Submitted:
03 July 2023
Posted:
04 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
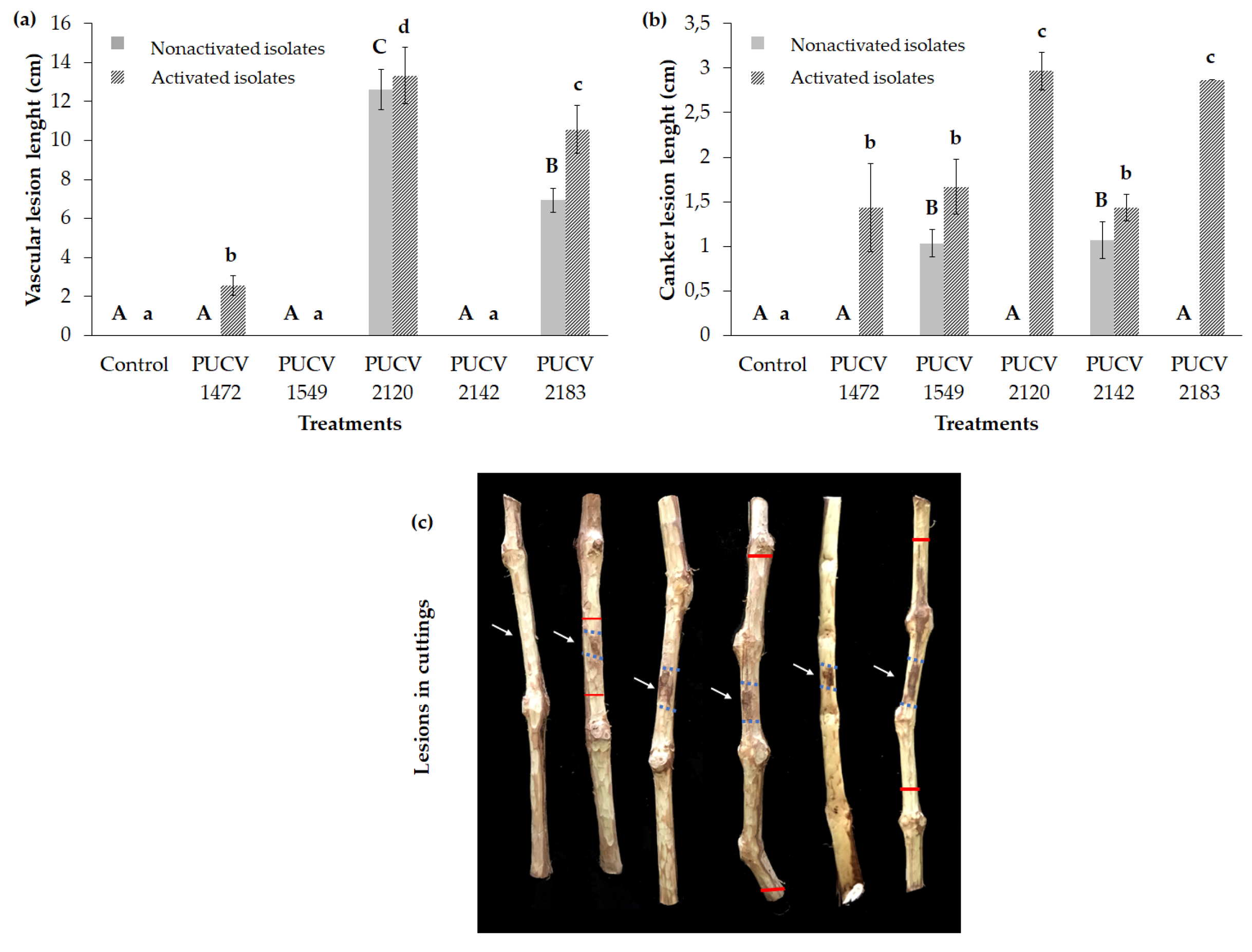
2.1. Pathogenicity Tests on Detached Canes (Nonactivated Isolates)
2.1.1. Damage Assessment and Recovery of Pathogens
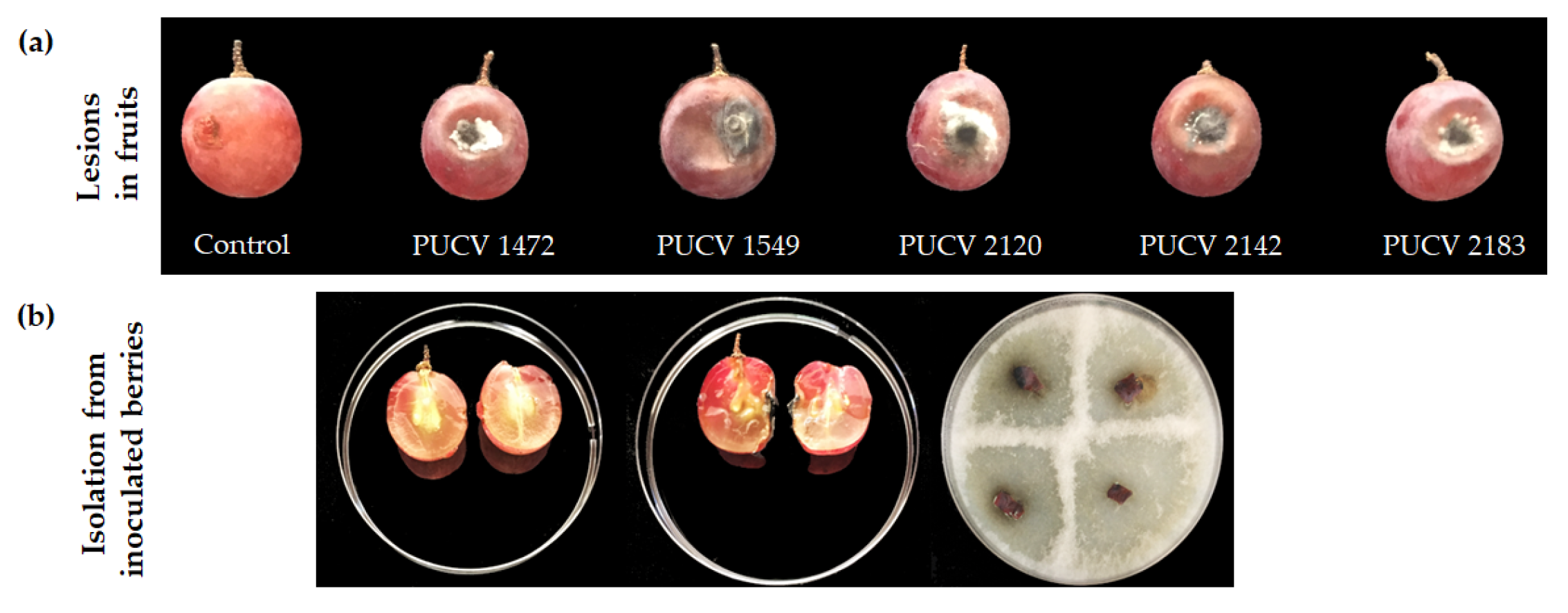
2.2. Pathogenicity Tests on Berry Grapes
2.2.1. Damage Assessment and Recovery of Pathogens
2.3. Pathogenicity Tests in Detached Canes (Activated Isolates)
2.3.1. Damage Assessment and Recovery of Pathogens
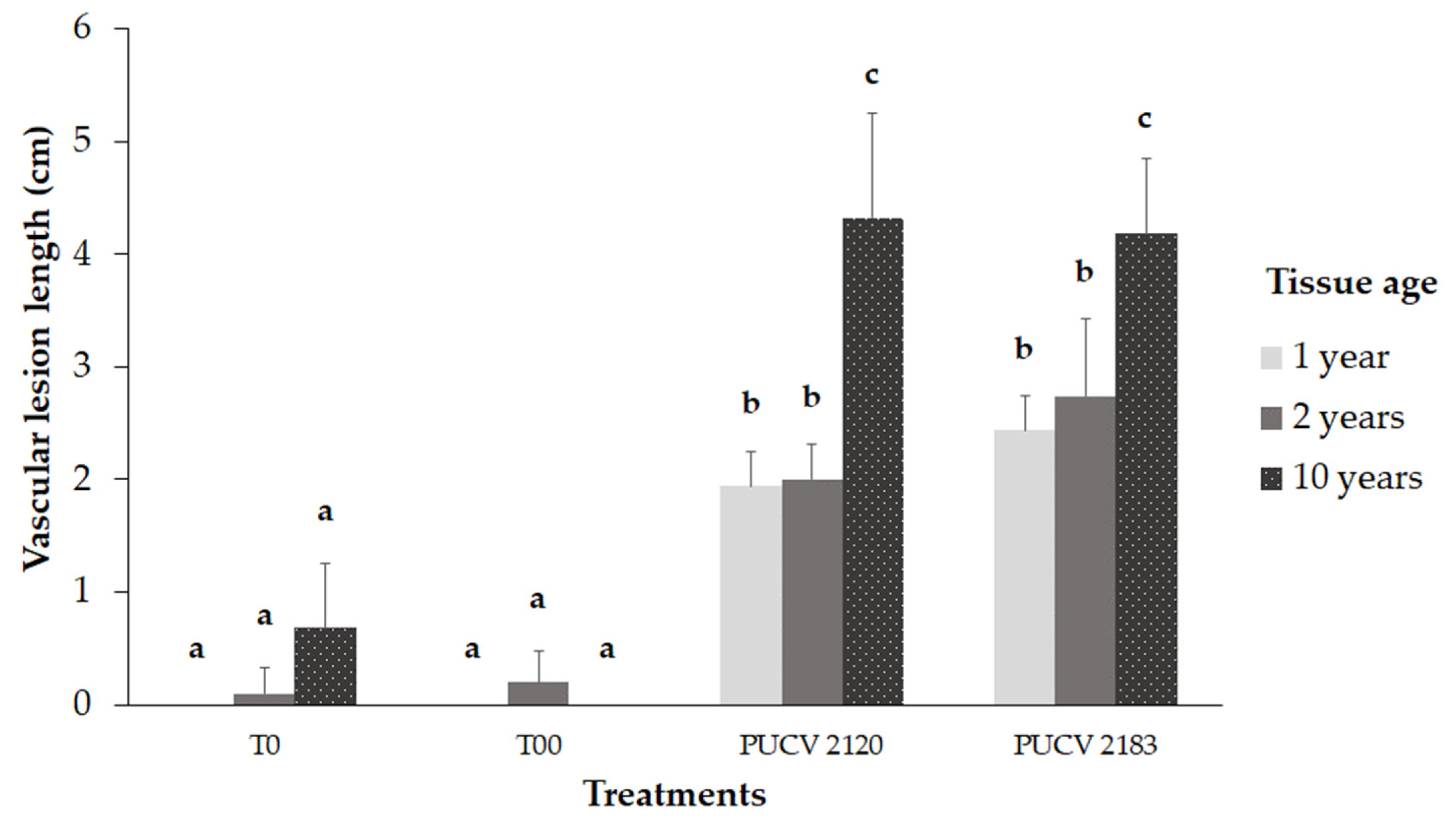
2.4. Pathogenicity Trial in the Field
2.4.1. Damage Assessment and Pathogen Recovery
2.5. Maximum Parsimony Analysis
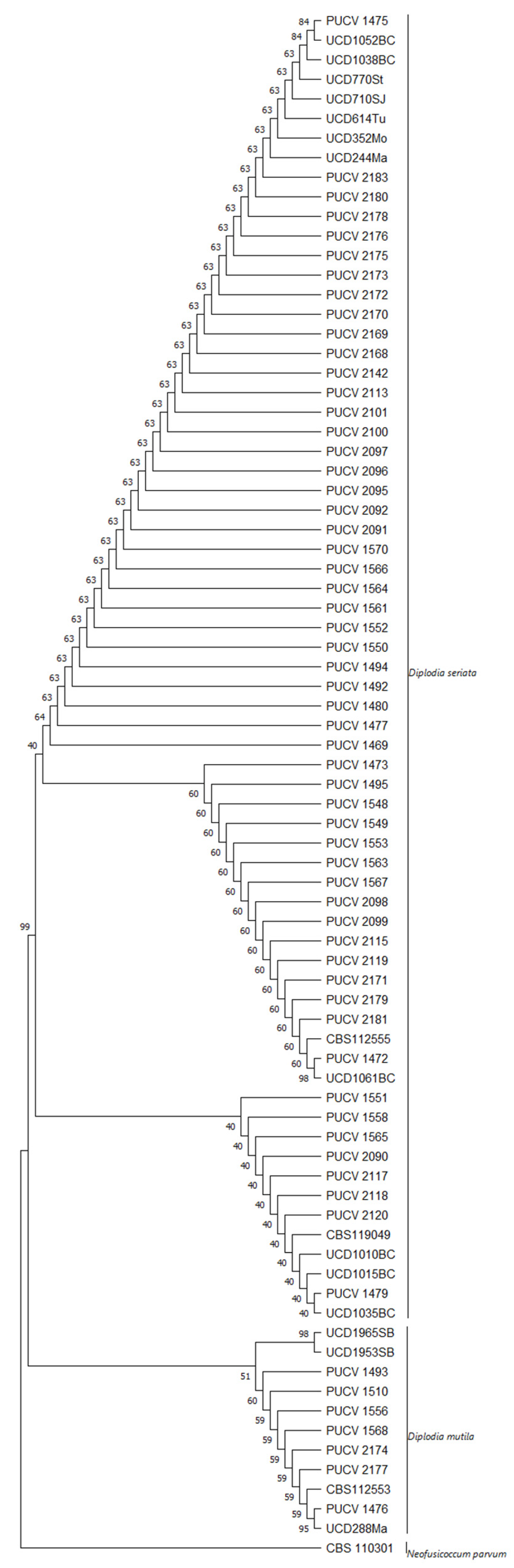
3. Discussion
4. Materials and Methods
4.1. Chemicals, Reagents, and Culture Media
4.2. Fungal Isolates
4.3. Pathogenicity Tests on Detached Canes (Nonactivated Isolates and Activated Isolates)
