Submitted:
03 July 2023
Posted:
04 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Stemness and cell differentiation are connected with mitochondrial dynamics and maintenance
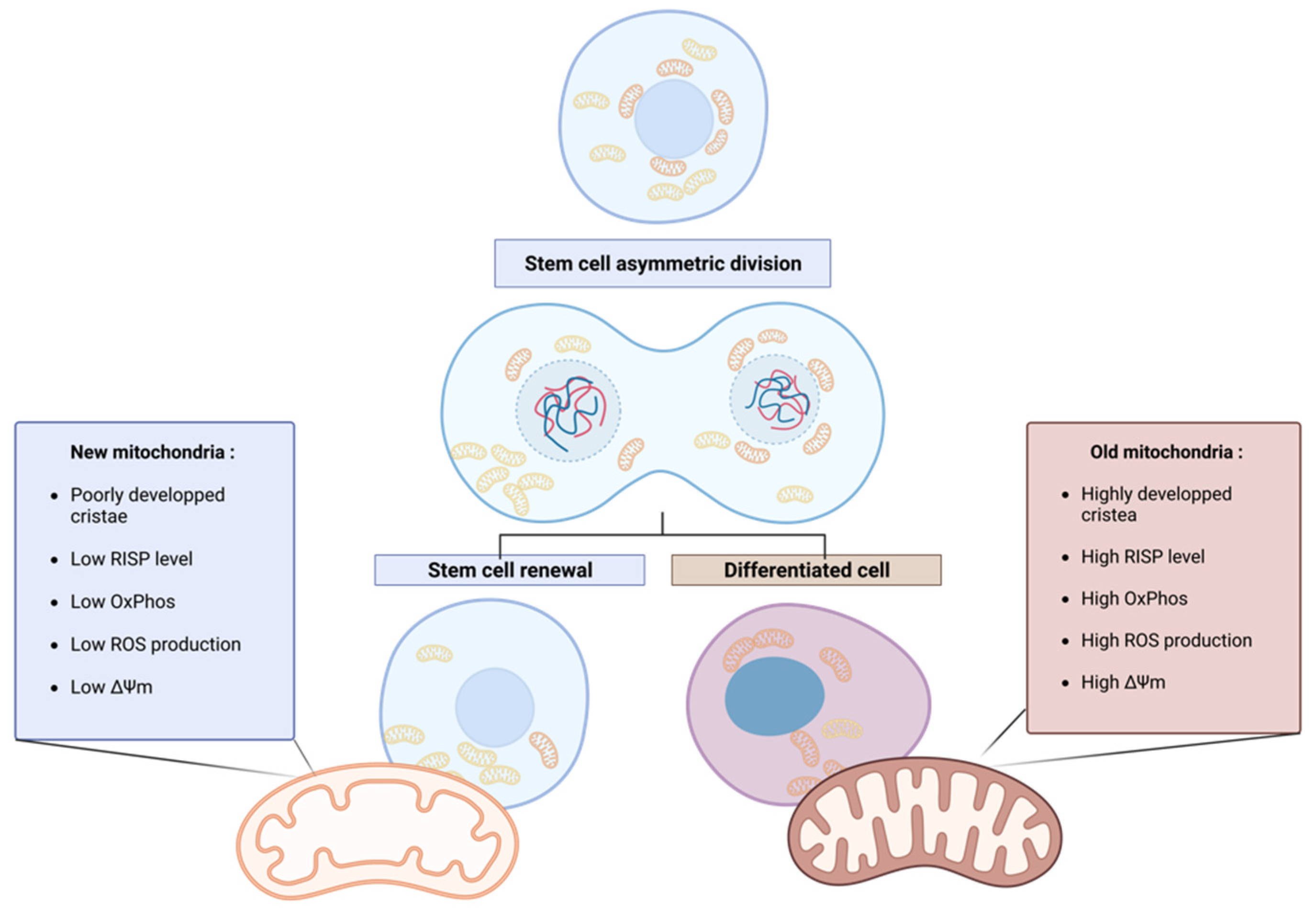
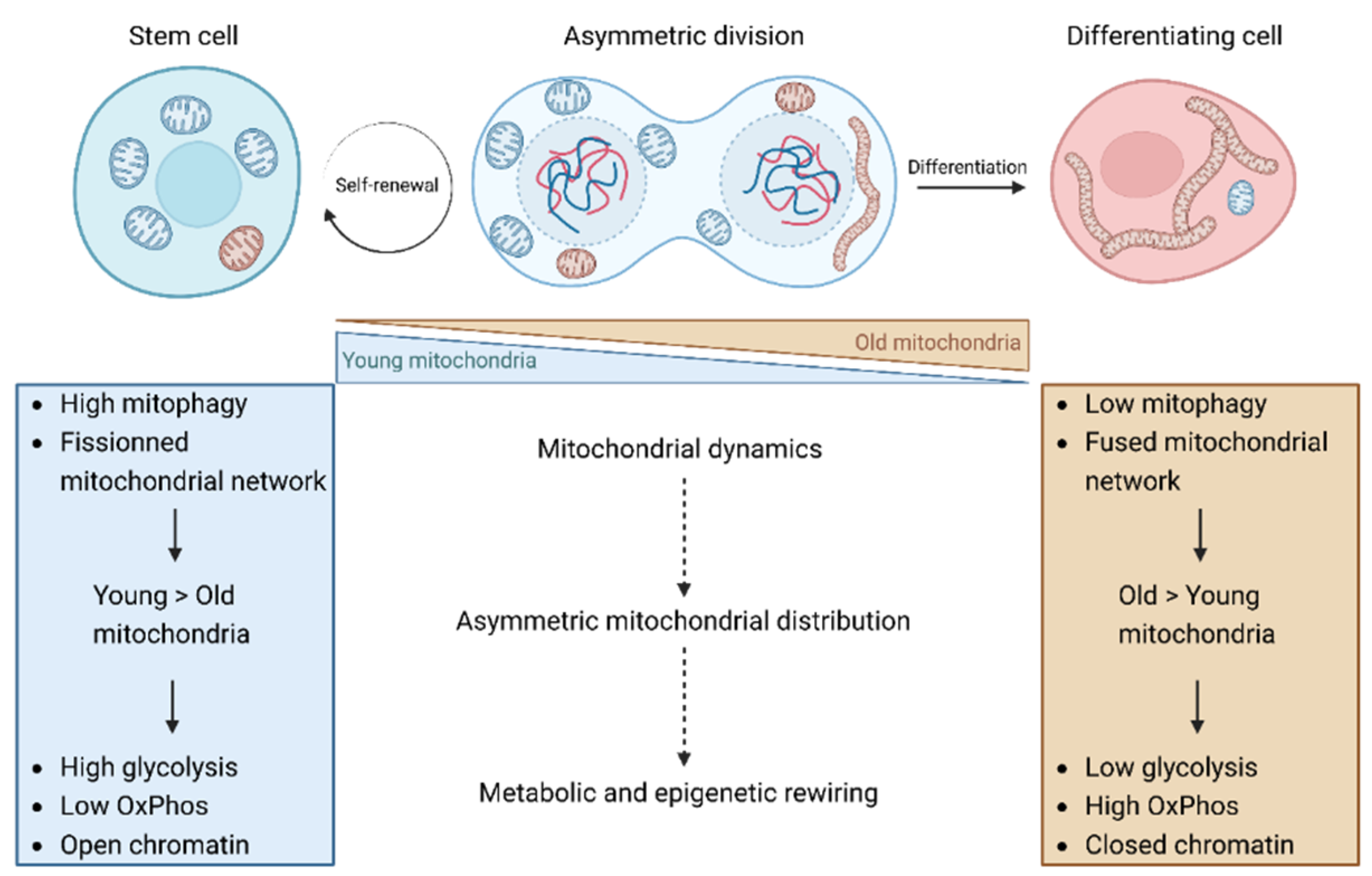
3. Asymmetric mitochondrial distribution and stem cell fate
4. The involvement of mitochondrial dynamics in asymmetric mitochondrial apportioning
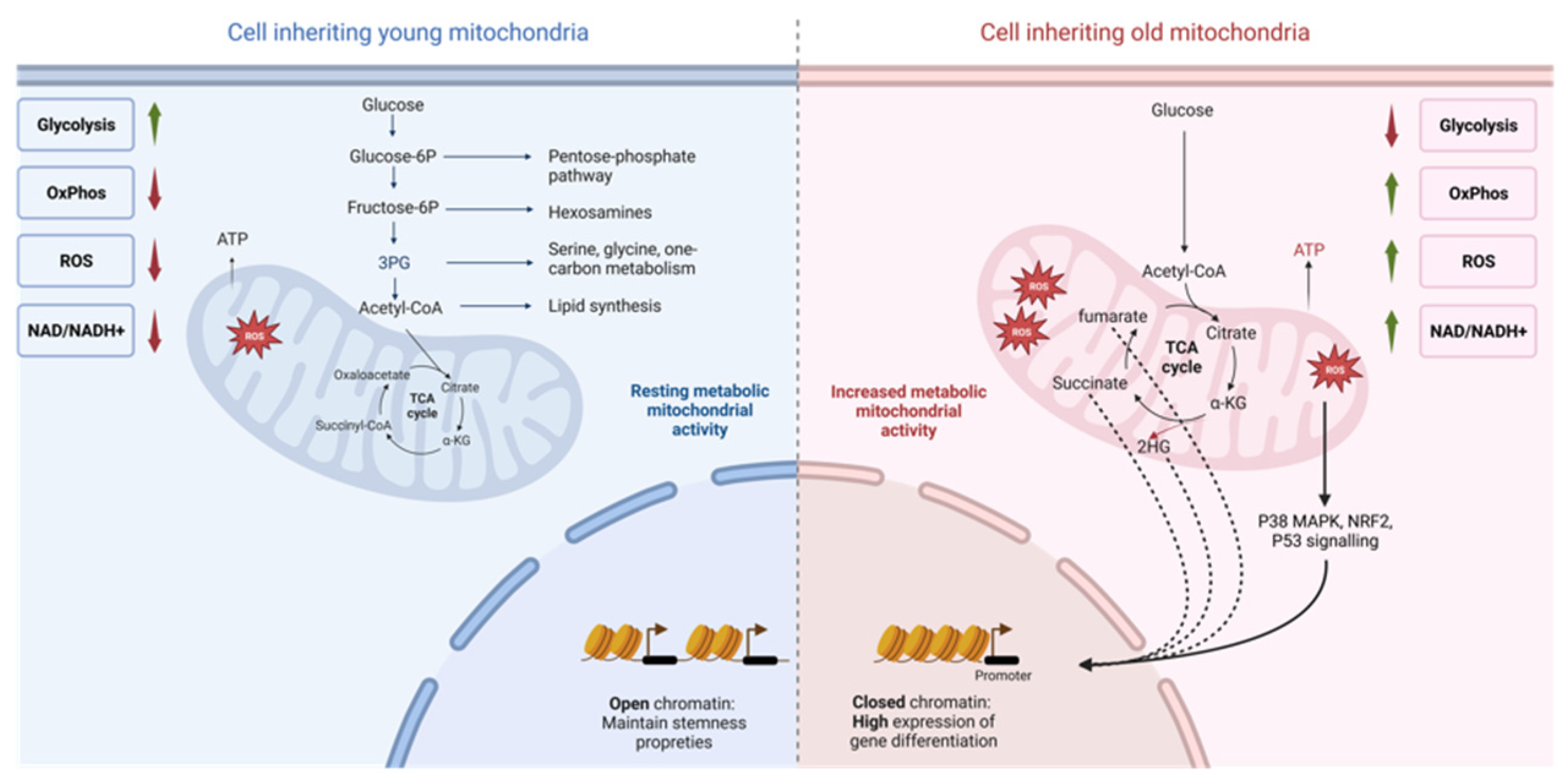
5. Metabolism of stem cells and progenitor cells
6. Benefits and regulation of glycolytic metabolism
7. Benefits and regulation of oxidative metabolism
8. Epigenetics changes and cell fate
9. Conclusion:
Funding
Conflicts of Interest
References
- Pevny, L.; Rao, M. S. The Stem-Cell Menagerie. Trends in Neurosciences 2003, 26 (7), 351–359. [CrossRef]
- Knoblich, J. A. Mechanisms of Asymmetric Stem Cell Division. Cell 2008, 132 (4), 583–597. [CrossRef]
- Schofield, R. The Relationship between the Spleen Colony-Forming Cell and the Haemopoietic Stem Cell. Blood Cells 1978, 4 (1–2), 7–25.
