M. Experimental infection of copepods and sticklebacks Gasterosteus aculeatus with small ensheathed and large third-stage larvae of Anisakis simplex (Nematoda, Ascaridoidea, Anisakidae). Parasitol. Res. 2001, 87, 32–36. [Google Scholar] [CrossRef]
- Lüthen, K. Mitteilung zum Auftreten von Anisakis sp. Larvae (Nematoda, Ascaridida) bei Plattfischen aus Gebieten der mittleren Ostsee. Wiss Z Padagog Hochschule Lisselotte Hermann Güstrow. 1989, 1, 83–87. [Google Scholar]
- Nadolna, K.; Podolska, M. Anisakid larvae in the liver of cod (Gadus morhua) L. from the southern Baltic Sea. J. Helminthol. 2014, 88, 237–246. [Google Scholar] [CrossRef]
- Buchmann, K.; Mehrdana, F. Effects of anisakid nematodes Anisakis simplex (s.l.), Pseudoterranova decipiens (s.l.) and Contracaecum osculatum (s.l.) on fish and consumer health. Food and Waterborne Parasiol. 2016, 4, 13–22. [Google Scholar] [CrossRef]
- Zuo, S.; Kania, P.W.; Mehrdana, F.; Marana, M.H.; Buchmann, K. Contracaecum osculatum and other anisakid nematodes in grey seals and cod in the Baltic Sea: molecular and ecological links. J. Helminthol. 2018, 92, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Nadolna-Ałtyn, K.; Szostakowska, B.; Podolska, M. Sprat (Sprattus sprattus) as a possible source of invasion of marine predators with Contracaecum osculatum in the Southern Baltic Sea. Russ. J. Mar. Biol. 2018, 44, 471–476. [Google Scholar] [CrossRef]
- Nadolna-Ałtyn, K.; Podolska, M.; Szostakowska, B. Great sandeel (Hyperoplus lanceolatus) as a putative transmitter of parasite Contracaecum osculatum (Nematoda: Anisakidae). Parasitol. Res. 2017, 116, 1931–1936. [Google Scholar] [CrossRef]
- Nadolna-Ałtyn, K.; Pawlak, J.; Podolska, M.; Lejk, A. Contracaecum osculatum and Pseudoterranova sp. in the liver of salmon (Salmo salar) from Polish marine waters. Fish. Aquatic. Life. 2023, 31, 44–53. [Google Scholar] [CrossRef]
- Lunneryd, S.G.; Boström, M.K.; Aspholm, P.E. Sealworm (Pseudoterranova decipiens) infection in grey seals (Halichoerus grypus), cod (Gadus morhua) and shorthorn sculpin (Myoxocephalus scorpius) in the Baltic Sea Parasito. Res. 2015, 114, 257–264. [Google Scholar] [CrossRef] [PubMed]
- McClelland, G.; Misra, R.K.; Martell, D.J. Larval anisakine nematodes in various fish species from Sable Island Bank and vicinity. Population biology of sealworm (Pseudoterranova decipiens) in relation to its invertebrate and seal hosts. Can. Bulletin Fish. Aquat. Sci. 1990, 222, 83–118. [Google Scholar]
- Klimpel, S.; Palm, H.W. Anisakid nematode (Ascaridoidea) life cycles and distribution: increasing zoonotic potential in the time of climate change. In Progress in Parasitology; Mehlhorn, H., Ed.; Parasitology Research Monographs; Springer: Berlin, Heidelberg, 2011; pp. 201–222. ISBN 978-3-642-21396-0. [Google Scholar]
- Herreras, M.V.; Balbuena, J.A.; Aznar, F.J.; Kaarstad, S.E.; Fernandez, M.; Raga, J.A. Population structure of Anisakis simplex (Nematoda) in harbor porpoises Phocoena phocoena off Denmark. J. Parasitol. 2004, 90, 933–938. [Google Scholar] [CrossRef]
- Fagerholm, H.P. Systematic position and delimitation of Ascaroid nematode parasites of the genus Contracaecum with a note on the superfamily Ascaridoidea; National Veterinary Institute: Helsinki, 1990. [Google Scholar]
