1. Introduction
The rapid spread of multidrug-resistant bacteria and the slow discovery of new classes of antibiotics is leading researchers to consider alternatives to classical antibiotics. Antimicrobial peptides (AMPs) represents one promising alternative [
1,
2]. AMPs are a ubiquitous part of the innate immune defence in all living organisms, from humans to bacteria themselves. They have been widely studied and today, more than 3000 natural AMPs have been reported and characterized [
3,
4,
5]. However, most natural AMPs are ill-suited as drug candidates due in part to either poor ADME-Tox properties (absorption, distribution, metabolism and toxicity) or overly complex chemistry [
6,
7]. Consequently,
de novo designed antimicrobial peptides (AMP) are being developed, with simplified sequences focusing on a small number of core residues [
8]. These synthetic AMPs combine intelligent design, microbiology, chemistry, and a knowledge of human metabolism in a series of iterations to provide highly efficient compounds. However, despite decades of AMP study, there is a lack of in-depth knowledge of their modes of action, including potential targets for the AMPs in live bacteria. Case in point: while a number of proposed modes have been put forth such as pore-formation, carpeting, and detergent-like, these models are often founded on observations in simplified model systems, at concentrations that are poorly representative of biological conditions – at least with regards to antimicrobial assays [
9,
10].
Currently, the largest class of AMPs contains a significant fraction of cationic residues [
11] . The net cationic charge of these peptides is believed to be essential to their mode of action and selectivity, with the cationic sites playing a crucial electrostatic role in the interaction between the AMPs and components of the bacterial membrane. Arginine and lysine are the most common acids in cationic AMPs with very similar overall abundances, still lysine is more common in AMPs meanwhile arginine is more common in antiviral peptides [
12,
13]. Studies with synthetic cationic AMPs either as derivatives of natural peptides or of
de novo design, have unequivocally shown that arginine analogues display higher antimicrobial efficacy than corresponding lysine analogues [
14,
15,
16]. While the effect of arginine on antimicrobial activity is well established, the underlying mechanism is not fully understood. For example, it has been observed that increased arginine content enhances peptide embedding in artificial POPC:POPG membranes [
15], with the ability of arginine to stabilize its charge in the bilayer [
17], or the ability of arginine to hydrogen bond with surrounding water when involved with cation-π interactions with tryptophan [
17,
18]. However, no consensus hypothesis of this phenomenon exists.
Another essential component of AMP activity is the hydrophobic volume of the peptide. The ratio of charged amino acids and hydrophobic volume is reported to correspond with the activity of AMPs on phospholipid based model membranes, an observation suggesting that the main mode of action for cationic AMPs is bilayer disruption [
19]. The antimicrobial peptide database – ADP3 shows that 80% of all entries possessing reported antimicrobial activities contain at least one of the hydrophobic phenylalanine or tryptophan [
20]. The hydrophobic bulk of these residues are believed to be important to the membrane disruption by positioning in the interfacial region of the bilayer [
10]. In particular, the paddle-like tryptophan leverages its unique indole moiety, forming hydrogen bonds with the lipid-tail carbonyl group and at the same time, its aromatic bulk is able to disturb the lipid packing. Indeed, it has been previously shown that tryptophan substitution with less hydrophobic amino acids can be used to fine-tune the natural lytic ability of rationally designed peptides [
19].
While the importance of the interplay between hydrophobicity and charge for membrane interaction and disruption is well ingrained in the consciousness of AMP research, what is often not considered is that the charge and lipophilic nature of AMPs are important properties for binding to the lipopolysaccharides (LPS) of Gram-negative bacteria. LPS is a major component of the outer membrane of Gram-negative bacteria and acts as a barrier that AMPs need to cross in order to reach the bacterial membrane [
21,
22,
23]. The sequence of initial LPS binding before membrane localization or intracellular binding cannot be avoided and thus initial LPS interaction acts as a major bottleneck for most AMPs. In this work, we have observed that the initial binding to LPS needs to be fine-tuned for cationic AMPs to attain their antimicrobial activity. Just as in Southey’s tale [
24], Goldilocks needed to find her ideal conditions, so the initial interaction between AMPs and the LPS needs to be ideal. Too strong LPS binding may prevent AMPs from reaching the membrane target or cause aggregation of peptides in the cell wall [
25]; LPS interaction that is too weak would reduce the amount of AMP available, and prevent adequate permeation through the tight network of LPS to the bacterial membrane. In short, AMPs need to find their ‘Goldilocks’ zone of LPS affinity, where the interaction is not too tight, not too loose, but "just right".
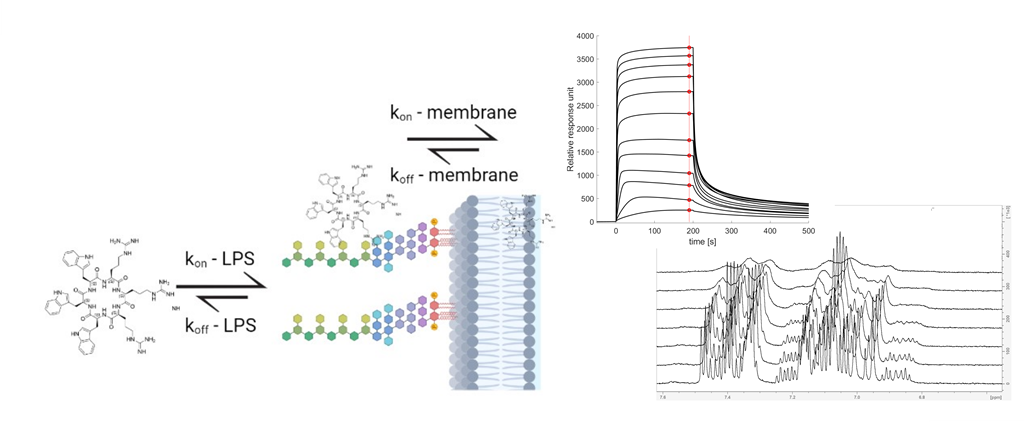
Here we present evidence that the interaction between AMPs and Gram-negative bacteria is driven by an initial binding to the LPS. All compounds reaching the bilayer, or an intracellular target, must first cross the LPS. In addition, we introduce a toolbox of suitable experiments that can be used to quantify this kinetic process – a combination of surface plasma resonance (SPR) and NMR experiments with live bacteria: live-cell NMR. We have tested this toolbox in the study of the interactions of a series of cyclic cationic AMPs (cAMPs) and several E. coli strains, including LPS-deficient and colistin-resistant strains. Using this toolbox, we can quantify the initial binding events towards several E. coli strains, and we are able to identify lysine as LPS binder in ‘Goldilocks’ zone of affinity.
2. Materials and Methods
The All the common chemicals are of analytical purity and supplied by Merck KGaA, Darmstadt, Germany. All the lipid samples had been supplied by Avanti Lipids (Alabaster, Alabama, US).
2.1. Peptide synthesis
The peptides were prepared from 2-Chlorotrityl chloride resin and Fmoc-Trp(Boc)-OH as the first amino acid using solid phase peptide synthesis strategy. The synthesis followed synthetic protocols previously reported [
26]. The side-chain protected linear peptide precursors were prepared by an automated peptide synthesiser (Biotage Initiator+ Alstra). Following resin cleavage, the peptides were head-to-tail cyclised in solution, side-chain deprotected and the crude peptides was purified by preparative reverse-phase high-performance liquid chromatography. Chemical analysis of synthesized peptides is available in Supplementary information.
2.2. MIC testing
The bacterial strains tested were
E. coli ATCC 25922,
E. coli CCUG 70562 and
E. coli NR-698. The MIC assays followed CLSI guidelines [
27]. Overnight cultures and MIC assays were performed in MHBII (212322, Becton, Dickson and Company, Sparks, MD, USA). In addition,
E. coli NR-698 was tested after cultivation with 4 µg/ml of colistin to induce alternation in surface charge.
2.3. Liposomes preparation
The large unilamellar liposomes of DMPC and DMPC:PG (95:5) were prepared for the SPR experiment using standard methods [
28]. In brief, lipid mixtures were weighted from dry powder (Avanti, USA) and dissolved in 3:1 dichloromethane:methanol mixtures. The organic mixture was dried in vacuum rotavapor to form a lipid film which was hydrated to 20 mM total concentration of lipids (for DMPC and DMPC:PG mixture) by adding 10 mM HEPES buffer pH 7.4 with 150 mM NaCl. For DMPC:LPS mixtures, dried LPS was dissolved in water and mixed with DMPC dissolved in dichloromethane. Isopropanol was added to the mixture until only one phase - dichloromethane:isopropanol:water was formed [
29]. Dry film was prepared and then hydrated using the same method as above to a concentration of 18 mM of DMPC and 10% (w/w) LPS in HEPES buffer. Lipid liposomes were extruded 13 times through 100 nm diameter pore using an Avanti Mini Extruder kit.
