1. Introduction
Multiple Sclerosis (MS) is a chronic, inflammatory, degenerative disease affecting both grey and white matter of the Central Nervous System [
1]. Cognitive impairment occurs in up to 70% of individuals with MS [
2,
3,
4], who typically show affected cognitive functioning in the domains of sustained attention, information processing speed, memory, and executive functions [
5,
6,
7].
Although cognitive impairment is one of the most frequent symptoms of MS, it is unclear why some patients do not show such dysfunctions [
8,
9], which usually appear in older individuals [
10] and in those with a longer history of disease (i.e., disease duration) [
11]. Recent longitudinal studies showed that higher motor disability, measured on the Expanded Disability Status Scale (EDSS) [
12], is correlated with lower cognitive functioning [
13,
14], even if the relationship between motor and cognitive impairment is still controversial [
15].
Recently, Cognitive Reserve (CR) has been considered a construct able to explain a possible variance of cognitive impairment in individuals with MS [
16,
17], similarly to what it does in healthy older populations at risk of dementia [
18]. CR allows to sustain cognitive performance when individuals have to face age-related changes, brain damage or disease [
19,
20,
21]). CR derives from lifetime experiences, intellectual activity, and environmental factors, all things that allow individuals to maintain good cognitive efficiency [
22]. Several proxies are used to quantify CR: education, occupational attainment, engagement in leisure and social activities, IQ and vocabulary size [
23,
24]. One of the most used tools to quantify CR, in a standardised way, is the Cognitive Reserve Index questionnaire (CRIq) [
25].
Some studies have examined the role of CR in modulating cognitive functioning in individuals with MS [
26,
27]. In general, results of such studies have shown that high levels of CR may be associated with less cognitive dysfunction [
28,
29,
30]. Instead, together with old age, low levels of CR are one of the most significant predictors of cognitive decline in MS [
31,
32].
However, only few studies have focused on the role of CR in the cognition of individuals with MS while also considering clinical indices such as disease duration, age of onset, level of motor disability. Rimkus et al. (2018) [
33] suggested that the protective effect of CR is strongest in the early years after disease onset when there is a reduced effect of inflammatory activity on cognition. A meta-analysis [
28] has highlighted the only effect of CR on cognitive performance and not on other clinical variables such as disease severity, disease duration, motor disability (measured with EDSS) and type of MS. Furthermore, Artemiadis et al. (2020) [
34] underlined that in individuals with the same level of motor disability, those with higher CR showed better cognitive performance.
These findings, however, are controversial and further investigation is needed. Indeed, CR was quantified through a heterogeneous pool of proxies and, in most cases, without a composite index. Tremblay et al.’s (2023) [
35] investigation considered a composite measure of CR using the standardised and reliable Cognitive Reserve Index questionnaire (CRIq) [
25]. The Authors found that CR moderated the relationship between EDSS and cognition, but they did not take into account disease duration.
The purpose of the present investigation is to verify whether a higher level of CR may interact with disease duration and motor disability in predicting cognitive outcomes. In particular, we hypothesised that, despite disease duration and motor impairment, a higher level of CR (quantified with the CRIq) may allow individuals with MS to maintain good cognitive efficiency. Due to the multiple factors considered in our hypothesis, we analysed the data through the Generalized Additive Models (GAMs) [
36], a statistical approach which allowed us to verify the complex interaction among multiple continuous variables.
2. Materials and Methods
2.1. Participants
In this cross-sectional study, 100 individuals with MS were enrolled, 88 with Relapsing-Remitting MS and 12 with progressive MS (according to the recent guidelines by Lublin et al., 2015) [
37]. Participants’ (72 F and 28 M, F: M=2.6:1) mean age was 50.9±9.25 (range 30-74), mean education was 12.5±3.53 years (range 5-21), disease duration ranged from 1 to 37 years (mean=14.82±9.28) and motor disability, measured through EDSS, ranged from 1.0 to 8.0 (mean=3±1.89). Most participants were treated with first- or second-line drugs, whereas 7% of them had no pharmacological therapy for a number of reasons (e.g., personal choice, no availability of appropriate drugs for the typology of MS).
