Submitted:
29 June 2023
Posted:
04 July 2023
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Definition of Long Covid
3. Prevalence of Long Covid
4. Clinical Symptoms & Syndromes
4.1. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS)
4.2. Cognitive disorders
5. The potential role of hypothalamic phospholipid liposomes in Long Covid
5.1. Pathophysiological mechanisms in Long-Covid and the pharmacology of hypothalamic phospholipid liposomes
5.1.1. The monoaminergic hypothesis
5.1.2. Neuroinflammation, demyelination and impaired neurogenesis
5.1.3. Cerebral hypometabolism
5.1.4. Male fertility alterations
5.2. Clinical evidence on hypothalamic phospholipid liposomes and its implications for Long Covid
| Long Covid | Hypothalamic phospholipid liposomes |
|---|---|
| Pathophysiology | Mechanism of action |
| Hypometabolic activity in certain brain areas [52] | Activation of cerebral metabolism (i.e., increased brain glucose content and phospholipid synthesis) [28] |
| ACE2-Dopa Decarboxylase co-expression which leads to impaired monoaminergic neurotransmission [30] | Increased catecholamine turnover and release, stimulation of tyrosine hydroxylase and dopamine dependent adenylyl cyclase, modification of monoaminergic receptor adaptation [28,29] |
| Neuroinflammation from CSF cytokine elevation (e.g., IL-1β, IL-6) and microglial reactivity [15,42,44] | Antagonizing effect on proinflammatory cytokines (IL-1β, IL-6, TNF-α) in different brain areas [28] |
| Demyelination and impaired neurogenesis [16,42] | Neurotrophic effect, increase in neurogenesis and dendritogenesis, as well as antagonizing effect of PE, PC and PS on demyelination [29,46,47] |
| Low testosterone [53,55] | PS increases plasma levels of testosterone compared to placebo and the testosterone to cortisol ratio in an exercise-related context [56,57] |
| Clinical manifestations | Clinical evidence |
| Fatigue | Improvement of asthenia [61,62] |
| Brain fog | PS: |
| Anxiety and depression | Improvement in the symptomatology of anxiety and depression as monotherapy or add-on to antidepressants [28,29] |
| Orthostatic intolerance | Antagonizing effect on hypotension and reflex tachycardia caused by trazodone [61] |
| Male sexual health problem | Phospholipids (PC in particular) improve erectile dysfunction and loss of libido [58] |
5. Conclusions
Conflicts of Interest
References
- Nurek, M.; Rayner, C.; Freyer, A.; Taylor, S.; Järte, L.; MacDermott, N.; Delaney, B. C. Recommendations for the Recognition, Diagnosis, and Management of Long COVID: A Delphi Study. Br J Gen Pract 2021, 71 (712), e815–e825. https://doi.org/10.3399/BJGP.2021.0265. [CrossRef]
- Soriano, J. B.; Murthy, S.; Marshall, J. C.; Relan, P.; Diaz, J. V. A Clinical Case Definition of Post-COVID-19 Condition by a Delphi Consensus. The Lancet Infectious Diseases 2022, 22 (4), e102–e107. https://doi.org/10.1016/S1473-3099(21)00703-9. [CrossRef]
- Montani, D.; Savale, L.; Noel, N.; Meyrignac, O.; Colle, R.; Gasnier, M.; Corruble, E.; Beurnier, A.; Jutant, E.-M.; Pham, T.; Lecoq, A.-L.; Papon, J.-F.; Figueiredo, S.; Harrois, A.; Humbert, M.; Monnet, X. Post-Acute COVID-19 Syndrome. Eur Respir Rev 2022, 31 (163), 210185. https://doi.org/10.1183/16000617.0185-2021. [CrossRef]
- Davis, H. E.; McCorkell, L.; Vogel, J. M.; Topol, E. J. Long COVID: Major Findings, Mechanisms and Recommendations. Nat Rev Microbiol 2023, 21 (3), 133–146. https://doi.org/10.1038/s41579-022-00846-2. [CrossRef]