- Aspholm, P.E.; Ugland, K.I.; Jødestøl, K.A.; Berland, B. Sealworm (Pseudoterranova decipiens) infection in common seals (Phoca vitulina) and potential intermediate fish hosts from the outer Oslofjord. Int. J. Parasitol. 1995, 25, 367–373. [Google Scholar] [CrossRef]
- Hauksson, E. The prevalence, abundance, and density of Pseudoterranova sp.(p) larvae in the flesh of cod (Gadus morhua) relative to proximity of grey seal (Halichoerus grypus) colonies on the coast off Drangar, Northwest Iceland. J. Mar. Biol. 2011, ID 235832, 1–8. [Google Scholar] [CrossRef]
- Køie, M.; Fagerholm, H.P. The life cycle of Contracaecum osculatum (Rudolphi, 1802) sensu stricto (Nematoda, Ascaridoidea, Anisakidae) in view of experimental infection. Parasitol. Res. 1995, 81, 481–489. [Google Scholar] [CrossRef]
- Klimpel, S.; Palm, H.W.; Rückert, S.; Piatkowski, U. The life cycle of Anisakis simplex in the Norwegian Deep (northern North Sea). Parasitol. Res. 2004, 94, 1–9. [Google Scholar] [CrossRef]
- Mouritsen, K.N.; Hederholm, R.; Schack, H.B.; Møller, L.N.; Storr-Paulsen, M.; Dzido, J.; Rokicki, J. Occurrence of anisakid nematodes in Atlantic cod (Gadus morhua) and Greenland cod (Gadus ogac) West Greenland. Acta Parasitol. 2010, 55, 81–89. [Google Scholar] [CrossRef]
- Martell, D.J.; McClelland, G. Transmission of Pseudoterranova decipiens (Nematoda: Ascaridoidea) via benthic macrofauna to sympatric flatfishes (Hippoglossoides platessoides, Pleuronectes ferrugineus, P. americanus) on Sable Island Bank, Canada. Mar. Biol 1995, 122, 129–135. [Google Scholar] [CrossRef]
- McClelland, G. Phocanema decipiens (Nematoda: Anisakidae): Experimental infections in marine copepods. Can. J. Zool. 1982, 60, 502–509. [Google Scholar] [CrossRef]
- Klimpel, S.; Kellermanns, E.; Palm, H.W. The role of pelagic swarm fish (Myctophidae: Teleostei) in the oceanic life cycle of Anisakis sibling species at the Mid-Atlantic Ridge, Central Atlantic. Parasitol. Res. 2008, 104, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Køie, M.; Berland, B.; Burt, M.D.B. Development to third-stage larvae occurs in the eggs of Anisakis simplex and Pseudoterranova decipiens (Nematoda, Ascaridoidea, Anisakidae). Can. J. Fish. Aquat. Sci. 1995, 52, 134–139. [Google Scholar] [CrossRef]
- Horbowy, J.; Podolska, M. Modelling infection of Baltic herring (Clupea harengus membras) by larval Anisakis simplex. ICES J. Mar. Sci. 2001, 58, 321–330. [Google Scholar] [CrossRef]
- Nadolna-Ałtyn, K.; Podolska, M.; Pawlak, J.; Szostakowska, B. Distribution of anisakid nematodes in the muscle tissue of cod (Gadus morhua) from the Norwegian Sea. Oceanologia. 2022, 64, 489–502. [Google Scholar] [CrossRef]
- Kent, A.J. Pert, C.C., Briers, R.A., Diele, K., Rueckert, S. Increasing intensities of Anisakis simplex third-stage larvae (L3) in Atlantic salmon of coastal waters of Scotland. Parasit. Vectors. 2020, 13, 62. [Google Scholar] [CrossRef]
- Wotten, R.; Yoon, G.H.; Bron, J.E. A Survey of Anisakid Nematodes in Scottish Wild Atlantic Salmon. FSAS Project S14008. Final Report 3rd February 2010.