2.4. SPR
The SPR experiments were performed at room temperature using the L1 chip and T200 Biacore instrument (GE Healthcare, Chicago, IL, USA). The experimental setup is the same as in Jakubec
et al. (2020) [
30]. Briefly, lipid liposomes were immobilized on a cleaned surface of the chip using a flowrate of 2 µl/min for 2400 s. The immobilisation efficiency was tested by injecting 0.2 mg/ml of bovine serum albumin (BSA, A7030, Sigma-Aldrich, Saint Louis, MO, USA) for 1 min at 30 µl/min. The change of RU less than 400 indicated sufficient coverage. Next, diluted peptides were injected over immobilized liposomes using flowrate 15 µl/min for 200 s with a subsequent 400 s dissociation phase. The peptide concentration was from 4 to 128 µM, except for poorly binding peptides c(LWwNKr) and c(WKWKWK), which were set from 24 to 768 µM to reach vesicle saturation. Samples were injected from low to high concentrations due to the possibility of sample retention in the liposomes. Between individual injections, liposomes were regenerated by three subsequent 30s injections of 10 mM NaOH at 30 µl/min flowrate. The control flow cell was treated the same way as the sample cells, except sample injections were replaced by HEPES buffer. The results were processed using in-laboratory written MATLAB scripts (MATLAB R2022a; scripts are available at [
31]). K
D was obtained Using
Equation 1 from a standard steady-state fit:
where RU is measured SPR response; RU
MAX is RU response during saturation; [P] is peptide concentration; and off is offset of response.
K
P was obtained from steady-state using a method developed by Figueira et al (2017) [
32] using
Equation 2:
where RU
S is the relative response of the solute (peptides); RU
L is the total lipid deposition response; γ
L is the molar volume of the lipids (average for mixtures); M
S and M
L are the molecular mass of the solute and lipid, respectively; and [S]
W is the concentration of solute in water. K
P is the partitioning constant, while σ is the lipid-to-solute ratio. The two latter are obtained from the fit.
The k
off rate was obtained from the first 200 s of dissociation using adapted formalism from Figueira
et al. (2017) using
Equation 3. Contributions from two populations were identified, so the average k
off response was calculated by
Equation 4.
where S
L is the linearised ratio of responses of the solute and lipid, which is plotted against the time of dissociation; α and β are individual populations; and S
L,r is the retained solute fraction.
The association rate k
on was obtained from experimentally measured K
D and k
off by
Equation 5:
2.5. NMR experiments
NMR spectra were acquired on a Bruker Avance III HD spectrometer operating at 600 MHz for
1H, equipped with an inverse TCl cryo probe. All NMR spectra were acquired at 298 K using 5 mm tubes using standard pulse programs. [
31]
2.5.1. Live cell NMR
E. coli strains ATCC 25922, CCUG 70662, and NR 698 were grown until OD
600 ≈ 0.6-0.8 in standard Lysogeny Broth at 37 °C from overnight colonies on agar plates. This allowed bacterial cultures to reach an exponential state of growth. CCUG 70662 was cultivated with and without 4 µg/ml of colistin in both agar and broth to allow for modification of the lipid A part of LPS [
33]. After that, cells were carefully centrifuged by 6000xg for 10 min, the supernatant was discarded and the cell pellet was resuspended in 10 mM Tris buffer, with 100 mM NaCl, and pH 7.4. The process was repeated two more times to wash out broth components and leftover colistin. Cells were then resuspended in 1 ml of Tris buffer and OD
600 was checked by dilution of 100 µl of the sample. The number of cells was estimated from OD
600 of the sample using the formulation presented by Beal
et al., (2022) [
34]. Furthermore, lipid concentration was estimated to be 50 mmol per bacterial cell [
35]. The part of samples was diluted to OD = 1 in Tris buffer and zeta potential was measured on Malvern Zetasizer Nano (Malvern Ins., Malvern, UK). The concentrated cell sample was then titrated (from 5 to 160 µl) into the 500 µl of peptide (1 mg/ml) in Tris buffer with 5% D
2O and
1H spectra were collected using double solvent suppression with excitation sculpting and presaturation (zqesgppepr) with extended delay d1=8s to allow for complete relaxation of nuclei.
Spectra were processed automatically using TopSpin 4.0.8 (Bruker, Germany) and by Matlab R2020b with Signal processing and Bioinformatics toolbox (USA, MA, Natick). The processing scripts are available at Github [
31].
K
D was estimated from individual signal integral by using
Equation 6 developed by Shortridge et al., (2008) [
36] for invisible binding states:
where B
expt is measured integral decrease; [Lip]
T is total lipid concentration estimated from the amount of titrated cells; [AMP]
T is total peptide concentration; and γ
B and γ
F are the line widths of bound and free AMP, respectively. Due to the nature of interaction and the invisibility of bound state line width ratio was estimated from fit and it was used for all proton signals. This value was different for each combination of AMP and bacterial sample and it is noted in supplementary.
2.5.2. Assignment
NOESY, TOCSY, DQF-COSY, 15N-HSQC, HSQC, HMBC, and H2BC NMR spectra were recorded with standard settings. NOESY and TOCSY spectra were recorded with mixing times of 100-400 ms and 80 ms, respectively. Spectra were processed with TopSpin 3.6.0, and assignments done manually. The full assignment is reported in Supporting Information.
3. Results and discussion
3.1. Cyclic peptides with balanced lipophilic bulk and cationic charge
In the present work, we have selected to limit the conformational freedom the peptides may adopt with head to tail cyclisation. The peptides designed for this study were cyclohexapeptides with an equal number of cationic residues (Arg or Lys) and bulky lipophilic residues (Trp), thus fulfilling the pharmacophore for short synthetic cationic antimicrobial peptides against both Gram-positive and Gram-negative bacteria [
26]. Two types of sequences were selected, either sequences where the cationic residues and tryptophan are alternating or a “clumped” sequence where the cationic residues are adjacent in the sequence and the tryptophan residues likewise. In addition, we have included a fifth peptide with a similar general structure – a cyclohexapeptide which proved to be both a very poor antimicrobial and lipid binder (
Figure 1).
Cyclic hexapeptides tend to adopt conformations where two opposing β-turns link two short antiparallel β-strands. Using the Dunitz-Waser concept [
27], there are two gross variants of the cyclic hexapeptide; One resembling the chair conformation of cyclohexane through the twisted βI turns, the other resembling the boat conformation of cyclohexane due to the presence of the flatter βII turns (
Figure 1B). An alternating sequence c(WR)3 or c(WK)3 would, due to the internal symmetry, give rise to only one structure within each gross conformation, whereas the clumped sequences, c(WWWRRR) and c(WWWKKK), would possibly form two different structures within each gross conformational category. section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn.
3.2. Antimicrobial activity of peptides
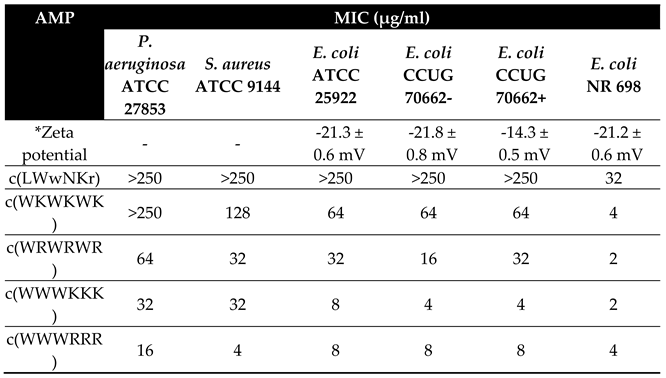
The antimicrobial activity of the five cyclic cationic AMPs was determined against
S. aureus, P. aeruginosa and three strains of
E. coli: ATCC 25922, LPS colistin resistant strain CCUG 70662 and LPS deficient strain NR 698 (
Table 1). CCUG 70662 was cultivated in two conditions, both in the absence (-), and in the presence (+) of colistin (4 µg/ml). Resistance development can be monitored by measuring changes in the ζ-potential, reflecting the overall charge of the bacteria. Indeed, cultivating CCUG 70662 in the presence of colistin caused a decrease in the ζ-potential [
33]. This change is due to the addition of phosphoethanolamine to the lipid A moiety of LPS, reducing the overall negative charge of the LPS [
33,
38]. In the NR 698 strain, translocation of LPS from the periplasmic space to the outer leaflet is hindered. This increases the susceptibility of the bacteria to bile salts, chlorobiphenyl-vancomycin, vancomycin, bacitracin, novobiocin, rifampicin and erythromycin, amongst others, suggesting that the poor LPS translocation results in a deficient outer membrane [
39]. The most active AMPs were those with clumped residues, c(WWWRRR) and c(WWWKKK), followed by the peptides with alternating sequences, c(WRWRWR) and c(WKWKWK). Subsequently, the AMP c(LWwNKr) was included as a negative control as it showed no detectable antimicrobial activity on
E. coli strains except the NR 698 strain and had only weak affinity towards lipid membranes during the preliminary experiments.
