Submitted:
29 June 2023
Posted:
30 June 2023
You are already at the latest version
Abstract
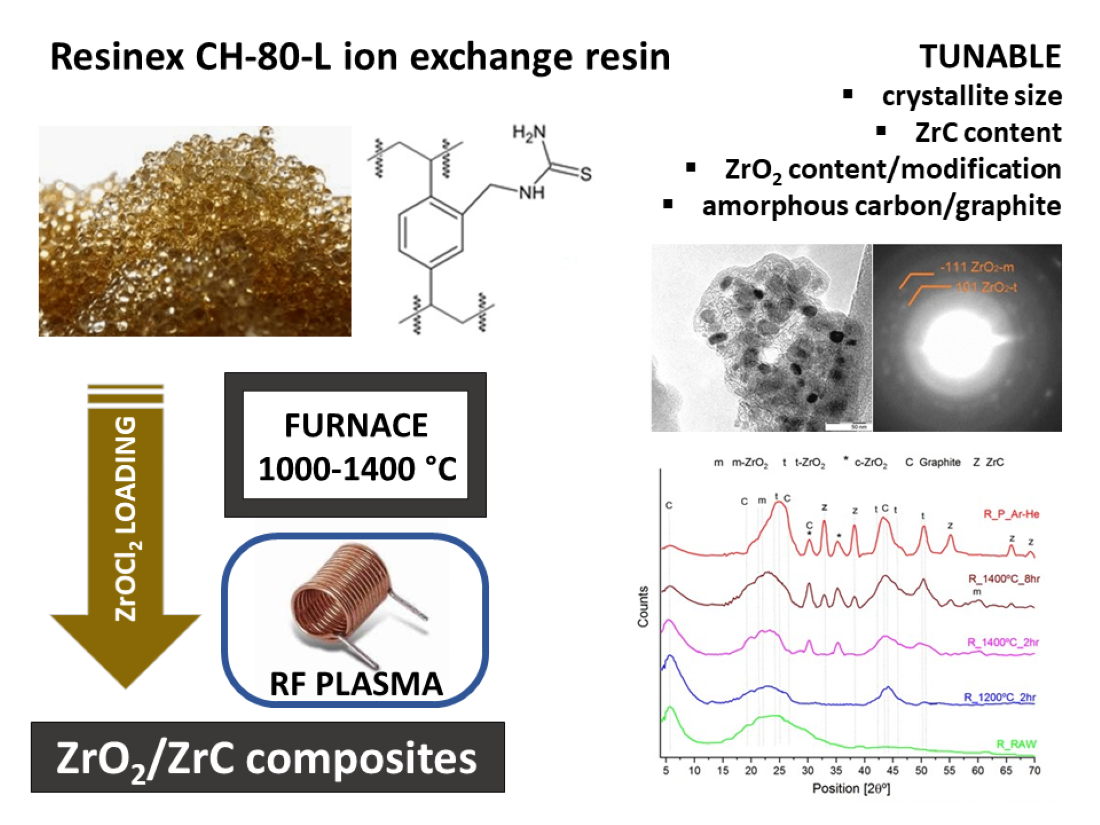
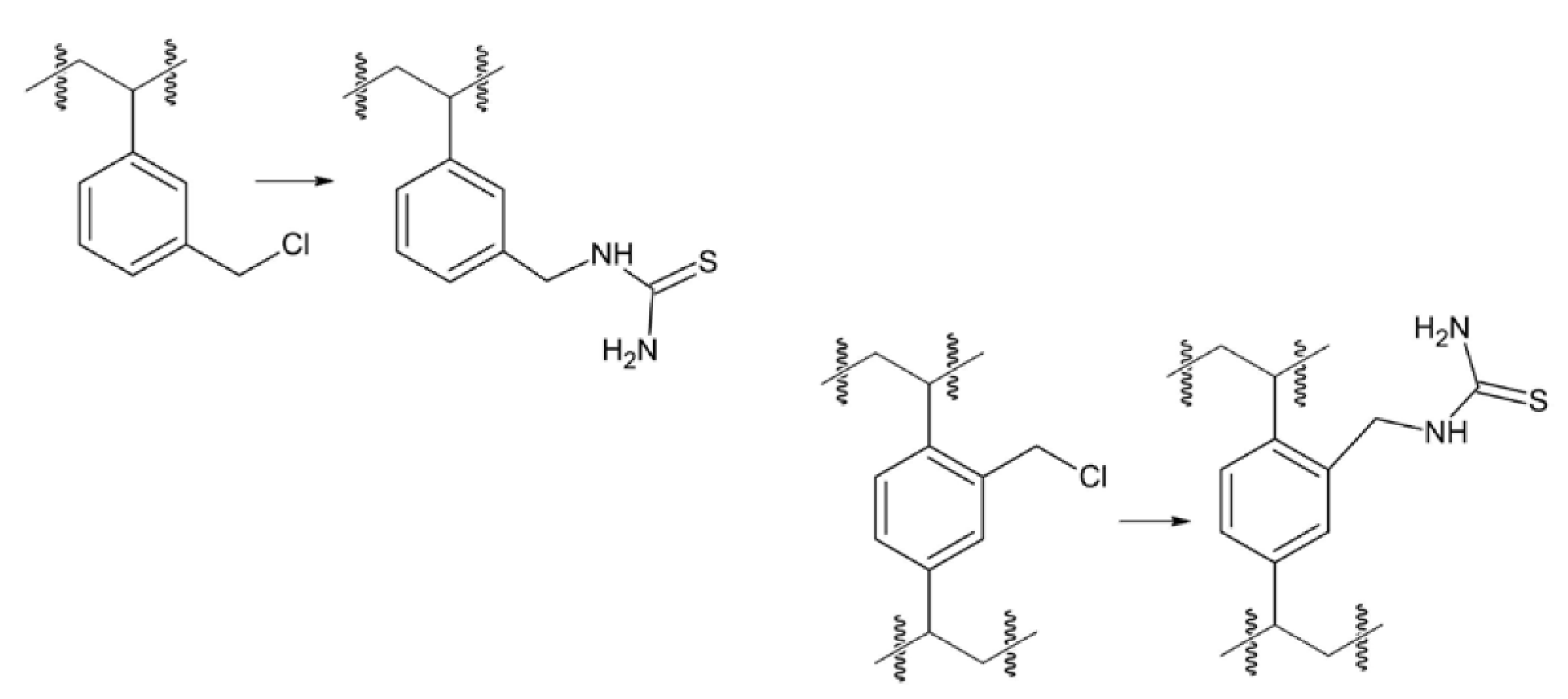
Keywords:
1. Introduction
2. Materials and Methods
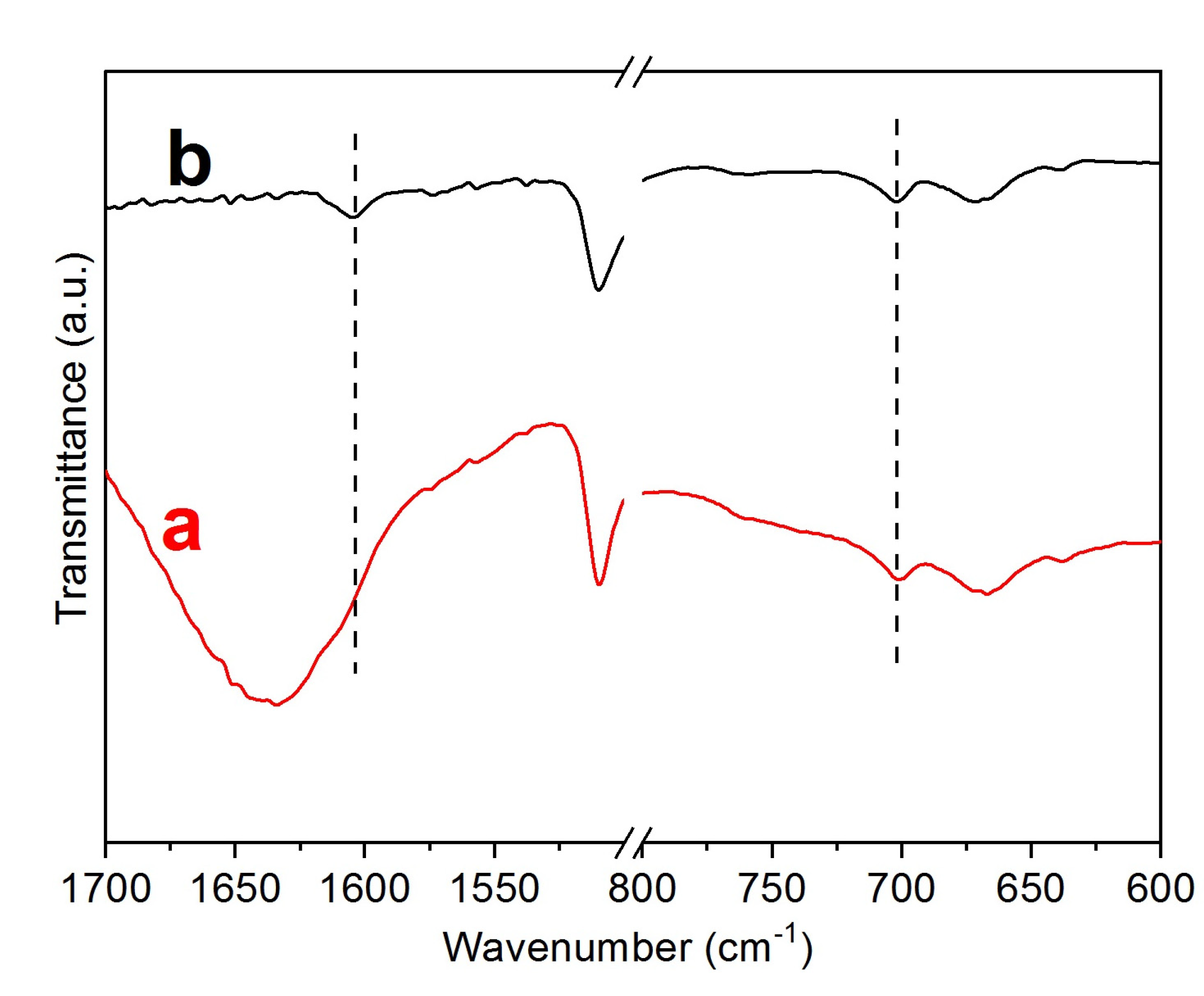
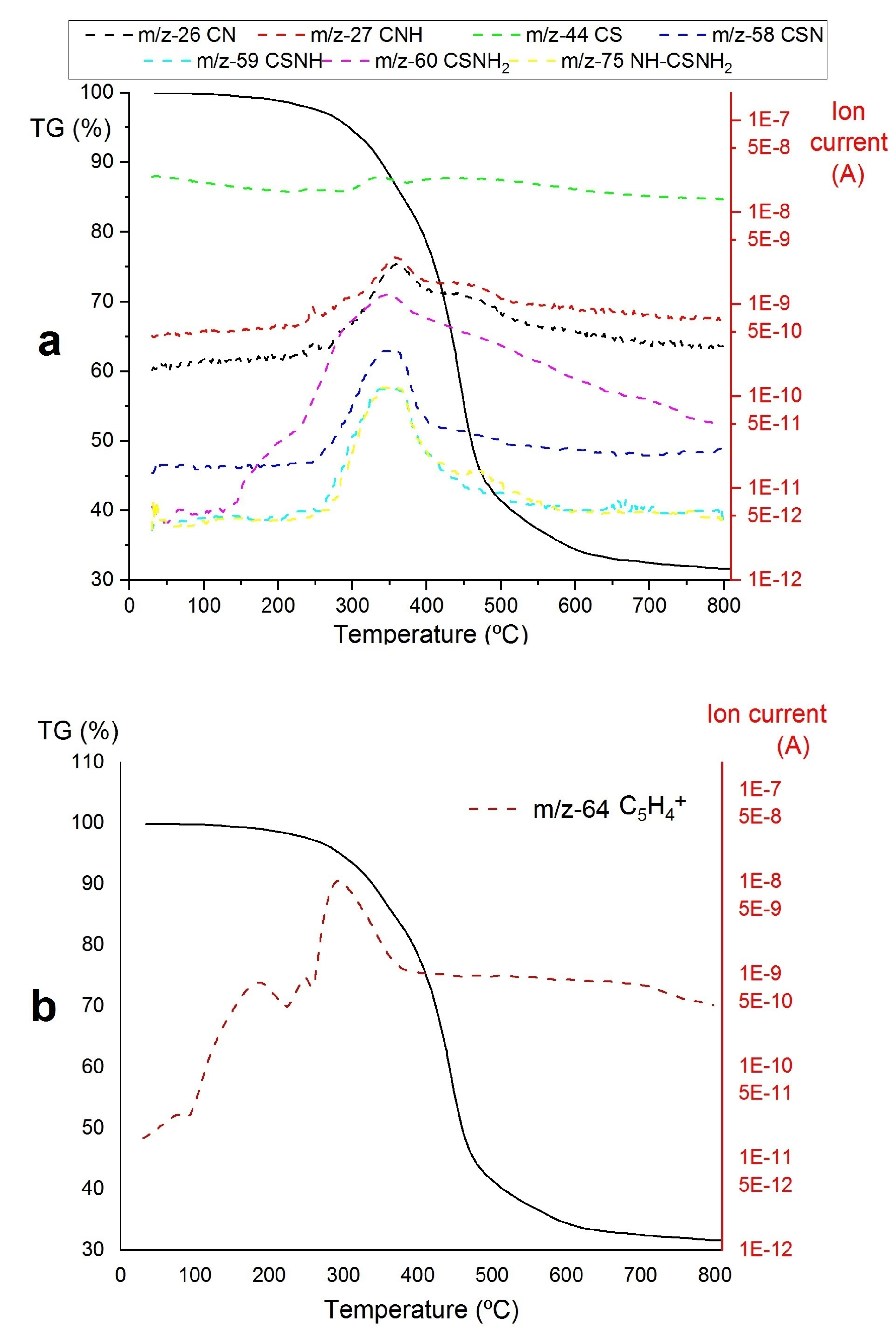
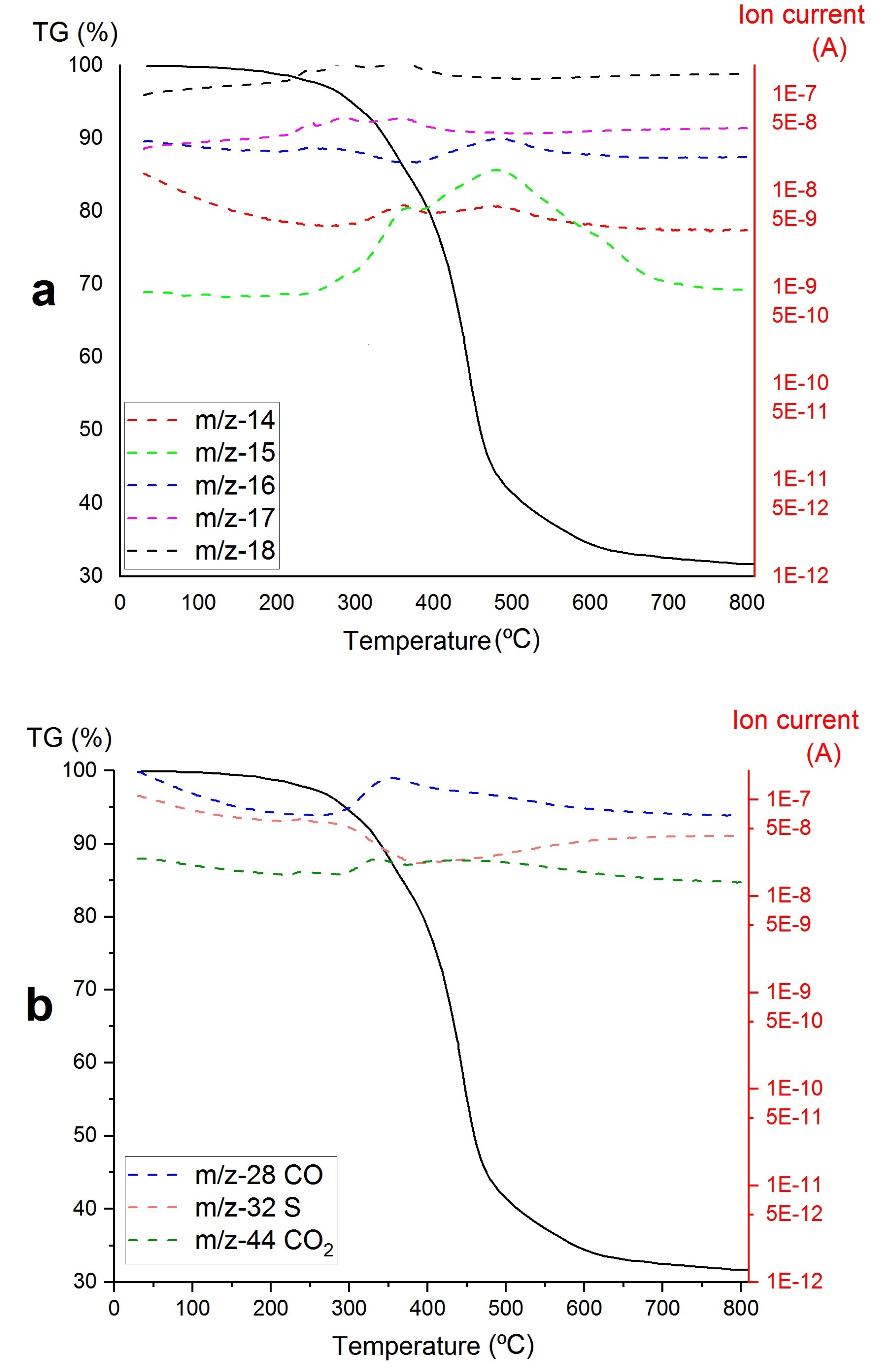
3. Results
- A)
- The decomposition of the Zr-loaded thiourea groups with partial depolymerization/degradation of the rings attached to the Zr-loaded thiourea functions
- B)
- The degradation of unfunctionalized thiourea groups with partial depolymerization and degradation of the rings attached to the unloaded thiourea groups
- C)
4. Conclusion
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Liu, G.; Cheng, L.; Li, K.; Chen, Z.; Xiong, X.; Luan, X. Damage behavior of atomic oxygen on zirconium carbide coating modified carbon/carbon composite. Ceram. Int. 2020, 3, 3324–3331. [Google Scholar] [CrossRef]
- Pierson, HO. Handbook of refractory carbides and nitrides: properties.; characteristics.; processing and applications. 1st ed.; Bergen: Noyes Publication, USA, 1997. [Google Scholar]
