Submitted:
13 July 2023
Posted:
14 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
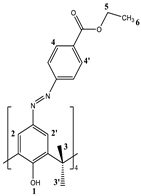
2.1. Structural Characterization of 5,11,17,23-Tetra[(4-ethylacetoxyphenyl) (azo)]calix[4]arene (CA-AZ)
2.1.1. 1H NMR Characterization of CA-AZ in DMSO-d6 and CDCl3 at 298 K.
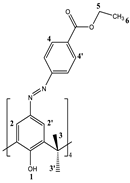
| δ (ppm) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Solvent | H-1 | H-2,2’ | H-3H-equatorial | H-3’H-axial | H-4 | H-4’ | H-5 | H-6 |
| CDCl3 | 10.25 | 7.85 | 3.87 | 4.40 | 8.13 | 8.14 | 4.39 | 1.4 |
| DMSO-d6 | - | 7.82 | 3.51 | 4.34 | 7.85 | 8.05 | 4.31 | 1.32 |
| ∆δ | - | -0.03 | -0.36 | -0.06 | -0.28 | -0.09 | -0.08 | -0.08 |
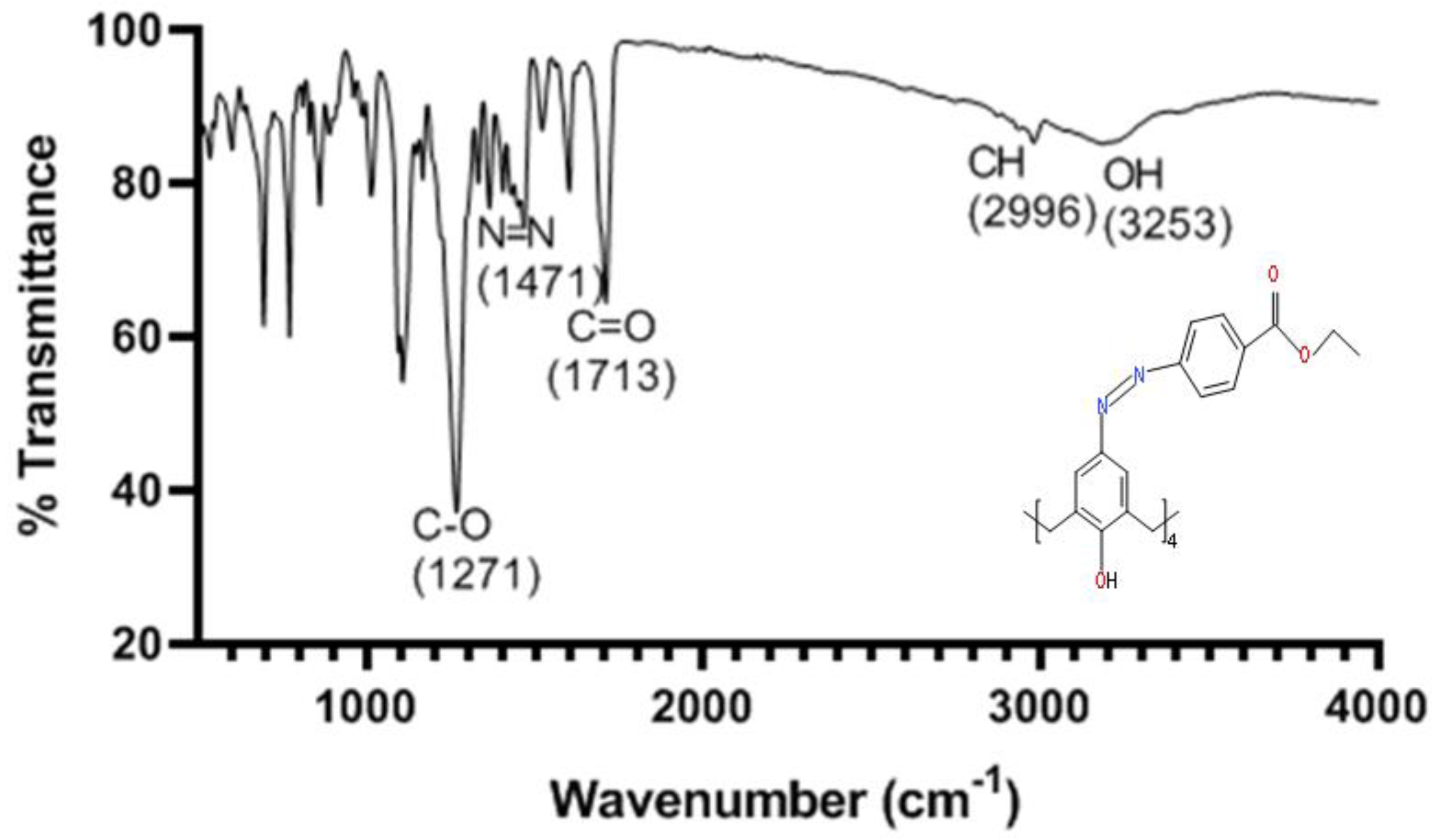
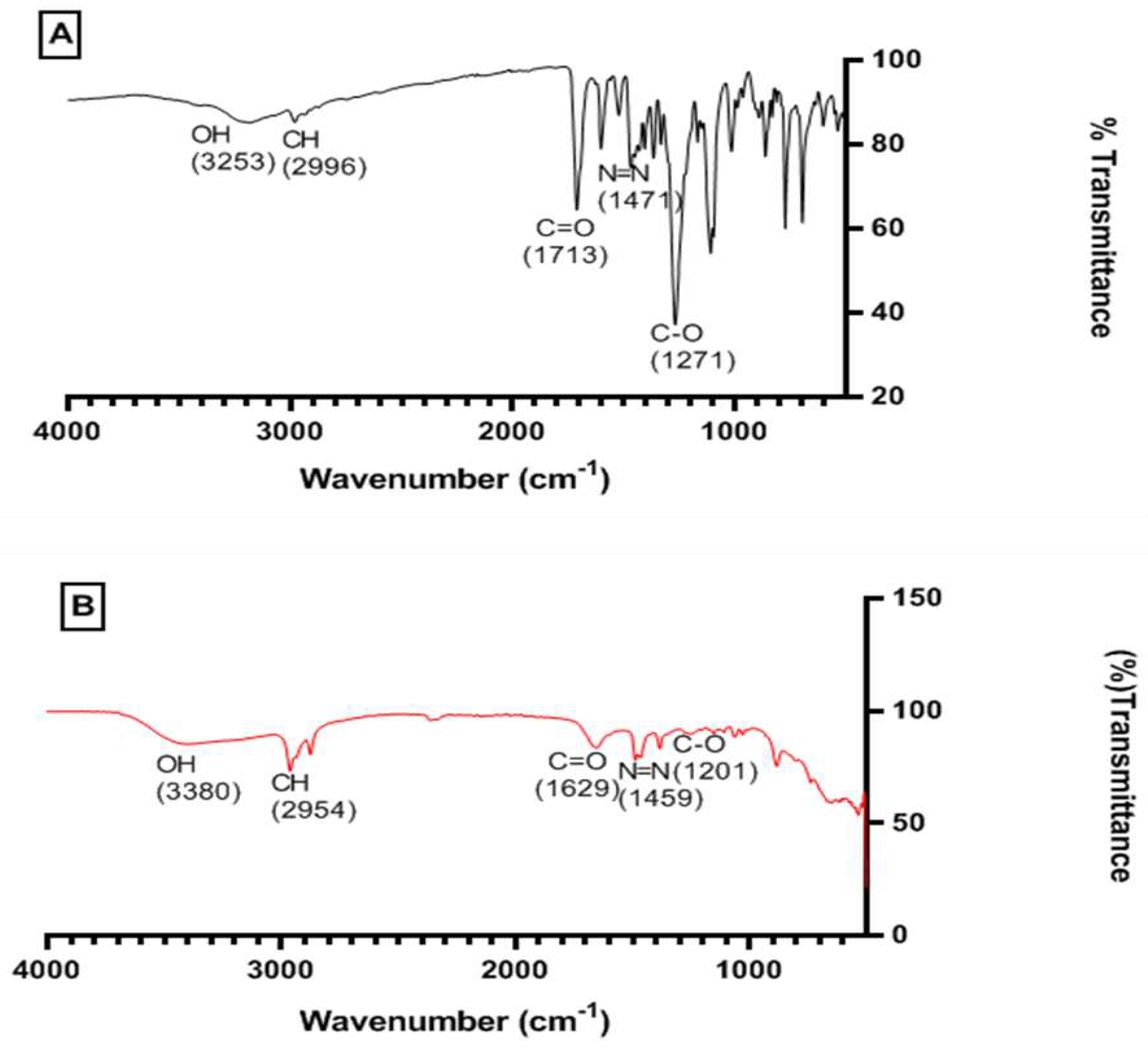
2.1.2. FT-ATR Characterisation of Para-Ester Diazophenylcalix[4]arene
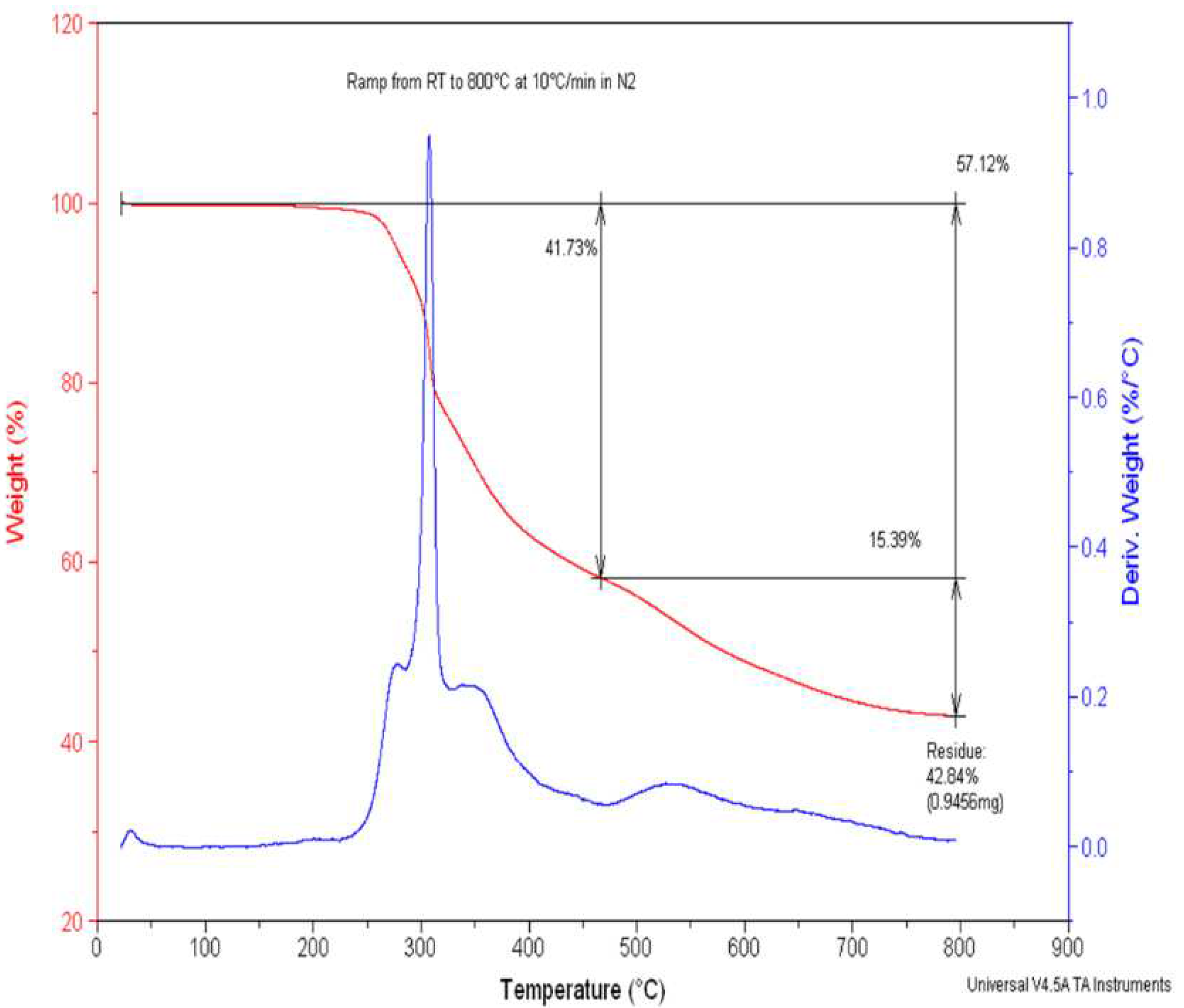
2.1.3. Thermal Stability of Para-Ester Diazophenylcalix[4]arene
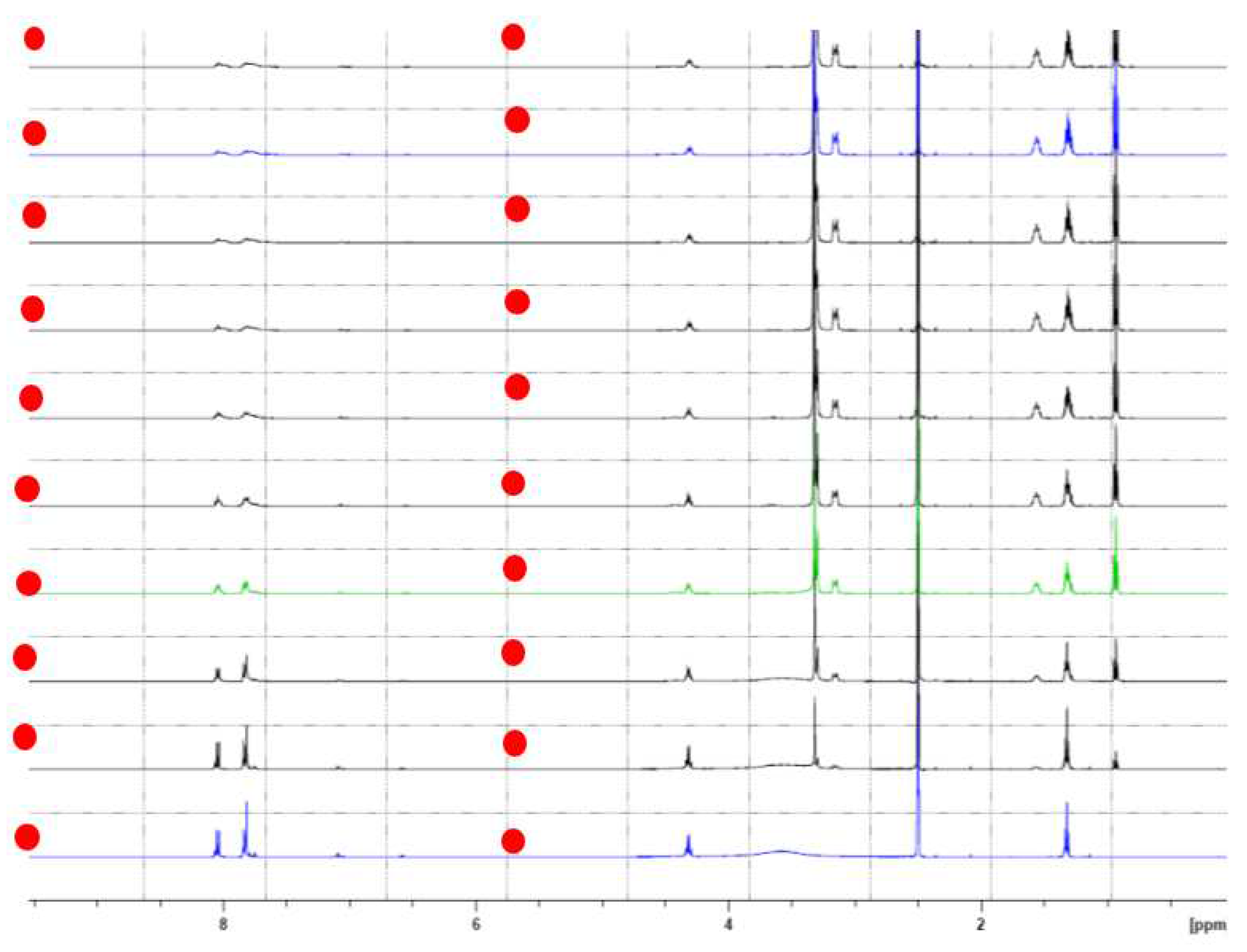
2.2. 1H NMR Complexation of CA-AZ with Anions in DMSO-d6 at 298 K.