4.3.1. Damage Assessment and Pathogen Recovery
4.4. Pathogenicity Tests on Berry Grapes
4.4.1. Fungal Isolates and Inoculation
4.4.2. Damage Assessment and Pathogen Recovery
4.5. Pathogenicity Trial in the Field
4.5.1. Fungal Isolates and Inoculation
4.5.2. Damage Assessment and Pathogen Recovery
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bruez, E.; Lecomte, P.; Grosman, J.; Doublet, B.; Bertsch, C.; Fontaine, F.; Ugaglia, A.; Teissedre, P.; Da Costa, J.; Guerin-Dubrana, L.; Rey, P. Overview of grapevine trunk diseases in France in the 2000s. Phytopathol Mediterr. 2013, 52(2), 262–275. [Google Scholar]
- Fontaine, F.; Pinto, C.; Vallet, J.; Clément, Ch.; Catarina, A.; Spagnolo, A. The effects of grapevine trunk diseases (GTDs) on vine physiology. Eur J Plant Pathol. 2016, 114, 707–721. [Google Scholar]
- Hillis, V.; Lubell, M.; Kaplan, J.; Doll, D.; Baumgartner, K. The role of pest control advisers in preventative management of grapevine trunk diseases. Phytopathol. 2016, 106(4), 339–47. [Google Scholar]
- Kaplan, J.; Travadon, R.; Cooper, M.; Hillis, V.; Lubell, M.; Baumgartner, K. Identifying Economic Hurdles to Early Adoption of Preventative Practices: The Case of Trunk Diseases in California Winegrape Vineyards. AgEcon Search 2016. [Google Scholar]
- Larach, A.; Torres, C.; Riquelme, N.; Valenzuela, M.; Salgado, E.; Seeger, M.; Besoain, X. Yield loss estimation and pathogen identification from (BD) in vineyards of Central Chile over two growing seasons. Phytopathol Mediterr. 2020, 59(3), 537–48. [Google Scholar]
- Kenfaoui, J.; Radouane, N.; Mennani, M.; Tahiri, A.;Ghadraoui, L.; Belabess, Z.; Fontaine, F.; El Hamss, H.; Amiri, S.; Lahlli, R.; Ait, Barka, E. A Panoramic View on Grapevine Trunk Diseases Threats: Case of Eutypa Dieback, (BD), and Esca Disease. J of Fungi. 2022, 8, 595. [Google Scholar]
- Úrbez-Torres, JR.; Gubler, WD. Susceptibility of grapevine pruning wounds to infection by Lasiodiplodia theobromae and Neofusicoccum parvum. Plant Pathol. 2011, 60(2), 261–70. [Google Scholar]
- Úrbez-Torres, JR.; Battany, M.; Bettiga, LJ.; Gispert, C.; McGourty, G.; Roncoroni, J.; Smith, R.; Verdegaal, P.; Gubler, W. Botryosphaeriaceae species spore-trapping studies in California vineyards. Plant Dis. 2010, 94(6), 717–24. [Google Scholar]
- Besoain, X. Grapevine Trunk Diseases (GTDs): Impact on Table Grapes and Wine Vineyards in Chile. In: Grapes and Wines - Advances in Production, Processing, Analysis and Valorization. InTech. 2018.