- Scales, N.; Chen, J.; Aughterson, RD.; Karatchevtseva, I.; Stopic, A.; Lumpkin, GR.; et al. Porous ZrC-carbon microspheres as potential Insoluble target matrices for production of 188W/188Re. J. Radioanal. Nucl. Chem. 2018, 318, 835–847. [Google Scholar] [CrossRef]
- Scales, N.; Chen, J.; Hanley, T.L.; Riley, D.P.; Lumpkin, G.R.; Luca, V. Hierarchically porous carbon–zirconium carbide spheres as potentially reusable transmutation targets. Microporous Mesoporous Mater. 2015, 212, 100–109. [Google Scholar] [CrossRef]
- Martiz, A.; Károly, Z.; Trif, L.; Mohai, M.; Bereczki, L.; Németh, P.; Zsombor, M.; Menyhárd, A.; Pawar, R. P.; Kótai, L. Plasma-assisted preparation of nano-(ZrC.; ZrO2)@carbon composites from Zr-loaded sulfonated styrene–divinylbenzene copolymers. J. Therm. Anal. Calorim 2022, 147, 9353–9365. [Google Scholar] [CrossRef]
- Martiz, A.; Károly, Z.; Domján, A.; Mohai, M.; Bereczki, L.; Trif, L.; Farkas, A.; László, K.; Menyhárd, A.; Kótai, L. Nano-ZrO2@C.; nano-(ZrC.; ZrO2)@C and nano-ZrC@C composites prepared by plasma-assisted carbonization of Zr-loaded iminodiacetate-functionalized styrene-divinylbenzene copolymers. Inorganics 2022, 10, 77. [Google Scholar] [CrossRef]