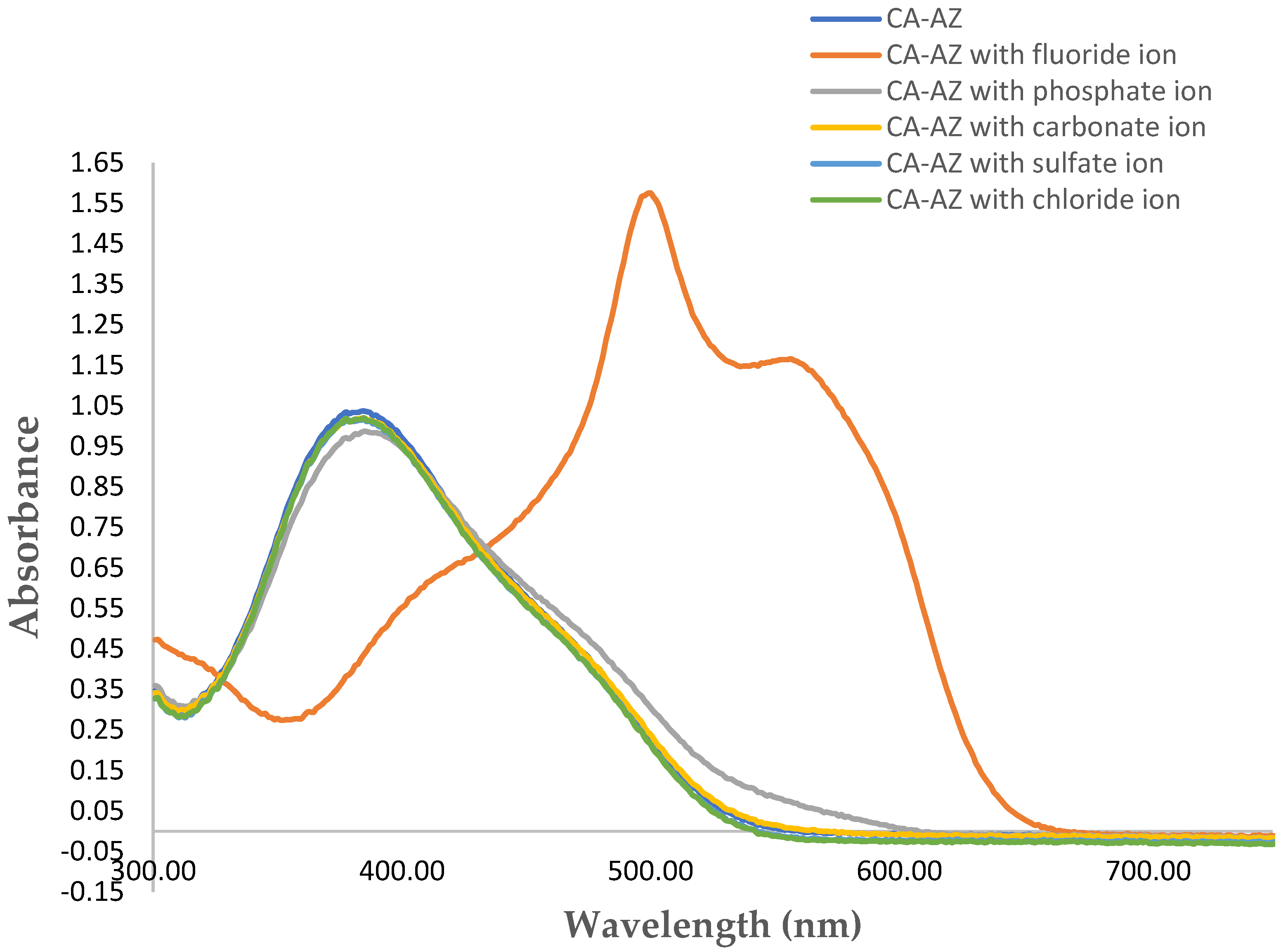
2.3. Chromogenic Properties and UV-VIS Experiments
2.4. 1H NMR Titration of Para-Ester Diazophenylcalix[4]arene with Fluoride Anion as Tetra-n-Butyl Ammonium in DMSO-d6
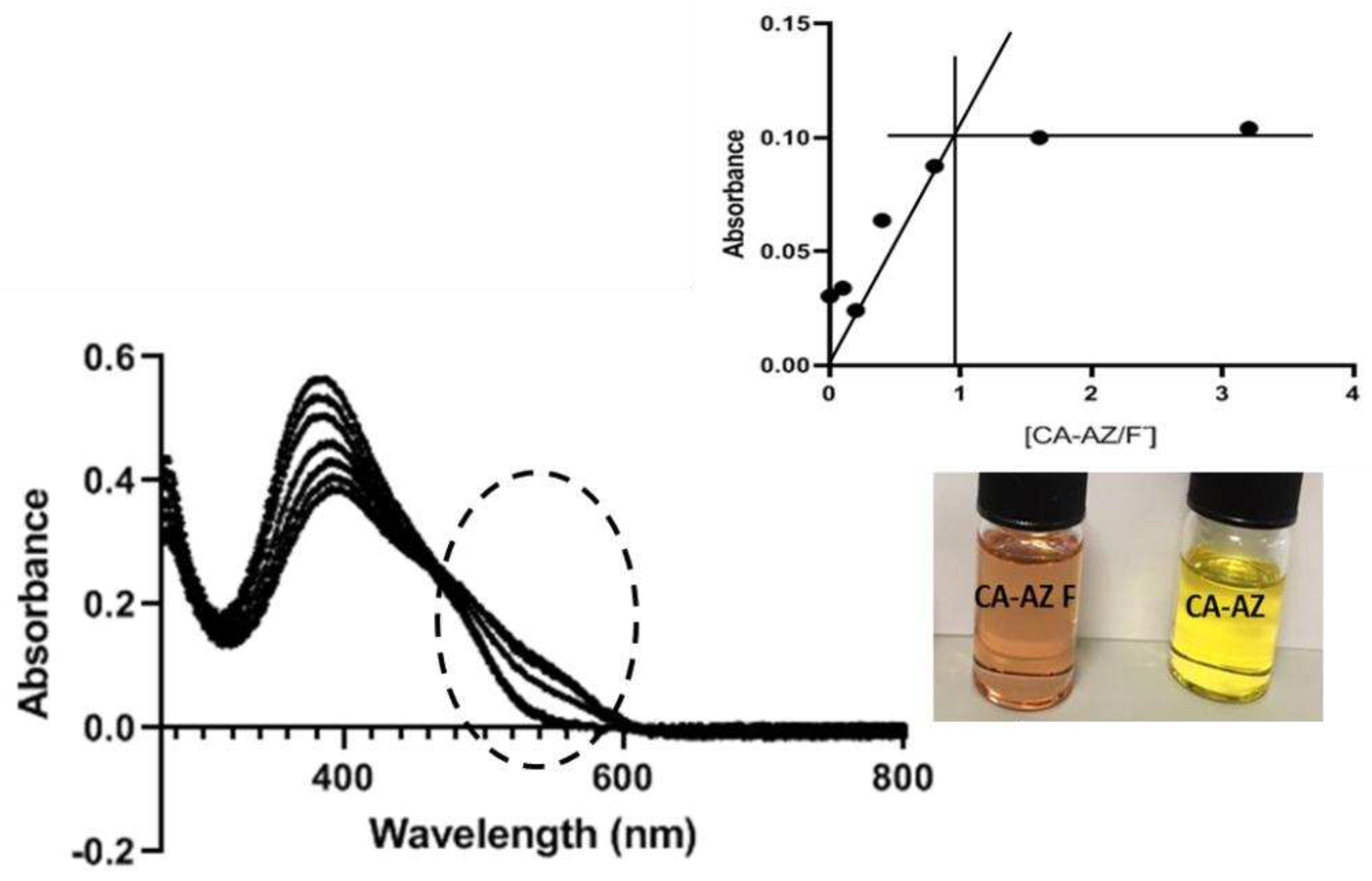
2.5. UV-VIS Titration of CA-AZ with the Fluoride Anion Salt in DMSO
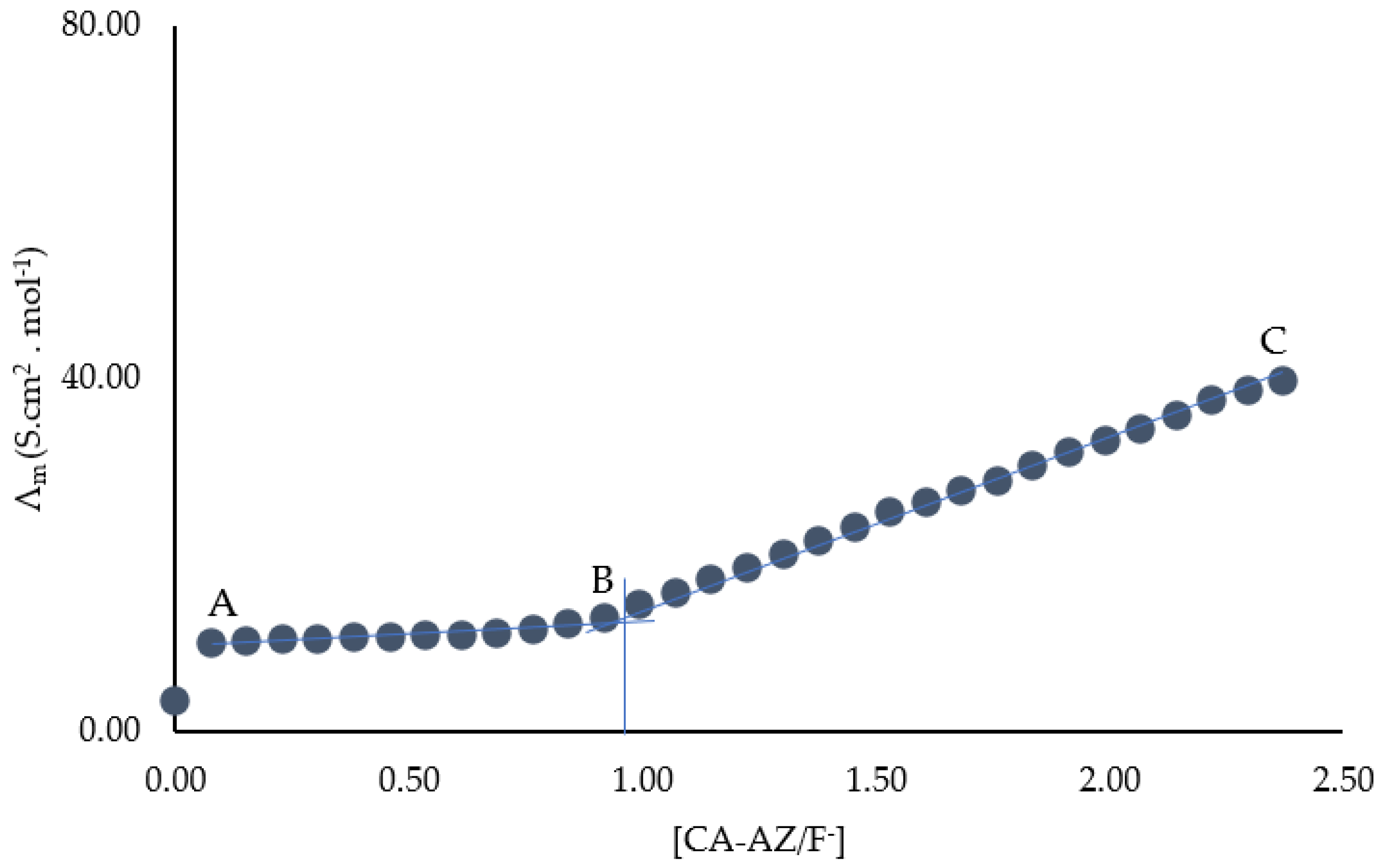
2.6. Conductometric Studies of CA-AZ Interacting with Fluoride Anion in DMSO at 298.15 K
2.7. Thermodynamics of Complexation of CA-AZ with Fluoride in DMSO at 298.15 K
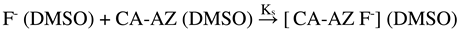
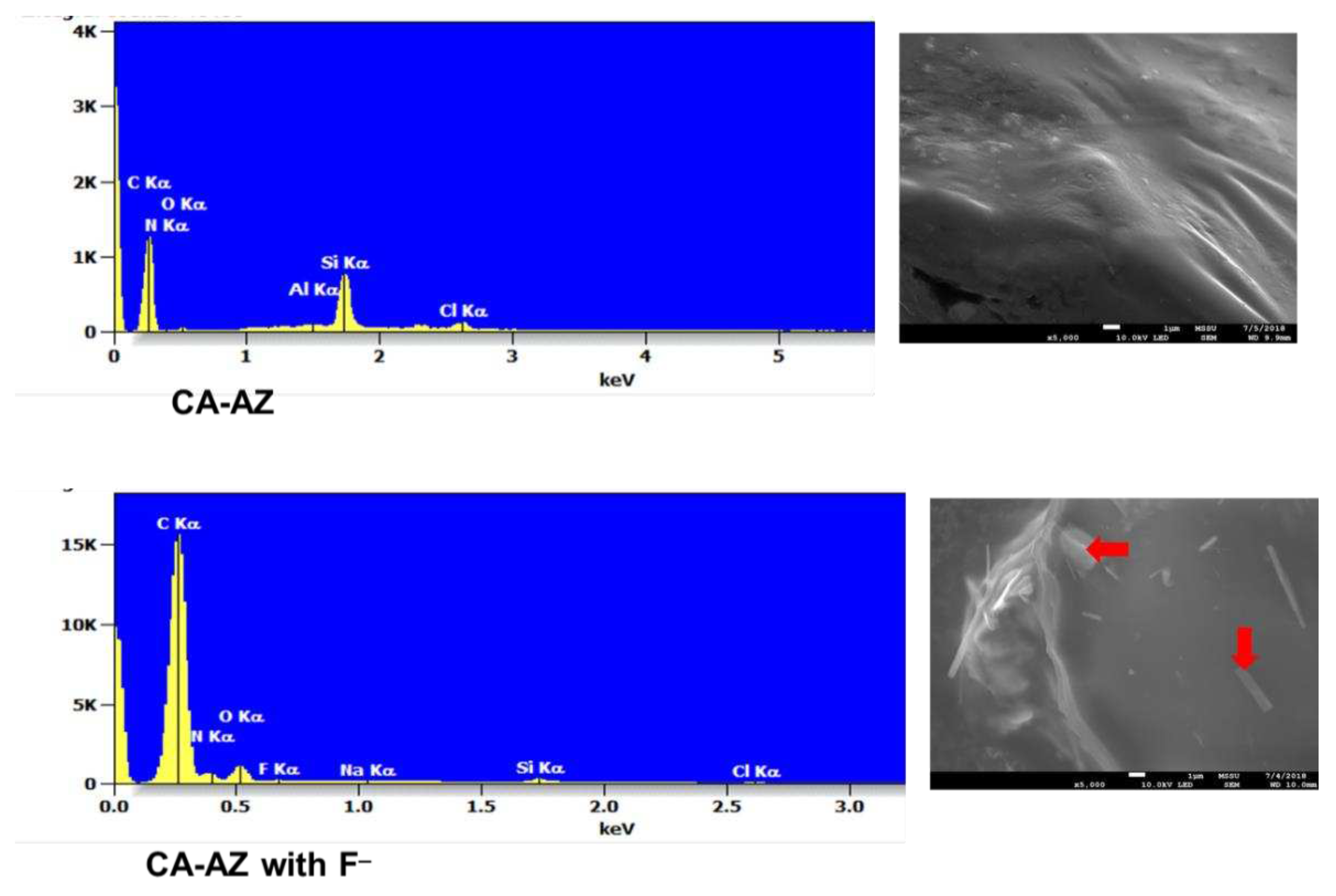
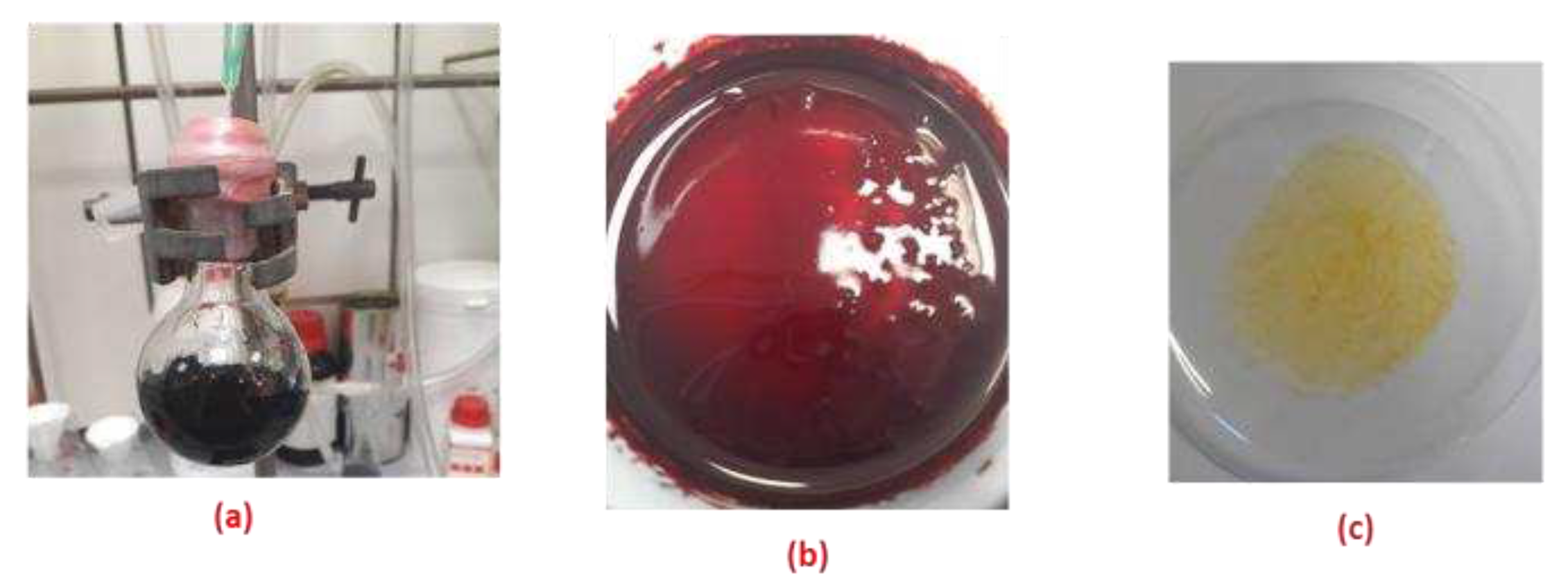
2.8. SEM-EDX and FT-IR Characterization of Bu4N [CA-AZ F]
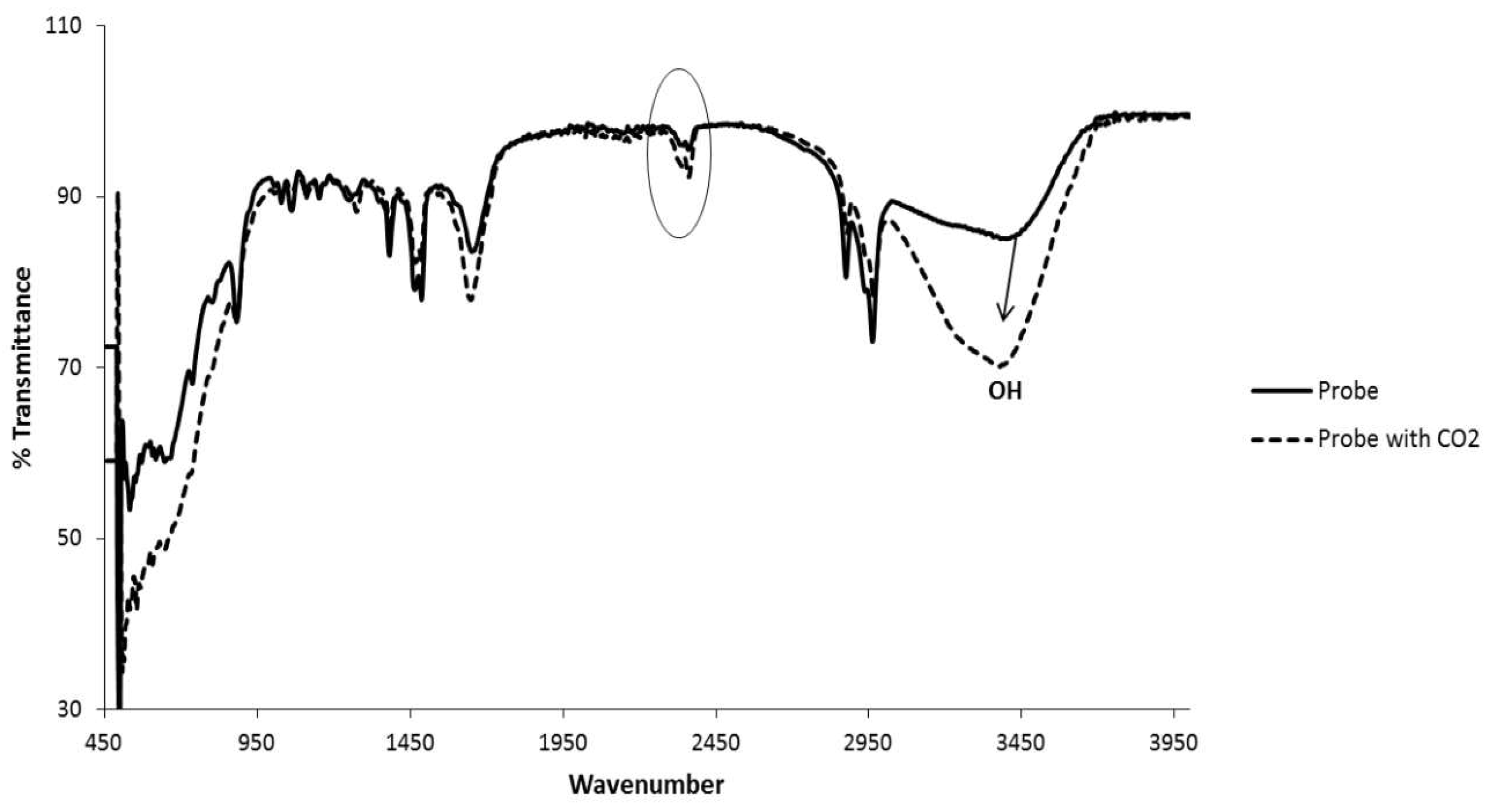
2.9. Interaction of Bu4N[CA-AZ F] with CO2
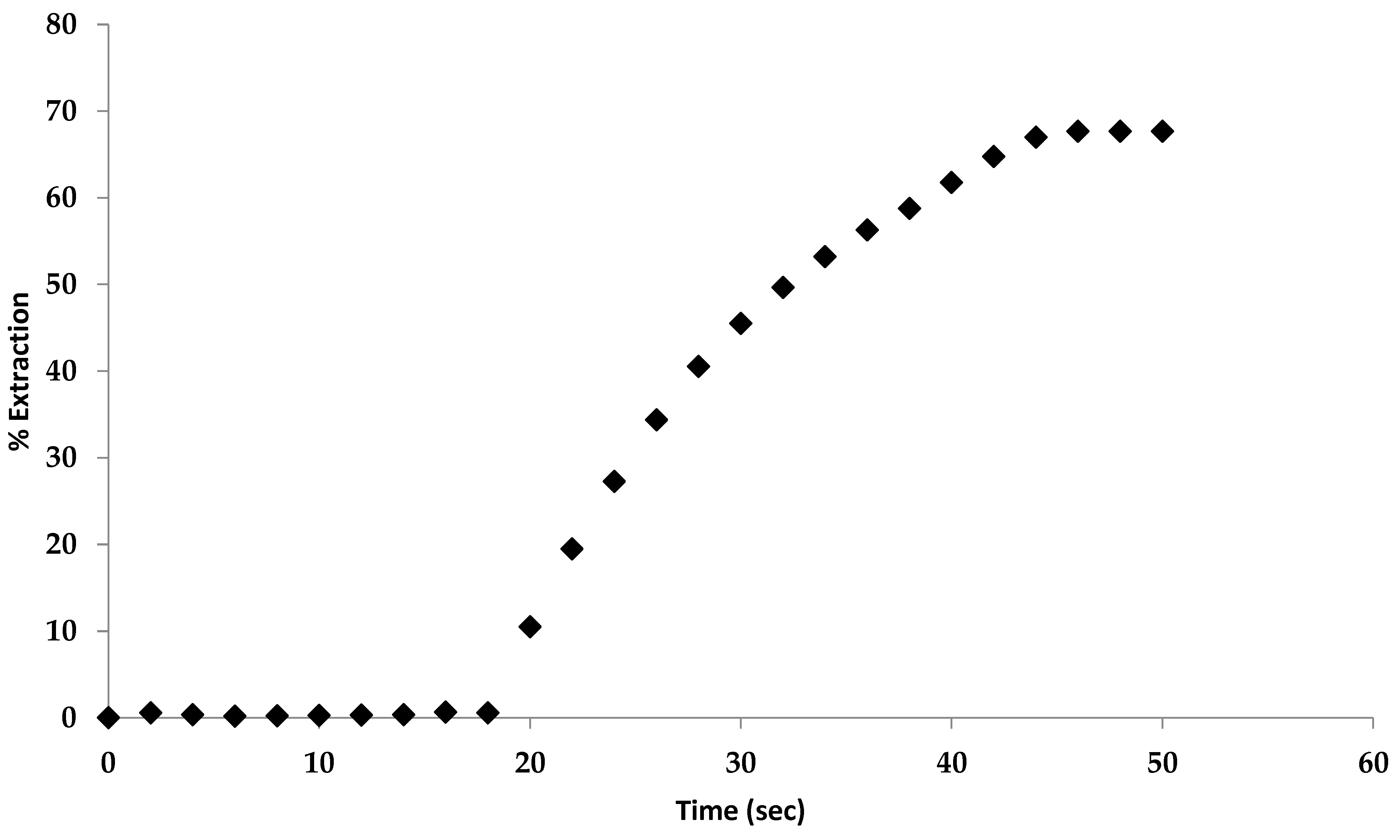
2.9.1. Extraction of Carbon Dioxide by the Solid Complex Salt
2.9.2. Recycling of the Complex
3. Materials and Methods
3.1. Chemicals
3.2.1. Synthesis of 25, 26, 27, 28-Tertrahydroxy Calix[4]arene via De-Tert-Butylation (Friedel Craft De-Tert-Butylation Reaction)
3.2.2. Preparation of 5,11,17,23-Tetra[(4-ethylacetoxyphenyl) (azo)]calix[4]arene, CA-AZ
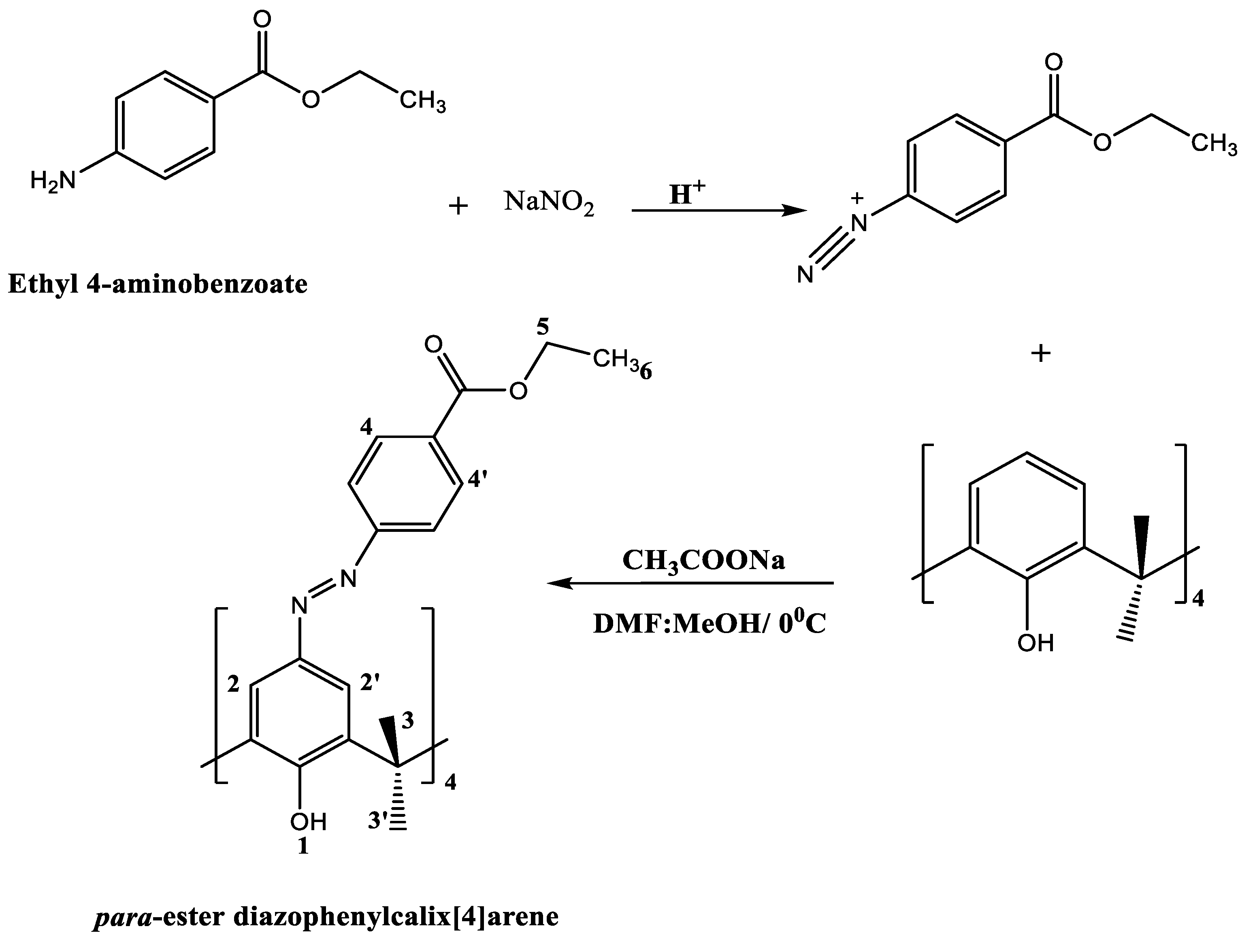
3.3. FT-IR, Thermogravimetric and SEM-EDX Analyses of CA-AZ and CA-AZ-F- Salt
3.4. 1H NMR Complexation Studies
3.5. UV-Visible Studies of CA-AZ and Ion Salts in Dimethyl Sulfoxide
3.6. 1H NMR Titration Studies
3.7. Conductometric Titrations of Para-Ester Diazophenylcalix[4]arene and Fluoride Anion in Dimethyl Sulfoxide at 298.15 K
3.8. Calorimetric Titration Experiments
