Submitted:
22 September 2023
Posted:
25 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Hyperinsulinemia exerts a similar effect on the cell cycle dynamics in ASC and HPC
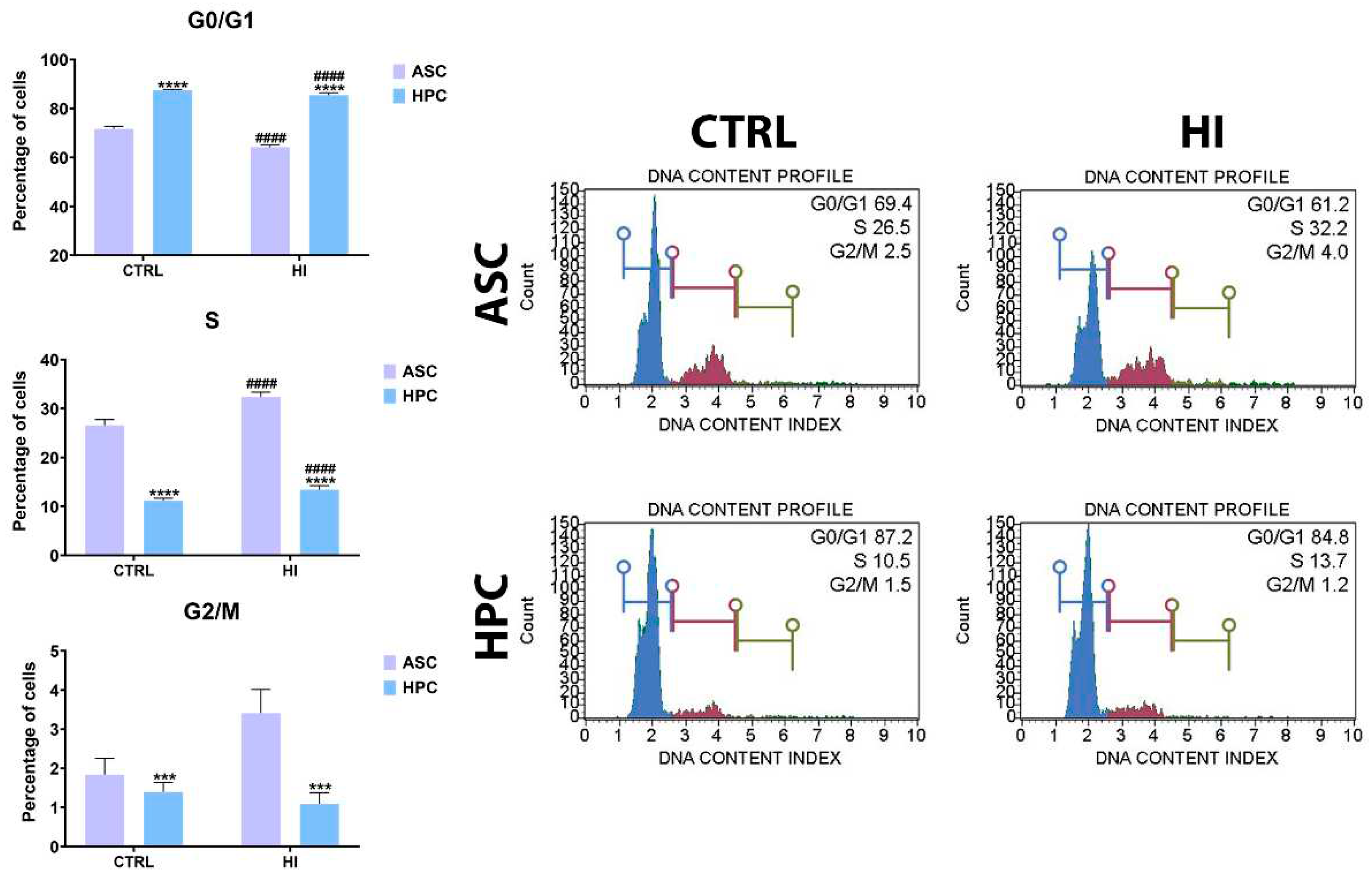
2.2. Mitochondrial membrane potential is not strongly influenced by hyperinsulinemia in ASC and HPC.
