Submitted:
06 July 2023
Posted:
07 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Recruitment and Clinical Investigation of Subjects
2.2. Whole-Genome Scan Using Markers and Linkage Assay
2.3. Sequence Analysis of the Genes Located at the Finely Mapped Locus
2.4. Production of Eukaryotic Gene Expression Vectors
2.5. Cellular Vector Transfection Followed by Dual-Reporter Assay
2.6. Assay of Electrophoretic Mobility of Mutant TBX20
2.7. Subcellular Distribution of TBX20 Mutants
2.8. Statistical Assessment
3. Results
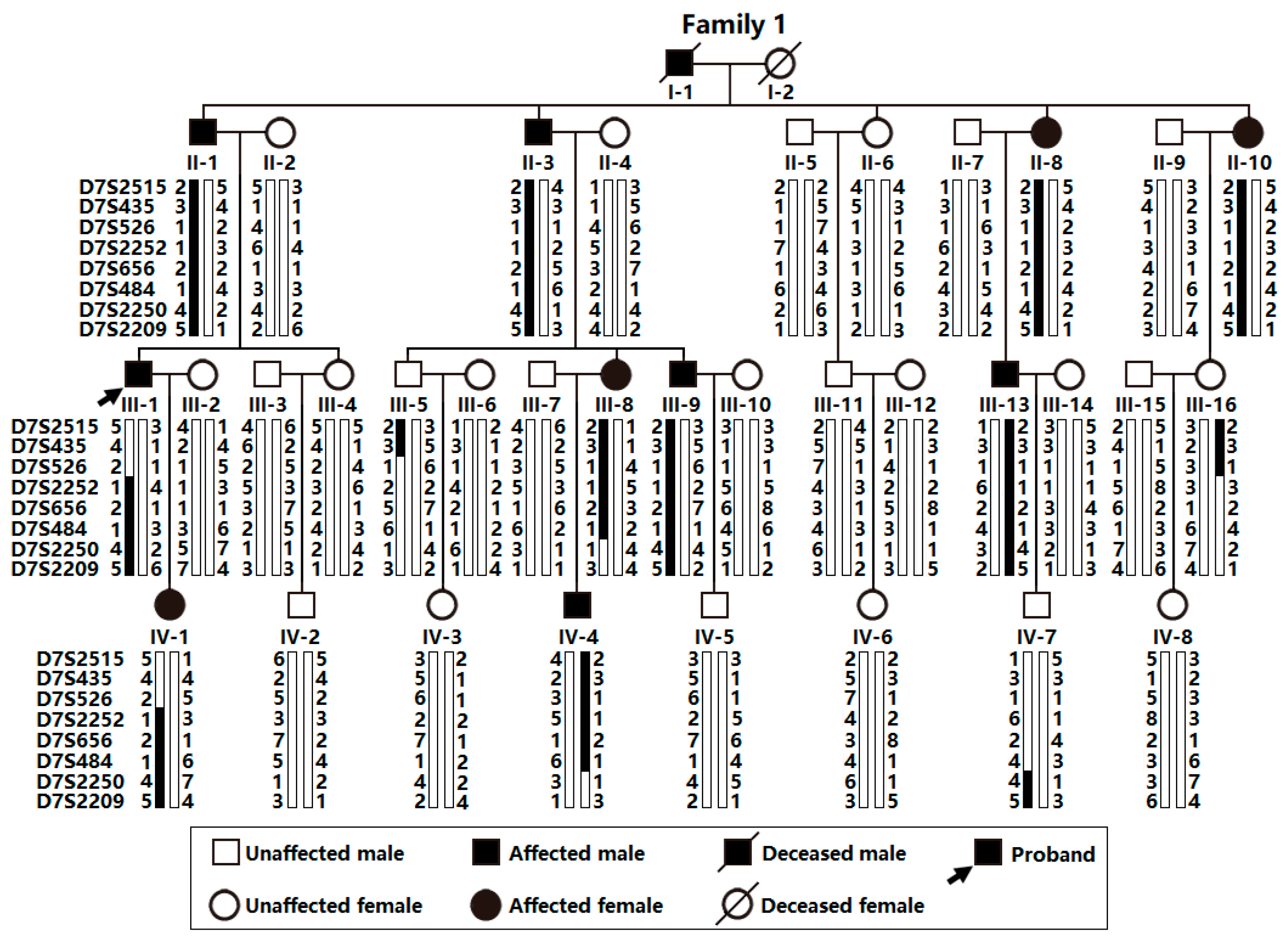
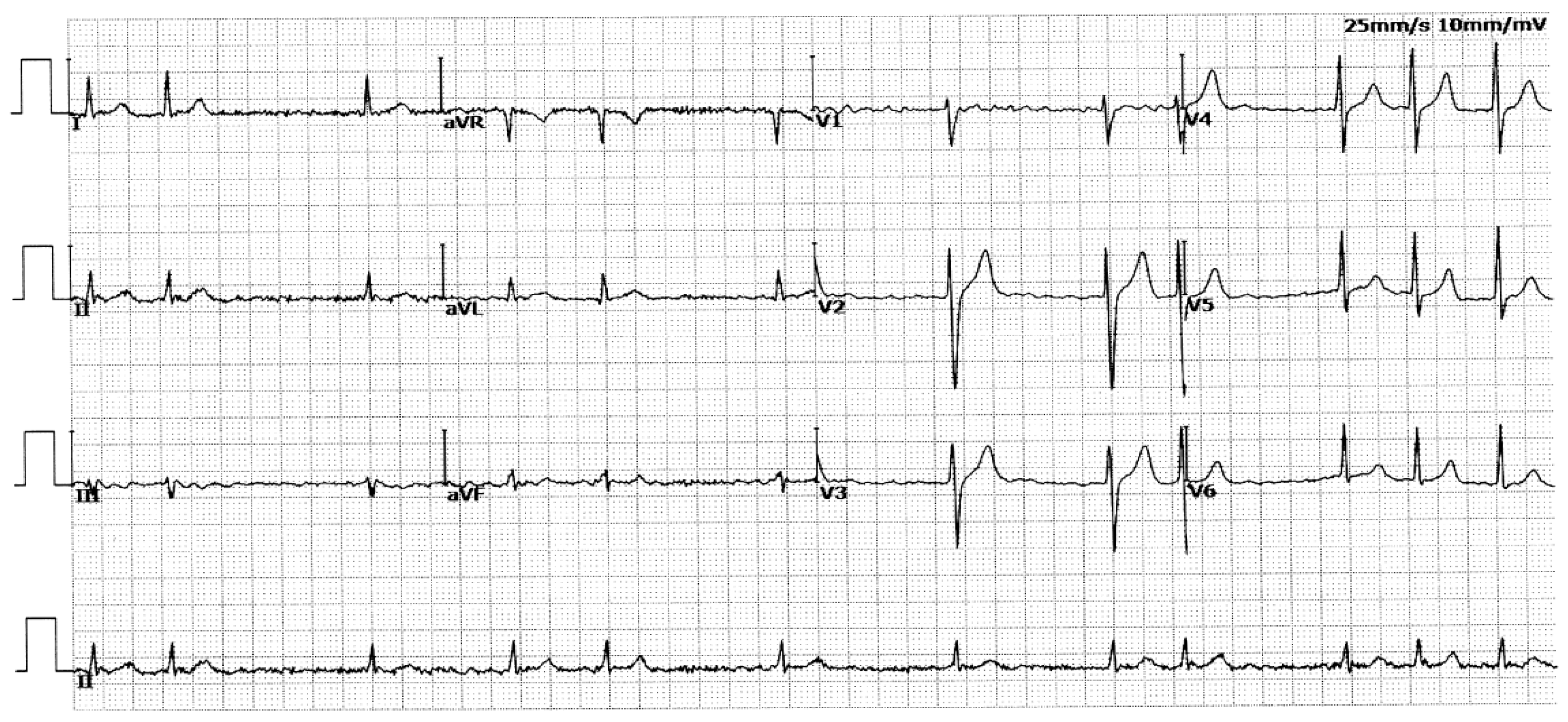
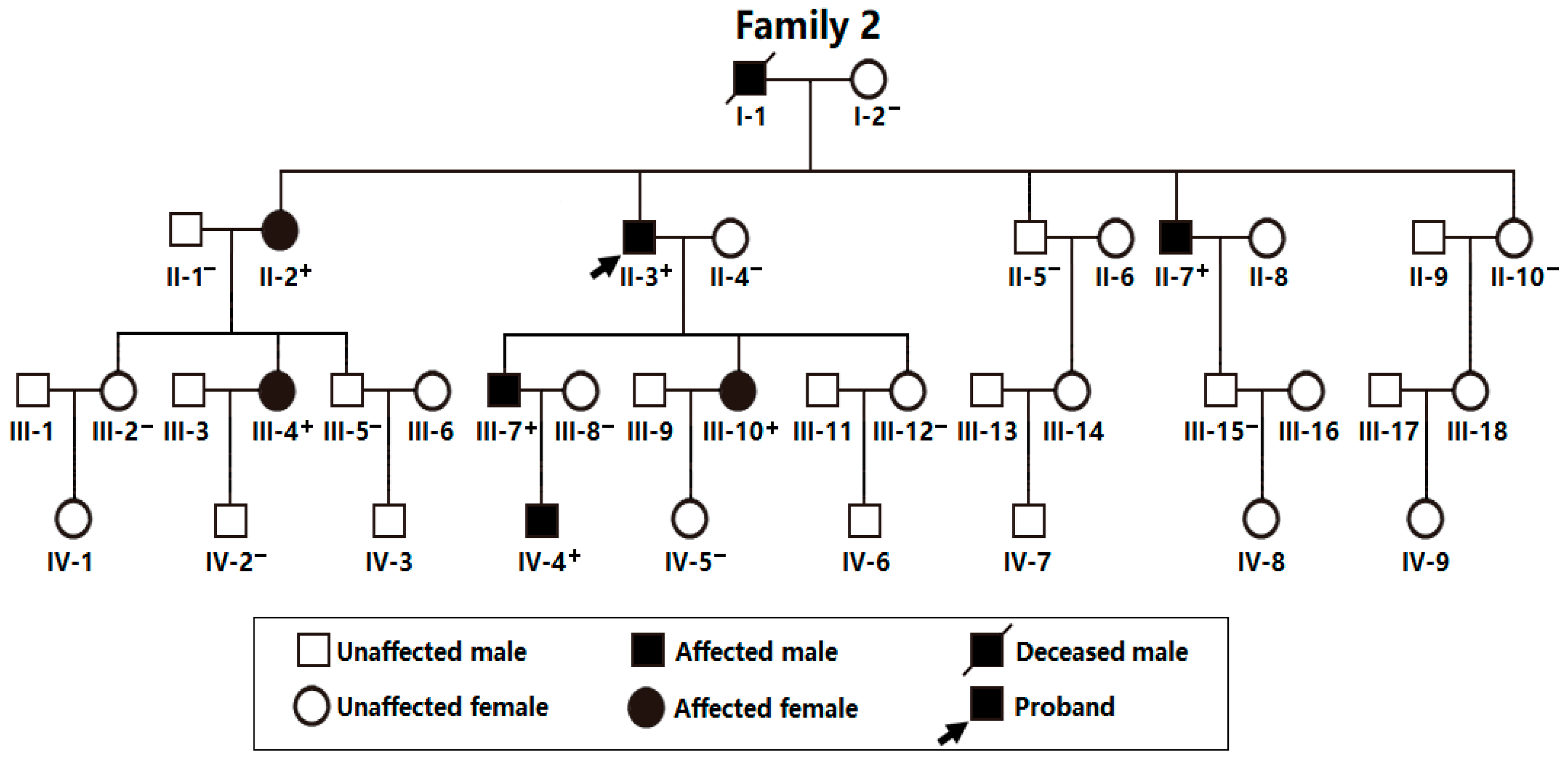
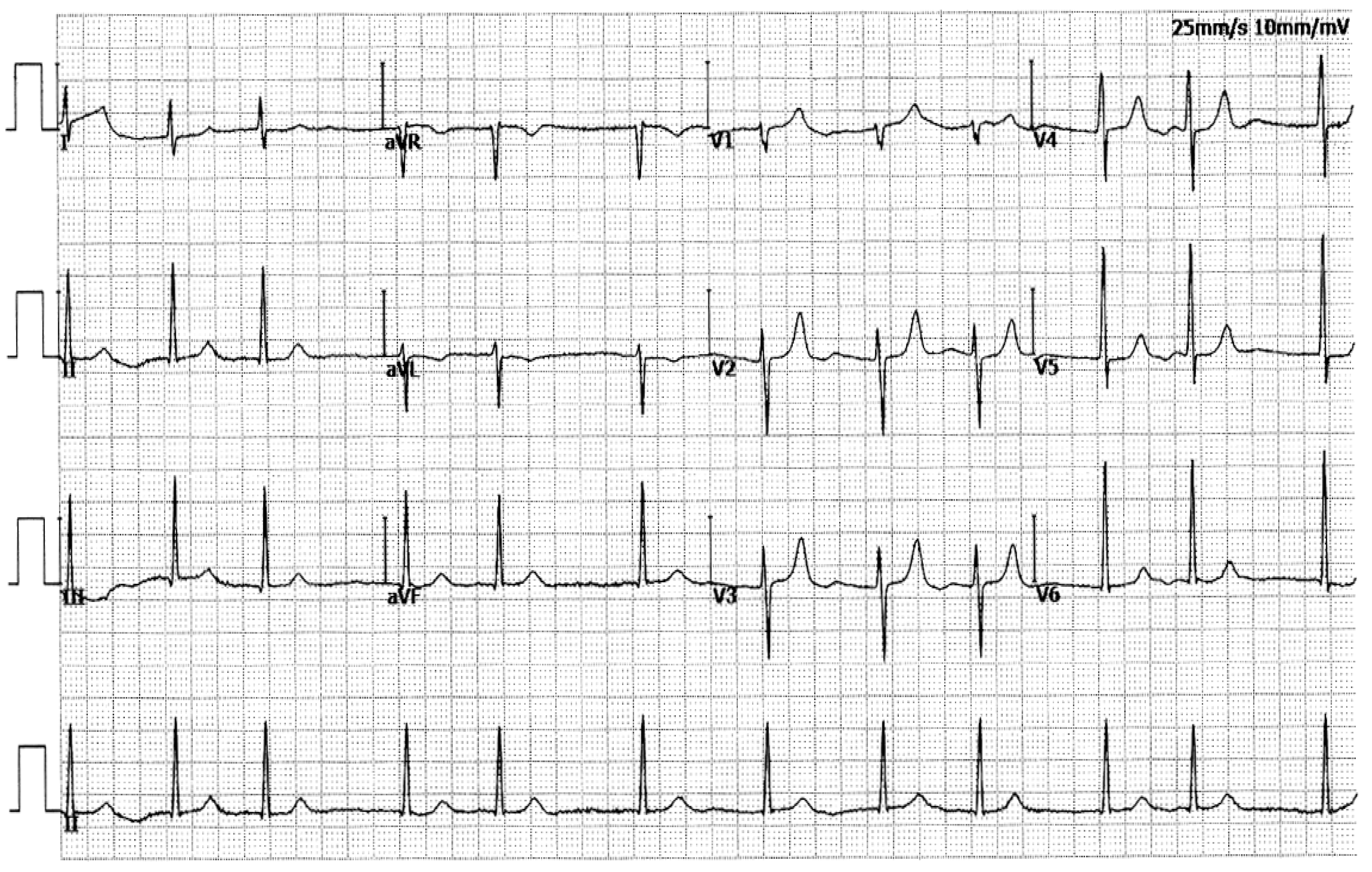
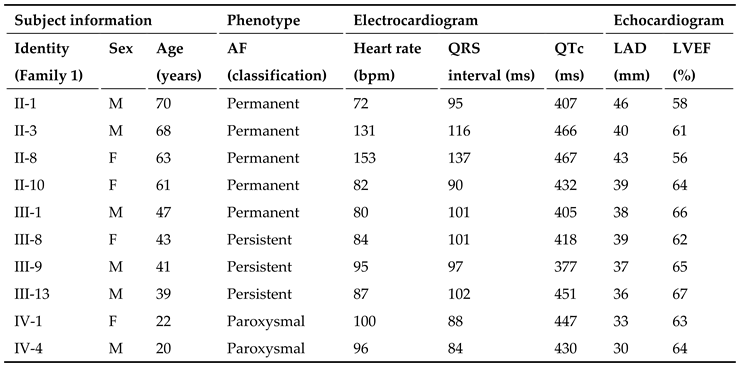
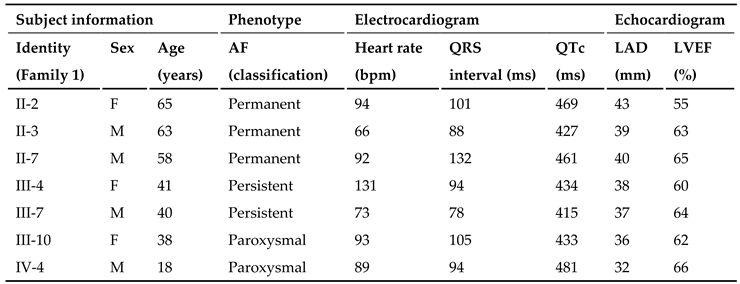
3.1. Phenotypic Characteristic Profiles of Study Participants
3.2. A New AF-causative Locus Mapped on Human Chromosome 7p14.2-p14.3
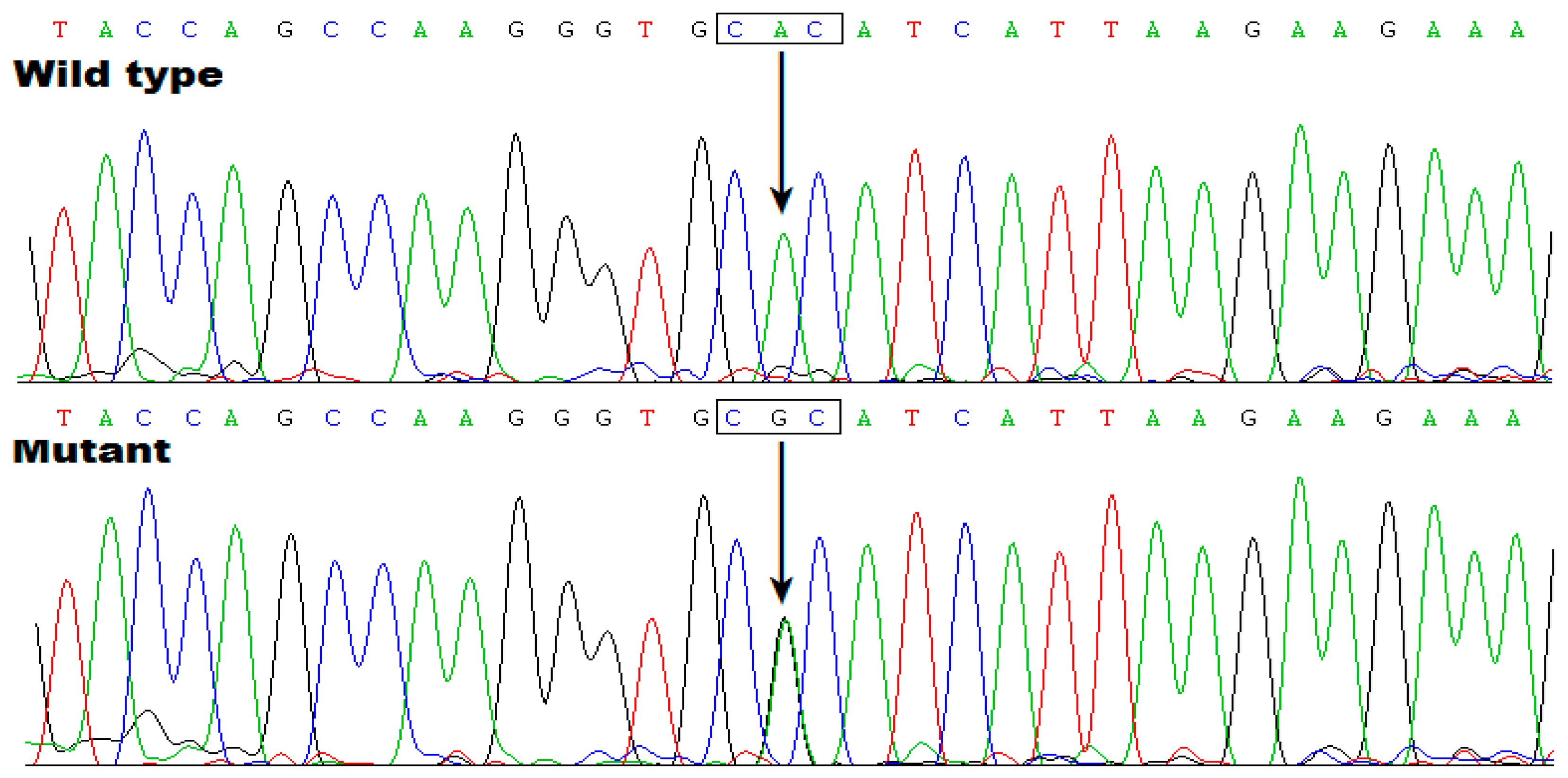
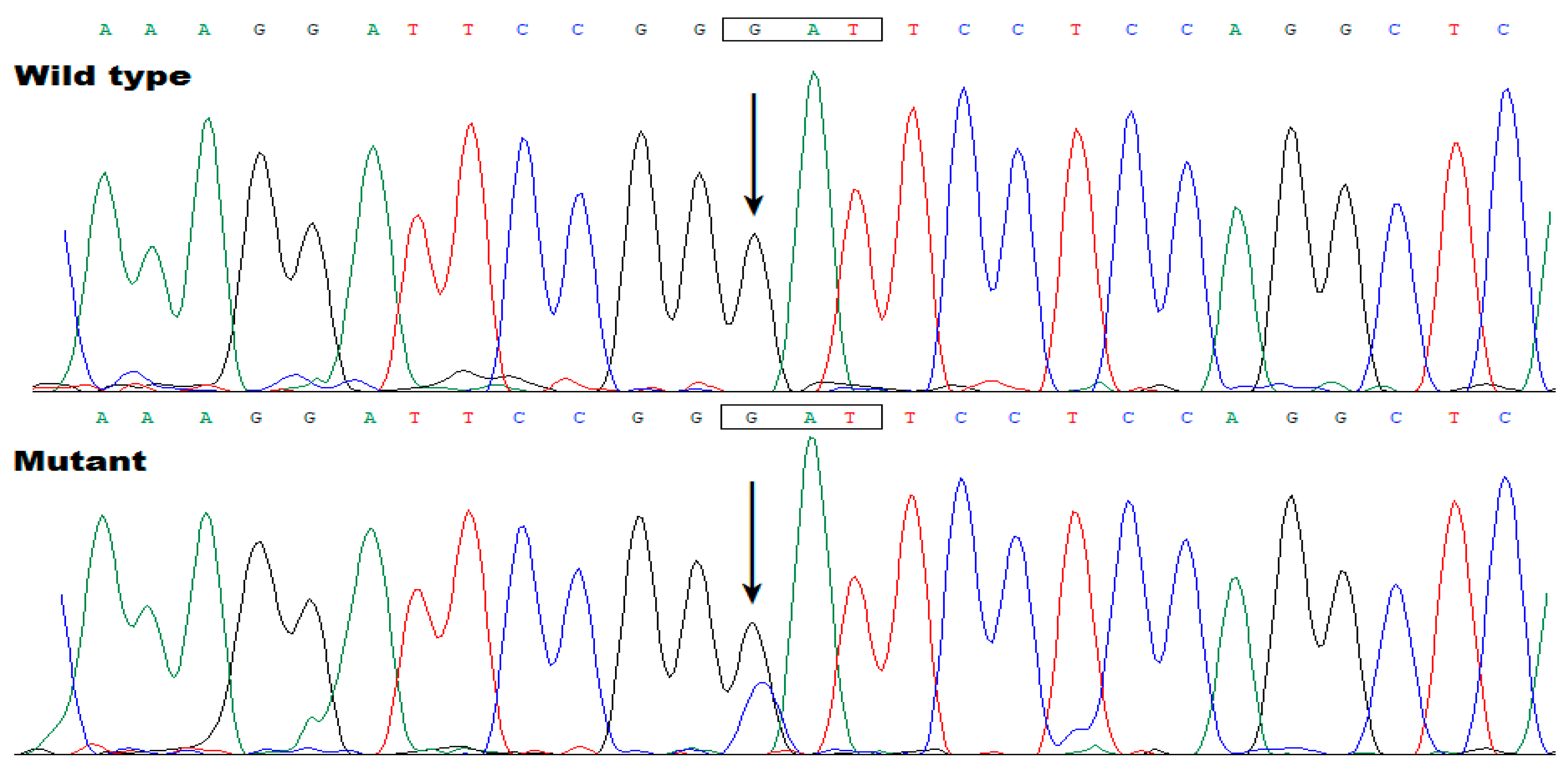
3.3. Discovery of TBX20 as a New AF-Causative Gene
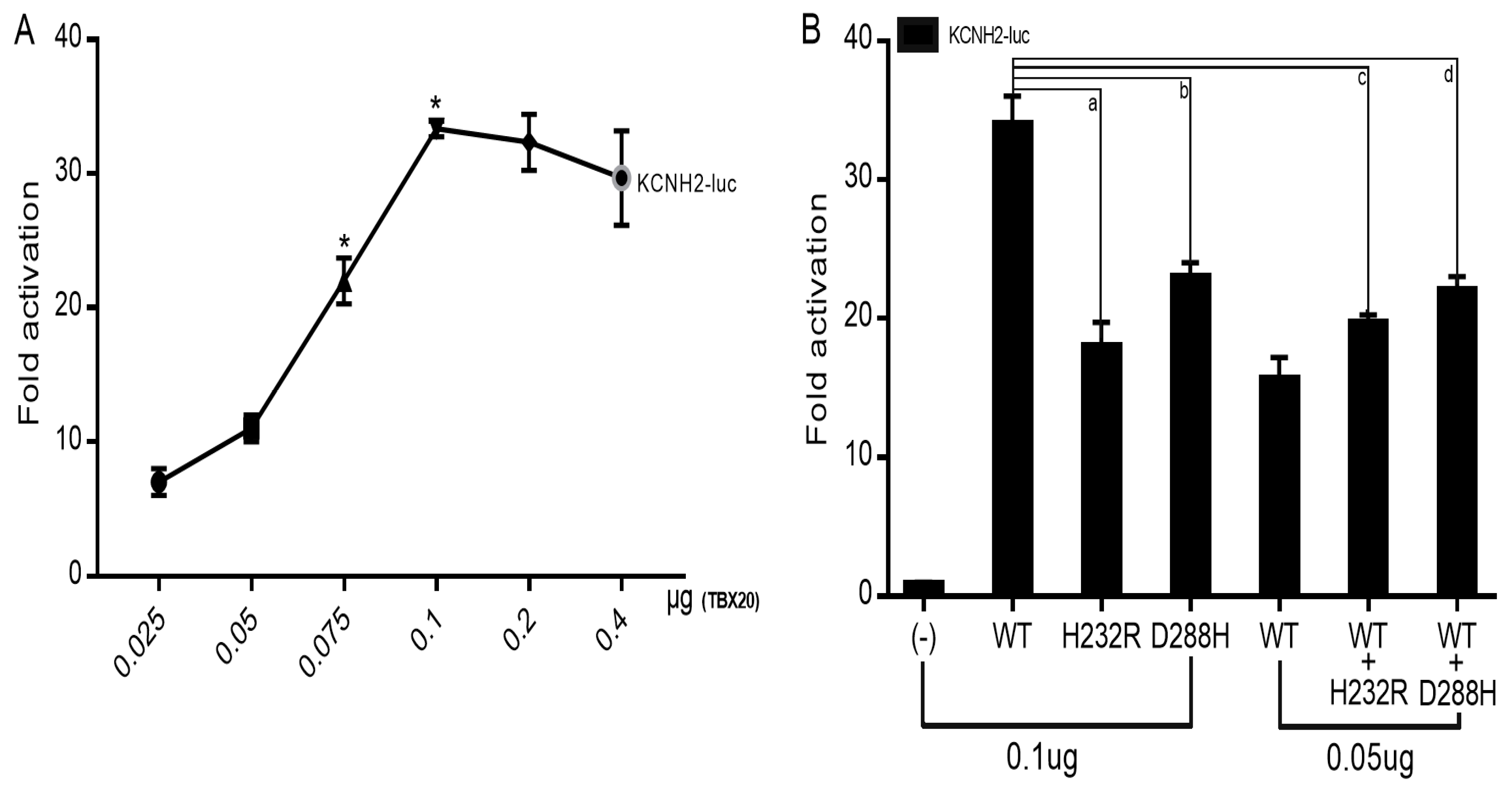
3.4. Reduced Transactivation of KCNH2 by Mutant TBX20
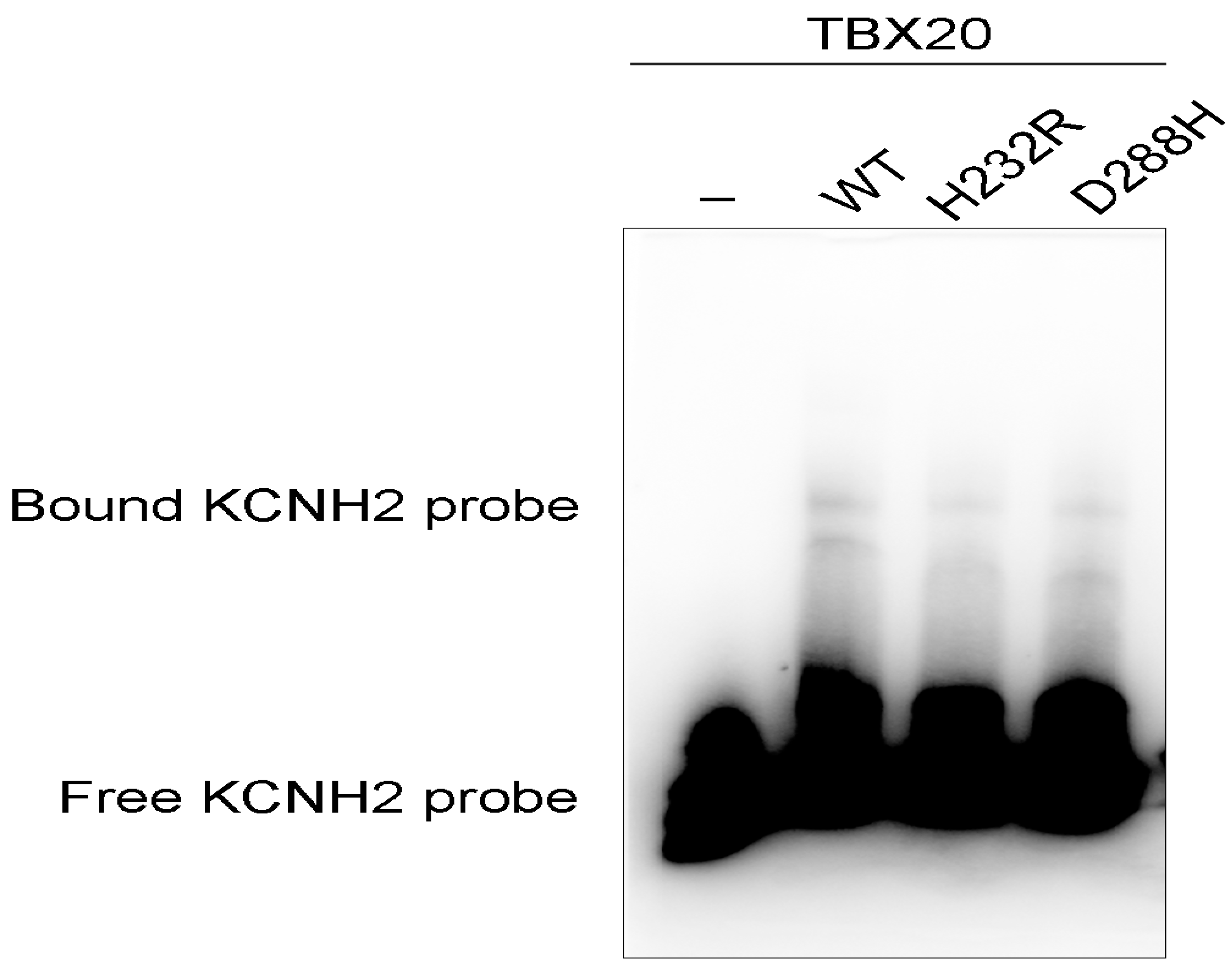
3.5. Diminished Ability of the TBX20 Mutants to Bind the KCNH2 Promoter
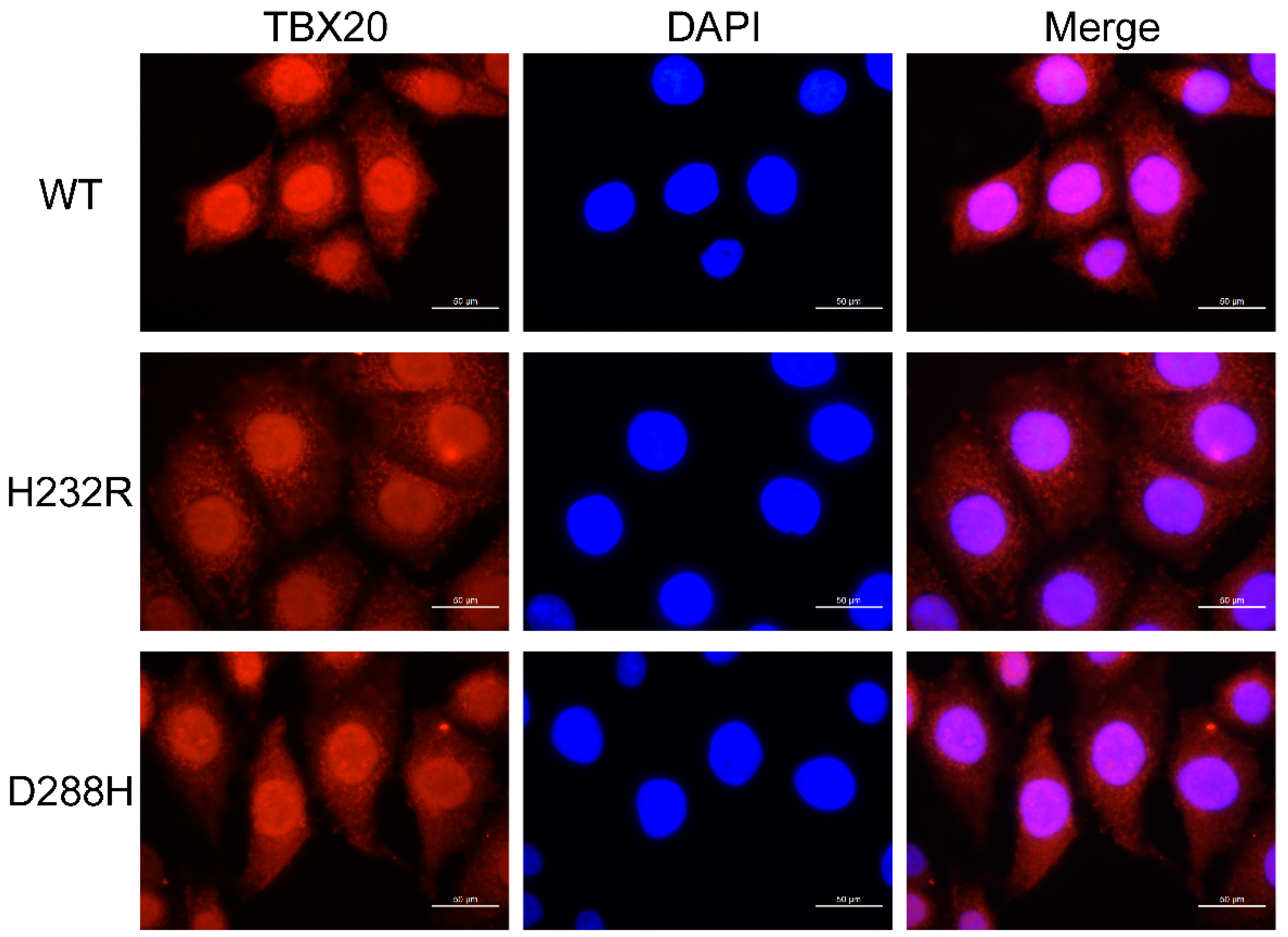
3.6. Subcellular Distribution of TBX20 Mutants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
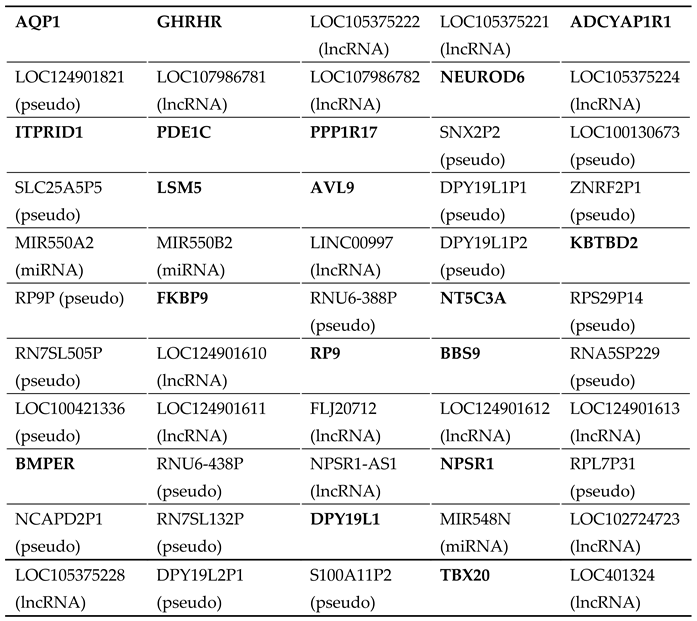 |
References
- January, C.T.; Wann, L.S.; Alpert, J.S.; Calkins, H.; Cigarroa, J.E.; Cleveland, J.C. Jr.; Conti, J.B.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Murray, K.T.; Sacco, R.L.; Stevenson, W.G.; Tchou, P.J.; Tracy, C.M.; Yancy, C.W.; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014, 130, e199–e267. [CrossRef]
- Sagris, M.; Vardas, E.P.; Theofilis, P.; Antonopoulos, A.S.; Oikonomou, E.; Tousoulis, D. Atrial Fibrillation: Pathogenesis, Predisposing Factors, and Genetics. Int. J. Mol. Sci. 2021, 23, 6. [CrossRef]
- Lloyd-Jones, D.M.; Wang, T.J.; Leip, E.P.; Larson, M.G.; Levy, D.; Vasan, R.S.; D'Agostino, R.B.; Massaro, J.M.; Beiser, A.; Wolf, P.A.; Benjamin, E.J. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation 2004, 110, 1042–1046. [CrossRef]
- Weng, L.C.; Preis, S.R.; Hulme, O.L.; Larson, M.G.; Choi, S.H.; Wang, B.; Trinquart, L.; McManus, D.D.; Staerk, L.; Lin, H.; Lunetta, K.L.; Ellinor, P.T.; Benjamin, E.J.; Lubitz, S.A. Genetic Predisposition, Clinical Risk Factor Burden, and Lifetime Risk of Atrial Fibrillation. Circulation 2018, 137, 1027–1038. [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; Fauchier, L.; Filippatos, G.; Kalman, J.M.; La Meir, M.; Lane, D.A.; Lebeau, J.P.; Lettino, M.; Lip, G.Y.H.; Pinto, F.J.; Thomas, G.N.; Valgimigli, M.; Van Gelder, I.C.; Van Putte, B.P.; Watkins, C.L.; ESC Scientific Document Group. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [CrossRef]
- Kiliszek, M.; Uziębło-Życzkowska, B.; Gorczyca, I.; Maciorowska, M.; Jelonek, O.; Wożakowska-Kapłon, B.; Wójcik, M.; Błaszczyk, R.; Gawałko, M.; Kapłon-Cieślicka, A.; Tokarek, T.; Rajtar-Salwa, R.; Bil, J.; Wojewódzki, M.; Szpotowicz, A.; Krzciuk, M.; Bednarski, J.; Bakuła-Ostalska, E.; Tomaszuk-Kazberuk, A.; Szyszkowska, A.; Wełnicki, M.; Mamcarz, A.; Krzesiński, P. Symptomatic and Asymptomatic Patients in the Polish Atrial Fibrillation (POL-AF) Registry. J. Clin. Med. 2021, 10, 1091. [CrossRef]
- Imberti, J.F.; Bonini, N.; Tosetti, A.; Mei, D.A.; Gerra, L.; Malavasi, V.L.; Mazza, A.; Lip, G.Y.H.; Boriani, G. Atrial High-Rate Episodes Detected by Cardiac Implantable Electronic Devices: Dynamic Changes in Episodes and Predictors of Incident Atrial Fibrillation. Biology 2022, 11, 443. [CrossRef]