- Subramanian, A.; Nirantharakumar, K.; Hughes, S.; Myles, P.; Williams, T.; Gokhale, K. M.; Taverner, T.; Chandan, J. S.; Brown, K.; Simms-Williams, N.; Shah, A. D.; Singh, M.; Kidy, F.; Okoth, K.; Hotham, R.; Bashir, N.; Cockburn, N.; Lee, S. I.; Turner, G. M.; Gkoutos, G. V.; Aiyegbusi, O. L.; McMullan, C.; Denniston, A. K.; Sapey, E.; Lord, J. M.; Wraith, D. C.; Leggett, E.; Iles, C.; Marshall, T.; Price, M. J.; Marwaha, S.; Davies, E. H.; Jackson, L. J.; Matthews, K. L.; Camaradou, J.; Calvert, M.; Haroon, S. Symptoms and Risk Factors for Long COVID in Non-Hospitalized Adults. Nat Med 2022, 28 (8), 1706–1714. https://doi.org/10.1038/s41591-022-01909-w. [CrossRef]
- Lam, I. C. H.; Wong, C. K. H.; Zhang, R.; Chui, C. S. L.; Lai, F. T. T.; Li, X.; Chan, E. W. Y.; Luo, H.; Zhang, Q.; Man, K. K. C.; Cheung, B. M. Y.; Tang, S. C. W.; Lau, C. S.; Wan, E. Y. F.; Wong, I. C. K. Long-Term Post-Acute Sequelae of COVID-19 Infection: A Retrospective, Multi-Database Cohort Study in Hong Kong and the UK. eClinicalMedicine 2023, 60, 102000. https://doi.org/10.1016/j.eclinm.2023.102000. [CrossRef]
- Mizrahi, B.; Sudry, T.; Flaks-Manov, N.; Yehezkelli, Y.; Kalkstein, N.; Akiva, P.; Ekka-Zohar, A.; Ben David, S. S.; Lerner, U.; Bivas-Benita, M.; Greenfeld, S. Long Covid Outcomes at One Year after Mild SARS-CoV-2 Infection: Nationwide Cohort Study. BMJ 2023, e072529. https://doi.org/10.1136/bmj-2022-072529. [CrossRef]
- Merad, M.; Blish, C. A.; Sallusto, F.; Iwasaki, A. The Immunology and Immunopathology of COVID-19. Science 2022, 375 (6585), 1122–1127. https://doi.org/10.1126/science.abm8108. [CrossRef]
- Nicolai, L.; Kaiser, R.; Stark, K. Thromboinflammation in Long COVID—the Elusive Key to Postinfection Sequelae? Journal of Thrombosis and Haemostasis 2023, S1538783623004002. https://doi.org/10.1016/j.jtha.2023.04.039. [CrossRef]
- Raveendran, A. V.; Jayadevan, R.; Sashidharan, S. Long COVID: An Overview. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 2021, 15 (3), 869–875. https://doi.org/10.1016/ j.dsx.2021.04.007. [CrossRef]
- Bateman, L.; Bested, A. C.; Bonilla, H. F.; Chheda, B. V.; Chu, L.; Curtin, J. M.; Dempsey, T. T.; Dimmock, M. E.; Dowell, T. G.; Felsenstein, D.; Kaufman, D. L.; Klimas, N. G.; Komaroff, A. L.; Lapp, C. W.; Levine, S. M.; Montoya, J. G.; Natelson, B. H.; Peterson, D. L.; Podell, R. N.; Rey, I. R.; Ruhoy, I. S.; Vera-Nunez, M. A.; Yellman, B. P. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Essentials of Diagnosis and Management. Mayo Clinic Proceedings 2021, 96 (11), 2861–2878. https://doi.org/10.1016/j.mayocp.2021.07.004. [CrossRef]
- Campos, M. C.; Nery, T.; Starke, A. C.; De Bem Alves, A. C.; Speck, A. E.; S Aguiar, A. Post-Viral Fatigue in COVID-19: A Review of Symptom Assessment Methods, Mental, Cognitive, and Physical Impairment. Neuroscience & Biobehavioral Reviews 2022, 142, 104902. https://doi.org/10.1016/j.neubiorev.2022.104902. [CrossRef]
- Sukocheva, O. A.; Maksoud, R.; Beeraka, N. M.; Madhunapantula, S. V.; Sinelnikov, M.; Nikolenko, V. N.; Neganova, M. E.; Klochkov, S. G.; Amjad Kamal, M.; Staines, D. R.; Marshall-Gradisnik, S. Analysis of Post COVID-19 Condition and Its Overlap with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Journal of Advanced Research 2022, 40, 179–196. https://doi.org/10.1016/j.jare.2021.11.013. [CrossRef]
- Retornaz, F.; Rebaudet, S.; Stavris, C.; Jammes, Y. Long-Term Neuromuscular Consequences of SARS-Cov-2 and Their Similarities with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Results of the Retrospective CoLGEM Study. J Transl Med 2022, 20 (1), 429. https://doi.org/10.1186/s12967-022-03638-7. [CrossRef]
- Castanares-Zapatero, D.; Chalon, P.; Kohn, L.; Dauvrin, M.; Detollenaere, J.; Maertens De Noordhout, C.; Primus-de Jong, C.; Cleemput, I.; Van Den Heede, K. Pathophysiology and Mechanism of Long COVID: A Comprehensive Review. Annals of Medicine 2022, 54 (1), 1473–1487. https://doi.org/10.1080/07853890.2022.2076901. [CrossRef]