One important observation is that the colistin resistant strain CCUG 70662 is still susceptible towards the tested hexapeptides even after cultivation with colistin which induced modification of lipid A. Colistin has been shown to bind LPS and displace divalent cations from lipid A. This will increase fluidity of LPS and causes major disruption of outer membrane and allow for colistin to reach the periplasmic space [
40,
41]. The following bacterial toxicity is not fully explained as outer membrane integrity is not crucial for bacteria survival. Currently, there are three major theories on colistin antimicrobial activity: direct disruption of the inner membrane, vesicle-vesicle contact pathways or targeting of lipid A in the periplasmic space [
40,
41,
42]. Regardless, these theories lead to the final disruption of inner membrane integrity and subsequent cell death. No change in activity against CCUG 70662+ with modified lipid A, suggests that the mode of action of the tested hexapeptides is not directly related to lipid A binding. Therefore, following these MIC results we have utilised surface plasmon resonance experiments to directly measure the compounds ability to translocate into lipid bilayers.
3.3. SPR and choice of lipid models
SPR is emerging as the method of choice for measuring partitioning of small compounds into lipid bilayers [
32,
43,
44]. In SPR, liposomes are immobilized on the surface of the SPR chip and AMPs are injected over the lipid layer at increasing concentrations. The measured change in the response of the plasmon wave allows for the determination of both the partitioning, K
P, which is defined as concentration of peptide in the lipid bilayer over the concentration of peptide in water ([AMPs] in lipid/[AMPs] in water), and the dissociation rate k
off [
32]. In addition, the dissociation constant (K
D – Supplementary) can be estimated from the steady state data using a generalised binding model. From the estimated K
D and the measured k
off values, the association rate k
on can be calculated (
Eq. 5). For the lipid models, DMPC and DMPC:DMPG (95:5) liposomes, as well as DMPC liposomes containing 10% LPS (isolates from
E. coli O111:B4) were used. The rationale behind the selection of models is their representation of key membranes. The DMPC liposomes possess a neutral membrane, akin to a eucaryotic membrane, and a useful probe of the lipophilic nature of the AMPs in the membrane interaction. The presence of 5% DMPG in the DMPC:DMPG liposomes provides a net anionic lipid surface, more similar to a procaryotic membrane. Finally, the LPS containing liposomes are used to model the outer membrane of Gram-negative bacteria, with LPS being a unique and abundant component of the Gram-negative outer membrane.
3.4. Antimicrobial activity broadly correlates with KP for DMPC liposomes
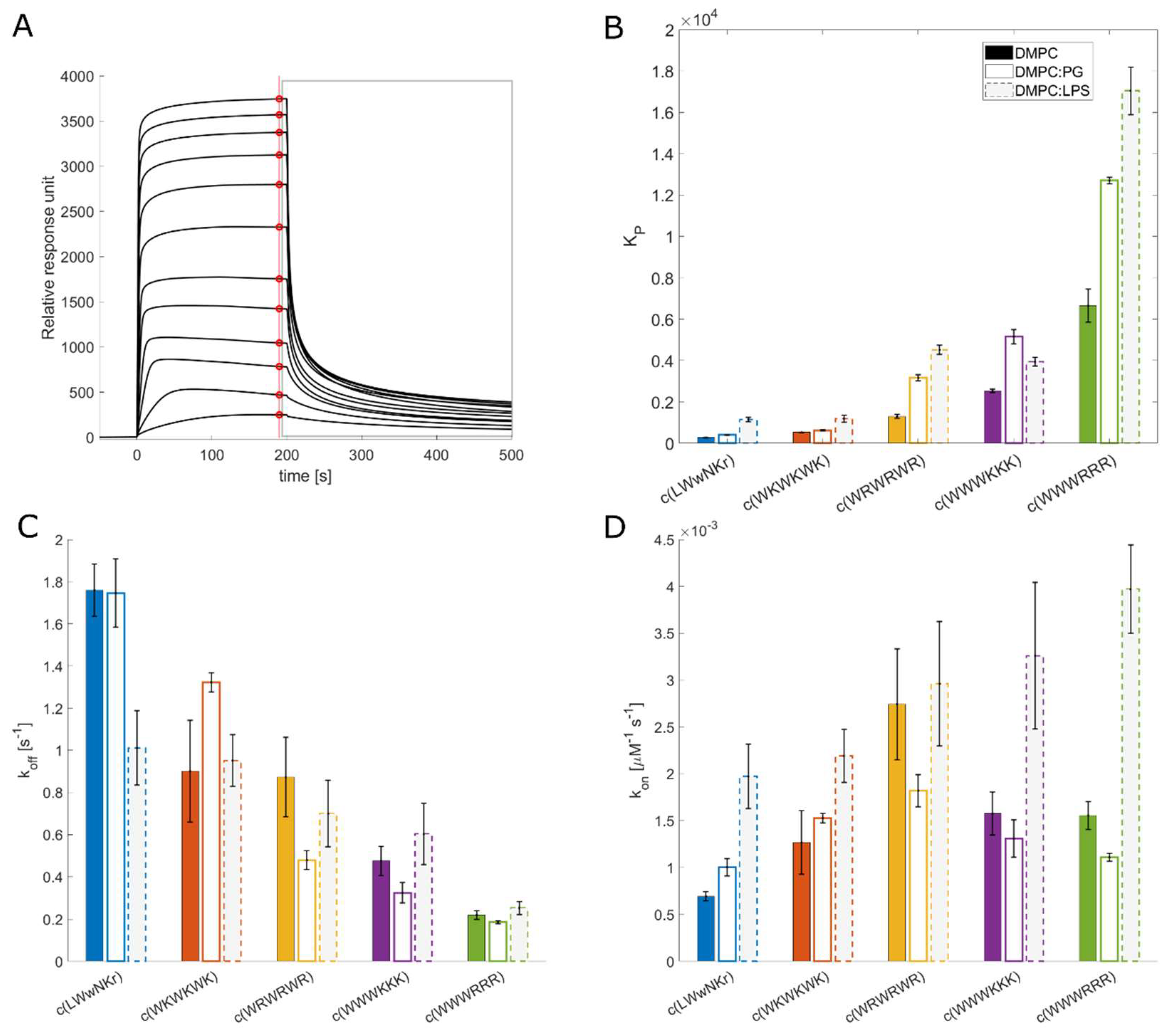
The measured K
P for the different peptide and lipid membrane combinations spans a wide range of values covering three orders of magnitude (
Figure 2). c(WWWRRR) strongly partitions into the DMPC lipid bilayer, with the highest K
P of the series - 6649, whereas the negative control peptide, c(LWwNKr), showed very poor preference for DMPC with a K
P of 278. Introducing 5% of a negatively charged component (DMPG) to the liposomes doubled the K
P of all peptides except c(WKWKWK), where the increase was less substantial.
The antimicrobial activity against Gram-positive
S. aureus closely follows the K
P in DMPC liposomes. However, the trend for MIC against Gram-negative bacteria was less clear. The K
P is calculated from steady-state conditions, so the value corresponds to the sum of possible interaction states including “coating” of surface membrane, self-aggregation and any penetration. This sum of values does not correspond closely with antibacterial activity against Gram-negative bacteria. This could be due to hindrance of peptide translocation through LPS loaded outer-membrane of Gram-negatives [
45]. Despite this, K
P can still be used for activity ranging against Gram-negatives, as the most active peptides have K
P values up to one order of magnitude higher than the inactive peptides.
3.5. The initial peptide:membrane association is affected by LPS
Both c(WWWRRR) and c(WWWKKK) display significantly different K
P and k
off values when measured against DMPC and DMPC:PG liposomes, a finding that was unexpected due to their similar MIC against
E. coli. As such, further explorations of the model space were warranted to achieve a better model for the Gram-negative. To this end 10% LPS was incorporated to the DMPC liposomes. LPS is a crucial component of the outer membrane of Gram-negative bacteria and is absent in Gram-positive bacteria. The ketodeoxyoctanic acid component in LPS confers an anionic charge to the DMPC:LPS construct [
46]. We have previously observed that the binding efficacy of the peptides was significantly enhanced by the introduction of negatively charged head groups, pointing to the importance of negative charge in the membrane for binding the cationic peptides. When 10% LPS was introduced to the DMPC membranes, an enhanced binding of the AMPs to the DMPC:LPS construct was observed - similar to the introduction of DMPG to the DMPC membranes.