- Sgreccia, D.; Manicardi, M.; Malavasi, V.L.; Vitolo, M.; Valenti, A.C.; Proietti, M.; Lip, G.Y.H.; Boriani, G. Comparing Outcomes in Asymptomatic and Symptomatic Atrial Fibrillation: A Systematic Review and Meta-Analysis of 81,462 Patients. J. Clin. Med. 2021, 10, 3979. [CrossRef]
- Sadlonova, M.; Senges, J.; Nagel, J.; Celano, C.; Klasen-Max, C.; Borggrefe, M.; Akin, I.; Thomas, D.; Schwarzbach, C.J.; Kleeman, T.; Schneider, S.; Hochadel, M.; Süselbeck, T.; Schwacke, H.; Alonso, A.; Haass, M.; Ladwig, K.H.; Herrmann-Lingen, C. Symptom Severity and Health-Related Quality of Life in Patients with Atrial Fibrillation: Findings from the Observational ARENA Study. J. Clin. Med. 2022, 11, 1140. [CrossRef]
- Rohrer, U.; Manninger, M.; Zirlik, A.; Scherr, D. Impact of Catheter Ablation for Atrial Fibrillation on Quality of Life. J. Clin. Med. 2022, 11, 4541. [CrossRef]
- Seki, Y.; Fujisawa, T.; Ikemura, N.; Ibe, S.; Tsuzuki, I.; Hashimoto, K.; Yamashita, T.; Miyama, H.; Niimi, N.; Suzuki, M.; Negishi, K.; Katsumata, Y.; Kimura, T.; Fukuda, K.; Kohsaka, S.; Takatsuki, S. Catheter ablation improves outcomes and quality of life in Japanese patients with early-stage atrial fibrillation: A retrospective cohort study. Heart Rhythm 2022, 19, 1076–1083. [CrossRef]
- Wazni, O.; Dandamudi, G.; Sood, N.; Hoyt, R.; Tyler, J.; Durrani, S.; Niebauer, M.; Makati, K.; Halperin, B.; Gauri, A.; Morales, G.; Shao, M.; Pouliot, E.; Kaplon, R.E.; Nissen, S.E.; STOP AF First Trial Investigators. Quality of life after the initial treatment of atrial fibrillation with cryoablation versus drug therapy. Heart Rhythm 2022, 19, 197–205. [CrossRef]
- Alves, L.S.; Bocchi, E.A.; Chizzola, P.R.; Castro, R.E.; Salemi, V.M.C.; de Melo, M.D.T.; Andreta, C.R.L.; Guimarães, G.V. Exercise training in heart failure with reduced ejection fraction and permanent atrial fibrillation: A randomized clinical trial. Heart Rhythm 2022, 19, 1058–1066. [CrossRef]
- Buckley, B.J.R.; Harrison, S.L.; Fazio-Eynullayeva, E.; Underhill, P.; Lane, D.A.; Thijssen, D.H.J.; Lip, G.Y.H. Exercise-Based Cardiac Rehabilitation and All-Cause Mortality Among Patients With Atrial Fibrillation. J. Am. Heart Assoc. 2021, 10, e020804. [CrossRef]
- Mujović, N.M.; Marinković, M.M.; Nedeljković, I.; Marković, N.; Banović, M.; Vučićević, V.; Stanković, G.; Potpara, T.S. Improvement of Maximal Exercise Performance After Catheter-Ablation of Atrial Fibrillation and Its Prognostic Significance for Long-Term Rhythm Outcome. J. Am. Heart Assoc. 2021, 10, e017445. [CrossRef]
- Mohanty, S.; Mohanty, P.; Trivedi, C.; Assadourian, J.; Mayedo, A.Q.; MacDonald, B.; Della Rocca, D.G.; Gianni, C.; Horton, R.; Al-Ahmad, A.; Bassiouny, M.; Burkhardt, J.D.; Di Biase, L.; Gurol, M.E.; Natale, A. Impact of Oral Anticoagulation Therapy Versus Left Atrial Appendage Occlusion on Cognitive Function and Quality of Life in Patients With Atrial Fibrillation. J. Am. Heart Assoc. 2021, 10, e019664. [CrossRef]
- Alam, A.B.; Kulshreshtha, A.; Li, L.; Subramanya, V.; Alonso, A. Associations of Atrial Fibrillation with Mild Cognitive Impairment and Dementia: An Investigation Using SPRINT Research Materials. J. Clin. Med. 2022, 11, 5800. [CrossRef]
- Rivard, L.; Friberg, L.; Conen, D.; Healey, J.S.; Berge, T.; Boriani, G.; Brandes, A.; Calkins, H.; Camm, A.J.; Yee Chen, L.; Lluis Clua Espuny, J.; Collins, R,.; Connolly, S.; Dagres, N.; Elkind, M.S.V.; Engdahl, J.; Field, T.S.; Gersh, B.J.; Glotzer, T.V.; Hankey, G.J.; Harbison, J.A.; Haeusler, K.G.; Hills, M.T.; Johnson, L.S.B.; Joung, B.; Khairy, P.; Kirchhof, P.; Krieger, D.; Li,p G.Y.H.; Løchen, M.L.; Madhavan, M.; Mairesse, G.H.; Montaner, J.; Ntaios, G.; Quinn, T.J.; Rienstra, M.; Rosenqvist, M.; Sandhu, R.K.; Smyth, B.; Schnabel, R.B.; Stavrakis, S.; Themistoclakis, S.; Van Gelder, I.C.; Wang, J.G.; Freedman, B. Atrial Fibrillation and Dementia: A Report From the AF-SCREEN International Collaboration. Circulation 2022, 145, 392–409. [CrossRef]