- Bai, L.; Yuan, F.; Fang, Z.; Wang, Q.; Ouyang, Y.; Jin, H.; He, J.; Liu, W.; Wang, Y. RF thermal plasma synthesis of ultrafine ZrB2-ZrC composite powders. Nanomaterials 2020, 10, 2497. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.B.; Wang, C.; Zhang, D.; Hernandez, A.; Nagle, D.C.; Mueller, T.; Spicer, J.B. Reactive laser synthesis of ultra-high-temperature ceramics HfC, ZrC, TiC, HfN, ZrN and TiN for additive manufacturing. Ceram. Int. 2023, 49, 11204–11229. [Google Scholar] [CrossRef]
- Martiz, A.; Károly, Z.; Bódis, E.; Fazekas, P.; Mohai, M.; Bertóti, I.; Keszler, A.M. In-flight synthesis of nanosized ZrC particles from various precursors in RF thermal plasma. Per. Polytech. Chem. Eng. 2021, 65, 331–342. [Google Scholar] [CrossRef]
- Saray, M. T.; Yurkiv, V.; Shahbazian-Yassar, R. In Situ Thermolysis of a Ni Salt on Amorphous Carbon and Graphene Oxide Substrates. Adv. Funct. Mater. 2023, 2213747. [Google Scholar] [CrossRef]
- Wei, Y.; FaZhan, X.; Xin, L.; Wei, H.; YongYue, Y.; DongChuang, W.; et al. Nickel and oxygen-containing functional groups co-decorated graphene-like shells as catalytic sites with excellent selective hydrogenation activity and robust stability. Chem. Eng. J. 2023, 452. [Google Scholar]
- Masoud, A.; Gholamhassan, A.; Mohammad, K. A. An on-line matrix separation and preconcentration procedure for ICP OES determination of Cd, Co, Cu, Mn and Pb traces in Zr and Zr–Nb alloys using a cation-exchange resin microcolumn. J. Anal. Atom. Spectrom. 2021, 36, 1074–1083. [Google Scholar]