- Scadden, D. T. Nice Neighborhood: Emerging Concepts of the Stem Cell Niche. Cell 2014, 157 (1), 41–50. [CrossRef]
- Walker, M.; Patel, K.; Stappenbeck, T. The Stem Cell Niche. The Journal of Pathology 2009, 217 (2), 169–180. [CrossRef]
- Arai, F.; Hirao, A.; Suda, T. Regulation of Hematopoiesis and Its Interaction with Stem Cell Niches. Int J Hematol 2005, 82 (5), 371–376. [CrossRef]
- Hayashi, K.; Surani, M. A. Self-Renewing Epiblast Stem Cells Exhibit Continual Delineation of Germ Cells with Epigenetic Reprogramming in Vitro. Development 2009, 136 (21), 3549–3556. [CrossRef]
- Yeung, T. M.; Chia, L. A.; Kosinski, C. M.; Kuo, C. J. Regulation of Self-Renewal and Differentiation by the Intestinal Stem Cell Niche. Cell. Mol. Life Sci. 2011, 68 (15), 2513–2523. [CrossRef]
- Tumbar, T.; Guasch, G.; Greco, V.; Blanpain, C.; Lowry, W. E.; Rendl, M.; Fuchs, E. Defining the Epithelial Stem Cell Niche in Skin. Science 2004, 303 (5656), 359–363. [CrossRef]
- Spradling, A.; Drummond-Barbosa, D.; Kai, T. Stem Cells Find Their Niche. Nature 2001, 414 (6859), 98–104. [CrossRef]
- Wanet, A.; Arnould, T.; Najimi, M.; Renard, P. Connecting Mitochondria, Metabolism, and Stem Cell Fate. Stem Cells and Development 2015, 24 (17), 1957–1971. [CrossRef]
- Ly, C. H.; Lynch, G. S.; Ryall, J. G. A Metabolic Roadmap for Somatic Stem Cell Fate. Cell Metabolism 2020, 31 (6), 1052–1067. [CrossRef]
- Zhang, Y.; Marsboom, G.; Toth, P. T.; Rehman, J. Mitochondrial Respiration Regulates Adipogenic Differentiation of Human Mesenchymal Stem Cells. PLoS ONE 2013, 8 (10), e77077. [CrossRef]
- Hsu, S.-H.; Chen, C.-T.; Wei, Y.-H. Inhibitory Effects of Hypoxia on Metabolic Switch and Osteogenic Differentiation of Human Mesenchymal Stem Cells. Stem Cells 2013, 31 (12), 2779–2788. [CrossRef]
- Wanet, A.; Remacle, N.; Najar, M.; Sokal, E.; Arnould, T.; Najimi, M.; Renard, P. Mitochondrial Remodeling in Hepatic Differentiation and Dedifferentiation. The International Journal of Biochemistry & Cell Biology 2014, 54, 174–185. [CrossRef]
- Katajisto, P.; Döhla, J.; Chaffer, C. L.; Pentinmikko, N.; Marjanovic, N.; Iqbal, S.; Zoncu, R.; Chen, W.; Weinberg, R. A.; Sabatini, D. M. Asymmetric Apportioning of Aged Mitochondria between Daughter Cells Is Required for Stemness. Science 2015, 348 (6232), 340–343. [CrossRef]
- Döhla, J.; Kuuluvainen, E.; Gebert, N.; Amaral, A.; Englund, J. I.; Gopalakrishnan, S.; Konovalova, S.; Nieminen, A. I.; Salminen, E. S.; Torregrosa Muñumer, R.; Ahlqvist, K.; Yang, Y.; Bui, H.; Otonkoski, T.; Käkelä, R.; Hietakangas, V.; Tyynismaa, H.; Ori, A.; Katajisto, P. Metabolic Determination of Cell Fate through Selective Inheritance of Mitochondria. Nat Cell Biol 2022, 24 (2), 148–154. [CrossRef]
- Ahlqvist, K. J.; Suomalainen, A.; Hämäläinen, R. H. Stem Cells, Mitochondria and Aging. Biochimica et Biophysica Acta (BBA) - Bioenergetics 2015, 1847 (11), 1380–1386. [CrossRef]
- Laird, P. W. Cancer Epigenetics. Human Molecular Genetics 2005, 14 (suppl_1), R65–R76. [CrossRef]
- Eccleston, A.; DeWitt, N.; Gunter, C.; Marte, B.; Nath, D. Epigenetics. Nature 2007, 447 (7143), 395–396.