- Giannone, M.E.; Filippini, T.; Whelton, P.K.; Chiari, A.; Vitolo, M.; Boriani, G.; Vinceti, M. Atrial Fibrillation and the Risk of Early-Onset Dementia: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2022, 11, e025653. [CrossRef]
- Lim, J.; Lee, S.R.; Choi, E.K.; Han, K.D.; Jung, J.H.; Ahn, H.J.; Yun, J.P.; Kwon, S.; Oh, S.; Lip, G.Y.H. Exercise and the Risk of Dementia in Patients with Newly Diagnosed Atrial Fibrillation: A Nationwide Population-Based Study. J. Clin. Med. 2021, 10, 3126. [CrossRef]
- Chen, Y.L.; Chen, J.; Wang, H.T.; Chang, Y.T.; Chong, S.Z.; Hsueh, S.; Chung, C.M.; Lin, Y.S. Sex Difference in the Risk of Dementia in Patients with Atrial Fibrillation. Diagnostics 2021, 11, 760. [CrossRef]
- Lip, G.Y.H.; Gue, Y.; Zhang, J.; Chao, T.F.; Calkins, H.; Potpara, T. Stroke prevention in atrial fibrillation. Trends Cardiovasc. Med. 2022, 32, 501–510. [CrossRef]
- Reading Turchioe, M.; Soliman, E.Z.; Goyal, P.; Merkler, A.E.; Kamel, H.; Cushman, M.; Soroka, O.; Masterson Creber, R.; Safford, M.M. Atrial Fibrillation and Stroke Symptoms in the REGARDS Study. J. Am. Heart Assoc. 2022, 11, e022921. [CrossRef]
- Singleton, M.J.; Yuan, Y.; Dawood, F.Z.; Howard, G.; Judd, S.E.; Zakai, N.A.; Howard, V.J.; Herrington, D.M.; Soliman, E.Z.; Cushman, M. Multiple Blood Biomarkers and Stroke Risk in Atrial Fibrillation: The REGARDS Study. J. Am. Heart Assoc. 2021, 10, e020157. [CrossRef]
- Paciaroni, M.; Caso, V.; Agnelli, G.; Mosconi, M.G.; Giustozzi, M.; Seiffge, D.J.; Engelter, S.T.; Lyrer, P.; Polymeris, A.A.; Kriemler, L.; Zietz, A.; Putaala, J.; Strbian, D.; Tomppo, L.; Michel, P.; Strambo, D.; Salerno, A.; Remillard, S.; Buehrer, M.; Bavaud, O.; Vanacker, P.; Zuurbier, S.; Yperzeele, L.; Loos, C.M.J.; Cappellari, M.; Emiliani, A.; Zedde, M.; Abdul-Rahim, A.; Dawson, J.; Cronshaw, R.; Schirinzi, E.; Del Sette, M.; Stretz, C.; Kala, N.; Reznik, M.; Schomer, A.; Grory, B.M.; Jayaraman, M.; McTaggart, R.; Yaghi, S.; Furie, K.L.; Masotti, L.; Grifoni, E.; Toni, D.; Risitano, A.; Falcou, A.; Petraglia, L.; Lotti, EM.; Padroni, M.; Pavolucci, L.; Lochner, P.; Silvestrelli, G.; Ciccone, A.;, Alberti, A.; Venti, M.; Traballi, L.; Urbini, C.; Kargiotis, O.; Rocco, A.; Diomedi, M.; Marcheselli, S.; Caliandro, P.; Zauli, A.; Reale, G.; Antonenko, K.; Rota, E.; Tassinari, T.; Saia, V.; Palmerini, F.; Aridon, P.; Arnao, V.; Monaco, S.; Cottone, S.; Baldi, A.; D'Amore, C.; Ageno, W.; Pegoraro, S.; Ntaios, G.; Sagris, D.; Giannopoulos, S.; Kosmidou, M.; Ntais, E.; Romoli, M.; Pantoni, L.; Rosa, S.; Bertora, P.; Chiti, A.; Canavero, I.; Saggese, C.E.; Plocco, M.; Giorli, E.; Palaiodimou, L.; Bakola, E.; Tsivgoulis, G.; Bandini, F.; Gasparro, A.; Terruso, V.; Mannino, M.; Pezzini, A.; Ornello, R.; Sacco, S.; Popovic, N.; Scoditti, U.; Genovese, A.; Denti, L.; Flomin, Y.; Mancuso, M.; Ferrari, E.; Caselli, M.C.; Ulivi, L.; Giannini, N.; De Marchis, G.M. Recurrent Ischemic Stroke and Bleeding in Patients With Atrial Fibrillation Who Suffered an Acute Stroke While on Treatment With Nonvitamin K Antagonist Oral Anticoagulants: The RENO-EXTEND Study. Stroke 2022, 53, 2620–2627. [CrossRef]
- Park, S.; Lee, S.; Kim, Y.; Lee, Y.; Kang, M.W.; Kim, K.; Kim, Y.C.; Han, S.S.; Lee, H.; Lee, J.P.; Joo, K.W.; Lim, C.S.; Kim, Y.S.; Kim, D.K. Atrial fibrillation and kidney function: a bidirectional Mendelian randomization study. Eur. Heart J. 2021, 42, 2816–2823. [CrossRef]