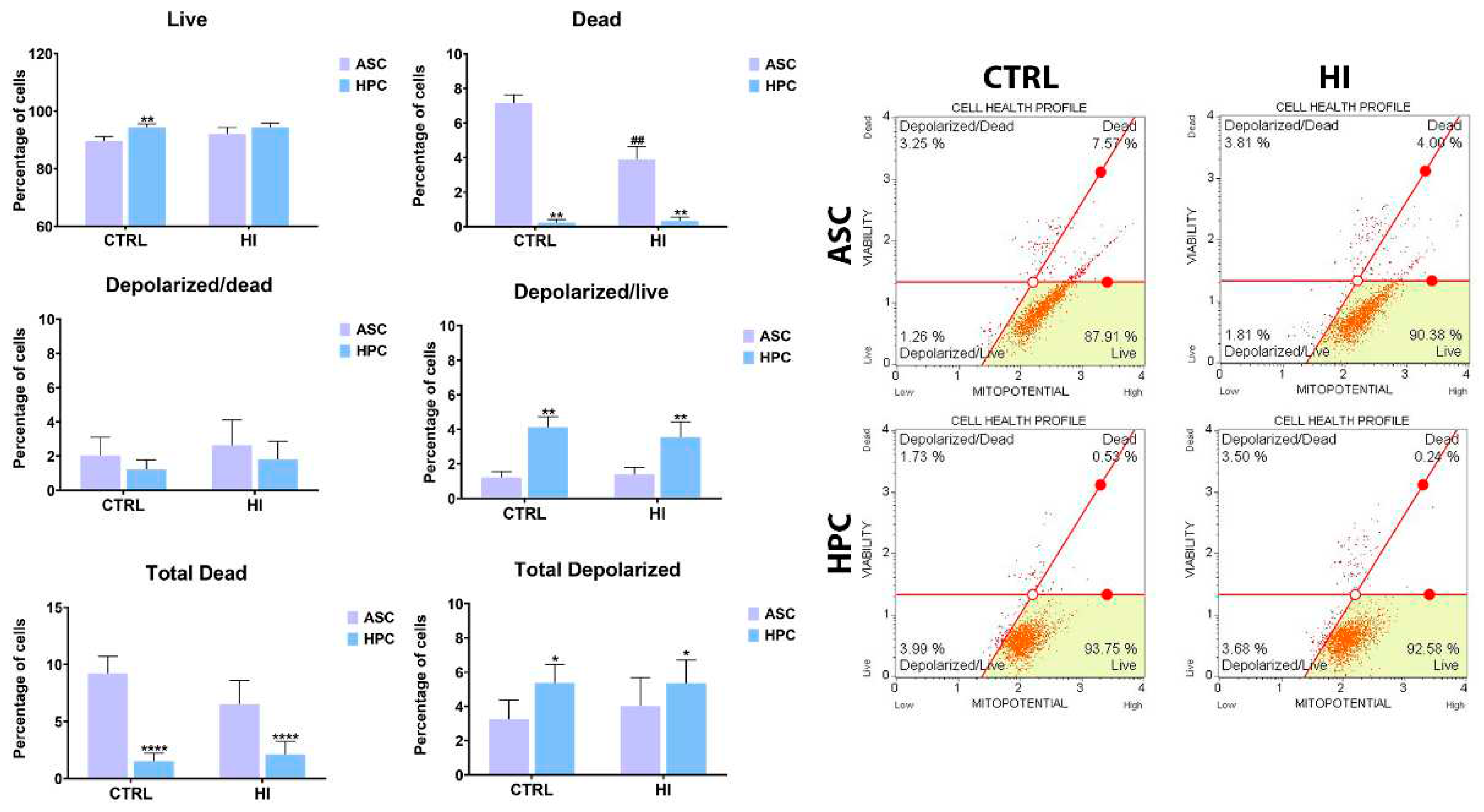
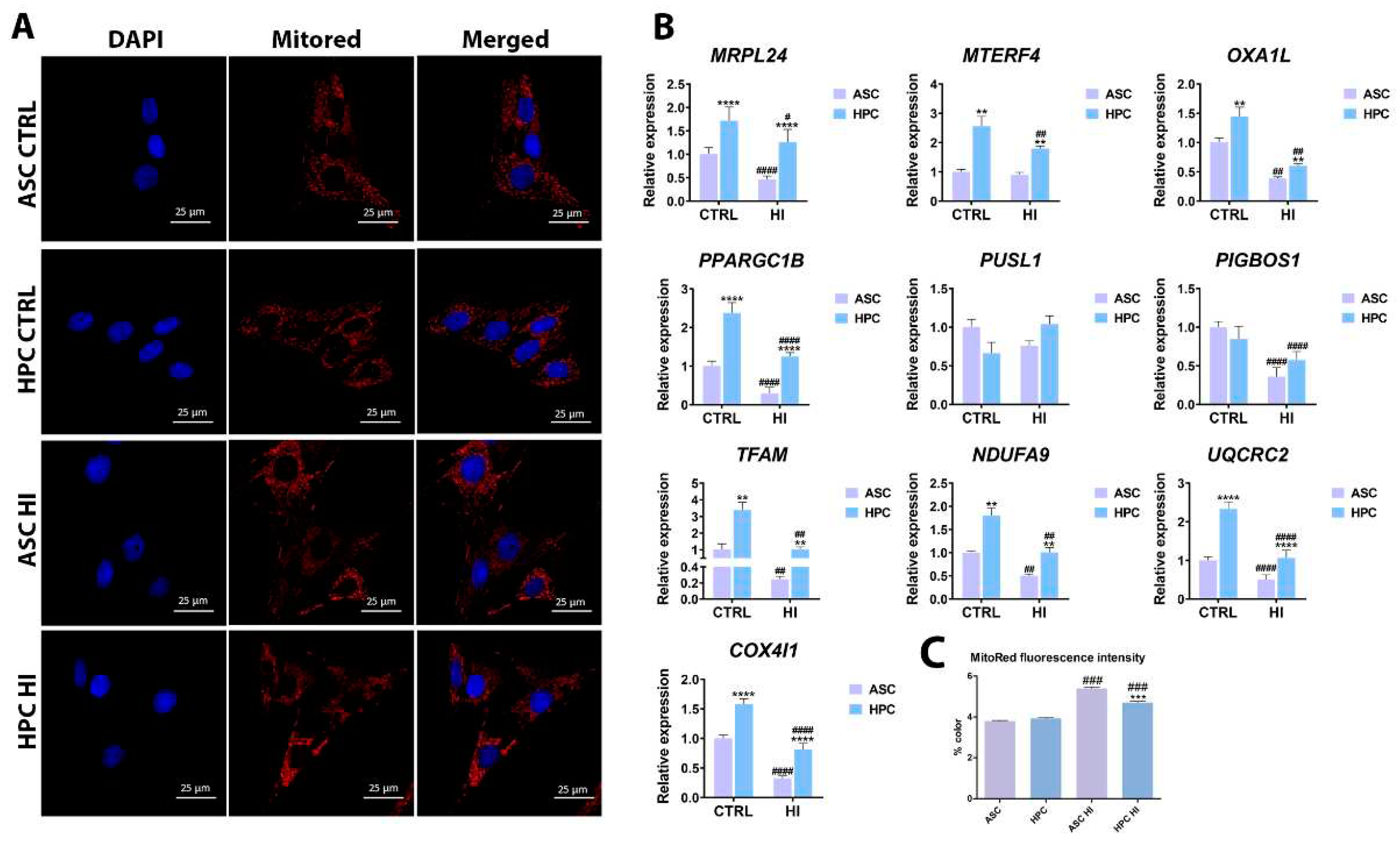
2.3. Hyperinsulinemia exerts a different effect on the mitochondrial metabolism of ASC and HPC
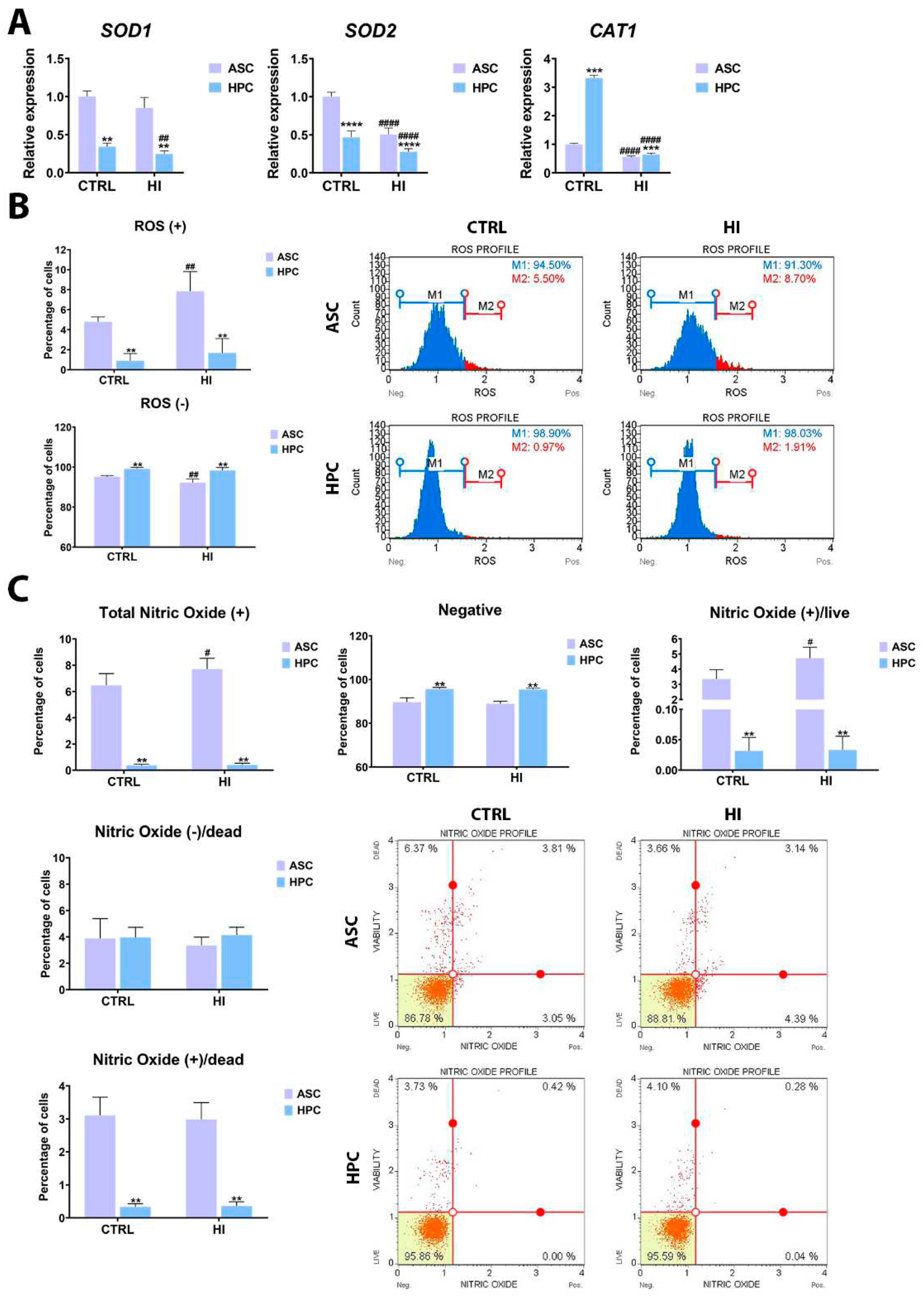