- Khodanovich, M.Y.; Kamaeva, D.A.; Naumova, A.V. Role of Demyelination in the Persistence of Neurological and Mental Impairments after COVID-19. Int. J. Mol. Sci. 2022, 23, 11291. https://doi.org/10.3390/ijms231911291. [CrossRef]
- Kell, D. B.; Laubscher, G. J.; Pretorius, E. A Central Role for Amyloid Fibrin Microclots in Long COVID/PASC: Origins and Therapeutic Implications. Biochemical Journal 2022, 479 (4), 537–559. https://doi.org/10.1042/BCJ20220016. [CrossRef]
- Davies, M. Long Covid Patients Travel Abroad for Expensive and Experimental “Blood Washing.” BMJ 2022, o1671. https://doi.org/10.1136/bmj.o1671. [CrossRef]
- Kurano, M.; Okamoto, K.; Jubishi, D.; Hashimoto, H.; Sakai, E.; Saigusa, D.; Kano, K.; Aoki, J.; Harada, S.; Okugawa, S.; Doi, K.; Moriya, K.; Yatomi, Y. Dynamic Modulations of Sphingolipids and Glycerophospholipids in COVID-19. Clinical & Translational Med 2022, 12 (10). https://doi.org/10.1002/ctm2.1069. [CrossRef]
- Kaur, G.; Ji, X.; Rahman, I. SARS-CoV2 Infection Alters Tryptophan Catabolism and Phospholipid Metabolism. Metabolites 2021, 11, 659. https://doi.org/10.3390/metabo11100659. [CrossRef]
- Clough, E.; Inigo, J.; Chandra, D.; Chaves, L.; Reynolds, J. L.; Aalinkeel, R.; Schwartz, S. A.; Khmaladze, A.; Mahajan, S. D. Mitochondrial Dynamics in SARS-COV2 Spike Protein Treated Human Microglia: Implications for Neuro-COVID. J Neuroimmune Pharmacol 2021, 16 (4), 770–784. https://doi.org/10.1007/s11481-021-10015-6. [CrossRef]
- Elkazzaz, M.; Ahmed, A.; Abo-Amer, Y.E.-E.; Hydara, T.; Haikal, A.; Razek, D.N.A.E.; Eltayb, W.A.;Wang, X.; Karpi´ nski, T.M.; Hamza, D.; et al. In Silico Discovery of GPCRs and GnRHRs as Novel Binding Receptors of SARS-CoV-2 Spike Protein Could Explain Neuroendocrine Disorders in COVID-19. Vaccines 2022, 10, 1500. https://doi.org/10.3390/vaccines10091500. [CrossRef]
- Hammad, R.; Elshafei, A.; Khidr, E. G.; El-Husseiny, A. A.; Gomaa, M. H.; Kotb, H. G.; Eltrawy, H. H.; Farhoud, H. Copeptin: A Neuroendocrine Biomarker of COVID-19 Severity. Biomarkers in Medicine 2022, 16 (8), 589–597. https://doi.org/10.2217/bmm-2021-1100. [CrossRef]
- Raony, Í.; De Figueiredo, C. S.; Pandolfo, P.; Giestal-de-Araujo, E.; Oliveira-Silva Bomfim, P.; Savino, W. Psycho-Neuroendocrine-Immune Interactions in COVID-19: Potential Impacts on Mental Health. Front. Immunol. 2020, 11, 1170. https://doi.org/10.3389/fimmu.2020.01170. [CrossRef]
- Jensterle, M.; Herman, R.; Janež, A.; Mahmeed, W.A.; Al-Rasadi, K.; Al-Alawi, K.; Banach, M.; Banerjee, Y.; Ceriello, A.; Cesur, M.; et al. The Relationship between COVID-19 and Hypothalamic–Pituitary–Adrenal Axis: A Large Spectrum from Glucocorticoid Insufficiency to Excess—The CAPISCO International Expert Panel. Int. J. Mol. Sci. 2022, 23, 7326. https://doi.org/10.3390/ ijms23137326. [CrossRef]
- Murga, I.; Aranburu, L.; Gargiulo, P. A.; Gómez Esteban, J. C.; Lafuente, J.-V. Clinical Heterogeneity in ME/CFS. A Way to Understand Long-COVID19 Fatigue. Front. Psychiatry 2021, 12, 735784. https://doi.org/10.3389/fpsyt.2021.735784. [CrossRef]
- Noor, N.; Urits, I.; Degueure, A.; Rando, L.; Kata, V.; Cornett, E. M.; Kaye, A. D.; Imani, F.; Narimani-Zamanabadi, M.; Varrassi, G.; Viswanath, O. A Comprehensive Update of the Current Understanding of Chronic Fatigue Syndrome. Anesth Pain Med 2021, 11 (3). https://doi.org/10.5812/aapm.113629. [CrossRef]
- Biggio, G.; Mostallino, M. C.; Giusti, P.; Zusso, M.; Toffano, G. Overview of the Pharmacological Properties and Therapeutic Efficacy of Phospholipid Liposomes (Liposom Forte®) in Patients with Depressive Disorders. Minerva Psychiatry 2018, 59 (1). https://doi.org/10.23736/S0391-1772.17.01955-0. [CrossRef]