3.9. Carbon Dioxide Detection Studies by Para-Ester Diazophenylcalix[4]arene-Fluoride Complex Using FTIR.
3.9.1. Extraction of CO2 by the Solid Complex Salt
4. Conclusions
- i)
- The conformational change that the receptor undergoes as a result of the medium effect and complexation with anions in DMSO.
- ii)
- The fast kinetics of anion complexation reflected in the immediate colour change resulting from the receptor-anion interaction easily detected by a naked eye
- iii)
- The selectivity of the receptor for fluoride is demonstrated to an extent that unlike for other anions, the stability of the fluoride complex was quantitatively assessed through titration calorimetry. Furthermore, the process is entropy controlled because of the de-solvation of the anion or receptor or both, upon complexation.
- iv)
- The mode of interaction of the receptor with the anion was established and consisted of deprotonation of one of the phenolic units in the narrow rim of the receptor followed by protonation of the functionalised upper rim which provide the sites of interaction with the anion
- v)
- The ability of the fully characterized anion salt to remove CO2 from the air is illustrated through the text, together with the recycling of the complex salt. This is a very important aspect to highlight given that so far, most reports on calixarene chemistry are centered on the complexation process but there is a need to explore further the practical applications of macrocyclic complex salts.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schrag, P.D. Preparing to capture Carbon. Science 2007, 315, 812–13. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhao, N.; Wei, W.; Sun, Y. A Review of research progress on CO2 capture, storage and utilization I Chinese Academy of Sciences. Fuel 2013, 108, 112–130. [Google Scholar] [CrossRef]