- Gramaje, D.; Urbez-Torres, JR.; Sosnowski, MR. Managing grapevine trunk diseases with respect to etiology and epidemiology: Current strategies and Future prospects. Plant Dis. 2018, 102(1), 12–39. [Google Scholar]
- Billones-Baaijens, R.; Savocchia, S. A review of Botryosphaeriaceae species associated with grapevine trunk diseases in Australia and New Zealand. Aust Plant Pathol. 2019, 48, 3–18. [Google Scholar]
- Rolshausen, P.; Baumgartner, K.; Travador, R.; Funjiyoshi, P.; Pouzoulet, J.; Wilcox, W. Identification of Eutypa spp. Causing Eutypa Dieback o Grapevine in Eastern North America. Plant Dis 2014, 483–491. [Google Scholar]
- Burruano, S.; Mondello, V.; Conigliario, G.; Alfonzo, A.; Spagnolo, A.; Mugnai, L. Grapevine decline in Italy caused by Lasiodiplodia theobromae. Phytopathol Mediterr. 2008, 47, 132–136. [Google Scholar]
- Reis, P.; Magnin-Robert, M.; Nascimento, T.; Spagnolo, A.; Abou-Mansour, E.; Fioretti, C.; Clément, Ch.; Rego, C.; Fontaine, F. Reproducing (BD) foliar symptoms in a simple model system. Plant Dis. 2016, 100(6), 1071–9. [Google Scholar] [PubMed]
- Morales, A.; Latore, B.; Piontelli, E.; Besoain, X. Botryosphaeriaceae Species affecting table grape vineyards in Chile and cultivar susceptibility. Cien Investig Agrar. 2012, 39(3), 445–445. [Google Scholar]
- Díaz, GA.; Auger, J.; Besoain, X.; Bordeu, E.; Latorre, B.A. Prevalence and pathogenicity of fungi associated with grapevine trunk diseases in Chilean vineyards. Cienc Investig Agrar. 2013, 40(2), 327–39. [Google Scholar]
- Savocchia, S.; Steel, CC.; Stodart, BJ.; Somers, A. Pathogenicity of Botryosphaeria species isolated from declining grapevines in sub-tropical regions of Eastern Australia. Vitis. 2007, 46. [Google Scholar]
- Burgess, T.; Wingfield, MJ. Quarantine is important in restricting the spread of exotic seed-borne tree pathogens in the southern hemisphere. International Foresty Review. 2002, 4, 56. [Google Scholar]
- Mohali, S.; Burgess, TI.; Wingfield, MJ. Diversity and host association of the tropical tree endophyte Lasiodiplodia theobromae revealed using simple sequence repeat markers. Path. 2005, 35, 385–396. [Google Scholar]
- Burgess, TI.; Barber, PA.; Mohali, S.; Pegg, G.; De Beer, W.; Wingfield, MJ. Three new Lasiodiplodia spp. from the tropics, recognized based on DNA sequence comparisons and morphology. Mycologia 2006, 98((3)), 423–435. [Google Scholar]
- Kuntzmann, P.; Villaume, S.; Larignon, P.; Bertsch, C. Esca, BDA and Eutypiosis: foliar symptoms, trunk lesions and fungi observed in diseased vinestocks in two vineyards in Alsace. Vitis. 2010, 49(2), 71–76. [Google Scholar]
- Valencia, D.; Torres, C.; Camps, R.; López, E.; Celis-Diez, JL.; Besoain, X. Dissemination of Botryosphaeriaceae conidia in vineyards in the semiarid Mediterranean climate of the Valparaíso Region of Chile. Phytopathol Mediterr. 2015, 54(2), 394–402. [Google Scholar]
- Burgess, T.; Pei, T.Y.; Garnas, J.; Edwards, J.; Scarlett, K.; Shuttleworth, L.; Daniel, R.; Dann, E.; Parkinson, L.; Dihn, Q.; Shivas, R.; Jami, F. Current status of the Botryosphaeriaceae in Australia. Aust Plant Pathology. 2018, 48, 35–44. [Google Scholar]
- Reis, P.; Gaspar, A.; Alves, A.; Fontaine, F.; Lourenco, I.; Saramago, J.; Mota, M.; Rego, C. Early season symptoms on stem, inflorescences and flowers of grapevine associated with Botryosphaeriaceae species. Plants. 2020, 11, 1–14. [Google Scholar]
- Castillo-Pando, M.; Somers, A.; Green, CD.; Priest, M.; Sriskanthades, M. Fungi associated with dieback of Semillon grapevines in the Hunter Valley of New South Wales. Aust Plant Pathology. 2001, 30, 59–63. [Google Scholar] [CrossRef]
- Pitt, WM.; Huang, R.; Steel, CC.; Savocchia, S. Identification, distribution and current taxonomy of Botryosphaeriaceae species associated with grapevine decline in New South Wales and South Australia. Aust J Grape Wine Res. 2010, 16(1), 258–71. [Google Scholar]
- Qiu, Y.; Steel, CC.; Ash, GJ.; Savocchia, S. Survey of Botryosphaeriaceae associated with grapevine decline in the Hunter Valley and Mudgee grape growing regions of New South Wales. Aust Plant Pathol. 2011, 40(1), 1–11. [Google Scholar]
- Taylor, A.; Hardy, G.E.S.J.; Wood, P.; Burgess, T. Identification and pathogenicity of Botryosphaeria species associated with grapevine decline in Western Australia. Aust Plant Pathol. 2005, 34(2), 187–95. [Google Scholar] [CrossRef]
- Úrbez-Torres, JR.; Leavitt, GM.; Voegel, TM.; Gubler, WD. Identification and distribution of Botryosphaeria spp. associated with grapevine cankers in California. Plant Dis. 2006, 90, 1490–503. [Google Scholar]
- Larignon, P.; Fulchic, R.; Cere, L.; Dubos, B. Observation on black arm in French vineyards. Phytopathol Mediterr. 2001, 40, 336–342. [Google Scholar]
- Van, Niekerk, JM.; Crous, PW.; Groenewald. JZ.; Fourie. PH.; Halleen, F. DNA Phylogeny, Morphology and Pathogenicity of Botryosphaeria species on Grapevines. Mycologia 2004, 96, 781–798.