- Li, F.; Huang, X.; Zhang, GJ. Scalable foaming assisted synthesis of ZrC nanopowder by carbothermal reduction. Ceram. Int. 2015, 41, 3335–3338. [Google Scholar] [CrossRef]
- Fazekas, P.; Czégény, Z.; Mink, J.; Bódis, E.; Klébert, S.; Németh, C.; Keszler, A.M.; et al. Decomposition of poly(vinyl chloride) in inductively coupled radiofrequency thermal plasma. Chem. Eng. J. 2016, 302, 163–171. [Google Scholar] [CrossRef]
- Szépvölgyi, J.; Mohai, I.; Karoly, Z.; Gál, L. Synthesis of nanosized ceramic powders in a radiofrequency thermal plasma reactor. J. Eur. Ceram. 2018, 28, 895–899. [Google Scholar] [CrossRef]
- Komarov, V.S.; Yatsevskaya, M.I.; Sycheva, O.A. Properties of activated carbon produced from spent ion-exchange resins. Dokl. Akad. Nauk. BSSR 1985, 29, 1010–1023. [Google Scholar]
- Chinthaka, Silva, G.W.; Kercher, A.A.; Hunn, J.D.; Martin, R.C.; Jellison, G.E.; Meyer, H.M. Characterization of zirconium carbides using electron microscopy, optical anisotropy, Auger depth profiles, X-ray diffraction and electron density calculated by charge flipping method. J. Solid State Chem 2012, 194, 91–99.