Inclusion criteria were: (i) age >18, (ii) no history/evidence of psychiatric or neurological disorders other than Multiple Sclerosis, (iii) no history of alcohol or drug abuse, (iv) no hearing difficulties, (v) Italian mother tongue.
2.2. Procedure
Participants were recruited between January and July 2021 at the Multiple Sclerosis Centre of the University Hospital of Padua, where they arrived for diagnosis or for clinical follow-ups. The entire procedure of this study was conducted remotely, by telephone. Each participant was informed about the research purpose and their consent was acquired via audio-recording. The examiner ensured that the room was quiet and distraction-free and that the telephone connection was stable.
All participants first answered the Cognitive Reserve Index questionnaire (CRIq) [
25] and then underwent the Tele-Global Examination of Mental State, version A (Tele-GEMS) [
38]. The whole administration lasted about 20 minutes.
The study was approved by the Ethical Committee of the Hospital of Padua (Prot. N 19669) and it was conducted in accordance with the Declaration of Helsinki.
2.3. Assessment and Materials
For each participant, Clinical variables, Cognitive reserve, and Neuropsychological measures were collected.
Clinical variables: included 1) Disease Duration (in years) registered in the clinical report, and personal anamnesis; 2) Motor disability, assessed through EDSS [
12] administered by trained neurologists. The other two tools were administered by a trained psychologist.
Cognitive Reserve: measured on the Cognitive Reserve Index Questionnaire (CRIq) [
25], which is a semi-structured interview to quantify a person's CR considering education (CRI-Education), working activity (CRI-WorkingActivity), and leisure time activities (CRI-LeisureTime) carried out during adulthood. The administration of CRIq provides the Index of cognitive reserve (CRI). A full description of the 20 items included in the CRIq and instructions for its administration are available on
https://www.cognitivereserveindex.org/.
Neuropsychological assessment. Cognitive functioning was assessed through Tele-Global Examination of Mental State (Tele-GEMS) [
38], a new cognitive screening tool that is administered remotely and consists of ten tasks investigating different cognitive domains: orientation, memory, working memory, spatial representation, language, auditory attention, verbal fluency, and pragmatic abilities. It provides an index of global cognitive functioning, which reflects the balanced contribution of each task to a total score that ranges from 0 to 100. The administration lasts about 10 minutes. Instructions, score sheets, and cut-offs according to age, education, and CR are available at
https://osf.io/r3ta5/.
2.4. Statistical Analysis
All statistical analyses were performed with R software [
39]. First, an independent sample t-test was carried out to analyse possible differences according to sex to verify whether to include this variable in the subsequent analyses. Descriptive correlation analyses among our predictors were calculated through Pearson’s
r. Generalized Additive Models (GAMs) [
36] of increasing complexity were used to investigate the interaction between clinical variables (i.e., EDSS and Disease Duration) and cognitive reserve (CRI) on the one side, and cognitive functioning (Tele-GEMS) on the other. Notably, GAMs are more flexible than the general linear models, allowing to test non-linear relationships between the independent and dependent variables. GAMs can also manipulate complex data, and their visualisation can help to understand the relationship between variables. In our analyses, the models were built from simpler to more complex in order to examine the best pattern of variables that would fit our data. The goodness of the models was assessed with Akaike Information Criterion (AICc) and GCV was used to check for any possible overfitting (GCV) [
40,
41]. We also calculated the Akaike Information Criterion Weights (AICw) which is the probability of a model being the best among a given set of models and, as such, it can be used for model selection. The best model was chosen according to AICw (R
2 and AIC are also reported) and a series of diagnostic tests were carried out to check the appropriateness of model characteristics. We also checked the quality of GAM models by visual inspection of the residuals.
3. Results
Descriptive statistics of our variables are reported in
Table 1.
Our sample did not show any difference between males and females on Tele-GEMS performance (t(98)=-0.79, p=0.43); thus, sex was not included as a predictor in GAM models.
Correlational analyses showed that Tele-GEMS correlated positively with CRI (
r=0.50,
p<0.001) and negatively with Age (
r=-0.29,
p=0.003), EDSS (
r=-0.44,
p<0.001) and Disease Duration (
r=-0.23,
p=0.02). Correlations among all our variables of interest (Tele-GEMS, CRI, Age, EDSS and Disease Duration) are reported in
Table 2.