- van der Burgh, A.C.; Geurts, S.; Ikram, M.A.; Hoorn, E.J.; Kavousi, M.; Chaker, L. Bidirectional Association Between Kidney Function and Atrial Fibrillation: A Population-Based Cohort Study. J. Am. Heart Assoc. 2022, 11, e025303. [CrossRef]
- Geurts, S.; van der Burgh, A.C.; Bos, M.M.; Ikram, M.A.; Stricker, B.H.C.; Deckers, J.W.; Hoorn, E.J.; Chaker, L.; Kavousi, M. Disentangling the association between kidney function and atrial fibrillation: a bidirectional Mendelian randomization study. Int. J. Cardiol. 2022, 355, 15–22. [CrossRef]
- Gabarin, M.; Hornik-Lurie, T.; Minha, S.; Omelchenko, A.; Barashi, R.; Arow, Z.; Assali, A.; Pereg, D. CHA2DS2-VASc Score, Mortality and Acute Myocardial Infarction in Patients With Nonvalvular Atrial Fibrillation. Am. J. Cardiol. 2022, 180, 24–28. [CrossRef]
- Camen, S.; Csengeri, D.; Geelhoed, B.; Niiranen, T.; Gianfagna, F.; Vishram-Nielsen, J.K.; Costanzo, S.; Söderberg, S.; Vartiainen, E.; Börschel, C.S.; Donati, M.B.; Løchen, M.L.; Ojeda, F.M.; Kontto, J.; Mathiesen, E.B.; Jensen, S.; Koenig, W.; Kee, F.; de Gaetano, G.; Zeller, T.; Jørgensen, T.; Tunstall-Pedoe, H.; Blankenberg, S.; Kuulasmaa, K.; Linneberg, A.; Salomaa, V.; Iacoviello, L.; Schnabel, R.B. Risk Factors, Subsequent Disease Onset, and Prognostic Impact of Myocardial Infarction and Atrial Fibrillation. J. Am. Heart Assoc. 2022, 11, e024299. [CrossRef]
- Belkouche, A.; Yao, H.; Putot, A.; Chagué, F.; Rochette, L.; Danchin, N.; Fauchier, L.; Zeller, M.; Cottin, Y. The Multifaceted Interplay between Atrial Fibrillation and Myocardial Infarction: A Review. J. Clin. Med. 2021, 10, 198. [CrossRef]
- Steinberg, B.A.; Li, Z.; O'Brien, E.C.; Pritchard, J.; Chew, D.S.; Bunch, T.J.; Mark, D.B.; Nabutovsky, Y.; Greiner, M.A.; Piccini, J.P. Atrial fibrillation burden and heart failure: Data from 39,710 individuals with cardiac implanted electronic devices. Heart Rhythm 2021, 18, 709–716. [CrossRef]
- Johnson, L.S.B.; Oldgren, J.; Barrett, T.W.; McNaughton, C.D.; Wong, J.A.; McIntyre, W.F.; Freeman, C.L.; Murphy, L.; Engström, G.; Ezekowitz, M.; Connolly, S.J.; Xu, L.; Nakamya, J.; Conen, D.; Bangdiwala, S.I.; Yusuf, S.; Healey, J.S. LVS-HARMED Risk Score for Incident Heart Failure in Patients With Atrial Fibrillation Who Present to the Emergency Department: Data from a World-Wide Registry. J. Am. Heart Assoc. 2021, 10, e017735. [CrossRef]
- Kawaji, T.; Ogawa, H.; Hamatani, Y.; Kato, M.; Yokomatsu, T.; Miki, S.; Abe, M.; Akao, M.; Fushimi AF Registry investigators. Fine Fibrillatory Wave as a Risk Factor for Heart Failure Events in Patients With Atrial Fibrillation: The Fushimi Atrial Fibrillation (AF) Registry. J. Am. Heart Assoc. 2022, 11, e024341. [CrossRef]
- Kim, Y.G.; Choi, Y.Y.; Han, K.D.; Min, K.; Choi, H.Y.; Shim, J.; Choi, J.I.; Kim, Y.H. Atrial fibrillation is associated with increased risk of lethal ventricular arrhythmias. Sci. Rep. 2021, 11, 18111. [CrossRef]
- Tanaka, Y.; Shah, N.S.; Passman, R.; Greenland, P.; Lloyd-Jones, D.M.; Khan, S.S. Trends in Cardiovascular Mortality Related to Atrial Fibrillation in the United States, 2011 to 2018. J. Am. Heart Assoc. 2021, 10, e020163. [CrossRef]
- Zhao, R.; Wang, Z.; Cao, F.; Song, J.; Fan, S.; Qiu, J.; Fan, X.; Yu, C. New-Onset Postoperative Atrial Fibrillation After Total Arch Repair Is Associated With Increased In-Hospital Mortality. J. Am. Heart Assoc. 2021, 10, e021980. [CrossRef]
- Brener, M.I.; George, I.; Kosmidou, I.; Nazif, T.; Zhang, Z.; Dizon, J.M.; Garan, H.; Malaisrie, S.C.; Makkar, R.; Mack, M.; Szeto, W.Y.; Fearon, W.F.; Thourani, V.H.; Leon, M.B.; Kodali, S.; Biviano, A.B. Atrial Fibrillation Is Associated With Mortality in Intermediate Surgical Risk Patients With Severe Aortic Stenosis: Analyses From the PARTNER 2A and PARTNER S3i Trials. J. Am. Heart Assoc. 2021, 10, e019584. [CrossRef]