- Gusev, A.I. Structural stability boundaries for nonstoichiometric compounds. Phys. Status Solidi A. 1989, 111, 443–450. [Google Scholar] [CrossRef]
- Mohai, M. XPS MultiQuant: multimodel XPS quantification software. Surf. Interface Anal. 2004, 36, 828–832. [Google Scholar] [CrossRef]
- Mohai, M.; Bertóti, I. Calculation of overlayer thickness on curved curfaces based on XPS intensities. Surf. Interface Anal. 2004, 36, 805–808. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, R.; Liao, L.; Duan, X. High-performance thin-film electronics based on inorganic nanostructures and composites. Nano Today 2013, 8, 514–530. [Google Scholar] [CrossRef]
- Basumatary, S. Transesterification with heterogeneous catalyst in production of biodiesel: A review. J. Chem. Pharm. 2013, 5, 1–7. [Google Scholar]
- Fedorov, P.P.; Yarotskaya, E.G. Zirconium dioxide. Review. Condens. Matter Interphases 2021, 23, 169–187. [Google Scholar] [CrossRef] [PubMed]
- Loubalová, I.; Kopel, P. Coordination Compounds of Cu, Zn, and Ni with Dicarboxylic Acids and N Donor Ligands, and Their Biological Activity: A Review. Molecules 2023, 28. [Google Scholar] [CrossRef]
- Swaminathan, K.; Irving, H.M.N.H. Infra-red absorption spectra of complexes of thiourea. J. Inorg. Nucl. Chem., V 1964, 26, 1291–1294. [Google Scholar] [CrossRef]
- Dunlap, C.J.; Mcneff, C.V.; Stoll, D.; Carr, P.W. Zirconia stationary phases for extreme separations. Anal. Chem. 2001, 73, 599A–607A. [Google Scholar] [CrossRef] [PubMed]
- Camps, M.; Chatzopoulos, M.; Camps, J.M.; Montheards, J.P. Chloromethylation of Polystyrenes and Styrene Copolymers. Applications. JMS-Rev. Macromol. Chem. Phys. 1987, 27, 505–557. [Google Scholar] [CrossRef]
- Yuchi, A.; Yoshida, N. Adsorption of tetravalent metal ions chelating resins containing iminodiacetic acid groups. Bull. Chem. Soc. Jpn. 2000, 73, 1841–1842. [Google Scholar] [CrossRef]
- Auer, B.M.; Skinner, J.L. IR and Raman spectra of liquid water: Theory and interpretation. J. Chem. Phys. 2008, 128, 224511. [Google Scholar] [CrossRef] [PubMed]
- Kotai, L.; Fodor, J.; Jakab, E.; Sajo, I. Szabo, P.; et al. A thermally induced low-temperature intramolecular redox reaction of bis(pyridine)silver(I) permanganateand its hemipyridinesolvate. Transit. Metal Chem. 2006, 31, 30–34. [Google Scholar] [CrossRef]
- Lee, G.; Park, S. I.; Shin, H. Y.; Joh, H. I.; Kim, S. S.; & Lee, S. Simultaneous reactions of sulfonation and condensation for high-yield conversion of polystyrene into carbonaceous material. J Ind Eng Chem 2023, 122, 426–436.
- Cheng, W.; Campolongo, MJ.; Tan, S.J.; Luo, D. Freestanding ultrathin nano-membranes via self-assembly. Nano Today 2009, 4, 482–493. [Google Scholar] [CrossRef]
- Kocsis, T.; May, Z.; Czégény, Z.; Sreedhar, B.; Pawar, R.P.; Kótai, L. Perspectives of magnetic and nanosized metal-containing amorphous carbon composite chemosorbents and catalysts. Nano Studies 2016, 14, 7–35. [Google Scholar]
- Fazekas, P.; Czégény, Z.; Mink, J.; Bódis, E.; Klébert, S.; Németh, C.; Keszler, A.M.; Károly, Z.; Szépvölgyi, J. Decomposition of poly(vinyl chloride) in inductively coupled radiofrequency thermal plasma. Chem. Eng. J. 2016, 302, 163–171. [Google Scholar] [CrossRef]
- Wen, Y.; Zhou, C.; Yu, L.; Zhang, Q.; He, W.; & Liu, Q. Preparation of nanometer zirconia by hydrothermal method: Influence of temperature and mechanism. Solid State Sci. 2023, 107237.