- Baskarathevan, J.; Jaspers, M.; Jones, EE.; Ridgway, HJ. Incidence and distribution of botryosphaeriaceous species in New Zealand vineyards. Eur J Plant Pathol. 2012, 132, 549–60. [Google Scholar]
- Bertsch, C.; Ramírez-Suero, M.; Magnin-Robert, M.; Larignon, P.; Chong, J.; Abou-Mansour, E.; Spagnolo, A.; Clément, A.; Fontaine, F. Grapevine trunk diseases: Complex and still poorly understood. Plant Pathol. 2013, 62, 243–65. [Google Scholar]
- Spagnolo, A.; Larignon, P.; Magnin-Robert, M.; Hovasse, A.; Cilindre, C.; Van Dorsselaer, A.; Clément, C.; Schaeffer-Reiss, C.; Fontaine, F. Flowering as the most highly sensitive period of grapevine (Vitis vinifera L. cv Mourvédre) to the (BD) agents Neofusicoccum parvum and Diplodia seriata infection. Int J Mol Sci 2014, 15((6)), 9644–9669. [Google Scholar] [PubMed]
- ODEPA. 2022. Oficina de Estudios y Políticas Agrarias. Statistics Productive. (accessed: February 2022, from www.odepa.cl). 20 February.
- Luque J, Elena G, Garcia-Figueres F, Reyes J, Barrios G, Legorburu FJ. Natural infections of pruning wounds by fungal trunk pathogens in mature grapevines in Catalonia (Northeast Spain). Aust J Grape Wine Res. 2014, 20, 134–143.
- Bellée, A.; Comont, G.; Nivault, A.; Abou-Mansour, E.; Coppin, C.; Dufour, MC.; Corio-Costet, M. Life traits of four Botryosphaeriaceae species and molecular responses of different grapevine cultivars or hybrids. Plant Pathol. 2017, 66(5), 763–76. [Google Scholar] [CrossRef]
- Úrbez-Torres, J.R.; Leavitt, G.M.; Guerrero, J.C.; Guevara., J.; Gubler, W.D. Identification and pathogenicity of Lasiodiplodia theobromae and Diplodia seriata, the causal agents of bot canker disease of Grapevines in Mexico. Plant Dis 2008, 92((4)), 519–529. [Google Scholar]
- Sosnowski, MR.; Ayres, MR.; McCarthy, MG.; Scott, ES. Winegrape cultivars (Vitis vinifera) vary in susceptibility to the grapevine trunk pathogens Eutypa lata and Diplodia seriata. Aust J Grape Wine. 2022, 28(1), 166–74. [Google Scholar]
- Van de Wouw, AP.; Howlett, BJ. Fungal pathogenicity genes in the age of “omics”. Mol Plant Pathol. 2011, 12(5), 507–14. [Google Scholar]
- Elena, G.; Garcia-Figueres, F.; Reigada, S.; Luque, J. Intraspecific variation in Diplodia seriata isolates occurring on grapevines in Spain. Plant Pathol. 2015, 64(3), 680–9. [Google Scholar]
- Boddy, L.; Rayner, A. Origins of decay in living deciduous trees: the role of moisture content and a re-appraisal of the expanded concept of tree decay. New Phytologist. 1983, 94(4), 623–41. [Google Scholar]