2.4. Hyperinsulinemia exerts a different effect on ASC and HPC oxidative stress levels
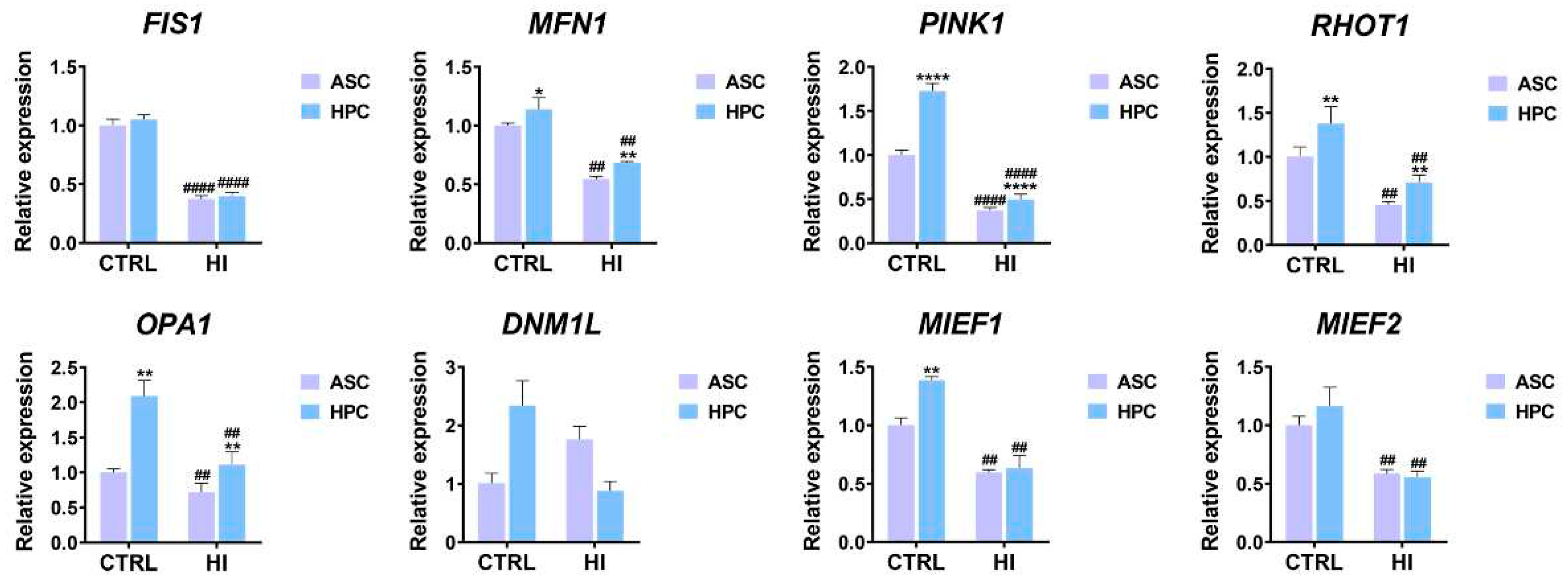
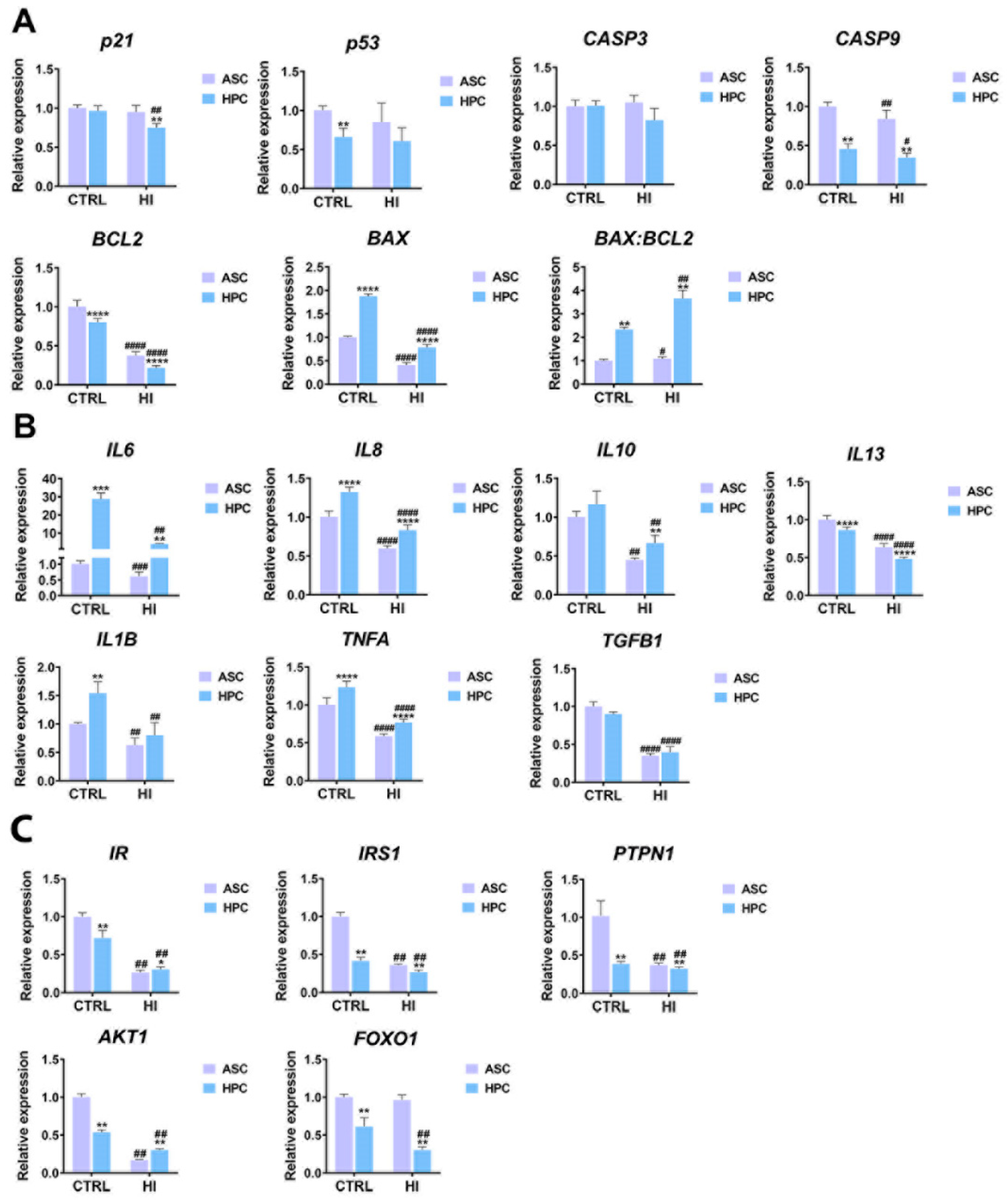
2.5. Apoptosis, immunomodulation and insulin signalling markers expression differs between ASC and HPC in standard culture conditions and hyperinsulinemia