- Fitzgerald, J.J.; Weiss, R.A. Cation-Anion and Cation-Cation Interactions in Sulfonated Polystyrene Ionomers Spectroscopic Studies of the Effects of Solvents. ACS Symp. Ser. 1986, 302, 35–53. [Google Scholar]
- El-NahhalI, I.M.; Zaggout, F.R.; Nassar, M.A.; El-Ashgar, N.M.; Maquet, J.; et al. Characterization and Applications of Immobilized Iminodiacetic Acid-Modified Silica. J. Sol-Gel Sci. Technol. 2003, 28, 255–265. [Google Scholar] [CrossRef]
- Jiang, J.; Renshaw, J.C.; Sarsfield, M.J.; Livens, FR.; Collison, D.; Charnock, J.M.; Eccles, H. Solution Chemistry of Uranyl Ion with Iminodiacetate and Oxydiacetate: A Combined NMR/EXAFS and Potentiometry/Calorimetry Study. Inorg. Chem. 2003, 42, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Busca, G.; Lorenzelli, V. Infrared spectroscopic identification of species arising from reactive adsorption of carbon oxides on metal oxide surfaces. Mater. Chem. 1982, 7, 89–126. [Google Scholar] [CrossRef]
- Choi, J.G. The Effect of Surface Properties of Carbon Nanomaterials on the Molecular Adsorption of Organophosphate. Graduate School of UNIST 2019. [Google Scholar]
- Komarov, V.S.; Yatsevskaya, M.I.; Sycheva, O.A. Properties of activated carbon produced from spent ion-exchange resins. Dokl. Akad. Nauk. BSSR 1985, 29, 1010–1023. [Google Scholar]
- Martiz, A.; Farkas, A.; Karoly, Z.; Franguelli, F.P.; Samaniego, S.K.; Menyhard, A.; Kotai, L. Raman studies on carbon-containing phases in nanosized-ZrO2/C and nanosized-(ZrC.; ZrO2)/C composites. Nano Studies 2022, 10, 21–22. [Google Scholar] [CrossRef]
- Lokshin, E.P.; Tareeva, O.A. Sorption of Zirconium from Nitrate and Sulfate Solutions. Theor. Found. Chem. Eng. 2019, 53, 688–692. [Google Scholar] [CrossRef]