- de Terwangne, C.; Lelubre, C.; Hanotier, P.; de Meester, A.; Descamps, O.; Duray, C.; Pannone, L.; Chierchia, G.B.; de Asmundis, C.; Nokerman, H.; Minette, P.; Ceccarelli, A.; Boland, B.; Sorgente, A. Prevalence and Impact of Atrial Fibrillation on Intra-Hospital Mortality in Patients Aged ≥75 Years. Am. J. Cardiol. 2022, 177, 40–47. [CrossRef]
- Bertini, M.; Pompei, G.; Tolomeo, P.; Malagù, M.; Fiorio, A.; Balla, C.; Vitali, F.; Rapezzi, C. Zero-Fluoroscopy Cardiac Ablation: Technology Is Moving Forward in Complex Procedures-A Novel Workflow for Atrial Fibrillation. Biology 2021, 10, 1333. [CrossRef]
- Sánchez de la Nava, A.M.; González Mansilla, A.; González-Torrecilla, E.; Ávila, P.; Datino, T.; Bermejo, J.; Arenal, Á.; Fernández-Avilés, F.; Atienza, F. Personalized Evaluation of Atrial Complexity of Patients Undergoing Atrial Fibrillation Ablation: A Clinical Computational Study. Biology 2021, 10, 838. [CrossRef]
- Makati, K.J.; Sood, N.; Lee, L.S.; Yang, F.; Shults, C.C.; DeLurgio, D.B.; Melichercik, J.; Gill, J.S.; Kaba, R.A.; Ahsan, S.; Weerasooriya, R.; Joshi, P.; Lellouche, N.; Blaauw, Y.; Zannis, K.; Sebag, F.A.; Gauri, A.; Zembala, M.O.; Tondo, C.; Steinberg, J.S. Combined epicardial and endocardial ablation for atrial fibrillation: Best practices and guide to hybrid convergent procedures. Heart Rhythm 2021, 18, 303–312. [CrossRef]
- Charitakis, E.; Metelli, S.; Karlsson, L.O.; Antoniadis, A.P.; Liuba, I.; Almroth, H.; Hassel Jönsson, A.; Schwieler, J.; Sideris, S.; Tsartsalis, D.; Dragioti, E.; Fragakis, N.; Chaimani, A. Comparing Efficacy and Safety in Catheter Ablation Strategies for Paroxysmal Atrial Fibrillation: A Network Meta-Analysis of Randomized Controlled Trials. Diagnostics 2022, 12, 433. [CrossRef]
- McCarthy, P.M.; Cox, J.L.; Kislitsina, O.N.; Kruse, J.; Churyla, A.; Malaisrie, S.C.; Mehta, C.K. Surgery and Catheter Ablation for Atrial Fibrillation: History, Current Practice, and Future Directions. J. Clin. Med. 2021, 11, 210. [CrossRef]
- Kim, Y.G.; Boo, K.Y.; Choi, J.I.; Choi, Y.Y.; Choi, H.Y.; Roh, S.Y.; Shim, J.; Kim, J.S.; Kim, Y.H. Early Recurrence Is Reliable Predictor of Late Recurrence After Radiofrequency Catheter Ablation of Atrial Fibrillation. JACC Clin. Electrophysiol. 2021, 7, 343–351. [CrossRef]
- Baalman, S.W.E.; Lopes, R.R.; Ramos, L.A.; Neefs, J.; Driessen, A.H.G.; van Boven, W.P.; de Mol, B.A.J.M.; Marquering, H.A.; de Groot, J.R. Prediction of Atrial Fibrillation Recurrence after Thoracoscopic Surgical Ablation Using Machine Learning Techniques. Diagnostics 2021, 11, 1787. [CrossRef]
- Istratoaie, S.; Vesa, Ș.C.; Cismaru, G.; Pop, D.; Roșu, R.; Puiu, M.; Pepine, D.; Ciobanu, C.; Minciuna, I.A.; Simu, G.; Zdrenghea, D.; Buzoianu, A.D. Value of Left Atrial Appendage Function Measured by Transesophageal Echocardiography for Prediction of Atrial Fibrillation Recurrence after Radiofrequency Catheter Ablation. Diagnostics 2021, 11, 1465. [CrossRef]
- Tachmatzidis, D.; Tsarouchas, A.; Mouselimis, D.; Filos, D.; Antoniadis, A.P.; Lysitsas, D.N.; Mezilis, N.; Sakellaropoulou, A.; Giannopoulos, G.; Bakogiannis, C.; Triantafyllou, K.; Fragakis, N.; Letsas, K.P.; Asvestas, D.; Efremidis, M.; Lazaridis, C.; Chouvarda, I.; Vassilikos, V.P. P-Wave Beat-to-Beat Analysis to Predict Atrial Fibrillation Recurrence after Catheter Ablation. Diagnostics 2022, 12, 830. [CrossRef]
- Chen, Y.; Wu, Y.; Chu, X.; Wang, M. Meta-analysis of the correlation between recurrence of atrial fibrillation and serum uric acid level after radiofrequencyablation. Am. J. Transl. Res. 2022, 14, 8793–8799.