- Dawson, R.; Cooper, A.I.; Adams, D.J. Chemistry functionalization strategies for carbon dioxide capture in microporous organic polymers. Polymer Int. 2013, 62, 335–522. [Google Scholar] [CrossRef]
- Perry, S.F.; Abdallah, S. Mechanisms and consequences of carbon dioxide sensing in fish. Respir. Phys. Neurobiol. 2012, 184, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Neethirajan, S.; Jayas, D.S. ; Sadistap, Carbon dioxide (CO2) sensors for the Agri-Food Industry- A Review. Food Bioprocess Technol. 2009, 2, 115–21. [Google Scholar] [CrossRef]
- Puligundla, P.; Jung, J.; Ko, S. Carbon dioxide sensors for Intelligent food packaging. Food Control 2012, 25, 328–333. [Google Scholar] [CrossRef]
- Mills, A.; Lepre, A.; Wild, L. Breath-by-Breath Measurements of carbon dioxide using a plastic film optical sensor, Sens. Actuators, B 1997, 38, 419. [Google Scholar] [CrossRef]
- Yasuda, T.; Yonemura, S.; Tani, A. Comparison of the characteristics of small commercial NDIR CO2 of a portable CO2 measurement device. Sensors 2012, 12, 3641–55. [Google Scholar] [CrossRef]
- Vorotyntsev, V.M.; Mochalov, G.M.; Baranova, I.V. ; Gas Chromatographic determination of admixtures of permanent gases CO, CO2 rand hydrocarbons in methyl silane. J. Anal. Chem. 2013, 68, 152–55. [Google Scholar] [CrossRef]
- Xie, X.; Bakker, E. Non-Severinghaus Potentiometric Dissolved CO2 sensor with improved characteristics. Anal. Chem. 2013, 85, 1332–36. [Google Scholar] [CrossRef]