- Clearfield, A. Structural aspects of zirconium chemistry. Rev. Pure Appl. Chem. 1964, 14, 91–108. [Google Scholar]
- Fathy, M.; Moghn, T.A.; Awadallah, A.E.; El-Bellihi, A.H. Nano Composites of Polystyrene Divinyl Benzene Resin Based on Oxidized Multi-Walled Carbon Nanotubes. Int. J. Modern Org. Chem. 2013, 2, 67–80. [Google Scholar]
- Shishlov, N.M.; Khurasan, S.L. Effect of ion interactions on the IR spectrum of benzenesulfonate ion. Restoration of sulfonate ion symmetry in sodium benzenesulfonate dimer. J. Mol. Struct. 2016, 1123, 360–366. [Google Scholar] [CrossRef]
- De, R.; Lee, H.; Das, B. Exploring the interactions in binary mixtures of polyelectrolytes: Influence of mixture composition.; concentration.; and temperature on counter-ion condensation. J. Mol. Liq. 2018, 251, 94–99. [Google Scholar] [CrossRef]
- Scales, N. Syntheses, structures and radionuclide extraction properties of porous ZrC-and Zr2SC-carbon composite sphere materials, PhD Thesis, University of Wollongong 2018. https://ro.uow.edu.au/theses1/343.
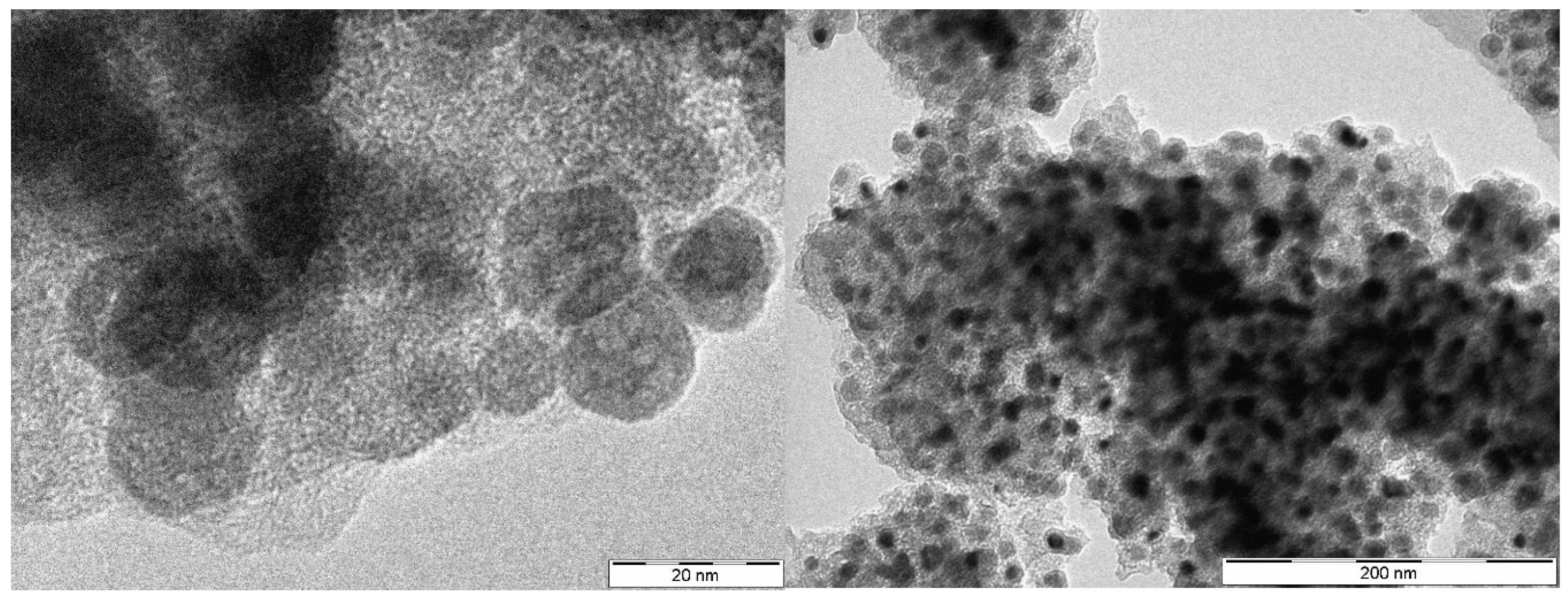
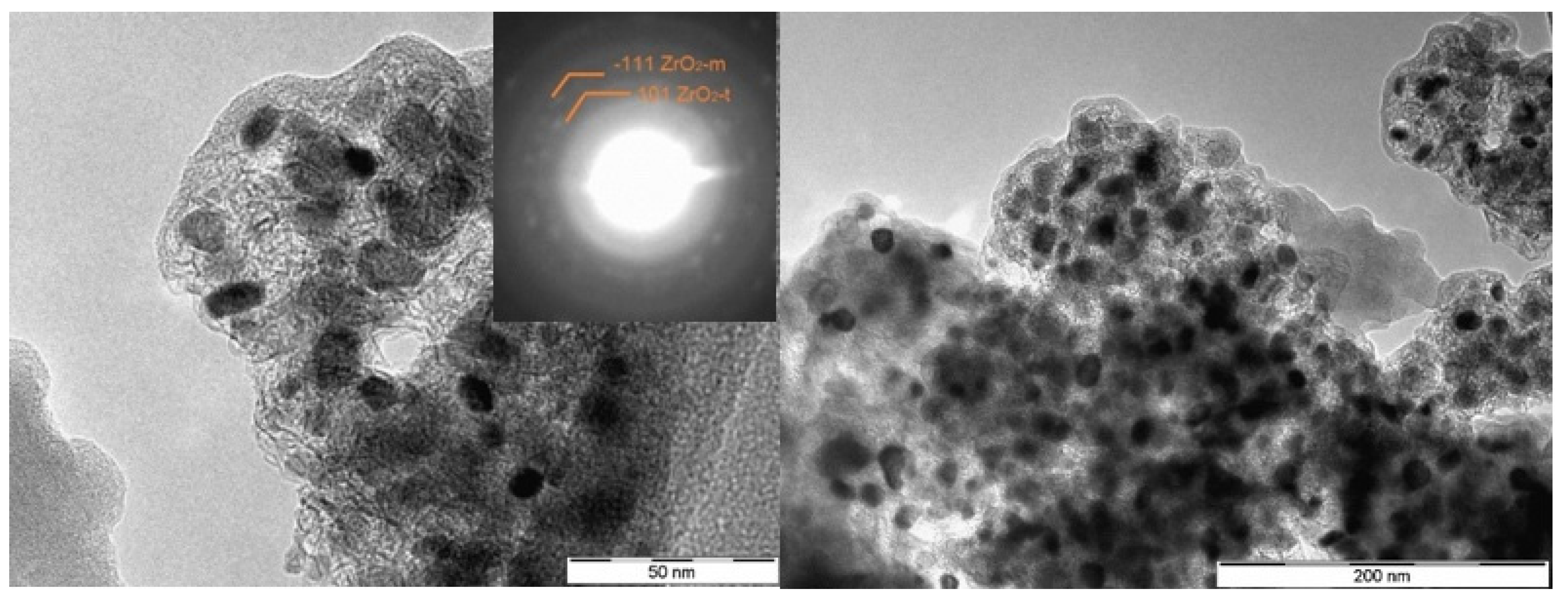
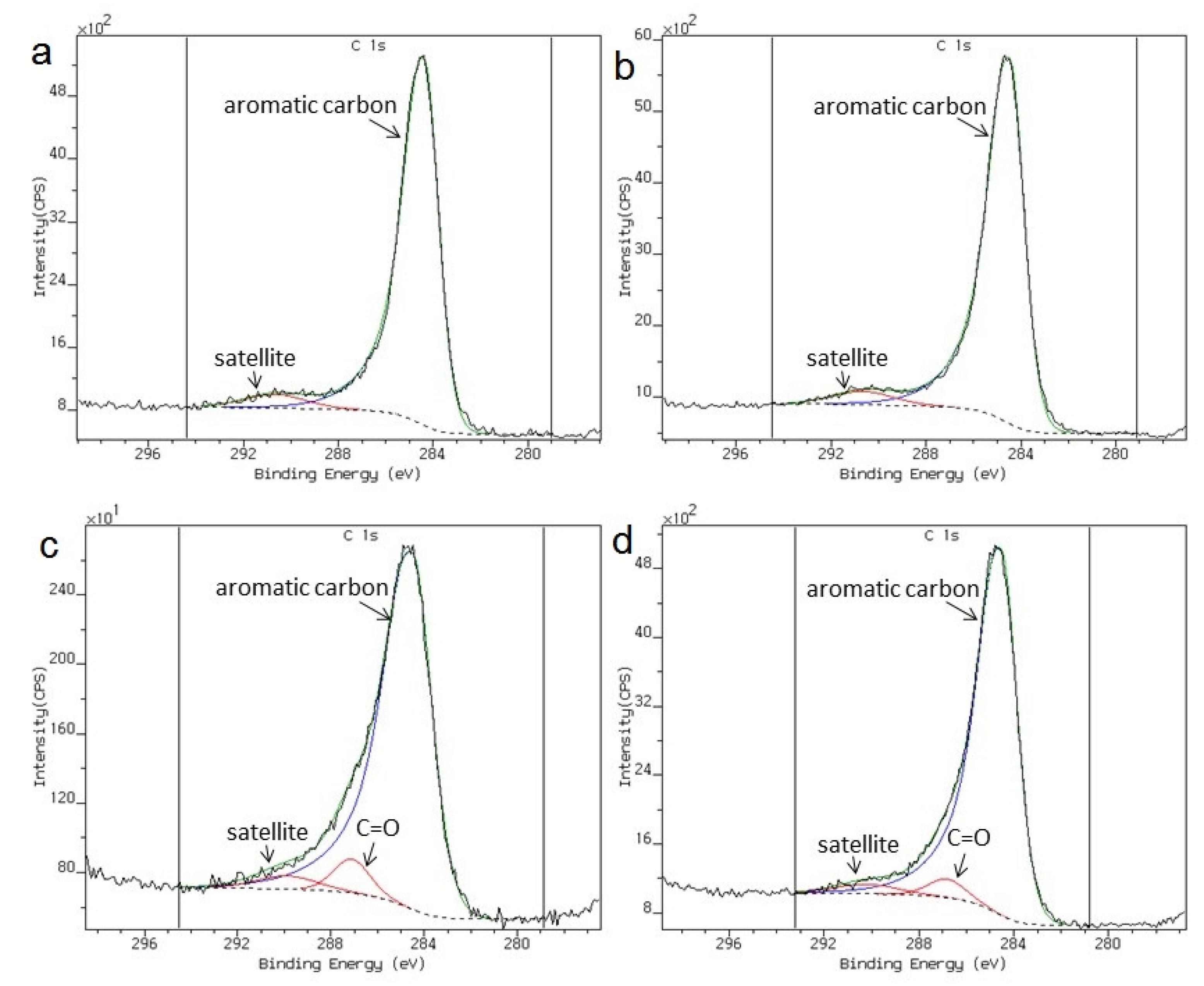
| Sample | Carbon content [wt%] | ZrO2 content ans size [wt%, nm] | ZrC content and size [wt%, nm] | |||
|---|---|---|---|---|---|---|
| Graphite | Amorphous carbon | Tetra | Mono | Cubic | ||
| R_1000C-2h | 30 | ~48 | 6 | 16 | ||
| R_1200C-2h | 26 | ~34 | 11 | 29 | ||
| R_1400C-2h | 38 | ~22 | 15 | 18 | 7 | |
| R_1400C-8h | 32 | ~10 | 18 | 15 | 15 | 8 |
| R_1000C_2h_P_Ar-He | >35 | -- | 27 | 11 | 11 | 16 |
| Sample | BET-specific surface area (m2/g) |
|---|---|
| R_1000C-2h | 15 |
| R_1200C-2h | 27 |
| R_1400C-2h | 43 |
| R_1400C-8h | 115 |
| R_1000C_2h_P_Ar-He | 278 |
| Functional group | Conditions | ZrO2 content and crystallite size | ZrC content and crystallite size | Ref. |