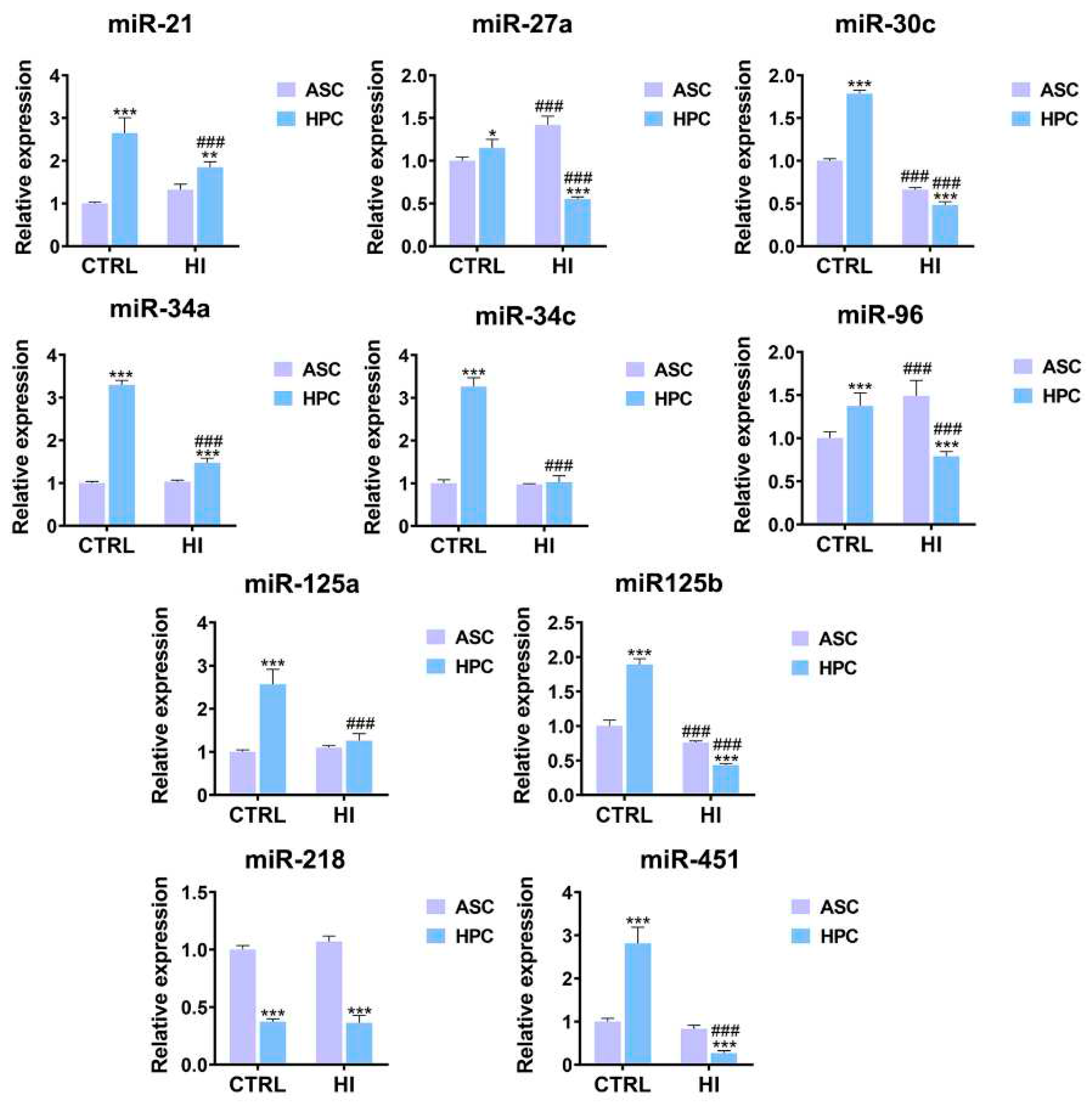
2.6. HPC and ASC are characterized by a disparate miRNome in standard culture conditions and hyperinsulinemia
3. Discussion
4. Materials and Methods
4.1. Sample Acquisition and Cell Isolation
4.2. Hyperinsulinemia model
4.3. Cell Imagining and Mitochondrial Network Visualization.
4.4. Microcapillary Flow Cytometry Analysis
4.5. RNA isolation and RT-qPCR
4.6. The isolation of mitochondria and mtRNA
4.7. Statistical analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- El-Husseiny, H.M.; Mady, E.A.; Helal, M.A.Y.; Tanaka, R. The Pivotal Role of Stem Cells in Veterinary Regenerative Medicine and Tissue Engineering. Vet. Sci. 2022, Vol. 9, Page 648 2022, 9, 648. [Google Scholar] [CrossRef]
- Chandra, V.; Mankuzhy, P.; Sharma G., T. Mesenchymal Stem Cells in Veterinary Regenerative Therapy: Basic Physiology and Clinical Applications. Curr. Stem Cell Res. Ther. 2021, 17, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Jovic, D.; Yu, Y.; Wang, D.; Wang, K.; Li, H.; Xu, F.; Liu, C.; Liu, J.; Luo, Y. A Brief Overview of Global Trends in MSC-Based Cell Therapy. Stem Cell Rev. Reports 2022 185 2022, 18, 1525–1545. [Google Scholar] [CrossRef]
- Hoang, D.M.; Pham, P.T.; Bach, T.Q.; Ngo, A.T.L.; Nguyen, Q.T.; Phan, T.T.K.; Nguyen, G.H.; Le, P.T.T.; Hoang, V.T.; Forsyth, N.R.; et al. Stem Cell-Based Therapy for Human Diseases. Signal Transduct. Target. Ther. 2022 71 2022, 7, 1–41. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem Cells: Past, Present, and Future. Stem Cell Res. Ther. 2019, 10, 1–22. [Google Scholar] [CrossRef]
- Guan, Y.T.; Xie, Y.; Li, D.S.; Zhu, Y.Y.; Zhang, X.L.; Feng, Y.L.; Chen, Y.P.; Xu, L.J.; Liao, P.F.; Wang, G. Comparison of Biological Characteristics of Mesenchymal Stem Cells Derived from the Human Umbilical Cord and Decidua Parietalis. Mol. Med. Rep. 2019, 20, 633–639. [Google Scholar] [CrossRef]
- Abu Kasim, N.H.; Govindasamy, V.; Gnanasegaran, N.; Musa, S.; Pradeep, P.J.; Srijaya, T.C.; Aziz, Z.A.C.A. Unique Molecular Signatures Influencing the Biological Function and Fate of Post-Natal Stem Cells Isolated from Different Sources. J. Tissue Eng. Regen. Med. 2015, 9, E252–E2662015. [Google Scholar] [CrossRef]
- Menicanin, D.; Bartold, P.M.; Zannettino, A.C.W.; Gronthos, S. Identification of a Common Gene Expression Signature Associated with Immature Clonal Mesenchymal Cell Populations Derived from Bone Marrow and Dental Tissues. https://home.liebertpub.com/scd 2010, 19, 1501–1510. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Horie, N.; Satoh, K.; Ishikawa, T.; Mori, T.; Maeda, H.; Fukuda, Y.; Ishizaka, S.; Hiu, T.; Morofuji, Y.; et al. Age of Donor of Human Mesenchymal Stem Cells Affects Structural andfunctional Recovery after Cell Therapy Following Ischaemic. J. Cereb. Blood Flow Metab. 2018, 38, 1199. [Google Scholar] [CrossRef]
- Oliva-Olivera, W.; Coin-Aragüez, L.; Lhamyani, S.; Clemente-Postigo, M.; Torres, J.A.; Bernal-Lopez, M.R.; El Bekay, R.; Tinahones, F.J. Adipogenic Impairment of Adipose Tissue–Derived Mesenchymal Stem Cells in Subjects With Metabolic Syndrome: Possible Protective Role of FGF2. J. Clin. Endocrinol. Metab. 2017, 102, 478–487. [Google Scholar] [CrossRef]
- Marzano, M.; Fosso, B.; Piancone, E.; Defazio, G.; Pesole, G.; De Robertis, M. Stem Cell Impairment at the Host-Microbiota Interface in Colorectal Cancer. Cancers 2021, Vol. 13, Page 996 2021, 13, 996. [Google Scholar] [CrossRef] [PubMed]
- Bogeska, R.; Mikecin, A.M.; Kaschutnig, P.; Fawaz, M.; Büchler-Schäff, M.; Le, D.; Ganuza, M.; Vollmer, A.; Paffenholz, S. V.; Asada, N.; et al. Inflammatory Exposure Drives Long-Lived Impairment of Hematopoietic Stem Cell Self-Renewal Activity and Accelerated Aging. Cell Stem Cell 2022, 29, 1273–1284.e8. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghadban, S.; Artiles, M.; Bunnell, B.A. Adipose Stem Cells in Regenerative Medicine: Looking Forward. Front. Bioeng. Biotechnol. 2022, 9, 837464. [Google Scholar] [CrossRef]
- Trzyna, A.; Bana´sbana´s-Z, A. ; Abczyk, ˛; Ong, W. K.; Sheard, J. Adipose-Derived Stem Cells Secretome and Its Potential Application in “Stem Cell-Free Therapy.” Biomol. 2021, Vol. 11, Page 878 2021, 11, 878. [Google Scholar] [CrossRef]
- Marycz, K.; Pielok, A.; Kornicka-Garbowska, K. Equine Hoof Stem Progenitor Cells (HPC) CD29 + /Nestin + /K15 + – a Novel Dermal/Epidermal Stem Cell Population With a Potential Critical Role for Laminitis Treatment. Stem Cell Rev. Reports 2021, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Marycz, K.; Weiss, C.; Śmieszek, A.; Kornicka, K. Evaluation of Oxidative Stress and Mitophagy during Adipogenic Differentiation of Adipose-Derived Stem Cells Isolated from Equine Metabolic Syndrome (EMS) Horses. Stem Cells Int. 2018, 2018. [Google Scholar] [CrossRef]
- Suagee, J.K.; Corl, B.A.; Geor, R.J. A Potential Role for Pro-Inflammatory Cytokines in the Development of Insulin Resistance in Horses. Anim. 2012, Vol. 2, Pages 243-260 2012, 2, 243–260. [Google Scholar] [CrossRef]
- Karikoski, N.P.; Horn, I.; McGowan, T.W.; McGowan, C.M. The Prevalence of Endocrinopathic Laminitis among Horses Presented for Laminitis at a First-Opinion/Referral Equine Hospital. Domest. Anim. Endocrinol. 2011, 41, 111–117. [Google Scholar] [CrossRef]
- Morgan, R.; Keen, J.; McGowan, C. Equine Metabolic Syndrome. Vet. Rec. 2015, 177, 173–179. [Google Scholar] [CrossRef]
- Frank, N.; Tadros, E.M. Insulin Dysregulation. Equine Vet. J. 2014, 46, 103–112. [Google Scholar] [CrossRef]
- de Laat, M.A.; McGree, J.M.; Sillence, M.N. Equine Hyperinsulinemia: Investigation of the Enteroinsular Axis during Insulin Dysregulation. Am. J. Physiol. - Endocrinol. Metab. 2015, 310, E61–E722015. [Google Scholar] [CrossRef] [PubMed]