- Esteller, M. Epigenetics Provides a New Generation of Oncogenes and Tumour-Suppressor Genes. Br J Cancer 2007, 96 Suppl, R26-30. [CrossRef]
- Lennartsson, A.; Ekwall, K. Histone Modification Patterns and Epigenetic Codes. Biochimica et Biophysica Acta (BBA) - General Subjects 2009, 1790 (9), 863–868. [CrossRef]
- Gibney, E. R.; Nolan, C. M. Epigenetics and Gene Expression. Heredity 2010, 105 (1), 4–13. [CrossRef]
- Lu, C.; Thompson, C. B. Metabolic Regulation of Epigenetics. Cell Metabolism 2012, 16 (1), 9–17. [CrossRef]
- Keating, S. T.; El-Osta, A. Epigenetics and Metabolism. Circ Res 2015, 116 (4), 715–736. [CrossRef]
- Kinnaird, A.; Zhao, S.; Wellen, K. E.; Michelakis, E. D. Metabolic Control of Epigenetics in Cancer. Nat Rev Cancer 2016, 16 (11), 694–707. [CrossRef]
- Dai, Z.; Ramesh, V.; Locasale, J. W. The Evolving Metabolic Landscape of Chromatin Biology and Epigenetics. Nat Rev Genet 2020, 21 (12), 737–753. [CrossRef]
- Intlekofer, A. M.; Finley, L. W. S. Metabolic Signatures of Cancer Cells and Stem Cells. Nat Metab 2019, 1 (2), 177–188. [CrossRef]
- Harvey, A.; Caretti, G.; Moresi, V.; Renzini, A.; Adamo, S. Interplay between Metabolites and the Epigenome in Regulating Embryonic and Adult Stem Cell Potency and Maintenance. Stem Cell Reports 2019, 13 (4), 573–589. [CrossRef]
- Seo, B. J.; Yoon, S. H.; Do, J. T. Mitochondrial Dynamics in Stem Cells and Differentiation. International Journal of Molecular Sciences 2018, 19 (12), 3893. [CrossRef]
- Fu, W.; Liu, Y.; Yin, H. Mitochondrial Dynamics: Biogenesis, Fission, Fusion, and Mitophagy in the Regulation of Stem Cell Behaviors. Stem Cells International 2019, 2019, e9757201. [CrossRef]
- Hoque, A.; Sivakumaran, P.; Bond, S. T.; Ling, N. X. Y.; Kong, A. M.; Scott, J. W.; Bandara, N.; Hernández, D.; Liu, G.-S.; Wong, R. C. B.; Ryan, M. T.; Hausenloy, D. J.; Kemp, B. E.; Oakhill, J. S.; Drew, B. G.; Pébay, A.; Lim, S. Y. Mitochondrial Fission Protein Drp1 Inhibition Promotes Cardiac Mesodermal Differentiation of Human Pluripotent Stem Cells. Cell Death Discovery 2018, 4 (1), 1–13. [CrossRef]
- Wang, L.; Zhang, T.; Wang, L.; Cai, Y.; Zhong, X.; He, X.; Hu, L.; Tian, S.; Wu, M.; Hui, L.; Zhang, H.; Gao, P. Fatty Acid Synthesis Is Critical for Stem Cell Pluripotency via Promoting Mitochondrial Fission. EMBO J 2017, 36 (10), 1330–1347. [CrossRef]
- Vazquez-Martin, A.; Haute, C. V. V. den; Cufí, S.; Corominas-Faja, B. C.; Cuyàs, E.; Lopez-Bonet, E.; Rodriguez-Gallego, E.; Fernández-Arroyo, S.; Joven, J.; Baekelandt, V.; Menendez, J. A. A. Mitophagy-Driven Mitochondrial Rejuvenation Regulates Stem Cell Fate. Aging 2016, 8 (7), 1330–1349. [CrossRef]
- Zhou, H.; Zhang, Y.; Hu, S.; Shi, C.; Zhu, P.; Ma, Q.; Jin, Q.; Cao, F.; Tian, F.; Chen, Y. Melatonin Protects Cardiac Microvasculature against Ischemia/Reperfusion Injury via Suppression of Mitochondrial Fission-VDAC1-HK2-MPTP-Mitophagy Axis. Journal of Pineal Research 2017, 63 (1), e12413. [CrossRef]
- Cribbs, J. T.; Strack, S. Reversible Phosphorylation of Drp1 by Cyclic AMP-dependent Protein Kinase and Calcineurin Regulates Mitochondrial Fission and Cell Death. EMBO Rep 2007, 8 (10), 939–944. [CrossRef]
- Zhong, Y.; Jin, C.; Han, J.; Zhu, J.; Liu, Q.; Sun, D.; Xia, X.; Peng, X. Inhibition of ER Stress Attenuates Kidney Injury and Apoptosis Induced by 3-MCPD via Regulating Mitochondrial Fission/Fusion and Ca2+ Homeostasis. Cell Biol Toxicol 2021, 37 (5), 795–809. [CrossRef]
- Todd, L. R.; Damin, M. N.; Gomathinayagam, R.; Horn, S. R.; Means, A. R.; Sankar, U. Growth Factor Erv1-like Modulates Drp1 to Preserve Mitochondrial Dynamics and Function in Mouse Embryonic Stem Cells. MBoC 2010, 21 (7), 1225–1236. [CrossRef]
- Adams, W. C.; Chen, Y.-H.; Kratchmarov, R.; Yen, B.; Nish, S. A.; Lin, W.-H. W.; Rothman, N. J.; Luchsinger, L. L.; Klein, U.; Busslinger, M.; Rathmell, J. C.; Snoeck, H.-W.; Reiner, S. L. Anabolism-Associated Mitochondrial Stasis Driving Lymphocyte Differentiation over Self-Renewal. Cell Reports 2016, 17 (12), 3142–3152. [CrossRef]