Generalized Additive Models analyses (GAMs) underlined that the best Model was the one having Age, CRI, EDSS, Disease Duration as covariates and including the interaction between CRI and EDSS and CRI and Disease Duration (Model 4). In particular, Model 4 had the highest R
2 (R
2= 0.458 and 50.9% Deviance explained) and the lowest AIC. We chose Model 4 as it was the best according to AICw (See
Table 3). A series of diagnostic tests (R function ‘gam.check’) showed appropriate characteristics for the tensors (for CRI*Disease Duration: k’=4,
p=0.78; for CRI*EDSS: k’=4,
p=0.99).
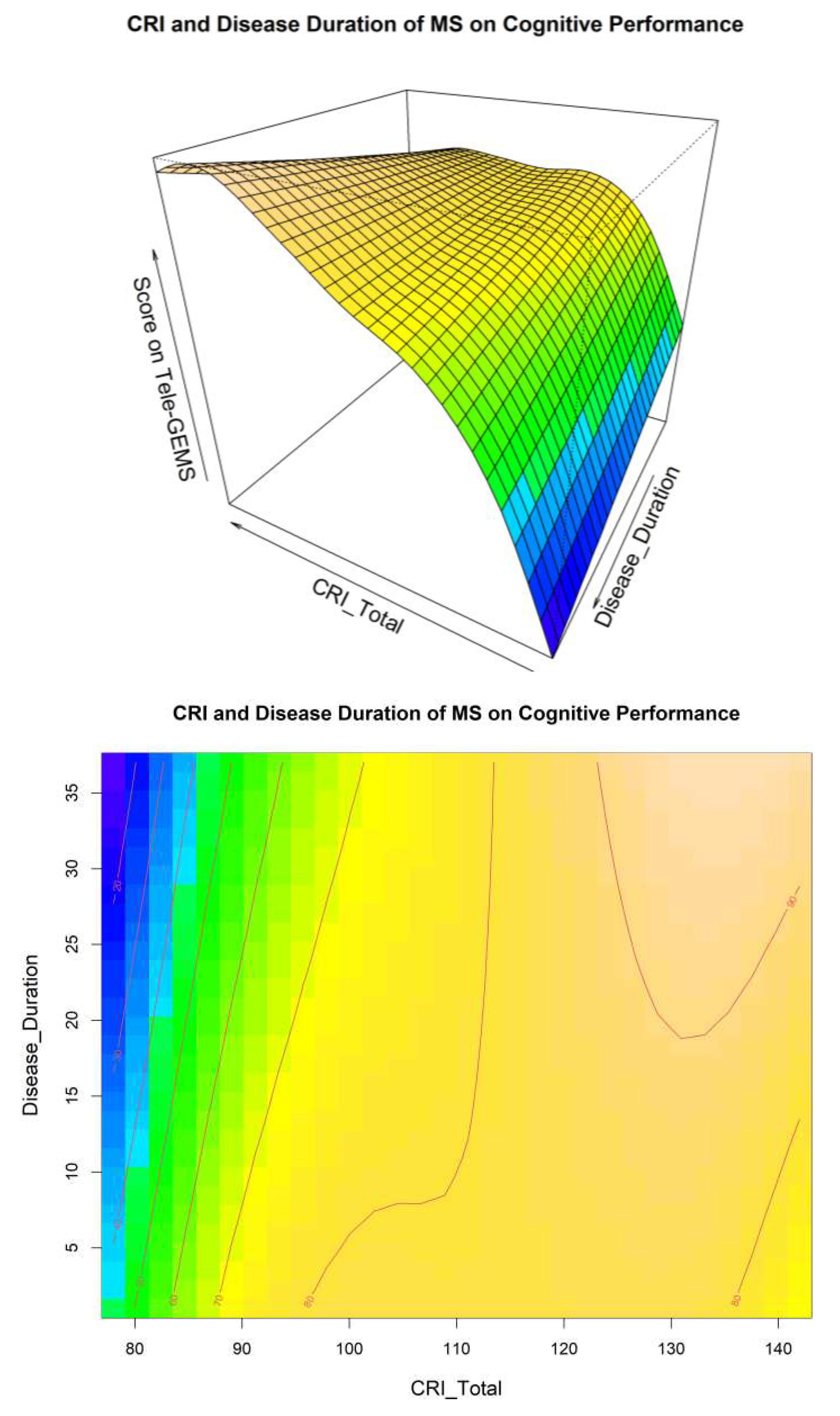
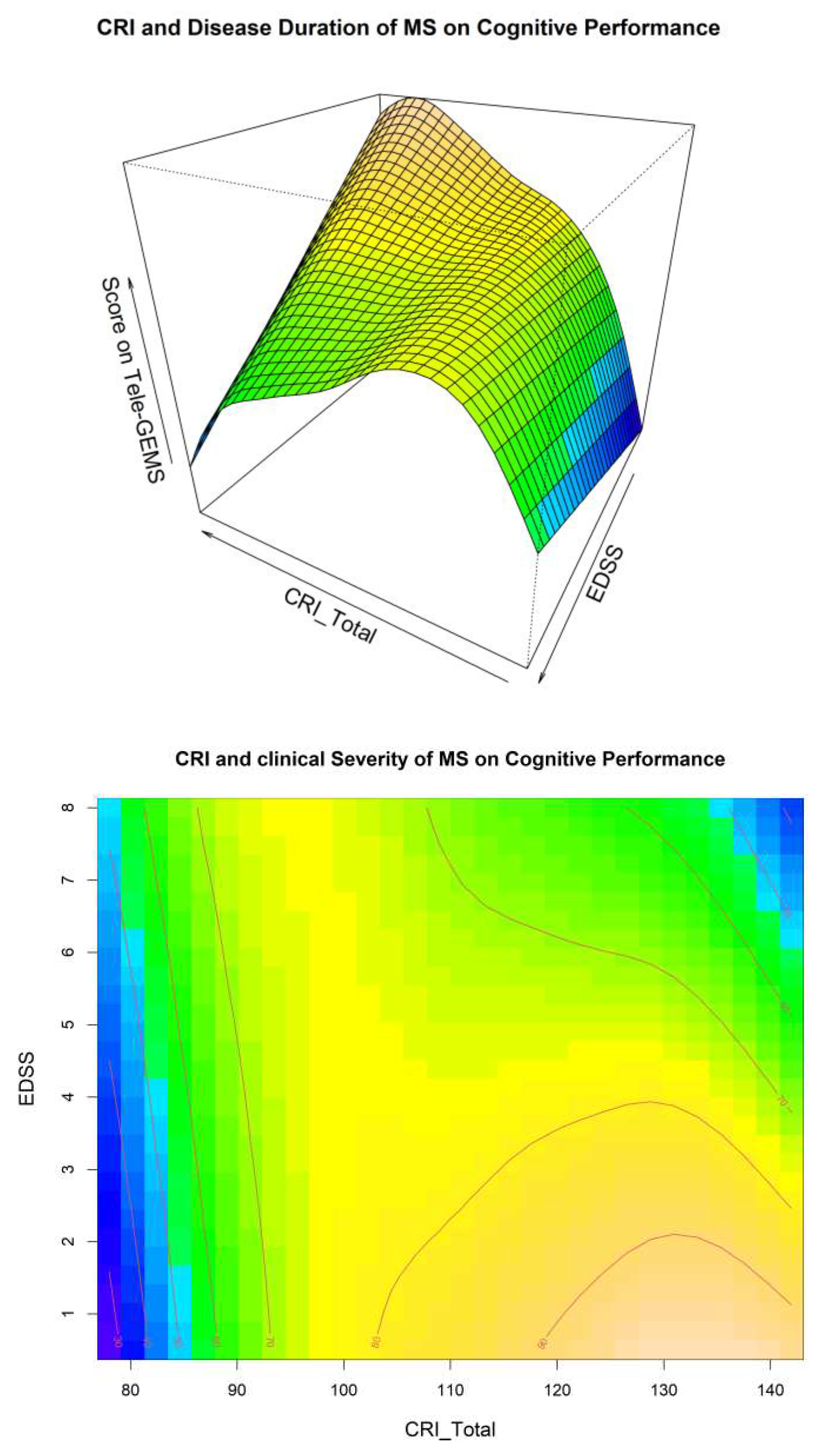
A visual inspection of Model 4 related to the interaction between CRI and Disease Duration is shown in
Figure 1, and related to the interaction between CRI and EDSS in
Figure 2. These two Figures give additional information on the interaction between CR EDSS and Disease Duration in predicting cognitive performance (i.e., Tele-GEMS). Tele-GEMS scores are represented with different colours: the darker shades of blue in the contour plot represent lower scores, while the darker shades of yellow represent higher scores.