- Asplin, K.E.; Sillence, M.N.; Pollitt, C.C.; McGowan, C.M. Induction of Laminitis by Prolonged Hyperinsulinaemia in Clinically Normal Ponies. Vet. J. 2007, 174, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Nanayakkara, S.N.; Rahnama, S.; Harris, P.A.; Anderson, S.T.; de Laat, M.A.; Bailey, S.; Sillence, M.N. Characterization of Insulin and IGF-1 Receptor Binding in Equine Liver and Lamellar Tissue: Implications for Endocrinopathic Laminitis. Domest. Anim. Endocrinol. 2019, 66, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Kullmann, A.; Weber, P.S.; Bishop, J.B.; Roux, T.M.; Norby, B.; Burns, T.A.; McCutcheon, L.J.; Belknap, J.K.; Geor, R.J. Equine Insulin Receptor and Insulin-like Growth Factor-1 Receptor Expression in Digital Lamellar Tissue and Insulin Target Tissues. Equine Vet. J. 2016, 48, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Serteyn, D.U. de L.-Ul.> D. clinique des animaux de compagnie et des équidés (DCA) > A. gén. et pathologie chirurg. des grds animaux; de la Rebière de Pouyade, G.U. de L.-Ul.> D. clinique des animaux de compagnie et des équidés (DCA) > A. gén. et pathologie chirurg. des grds animaux; Sandersen, C.U. de L.-Ul.> D. clinique des animaux de compagnie et des équidés (DCA) > A. et réanimation vétérinaires; Salciccia, A.U. de L.-Ul.> D. clinique des animaux de compagnie et des équidés (DCA) > D. clinique des animaux de compagnie et des équidés (DCA); Grulke, S.U. de L.-Ul.> D. clinique des animaux de compagnie et des équidés (DCA) > D. clinique des animaux de compagnie et des équidés (DCA); Mouithys-Mickalad, A.U. de L.-Ul.> C. de l’oxygène : R. et développement (C. O.R.D..; Franck, T.U. de L.-Ul.> C. de l’oxygène : R. et développement (C. O.R.D..; Lejeune, J.-P.U. de L.-Ul.> D. clinique des animaux de compagnie et des équidés (DCA) > A. gén. et pathologie chirurg. des grds animaux; Ceusters, J.U. de L.-Ul.> C. des grands animaux (chirurgie) Muscle Mitochondrial Dysfunction in Horses Affected by Acute Laminitis. Bioenergetics 2014, 03. [Google Scholar] [CrossRef]
- de Laat, M.A.; McGowan, C.M.; Sillence, M.N.; Pollitt, C.C. Equine Laminitis: Induced by 48 h Hyperinsulinaemia in Standardbred Horses. Equine Vet. J. 2010, 42, 129–135. [Google Scholar] [CrossRef]
- Pielok, A.; K˛ Epska, M.; Steczkiewicz, Z.; Grobosz, S.; Bourebaba, L.; Marycz, K. Equine Hoof Progenitor Cells Display Increased Mitochondrial Metabolism and Adaptive Potential to a Highly Pro-Inflammatory Microenvironment. Int. J. Mol. Sci. 2023, Vol. 24, Page 11446 2023, 24, 11446. [Google Scholar] [CrossRef]
- Li, P.; Wei, J.; Gao, X.; Wei, B.; Lin, H.; Huang, R.; Niu, Y.; Lim, K.; Jing, K.; Chu, J. Insulin Promotes the Proliferation of Human Umbilical Cord Matrix-Derived Mesenchymal Stem Cells by Activating the Akt-Cyclin D1 Axis. Stem Cells Int. 2017, 2017. [Google Scholar] [CrossRef]
- Brown, L.D.; Wesolowski, S.R.; Kailey, J.; Bourque, S.; Wilson, A.; Andrews, S.E.; Hay, W.W.; Rozance, P.J. Chronic Hyperinsulinemia Increases Myoblast Proliferation in Fetal Sheep Skeletal Muscle. Endocrinology 2016, 157, 2447–2460. [Google Scholar] [CrossRef]
- Zheng, X. rong; Pan, X.; Zhang, J.; Cao, X. Hyperinsulinemia-Induced PAX6 Expression Promotes Endometrial Epithelial Cell Proliferation via Negatively Modulating P27 Signaling. Biomed. Pharmacother. 2018, 97, 802–808. [Google Scholar] [CrossRef]
- Tran, T.T.; Naigamwalla, D.; Oprescu, A.I.; Lam, L.; McKeown-Eyssen, G.; Bruce, W.R.; Giacca, A. Hyperinsulinemia, But Not Other Factors Associated with Insulin Resistance, Acutely Enhances Colorectal Epithelial Proliferation in Vivo. Endocrinology 2006, 147, 1830–1837. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Li, Y.; Li, X.; Zhang, S. Hyperinsulinemia Impairs Functions of Circulating Endothelial Progenitor Cells. Acta Diabetol. 2019, 56, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, E.; Sorice, A.; Capone, F.; Stor Ti, G.; Colonna, G.; Ciliberto, G.; Costantini, S. Combining Doxorubicin with a Phenolic Extract from Flaxseed Oil: Evaluation of the Effect on Two Breast Cancer Cell Lines. Int. J. Oncol. 2017, 50, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J.; Bliek, A.M. Van Der; Complementation, F.P.; Mitochondria, B.D.; Fusion, M.; Proteins, F. REVIEW Mitochondrial Fission, Fusion, and Stress. 2012, 337, 1062–1066. [Google Scholar] [PubMed]
- Zorzano, A.; Liesa, M.; Palacin, M. Mitochondrial Dynamics as a Bridge between Mitochondrial Dysfunction and Insulin Resistance. 2009, 115, 1–12. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, H.; Deng, J.; Fan, D. Ginsenoside Rg5 Improves Insulin Resistance and Mitochondrial Biogenesis of Liver via Regulation of the Sirt1/PGC-1α Signaling Pathway in Db/Db Mice. J. Agric. Food Chem. 2021, 69, 8428–8439. [Google Scholar] [CrossRef]
- Mazibuko-Mbeje, S.E.; Mthembu, S.X.H.; Dludla, P. V.; Madoroba, E.; Chellan, N.; Kappo, A.P.; Muller, C.J.F. Antimycin A-Induced Mitochondrial Dysfunction Is Consistent with Impaired Insulin Signaling in Cultured Skeletal Muscle Cells. Toxicol. Vitr. 2021, 76, 105224. [Google Scholar] [CrossRef]
- Cooper, I.D.; Brookler, K.H.; Kyriakidou, Y.; Elliott, B.T.; Crofts, C.A.P. Metabolic Phenotypes and Step by Step Evolution of Type 2 Diabetes: A New Paradigm. Biomed. 2021, Vol. 9, Page 800 2021, 9, 800. [Google Scholar] [CrossRef]
- Bourebaba, N.; Kornicka-Garbowska, K.; Marycz, K.; Bourebaba, L.; Kowalczuk, A. Laurus Nobilis Ethanolic Extract Attenuates Hyperglycemia and Hyperinsulinemia-Induced Insulin Resistance in HepG2 Cell Line through the Reduction of Oxidative Stress and Improvement of Mitochondrial Biogenesis – Possible Implication in Pharmacotherapy. Mitochondrion 2021, 59, 190–213. [Google Scholar] [CrossRef]
- Shan, Z.; Fa, W.H.; Tian, C.R.; Yuan, C.S.; Jie, N. Mitophagy and Mitochondrial Dynamics in Type 2 Diabetes Mellitus Treatment. Aging (Albany. NY). 2022, 14, 2902–2919. [Google Scholar] [CrossRef]
- Zhou, P.; Xie, W.; Meng, X.; Zhai, Y.; Dong, X.; Zhang, X.; Sun, G.; Sun, X. Notoginsenoside R1 Ameliorates Diabetic Retinopathy through PINK1-dependent Activation of Mitophagy. Cells 2019, 8. [Google Scholar] [CrossRef]
- He, F.; Huang, Y.; Song, Z.; Zhou, H.J.; Zhang, H.; Perry, R.J.; Shulman, G.I.; Min, W. Mitophagy-Mediated Adipose Inflammation Contributes to Type 2 Diabetes with Hepatic Insulin Resistance. J. Exp. Med. 2021, 218. [Google Scholar] [CrossRef]
- Sun, D.; Wang, J.; Toan, S.; Muid, D.; Li, R.; Chang, X.; Zhou, H. Molecular Mechanisms of Coronary Microvascular Endothelial Dysfunction in Diabetes Mellitus: Focus on Mitochondrial Quality Surveillance. Angiogenesis 2022, 25, 307–329. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, Y.; Li, W.; Chen, H.; Du, L.; Liu, D.; Wang, X.; Xu, T.; Liu, L.; Chen, Q. Deficiency of Mitophagy Receptor FUNDC1 Impairs Mitochondrial Quality and Aggravates Dietary-Induced Obesity and Metabolic Syndrome. Autophagy 2019, 15, 1882–1898. [Google Scholar] [CrossRef] [PubMed]