- Rey, P.; Lecomte, P.; Guérin-Dubrana, L.; Corio-Costel, F.; Fonteine, F.; Gomes, E.; Goutouly, JP.; Hofdtetter, V.; Legorburu, X.; De La Rocque, B. Maladies du bois de la vigne situation enjeux et perpective. Phytoma. 2011, 640. [Google Scholar]
- Hofstetter, V.; Buyck, B.; Croll, D.; Viret, O.; Couloux, A.; Gindro, K. What if esca disease of grapevine were not a fungal disease? Fungal Divers. 2012, 54, 51–67. [Google Scholar]
- Andreolli, M.; Lampis, S.; Zapparoli, G.; Angelini, E.; Vallini, G. Diversity of bacterial endophytes in 3- and 15-year-old grapevines of Vitis vinifera cv. Corvina and their potential for plant growth promotion and phytopathogen control. Microbiological. 2016, 183, 42–52. [Google Scholar]
- Claverie, M.; Notaro, M.; Fontaine, F.; Wery, J. Current knowledge on Grapevine Trunk Diseases with complex etiology: a systemic approach. Phytopathol Mediterr. 2020, 59, 29–53. [Google Scholar] [CrossRef]
- Úrbez-Torres, JR.; Bruez, E.; Hurtado, J.; Gubler, WD. Effect of temperature on conidial germination of Botryosphaeriaceae species infecting grapevines. Plant Dis. 2010, 94(12), 1476–84. [Google Scholar]
- Amalfitano, C.; Evidente, A.; Surico, G.; Tegli, S.; Bertelli, E.; Mugnai, L. Phenols and Stilbene Polyphenols in the Wood of Esca-Diseased Grapevines. Phytopathol Mediterr. 2000, 39, 178–183. [Google Scholar]
- Del Rio, J.; Gonzalez, A.; Fuster, M.; Botia, J.; Gomez, P.; Frias, V.; Ortuño, A. Tylose formation and changes in phenolic compounds of grape roots infected with Phaeomoniella chlamydospore and Phaeocremonium species. Phytopathol Mediterr. 2001, 40, 394–399. [Google Scholar]
- Bruno, G.; Sparapano, L. Effects of three esca-associated fungi on Vitis vinifera L.: I. Characterization of secondary metabolites in culture media and host responses to the pathogens in calli. Physiol Mol Plant Pathol 2006, 69, 209–223. [Google Scholar]
- Martin, N.; Vesentini, D.; Rego, C.; Monteiro, S.; Oliveira, H.; Ferreira, RB. Phaeomoniella chlamydospora infection induces changes in phenolic compounds content in Vitis vinifera. Phytopathol Mediterr. 2009, 48, 101–116. [Google Scholar]
- Spagnolo, A.; Magnin-Robert, M.; Alayi, TD.; Cilindre, C.; Schaeffer-Reiss, C.; Van Dorsselaer, A.; Clément, C.; Larignon, P.; Ramirez-Suero, M.; Chong, J.; Bertsch, C.; Abou-Monsour, E.; Fontaine, F. Differential responses of three grapevine cultivars to (BD). Phytopathol 2014, 104, 1021–1031. [Google Scholar]
- Gómez, P.; Báidez, AG.; Ortuño, A.; Del Río, JA. Grapevine xylem response to fungi involved in trunk diseases. Annals of Applied Biology. 2016, 169(1), 116–24. [Google Scholar]
- Pearce, RB. Antimicrobial defences in the wood of living trees. New Phytologist. 1996, 132, 203–33. [Google Scholar]