- Beyenal, H.; Davis, C.C.; Lewandowski, Z. An improved Severinghaus type carbon dioxide microelectrode for use in biofilms. Sens. Actuators, B 2004, 97, 202–210. [Google Scholar] [CrossRef]
- Lee, M.; Jo, S.; Lee, D.; Xu, Z.; Yoon, J. A new naphtalimide derivative as a selective fluorescent and colorimetric sensor for fluoride, cyanide and CO2. J. Dyes and Pigments 2015, 120, 288–292. [Google Scholar] [CrossRef]
- Gupta, R.C.; Ali, R.; Razi, S.S.; Srivastava, P.; Dwivedi, S.K.; Misra, A. Synthesis and Applications of a new class of D-π-A type charge transfer probe containing imidazole-naphthalene units for detection of F- and CO2. RSC Adv. 2017, 7, 4941–49. [Google Scholar] [CrossRef]
- Xia, G.; Ruan, C.; Wang, H. Highly sensitive detection of carbon dioxide by a pyramido (1,2-a) benzimidazole derivative combining experimental and theoretical studies. Analyst 2015, 140, 5099–5104. [Google Scholar] [CrossRef]
- Xia, G.; Liu, Y.; Ye, B.; Sun, J.; Wang, H. A squarine-based colorimetric and F- dependent chemo sensor for recyclable CO2 gas detection: highly sensitive off-on-off response. J Chem. Soc. Chem. Commun. 2015, 51, 13802–805. [Google Scholar] [CrossRef]
- Gutsche, C.D. Calixarenes. The Royal Society of Chemistry 1969. [Google Scholar]
- Danil de Namor, A.F.; Cleverley, R.M.; Zapata Ormachea, M.L. Thermodynamics of Calixarene Chemistry. Chem. Reviews 1998, 98, 2495–2525. [Google Scholar] [CrossRef]
- Danil de Namor, A.F.; Chahine, S.; Castellano, E.E.; Piro, O.E.; Jenkins, H.D.B. A Preliminary Observation of Additive Thermodynamic Contribution of Pendant Arms to the Complexation of Calixarene Derivatives with Mercury (II). J. Chem. Soc; Chem.Comm 2005, 3844-3846.