- Cairns, G.; Thumiah-Mootoo, M.; Burelle, Y.; Khacho, M. Mitophagy: A New Player in Stem Cell Biology. Biology 2020, 9 (12), 481. [CrossRef]
- Ho, T. T.; Warr, M. R.; Adelman, E. R.; Lansinger, O. M.; Flach, J.; Verovskaya, E. V.; Figueroa, M. E.; Passegué, E. Autophagy Maintains the Metabolism and Function of Young and Old Stem Cells. Nature 2017, 543 (7644), 205–210. [CrossRef]
- Mortensen, M.; Watson, A. S.; Simon, A. K. Lack of Autophagy in the Hematopoietic System Leads to Loss of Hematopoietic Stem Cell Function and Dysregulated Myeloid Proliferation. Autophagy 2011, 7 (9), 1069–1070. [CrossRef]
- Song, B.; Chi, Y.; Li, X.; Du, W.; Han, Z.-B.; Tian, J.; Li, J.; Chen, F.; Wu, H.; Han, L.; Lu, S.; Zheng, Y.; Han, Z. Inhibition of Notch Signaling Promotes the Adipogenic Differentiation of Mesenchymal Stem Cells Through Autophagy Activation and PTEN-PI3K/AKT/MTOR Pathway. CPB 2015, 36 (5), 1991–2002. [CrossRef]
- Klecker, T.; Westermann, B. Asymmetric Inheritance of Mitochondria in Yeast. Biological Chemistry 2020, 401 (6–7), 779–791. [CrossRef]
- Dalton, C. M.; Carroll, J. Biased Inheritance of Mitochondria during Asymmetric Cell Division in the Mouse Oocyte. Journal of Cell Science 2013, 126 (13), 2955–2964. [CrossRef]
- Loeffler, D.; Schneiter, F.; Wang, W.; Wehling, A.; Kull, T.; Lengerke, C.; Manz, M. G.; Schroeder, T. Asymmetric Organelle Inheritance Predicts Human Blood Stem Cell Fate. Blood 2022, 139 (13), 2011–2023. [CrossRef]
- Hinge, A.; He, J.; Bartram, J.; Javier, J.; Xu, J.; Fjellman, E.; Sesaki, H.; Li, T.; Yu, J.; Wunderlich, M.; Mulloy, J.; Kofron, M.; Salomonis, N.; Grimes, H. L.; Filippi, M.-D. Asymmetrically Segregated Mitochondria Provide Cellular Memory of Hematopoietic Stem Cell Replicative History and Drive HSC Attrition. Cell Stem Cell 2020, 26 (3), 420-430.e6. [CrossRef]
- Vannini, N.; Campos, V.; Girotra, M.; Trachsel, V.; Rojas-Sutterlin, S.; Tratwal, J.; Ragusa, S.; Stefanidis, E.; Ryu, D.; Rainer, P. Y.; Nikitin, G.; Giger, S.; Li, T. Y.; Semilietof, A.; Oggier, A.; Yersin, Y.; Tauzin, L.; Pirinen, E.; Cheng, W.-C.; Ratajczak, J.; Canto, C.; Ehrbar, M.; Sizzano, F.; Petrova, T. V.; Vanhecke, D.; Zhang, L.; Romero, P.; Nahimana, A.; Cherix, S.; Duchosal, M. A.; Ho, P.-C.; Deplancke, B.; Coukos, G.; Auwerx, J.; Lutolf, M. P.; Naveiras, O. The NAD-Booster Nicotinamide Riboside Potently Stimulates Hematopoiesis through Increased Mitochondrial Clearance. Cell Stem Cell 2019, 24 (3), 405-418.e7. [CrossRef]
- Wu, M.-J.; Chen, Y.-S.; Kim, M. R.; Chang, C.-C.; Gampala, S.; Zhang, Y.; Wang, Y.; Chang, C.-Y.; Yang, J.-Y.; Chang, C.-J. Epithelial-Mesenchymal Transition Directs Stem Cell Polarity via Regulation of Mitofusin. Cell Metabolism 2019, 29 (4), 993-1002.e6. [CrossRef]
- Mahendralingam, M. J.; Kim, H.; McCloskey, C. W.; Aliar, K.; Casey, A. E.; Tharmapalan, P.; Pellacani, D.; Ignatchenko, V.; Garcia-Valero, M.; Palomero, L.; Sinha, A.; Cruickshank, J.; Shetty, R.; Vellanki, R. N.; Koritzinsky, M.; Stambolic, V.; Alam, M.; Schimmer, A. D.; Berman, H. K.; Eaves, C. J.; Pujana, M. A.; Kislinger, T.; Khokha, R. Mammary Epithelial Cells Have Lineage-Rooted Metabolic Identities. Nat Metab 2021, 3 (5), 665–681. [CrossRef]
- Gustafsson, Å. B.; Dorn, G. W. Evolving and Expanding the Roles of Mitophagy as a Homeostatic and Pathogenic Process. Physiological Reviews 2019, 99 (1), 853–892. [CrossRef]
- Joshi, A.; Kundu, M. Mitophagy in Hematopoietic Stem Cells: The Case for Exploration. Autophagy 2013, 9 (11), 1737–1749. [CrossRef]
- Naik, P. P.; Birbrair, A.; Bhutia, S. K. Mitophagy-Driven Metabolic Switch Reprograms Stem Cell Fate. Cell. Mol. Life Sci. 2019, 76 (1), 27–43. [CrossRef]
- Teslaa, T.; Teitell, M. A. Pluripotent Stem Cell Energy Metabolism: An Update. EMBO J 2015, 34 (2), 138–153. [CrossRef]
- Prigione, A.; Adjaye, J. Modulation of Mitochondrial Biogenesis and Bioenergetic Metabolism upon in Vitro and in Vivo Differentiation of Human ES and IPS Cells. Int. J. Dev. Biol. 2010, 54 (11–12), 1729–1741. [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126 (4), 663–676. [CrossRef]