- Scheele, C.; Nielsen, A.R.; Walden, T.B.; Sewell, D.A.; Fischer, C.P.; Brogan, R.J.; Petrovic, N.; Larsson, O.; Tesch, P.A.; Wennmalm, K.; et al. Altered Regulation of the PINK1 Locus: A Link between Type 2 Diabetes and Neurodegeneration? FASEB J. 2007, 21, 3653–3665. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.A.; Fumoto, T.; Iwai, K.; Takeshita, S.; Ito, M.; Shimohata, N.; Aburatani, H.; Taketani, S.; Lelliott, C.J.; Vidal-Puig, A.; et al. Coordination of PGC-1β and Iron Uptake in Mitochondrial Biogenesis and Osteoclast Activation. Nat. Med. 2009 153 2009, 15, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Puig, A.J. Metabolic Characterisation of PGC1b Ko Mice. FASEB J. 2007, 21, A91–A912007. [Google Scholar] [CrossRef]
- Sparks, L.M.; Xie, H.; Koza, R.A.; Mynatt, R.; Hulver, M.W.; Bray, G.A.; Smith, S.R. A High-Fat Diet Coordinately Downregulates Genes Required for Mitochondrial Oxidative Phosphorylation in Skeletal Muscle. Diabetes 2005, 54, 1926–1933. [Google Scholar] [CrossRef]
- Miao, H.; Ou, J.; Ma, Y.; Guo, F.; Yang, Z.; Wiggins, M.; Liu, C.; Song, W.; Han, X.; Wang, M.; et al. Macrophage CGI-58 Deficiency Activates ROS-Inflammasome Pathway to Promote Insulin Resistance in Mice. Cell Rep. 2014, 7, 223–235. [Google Scholar] [CrossRef]
- Lopez Sanchez, M.I.G.; Krüger, A.; Shiriaev, D.I.; Liu, Y.; Rorbach, J. Human Mitoribosome Biogenesis and Its Emerging Links to Disease. Int. J. Mol. Sci. 2021, 22. [Google Scholar] [CrossRef]
- Stiburek, L.; Fornuskova, D.; Wenchich, L.; Pejznochova, M.; Hansikova, H.; Zeman, J. Knockdown of Human Oxa1l Impairs the Biogenesis of F1Fo-ATP Synthase and NADH:Ubiquinone Oxidoreductase. J. Mol. Biol. 2007, 374, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Liang, Y.; Zhuang, Y.; Yuan, Z. Identification of MiRNA-MRNA Regulatory Networks Associated with Diabetic Retinopathy Using Bioinformatics Analysis. Endocr. Metab. Immune Disord. Drug Targets 2023, 23. [Google Scholar] [CrossRef] [PubMed]
- Matyal, R.; Sakamuri, S.; Huang, T.; Owais, K.; Parikh, S.; Khabbaz, K.; Wang, A.; Sellke, F.; Mahmood, F. Oxidative Stress and Nerve Function after Cardiopulmonary Bypass in Patients with Diabetes. Ann. Thorac. Surg. 2014, 98, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- Chu, Q.; Martinez, T.F.; Novak, S.W.; Donaldson, C.J.; Tan, D.; Vaughan, J.M.; Chang, T.; Diedrich, J.K.; Andrade, L.; Kim, A.; et al. Regulation of the ER Stress Response by a Mitochondrial Microprotein. Nat. Commun. 2019 101 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Lee, K.-U.; Harris, R.A.; Lee, I.-K.; Roy, S. Mitochondria and Endoplasmic Reticulum in Diabetes and Its Complications Experimental Diabetes Research.
- Abhijit, S.; Bhaskaran, R.; Narayanasamy, A.; Chakroborty, A.; Manickam, N.; Dixit, M.; Mohan, V.; Balasubramanyam, M. Hyperinsulinemia-Induced Vascular Smooth Muscle Cell (VSMC) Migration and Proliferation Is Mediated by Converging Mechanisms of Mitochondrial Dysfunction and Oxidative Stress. Mol. Cell. Biochem. 2013, 373, 95–105. [Google Scholar] [CrossRef]
- Liu, H.Y.; Hong, T.; Wen, G.B.; Han, J.; Zuo, D.; Liu, Z.; Cao, W. Increased Basal Level of Akt-Dependent Insulin Signaling May Be Responsible for the Development of Insulin Resistance. Am. J. Physiol. - Endocrinol. Metab. 2009, 297, 898–906. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef]
- Roberts, C.K.; Barnard, R.J.; Sindhu, R.K.; Jurczak, M.; Ehdaie, A.; Vaziri, N.D. Oxidative Stress and Dysregulation of NAD(P)H Oxidase and Antioxidant Enzymes in Diet-Induced Metabolic Syndrome. Metabolism 2006, 55, 928–934. [Google Scholar] [CrossRef]
- Rains, J.L.; Jain, S.K. Oxidative Stress, Insulin Signaling, and Diabetes. Free Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef]
- Turina, M.; Miller, F.N.; Tucker, C.; Polk, H.C. Effects of Hyperglycemia, Hyperinsulinemia, and Hyperosmolarity on Neutrophil Apoptosis. https://home.liebertpub.com/sur 2006, 7, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.R.; Sun, Z.J.; Hu, G.H.; Wang, R.H. High Concentration of Insulin Promotes Apoptosis of Primary Cultured Rat Ovarian Granulosa Cells Via Its Increase in Extracellular HMGB1. 2014, 22, 271–277. [Google Scholar] [CrossRef]
- Sifuentes-Franco, S.; Padilla-Tejeda, D.E.; Carrillo-Ibarra, S.; Miranda-Díaz, A.G. Oxidative Stress, Apoptosis, and Mitochondrial Function in Diabetic Nephropathy. Int. J. Endocrinol. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.A.; Blue, R.E.; Andres, S.F.; Mah, A.T.; Van Landeghem, L.; Lund, P.K. Obesity and Intestinal Epithelial Deletion of the Insulin Receptor, but Not the IGF 1 Receptor, Affect Radiation-Induced Apoptosis in Colon. Am. J. Physiol. - Gastrointest. Liver Physiol. 2015, 309, G578–G5892015. [Google Scholar] [CrossRef]
- Di Sebastiano, K.M.; Pinthus, J.H.; Duivenvoorden, W.C.M.; Mourtzakis, M. Glucose Impairments and Insulin Resistance in Prostate Cancer: The Role of Obesity, Nutrition and Exercise. Obes. Rev. 2018, 19, 1008–1016. [Google Scholar] [CrossRef]
- Püschel, G.P.; Klauder, J.; Henkel, J. Macrophages, Low-Grade Inflammation, Insulin Resistance and Hyperinsulinemia: A Mutual Ambiguous Relationship in the Development of Metabolic Diseases. J. Clin. Med. 2022, Vol. 11, Page 4358 2022, 11, 4358. [Google Scholar] [CrossRef]
- Ruge, T.; Lockton, J.A.; Renstrom, F.; Lystig, T.; Sukonina, V.; Svensson, M.K.; Eriksson, J.W. Acute Hyperinsulinemia Raises Plasma Interleukin-6 in Both Nondiabetic and Type 2 Diabetes Mellitus Subjects, and This Effect Is Inversely Associated with Body Mass Index. Metabolism 2009, 58, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Watts, M.R.; Hegedus, O.C.; Eades, S.C.; Belknap, J.K.; Burns, T.A. Association of Sustained Supraphysiologic Hyperinsulinemia and Inflammatory Signaling within the Digital Lamellae in Light-Breed Horses. J. Vet. Intern. Med. 2019, 33, 1483–1492. [Google Scholar] [CrossRef]
- Straczkowski, M.; Dzienis-Straczkowska, S.; Stêpieñ, A.; Kowalska, I.; Szelachowska, M.; Kinalska, I. Plasma Interleukin-8 Concentrations Are Increased in Obese Subjects and Related to Fat Mass and Tumor Necrosis Factor-α System. J. Clin. Endocrinol. Metab. 2002, 87, 4602–4606. [Google Scholar] [CrossRef]
- Zozulinska, D.; Majchrzak, A.; Sobieska, M.; Wiktorowicz, K.; Wierusz-Wysocka, B. Serum Interleukin-8 Level Is Increased in Diabetic Patients [1]. Diabetologia 1999, 42, 117–118. [Google Scholar] [CrossRef]
- van Niekerk, G.; Christowitz, C.; Conradie, D.; Engelbrecht, A.M. Insulin as an Immunomodulatory Hormone. Cytokine Growth Factor Rev. 2020, 52, 34–44. [Google Scholar] [CrossRef]
- Zhu, Z.; Hu, T.; Wang, Z.; Wang, J.; Liu, R.; Yang, Q.; Zhang, X.; Xiong, Y. Anti-Inflammatory and Organ Protective Effect of Insulin in Scalded MODS Rats without Controlling Hyperglycemia. Am. J. Emerg. Med. 2018, 36, 202–207. [Google Scholar] [CrossRef]