- Setyawan, A.C.; Zuo, S.; Kania, P.W.; Buchmann, K. Endoparasitic helminths in Baltic salmon Salmo salar: ecological implications. Dis. Aquat. Org. 2019, 135, 193–199. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Scientific opinion on risk assessment of parasites in fishery products. Panel on Biological Hazards (BIOHAZ). EFSA J. 2010, 8, 1543. [Google Scholar] [CrossRef]
- Hochberg, N.S.; Hamer, D.H.; Hughes, J.M.; Wilson, M.E. Anisakidosis: Perils of the Deep. Clin. Infect. Dis. 2010, 51(7), 806–812. [Google Scholar] [CrossRef] [PubMed]
- Ishikura, H.; Kikuchi, K.; Nagasawa, K.; Ooiwa, T.; Takamiya, H.; Sato, N.; Sugane, K. Anisakidae and anisakidosis. Prog. Clin. Parasitol. 1993, 3, 43–102. [Google Scholar] [CrossRef]
- Wharton, D.A.; Aalders, O. The response of Anisakis larvae to freezing. J. Helminthol. 2002, 76, 363–368. [Google Scholar] [CrossRef]
- Audicana, M.T.; Ansotegui, I.J.; de Corres, L.F.; Kennedy, M.W. Anisakis simplex: dangerous – dead and alive? Trends Parasitol. 2002, 18, 20–25. [Google Scholar] [CrossRef]
- Moneo, I.; Caballero, M.L.; Gonzalez-Munoz, M.; Rodriguez-Mahillo, A.I.; Rodriguez-Perez, R.; Silva, A. Isolation of a heat-resistant allergen from the fish parasite Anisakis simplex. Parasitol. Res. 2005, 96, 285–289. [Google Scholar] [CrossRef]
- Berland, B. Nematodes from some Norwegian marine fishes. Sarsia. 1961, 2, 1–50. [Google Scholar] [CrossRef]
- Berland, B. Identification of larval nematodes from fish. Nematode problems in North Atlantic fish, Möller, H., Eds.; Report from a workshop in Kiel, 3–4 April 1989. International Council for the Exploration of the Sea, C.M./F6:16–22; pp. 16–22.
- Fagerholm, H.P. Parasites of fish in Finland. VI. Nematodes. Acta Acadamiae Aboensis, Series B 40, 1982; pp. 1–128. ISBN 9516488498.
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef]
- Zhu, X.; D’Amelio, S.; Paggi, L.; Gasser, R.B. Assessing sequence variation in the internal transcribed spacers of ribosomal DNA within and among members of the Contracaecum osculatum complex (Nematoda: Ascaridoidea: Anisakidae). Parasitol. Res. 2000, 86, 677–683. [Google Scholar] [CrossRef]
- Zhu, X.; D’Amelio, S.; Palm, H.W.; Paggi, L.; George-Nascimento, M.; Gasser, R.B. SSCP-based identification of members within the Pseudoterranova decipiens complex (Nematoda: Ascaridoidea: Anisakidae) using genetic markers in the internal transcribed spacers of ribosomal DNA. Parasitol. 2002, 124, 615–623. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Scientific opinion on assessment of epidemiological data in relation to the health risks resulting from the presence of parasites in wild caught fish from fishing grounds in the Baltic Sea. Panel on Biological Hazards (BIOHAZ). EFSA J. 2011, 9, 2320. [Google Scholar] [CrossRef]
- Grabda, J. The occurence of Anisakidae nematode larvae in Baltic cod (Gadus morhua callarias L.) and the dynamics of their invasion. Acta Ichthyol. Piscat. 1976, 6, 4–22. [Google Scholar] [CrossRef]
- Myjak, P.; Szostakowska, B.; Wojciechowski, J.; Pietkiewicz, H.; Rokicki, J. Anisakidae larvae in cod from the southern Baltic Sea. Arch. Fish. Mar. Res. 1994, 42, 149–161. [Google Scholar]
- Szostakowska, B.; Myjak, P.; Wyszyński, M.; Pietkiewicz, H.; Rokicki, J. Prevalence of anisakin nematodes in fish from Southern Baltic Sea. Pol. J. Microbiol. 2005, 54, 41–45. [Google Scholar] [PubMed]
- Sobecka, E. Pasożyty dorsza atlantyckiego z podgatunków Gaadus morhua morhua L. i Gadus morhua callarias L. z wybranych rejonów Atlantyku i Bałtyku (in polish). Habilitation thesis no. 245, Agricultural Academy in Szczecin, 2007.