Figure 1 shows the interaction between CRI and Disease Duration, highlighting that high levels of CRI (on the x-axis) are associated with better Tele-GEMS (on the y-axis) even when the Disease Duration (on the z-axis) is very high. However, a negative effect of Disease Duration is found on Tele-GEMS in persons with low CR.
Figure 2 shows the interaction between CRI and EDSS, indicating that high levels of CRI (on the x-axis) correspond to higher Tele-GEMS (on the y-axis), but that such a positive effect of CRI is significantly attenuated when EDSS (on the z-axis) is very high (i.e., severe motor disability). This is clear in
Figure 2 (right side) in correspondence of the dark blue in the upper right-hand side corner. In the case of low CR, instead, Tele-GEMS is low at any level of EDSS.
4. Discussion
The present study aimed to investigate the impact of factors such as CR, disease duration and motor disability on the cognitive efficiency of people with Multiple Sclerosis. It is known that high levels of CR are associated with better cognitive performance in both healthy [
42] and pathological people, such as those who have dementia [
43] or MS [
30]. Our data, obtained with a sample of 100 people with MS, confirmed what is reported in the literature [
11,
14]: cognitive efficiency is negatively correlated with disease duration and with motor disability, but positively correlated with CR.
The added value of the present study is the methodological approach adopted. Indeed, the use of GAMs [
36] allowed us to consider the potentially complex interaction between different clinical variables and socio-demographic variables, at the same time disentangling their respective effects. In the present study, motor disability and disease duration in interaction with CR and age were considered as predictors of cognitive performance. In the literature, these same variables are rarely considered together in interaction. Our results confirm the positive effect of CR in predicting a better cognitive profile regardless of disease duration and motor disability, but not in the case of severe motor impairment (i.e., EDSS= 6-7, corresponding to needed bilateral assistance to walk 100 m.). This is in contrast with a recent paper [
33] whose results confined a positive effect of CR to the first five years of the disease. Further research is needed to clarify how CR may modulate cognitive performance in individuals with MS at different stages of the disease. As MS is a degenerative disorder, our findings (i.e., an extended positive effect of CR throughout the disease) are crucial, since they suggest that patients with high CR may maintain a suitable quality of life for longer. However, at a high level of motor impairment CR is no longer protective and, at this point of the disease, a cognitive decline appears inevitable. In line with our results, Tremblay et al.’s (2023) [
35] research highlighted the modulating effect of CR on motor disability and underlined its effect on specific cognitive domains (i.e., visuospatial memory and processing speed); however, they had not considered disease duration, which we found is an important clinical variable.
In this context, Amato et al. (2019) [
31] reported similar conclusions, but on child and adolescent patients with MS. In their research, they found a positive relationship between physical activity and CR in predicting cognitive performance, a relationship that has been gaining more and more attention in the context of CR [
44,
45]. These findings suggest the potential benefits of training focused on both cognitive and physical activities in enhancing CR and, consequently, reducing possible decline.
This cross-sectional investigation is not, however, exempt from limitations. First of all, MS is a progressive disorder and any possible effect of CR over time should be further investigated through longitudinal observations, in which the clinical changes in MS patients would be systematically monitored. Secondly, neuroimaging data were not available for this study. However, if on the one hand the use of such data would have allowed us to better characterise brain deterioration, on the other, GAMs provided a solid pool of information accounting for the complex interaction among different clinical variables.
Other important factors possibly at play in determining cognitive efficiency in our sample would be perceived fatigue, type of medication, mood deflection and other psychological traits. These characteristics have not been considered in the present study and further investigation would prove helpful to examine the interaction between CR and clinical MS manifestations.