All active peptides demonstrated an increase in KP to the DMPC:LPS membranes compared to DMPC. A greater increase in binding to the LPS-membrane relative to the negatively charged DMPC:PG membranes was also generally observed. However, two noteworthy exceptions can be identified. Firstly, c(LWwNKr) sees a four-fold increase in KP compared to the DMPC and DMPC:PG constructs, suggesting a strong preference for the LPS. Secondly, although c(WWWKKK), sees an enhanced KP when LPS is present, this effect is less pronounced than in DMPG:DMPC membranes. This indicates that the anionic charge of the lipid head group is a greater driver of the interaction between the AMP and the lipid bilayer than LPS. The presence of LPS has no effect on koff for any of the active peptides. Instead, LPS significantly increases their association rate kon. This is coherent with the proposal that LPS is a driving force for the initial interactions of AMPs, but that it is not the final destination, this remains the lipid bilayer. Hence, the introduction of LPS in the bilayer boosts kon while koff remains largely unchanged.
LPS forms a unique combination of two barriers: a hydrophilic barrier is provided by a densely packed oligosaccharide core, and a hydrophobic barrier is provided by the hydrocarbon chain region of lipid A buried in the phospholipid membrane [
47]. During the initial interaction between AMPs and LPS, the oligosaccharides can hinder the cationic AMPs and slow their localization into the membrane. This has been shown previously with the linear peptide K
5L
7, where its association with LPS prevents it from reaching its target reducing its activity [
21]. Meanwhile, its diastereomer 4D-K
5L
7, which can penetrate LPS has higher activity. This modification of peptide configuration changes its binding mode with LPS without affecting the lipophilicity of the peptide and renders one peptide active and the other inactive. The difference in K
P for the clumped c(WWWRRR) and c(WWWKKK) is not reflected in their antimicrobial activity and can be interpreted as a consequence of the same LPS-induced phenomenon. In the presence of LPS a much stronger association of c(WWWRRR) than c(WWWKKK) to the DMPC:LPS liposomes can be observed. If the activity was to follow affinity directly, c(WWWRRR) would be expected to be the most active of the two, however, both peptides have similar MIC values across all strains. This suggests that the antimicrobial activity of c(WWWRRR) is hindered by LPS association; that is to say, it is bound too tightly and strays out of the goldilocks zone of LPS binding. The loss of activity in the presence of LPS can also be observed on the less lipophilic peptides (with lower K
P) c(LWwNKr), c(WKWKWK), and c(WRWRWR) as the MIC of these peptides is improved against NR-698. This strain has deficient LPS transport to the outer membrane, and as a result there is a lower abundance of LPS in the outer membrane, which are replaced by phospholipids [
39].
The SPR results taken as a whole suggest that peptides with a good combination of hydrophobicity and hydrophilicity, translating into a beneficial combination of k
on and k
off rates, will pass the LPS and outer membrane unperturbed reaching their antimicrobial target - either the inner membrane or an intracellular binding partner. From the tested peptides, the best combination of these rates is observed for peptide c(WWWKKK) – high k
on towards LPS allows efficient entry, but relatively high k
off allows for translocation towards the inner bilayer and prevents peptide sequestration in the outer membrane. Indeed, of the tested AMPs, c(WWWKKK) seems to have k
off and k
on rates within the most efficient combined range, following the Goldilocks principle. However, lysine is known to be generally less destructive towards lipid bilayers [
17].
3.6. NMR Line shape analysis can be used to probe binding strength of AMPs towards lipid liposomes
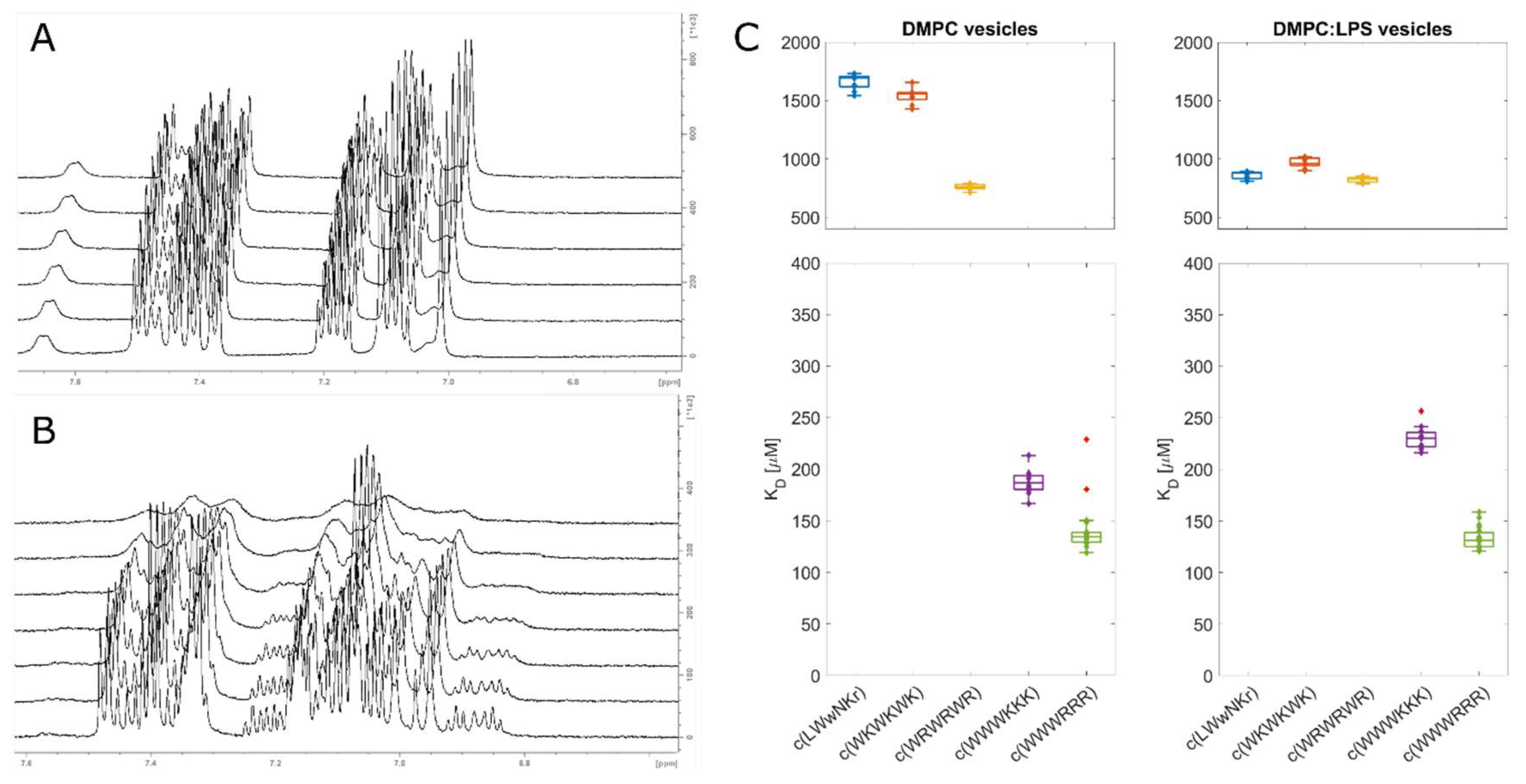
In addition to SPR, 1D
1H line shape NMR analysis was performed by titration of lipid liposomes into a fixed concentration of peptide. The size of vesicle/AMP complex will result in line broadening and signal loss of the monitored compound with increasing concentration of liposomes. This loss of the signal can be quantified and the dissociation constant, K
D, can be estimated [
36,
48]. This method has previously been used to monitor the kinetics of enzymes and various inhibitors [
49], to monitor protein binding to different lipid models [
30] or in the qualitative assessment of AMP binding towards bacterial cells [
50]. However, it is important to note that signal attenuation and line broadening are caused by relaxation, sample inhomogeneity as well as contributions from chemical exchange in the NMR time scale, which can vary for different molecules, different interactions and different concentration ranges. Nevertheless, SPR showed that all five tested peptides have relatively slow off-rates, k
off< 10 s
-1 [
51]. If we assume that the off-rates for free liposomes in solution are comparable to those of immobilized and partially fused liposomes to the SPR chips [
32], then the contributions from on/off exchange processes are not expected to significantly affect the effective relaxation, and the major source of attenuation is fast relaxation caused by the slow correlation time of the peptides in complex with the lipid bilayer or LPS (
Figure 3).