|---|---|---|---|---|
| -SO3H, ZrOCl2 loaded | 1400 °C, 8 h, 2% DVB | 7%tetragonal and 11% monoclinic, each size was 32 nm | No ZrC formation | [5] |
| 1400 °C, 8 h, 8% DVB | <5%tetragonal and <5% monoclinic, 20 and 27 nm, respectively | No ZrC formation | [5] | |
| RF Plasma, He | 16% tetragonal, 13% monoclinic, <5% cubic, 20, 27 and 27 nm, respectively | 11% ZrC, 23 nm >55% graphite content |
[5] | |
| RF Plasma, H2 | 17% tetragonal, 10% monoclinic, <5% cubic, 18, 27, and 22 nm, respectively | 13% ZrC, 21 nm >55% graphite content |
[5] | |
| -CH2-NHC(S)NH2 | 1400 °C, 8 h, 2% DVB | 18% tetragonal, 15% monoclinic, 15% cubic, 71, 66, and 52 nm, respectively | 8% ZrC content, 44 nm (32 and 10% graphite and amorphous C content) | |
| RF plasma, He | 27% tetragonal, 15% monoclinic, 11% cubic, 65, 69, and 53 nm, respectively | 16% ZrC content, 41 nm (only graphite is present as carbonaceous phase) | ||
| -CH2-N(CH2COOH)2, ZrOCl2 loaded | 1200 °C, 2 h | 15% tetragonal, 11 nm | 75% ZrC, 10 nm (no graphite and 10% amorphous C content) |
[6] |
| -CH2-N(CH2COOH)2, ZrOCl2 loaded | 1400 °C, 8 h | 5% tetragonal/monoclinic=1.92, 26 nm | 85% ZrC, 14 nm (no graphite and 10% amorphous C content) |
[6] |
| -CH2-N(CH2COOH)2, Zr-sulphate loaded | 1400 °C, 8 h | 5% monoclinic/cubic=1.05, 31 nm | 50% ZrC, 56 nm (no graphite and 45% amorphous C content) |
[6] |
| -CH2-N(CH2COOH)2, Zr-nitrate loaded | 1400 °C, 8 h | No ZrO2 | 95% ZrC, 18 nm (5% amorphous C content) |
[6] |
| -CH2-N(CH2COOH)2, Zr-nitrate loaded | RF plasma, He | No ZrO2 | 95% ZrC, 62 nm (5% graphite) |
[6] |
| -CH2-N(CH2COOH)2, Zr-nitrate–loaded | RF plasma, H2 | No ZrO2 | 90% ZrC, 35 nm (10% graphite) |
[6] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