- Nishimura, K.; Fukuda, A.; Hisatake, K. Mechanisms of the Metabolic Shift during Somatic Cell Reprogramming. International Journal of Molecular Sciences 2019, 20 (9), 2254. [CrossRef]
- Yu, J.; Vodyanik, M. A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J. L.; Tian, S.; Nie, J.; Jonsdottir, G. A.; Ruotti, V.; Stewart, R.; Slukvin, I. I.; Thomson, J. A. Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells. Science 2007, 318 (5858), 1917–1920. [CrossRef]
- Panopoulos, A. D.; Ruiz, S.; Yi, F.; Herrerías, A.; Batchelder, E. M.; Belmonte, J. C. I. Rapid and Highly Efficient Generation of Induced Pluripotent Stem Cells from Human Umbilical Vein Endothelial Cells. PLOS ONE 2011, 6 (5), e19743. [CrossRef]
- Folmes, C. D. L.; Dzeja, P. P.; Nelson, T. J.; Terzic, A. Metabolic Plasticity in Stem Cell Homeostasis and Differentiation. Cell Stem Cell 2012, 11 (5), 596–606. [CrossRef]
- Takubo, K.; Nagamatsu, G.; Kobayashi, C. I.; Nakamura-Ishizu, A.; Kobayashi, H.; Ikeda, E.; Goda, N.; Rahimi, Y.; Johnson, R. S.; Soga, T.; Hirao, A.; Suematsu, M.; Suda, T. Regulation of Glycolysis by Pdk Functions as a Metabolic Checkpoint for Cell Cycle Quiescence in Hematopoietic Stem Cells. Cell Stem Cell 2013, 12 (1), 49–61. [CrossRef]
- Kohli, L.; Passegué, E. Surviving Change: The Metabolic Journey of Hematopoietic Stem Cells. Trends in Cell Biology 2014, 24 (8), 479–487. [CrossRef]
- Suda, T.; Takubo, K.; Semenza, G. L. Metabolic Regulation of Hematopoietic Stem Cells in the Hypoxic Niche. Cell Stem Cell 2011, 9 (4), 298–310. [CrossRef]
- Shyh-Chang, N.; Daley, G. Q. Lin28: Primal Regulator of Growth and Metabolism in Stem Cells. Cell Stem Cell 2013, 12 (4), 395–406. [CrossRef]
- Mohyeldin, A.; Garzón-Muvdi, T.; Quiñones-Hinojosa, A. Oxygen in Stem Cell Biology: A Critical Component of the Stem Cell Niche. Cell Stem Cell 2010, 7 (2), 150–161. [CrossRef]
- Semenza, G. L.; Wang, G. L. A Nuclear Factor Induced by Hypoxia via de Novo Protein Synthesis Binds to the Human Erythropoietin Gene Enhancer at a Site Required for Transcriptional Activation. Molecular and Cellular Biology 1992, 12 (12), 5447–5454. [CrossRef]
- Prigione, A.; Rohwer, N.; Hoffmann, S.; Mlody, B.; Drews, K.; Bukowiecki, R.; Blümlein, K.; Wanker, E. E.; Ralser, M.; Cramer, T.; Adjaye, J. HIF1α Modulates Cell Fate Reprogramming Through Early Glycolytic Shift and Upregulation of PDK1–3 and PKM2. Stem Cells 2014, 32 (2), 364–376. [CrossRef]
- Rodrigues, A. S.; Correia, M.; Gomes, A.; Pereira, S. L.; Perestrelo, T.; Sousa, M. I.; Ramalho-Santos, J. Dichloroacetate, the Pyruvate Dehydrogenase Complex and the Modulation of MESC Pluripotency. PLOS ONE 2015, 10 (7), e0131663. [CrossRef]
- Forristal, C. E.; Wright, K. L.; Hanley, N. A.; Oreffo, R. O. C.; Houghton, F. D. Hypoxia Inducible Factors Regulate Pluripotency and Proliferation in Human Embryonic Stem Cells Cultured at Reduced Oxygen Tensions. Reproduction 2010, 139 (1), 85–97. [CrossRef]
- Arthur, S. A.; Blaydes, J. P.; Houghton, F. D. Glycolysis Regulates Human Embryonic Stem Cell Self-Renewal under Hypoxia through HIF-2α and the Glycolytic Sensors CTBPs. Stem Cell Reports 2019, 12 (4), 728–742. [CrossRef]
- Shapira, S. N.; Christofk, H. R. Metabolic Regulation of Tissue Stem Cells. Trends in Cell Biology 2020, 30 (7), 566–576. [CrossRef]
- Maryanovich, M.; Zaltsman, Y.; Ruggiero, A.; Goldman, A.; Shachnai, L.; Zaidman, S. L.; Porat, Z.; Golan, K.; Lapidot, T.; Gross, A. An MTCH2 Pathway Repressing Mitochondria Metabolism Regulates Haematopoietic Stem Cell Fate. Nat Commun 2015, 6 (1), 7901. [CrossRef]
- Jones, W.; Bianchi, K. Aerobic Glycolysis: Beyond Proliferation. Frontiers in Immunology 2015, 6. [CrossRef]
- Ferreira, L. M. R.; Li, A. M.; Serafim, T. L.; Sobral, M. C.; Alpoim, M. C.; Urbano, A. M. Intermediary Metabolism: An Intricate Network at the Crossroads of Cell Fate and Function. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 2020, 1866 (10), 165887. [CrossRef]