- Pouzoulet, J.; Pivovaroff, AL.; Santiago, LS.; Rolshausen, PE. Can vessel dimension explain tolerance toward fungal vascular wilt diseases in woody plants? Lessons from Dutch elm disease and esca disease in grapevine. Front Plant Sci 2014, 5, 253. [Google Scholar]
- Ramírez, M.; Pérez, L.M.; Montealegre, J.R. Susceptibility of different grapevine (Vitis vinifera L.) cultivars to Diplodia seriata and Diplodia mutila. Cien. Inv. Agr. 2018, 45, 93–98. [Google Scholar]
- Pouzoulet, J.; Scudiero, E.; Schiavon, M.; Rolshausen, P.E. Xylem Vessel Diameter Affects the Compartmentalization of the Vascular Pathogen Phaeomoniella chlamydospora in Grapevine. Front Plant Sci 2017, 21, 1442. [Google Scholar]
- Amponsah, NT.; Jones, EE.; Ridgway, HJ.; Jaspers, M. Infection and disease progression of Neofusicoccum luteum in grapevine plants. Plant Health Management:An Integrated Approach 2009, 70. [Google Scholar]
- Moral, J.; Muñoz-Díez, C.; González, N.; Trapero, A.; Michailides, TJ. Characterization and pathogenicity of Botryosphaeriaceae species collected from olive and other hosts in Spain and California. Phytopathol. 2010, 100(12), 1340–51. [Google Scholar] [CrossRef]
- Reveglia, P.; Savocchia, S.; Billones-Baaijens, R.; Masi, M.; Cimmino, A.; Evidente, A. Phytotoxic metabolites by nine species of Botryosphaeriaceae involved in grapevine dieback in Australia and identification of those produced by Diplodia mutila, Diplodia seriata, Neofusicoccum australe and Neofusicoccum luteum. Nat Prod Res. 2019, 33(15), 2223–9. [Google Scholar]
- Martos, S.; Andolfi, A.; Luque, J.; Mugnai, L.; Surico, G.; Evidente, A. Production of phytotoxic metabolites by five species of Botryosphaeriaceae causing decline on grapevines, with special interest in the species Neofusicoccum luteum and N. parvum. Eur J Plant Pathol. 2008, 121(4), 451–61. [Google Scholar]
- Ramírez, M.; Pérez, LM.; Montealegre, JR. Susceptibility of different grapevine (Vitis vinifera L.) cultivars to Diplodia seriata and Diplodia mutila. Cienc Investig Agrar 2018, 45, 93–98. [Google Scholar] [CrossRef]

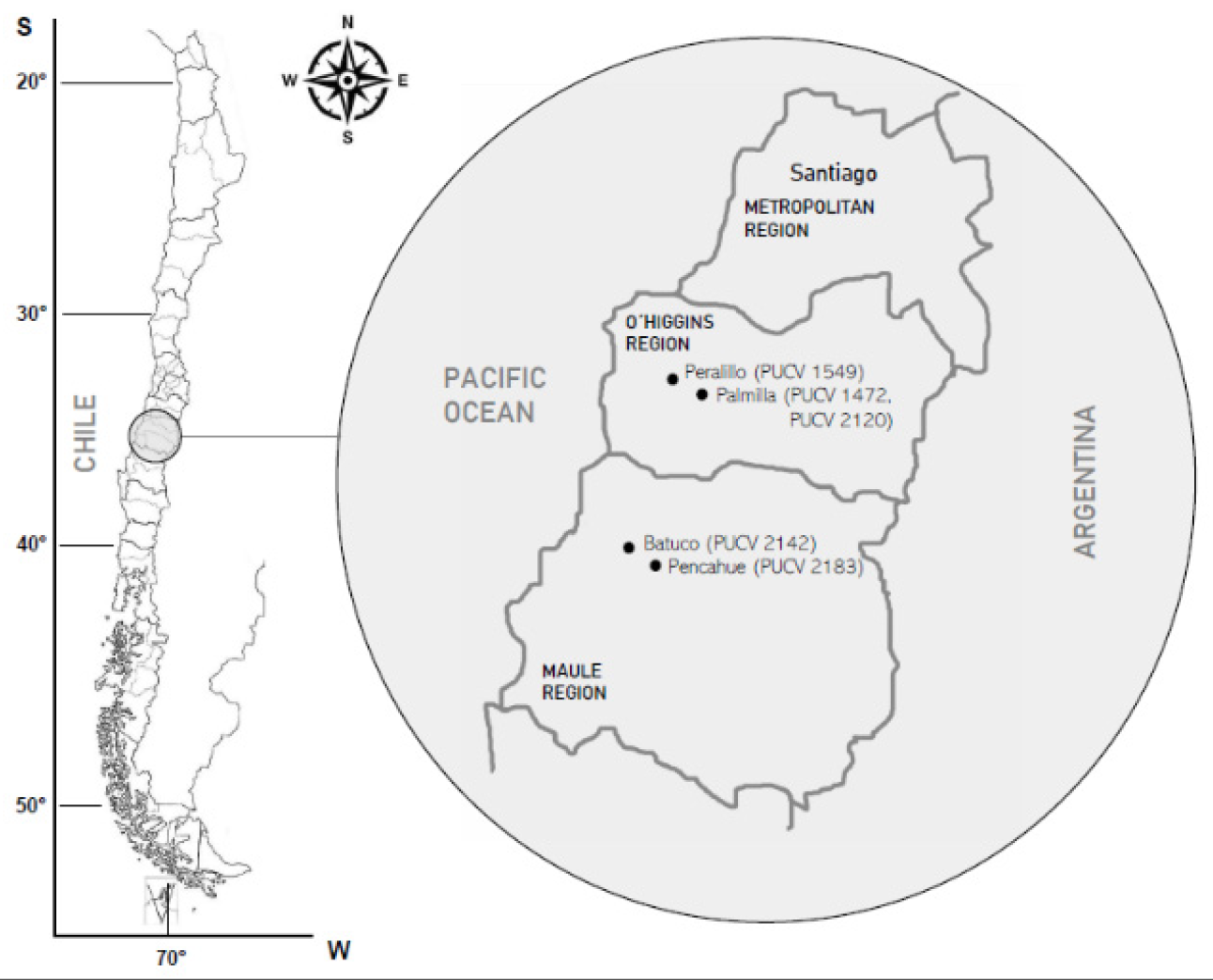

| Isolate of Diplodia seriata* | Year of Collection | GenBank Access No. | ||
|---|---|---|---|---|
| ITS | BT | EF1-α | ||
| PUCV 1472 | 2010 | KM372581 | KP762454 | - |
| PUCV 1549 | 2010 | KM580514 | KP762464 | - |
| PUCV 2120 | 2018 | MT023573 | MT063140 | MT120827 |
| PUCV 2142 | 2018 | MT023574 | MT063141 | MT120827 |
| PUCV 2183 | 2018 | MT023587 | MT063154 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