- Biggio, G.; Mostallino, M. C.; Biggio, F.; Minervino, A.; Giannetti, F. Therapeutic Efficacy and Tolerability of Phospholipid Liposomes (Liposom Forte®) for the Management of Depressive Disorders in Elderly Patients. Evidence-Based Psychiatric Care 2020, 6 (2), 76–91. https://doi.org/10.36180/2421-4469-2020-13. [CrossRef]
- Nataf, S. An Alteration of the Dopamine Synthetic Pathway Is Possibly Involved in the Pathophysiology of COVID-19. J Med Virol 2020, 92 (10), 1743–1744. https://doi.org/10.1002/jmv.25826. [CrossRef]
- Antonini, A.; Leta, V.; Teo, J.; Chaudhuri, K. R. Outcome of Parkinson’s Disease Patients Affected by COVID -19. Mov Disord 2020, 35 (6), 905–908. https://doi.org/10.1002/mds.28104. [CrossRef]
- Pawlak, R.; Napiorkowska-Pawlak, D.; Takada, Y.; Urano, T.; Nagai, N.; Ihara, H.; Takada, A. The Differential Effect of Angiotensin II and Angiotensin 1-7 on Norepinephrine, Epinephrine, and Dopamine Concentrations in Rat Hypothalamus: The Involvement of Angiotensin Receptors. Brain Research Bulletin 2001, 54 (6), 689–694. https://doi.org/10.1016/S0361-9230(01)00489-0. [CrossRef]
- Klempin, F.; Mosienko, V.; Matthes, S.; Villela, D. C.; Todiras, M.; Penninger, J. M.; Bader, M.; Santos, R. A. S.; Alenina, N. Depletion of Angiotensin-Converting Enzyme 2 Reduces Brain Serotonin and Impairs the Running-Induced Neurogenic Response. Cell. Mol. Life Sci. 2018, 75 (19), 3625–3634. https://doi.org/10.1007/s00018-018-2815-y. [CrossRef]
- Bauer, L.; Laksono, B. M.; De Vrij, F. M. S.; Kushner, S. A.; Harschnitz, O.; Van Riel, D. The Neuroinvasiveness, Neurotropism, and Neurovirulence of SARS-CoV-2. Trends in Neurosciences 2022, 45 (5), 358–368. https://doi.org/10.1016/j.tins.2022.02.006. [CrossRef]
- Lu, Y.; Zhu, Q.; Fox, D. M.; Gao, C.; Stanley, S. A.; Luo, K. SARS-CoV-2 down-Regulates ACE2 through Lysosomal Degradation. MBoC 2022, 33 (14), ar147. https://doi.org/10.1091/mbc.E22-02-0045. [CrossRef]
- Attademo, L.; Bernardini, F. Are Dopamine and Serotonin Involved in COVID-19 Pathophysiology? The European Journal of Psychiatry 2021, 35 (1), 62–63. https://doi.org/10.1016/j.ejpsy.2020.10.004. [CrossRef]
- Cordeiro, L. M. S.; Rabelo, P. C. R.; Moraes, M. M.; Teixeira-Coelho, F.; Coimbra, C. C.; Wanner, S. P.; Soares, D. D. Physical Exercise-Induced Fatigue: The Role of Serotonergic and Dopaminergic Systems. Braz J Med Biol Res 2017, 50 (12), e6432. https://doi.org/10.1590/1414-431x20176432. [CrossRef]
- Nizzo, M. C.; Tegos, S.; Gallaminia, A.; Toffano, G.; Polleri, A.; Massarottib, M. Brain Cortex Phospholipids Liposomes Effects on CSF HVA, 5-HIAA and on Prolactin and Somatotropin Secretion in Man. J. Neural Transmission 1978, 43 (2), 93–102. https://doi.org/10.1007/BF01579068. [CrossRef]
- Toffano, G.; Bruni, A. Pharmacological Properties of Phospholipid Liposomes. Pharmacological Research Communications 1980, 12 (9), 829–845. https://doi.org/10.1016/S0031-6989(80)80046-4. [CrossRef]
- Canonico, P. L.; Annunziato, L.; Toffano, G.; Bernardini, R.; Stanzani, S.; Foti, M.; Clementi, G.; Drago, F.; Scapagnini, U. In Vivo and in Vitro Interference of Phosphatidylserine Liposomes on Prolactin Secretion in the Rat. Neuroendocrinology 1981, 33 (6), 358–362. https://doi.org/10.1159/000123261. [CrossRef]
- Rao, F.; Zhang, L.; Wessel, J.; Zhang, K.; Wen, G.; Kennedy, B. P.; Rana, B. K.; Das, M.; Rodriguez-Flores, J. L.; Smith, D. W.; Cadman, P. E.; Salem, R. M.; Mahata, S. K.; Schork, N. J.; Taupenot, L.; Ziegler, M. G.; O’Connor, D. T. Tyrosine Hydroxylase, the Rate-Limiting Enzyme in Catecholamine Biosynthesis: Discovery of Common Human Genetic Variants Governing Transcription, Autonomic Activity, and Blood Pressure In Vivo. Circulation 2007, 116 (9), 993–1006. https://doi.org/10.1161/CIRCULATIONAHA.106.682302. [CrossRef]