- ICES. 2020. Working Group on Pathology and Diseases of Marine Organisms (WGPDMO). ICES Scientific Reports. 2:53. 23 pp. [CrossRef]
- Podolska, M.; Horbowy, J.; Wyszyński, M. Discrimination of Baltic herring populations with respect to Anisakis simplex larvae infection. J. Fish. Biol. 2006, 68, 1241–1256. [Google Scholar] [CrossRef]
- Rolbiecki, L.; Janc, A.; Rokicki, J. Stickleback as a potential paratenic host in the Anisakis simplex life cycle in the Baltic Sea: results of experimental infection. Wiad. Parazytol. 2001, 47, 257–262. [Google Scholar]
- Skrzypczak, M.; Rolbiecki, L. Endoparasitic helminths of the European Sprat, Sprattus sprattus (Linnaeus, 1758) from the Gulf of Gdansk (the Southern Baltic Sea) with a checklist of its parasites, Russ. J. Mar. Biol. 2015, 41, 167–175. [Google Scholar] [CrossRef]
- Nadolna-Ałtyn, K.; Kucińki, M.; Mirny, Z.; Góra, A.; Pawlak, J. Anisakidae nematodes in the liver of European flounder (Platichthys flesus) from the southern Baltic Sea. The 21st International Conference on Diseases of Fish and Shellfish (EAFP XXI), Aberdeen, Scotland, 11-14.09.2023.
- HELCOM. Baltic grey seal censuses in 2020. 15th Meeting of HELCOM Expert Group on Marine Mammals. Online, 14-16 September 2021.
- Skrzypczak, M.; Rokicki, J.; Pawliczka, I.; Najda, K.; Dzido, J. Anisakids of seals found on the southern coast of Baltic Sea. Acta Parasitol. 2014, 59, 165–172. [Google Scholar] [CrossRef]
- Herreras, M.V.; Montero, F.E.; Marcogliese, D.J.; Raga, J.A.; Balbuena, J.A. Phenotypic tradeoffs between egg number and egg size in three parasitic anisakid nematodes. Oikos 2007, 116, 1737–1747. [Google Scholar] [CrossRef]
- Measures, L.N. Effect of temperature and salinity on development and survival of eggs and free-living larvae of sealworm (Pseudoterranova decipiens). Can J Fish Aquat Sci. 1996, 53, 2804–2807. [Google Scholar] [CrossRef]
- Valtonen, E.T.; Fagerholm, H.P.; Helle, E. Contracaecum osculatum (Nematoda: Anisakidae) in fish and seals in Bothnian Bay (northeastern Baltic Sea). Int. J. Parasitol. 1988, 18, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Gabel, M.; Theisen, S.; Palm, H.W.; Dähne, M.; Unger, P. Nematode Parasites in Baltic Sea Mammals, Grey Seal (Halichoerus grypus (Fabricius, 1791)) and Harbour Porpoise (Phocoena phocoena (L.)), from the German Coast. Acta Parasitol. 2021, 66, 26–3. [Google Scholar] [CrossRef]
- Fiorenza, E.A.; Wendt, C.A; Dobkowski, K.A; King, T.L.; Pappaionou, M.; Rabinowitz, P.; Samhouri, J.S.; Wood, C.L. It’s a wormy world: Meta-analysis reveals several decades of change in the global abundance of the parasitic nematodes Anisakis spp. and Pseudoterranova spp. in marine fishes and invertebrates. Glob. Change. Biol. 2020, 26, 2854–2866. [Google Scholar] [CrossRef]
- Unger, P.; Klimpel, S.; Lang, T.; Palm, H.W. Metazoan parasites from herring (Clupea harengus L.) as biological indicators in the Baltic Sea. Acta. Parasitol. 2014, 3, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, J.; Nadolna-Ałtyn, K.; Szostakowska, B.; Pachur, M.; Bańkowska, A.; Podolska, M. First evidence of the presence of Anisakis simplex in Crangon crangon and Contracaecum osculatum in Gammarus sp. by in situ examination of the stomach contents of cod (Gadus morhua) from the southern Baltic Sea. Parasitol. 2019, 146, 1699–1706. [Google Scholar] [CrossRef] [PubMed]
- Pachur, M.E.; Horbowy, J. Food composition and prey selection of cod, Gadus morhua (Actinopterygii: Gadiformes: Gadidae), in the southern Baltic Sea. Acta Ichthyol. Piscat. 2013, 43, 109–118. [Google Scholar] [CrossRef]
- Haase, K.; Orio, A.; Pawlak, J.; Pachur, M.; Casini, M. Diet of dominant demersal fish species in the Baltic Sea: Is flounder stealing benthic food from cod? Mar. Ecol. Prog. Ser. 2020, 645, 159–170. [Google Scholar] [CrossRef]