The results from the line shape analysis can be used to rate the peptides in the same order as SPR from highest to lowest affinity: c(WWWRRR)> c(WWWKKK)≫ c(WRWRWR)> c(WKWKWK)> c(LWwNKr). When LPS was present, the c(LWwNKr) and c(WKWKWK) peptides were most affected by the change with a notable decrease in KD. The most efficiently bound peptides, c(WWWRRR)> c(WWWKKK), were not affected by the LPS addition. The NMR line shape analysis data follows the same general qualitative trend of the SPR with minor quantitative differences which could be caused by the different states of the lipid bilayer between the NMR and SPR experiments. Nevertheless, line shape analysis offers simple, alternative method to explore peptide binding towards model lipid membranes.
3.7. Live cell NMR highlights strain differences
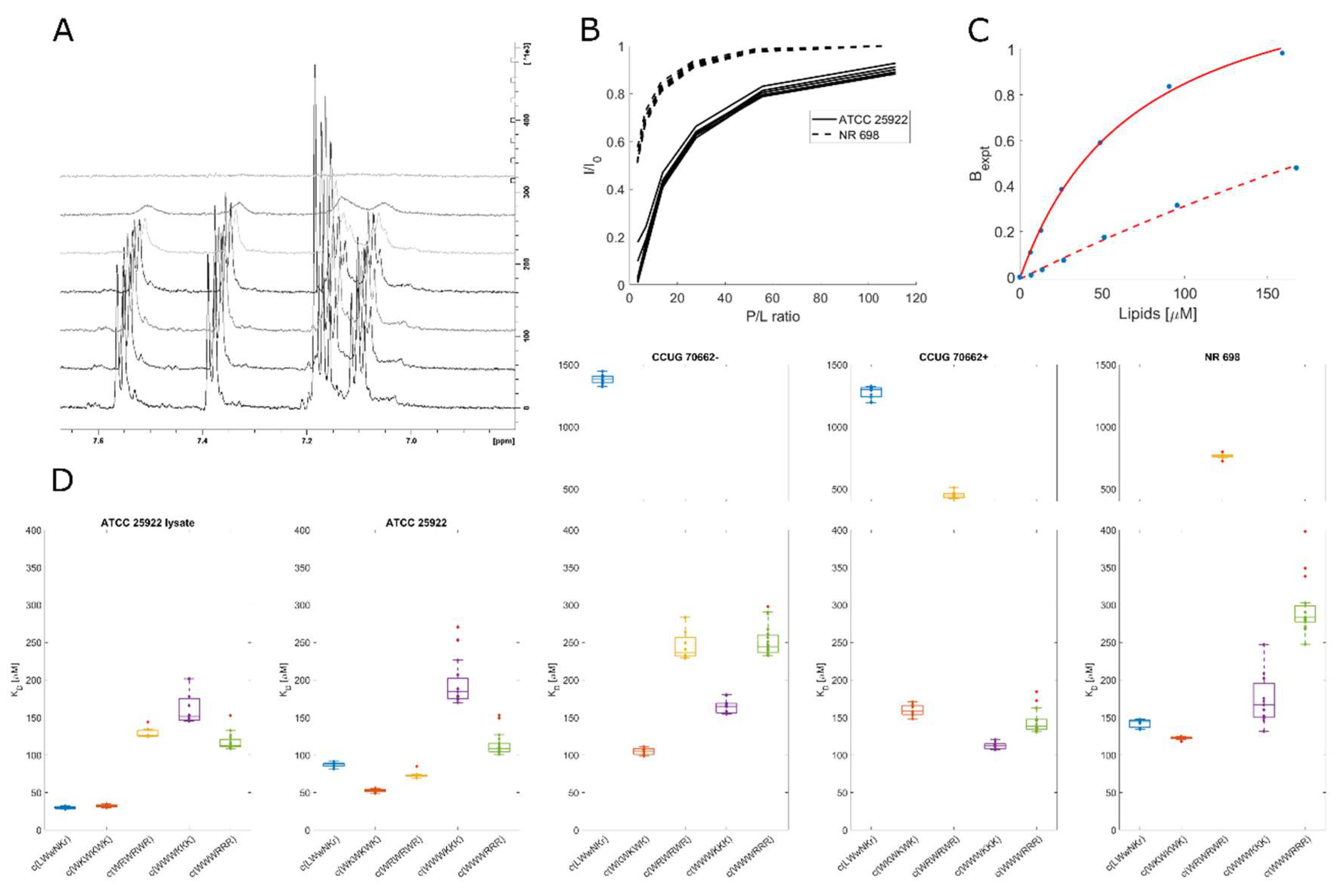
While binding of the AMPs is a necessity to achieve bacterial killing, it may neither be sufficient to explain the differences between the activities of individual compounds, nor the main factor for the mode of action. We aimed to expand the NMR toolbox to include live cells with the aim to connect observed lipid partitioning, MIC activities and mode of action. To our knowledge, this is the first time the live cell NMR approach has been used to quantify peptide:bacterial cell binding.
For the live cell NMR the same
E. coli strains as for the MIC testing (see above) were used. In addition, the ATCC 25922 cell lysate was used to observe the effect the release of intracellular components has on peptide binding. To measure the initial AMP:bacterial cell binding, we titrated an increasing number of cells into a known and fixed concentration of AMP and monitored the signal attenuation of the peptide
(Figure 4A-B). From this, we have extracted the K
D for each AMPs towards the bacteria using the method developed by Shortridge
et al. (2008) [
36] (
Figure 4C), which allows us to estimate the binding affinity based on signal broadening (see Method section). There were major affinity differences for individual strains tested, as summarised in
Figure 4D.
3.8. Inactive peptide c(LWwNKr) binds strongly ATCC 25922 and NR 698
No direct correlation between the live cell affinity and MIC activity is seen in the data. Furthermore, there were significant differences between the different E. coli cell lines, highlighting AMP:strain specificity. Accordingly, conservative interpretation of the differences of binding affinity between different strains is called for. However, interesting observations of the differences in AMP binding within each individual strains exists. For example, the SPR results showed that peptide c(LWwNKr) has poor partitioning and retention in a lipid bilayer, which is only slightly improved by the presence of LPS. Now, the markedly different affinity of c(LWwNKr) to different strains suggests that this peptide can interact with an unknown component (possibly membrane protein) in the outer part of the bacterium, different than phospholipids and LPS, which is more abundant in ATCC 25922 and NR 698 and less present or mutated in CCUG 70662. In addition, the KD is decreased for the lysate ATCC 25922 sample, suggesting non-specific binding with intracellular components (larger surface area) or increased availability of alternate binding sites. Still, binding of the c(LWwNKr) peptide provides no or very inefficient antimicrobial effect as shown by its high MIC values.
3.9. Active peptides are less affected by changes in membrane composition
There is no significant change in K
D for c(WWWRRR), c(WWWKKK), and c(WKWKWK) between the lysate and live cell ATCC 25922. This suggests that these peptides are able to interact with their target in intact cells and are not affected by the presence of free intracellular components. Meanwhile, with c(WRWRWR) there is a two-fold increase in K
D (a decrease in affinity after sonication). This may be caused by the partial disintegration of LPS molecules by sonication [
52], and so eliminating the major binding site of c(WRWRWR).
The preference of c(WRWRWR) towards LPS can also be seen from a decreased affinity towards the activated CCUG 70662+, where lipid A is modified, compared to the native CCUG 70662-, highlighting the impact of the charged component of LPS on the binding of c(WRWRWR). The decrease in affinity may also be accompanied by a decrease in antimicrobial activity, with a single titre increase in MIC against CCUG 70662+ (
Table 1). Taken together, this may indicate that c(WRWRWR) derives its activity by binding to the lipid A component of LPS. An increase in K
D is also observed in c(WKWKWK) when lipid A is modified, though this is less pronounced in comparison to c(WRWRWR). In opposition to this trend, both clumped peptides had decreased K
D when lipid A was modified. These results point to the main driving force of clumped peptides being hydrophobic interactions, while the alternating peptides are driven by charge. The SPR results support the observation that arginine is a more proficient LPS binder than lysine, suggesting that arginine containing peptides should be most affected by the LPS modification. c(WWWRRR) is able to compensate for the reduced LPS affinity with increased hydrophobicity, which allows the peptide to efficiently partition into the lipid bilayer. However, this advantage is lost in the alternating peptide c(WRWRWR) and this is evidenced by the large increase in K
D towards the modified LPS strain.
Due to strain differences, it is not possible to compare ATCC 25922 and NR 698 directly. However, c(WRWRWR) demonstrated K
D differences between the two strains that were not evident for the other peptides. For ATCC 25922 the K
D of c(WRWRWR) is in a similar range to the other peptides (~50-200 µM) and for NR 698 it moves beyond 500 µM. This could be explained by the decrease of LPS in the outer membrane of NR 698. The notion that c(WRWRWR) binds lipid A may be further strengthened by the fact that there is reduced affinity towards the NR-698 strain, as the reduced transport of LPS to the outer membrane results in lower availability of lipid A [
39].