- Soung, A. L.; Vanderheiden, A.; Nordvig, A. S.; Sissoko, C. A.; Canoll, P.; Mariani, M. B.; Jiang, X.; Bricker, T.; Rosoklija, G. B.; Arango, V.; Underwood, M.; Mann, J. J.; Dwork, A. J.; Goldman, J. E.; Boon, A. C. M.; Boldrini, M.; Klein, R. S. COVID-19 Induces CNS Cytokine Expression and Loss of Hippocampal Neurogenesis. Brain 2022, 145 (12), 4193–4201. https://doi.org/10.1093/brain/awac270. [CrossRef]
- Leng, A.; Shah, M.; Ahmad, S.A.; Premraj, L.; Wildi, K.; Li Bassi, G.; Pardo, C.A.; Choi, A.; Cho, S.-M. Pathogenesis Underlying Neurological Manifestations of Long COVID Syndrome and Potential Therapeutics. Cells 2023, 12, 816. https://doi.org/ 10.3390/cells12050816. [CrossRef]
- Fernández-Castañeda, A.; Lu, P.; Geraghty, A. C.; Song, E.; Lee, M.-H.; Wood, J.; O’Dea, M. R.; Dutton, S.; Shamardani, K.; Nwangwu, K.; Mancusi, R.; Yalçın, B.; Taylor, K. R.; Acosta-Alvarez, L.; Malacon, K.; Keough, M. B.; Ni, L.; Woo, P. J.; Contreras-Esquivel, D.; Toland, A. M. S.; Gehlhausen, J. R.; Klein, J.; Takahashi, T.; Silva, J.; Israelow, B.; Lucas, C.; Mao, T.; Peña-Hernández, M. A.; Tabachnikova, A.; Homer, R. J.; Tabacof, L.; Tosto-Mancuso, J.; Breyman, E.; Kontorovich, A.; McCarthy, D.; Quezado, M.; Vogel, H.; Hefti, M. M.; Perl, D. P.; Liddelow, S.; Folkerth, R.; Putrino, D.; Nath, A.; Iwasaki, A.; Monje, M. Mild Respiratory COVID Can Cause Multi-Lineage Neural Cell and Myelin Dysregulation. Cell 2022, 185 (14), 2452-2468.e16. https://doi.org/ 10.1016/j.cell.2022.06.008. [CrossRef]
- Fledrich, R.; Abdelaal, T.; Rasch, L.; Bansal, V.; Schütza, V.; Brügger, B.; Lüchtenborg, C.; Prukop, T.; Stenzel, J.; Rahman, R. U.; Hermes, D.; Ewers, D.; Möbius, W.; Ruhwedel, T.; Katona, I.; Weis, J.; Klein, D.; Martini, R.; Brück, W.; Müller, W. C.; Bonn, S.; Bechmann, I.; Nave, K. A.; Stassart, R. M.; Sereda, M. W. Targeting Myelin Lipid Metabolism as a Potential Therapeutic Strategy in a Model of CMT1A Neuropathy. Nat Commun 2018, 9 (1), 3025. https://doi.org/10.1038/s41467-018-05420-0. [CrossRef]
- Monastra, G.; Cross, A. H.; Bruni, A.; Raine, C. S. Phosphatidylserine, a Putative Inhibitor of Tumor Necrosis Factor, Prevents Autoimmune Demyelination. Neurology 1993, 43 (1 Part 1), 153–153. https://doi.org/10.1212/WNL.43.1_Part_1.153. [CrossRef]
- Karimi-Galougahi, M.; Yousefi-Koma, A.; Bakhshayeshkaram, M.; Raad, N.; Haseli, S. 18FDG PET/CT Scan Reveals Hypoactive Orbitofrontal Cortex in Anosmia of COVID-19. Academic Radiology 2020, 27 (7), 1042–1043. https://doi.org/ 10.1016/j.acra.2020.04.030. [CrossRef]
- Sollini, M.; Morbelli, S.; Ciccarelli, M.; Cecconi, M.; Aghemo, A.; Morelli, P.; Chiola, S.; Gelardi, F.; Chiti, A. Long COVID Hallmarks on [18F]FDG-PET/CT: A Case-Control Study. Eur J Nucl Med Mol Imaging 2021, 48 (10), 3187–3197. https://doi.org/10.1007/s00259-021-05294-3. [CrossRef]
- Guedj, E.; Campion, J. Y.; Dudouet, P.; Kaphan, E.; Bregeon, F.; Tissot-Dupont, H.; Guis, S.; Barthelemy, F.; Habert, P.; Ceccaldi, M.; Million, M.; Raoult, D.; Cammilleri, S.; Eldin, C. 18F-FDG Brain PET Hypometabolism in Patients with Long COVID. Eur J Nucl Med Mol Imaging 2021, 48 (9), 2823–2833. https://doi.org/10.1007/s00259-021-05215-4. [CrossRef]
- Donegani, M. I.; Miceli, A.; Pardini, M.; Bauckneht, M.; Chiola, S.; Pennone, M.; Marini, C.; Massa, F.; Raffa, S.; Ferrarazzo, G.; Arnaldi, D.; Sambuceti, G.; Nobili, F.; Morbelli, S. Brain Metabolic Correlates of Persistent Olfactory Dysfunction after SARS-Cov2 Infection. Biomedicines 2021, 9 (3), 287. https://doi.org/10.3390/biomedicines 9030287. [CrossRef]