- Leffler, M.; Hrach, T.; Stuerzl, M.; Horch, R.E.; Herndon, D.N.; Jeschke, M.G. Insulin Attenuates Apoptosis and Exerts Anti-Inflammatory Effects in Endotoxemic Human Macrophages. J. Surg. Res. 2007, 143, 398–406. [Google Scholar] [CrossRef]
- Brix-Christensen, V.; Andersen, S.K.; Andersen, R.; Mengel, A.; Dyhr, T.; Andersen, N.T.; Larsson, A.; Schmitz, O.; Ørskov, H.; Tønnesen, E. Acute Hyperinsulinemia Restrains Endotoxin-Induced Systemic Inflammatory ResponseAn Experimental Study in a Porcine Model. Anesthesiology 2004, 100, 861–870. [Google Scholar] [CrossRef]
- Van Exel, E.; Gussekloo, J.; De Craen, A.J.M.; Frölich, M.; Wiel, A.B. Van Der; Westendorp, R.G.J. Low Production Capacity of Interleukin-10 Associates with the Metabolic Syndrome and Type 2 Diabetes: The Leiden 85-plus Study. Diabetes 2002, 51, 1088–1092. [Google Scholar] [CrossRef]
- Jiang, L.Q.; Franck, N.; Egan, B.; Sjögren, R.J.O.; Katayama, M.; Duque-Guimaraes, D.; Arner, P.; Zierath, J.R.; Krook, A. Autocrine Role of Interleukin-13 on Skeletal Muscle Glucose Metabolism in Type 2 Diabetic Patients Involves MicroRNA Let-7. Am. J. Physiol. - Endocrinol. Metab. 2013, 305, 1359–1366. [Google Scholar] [CrossRef]
- Martínez-Reyes, C.P.; Gómez-Arauz, A.Y.; Torres-Castro, I.; Manjarrez-Reyna, A.N.; Palomera, L.F.; Olivos-García, A.; Mendoza-Tenorio, E.; Sánchez-Medina, G.A.; Islas-Andrade, S.; Melendez-Mier, G.; et al. Serum Levels of Interleukin-13 Increase in Subjects with Insulin Resistance but Do Not Correlate with Markers of Low-Grade Systemic Inflammation. J. Diabetes Res. 2018, 2018. [Google Scholar] [CrossRef]
- Calera, M.R.; Vallega, G.; Pilch, P.F. Dynamics of Protein-Tyrosine Phosphatases in Rat Adipocytes. J. Biol. Chem. 2000, 275, 6308–6312. [Google Scholar] [CrossRef]
- Nakae, J.; Kitamura, T.; Kitamura, Y.; Biggs, W.H.; Arden, K.C.; Accili, D. The Forkhead Transcription Factor Fox01 Regulates Adipocyte Differentiation. Dev. Cell 2003, 4, 119–129. [Google Scholar] [CrossRef]
- Tsuchida, A.; Yamauchi, T.; Ito, Y.; Hada, Y.; Maki, T.; Takekawa, S.; Kamon, J.; Kobayashi, M.; Suzuki, R.; Hara, K.; et al. Insulin/Foxo1 Pathway Regulates Expression Levels of Adiponectin Receptors and Adiponectin Sensitivity. J. Biol. Chem. 2004, 279, 30817–30822. [Google Scholar] [CrossRef]
- Okabayashi, Y.; Maddux, B.A.; McDonald, A.R.; Logsdon, C.D.; Williams, J.A.; Goldfine, I.D. Mechanisms of Inslulin-Induced Insulin-Receptor Downregulation. Decrease of Receptor Biosynthesis and MRNA Levels. Diabetes 1989, 38, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Cen, H.H.; Hussein, B.; Botezelli, J.D.; Wang, S.; Zhang, J.A.; Noursadeghi, N.; Jessen, N.; Rodrigues, B.; Timmons, J.A.; Johnson, J.D. Human and Mouse Muscle Transcriptomic Analyses Identify Insulin Receptor MRNA Downregulation in Hyperinsulinemia-Associated Insulin Resistance. FASEB J. 2022, 36. [Google Scholar] [CrossRef] [PubMed]
- Mayer, C.M.; Belsham, D.D. Central Insulin Signaling Is Attenuated by Long-Term Insulin Exposure via Insulin Receptor Substrate-1 Serine Phosphorylation, Proteasomal Degradation, and Lysosomal Insulin Receptor Degradation. Endocrinology 2010, 151, 75–84. [Google Scholar] [CrossRef]
- Durham, A.E.; Frank, N.; McGowan, C.M.; Menzies-Gow, N.J.; Roelfsema, E.; Vervuert, I.; Feige, K.; Fey, K. ECEIM Consensus Statement on Equine Metabolic Syndrome. J. Vet. Intern. Med. 2019, 33, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, C.; Holmes, E.; Beadle, R.; Kearney, M.; Eades, S. Comparison of Insulin-Induced Digital Vessel Ring Responses of Laminitic and Clinically Healthy Horses. J. Equine Vet. Sci. 2014, 34, 998–1002. [Google Scholar] [CrossRef]
- Carter, R.A.; Treiber, K.H.; Geor, R.J.; Douglass, L.; Harris, P.A. Prediction of Incipient Pasture-Associated Laminitis from Hyperinsulinaemia, Hyperleptinaemia and Generalised and Localised Obesity in a Cohort of Ponies. Equine Vet. J. 2009, 41, 171–178. [Google Scholar] [CrossRef]
- Martin, E. C. , Qureshi, A. T., Dasa, V., Freitas, M. A., Gimble, J. M., & Davis, T.A. MicroRNA Regulation of Stem Cell Differentiation and Diseases of the Bone and Adipose Tissue. Biochimie. 2014, 124, 98–111. [Google Scholar] [CrossRef]
- Włodarski, A.; Strycharz, J.; Wróblewski, A.; Kasznicki, J.; Drzewoski, J.; Śliwińska, A. The Role of MicroRNAs in Metabolic Syndrome-Related Oxidative Stress. Int. J. Mol. Sci. 2020, Vol. 21, Page 6902 2020, 21, 6902. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, L.; Xing, L.; Chen, D. MicroRNA-204 Regulates Runx2 Protein Expression and Mesenchymal Progenitor Cell Differentiation. Stem Cells 2010, 28, 357–364. [Google Scholar] [CrossRef]
- Xu, S.; Cecilia Santini, G.; De Veirman, K.; Vande Broek, I.; Leleu, X.; De Becker, A.; Van Camp, B.; Vanderkerken, K.; Van Riet, I. Upregulation of MiR-135b Is Involved in the Impaired Osteogenic Differentiation of Mesenchymal Stem Cells Derived from Multiple Myeloma Patients. PLoS One 2013, 8, e797522013–8. [Google Scholar] [CrossRef]
- Tay, Y.; Zhang, J.; Thomson, A.M.; Lim, B.; Rigoutsos, I. MicroRNAs to Nanog, Oct4 and Sox2 Coding Regions Modulate Embryonic Stem Cell Differentiation. Nat. 2008 4557216 2008, 455, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Marson, A.; Levine, S.S.; Cole, M.F.; Frampton, G.M.; Brambrink, T.; Johnstone, S.; Guenther, M.G.; Johnston, W.K.; Wernig, M.; Newman, J.; et al. Connecting MicroRNA Genes to the Core Transcriptional Regulatory Circuitry of Embryonic Stem Cells. Cell 2008, 134, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, Y.; Yagi, K.; Tokuzawa, Y.; Kanesaki-Yatsuka, Y.; Suda, T.; Katagiri, T.; Fukuda, T.; Maruyama, M.; Okuda, A.; Amemiya, T.; et al. MiR-125b Inhibits Osteoblastic Differentiation by down-Regulation of Cell Proliferation. Biochem. Biophys. Res. Commun. 2008, 368, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Boissart, C.; Nissan, X.; Giraud-Triboult, K.; Peschanski, M.; Benchoua, A. MiR-125 Potentiates Early Neural Specification of Human Embryonic Stem Cells. Development 2012, 139, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.S.; Xu, Q. Neuronal Differentiation of Human Mesenchymal Stem Cells Using Exosomes Derived from Differentiating Neuronal Cells. PLoS One 2015, 10. [Google Scholar] [CrossRef]
- Zhang, L.; Stokes, N.; Polak, L.; Fuchs, E. Specific MicroRNAs Are Preferentially Expressed by Skin Stem Cells to Balance Self-Renewal and Early Lineage Commitment. Cell Stem Cell 2011, 8, 294–308. [Google Scholar] [CrossRef]