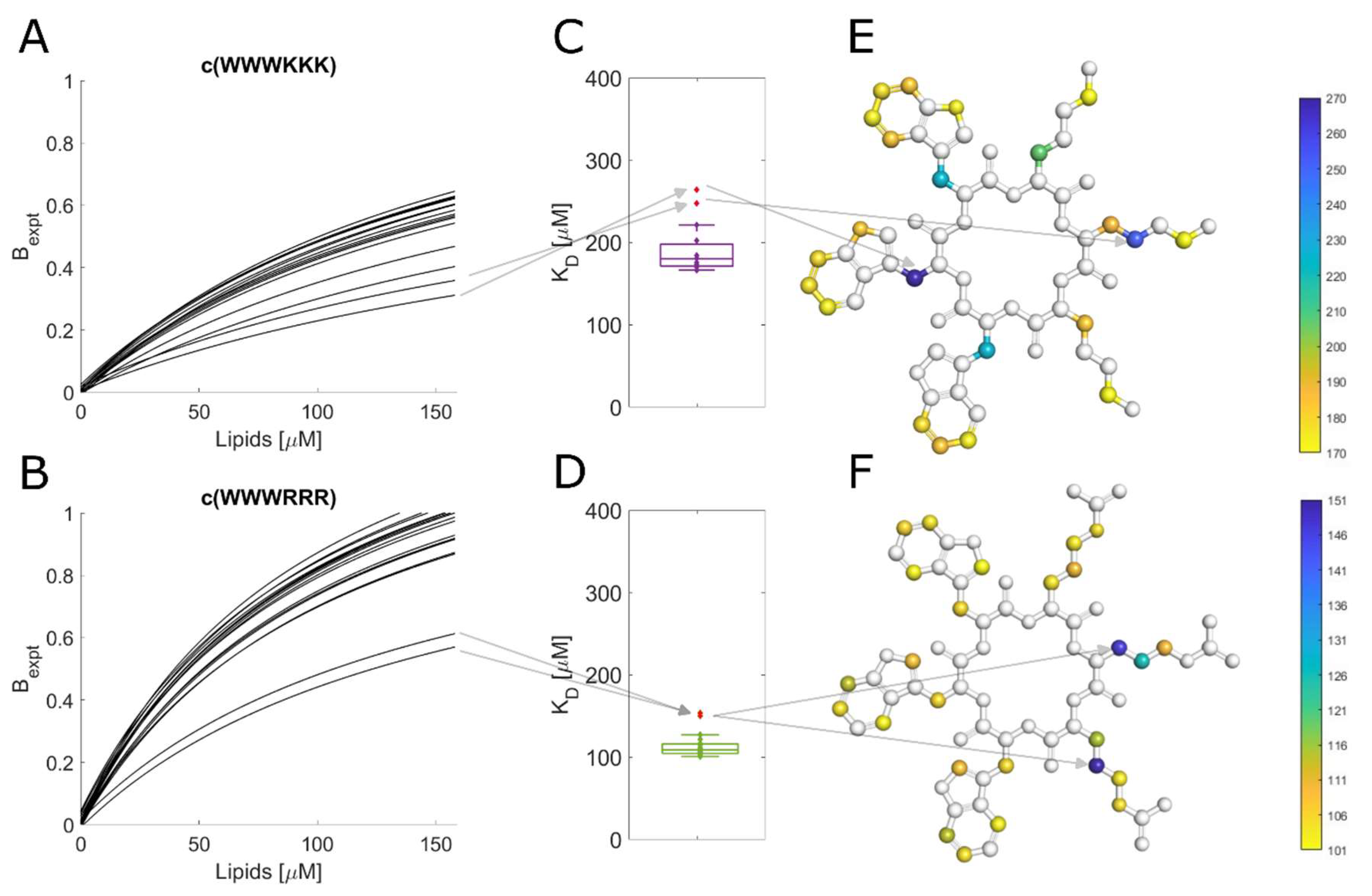
3.10. Different binding modes of hexapeptides towards ATCC 25922
The protons of most of the tested peptides experienced similar attenuation through the whole peptide, this led to uniform K
D values, as shown in
Figure 5D. This is especially true for peptides with alternating sequence where due to their molecule symmetry we can only observe average proton signal for dipeptides units (WR or WK). However, some combinations of the clumped peptides and strains showed outliers for specific protons. These outliers suggest that there is a shift in the average binding of the peptide i.e., a preference for a particular orientation/conformation of the peptide or change in internal dynamics of the ring. A breakdown of these outliers for binding towards ATCC 25922 is in
Figure 5. For c(WWWKKK) the highest K
D was observed for the protons on Cβ of the three tryptophan residues (
Figure 5E). Meanwhile, the highest K
D for c(WWWRRR) was localized on Cβ of Arg2 and Cγ of Arg3 (
Figure 5F). This shows that, on average, there is a markedly different type of interaction between these two peptides and the bacteria. This deviation could be due to either structural differences or in different interaction dynamics of the peptides – suggesting difference in binding modes.
Arginine and lysine are both cationic residues with very similar properties. They are both basic amino acids with similar pK
A in an aqueous environment (∼12.5 for Arg [
53] and ∼10.5 for Lys [
54]), which enables them to be charged in most physiological circumstances. It has been shown that these amino acids can be, to some extent, exchangeable without affecting the peptide's secondary structure [
15,
55]. However, it has also been shown that Arg is more lipophilic as poly-Arg can cross cells more efficiently than oligomers of Lys [
56]. In fact, the substitution of Arg for Lys has been shown to decrease the translocation efficiency of host defensive peptides [
57,
58]. Li
et al. (2013) [
17], showed that this enhanced lipophilicity could be explained by Arg ability to maintain its diffused charge even in the bilayer, unlike Lys which seems to cross the bilayer deprotonated, or by the ability of Arg to form hydrogen bonds and cation-π interaction at the same time [
18].
This difference in the nature of the arginine and lysine interactions with the lipid bilayer could explain some differences in signal attenuation observe in the live cell NMR. Where the poorer affinity of Lys will cause c(WWWKKK) to bind with preferably sidechains, meanwhile c(WWWRRR) will quickly orient itself in the bilayer, burying its hydrophobic groups with the Arg chains available for exchange, closer to the lipid headgroups; this will lead to the higher disruption effect of arginine. Arg has a strong killing efficacy, however it can be sequestrated in the LPS layer [
59]. Opposingly, Lys is able to reach the bilayer unhindered, though it is not as efficient in its disruption. The combination of these effects could lead to similar antimicrobial activity of c(WWWKKK) and c(WWWRRR).
4. Conclusions
Using lipid model systems, we have observed a correlation of AMPs amino acid composition and its binding towards DMPC liposomes, and its correlation of lipid affinity with antimicrobial activity. Arginine has shown to be a more efficient lipid binder than lysine (c(WWWRRR) > c(WWWKKK)), and the clumped hydrophobic bulk of peptides formed by tryptophan showed to be more efficient than alternating sequences (c(WWWRRR) > c(WRWRWR)). This trend was further enhanced when we introduced 5% of DMPG in liposomes, highlighting the increased selectivity of cationic peptides towards anionic membranes.
However, when moving from these simple models to more complex bacterial membranes, the complexity of interactions radically increases, and the simple correlation of binding = activity is lost. The presence of only one additional component, LPS, shows drastic changes in AMPs rating, partially explaining why peptides c(WWWRRR) and c(WWWKKK) have different lipid partitioning but the same MIC for Gram-negative bacteria; LPS efficiently binds to c(WWWRRR), lowering its potential.
Nevertheless, it is within live cells where activity takes place, and these environments should be explored further. Live cell NMR (line shape analysis on live bacterial cells) could help bridge this gap and offer a simple tool to measure complex interaction processes. It has already helped to identify several potential binding partners, such as c(WRWRWR) and lipid A binding or c(LWwNKr) and protein interactions. Further investigations are required to fully understand the mode of action of these AMPs, but the identified targets provide a starting point.
Supplementary Materials
Figure S1: K
D extracted from steady state SPR analysis; Figure S2: The five peptides with numbered amino acids for structure elucidation; Figure S3: Labelled amino acids. Table S1: K
P and k
off values used in
Figure 1; Table S2: γB/γF ratios; Table S3: Full assignment of c(WWWKKK); Table S4: Full assignment of c(WKWKWK); Table S5: Full assignment of c(WRWRWR); Table S6: Full assignment of c(WWWRRR); Table S7: Full assignment of c(LWwNKr) .