- Rudroff, T.;Workman, C.D.; Ponto, L.L.B. 18F-FDG-PET Imaging for Post-COVID-19 Brain and Skeletal Muscle Alterations. Viruses 2021, 13, 2283. https://doi.org/10.3390/v13112283. [CrossRef]
- Salonia, A.; Pontillo, M.; Capogrosso, P.; Gregori, S.; Carenzi, C.; Ferrara, A. M.; Rowe, I.; Boeri, L.; Larcher, A.; Ramirez, G. A.; Tresoldi, C.; Locatelli, M.; Cavalli, G.; Dagna, L.; Castagna, A.; Zangrillo, A.; Tresoldi, M.; Landoni, G.; Rovere-Querini, P.; Ciceri, F.; Montorsi, F. Testosterone in Males with COVID-19: A 7-month Cohort Study. Andrology 2022, 10 (1), 34–41. https://doi.org/10.1111/andr.13097. [CrossRef]
- Uckert, S.; Fuhlenriede, M. H.; Becker, A. J.; Stief, C. G.; Scheller, F.; Knapp, W. H.; Jonas, U. Is There an Inhibitory Role of Cortisol in the Mechanism of Male Sexual Arousal and Penile Erection? Urological Research 2003, 31 (6), 402–406. https://doi.org/ 10.1007/s00240-003-0359-5. [CrossRef]
- Adeyemi, D. H.; Odetayo, A. F.; Hamed, M. A.; Akhigbe, R. E. Impact of COVID 19 on Erectile Function. The Aging Male 2022, 25 (1), 202–216. https://doi.org/10.1080 /13685538.2022.2104833. [CrossRef]
- Leema Rose, A.; Manickam, A.; Agrawal, M. A Mathematical Model For The Special Effects Of Phosphatidylserine On Endocrine Reaction To Reasonable Concentration Exercise In Healthy Male Subjects. TURCOMAT 2021, 12 (3), 3555–3559. https://doi.org/10.17762/turcomat.v12i3.1632. [CrossRef]
- Starks, M. A.; Starks, S. L.; Kingsley, M.; Purpura, M.; Jäger, R. The Effects of Phosphatidylserine on Endocrine Response to Moderate Intensity Exercise. Journal of the International Society of Sports Nutrition 2008, 5 (1), 11. https://doi.org/10.1186/1550-2783-5-11. [CrossRef]
- Kiriakova, N.; Kiriakov, A.; Schneider, E.; Bonev, A. Therapeutic Effect of Essential Phospholipids on Functional Sexual Disorders in Males. J Eur Acad Dermatol Venerol 1998, 11 (2), 191–193. https://doi.org/10.1111/j.1468-3083.1998.tb00783.x. [CrossRef]
- Casacchia, M.; Marola, W.; Meco, G.; Pirro, R.; Di Cesare, E.; Allegro, A.; Cusimano, G. Phospholipid Liposomes in Depression: A Double-Blind Study versus Placebo. Int Pharmacopsychiatry 1982, 17 (4), 274–279. https://doi.org/10.1159/000468583. [CrossRef]
- Aguglia, E.; Calandra, C.; Rapisarda, V.; Maugeri, D. The Effect of Hypothalamic Phospholipid Liposomes in Patients Treated with Sulpiride or Haloperidol. Acta Ther 1984, 10, 133–144.
- Giannelli, A.; Rabboni, M.; Zarattini, F.; Malgeri, C.; Magnolfi, G. A Combination of Hypothalamic Phospholipid Liposomes with Trazodone for Treatment of Depression.: An Open Controlled Study. Acta Psychiatr Scand 1989, 79 (1), 52–58. https://doi.org/10.1111/j.1600-0447.1989.tb09234.x. [CrossRef]
- Rachev, E.; Nalbansky, B.; Kolarov, G.; Agrosì, M. Efficacy and Safety of Phospholipid Liposomes in the Treatment of Neuropsychological Disorders Associated with the Menopause: A Double-Blind, Randomised, Placebo-Controlled Study. Curr Med Res Opin 2001, 17 (2), 105–110.
- Bruni, A.; Toffano, G.; Leon, A.; Boarato, E. Pharmacological Effects of Phosphatidylserine Liposomes. Nature 1976, 260 (5549), 331–333. https://doi.org/ 10.1038/260331a0. [CrossRef]
- Kang, E. Y.; Cui, F.; Kim, H. K.; Nawaz, H.; Kang, S.; Kim, H.; Jang, J.; Go, G. Effect of Phosphatidylserine on Cognitive Function in the Elderly: A Systematic Review and Meta-Analysis. Korean Journal of Food Science and Technology 2022, 54 (1), 52–58. https://doi.org/10.9721/KJFST.2022.54.1.52. [CrossRef]
- US Food and Drug Administration. Letter Updating the Phosphatidylserine and Cognitive Function and Dementia Qualified Health Claim, 2004. http://wayback.archive-it.org/7993/20171114183737/https://www.fda.gov/Food/IngredientsPackagingLabeling/LabelingNutrition/ucm072999.htm (accessed 2023-06-06).