- Ma, S.; Wang, D.D.; Ma, C.Y.; Zhang, Y.D. MicroRNA-96 Promotes Osteoblast Differentiation and Bone Formation in Ankylosing Spondylitis Mice through Activating the Wnt Signaling Pathway by Binding to SOST. J. Cell. Biochem. 2019, 120, 15429–15442. [Google Scholar] [CrossRef]
- Karvande, A.; Kushwaha, P.; Ahmad, N.; Adhikary, S.; Kothari, P.; Tripathi, A.K.; Khedgikar, V.; Trivedi, R. Glucose Dependent MiR-451a Expression Contributes to Parathyroid Hormone Mediated Osteoblast Differentiation. Bone 2018, 117, 98–115. [Google Scholar] [CrossRef]
- Shi, L.; Feng, L.; Liu, Y.; Duan, J. qiang; Lin, W. ping; Zhang, J. fang; Li, G. MicroRNA-218 Promotes Osteogenic Differentiation of Mesenchymal Stem Cells and Accelerates Bone Fracture Healing. Calcif. Tissue Int. 2018, 103, 227–236. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, N.; Chen, T.; Chen, W.; Kong, J.; Zheng, W.; Ruan, J. Triptolide Suppressed the Microglia Activation to Improve Spinal Cord Injury Through MiR-96/IKKβ/NF-ΚB Pathway. Spine (Phila. Pa. 1976). 2019, 44, E707–E7142019. [Google Scholar] [CrossRef]
- Wu, P.; Cao, Y.; Zhao, R.; Wang, Y. MiR-96-5p Regulates Wound Healing by Targeting BNIP3/FAK Pathway. J. Cell. Biochem. 2019, 120, 12904–12911. [Google Scholar] [CrossRef]
- Uwiera, R.R.E.; Egyedy, A.F.; Ametaj, B.N. Laminitis: A Multisystems Veterinary Perspective with Omics Technologies. Periparturient Dis. Dairy Cows A Syst. Biol. Approach 2017, 185–200. [Google Scholar] [CrossRef]
- Mobasheri, A.; Critchlow, K.; Clegg, P.D.; Carter, S.D.; Canessa, C.M. Chronic Equine Laminitis Is Characterised by Loss of GLUT1, GLUT4 and ENaC Positive Laminar Keratinocytes. Equine Vet. J. 2004, 36, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xu, Z.; Shao, J.; Fu, P.; Wu, H. MicroRNA-218 Promotes Early Chondrogenesis of Mesenchymal Stem Cells and Inhibits Later Chondrocyte Maturation. BMC Biotechnol. 2019, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Peng, R.; Peng, H.; Liu, H.; Wen, L.; Wu, T.; Yi, H.; Li, A.; Zhang, Z. MiR-451 Suppresses the NF-KappaB-Mediated Proinflammatory Molecules Expression through Inhibiting LMP7 in Diabetic Nephropathy. Mol. Cell. Endocrinol. 2016, 433, 75–86. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, H. MiR-451 Elevation Relieves Inflammatory Pain by Suppressing Microglial Activation-Evoked Inflammatory Response via Targeting TLR4. Cell Tissue Res. 2018, 374, 487–495. [Google Scholar] [CrossRef]
- Choi, Y.J.; Lin, C.P.; Ho, J.J.; He, X.; Okada, N.; Bu, P.; Zhong, Y.; Kim, S.Y.; Bennett, M.J.; Chen, C.; et al. MiR-34 MiRNAs Provide a Barrier for Somatic Cell Reprogramming. Nat. Cell Biol. 2011 1311 2011, 13, 1353–1360. [Google Scholar] [CrossRef]
- Alvarez, M.L.; Khosroheidari, M.; Eddy, E.; Done, S.C. MicroRNA-27a Decreases the Level and Efficiency of the LDL Receptor and Contributes to the Dysregulation of Cholesterol Homeostasis. Atherosclerosis 2015, 242, 595–604. [Google Scholar] [CrossRef]
- Karolina, D.S.; Tavintharan, S.; Armugam, A.; Sepramaniam, S.; Pek, S.L.T.; Wong, M.T.K.; Lim, S.C.; Sum, C.F.; Jeyaseelan, K. Circulating MiRNA Profiles in Patients with Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2012, 97, E2271–E22762012. [Google Scholar] [CrossRef]
- Yao, F.; Yu, Y.; Feng, L.; Li, J.; Zhang, M.; Lan, X.; Yan, X.; Liu, Y.; Guan, F.; Zhang, M.; et al. Adipogenic MiR-27a in Adipose Tissue Upregulates Macrophage Activation via Inhibiting PPARγ of Insulin Resistance Induced by High-Fat Diet-Associated Obesity. Exp. Cell Res. 2017, 355, 105–112. [Google Scholar] [CrossRef]
- Bridge, G.; Monteiro, R.; Henderson, S.; Emuss, V.; Lagos, D.; Georgopoulou, D.; Patient, R.; Boshoff, C. The MicroRNA-30 Family Targets DLL4 to Modulate Endothelial Cell Behavior during Angiogenesis. Blood 2012, 120, 5063–5072. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Fukuda, D.; Koga, J.I.; Aikawa, M. Delta-Like Ligand 4-Notch Signaling in Macrophage Activation. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2038–2047. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Sun, L.; Lodish, H.F. Targeting MicroRNAs in Obesity. Expert Opin. Ther. Targets 2009, 13, 1227–1238. [Google Scholar] [CrossRef] [PubMed]
- Purvis, N.; Kumari, S.; Chandrasekera, D.; Bellae Papannarao, J.; Gandhi, S.; van Hout, I.; Coffey, S.; Bunton, R.; Sugunesegran, R.; Parry, D.; et al. Diabetes Induces Dysregulation of MicroRNAs Associated with Survival, Proliferation and Self-Renewal in Cardiac Progenitor Cells. Diabetologia 2021, 64, 1422–1435. [Google Scholar] [CrossRef]
- Ling, H.Y.; Hu, B.; Hu, X.B.; Zhong, J.; Feng, S.D.; Qin, L.; Liu, G.; Wen, G.B.; Liao, D.F. MiRNA-21 Reverses High Glucose and High Insulin Induced Insulin Resistance in 3T3-L1 Adipocytes through Targeting Phosphatase and Tensin Homologue. Exp. Clin. Endocrinol. Diabetes 2012, 120, 553–559. [Google Scholar] [CrossRef]
- Jeong Kim, Y.; Jin Hwang, S.; Chan Bae, Y.; Sup Jung, J. MiR-21 Regulates Adipogenic Differentiation through the Modulation of TGF-β Signaling in Mesenchymal Stem Cells Derived from Human Adipose Tissue. Stem Cells 2009, 27, 3093–3102. [Google Scholar] [CrossRef]
- Yang, Q.; Pinto, V.M.R.; Duan, W.; Paxton, E.E.; Dessauer, J.H.; Ryan, W.; Lopez, M.J. In Vitro Characteristics of Heterogeneous Equine Hoof Progenitor Cell Isolates. Front. Bioeng. Biotechnol. 2019, 7, 155. [Google Scholar] [CrossRef]
- Marędziak, M.; Marycz, K.; Lewandowski, D.; Siudzińska, A.; Śmieszek, A. Static Magnetic Field Enhances Synthesis and Secretion of Membrane-Derived Microvesicles (MVs) Rich in VEGF and BMP-2 in Equine Adipose-Derived Stromal Cells (EqASC)—a New Approach in Veterinary Regenerative Medicine. Vitr. Cell. Dev. Biol. - Anim. 2015, 51, 230–240. [Google Scholar] [CrossRef]
- CHOMZYNSKI, P. Single-Step Method of RNA Isolation by Acid Guanidinium Thiocyanate–Phenol–Chloroform Extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Mularczyk, M.; Bourebaba, N.; Marycz, K.; Bourebaba, L. Astaxanthin Carotenoid Modulates Oxidative Stress in Adipose-Derived Stromal Cells Isolated from Equine Metabolic Syndrome Affected Horses by Targeting Mitochondrial Biogenesis. Biomol. 2022, Vol. 12, Page 1039 2022, 12, 1039. [Google Scholar] [CrossRef]
- Suszynska, M.; Poniewierska-Baran, A.; Gunjal, P.; Ratajczak, J.; Marycz, K.; Kakar, S.S.; Kucia, M.; Ratajczak, M.Z. Expression of the Erythropoietin Receptor by Germline-Derived Cells - Further Support for a Potential Developmental Link between the Germline and Hematopoiesis. J. Ovarian Res. 2014, 7, 66. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