- Podolska, M.; Pawlikowski, B.; Nadolna-Ałtyn, K.; Pawlak, J.; Komar-Szymczak, K.; Szostakowska, B. How effective is freezing at killing Anisakis simplex, Pseudoterranova krabbei, and P. decipiens larvae? An experimental evaluation of timetemperature conditions. Parasitol. Res. 2019, 118, 2139–2147. [Google Scholar] [CrossRef]
- Ludovisi, A.; Di Felice, G.; Carballeda-Sangiao, N.; Barletta, B.; Butteroni, C.; Corinti, S.; Marucci, G.; González-Muñoz, M.; Pozio, E.; Gómez-Morales, M.A. Allergenic activity of Pseudoterranova decipiens (Nematoda: Anisakidae) in BALB/c mice. Parasit. Vectors. 2017, 10, 290. [Google Scholar] [CrossRef]
- Nordholm, A.; Kurtzhals, J.A.L.; Karami, A.M.; Kania, P.W.; Buchmann, K. Nasal localization of a Pseudoterranova decipiens larva in a Danish patient with suspected allergic rhinitis. Helminthol. 2020, 94, E187. [Google Scholar] [CrossRef] [PubMed]
- Strøm, S.B.; Haarder, S.; Korbut, R.; Mejer, H.; Thamsborg, S.M.; Kania, P.W.; Buchmann, K. Third-stage nematode larvae of Contracaecum osculatum from Baltic cod (Gadus morhua) elicit eosinophilic granulomatous reactions when penetrating the stomach mucosa of pigs. Parasitol. Res. 2015, 114, 1217–1220. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, A.R.; Kiani, B.; Afshari, A.; Moghaddas, E.; Williams, M.; Shamsi, S. World-wide prevalence of Anisakis larvae in fish and its relationship to human allergic anisakiasis: a systematic review. Parasitol. Res. 2020, 119, 3585–3594. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, A.R.; Moghaddas, E.; Kiani, B.; Afshari, A.; Williams, M.; Shamsi, S. Anisakis allergy: unjustified social alarm versus healthy diet; commentary to the “Letter to the Editor” of Drs Daschner, Levsen, Cipriani, and del Hoyo, referencing to “World- wide prevalence of Anisakis larvae in fish and its relationship to human allergic anisakiasis: a systematic review. Parasitol. Res. 2020, 120, 1921–1923. [Google Scholar] [CrossRef]
- Kołodziejczyk, L.; Szostakowska, B.; Sobecka, E.; Szczucki, K.; Stankiewicz, K. First case of human anisakiasis in Poland. Parasitol Int. 2020, 76, 102073. [Google Scholar] [CrossRef]
- Takei, H.; Powell, S.Z. Intestinal anisakidosis (anisakiosis). Ann. Diagn. Pathol. 2007, 11, 350–352. [Google Scholar] [CrossRef]
- Shamsi, S. Seafood-Borne Parasitic Diseases: A “One-Health” Approach Is Needed. Fishes. 2019, 4, 1–11. [Google Scholar] [CrossRef]
- Levsen, A.; Berland, B. Anisakis species. In Fish Parasites, Pathobiology and Protection; Woo, P.T.K., Buchmann, K., Eds.; CAB International, 2012; pp. 298–309.
- Beck, M.; Evans, R.; Feist, S.W.; Stebbing, P.; Longshaw, M.; Harris, E. Anisakis simplex sensu lato associated with red vent syndrome in wild Atlantic salmon Salmo salar in England and Wales. Dis. Aquat. Org. 2008, 82, 61–65. [Google Scholar] [CrossRef]
- Noguera, P.; Collins, C.; Bruno, D.; Pert, C.; Turnbull, A.; McIntosh, A.; Lester, K.; Bricknell, I.; Wallace, S.; Cook, P. Red vent syndrome in wild Atlantic salmon Salmo salar in Scotland is associated with Anisakis simplex sensu stricto (Nematoda: Anisakidae). Dis. Aquat. Org. 2009, 87, 199–215. [Google Scholar] [CrossRef]
- Haarder, S.; Kania, P.W.; Bahlool, Q.M.; Buchmann, K. Expression of immune relevant genes in rainbow trout following exposure to live Anisakis simplex larvae. Exp Parasitol. 2013, 135, 564–569. [Google Scholar] [CrossRef]
- Horbowy, J.; Podolska, M.; Nadolna-Ałtyn, K. Increasing occurrence of Anisakid nematodes in the liver of cod (Gadus morhua) from the Baltic Sea: does infection affect the condition and mortality of fish? Fish. Res. 2016, 179, 98–103. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