- Zhang, Y. Y.; Yang, L. Q.; Guo, L. M. Effect of Phosphatidylserine on Memory in Patients and Rats with Alzheimer’s Disease. Genet. Mol. Res. 2015, 14 (3), 9325–9333. https://doi.org/10.4238/2015.August.10.13. [CrossRef]
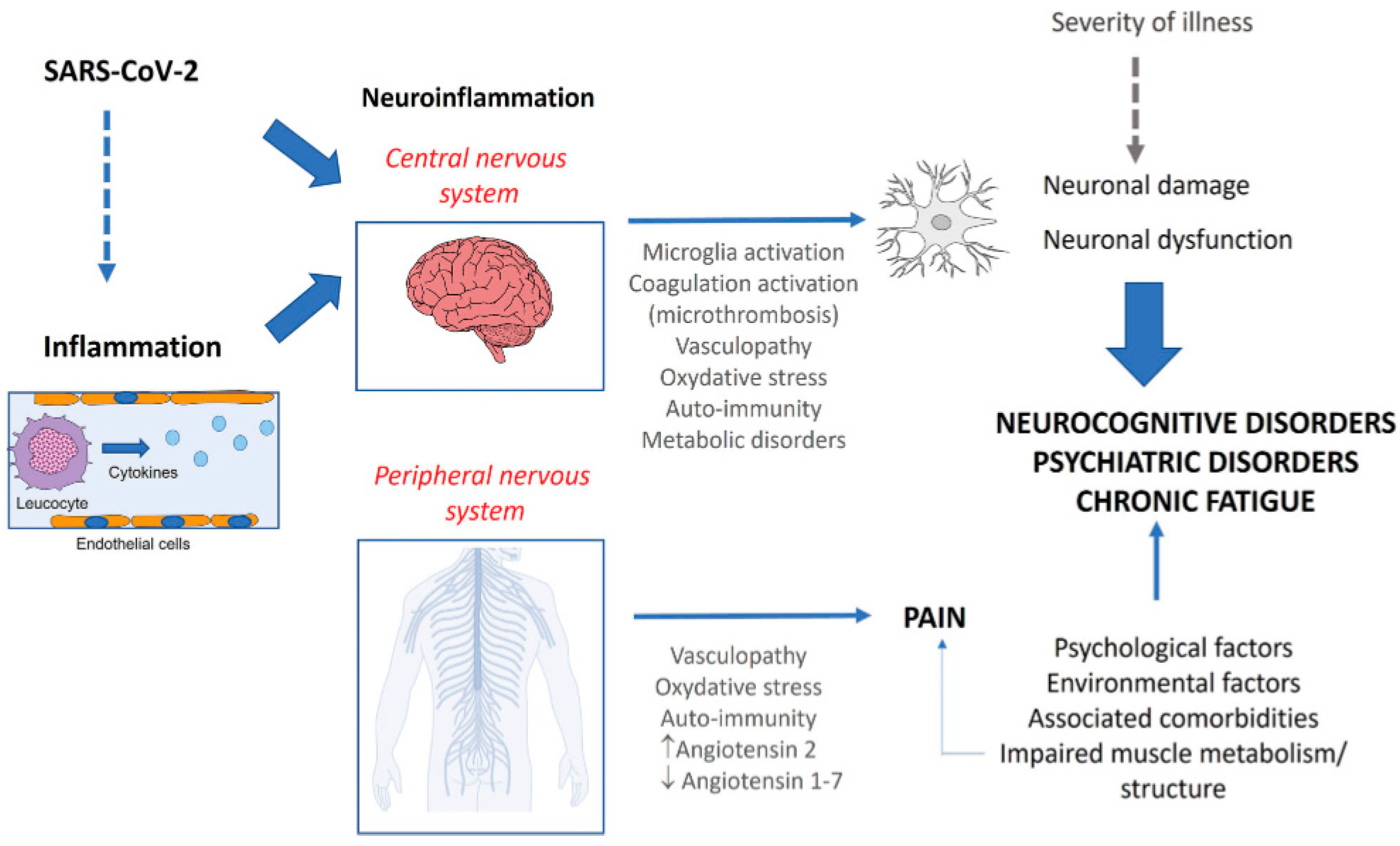
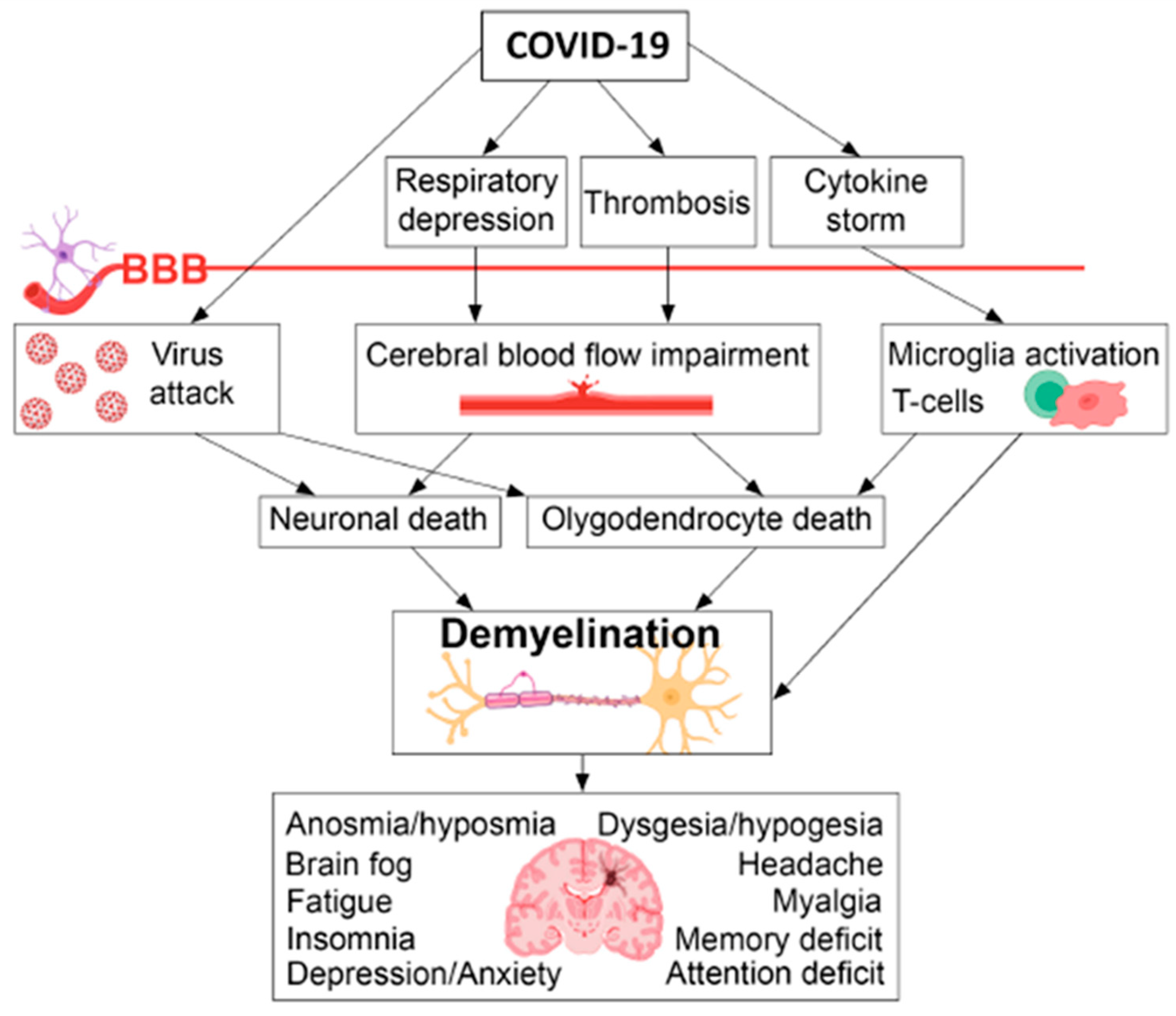
| Post COVID syndrome | Clinical manifestations | Comment |
|---|---|---|
| Post COVID fatigue syndrome | Profound fatigue, post-exertion malaise and/or poor resistance | Rule out causes like anemia, electrolyte imbalance, hypothyroidism, |
| Post COVID cardio-respiratory Syndrome | Cough, dyspnea or increased fatigue, low grade fever, chest pain, orthostatic hypotension, palpitations and tachycardia | Sudden worsening of dyspnea: Consider tension pneumothorax, pulmonary embolism, coronary artery disease or heart failure |
| Post COVID neuro-psychiatric Syndrome | Headaches, anosmia or dysgeusia, cognitive impairment or "brain fog", depression and other mood changes, paresthesias, insomnia and other sleep difficulties, dizziness | If acute onset neurological symptoms also consider vasculitis, thrombosis or demyelination. Properly evaluate post-covid psychological problems. |
| Post COVID gastro-intestinal Syndrome | Abdominal discomfort, diarrhea, constipation, vomiting | GI symptoms can be a sequalae of the disease or therapy-related side effects |