- Danil de Namor, A.F.; Chahine, S. The Effect of Solvation of Guest, Supramolecular Host and Host-Guest Complex on the Thermodynamic Selectivity of Calix [4] arene Derivatives and Soft Metal Cations. J. Phys. Chem 2005, 109, 18096–112. [Google Scholar] [CrossRef]
- Danil de Namor, A.F.; El Gamouz, A.; Alharthi, S.; Al Hakawati, N.; Varcoe, J.R. A Ditopic Calix [4] pyrrole Amide Derivative: Highlighting the Importance of Fundamental Studies and the Use of NaPh4B as Additive in the Design and Applications of Mercury (II) Ion Selective Electrodes. J. Mater. Chem. A 2015, 3, 13016–13030. [Google Scholar] [CrossRef]
- Danil de Namor, A.F.; Alharthi, S.; El Gamouz, A.; Al Hakawati, N.; Cox, B.G. Calix [4] Based Hg (II) Ion Selective Electrodes: A Thermodynamic Protocol to Address the Selectivity versus the hosting capacity paradigm in the selection of the carrier. Electrochimica Acta 2018, 290, 686–694. [Google Scholar] [CrossRef]
- Benkhaya, S.; M’rabet, S.; El Harfi, A. Classifications, properties, recent synthesis and applications of azo dyes. Heliyon 2020, 6, e03271. [Google Scholar] [CrossRef] [PubMed]
- Benkhaya, S.; Cherkaoui, O.; Assouag, M.; Mrabet, S.; Rafik, M.; El Harfi, A. Synthesis of a New Asymmetric Composite Membrane with Bi-Component Collodion: Application in the Ultra filtration of Baths of Reagent Dyes of Fabric Rinsing/Padding. J. Mater. Environ. Sci. 2016, 7, 4556–4569. [Google Scholar]
- Georgiev, A.; Stoilova, A.; Dimov, D.; Yordanov, D.; Zhivkov, I.; Weiter, M. Synthesis and photochromic properties of some N-phthalimide azo-azomethine dyes. A DFT quantum mechanical calculation on imine-enamine tautomerism and trans-cis photoisomerization. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 210, 230–244. [Google Scholar] [CrossRef] [PubMed]
- Sahan, F.; Kose, M.; Hepokur, C.; Karakas, D.; Kurtoglu, M. New azo-azomethine-based transition metal complexes: Synthesis, spectroscopy, solid-state structure, density functional theory calculations and anticancer studies. Appl. Organomet. Chem. 2019, 33, e4954. [Google Scholar] [CrossRef]
- Oueslati, F.; Dumazet-Bonnamour, I.; Lamartine, R. New Azothiacalix[4]arenes Containing Biheterocyclic Subunits: Extraction and Complexation Properties. Supramol. Chem. 2004, 227–232. [Google Scholar] [CrossRef]
- Gunnlaugsson, T.; Leonard, J.P.; Murray, N.S. Highly selective colorimetric naked-eye Cu (II) detection using an azobenzene chemosensor. Org. Lett. 2004, 6, 1557–1560. [Google Scholar] [CrossRef]
- Lee, S.J.; Jung, J.H.; Seo, J.; Yoon, I.; Park, K.M.; Lindoy, L.F.; Lee, S.S. A Chromogenic Macrocycle Exhibiting Cation-Selective and Anion-Controlled Colour Change: An Approach to Understanding Structure-Color Relationships. Organic. Letts. 2006, 8, 1641–1643. [Google Scholar] [CrossRef]
- Cheng, Y.F.; Zhao, D.T.; Zhang, M.; Liu, Z.Q.; Zhou, Y.F.; Shu, T.M.; Li, F.Y.; Yi, T.; Huang, C.H. Azo 8-hydroxyquinoline benzoate as selective chromogenic chemo sensor for Hg2+ and Cu2+. Tetrahedron Lett., 2006, 47, 6413–6416. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, S.S.; Jeong, I.Y.; Lee, J.Y.; Jung, J.H. Azobenzene coupled chromogenic receptors for the selective detection of copper (II) and its application as a chemo sensor kit24. Tetrahedron Lett. 2007, 48, 393–396. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, H.Y.; Lee, D.H.; Hong, J.I. Fluoride-selective chromogenic sensors based on azophenol. Tetrahedron Lett. 2001, 42, 5447–49. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, K.H.; Hong, J.I. An Azophenol-based Chromogenic Anion Sensor. Org. Lett. 2001, 3, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Lee, H.Y.; Lee, K.H.; Hong, J.I. Selective anion sensing based on a dual-chromophore approach. Chem. Commun. 2001, 1188.-89.