- Bhat, T.; Baydoun, H.; Asti, D.; Rijal, J.; Teli, S.; Tantray, M.; Bhat, H.; Kowalski, M. Major complications of cryoballoon catheter ablation for atrial fibrillation and their management. Expert Rev. Cardiovasc. Ther. 2014, 12, 1111–1118. [CrossRef]
- Zhang, J.; Johnsen, S.P.; Guo, Y.; Lip, G.Y.H. Epidemiology of Atrial Fibrillation: Geographic/Ecological Risk Factors, Age, Sex, Genetics. Card. Electrophysiol. Clin. 2021, 13, 1–23. [CrossRef]
- Kim, J.A.; Chelu, M.G.; Li, N. Genetics of atrial fibrillation. Curr. Opin. Cardiol. 2021, 36, 281–287. [CrossRef]
- Kany, S.; Reissmann, B.; Metzner, A.; Kirchhof, P.; Darbar, D.; Schnabel, R.B. Genetics of atrial fibrillation-practical applications for clinical management: if not now, when and how? Cardiovasc. Res. 2021, 117, 1718–1731. [CrossRef]
- Wabich, E.; Zienciuk-Krajka, A.; Nowak, R.; Raczak, A.; Daniłowicz-Szymanowicz, L. Comprehensive Echocardiography of Left Atrium and Left Ventricle Using Modern Techniques Helps in Better Revealing Atrial Fibrillation in Patients with Hypertrophic Cardiomyopathy. Diagnostics 2021, 11, 1288. [CrossRef]
- Fragão-Marques, M.; Barroso, I.; Farinha, R.; Miranda, I.M.; Martins, D.; Mancio, J.; Rocha-Neves, J.; Guimarães, J.T.; Leite-Moreira, A.; Falcão-Pires, I. Pericardial NT-Pro-BNP and GDF-15 as Biomarkers of Atrial Fibrillation and Atrial Matrix Remodeling in Aortic Stenosis. Diagnostics 2021, 11, 1422. [CrossRef]
- Gaibazzi, N.; Martini, C.; Benatti, G.; Palumbo, A.A.; Cacciola, G.; Tuttolomondo, D. Atrial Fibrillation and Peri-Atrial Inflammation Measured through Adipose Tissue Attenuation on Cardiac Computed Tomography. Diagnostics 2021, 11, 2087. [CrossRef]
- Papathanasiou, K.A.; Giotaki, S.G.; Vrachatis, D.A.; Siasos, G.; Lambadiari, V.; Iliodromitis, K.E.; Kossyvakis, C.; Kaoukis, A.; Raisakis, K.; Deftereos, G.; Papaioannou, T.G.; Giannopoulos, G.; Avramides, D.; Deftereos, S.G. Molecular Insights in Atrial Fibrillation Pathogenesis and Therapeutics: A Narrative Review. Diagnostics 2021, 11, 1584. [CrossRef]
- Malagù, M.; Marchini, F.; Fiorio, A.; Sirugo, P.; Clò, S.; Mari, E.; Gamberini, M.R.; Rapezzi, C.; Bertini, M. Atrial Fibrillation in β-Thalassemia: Overview of Mechanism, Significance and Clinical Management. Biology 2022, 11, 148. [CrossRef]
- Charalampidis, P.; Teperikidis, E.; Boulmpou, A.; Papadopoulos, C.E.; Potoupni, V.; Tsioni, K.; Rakitzi, P.; Karamitsos, T.; Vassilikos, V. Homocysteine as a Predictor of Paroxysmal Atrial Fibrillation-Related Events: A Scoping Review of the Literature. Diagnostics 2022, 12, 2192. [CrossRef]
- Giannopoulos, G.; Anagnostopoulos, I.; Kousta, M.; Vergopoulos, S.; Deftereos, S.; Vassilikos, V. Alcohol Consumption and the Risk of Incident Atrial Fibrillation: A Meta-Analysis. Diagnostics 2022, 12, 479. [CrossRef]
- Ke, Z.P.; Zhang, G.F.; Guo, Y.H.; Sun, Y.M.; Wang, J.; Li, N.; Qiu, X.B.; Xu, Y.J.; Yang, Y.Q. A novel PRRX1 loss-of-function variation contributing to familial atrial fibrillation and congenital patent ductus arteriosus. Genet. Mol. Biol. 2022, 45, e20210378. [CrossRef]
- Chalazan, B.; Mol, D.; Darbar, F.A.; Ornelas-Loredo, A.; Al-Azzam, B.; Chen, Y.; Tofovic, D.; Sridhar, A.; Alzahrani, Z.; Ellinor, P.; Darbar, D. Association of Rare Genetic Variants and Early-Onset Atrial Fibrillation in Ethnic Minority Individuals. JAMA Cardiol. 2021, 6, 811–819. [CrossRef]
- Yoneda, Z.T.; Anderson, K.C.; Quintana, J.A.; O'Neill, M.J.; Sims, R.A.; Glazer, A.M.; Shaffer, C.M.; Crawford, D.M.; Stricker, T.; Ye, F.; Wells, Q.; Stevenson, L.W.; Michaud, G.F.; Darbar, D.; Lubitz, S.A.; Ellinor, P.T.; Roden, D.M.; Shoemaker, M.B. Early-Onset Atrial Fibrillation and the Prevalence of Rare Variants in Cardiomyopathy and Arrhythmia Genes. JAMA Cardiol. 2021, 6, 1371–1379. [CrossRef]
- Hateley, S.; Lopez-Izquierdo, A.; Jou, C.J.; Cho, S.; Schraiber, J.G.; Song, S.; Maguire, C.T.; Torres, N.; Riedel, M.; Bowles, N.E.; Arrington, C.B.; Kennedy, B.J.; Etheridge, S.P.; Lai, S.; Pribble, C.; Meyers, L.; Lundahl, D.; Byrnes, J.; Granka, J.M.; Kauffman, C.A.; Lemmon, G.; Boyden, S.; Scott Watkins, W.; Karren, M.A.; Knight, S.; Brent Muhlestein, J.; Carlquist, J.F.; Anderson, J.L.; Chahine, K.G.; Shah, K.U.; Ball, C.A.; Benjamin, I.J.; Yandell, M.; Tristani-Firouzi, M. The history and geographic distribution of a KCNQ1 atrial fibrillation risk allele. Nat. Commun. 2021, 12, 6442. [CrossRef]
- Li, R.G.; Xu, Y.J.; Ye, W.G.; Li, Y.J.; Chen, H.; Qiu, X.B.; Yang, Y.Q.; Bai, D. Connexin45 (GJC1) loss-of-function mutation contributes to familial atrial fibrillation and conduction disease. Heart Rhythm 2021, 18, 684–693. [CrossRef]
- Guo, X.J.; Qiu, X.B.; Wang, J.; Guo, Y.H.; Yang, C.X.; Li, L.; Gao, R.F.; Ke, Z.P.; Di, R.M.; Sun, Y.M.; Xu, Y.J.; Yang, Y.Q. PRRX1 Loss-of-Function Mutations Underlying Familial Atrial Fibrillation. J. Am. Heart Assoc. 2021, 10, e023517. [CrossRef]
- Abou Ziki, M.D.; Bhat, N.; Neogi, A.; Driscoll, T.P.; Ugwu, N.; Liu, Y.; Smith, E.; Abboud, J.M.; Chouairi, S.; Schwartz, M.A.; Akar, J.G.; Mani, A. Epistatic interaction of PDE4DIP and DES mutations in familial atrial fibrillation with slow conduction. Hum. Mutat. 2021, 42, 1279–1293. [CrossRef]
- Clausen, A.G.; Vad, O.B.; Andersen, J.H.; Olesen, M.S. Loss-of-Function Variants in the SYNPO2L Gene Are Associated With Atrial Fibrillation. Front. Cardiovasc. Med. 2021, 8, 650667. [CrossRef]
- Li, N.; Xu, Y.J.; Shi, H.Y.; Yang, C.X.; Guo, Y.H.; Li, R.G.; Qiu, X.B.; Yang, Y.Q.; Zhang, M. KLF15 Loss-of-Function Mutation Underlying Atrial Fibrillation as well as Ventricular Arrhythmias and Cardiomyopathy. Genes 2021, 12, 408. [CrossRef]
- van Wijk, S.W.; Su, W.; Wijdeveld, L.F.J.M.; Ramos, K.S.; Brundel, B.J.J.M. Cytoskeletal Protein Variants Driving Atrial Fibrillation: Potential Mechanisms of Action. Cells 2022, 11, 416. [CrossRef]
- Vad, O.B.; Yan, Y.; Denti, F.; Ahlberg, G.; Refsgaard, L.; Bomholtz, S.H.; Santos, J.L.; Rasmussen, S.; Haunsø, S.; Svendsen, J.H.; Christophersen, I.E.; Schmitt, N.; Olesen, M.S.; Bentzen, B.H. Whole-Exome Sequencing Implicates Neuronal Calcium Channel with Familial Atrial Fibrillation. Front. Genet. 2022, 13, 806429. [CrossRef]
- Malakootian, M.; Jalilian, M.; Kalayinia, S.; Hosseini Moghadam, M.; Heidarali, M.; Haghjoo, M. Whole-exome sequencing reveals a rare missense variant in DTNA in an Iranian pedigree with early-onset atrial fibrillation. BMC Cardiovasc. Disord. 2022, 22, 37. [CrossRef]
- Lin, L.; Li, K.; Tian, B.; Jia, M.; Wang, Q.; Xu, C.; Xiong, L.; Wang, Q.K.; Zeng, Y.; Wang, P. Two Novel Functional Mutations in Promoter Region of SCN3B Gene Associated with Atrial Fibrillation. Life 2022, 12, 1794. [CrossRef]
- Guo, Y.H.; Yang, Y.Q. Atrial Fibrillation: Focus on Myocardial Connexins and Gap Junctions. Biology 2022, 11, 489. [CrossRef]
- Caballero, R.; Utrilla, R.G.; Amorós, I.; Matamoros, M.; Pérez-Hernández, M.; Tinaquero, D.; Alfayate, S.; Nieto-Marín, P.; Guerrero-Serna, G.; Liu, Q.H.; Ramos-Mondragón, R.; Ponce-Balbuena, D.; Herron, T.; Campbell, K.F.; Filgueiras-Rama, D.; Peinado, R.; López-Sendón, J.L.; Jalife, J.; Delpón, E.; Tamargo, J. Tbx20 controls the expression of the KCNH2 gene and of hERG channels. Proc. Natl. Acad. Sci. U. S. A. 2017, 114, E416–E425. [CrossRef]
- Hong, K.; Bjerregaard, P.; Gussak, I.; Brugada, R. Short QT syndrome and atrial fibrillation caused by mutation in KCNH2. J. Cardiovasc. Electrophysiol. 2005, 16, 394–396. [CrossRef]
- Sinner, M.F.; Pfeufer, A.; Akyol, M.; Beckmann, B.M.; Hinterseer, M.; Wacker, A.; Perz, S.; Sauter, W.; Illig, T.; Näbauer, M.; Schmitt, C.; Wichmann, H.E.; Schömig, A.; Steinbeck, G.; Meitinger, T.; Kääb, S. The non-synonymous coding IKr-channel variant KCNH2-K897T is associated with atrial fibrillation: results from a systematic candidate gene-based analysis of KCNH2 (HERG). Eur. Heart J. 2008, 29, 907–914. [CrossRef]
- Wang, Q.S.; Wang, X.F.; Chen, X.D.; Yu, J.F.; Wang, J.; Sun, J.; Lu, S.B.; Shen, M.Y.; Lu, M.; Li, Y.G.; Jin, L. Genetic polymorphism of KCNH2 confers predisposition of acquired atrial fibrillation in Chinese. J. Cardiovasc. Electrophysiol. 2009, 20, 1158–1162. [CrossRef]
- Darbar, D.; Hardy, A.; Haines, J.L.; Roden, D.M. Prolonged signal-averaged P-wave duration as an intermediate phenotype for familial atrial fibrillation. J. Am. Coll. Cardiol. 2008, 51, 1083–1089. [CrossRef]