Author Contributions
MJ conceived the research questions and designed the experiments. MS, TK and JSMS established peptide synthesis. MJ and MB collected and analysed the SPR data. MJ, FGR, PR and JMI collected and analysed the NMR data. ER and JUE obtained bacterial strains and collected MIC values. MJ, FGR and PR prepared figures. JUE, JMI and JSMS conceived, designed and wrote original the grant proposal and obtained funding. All authors interpreted data and commented on the final version of the manuscript.
Funding
This project received funding from the DigiBiotics project (ID 269425) and by the nanoAMP project (ID 275186), both granted by the Research Council of Norway. The APC was covered by the open access publishing fund, UiT.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All processed data can be found in the paper and supporting information. The scripts utilized for data processing are available at [
31]. Raw data can be obtained upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic Resistance: A Rundown of a Global Crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.W.; Sahl, H.G. Antimicrobial and Host-Defense Peptides as New Anti-Infective Therapeutic Strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Ageitos, J.M.; Sánchez-Pérez, A.; Calo-Mata, P.; Villa, T.G. Antimicrobial Peptides (AMPs): Ancient Compounds That Represent Novel Weapons in the Fight against Bacteria. Biochem. Pharmacol. 2017, 133, 117–138. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M. Antimicrobial Peptides of Multicellular Organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mant, C.T.; Farmer, S.W.; Hancock, R.E.W.; Vasil, M.L.; Hodges, R.S. Rational Design of α-Helical Antimicrobial Peptides with Enhanced Activities and Specificity/Therapeutic Index. J. Biol. Chem. 2005, 280, 12316–12329. [Google Scholar] [CrossRef]
- Dijksteel, G.S.; Ulrich, M.M.W.; Middelkoop, E.; Boekema, B.K.H.L. Review: Lessons Learned From Clinical Trials Using Antimicrobial Peptides (AMPs). Front. Microbiol. 2021, 12. [Google Scholar] [CrossRef]
- Luo, X.; Chen, H.; Song, Y.; Qin, Z.; Xu, L.; He, N.; Tan, Y.; Dessie, W. Advancements, Challenges and Future Perspectives on Peptide-Based Drugs: Focus on Antimicrobial Peptides. Eur. J. Pharm. Sci. 2023, 181, 106363. [Google Scholar] [CrossRef]
- Fjell, C.D.; Hiss, J.A.; Hancock, R.E.W.; Schneider, G. Designing Antimicrobial Peptides: Form Follows Function. Nat. Rev. Drug Discov. 2012, 11, 37–51. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Yan, Z. Bin; Meng, Y.M.; Hong, X.Y.; Shao, G.; Ma, J.J.; Cheng, X.R.; Liu, J.; Kang, J.; Fu, C.Y. Antimicrobial Peptides: Mechanism of Action, Activity and Clinical Potential. Mil. Med. Res. 2021 81 2021, 8, 1–25. [Google Scholar] [CrossRef]
- Wimley, W.C. Describing the Mechanism of Antimicrobial Peptide Action with the Interfacial Activity Model. ACS Chem. Biol. 2010, 5, 905–917. [Google Scholar] [CrossRef]
- Pirtskhalava, M.; Amstrong, A.A.; Grigolava, M.; Chubinidze, M.; Alimbarashvili, E.; Vishnepolsky, B.; Gabrielian, A.; Rosenthal, A.; Hurt, D.E.; Tartakovsky, M. DBAASP v3: Database of Antimicrobial/Cytotoxic Activity and Structure of Peptides as a Resource for Development of New Therapeutics. Nucleic Acids Res. 2021, 49, D288–D297. [Google Scholar] [CrossRef]
- Mishra, B.; Wang, G. The Importance of Amino Acid Composition in Natural Amps: An Evolutional, Structural, and Functional Perspective. Front. Immunol. 2012, 3, 31946. [Google Scholar] [CrossRef] [PubMed]
- You, Y.H.; Liu, H.Y.; Zhu, Y.Z.; Zheng, H. Rational Design of Stapled Antimicrobial Peptides. Amin. Acids 2023 554 2023, 55, 421–442. [Google Scholar] [CrossRef]
- Clark, S.; Jowitt, T.A.; Harris, L.K.; Knight, C.G.; Dobson, C.B. The Lexicon of Antimicrobial Peptides: A Complete Set of Arginine and Tryptophan Sequences. Commun. Biol. 2021 41 2021, 4, 1–14. [Google Scholar] [CrossRef]
- Cutrona, K.J.; Kaufman, B.A.; Figueroa, D.M.; Elmore, D.E. Role of Arginine and Lysine in the Antimicrobial Mechanism of Histone-Derived Antimicrobial Peptides. FEBS Lett. 2015, 589, 3915–3920. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Brady, A.; Young, A.; Rasimick, B.; Chen, K.; Zhou, C.; Kallenbach, N.R. Length Effects in Antimicrobial Peptides of the (RW)n Series. Antimicrob. Agents Chemother. 2007, 51, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Vorobyov, I.; Allen, T.W. The Different Interactions of Lysine and Arginine Side Chains with Lipid Membranes. J. Phys. Chem. B 2013, 117, 11906–11920. [Google Scholar] [CrossRef]
- Chan, D.I.; Prenner, E.J.; Vogel, H.J. Tryptophan- and Arginine-Rich Antimicrobial Peptides: Structures and Mechanisms of Action. Biochim. Biophys. Acta - Biomembr. 2006, 1758, 1184–1202. [Google Scholar] [CrossRef]
- Khemaissa, S.; Walrant, A.; Sagan, S. Tryptophan, More than Just an Interfacial Amino Acid in the Membrane Activity of Cationic Cell-Penetrating and Antimicrobial Peptides. Q. Rev. Biophys. 2022, 55, e10. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Wang, Z. APD3: The Antimicrobial Peptide Database as a Tool for Research and Education. Nucleic Acids Res. 2016, 44, D1087. [Google Scholar] [CrossRef]
- Papo, N.; Shai, Y. A Molecular Mechanism for Lipopolysaccharide Protection of Gram-Negative Bacteria from Antimicrobial Peptides. J. Biol. Chem. 2005, 280, 10378–10387. [Google Scholar] [CrossRef] [PubMed]
- Papo, N.; Oren, Z.; Pag, U.; Sahl, H.G.; Shai, Y. The Consequence of Sequence Alteration of an Amphipathic α-Helical Antimicrobial Peptide and Its Diastereomers. J. Biol. Chem. 2002, 277, 33913–33921. [Google Scholar] [CrossRef] [PubMed]
- Needham, B.D.; Trent, M.S. Fortifying the Barrier: The Impact of Lipid A Remodelling on Bacterial Pathogenesis. Nat. Rev. Microbiol. 2013 117 2013, 11, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Southey, R. The Story of the Three Bears, W. NICOL 60, PALL-MALL. 1839.
- Joo, H.S.; Fu, C.I.; Otto, M. Bacterial Strategies of Resistance to Antimicrobial Peptides. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371. [Google Scholar] [CrossRef]
- Eikås, K.D.R.; Krupová, M.; Kristoffersen, T.; Beerepoot, M.T.P.; Ruud, K. Can the Absolute Configuration of Cyclic Peptides Be Determined with Vibrational Circular Dichroism? Phys. Chem. Chem. Phys. 2023, 25, 14520–14529. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; 2006; ISBN 1562386263. [Google Scholar]
- Torchilin, V.; Weissig, V. Liposomes: A Practical Approach; Oxford University Press: Oxford, 2003; ISBN 0-19-963654-0. [Google Scholar]
- Palusińska-Szysz, M.; Zdybicka-Barabas, A.; Luchowski, R.; Reszczyńska, E.; Śmiałek, J.; Mak, P.; Gruszecki, W.I.; Cytryńska, M. Choline Supplementation Sensitizes Legionella Dumoffii to Galleria Mellonella Apolipophorin III. Int. J. Mol. Sci. 2020, 21, 1–23. [Google Scholar] [CrossRef]
- Jakubec, M.; Bariås, E.; Furse, S.; Govasli, M.L.; George, V.; Turcu, D.; Iashchishyn, I.A.; Morozova-Roche, L.A.; Halskau, Ø. Cholesterol-containing Lipid Nanodiscs Promote an A-synuclein Binding Mode That Accelerates Oligomerization. FEBS J. 2020, febs.15551. [Google Scholar] [CrossRef] [PubMed]
- Jakubec, M. GitHub - Martin Jakubec Available online:. Available online: https://github.com/MarJakubec (accessed on 21 June 2023).