- Pereira, S. L.; Grãos, M.; Rodrigues, A. S.; Anjo, S. I.; Carvalho, R. A.; Oliveira, P. J.; Arenas, E.; Ramalho-Santos, J. Inhibition of Mitochondrial Complex III Blocks Neuronal Differentiation and Maintains Embryonic Stem Cell Pluripotency. PLOS ONE 2013, 8 (12), e82095. [CrossRef]
- Tormos, K. V.; Anso, E.; Hamanaka, R. B.; Eisenbart, J.; Joseph, J.; Kalyanaraman, B.; Chandel, N. S. Mitochondrial Complex III ROS Regulate Adipocyte Differentiation. Cell Metabolism 2011, 14 (4), 537–544. [CrossRef]
- Ansó, E.; Weinberg, S. E.; Diebold, L. P.; Thompson, B. J.; Malinge, S.; Schumacker, P. T.; Liu, X.; Zhang, Y.; Shao, Z.; Steadman, M.; Marsh, K. M.; Xu, J.; Crispino, J. D.; Chandel, N. S. The Mitochondrial Respiratory Chain Is Essential for Haematopoietic Stem Cell Function. Nat Cell Biol 2017, 19 (6), 614–625. [CrossRef]
- Mailloux, R. J. An Update on Mitochondrial Reactive Oxygen Species Production. Antioxidants 2020, 9 (6), 472. [CrossRef]
- Nickel, A.; Kohlhaas, M.; Maack, C. Mitochondrial Reactive Oxygen Species Production and Elimination. Journal of Molecular and Cellular Cardiology 2014, 73, 26–33. [CrossRef]
- Schieber, M.; Chandel, N. S. ROS Function in Redox Signaling and Oxidative Stress. Current Biology 2014, 24 (10), R453–R462. [CrossRef]
- Bigarella, C. L.; Liang, R.; Ghaffari, S. Stem Cells and the Impact of ROS Signaling. Development 2014, 141 (22), 4206–4218. [CrossRef]
- Cao, Y.; Fang, Y.; Cai, J.; Li, X.; Xu, F.; Yuan, N.; Zhang, S.; Wang, J. ROS Functions as an Upstream Trigger for Autophagy to Drive Hematopoietic Stem Cell Differentiation. Hematology 2016, 21 (10), 613–618. [CrossRef]
- Ludin, A.; Gur-Cohen, S.; Golan, K.; Kaufmann, K. B.; Itkin, T.; Medaglia, C.; Lu, X.-J.; Ledergor, G.; Kollet, O.; Lapidot, T. Reactive Oxygen Species Regulate Hematopoietic Stem Cell Self-Renewal, Migration and Development, As Well As Their Bone Marrow Microenvironment. Antioxidants & Redox Signaling 2014, 21 (11), 1605–1619. [CrossRef]
- Wany, A.; Foyer, C. H.; Gupta, K. J. Nitrate, NO and ROS Signaling in Stem Cell Homeostasis. Trends in Plant Science 2018, 23 (12), 1041–1044. [CrossRef]
- Ryu, J. M.; Lee, H. J.; Jung, Y. H.; Lee, K. H.; Kim, D. I.; Kim, J. Y.; Ko, S. H.; Choi, G. E.; Chai, I. I.; Song, E. J.; Oh, J. Y.; Lee, S.-J.; Han, H. J. Regulation of Stem Cell Fate by ROS-Mediated Alteration of Metabolism. International Journal of Stem Cells 2015, 8 (1), 24–35. [CrossRef]
- Brown, G. C.; Borutaite, V. There Is No Evidence That Mitochondria Are the Main Source of Reactive Oxygen Species in Mammalian Cells. Mitochondrion 2012, 12 (1), 1–4. [CrossRef]
- McGuire, V. A.; Arthur, J. S. C. Stress-Induced Haematopoietic Stem Cell Proliferation: New Roles for P38α and Purine Metabolism. Stem Cell Investig 2016, 3, 64. [CrossRef]
- Canovas, B.; Nebreda, A. R. Diversity and Versatility of P38 Kinase Signalling in Health and Disease. Nat Rev Mol Cell Biol 2021, 22 (5), 346–366. [CrossRef]
- Bhattacharya, D.; Czechowicz, A.; Ooi, A. G. L.; Rossi, D. J.; Bryder, D.; Weissman, I. L. Niche Recycling through Division-Independent Egress of Hematopoietic Stem Cells. Journal of Experimental Medicine 2009, 206 (12), 2837–2850. [CrossRef]
- Pallafacchina, G.; François, S.; Regnault, B.; Czarny, B.; Dive, V.; Cumano, A.; Montarras, D.; Buckingham, M. An Adult Tissue-Specific Stem Cell in Its Niche: A Gene Profiling Analysis of in Vivo Quiescent and Activated Muscle Satellite Cells. Stem Cell Research 2010, 4 (2), 77–91. [CrossRef]
- Lee, Y. K.; Chung, Y. S.; Lee, J. H.; Chun, J. M.; Park, J. H. The Intricate Role of P53 in Adipocyte Differentiation and Function. Cells 2020, 9 (12), 2621. [CrossRef]
- Kärkkäinen, V.; Pomeshchik, Y.; Savchenko, E.; Dhungana, H.; Kurronen, A.; Lehtonen, S.; Naumenko, N.; Tavi, P.; Levonen, A.-L.; Yamamoto, M.; Malm, T.; Magga, J.; Kanninen, K. M.; Koistinaho, J. Nrf2 Regulates Neurogenesis and Protects Neural Progenitor Cells Against Aβ Toxicity. Stem Cells 2014, 32 (7), 1904–1916. [CrossRef]