5. Conclusions
In conclusion, this study reveals that accounting for the interplay between CR and disease duration, as well as between CR and motor disability, may explain a significant degree of variance in cognitive performance. Our results, which we hope will be of use to clinicians and researchers, indicate that high levels of CR are related to improved cognitive performance even in prolonged disease duration, although severe motor disability may, at some point, attenuate this “protective effect”.
Author Contributions
Conceptualization, Sa. Mo., and A.R; methodology, So.Mo., G.A., and V.P., formal analysis, So.Mo., G.A., and V.P.; investigation, Sa. Ma., data curation, Sa.Ma. and V.P.; writing—original draft preparation Sa.Mo., V.P., and Sa.Ma.; writing—review and editing, all authors; visualization, So.Mo. and G.A., supervision, Sa.Mo and P.G.; All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Hospital of Padua (Prot. N 19669).
Informed Consent Statement
Informed consent was obtained from all patients involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author under a reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dobson, R., Giovannoni, G. Multiple sclerosis - a review. Eur J Neurol 2019, 26(1), 27–40.
- Oreja-Guevara, C., Ayuso Blanco, T., Brieva Ruiz, L., Hernández Pérez, M.Á., Meca-Lallana, V., Ramió-Torrentà, L. Cognitive Dysfunctions and Assessments in Multiple Sclerosis. Front neurol 2019, 10, 581.
- Sumowski, J.F., Benedict, R., Enzinger, C., Filippi, M., Geurts, J.J., Hamalainen, P., Hulst, H., Inglese, M., Leavitt, V.M., Rocca, M.A., Rosti-Otajarvi, E.M., Rao, S. Cognition in multiple sclerosis: State of the field and priorities for the future. Neurol 2018, 90(6), 278–288.
- Chiaravalloti, N.D., DeLuca, J. Cognitive impairment in multiple sclerosis. Lancet Neurol 2008, 7(12), 1139–1151.
- Benedict, R.H.B., Amato, M.P., DeLuca, J., Geurts, J.J.G. Cognitive impairment in multiple sclerosis: clinical management, MRI, and therapeutic avenues. Lancet Neurol 2020, 19(10), 860–871.
- Grzegorski, T., Losy, J. Cognitive impairment in multiple sclerosis - a review of current knowledge and recent research. Rev Neurosci 2017, 28(8), 845–860.
- Rocca, M.A., Amato, M.P., De Stefano, N., Enzinger, C., Geurts, J.J., Penner, I.K., Rovira, A., Sumowski, J.F., Valsasina, P., Filippi, M., MAGNIMS Study Group. Clinical and imaging assessment of cognitive dysfunction in multiple sclerosis. Lancet Neurol 2015, 14(3), 302–317.
- Ghaffar, O., Fiati, M., Feinstein, A. Occupational attainment as a marker of cognitive reserve in multiple sclerosis. PloSone 2012, 7(10), e47206.
- Benedict, R. H., Zivadinov, R. Risk factors for and management of cognitive dysfunction in multiple sclerosis. Nat Rev Neurol 2011, 7(6), 332–342. 6.
- Brochet, B., Ruet, A. Cognitive Impairment in Multiple Sclerosis With Regards to Disease Duration and Clinical Phenotypes. Front neurol 2019, 10, 261.
- Tremblay, A., Charest, K., Brando, E., Roger, E., Duquette, P., Rouleau, I. The effects of aging and disease duration on cognition in multiple sclerosis. Brain Cog 2020, 146, 105650.
- Kurtzke J.F. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurol 1983, 33(11), 1444–1452.
- Yigit, P., Acikgoz, A., Mehdiyev, Z., Dayi, A., Ozakbas, S. The relationship between cognition, depression, fatigue, and disability in patients with multiple sclerosis. Ir J Med Sci 2021, 190(3), 1129–1136.
- Heled, E., Aloni, R., Achiron, A. Cognitive functions and disability progression in relapsing-remitting multiple sclerosis: A longitudinal study. Appl neuropsychol. Adult 2021, 28(2), 210–219.
- Amato, M.P., Portaccio, E., Goretti, B., Zipoli, V., Iudice, A., Della Pina, D., Malentacchi, G., Sabatini, S., Annunziata, P., Falcini, M., Mazzoni, M., Mortilla, M., Fonda, C., De Stefano, N., TuSCIMS Study Group. Relevance of cognitive deterioration in early relapsing-remitting MS: a 3-year follow-up study. Mult scler J 2010, 16(12), 1474–1482.