- Figueira, T.N.; Freire, J.M.; Cunha-Santos, C.; Heras, M.; Gonçalves, J.; Moscona, A.; Porotto, M.; Salomé Veiga, A.; Castanho, M.A.R.B. Quantitative Analysis of Molecular Partition towards Lipid Membranes Using Surface Plasmon Resonance. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Li, B.; Yin, F.; Zhao, X.; Guo, Y.; Wang, W.; Wang, P.; Zhu, H.; Yin, Y.; Wang, X. Colistin Resistance Gene Mcr-1 Mediates Cell Permeability and Resistance to Hydrophobic Antibiotics. Front. Microbiol. 2020, 10, 3015. [Google Scholar] [CrossRef]
- Beal, J.; Farny, N.G.; Haddock-Angelli, T.; Selvarajah, V.; Baldwin, G.S.; Buckley-Taylor, R.; Gershater, M.; Kiga, D.; Marken, J.; Sanchania, V.; et al. Robust Estimation of Bacterial Cell Count from Optical Density. Commun. Biol. 2020 31 2020, 3, 1–29. [Google Scholar] [CrossRef]
- Lee, S.; Bayley, H.; Lee, S.; Bayley, H. Reconstruction of the Gram-Negative Bacterial Outer-Membrane Bilayer. Small 2022, 18, 2200007. [Google Scholar] [CrossRef] [PubMed]
- Shortridge, M.D.; Hage, D.S.; Harbison, G.S.; Powers, R. Estimating Protein-Ligand Binding Affinity Using High-Throughput Screening by NMR. J. Comb. Chem. 2008, 10, 948–958. [Google Scholar] [CrossRef] [PubMed]
- Jwad, R.; Weissberger, D.; Hunter, L. Strategies for Fine-Tuning the Conformations of Cyclic Peptides. Chem. Rev. 2020, 120, 9743–9789. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Liang, W.; Li, J.; Chen, Y.; Zhou, D.; Liang, L.; Lin, D.; Li, Y.; Zhao, H.; Du, H.; et al. MCR-1-Dependent Lipid Remodelling Compromises the Viability of Gram-Negative Bacteria. Emerg. Microbes Infect. 2022, 11, 1236–1249. [Google Scholar] [CrossRef]
- Krüger, M.; Richter, P.; Strauch, S.M.; Nasir, A.; Burkovski, A.; Antunes, C.A.; Meißgeier, T.; Schlücker, E.; Schwab, S.; Lebert, M. What an Escherichia Coli Mutant Can Teach Us About the Antibacterial Effect of Chlorophyllin. Microorganisms 2019, 7. [Google Scholar] [CrossRef]
- Aghapour, Z.; Gholizadeh, P.; Ganbarov, K.; Bialvaei, A.Z.; Mahmood, S.S.; Tanomand, A.; Yousefi, M.; Asgharzadeh, M.; Yousefi, B.; Kafil, H.S. <p>Molecular Mechanisms Related to Colistin Resistance in Enterobacteriaceae</P>. Infect. Drug Resist. 2019, 12, 965–975. [Google Scholar] [CrossRef]
- Rhouma, M.; Beaudry, F.; Thériault, W.; Letellier, A. Colistin in Pig Production: Chemistry, Mechanism of Antibacterial Action, Microbial Resistance Emergence, and One Health Perspectives. Front. Microbiol. 2016, 7, 1789. [Google Scholar] [CrossRef]
- Elias, R.; Duarte, A.; Perdigão, J. A Molecular Perspective on Colistin and Klebsiella Pneumoniae: Mode of Action, Resistance Genetics, and Phenotypic Susceptibility. Diagnostics 2021, 11, 1165. [Google Scholar] [CrossRef]
- Juskewitz, E.; Mishchenko, E.; Dubey, V.K.; Jenssen, M.; Jakubec, M.; Rainsford, P.; Isaksson, J.; Andersen, J.H.; Ericson, J.U. Lulworthinone: In Vitro Mode of Action Investigation of an Antibacterial Dimeric Naphthopyrone Isolated from a Marine Fungus. Mar. Drugs 2022, 20, 277. [Google Scholar] [CrossRef]
- Rainsford, P.; Rylandsholm, F.G.; Jakubec, M.; Silk, M.; Juskewitz, E.; Engh, R.A.; Isaksson, J. Label-Free Measurement of Antimicrobial Peptide Interactions with Lipid Vesicles and Nanodiscs Using Microscale Thermophoresis. 2022. [Google Scholar] [CrossRef]
- Malanovic, N.; Lohner, K. Gram-Positive Bacterial Cell Envelopes: The Impact on the Activity of Antimicrobial Peptides. Biochim. Biophys. Acta - Biomembr. 2016, 1858, 936–946. [Google Scholar] [CrossRef]
- Rietschel, E.T.; Kirikae, T.; Schade, F.U.; Mamat, U.; Schmidt, G.; Loppnow, H.; Ulmer, A.J.; Zähringer, U.; Seydel, U.; Padova, F. Di; et al. Bacterial Endotoxin: Molecular Relationships of Structure to Activity and Function. FASEB J. 1994, 8, 217–225. [Google Scholar] [CrossRef]
- Snyder, S.; Kim, D.; McIntosh, T.J. Lipopolysaccharide Bilayer Structure: Effect of Chemotype, Core Mutations, Divalent Cations, and Temperature. Biochemistry 1999, 38, 10758–10767. [Google Scholar] [CrossRef]
- Waudby, C.A.; Alfonso, I. An Introduction to One- and Two-Dimensional Lineshape Analysis of Chemically Exchanging Systems. J. Magn. Reson. Open 2023, 16–17, 100102. [Google Scholar] [CrossRef]
- Luchinat, E.; Barbieri, L.; Cremonini, M.; Nocentini, A.; Supuran, C.T.; Banci, L. Drug Screening in Human Cells by NMR Spectroscopy Allows the Early Assessment of Drug Potency. Angew. Chemie Int. Ed. 2020, 59, 6535–6539. [Google Scholar] [CrossRef]
- Pandit, G.; Biswas, K.; Ghosh, S.; Debnath, S.; Bidkar, A.P.; Satpati, P.; Bhunia, A.; Chatterjee, S. Rationally Designed Antimicrobial Peptides: Insight into the Mechanism of Eleven Residue Peptides against Microbial Infections. Biochim. Biophys. Acta - Biomembr. 2020, 1862, 183177. [Google Scholar] [CrossRef] [PubMed]
- Waudby, C.A.; Ramos, A.; Cabrita, L.D.; Christodoulou, J. Two-Dimensional NMR Lineshape Analysis. Sci. Reports 2016 61 2016, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Komuro, T.; Murai, T.; Kawasaki, H. Effect of Sonication on the Dispersion State of Lipopolysaccharide and Its Pyrogenicity in Rabbits. Chem. Pharm. Bull. 1987, 35, 4946–4952. [Google Scholar] [CrossRef] [PubMed]
- Angyal, S.J.; Warburton, W.K. The Basic Strengths of Methylated Guanidines. J. Chem. Soc. 1951, 2492–2494. [Google Scholar] [CrossRef]
- Harms, M.J.; Schlessman, J.L.; Chimenti, M.S.; Sue, G.R.; Damjanovic´, A.; Damjanovic´, D.; Garci´a, B.; Garci´a-Moreno, G. A Buried Lysine That Titrates with a Normal PKa: Role of Conformational Flexibility at the Protein–Water Interface as a Determinant of PKavalues. Protein Sci. 2008, 17, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Koo, Y.S.; Kim, J.M.; Park, I.Y.; Yu, B.J.; Jang, S.A.; Kim, K.S.; Park, C.B.; Cho, J.H.; Kim, S.C. Structure–Activity Relations of Parasin I, a Histone H2A-Derived Antimicrobial Peptide. Peptides 2008, 29, 1102–1108. [Google Scholar] [CrossRef]
- Mitchell, D.J.; Steinman, L.; Kim, D.T.; Fathman, C.G.; Rothbard, J.B. Polyarginine Enters Cells More Efficiently than Other Polycationic Homopolymers. J. Pept. Res. 2000, 56, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Åmand, H.L.; Fant, K.; Nordén, B.; Esbjörner, E.K. Stimulated Endocytosis in Penetratin Uptake: Effect of Arginine and Lysine. Biochem. Biophys. Res. Commun. 2008, 371, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Zou, G.; De Leeuw, E.; Li, C.; Pazgier, M.; Li, C.; Zeng, P.; Lu, W.Y.; Lubkowski, J.; Lu, W. Toward Understanding the Cationicity of Defensins: ARG AND LYS VERSUS THEIR NONCODED ANALOGS. J. Biol. Chem. 2007, 282, 19653–19665. [Google Scholar] [CrossRef]
- Savini, F.; Loffredo, M.R.; Troiano, C.; Bobone, S.; Malanovic, N.; Eichmann, T.O.; Caprio, L.; Canale, V.C.; Park, Y.; Mangoni, M.L.; et al. Binding of an Antimicrobial Peptide to Bacterial Cells: Interaction with Different Species, Strains and Cellular Components. Biochim. Biophys. Acta - Biomembr. 2020, 1862, 183291. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).