- Brugada, R.; Tapscott, T.; Czernuszewicz, G.Z.; Marian, A.J.; Iglesias, A.; Mont, L.; Brugada, J.; Girona, J.; Domingo, A.; Bachinski, L.L.; Roberts, R. Identification of a genetic locus for familial atrial fibrillation. N. Engl. J. Med. 1997, 336, 905–911. [CrossRef]
- Chen, Y.H.; Xu, S.J.; Bendahhou, S.; Wang, X.L.; Wang, Y.; Xu, W.Y.; Jin, H.W.; Sun, H.; Su, X.Y.; Zhuang, Q.N.; Yang, Y.Q.; Li, Y.B.; Liu, Y.; Xu, H.J.; Li, X.F.; Ma, N.; Mou, C.P.; Chen, Z.; Barhanin, J.; Huang, W. KCNQ1 gain-of-function mutation in familial atrial fibrillation. Science 2003, 299, 251–254. [CrossRef]
- Ellinor, P.T.; Shin, J.T.; Moore, R.K.; Yoerger, D.M.; MacRae, C.A. Locus for atrial fibrillation maps to chromosome 6q14-16. Circulation 2003, 107, 2880–2883. [CrossRef]
- Han, X.; Cao, X.; Cabrera, R.M.; Pimienta Ramirez, P.A.; Zhang, C.; Ramaekers, V.T.; Finnell, R.H.; Lei, Y. KDM6B Variants May Contribute to the Pathophysiology of Human Cerebral Folate Deficiency. Biology 2022, 12, 74. [CrossRef]
- Azzarà, A.; Risi Ambrogioni, L.; Cassano, I.; Lintas, C.; Longo, U.G.; Denaro, V.; Gurrieri, F. Genetic Characterization in Familial Rotator Cuff Tear: An Exome Sequencing Study. Biology 2022, 11, 1565. [CrossRef]
- Iqbal, Z.; Absar, M.; Akhtar, T.; Aleem, A.; Jameel, A.; Basit, S.; Ullah, A.; Afzal, S.; Ramzan, K.; Rasool, M.; Karim, S.; Mirza, Z.; Iqbal, M.; AlMajed, M.; AlShehab, B.; AlMukhaylid, S.; AlMutairi, N.; Al-Anazi, N.; Sabar, M.F.; Arshad, M.; Asif, M.; Shammas, M.; Mahmood, A. Integrated Genomic Analysis Identifies ANKRD36 Gene as a Novel and Common Biomarker of Disease Progression in Chronic Myeloid Leukemia. Biology 2021, 10, 1182. [CrossRef]
- Pan, Y.; Geng, R.; Zhou, N.; Zheng, G.F.; Zhao, H.; Wang, J.; Zhao, C.M.; Qiu, X.B.; Yang, Y.Q.; Liu, X.Y. TBX20 loss-of-function mutation contributes to double outlet right ventricle. Int. J. Mol. Med. 2015, 35, 1058–1066. [CrossRef]
- Guo, Y.H.; Wang, J.; Guo, X.J.; Gao, R.F.; Yang, C.X.; Li, L.; Sun, Y.M.; Qiu, X.B.; Xu, Y.J.; Yang, Y.Q. KLF13 Loss-of-Function Mutations Underlying Familial Dilated Cardiomyopathy. J. Am. Heart Assoc. 2022, 11, e027578. [CrossRef]
- Plageman, T.F. Jr; Yutzey, K.E. T-box genes and heart development: putting the "T" in heart. Dev. Dyn. 2005, 232, 11–20. [CrossRef]
- Huang, R.T.; Wang, J.; Xue, S.; Qiu, X.B.; Shi, H.Y.; Li, R.G.; Qu, X.K.; Yang, X.X.; Liu, H.; Li, N.; Li, Y.J.; Xu, Y.J.; Yang, Y.Q. TBX20 loss-of-function mutation responsible for familial tetralogy of Fallot or sporadic persistent truncus arteriosus. Int. J. Med. Sci. 2017, 14, 323–332. [CrossRef]
- Greulich, F.; Rudat, C.; Kispert, A. Mechanisms of T-box gene function in the developing heart. Cardiovasc. Res. 2011, 91, 212–222. [CrossRef]
- Chen, Y.; Xiao, D.; Zhang, L.; Cai, C.L.; Li, B.Y.; Liu, Y. The Role of Tbx20 in Cardiovascular Development and Function. Front. Cell Dev. Biol. 2021, 9, 638542. [CrossRef]
- Brown, D.D.; Martz, S.N.; Binder, O.; Goetz, S.C.; Price, B.M.; Smith, J.C.; Conlon, F.L. Tbx5 and Tbx20 act synergistically to control vertebrate heart morphogenesis. Development 2005, 132, 553–563. [CrossRef]
- Stennard, F.A.; Costa, M.W.; Elliott, D.A.; Rankin, S.; Haast, S.J.; Lai, D.; McDonald, L.P.; Niederreither, K.; Dolle, P.; Bruneau, B.G.; Zorn, A.M.; Harvey, R.P. Cardiac T-box factor Tbx20 directly interacts with Nkx2-5, GATA4, and GATA5 in regulation of gene expression in the developing heart. Dev. Biol. 2003, 262, 206–224. [CrossRef]
- Hodgson-Zingman, D.M.; Karst, M.L.; Zingman, L.V.; Heublein, D.M.; Darbar, D.; Herron, K.J.; Ballew, J.D.; de Andrade, M.; Burnett, J.C. Jr; Olson, T.M. Atrial natriuretic peptide frameshift mutation in familial atrial fibrillation. N. Engl. J. Med. 2008, 359, 158–165. [CrossRef]
- Gollob, M.H.; Jones, D.L.; Krahn, A.D.; Danis, L.; Gong, X.Q.; Shao, Q.; Liu, X.; Veinot, J.P.; Tang, A.S.; Stewart, A.F.; Tesson, F.; Klein, G.J.; Yee, R.; Skanes, A.C.; Guiraudon, G.M.; Ebihara, L.; Bai, D. Somatic mutations in the connexin 40 gene (GJA5) in atrial fibrillation. N. Engl. J. Med. 2006, 354, 2677–2688. [CrossRef]
- Sun, Y.; Yang, Y.Q.; Gong, X.Q.; Wang, X.H.; Li, R.G.; Tan, H.W.; Liu, X.; Fang, W.Y.; Bai, D. Novel germline GJA5/connexin40 mutations associated with lone atrial fibrillation impair gap junctional intercellular communication. Hum. Mutat. 2013, 34, 603–609. [CrossRef]
- Noureldin, M.; Chen, H.; Bai, D. Functional Characterization of Novel Atrial Fibrillation-Linked GJA5 (Cx40) Mutants. Int. J. Mol. Sci. 2018, 19, 977. [CrossRef]
- Postma, A.V.; van de Meerakker, J.B.; Mathijssen, I.B.; Barnett, P.; Christoffels, V.M.; Ilgun, A.; Lam, J.; Wilde, A.A.; Lekanne Deprez, R.H.; Moorman, A.F. A gain-of-function TBX5 mutation is associated with atypical Holt-Oram syndrome and paroxysmal atrial fibrillation. Circ. Res. 2008, 102, 1433–1442. [CrossRef]
- Ma, J.F.; Yang, F.; Mahida, S.N.; Zhao, L.; Chen, X.; Zhang, M.L.; Sun, Z.; Yao, Y.; Zhang, Y.X.; Zheng, G.Y.; Dong, J.; Feng, M.J.; Zhang, R.; Sun, J.; Li, S.; Wang, Q.S.; Cao, H.; Benjamin, E.J.; Ellinor, P.T.; Li, Y.G.; Tian, X.L. TBX5 mutations contribute to early-onset atrial fibrillation in Chinese and Caucasians. Cardiovasc. Res. 2016, 109, 442–450. [CrossRef]
- Wang, Z.C.; Ji, W.H.; Ruan, C.W.; Liu, X.Y.; Qiu, X.B.; Yuan, F.; Li, R.G.; Xu, Y.J.; Liu, X.; Huang, R.T.; Xue, S.; Yang, Y.Q. Prevalence and Spectrum of TBX5 Mutation in Patients with Lone Atrial Fibrillation. Int. J. Med. Sci. 2016, 13, 60–67. [CrossRef]
- Yang, Y.Q.; Wang, M.Y.; Zhang, X.L.; Tan, H.W.; Shi, H.F.; Jiang, W.F.; Wang, X.H.; Fang, W.Y.; Liu, X. GATA4 loss-of-function mutations in familial atrial fibrillation. Clin. Chim. Acta 2011, 412, 1825–1830. [CrossRef]
- Jiang, J.Q.; Shen, F.F.; Fang, W.Y.; Liu, X.; Yang, Y.Q. Novel GATA4 mutations in lone atrial fibrillation. Int. J. Mol. Med. 2011, 28, 1025–1032. [CrossRef]
- Wang, J.; Sun, Y.M.; Yang, Y.Q. Mutation spectrum of the GATA4 gene in patients with idiopathic atrial fibrillation. Mol. Biol. Rep. 2012, 39, 8127–8135. [CrossRef]
- Wang, X.H.; Huang, C.X.; Wang, Q.; Li, R.G.; Xu, Y.J.; Liu, X.; Fang, W.Y.; Yang, Y.Q. A novel GATA5 loss-of-function mutation underlies lone atrial fibrillation. Int. J. Mol. Med. 2013, 31, 43–50. [CrossRef]
- Gu, J.Y.; Xu, J.H.; Yu, H.; Yang, Y.Q. Novel GATA5 loss-of-function mutations underlie familial atrial fibrillation. Clinics 2012, 67, 1393–1399. [CrossRef]
- Huang, R.T.; Xue, S.; Xu, Y.J.; Zhou, M.; Yang, Y.Q. A novel NKX2.5 loss-of-function mutation responsible for familial atrial fibrillation. Int. J. Mol. Med. 2013, 31, 1119–1126. [CrossRef]
- Xie, W.H.; Chang, C.; Xu, Y.J.; Li, R.G.; Qu, X.K.; Fang, W.Y.; Liu, X.; Yang, Y.Q. Prevalence and spectrum of Nkx2.5 mutations associated with idiopathic atrial fibrillation. Clinics 2013, 68, 777–784. [CrossRef]
- Yu, H.; Xu, J.H.; Song, H.M.; Zhao, L.; Xu, W.J.; Wang, J.; Li, R.G.; Xu, L.; Jiang, W.F.; Qiu, X.B.; Jiang, J.Q.; Qu, X.K.; Liu, X.; Fang, W.Y.; Jiang, J.F.; Yang, Y.Q. Mutational spectrum of the NKX2-5 gene in patients with lone atrial fibrillation. Int. J. Med. Sci. 2014, 11, 554–563. [CrossRef]
- Mittal, A.; Sharma, R.; Prasad, R.; Bahl, A.; Khullar, M. Role of cardiac TBX20 in dilated cardiomyopathy. Mol. Cell. Biochem. 2016, 414, 129–136. [CrossRef]
- Wolf, C.M.; Berul, C.I. Inherited conduction system abnormalities—one group of diseases, many genes. J. Cardiovasc. Electrophysiol. 2006, 17, 446–455. [CrossRef]
- Mangoni, M.E.; Nargeot, J. Genesis and regulation of the heart automaticity. Physiol, Rev. 2008, 88, 919–982. [CrossRef]
- Christoffels, V.M.; Mommersteeg, M.T.; Trowe, M.O.; Prall, O.W.; de Gier-de Vries, C.; Soufan, A.T.; Bussen, M.; Schuster-Gossler, K.; Harvey, R.P.; Moorman, A.F.M.; Kispert, A. Formation of the venous pole of the heart from an Nkx2-5-negative precursor population requires Tbx18. Circ. Res. 2006, 98, 1555–1563. [CrossRef]
- Munshi, N.V. Gene regulatory networks in cardiac conduction system development. Circ. Res. 2012, 110, 1525–1537. [CrossRef]
- van Weerd, J.H.; Christoffels, V.M. The formation and function of the cardiac conduction system. Development 2016, 143, 197–210. [CrossRef]