- Lee, D.H.; Im, J.H.; Son, S.U.; Chung, Y.K.; Hong, J.I. An azophenol-based chromogenic pyrophosphate sensor in water. J. Am. Chem. Soc. 2003, 125, 7752–7753. [Google Scholar] [CrossRef] [PubMed]
- Sancenón, F.; Martínez-Máñez, R.; Soto, J. A Selective Chromogenic Reagent for Nitrate. Angew. Chem. Int. Ed. 2002, 41, 1416–1417. [Google Scholar] [CrossRef]
- Cho, E.J.; Ryu, B.J.; Lee, Y.J.; Nam, K.C. Visible Colorimetric Fluoride Ion Sensors. Org. Lett. 2005, 7, 2607–2609. [Google Scholar] [CrossRef]
- Farinha, A.S.F.; Tome, A.C.; Cavaleiro, J.A.S. Synthesis of new calix[4]pyrrole derivatives via 1,3-dipolar cycloadditions. Tetrahedron 2010, 66, 7595–7599. [Google Scholar] [CrossRef]
- Yoo, J.; Kim, M.S.; Hong, S.J.; Sessler, J.L.; Lee, C.H. Selective Sensing of Anions with Calix[4]pyrroles Strapped with Chromogenic Dipyrrolylquinoxalines. J. Org. Chem. 2009, 74, 1065–1069. [Google Scholar] [CrossRef]
- Suksai, C.; Tuntulani, T. Chromogenic anion sensors. Chem. Soc. Rev. 2003, 32, 192–202. [Google Scholar] [CrossRef]
- Chang, K.C.; Su, I.H.; Wang, Y.Y.; Chung, W.S. A Bifunctional Chromogenic Calix[4]arene Chemo sensor for Both Cations and Anions: A Potential Ca2+ and F- Switched Inhıbıt Logic Gate with a YES Logic Function. Eur. J. Org. Chem. 2010, 4700–4704. [Google Scholar] [CrossRef]
- Quinlan, E.; Matthews, S.E.; Gunnlaugsson, T.J. Colorimetric Recognition of Anions Using Preorganized Tetra-Amidourea Derived Calix[4]arene Sensors. Org. Chem. 2007, 72, 7497–7503. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, S.K.; Lee, J.Y.; Kim, J.S. Fluoride-Sensing Calix-luminophores Based on Regioselective Binding. J. Org. Chem. 2006, 71, 6611–6614. [Google Scholar] [CrossRef]
- Wagner-Wysiecka, E.; Lukasik, N.; Biernat, J.F.; Luboch, E. Azo group(s) in selected macrocyclic compounds. J Incl. Phenom. and Macrocycl. Chem. 2018, 90, 189–257. [Google Scholar] [CrossRef]
- Browne, D.; Whelton, H.; Mullane, D.O. Fluoride metabolism and fluorosis. J. Dent., 2005, 33, 177–86. [Google Scholar] [CrossRef]
- Schwarzenbach, B.I.; Escher, K.; Fenner, T.B.; Hofstetter, C.A.; Johnson, U.; von Gunten, B. ; Wehrli, The challenge of micropollutants in aquatic systems. Science 2006, 313, 1072–77. [Google Scholar] [CrossRef] [PubMed]
- Bassin, E.B.; Wypij, D.; Davis, R.B. Age-specific fluoride exposure in drinking water and osteosarcoma (United States). Canc. Causes Contr. 2006, 17, 421–28. [Google Scholar] [CrossRef] [PubMed]
- Danil de Namor, A.F. The Fluoride Dilemma: Thermodynamics of Anion Complexation by Calixpyrroles. J. Therm. Anal 2007, 87, 7–14. [Google Scholar] [CrossRef]
- Bazzicalupi, C.; Bencini, A.; Bencini, A.; Bianchi, A.; Corana, F.; Fusi, V.; Giorgi, C.; Paoli, P.; Paoletti, P.; Valtancoli, B.; Zanchini, C. CO2 fixation by novel copper (II) and zinc (II) macrocyclic complexes. A solution and solid state study. Inorg. Chem. 1996, 35, 5540–5548. [Google Scholar] [CrossRef] [PubMed]
- Gaber, M.; El-Sayed, Y.S.; El-Baradie, K.; Fahmy, R.M. Cu (II) complexes of monobasic bi-or tridentate (NO, NNO) azo dye ligands: Synthesis, characterization, and interaction with Cu-nanoparticles. J. Mol. Struct. 2013, 1032, 185–194. [Google Scholar] [CrossRef]
- Dincalp, H.; Toker, F.; Durucasu, I.; Avcibasi, N.; Icli, S. New thiophene-based azo ligands containing azo methine group in the main chain for the determination of copper(II) ions. Dyes and Pigments 2007, 75, 11–24. [Google Scholar] [CrossRef]
- Chawla, H.M.; Hundal, G.; Singh, S.P.; Upreti, S. Conformational morphosis in azocalix[4]arenes. CrystEngComm, 2007, 9, 119–122. [Google Scholar] [CrossRef]
- Ho, I.T.; Lee, G.H.; Chung, W.S. Synthesis of Upper-Rim Allyl- and p-Methoxyphenylazocalix[4]arenes and Their Efficiencies in Chromogenic Sensing of Hg2+ Ion. J. Org. Chem. 2007, 72, 2434–2442. [Google Scholar] [CrossRef]
- Kim, J.S.; Shon, O.J.; Lee, J.K.; Lee, S.H.; Kim, J.Y.; Park, K.M.; Lee, S.S. Chromogenic Azo-Coupled Calix[4]arenes. J. Org. Chem. 2002, 67, 1372–1375. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Kim, G.; Kim, C.R.; Lee, S.H.; Lee, J.H.; Kim, J.S. UV Band Splitting of Chromogenic Azo-Coupled Calix[4]crown upon Cation Complexation. J. Org. Chem. 2003, 68, 1933–1937. [Google Scholar] [CrossRef] [PubMed]
- Karci, F.; Sener, I.; Deligoz, H. Azocalixarenes. 1: synthesis, characterization and investigation of the absorption spectra of substituted azocalix[4]arenes. Dyes Pigments 2003, 59, 53–61. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, S.H.; Tan, L.V.; Seo, Y.J.; Park, S.Y.; Kim, H.; Kim, J.S. Transition Metal Ion Selective Ortho-Ester Diazophenylcalix[4]arene. Talanta 2007, 71, 1294–1297. [Google Scholar] [CrossRef]
- Chawla, H.; Gupta, T. Design, synthesis and evaluation of a new calix[4]arene based molecular receptor for multiple ion selectivity. J. Incl Phenom Macrocycl Chem. 2015, 81, 49–56. [Google Scholar] [CrossRef]
- Lӧhr, H.G.; Vӧgtle, F. Acc. Chem. Rev. 1985, 18, 65.
- Chen, C.F.; Chen, Q.Y. Azocalix[4]arene-based chromogenic anion probes. New J. Chem. 2006, 30, 143–147. [Google Scholar] [CrossRef]
- Thiampanya, P.; Muangsin, N.; Pulpoka, B. Azocalix[4]arene Strapped Calix[4]pyrrole: A Confirmable Fluoride Sensor. Org. Lett. 2012, 14, 4050–4053. [Google Scholar] [CrossRef]
- Zheng,Y. L.; Hong, K. S.; Hong, X.; Tong, Y. Y.; Tangxin, X.; Xiao, Q. S.; Juli, J.; Leyong, W. Calix[4]arene containing thiourea and coumarin functionality as highly selective fluorescent and colorimetric chemosensor for fluoride ion. Spectrochimic Acta Part A: Molecular and Biomolecular Spectroscopy 2018, 200, 307–312. [Google Scholar] [CrossRef]
- Hellwig, P. Infrared spectroscopic markers of quinones in proteins from the respiratory chain. Biochemica et Biohysica Acta 2015, 1847, 126–133. [Google Scholar] [CrossRef]
- Bocchi, V.; Foina, D.; Pochini, A.; Hứngaro, R.; Andreetti, G.D. Synthesis, 1H NMR, 13C NMR spectra and conformational preference of open chain ligands on lipophilic macrocycles. Tetrahedron 1982, 38, 373–378. [Google Scholar] [CrossRef]
- Ungaro, R.; Arduini, A.; Pochini, A.; Reverberi, S. p-t-Butyl-calix[4]arene tetracarboxylic acid. A water soluble calixarene in a cone structure. J. Chem.Soc. Chem.Commun. 1984, 981–982. [Google Scholar]
- Shinkai, S.; Araki, K.; Shibata, J.; Tsugawa, D.; Manabe, Autoaccelerative diazo coupling with calix[4]arene: substituent effects on the unusual co-operativity of the OH groups. O. J. Chem. Soc. Perkin Trans. 1990, 1, 3333–3337. [Google Scholar] [CrossRef]
- Danil de Namor, A.F.; El Gamouz, A.; Alharthi, S.; Al Hakawati, N.; Varcoe, J. R. A ditopic calix[4]pyrrole amide derivative: highlighting the importance of fundamental studies and the use of NaPh4B as additive in the design and applications of mercury(II) ion selective electrodes. J. Mater. Chem. A 2015, 3, 13016–13030. [Google Scholar] [CrossRef]
- Chaaban, J. K.; Al Hakawati, N.; Howlin, B.; Bance-Soualhi, R.; Danil de Namor, A.F. An asymmetric N-rim partially substituted calix[4]pyrrole: Its affinity for Ag(I) and its destruction by Hg(II). Arab. J. Chem. 2020, 13, 4824–4834. [Google Scholar] [CrossRef]

| δ (ppm) | ||||||||
|---|---|---|---|---|---|---|---|---|
| H-1 | H-2 | H-3 | H-3’ | H-4 | H-4’ | H-5 | H-6 | |
| δ Ref | - | 7.82 | 3.51 | 4.34 | 7.85 | 8.05 | 4.3 | 1.32 |
| F- | - | - | -0.19 | -0.64 | - | - | - | -0.39 |
| CO32- | - | -0.2 | 0.12 | 0.28 | -0.1 | -0.08 | 0.0 | -0.01 |
| H2PO4- | - | -0.17 | - | 0.3 | -0.09 | -0.06 | 0.0 | -0.39 |
| HSO4- | - | -0.01 | -0.18 | 0.6 | 0.0 | 0.0 | 0.0 | -0.39 |
| Cl- | - | 0.02 | 0.17 | 0.08 | 0.0 | 0.0 | -0.08 | -0.39 |
|
para-ester diazophenylcalix[4]arene |
|||||
|---|---|---|---|---|---|
| Anion | (CA-AZ: ) | log KS | (kJ mol-1) | (kJ mol-1) |
(J mol-1 K-1) |
| F- | 1:1 | 5.9±0.1 | -33.7±0.2 | -12 ±0.3 | 72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