- Murakami, S.; Motohashi, H. Roles of Nrf2 in Cell Proliferation and Differentiation. Free Radical Biology and Medicine 2015, 88, 168–178. [CrossRef]
- Wu, H.; Sun, Y. E. Epigenetic Regulation of Stem Cell Differentiation. Pediatr Res 2006, 59 (4), 21–25. [CrossRef]
- Spivakov, M.; Fisher, A. G. Epigenetic Signatures of Stem-Cell Identity. Nat Rev Genet 2007, 8 (4), 263–271. [CrossRef]
- Ji, H.; Ehrlich, L. I. R.; Seita, J.; Murakami, P.; Doi, A.; Lindau, P.; Lee, H.; Aryee, M. J.; Irizarry, R. A.; Kim, K.; Rossi, D. J.; Inlay, M. A.; Serwold, T.; Karsunky, H.; Ho, L.; Daley, G. Q.; Weissman, I. L.; Feinberg, A. P. Comprehensive Methylome Map of Lineage Commitment from Haematopoietic Progenitors. Nature 2010, 467 (7313), 338–342. [CrossRef]
- Hawkins, R. D.; Hon, G. C.; Lee, L. K.; Ngo, Q.; Lister, R.; Pelizzola, M.; Edsall, L. E.; Kuan, S.; Luu, Y.; Klugman, S.; Antosiewicz-Bourget, J.; Ye, Z.; Espinoza, C.; Agarwahl, S.; Shen, L.; Ruotti, V.; Wang, W.; Stewart, R.; Thomson, J. A.; Ecker, J. R.; Ren, B. Distinct Epigenomic Landscapes of Pluripotent and Lineage-Committed Human Cells. Cell Stem Cell 2010, 6 (5), 479–491. [CrossRef]
- Ryall, J. G.; Cliff, T.; Dalton, S.; Sartorelli, V. Metabolic Reprogramming of Stem Cell Epigenetics. Cell Stem Cell 2015, 17 (6), 651–662. [CrossRef]
- Katada, S.; Imhof, A.; Sassone-Corsi, P. Connecting Threads: Epigenetics and Metabolism. Cell 2012, 148 (1), 24–28. [CrossRef]
- Brunet, A.; Rando, T. A. Interaction between Epigenetic and Metabolism in Aging Stem Cells. Current Opinion in Cell Biology 2017, 45, 1–7. [CrossRef]
- Imai, S.; Guarente, L. Ten Years of NAD-Dependent SIR2 Family Deacetylases: Implications for Metabolic Diseases. Trends in Pharmacological Sciences 2010, 31 (5), 212–220. [CrossRef]
- Bahat, A.; Gross, A. Mitochondrial Plasticity in Cell Fate Regulation. Journal of Biological Chemistry 2019, 294 (38), 13852–13863. [CrossRef]
- Fang, Y.; Li, X. Chapter 2 - Sirtuins in Metabolic and Epigenetic Regulation of Stem Cells. In Sirtuin Biology in Cancer and Metabolic Disease; Maiese, K., Ed.; Academic Press, 2021; pp 25–37. [CrossRef]
- Cha, Y.; Han, M.-J.; Cha, H.-J.; Zoldan, J.; Burkart, A.; Jung, J. H.; Jang, Y.; Kim, C.-H.; Jeong, H.-C.; Kim, B.-G.; Langer, R.; Kahn, C. R.; Guarente, L.; Kim, K.-S. Metabolic Control of Primed Human Pluripotent Stem Cell Fate and Function by the MiR-200c–SIRT2 Axis. Nat Cell Biol 2017, 19 (5), 445–456. [CrossRef]
- Hsu, Y.-C.; Wu, Y.-T.; Tsai, C.-L.; Wei, Y.-H. Current Understanding and Future Perspectives of the Roles of Sirtuins in the Reprogramming and Differentiation of Pluripotent Stem Cells. Exp Biol Med (Maywood) 2018, 243 (6), 563–575. [CrossRef]
- Zwaka, T. P. Breathing Chromatin in Pluripotent Stem Cells. Developmental Cell 2006, 10 (1), 1–2. [CrossRef]
- Xiao, M.; Yang, H.; Xu, W.; Ma, S.; Lin, H.; Zhu, H.; Liu, L.; Liu, Y.; Yang, C.; Xu, Y.; Zhao, S.; Ye, D.; Xiong, Y.; Guan, K.-L. Inhibition of α-KG-Dependent Histone and DNA Demethylases by Fumarate and Succinate That Are Accumulated in Mutations of FH and SDH Tumor Suppressors. Genes Dev. 2012, 26 (12), 1326–1338. [CrossRef]
- Zhang, J.; Jing, L.; Li, M.; He, L.; Guo, Z. Regulation of Histone Arginine Methylation/Demethylation by Methylase and Demethylase (Review). Molecular Medicine Reports 2019, 19 (5), 3963–3971. [CrossRef]
- Zheng, Y.-C.; Ma, J.; Wang, Z.; Li, J.; Jiang, B.; Zhou, W.; Shi, X.; Wang, X.; Zhao, W.; Liu, H.-M. A Systematic Review of Histone Lysine-Specific Demethylase 1 and Its Inhibitors. Medicinal Research Reviews 2015, 35 (5), 1032–1071. [CrossRef]
- Langer, M. R.; Fry, C. J.; Peterson, C. L.; Denu, J. M. Modulating Acetyl-CoA Binding in the GCN5 Family of Histone Acetyltransferases *. Journal of Biological Chemistry 2002, 277 (30), 27337–27344. [CrossRef]
- Motohashi, N.; Asakura, A. Muscle Satellite Cell Heterogeneity and Self-Renewal. Frontiers in Cell and Developmental Biology 2014, 2. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