- Sotoodehnia, N.; Isaacs, A.; de Bakker, P.I.; Dörr, M.; Newton-Cheh, C.; Nolte, I.M.; van der Harst, P.; Müller, M.; Eijgelsheim, M.; Alonso, A.; Hicks, A.A.; Padmanabhan, S.; Hayward, C.; Smith, A.V.; Polasek, O.; Giovannone, S.; Fu, J.; Magnani, J.W.; Marciante, K.D.; Pfeufer, A.; Gharib, S.A.; Teumer, A.; Li, M.; Bis, J.C.; Rivadeneira, F.; Aspelund, T.; Köttgen, A.; Johnson, T.; Rice, K.; Sie, M.P.; Wang, Y.A.; Klopp, N.; Fuchsberger, C.; Wild, S.H.; Mateo Leach, I.; Estrada, K.; Völker, U.; Wright, A.F.; Asselbergs, F.W.; Qu, J.; Chakravarti, A.; Sinner, M.F.; Kors, J.A.; Petersmann, A.; Harris, T.B.; Soliman, E.Z.; Munroe, P.B.; Psaty, B.M.; Oostra, B.A.; Cupples, L.A.; Perz, S.; de Boer, R.A.; Uitterlinden, A.G.; Völzke, H.; Spector, T.D.; Liu, F.Y.; Boerwinkle, E.; Dominiczak, A.F.; Rotter, J.I.; van Herpen, G.; Levy, D.; Wichmann, H.E.; van Gilst, W.H.; Witteman, J.C.; Kroemer, H.K.; Kao, W.H.; Heckbert, S.R.; Meitinger, T.; Hofman, A.; Campbell, H.; Folsom, A.R.; van Veldhuisen, D.J.; Schwienbacher, C.; O'Donnell, C.J.; Volpato, C.B.; Caulfield, M.J.; Connell, J.M.; Launer, L.; Lu, X.; Franke, L.; Fehrmann, R.S.; te Meerman, G.; Groen, H.J.; Weersma, R.K.; van den Berg, L.H.; Wijmenga, C.; Ophoff, R.A.; Navis, G.; Rudan, I.; Snieder, H.; Wilson, J.F.; Pramstaller, P.P.; Siscovick, D.S.; Wang, T.J.; Gudnason, V.; van Duijn, C.M.; Felix, S.B.; Fishman, G.I.; Jamshidi, Y.; Stricker, B.H.; Samani, N.J.; Kääb, S.; Arking, D.E. Common variants in 22 loci are associated with QRS duration and cardiac ventricular conduction. Nat. Genet. 2010, 42, 1068–1076. [CrossRef]
- Evans, D.S.; Avery, C.L.; Nalls, M.A.; Li, G.; Barnard, J.; Smith, E.N.; Tanaka, T.; Butler, A.M.; Buxbaum, S.G.; Alonso, A.; Arking, D.E.; Berenson, G.S.; Bis, J.C.; Buyske, S.; Carty, C.L.; Chen, W.; Chung, M.K.; Cummings, S.R.; Deo, R.; Eaton, C.B.; Fox, E.R.; Heckbert, S.R.; Heiss, G.; Hindorff, L.A.; Hsueh, W.C.; Isaacs, A.; Jamshidi, Y.; Kerr, K.F.; Liu, F.; Liu, Y.; Lohman, K.K.; Magnani, J.W.; Maher, J.F.; Mehra, R.; Meng, Y.A.; Musani, S.K.; Newton-Cheh, C.; North, K.E.; Psaty, B.M.; Redline, S.; Rotter, J.I.; Schnabel, R.B.; Schork, N.J.; Shohet, R.V.; Singleton, A.B.; Smith, J.D.; Soliman, E.Z.; Srinivasan, S.R.; Taylor, H.A. Jr; Van Wagoner, D.R.; Wilson, J.G.; Young, T.; Zhang, Z.M.; Zonderman, A.B.; Evans, M.K.; Ferrucci, L.; Murray, S.S.; Tranah, G.J.; Whitsel, E.A.; Reiner, A.P.; CHARGE QRS Consortium; Sotoodehnia, N. Fine-mapping, novel loci identification, and SNP association transferability in a genome-wide association study of QRS duration in African Americans. Hum. Mol. Genet. 2016, 25, 4350–4368. [CrossRef]
- Shen, T.; Aneas, I.; Sakabe, N.; Dirschinger, R.J.; Wang, G.; Smemo, S.; Westlund, J.M.; Cheng, H.; Dalton, N.; Gu, Y.; Boogerd, C.J.; Cai, C.L.; Peterson, K.; Chen, J.; Nobrega, M.A.; Evans, S.M. Tbx20 regulates a genetic program essential to adult mouse cardiomyocyte function. J. Clin. Invest. 2011, 121, 4640–4654. [CrossRef]
- Sakabe, N.J.; Aneas, I.; Shen, T.; Shokri, L.; Park, S.Y.; Bulyk, M.L.; Evans, S.M.; Nobrega, M.A. Dual transcriptional activator and repressor roles of TBX20 regulate adult cardiac structure and function. Hum. Mol. Genet. 2012, 21, 2194–2204. [CrossRef]
- Priori, S.G.; Napolitano, C. Role of genetic analyses in cardiology: part I: mendelian diseases: cardiac channelopathies. Circulation 2006, 113, 1130–1135. [CrossRef]
- Lehnart, S.E.; Ackerman, M.J.; Benson, D.W. Jr; Brugada, R.; Clancy, C.E.; Donahue, J.K.; George, A.L. Jr; Grant, A.O.; Groft, S.C.; January, C.T.; Lathrop, D.A.; Lederer, W.J.; Makielski, J.C.; Mohler, P.J.; Moss, A.; Nerbonne, J.M.; Olson, T.M.; Przywara, D.A.; Towbin, J.A.; Wang, L.H.; Marks, A.R. Inherited arrhythmias: a National Heart, Lung, and Blood Institute and Office of Rare Diseases workshop consensus report about the diagnosis, phenotyping, molecular mechanisms, and therapeutic approaches for primary cardiomyopathies of gene mutations affecting ion channel function. Circulation 2007, 116, 2325–2345. [CrossRef]
- Roberts J.D.; Gollob, M.H. The genetic and clinical features of cardiac channelopathies. Future Cardiol. 2010, 6, 491–506. [CrossRef]
- Trudeau, M.C.; Warmke, J.W.; Ganetzky, B.; Robertson, G.A. HERG, a human inward rectifier in the voltage-gated potassium channel family. Science 1995, 269, 92–95. [CrossRef]
- Kekenes-Huskey, P.M.; Burgess, D.E.; Sun, B.; Bartos, D.C.; Rozmus, E.R.; Anderson, C.L.; January, C.T.; Eckhardt, L.L.; Delisle, B.P. Mutation-Specific Differences in Kv7.1 (KCNQ1) and Kv11.1 (KCNH2) Channel Dysfunction and Long QT Syndrome Phenotypes. Int. J. Mol. Sci. 2022, 23, 7389. [CrossRef]
- Ono, M.; Burgess, D.E.; Schroder, E.A.; Elayi, C.S.; Anderson, C.L.; January, C.T.; Sun, B.; Immadisetty, K.; Kekenes-Huskey, P.M.; Delisle, B.P. Long QT Syndrome Type 2: Emerging Strategies for Correcting Class 2 KCNH2 (hERG) Mutations and Identifying New Patients. Biomolecules 2020, 10, 1144. [CrossRef]
- Heijman, J.; Voigt, N.; Nattel, S.; Dobrev, D. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circ. Res. 2014, 114, 1483–1499. [CrossRef]
- Nattel, S.; Dobrev, D. Electrophysiological and molecular mechanisms of paroxysmal atrial fibrillation. Nat. Rev. Cardiol. 2016, 13, 575–590. [CrossRef]
- Yang, Y.; Xia, M.; Jin, Q.; Bendahhou, S.; Shi, J.; Chen, Y.; Liang, B.; Lin, J.; Liu, Y.; Liu, B.; Zhou, Q.; Zhang, D.; Wang, R.; Ma, N.; Su, X.; Niu, K.; Pei, Y.; Xu, W.; Chen, Z.; Wan, H.; Cui, J.; Barhanin, J.; Chen, Y. Identification of a KCNE2 gain-of-function mutation in patients with familial atrial fibrillation. Am. J. Hum. Genet. 2004, 75, 899–905. [CrossRef]
- Nielsen, J.B.; Bentzen, B.H.; Olesen, M.S.; David, J.P.; Olesen, S.P.; Haunsø, S.; Svendsen, J.H.; Schmitt, N. Gain-of-function mutations in potassium channel subunit KCNE2 associated with early-onset lone atrial fibrillation. Biomark. Med. 2014, 8, 557–570. [CrossRef]
- Newton-Cheh, C.; Guo, C.Y.; Larson, M.G.; Musone, S.L.; Surti, A.; Camargo, A.L.; Drake, J.A.; Benjamin, E.J.; Levy, D.; D'Agostino, R.B. Sr; Hirschhorn, J.N.; O'donnell, C.J. Common genetic variation in KCNH2 is associated with QT interval duration: the Framingham Heart Study. Circulation 2007, 116, 1128–1136. [CrossRef]
- Rossenbacker, T.; Mubagwa, K.; Jongbloed, R.J.; Vereecke, J.; Devriendt, K.; Gewillig, M.; Carmeliet, E.; Collen, D.; Heidbüchel, H.; Carmeliet, P. Novel mutation in the Per-Arnt-Sim domain of KCNH2 causes a malignant form of long-QT syndrome. Circulation 2005, 111, 961–968. [CrossRef]
- Partemi, S.; Cestèle, S.; Pezzella, M.; Campuzano, O.; Paravidino, R.; Pascali, V.L.; Zara, F.; Tassinari, C.A.; Striano, S.; Oliva, A.; Brugada, R.; Mantegazza, M.; Striano, P. Loss-of-function KCNH2 mutation in a family with long QT syndrome, epilepsy, and sudden death. Epilepsia 2013, 54, e112–e116. [CrossRef]
- Eldstrom, J.; Fedida, D. The voltage-gated channel accessory protein KCNE2: multiple ion channel partners, multiple ways to long QT syndrome. Expert Rev. Mol. Med. 2011, 13, e38. [CrossRef]
- Kirk, E.P.; Sunde, M.; Costa, M.W.; Rankin, S.A.; Wolstein, O.; Castro, M.L.; Butler, T.L.; Hyun, C.; Guo, G.; Otway, R.; Mackay, J.P.; Waddell, L.B.; Cole, A.D.; Hayward, C.; Keogh, A.; Macdonald, P.; Griffiths, L.; Fatkin, D.; Sholler, G.F.; Zorn, A.M.; Feneley, M.P.; Winlaw, D.S.; Harvey, R.P. Mutations in cardiac T-box factor gene TBX20 are associated with diverse cardiac pathologies, including defects of septation and valvulogenesis and cardiomyopathy. Am. J. Hum. Genet. 2007, 81, 280–291. [CrossRef]
- Qian, L.; Mohapatra, B.; Akasaka, T.; Liu, J.; Ocorr, K.; Towbin, J.A.; Bodmer, R. Transcription factor neuromancer/TBX20 is required for cardiac function in Drosophila with implications for human heart disease. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 19833–19838. [CrossRef]
- Zhao, C.M.; Sun, B.; Song, H.M.; Wang, J.; Xu, W.J.; Jiang, J.F.; Qiu, X.B.; Yuan, F.; Xu, J.H.; Yang, Y.Q. TBX20 loss-of-function mutation associated with familial dilated cardiomyopathy. Clin. Chem. Lab. Med. 2016, 54, 325–332. [CrossRef]
- Zhou, Y.M.; Dai, X.Y.; Huang, R.T.; Xue, S.; Xu, Y.J.; Qiu, X.B.; Yang, Y.Q. A novel TBX20 loss-of-function mutation contributes to adult-onset dilated cardiomyopathy or congenital atrial septal defect. Mol. Med. Rep. 2016, 14, 3307–3314. [CrossRef]
 |
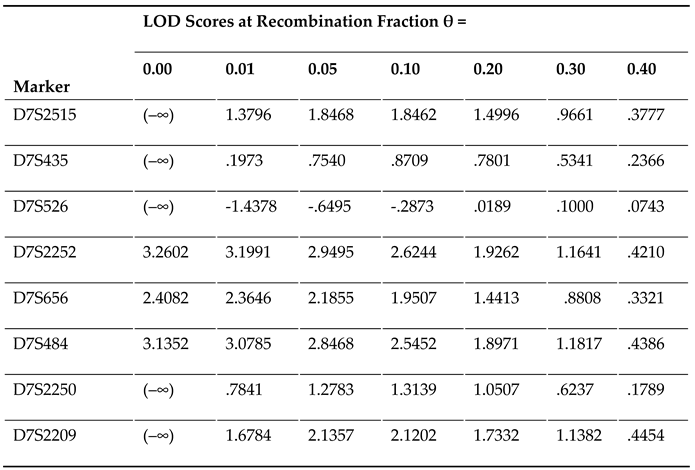
 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