| Post COVID hepato-biliary Syndrome | Nausea, jaundice, Liver Function Tests alterations | Drugs used in the treatment of COVID-19 can cause hepatic impairment. |
| Post COVID musculo-skeletal Syndrome | Arthralgia, myalgia, muscle weakness | Causes include: Covid-19 disease, prolonged ICU care, neurological problems, myopathy or electrolyte imbalance. Usually subside during follow up. Inflammatory arthralgia has to be differentiated from other causes like Systemic Lupus Erithematosus, Rheumatoid Arthritis. |
| Post COVID thromboembolic Syndrome | Depending upon the vascular territory of involvement dyspnea in Pulmonary Embolism, chest pain in Coronay Artery Disease and limb weakness and neurological deficit in stroke | Early diagnosis and treatment is lifesaving. Follow the standard treatment protocol. |
| Post COVID multisystem inflammatory syndrome/post COVID autoimmune syndrome | Fever, gastrointestinal symptoms, rash, chest pain, Palpitations | Elevated levels of markers of inflammation. |
| Post COVID genito-urinary Symptoms | Proteinuria, hematuria, development of kidney injury, menstrual cycle irregularities, erectile dysfunction | |
| Post COVID dermatological Syndrome | Vesicular, maculopapular, urticarial, or chilblain-like lesions on the extremities (COVID toe) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