- Martins Da Silva, A., Cavaco, S., Moreira, I., Bettencourt, A., Santos, E., Pinto, C., Gonçalves, A., Coutinho, E., Samões, R., Dias, C.C., Teixeira-Pinto, A., Da Silva, B.M., Montalban, X. Cognitive reserve in multiple sclerosis: Protective effects of education. Mult scler J 2015, 21(10), 1312–1321.
- Amato, M.P., Razzolini, L., Goretti, B., Stromillo, M.L., Rossi, F., Giorgio, A., Hakiki, B., Giannini, M., Pastò, L., Portaccio, E., De Stefano, N. Cognitive reserve and cortical atrophy in multiple sclerosis: a longitudinal study. Neurol 2015, 80(19), 1728–1733.
- Mondini, S., Pucci, V., Montemurro, S., Rumiati, R.I. Protective factors for subjective cognitive decline individuals: trajectories and changes in a longitudinal study with Italian elderly. Eur J Neurol 2022, 29(3), 691–697.
- Stern, Y., Arenaza-Urquijo, E.M., Bartrés-Faz, D., Belleville, S., Cantilon, M., Chetelat, G., Ewers, M., Franzmeier, N., Kempermann, G., Kremen, W.S., Okonkwo, O., Scarmeas, N., Soldan, A., Udeh-Momoh, C., Valenzuela, M., Vemuri, P., Vuoksimaa, E., the Reserve, Resilience and Protective Factors PIA Empirical Definitions and Conceptual Frameworks Workgroup. Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers dement: the journal of the Alzheimer's Association 2020, 16(9), 1305–1311.
- Pettigrew, C., Soldan, A. Defining Cognitive Reserve and Implications for Cognitive Aging. Curr Neurol Neurosci Rep 2019, 19(1), 1.
- Barulli, D., Stern, Y. Efficiency, capacity, compensation, maintenance, plasticity: emerging concepts in cognitive reserve. Trends cog sci 2013, 17(10), 502–509.
- Stern, Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsycol Soc 2002, 8(3), 448–460. [Google Scholar] [CrossRef]
- Grotz, C.; Seron, X.; Van Wissen, M.; Adam, S. How should proxies of cognitive reserve be evaluated in a population of healthy older adults? Int psychogeriatr 2017, 29(1), 123–136. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.L., Sajjad, A., Bramer, W.M., Ikram, M.A., Tiemeier, H., Stephan, B.C. Exploring strategies to operationalize cognitive reserve: A systematic review of reviews. J clin exp neuropsychol 2015, 37(3), 253–264.
- Nucci, M., Mapelli, D., Mondini, S. Cognitive Reserve Index questionnaire (CRIq): a new instrument for measuring cognitive reserve. Aging clin exp res 2012, 24(3), 218–226.
- Luerding, R.; Gebel, S.; Gebel, E.M.; Schwab-Malek, S.; Weissert, R. Influence of Formal Education on Cognitive Reserve in Patients with Multiple Sclerosis. Front neurol 2016, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Sumowski, J.F., Rocca, M.A., Leavitt, V.M., Riccitelli, G., Meani, A., Comi, G., Filippi, M. Reading, writing, and reserve: Literacy activities are linked to hippocampal volume and memory in multiple sclerosis. Mult scler J 2016, 22(12), 1621–1625.
- Santangelo, G., Altieri, M., Enzinger, C., Gallo, A., Trojano, L. Cognitive reserve and neuropsychological performance in multiple sclerosis: A meta-analysis. Neuropsychol 2019, 33(3), 379–390.
- Ifantopoulou, P., Artemiadis, A. K., Bakirtzis, C., Zekiou, K., Papadopoulos, T.S., Diakogiannis, I., Hadjigeorgiou, G., Grigoriadis, N., Orologas, A. Cognitive and brain reserve in multiple sclerosis--A cross-sectional study. Mul scler relat disord 2019, 35, 128–134.
- Sumowski, J.F.; Leavitt, V.M. Cognitive reserve in multiple sclerosis. Mult scler J 2013, 19(9), 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.P., Prestipino, E., Bellinvia, A., Niccolai, C., Razzolini, L., Pastò, L., Fratangelo, R., Tudisco, L., Fonderico, M., Mattiolo, P.L., Goretti, B., Zimatore, G.B., Losignore, N.A., Portaccio, E., Lolli, F. Cognitive impairment in multiple sclerosis: An exploratory analysis of environmental and lifestyle risk factors. PloSone 2019, 14(10), e0222929.
- Nunnari, D., De Cola, M.C., Costa, A., Rifici, C., Bramanti, P., Marino, S. Exploring cognitive reserve in multiple sclerosis: New findings from a cross-sectional study. J clin exp neuropsychol 2016, 38(10), 1158–1167.
- Rimkus, C.M.; Avolio, I.M.B.; Miotto, E.C.; Pereira, S.A.; Mendes, M.F.; Callegaro, D.; Leite, C.D.C. The protective effects of high-education levels on cognition in different stages of multiple sclerosis. Mult scler relat disord 2018, 22, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Artemiadis, A.; Bakirtzis, C.; Ifantopoulou, P.; Zis, P.; Bargiotas, P.; Grigoriadis, N.; Hadjigeorgiou, G. The role of cognitive reserve in multiple sclerosis: A cross-sectional study in 526 patients. Mult scler relat disord 2020, 41, 102047. [Google Scholar] [CrossRef] [PubMed]
- Trembley, A.; Charest, K.; Brando, E.; Roger, E.; Duquette, P.; Rouleau, I. Cognitive reserve as a moderating factor between EDSS and cognition in multiple sclerosis. Mult scler relat disord 2023, 70, 104482. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.N. Generalized additive models: An introduction with R, second edition 2017. In Generalized Additive Models: An Introduction with R, Second Edition.
- Lublin, F.D., Reingold, S.C., Cohen, J.A., Cutter, G.R., Sørensen, P.S., Thompson, A.J., Wolinsky, J.S., Balcer, L.J., Banwell, B., Barkhof, F., Bebo, B., Calabresi, P.A., Clanet, M., Comi, G., Fox, R.J., Freedman, M.S., Goodman, A.D., Inglese, M., Kappos, L., Kieseier, B.C., … Polman, C.H. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurol 2014, 83(3), 278–286.
- Montemurro, S.; Mondini, S.; Pucci, V.; Durante, G.; Riccardi, A.; Maffezzini, S.; Scialpi, G.; Signorini, M.; Arcara, G. Tele-Global Examination of Mental State (Tele-GEMS): an open tool for the remote neuropsychological screening. Neurol sci 2023, 1–10. [Google Scholar]
- CIR Core Team, “R: A language and environment for statistical computing.” R Foundation for Statistical Computing, Vienna, 2022, [Online]. Available: https://www.r-project.org/.
- Burham, K.P., Anderson D.R. Model selection and multimodel inference: a practical information-theoretic approach. Springer-Verlag 2002, New York.
- Symonds, M.R.E.; Moussalli, A. A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behav Ecol Sociobiol 2011, 65, 13–21. [Google Scholar] [CrossRef]
- Opdebeeck, C., Martyr, A., Clare, L. Cognitive reserve and cognitive function in healthy older people: a meta-analysis. Aging Neuropsychol Cog 2016, 23(1), 40-60.
- Nelson, M.E., Jester, D.J., Petkus, A. J., Andel, R. Cognitive Reserve, Alzheimer's Neuropathology, and Risk of Dementia: A Systematic Review and Meta-Analysis. Neuropsychol rev 2021, 31(2), 233–250.
- Pucci, V.; Guerra, C.; Barsi, A.; Nucci, M.; Mondini, S. How long have you exercised in your life? The effect of motor reserve and current physical activity on cognitive performance. J Int Neuropsychol Soc 2023, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, L.; Olivito, G.; Urbini, N.; Silveri, M.C.; Leggio, M. "Mens Sana in Corpore Sano": The Emerging Link of Motor Reserve with Motor and Cognitive Abilities and Compensatory Brain Networks in SCA2 Patients. Biomedicines 2022, 10(9), 2166. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).